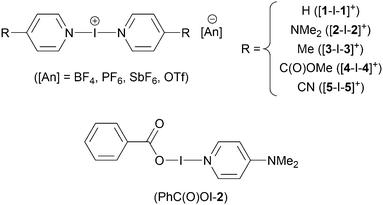
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Iodination of antipyrine with [N–I–N]+ and carbonyl hypoiodite iodine(I) complexes†
Laura M. E.
Wilson
 ,
Kari
Rissanen
,
Kari
Rissanen
 * and
Jas S.
Ward
* and
Jas S.
Ward
 *
*
University of Jyvaskyla, Department of Chemistry, Jyväskylä, 40014, Finland. E-mail: kari.t.rissanen@jyu.fi; james.s.ward@jyu.fi
First published on 12th January 2023
Abstract
A series of iodine(I) complexes, both known and new, were synthesised and the dependence of iodination reactivity on the identity of the Lewis bases and anions present was investigated. Using a previously established screening protocol based on the iodination of antipyrine to iodo-antipyrine, the capability of the iodine(I) species to perform the iodination was tested and compared, especially in relation to Barluenga's reagent, [I(pyridine)2]BF4. The results indicated that the identity of both the Lewis bases and the anion influence the iodination capability of the iodine(I) species, and that the less efficient reagents can deliver favourably comparable percentage conversions with longer reaction times.
Introduction
The study of halogen(I) chemistry continues to be at the forefront of current research, with recent developments including the first asymmetric,1 heteroleptic,2 halogen-bonded organic framework (XOF),3 and nucleophilic interactions of iodine(I) complexes being reported.4–6 Owing to favourable characteristics of halogen-bonding, such as the consistent, high-degree of linear directionality, halogen-bonding has fruitfully been implemented in self-assembling processes to generate supramolecular architectures,7–9 as well as in the synthesis of functional materials (magnetic, porous, phosphorescent, liquid crystals).10Halogen(I) (or halonium) complexes, of the form [L–X–L][anion] (X = Cl, Br, I),2,11–13 are species which contain a halogen element ionised to a formally cationic state, X+, stabilised by two Lewis bases (L). The stabilising Lewis bases are most commonly N-heterocyclic aromatic amines, though alkyl amines and other donor atoms have been successfully implemented in this role.14–18 The stability of halogen(I) complexes follows the trend: I+ > Br+ ≫ Cl+,19–21 which can be attributed to the X+ halogen-bonds electronically originating from a σ-hole interaction.22,23
The [N–I–N]+ structure of iodine(I) complexes was first identified in the 1960s,24,25 but these oddities were largely overlooked until Barluenga recognised and demonstrated their use as mild iodinating and oxidising reagents,26,27 predominantly utilising his now eponymous reagent [I(pyridine)2]BF4, which features a [N–I–N]+ three-centre four-electron bond.
Interest in halogen(I) species has persisted over time, not only due to their intriguing structures and properties, but because of their use as synthetic reagents that can perform unique, and sometimes otherwise unobtainable, organic transformations. Through the efforts of Barluenga and co-workers, a number of invaluable transformations were demonstrated utilising halogen(I) species,28 including the electrophilic iodination of un-activated arenes (obviating the need for protecting group chemistry, thereby increasing atom economy), the promotion of C–C and C–X bond formation, and the selective direct iodination of peptides,29–32 which often cannot otherwise be accessed without tedious multistep synthetic procedures, if at all. It should be noted that the analogous anionic dioxoiodane complexes, [O–I–O]−, are also known and have been previously utilised as organic reagents.33 As a result, Barluenga's reagent is today commercially available and, along with other halogen(I) species, routinely used to enact a myriad of organic transformations, making halogen(I) species an essential tool in the synthetic chemists’ toolbox.
Similarly, in situ generated hypoiodites (R–OI; R = alkyl) also have an established track-record as iodination reagents,34,35 though the exact species responsible for the iodination are still under debate (cf. “tBuOI”),36,37 making structure-reactivity relationships difficult to discern. Whilst iodination reagents like the carbonyl hypoiodite iodine monoacetate, CH3C(O)OI, have only been observed by 1H NMR spectroscopy and spectrophotometrically,38,39 a renewed interest in Lewis base stabilised carbonyl hypoiodites has offered many potential new iodination reagent candidates that have well-defined structures and can be isolated in their pure forms.40,41 Recent single crystal X-ray diffraction studies have confirmed the similarities of the I–N bond lengths between cationic iodine(I) complexes, [N–I–N]+, and neutral stabilised carbonyl hypoiodites, O–I–N, in the solid state.40,41 Such observations would therefore offer a route to better understanding the structure–reactivity relationships of carbonyl hypoiodite species and their use as iodination reagents.
Given the overwhelming successes of Barluenga's reagent, hereafter referred to as [1–I–1]BF4, it has become ubiquitous in the field of halogen(I) chemistry and therefore dominated research into the utility of iodine(I) species. Usually considered to be stable under ambient conditions, [1–I–1]BF4 is a white solid in pure form. However, over time the reagent degrades upon reaction with water to form hypoiodous acid (HOI), granting a strong orange colour to the solid, which is routinely observed in even relatively fresh commercial samples despite their storage conditions (see ESI,† Fig. S2). Therefore, if other more resilient iodine(I) species could be identified, with reactivity that rivalled or surpassed [1–I–1]BF4, this would have important implications in the field of iodine(I) chemistry. The flurry of new iodine(I) species reported in recent years offer a wide range of potential iodination reagents to pursue, which might offer alternatives and advantages desirable to the modern synthetic chemist.
Results and discussion
Preparation and characterization of iodine(I) complexes
The known Barluenga complexes [1–I–1]BF4,26 [1–I–1]PF6,2 [2–I–2]PF62 and carbonyl hypoiodite complex (PhC(O)OI–2)40 were prepared according to literature procedures (Scheme 1). In addition, the new complexes [2–I–2]BF4, [2–I–2]SbF6, [2–I–2]OTf, [3–I–3]PF6 [4–I–4]PF6 and [5–I–5]PF6 were also prepared in an analogous fashion.The 1H–15N HMBC determined 15N NMR chemical shifts for the three [2–I–2]+ complexes, with BF4 (−215.9 ppm), SbF6 (−215.8 ppm), and OTf (trifluoromethanesulfonate; −215.8 ppm) anions were all effectively identical to the previously reported PF6 analogue (−216.1 ppm), consistent with previous studies on the anion influence of halogen(I) complexes.11 Similarly, the 15N NMR chemical shifts for [3–I–3]PF6 (−182.2 ppm) and [4–I–4]PF6 (−164.5 ppm) were also reminiscent of related complexes,2,5 whilst the resonance for [5–I–5]PF6 were not observed due to broadening in the 1H NMR spectrum. The solid-state structures were also obtained for [2–I–2]SbF6, [3–I–3]PF6, [4–I–4]PF6, and [5–I–5]PF6 (Fig. 1) all of which exhibited I–N bond lengths within the narrow range of 2.235(4)–2.284(4) Å, which is a common trait for [N–I–N]+ iodine(I) complexes despite the variety of electron donating (NMe2, Me) and withdrawing (C(O)OMe, CN) groups present in the 4-position of the pyridine-based ligands.13,42
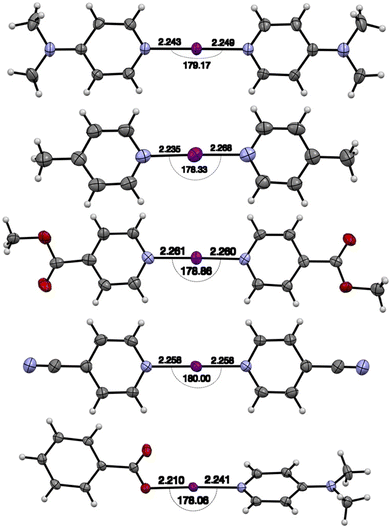 | ||
| Fig. 1 The X-ray structures of [2–I–2]SbF6, [3–I–3]PF6, [4–I–4]PF6, [5–I–5]PF6 and (PhC(O)OI–2)39 (CSD ref. code BZPRIA01) (from top to bottom; anisotropic displacement parameters at the 50% probability level). | ||
Iodination studies
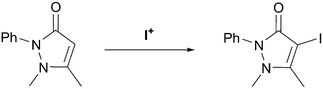
All complexes were thoroughly washed to ensure complete removal of any excess I2 from their synthesis which might taint the subsequent reactivity studies (given that the I2 used in the synthesis of iodine(I) complexes can itself act as a source of I+), and were utilised for further studies within hours of being freshly prepared. The complexes were also prepared in bulk to minimise any discrepancies in the reactivity studies due to minor variations from batch to batch. However, these somewhat strict requirements did necessitate some prospective iodine(I) species to be eliminated from this study due to an inability to reliably prepare or purify bulk samples, which would be detrimental to the quality of the reactivity studies, and moreover were seen as undesirable qualities in terms of future commercial viability.As a test of the proof-of-concept, the iodine(I) complexes were subjected to the iodination reaction of antipyrine to iodo-antipyrine (Scheme 2, X-ray structure, see ESI,† Fig. S1). This reaction had been previously used for this purpose with [1–I–1]BF4,43 offering a simple reaction with no alternative positions for iodination or decomposition, and which offered a straightforward workup of the iodination product, thereby providing qualitative comparisons of the reaction yields. The parent study, which reported a 94% conversion to the iodo-antipyrine utilising [1–I–1]BF4 with a 2 hour reaction time, was repeated and used as a benchmark for comparisons.
The results of the iodination studies found that [1–I–1]BF4 performed the best out of those tested with a 93% conversion of the antipyrine, in near perfect agreement to the parent study (Table 1), closely followed by [5–I–5]PF6 (89%) and [4–I–4]PF6 (85%). This was unsurprising given that 1, 4, and 5 were the least nucleophilic ligands, and their participation in an iodine(I) complex would be expected to contribute to greater reactivity due to increased lability of the corresponding complexes, which is a mechanistic necessity to the iodination process.44 The utilisation of [4–I–4]PF6 or [5–I–5]PF6, instead of [1–I–1]BF4, could offer advantages such as the presence of less nucleophilic pyridine ligands in the reaction mixture, or the possible desirability of having the additional functional groups of 4 (COOMe) or 5 (CN) being present in the reaction mixture. Additional considerations include the use of stabilising ligands which are solids (i.e., 2 and 5; the free pyridine, 1, of Barluenga's reagent is a liquid at ambient conditions), a factor which may become more important given the recent preference toward environmentally-friendly techniques, such as mechanochemistry and solid-state NMR spectroscopy, for the preparation and use of reagents like those described herein.
The study of the strongly nucleophilic DMAP derivatives, [2–I–2]+, with various anions, however, did reveal some interesting results. No major difference in the reactivity was anticipated due to variation of the anion, as previous NMR studies on the effect of the anion in [N–I–N]+ complexes had noted little to no influence,11 though differences could be expected if reagent solubilities were found to noticeably differ as a result of anion identity. However, despite all complexes except [5–I–5]PF6 exhibiting good CH2Cl2 solubility, the identity of the anion accompanying the [2–I–2]+ cation was found to influence the iodo-antipyrine conversion in the order: OTf (63%) > PF6 (58%) > BF4 (50%) > SbF6 (49%). However, this trend of greater reactivity with the PF6 anion was not observed for [1–I–1]PF6 (76%), which performed noticeably poorer than [1–I–1]BF4 (93%). These observations support that the identity of the anion does indeed influence the reactivity of the iodine(I) species, but also that the outcome might be dependent on additional factors like the characteristics of the cation it is paired with, or the stability of the [I(Lewis base)][Anion] transition state upon dissociation of the second Lewis base.44 Elemental iodine was tested and found to give a similarly lacklustre conversion (55%) as the aforementioned DMAP (2) derivatives.
To complement the [L–X–L][Anion] complexes reported above, for the first time the iodination reaction with a neutral O–I–N stabilised carbonyl hypoiodite compound was investigated. In contrast to their in situ generated alkyl and non-stabilised analogues, the carbonyl hypoiodites are isolable and often amenable to solid-state studies like X-ray diffraction.39,40,45 Their charge-neutral composition offers advantages of greater solubility in a wider range of organic solvents over the Barluenga-type [N–I–N]+ iodine(I) complexes. This is particularly true in the upcoming pursuit of multi-iodine(I) species where the solubility of bis([N–I–N]+) complexes is reduced in organic media,1,45 whilst the bis(O–I–N) and the recently reported first examples of tris(O–I–N) compounds still maintain good solubility.41,46 Whilst the mono(O–I–N) carbonyl hypoiodite, PhC(O)OI–2, was able to enact the conversion, despite its stabilisation, it was found to give the worst conversion (37%) of the iodine(I) species tested.
It should be noted that the description of stabilised carbonyl hypoiodites as halogen(I) compounds has only recently been established,40 with this being the first report of their iodination reactivity. Whilst the active I+ species generated from the homoleptic [L–I–L]+ (L = 1–5) species tested, i.e. [L–I]+via the loss of L, the active I+ species for stabilised carbonyl hypoiodites is unknown at this time (as previously described for in situ generated hypoiodites). Due to the heteroleptic nature of PhC(O)OI–2 there are two possibilities for the active I+ species, via the loss of [PhC(O)O]− or 2 to give either [2–I]+ (analogous to the homoleptic [L–I–L]+ complexes) or PhC(O)OI, respectively. If the active species was [2–I]+ from loss of [PhC(O)O]−, then analogous reactivity to the [2–I–2]+ species might have been expected, which was not found to be the case. However, given the largely organic nature of the [PhC(O)O]− anion, the lower reactivity might simply by a reflection of the aforementioned anion effect noted for the series of [2–I–2]+ complexes.
To determine if some of the iodination reagents were feasible alternatives to Barluenga's reagent despite their reduced kinetics, with the potential to deliver comparable percentage conversions as [1–I–1]BF4, the poorer performing species were re-tested over the longer period of 22 hours (Table 2). In all four instances, the percentage conversions of antipyrine to iodo-antipyrine were found to greatly increase in the range of 21–35%, with [2–I–2]PF6 having the smallest increase and I2 demonstrating the largest improvement. These results highlight that iodine(I) species with better shelf lives such as those based on the [2–I–2]+ cation, for which no degradation has been observed over much longer timeframes (>6 months) than with those based on the [1–I–1]+ cation that does experience such issues, are indeed viable alternatives as iodination reagents. Species like [2–I–2]PF6 would indeed be better suited for research groups that only periodically utilise iodination reagents, as it is amenable to being prepared on a large scale and can be reliably stored over the long periods between uses, without the necessity of freshly preparing or purchasing it each time it is required. A reliable shelf life is also an important consideration for researchers performing reactions that are sensitive (e.g., to acids or bases) to the potential decomposition products of [1–I–1]BF4 (HOI, pyridine).
| Iodination reagent | % Conversion (2 hours) | % Conversion (22 hours) |
|---|---|---|
| I2 | 55 | 90 |
| [2–I–2]BF4 | 50 | 78 |
| [2–I–2]PF6 | 58 | 79 |
| PhC(O)OI–2 | 37 | 68 |
Conclusions
A variety of iodine(I) complexes, both known and new, were synthesised and thoroughly purified to test the dependence of iodination reactivity on the identity of the Lewis bases and anions present. These results would indicate that [1–I–1]BF4 merits it popularity as an iodination reagent, despite its limited shelf life, with it proving to be the best iodination reagent tested in terms of percentage conversion. However, several viable alternatives of comparable performance ([4–I–4]PF6, [5–I–5]PF6) were identified, which could offer advantages such as less nucleophilic pyridine-based ligands being present in the reaction mixture. However, iodination studies with longer reaction times found that even the more stable, and thus poorer performing, iodination reagents could yield respectable percentage conversions, overcoming the reduced kinetics their increased stabilisation imparts. Despite their lower efficiency, the greater stability and shelf life would make iodination reagents like [2–I–2]PF6 viable alternatives for more practical considerations. These considerations will appeal to researchers that only sparingly utilise such reagents, or where expensive and hard to replace starting materials might be jeopardised through the use of potentially degraded samples of [1–I–1]BF4.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the Academy of Finland (K. R. grant no. 351121), the Magnus Ehrnrooth Foundation (J. S. W.), and the University of Jyväskylä, Finland for financial support.Notes and references
- A. Vanderkooy, A. K. Gupta, T. Földes, S. Lindblad, A. Orthaber, I. Pápai and M. Erdélyi, Angew. Chem., Int. Ed., 2019, 58, 9012–9016 CrossRef CAS PubMed.
- J. S. Ward, G. Fiorini, A. Frontera and K. Rissanen, Chem. Commun., 2020, 56, 8428–8431 RSC.
- G. Gong, S. Lv, J. Han, F. Xie, Q. Li, N. Xia, W. Zeng, Y. Chen, L. Wang, J. Wang and S. Chen, Angew. Chem., Int. Ed., 2021, 60, 14831–14835 CrossRef CAS PubMed.
- S. Yu, P. Kumar, J. S. Ward, A. Frontera and K. Rissanen, Chem, 2021, 7, 948–958 CAS.
- J. S. Ward, A. Frontera and K. Rissanen, Inorg. Chem., 2021, 60, 5383–5390 CrossRef CAS PubMed.
- S. Wilcox, D. Sethio, J. S. Ward, A. Frontera, R. Lindh, K. Rissanen and M. Erdélyi, Chem. Commun., 2022, 58, 4977–4980 RSC.
- L. C. Gilday, S. W. Robinson, T. A. Barendt, M. J. Langton, B. R. Mullaney and P. D. Beer, Chem. Rev., 2015, 115, 7118–7195 CrossRef CAS PubMed.
- L. Turunen, U. Warzok, R. Puttreddy, N. K. Beyeh, C. A. Schalley and K. Rissanen, Angew. Chem., Int. Ed., 2016, 55, 14033–14036 CrossRef CAS PubMed.
- L. Turunen, U. Warzok, C. A. Schalley and K. Rissanen, Chem, 2017, 3, 861–869 CAS.
- G. Cavallo, P. Metrangolo, R. Milani, T. Pilati, A. Priimagi, G. Resnati and G. Terraneo, Chem. Rev., 2016, 116, 2478–2601 CrossRef CAS PubMed.
- M. Bedin, A. Karim, M. Reitti, A.-C. C. Carlsson, F. Topić, M. Cetina, F. Pan, V. Havel, F. Al-Ameri, V. Sindelar, K. Rissanen, J. Gräfenstein and M. Erdélyi, Chem. Sci., 2015, 6, 3746–3756 RSC.
- A.-C. C. Carlsson, K. Mehmeti, M. Uhrbom, A. Karim, M. Bedin, R. Puttreddy, R. Kleinmaier, A. A. Neverov, B. Nekoueishahraki, J. Gräfenstein, K. Rissanen and M. Erdélyi, J. Am. Chem. Soc., 2016, 138, 9853–9863 CrossRef CAS PubMed.
- J. S. Ward, K.-N. Truong, M. Erdélyi and K. Rissanen, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, Elsevier, 2022, DOI:10.1016/B978-0-12-823144-9.00043-1.
- C. P. Brock, Y. Fu, L. K. Blair, P. Chen and M. Lovell, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1988, 44, 1582–1585 CrossRef.
- J. S. Ward, A. Frontera and K. Rissanen, Dalton Trans., 2021, 50, 8297–8301 RSC.
- S. Lindblad, F. B. Németh, T. Földes, A. Vanderkooy, I. Pápai and M. Erdélyi, Chem. Commun., 2020, 56, 9671–9674 RSC.
- G. H.-Y. Lin and H. Hope, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, 28, 643–646 CrossRef CAS.
- F. Demartin, P. Deplano, F. A. Devillanova, F. Isaia, V. Lippolis and G. Verani, Inorg. Chem., 1993, 32, 3694–3699 CrossRef CAS.
- E. S. Stoyanov, I. V. Stoyanova, F. S. Tham and C. A. Reed, J. Am. Chem. Soc., 2010, 132, 4062–4063 CrossRef CAS PubMed.
- A. Karim, M. Reitti, A.-C. C. Carlsson, J. Gräfenstein and M. Erdélyi, Chem. Sci., 2014, 5, 3226–3233 RSC.
- L. Turunen and M. Erdélyi, Chem. Soc. Rev., 2020, 49, 2688–2700 RSC.
- M. H. Kolář and P. Hobza, Chem. Rev., 2016, 116, 5155–5187 CrossRef PubMed.
- J. Pancholi and P. D. Beer, Coord. Chem. Rev., 2020, 416, 213281 CrossRef CAS.
- J. A. Creighton, I. Haque and J. L. Wood, Chem. Commun., 1966, 229 RSC.
- I. Haque and J. L. Wood, J. Mol. Struct., 1968, 2, 217–238 CrossRef CAS.
- J. Barluenga, J. M. González, P. J. Campos and G. Asensio, Angew. Chem., Int. Ed. Engl., 1985, 24, 319–320 CrossRef.
- J. Barluenga, Pure Appl. Chem., 1999, 71, 431–436 CrossRef CAS.
- J. M. Chalker, A. L. Thompson, B. G. Davis, N. Zaware and P. Wipf, Org. Synth., 2010, 87, 288–298 CrossRef CAS.
- J. Barluenga, J. M. González, M. A. Garcia-Martin, P. J. Campos and G. Asensio, J. Chem. Soc., Chem. Commun., 1992, 1016–1017 RSC.
- J. Ezquerra, C. Pedregal, C. Lamas, J. Barluenga, M. Pérez, M. A. García-Martín and J. M. González, J. Org. Chem., 1996, 61, 5804–5812 CrossRef CAS.
- G. Espuña, G. Arsequell, G. Valencia, J. Barluenga, M. Pérez and J. M. González, Chem. Commun., 2000, 1307–1308 RSC.
- J. Barluenga, F. González-Bobes, M. C. Murguía, S. R. Ananthoju and J. M. González, Chem. – Eur. J., 2004, 10, 4206–4213 CrossRef CAS PubMed.
- K. Muñiz, B. García, C. Martínez and A. Piccinelli, Chem. – Eur. J., 2017, 23, 1539–1545 CrossRef PubMed.
- D. D. Tanner and G. C. Gidley, J. Am. Chem. Soc., 1968, 90, 808–809 CrossRef CAS.
- J. Barluenga, F. González-Bobes and J. M. González, Angew. Chem., Int. Ed., 2002, 41, 2556–2558 CrossRef CAS PubMed.
- R. Montoro and T. Wirth, Org. Lett., 2003, 5, 4729–4731 CrossRef CAS PubMed.
- D. D. Tanner, G. C. Gidley, N. Das, J. E. Rowe and A. Potter, J. Am. Chem. Soc., 1984, 106, 5261–5267 CrossRef CAS.
- J. L. Courtneidge, J. Lusztyk and D. Pagé, Tetrahedron Lett., 1994, 35, 1003–1006 CrossRef CAS.
- T. Hokamp, A. T. Storm, M. Yusubov and T. Wirth, Synlett, 2018, 415–418 CAS.
- S. Yu, J. S. Ward, K.-N. Truong and K. Rissanen, Angew. Chem., Int. Ed., 2021, 60, 20739–20743 CrossRef CAS PubMed.
- E. Kramer, S. Yu, J. S. Ward and K. Rissanen, Dalton Trans., 2021, 50, 14990–14993 RSC.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS PubMed.
- P. J. Campos, J. Arranz and M. A. Rodríguez, Tetrahedron Lett., 1997, 38, 8397–8400 CrossRef CAS.
- D. von der Heiden, F. B. Németh, M. Andreasson, D. Sethio, I. Pápai and M. Erdélyi, Org. Biomol. Chem., 2021, 19, 8307–8323 RSC.
- E. Taipale, M. Siepmann, K.-N. Truong and K. Rissanen, Chem. – Eur. J., 2021, 27, 17412–17419 CrossRef CAS PubMed.
- J. S. Ward, J. Martõnova, L. M. E. Wilson, E. Kramer, R. Aav and K. Rissanen, Dalton Trans., 2022, 51, 14646–14653 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, NMR and X-ray crystallographic details. CCDC 2064895, 2208422–2208425. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2nj05349g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |