Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of a novel perovskite-carbon aerogel hybrid adsorbent with multiple metal-Lewis active sites for the removal of dyes from water: experimental and DFT studies†
Daryoush
Sanaei
a,
Mohammad Hadi
Dehghani
bc,
Hamidreza
Sharifan
 d,
Monika
Jain
e,
Bahram
Roshan
a,
Javier A.
Arcibar-Orozco
d,
Monika
Jain
e,
Bahram
Roshan
a,
Javier A.
Arcibar-Orozco
 *f and
Vassilis J.
Inglezakis
*f and
Vassilis J.
Inglezakis
 *g
*g
aDepartment of Environmental Health Engineering, Faculty of Public Health and Safety, Shahid Beheshti University of Medical Sciences, Tehran, Iran
bDepartment of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
cInstitute for Environmental Research, Center for Solid Waste Research, Tehran University of Medical Sciences, Tehran, Iran
dDepartment of Natural Science, Albany State University, Georgia, USA
eDepartment of Natural Resource Management, College of Forestry, Banda University of Agriculture and Technology, Banda, India
fResearch Department, CIATEC A.C. Centro de Innovación Aplicada en Tecnologías Competitivas, León, Mexico
gDepartment of Chemical & Process Engineering, University of Strathclyde, Glasgow, UK. E-mail: vasileios.inglezakis@strath.ac.uk
First published on 30th January 2023
Abstract
Mixed perovskites have vast industrial potential, but some challenges (i.e., aggregation and chemical instability) limit their applications. Herein, a novel environment-friendly carbon aerogel (CAg) synthesized from sodium alginate (SA) was used as a precursor to create a double-B-site perovskite/carbon aerogel hybrid adsorbent ((Sr0.7Mn0.3Co0.5Fe0.5O3−δ)/CAg) (DB-perovskite/CAg hybrid). The adsorbent was extensively characterized via different techniques, including X-ray photoelectron spectroscopy and Fourier transform infrared (FTIR) spectroscopy. The removal efficiency for crystal violet (CV) and acid yellow 17 (AY17) was conducted over various pH, adsorbent/adsorbate dosages, and reaction times in an aqueous system. The maximum adsorbed concentration (Qmax) recorded by 206 mg g−1 and 113 mg g−1 for CV and AY17, respectively, and compared to the performance of only DB-perovskite (114 mg g−1 and 59 mg g−1), respectively. The adsorption site energy distribution was studied by applying the density functional theory (DFT). The adsorption on the DB-perovskite/CAg hybrid was significantly regulated by pH change. The cooperative metal active/Lewis acid sites of the DB-perovskite/CAg adsorbent led to a faster and higher adsorption capacity toward CV and AY 17. The doping of Mn indicated a synergistic effect in improving the adsorption of either dye through the introduction of abundant active sites and strengthening of metal-functional groups (–C–O–C, –COOH, and C–OH/O–H)–π aromatic bonding, confirmed by DFT calculations.
Introduction
The removal of organic dyes from industrial wastewater has been a challenging issue since they are highly toxic, with an annual production rate of 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons.1,2 A variety of industries, such as cosmetics, paint, paper, and textile, release a significant amount of dyes into wastewater annually. Textile industries are the primary source of dye pollution, with over 140
000 metric tons.1,2 A variety of industries, such as cosmetics, paint, paper, and textile, release a significant amount of dyes into wastewater annually. Textile industries are the primary source of dye pollution, with over 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of annual discharged dyes worldwide.3,4 Dye treatment and removal primarily depend on the physiochemical properties of dyes. The two types of dyes that have been frequently reported due to removal challenge, persistency, and high health risk in the aqueous systems are acid yellow 17 (AY 17), a monoazo dye (disodium 2,5-dichloro-4-[5-hydroxy-3-methyl-4-(sulphophenylazo)pyrazol-1-yl] benzenesulphonate), and crystal violet (CV), a triarylmethane dye (hexamethyl pararosaniline chloride).5 Inefficient treatment of dyes from wastewater leads to the build-up of a toxic concentration of organic dyes, which causes respiratory problems, genotoxicity, cytotoxicity, and carcinogenicity.6 Due to the photostability, thermal resistance, and poor biodegradability nature of organic dyes,1 low-cost, renewable, and efficient treatment techniques are in high demand. Conventional wastewater treatment processes often fail to efficiently remove dyes and their by-products from effluents.7 Among the numerous proposed dye removal techniques, adsorption has demonstrated promising applications due to its high potential to remove dyes with different physiochemical properties from an aqueous system.
000 tons of annual discharged dyes worldwide.3,4 Dye treatment and removal primarily depend on the physiochemical properties of dyes. The two types of dyes that have been frequently reported due to removal challenge, persistency, and high health risk in the aqueous systems are acid yellow 17 (AY 17), a monoazo dye (disodium 2,5-dichloro-4-[5-hydroxy-3-methyl-4-(sulphophenylazo)pyrazol-1-yl] benzenesulphonate), and crystal violet (CV), a triarylmethane dye (hexamethyl pararosaniline chloride).5 Inefficient treatment of dyes from wastewater leads to the build-up of a toxic concentration of organic dyes, which causes respiratory problems, genotoxicity, cytotoxicity, and carcinogenicity.6 Due to the photostability, thermal resistance, and poor biodegradability nature of organic dyes,1 low-cost, renewable, and efficient treatment techniques are in high demand. Conventional wastewater treatment processes often fail to efficiently remove dyes and their by-products from effluents.7 Among the numerous proposed dye removal techniques, adsorption has demonstrated promising applications due to its high potential to remove dyes with different physiochemical properties from an aqueous system.
As a class of widespread natural materials, perovskites have attracted considerable attention due to their unique properties, such as abundant active sites, high chemical and thermal durability, and tunable structure. Researchers reported the broad applicability of perovskite nanomaterials from catalysis to the adsorption process due to their excellent optical, magnetic, thermal, and electric characteristics.8,9 The high adsorption capacity and fast kinetics of perovskites make them suitable adsorbent candidates in industrial wastewater bearing high levels of organic dyes.10–12 For instance, Cuijin Pei et al. 2016 showed a remarkable adsorption capacity (7154 mg g−1 in 40 min) for Acid Fuschia (AF) using a 3-dimensional hierarchical ZnO adsorbent.13 Perovskites are ceramic materials with the formula of ABO3, classified as metal oxides. Cationic sites of A and B are commonly alkaline-earth/rare-earth metals and transition metals with partially filled d-orbitals, respectively. The redox reactions usually occur at the B-site, which plays a primary role in pollutant removal. Several researchers reported that these perovskite nanomaterials could be used in many applications from catalysis to adsorption process due to good optical, magnetic, thermal, and electric characteristics.8,9,14 Several synthetic methods have been reported for preparing perovskites that included sol–gel, Pechini, co-precipitation, chelating complex, and pyrolysis.15–18 The perovskites exhibit not only higher adsorption capacity and fast kinetics but also act as suitable adsorbents toward industrial wastewater with high concentration.10–13 Moreover, the perovskites contain a larger number of active sites than that of conventional materials.19–21 However, B-site perovskite suffers from slow electron transfer, low electrical conductivity, and poor and unstable active sites. Therefore, engineering modification of B-sites with appropriate substitution can enhance perovskite adsorption capacity. For example, the B-site of cobalt-doped perovskite oxide (BaCoxNi1−xO3−δ) significantly increased the oxygen adsorption capacity by 49.5 mg g−1.22 H. Sun et al. 2017 reported outstanding oxygen evolution reaction (OER) electrocatalyst of a double-B-site perovskite oxide compared with those of single perovskite oxides.23 However, the literature is minimal on dual-B site perovskites as adsorbents for persistent organic pollutants, particularly cationic and anionic dyes.
To optimize the double-B-site performance, a novel integration of the chemical properties of several key elements is of high interest. This study formulated a hybrid adsorbent on carbon-based support to improve the stability and homogenous dispersion of double-B-sites (DB) perovskite. Carbon Aerogel (CAg) with a nanoporous structure provides support with excellent porosity, high specific surface area, and conductivity. Also, it reinforces the overall thermal and mechanical stability of the perovskite. The hybrid would further lead to great treatment efficiency in a cost-effective manner for the purification of effluents/wastewater. CAg has been investigated as an adsorbent for several pollutants such as inorganic (especially metal ions), pharmaceuticals, and various gases; also, its used for the removal of organic dyes has been widely reported (R).
This environment-friendly hybrid adsorbent would prevent the aggregation of nanoparticles owing to high porosity and large surface area. To the best of our knowledge, this is the first report on the synthesis of DB-perovskite/CAg hybrid (Sr0.7Mn0.3Co0.5Fe0.5O3−δ/CAg). This study aims to evaluate the efficiency, application, and durability of a DB-perovskite/CAg hybrid for the removal of two commercial dyes of CV and AY 17 from an aqueous system. The relationship between the functionality and composition of the DB-perovskite/CAg was investigated through extensive material characterization such as X-ray photoelectron spectroscopy (XPS) and density functional theory (DFT) calculations. The designed hybrid adsorbent exhibits the advantage of single perovskite with mixed oxidation states of Fe, Co, and Mn, and those of the double B-site perovskite with arranged and multi-active sites for enhancing the adsorption of CV and AY17 molecules in aqueous solution. This study provides a baseline for applying novel hybrid perovskites and removing dyes with various physiochemical properties.
Materials and methods
Reagents
Iron(III) nitrate nano hydrate (Fe(NO3)3·9H2O, ACS reagent ≥98%), manganese(II) nitrate tetrahydrate (Mn(NO3)2·4H2O, ≥97.0% (KT)), strontium nitrate (Sr(NO3)2, 99.995% trace metals basis), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, ACS reagent, ≥98%), citric acid (C6H8O7), and ethylenediaminetetraacetic acid (EDTA) were purchased from Fisher Scientific Company, USA. Formaldehyde solution (HCOH, 37%) and resorcinol (C6H4(OH)2, 99.9%) were obtained from Merck Millipore. Crystal violet (C25H30ClN3, C.I. 42555), Acid Yellow 17 (C16H10Cl2N4Na2O7S2, dye content 60%), and sodium alginate were supplied by Sigma Aldrich, USA. Anhydrous calcium chloride was bought from Aladdin Chemical Reagent Co. Ltd (Shanghai, China).Preparation of environment-friendly carbon aerogel (CAg)
To prepare the carbon aerogel (CAg), 1 g sodium alginate (SA) was added to 50 mL distilled H2O to produce a viscous solution, followed by stirring at 45 °C for 30 min. The SA solution was slowly dropped into 200 mL 2.5 wt%. CaCl2 solution under magnetic stirring using a 5 mL syringe. After 1 h, the dispersed granular hydrogel was transferred to a 0.2% CaCl2 solution to promote solidification. The resulting SA hydrogel was washed with distilled water and 96% ethanol to remove any impurities and unbound CaCl2 from the surface. Subsequently, the prepared hydrogel was frozen in a −80 °C for 2 h. To form SA aerogel from SA hydrogel, the vacuum freeze-dryer technology (FD-1-50, Boyikang Laboratory Apparatus Co., Ltd, Beijing, China) was used. The resulting aerogel was heated in a tube furnace (GSL-1700X, MTI Corporation, USA) for 30 min at 800 °C under nitrogen atmosphere with a 10 °C min−1 as the heating rate to form the SA-based carbon aerogel.Preparation of double-B-site perovskite
The double-B-site perovskite (Sr0.7Mn0.3Co0.5Fe0.5O3−δ) was synthesized by the modified Pechini method known as the EDTA-CA-complexing sol–gel.24–26 Briefly, 3.7 g Sr, 1.88 g Mn, 5.05 g Fe, and 3.63 g Co were dissolved in 25 mL deionized water. Next, citric acid and ethylene diamine tetra-acetic acid (EDTA) as the complexing agents were added to the solution with a molar ratio of citric acid/EDTA/total metal ions of 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. pH was adjusted to 6.5 by the dropwise addition of ammonia solution (25 wt%). The mixed solution was vigorously shaken at 95 °C for 3 h (Heidolph Instruments, Unimax 2010) to form a transparent gel that was dried in an oven overnight. The dried gel was thermally treated in two steps first, pre-calcination at 450 °C for 8 h, and calcination at 800 °C for 7 h. The double-B-site perovskite (Sr0.7Mn0.3Co0.5Fe0.5O3−δ) was kept in a desiccator for further experiments and characterization. The flow diagram of preparation is depicted in the (ESI†).
1. pH was adjusted to 6.5 by the dropwise addition of ammonia solution (25 wt%). The mixed solution was vigorously shaken at 95 °C for 3 h (Heidolph Instruments, Unimax 2010) to form a transparent gel that was dried in an oven overnight. The dried gel was thermally treated in two steps first, pre-calcination at 450 °C for 8 h, and calcination at 800 °C for 7 h. The double-B-site perovskite (Sr0.7Mn0.3Co0.5Fe0.5O3−δ) was kept in a desiccator for further experiments and characterization. The flow diagram of preparation is depicted in the (ESI†).
Preparation of the DB-perovskite/CAg hybrid
For DB-perovskite/CAg hybrid synthesis, 1.22 g CAg and 0.5 g perovskite were mixed for 30 min. Then, 140 mL sodium hydroxide (1.5 M) was added to the prepared solution for 60 min under vigorous stirring at room temperature for 24 h. After that, 100 mL sodium hydroxide (3 M) was added to the solution for another 4 h. The obtained DB-perovskite (Sr0.7Mn0.3Co0.5Fe0.5O3−δ)/CAg was vacuum filtered and washed several times with distilled water to achieve the pH of 7–8 and dried in an oven overnight. The resulting powder was heated in a tube furnace (GSL-1700X, MTI Corporation, USA) for 1 h at 800 °C under a heating rate of 10 °C min−1 at a nitrogen flow of 120 cm3 min−1.Characterization of the perovskite
The perovskite was extensively characterized by X-ray photoelectron spectroscopy (XPS) using an Omicron ESCA 250 (Scienta Omicron GmbH, Germany) using monochromatic Al Kα (1486.6 eV). The composition was analyzed at 225 W, passing an energy of 100 eV (survey scans) and 50 eV (specific regions). A Gaussian function fitted the peaks using the Origin software 2017 (OriginLab, Northampton, MA). In addition, FT-IR spectroscopy quantified the bonding arrangements within 4000 to 400 cm−1 (Thermo Scientific; model Nicolet iS10). The N2 adsorption–desorption isotherms were applied to estimate the specific surface area (SBET), total pore volume (Vt), and pore size distributions (ASAP 2020 M, Micromeritics Inc., USA).Experimental design
The adsorption of two cationic dyes of crystal violet (CV) and acid yellow 17 (AY17) onto the DB-perovskite/CAg hybrid was performed in batch reactors. Briefly, different doses of the adsorbent (0.005 g to 0.05 g for CV and 0.02 g to 0.14 g for AY17) were added in Erlenmeyer flasks containing different concentrations of CV and AY17 (5 to 40 mg L−1) in duplicate. In each series of experiments, a control was used without the addition of the adsorbent (only dye). The flasks were shaken at 180 rpm for 1 h. Right after this, the adsorbent in each flask was separated by a membrane filter syringe (0.45 microns), and the concentration of dyes in the supernatant was measured by a UV-Vis spectrophotometer (λmax = 590 nm for CV and λmax = 402 nm for AY17). The adsorption capacity of CV and AY17 was calculated using eqn (1).27–29 | (1) |
 | (2) |
Results and discussion
Structural and morphological characterization
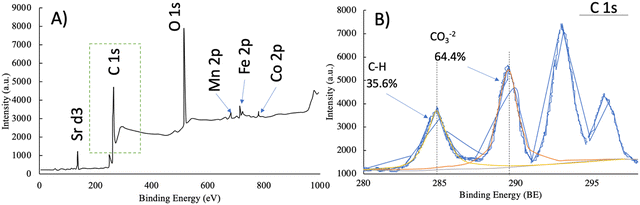
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of aliphatic and aromatic structures. The two peaks at BE of 286.6 eV and 288.1 eV denote C
C groups of aliphatic and aromatic structures. The two peaks at BE of 286.6 eV and 288.1 eV denote C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in ketone and carbonyl and O–C
O in ketone and carbonyl and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic groups, respectively. The peak at a binding energy of 289.6 eV signifies plasmon losses or shake-up peak of π–π* bonding stemming from π-electrons in aromatic rings.30 The surface characteristics (relative concentration) of the adsorbent hybrid calculated from the XPS spectra is provided in Table S1 (ESI†).
O in carboxylic groups, respectively. The peak at a binding energy of 289.6 eV signifies plasmon losses or shake-up peak of π–π* bonding stemming from π-electrons in aromatic rings.30 The surface characteristics (relative concentration) of the adsorbent hybrid calculated from the XPS spectra is provided in Table S1 (ESI†).
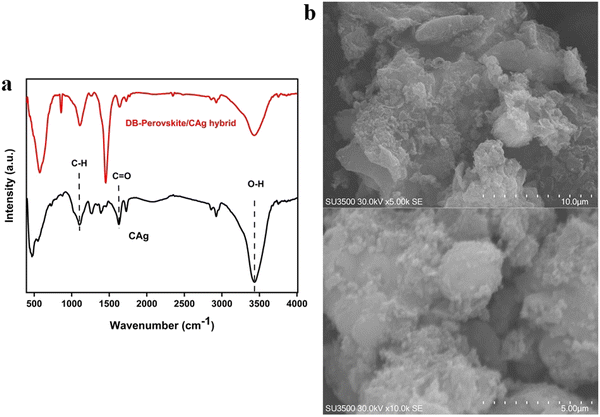
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the carboxylic, ester, and lactonic groups); 1389 and 1102 cm−1 (C–O stretch in secondary alcohol). The peak at 1108.6 cm−1 pertains to three-dimensional M–O–M stretching vibrations. Peaks at 574 and 860 cm−1 correspond to the formation of M–O bonds originated by the intrinsic nature of vibrations at tetrahedral places of the metal. The spectra suggested the presence of active sites on the adsorbent surface. The sharp peak observed at 1452 cm−1 only in the DB-perovskite/CAg hybrid but not in the CAg spectra signifies the bending vibration of hydroxyl groups on the surface of Sr0.7Mn0.3Co0.5Fe0.5O3−δ (M–OH). The peak position and also intensities were shifted, implying an interaction between the surface OH groups with both AY17 and CV after adsorption. Moreover, the formation of new bands (574 and 860 cm−1) in lower frequencies supported the adsorption of CV and AY17 through ion-exchange interactions.32–34 The morphology and microstructure of the as-prepared adsorbent are depicted by SEM analysis (Fig. 2b). Alginate that comprised B-D-mannonate (M) and α-L-guluronate (G) monomers can be used as a precursor to prepare a carbon aerogel by dispersing wet spun sodium alginate into a coagulating bath of 2.5 wt%. CaCl2 aqueous solution. Calcium cations can induce an interchain between the long structure of G-units and gel junction zones, whose structure has been known as an egg-box structure. After drying and converting it to aerogel, it is carbonized at high temperature in N2 atmosphere to prepare carbon aerogel nanocomposite or sheet, wherein the DB-perovskite nanoparticles can be encapsulated or distributed well by the carbonized alginate. As can be seen, the well distributed DB-perovskite over CAg and also the presence of a rough surface can enhance the contact active area between the DB-perovskite/CAg hybrid and dyes in aqueous solution and also provide extra binding sites, which is beneficial for the quick adsorption of the target dye molecules and further enhancing the adsorption capacity of the DB-perovskite/CAg hybrid for dye molecules.
O in the carboxylic, ester, and lactonic groups); 1389 and 1102 cm−1 (C–O stretch in secondary alcohol). The peak at 1108.6 cm−1 pertains to three-dimensional M–O–M stretching vibrations. Peaks at 574 and 860 cm−1 correspond to the formation of M–O bonds originated by the intrinsic nature of vibrations at tetrahedral places of the metal. The spectra suggested the presence of active sites on the adsorbent surface. The sharp peak observed at 1452 cm−1 only in the DB-perovskite/CAg hybrid but not in the CAg spectra signifies the bending vibration of hydroxyl groups on the surface of Sr0.7Mn0.3Co0.5Fe0.5O3−δ (M–OH). The peak position and also intensities were shifted, implying an interaction between the surface OH groups with both AY17 and CV after adsorption. Moreover, the formation of new bands (574 and 860 cm−1) in lower frequencies supported the adsorption of CV and AY17 through ion-exchange interactions.32–34 The morphology and microstructure of the as-prepared adsorbent are depicted by SEM analysis (Fig. 2b). Alginate that comprised B-D-mannonate (M) and α-L-guluronate (G) monomers can be used as a precursor to prepare a carbon aerogel by dispersing wet spun sodium alginate into a coagulating bath of 2.5 wt%. CaCl2 aqueous solution. Calcium cations can induce an interchain between the long structure of G-units and gel junction zones, whose structure has been known as an egg-box structure. After drying and converting it to aerogel, it is carbonized at high temperature in N2 atmosphere to prepare carbon aerogel nanocomposite or sheet, wherein the DB-perovskite nanoparticles can be encapsulated or distributed well by the carbonized alginate. As can be seen, the well distributed DB-perovskite over CAg and also the presence of a rough surface can enhance the contact active area between the DB-perovskite/CAg hybrid and dyes in aqueous solution and also provide extra binding sites, which is beneficial for the quick adsorption of the target dye molecules and further enhancing the adsorption capacity of the DB-perovskite/CAg hybrid for dye molecules.
Adsorption behavior. As shown in Table 1 and Fig. 3a, the Kd value of the DB-perovskite/CAg hybrid was higher for CV than AY17 under an initial concentration of 50 mg L−1, indicating that the DB-perovskite/CAg hybrid is considered to be an excellent adsorbent for CV. The Kd value of Sr0.7Mn0.3Co0.5Fe0.5O3−δ/CAg was higher in comparison to other adsorbents in the literature.7,35–40 It was found that the DB-perovskite/CAg hybrid leads to a higher Kd than other adsorbents, suggesting higher retention and low mobility of CV and AY17 in an aqueous solution. The CV molecules have smaller sizes than AY17. Therefore, their mobility through the cavity of DB-perovskite/CAg hybrid is relatively faster (Fig. 3b). Hence, it leads to enhanced adsorption activity than that AY17.
| Type of adsorbent | Type of pollutants | Selectivity coefficient (mL g−1) | Ref. |
|---|---|---|---|
| a Adsorptive removal of methylene blue from colored effluents on Fuller's earth. b Low-spin-state hematite with superior adsorption of anionic contaminations for water purification. c Adsorption of the Sudan dye(III) in methanol using activated carbon. | |||
| DB-perovskite/CAg hybrid | CV | 985 | This study |
| AY17 | 440 | This study | |
| Fuller's earth (FE)a | Methylene blue (MB) | 600 | 1 |
| Activated carbon | Rhodamine B | 302 | 2 |
| MCM-41b | Rhodamine B | 56.2 | 2 |
| TM | Rhodamine B | 31.7 | 2 |
| Bulk Fe2O3 | Rhodamine B | 177 | 2 |
| Activated carbonc | Sudan dye(III) | 120 | 3 |
| Bamboo biochar | Red 46 dye | 600 | 4 |
| Rice straw biochar | Red 46 dye | 245 | 4 |
| UiO-66 | Methyl orange | 1025 | 5 |
| UiO-66 | Malachite green | 225 | 5 |
The key parameters affecting adsorption performance. Regarding the removal of CV, the adsorption rate was faster, reaching equilibrium after 15 min. For the AY17 (Fig. 3c and d), the adsorption rate slowly increased and reached equilibrium within 60 min. As illustrated in Fig. 3c and d, for removing CV, when the dosage was increased from 0.005 g to 0.05 g/50 mL, CV removal increased step-wise by 41%, 63%, 95%, and 98%, respectively. In contrast, by increasing DB-perovskite/CAg hybrid concentration from 0.02 to 0.14 g/50 mL, the removal of AY17 increased from 74% to 93.5% at 0.1 g and it then gradually decreased to 91% at 0.14 g/50 mL. The higher DB-perovskite/CAg dose likely corresponds to pH-dependent adsorption mechanisms. Moreover, as the initial concentration and volume of dyes are constant, the number of contacted and adsorbed dye molecules or ions by the DB-perovskite/CAg Hybrid per unit mass decreases as the adsorbent dosage increases, and the active sites of DB-perovskite/CAg Hybrid are not fully occupied.
Overall, the DB-perovskite/CAg hybrid is highly sustainable compared to other pH-sensitive adsorbents. For instance, for tea leaves modified with polyethyleneimine (PEI-STL),41,42 efficient adsorption took place at pH 3 and for the almond shell,43 the optimal removal of cationic dyes occurred at pH 8–10. More details about the influence of the experimental conditions on the removal performance are presented in Fig. S1 (ESI†).
Adsorption isotherm and kinetics evaluation. The adsorption isotherm models fitted by Langmuir, Freundlich, Temkin, and DR are shown in Fig. 4a (also Fig. S2 andTable S2, ESI†). The interaction between CV and DB-perovskite/CAg hybrid surface was better elucidated by the Langmuir model (Fig. 4a). It represents monolayer adsorption, suggesting the monolayer coverage of dye molecules on a homogenous adsorbent surface with the same and equivalent adsorption active sites. While the Dubinin–Radushkevich (D–R) and Temkin models are based on the higher R2 and lower ARE, x2 and Δq were fitted better for the adsorption of AY17 on DB-perovskite/CAg hybrid (Fig. 4a). For the adsorption of CV and AY17, the maximum adsorption capacity (Qmax) was 206 mg g−1 and 113.54 mg g−1, respectively, compared to solely DB-perovskite (114 mg g−1 and 59 mg g−1, respectively).
The kinetics results of DB-perovskite/CAg hybrid on the adsorption of dyes are shown in Fig. 4b and Table S3 (ESI†). It can be seen that the adsorption capacity (qe) of AY17 was 26.11 mg g−1, which is smaller than that for CV (29.55 mg g−1) in the pseudo-first order model. The results indicated that the adsorption data fitted better with the Elovich model based on coefficient (R2) and lower error analysis (ARE, x2, and Δq) than pseudo-first order and pseudo-second order kinetics. This result was based on the higher correlation coefficient (R2) and lower error analysis (ARE, x2, and Δq) values than pseudo-first and pseudo-second order models (Table S3, ESI†). The Elovich model assumes that the entire adsorbent surface is highly heterogeneous. As the initial concentration is increased, the parameter α would increase and constant β would decrease.44 The constant β is related to the extent of surface coverage, increasing the reaction rate of adsorption as the initial concentration decreases, which might be related to the expansion of the available sites for the adsorbate.
Rate-determining mechanism for adsorption.
Boyd's film diffusion model. The first step in the adsorption process is the transport of dye molecules within the pores of the DB-perovskite/CAg hybrid. A linear or non-linear plot was obtained by plotting the graph between Btvs. t (min), called the Boyd plot. If the graph passes through the origin or not-origin, then the adsorption process is controlled by intraparticle diffusion only or both film and intraparticle resistance, respectively. The Boyd plot was obtained at different concentrations, as depicted in Fig. 5a. The plot at the lower initial concentration (5 mg L−1) is completely non-linear and does not pass through the origin for CV, while it is linear and passed through the origin for the AY 17. The pattern suggested that the adsorption of CV onto DB-perovskite/CAg hybrid is mainly controlled by film diffusion and intraparticle resistance, whereas in AY 17, it is controlled by film diffusion. However, the plot appeared linear when the initial concentration was increased for both CV and AY17. However, the plot for CV at higher concentrations became non-linear. Overall, it was concluded that the adsorption process for CV is controlled by both film and intraparticle resistance, while the film diffusion is dominated by diffusion mechanisms for AY17 adsorption.
 | ||
| Fig. 5 (a) Boyd model; (b) intraparticle diffusion, and (c) Bangham model for the adsorption of CV and AY17 on the DB-perovskite/CAg hybrid at 303.15 K. | ||
Intraparticle diffusion model. The intraparticle diffusion model developed by Weber and Morris and McKay and Poots, calculating diffusion rate from the plots of qtvs. t1/2 (Fig. 5b), is depicted by the following eqn (3).45,46
| qt = kidt1/2 + C | (3) |
 | (4) |
 | (5) |
Bingham's model. Bingham's model was applied to understand the rate-controlling step in the adsorption process (Fig. 5c). The Bingham model showed limited adsorption kinetics with pore diffusion. It was observed that the α decreased from 0.305 to 0.18 for CV and from 0.368 to 0.179 for AY17, and the k0 values increased from 11.19 to 22.21 mL g−1 L−1 for CV and from 11.19 to 19.99 mL g−1 L−1 for AY17, when the initial concentration of the dyes was increased. This change confirmed that the pore diffusion of CV and AY17 molecules drives the adsorption process by Sr0.7Mn0.3Co0.5Fe0.5O3−δ. For choosing the best kinetic model and adsorption mechanism, generally, correlation coefficient (R2) is not the only appropriate criterion. Thus, for comparison of models, three error analysis (Δq %, ARE, and x2) (eqn (6)–(8)) Table S4, ESI†) was done. The results showed that the correlation coefficient and error parameters for the intraparticle diffusion and Bingham's models for both the dyes (AY17 and CV) fitted better than the other models. According to higher R2 value and error analysis for the intraparticle and Bingham's curves, the diffusion of CV and AY 17 into the internal pores of the DB-perovskite/CAg hybrid was considered as the slowest step in the adsorption mechanism. As a result, various mechanisms translate the kinetic adsorption of CV and AY17 on the DB-perovskite/CAg hybrid. On the other hand, adsorption process is a heterogeneous process that happened at the liquid–solid boundary for AY17 and the mixing of pore diffusion and film (intraparticle) diffusion for both the dyes, called as “complex mechanisms”.50–52 However, for describing the satisfactory implementation of the kinetics/equilibrium models, a rough estimation of solid phase diffusion coefficients is acceptable, and equilibrium constants and conclusions on the underlying mechanisms should be treated with caution.53,54
 | (6) |
 | (7) |
 | (8) |
The adsorption behavior at different pH. The adsorption potential of the DB-perovskite/CAg hybrid for the removal of both dyes is illustrated in Fig. 6 at two different pH values. The DB-perovskite/CAg hybrid is an effective material to treat dye laden wastewater under acidic, neutral, and weak alkaline conditions. As can be explained, the adsorption of CV on the DB-perovskite/CAg hybrid was not significantly affected by pH. At higher pH, the adsorption of CV was slightly decreased. The pH may affect the adsorption by multifarious electrostatic interaction, electrophilic–nucleophilic interactions, hydrogen bonding, and proton transfer reaction. In the acidic condition, all three N atoms in CV can be fully protonated, sparking the interaction with the negatively charged surface (OH, carboxylic acid, and oxygen) of the DB-perovskite/CAg hybrid.55 When pH becomes alkaline, the interaction between CV and DB-perovskite/CAg hybrid is dominated by electrophilic–nucleophilic (Lewis's acid-base reaction) mechanism. The introduction of DB-perovskite into the porous networks of CAg creates additional metal-active sites. Moreover, the DB-perovskite can directly bind with the inner atoms of CAg such as N and O, providing M–O or M–N/C bond (M = Fe, Co, Mn) that can serve as the active sites for the adsorption of CV. Such active sites consist of a cloud of electrons of the Sigma band. As a result of this cloud of electrons, the DB-perovskite/CAg hybrid can be considered as nucleophilic. The nucleophilic DB-perovskite/CAg hybrid interacts with electrophilic central carbon/nitrogen of CV. Under weak alkaline conditions (pH = 8.5), the relative content of OH ions as the nucleophile is less competitive with the proposed nucleophile (DB-perovskite/CAg hybrid). However, the adsorption efficiency decreases at higher pH, which allows to predict a more decreasing rate at alkaline pH with increasing OH ions.56,57
 | ||
| Fig. 6 The potential interactions driving dye adsorption on the DB-perovskite/CAg hybrid at wide ranges of pH, including electrostatic attachment and hydrogen bonding. | ||
The introduction of DB-perovskite into the porous structure of CAg can create various surface functional groups from basic groups to acidic groups and active metal sites. The electrostatic interactions between AY 17 and DB-perovskite/CAg hybrid were considered major mechanisms related to pH in the adsorption process.58,59 However, pH changes in the solution might create changes in the AY17 structure and adsorption mechanisms by the DB-perovskite/CAg hybrid. At solution pH lower than the pKa of AY17 (5.5),60–63 the AY 17 dye exists primarily in its acidic form; as a result, the relatively negatively charged surface of the DB-perovskite/CAg hybrid can adsorb AY 17 with a dominant electrostatic mechanism. At higher pH, above the pKa of AY 17, the decrease in the adsorption capacity was lower than acidic pH owing to relatively strong electrostatic interaction than that of other mechanisms such as Lewis-acid-base interaction, London dispersive interaction, and hydrogen bonding that occurred at a higher pH of solution. Unsaturated metals (Perovskite = Fe, Co, and Mn) coordinated with oxygen, carbon (or N) in the DB-perovskite/CAg hybrid can serve as excellent active Lewis-acid sites that can accept and share an electron pair and react with l pair of oxygen-containing groups of AY17, thereby forming Lewis acid-base complexes, and successfully led to the adsorption of AY 17 by the DB-perovskite/CAg hybrid. In the DB-perovskite/CAg hybrid, positively charged metals are in fixed positions on the carbon matrix that are surrounded by delocalized electrons.64 Thus, the π electrons in the AY17 molecules are accessible to create interactions with the delocalized electrons of the DB-perovskite/CAg hybrid; as a result, the AY 17 molecules are successfully adsorbed on the DB-perovskite/CAg hybrid at higher pH of the solution beside other interaction mechanisms.65 Hence, the multitudinous and multifarious active groups and sites of the DB-perovskite/CAg hybrid that confirmed the obtained result allow describing the different magnitude of the interactions between AY17 or CV and DB-perovskite/CAg hybrid as a function of pH.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (KT) vs. 1/T, which are depicted in Fig. 7 and Table 2. The adsorption was facilitated at higher temperatures. The rate of diffusion increased at higher temperatures, and the adsorption of both dyes on DB-perovskite/CAg hybrid was an exothermic and spontaneous process. The negative ΔG values at all three temperatures suggested that the adsorption was spontaneous. The ΔG value for both the dyes was reduced with the increase in temperature. At 298 K, the ΔG value for CV was calculated as −0.92 kJ mol−1, which further decreased to −1.94 kJ mol−1 at 313 K. The change in the ΔG value was lower in AY, in which the ΔG value was reduced by 0.89 kJ mol−1 over the same range of temperature by 15 °C increase.
(KT) vs. 1/T, which are depicted in Fig. 7 and Table 2. The adsorption was facilitated at higher temperatures. The rate of diffusion increased at higher temperatures, and the adsorption of both dyes on DB-perovskite/CAg hybrid was an exothermic and spontaneous process. The negative ΔG values at all three temperatures suggested that the adsorption was spontaneous. The ΔG value for both the dyes was reduced with the increase in temperature. At 298 K, the ΔG value for CV was calculated as −0.92 kJ mol−1, which further decreased to −1.94 kJ mol−1 at 313 K. The change in the ΔG value was lower in AY, in which the ΔG value was reduced by 0.89 kJ mol−1 over the same range of temperature by 15 °C increase.
Mechanisms of the adsorption process. According to the literature,68–71 various mechanisms, namely, hydrogen bonding, pore-filling, van der Waals force, n–π interaction, and π–π interaction can be considered as the main or secondary adsorption mechanism. Given below is a brief description of the plausible adsorption mechanisms.
Pore filling. The adsorption and desorption isotherms of the CAg and DB-perovskite/CAg hybrid composite under N2 atmosphere are depicted in Fig. S3 (ESI†). The type IV isotherms are shown in CAg curves with an H4 hysteresis loop that is usual for micro and mesoporous compounds. The specific surface area of CAg was 714.9 m2 g−1, with a pore volume of 1.1 cm3 g−1, while on introducing DB-perovskite into CAg, a decrease un the specific area and pore volume to 390 m2 g−1 and 0.7 cm3 g−1, respectively, were observed. As can be seen, the introduction of Sr0.7Mn0.3Co0.5Fe0.5O3−δ increased the mesostructural properties of CAg. On the other hand, the porosity of CAg after embedding with Sr0.7Mn0.3Co0.5Fe0.5O3−δ improved. The pore size distribution of CAg and DB-perovskite/CAg hybrid composite is depicted in Fig. 8a. CA showed a bimodal distribution with microspore region, removing these microspores by incorporating Sr0.7Mn0.3Co0.5Fe0.5O3−δ into CAg that generated a single-modal distribution. The obtained parameters from the isotherms and the information in detail are summarized in Tables S5 and S6 (ESI†). The hybrid adsorbent provided great access sites for the adsorption of CV and AY17 because of the existence of many micropores and mesopores, thus improving the adsorption capacity. The average pore size of the DB-perovskite/CAg hybrid composite increased from 6.51 to 24.88 nm due to the removal of micropores. The pore size of the mesopores somehow decreased due to changing the pore size distribution to the large pore size shown in Fig. 8a.
Hydrogen bonding, n–π interaction, and π–π interaction. As shown in Fig. 2, a sharp peak was obtained at nearly 1452 cm−1 in the DB-perovskite/CAg hybrid, suggesting the presence of hydroxyl bonding on the surface (M–OH). Both the shifting of the peak and intensity bands also confirmed the interaction between hydroxyl groups and dyes after adsorption occurred. The π–π stacking interaction is another major mechanism in which sp2 hybridized C atoms and high chemical/thermal stability of metal oxide materials are involved. A strong interaction would occur between the DB-perovskite/CAg hybrid and the delocalized π bonds of dye molecules.
To provide a better insight into underlying mechanisms in the adsorption of CV and AY17 onto the DB-perovskite/CAg hybrid, the XPS spectra after and before adsorption were recorded. The XPS analysis led to the determination of the surface chemistry and calculated the proportion of oxygen-including functional groups on the surface of the DB-perovskite/CAg hybrid. The C 1s region curves of the DB-perovskite/CAg hybrid are displayed in Fig. 1b. The main peak at a binding energy (BE) of 283.8 eV was noticed, which could be assigned to the CHx, C–C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C groups of aliphatic and aromatic structures. The two peaks at BE of 286.6 eV and 288.1 eV indicated the presence of C
C groups of aliphatic and aromatic structures. The two peaks at BE of 286.6 eV and 288.1 eV indicated the presence of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in ketone and carbonyl and O–C
O in ketone and carbonyl and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in carboxylic groups, respectively. Also, one more peak at a binding energy of 289.6 eV is assigned to the Plasmon losses or shake-up peak of π–π* due to the abundance of π-electrons in the aromatic rings.72,73 Three major peaks appeared in the O 1s spectra (Fig. 8b), which were ascribed to C–O–C, –COOH, and C–OH/O–H.74 After the adsorption, the interactions occurred between oxygen-containing groups (–O– binds) and CV and AY17 molecules due to the decrease and shifting of the position of the O 1s peaks. Moreover, the new peaks exhibited in the DB-perovskite/CAg hybrid after adsorption suggested the presence of related reactions with CV and AY 17. Thus, it was concluded from the XPS measurement that π–π stacking or metal–π interactions might have occurred between the positive metal ions and the aromatic ring of CV and AY 17.
O in carboxylic groups, respectively. Also, one more peak at a binding energy of 289.6 eV is assigned to the Plasmon losses or shake-up peak of π–π* due to the abundance of π-electrons in the aromatic rings.72,73 Three major peaks appeared in the O 1s spectra (Fig. 8b), which were ascribed to C–O–C, –COOH, and C–OH/O–H.74 After the adsorption, the interactions occurred between oxygen-containing groups (–O– binds) and CV and AY17 molecules due to the decrease and shifting of the position of the O 1s peaks. Moreover, the new peaks exhibited in the DB-perovskite/CAg hybrid after adsorption suggested the presence of related reactions with CV and AY 17. Thus, it was concluded from the XPS measurement that π–π stacking or metal–π interactions might have occurred between the positive metal ions and the aromatic ring of CV and AY 17.
Electrostatic interaction. According to the obtained results (Fig. S4, ESI†), the adsorption of CV and AY 17 is primarily governed by the DB-perovskite/CAg hybrid properties, dye structure, and surface charge. The AY17 and CV molecules have a linear and non-linear structure that could be responsible for the adsorption of AY 17 by the porous DB-perovskite/CAg hybrid because the linear molecules could easily penetrate into the DB-perovskite/CAg void spaces. The introduction of Sr0.7Mn0.3Co0.5Fe0.5O3−δ into CAg resulted in surface charge (as observed from zeta potential measurements), revealing an increasing positive charge on the surface DB-perovskite/CAg Hybrid than that of CAg (from −48.1 mV (CAg) to −24.4 mV). It was thought that the presence of metal oxides on the surface of CAg could increase electrostatic repulsion between the DB-perovskite/CAg hybrid and both dyes.
Hydrophobic interactions. The CAg represents amphipathic characteristics due to the unique properties of organic and inorganic materials (Sr0.7Mn0.3Co0.5Fe0.5O3−δ). The presence of oxygen means CAg is polar in nature; therefore, water is adsorbed predominantly. On the surface of the adsorbent, the water are adsorbed via hydrogen bonding on oxygen-containing functional groups; in turn, the additional water molecules are clustered at these sites.75,76 The hydrophobic nature of CAg causes a decrease in the polar groups in the inner pore structure (active sites of adsorbent). As a result, the accessibility and affinity of the CV and AY17 to the inner pore structure may increase. In turn, the hydrophobic mechanisms might play the main role in CV and AY17 adsorption on the DB-perovskite/CAg hybrid. The proposed mechanisms that could be conceptualized in the adsorption process of AY17 and CV with the DB-perovskite/CAg hybrid composite are given in Fig. 8c.
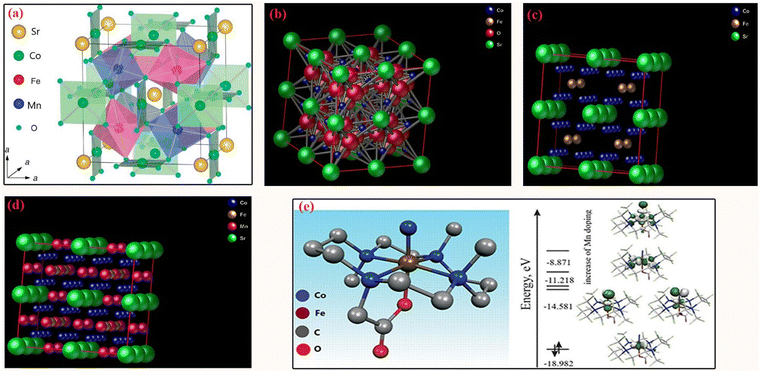 | ||
| Fig. 9 The DFT-optimized atomic configurations of (a–d) DB-perovskite/CAg hybrid; and (e) free energy diagrams of enhanced CV and AY17 adsorption process by the doping of Mn. | ||
| Rank | Bond | d [Å] | d-ratio |
|---|---|---|---|
| 1 | Fe–O | 0.82265 | 0.43298 |
| 2 | Fe–O | 0.88265 | 0.43298 |
| 3 | Fe–O | 2.28378 | 0.43298 |
| 4 | Fe–O | 2.38378 | 0.43298 |
| 5 | Co–O | 1.06967 | 0.43298 |
| 6 | Co–O | 1.12856 | 0.43298 |
| 7 | Co–O | 1.84761 | 0.43298 |
| 8 | Co–O | 2.41563 | 0.43298 |
| 9 | Sr–O | 2.28627 | 0.43298 |
| 10 | Sr–O | 2.34220 | 0.43298 |
Desorption and regeneration experiments
The regeneration and reusability of the adsorbent are considered as key characteristics to evaluate the adsorption performance of dyes for field application.77–79 Therefore, the regeneration and reusability of CV and AY17 were studied using 0.1 N HCl and 0.1 N HNO3. After the desorption of dyes from the adsorbent, the regenerated DB-perovskite/CAg hybrid was washed with distilled water and dried in the oven at 50 °C. Then, the reused adsorbent was used for four cycles of desorption–adsorption. As shown in Fig. 10, no remarkable difference was observed between the adsorption–desorption cycles of 0.1 N HCl and 0.1 N HNO3. As per the results, the regeneration from the first cycle was 98% and 92% for CV and AY17, respectively. After that, the regenerative capacity slightly decreased and reached 93% after four cycles for CV, but the performance of adsorption–desorption for AY17 was significantly reduced to 81%, and decreased sharply with a further increase in the number of cycles. The decrease in AY17 adsorption–desorption performance might be related to net adsorption energy at various contact places between large AY17 molecules and DB-perovskite/CAg hybrid. Moreover, the adsorption capacity of the DB-perovskite/CAg hybrid in the adsorption of CV and AY17 decreased from 14.7 mg g−1 to 14 mg g−1 and 6.9 mg g−1 to 6 mg g−1, respectively, by the end of the 4th regeneration cycle.Conclusion
A sustainable and efficient DB-perovskiteDB-perovskite (Sr0.7Mn0.3Co0.5Fe0.5O3−δ) embedded into carbon aerogel (CAg) was successfully synthesized by a pyrolysis method. The applied models indicated that the adsorption of CV and AY17 on the surface of the DB-perovskite/CAg hybrid was controlled by both external diffusion (film diffusion) and internal diffusion (particle or pore diffusion). The DB-perovskite/CAg hybrid led to a fast and sustainable adsorption capacity for both dyes in a wide range of pH. Thermodynamic analysis showed that the adsorption of CV and AY17 on the DB-perovskite/CAg hybrid is endothermic, indicating that more active sites are available for bonding interactions with the increase in temperature. The higher temperatures increased the static dipole polarizability and promoted the stability of electron acceptors–donors in turn; the interactions between the dyes and functional groups of the DB-perovskite/CAg hybrid were strengthened, indicating increasing weighted mean of site energy distribution at higher temperatures. The Mn atoms exhibited active sites in the DB-perovskite/CAg hybrid due to lower electronegativity than Fe and Mn atoms but still played a critical role in enhancing the valence state of metal ions and strengthened the metals–O bond for the adsorption of CV and AY 17 molecules. DB-perovskite/CAg hybrid acts as a renewable and cost-effective candidate to eliminate cationic and anionic dyes from aqueous solutions because of its higher stability, adsorption capacity, and reusability. Further studies can reveal the application of DB-perovskiteDB-perovskite/CAg hybrid on different dyes and other persistent organic compounds. Another suggestion is the study of the adsorption/desorption cycles and regeneration. This study exhibited that the perovskite-carbon aerogel hybrid adsorbents are competitive for the removal of dyes; the next step is the study of the removal of heavy metals.Conflicts of interest
There are no conflicts to declare.References
- J. Gao, Q. Zhang, K. Su, R. Chen and Y. Peng, J. Hazard. Mater., 2010, 174, 215–225 CrossRef CAS PubMed
.
- M. Sarmadi, M. Foroughi, H. N. Saleh, D. Sanaei, A. A. Zarei, M. Ghahrchi and E. Bazrafshan, Environ. Sci. Pollut. Res., 2020, 1–17 Search PubMed
.
- J. Khan, M. Sayed, F. Ali and H. M. Khan, Z. Phys. Chem., 2018, 232, 507–525 CrossRef CAS
.
- W. Ahlawat, N. Kataria, N. Dilbaghi, A. A. Hassan, S. Kumar and K.-H. Kim, Environ. Res., 2020, 181, 108904 CrossRef CAS PubMed
.
- J.-M. Yang, R.-J. Ying, C.-X. Han, Q.-T. Hu, H.-M. Xu, J.-H. Li, Q. Wang and W. Zhang, Dalton Trans., 2018, 47, 3913–3920 RSC
.
- S. Chang, Q. Zhang, Y. Lu, S. Wu and W. Wang, Sep. Purif. Technol., 2020, 238, 116400 CrossRef CAS
.
- I. Belbachir and B. Makhoukhi, J. Taiwan Inst. Chem. Eng., 2017, 75, 105–111 CrossRef CAS
.
- M. A. Maksoud, R. A. Fahim, A. G. Bedir, A. I. Osman, M. M. Abouelela, G. S. El-Sayyad, M. Abd Elkodous, A. S. Mahmoud, M. M. Rabee and H. Ala’a, Environ. Chem. Lett., 2021, 1–44 Search PubMed
.
- S. Banerjee, A. Debnath, B. K. Allam and N. Musa, Environ. Challenges, 2021, 5, 100240 CrossRef CAS
.
- L. Wang, C. Shi, L. Pan, X. Zhang and J.-J. Zou, Nanoscale, 2020, 12, 4790–4815 RSC
.
- N. G. Deshpande, C. H. Ahn, D. S. Kim, S. H. Jung, Y. B. Kim and H. K. Cho, Catal. Sci. Technol., 2020, 10, 6188–6197 RSC
.
- S. Zhai, R. Chen, J. Liu, J. Xu and H. Jiang, J. Taiwan Inst. Chem. Eng., 2021, 120, 161–168 CrossRef CAS
.
- C. Pei, G. Han, Y. Zhao, H. Zhao, B. Liu, L. Cheng, H. Yang and S. Liu, J. Hazard. Mater., 2016, 318, 732–741 CrossRef CAS PubMed
.
- C. B. Njoku, E. Oseghe and T. A. Msagati, J. Mol. Liq., 2022, 346, 118232 CrossRef CAS
.
- X. Li, H. Zhao, J. Liang, Y. Luo, G. Chen, X. Shi, S. Lu, S. Gao, J. Hu and Q. Liu, J. Mater. Chem. A, 2021, 9, 6650–6670 RSC
.
- X. Ye, X. Wang, Z. Liu, B. Zhou, L. Zhou, H. Deng and Y. Long, Dalton Trans., 2022, 51, 1745–1753 RSC
.
- H. Wang, Q. Zhang, M. Qiu and B. Hu, J. Mol. Liq., 2021, 334, 116029 CrossRef CAS
.
- É. Nascimento, A. Garrido Pedrosa and M. Souza, Water Sci. Technol., 2021, 83, 2793–2808 CrossRef PubMed
.
- K. Gupta, P. Joshi, R. Gusain and O. P. Khatri, Coord. Chem. Rev., 2021, 445, 214100 CrossRef CAS
.
- A. Ruhaimi, M. Aziz and A. Jalil, J. CO2 Util., 2020, 101357 Search PubMed
.
- R. A. Gopal, M. Song, D. Yang, T. Lkhagvaa, S. Chandrasekaran and D. Choi, Environ. Pollut., 2020, 267, 115498 CrossRef CAS PubMed
.
- Y. Jiang, Q. Shen, S. Li, G. Yang and N. Huang, New J. Chem., 2020, 44, 6003–6009 RSC
.
- H. Sun, G. Chen, Y. Zhu, B. Liu, W. Zhou and Z. Shao, Chem. – Eur. J., 2017, 23, 5722–5728 CrossRef CAS PubMed
.
- H. Mersian and M. Alizadeh, Ceram. Int., 2020, 46, 17197–17208 CrossRef CAS
.
- B. C. Mastoroudes, J. Markgraaff, J. B. Wagener and E. J. Olivier, Chem. Phys., 2020, 537, 110816 CrossRef CAS
.
- N. Choudhary, M. K. Verma, N. D. Sharma, S. Sharma and D. Singh, Mater. Chem. Phys., 2020, 242, 122482 CrossRef CAS
.
- A. H. Mahvi, M. Sarmadi, D. Sanaei and H. Abdolmaleki, Desalin. Water Treat., 2020, 200, 205–216 CrossRef CAS
.
- M. H. Dehghani, M. Sarmadi, M. R. Alipour, D. Sanaei, H. Abdolmaleki, S. Agarwal and V. K. Gupta, Microchem. J., 2019, 146, 1043–1053 CrossRef CAS
.
- B. B. Mohammed, A. Hsini, Y. Abdellaoui, H. Abou Oualid, M. Laabd, M. El Ouardi, A. A. Addi, K. Yamni and N. Tijani, J. Environ. Chem. Eng., 2020, 8, 104419 CrossRef
.
- M. H. Dehghani, D. Sanaei, I. Ali and A. Bhatnagar, J. Mol. Liq., 2016, 215, 671–679 CrossRef CAS
.
- P. Song, B. Liu, C. Liang, K. Ruan, H. Qiu, Z. Ma, Y. Guo and J. Gu, Nano-Micro Lett., 2021, 13, 1–17 CrossRef PubMed
.
- C. Duan, T. Ma, J. Wang and Y. Zhou, J. Water Process Eng., 2020, 37, 101339 CrossRef
.
- J. Liu, X. Yang, H. Liu, W. Cheng and Y. Bao, Colloids Surf., A, 2020, 601, 124960 CrossRef CAS
.
- S. Sun, Z. Yang, J. Cao, Y. Wang and W. Xiong, J. Solid State Chem., 2020, 285, 121219 CrossRef CAS
.
- Y. Gao, R. Kang, J. Xia, G. Yu and S. Deng, J. Colloid Interface Sci., 2019, 535, 159–168 CrossRef CAS PubMed
.
- M. A. Al-Ghouti and D. A. Da'ana, J. Hazard. Mater., 2020, 393, 122383 CrossRef CAS PubMed
.
- Q. T. N. Le and K. Cho, J. Colloid Interface Sci., 2021, 581, 741–750 CrossRef CAS PubMed
.
- D. Panchal, A. Sharma, P. Mondal, O. Prakash and S. Pal, Appl. Surf. Sci., 2021, 553, 149577 CrossRef CAS
.
- M. T. Amin, A. A. Alazba and M. Shafiq, Sustainability, 2021, 13, 3600 CrossRef CAS
.
- A. A. Khan, A. Molla, A. Chowdhury, S. Kumari and S. Hussain, ACS Appl. Nano Mater., 2021, 4, 4114–4128 CrossRef CAS
.
- S. Wong, H. H. Tumari, N. Ngadi, N. B. Mohamed, O. Hassan, R. Mat and N. A. S. Amin, J. Cleaner Prod., 2019, 206, 394–406 CrossRef CAS
.
- S. Wong, N. Abd Ghafar, N. Ngadi, F. A. Razmi, I. M. Inuwa, R. Mat and N. A. S. Amin, Sci. Rep., 2020, 10, 1–13 CrossRef PubMed
.
- M. Zbair, Z. Anfar, H. A. Ahsaine, N. El Alem and M. Ezahri, J. Environ. Manage., 2018, 206, 383–397 CrossRef CAS PubMed
.
- M. Massoudinejad, H. Rasoulzadeh and M. Ghaderpoori, Carbohydr. Polym., 2019, 206, 844–853 CrossRef CAS PubMed
.
- L. M. Silva, M. J. Muñoz-Peña, J. R. Domínguez-Vargas, T. González and E. M. Cuerda-Correa, Surf. Interfaces, 2021, 22, 100791 CrossRef CAS
.
- R. Leyva-Ramos, J. Salazar-Rábago and R. Ocampo-Pérez, Chem. Eng. Res. Des., 2021, 172, 43–52 CrossRef CAS
.
- G. Bufalo, F. Di Nezza, M. Perna, S. Salvestrini and L. Ambrosone, Appl. Sci., 2021, 11, 3096 CrossRef CAS
.
- J.-J. Lee, Clean Technol., 2021, 27, 182–189 Search PubMed
.
- K. A. M. Said, A. Ismail, A. Zulhairun, M. Abdullah, J. Usman, M. A. Azali and M. A. Azali, J. Environ. Chem. Eng., 2021, 9, 105036 CrossRef
.
- H. Feng, Z. Feng, W. Wang, Z. Deng and B. Zheng, Constr. Build. Mater., 2021, 292, 123285 CrossRef CAS
.
- H. Lei, Y. Muhammad, K. Wang, M. Yi, C. He, Y. Wei and T. Fujita, J. Hazard. Mater., 2021, 406, 124292 CrossRef CAS PubMed
.
- F. M. Mpatani, R. Han, A. A. Aryee, A. N. Kani, Z. Li and L. Qu, Sci. Total Environ., 2021, 146629 CrossRef CAS PubMed
.
- V. Inglezakis, M. Fyrillas and J. Park, J. Hazard. Mater., 2019, 367, 224–245 CrossRef CAS PubMed
.
- A. Z. Baimenov, D. Berillo, K. Moustakas and V. Inglezakis, J. Hazard. Mater., 2020, 399, 123056 CrossRef CAS PubMed
.
- E. Q. Adams and L. Rosenstein, J. Am. Chem. Soc., 1914, 36, 1452–1473 CrossRef CAS
.
- D. Brunel and F. Dumur, New J. Chem., 2020, 44, 3546–3561 RSC
.
-
D. R. Waring and G. Hallas, The chemistry and application of dyes, Springer Science & Business Media, 2013 Search PubMed
.
-
P. F. Gordon and P. Gregory, Organic chemistry in colour, De Gruyter, 2022 Search PubMed
.
- H. Shindy, Dyes Pigm., 2017, 145, 505–513 CrossRef CAS
.
- M. Teli and G. T. Nadathur, J. Environ. Chem. Eng., 2018, 6, 7257–7272 CrossRef CAS
.
- M. Pérez-Urquiza and J. Beltrán, J. Chromatogr. A, 2001, 917, 331–336 CrossRef PubMed
.
- J.-F. Gao, Q. Zhang, K. Su and J.-H. Wang, Bioresour. Technol., 2010, 101, 5793–5801 CrossRef CAS PubMed
.
- R. Srinivasan, M. N. Kathiravan and K. P. Gopinath, Bioresour. Technol., 2011, 102, 2242–2247 CrossRef CAS PubMed
.
- S. Parvez, M. J. Long, J. R. Poganik and Y. Aye, Chem. Rev., 2018, 118, 8798–8888 CrossRef CAS PubMed
.
- S. Shakra, H. Hanna and A. Hebeish, Angew. Makromol. Chem., 1979, 75, 53–62 CrossRef CAS
.
- D. O. Omokpariola, J. Chem. Lett., 2021, 2, 9–24 Search PubMed
.
- B. Priyadarshini, T. Patra and T. R. Sahoo, J. Magnesium Alloys, 2021, 9, 478–488 CrossRef CAS
.
- J. Zhao, C. Wang, S. Wang and Y. Zhou, J. Ind. Eng. Chem., 2020, 83, 111–122 CrossRef CAS
.
- S. Shang, Y. Liu, M. Liu, Y. Bai, X. Wang, B. Wu, J. Chen, J. Dong and Y. Liu, J. Hazard. Mater., 2022, 421, 126796 CrossRef CAS PubMed
.
- H. Rasoulzadeh, A. Sheikhmohammadi, M. Abtahi, B. Roshan and R. Jokar, J. Environ. Chem. Eng., 2021, 9, 105954 CrossRef CAS
.
- H. Rasoulzadeh, A. Sheikhmohammadi, M. Abtahi, M. Alipour and B. Roshan, Int. J. Environ. Anal. Chem., 2021, 1–14 Search PubMed
.
- L. Zhang, Y. Liu, Y. Wang, X. Li and Y. Wang, Appl. Surf. Sci., 2021, 557, 149838 CrossRef CAS
.
- S. Hussain, M. Kamran, S. A. Khan, K. Shaheen, Z. Shah, H. Suo, Q. Khan, A. B. Shah, W. U. Rehman and Y. O. Al-Ghamdi, Int. J. Biol. Macromol., 2021, 168, 383–394 CrossRef CAS PubMed
.
- Y. Tanimoto and S.-I. Noro, RSC Adv., 2021, 11, 23707–23713 RSC
.
- K. Belkassa, M. Khelifa, I. Batonneau-Gener, K. Marouf-Khelifa and A. Khelifa, J. Hazard. Mater., 2021, 415, 125656 CrossRef CAS PubMed
.
- I. V. Melnyk, V. V. Tomina, N. V. Stolyarchuk, G. A. Seisenbaeva and V. G. Kessler, J. Mol. Liq., 2021, 336, 116301 CrossRef CAS
.
- N. S. A. Mubarak, T. Chuan, H. Khor, A. H. Jawad, L. Wilson and S. Sabar, J. Polym. Environ., 2021, 29, 1050–1062 CrossRef
.
- J. Zhou, Y. Zhang, G. Jia, Z. Chen, Y. Yang and L. Zhang, New J. Chem., 2021, 45, 4835–4842 RSC
.
- B. F. do Nascimento, C. M. B. de Araujo, A. C. do Nascimento, G. R. B. da Costa, B. F. M. L. Gomes, M. P. da Silva, R. K. da Silva Santos and M. A. da Motta Sobrinho, Bioresour. Technol. Rep., 2021, 100767 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj05646a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |