Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synergism of Co/Na in BiVO4 microstructures for visible-light driven degradation of toxic dyes in water†
Muhammad Zeeshan
Abid
 a,
Khezina
Rafiq
a,
Khezina
Rafiq
 a,
Abdul
Rauf
a,
Syed Shoaib
Ahmad Shah
a,
Abdul
Rauf
a,
Syed Shoaib
Ahmad Shah
 b,
Rongchao
Jin
b,
Rongchao
Jin
 *c and
Ejaz
Hussain
*c and
Ejaz
Hussain
 *ac
*ac
aInstitute of Chemistry, Inorganic Materials Laboratory 52S, The Islamia University of Bahawalpur-63100, Pakistan. E-mail: ejaz.hussain@iub.edu.pk; Tel: +92 3026500254
bDepartment of Chemistry, School of Natural Sciences, National University of Sciences and Technology, Islamabad-24090, Pakistan
cDepartment of Chemistry, Carnegie Mellon University, Pittsburgh, Pennsylvania-15213, USA. E-mail: rongchao@andrew.cmu.edu
First published on 27th March 2023
Abstract
In this work, we report a synergism of Co/Na in Co@Na–BiVO4 microstructures to boost the photocatalytic performance of bismuth vanadate (BiVO4) catalysts. A co-precipitation method has been employed to synthesize blossom-like BiVO4 microstructures with incorporation of Co and Na metals, followed by calcination at 350 °C. The structure and morphology of the as-prepared photocatalysts are characterized by XRD, Raman, FTIR, SEM, EDX, AFM, UV-vis/DRS and PL techniques. Dye degradation activities are evaluated by UV-vis spectroscopy, in which methylene blue, Congo red and rhodamine B dyes are chosen for comparative study. The activities of bare BiVO4, Co–BiVO4, Na–BiVO4, and Co@Na–BiVO4 are compared. To evaluate the ideal conditions, various factors that affect degradation efficiencies have been investigated. The results of this study show that the Co@Na–BiVO4 photocatalysts exhibit higher activity than bare BiVO4, Co–BiVO4 or Na–BiVO4. The higher efficiencies were attributed to the synergistic role of Co and Na contents. This synergism assists in better charge separation and more electron transportation to the active sites during the photoreaction.
1. Introduction
Water is the main natural resource covering over 70% of the earth's surface. The accessibility of safe drinking water (0.5%) is crucial not only for humans but also for the survival of other living organisms. Annually, 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of toxic dyes are dumped into water bodies.1 The textile, paper, leather, paint and cosmetics industries are the major contributors of these contaminants (i.e. toxic dyes). Such dyes are hazardous, carcinogenic and non-biodegradable effluents which cause damage to human health, plant species, environment and soil even at very low concentrations (i.e. less than 1 ppm).2
000 tons of toxic dyes are dumped into water bodies.1 The textile, paper, leather, paint and cosmetics industries are the major contributors of these contaminants (i.e. toxic dyes). Such dyes are hazardous, carcinogenic and non-biodegradable effluents which cause damage to human health, plant species, environment and soil even at very low concentrations (i.e. less than 1 ppm).2
Common ways to remove the dyes from water are through adsorption and coagulation.3 However, these methods produce secondary pollutants because dyes are only changed from the liquid to the solid phase during adsorption and coagulation.4 To resolve this issue, an environment-friendly and cost-effective approach is urgently required that can utilize visible light for the removal of organic dyes from aqueous solutions.5 Photocatalysts accelerate the photoreactions and convert photons into chemical energy. Photocatalysts are now widely used in agriculture, medicine, electrical appliances, photoelectrochemical reactions, environmental and energy fields. A number of metal-element-doped semiconductor materials have been employed for visible light induced dye degradation for water purification purposes.6
BiVO4 and TiO2 are considered the most advantageous photocatalysts because of their photocatalytic activity, stability and non-toxicity. However, TiO2 only responds to ultraviolet radiations, which restricts its further application.7 In this regard, photocatalysts that can efficiently work in the visible range are more desired. Unfortunately some visible light responsive photocatalysts have stability issues; for example, CdS is active under visible light but not stable in an aqueous system due to photocorrosion.8 Among the photocatalysts, BiVO4 has been reported to be a promising candidate.9 Various techniques have been introduced for the development of BiVO4 nanostructures, including the solid-state reaction,10 hydrolysis of metal alkoxides,11 co-precipitation12 and hydrothermal synthesis.13 Nanostructures obtained by different methods often have different crystal structures and defects that influence the photocatalytic efficiencies. Among the common synthetic protocols, the co-precipitation method was found to deliver higher yields with high purity without using organic solvents.14 Moreover, this strategy is cost effective and straight forward.
One of the common problems with BiVO4 is the rapid recombination of photogenerated electrons with holes, which reduces the overall activities. However, this problem could be reduced by generating more active centers in the form of transition metal dopants. Similarly, the BiVO4 activity can also be enhanced by doping with non-metals, metals, metal cations and non-metal anions or by fusion with another oxide. For combination with BiVO4, noble metals such as Pt, Au, Pd and Ag serve as excellent cocatalysts in photocatalysis.15 But due to high cost, these metals are less common in dye degradation applications, although these metals enhance electron–hole separation (due to electron quenching abilities) and increase light absorption. The separation of charge carriers and their lifetime can also be enhanced by developing composites via incorporating suitable dopants. Many composites of metals or metals oxides, such as Au, CeO2, WO3, CdS, SnO2 and TiO2, still have some drawbacks and their photocatalytic efficiencies are still unsatisfactory.16–19 Among the dopants, cobalt (Co) doped20,21 and alkali (Na+ and K+) doped BiVO4 nanostructures have been reported for better charge transfer in photocatalysis.22,23
To demonstrate the advantage of an alkali metal oxide (Na2O) with a transition metal co-catalyst, Co and Na contents were in situ employed and incorporated into BiVO4. This work was designed to promote the charge transfer from the semiconductor (BiVO4) to the co-catalyst active sites by shifting the Fermi level. The Co in the form of Co3O4 acts as excellent active sites for dye degradation. Another advantage of Co is to enhance the photon absorption surfaces of the semiconductor support. The approach employed in this study exhibits various other advantages like the excellent stability of the synthesized photocatalysts, higher dispersion rather than agglomeration and excellent dye degradation efficiencies. This work demonstrates the extended absorption in visible light that attributes to higher dye degradation efficiencies of various toxic dyes (e.g. methylene blue, Congo red and rhodamine B) under solar radiations.
2. Experimental
2.1 Materials used
The following chemicals were used for the synthesis of photocatalysts, bismuth nitrate pentahydrate (Bi(NO3)3·5H2O, Sigma Aldrich 98%), ammonium metavanadate (NH4VO3, Sigma Aldrich 98%), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O, Sigma Aldrich 98%), sodium hydroxide (NaOH, Sigma Aldrich 98%) and sodium borohydride (NaBH4, Sigma Aldrich 98%). To monitor the degradation activities, methylene blue (MB) dye (C16H18ClN3S, Sigma Aldrich), Congo red (CR) dye (C32H22N6Na2O6S2), and rhodamine B (C28H31ClN2O3, Sigma Aldrich) were used.2.2 Catalysts preparation
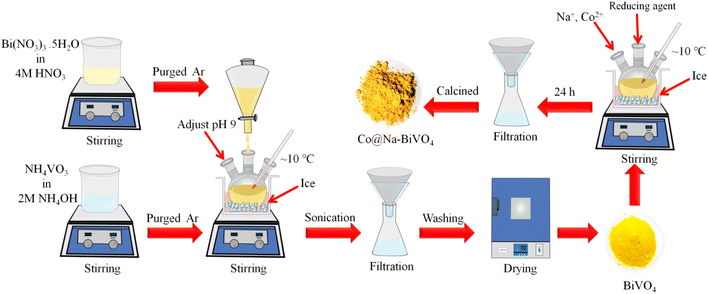
BiVO4 was synthesized by the co-precipitation method with optimized timescale strategy. Briefly, Bi(NO3)3·5H2O solution (7 mM) was prepared by dissolving 3.396 g of Bi(NO3)3·5H2O (Sigma Aldrich, 99%) in 50 mL of 4 M HNO3 solution. In the next step, NH4VO3 solution (7 mM) was prepared by adding 0.819 g of NH4VO3 (Sigma Aldrich, 99%) to 50 mL of 2 M NH4OH solution. To remove the dissolved oxygen, both solutions were purged with high purity argon (Ar) for 30 min. After that, the Bi(NO3)3·5H2O solution was added dropwise into the NH4VO3 solution with vigorous stirring for 12 h while maintaining the pH = 9 at a temperature of 10 °C. The precipitate mixture was then sonicated for 20 min by adjusting the sweep frequency (37 kHz, 300 W). The yellow precipitate was filtered out using high grade filter paper (WHA 1001325, Grade-1). The solid product was washed thoroughly with deionized water and then with absolute ethanol. After that, the product was dried at 94 °C overnight in an oven (Sanyo/MOV-112 Japan).24 For the synthesis of Co@Na–BiVO4 microstructures, an optimized amount of BiVO4 (155 mg) powder was transferred into a three-neck, round bottom flask, followed by addition of 50 mL of high purity deionized water (PIAS-GW1-Z) to produce a uniform slurry. After 15 min of purging with argon, Co and Na ions (from Co (NO3)2·6H2O & NaOH/NaCl) were dispersed and in situ incorporated into BiVO4 by using a chemical method. The amounts of Co and Na metal contents were fixed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and overall 2% metal contents (2% w/w). To grow the Co@Na–BiVO4 microstructures, the above mixture solution was under optimized stirring (150 rpm) at approximately 10 °C for 24 h. The final suspension was filtered and dried at 94 °C. The synthesis of Co–BiVO4 and Na–BiVO4 are discussed in the ESI.†
1 and overall 2% metal contents (2% w/w). To grow the Co@Na–BiVO4 microstructures, the above mixture solution was under optimized stirring (150 rpm) at approximately 10 °C for 24 h. The final suspension was filtered and dried at 94 °C. The synthesis of Co–BiVO4 and Na–BiVO4 are discussed in the ESI.†
2.3 Catalysts characterization
UV-vis/DRS for the as synthesized photocatalysts was obtained over the wavelength range of 265–850 nm on a PerkinElmer (λ-850+/Tungsten-Halogen) spectrophotometer. Powdered XRD analysis was conducted on an advanced XRD system (Bruker D2-phaser) equipped with a LYNXEYE XE-T Detector, 220 V/60 Hz. Using the Scherer equation, particle sizes were measured having D ≈ 0.9λ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ); the Cu Kα operational X-ray source is (λ = 1.54 Å, 40 kV, 40 mA). The 2θ range was fixed from 15° to 80° (step: 0.05° and scan rate: 2° min−1), Fourier transform infrared (FT-IR) analysis was performed on a BrukerTensor-27. The SEM results were obtained using an FEI-Nova NanoSEM-450 electron microscope. The elemental composition of Co@Na–BiVO4 NPs was obtained using a SEM equipped with an energy dispersive X-ray (EDX) accessory. The AFM results were obtained using an Agilent 5500 SPM/AFM. The photoluminescence results were recorded on a spectrometer (LS-45, PerkinElmer). Photocatalytic dye degradation efficiencies were recorded using a UV/vis-spectrophotometer (PerkinElmer/λ-365).
θ); the Cu Kα operational X-ray source is (λ = 1.54 Å, 40 kV, 40 mA). The 2θ range was fixed from 15° to 80° (step: 0.05° and scan rate: 2° min−1), Fourier transform infrared (FT-IR) analysis was performed on a BrukerTensor-27. The SEM results were obtained using an FEI-Nova NanoSEM-450 electron microscope. The elemental composition of Co@Na–BiVO4 NPs was obtained using a SEM equipped with an energy dispersive X-ray (EDX) accessory. The AFM results were obtained using an Agilent 5500 SPM/AFM. The photoluminescence results were recorded on a spectrometer (LS-45, PerkinElmer). Photocatalytic dye degradation efficiencies were recorded using a UV/vis-spectrophotometer (PerkinElmer/λ-365).
2.4 Photocatalytic degradation experiments
To investigate comparative efficiencies, degradation experiments of selected dyes (MB, CR and RhB) were performed on a spectrophotometer (PerkinElmer/λ-365). Dye degradation experiments were performed in a Pyrex reactor of size 6.1 × 4.3 × 4.2 inches (100 mL/silicon septum, ChemGlass AF-0528-06). For photocatalytic experiments, the photoreaction set up was made to elucidate the photolysis reaction of dyes, pure BiVO4, Na–BiVO4, Co–BiVO4 and Co@Na–BiVO4 microstructures. Photocatalytic activities of all as-synthesized photocatalysts were investigated using direct sunlight. All photoreactions have been carried out on consecutive days under a clear sky to ensure accurate photon absorption. The average photon flux was measured to be 1.388 × 103 W m−2 using a light meter (Extech/LT-300 USA). For each photoreaction, 10 mg of the photocatalyst was optimized, and treated with 50 mL of 5 ppm solution of each dye. Before starting the photoreaction, the analyte solution was stirred in the absence of light for 30 min to soak the dye with photocatalysts. At regular intervals, 3.5 mL reaction mixture was pipetted out and centrifuged to get a clear solution for UV-vis measurements.3. Results and discussion
The synthesis scheme of all photocatalysts is demonstrated in Fig. 1 (see details in the Experimental section). To remove the impurities and enhance the crystallinity, the as-synthesized photocatalysts were calcined at 350 °C for 3 h.3.1 XRD
XRD is an excellent tool for the detection or identification of crystal phases in solid-state materials.25 The XRD patterns of the as-synthesized plain BiVO4, Na–BiVO4, Co–BiVO4, and Co@Na–BiVO4 are shown in Fig. 2(a). The synthesized BiVO4 is monoclinic in phase (c.f. JCPDS#14-0688), with XRD peaks observed at 18.66°, 18.98°, 28.94°, 30.54°, 34.49°, 35.22°, 39.78°, 42.46°, 46.71°, 47.30°, 50.31°, 53.31° and 58.53°, corresponding to (110), (011), (121), (040), (200), (002), (211), (051), (240), (042), (202), (161) and (321) crystallographic planes, respectively.25 No other diffractions were seen, confirming the phase purity of BiVO4. Co metal exists in the form of cobalt oxide (Co3O4, cubic phase), with major diffraction peaks at 36.8 and 65.2° (PDF#43-1003). The ionic radius of Na+ (1.02 Å) is almost same as that of the Bi3+ ion (1.03 Å), which favors the substitution of Na+ on Bi3+, while most of the Na exists as sodium oxide Na2O (cubic), with major peaks observed at 27.7, 32.1 and 46.1°, in accordance with PDF#23-0528. By employing the reported method, the crystallite size was measured to be 19.7 nm. The results showed that intrinsic catalytic activity of the photocatalyst is not a sole factor of particle sizes; however, the rate of dye degradation is mainly due to metal cocatalysts over BiVO4 surfaces.26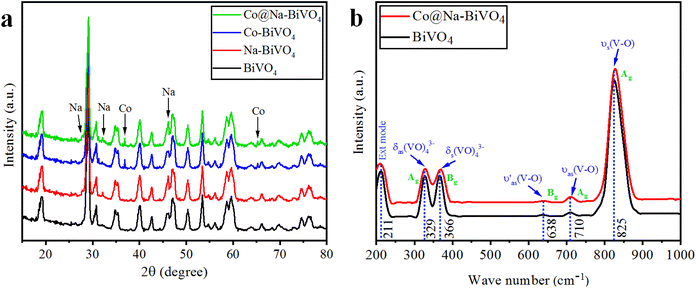 | ||
| Fig. 2 (a) Powder XRD patterns of BiVO4, Na–BiVO4, Co–BiVO4 and Co@Na–BiVO4 (b) Raman vibrations of BiVO4 and Co@Na–BiVO4 microstructures. | ||
3.2 Raman spectroscopy
The structure, chemical composition and bonding of the as-synthesized materials were further analyzed by Raman spectroscopy as illustrated in Fig. 2(b). The vibration bands observed at 211, 329, 366, 638, 710, and 825 cm−1 were attributed to the VO4 tetrahedron. The strong vibrations at 825 cm−1 were allocated to the V–O symmetric stretching mode (Ag). At 710 and 638 cm−1, weak asymmetric stretching bands (Ag and Bg) of V–O were observed. The asymmetric and symmetric bending vibrations of the VO4 tetrahedron were observed at 327 and 367 cm−1, respectively. In Co@Na–BiVO4, the intense vibration mode shifted to 828 cm−1 from 826 cm−1. In the presence of Co and Na, a shift was observed due to the decrease in the V–O bond length. Moreover, Raman band intensities and the FWHM also shortens: this behavior corresponds to crystallinity or flaws.27,283.3 SEM and EDX
The structural and surface morphology of the Co@Na–BiVO4 photocatalysts were investigated by scanning electron microscopy (SEM). Fig. 3(a–d) shows the typical SEM images of the Co@Na–BiVO4 photocatalysts. The microstructures are blossom-like due to our new synthesis, rather than the conventional structures.29 The SEM results are in good agreement that Co (in the form of Co3O4) and Na (in the form of Na2O) enhance the surface area of BiVO4. The novelty of our work is that the blossom-like structures were developed using the time-scaled low temperature co-precipitation method (24 h, 10 °C), which led to an enhancement in the surface area and active sites. An EDX accessory was used to elucidate the elemental composition of the as-synthesized Co@Na–BiVO4 photocatalysts Fig. 3(e), which confirms the presence of Co and Na contents with BiVO4. The weights and atomic percentages of elements are tabulated in Table S1.†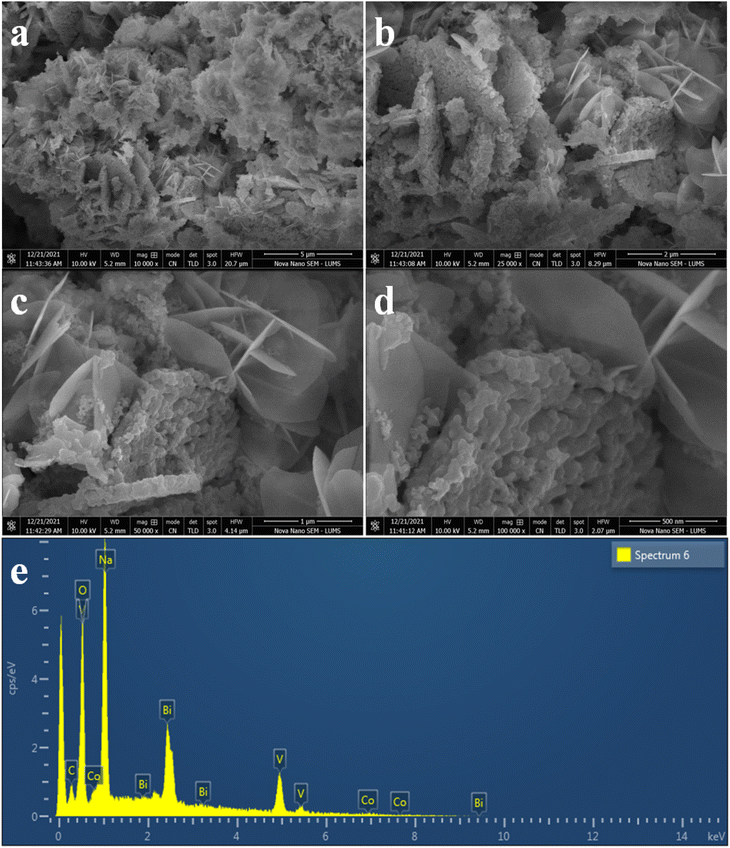 | ||
| Fig. 3 SEM images of Co@Na–BiVO4 microstructures (a) 5 μm (b) 2 μm (c) 1 μm and (d) 500 nm, whereas (e) represents the EDX analysis. | ||
3.4 AFM
Atomic force microscopy (AFM) is a characterization technique that provides information about the topography, surface roughness, size distribution, and morphology of materials at a high resolution.30 The Co@Na–BiVO4 photocatalysts were studied using AFM; the two-dimensional and three-dimensional topographical images are shown in Fig. 4(a–d). The scanning area is 9 × 9 μm, The AFM results revealed the surface morphology of Co@Na–BiVO4 particles with an average size of 18.4 nm, as shown in Fig. S1.† The size distribution of particles ranges from 9 to 20.5 nm.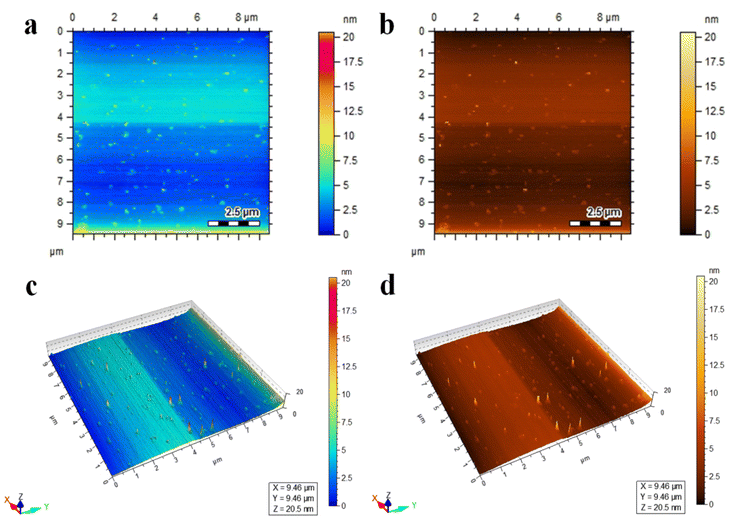 | ||
| Fig. 4 AFM images of Co@Na–BiVO4 (a and b) topographical images, and (c and d) three-dimensional view. | ||
3.5 UV-vis/DRS
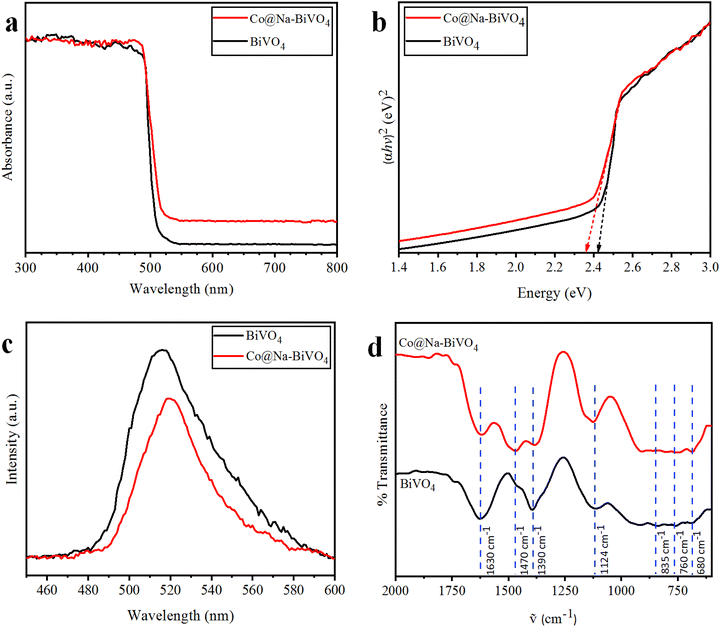
To evaluate the optical properties and band gap, UV-vis/DRS results of the as-synthesized photocatalysts were obtained (Fig. 5(a)). Plain BiVO4 shows maximum absorption at 512 nm, corresponding to its optical band gap Eg = 2.42 eV. For the Co@Na–BiVO4 microstructure, the absorption edge is slightly red-shifted to 525 nm (Eg = 2.36 eV), showing an excellent visible light response. The absorption of the Co@Na–BiVO4 microstructure further extends to 800 nm, which is due to Co d–d transitions31 and synergism of metal cocatalysts over BiVO4.323.6 PL
It is worth mentioning that our photoluminescence results confer the essential information about excitation, trapping and transfer of the charge carriers (e− & h+). Higher recombination of e− and h+ will result in higher emission intensities of PL signals.33 In this work, the high PL intensities (Fig. 5(c)) indicate a higher rate of charge recombination. These results indicate the higher charge separation in Co@Na–BiVO4 as compared to that in bulk BiVO4. As clearly seen in Fig. 5(c), Co@Na–BiVO4 intensity is weaker than that of BiVO4 indicating that recombination of charges is suppressed due to the presence of Co and Na metal oxides.3.7 FT-IR
The FT-IR results of as-synthesized BiVO4 and Co@Na–BiVO4 microstructures exhibited the intense and broad absorption bands (Fig. 5(d)), due to V–O vibration at 760 cm−1 and 835 cm−1, respectively, whereas Bi–O bending vibration was observed at 680 cm−1. Additional bands are observed at 1390 cm−1 and 1470 cm−1, which are characteristic of stretching vibrations of the absorbed atmospheric and adventitious CO2 of the instrument. The band at 1630 cm−1 corresponds to the bending vibration of water due to the moisture content in KBr used in sample preparation for FT-IR analysis. In the FT-IR spectrum of Co@Na–BiVO4, Co and Na metal content slightly narrowed the typical absorption band.343.8 Photocatalytic degradation studies of MB, CR and RhB
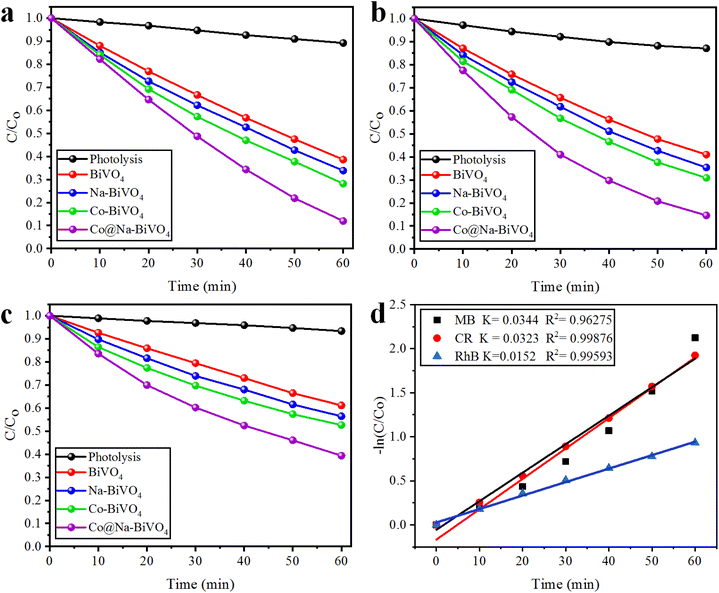
Photocatalytic dye degradation efficiencies are compared and quantified on the basis of the measured absorbance. It is important to select a suitable wavelength at which the dye solution shows the maximum absorbance. Using the Beer–Lambert law, the amount of dye was deliberated quantitatively with the absorption of light at λmax (specific to each dye).35 The absorbance of dyes was observed to decrease during the photocatalytic reaction, indicating photocatalytic degradation. The efficiencies of photocatalytic degradation of each dye are determined by using the following formula:where C0 and C represent the concentrations before and after irradiation, respectively. In this work, MB, CR, and RhB dyes were selected for photocatalytic performances of the synthesized catalysts. For the photoreactions, 5 ppm concentration of each dye was optimized. For MB, CR, and RhB dyes, the maximum absorbance was measured at wavelengths of 664, 498 and 552 nm, respectively (Fig. 6(a)). The absorbance peak of MB at 664 nm is due to its azo groups as well as π–π* electronic excitations.36 The UV-vis absorption of CR exhibits two dominant absorption peaks observed at 347 and 498 nm, and a peak observed at 347 nm, attributed to the predominant absorption of a benzene group.37,38 The peak at 498 nm is due to the naphthalene group.39 Similarly, the prime peak for RhB is attributed to the combined effect of four N-ethyl groups in the xanthene ring structure.40 Based on our UV-vis results (Fig. 6(b and c)), it is confirmed that MB, CR, and RhB were degraded with efficiencies of 88%, 85% and 60%, respectively. Overall, higher photocatalytic degradation activities over Co@Na–BiVO4 were recorded for MB and CR dyes. The degradation of RhB was found to be less than 60%, due to the stable configuration of the xanthene ring. The blue shift indicates the de-ethylation and organic intermediates produced during the photoreaction.41 Photocatalytic activities of as-synthesized Co@Na–BiVO4 were compared with those of Co–BiVO4, Na–BiVO4, bulk BiVO4 and photolysis as well (i.e. photoreaction of dyes without any catalyst) as shown in Fig. 7(a–c). The specialty of Co@Na–BiVO4 is that it gives the highest degradation activity (88%) than previously reported works (see Table S2†). Similarly, 85% efficiency was noted for the CR dye that is almost slightly less than that in the case of MB. BiVO4 gives 60% activity for CR and direct photolysis exhibits 13% only. Co@Na–BiVO4 showed 60% degradation efficiency for RhB, whereas BiVO4 (i.e. without Co & Na) exhibits 39% only. It has been observed that Na–BiVO4 is active and gives 64.6% degradation individually for the CR dye, whereas the Co–BiVO4 catalyst exhibits 69.1% activity for CR. The catalysts having both Co and Na contents exhibit 85% dye degradation activity that is almost two times higher than that of BiVO4 or that of individual Co/Na over BiVO4 surfaces. These results demonstrate that the existence of Co in the form of Co3O4 over BiVO4 is more favorable than the existence of alkali metal oxides (i.e. Na2O), because due to Na and Co, synergism was established for charge transportation to active sites. Due to synergism, electrons and holes can build equilibrium for migration and transfer from the support to active sites. On the basis of photocatalytic activities, it can be concluded that the presence of Co and Na oxides offers an effective synergism during the photoreaction. Table S1† demonstrates the comparison of the efficiencies of as-synthesized Co@Na–BiVO4 (the most active) with those of the reported photocatalysts. Fig. 7(d) demonstrates the photocatalytic apparent rate constant k for the degradation of MB, CR and RhB dyes using the Co@Na–BiVO4 catalyst.
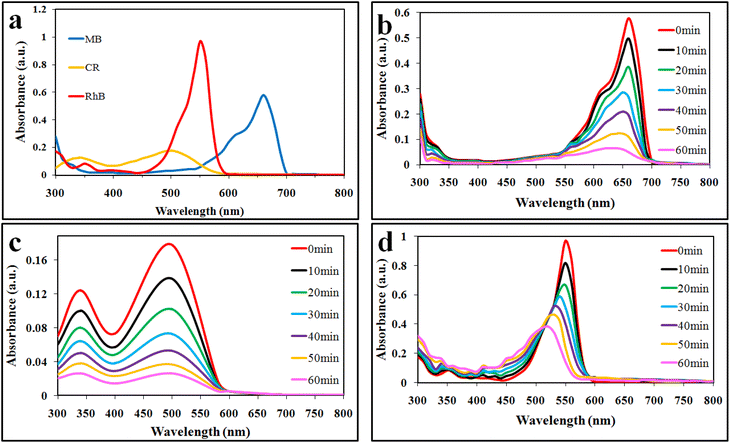 | ||
| Fig. 6 UV-vis spectrophotometry results: (a) lambda max, (b) MB, (c) CR and (d) RhB obtained over Co@Na–BiVO4 photocatalysts. | ||
3.9 Recyclability of the photocatalyst
The recyclability tests of Co@Na–BiVO4 microstructures were further investigated (Table S3† and Fig. 8(a–d)). The results of most active photocatalysts demonstrate the highest stability (each run: up to 60 min for each cycle), and only a small loss of 2.6%, 3.4%, 3.1% was observed for MB, CR and RhB, respectively. The slight decrease in efficiency is actually due to the deposition of the photocatalyst particles on the reactor wall, which causes the loss of active sites. Thus, results obtained during recyclability tests assure the stability and continuous use of Co@Na–BiVO4 for dye degradation and other photocatalytic applications as well.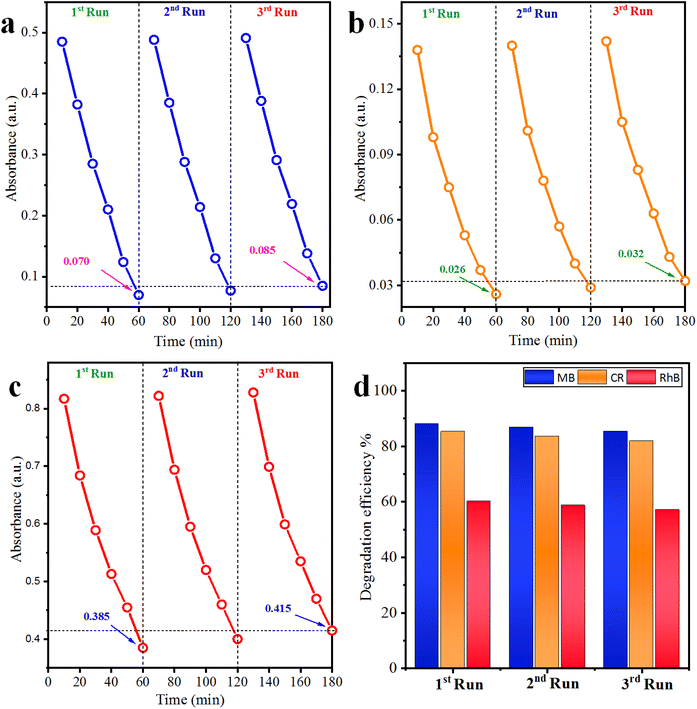 | ||
| Fig. 8 Recyclability of the Co@Na–BiVO4 catalyst for (a) MB, (b) CR and (c) RhB, whereas (d) represents the overall% degradation efficiency. | ||
3.10 Effect of pH
The pH of the dye solution greatly affects the degradation of cationic (MB and RhB) as well as anionic (CR) dyes. The effect of pH was investigated in the range of 4–10 for our selected dyes using 10 mg of the Co@Na–BiVO4 catalyst for each photoreaction. The results for percentage dye removal are illustrated in Fig. 9(a). The results show that degradation of cationic (MB and RhB) dyes is increased under slightly acidic conditions due to their strong affinities with the surface of the catalyst; however, at below pH 5, photocatalytic degradation is inhibited by reducing the number of available electrons. Moreover, anionic (CR) dye degradation is increased at a slightly basic pH, while under highly basic conditions (>9), the formation of hydroxyl radicals hinders the degradation process by reducing the number of available holes.42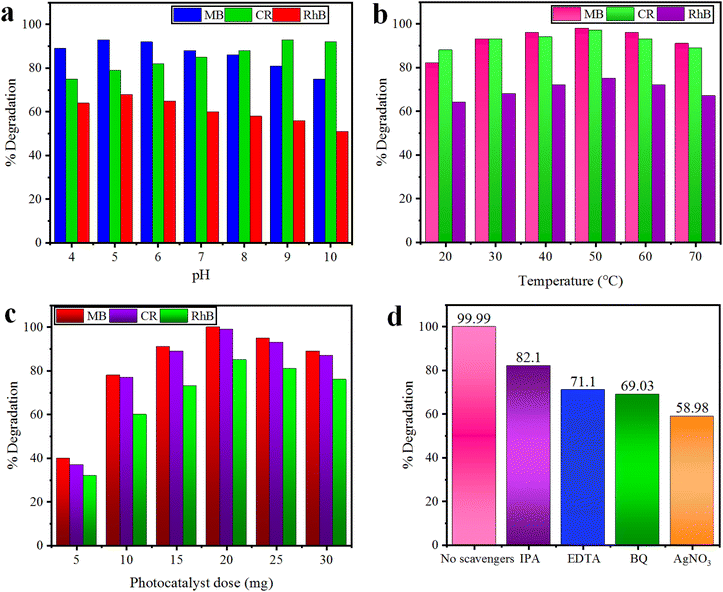 | ||
| Fig. 9 Effect of (a) pH, (b) temperature and (c) photocatalyst dose on MB, CR, and RhB dyes using the Co@Na–BiVO4 catalyst, whereas (d) represents the role of major active species. | ||
3.11 Effect of temperature
The optimized pH for each dye solution was used to evaluate the effect of temperature during the photodegradation reaction. The 20–70 °C temperature range was set using 10 mg of the Co@Na–BiVO4 catalyst for each photoreaction. The results indicate that 50 °C (±2) is the optimum temperature that facilitates the effective collisions of photocatalysts and dyes molecules. When the temperature is further increased, the adsorption of dye molecules on the catalyst becomes less which reduced the photodegradation efficiency.43 In contrast, the results (Fig. 9(b)) illustrate that at low temperature, slower desorption and less collisions between dye molecules inhibits the photoreaction.3.12 Effect of the photocatalyst dose
The photocatalyst doses have significant effect on the degradation of dyes; the effect of the dose was monitored using 5.0–30 mg Co@Na–BiVO4, at an optimized temperature and pH for each dye. The results illustrated in Fig. 9(c) prove that a high photocatalyst dose leads to the generation of more active sites and active species (˙O2− and ˙OH), which results in a higher degradation rate.44 However, addition of more than 20 mg of photocatalysts has negative effects on the photocatalytic degradation process. A high dose can initiate the formation of photocatalyst aggregates, which reduces the available surface area, blocks sunlight, and reduces efficiency. In addition to this, a higher photocatalyst dose can cause increased costs, and environmental concerns. Consequently, 20 mg was chosen as the optimal catalyst amount using a 150 mL Pyrex reactor.3.13 Role of sacrificial agents
To investigate the role of sacrificial agents, radical quenching experiments were carried out using the Co@Na–BiVO4 photocatalyst, and MB dye was chosen. Radical scavenging agents used during this study are p-benzoquinone (BQ, 0.001 mol L−1 for ˙O2− radicals), isopropyl alcohol (IPA, 0.01 mol L−1 for ˙OH), AgNO3 (0.001 mol L−1 for e−) and ethylene diamine tetra acetate (EDTA, 0.01 mol L−1 for h+).45 The degradation efficiency was considerably suppressed by using BQ, EDTA, and IPA, as illustrated in Fig. 9(d). These results demonstrate that e−, ˙O2− and h+ are the major active species. However, the addition of AgNO3 decreases the activities only a little, which discloses that e− plays a very minor role in dye degradation.3.14 Mechanism of the photoreaction
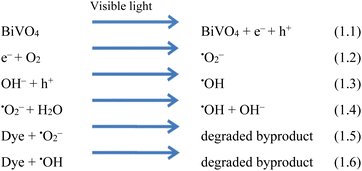
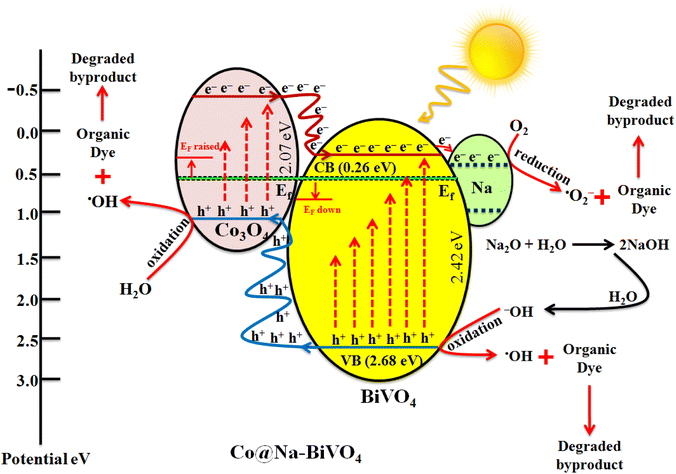
Understanding the mechanism is of vital importance in order to understand the overall efficiencies of photocatalyts.46 For the efficiency of any photocatalyst, it is essential to have a band gap less than 3 eV in order to extend light absorption and harvest maximum solar energy.47 It has been observed that the synergism between Co and Na enhances the redox sites for dye degradation due to their inherent characteristics during the photoreaction. The mechanism, band energy levels and charge transport (h+/e−) due to synergism over Co@Na–BiVO4 photocatalysts are illustrated in Fig. 7 and Scheme 1. The dye degradation results show that bare BiVO4 catalysts (i.e. in the absence of Co/Na) are not capable of transporting the photo induced charges to the active centres. Co3O4 is a p-type semiconductor, and when it is assembled with n-type BiVO4, the Fermi energy of both semiconductor systems aligned and shifted to new energy levels. Fermi level (EF) of Co3O4 lies near to the valence bands. During photoreaction, it gets a new position (raised) that makes the charge transportation more convenient.48 The EF of BiVO4 present near the conduction band shifted down till equilibrium. When the photoreaction starts, electron–hole pairs started to migrate to photocatalyst surfaces where they are actually involved in the photodegradation of dyes. Due to higher CB levels of Co3O4 compared to those of BiVO4, the photogenerated e− of Co3O4 preferably transfer to the CB of BiVO4 support. Electrons are further trapped by Na dopants where these are utilized by dissolved O2 to generate superoxide anion radicals (˙O2−),23 and thus, synergism starts to be established between Co/Na and the BiVO4 support. Meanwhile, holes (h+) migrate from the valence band of BiVO4 to the valence band of Co3O4, where they react with water molecules to generate ˙OH radicals. These ˙OH radicals are further utilized for dye degradation reactions. Overall higher dye degradation results were obtained in the presence of both Co & Na contents, because Na in the form of Na2O, when reacted with water produces NaOH (alkali). This NaOH increases the concentration of hydroxyl groups (−OH). The hydroxyl groups are further consumed by the holes at the surfaces of BiVO4 supports.49,50 The holes behave as electrophiles that convert –OH groups into radicals (˙OH). It has been observed that due to the presence of Na contents, a higher concentration of ˙OH is inevitable that is impossible in the case of bare BiVO4 or Co–BiVO4. The higher number of ˙OH radicals lead to the increased degradation of dyes that is only possible due to the existence of Na contents. In the presence of synergy, electrons of cobalt oxides migrated to the BiVO4 system and are trapped by doped Na, whereas holes migrate from BiVO4 to the Co3O4 system. Due to the synergistic effect, photocatalytic performance of BiVO4 has been increased mainly by (a) higher charge transportation to the active centres, (b) higher absorption of visible light, (c) efficient charge separation and (d) availably of more active sites (˙OH) due to Na. Without co-catalysts (Co and Na), BiVO4 is not efficient for dye degradation. Overall, as-synthesized Co@Na–BiVO4 leads to more active sites, which enhances the photocatalytic activity (Fig. 10).4. Conclusion
Blossom-like BiVO4 microstructures have been successfully synthesized by the co-precipitation method with an optimized timescale strategy. Co and Na were in situ incorporated into BiVO4via low temperature chemical reduction followed by calcination. Powdered XRD and EDX analyses confirmed the existence of incorporated Co & Na over the BiVO4 support. The Raman and FT-IR results revealed the presence of active centers where dye degradation reactions occur. The UV-vis/DRS results revealed an extension in absorption to the visible spectrum, that is, from 512 nm to 530 nm due to Co/Na contents. The PL results indicate the suppressed recombination of photo-induced charges that is due to the synergism and continuous transfer of charges to active sites. Co@Na–BiVO4 exhibits a higher photocatalytic activity as compared to BiVO4, Co–BiVO4 or Na–BiVO4. Overall, the higher activities are attributed to synergistic charge transfer. Thus, the introduction of Co & Na in this work offers a new approach for photocatalysis applications. Furthermore, the recyclability test assures the structural stability of the assynthesized catalysts. Although various morphologies of BiVO4 have been designed for dye degradation, the introduction of Co and Na contents over blossom-like BiVO4 microstructures delivers higher dye degradation activities.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Higher Education Commission (HEC) of Pakistan (No. 377/IPFP-II) SRGP/NAHE/HEC/2020/27 Islamabad. Synthesis work and dye degradation activities were conducted in the Inorganic Materials Laboratory (52S), Institute of Chemistry, The Islamia University of Bahawalpur. Dr Ejaz Hussain acknowledges the Carnegie Mellon University (CMU) of USA and Syed Babar Ali School of Science and engineering, Lahore University of Management Sciences (LUMS) to facilitate for sample characterization.References
- F. D. Chequer, et al., Textile dyes: dyeing process and environmental impact, Eco-Friendly Text. Dyeing Finish., 2013, 6(6), 151–176 Search PubMed.
- M. Rauf and S. S. Ashraf, Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution, Chem. Eng. J., 2009, 151(1–3), 10–18 Search PubMed.
- U. Quyyum, et al., Tunable sulphur doping in CuFe2O4 for the efficient removal of arsenic through arsenomolybdate complex adsorption: kinetics, isothermal and mechanistic studies, Environ. Sci.: Water Res. Technol., 2023, 9, 1147–1160 Search PubMed.
- P. S. Kumar, et al., A critical review on recent developments in the low-cost adsorption of dyes from wastewater, Desalin. Water Treat., 2019, 172, 395–416 Search PubMed.
- S. Kohtani, et al., Photooxidation reactions of polycyclic aromatic hydrocarbons over pure and Ag-loaded BiVO4 photocatalysts, Appl. Catal., B, 2005, 58(3–4), 265–272 Search PubMed.
- H. Kato, et al., Construction of Z-scheme type heterogeneous photocatalysis systems for water splitting into H2 and O2 under visible light irradiation, Chem. Lett., 2004, 33(10), 1348–1349 Search PubMed.
- B. Pant, et al., General one-pot strategy to prepare Ag–TiO2 decorated reduced graphene oxide nanocomposites for chemical and biological disinfectant, J. Alloys Compd., 2016, 671, 51–59 Search PubMed.
- X. Ning, et al., Inhibition of photocorrosion of CdS via assembling with thin film TiO2 and removing formed oxygen by artificial gill for visible light overall water splitting, Appl. Catal., B, 2017, 212, 129–139 Search PubMed.
- S. Kohtani, et al., Photocatalytic degradation of 4-n-nonylphenol under irradiation from solar simulator: comparison between BiVO4 and TiO2 photocatalysts, Chem. Lett., 2002, 31(7), 660–661 Search PubMed.
- T. Lu and B. Steele, Electrical conductivity of polycrystalline BiVO4 samples having the scheelite structure, Solid State Ionics, 1986, 21(4), 339–342 Search PubMed.
- L. Zhou, et al., A sonochemical route to visible-light-driven high-activity BiVO4 photocatalyst, J. Mol. Catal. A: Chem., 2006, 252(1–2), 120–124 Search PubMed.
- P. Wood and F. Glasser, Preparation and properties of pigmentary grade BiVO4 precipitated from aqueous solution, Ceram. Int., 2004, 30(6), 875–882 Search PubMed.
- J. Liu, et al., Hydrothermal preparation of BiVO4 powders, Mater. Sci. Eng. B, 2003, 104(1–2), 36–39 Search PubMed.
- M. Nawaz, et al., Magnetic and pH-responsive magnetic nanocarriers, in Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications, Elsevier, 2019, pp. 37–85 Search PubMed.
- C. V. Reddy, et al., Effect of noble metal ions dopants on solar photoelectrochemical water splitting and electrochemical supercapacitive performance of BiVO4 hollow tubes, Sol. Energy Mater. Sol. Cells, 2021, 226, 111056 Search PubMed.
- S.-W. Cao, et al., Preparation of Au-BiVO4 heterogeneous nanostructures as highly efficient visible-light photocatalysts, ACS Appl. Mater. Interfaces, 2012, 4(1), 418–423 Search PubMed.
- N. Wetchakun, et al., BiVO4/CeO2 nanocomposites with high visible-light-induced photocatalytic activity, ACS Appl. Mater. Interfaces, 2012, 4(7), 3718–3723 Search PubMed.
- Y. Pihosh, et al., Photocatalytic generation of hydrogen by core-shell WO3/BiVO4 nanorods with ultimate water splitting efficiency, Sci. Rep., 2015, 5(1), 1–10 Search PubMed.
- L. Zou, H. Wang and X. Wang, High efficient photodegradation and photocatalytic hydrogen production of CdS/BiVO4 heterostructure through Z-scheme process, ACS Sustainable Chem. Eng., 2017, 5(1), 303–309 Search PubMed.
- C. Regmi, et al., Cobalt-doped BiVO4 (Co-BiVO4) as a visible-light-driven photocatalyst for the degradation of malachite green and inactivation of harmful microorganisms in wastewater, Res. Chem. Intermed., 2017, 43, 5203–5216 Search PubMed.
- B. Zhou, et al., Visible-light sensitive cobalt-doped BiVO4 (Co-BiVO4) photocatalytic composites for the degradation of methylene blue dye in dilute aqueous solutions, Appl. Catal., B, 2010, 99(1–2), 214–221 Search PubMed.
- U. Prasad, et al., Role of alkali metal in BiVO4 crystal structure for enhancing charge separation and diffusion length for photoelectrochemical water splitting, ACS Appl. Mater. Interfaces, 2020, 12(47), 52808–52818 Search PubMed.
- J. Kang, et al., The enhanced peroxymonosulfate-assisted photocatalytic degradation of tetracycline under visible light by g-C3N4/Na-BiVO4 heterojunction catalyst and its mechanism, J. Environ. Chem. Eng., 2021, 9(4), 105524 Search PubMed.
- C. Ravidhas, et al., Facile synthesis of nanostructured monoclinic bismuth vanadate by a co-precipitation method: Structural, optical and photocatalytic properties, Mater. Sci. Semicond. Process., 2015, 30, 343–351 Search PubMed.
- Y. Zhao, et al., Significance of crystal morphology controlling in semiconductor-based photocatalysis: a case study on BiVO4 photocatalyst, Cryst. Growth Des., 2017, 17(6), 2923–2928 Search PubMed.
- C. Feng, et al., Ultrathin NiCo2O4 nanosheets with dual-metal active sites for enhanced solar water splitting of a BiVO4 photoanode, J. Mater. Chem. A, 2019, 7(39), 22274–22278 Search PubMed.
- C. Yu, et al., Design and fabrication of microsphere photocatalysts for environmental purification and energy conversion, Chem. Eng. J., 2016, 287, 117–129 Search PubMed.
- E. Hussain, et al., Titania-Supported Palladium/Strontium Nanoparticles (Pd/Sr-NPs@P25) for Photocatalytic H2 Production from Water Splitting, J. Phys. Chem. C, 2016, 120(31), 17205–17213 Search PubMed.
- M. Shang, et al., Nanosized BiVO4 with high visible-light-induced photocatalytic activity: ultrasonic-assisted synthesis and protective effect of surfactant, J. Hazard. Mater., 2009, 172(1), 338–344 Search PubMed.
- G. Li, et al., Photocatalytic behaviors of epitaxial BiVO4 (010) thin films, Appl. Catal., B, 2019, 248, 115–119 Search PubMed.
- C. Qin, et al., A Near-Infrared cis-Configured Squaraine Co-Sensitizer for High-Efficiency Dye-Sensitized Solar Cells, Adv. Funct. Mater., 2013, 23(30), 3782–3789 Search PubMed.
- S. Chen, et al., In-situ synthesis of facet-dependent BiVO4/Ag3PO4/PANI photocatalyst with enhanced visible-light-induced photocatalytic degradation performance: Synergism of interfacial coupling and hole-transfer, Chem. Eng. J., 2020, 382, 122840 Search PubMed.
- E. Hussain, et al., Remarkable effect of BaO on photocatalytic H2 evolution from water splitting via TiO2 (P25) supported palladium nanoparticles, J. Environ. Chem. Eng., 2019, 7(1), 102729 Search PubMed.
- I. Khan, et al., Sonochemical assisted hydrothermal synthesis of pseudo-flower shaped Bismuth vanadate (BiVO4) and their solar-driven water splitting application, Ultrason. Sonochem., 2017, 36, 386–392 Search PubMed.
- R. Dariani, et al., Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles, Optik, 2016, 127(18), 7143–7154 Search PubMed.
- P. Kumar, et al., C3N5: a low bandgap semiconductor containing an azo-linked carbon nitride framework for photocatalytic, photovoltaic and adsorbent applications, J. Am. Chem. Soc., 2019, 141(13), 5415–5436 Search PubMed.
- S. S. Bhat and N. G. Sundaram, Efficient visible light photocatalysis of Bi4TaO8Cl nanoparticles synthesized by solution combustion technique, RSC Adv., 2013, 3(34), 14371–14378 Search PubMed.
- M. A. Sayed, et al., A facile hydrothermal synthesis of novel CeO2/CdSe and CeO2/CdTe Nanocomposites: Spectroscopic investigations for economically feasible photocatalytic degradation of Congo red dye, Inorg. Chem. Commun., 2021, 130, 108750 Search PubMed.
- S. Erdemoğlu, et al., Photocatalytic degradation of Congo Red by hydrothermally synthesized nanocrystalline TiO2 and identification of degradation products by LC–MS, J. Hazard. Mater., 2008, 155(3), 469–476 Search PubMed.
- C. Chen, et al., Photosensitized degradation of dyes in polyoxometalate solutions versus TiO2 dispersions under visible-light irradiation: mechanistic implications, Chem.–Eur. J., 2004, 10(8), 1956–1965 Search PubMed.
- Y.-H. Chiu, et al., Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts, Catalysts, 2019, 9(5), 430 Search PubMed.
- R. Coronado-Castañeda, et al., Photocatalytic degradation and toxicity reduction of isoniazid using β-Bi2O3 in real wastewater, Catal. Today, 2020, 341, 82–89 Search PubMed.
- M. Malekkiani, et al., Facile fabrication of ternary MWCNTs/ZnO/Chitosan nanocomposite for enhanced photocatalytic degradation of methylene blue and antibacterial activity, Sci. Rep., 2022, 12(1), 1–22 Search PubMed.
- A. Ilyas, et al., Growth of villi-microstructured bismuth vanadate (Vm-BiVO4) for photocatalytic degradation of crystal violet dye, RSC Adv., 2023, 13(4), 2379–2391 Search PubMed.
- Z. Sha and J. Wu, Enhanced visible-light photocatalytic performance of BiOBr/UiO-66(Zr) composite for dye degradation with the assistance of UiO-66, RSC Adv., 2015, 5(49), 39592–39600 Search PubMed.
- A. O. Ibhadon and P. Fitzpatrick, Heterogeneous Photocatalysis: Recent Advances and Applications, Catalysts, 2013, 3(1), 189–218 Search PubMed.
- J. Yu and A. Kudo, Effects of Structural Variation on the Photocatalytic Performance of Hydrothermally Synthesized BiVO4, Adv. Funct. Mater., 2006, 16(16), 2163–2169 Search PubMed.
- X. Chang, et al., Enhanced surface reaction kinetics and charge separation of p–n heterojunction Co3O4/BiVO4 photoanodes, J. Am. Chem. Soc., 2015, 137(26), 8356–8359 Search PubMed.
- X. Zhou, et al., Highly efficient Ag2O/Na-g-C3N4 heterojunction for photocatalytic desulfurization of thiophene in fuel under ambient air conditions, Appl. Catal., B, 2022, 316, 121614 Search PubMed.
- H. Golmojdeh and M. Zanjanchi, A facile approach for synthesis of BiVO4 nano-particles possessing high surface area and various morphologies, Cryst. Res. Technol., 2012, 47(9), 1014–1025 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na00048f |
| This journal is © The Royal Society of Chemistry 2023 |