Electrifying H2O2 synthesis with g-C3N4-based single atom catalysts†
Jungki
Ryu
 *
*
School of Energy and Chemical Engineering, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, Republic of Korea. E-mail: jryu@unist.ac.kr
Abstract
This article highlights the recent work of Zhang, Peng et al. (Nanoscale Horiz., 2023, 8, 695, https://doi.org/10.1039/D2NH00564F) on transition metal single atom embedded graphitic carbon nitride nanosheets for the neutral electrosynthesis of hydrogen peroxide.
Electrifying the synthesis of commodity chemicals can play a critical role in achieving carbon neutrality, as well as addressing global energy and environmental problems. Among the diverse range of chemicals, hydrogen peroxide (H2O2) has emerged as a promising green oxidant and liquid hydrogen carrier.1 However, H2O2 is currently produced by the anthraquinone autooxidation process under harsh conditions with huge energy consumption.2 Consequently, researchers have been actively exploring alternative approaches for the synthesis of H2O2 using renewable resources under milder conditions.
In this regard, extensive studies have focused on the green synthesis of H2O2 using renewable electricity and employing either electrocatalysts3 or photocatalysts4 directly powered by sunlight. Most conventional studies on electrochemical H2O2 production have been conducted under alkaline conditions, which are known to facilitate efficient H2O2 production. However, it is important to note that H2O2 becomes unstable at high pHs.5 Moreover, from an environmental standpoint, there is a strong desire to develop electrocatalysts that can operate at neutral pH.
In this context, a recent paper by Yang et al. reports very interesting results (https://doi.org/10.1039/D2NH00564F). The researchers prepared graphitic carbon nitride (g-C3N4) nanosheets (CNNS) embedded with various transition metal single atoms (TM SAs) and discovered that TM SA-embedded CNNS show high electrocatalytic activity for H2O2 production at neutral pHs (Fig. 1). Among the various TM SAs tested, Ni SAs on CNNS were particularly effective and showed the highest mass-specific activity of ∼503 mmol gcat−1 h−1 and H2O2 selectivity of ∼98%. According to their mechanistic analysis, the introduction of TM SAs promotes the formation of N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N sites, which are beneficial for H2O2 production via a two-electron oxygen reduction reaction (2e− ORR), while suppressing the formation of C–C/C
N sites, which are beneficial for H2O2 production via a two-electron oxygen reduction reaction (2e− ORR), while suppressing the formation of C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C sites, which are beneficial for H2O production via a 4e− ORR. This suggests the excellent function of g-C3N4 as a support for TM SAs in selectively producing H2O2.
C sites, which are beneficial for H2O production via a 4e− ORR. This suggests the excellent function of g-C3N4 as a support for TM SAs in selectively producing H2O2.
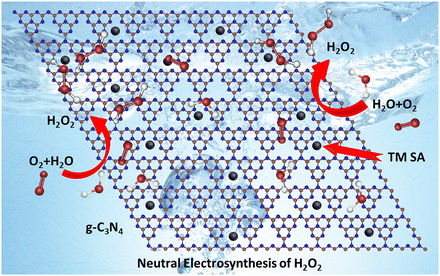 | ||
| Fig. 1 Schematic of H2O2 production from H2O and O2 on a transition metal embedded graphitic carbon nitride sheet. Reproduced from https://doi.org/10.1039/D2NH00564F with permission from the Royal Society of Chemistry. | ||
Notably, this paper is also intriguing from an academic perspective, as it demonstrates the efficient use of g-C3N4 as a support material for electrocatalysts, deviating from its traditional application as a photocatalyst in conventional studies.6 The findings offer new insights into the potential of g-C3N4 in catalytic systems and open avenues for further research in the field of sustainable chemical synthesis.
Acknowledgements
This work was also supported by the Basic Science Research Program (2021R1A2C2013684) and the Regional Leading Research Center (RLRC) (RS-2023-00217778) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT of Korea.References
- J. Y. Tang, T. S. Zhao, D. Solanki, X. B. Miao, W. G. Zhou and S. Hu, Joule, 2021, 5, 1432–1461 CrossRef CAS.
- J. M. Campos-Martin, G. Blanco-Brieva and J. L. G. Fierro, Angew. Chem., Int. Ed., 2006, 45, 6962–6984 CrossRef CAS PubMed.
- S. C. Perry, D. Pangotra, L. Vieira, L.-I. Csepei, V. Sieber, L. Wang, C. P. de León and F. C. Walsh, Nat. Rev. Chem., 2019, 3, 442–458 CrossRef CAS.
- H. Hou, X. Zeng and X. Zhang, Angew. Chem., Int. Ed., 2020, 59, 17356–17376 CrossRef CAS PubMed.
- H. M. Cota, T. Katan, M. Chin and F. J. Schoenweis, Nature, 1964, 203, 1281 CrossRef CAS.
- L. Xie, X. Wang, Z. Zhang, Y. Ma, T. Du, R. Wang and J. Wang, Small, 2023, 19, 2301007 CrossRef CAS PubMed.
Footnote |
| † This content was originally published on our blogs platform at https://blogs.rsc.org/nh/2023/08/22/electrifying-h2o2-synthesis-with-g-c3n4-based-single-atom-catalysts/. |
| This journal is © The Royal Society of Chemistry 2023 |