One-pot Mannich/aza-Wittig/deaminative aromatization reactions for the synthesis of 1,2,3,4-tetrahydroacridines and cyclohepta[b]quinolines†
Wensheng Zhanga,
Wei Zhenga,
Guoqiang Zuoa,
Xiaole Lia,
Xiaofeng Zhang *bc and
Wei Zhang
*bc and
Wei Zhang *b
*b
aJiyuan Vocational and Technical College, 88 Jiyuan Avenue, Jiyuan 459000, China
bDepartment of Chemistry, University of Massachusetts Boston, 100 Morrissey Boulevard, Boston, MA 02125, USA. E-mail: wei2.zhang@umb.edu
cDepartment of Cancer Biology, Dana-Farber Cancer Institute and Harvard Medical School, Boston, MA 02215, USA. E-mail: xfxiaofengzhang@gmail.com
First published on 16th November 2022
Abstract
A one-pot synthesis for the preparation of 1,2,3,4-tetrahydroacridines involving a Zr(IV)-catalyzed Mannich reaction of o-azidobenzaldehydes and arylamines with cycloketones, followed by aza-Witting cyclization and deaminative aromatization, is developed. This method can be extended for the synthesis of cyclohepta[b]quinolines.
Introduction
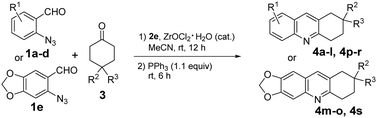
Acridine is a privileged ring which could be found in natural products, such as plakinidine A and B1a and dercitin,1b as well as drug molecules, such as amsacrine (cytotoxic, antiviral),2a,b botiacrine (antiparkinasonian),2c,d clomacran (tranquilizer),2e,f and monometacrine (antidepressant),2g,h antitumor agents,3a acetylcholinesterase inhibitors,3b anticancer agents,3c and DNA binding agents (Fig. 1).3d 1,2,3,4-Tetrahydroacridine is an analogue of partially reduced acridine. The development of efficient methods for the synthesis of 1,2,3,4-tetrahydroacridine is desirable.3b,c,4 The Friedländer-type reaction between o-aminobenzaldehyde and cyclohexanone is a traditional approach for 1,2,3,4-tetrahydroacridines (Scheme 1, A).4 The Katritzky group developed a one-pot synthesis involving the addition of a Vilsmeier-type reagent (benzotriazole iminium salt) to N-arylcyclohexanimines, followed by cyclization and elimination reactions (Scheme 1, B).5 The Basavaiah group utilized Baylis–Hillman alcohols (B–H alcohols) derived from 2-nitrobenzaldehydes and cyclohex-2-enone for the synthesis of 1-acetoxy-1,2,3,4-tetrahydroacridines (Scheme 1, C).6 The Ramachary group reported the List–Lerner–Barbas aldol (LLB-A) reaction of 2-azidobenzaldehydes and cyclohexanones for the synthesis of 1,2,3,4-tetrahydroacridines (Scheme 2, A).7 The iminophosphorane intermediate generated from tributylphosphine and the azido group underwent an intramolecular aza-Wittig reaction, followed by dehydroxylative aromatization, to generate bioactive tetrahydroacridines.Readily available o-azidobenzaldehydes are a versatile synthon for constructing nitrogen-containing heterocycles.8 We have utilized them in the synthesis of quinolines,9 tetrahydroquinolines,10 benzodiazepines,11 benzodiazepinones,12 triazo-fused benzodiazepines13 and dihydroquinazolinethiones.14 We have also reported the synthesis of 4-hydroxyquinolines through a cascade Knoevenagel reaction of 2-azidobenzaldehydes and α-fluoro-β-ketoesters and an intramolecular aza-Wittig reaction (Scheme 2, B)9b and the construction of 4-aminoquinolines through a cascade Mannich reaction of 2-azidobenzaldehydes, amines and α-fluoro-β-ketoesters, followed by aza-Wittig cyclization (Scheme 2, C).9c Literature survey reveals that there is no report on the deaminative aromatization for preparing quinolines after the intramolecular aza-Wittig reaction. We decided to explore the reaction of o-azidobenzaldehydes 1, amines 2 and cycloketones 3 in sequential Mannich/aza-Wittig/deaminative aromatization reactions to construct biologically interesting tetrahydroacridines 4 (Scheme 3).
Results and discussion
The development of reaction conditions was carried out using 2-azidobenzaldehyde 1a (1 equiv.), aniline 2a (1.05 equiv.) and cyclohexanone 3a (1.5 equiv.) as the substrates. The Mannich reaction catalysed by 15 mol% of ZrOCl2·8H2O was performed in MeCN (5 mL) at rt for 12 h following the literature procedures.15 Without isolation of the intermediate, the Mannich product was treated with PPh3 (1.2 equiv.) for the intramolecular aza-Wittig reaction, followed by sequential deaminative aromatization at rt for 6 h to afford product 4a in 73% yield (Table 1, entry 1). Screening of a number of arylamines 2c–f indicated that 4-chloroaniline 2e is the best choice which gave product 4a in 87% yield (entries 2–6). Other conditions such as a higher reaction temperature and a prolonged reaction time failed to improve the yields (entries 7 and 8).| Entry | R-NH2 (2) | T (°C) | t (h) | 4a (%) |
|---|---|---|---|---|
| a Reaction conditions: (1) 1a (0.5 mmol), 2 (0.525 mmol/1.05 eq.), 3a (0.75 mmol/1.5 equiv.), ZrOCl2·8H2O (0.075 mmol/0.15 eq.), MeCN (5 mL), rt, 12 h; (2) PPh3 (0.6 mmol/1.2 eq.), rt, 6 h. b Isolated yield based on 1a. | ||||
| 1 | Ph-NH2, 2a | rt | 6 | 73 |
| 2 | Bn-NH2, 2b | rt | 6 | 23 |
| 3 | 4-Me-Ph-NH2, 2c | rt | 6 | 76 |
| 4 | 4-MeO-Ph-NH2, 2d | rt | 6 | 81 |
| 5 | 4-Cl-Ph-NH2, 2e | rt | 6 | 87 |
| 6 | 4-CO2Me-Ph-NH2, 2f | rt | 6 | 80 |
| 7 | 4-Cl-Ph-NH2, 2e | 105 | 6 | 85 |
| 8 | 4-Cl-Ph-NH2, 2e | rt | 12 | 88 |
The scope of this new reaction process was evaluated under the optimized conditions using different o-azidobenzaldehydes 1 and cyclohexanones 3 (Table 2). It was found that Cl and Br on the benzene ring of 1 and Me at the 4-position of cyclohexanone 3 had little impact on the product yields. For example, reactions of 3a with halogen-containing 1b, 1c and 1d proceeded smoothly to produce 4d, 4g and 4j in 91%, 80% and 84%, respectively (entries 4, 7 and 10). These products could be further derivatized through metal-catalysed cross-coupling reactions. The reaction of 4-substituted cyclohexanones 3 gave products 4e–f, 4h–4i and 4k–l in 78–86% yields (entries 5, 6, 8, 9, 10 and 12). Reactions of 1e bearing an electron donating group also afforded the corresponding products 4m–4o in 83–87% yields (entries 13–15). However, the reaction of 4,4-difluorocyclohexan-1-one 3d afforded products 4p–4s in relatively low yields of 53–63% (entries 16–19).
| Entry | Aldehyde 1 | Cyclic ketone 3 | 4 (%) | |||
|---|---|---|---|---|---|---|
| R1 | 1 | R2 | R3 | 3 | ||
| a Reaction conditions: (1) 1 (0.5 mmol), 2e (0.525 mmol/1.05 eq.), 3 (0.75 mmol/1.5 equiv.), ZrOCl2·8H2O (0.075 mmol/0.15 equiv.), MeCN (5 mL), rt, 12 h; (2) PPh3 (0.6 mmol/1.2 equiv.), rt, 6 h. b Isolated yield based on 1. | ||||||
| 1 | H | 1a | H | H | 3a | 4a, 87 |
| 2 | H | 1a | H | Me | 3b | 4b, 84 |
| 3 | H | 1a | Me | Me | 3c | 4c, 85 |
| 4 | 5-Cl | 1b | H | H | 3a | 4d, 91 |
| 5 | 5-Cl | 1b | H | Me | 3b | 4e, 84 |
| 6 | 5-Cl | 1b | Me | Me | 3c | 4f, 86 |
| 7 | 5-Br | 1c | H | H | 3a | 4g, 80 |
| 8 | 5-Br | 1c | H | Me | 3b | 4h, 82 |
| 9 | 5-Br | 1c | Me | Me | 3c | 4i, 78 |
| 10 | 4-Cl | 1d | H | H | 3a | 4j, 84 |
| 11 | 4-Cl | 1d | H | Me | 3b | 4k, 85 |
| 12 | 4-Cl | 1d | Me | Me | 3c | 4l, 79 |
| 13 | 1e | H | H | 3a | 4m, 87 | |
| 14 | 1e | H | Me | 3b | 4n, 86 | |
| 15 | 1e | Me | Me | 3c | 4o, 83 | |
| 16 | 5-Cl | 1b | F | F | 3d | 4p, 63 |
| 17 | 5-Br | 1c | F | F | 3d | 4q, 56 |
| 18 | 4-Cl | 1d | F | F | 3d | 4r, 60 |
| 19 | 1e | F | F | 3d | 4s, 53 | |
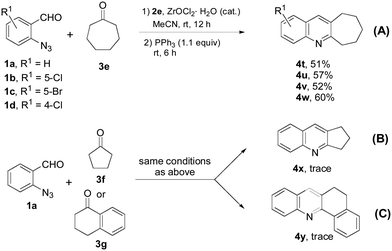
Other than 6-membered cyclic ketones, we also investigated the reaction of cycloheptanone 3e with 1a–d and obtained cyclohepta[b]quinolines 4t–4w in 51–60% yields. The lower yields from the reaction of cycloheptanone could have resulted from the steric effect of the 7-membered ring (Scheme 4, a). Under the same conditions, reaction 1a with cyclopentanone 3f (Scheme 4, b) or benzocyclohexanone 3g (Scheme 4, c) gave trace amounts of products 4x and 4y.
Control experiments were carried out to gain information for understanding the mechanism of the cascade reaction process and the role of aryl amines 2. Without using ZrOCl2·8H2O or in the presence of ZrOCl2·8H2O but without amines 2e, no product was detected from the reaction mixture. The results suggested that the formation of a Mannich base as a reaction intermediate is indispensable. A plausible mechanism to rationalize the formation of 4 is proposed (Scheme 5). The Mannich reaction of 1, 2 and 3 under the catalysis of ZrOCl2·8H2O provides intermediate A. Aza-Wittig cyclization of A in the presence of PPh3 affords N-substituted-9-aminohexahydroacridine intermediate B, followed by in situ release of aryl amine 2 to give product 4.
Conclusions
In summary, we have developed a 4-chloroaniline-mediated highly efficient and simple one-pot and two-step synthesis of 1,2,3,4-tetrahydroacridines and cyclohepta[b]quinolines using readily available o-azidobenzaldehydes and cycloketones. The reaction process involves sequential Zr(IV)-catalysed Mannich/intramolecular aza-Wittig/deaminative aromatization reactions to give the desired products in moderate to good yields. The one-pot reaction has green chemistry advantages of pot economy, resource efficiency, and elimination of the waste generated from intermediate purification.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the Program for Science and Technology Development of Henan (212102310883) and the Key Natural Science Research Program of Education Department of Henan (21B150013).Notes and references
- (a) W. D. Inman, M. O’Neill-Johnson and P. Crews, J. Am. Chem. Soc., 1990, 112, 1–4 CrossRef CAS; (b) G. P. Gunawardana, S. Kohmoto, S. P. Gunasekara, O. J. McConnell and F. E. Koehn, J. Am. Chem. Soc., 1988, 110, 4856–4858 CrossRef CAS.
- (a) Dictionary of Drugs, ed. J. Elks and C. R. Ganellin, Chapman and Hall, London, 1st edn, 1990, p. 84 Search PubMed; (b) B. F. Cain, G. J. Atwell and W. A. Denny, J. Med. Chem., 1975, 18, 1110–1117 CrossRef CAS; (c) Dictionary of Drugs, ed. J. Elks and C. R. Ganellin, Chapman and Hall, London, 1st edn, 1990, p. 170 Search PubMed; (d) I. Molnar, T. Wagner-Jauregg and U. Jahn, US Pat., 3830918, Search PubMed(Chem. Abstr., 1975, 83, 58674c); (e) Dictionary of Drugs, ed. J. Elks and C. R. Ganellin, Chapman and Hall, London, 1st edn, 1990, p. 297 Search PubMed; (f) C. L. Zirkle, German Pat., 1470245, Search PubMed(Chem. Abstr., 1973, 78, 147825s); (g) Dictionary of Drugs, ed. J. Elks and C. R. Ganellin, Chapman and Hall, London, 1st edn, 1990, p. 835 Search PubMed; (h) A.-G. Siegfried, French Pat., 1438357, Search PubMed(Chem. Abstr., 1967, 66, 10856j).
- (a) K. Dzierzbicka, A. M. Kolodziejczyk, B. Wysocka-Skrzela, A. Myśliwski and D. Sosnowska, J. Med. Chem., 2001, 44, 3606–3615 CrossRef CAS PubMed; (b) F. Gatta, M. R. Del Giudice, M. Pomponi and M. Marta, Heterocycles, 1992, 34, 991–1004 CrossRef CAS; (c) S. A. Gamage, J. A. Spicer, G. J. Atwell, G. J. Finlay, B. C. Baguley and W. A. Denny, J. Med. Chem., 1999, 42, 2383–2393 CrossRef CAS; (d) T. Bentin and P. E. Nielsen, J. Am. Chem. Soc., 2003, 125, 6378–6379 CrossRef CAS.
- (a) B. R. McNaughton and B. L. Miller, Org. Lett., 2003, 5, 4257–4259 CrossRef CAS; (b) R. Goossens, M. Smet and W. Dehaen, Tetrahedron Lett., 2002, 43, 6605–6608 CrossRef CAS; (c) J. Dinesen, J. P. Jacobsen, F. P. Hansen, E. B. Pedersen and H. Eggert, J. Med. Chem., 1990, 33, 93–97 CrossRef CAS PubMed; (d) S. S. Palimkar, S. A. Siddiqui, T. Daniel, R. J. Lahoti and K. V. Srinivasan, J. Org. Chem., 2003, 68, 9371–9378 CrossRef CAS; (e) A. Arcadi, M. Chiarini, S. D. Giuseppe and F. Marinelli, Synlett, 2003, 203–206 CrossRef CAS; (f) C. S. Cho, B. T. Kim, T.-J. Kim and S. C. Shim, Chem. Commun., 2001, 2576–2577 RSC; (g) L. Ren, T. Lei and L. Z. Gong, Chem. Commun., 2011, 47, 11683–11685 RSC; (h) R. Martínez, D. J. Ramón and M. Yus, J. Org. Chem., 2008, 73, 9778–9780 CrossRef; (i) A. Bañón-Caballero, G. Guillena and C. Nájera, J. Org. Chem., 2013, 78, 5349–5356 CrossRef; (j) N. Anand, S. Koley, B. J. Ramulu and M. S. Singh, Org. Biomol. Chem., 2015, 13, 9570–9574 RSC; (k) L. Li and D. Seidel, Org. Lett., 2010, 12, 5064–5067 CrossRef CAS PubMed.
- A. R. Katritzky and M. J. Arend, Org. Chem., 1998, 63, 9989–9991 CrossRef CAS.
- D. Basavaiah, J. S. Rao and R. J. Reddy, J. Org. Chem., 2004, 69, 7379–7382 CrossRef CAS.
- D. B. Ramachary and K. S. Shruth, J. Org. Chem., 2016, 81, 2405–2419 CrossRef CAS PubMed.
- (a) S. J. Gharpure, S. K. Nanda, P. A. Adate and Y. G. Shelke, J. Org. Chem., 2017, 82, 2067–2080 CrossRef CAS PubMed; (b) J. G. Harrison, O. Gutierrez, N. Jana, T. G. Driver and D. J. Tantillo, J. Am. Chem. Soc., 2016, 138, 487–490 CrossRef CAS PubMed; (c) F. Qu, P. He, R. F. Hu, X. H. Cheng, S. Wang and J. Wu, Synth. Commun., 2015, 45, 2802–2809 CrossRef CAS; (d) K. G. Guggenheim, H. Toru and M. J. Kurth, Org. Lett., 2012, 14, 3732–3735 CrossRef CAS PubMed; (e) H. H. Nguyen, T. A. Palazzo and M. J. Kurth, Org. Lett., 2013, 15, 4492–4495 CrossRef CAS PubMed; (f) C. Xu, F. C. Jia, Z. W. Zhou, S. J. Zheng, H. Li and A. X. Wu, J. Org. Chem., 2016, 81, 3000–3006 CrossRef CAS.
- (a) X. F. Zhang, G. Dhawan, A. Muthengi, S. Liu, W. Wang, M. Legris and W. Zhang, Green Chem., 2017, 19, 3851–3855 RSC; (b) X. F. Zhang, X. M. Ma, W. Q. Qiu, J. Evansa and W. Zhang, Green Chem., 2019, 21, 349–354 RSC; (c) X. F. Zhang, X. M. Ma, W. Q. Qiu, J. M. Awad, J. Evans and W. Zhang, Adv. Synth. Catal., 2020, 362, 5513–5517 CrossRef CAS.
- X. F. Zhang, K. Pham, S. Liu, M. Legris, A. Muthengi and W. Zhang, Beilstein J. Org. Chem., 2016, 12, 2204–2210 CrossRef CAS PubMed.
- (a) X. M. Ma, X. F. Zhang, J. Award, G. Xie, W. Q. Qiu and W. Zhang, Green Chem., 2019, 21, 4489–4494 RSC; (b) X. M. Ma, X. F. Zhang, J. Award, G. Xie, W. Q. Qiu, R. E. Muriph and W. Zhang, Tetrahedron Lett., 2020, 61, 151392 CrossRef CAS.
- A. Muthengi, J. Erickson, R. E. Muriph and W. Zhang, J. Org. Chem., 2019, 84, 5927–5935 CrossRef CAS PubMed.
- (a) X. F. Zhang, S. J. Zhi, W. Wang, S. Liu, J. P. Jasinski and W. Zhang, Green Chem., 2016, 18, 2642–2646 RSC; (b) X. M. Ma, X. F. Zhang, W. Q. Qiu, W. Zhang, B. Wan, J. Evans and W. Zhang, Molecules, 2019, 24, 601–607 CrossRef PubMed.
- (a) W. S. Zhang, X. F. Zhang, X. M. Ma and W. Zhang, Tetrahedron Lett., 2018, 59, 3845–3847 CrossRef CAS; (b) W. S. Zhang, Y. Li, H. Y. Zhou, X. L. Su, X. F. Zhang and W. Zhang, Tetrahedron Lett., 2021, 81, 153361 CrossRef CAS.
- B. Eftekhari-Sis, A. Abdollahifar, M. M. Hashemi and M. Zirak, Eur. J. Org. Chem., 2006, 5152–5157 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, compound characterization, and NMR spectra. See DOI: https://doi.org/10.1039/d2nj04082d |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2023 |