Open Access Article
Open Access ArticleSuperparamagnetic cobalt ferrite nanoparticles synthesized by gamma irradiation†
Amel
Zorai
 ab,
Abdelhafid
Souici
ab,
Abdelhafid
Souici
 *a,
Diana
Dragoe
c,
Eric
Rivière
*a,
Diana
Dragoe
c,
Eric
Rivière
 c,
Salim
Ouhenia
a,
Jacqueline
Belloni
b and
Mehran
Mostafavi
c,
Salim
Ouhenia
a,
Jacqueline
Belloni
b and
Mehran
Mostafavi
 *b
*b
aLaboratoire de Physico-Chimie des Matériaux et Catalyse, Faculté des Sciences Exactes, Université de Bejaia, Bejaia 06000, Algeria
bInstitut de Chimie Physique, UMR 8000, CNRS, Université Paris-Saclay, Bâtiment 349, Campus d'Orsay, 15 Avenue Jean Perrin, 91405, Orsay Cedex, France
cInstitut de Chimie Moléculaire et des Matériaux d’Orsay, UMR 8182, CNRS, Université Paris-Saclay, Bâtiment Henri Moissan, 19 avenue des Sciences, 91400, Orsay, France
First published on 27th December 2022
Abstract
The radiolytic method is used to synthesize ultrasmall cobalt ferrite nanoparticles, CoFe2O4, exhibiting superparamagnetic properties. These systems are investigated by studying their properties at increasing dose of the irradiation process. These magnetic nanoparticles (MNPs) are characterized by X-ray diffraction (XRD), high-resolution transmission electronic microscopy (HRTEM), energy dispersive spectroscopy (EDS), and X-ray photoelectron spectroscopy (XPS). The XRD analysis and HRTEM observations confirm the formation of ultra-small cobalt ferrite nanoparticles (NPs) of CoFe2O4 (6–9 nm) in the cubic spinel structure. The temperature-dependent magnetic measurements reveal that the NPs exhibit superparamagnetic properties with a high magnetization Ms ∼ 70 Am2 kg−1 at 300 K and a very low coercivity (0.9 mT).
Introduction
Magnetic nanoparticles (MNPs) exhibit interesting physical and chemical properties for a wide range of technological applications, principally in catalysis,1 magnetic storage technology,2,3 spintronic devices,4 and biomedicine.5,6 When the magnetic behaviour is observed at temperature higher than the blocking temperature (TB), the material is superparamagnetic.7Superparamagnetic cobalt ferrite NPs CoFe2O4 (or mixed iron and cobalt oxides) exhibit excellent properties of high saturation magnetization (Ms) and magnetocrystalline anisotropy, low coercivity (HC) and remanent magnetization (Mr), and high chemical stability and mechanical hardness.8 They have thus been used in nanomedicine in both diagnostic and therapeutic applications such as magnetic resonance imaging (MRI),9 drug delivery,10 and especially magnetic hyperthermia therapy (MHT) for cancer treatment.11–13
The synthesis of cobalt ferrite CoFe2O4 has been extensively explored by various methods including co-precipitation,14,15 sol–gel,16 solvothermal,17 hydrothermal,18 thermal decomposition,19,20 reduction in boiling polyol,21 or alkalide in ethers (Table S1, ESI†).22
Over the past 40 years, radiation-induced synthesis has been extensively used to prepare metal and semiconductor nanoscale particles,23–25 including metal sulfides.26,27 This method at ambient temperature and pressure, avoiding the use of reducing chemical agents that could contaminate the product and lead to side reactions, offers a powerful alternative way for the synthesis of nanoparticles with high dispersity. This method allows controlling particle nucleation and the final size of the particles. The room temperature conditions aid in preventing sintering. In addition, the pulse radiolysis technique offers the possibility of studying in real time the different growth steps of the metal.28,29
Recently, the radiolytic route has been used for the synthesis of monodisperse small and MNPs of iron oxide Fe3O4,30–32 manganese oxide Mn3O4,33,34 cobalt oxide Co3O4,35 and cobalt hydroxide α-Co(OH)2.36 However, mixed cobalt and iron oxide CoFe2O4 (or cobalt ferrite) has not yet been synthesized by the radiolytic method.
The aim of the current study is to give particular attention to the γ-induced reduction of cobaltic hydroxide in a mixed solution with ferric iron hydroxide and the mechanism of synthesis of ultra-small cobalt ferrite nanoparticles in the presence of polyvinylpyrrolidone (PVP) as a biocompatible stabilizer molecule,37 and to investigate their magnetic properties. In fact, our work aims to obtain efficient MNPs for MHT for cancer treatment which is a potential application. Therefore, we report here the influence of the dose and the size of MNPs on their magnetic properties.
Experimental section
Materials
All chemical reagents were of high grade and purity and were purchased from Sigma-Aldrich.General procedure for radiation-induced synthesis
Analogous to the radiolytic synthesis of iron oxide Fe3O4,27–31 this method was also used to prepare cobalt ferrite nanoparticles. Because of their high energy, the radiation penetrates throughout the precursor solution, and, under selective conditions, generates from water, at room temperature and without any added chemical agent, strongly reducing species that are homogeneously distributed. At an adequate dose, one-third of the trivalent cations are thus reduced into divalent ions with the same distribution. Compared to chemical reduction at high temperature, the final nanoparticles are free of any chemical agent and smaller because they do not undergo sintering. The one pot radiolytic method has been successfully used for reduction into metal nanoparticles,23 and more recently, for the reduction of trivalent hydroxides into Me3O4.27–31Cobalt ferrite is a mixed oxide of trivalent iron and divalent cobalt ions. To synthesize these ions in an intimate mixture, our approach is to oxidize first, under aerated basic conditions (NH4OH), the cobalt ions from CoII to CoIII in a solution containing CoII and FeIII. An ammoniated complex of trivalent cobalt ions is formed before being precipitated together with trivalent iron ions. Then, the penetrating γ-radiation in solution reduces the CoIII ions back into CoII homogeneously inside the mixed hydroxide particles at room temperature.
For this purpose, the solutions were prepared by mixing chloride hexahydrate iron(III) (FeCl3, 6H2O at 4 × 10−3 mol L−1), and cobalt(II) chloride hexahydrate (CoCl2, 6H2O, at 2 × 10−3 mol L−1). Therefore, the stoichiometry of the metal atoms in the precursor solution is Fe/Co = 2 as required by the synthesis of cobalt ferrite. The solution was mixed with 2-propanol ((CH3)2CHOH) at 0.13 mol L−1 in order to scavenge the radiolytic oxidizing radicals OH• (much more efficiently than the diluted Cl− ions), and with polyvinylpyrrolidone (PVP) M.W. ∼ 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, as the particle stabilizer. PVP is attractive for medical applications because it is biodegradble, non-poisonous, pH-stable and heat-resistant.38 The PVP concentration was 10−3 mol L−1 only in order to avoid any cross-linking by radical scavenging. The solution pH was finally adjusted to 11.8 by adding ammonium hydroxide (NH4OH) to produce a colloidal suspension of trivalent iron and cobalt hydroxides intimately mixed. Without the cobalt salt, the FeIII solution is readily replaced by a light rust-orange colloidal suspension, whereas, without the iron salt, the pink CoII solution would be oxidized to a clear brown solution of CoIII hydroxide. When NH4OH is added to the mixed solution of both ions, the colloid becomes orange-brown and the supernatant solution becomes colorless, indicating the formation of colloidal particles of cobalt and ferric hydroxides.
000, as the particle stabilizer. PVP is attractive for medical applications because it is biodegradble, non-poisonous, pH-stable and heat-resistant.38 The PVP concentration was 10−3 mol L−1 only in order to avoid any cross-linking by radical scavenging. The solution pH was finally adjusted to 11.8 by adding ammonium hydroxide (NH4OH) to produce a colloidal suspension of trivalent iron and cobalt hydroxides intimately mixed. Without the cobalt salt, the FeIII solution is readily replaced by a light rust-orange colloidal suspension, whereas, without the iron salt, the pink CoII solution would be oxidized to a clear brown solution of CoIII hydroxide. When NH4OH is added to the mixed solution of both ions, the colloid becomes orange-brown and the supernatant solution becomes colorless, indicating the formation of colloidal particles of cobalt and ferric hydroxides.
| 2FeCl3 + CoCl3 + 9OH− → (FeIII(OH)3)2, CoIII(OH)3 + 9Cl− | (1) |
Before irradiation, the samples were thoroughly deaerated in small flasks (10 ml) for approximately 10 min by flushing with nitrogen to remove the O2. The samples were then exposed to a panoramic γ-60Co source at a dose rate of ≈2.3 kGy h−1.
During the colloidal solution irradiation, the major part of the energy is absorbed by the most abundant water molecules (reaction (1)):
 | (2) |
(The radiolytic yields of the products of water radiolysis in 10−7 mol J−1 unit are in brackets).39
Under basic conditions (pK = 9.6), the H˙ radicals are scavenged by the anions OH− and are replaced by e−aq:
| H˙ + OH− → e−aq | (3) |
The radicals OH˙ are scavenged by isopropanol, and the strongly reducing radicals (CH3)2C˙OH are formed (reactions (3)):
| OH˙ + (CH3)2CHOH → (CH3)2C˙OH + H2O | (4) |
Therefore, under these conditions of irradiation with the formation of e−aq and (CH3)2C˙OH radicals, the medium under irradiation is strongly reductive.
Characterization of cobalt ferrite nanoparticles
The UV-visible spectra of the solutions were recorded before and after irradiation using a Hewlett-Packard 8453A UV-visible spectrophotometer.The crystalline structures were evaluated by grazing incidence (3°) X-ray diffraction performed on a Panalytical X’pert pro MRD diffractometer with Cu Kα radiation (λ = 0.15418 nm). The patterns were obtained by step scanning from 15° to 90° in 2θ with an increment of 0.0752° and a counting time of 1800 s per step. Crystallographic phases were identified according to JCPDS files No. 22-1086 and fitted by MAUD Rietveld refinement software.40 The LaB6-SRM660a standard was used to calibrate the instrument broadening. An isotropic model was used to refine the mean nanoparticle size. During Rietveld refinement, the microstrains and cell parameters were refined to obtain the best reliability factors.
The magnetic measurements of the CoFe2O4 nanoparticles were performed using a Superconducting Quantum Interference Device (SQUID) magnetometer (MPMS XL7 Quantum Design). Zero-field cooling (ZFC) and field cooling (FC) magnetization curves were measured at different temperatures with a constant temperature sweep rate of 2 K min−1 and a magnetic field of 5 mT. Isothermal magnetization studies were also performed at temperatures of 5 and 300 K by measuring the hysteresis curves in the range of −5 T ≤ H ≤ 5 T. The samples were blocked with glue to avoid orientation with respect to the magnetic field.
The cobalt ferrite nanoparticle morphology, size distribution and stoichiometry were investigated by high-resolution transmission electron microscopy (HRTEM), selected area electron diffraction (SAED), and energy dispersive spectroscopy (EDS) using a JEOL 2100 Plus instrument working at 200 kV fitted with a GATAN Rio 16 camera and an IDFix EDS system from SAMx.
X-ray photoelectron spectroscopy (XPS) measurements were performed using a Thermo Fisher Scientific instrument with a monochromatic Al-Kα X-ray source (energy 1486.7 eV). The samples were measured as compacted powders and fixed using an aluminium foil mask. The base pressure was approximately 5 × 10−9 mbar and the diameter of the X-ray beam spot was 200 μm, corresponding to an irradiated surface of approximately 0.5 mm2. The hemispherical analyzer was operated at a 0° take-off angle in Constant Analyzer Energy (CAE) mode. Wide-scan spectra were recorded at a pass energy of 200 eV and an energy step of 1 eV, while narrow-scan spectra were recorded at pass energies of 50 eV and 20 eV with an energy step of 0.1 eV. Charge compensation was achieved with the help of a “dual beam” flood gun using low-energy electrons (<5 eV) and argon ions. The binding energy scale was calibrated on neutral carbon set at 285 eV, which corresponds to a value of 529.9 eV for the oxide component in the O 1s core-level spectrum.
Results and discussion
Synthesis of cobalt ferrite nanoparticles
Under reducing conditions, the cobaltic ions, preferentially over the ferric ions, are reduced by both hydrated electrons and isopropyl radicals:| e−aq + CoIII(OH)3, (FeIII(OH)3)2 → CoII(OH)2, 2FeIII(OH)3, + OH− | (5) |
| (CH3)2C˙OH + CoIII(OH)3, (FeIII(OH)3)2 → CoII(OH)2, (FeIII(OH)3)2 + (CH3)2CO + H2O | (6) |
The ferric ions may also be reduced as well:
| e−aq + CoIII(OH)3, (FeIII(OH)3)2 → CoIII(OH)2, FeII(OH)2, FeIII(OH)3 + OH− | (7) |
| (CH3)2C˙OH + CoIII(OH)3, (FeIII(OH)3)2 → CoII(OH)2, FeII(OH)2, FeIII(OH)3, (CH3)2CO + H2O | (8) |
However, the valency II of cobalt ions is more stable than that of iron ions, and internal electron transfer occurs from FeII to CoIII. Therefore, cobalt ions are preferentially reduced:
| CoIII(OH)3, FeII(OH)2, FeIII(OH)3 → CoII(OH)2, (FeIII(OH)3)2 | (9) |
The cobalt-ferric hydroxide progressively loses water molecules and precipitates into black nanoparticles of the cobalt ferrite CoFe2O4, stabilized by their interactions with PVP:
| CoII(OH)2, (FeIII(OH)3)2 →⋯→ CoII(OH)2, 2FeO(OH)2 + H2 → CoFe2O4 + 4H2O | (10) |
It is like that hydroxide anions OH− are still adsorbed on the nanoparticle surface.
First, it is worth noting that the cobalt ferrite nanoparticles are very stable with respect to oxidation, especially when the flasks are open to air, in contrast to the Fe3O4 nanoparticles which were partially reoxidized.41 Then, the characterization measurements were found to be constant during aging.
Optical properties
The basic solution of FeIII and CoIII is initially orange-brown and turns progressively pale gray at increasing doses. After approximately 8 kGy, small black particles were immediately attracted by a magnet across the glass wall of the flask (Fig. 1). Thus, these particles display magnetic properties. Without agitation or magnetic attraction, the particles precipitate slowly.The formation and growth of nanoparticles were followed by UV-visible measurements up to irradiation doses of 60 kGy. Before irradiation, the solution presented a broad absorbance band between 300 and 500 nm, which was assigned to mixed FeIII and CoIII hydroxide formed by adding ammonia to the iron(III) chloride and cobalt(II) chloride solutions.
The intensity of the absorbance band at 306 nm, which is assigned to the FeIII complex, first decreases to a minimum at 8 kGy, whereas the broad band above 400 nm increases with an isosbestic point at ≈ 390 nm (Fig. S1, ESI†). The minimum is assigned to the complete formation of cobalt ferrite particles absorbing widely up to 800 nm. In the second step, after 8 kGy, the absorbance increases at any wavelength because of the increasing light scattering of growing agglomerates between the magnetic particles into larger agglomerates.
The radiolytic yield corresponding to the complete reduction of CoIII ions at 8 kGy is equal to G(CoII) = 2 × 10−3 mol L−1/8 × 103 Gy = 2.5 × 10−7 mol J−1. This value is less than the total reducing yield (6.2 × 10−7 mol J−1) (eqn (2) because the concentration of hydroxide colloidal particles is much lower than that of CoIII, and the radical scavenging competes with radical recombination.
Crystallographic structure
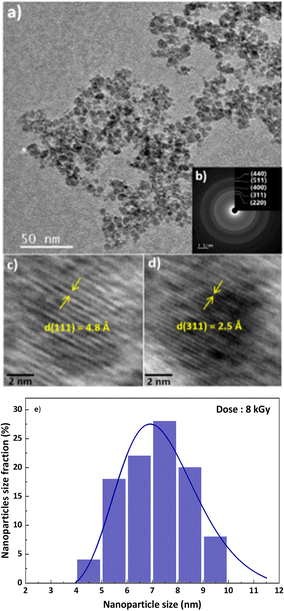
Fig. 2 presents the XRD patterns of the nanoparticles synthesized at doses of 6, 8, 16, and 60 kGy.The XRD patterns demonstrate that the diffraction peaks situated at the positions 18.32°, 30.13°, 35.49°, 43.14°, 57.05°, and 62.65° correspond to the (111), (220), (311), (400), (511), and (440) planes, respectively. At the three doses ≥ 8 kGy, these results correspond to the spinel structure of the CoFe2O4 compound with space group Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m (No 227) and a lattice parameter a = 8.43 Å, very close to that of JCPDS card No. 22-1086 with a lattice parameter a = 8.392 Å.
m (No 227) and a lattice parameter a = 8.43 Å, very close to that of JCPDS card No. 22-1086 with a lattice parameter a = 8.392 Å.
The anisotropic nanoparticle diameter obtained by the Rietveld refinement is 6–8 nm (Table 1). It is observed that the nanoparticle size increases slightly with an increasing dose. Moreover, the XRD patterns are similar at all the doses except 6 kGy, indicating that the reduction is completed only for doses ≥ 8 kGy (Fig. 2).
| Dose (kGy) | D (nm) (XRD) | D (nm) (HRTEM) | a (Å) (XRD)a | Fe/Co (EDS) | Fe3+/Co2+ (XPS) |
|---|---|---|---|---|---|
| a Lattice parameter of the spinel structure in JCPDS card No. 22-1086: a = 8.392 Å. b See size distribution (fig. S2, ESI). | |||||
| 6 | — | — | — | — | 1.9 |
| 8 | 6.7 (0.2) | 7.3 (1.5) | 8.43 (0.01) | 2.1 | 1.4 |
| 16 | 7.0 (0.1) | — | 8.43 (0.03) | 1.9 | 0.9 |
| 20 | 7.9 (0.3) | 9.6 (1.0)b | 8.40 (0.02) | 1.8 | 1.1 |
| 60 | 8.4 (0.1) | 10.1 (1.5)b | 8.43 (0.01) | 2.0 | 1.0 |
This dose also corresponds to the minimum observed in the optical absorption spectrum (Fig. S1, ESI†). Actually, the concentration of the colloidal particles CoIII(OH)3, (FeIII(OH)3)2 is several times lower than the precursor CoIII concentration and their radical scavenging undergoes a significant competition with the radical recombination. Another remarkable feature is that the XRD spectrum of the spinel structure is identical in the range of 8–60 kGy (Fig. 2). The further reduction in the nanoparticles is thus negligible, because in the flask bottom, they are much less subjected to the attack of the radicals formed in the bulk. In addition, geminate ion recombination in these crystallites after their direct irradiation hinders further reduction.
Microscopy imaging of radiation-induced cobalt ferrite nanoparticles
The morphological properties and size distribution of the synthetized nanoparticles were investigated using transmission electron microscopy (TEM) and high-resolution transmission electron microscopy (HRTEM). The images in Fig. 3 illustrate the morphology of the nanoparticles formed at 8 kGy. The CoFe2O4 nanosized particles stabilized by the PVP agent are monodisperse with a spherical- shape. However, it appears on the images that a few of them are isolated, very small, and at the limit of the TEM observation, while the majority of the nanoparticles are agglomerated, probably because of the powerful dipole–dipole interactions between the magnetic nanoparticles, which may facilitate their agglomeration. The corresponding SAED diagram (Fig. 3b) confirms the spinel structure observed by XRD. In the HRTEM images, the distances between the planes are d(311) = 2.5 Å (Fig. 3c) and d(211) = 4.8 Å (Fig. 3d). The lognormal distribution fit of the nanoparticles is 6–8 nm for an irradiation dose of 8 kGy (Fig. 3e), which is in agreement with the XRD observations.The stoichiometry of the cobalt ferrite nanoparticles analysed using EDS is shown in Table 1. The ratio of Fe and Co ions is close to Fe/Co = 2. These results clearly demonstrate the radiolytic formation in ammonia solutions and in the presence of PVP of the ultra-small cobalt ferrite CoFe2O4 nanoparticles. Notably, the Fe/Co ratio is the same at 8 kGy, where the reduction is complete, according to the dose-dependence of the absorption spectrum (Fig. S1, ESI†), and at higher doses. It seems that the precipitation of particles of CoFe2O4 to the flask bottom protects them from a further reduction by the radicals. Even then, they are still directly irradiated, and the recombination of charges in the small solid crystals avoids the reduction.
X-ray photoelectron spectroscopy analysis
XPS measurements were conducted to reveal the surface information of radiation-induced cobalt ferrite nanoparticles. The XPS survey spectrum (Fig. 4a) shows peaks corresponding to the Co 2p, Fe 2p, O 1s, C 1s, N 1s, and Auger signals. The Co 2p core-level spectrum (Fig. 4b) is composed of two main peaks located at 780.4 eV and 796.1 eV corresponding to Co 2p3/2 and Co 2p1/2. The presence of a satellite peak at ∼6 eV from the main Co 2p3/2 peak and its intensity suggest that Co is present on the surface mainly as Co2+, as also indicated by the spin-orbit splitting which is 15.8 eV.42,43 The Fe 2p core-level spectra shown in Fig. 4c show the spin–orbit doublet Fe 2p3/2 and Fe 2p1/2 located at 711 eV and 724.5 eV, respectively, with the shake-up satellite peak at 719.3 eV present in all spectra, confirming the valence state 3+.44–48 These spectra confirm the presence of Co2+ and Fe3+ ions located at octahedral and tetrahedral sites in the CoFe2O4 lattice.49 There is reciprocal interference between the Co et Fe 2p lines and Auger signals. An attempt was made to subtract the Auger signals, as in ref. 49 and the results are shown in Fig. S3 (ESI†). No further attempt to decompose the Fe 2p and Co 2p difference spectra was made as this subtraction represents an additional source of error. The O 1s core-level spectrum in Fig. 4d may be decomposed into two main contributions, one located at approximately 529.9 eV corresponding to the lattice oxygen in the Co/Fe-oxygen framework, and the second at 531.7 eV attributed to the oxygen originating from the surface hydroxide and to oxygen in polyvinylpyrrolidone. | ||
| Fig. 4 XPS spectra of radiation-induced cobalt ferrite nanoparticles. (a) XPS survey. (b) Co 2p. (c) Fe 2p. (d) O 1s. (e) N 1s. | ||
The N1s core-level spectrum (Fig. 4e) centered at 400 eV confirms the presence of the PVP coating on the cobalt ferrite particle surface. The Fe/Co surface ratio at the particles surface, as measured by XPS with increasing dose from 6 to 16 kGy, is reported in Table 1. Remarkably, it decreases, whereas it is confirmed by EDS that the ions contained in the particles have the same ratio of 2 as the salt precursors. It seems that, slowly under irradiation, an exchange occurs between the inner Co2+ and the outer Fe3+.50
Magnetic properties of radiation-induced cobalt ferrite nanoparticles
The magnetic properties of small cobalt ferrite nanoparticles were studied using a SQUID under a low external magnetic field (H = 5 mT) as a function of temperature.Fig. 5 presents the zero-field-cooled (ZFC) and field cooled (FC) temperature-dependent magnetization curves between 10 and 400 K for 8 and 60 kGy. The maximum of the ZFC curve corresponds to the blocking temperature (TB). Tirr corresponds to the irreversibility temperature at which the ZFC and FC curves merge and where the magnetic moments are unblocked. Above this temperature, superparamagnetic behavior is observed.51 Below T ≈ 200 K, the FC curves show saturated magnetization with temperature-independent behavior, which confirms the presence of the dipolar and interparticle coupling interaction effects.52 As illustrated in Fig. 5, it is clear from the ZFC and FC curves that the blocking temperatures TB for 8 and 60 kGy are 273 and 315 K, respectively. The increasing of the blocking temperature with the increasing dose is due to the increasing magnetocrystalline anisotropy associated with the spin-orbital coupling effect of Co2+.53 For both doses, the difference between Tirr and TB suggests the presence of particle interactions due to surface spin disorder.54 The hysteresis loop curves of radiation-induced cobalt ferrite nanoparticles, CoFe2O4, formed after 8, 20, and 60 kGy were recorded at 5, 100, and 300 K (Fig. 6 and Fig. S4, ESI†).
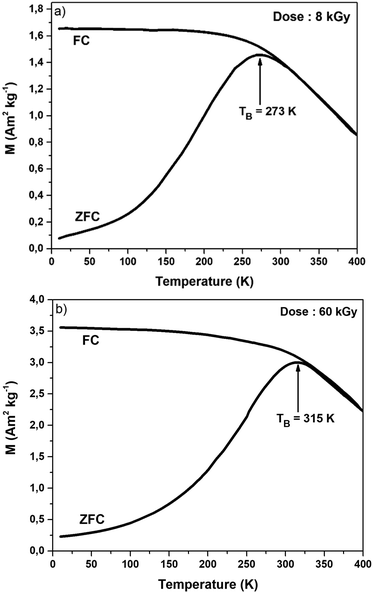 | ||
| Fig. 5 DC magnetization curves obtained for CoFe2O4, formed at 8 and 60 kGy, respectively, under zero-field-cooled (ZFC) and field cooled (FC) conditions. Magnetic field H = 50 Oe. | ||
The magnetic parameters, TB, Tirr, remanence magnetization (Mr), coercive field (Hc), saturation magnetization (Ms), magnetic anisotropy (K), and the squareness ratio R = Mr/MS, are summarized in Table 2.
| Dose (kGy) | T B (K) | T irr (K) | K (105 J m−3) | H 5C K (T) | M 5r K (Am2 kg−1) | M 5s K (Am2 kg−1) | R 5 K | H 300C K (10−4 T) | M 300r K (Am2 kg−1) | M 300s K (Am2 kg−1) | R 300 K |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 273 | 310 | 5.98 | 1.06 | 33.3 | 57.9 | 0.57 | 17.8 | 0.47 | 50.9 | 0.009 |
| 20 | — | — | — | 1.02 | 39.5 | 66.7 | 0.59 | 16.3 | 0.46 | 52.8 | 0.009 |
| 60 | 315 | 395 | 3.55 | 0.76 | 50.3 | 92.5 | 0.54 | 9.1 | 0.38 | 69.2 | 0.005 |
At a very low temperature of 5 K (Fig. 6), the radiation-induced CoFe2O4 NPs exhibit ferrimagnetic behavior with a large coercivity. According to the Stoner-Wohlfarth model, the HC values depend not only on the nanoparticle size and shape, but also on the surface spin and interparticle interaction.55,56 By increasing the dose up to 60 kGy, the HC value decreases.57 It has been reported that gamma radiation softens the materials by reducing coercivity. Remarkably, the saturation magnetization value of CoFe2O4 NPs at 5 K, MS = 92.5 Am2 kg−1 at 60 kGy, is as high as for the bulk (Ms = 93 Am2 kg−1).
The ratio R is 0.54–0.59 for the nanoparticles formed at 8–60 kGy, respectively. The corresponding values of R are slightly larger than those obtained for noninteracting single-domain NPs with uniaxial anisotropy (R = 0.50) and less than those of NPs with cubic magnetocrystalline anisotropy (R = 0.83). Even though cobalt ferrite in the bulk has cubic anisotropy, the existence of an effective uniaxial anisotropy in magnetic nanoparticles has been attributed to surface effects at smaller crystallite sizes.22
Therefore, in a first approximation, assuming a noninteracting system of nanoparticles with uniaxial anisotropy, the magnetic anisotropy constant K may be estimated as a function of size at TB using the equation K = 25kBTB/Vm where kB is the Boltzmann constant and Vm is the median volume.22,58 The corresponding anisotropy constant values of 5.98 × 105 J m−3 for nanoparticles formed at 8 kGy is significantly higher than that of CoFe2O4 bulk materials (3.0 × 105 J m−3). However, by increasing the dose, the magnetic anisotropy decreases and approaches the bulk value. The same phenomenon was reported for CoFe2O4 NPs synthesized using the sol-gel auto-combustion method and then irradiated at 50 and 100 kGy.59 It is explained that γ-irradiation modifies the surface and the interparticle contact, which has a direct relationship with dipolar and exchange mechanisms, and therefore magnetic anisotropy.
The observed negligible coercivity HC and remanence magnetization Mr at 300 K indicate that the CoFe2O4 NPs are superparamagnetic. Superparamagnetism is an essential feature for biomedical applications of these nanoparticles. The significant decrease of the ratio values up to 0.005 confirms a change in the magnetocrystalline anisotropy from cubic to uniaxial in a single magnetic domain.60 The coercivity value at 60 kGy is less than that at 8 and 20 kGy, because the monodomain responsible for the superparamagnetism is larger.
The saturation magnetization increases with dose from 50.9 Am2 kg−1 at 8 kGy to 69.2 Am2 kg−1 at 60 kGy (Table 2), in parallel with the slight increase of the nanoparticle size (Table 1). Gamma irradiation as a post-treatment to synthesis has been indeed reported to play an important role in the redistribution of cations in ferrite structures,16 and thus, to increase the magnetization values with the dose.
The Ms values for CoFe2O4 nanoparticles at 300 K (69.2 Am2 kg−1) are close to those of the CoFe2O4 bulk synthesized by the organic phase process (80 Am2 kg−1).53 It is suggested that the magnetic moment disorder at the particle surface is responsible for the magnetization lowering with respect to the bulk.53 Moreover, by the radiation-induced method in water without any heat treatment, the Ms values are among the highest values found for nanoparticles of cobalt ferrite of a similar size prepared by other methods, but after heating and/or annealing (Table S1, ESI†) such as coprecipitation,61,62 hydrothermal,18 thermal decomposition,20,63 and polyol method21 (Table S1, ESI†). Moreover, in radiation-induced synthesis, any possible adsorption of toxic additives is avoided in view of medical applications.
Conclusion
Using the gamma-radiolysis technique, we successfully synthesized for the first time ultra-small magnetic CoFe2O4 nanoparticles with sizes less than 10 nm. Cobalt(III) cations were reduced, without heat or chemical agents, in the presence of iron(III) cations and PVP as a biocompatible stabilizer polymer. The nanoparticles were highly stable during aging. The XRD analysis confirmed the spinel structure of the CoFe2O4 nanoparticles obtained at doses between 8 and 60 kGy. An Fe/Co ratio of 2 was measured using EDS. SQUID magnetic measurements revealed that the nanoparticles have superparamagnetic properties above 300 K with low coercivity HC = 0.9 mT and a very high magnetization Ms close to 70 Am2 kg−1. This magnetization value is among the highest values reported in the literature but obtained by using various preparation techniques requiring heating and/or annealing treatment. The radiation-induced reduction method under normal temperature and pressure conditions has proven to be extremely efficient to synthesize ultra-small superparamagnetic nanoparticles of cobalt ferrite. The magnetic properties of these NPs can be exploited for MHT processes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge Antoine Raison and François Brisset from ICMMO for their help in XRD measurements and HRTEM analysis.References
- R. Hudson, Y. Feng, R. S. Varma and A. Moores, Green Chem., 2014, 16, 4493–4505 RSC.
- E. Raymenants, O. Bultynck, D. Wan, T. Devolder, K. Garello, L. Souriau, A. Thiam, D. Tsvetanova, Y. Canvel and D. Nikonov, Nat. Electron., 2021, 4, 392–398 CrossRef.
- G. Reiss and A. Hütten, Nat. Mater., 2005, 4, 725–726 CrossRef CAS PubMed.
- B. Dieny, I. L. Prejbeanu, K. Garello, P. Gambardella, P. Freitas, R. Lehndorff, W. Raberg, U. Ebels, S. O. Demokritov and J. Akerman, Nat. Electron., 2020, 3, 446–459 CrossRef.
- M. M. Yallapu, S. F. Othman, E. T. Curtis, B. K. Gupta, M. Jaggi and S. C. Chauhan, Biomaterials, 2011, 32, 1890–1905 CrossRef CAS PubMed.
- R. A. Frimpong and J. Z. Hilt, Nanomedicine, 2010, 5, 1401–1414 CrossRef CAS PubMed.
- I. J. Bruvera, P. Mendoza Zélis, M. Pilar Calatayud, G. F. Goya and F. H. Sánchez, J. Appl. Phys., 2015, 118, 184304 CrossRef.
- H. Gavilán, S. K. Avugadda, T. Fernández-Cabada, N. Soni, M. Cassani, B. T. Mai, R. Chantrell and T. Pellegrino, Chem. Soc. Rev., 2021, 50, 11614–11667 RSC.
- H. M. Joshi, Y. P. Lin, M. Aslam, P. Prasad, E. A. Schultz-Sikma, R. Edelman, T. Meade and V. P. Dravid, J. Phys. Chem. C, 2009, 113, 17761–17767 CrossRef CAS PubMed.
- G. Wang, F. Zhou, X. Li, J. Li, Y. Ma, J. Mu, Z. Zhang, H. Che and X. Zhang, Ceram. Int., 2018, 44, 13588–13594 CrossRef CAS.
- J. Pan, P. Hu, Y. Guo, J. Hao, D. Ni, Y. Xu, Q. Bao, H. Yao, C. Wei and Q. Wu, ACS Nano, 2020, 14, 1033–1044 CrossRef CAS PubMed.
- W. M. O. De Santana, S. Abramson, R. Fini, B. L. Caetano, C. Ménager, S. H. Pulcinelli and C. V. Santilli, ACS Appl. Polym. Mater., 2021, 3, 4837–4848 CrossRef CAS.
- S. G. Mendo, A. F. Alves, L. P. Ferreira, M. M. Cruz, M. H. Mendonça, M. Godinho and M. D. Carvalho, New J. Chem., 2015, 39, 7182–7193 RSC.
- J. Thomas, N. Thomas, F. Girgsdies, M. Beherns, X. Huang, V. Sudheesh and V. Sebastian, New J. Chem., 2017, 41, 7356–7363 RSC.
- S. F. Shams, M. Kashefi and C. Schmitz-Antoniak, New J. Chem., 2018, 42, 3050–3062 RSC.
- A. V. Raut, D. Kurmude, D. Shengule and K. Jadhav, Mater. Res. Bull., 2015, 63, 123–128 CrossRef CAS.
- M. Sanna Angotzi, A. Musinu, V. Mameli, A. Ardu, C. Cara, D. Niznansky, H. L. Xin and C. Cannas, ACS Nano, 2017, 11, 7889–7900 CrossRef CAS PubMed.
- P. Palade, C. Comanescu, A. Kuncser, D. Berger, C. Matei, N. Iacob and V. Kuncser, Nanomaterials, 2020, 10, 476 CrossRef CAS PubMed.
- H. Zhang, L. Liu and X. Liu, ACS Nano, 2017, 11, 3614–3631 CrossRef CAS PubMed.
- R. A. Bohara, N. D. Thorat, H. M. Yadav and S. H. Pawar, New J. Chem., 2014, 38, 2979–2986 RSC.
- T. Gaudisson, M. Artus, U. Acevedo, F. Herbst, S. Nowak, R. Valenzuela and S. Ammar, J. Magn. Magn. Mater., 2014, 370, 87–95 CrossRef CAS.
- K. E. Mooney, J. A. Nelson and M. J. Wagner, Chem. Mater., 2004, 16, 3155–3161 CrossRef CAS.
- J. Marignier, J. Belloni, M. Delcourt and J. Chevalier, Nature, 1985, 317, 344–345 CrossRef CAS.
- J. Belloni and M. Mostafavi, Studies in Physical and Theoretical Chemistry, Elsevier, 2001, vol. 87, pp. 411–452 Search PubMed.
- J. Belloni, J.-L. Marignier and M. Mostafavi, Radiat. Phys. Chem., 2020, 169, 107952 CrossRef CAS.
- A. Souici, N. Keghouche, J. Delaire, H. Remita, A. Etcheberry and M. Mostafavi, J. Phys. Chem. C, 2009, 113, 8050–8057 CrossRef CAS.
- A. Souici, K.-L. Wong, V. De Waele, J. Marignier, T. H. Metzger, N. Keghouche, S. Mintova and M. Mostafavi, J. Phys. Chem. C, 2014, 118, 6324–6334 CrossRef CAS.
- J. Belloni, M. Mostafavi, H. Remita, J.-L. Marignier and M.-O. Delcourt, New J. Chem., 1998, 22, 1239–1255 RSC.
- P. Mulvaney and A. Henglein, J. Phys. Chem., 1990, 94, 4182–4188 CrossRef CAS.
- I. Marić, G. Dražić, G. Štefanić, K. Zadro, M. Gotić and T. Jurkin, Mater. Charact., 2020, 159, 110038 CrossRef.
- T. Jurkin, M. Gotić, G. Štefanić and I. Pucić, Radiat. Phys. Chem., 2016, 124, 75–83 CrossRef CAS.
- S. Wang and H. Xin, Radiat. Phys. Chem., 1999, 56, 567–572 CrossRef CAS.
- Z. Li, Y. Yang, A. Relefors, X. Kong, G. M. Siso, B. Wickman, Y. Kiros and I. L. Soroka, J. Colloid Interface Sci., 2021, 583, 71–79 CrossRef CAS PubMed.
- P. Mulvaney, L. Denison, F. Grieser, R. Cooper, J. V. Sanders and D. Meisel, J. Colloid Interface Sci., 1988, 121, 70–80 CrossRef CAS.
- L. Alrehaily, J. Joseph, M. Biesinger, D. Guzonas and J. Wren, Phys. Chem. Chem. Phys., 2013, 15, 1014–1024 RSC.
- S.-W. Kim, B.-J. Kwon, J.-H. Park, M.-G. Hur, S.-D. Yang and H. Jung, Bull. Korean Chem. Soc., 2010, 31, 910–914 CrossRef CAS.
- S.-H. Choi, Y.-P. Zhang, A. Gopalan, K.-P. Lee and H.-D. Kang, Colloids Surf., A, 2005, 256, 165–170 CrossRef CAS.
- M. Kurakula and G. K. Rao, Drug Delivery Sci. Technol., 2020, 60, 102046 CrossRef CAS PubMed.
- G. V. Buxton, C. L. Greenstock, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886 CrossRef CAS.
- L. Lutterotti, Nucl. Instrum. Methods Phys. Res., Sect. B, 2010, 268, 334–340 CrossRef CAS.
- I. Marić, M. Gotić, G. Štefanić, A. Pustak and T. Jurkin, Radiat. Phys. Chem., 2020, 170, 108648 CrossRef.
- D. Gallant, M. Pezolet and S. Simard, J. Phys. Chem. B, 2006, 110, 6871–6880 CrossRef CAS PubMed.
- W. Wei, W. Chen and D. G. Ivey, Chem. Mater., 2008, 20, 1941–1947 CrossRef CAS.
- N. McIntyre and D. Zetaruk, Anal. Chem., 1977, 49, 1521–1529 CrossRef CAS.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254, 2441–2449 CrossRef CAS.
- H. Liu, G. Wei, Z. Xu, P. Liu and Y. Li, Appl. Surf. Sci., 2016, 389, 438–446 CrossRef CAS.
- A. Grosvenor, B. Kobe, M. Biesinger and N. McIntyre, Surf. Interface Anal., 2004, 36, 1564–1574 CrossRef CAS.
- R. D. Diehl and R. McGrath, Surf. Sci. Rep., 1996, 23, 43–171 CrossRef CAS.
- P. M. Kouotou, H. Vieker, Z. Tian, P. T. Ngamou, A. El Kasmi, A. Beyer, A. Gölzhäuser and K. Kohse-Höinghaus, Catal. Sci. Technol., 2014, 4, 3359–3367 RSC.
- G. Allen and K. Hallam, Appl. Surf. Sci., 1996, 93, 25–30 CrossRef CAS.
- G. Barrera, P. Tiberto, P. Allia, B. Bonelli, S. Esposito, A. Marocco, M. Pansini and Y. Leterrier, Appl. Sci., 2019, 9, 212 CrossRef CAS.
- B. Cai, M. Zhao, Y. Ma, Z. Ye and J. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 1327–1333 CrossRef CAS PubMed.
- D. Li, H. Yun, B. T. Diroll, V. V. Doan-Nguyen, J. M. Kikkawa and C. B. Murray, Chem. Mater., 2016, 28, 480–489 CrossRef CAS.
- S. Chandra, H. Khurshid, W. Li, G. Hadjipanayis, M. Phan and H. Srikanth, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 014426 CrossRef.
- S. M. Ansari, B. B. Sinha, D. Phase, D. Sen, P. U. Sastry, Y. D. Kolekar and C. V. Ramana, ACS Appl. Nano Mater., 2019, 2, 1828–1843 CrossRef CAS.
- C. Tannous and J. Gieraltowski, Eur. J. Phys., 2008, 29, 475 CrossRef CAS.
- K. L. Routray, D. Sanyal and D. Behera, Mater. Res. Bull., 2019, 110, 126–134 CrossRef CAS.
- T. Hyeon, Y. Chung, J. Park, S. S. Lee, Y.-W. Kim and B. H. Park, J. Phys. Chem. B, 2002, 106, 6831–6833 CrossRef CAS.
- A. Raut, D. Kurmude, S. Jadhav, D. Shengule and K. Jadhav, J. Alloys Compd., 2016, 676, 326–336 CrossRef CAS.
- N. Moumen and M. Pileni, J. Phys. Chem., 1996, 100, 1867–1873 CrossRef CAS.
- M. S. Darwish, H. Kim, H. Lee, C. Ryu, J. Y. Lee and J. Yoon, Nanomaterials, 2019, 9, 1176 CrossRef CAS PubMed.
- P. Kumar, S. Pathak, A. Singh, H. Khanduri, G. Basheed, L. Wang and R. Pant, Nanoscale Adv., 2020, 2, 1939–1948 RSC.
- A. P. Herrera, L. Polo-Corrales, E. Chavez, J. Cabarcas-Bolivar, O. N. Uwakweh and C. Rinaldi, J. Magn. Magn. Mater., 2013, 328, 41–52 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2nj05433g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |