Neuronal maturation-dependent nano–neuro interaction and modulation†
Prashant
Gupta
 a,
Priya
Rathi
a,
Rohit
Gupta
a,
Harsh
Baldi
a,
Quentin
Coquerel
b,
Avishek
Debnath
a,
Hamed Gholami
Derami
a,
Baranidharan
Raman
a,
Priya
Rathi
a,
Rohit
Gupta
a,
Harsh
Baldi
a,
Quentin
Coquerel
b,
Avishek
Debnath
a,
Hamed Gholami
Derami
a,
Baranidharan
Raman
 *b and
Srikanth
Singamaneni
*b and
Srikanth
Singamaneni
 *a
*a
aDepartment of Mechanical Engineering and Materials Science, and Institute of Materials Science and Engineering, Washington University in St. Louis, St. Louis, MO 63130, USA. E-mail: singamaneni@wustl.edu
bDepartment of Biomedical Engineering, Washington University in St. Louis, St. Louis, MO 63130, USA. E-mail: barani@wustl.edu
First published on 29th August 2023
Abstract
Nanotechnology-enabled neuromodulation is a promising minimally-invasive tool in neuroscience and engineering for both fundamental studies and clinical applications. However, the nano–neuro interaction at different stages of maturation of a neural network and its implications for the nano–neuromodulation remain unclear. Here, we report heterogeneous to homogeneous transformation of neuromodulation in a progressively maturing neural network. Utilizing plasmonic-fluors as ultrabright fluorescent nanolabels, we reveal that negative surface charge of nanoparticles renders selective nano–neuro interaction with a strong correlation between the maturation stage of the individual neurons in the neural network and the density of the nanoparticles bound on the neurons. In stark contrast to homogeneous neuromodulation in a mature neural network reported so far, the maturation-dependent density of the nanoparticles bound to neurons in a developing neural network resulted in a heterogeneous optical neuromodulation (i.e., simultaneous excitation and inhibition of neural network activity). This study advances our understanding of nano–neuro interactions and nano–neuromodulation with potential applications in minimally-invasive technologies for treating neuronal disorders in parts of the mammalian brain where neurogenesis persists throughout aging.
New conceptsFor the first time, we reveal maturation-dependent binding of negatively charged nanoparticles to neurons cultured in vitro. We observed a progressive increase in the fraction of neurons tagged with nanoparticles and number of nanoparticles per neuron with an increase in the number of cultured days in vitro. In contrast to our current understanding, we note that the electrical activity of the neurons does not govern the binding of the nanoparticles to neurons; instead, the surface charge state of the neurons might play a critical role in nano–neuro interactions. This heterogeneous binding of the nanoparticles to neurons in a maturing network results in a heterogeneous modulation characterized by simultaneous excitation and inhibition of electrical activity under photothermal stimulation. In contrast, a matured neural network, comprised of neurons that are uniformly tagged with nanoparticles, exhibited homogeneous inhibition of electrical activity under optical stimulation that is completely reversible. Overall, our findings provide a better understanding of the interaction of the nanoparticles with neurons and provide critical guidelines in the design of nanoparticles for recording and stimulating neural activity. |
One of the major goals of modern biomedical research is to understand the working principles of the nervous system.1 As a rapidly growing technique, neuromodulation has proved to be of paramount importance in answering fundamental neuroscience questions and in devising advanced treatments of various neurological disorders.2 Electrical neuromodulation-based implantable devices developed over the past few decades have proved effective in the treatment of many debilitating medical conditions including Parkinson's disease, clinical depression, and epilepsy.3 However, the use of these devices (usually metal electrodes), owing to their bulkiness, mechanical invasiveness and inability to target individual neurons and neuronal circuits, is often limited.4,5 Optogenetics, involving genetic modification to control cellular activity via optical stimuli, has emerged as an attractive alternative tool over the past two decades.6,7 Although optogenetics overcomes many of the aforementioned issues associated with physical electrodes,7,8 it relies on genetic modification of neurons, which is irreversible and difficult to implement in model organisms without a rich repertoire of genetic tools.9 As such, nanomaterial based non-genetic neuromodulation approaches, which can be administered in a drug-like fashion, have been explored in recent years.1,10 Nano-enabled neuromodulation involves harvesting energy from an external source by the nanomaterials in a wireless manner and transducing it into physiologically relevant stimuli in a localized region (down to single neurons or subcellular compartments) for neural stimulation.11 Nano–neuromodulation also provides additional flexibility towards stimulation modes based on the energy sources employed in conjunction with specific transducing nanostructures such as optical,12 acoustic13 and magnetic14 stimulation. Among these, optical stimulation via photothermal nano-transducers (such as plasmonic nanostructures, graphene, polydopamine nanoparticles, etc.) has shown great promise and versatility.15–23 Moreover, recent advances in the development of efficient strategies for the transport of nanomaterials across the blood–brain barrier (BBB) to the brain parenchyma, via either receptor-mediated endocytosis, physical disruption of the BBB or local delivery, have demonstrated tremendous potential in clinical translation of nanomaterial-assisted neuromodulation for neurotherapeutics.24–27
Majority of the photothermal neuromodulation studies involve primary neuron culture close to its complete maturation stage as the model system. Although in most brain regions, neurogenesis (process of generating new functional neurons from precursors) has been confined to a discrete developmental period, life-long neurogenesis has been observed in both the hippocampus and subventricular zone of almost all mammals, including humans.28 The addition of new neurons to the complex circuitry of the adult brain plays a crucial role in memory and behavior.29 Interestingly, these immature neurons exhibit high excitability, reduced GABAergic inhibition and a lower threshold for the induction of long-term potentiation, which allows them to spike despite their developing glutamatergic inputs and participate in information processing before reaching a complete maturation stage.29 The interaction of nanomaterials with these young neurons, if any, in the heterogeneous neural network comprising of both young and mature neurons, and its implications for the nano–neuromodulation are yet to be elucidated. This improved understanding would pave the way in designing minimally invasive non-genetic nanomaterial-based neuromodulation techniques for both fundamental studies and clinical applications.
Recently, Dante et al. described the critical role of the surface charge of nanoparticles in their selective binding to neurons.30 They demonstrated that negatively charged nanoparticles, irrespective of shape, size and material composition of the nanomaterial, exclusively bind to excitable neuronal cells and never to non-excitable glial cells, whereas positively charged and neutral particles never spontaneously bind to neurons. Moreover, Walters et al.,31 using zwitterionic coatings, have systematically demonstrated that the surface charge of nanoparticles plays an important role in nano–neuro interaction rather than the specific chemical functional group. In this study, we harness plasmonic fluors-IR650 (PFs), ultrabright fluorescent nanoconstructs recently developed by our research group,32 to unveil the neuronal maturation-dependent nano–neuro interactions (Fig. 1). Building on these findings, we rationalize the nongenetic optical neuromodulation in both heterogeneous neural network (comprising of both young and mature neurons) and homogeneous neural network (majorly comprising of mature neurons) utilizing a commonly employed plasmonic photothermal nanotransducer, gold nanorods.15,17,19,21,22
Results
Role of the surface charge of nanoparticles in binding to neurons
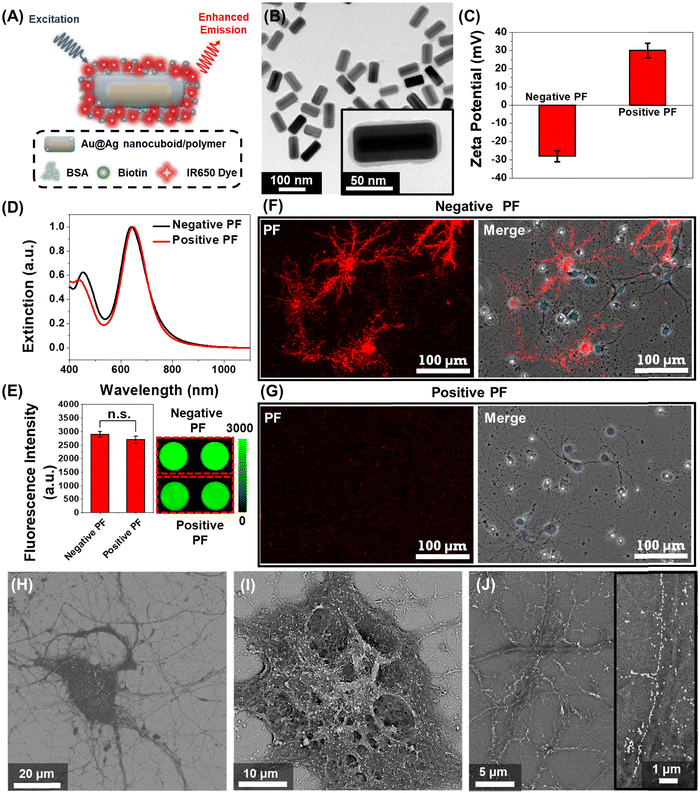
We employed plasmonic-fluors comprised of a near infrared dye IR-650 (PF-650) as model nanostructures to understand the interactions between nanoparticles and neurons. We have recently introduced plasmonic-fluors as ultrabright fluorescent nanoconstructs that are nearly 7000-fold brighter compared to the corresponding molecular fluorophores.32 PF-650 is comprised of Au@Ag nanocuboids as plasmonic nanoantenna, a siloxane copolymer layer as a dielectric spacer and BSA-biotin-IR650 conjugates (Fig. 1A). The transmission electron microscopy (TEM) image depicts the Au@Ag nanocuboids with a length 98 ± 5 nm and a width 42 ± 2.5 nm and the polymer and BSA-biotin-IR650 coating of 3 ± 1 nm (Fig. 1B). Owing to the presence of BSA on the surface, under physiological pH conditions, the PFs are negatively charged (with a ζ-potential of −28 ± 3 mV), henceforth termed as negatively charged PFs (Fig. 1C). The positively charged PFs (with a ζ-potential of +30 ± 4 mV) were obtained by coating negative PFs with poly(allylamine hydrochloride). The positively charged PFs showed no sign of aggregation as evidenced by the absence of broadening of the localized surface plasmon resonance (LSPR) band in the extinction spectrum and the retained florescence intensity (Fig. 1D and E). We then assessed the stability of both negatively charged PFs and positively charged PFs in the culture medium (NbActiv4) used for neural culture. We observed that both the particles exhibited stable dispersion in the medium after 1 hour of incubation assessed via extinction spectra as well as maintained their surface charge assessed via ζ-potential measurements (Fig. S1, ESI†). To investigate the interaction of nanoparticles with the neurons, primary hippocampal neuronal culture at DIV 14 was incubated with the negative and positive PFs for 1 hour in NbActiv4 medium, which is a serum-free medium. The absence of serum in the medium precludes the formation of protein corona on the nanoparticles, thus preserving their surface state. We observed that the negatively charged PFs readily bind to the neurons as evidenced by the co-localization of the PF fluorescence signal (λemission = 650 nm) with the neurons (Fig. 1F). On the other hand, the positively charged particles do not bind to the neurons (Fig. 1G), as reported previously.30 This observation suggests that the negative surface charge is a necessary condition for the spontaneous binding (i.e., without any specific targeting moiety) of the nanoparticles to the neurons. The PFs uniformly decorated the soma and the neurites of the neurons. The anisotropic nanostructures (i.e., nanocuboids) bound on the soma and thicker regions of neurites exhibited random orientation whereas those bound on the thinner regions of neurites were oriented along the length of the neurites (Fig. 1H–J). Notably, in most cases, the PFs bound to the thinner region of neurites formed a single-particle wide linear array. Considering that the lateral dimension of the neurites is 100–1000 nm, the longitudinal alignment of PFs possibly stems from the maximal interfacial contact area of the PFs with the neurites under this orientation.33–35Effect of nanoparticle binding on the electrical activity of the neural network
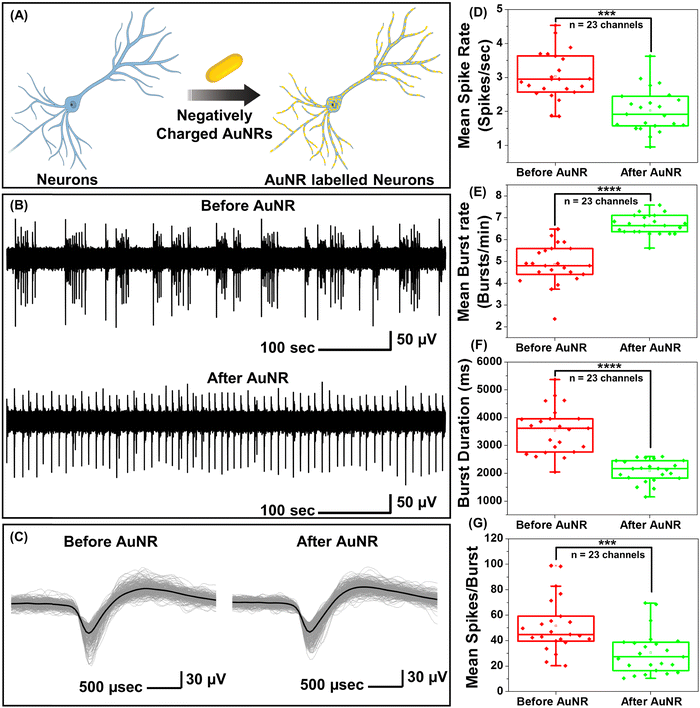
While PFs are extremely bright fluorescent nanoconstructs that serve as ideal nanolabels to monitor the binding of the nanostructures to neurons, they are not commonly employed for optical neuromodulation. Owing to the facile tunability of the LSPR wavelength over a broad range and their large extinction cross-section, gold nanorods (AuNRs) are highly attractive photothermal nanotransducers for optical neuromodulation.15,17,19,21,22 We set out to investigate the effect of the binding of the negatively charged AuNRs on the electrical activity of the neurons (Fig. S2A, ESI†). Negatively charged AuNRs were obtained by coating the as-synthesized AuNRs with polystyrene sulfonate (PSS). Following the PSS coating, the AuNRs exhibited a blue shift (of 10 nm) in the LSPR wavelength and a zeta-potential of −34 ± 4 mV (Fig. S2B, ESI†). Negatively charged AuNRs exhibited similar nano–neuro interaction as compared to negatively charged PFs (Fig. S3, ESI†). To investigate the change in the electrical activity of neurons in response to nanoparticle binding, hippocampal neurons were cultured on microelectrode arrays (MEAs) consisting of 60 electrodes. The extracellular activity of neurons was recorded with and without AuNR incubation (Fig. 2A). Neurons cultured on MEAs formed a dense network of neurites around TiN recording electrodes (Fig. S4, ESI†). To investigate the effect of nano–neuro interaction on the electrical activity of the primary hippocampal cultured neurons, the extracellular activity was recorded for 10 min prior to AuNR binding at 14 days-in vitro (DIV 14) (Fig. 2B). The neurons cultured on MEAs were then incubated with AuNRs (at a final concentration corresponding to optical density (O.D.) ∼ 0.5 at the LSPR wavelength) for 1 hour followed by rinsing with the medium. Following the binding of AuNRs to the neurons, although there is a significant change in the spontaneous electrical activity of the neuronal network, the spike shape and amplitude remained unaltered (Fig. 2B, C, Fig. S5 and S6, ESI†). The mean spike rate of the network measured over a period of 10 min reduced significantly while the burst activity significantly increased (Fig. 2D and E). Note that the duration of the burst events and number of spikes per burst decreased significantly after binding of the AuNRs (Fig. 2F and G).Homogeneous and heterogeneous modulation of neuronal activity through photothermal stimulation
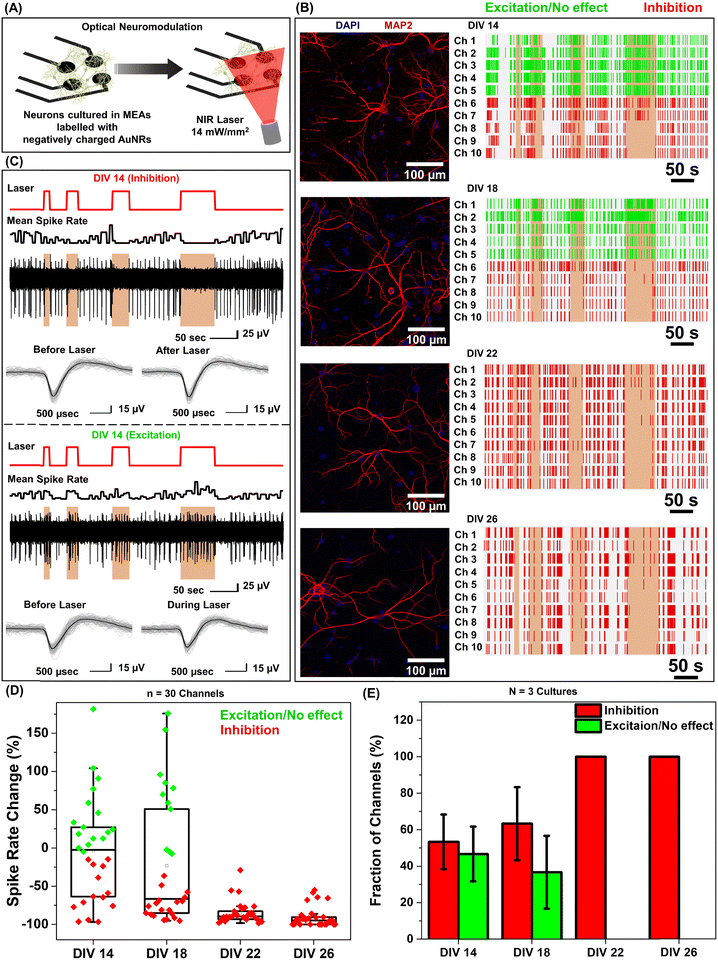
Under near infrared laser illumination, the plasmonic nanostructures bound on the neurons result in localized temperature rise, which in turn either reversibly alters the electrical capacitance and therefore the excitability of the neurons or reversibly activates the temperature sensitive TREK-1 ion channels and consequently reduces the discharge of action potentials.17,36,37 Owing to their ability to readily bind to neurons (Fig. 1F), we employed negatively charged AuNRs to understand the effect of maturation stage of the neuronal network on photothermal neuromodulation. Hippocampal neurons cultured on MEAs were incubated with negatively charged AuNRs (76.2 pM final concentration) for 1 hour at DIV 14, 18, 22 and 26, followed by washing with the NbActiv4 medium. Different MEAs were utilized at different DIVs, so as to avoid any interference from nanoparticle-induced neuronal membrane depolarization in the neuron maturation process.30 Note that the kinetics of the neuronal maturation is significantly modulated by local network activity.38The AuNR localized neurons were subjected to repeated irradiation of an 808 nm laser at a power density of 14 mW mm−2 for different durations (10, 20, 30 and 60 seconds) in a back-to-back pulsatile fashion (Fig. 3A). The extracellular activity of the neurons was recorded before, during, and after the photothermal treatment. The extracellular signal recorded by each of the MEA channels corresponds to a group of neurons on and around the channels that are irradiated by the NIR laser. At DIV 14 and 18, a small fraction of channels (10 to 30%) exhibited complete inhibition of neural activity in response to photothermal stimulation (observed from the mean spike rate before, during and after laser illumination) which may be attributed to the membrane-localized photothermal heating via AuNRs. However, most of the electrodes depicted partial reduction, enhancement or no change in spiking activity (Fig. 3B and D). In the channels where spiking activity was suppressed during photothermal treatment, the shape and amplitude of the remnant spikes remained unaltered before and after laser illumination suggesting the reversibility of the neuromodulation (Fig. 3C, top panel). In addition, no significant difference in the spike shape and amplitude was observed in the channels exhibiting excitation during laser stimuli (Fig. 3C, bottom panel). Furthermore, for all cells, no significant difference in the mean spike rate was observed before and after photothermal neuromodulation, which further confirms the complete reversibility of the nano–neuromodulation (Fig. S7, ESI†). At DIV 22 and 26, the photothermal neuromodulation resulted in nearly complete inhibition of spiking activity (Fig. 3B and D). Moreover, with an increase in DIV from 14 to 18, a larger fraction of the electrodes exhibited inhibition in response to photothermal stimulation, finally reaching 100% at DIV 22 and above (Fig. 3E). These results highlight the transformation of the photothermal neuromodulation from a heterogeneous (in early stages of DIV 14 and 18) to homogeneous (in later stages of DIV 22 and 26) change in electrical activity.
Heterogeneous nano–neuro interaction
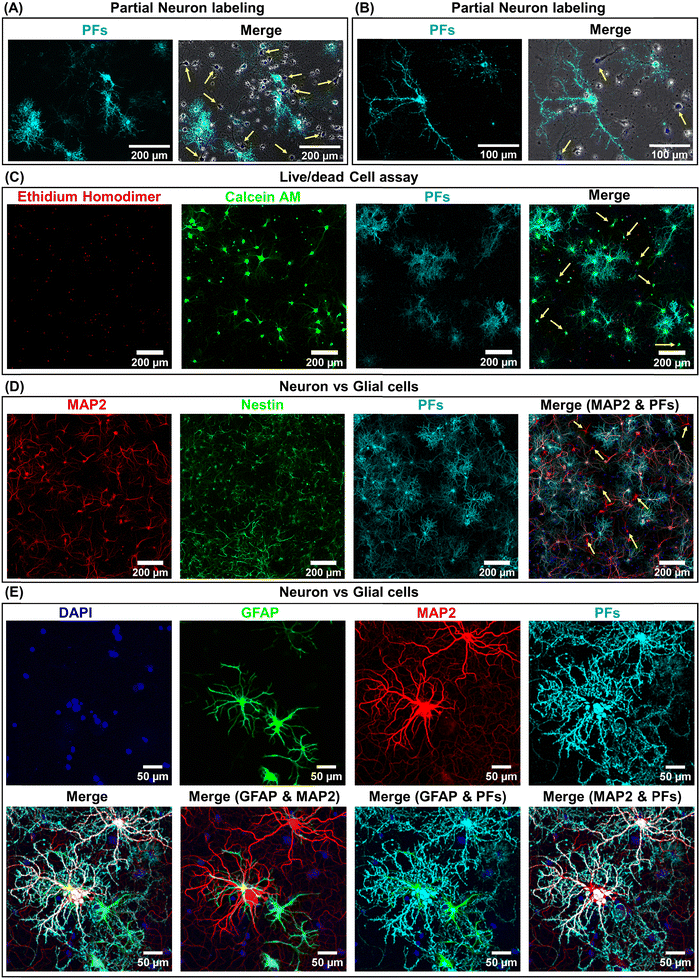
We hypothesized that the heterogeneity in the nano–neuromodulation at early stages of the neuronal network maturation is associated with the heterogeneity in the nano–neuro interaction. To test this hypothesis, we examined the binding of negative PFs to neurons at DIV 14, a time point at which we observed heterogeneous neuromodulation (Fig. 3E). We observed that only a small fraction of the cells are tagged with negative PFs while most of the cells are devoid of nanoparticles (Fig. 4A, untagged cells indicated by yellow arrows). This observation suggested that at DIV 14, there is indeed a heterogeneous binding of nanoparticles in the neural network. We investigated the viability of the cells that are not tagged with negative PFs at DIV 14 using calcein AM and ethidium homodimer staining (i.e. live/dead cell assay). We found that even the cells that are not tagged with negative PFs are alive, as confirmed by the confocal fluorescence images (Fig. 4C and Fig. S8 (ESI†), yellow arrows indicate untagged viable cells). We then employed MAP2 as a neuronal marker39 and nestin as progenitor cell marker of both neuronal and glial lineage40 to differentiate between neurons and glial cells in the culture (neuronal cells expressed both MAP2 (red) and nestin (green) markers while glial cells expressed only the nestin (green) marker) and subsequently investigated the presence of unlabeled neurons in the cultured neural network at DIV 14. We observed that a significant fraction of untagged cells (absence of PFs, cyan) expressed MAP2 (red), confirming the heterogeneous binding of negatively charged nanoparticles to the cultured neural network at earlier stages (DIV 14) (Fig. 4D). We further investigated the selectivity of negatively charged nanostructures to neurons by employing GFAP as a glial cell marker41 in conjunction with MAP2. We found that negative PFs (cyan) specifically bind to neurons (MAP2, red) and completely avoid the glial cells (GFAP, green) (Fig. 4E). This suggests that the interaction of negatively charged nanoparticles with the neurons is both specific to neurons and heterogeneous across neurons.Neuron maturation-dependent nano–neuro interaction
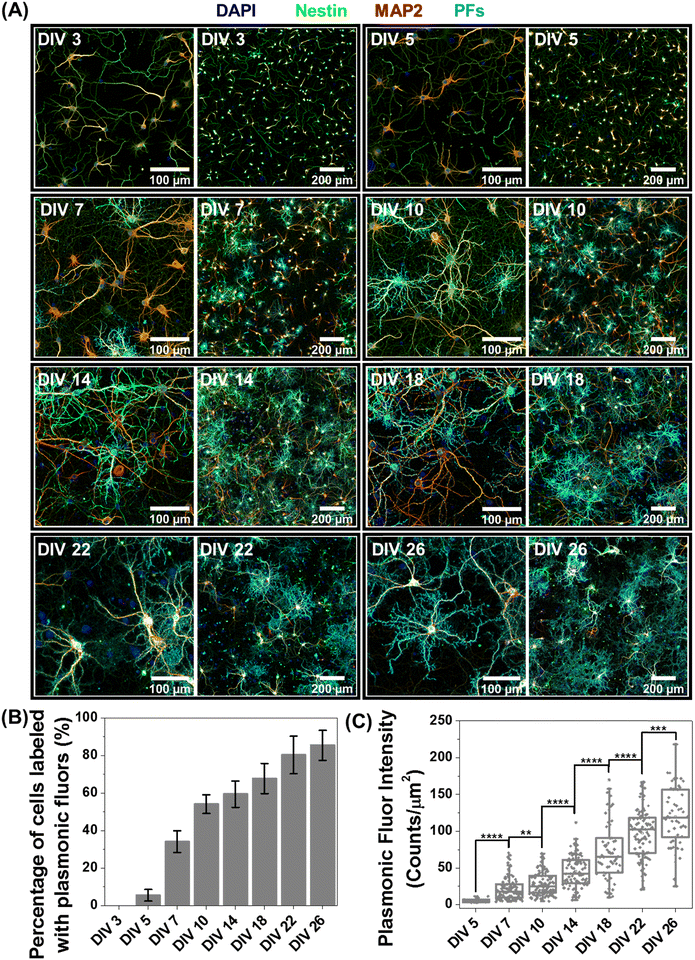
Based on the observation that a significant fraction of live neurons remain untagged at DIV 14, we hypothesized that this heterogeneity in the nano–neuro interaction is responsible for the heterogeneous neuromodulation in earlier stages (DIV 14 and 18, Fig. 3) and homogeneous neuromodulation in later stages (DIV 22 and 26, Fig. 3). To test this hypothesis, we employed negative PFs to investigate the neuronal maturation-dependent nano–neuro interaction. We monitored the binding of the negatively charged PFs to neurons at various DIVs (DIV 3, 5, 7, 10, 14, 18, 22 and 26) (Fig. 5A, individual channels of fluorescence images presented in Fig. S9–S24, ESI†). After nanoparticle binding, the neurons were co-stained with MAP2 and nestin post-fixation to distinguish neuronal cells from glial cells. We did not observe discernable binding of negatively charged nanostructures to neurons till DIV 5, suggesting that the nanostructures do not interact with young neurons. However, as the DIV increased above 7, the fraction of neurons tagged with the negatively charged PFs (cyan) and the florescence intensity (representing the number of PFs bound to the neurons) associated with the tagged neurons increased (Fig. 5B and C). The progressive increase in the nanoparticle binding to the neurons with an increase in DIV may be attributed to the progressive transformation of the neuronal network from young to developing to a finally mature state. Considering that the neuron maturation process is heterogeneous in nature,42 young, developing and mature neurons co-exist over the DIV range studied here. However, with an increase in DIV the fraction of young neurons decreases and that of developing neurons and mature neurons increases, until all the neurons in the network mature. These observations suggest that neuron maturation plays a critical role in nanoparticle binding in the in vitro neural network, which in turn affects the nano–neuromodulation. We believe that the transformation from heterogeneous response to photothermal stimulation at early stages of the network (DIV 14 and 18) to homogeneous response at later stages (DIV 22 and 26) is a direct manifestation of maturation-dependent tagging of neurons with negatively charged plasmonic nanostructures (Fig. 3).Role of neural network electrophysiological activity in nano–neuro interaction
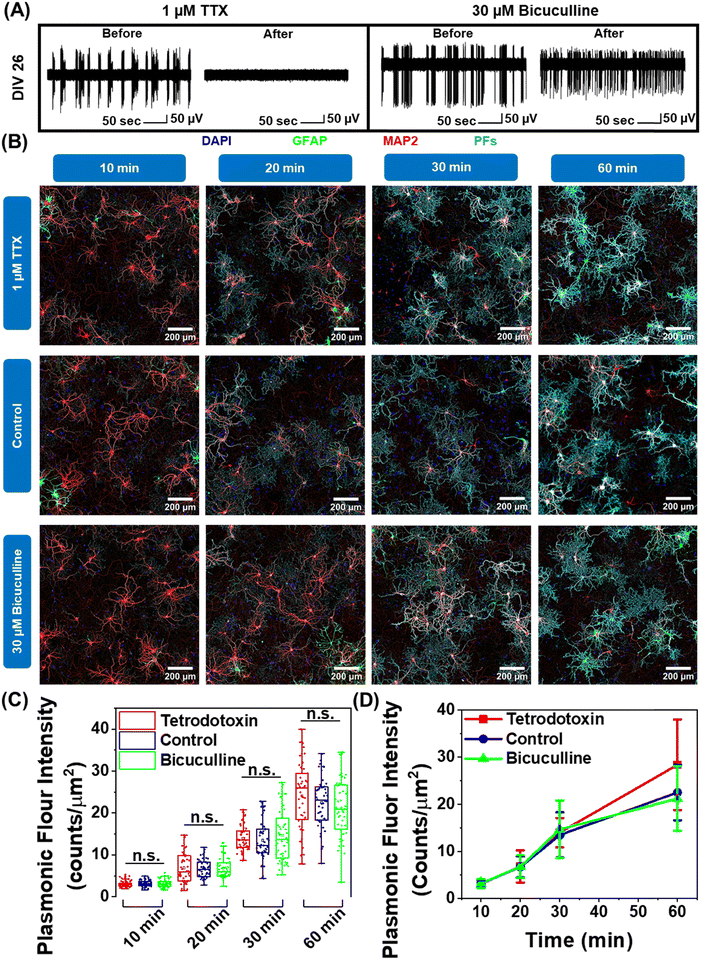
Neuron maturation is a pivotal process through which neurons gain their electrophysiological, morphological, and molecular characteristics to evolve into functioning units of the neural network.43 Based on our observation that negatively charged nanoparticles selectively bind to neurons and positively charged nanoparticles do not interact with neurons (Fig. 1F and G), we hypothesized that this electrostatic nature of nano–neuro interaction might be a direct consequence of the evolution of electrophysiological activity of neurons during the maturation process. To test this hypothesis, we employed pharmacological agents tetrodotoxin and bicuculline to suppress and increase the electrical activity of the primary cultured neural network, respectively, at DIV 14 (network comprising of both mature and young neurons) and DIV 26 (network comprising of majorly mature neurons) (Fig. S25A, B, ESI† and Fig. 6A).30 We then monitored the interaction of negatively charged PFs with neurons under these pharmacologically manipulated electrophysiological conditions. We observed no significant effect of electrical activity of the network on nano–neuro interaction at either early or later stages of maturation (Fig. S25C, D, ESI†). This suggests that electrophysiological activity of the neurons is not a governing factor for nano–neuro interaction. Owing to the electrostatic nature of nano–neuro interaction, we then speculated that the electrophysiological activity of the neurons might possibly regulate the kinetics of nanoparticle binding on the neurons. As such, we systematically investigated the binding kinetics of PFs to neurons under pharmacologically manipulated conditions. The fluorescence intensity of PFs, which represents the density of these nanostructures, progressively increased with an increase in the incubation time across all the groups. The difference in the density of PFs between the control and pharmacologically manipulated groups at any of the time points was not statistically significant (Fig. 6B–D). These observations reveal the unimportant role of neural network electrophysiological activity in nano–neuro interaction.Correlation between morphological maturation parameters of neurons and nano–neuro interaction
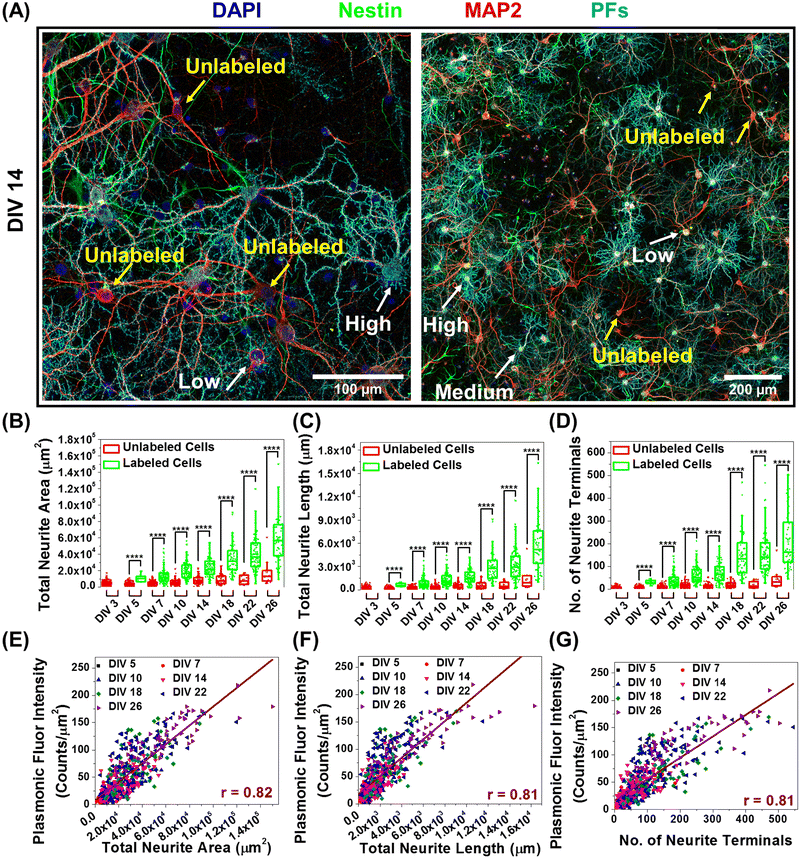
In further delving into the maturation-dependent nano–neuro interaction, we made two important observations: (i) at different DIVs, a varying fraction of neurons within the network were not tagged with negatively charged PFs (Fig. 7A, pointed with yellow arrows) and (ii) the localized nanoparticle density varies over a wide range among the labelled neurons (Fig. 7A, pointed with white arrows identifying low, medium and high density of PFs). We hypothesized that this graded binding of the nanostructures to neurons might be correlated with the neuron-maturation state (i.e., higher in mature neurons and lower in young neurons). To quantify this phenomenon, we employed the filament tracer module of IMARIS software (Oxford Instruments) to extract the morphological parameters of neurons (Fig. S26, ESI†).44 We selected total neurite area, total neurite length and number of neurite terminals extracted using filament tracking analysis as the morphological maturation parameters to examine the correlation between neuron maturation and nanoparticle binding.45,46 The image corresponding to the MAP2 channel representing all the neurons in the culture was utilized for comparing the morphological parameters of labeled and unlabeled neurons, while the image corresponding to the PF channel was only utilized to spot the neurons with and without nanoparticles. We observed that the neurons tagged with negative PFs exhibited significantly higher morphological maturation parameters as compared to the neurons without PFs (Fig. 7B–D), suggesting that nanoparticle localization is highly dependent on the maturation state of the neurons. Further, the density of nanoparticles localized on the neurons (measured as fluorescence intensity of PFs) exhibited strong correlation (Pearson's r value of 0.81) with the morphological maturation parameters of neurons (Fig. 7E–G). These observations suggest that the morphological maturation stage of the neurons strongly correlates to the interactions between nanoparticles and neurons, with higher binding on mature neurons and lower binding on maturing neurons.Discussion
We elucidate the effect of nanoparticle binding to neurons on the electrical activity in a neuronal network as well as the effect of maturation-dependent nano–neuro interaction on the nano–neuromodulation. The negative surface charge of the nanoparticles is a necessary condition for spontaneous binding of the nanoparticles to neurons in a culture. These results are in agreement with a recent report that highlighted the importance of the surface charge of nanoparticles in nano–neuro interactions and the relative insignificance of the size, shape, and composition of the nanostructures.30 It has been reported that the nano–neuro interaction depolarizes the neuronal membrane potential, resulting in increased excitability and firing rate of individual neurons.47,48 The increase in the excitability of the neurons results in increased probability of burst discharge instead of a single firing event.49 Likewise, in the present study, as a result of nanoparticle binding to a fraction of neurons in the neuronal network, the excitability of only the neurons tagged with nanoparticles is expected to increase. The increased excitability of tagged neurons in turn significantly increased the bursting activity of the network. Consequently, this heterogeneous nano–neuro interaction resulted in faster, regular and smaller burst discharge as compared to slower, irregular and longer burst discharge in the absence of nanoparticles.Neuronal maturation is a dynamic and heterogeneous process in which the neuron undergoes well-defined transition in morphology, excitability and connectivity in the pathway toward a fully mature phenotype.42 During the neuron maturation process, the dendritic length and the number of dendritic terminals of the neurons increase. Moreover, during this maturation process, the electrophysiological properties of neurons transform from high input resistance, relatively depolarized resting membrane potential and small action potentials in the case of young neurons to low input resistance, relatively hyperpolarized resting membrane potential and large action potentials in the case of mature neurons.50,51 In the current study, we have unveiled the critical role of neuronal maturation in the nano–neuro interactions. Dante et al. indicated that the neuronal spiking activity causes the spontaneous binding of the negatively charged nanoparticles to the surface of the electrically active neurons.30 We have also observed the selective binding of negatively charged particles to the neurons, which is in agreement with the previously reported work. However, upon pharmacologically manipulating the electrophysiological activity of the neural network using bicuculline (BICU) and tetrodotoxin (TTX) for increasing and suppressing the spiking activity respectively, we observed no significant effect on the nano–neuro interaction (Fig. 6 and Fig. S25, ESI†). This suggests that electrical activity of the neurons is not the governing factor for selective binding of negatively charged nanoparticles to neurons.
Although we noted a strong correlation between morphological maturity of neurons and the binding of the negatively charged nanoparticles to the neurons, the underlying electrophysiological and/or cell surfaceome factors responsible for this maturation-dependent nano–neuro interaction still remain unclear. Oostrum et al. recently demonstrated the reorganization of neuronal surface proteins during maturation in culture, which is proteostasis-independent, and this regulation affects the quantitative surface abundance of the surfaceome with very few qualitative alterations.52 Considering the unimportance of electrical activity of neurons in the binding of negatively charged nanoparticles to mature neurons, we speculate that the selective nano–neuro interaction might be attributed to this surfaceome reorganization with maturation, which might lead to change in the surface charge of the neurons from neutral in early stages to positively charged with maturation. It is important to note that the interaction of positively charged and negatively charged nanoparticles with the cells is still considered as a standard technique to estimate the surface charge of living as well as fixed cells,53,54 which is similar to the experimental protocol employed in this work, suggesting the progressive change in the neuronal surface charge with maturation. This change in the neuronal surface charge with maturation might be responsible for the maturation-dependent graded nano–neuro interaction of negatively charged nanoparticles. Considering that a fine control over nano–neuro interactions is critical for efficacious nano–neuromodulation, the mechanistic aspects of interaction between negatively charged particles and the neuronal membrane need to be further investigated.
One of the major challenges in the development of nanomaterial-assisted neurotherapeutics is the transport of nanomaterials across the BBB.24,25 Many approaches that enable nanomaterials to cross the BBB have been developed. However, target-specific nanomaterial delivery to brain parenchymal tissue remains challenging owing to the distinct and highly regulated transport across the BBB. Although positively charged particles are preferable for BBB crossing via adsorptive transcytosis,55 our study indicates that these particles do not readily interact with neurons. Alternatively, optimizing the ligand density on the nanoparticle surface to achieve efficient receptor-mediated transcytosis while maintaining the overall negative surface charge of the nanoparticles could potentially enable BBB crossing as well maintain the selectivity of nanoparticles towards neurons. Additionally, various other techniques have also been developed that rely on increasing transient permeability in the BBB paracellular pathway via ultrasound/microbubbles or osmotic pressure. However, these strategies are plagued by non-discriminate entry of compounds into the brain, which could lead to cerebral toxicity.25–27,56 While our results demonstrate necessary physicochemical properties of nanomaterials to achieve selective targeting of neurons required for efficient neuromodulation, further studies are needed to understand and control the transport of nanomaterials across the BBB.
Regardless, this heterogeneous nano–neuro interaction has major implications for the optical neuromodulation of the cultured neurons. Plasmonic nanostructures have been widely investigated for neuromodulation, which resulted in either inhibition of spiking activity under continuous wave laser stimulation or an increase in spiking activity via pulsed laser illumination.12,17 In the case of the pulsed laser, a likely mechanism for the increase in spiking activity is the photothermally induced membrane capacitance changes resulting in cell depolarization.57,58 Another possible mechanism is the alteration of cell membrane properties via short thermal pulses. Potentially, short thermal pulses result in transient nanopores in the cell membrane, which in turn can increase cell membrane fluidity, thereby altering the cell potential and activating the voltage-gated ion channels.59–61 On the other hand, the inhibition of spiking activity in response to continuous wave lasers likely stems from the thermo-sensitive potassium channel – TREK-1.17 In stark contrast to the earlier observations, for the first time, we report a heterogeneous modulation of neuronal activity at the network level, i.e., simultaneous excitation and inhibition of electrical activity under optical stimulus. The heterogeneous optical modulation in the early stages (i.e. at DIV 14 and 18) of neuronal maturation may be attributed to heterogeneous binding of the nanostructures to neurons (∼65–70% of the neurons in the culture tagged with nanoparticles). We speculate that the channels of MEAs exhibiting partial inhibition or no change in spiking activity majorly comprise of maturing neurons, which do not respond to photothermal stimulation because of the absence or low density of photothermal nanostructures on their surface.29 On the other hand, the electrodes exhibiting increased spiking activity might be majorly surrounded by untagged young neurons. Since these untagged neurons are part of a larger neuronal network, photothermal stimulation could turn down inhibitory inputs received from maturing or matured neurons thereby resulting in an increased spiking activity of these young neurons.29 Similarly, there can be many other combinations of young, maturing and mature neurons that can result in the observed heterogeneous response to photothermal stimulation. In contrast, as majority of the neurons reach advanced states of maturity or are completely matured, the photothermal stimulation resulted in nearly complete inhibition of spiking activity as observed in the case of DIV 22 and 26. This observation suggests that till DIV 18, a major fraction of neurons in the network are still young or in the early maturation stage while after DIV 22, majority of the neurons reached the maturing or matured state. This is also evident from the fluorescence images demonstrating the binding of negatively charged PFs on the neurons at corresponding DIVs. The graded and selective binding of negatively charged nanoparticles on neurons demonstrated here opens novel avenues in the minimally invasive nanomaterial-based non-genetic neuromodulation approach for treatments of neural disorders in the complex environment of the large mammalian brain.
Outlook
In summary, we unveil neuronal maturation-dependent nano–neuro interaction and nano–neuromodulation with a transformation from heterogeneous to homogeneous change in electrical activity with photothermal stimulation. We found that the nano–neuro interaction not only depends on the surface charge of the nanoparticles but also strongly correlates with the maturation stage of each individual neuron in the network, which in turn determines the homogeneity of nano–neuromodulation in a maturing neural network. Our results have broad implications for both neuroscience research and clinical applications. Recent advances in nanotechnology have revolutionized the field of neuroscience research by enabling various neuromodulation modalities (viz. electrical, optical, acoustic, magnetic, chemical).1,10,62 A comprehensive understanding of the factors influencing nano–neuro interaction will greatly advance our capability to seamlessly integrate nanomaterials with the nervous system and could help shape the future of neuromodulation therapy. Furthermore, we envisage that the ability to achieve precise and selective nano–neuro interaction could potentially alleviate the incessant bottleneck in the deployment of nanoprobes such as plasmonic NPs, upconversion nanoparticles, quantum dots, and nanodiamonds for both recording and manipulating complex neural circuits in vitro and in vivo.63–67Considering the critical role of nanoparticle surface charge in nano–neuro interaction, the heterogeneity observed in nano–neuromodulation, stemming from the heterogeneous binding of nanoparticles in a maturing neural network, possibly applies to a wide repertoire of nano-enabled neuromodulation modalities. Moving forward, we envision that a better understanding of the cell surface proteins responsible for the maturation-dependent electrostatic state of the neurons, possibly governing the maturation-dependent nano–neuro interactions presented here, could prove as an additional tool in the nanotechnology toolkit for the development of next-generation neuromodulation modalities with unprecedented spatiotemporal resolution. In clinical applications, owing to the fact that neurogenesis persists throughout aging in the human hippocampus,28,29,42 the maturation-dependent graded and selective nano–neuro interaction offers an additional handle in developing nano–neuromedicine for addressing neurological disorders. Moreover, the change in the firing pattern of the neural network stemming from nano–neuro interaction could serve as a non-invasive treatment for diseases that are characterized by erratic electrical activity in parts of the brain, such as epilepsies and seizures.68,69 Collectively, our findings facilitate the development of new nanotechnologies for the nano–neuro interface, which may be broadly applicable to both understanding neural pathways and minimally invasive nano-enabled drug-like administrable neurotherapeutics.
Methods
Cell culture
All procedures have been approved by the Institutional Animal Care and Use Committee (IACUC) at Washington University in St. Louis. The hippocampal tissues were manually isolated from day E18 embryos of pregnant Sprague Dawley rat brains (Charles River, USA) in Hibernate EB medium (HEB, BrainBits, USA) as previously described.23 The isolated tissues were incubated in cell dissociation solution comprising of 6 mg of papain (P4762, Sigma, USA) in 3 ml of Hibernate E-Ca (HE-Ca, BrainBits, USA) for 10 minutes at 30 °C. Subsequently, the tissues were mechanically dissociated via trituration with a fire-polished Pasteur pipette after replacing the cell dissociation solution with HEB medium to obtain the single cell suspension. The resultant cell suspension was centrifuged at 200 × g for 1 minute and the supernatant was decanted, and the pellets were resuspended in NbActiv4 medium (BrainBits, USA). Prior to plating the cells, the substrates were coated with poly(ethyleneimine) solution (0.1% in water, P3143, Sigma, USA) for 30 minutes followed by rinsing with water, air drying and sterilization under UV light exposure for 1 hour. Subsequently, the substrates were further treated with laminin solution (20 μg ml−1 in NbActiv4 medium, L2020, Sigma, USA) for 30 minutes to promote cell adhesion and neurite outgrowth.70 After decanting the excess laminin solution from the substrates, the cells were plated onto glass bottom Petri dishes (35 mm glass bottom dish with 14 mm micro-well #1 cover glass, D35-14-1-N, Cellvis, CA, USA) at a density of 120–160 cells per mm2 for use in microscopy experiments and onto the microelectrode array (MEA, Multichannel Systems, Germany) at a density of 500–1000 cells per mm2 for electrophysiology measurements. The neurons were maintained in a humidified incubator under 5% CO2 and 37 °C conditions. At DIV 3, half of the culture medium (NbActiv4) was replaced with fresh culture medium and subsequently replaced regularly every 7 days in the case of glass bottom Petri dishes and every 2 days in the case of MEAs.Synthesis of positively charged PFs
PF-650 with IR-650 as the molecular fluorophore and Au@Ag nanocuboids as the plasmonic core were generously provided by Auragent Bioscience, MO, USA. The PFs were synthesized according to a procedure we recently reported.32 Owing to the presence of BSA on the surface of the PFs, the PFs are inherently negatively charged under physiological pH. To realize positively charged PFs, the surface of these negatively charged PFs was coated with a cationic polyelectrolyte, poly(allylamine hydrochloride) (PAH, 43092, Alfa Aesar, USA), via electrostatic interaction. Briefly, 10 ml of PFs (O.D. ∼ 2) was washed 3 times using alkaline nanopure water (pH = 10) via centrifugation at 6000 rpm to remove excess salt present in the storage buffer of PFs and re-dispersed in 10 ml of nanopure water (pH = 10). Subsequently, the purified PFs were added dropwise to 10 ml of PAH solution (0.2% W/V in water, pH adjusted to 10 using 1 M NaOH) under vigorous stirring and sonicated for 1 hour at room temperature under dark conditions. Finally, PAH-coated PFs were washed with nanopure deionized (DI) water (resistivity > 18.2 MΩ cm) twice by centrifugation at 6000 rpm and re-dispersed in DI water for further use.Synthesis of negatively charged AuNRs
The localized surface plasmon resonance (LSPR) wavelength of the AuNRs can be easily tuned over a wide range by varying their aspect ratio.71–73 Considering the wavelength of the NIR light source (808 nm) utilized for photothermal modulation in the present work, the AuNRs with a LSPR wavelength of 820 nm were synthesized via a previously reported seed-mediated approach.71,72,74 Briefly, the gold seed solution was first prepared by adding 0.6 ml of ice-cold 10 mM NaBH4 solution (71321, Sigma, USA) into a magnetically stirred (800 rpm) solution comprising of 0.25 ml of 10 mM HAuCl4 (520918, Sigma, USA) and 9.75 ml of 0.1 M hexadecyltrimethylammonium bromide (CTAB) (H5882, Sigma, USA) at room temperature for 10 min. Consequently, the solution color changed from orange to brown, indicating the Au seed formation. Subsequently, the growth solution was prepared by sequential addition of 2 ml of 10 mM HAuCl4 aqueous solution, 38 ml of 0.1 M CTAB solution, 0.4 ml of 10 mM AgNO3 (204390, Sigma, USA) and 0.22 ml of 0.1 M ascorbic acid (A92902, Sigma, USA) followed by gentle homogenization via inversion, rendering the growth solution change its color from orange to colorless. Finally, 48 μl of freshly prepared gold seed solution was added to the growth solution, mixed via inversion and left undisturbed in the dark at room temperature for 24 hours. The AuNRs were collected via centrifugation at 9000 rpm for 30 min to remove the supernatant and re-dispersed in DI water for further use.Owing to the presence of CTAB on the surface of AuNRs, the AuNRs are inherently positively charged. To realize negatively charged AuNRs, the positively charged AuNRs were coated with an anionic polyelectrolyte, poly(sodium 4-styrenesulfonate) (PSS, 434574, Sigma, USA), via electrostatic interaction. Briefly, 10 ml of AuNRs (O.D. ∼ 2) was washed once with DI water via centrifugation at 9000 rpm to remove excess CTAB and re-dispersed in 10 ml of nanopure deionized (DI) water (resistivity > 18.2 MΩ cm). Subsequently, the purified AuNRs were added dropwise to 10 ml of PSS solution (0.5% W/V in water) under vigorous stirring and sonicated for 1 hour at room temperature. Finally, the PSS-coated AuNRs were washed with DI water twice by centrifugation at 9000 rpm and re-dispersed in DI water for further use.
Material characterization
Transmission electron microscopy (TEM) micrographs were acquired using a JEOL JEM-2100F field emission microscope. A drop of plasmonic nanostructure aqueous dispersion was cast onto the copper grids (Carbon Type-B, 200 mesh, Ted Pella, USA). The extinction spectra of plasmonic nanostructures were acquired using a Shimadzu UV-1800 spectrophotometer. The zeta potential measurements were performed using a Malvern Zetasizer (Nano ZS). Large area fluorescence mappings were obtained using the LI-COR Odyssey CLx imaging system.Neuron electrophysiology experiments
Confocal fluorescence microscopy experiments
Scanning electron microscopy
Negatively charged PFs dispersed in Nbactiv4 medium were added to the cultured neurons on DIV 14 ad 26 at a final concentration of O.D. ∼ 0.5 and let to incubate for 1 hour at 37 °C and 5% CO2. Subsequently, the cells were washed once with PBS and fixed with PFA overnight at room temperature. The cells were dehydrated with ethanol and vacuum dried before being sputter coated with 10 nm of gold metal. Scanning electron micrographs (SEM) were acquired using a JEOL JSM-7001 LVF field emission scanning electron microscope.Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.Author contributions
S. S., B. R., P. G. and H. G. D. conceived the project. S. S., B. R., P. G., P. R. and Q. C. designed the experiments. P. G., P. R. and H. B. performed the primary neuron cell culture for all the experiments. P. G. and P. R. performed all neuron electrophysiology and fluorescence imaging experiments. R. G. synthesized the positively charged plasmonic fluors. P. G. performed the TEM imaging. P. G. and H. B. prepared samples for SEM of nanoparticles labelled neuron samples. A. D. performed the SEM imaging. P. G. performed all the neuron electrophysiology data analysis and the confocal fluorescence image analysis. Q. C. provided support and input on all experiments. S. S. and B. R. directed the research. P. G. S. S. and B. R. co-wrote the paper. All authors reviewed and commented on the manuscript.Conflicts of interest
The authors declare the following competing financial interests: S. S. is one of the inventors on a pending patent related to plasmonic fluor technology and the technology has been licensed by the Office of Technology Management at Washington University in St Louis to Auragent Bioscience LLC, which is developing plasmonic fluor products. S. S. is one of the co-founders and shareholders of Auragent Bioscience LLC. These potential conflicts of interest have been disclosed and are being managed by Washington University in St Louis.Acknowledgements
The authors acknowledge support from Air Force Office of Scientific Research (#FA95501910394 (SS and BR)). The authors thank the Institute of Materials Science and Engineering at Washington University for providing access to electron microscopy facilities. The authors also thank Dr Jai Rudra for providing access to the Lionheart FX Automated Microscope. Confocal fluorescence imaging using a Zeiss LSM 880 Airyscan Confocal Microscope and subsequent image analysis using IMARIS were performed in part through the use of Washington University Center for Cellular Imaging (WUCCI) supported by Washington University School of Medicine, The Children's Discovery Institute of Washington University and St. Louis Children's Hospital (CDI-CORE-2015-505 and CDI-CORE-2019-813) and the Foundation for Barnes-Jewish Hospital (3770 and 4642). Confocal data were generated on a Zeiss LSM 880 Airyscan Confocal Microscope which was purchased with support from the Office of Research Infrastructure Programs (ORIP), a part of the NIH Office of the Director under grant OD021629.References
- H. Acarón Ledesma, X. Li, J. L. Carvalho-de-Souza, W. Wei, F. Bezanilla and B. Tian, Nat. Nanotechnol., 2019, 14, 645–657 CrossRef PubMed.
- C. Yang and S. Park, Biomed. Eng. Lett., 2021, 11, 163–170 CrossRef PubMed.
- K. Ashkan, P. Rogers, H. Bergman and I. Ughratdar, Nat. Rev. Neurol., 2017, 13, 548–554 CrossRef PubMed.
- J. W. Salatino, K. A. Ludwig, T. D. Y. Kozai and E. K. Purcell, Nat. Biomed. Eng., 2017, 1, 862–877 CrossRef CAS PubMed.
- W. Zhou, X. Dai and C. M. Lieber, Rep. Prog. Phys., 2016, 80, 016701 CrossRef PubMed.
- E. S. Boyden, F. Zhang, E. Bamberg, G. Nagel and K. Deisseroth, Nat. Neurosci., 2005, 8, 1263–1268 CrossRef CAS PubMed.
- K. Deisseroth, Nat. Methods, 2011, 8, 26–29 CrossRef CAS PubMed.
- O. A. Shemesh, D. Tanese, V. Zampini, C. Linghu, K. Piatkevich, E. Ronzitti, E. Papagiakoumou, E. S. Boyden and V. Emiliani, Nat. Neurosci., 2017, 20, 1796–1806 CrossRef CAS PubMed.
- A. M. Packer, B. Roska and M. Häusser, Nat. Neurosci., 2013, 16, 805–815 CrossRef CAS PubMed.
- Y. Wang and L. Guo, Front. Neurosci., 2016, 10, 69 Search PubMed.
- F. Benfenati and G. Lanzani, Nat. Rev. Mater., 2021, 6, 1–4 Search PubMed.
- J. L. Carvalho-de-Souza, B. I. Pinto, D. R. Pepperberg and F. Bezanilla, Biophys. J., 2018, 114, 283–288 CrossRef CAS PubMed.
- J. Kubanek, P. Shukla, A. Das, S. A. Baccus and M. B. Goodman, J. Neurosci., 2018, 38, 3081–3091 CrossRef CAS PubMed.
- M. Hallett, Nature, 2000, 406, 147–150 CrossRef CAS PubMed.
- S. Yoo, J.-H. Park and Y. Nam, ACS Nano, 2018, 13, 544–551 CrossRef PubMed.
- S. K. Rastogi, R. Garg, M. G. Scopelliti, B. I. Pinto, J. E. Hartung, S. Kim, C. G. E. Murphey, N. Johnson, D. San Roman, F. Bezanilla, J. F. Cahoon, M. S. Gold, M. Chamanzar and T. Cohen-Karni, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 13339 CrossRef CAS PubMed.
- S. Yoo, S. Hong, Y. Choi, J.-H. Park and Y. Nam, ACS Nano, 2014, 8, 8040–8049 CrossRef CAS PubMed.
- J. L. Carvalho-de-Souza, J. S. Treger, B. Dang, S. B. H. Kent, D. R. Pepperberg and F. Bezanilla, Neuron, 2015, 86, 207–217 CrossRef CAS PubMed.
- H. Kang, G.-H. Lee, H. Jung, J. W. Lee and Y. Nam, ACS Nano, 2018, 12, 1128–1138 CrossRef CAS PubMed.
- J. W. Lee, H. Jung, H. H. Cho, J. H. Lee and Y. Nam, Biomaterials, 2018, 153, 59–69 CrossRef CAS PubMed.
- K. Eom, J. Kim, J. M. Choi, T. Kang, J. W. Chang, K. M. Byun, S. B. Jun and S. J. Kim, Small, 2014, 10, 3853–3857 CrossRef CAS PubMed.
- S. Yoo, R. Kim, J.-H. Park and Y. Nam, ACS Nano, 2016, 10, 4274–4281 CrossRef CAS PubMed.
- H. Gholami Derami, P. Gupta, K. C. Weng, A. Seth, R. Gupta, J. R. Silva, B. Raman and S. Singamaneni, Adv. Mater., 2021, 33, 2008809 CrossRef CAS PubMed.
- D. E. Tylawsky, H. Kiguchi, J. Vaynshteyn, J. Gerwin, J. Shah, T. Islam, J. A. Boyer, D. R. Boué, M. Snuderl, M. B. Greenblatt, Y. Shamay, G. P. Raju and D. A. Heller, Nat. Mater., 2023, 22, 391–399 CrossRef CAS PubMed.
- T. Kim, H. J. Kim, W. Choi, Y. M. Lee, J. H. Pyo, J. Lee, J. Kim, J. Kim, J.-H. Kim, C. Kim and W. J. Kim, Nat. Biomed. Eng., 2023, 7, 149–163 CrossRef CAS PubMed.
- J. Xie, Z. Shen, Y. Anraku, K. Kataoka and X. Chen, Biomaterials, 2019, 224, 119491 CrossRef CAS PubMed.
- Q. Tan, S. Zhao, T. Xu, Q. Wang, M. Zhang, L. Yan, X. Chen and M. Lan, Coord. Chem. Rev., 2023, 494, 215344 CrossRef CAS.
- N. Urbán and F. Guillemot, Front. Cell. Neurosci., 2014, 8, 396 Search PubMed.
- W. Deng, J. B. Aimone and F. H. Gage, Nat. Rev. Neurosci., 2010, 11, 339–350 CrossRef CAS PubMed.
- S. Dante, A. Petrelli, E. M. Petrini, R. Marotta, A. Maccione, A. Alabastri, A. Quarta, F. De Donato, T. Ravasenga, A. Sathya, R. Cingolani, R. Proietti Zaccaria, L. Berdondini, A. Barberis and T. Pellegrino, ACS Nano, 2017, 11, 6630–6640 CrossRef CAS PubMed.
- R. Walters, I. L. Medintz, J. B. Delehanty, M. H. Stewart, K. Susumu, A. L. Huston, P. E. Dawson and G. Dawson, ASN Neuro, 2015, 7, 1759091415592389 CrossRef PubMed.
- J. Luan, A. Seth, R. Gupta, Z. Wang, P. Rathi, S. Cao, H. Gholami Derami, R. Tang, B. Xu, S. Achilefu, J. J. Morrissey and S. Singamaneni, Nat. Biomed. Eng., 2020, 4, 518–530 CrossRef CAS PubMed.
- J. Chen, N. Sawyer and L. Regan, Protein Sci., 2013, 22, 510–515 CrossRef CAS PubMed.
- C. L. Call and D. E. Bergles, Nat. Commun., 2021, 12, 4767 CrossRef CAS PubMed.
- W. P. Bartlett and G. A. Banker, J. Neurosci., 1984, 4, 1954 CrossRef CAS PubMed.
- Mikhail G. Shapiro, Kazuaki Homma, Sebastian Villarreal, C.-P. Richter and F. Bezanilla, Nat. Commun., 2012, 3, 736 CrossRef PubMed.
- F. Maingret, I. Lauritzen, A. J. Patel, C. Heurteaux, R. Reyes, F. Lesage, M. Lazdunski and E. Honoré, EMBO J., 2000, 19, 2483–2491 CrossRef CAS PubMed.
- V. C. Piatti, M. G. Davies-Sala, M. S. Espósito, L. A. Mongiat, M. F. Trinchero and A. F. Schinder, J. Neurosci., 2011, 31, 7715 CrossRef CAS PubMed.
- E. Kruminis-Kaszkiel, A. Osowski, E. Bejer-Oleńska, M. Dziekoński and J. Wojtkiewicz, Cells, 2020, 9, 739 CrossRef CAS PubMed.
- J. Liu, C. Reeves, T. Jacques, A. McEvoy, A. Miserocchi, P. Thompson, S. Sisodiya and M. Thom, Glia, 2018, 66, 62–77 CrossRef PubMed.
- H. S. Günther, S. Henne, J. Oehlmann, J. Urban, D. Pleizier, N. Renevier, C. Lohr and C. Wülfing, Sci. Rep., 2021, 11, 13322 CrossRef PubMed.
- N. Toni, D. A. Laplagne, C. Zhao, G. Lombardi, C. E. Ribak, F. H. Gage and A. F. Schinder, Nat. Neurosci., 2008, 11, 901–907 CrossRef CAS PubMed.
- Z. He and Q. Yu, BMC Genomics, 2018, 19, 262 CrossRef PubMed.
- J. Wahis, A. Baudon, F. Althammer, D. Kerspern, S. Goyon, D. Hagiwara, A. Lefevre, L. Barteczko, B. Boury-Jamot, B. Bellanger, M. Abatis, M. Da Silva Gouveia, D. Benusiglio, M. Eliava, A. Rozov, I. Weinsanto, H. S. Knobloch-Bollmann, M. K. Kirchner, R. K. Roy, H. Wang, M. Pertin, P. Inquimbert, C. Pitzer, J. Siemens, Y. Goumon, B. Boutrel, C. M. Lamy, I. Decosterd, J.-Y. Chatton, N. Rouach, W. S. Young, J. E. Stern, P. Poisbeau, R. Stoop, P. Darbon, V. Grinevich and A. Charlet, Nat. Neurosci., 2021, 24, 529–541 CrossRef CAS PubMed.
- C. Zhao, E. M. Teng, R. G. Summers, G.-L. Ming and F. H. Gage, J. Neurosci., 2006, 26, 3–11 CrossRef CAS PubMed.
- G. Y. Wu, D. J. Zou, I. Rajan and H. Cline, J. Neurosci., 1999, 19, 4472–4483 CrossRef CAS PubMed.
- K. Salinas, Z. Kereselidze, F. DeLuna, X. G. Peralta and F. Santamaria, J. Nanobiotechnol., 2014, 12, 31 CrossRef PubMed.
- S. Jung, M. Bang, B. S. Kim, S. Lee, N. A. Kotov, B. Kim and D. Jeon, PLoS One, 2014, 9, e91360 CrossRef PubMed.
- G. Buzsáki, J. Csicsvari, G. Dragoi, K. Harris, D. Henze and H. Hirase, Cereb. Cortex, 2002, 12, 893–899 CrossRef PubMed.
- Y.-B. Liu, P. A. Lio, J. F. Pasternak and B. L. Trommer, J. Neurophysiol., 1996, 76, 1074–1088 CrossRef CAS PubMed.
- X.-S. Liu, S. Tilwalli, G.-L. Ye, P. A. Lio, J. F. Pasternak and B. L. Trommer, Brain Res., 2000, 856, 202–212 CrossRef CAS PubMed.
- M. van Oostrum, B. Campbell, C. Seng, M. Müller, S. Tom Dieck, J. Hammer, P. G. A. Pedrioli, C. Földy, S. K. Tyagarajan and B. Wollscheid, Nat. Commun., 2020, 11, 4990 CrossRef CAS PubMed.
- M. Nishino, I. Matsuzaki, F. Y. Musangile, Y. Takahashi, Y. Iwahashi, K. Warigaya, Y. Kinoshita, F. Kojima and S. I. Murata, PLoS One, 2020, 15, e0236373 CrossRef CAS PubMed.
- L. Ouyang, R. Shaik, R. Xu, G. Zhang and J. Zhe, Cells, 2021, 10, 1519 CrossRef CAS PubMed.
- V. Ceña and P. Játiva, Nanomedicine, 2018, 13, 1513–1516 CrossRef PubMed.
- R. A. Vega, Y. Zhang, C. Curley, R. L. Price and R. Abounader, Neurosurgery, 2016, 63, 210 CrossRef.
- M. G. Shapiro, K. Homma, S. Villarreal, C.-P. Richter and F. Bezanilla, Nat. Commun., 2012, 3, 1–11 Search PubMed.
- J. Wells, C. Kao, P. Konrad, T. Milner, J. Kim, A. Mahadevan-Jansen and E. D. Jansen, Biophys. J., 2007, 93, 2567–2580 CrossRef CAS PubMed.
- H. T. Beier, G. P. Tolstykh, J. D. Musick, R. J. Thomas and B. L. Ibey, J. Neural Eng., 2014, 11, 066006 CrossRef PubMed.
- A. J. Walsh, G. P. Tolstykh, S. Martens, B. L. Ibey and H. T. Beier, Neurophotonics, 2016, 3, 040501 CrossRef PubMed.
- P. Bazard, R. D. Frisina, J. P. Walton and V. R. Bhethanabotla, Sci. Rep., 2017, 7, 7803 CrossRef PubMed.
- X. Yang, E. McGlynn, R. Das, S. P. Paşca, B. Cui and H. Heidari, Adv. Mater., 2021, 33, 2103208 CrossRef CAS PubMed.
- S. Haziza, N. Mohan, Y. Loe-Mie, A.-M. Lepagnol-Bestel, S. Massou, M.-P. Adam, X. L. Le, J. Viard, C. Plancon, R. Daudin, P. Koebel, E. Dorard, C. Rose, F.-J. Hsieh, C.-C. Wu, B. Potier, Y. Herault, C. Sala, A. Corvin, B. Allinquant, H.-C. Chang, F. Treussart and M. Simonneau, Nat. Nanotechnol., 2017, 12, 322–328 CrossRef CAS PubMed.
- A. L. Efros, J. B. Delehanty, A. L. Huston, I. L. Medintz, M. Barbic and T. D. Harris, Nat. Nanotechnol., 2018, 13, 278–288 CrossRef CAS PubMed.
- K. Park, Y. Kuo, V. Shvadchak, A. Ingargiola, X. Dai, L. Hsiung, W. Kim, Z. H. Zhou, P. Zou and A. J. Levine, Sci. Adv., 2018, 4, e1601453 CrossRef PubMed.
- A. Garcia-Etxarri and R. Yuste, Nat. Methods, 2021, 18, 1287–1293 CrossRef CAS PubMed.
- W. R. Adams, R. Gautam, A. Locke, L. E. Masson, A. I. Borrachero-Conejo, B. R. Dollinger, G. A. Throckmorton, C. Duvall, E. D. Jansen and A. Mahadevan-Jansen, Biophys. J., 2022, 121, 1525–1540 CrossRef CAS PubMed.
- T. I. Netoff, R. Clewley, S. Arno, T. Keck and J. A. White, J. Neurosci., 2004, 24, 8075 CrossRef CAS PubMed.
- K. Autar, X. Guo, J. W. Rumsey, C. J. Long, N. Akanda, M. Jackson, N. S. Narasimhan, J. Caneus, D. Morgan and J. J. Hickman, Stem Cell Rep., 2022, 17, 96–109 CrossRef CAS PubMed.
- L. Luckenbill-Edds, Brain Res. Rev., 1997, 23, 1–27 CrossRef CAS PubMed.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957–1962 CrossRef CAS.
- A. Gole and C. J. Murphy, Chem. Mater., 2004, 16, 3633–3640 CrossRef CAS.
- L. Tian, E. Chen, N. Gandra, A. Abbas and S. Singamaneni, Langmuir, 2012, 28, 17435–17442 CrossRef CAS PubMed.
- D. K. Smith and B. A. Korgel, Langmuir, 2008, 24, 644–649 CrossRef CAS PubMed.
- C. R. Legendy and M. Salcman, J. Neurophysiol., 1985, 53, 926–939 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S24. See DOI: https://doi.org/10.1039/d3nh00258f |
| This journal is © The Royal Society of Chemistry 2023 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=