Open Access Article
Open Access ArticleAccounts of applied molecular rotors and rotary motors: recent advances
Anup
Singhania
 ab,
Sudeshna
Kalita
ab,
Sudeshna
Kalita
 ab,
Prerna
Chettri
ab,
Prerna
Chettri
 ab and
Subrata
Ghosh
ab and
Subrata
Ghosh
 *ab
*ab
aNatural Product Chemistry Group, Chemical Sciences & Technology Division, CSIR-North East Institute of Science & Technology, Jorhat, 785006, Assam, India. E-mail: ocsgin@gmail.com
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad, 201002, India
First published on 22nd May 2023
Abstract
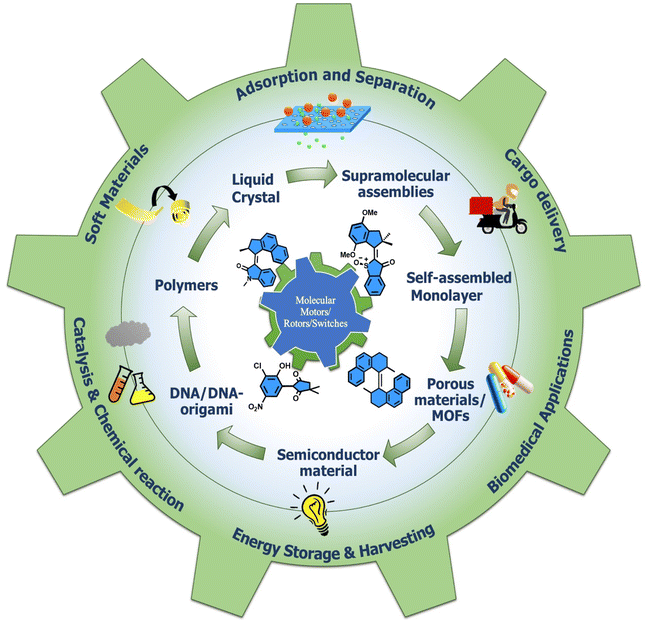
Molecular machines are nanoscale devices capable of performing mechanical works at molecular level. These systems could be a single molecule or a collection of component molecules that interrelate with one another to produce nanomechanical movements and resulting performances. The design of the components of molecular machine with bioinspired traits results in various nanomechanical motions. Some known molecular machines are rotors, motors, nanocars, gears, elevators, and so on based on their nanomechanical motion. The conversion of these individual nanomechanical motions to collective motions via integration into suitable platforms yields impressive macroscopic output at varied sizes. Instead of limited experimental acquaintances, the researchers demonstrated several applications of molecular machines in chemical transformation, energy conversion, gas/liquid separation, biomedical use, and soft material fabrication. As a result, the development of new molecular machines and their applications has accelerated over the previous two decades. This review highlights the design principles and application scopes of several rotors and rotary motor systems because these machines are used in real applications. This review also offers a systematic and thorough overview of current advancements in rotary motors, providing in-depth knowledge and predicting future problems and goals in this area.
1. Introduction
1.1. Translation of chemical structure to molecular machine
Chemical structures often account for the execution of chemical reactions associated with the chemical state change of the molecules; nature's kitchen uses chemical reactions and biosynthesis processes to produce an innumerable number of molecules. Besides these, nature has specific ways of organizing biological processes with various regulatory tools.1–3 One such tool is biomolecular machines that execute jobs by changing their conformational states and using their programmed mechanical dynamics. Though there is hardly any difference in the chemical structures of machines and nonmachines, these molecular machines show real-time operations to execute many routine biological jobs. The most miniature proton-driven biomolecular motor is F1-ATPase, a γ-subunit of ATP synthetase enzyme that works as a central rotor. It processes unrestricted angular rotatory motion of the subunit surrounding a α3β3stator and reverses ATP synthesis and hydrolysis.4 Thereby, the knowledge of molecular machines came, and it categorically refers to groups of chemical structures with remarkable potential to produce mechanical forces and accomplish measurable physical works. Molecular machines5,6 are generally subclassed into rotors, motors,7 rotaxanes, catenanes,8,9 and some typical switches.10 Synthetic chemists started mimicking the biological molecular machines and using biomimetic nanomechanical mechanisms to build artificial molecular machines. They focused on the control of the directional movement processes of the molecule. Investigations have shown the different modes of operation and control of molecular machines in different environments.11–13The advancement was significantly accelerated when scientists learned to synthesize mechanically interlocked molecules.14 The concept of nanotechnology by Richard Feynman was well-established by Jean-Pierre Sauvage, Sir Fraser Stoddart, and B. L. Feringa through their contribution to molecular machines.15–18 We found several biomachines in nature, but deciphering their exact mechanisms is complex. However, researchers have successfully decoded the mode of action of natural motor proteins in the last few decades. Depending on the unique properties of biomachines, the molecular systems were classified6,8,19 earlier based on the types of work they served. For example, a switch-based molecule has reversible movements, and the relative positions of different molecular parts influence the state of the switch. The molecule can undo the switch effect when the molecular counterparts return to the initial position. A molecular motor impacts systems by operating their components or a substrate over the trajectory. Motor function is repetitive and progressive in a system.6,19
However, the switches can be a motor in a particular condition if the “forward” transition between two states takes a different route than the “backward” transition.8 In this context, Feringa's unidirectional overcrowded alkene motor is an example.
1.2. Molecule motors and their motion
After the discovery of Brownian motion in 1827 by the famous botanist Robert Brown, Einstein and Perrin proved that the bombardment by the particles available for collision with the molecule instigated Brownian motion.20–23 With the growing interest in building molecular machines, scientists have made numerous efforts to understand artificial molecular machines and their modes of action by synthesis and characterization. Brownian motor-type machinery is found in nature in the curved movement of eggs in the fallopian tube.24–26 The egg progression occurs by systematically regulating the outer and inner dynein arms in the fallopian tube. Three biomolecular motor classes in eukaryotic cells generate power via their stochastic linear movements: actin-based myosin band motor proteins, tubulin-based dyneins, and kinesin motor proteins. These proteins bind to the specific nucleotide, leading to a change in the conformation due to ATP hydrolysis.27–30 Actin-based protein myosin hydrolyzes ATP; it generates force through the power stroke (PS) mechanism in skeletal muscle that walks toward the positive end of an actin filament and causes muscle contraction.31,32 Similarly, other motor proteins play a vital role in cellular functions: transporting intracellular cargo to the specific site, synthesizing proteins, replicating DNAs, and decoding the DNA strands.33–36Motor proteins have been extensively used in nanofabrication and as building blocks for generating hybrid systems by engineering synthetic molecules.37–39 These biomolecular machines comprise amino acid chains that undergo self-assembly or folding utilizing ATP as fuel. DNA scaffolds are used as a tool to arrange/self-assemble the molecular motors, where DNA serves as fuel.40 Notably, different molecular motors/rotors are linked with DNA origami, where DNA is used to template or drive the assembly of molecular motors. Thus, taking advantage of the complementary base–pair interaction in oligonucleotides and the self-assembly process, DNA-based machines have been developed.
This review focuses on information about the molecular motors' physical and chemical characteristics. We emphasize the development of various rotary motor systems with mechanical properties and their microscopic and macroscopic works (Chart 1). We discuss how the molecular engineering of these motors can allow the building of new hybrid nanodevices that deliver relevant outcomes. Moreover, the collective macroscopic works are highlighted upon integrating with different surfaces or interfaces (such as polymers and 3D porous materials). We kept our discussion centered on rotary motor systems because we found they have the most practical applications. Therefore, some current reviews6,8,41–43 by eminent contributors have been discussed in depth for the readers' interest.
1.3. Brownian ratchet and power stroke motions
The first and second laws of thermodynamics gave us the knowledge of energy transportation that determines the direction of motion of a body; these laws are valid for macroscopic systems. However, many microscopic biomolecular motors govern the net directional motion in a biological system; thus, it is doubtful whether these two laws are relevant in the molecular domain. A motor protein uses the thermal energy, kT, to produce the directed motion, which conflicts with the second law of thermodynamics. Here, a ratchet mechanism is introduced to describe the phenomenon that governs biomolecular motors' function, generally mentioned as Brownian ratchet (BR) and power stroke (PS) rotors.44 This mechanism results in a net directional motion when the system is in symmetric potential with directionless applied force. Therefore, the question is how far these macroscopic laws govern the nanomachines in a nonequilibrium environment. The experimental and theoretical investigations strengthen the field of Brownian rotor that gives an insight into the mechanism.45 Several other published reviews6,8,19,46 on molecular machines have vividly consolidated the discussion on these motors' design and working mechanisms. Therefore, we would like to give brief details about some selected interpretations of the same and extend further toward their working principle, potential applications, and future outlook.A BR is a conceptual machine where an axel connected to a ratchet wheel allows the wheel system to rotate 360° around the axis in the forward direction with a reverse rotation restriction. Such ratchets get power by absorbing random thermal energy from the available sources. However, we may come to specific points where such motion appears highly unrealistic, although the working mechanism is the same. In nature, the BR mechanism assists many intracellular parasites in moving forward in their host system. The motion of a motile bacteria that move forward in the suspension fluid occurs at a low Reynolds number. The working principle of the F0F1 rotary motor of ATP synthase was explained with a model that shows how two BR states of the F1 motor utilize the elastic energy to induce the production of ATP, competing with the F0 motor to perform complex tasks.47 The F1 motor works as a PS using ATP as its fuel. In contrast, the F0 motor hydrolyzes the ATP and uses the chemical energy against the concentration gradient to rectify the F1 rotational motion. Efforts have been made to build artificial molecular motors by mimicking these ratchet mechanisms; however, the complexity and design of the biological rotors are stochastic and event-based. Hence, theoretical investigations are helpful and offer insight into the fundamental principles of making the molecules motorized. In another study, Astumian described a unidirectional Brownian motor motion of a large ring that became operational in adiabatic conditions at near equilibrium at every instant,48 resulting in about efficiency 100%. Eventually, two basic parameters are required for the molecule to become a Brownian rotor: one is self-propelled motion at a low Reynold number, and the other is that the motor should utilize Brownian motion rather than fighting against it because the temperature is not an adequate parameter as the velocity of the system is proportional to the square root of the temperature.49 Thus, an applied torque is needed for the rotary motor to perform a rotational motion and to repeat mechanical performance. These features must be fitted in a system to design a motor or rotor. Thus, a molecular motor will perform the net-directed motion in potential asymmetric barriers by combining the effect of structural asymmetry and diffusion motion by manipulating the energy barrier at nonequilibrium.50–52
The role of kinetic parameters is demonstrated by a comparative study between macroscopic and microscopic tasks, where an example of the movement of the brick from the lower staircase to the upper staircase is performed in two mechanistic ways.53 Moving the brick from white to black must work against friction that causes energy dissipation irreversibly, whereas lifting the brick into the next staircase is done reversibly (Fig. 1a–c). The modulation of surface friction manipulates this energy dissipation, and this process can be correlated with the modulation of microscopic work exploiting thermal noise and diffusion. The microscopic brick moves forward due to diffusion; moreover, the thermal activation causes the brick to move into the next staircase, whereas chemical energy is utilized to block the backward movement. However, the initial state of the brick is thermodynamically favorable compared to the kinetically stable final state, ascribed to the modulation of the energy barrier, known as the information ratchet. This information ratchet of the Brownian motor is explained by the catalytically driven state of the motor undergoing thermal activation, and diffusion is depicted.
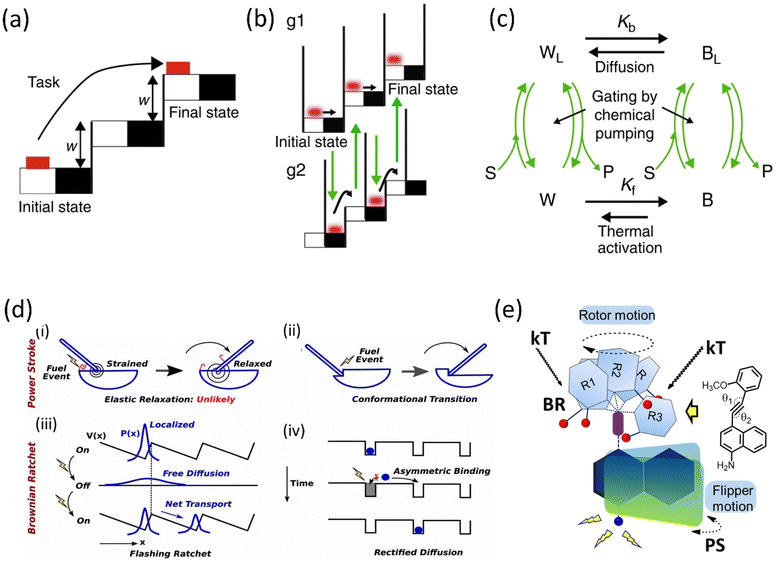 | ||
| Fig. 1 Graphical representation of (a) a macroscopic task with an example of the movement of a brick and (b) a microscopic brick information ratchet. (c) A cyclic four-state mechanism for the Brownian machine is shown (where W = white, B = black, S = substrate, P = product, and L implies motor bound to S and P) Kb and Kf are equilibrium constants between the black and white stairs. Reproduced with permission.53 Copyright 2019, The Authors, Published by Springer Nature. (d) Mechanisms of Power stroke (i) elastic relaxation. A fuel processing event (e.g., binding of an ATP or releasing a hydrolysis product, denoted by a lightning symbol): leads to the release of elastic energy. (ii) Conformational transition. Due to the conformational change of the motor, a fuel-processing event leads to a variation in the mechanical element's equilibrium position (denoted by the swinging rod). Before and after the stroke, the motor is not strained; Brownian ratchet: (iii) BR follows the flashing ratchet model; the potential V(x) barrier could change externally by supplying energy and generating a net current. (iv) Rectified diffusion model (based on the concept of information ratchet). Reproduced with permission.55 Copyright 2019, National Academy of Sciences. (e) A power-harvesting double ratchet motor: fusion of power stroke and Brownian ratchet.58 Adapted with permission from ref. 89. Copyright 2023, American Chemical Society. | ||
The complex performance of a molecular machine needs to have multiple functional components collectively work in coherence. Different parts of the machine may work in a parallel or stepwise manner. For example, when the ratchet mechanism has different characteristics, i.e., PS and BR, depending on their periodicity and energy inputs, multiple ratchets can possibly combine in a single system to build a motor that works to produce complex tasks (Fig. 1d).54,55 Biological motor proteins, e.g., Kinesin-1, Myosin-V, and F1-ATPase, employ these PS and BR mechanisms to a different extent to perform individual biological functions. Thus, combining these two mechanisms makes it possible to deduce a complex motion in a synthetic motor. Generally, we discuss PS and BR mechanisms as two separate ratchet behaviors of a motor protein. However, a clear consensus on their exact nature of work is yet to be known.
1.4. Rationale of double ratchet rotor/motor technology
We define a PS as a significant downhill free energy gradient generated over a distance more significant than the step size.56 A protein with this sufficiently large gradient may show a strong bias to move against the load.57 Therefore, the forward transition of a PS is nearly an irreversible process. It is similar to a relaxation of stored elastic potential energy from a strained state along with a nanometer-scale conformation change. Moreover, a BR mechanism follows a thermal gradient for forwarding movement. It stabilizes the position in a stepwise manner via the corresponding conformational changes utilizing the fuel processing events.54,55 However, a small backstroke or limited reversibility produces a stepper motion. An artificial molecular motor is thus created by combining PS and BR functional units in a single system, resulting in a double ratchet rotor (Fig. 1e).58 These two modules are connected so that BR independently diffuses to a stepper movement within a long temperature range. Such a BR motion can harvest natural energy from thermal fluctuation and engenders a favorable situation to store the energy. For this reason, the adjoint PS module can use the energy as elastic energy to favor the PS to move. Thus, the overall system behaves like a motor running under a long-range free energy gradient. In this current rotor, BR and PS modules are divided into two separate aromatic planes connected through a carbon–carbon triple bond in the current system. The naphthalene plane acts as a string in the PS module and undergoes elastic relaxation using the stored potential energy.2. Chronology of molecular rotary motor development
2.1. Molecular rotors and motors: from switches to rotors
It is possible to convert typical molecular switches to specific molecular machines with rotary functions when they are toggled between more than two states. Often, rotary motion is produced by applying external stimuli, such as light, heat, or electric current. Such switches have molecular components, which change their relative positions (or conformations) in response to these stimuli, producing a change in the overall structure and property of the molecule. These switches, which differ in the directionality for building molecular motors, are explored.6–8,19 Energy inputs can continuously drive conformations away from chemical equilibrium. Although rotation around a single bond is now well-known, controlling the net directional rotation is highly challenging and elusive. The transitional orientational changes around a single bond axis are an easy trick to convert a switch to a unidirectional rotor.In the present context, Kelly published a chemically driven system59 that performs 120° unidirectional rotation around a single covalent bond based on Feynman's ‘ratchet-pawl’ model in 1999. Nevertheless, the molecular system could not complete a cyclic and fast rotation. Yet, the studies contribute to understanding the design principles of molecular motors. Apart from a single bond, the double bond for molecular switches was exploited to build molecular motors. The inclusion of a sterically bulky unit in a stilbene switch results in helical chirality, which is further explored to develop chiroptical switches.60 The works on light-activated switches laid the foundation for developing light-activated molecular motors.61 Based on this, Feringa reported the first unidirectional light-activated overcrowded alkene molecular motor in 1999.62 This motor, featuring two identical halves connected by a double bond, executes 360° unidirectional rotations with four discreet isomerization conformations (Fig. 2a). Both halves contain a chiral point attributed to two energetically uphill cis–trans isomerization and two energetically downhill thermal helix inversions (THI), which assist in blocking the reverse rotation because of the steric effect. Therefore, it was a significant step toward building a unidirectional rotary motor for the first time. Afterward, the second and third-generation overcrowded motors were developed, and their properties were investigated extensively.61,63,64 Feringa's group also extensively worked on various key parameters to upgrade overcrowded alkene rotary motors.6,8,10,19,65
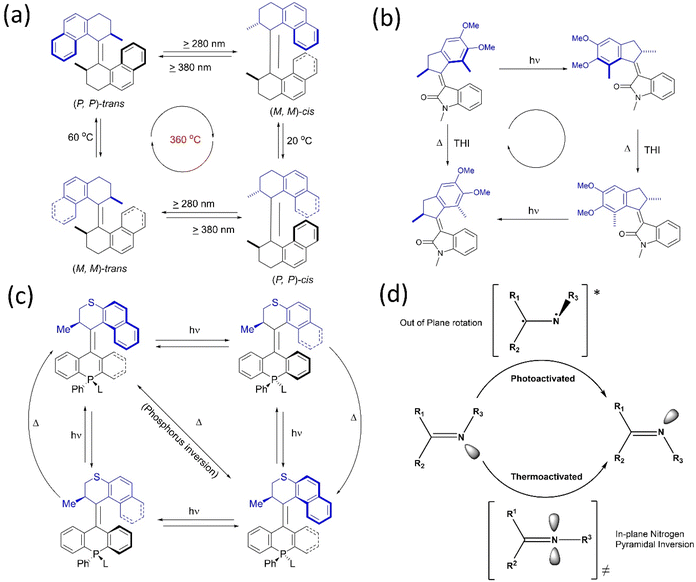 | ||
| Fig. 2 (a) First light-activated rotary motor.62 (b) Full 360° rotational cycle of oxindole-based molecular motor.67 (c) The four-step rotary motion of the phosphine-based molecular motor under photochemical conditions shows a three-step rotary cycle at elevated temperature.68 (d) Cyclic representation of the isomerization process of the imine rotor that undergoes an E–Z transition state.70 | ||
Recently, Feringa and coworkers developed a visible light-activated rotary motor based on the N-methyloxindole motif synthesized by Knoevenagel condensation.66 However, due to these motors' low photoisomerization quantum yield, a new oxindole-based motor was developed, which showed a better charge transfer process with increased four-fold photoisomerization (10%) quantum yield (Fig. 2b).67 In 2020, they developed solely photochemically-driven second-generation motors by the modification of stator parts, possessing four-step photochemically-induced unidirectional motion.68 A tetrahedral chiral phosphorus stereo element was introduced in the motor's lower half, which could ligate to a gold surface. Moreover, this system could perform unidirectional rotation in three rather than four steps as the chiral phosphorus center undergoes inversion in a thermal environment, which is the conventional isomerization cycle (Fig. 2c). This system could be functionalized to integrate into different surfaces/interfaces to generate smart materials to be applied in various fields.6,8,10,19,42,65
Another photoswitch, a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond bearing two asymmetrical motifs, undergoes E–Z isomerization like an alkene under photochemical and thermal conditions.69 However, in silico studies suggested that an out-of-plane rotation was observed around the C
N bond bearing two asymmetrical motifs, undergoes E–Z isomerization like an alkene under photochemical and thermal conditions.69 However, in silico studies suggested that an out-of-plane rotation was observed around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N asymmetrical bond through a singlet or triplet excited state on exposure to photochemical conditions when E to Z interconversion occurs (Fig. 2d).70 On the contrary, the thermal process occurs via the inversion of the N motif with a linear transition state instead of a rotational process. In 2014, Lehn and coworkers confirmed the thermal N-inversion path by studying the photothermal E/Z isomerization of camphorquinone-derived imines.71 Later on, they described that the chiral imine incorporated rotor shows a four-step rotation instead of a two-step rotation depending on the conformational flexibility of the C-stator part in the rotor72 as two energetically uphill photochemical isomerization processes, followed by a thermal ring inversion process.
N asymmetrical bond through a singlet or triplet excited state on exposure to photochemical conditions when E to Z interconversion occurs (Fig. 2d).70 On the contrary, the thermal process occurs via the inversion of the N motif with a linear transition state instead of a rotational process. In 2014, Lehn and coworkers confirmed the thermal N-inversion path by studying the photothermal E/Z isomerization of camphorquinone-derived imines.71 Later on, they described that the chiral imine incorporated rotor shows a four-step rotation instead of a two-step rotation depending on the conformational flexibility of the C-stator part in the rotor72 as two energetically uphill photochemical isomerization processes, followed by a thermal ring inversion process.
Another type of molecular motor hemithioindigo-based system was designed and developed.73 Henry Dube and coworkers first reported the motor as mentioned above by including sulfoxide and helical twisting around a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond that performs high speed 360° unidirectional rotation through photoisomerization and thermal helix inversion under visible light.74 The fast rotation of this motor can slow down by making the system sterically more hindered, providing direct evidence of all four intermediate states of the rotational cycle. In-depth studies of the operational mechanism of the hemithioindigo motor revealed that the unwanted tripled pathway hampered E–Z isomerization, and the dynamic can be more rapid if the quantum yield of isomerization increases.75 Notably, thermal ratcheting is essential in converting unstable isomers to stable ones; hence, directional motion is achieved. But this pathway did not proceed at low temperatures, resulting in a motor becoming a light-gated switch. The same group reported another hemithioindigo motor76 that can perform all the rotational steps more efficiently at low temperatures under a photoreaction (Fig. 3a). These findings by Dube's group provide new insights into the designing principles of light-driven molecular motors.
C bond that performs high speed 360° unidirectional rotation through photoisomerization and thermal helix inversion under visible light.74 The fast rotation of this motor can slow down by making the system sterically more hindered, providing direct evidence of all four intermediate states of the rotational cycle. In-depth studies of the operational mechanism of the hemithioindigo motor revealed that the unwanted tripled pathway hampered E–Z isomerization, and the dynamic can be more rapid if the quantum yield of isomerization increases.75 Notably, thermal ratcheting is essential in converting unstable isomers to stable ones; hence, directional motion is achieved. But this pathway did not proceed at low temperatures, resulting in a motor becoming a light-gated switch. The same group reported another hemithioindigo motor76 that can perform all the rotational steps more efficiently at low temperatures under a photoreaction (Fig. 3a). These findings by Dube's group provide new insights into the designing principles of light-driven molecular motors.
 | ||
| Fig. 3 (a) Molecular structures of hemithioindigo motor with four stable diastereoisomers and their cyclic motion. The “rotors” are colored green.76 (b) Structure and mechanism of directional rotation in the chemically-fueled motor.80 | ||
Inspired by nature, scientists have designed and reported chemically-fueled motors apart from the light-driven molecular motor. In this context, Feringa and coworkers described the directional motion of a motor system around a single covalent bond at the expense of a chemical reaction in four steps.77 The suitable chemical supply drives the phenyl rotor to rotate relative to a naphthyl stator to afford unidirectional motion in four district steps. However, the requirement of sequential chemical supplies is one of the drawbacks of the autonomous motor; hence, scientists explored and reported better chemically-driven autonomous molecular motors. The same group reported another motor based on an organopalladium motif that provides 360° rotation around a single bond upon supplying chemical fuel.78 Recently, Feringa's group described a biaryl motor comprised of a carboxylic side chain-integrated aryl rotor that accomplished full cyclic rotation in six steps.79 Moreover, Leigh and coworkers also reported a chemically driven motor that rotates around the N–C bond,80 composed of a pyrrole-2-carboxylic acid connected to a different substituent of benzoic acid. Upon the addition of chiral carbodiimide to the diacid system, it is converted into an anhydride system that later settles in a stable conformation (Fig. 3b). Further, the hydrolysis of this intramolecular anhydride motor with an asymmetric catalyst leads to its diacid form. Both reactions were kinetically gate and biased the directional motion, resulting in 360° rotation. Overall, this chemical engine system is an example of a Brownian information ratchet mechanism where the motor performs the task by the consumption of fuels. The authors envision that this first-generation autonomous single-bond rotary motor could be useful in catalytic transformation. These autonomous motors have a high potential to serve as chemical engines.
Frutos and colleagues conceptualized the design of a photoactive unidirectional molecular motor based on chiral hydrogen bond generation.81 Introducing the hydroxyl and amine group around the double bond of two sides forming hydrogen bonding induced the chiral environment, and isomerization occurs within 1.0 picosecond without forming any intermediate due to the hydrogen bond strength that allows rotation to overcome torsion. Several research teams designed other types of molecular motors systems based on azogroup82 and fulgides.83 Further improvement by structural modifications is necessary, and the applied molecular motors in various fields are discussed here.
2.2. Dynamics and controls of molecular rotor and motor
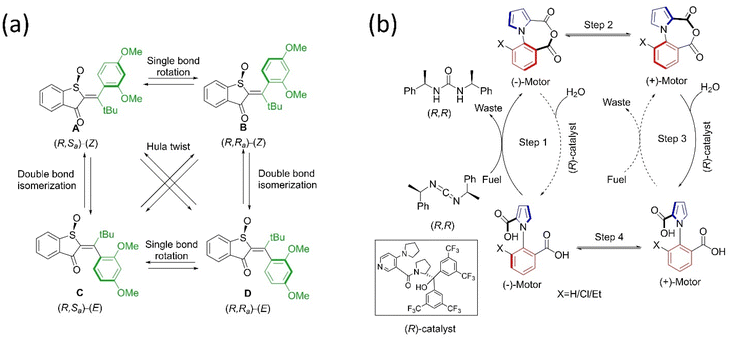
One of the main goals of molecular motor engineering is to regulate the mechanical motion that enables measurable outcomes in a controlled manner. An artificial molecular motor should ideally perform practical work reversibly and regulate mechanical motion.84 Although researchers have successfully built complex molecular rotors and motors, controlling the rotational motion at multistage by tuning different frequencies is yet to be successful, especially other than thermal stimuli. Different techniques are adopted to control the mechanical motion of artificial molecular motors, for instance, cooperative switching, allosteric interaction, pH, and metal ion exchange. Herein, we briefly highlighted a few approaches for controlling dynamic motions with selected examples in the solution and solid phase.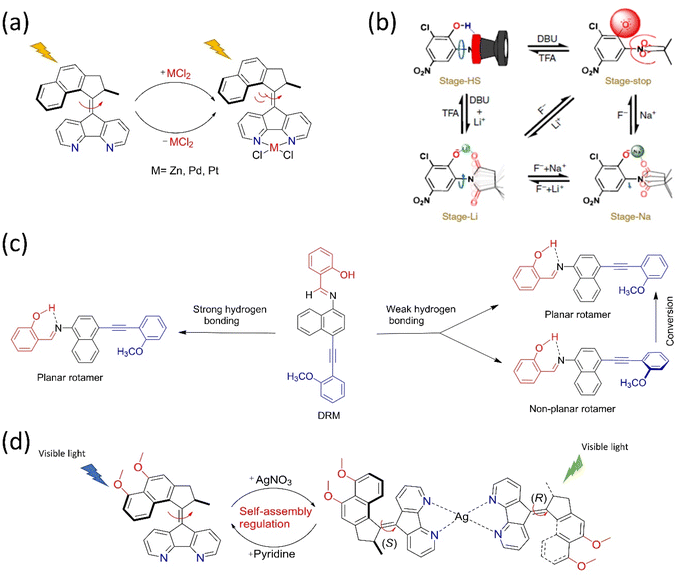 | ||
| Fig. 4 Approaches for controlling the dynamic motions in molecular motors. (a) Allosteric regulation of the overcrowded alkene-based motor by incorporating Zn, Pd, and Pt metal chlorides under light.85 (b) Schematic representation of a multistage modulated rotor, in which the four dynamic stages of the rotors are achieved with acid–base and allosteric interaction. Reproduced with permission.87 Copyright 2018, The Authors, published by Springer Nature. (c) The rotational control of DRM by weak and strong hydrogen bonding. The “BR”, “PS”, and “AM” moieties are colored blue, black, and red, respectively.89 (d) Coordination-directed self-assembly of a motorized nanocar.92 | ||
All the studies on regulating molecular motion in the range of too slow to ultrafast in various steps reveal that the precise regulation of the dynamic motion of the synthetic molecular rotor/motor is still challenging. Wu et al. reported the multistage-modulated rotational speed of a molecular rotor that is controlled by pH and metal cations Na+ and Li+ cations, which are reversible.87 This molecular rotor was integrated with succinimide, and phenol possesses a four-stage rotational speed ranging from slow-fast-stop to ultrafast rotational frequency at the same temperature (Fig. 4b). Generally, the OH-group rotates fast. Still, the rotor stops upon deprotonation because of electrostatic repulsion between the carbonyl motifs of succinimide and phenolate anion. The speed could be restored by adding metal ions, which can be monitored reversibly between high speeds to stop employing redox properties. These studies open the way for multistage speed-controlled rotor development by coordinating metal–ligand interactions. In another study, Feringa's rotary motor was modified with a biphenyl system that exhibits slow rotational motion on adding diamine. Consequently, it speeds up as the acid is added to the system.88 The rotation of a Brownian motor in a double ratchet rotary motor can be controlled by introducing a pawl-like functional unit that can manipulate the electronic distribution of the system. When embedded with the double ratchet motor's PS, an electron-deficient molecule possessing an intramolecular hydrogen bond controls the electronic flow from the BR to the PS (Fig. 4c).89 The motor operation stops when the intramolecular hydrogen bonding is strong. Thus, this work described that electronic gating could be one strategy to program the double ratchet rotary motors.
The structural modification of the motors by integrating different functional groups is one approach to regulating the motion. For example, the light-gated modulation of the motor's rotation was achieved by introducing a molecular switch in the system. The first multiphotochromic motor was designed based on Feringa's second-generation motor embedded with a DAE photoswitch, which controlled the rotary motion by the light source in a noninvasive manner.90 The motor becomes operational at 455 nm and stops if the DAE is in a closed state. However, the green light irradiation causes DAE to adopt an open state, allowing the motor to rotate. Pfeifer et al. reported a mechanistic pathway to control the rotary motion of a second-generation motor by upgrading it to a push–pull type system by introducing an electron pulling and pushing the group in the lower and upper halves, respectively.91 Interestingly, this motor follows the function of a motor and a switch based on a polar or apolar environment. Such systems are advantageous if fabricated in different types of frameworks where the function of the motor or switch can be altered as per the situation by varying solvent polarity.
Zeng's group recently developed a molecular nanocar based on the concept of metal-coordination-driven self-assembly, which became operational with green light (Fig. 4d).92 Here, two chiral molecular motors act as a wheel, self-assembled via Ag metal. The dynamics of this motorized system are controlled by adding pyridine, which breaks down the system into two individual motors. This triplet–triplet intramolecular charge transfer sensitization and push–pull features of the nanocar make the system operational at 500 nm, whereas the individual motor is functional in the blue region. Moreover, this is the first example of coordination directed self-assembly to initiate motor conversion into a nanocar and easily dismantled by adding chemical stimuli to realize the goal of metal-driven transportation and release.
The same group reported a porous molecular crystal comprising rotors linked through charge-assisted hydrogen bonds, and their dynamic was switchable through gas adsorption/desorption.97 In another report, they developed a metal–organic framework (MOF) fabricated with a rotor that exhibits fast rotation.98 Herein, a zinc-based framework was composed of a rigid bicyclopentane–dicarboxylate linker where the bicyclic unit serves as the rotor. The carboxylic stator motif aligned perpendicularly in the cubic unit of zinc-based metal frameworks enables ultrafast rotation, which is attributed to high torsional flexibility. This aliphatic linker achieved unidirectional rotation with a speed of 1010 Hz even at temperatures below 2 K. This system described rotary motion at low temperatures by continuous unhindered molecular wheel rotation for several turns. This could be attributed to minimized thermal energy as the temperature becomes low. Although molecular motion usually freezes at low temperatures, no such hindrance was observed in this fast rotor, exhibiting low torsional barriers compared to their thermal energy. Thus, these rotors could be implemented for working in low-temperature ranges. Horike and his group reported another type of work in which the precise regulation of the rotational motion of a rotor in the crystalline phase could be achieved using a solid-solution approach.99 The variation of solid/solution ratios would modulate the volume of cells in porous materials, which led to changes in the rotational barrier for pillar rotors; therefore, rotational frequency control requires no thermal stimuli. However, the speed could be mainly regulated from ultrafast to fast; thus, the challenges still need to be addressed.
2.3. Exploring working motor-based device prototypes
Macroscopic machines assemble many complementary parts to perform a specific task.100 A molecular machine is capable of complicated operations comparable to a macrodevice, except that the molecule's mode of operation differs and the device's power source is irrelevant. Top-down approaches have delivered many solid-state miniaturized devices. Although targeting sustainable molecular level precision, this has not gone well. The concept of miniaturization of components is exploited by the bottom-up strategy that offers to manipulate the molecules in the nanodomain.101 The development of supramolecular chemistry plays a crucial role, allowing molecular machines to build smart materials and supramolecular architecture-based devices, hence offering practically limitless potential.102 Researchers attempt to produce smaller semiconductors with photolithographic and chemical etching techniques. The semiconductor devices from 65 nm to 14 nm chip have been developed, and now the 7–2 nm chip has been explored.103–105 However, there are a few hurdles associated with the molecular level device design, such as the Fermi energy level of the electrode, which should be appropriately aligned with the molecular orbital of the molecules to develop a molecular transistor.106 Scientists have attempted to develop a single molecular device that can overcome the limitation of Moore's law.107 The significance and development of single-molecule electronics are outlined owing to the limitation of Si-based devices.108 Several methods have been developed to study single molecular electronic devices in the last few decades. Molecular motors/switches can exhibit different states beneficial to developing high-density molecular electronics. Tour and his team have recently developed a chip integrating a single molecule in a circuit, resulting in a programmable biosensor.109 This semiconductor chip consists of a scalable sensor array architecture, and an electric meter is integrated with each array that records the current flow through the molecular wire. The desired molecule is connected to the wire via a conjugation site that offers a real-time electronic readout of molecular interactions of the probe.3. The use of molecular rotors and motors
3.1. Uses in energy conversion harvesting and transfer
The ability of motor proteins to transform chemical energy into mechanical motion represents energy conversion and storage in molecules. Because the motor protein has the additional property of dynamic density of states (DOS), the mechanical movements of a molecular motor may lead to dynamic switching states in a device. It is a significant advantage for using such molecules to advance molecular electronics, energy transfer, and energy harvesting from available solar, thermal, or any other form of energy. For this purpose, different molecular systems are engineered with the semiconducting surface, resulting in an energy harvesting device. For example, Feringa's group developed a visible light-driven motor integrated MOF, where two components are palladium-coordinated porphyrin and bipyridyl-functionalized overcrowded motor.110 The porphyrin moiety absorbs visible light energy to run the motor by transferring the energy to the motor struts (Fig. 5a). Irrespective of the solution and solid state, this advanced photochemical process of the motor was observed to be unchanged. Thus, the system functions as an energy harvesting unit where the photon energy is utilized to result in nanomechanical motion within the nanocage of MOF. Such a MOF-nanopocket is useful in improving the catalytic reaction kinetics.111 In the context of the energy transfer process, Bojinov and his group synthesized a multicomponent light-harvesting molecular rotor that can also sense pH and viscosity.112 The system comprises a switch, rotor, and a light-harvesting motif that transports up to 99% energy through a fluorescence resonance-energy transfer process. Moreover, a radiofrequency-guided dual ratchet motor can convert available thermal energy to power under applied bias when integrated on a highly oriented pyrolytic graphite (HOPG) surface (Fig. 5b). The Brownian rotor mechanism of the motors uses available thermal noise, and the chip harvests an excess power than the input bias.58 The system can harvest noise when dipped in a suitable solvent and exposed to electromagnetic radiation. Thus, Brownian rotors absorb noise (kT), and the produced thermal fluctuation in diffused solvent molecules leads to energy conversion.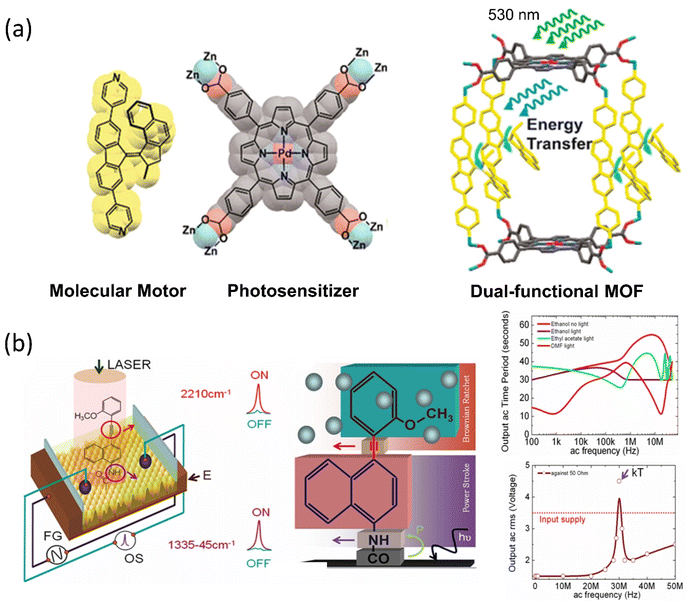 | ||
| Fig. 5 (a) Design and molecular structure of visible light-driven molecular motors in MOF. Reproduced with permission.110 Copyright 2020, American Chemical Society. (b) The dual rachet motor on the HOPG chip and the graph of power production as a function of AC applied frequency. Reproduced with permission.58 Copyright 2020, American Chemical Society. | ||
In 2015, a new photoswitch hemithioindigo113 exhibited superior photophysical properties and was used to build a molecular motor.114 Herein, the sunlight energy was supplied to the motor to perform the unidirectional motion. Henry Dube and his group reported several hemithioindigo motor systems115,116 that displayed an efficient visible light-induced photoisomerization process in the last few years. These design strategies for constructing green molecular motors could be used as power sources or as active materials in solar cells for better solar energy conversion.117,118 The molecular motors are coupled with the polymeric chain to harness the energy used to perform mechanical work at a macroscopic level. Giuseppone and coworkers have demonstrated that the contraction of the gel made of overcrowded alkene motor is integrated with polymers owing to the dynamic motion of the rotary motor.119 Thus, the rotational motion of the motor stores the light energy in the form of potential energy in the polymer. However, the stored energy cannot be accomplished again owing to the irreversibility of braiding the polymer under light. Thereby, the group has introduced a diarylethene switch that serves as a modulator in the gel system that assists in overcoming the abovementioned issue.120 These molecular motors are eligible for energy harvesting or storage, which could be used for future purposes; however, more challenges exist in developing a power harvesting device.
3.2. Possible uses of shaft-based rotary motors as wheel and propeller
Tour developed the first nanocar bears wheel-like C60 fullerenes and various molecular motor-based vehicles that show collective motion on the solid surface, for instance, nanotrucks, carborane nanocars, and light-powered nanocars.121–123 In 2016, Grill et al. showed the translational motion of a three-wheeler nanocar based on the rotary motions from two adamantane moieties and a Feringa motor as a third wheel.124 The nanocar was analyzed on the Cu(111) surface by STM, illuminating a 355 nm light for photoisomerization and then 161 K temperature for helix inversion (Fig. 6a).125 Thermal-induced helix inversion was crucial as these steps led to the nanocar to perform lateral translational motion. The mean-squared displacement of the motor without light was 3.37 ± 1.00 nm2, increasing to 10.48 ± 4.10 nm2 under light exposure of the mean squared displacement of the light. All these reported nanocars were investigated at the molecular level under STM, which requires a conducting surface. Taking it one step further, the group incorporated the nanocar with a fluorescent dye, which could be studied in single-molecule fluorescence microscopy on a nonconducting glass surface. Feringa's group upgraded the various motorized nanocars by incorporating BODIPY in different positions to control the net directional motion.126,127 | ||
| Fig. 6 (a) Molecular structure of light-activated motorized nanocar. The photoinduced motion of a molecular rotor on Cu(111) surface at 161 K. Reproduced with permission.125 Copyright 2016, American Chemical Society. Line-scan imaging protocol (b) nanocar 1 & 2 equipped with adamantane wheels and BODIPY. (c) Conventional 2D photograph of nanocar 2 on the air-glass interface. (a and b) The line-scan images of nanocars 1 and 2 where nanocar 2 is in motion while nanocar 1 is immobilized. Reproduced with permission.126 Copyright 2018, American Chemical Society. | ||
Although, owing to higher THI, the rotational rate of the motor decreases compared to the motor without BODIPY, which isomerized up to 91%. The structural redesign of the nanocar addressed this problem as the system avoids the energy transfer process, which lessens the photoisomerization process. In addition, the motor with more photostable BODIPY dye compared to the least stable Cy5 dye is a better choice to study on the glass-air surface.128,129 Later, with these structural advantages, a line scan imaging protocol was developed that facilitates the four-wheeled BODIPY-functionalized nanocars gliding in a quasi-random 2D manner on the glass surface. Nanocar 1 shows better performance as it is able to maintain a linear trajectory and move on the air-glass interface with a quasi-random 2D diffusion mode (Fig. 6b and c).126 This fast-line scanning imaging protocol accurately estimates the diffusion coefficient of molecules. In 2017, Tour, Grill, and coworkers reported the surface rolling motion of the nanocar,130 where the effect of the dipole moment upon the electric field was described. The nanocar was comprised of electron push–pull groups in an aryl ring, namely, dimethylamine and –NO2 group, along with two adamantanes as a wheel. The dipolar nanocar was manipulated with the electric energy in an STM that exploited the dipole molecules and induced the unidirectional motion. These systems were highly oriented on the Ag surface with high precision due to the dipole moment of a nitro moiety that interacts with the surface, causing translation motion (Fig. 7a and b). Thus, the nanocar's design and surface rolling properties with various surfaces to afford better directional property is challenging but essential.131,132 Jacobson et al. described Feringa-developed motor adsorption on the TiO2 (110) surface trapped by the –OH group, resulting in a charge transfer process.133 These outcomes could assist in developing motor-incorporated organic–inorganic nanodevice for a wide range of trafficking on the nanosurfaces.
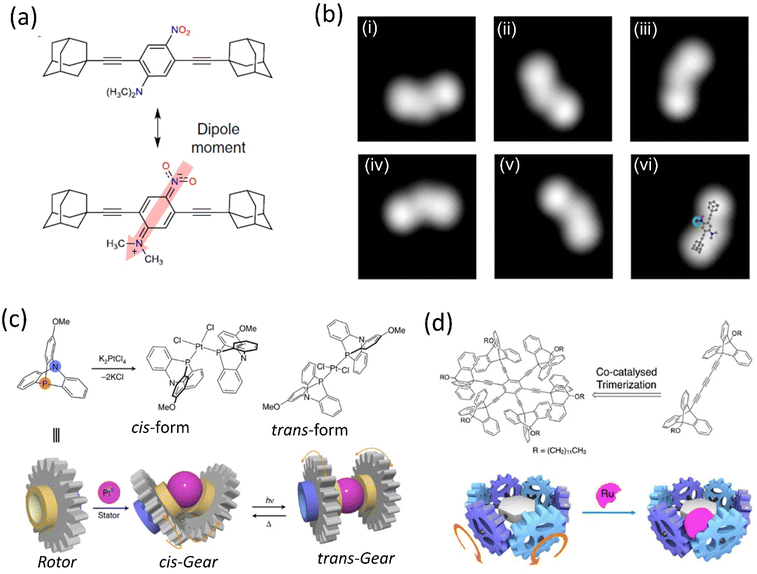 | ||
| Fig. 7 (a) Chemical structure of polar motor. (b) (i)–(vi) STM images of the molecule showing clockwise rotation upon applied voltage pulse. Reproduced with permission.130 Copyright 2019, The Authors, published by Springer Nature. (c) Schematic representation of a PtII-centered molecular gear exhibiting cis–trans isomers could be achieved upon ultraviolet irradiation at 360 nm and heating. Reproduced with permission.134 Copyright 2017, The Authors, published by Springer nature. (d) Ru-centered sextupled triptycene gear. Reproduced with permission.135 Copyright 2017, American Chemical Society. | ||
Metal ions have been observed to act as a controller of molecular motions due to their reversible ligand binding nature, ability to change coordination geometry, ligand exchange, and reactivity to external stimuli. Metal-centered molecular machines capable of switching rotary motion either through metallation/demetallation or by changing the coordination number of the metal center have been facilitated by such properties, albeit the latter remains mainly unexplored. For instance, Ube et al. reported a Pt(II)-centered molecular rotary gear constructed using two azaphosphatriptycenes as rotators and one Pt(II) ion as a stator (Fig. 7c).134 The molecular rotary motion transmitter demonstrated control of engagement and disengagement in the rotor. It was in accordance with the isomerization of the cis- and trans-forms initiated by photochemical and thermal conditions. The repeatable isomerization between a disengaged trans-form and an engaged cis-form was found to be reversibly navigated by heat and photoirradiation of ultraviolet light. They suggested that such mechanical switching based on cis–trans isomerization of the Pt(II) ion center operated by photo and thermal motion would yield a selector switch for more complicated molecular machines.
In 2017, they reported another gear system consisting of six triptycene gear units in a circular arrangement around a benzene ring (Fig. 7d)135 through an ethynyl linker. These six triptycene gears had a close engagement; coordinating a bulky RuCp* complex to any of the six gears, they observed high restriction on the overall movement of the six gears within the molecule, although the movement was not completely stopped. This RuCp*-bound multigear molecule is chiral; thus, despite having nondirectional motion, the rotational rates of the different gears from one another were different. This multigear molecule provides a structural basis for the long-distance transmission of molecular gear systems containing an on/off function. Recently, Asato et al. developed an electron-triggered Ru-based molecular motor integrated photoresponsive brake and studied dynamic motion on the surface employing light.136 This dual responsive system shows clockwise and anticlockwise directions. Recently, Dube's group reported the photogearing motion of a hemithioindigo-based molecular gear that can transfer the rotational motion, i.e., the isomerization around the double bond results in 180° rotation.137 As a result, the associated single bond with the double bond undergoes 120° rotation. This photogearing process at the nanoscale can be correlated with the bevel-gear system. Moreover, light as an external stimulus for gearing motion before a thermal-driven motion has several advantages.
The gear and axle development would improve the molecular motor's design, enabling better control of the nanorobotics' movement.138–140 Hla's group designed a molecule propeller consisting of three molecular blades141 connected to a ruthenium atom in a facial mode connected to the stator. STM studies clearly showed each molecular propeller's motion on applying electrical voltage. The system was grafted into the Au surface and manipulated in STM by different techniques to analyze the unidirectional rotation. Moreover, the 2D nature of the gold surface and ratchet shape gear in the system introduces the chirality that initiates 360° rotation that splits into 15 steps when the system is energized in STM. The generation of chirality by synchronizing the ratchet mechanism is the crucial factor for the movement, and this concept could potentially develop a wide range of applications.
3.3. Possible use of motor in cargo delivery
Amphiphilic molecules could be arranged from the nanoscale to the macroscopic domain through 3D supramolecular assembly on applying the external stimuli. Tian and coworkers described the preparation of a tetraethylene glycol-functionalized amphiphile cargo based on an overcrowded alkene system (OAS) capable of forming well-defined vesicles in a water-based solution (Fig. 8a and b).142 When the vesicles were exposed to light, the nanocontainer contracted due to the isomerization of alkene between its cis- and trans-states. As a result, the vehicle's internal volume will alter, allowing them to wrap cargo in its cavities and release it depending on the situation. Examining the ability of vesicles to encapsulate indicates that under light, calcein-loaded trans-vesicles release at a high pace, as measured by fluorescence intensity. Thus, these photoresponsive vesicle-based smart nanocontainers could facilitate the construction of remote drug delivery systems in the future, which may further inspire the development of motor-based vesicles.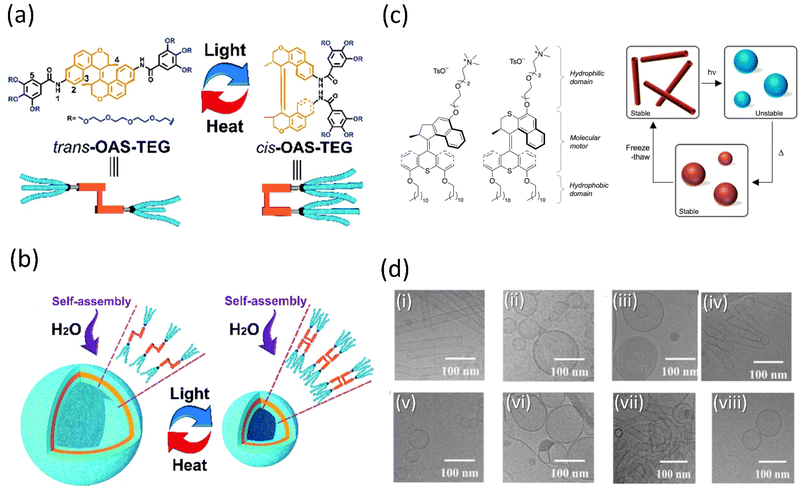 | ||
Fig. 8 (a) Interconversion of OAS-TEG upon light and thermal irradiation. (b) Self-assembly of OAS-TEG in an aqueous medium to form vesicles. Reproduced with permission.142 Copyright 2016, Royal Society of Chemistry. (c) Amphiphilic molecular motors 1 (fast) and 2 (slow) and their change in aggregation upon the isomerization of self-assembled molecular motor constituent. (d) Reversible isomerization of motor 2 and DOPC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (i) starting point, (ii) after irradiation, (iii) after heating, (iv) after three freeze–thaw cycles, (v) after irradiation, (vi) after heating, (vii) after three freeze–thaw cycles, (viii) after irradiation. Reproduced with permission.143 Copyright 2011, Springer Nature. 1) (i) starting point, (ii) after irradiation, (iii) after heating, (iv) after three freeze–thaw cycles, (v) after irradiation, (vi) after heating, (vii) after three freeze–thaw cycles, (viii) after irradiation. Reproduced with permission.143 Copyright 2011, Springer Nature. | ||
The complex yet fascinating self-assembled polymeric molecules in nature have inspired Feringa and his group to put forward a novel concept of creating a unique molecular motor system in water that is amphiphilic and self-assembles into well-defined supramolecular nanotubes based on their earlier report.143 These self-assembled polymers changed their morphologies back and forth when exposed to external stimuli as the motor isomerized. It was the first example of a molecular rotary motor that self-assembled in an aqueous medium without losing its functionality (Fig. 8c and d), alternating the photo and thermally-induced steps, an isomerization process-controlled reshuffling between the nanotubes and vesicles. Thus, it provided access to the new future of water-soluble motors and opened the pathway to produce highly progressive and dynamic artificial nanosystems in water. Although the cargo activity of the aforementioned self-assembled vesicles and nanotubes is not reported, owing to their aqueous solubility, they may develop certain properties that would help cargo delivery.
Chen and coworkers reported that dual light controls dynamic supramolecular assemblies comprised of Feringa's first-generation motor, which bears both hydrophobic and hydrophilic chains and exhibits controlled foaming abilities in water at the macroscopic level.144 The design was such that trans-isomer form with lower packing parameters than the cis-isomer displays precise control over the aggregation properties (Fig. 9a and b). The system exhibits four states that systematically control the geometrical structure from the nanodomain to the macroscopic domain as two distinguishable worm-like micelles, resulting from stable trans-isomers and stable cis-isomers associated with vesicles. At the same time, unstable isomers exhibit a mixture of geometries. The reversible foaming-property switching could be achieved until ten cycles upon periodic light and heat irradiation. The further exploitation of this field with a dynamic system will unveil other possibilities.
 | ||
| Fig. 9 (a) Light-responsive molecular motor amphiphile (MA) and its reversible photoisomerization process. (b) The alteration in visible macroscopic foaming processes brought structural changes in the supramolecular assembly. Stable synthetically purified cis-MA is used to create state 4. Reproduced with permission.144 Copyright 2020, American Chemical Society. | ||
To maintain the physiological functionalities of living cells, the transportation of ions across the lipid membranes is necessary. Nature carried out these cargo tasks selectively employing biological motors under chaotic environments with chemical fuel or light absorption. However, mimicking such a natural system to develop synthetic molecular motors are highly challenging and desirable at the same time. Wen and colleagues reported an artificial motor system embedded with azobenzene (AB) that transports cyclodextrin molecules across the synthetic nanochannels upon exposure to light.145 Here, the light energy initiated the rotational inversion of the AB molecular motor along with hydrophobicity and reversible photoreaction of the system, enabling the transport of the molecules across the nanochannels. Recently, Giuseppone and coworkers synthesized the light-powered hydrophobic rotary motor that integrated with two macrocycles and investigated their ion transport properties in a phospholipid membrane.146,147 The motor system was also embedded with a urea motif that forms a hydrogen bond, which induces the supramolecular self-assembled with the phospholipid membrane. The crown ether macrocycle can encapsulate the alkali metal ion. The fluorescence assays and patch clamp investigations revealed ion channel formation with the rotary motors system. Furthermore, the transportation could be enhanced up to 400% on exposure to UV light, which could be attributed to the photoactuation of the rotary motor that provides sufficient energy to the passage of the alkali metal along the artificial pore.
3.4. Biomedical uses of rotors and motors
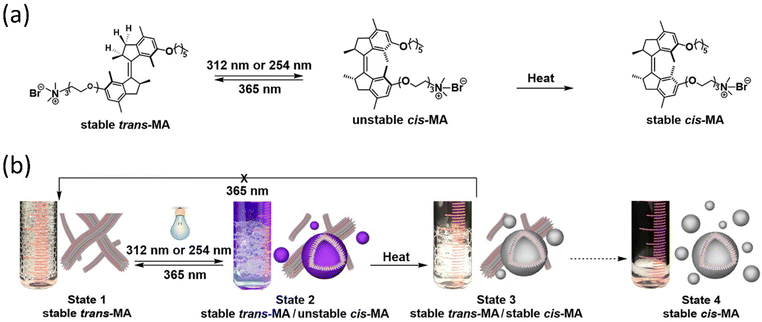
Tamaoki and colleagues presented a peptide-integrated AB machine that disables the function of the kinesin microtubule.148 The system comprises an electron-withdrawing and an electron-releasing group to decrease the lifetime of the cis-isomer by shifting the π–π* band toward the visible region. The transformation of the machinery mentioned above disables the kinesin microtube's motility without hampering the other filaments, but the cis-form enables the movements. As a result of the rapid thermal relaxation from cis-to trans-interconversion, the machine was efficiently functioning in a single wavelength to control kinesin movement. This push–pull machinery is an excellent example of engineering in developing a synthetic machine incorporating biomolecular motors for better results.It is reported that the double ratchet motors were used in a nanoplatform to develop higher-level nanomachines (PCMS and PCBM).149–151 PCMS nanomachine comprised of PAMAM dendrimer as matrix molecules has been found to target cancer cells specifically. The 3G (400 MHz–3 GHz) mobile radiation was used to activate it, which sensed the microsatellite instability and disintegrated the nucleic acids through the energy transfer process by the motors.149 The working principle of PCMS was showed that when the GHz signal was shone on PCMS, it generated oscillation frequencies matching the microsatellite instability of cancer cells. On the other hand, PCMS resonance oscillation could also be matched to Alzheimer-related beta plaques at room temperature (Fig. 10a–c). This standalone system performed its job as a purely mechanical drug via a triangular energy transmission path (S → C → M); therefore, the physical mode of interaction of PCMS could avoid or minimize the side effects compared to chemical interaction. Further, the PCBM crypto nanobot resonance drug was built based on the information from the motor-functionalized dendritic system and investigated the various parameters, which will not be affected by proteolytic enzymes.151 This cryptosystem comprises a double ratchet motor and streptavidin marker protein-specific biotin (Fig. 10d). The study of PCMS and PCBM demonstrated the possibility of replacing generic cancer medications as well as paving the way for developing a more dynamic system with a novel molecular rotor that might be used in the medical area to produce physical and mechanical drugs.
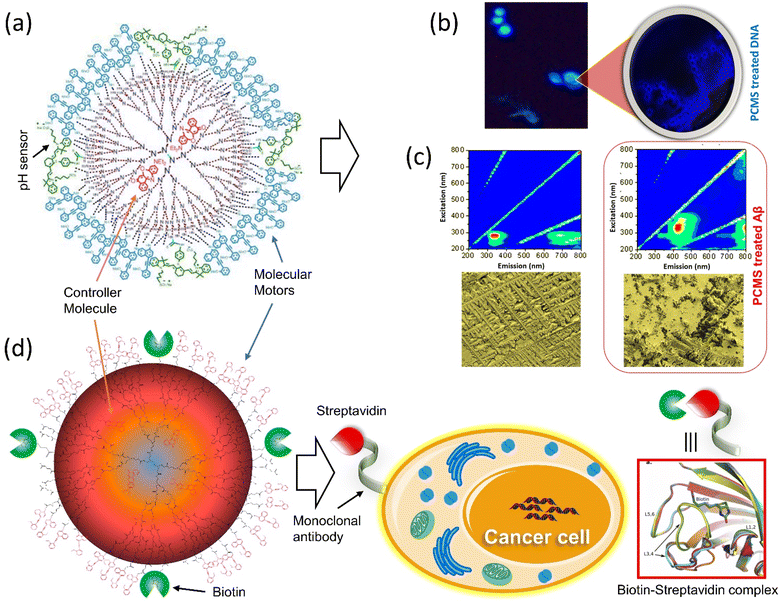 | ||
| Fig. 10 Dual ratchet motorized PCMS. (a) Molecular structure of PCMS. (b) The nanomechanical action of PCMS destroys cancer cells and (c) the nanomechanical action of PCMS disintegrates αβ-plaques.149 (d) The target-specific crypto nanoassembly system.151 | ||
Overcrowded alkene-based motors are primarily employed in biology and show remarkable outcomes. Tour's group created light-driven versions of molecular machines that can pierce biological membranes and use them for target-specific cell tracking by tagging fluorophore-integrated motors, peptide-linked motors, etc.152 The group discovered that a biocompatible-functionalized light-activated Feringa's motor could adsorb onto lipid bilayers and when activated, drill into the cell membranes. They demonstrated the in vitro applications of the simple to a target-specific molecular machine that induces necrosis, such as human prostate cancer cells targeting motor functionalized SNTRVAP-peptide specific to the 78 kDa glucose-regulated protein (GRP78), were developed. Notably, this activated motor enhances necrosis by 40%, and a simple untargeted motor fastens necrosis by 50% compared to the necrosis induced by solely UV light (Fig. 11). These findings confirmed that molecular machines could function at the cellular level. However, the disadvantage of requiring harmful UV light to activate the motors must be addressed. Later, the same group described how near-infrared–triggered two-photon excitation (2PE)–activated molecular machines that could drill through the cell membrane to destroy cancer cells,153 which is superior in the in vitro application compared to an earlier report. These molecular machines could make targets specific to cells by tagging the specific peptide chain or antibody, possibly potential cancer cells (Fig. 12a and b). The same group tagged fluorescent dye Cy-5 with the machine for monitoring synthetic bilayer vesicles. They studied cell death in different cells using untargeted and targeted nanomachines, whereas the untargeted nanomachine initiated necrosis at a much higher rate than the UV-activated necrosis. A similar observation was reported for the SNTRVAP peptide-functionalized motor. The MCF-7–targeted peptide sequence DMPGTVLP functionalized with the machine displayed necrosis at the rate of 63% within 3 min in the in vitro test. However, this method requires that the penetration level of cells be limited.
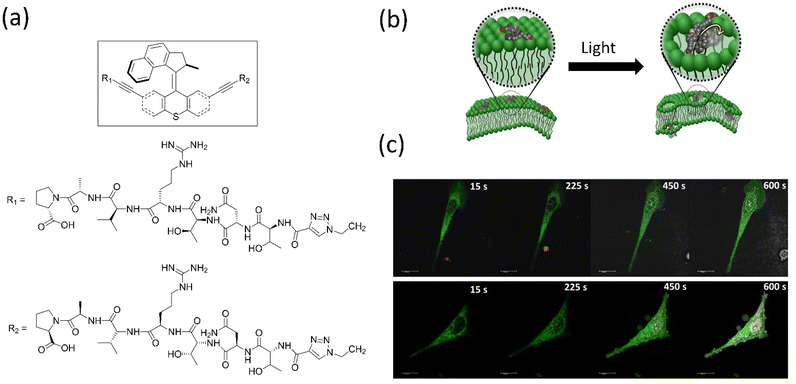 | ||
| Fig. 11 (a) Structure of molecular motor along with the peptide SNTRVAP sequence. (b) A representative diagram of a molecular machine that opens a cell membrane upon light irradiation. (c) Photographs showing the action of a SNTRVAP-functionalized molecular motor on PC-3 cell. Necrosis induced by the motor upon light exposure within a time interval is presented. The scale of all photographs is 20 μm. Reproduced with permission.152 Copyright 2017, Springer Nature. | ||
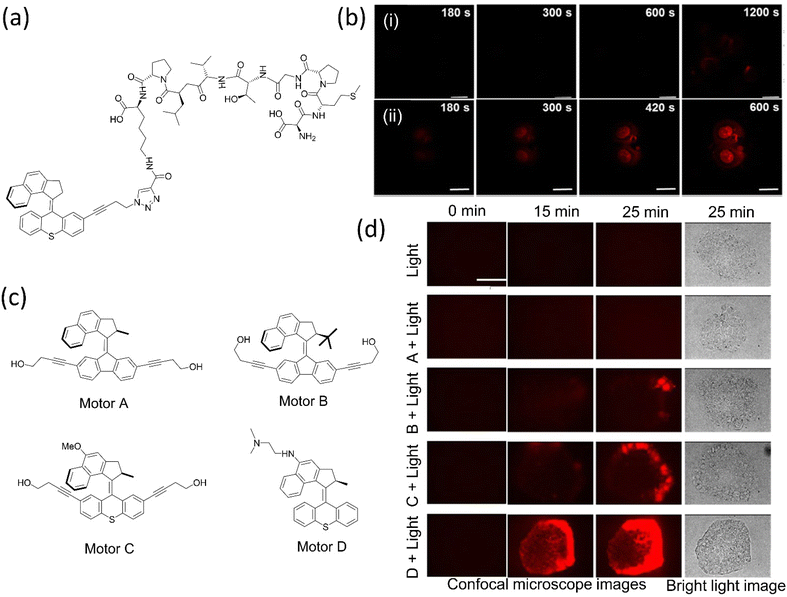 | ||
| Fig. 12 (a) 2PE NIR-activated fast molecular nanomachine integrated with mono-DMPGTVLP induces cell death. (b) MCF-7–targeted nanomachine cause morphological changes and necrotic cell death on exposure to light, (i) without nanomachine, (ii) with targeted nanomachine; the scale bar in the images is 20 μm. Reproduced with permission.153 Copyright 2019, American Chemical Society. (c) The chemical structure of visible light-activated molecular machine causes cell death through mechanical action. Among all, C and D are fast rotors. (d) Nanomechanical action of the motors on pancreatic cancer cells induces cell death. The scale bar for all the photographs is 100 μm. Reproduced with permission.154 Copyright 2020, American Chemical Society. | ||
In 2019, the same group upgraded the nanomotor that destroyed pancreatic cancer cells upon activation with 405 nm light.154 As structural modifications on the motors change the rotary rate, consequently (Fig. 12c and d), fast rotor causes cell death within 5 min, whereas the slow and medium motors need 20–15 min. These mechanical motors inhibit the production of the reactive oxygen species responsible for cancer cell death. However, these systems fail to penetrate deeper into the tumor yet can treat diseases such as shallow oral, gastrointestinal, and genitourinary cancers. Moreover, the fast mots among these motors are able to raise the mortality rate in C. elegans by 70% and in daphnia by 100% and are also potent in destroying the prokaryotic cell, eukaryotic cell membrane, and many physiological functions of multicellular eukaryotes in vivo and in vitro.155 Such a system could treat diseases such as skin cancer, cosmetic application, and parasite controls.
Inspired by actin filaments and myosin, Zheng et al. reported a molecular machine incorporated with two triethylene glycol chain polymers that can exert force at the cellular level equivalent to 12 kT and induce mechanotransduction processes.156 The light-induced rotation of the motor is braided into the polymer chains to minimize the receptor-interface linkage. Consequently, a mechanical force is applied to the cell membrane receptor, which causes mechanotransduction. These works were related to force-dependent cellular works such as T-cell activation and focal adhesion (FA) maturation. An alternative approach to achieve visible light-driven rotation of these molecules is to take advantage of intramolecular or intermolecular sensitization through triplet energy transfer from a second chromophore. Pfeifer et al. developed a NIR range-activated molecular motor integrated with a two-photon absorption (2PA) sensitizer dye.157 The motor was activated with low-intensity NIR light via the resonance energy transfer process, which is more convenient in biological studies, such as in vivo studies. NIR light first activated the sensitizer, conveying this energy to the rotor for rotation. These findings lead to the development three-photon activator systems that may hold more efficiency and be more compatible with the environment. Stimuli-responsive transmembrane transport proteins play an important role in biological systems, and defects in them cause serious diseases. Various transporters are developed, yet stimuli-responsive materials are highly desirable.
Wezenberg and coworkers developed an anion-binding stiff stilbene system modulated by light.158 These anion receptors are modified based on their previous work,159 where the system shows a high affinity toward acetate and dihydrogen phosphate.160 The Z-isomer exhibits high affinity toward chloride anion and high activity owing to the close arrangement of compare to urea units compared to the E-isomer in several membrane transport experiments. These photomodulation systems are intriguing in developing physiological tools to study diseases related to defective transport and optopharmacological tools to stimulate neuronal activity such as halorhodopsin and rhodopsin.
3.5. Motor applications in catalysis and chemical syntheses
Enzyme catalytic reactions are well-known for their high selectivity and precision in nature. To attain this, the dynamic behavior of motors is conceivably used in catalytic reactions for better selectivity or chemical transformation. The orthogonally-responsive molecular machine as a catalyst with proper ligand arrangement anticipates the machine's future in chemical reactions. Developing a machinery-based catalyst is challenging as properly incorporating ligands with accurate dynamics must be fitted within the system, such as enzymes. The benefit of a stimuli-responsive catalyst is one that can regulate the catalytic activity as these systems offer the modulation of geometrically-distinct conformational states. Feringa's group161 and Fletcher's group162 separately reviewed the dynamic system's role in catalysis. Because of the extraordinary advancements in this discipline over the past 20 years, very innovative designs have been created that incorporate responsive units into complex functional molecules.Over a decade, various groups have worked on advancing motorized catalysts considering different parameters. For instance, Gilissen et al. developed a macrocycle system, incorporating the second-generation motor in a porphyrin system that could be used as photoswitchable catalysts exhibiting various chirality elements.163 This motor modulates its helicity and the chiral environment, which could be used in writing binary codes 0 and 1 based on different chiralities. The inclusion of viologen guests in the motor above affected the synthesized diastereomers, which led to an acceleration of the rotational dynamics. Feringa and coworkers described the catalytic activity of the biofunctionalized first-generation motor and absolute stereoselectivity in an asymmetric conversion for the first time.164 The stator part was tagged with thiourea, and the rotor incorporated with dimethylaminopyridine led to a bifunctional organocatalyst used in Michael additions of a 2-methoxythiophenol to 2-cyclohexene-1-one (Fig. 13a). The chirality of the motor ruled the directionality of the rotation that governed the catalytic's orientation, which determines the stereogenic center of the product. The S-product was obtained employing (M, M)-Z isomer, and the R-product with a higher yield resulted while helicity was changed from M to P. However, this catalyst shows low activity in the Henry reaction; thus, the group customized it by connecting the motor to the catalytic unit without the phenyl spacer.165 The Z-isomer of the system affords the desired product with enantioselectivity up to e.e. 86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14. Later, to understand the role of stereoselectivity and catalyst structure, the authors developed two thiourea-functionalized catalysts for asymmetric Henry reactions (Fig. 13b).166 The rotor tagged with an aliphatic catalytic unit showed no enantioselective control over the reaction. All the findings dictated that the catalyst structure plays a crucial role along with the rotational dynamics of the motor.
14. Later, to understand the role of stereoselectivity and catalyst structure, the authors developed two thiourea-functionalized catalysts for asymmetric Henry reactions (Fig. 13b).166 The rotor tagged with an aliphatic catalytic unit showed no enantioselective control over the reaction. All the findings dictated that the catalyst structure plays a crucial role along with the rotational dynamics of the motor.
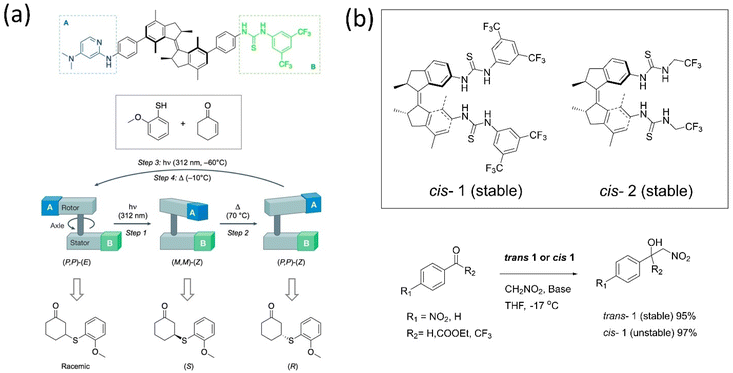 | ||
| Fig. 13 (a) A bifunctional motor-based catalyst assisted the Michael addition.164 Adapted with permission from ref. 8. Copyright 2017, Springer Nature. (b) Stable cis and trans conformer of thiourea-based catalyst. The stable and unstable catalysts 1 and 2 are employed in the Henry reaction.166 | ||
Transferring the specific chirality to the different elements of chirality allows the molecular motor to perform the catalytic activity.167–170 For example, Feringa's group developed a light-driven motor-based catalyst composed of the bis(2-phenol) unit that changes its axial chirality to helical chirality (Fig. 14a).171 The (R)-state of the catalyst was employed in the enantioselective addition of the diethylzinc unit into aromatic aldehyde derivatives, bringing out an enantioselective reversal product with 68% ee, and the yield reported was 87%. The results supported that the catalyst's chirality transfer process and allowed controlling the dynamic chirality from central to helical to axial chirality.
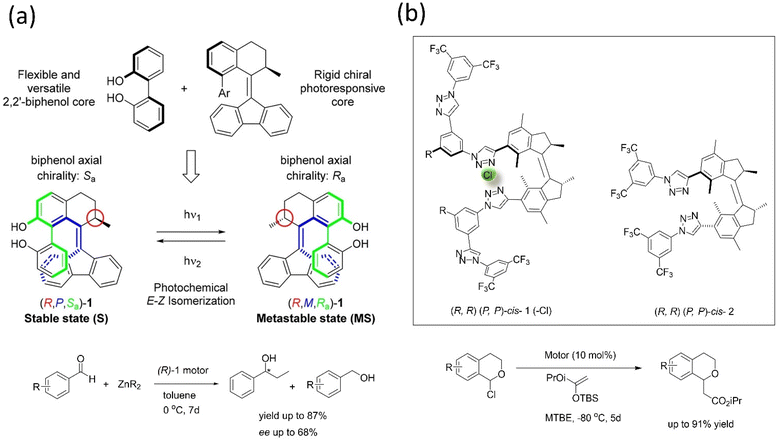 | ||
| Fig. 14 (a) Dual stereo control biaryl-substituted Feringa's motor catalyst that shows an internal transfer of chirality to the coordinated metal site in an organocatalysis reaction under the light. The catalyst-mediated enantioselective addition of organozinc to aromatic aldehydes. Reproduced with permission.171 Copyright 2018, American Chemical Society. (b) Molecular structure of the two motorized systems for stereodivergent chloride anion binding catalysis. Addition of silyl ketene acetal to 1-chloroisochroman.172 | ||
Dorel et al. reported a photoresponsive chiral catalyst based on a unidirectional molecular motor, functionalized with two oligo triazole units in each site of the system applied in anion binding.172 These anion-binding receptor sites induce chirality in each motor's unidirectional rotation step. This motorized catalyst was employed in adding silyl ketene acetal to 1-chloroisochroman (Fig. 14b); this catalyst assists in the divergent stereoaddition of silyl ketene up to 142% Δee. The (M, M)-cis isomer of the catalyst produces up to 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 e.r., whereas (P, P)-cis state is 5
20 e.r., whereas (P, P)-cis state is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 e.r. Herges and coworkers reported a novel approach to synthesizing cyclic tetravanadate via endogenic condensation employing an AB-based machine.173 This system was designed by attaching two Zn-cyclene units to the AB through methylene that was bound efficiently with the reactant monovanadate, leading to the formation of the tetravanadate ring. While the system interconverts into a cis-isomer, it eliminates the cyclic product tetravanadate via hydrolysis, although it reverts to monovanadate owing to the system's stability. This example indicates that artificial molecular assemblers can change chemical synthesis paradigms such as bioproteins using the mechano-synthesis process. Based on the concept of the supramolecular process, Dube's group designed and reported a reaction of Michael addition using a hemithioindigo-based motor under visible light.174 This motor interacted through the hydrogen bonding with the organocatalyst, namely, Schreiner's thiourea and squaramide (Fig. 15), which stopped the transformation. Still, the catalysis starts as soon as the motor adopts the E-conformer.
95 e.r. Herges and coworkers reported a novel approach to synthesizing cyclic tetravanadate via endogenic condensation employing an AB-based machine.173 This system was designed by attaching two Zn-cyclene units to the AB through methylene that was bound efficiently with the reactant monovanadate, leading to the formation of the tetravanadate ring. While the system interconverts into a cis-isomer, it eliminates the cyclic product tetravanadate via hydrolysis, although it reverts to monovanadate owing to the system's stability. This example indicates that artificial molecular assemblers can change chemical synthesis paradigms such as bioproteins using the mechano-synthesis process. Based on the concept of the supramolecular process, Dube's group designed and reported a reaction of Michael addition using a hemithioindigo-based motor under visible light.174 This motor interacted through the hydrogen bonding with the organocatalyst, namely, Schreiner's thiourea and squaramide (Fig. 15), which stopped the transformation. Still, the catalysis starts as soon as the motor adopts the E-conformer.
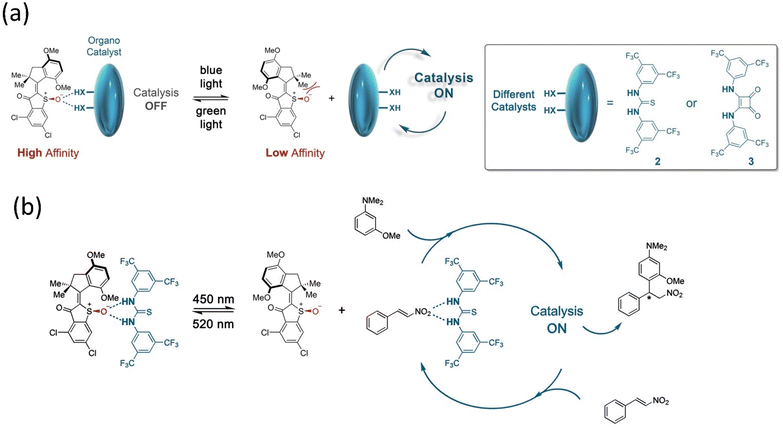 | ||
| Fig. 15 (a and b) Organocatalysis of the hemithioindigo-based motor. Reproduced with permission.174 Copyright 2020, American Chemical Society. | ||
Leigh and coworkers described a motorized system capable of moving a substrate to different sites, leading to different diastereoisomer products in a chemical transformation.175 This system comprises terminal alkene-incorporated acyl hydrazone rotor and quinoline stator bearing two prolinol silyl ethers connected by triazole linkages. The novelty of this machine is that it can stereoselectively produce one diastereomer in excess over the four products, which could not be achieved via the conventional method. These methods have several merits over conventional synthetic procedures, such as control over product formation. Hence, it laid the foundation for next-generation molecular machines and motors. Significant progress has been made in molecular motor-based catalysis in the last two decades, yet this field is young. The careful selection of motors with controlled dynamic motion and compatibility of the catalyst geometry are two basic parameters that offer unmatched opportunities to control a catalyst's activity and selectivity. Directing the chemical synthesis by controlling the dynamics of molecular motion will need to be explored more with structural modification, switching efficiency, and new motor design to take this sector to the next level.
3.6. Uses of molecular motors and rotors in the development of stimuli-responsive crystalline and porous materials
Porous materials provide a unique architecture for molecular motors to transform the motion at a larger scale in a solid state. The incorporation of molecular rotors and motors in the solid phase is discussed briefly in Section 2.2.2. These materials offer opportunities to develop photoresponsive materials by harnessing collective motion; however, the topological change in the molecular motor may hamper the robustness of the porous materials.176 Selectivity and separation factor might be increased by including appropriate stimuli-responsive molecules and adjusting the pore size of MOF or SURMOF (surface-mounted metal–organic framework). As a result, 3D motorized solid materials could be employed for medicine delivery, gas separation, or Li-ion battery applications. Molecular motors with ultrafast dynamics were engineered into shape-consistent, low-density porous molecular materials.94,95,97,98 Consequently, host–guest interactions offer them to control the motor's rotational speed by interacting with diffused chemical species such as gas, liquids, or vapors.177,178Perego et al. reported a MOF architecture integrated with two ultrafast rotors exhibiting fast mechanical motion even at 2 K temperature.179 The two distinct rotors, bipyridine as a pillar and bicyclo[1.1.1]pentanedicarboxylate arranged in 2D layers, coordinated with the Zn-paddle wheel node, resulted in a multidynamic system. The disorder-to-order phase transitions are ascribed to the 180° flip rotation of the bipyridine (Bipy) rotor and bicyclo[1.1.1]pentanedicarboxylate (BCP) rotor that initiate multiple configurations of geared and anti-geared rotators (Fig. 16a). Notably, the low energy barrier of BCP enabled the conversation of low thermal energy into rotary motion, which could be controlled by low-pressure CO2 gas or DMF.
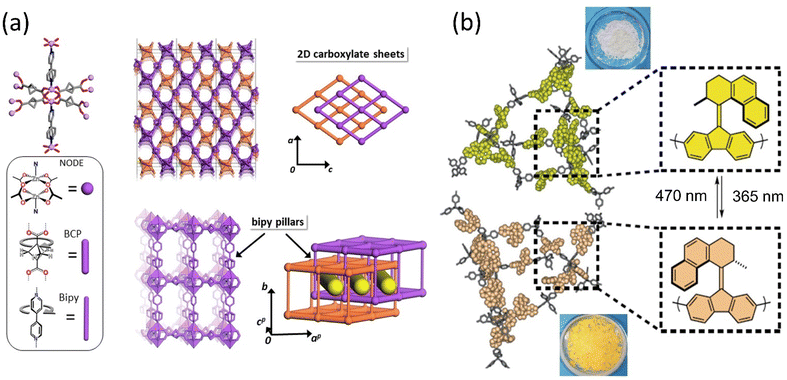 | ||
| Fig. 16 (a) The component of ultrafast motorized MOF consists of Zn-paddlewheel node and molecular structures of BCP and bipy linkers and their arrangements. Reproduced with permission.179 Copyright 2020, Springer Nature. (b) Rotary motor functionalized in MOF that changes color upon isomerization. Reproduced with permission.180 Copyright 2020, Springer Nature. | ||
Feringa, Comotti and coworkers incorporated a photoresponsive overcrowded alkene motor into porous material through the stator part to control the free volume available within the system to modulate as per conditions (Fig. 16b).180 The system varies with inducing heat and light, leading to bulk isomerization due to the bistable rotor in the framework, which could uptake N2 and CO2, releasing 20% of the initial volume at a relative pressure (P/P0) of 0.6 bar. In 2019, the group of Feringa developed a zinc pillared-paddlewheel 3D solid MOF integrating UV light-activating motors.181 Interestingly, the unidirectional movement of the motor in the solution state is retained in the solid-state MOF as well; hence, one could expect that this system would be applicable in gas diffusion or microfluidic pumps. Different sizes of 3D metallocycles were developed by fabricating bis-pyridyl-based MPY electron-rich donor rotor with di-Pt(II) acceptors described. The metal-driven self-assembly 3D metallocycles produce a more efficient push–pull type under visible light, providing strategies for a biomimetic hierarchical system potential for generating collective motion.182 Owing to the densely packed structure of the motor, motions are limited in the crystalline phase; however, amphidynamic crystals with molecular rotors promise solid materials that might allow conformational changes along with a change in the properties.183 In this regard, a binuclear emissive crystalline rotor integrated with a pyrazine rotator connected to Cu or Au metal through stator NHC carbene was developed by Mingoo Jin and coworkers.184 The length of the rotational axle connected to Au or Cu played a crucial role in controlling the rotation barrier owing to the π-accepting ability of the rotor molecule. The Cu(I) rotor complex possesses higher rotational energy and electronic delocalization than the Au (rotor), which is attributed to the higher steric interaction in the Cu(I) complex than the latter, causing a red-shifted emission in the solid state (Fig. 17). Although this system's application is not reported, these physical properties of amphidynamic crystals could be employed in pharmaceuticals, sensors, and adsorption/desorption.185,186
 | ||
| Fig. 17 Structure of the crystalline molecular rotor, the rotators are linked by metal atoms (Cu or Au). Reproduced with permission.184 Copyright 2021, American Chemical Society. | ||
3.7. Motor use in building stimuli-responsive soft materials and soft robotics
Through the transmission of the molecular-level nanomotion of molecular machines onto the collective motion by integrating with well-arranged networks, one could envision achieving motion such as myosin and actin filaments. Soft materials are unique functional materials that are distorted under the influence of external input and exhibit versatile features. For instance, a hydrogel could undergo shape irregularity by expansion/contraction, which is ascribed to the water releasing or retaining capacity. Stimuli-responsive and programmable shape morphing systems hold tremendous potential in biomedical, soft robotics, and biomimetic systems, thus attracting great interest. These materials are mainly functionalized with polymer, supramolecular materials, liquid crystals (LC), etc., resulting in hierarchical, shape-memory, hydrogel, self-oscillating structures, and so on, especially LC-based materials and hydrogels.Several groups successfully connected the molecular machine with supramolecular polymers and showed motion amplification at the macroscopic scale.8,42 In 2017, a photoresponsive Feringa-created motor was incorporated into a hierarchical supramolecular assembly that shows macroscopic contractile motion.187 An amphiphilic motor integrated with a dodecyl chain through the rotor part and stator was tagged with the carboxyl group to increase water solubility. This system undergoes a hierarchical assembly that is first assembled into the nanofiber bundle, which finally generates the long string (Fig. 18). When this string was generated under the shear flow method in an aqueous CaCl2 medium, a unidirectional alignment of nanofibers and UV light exposure made the string bend toward the light. The string was able to perform a flexion angle of 90° within the 60 s in an aqueous medium with an actuation speed of 1.5 ± 0.02° s−1 and with a speed of 1.8 ± 0.07° s−1 in the air. The isomerization of the motor varies by local packing environment resulted in lifting a 0.4 g piece of paper on exposure to UV light. This noninvasive, light-triggered nanoscale motion is transferred to collective motion in the supramolecular assembly in water and air is envisioned to create soft robotics. Molecular motors ideally require effective chirality transfer to function from the motor part to the unit responsible for carrying out the required operation.188 Feringa and his team provided the first example of using an overcrowded chiral alkene as the dopant by studying the transfer of photoswitchable chirality from a light-responsive molecular motor to a dynamic helical polymer to control the polymer properties. The transfer mechanism was found to be a result of the ionic interactions involving the molecular motor and helical polymers. In 2017, they reported that a dopant with photoswitchable chirality was able to bring about a preferred helicity in a poly(phenylacetylene) polymer, which could be altered in situ using light stimuli.189
 | ||
| Fig. 18 (a) Graphical representation of the self-assembly of the photoresponsive molecular rotor into nanofibers, which generate a string that undergoes deformation upon exposure to UV light. (b) Photochemical and thermal helix inversion steps of the motor. Light irradiation images of different supramolecular self-assembly. (c) (i) A supramolecular string in water bends toward the direction of the UV light from 0° to 90° within 60 s; (ii) a motor string bends toward the UV light to 90° within 1 min toward the right direction; (iii) photo and thermal actuation of the motor string. The scale bars for all photographs is 0.5 cm. Reproduced with permission.187 Copyright 2017, Springer Nature. | ||
The bottom-up approaches of integrating responsive systems with supramolecular assembly generate 3D soft matter that could amplify the molecular motion to macroscopic motion with molecular level precession. One of the approaches to achieve this is fabricating molecular rotary motors with a supramolecular assembly that combines with covalent frameworks to provide a hybrid polymer system that enables mechanical actuation at a larger scale. Giuseppone described UV-light mediated contraction of a gel framework comprising Feringa's motor.119 These motors with a PEG chain of different molecular weights were developed, which could be used to generate eight-shaped and crosslinked gel depending upon the concentration. These systems show macroscopic work on account of the motor's rotation through the polymers' braids. However, its reversibility becomes complex as it demands massive chemical changes. In this context, the authors developed a dual light-activated system by integrating with an extra photoswitch DAE, which works overall on two different wavelengths.120 The DAE modulator adopts a cyclic or closed form that releases the stored energy on demand. The motor stops its rotation upon irradiation with visible light. The photoswitch adopts an open form that generates free rotation around the C–C bond (Fig. 19). It releases elastic energy stored in the entangled polymer chains as kinetic energy can initiate the rotation until the system achieves thermodynamic equilibrium. The elasticity of the polymer network and osmotic pressure governed the reversible process. Taking inspiration from whirligig crafts, recently, the same group developed another system comprising a second-generation motor integrated with two polymer chains that released elastic energy from a twisted state through the reverse rotation of the strained state to a relaxed state.190 Thus, these motorized polymeric gel systems are related to biomolecular motor-integrated muscular tissues and continuously drive away from thermodynamic equilibrium, which is more advantageous compared to molecular switch-based gel.191
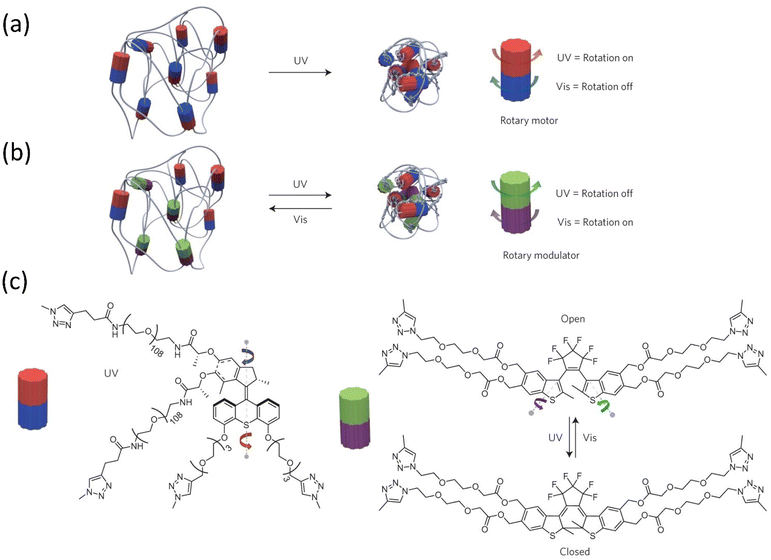 | ||
| Fig. 19 Dual light control motorized modulator. Graphical representation of (a) a reticulated polymer-motor gel under UV light. (b) A polymer–motor–modulator gel. (c) Dual light control of the polymer–motor–modulator system based on enantiopure overcrowded alkenes rotor and photoswitchable dithienylethenes as the modulators. Reproduced with permission.120 Copyright 2017, Springer Nature. | ||
Colin-Molina et al. developed a thermosalient rotor with DABCO and carbazole, showing cocrystal reversible phase transition.192 In this system, the DABCO motif acts as a rotator, which shows fast rotation at low temperature with an activation energy of 2.6 kcal mol−1. Single crystal analysis implied the phase transition process as a collective molecular displacement into macroscopic motion. The crystals were found to jump off the surface above 316 K because of the phase transition process, resulting in changes in the rhomboid lattice of the two crystals. Thus, phase transition causes structural change, which ultimately varies the dynamics. It is the first example of a crystalline molecular machine capable of showing dynamics at the macroscopic level. Thermosensors can be constructed from such a light- and temperature-responsive material. Overall, the aforementioned working principles could be applied to create soft-phase transition materials based on rotary motors for use in molecular actuators and energy storage.193,194
The LC materials exist between the crystalline solid-state and liquid-state, and their disordered arrangement could be aligned by applying lower energy stimuli. The long-range spatial arrangements of these thermodynamically stable LC could be achieved by interacting/combining the dynamic motion of molecular machines, which translate the nanomotion to collective motion at a certain length. It is worth mentioning that LC-based actuators can show reversible shape change in a solid state without an aqueous environment, unlike hydrogel, if they are precisely integrated with aligned molecular systems.195 Hence, these LC-based stimuli-responsive soft materials that can magnify the external inputs into mechanical work and the future could be anticipated as an evolution of such collective motion in soft actuators, which would assist innovation in soft robotics, photonics, microfluidics, and biomedical applications.196 For example, Katsonis and colleagues demonstrated an oscillating pattern of the motor-doped achiral LC.197 A passive codopant was utilized to prevent the chiral-dopants' helical unwinding under light, which could produce geometrical frustration (Fig. 20a and b). When a film of the helix is enclosed between two glass slides, stimulating perpendicular orientation, this supramolecular structure was observed to impart angle-dependent propagation as the axis symmetry was disturbed. These outcomes might hold potential for developing autonomous motors that could harness light to work. Feringa's group described that a first-generation motor could transfer helices chirality into LC networks.198 The different chiral states of the rotary motor under light can alter the geometry of the LC matrix. The system has four isomeric steps compared to the second-generation motor, which shows only two isomeric steps.199 Thus, light can trigger the rotational steps of the motor reversibly in the macroscopic domain. The LC functionalized with a second-generation alkene motor that results in helical twisting under a single wavelength racemic motor-embedded polymeric film shows bending when induced by UV light and reverts to the initial state upon removing the source (Fig. 20c–e). Thus, a small film of the LC polymer irradiated with light results in walking or bending toward the direction of the light.
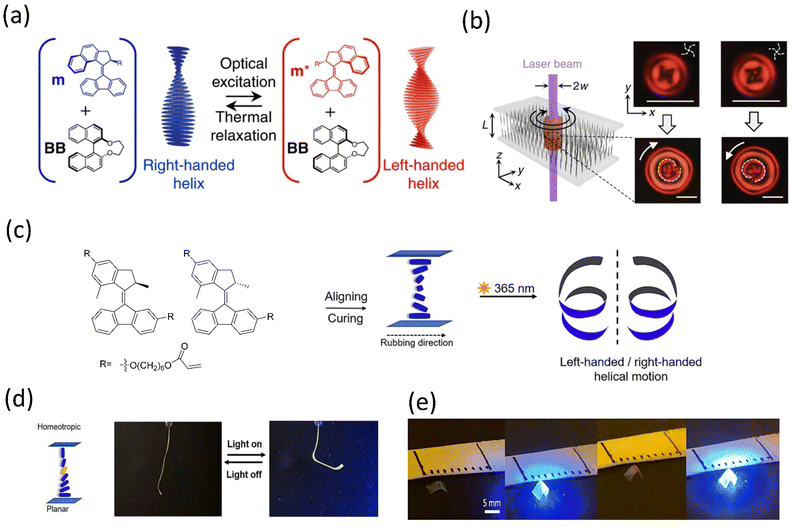 | ||
| Fig. 20 (a) Molecular motor with Binol (BB) doped in LC that shows winding under light irradiation. (b) Axisymmetric chiral patterns with opposite handedness were observed by polarized optical microscopy between crossed linear polarizers. Reproduced with permission.197 Copyright 2018, Springer Nature. (c) An enantiomerically pure molecular rotor and a mixture of these motors with LC monomer result in an indifferent helical motion. (d) Arrangement of the motor in LC monomer and its bending motion under exposure to light. (e) photoactuation of the LC ribbon on a glass surface. Reproduced with permission.199 Copyright 2021, The Authors, published by Wiley-VCH. | ||
Recently, Feringa, Chen, and coworkers investigated the light-driven intricate and programmable motion of the second-generation rotary motor with an LC network.200 The system showed fast motility and helical motion with different handedness. LC-based polymeric ribbon having four parts after UV light irradiation produced a fast wavy motion, and the motion was either against or toward the light source (Fig. 21). This actuation was due to the two different orientations of the racemic mixture of motors contained within the LCs network. Here, the motors play a crosslinking role in actuators; however, when enantiomerically pure motors are employed as a dopant for the LC matrix, the spiral motion was accomplished owing to the remarkable special axial chirality of the pure motor. These mentioned studies are performed employing photolithography techniques that allow the well-defined orientation of the motor and LC monomers. Hence, these studies provide the designing principles for advancing soft robotics and actuators that could generate more complex motions.
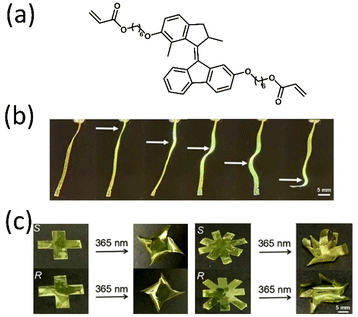 | ||
| Fig. 21 (a) Molecular structure of a rotary motor. Light-powered (b) wavy motion and (c) helical motion of the motorized LC film (scale bar is 5.0 mm). Reproduced with permission.200 Copyright 2022, The Authors, published by American Chemical Society. | ||
4. Some of the scope and crucial hurdles in designing artificial molecular machinery for practical use
Currently, chemists attempt to mimic autonomous natural motors to build different functional nanodevices. However, whatever molecular machines are reported to date are unlike the natural biomolecular motors and practically nonautonomous, apart from a few. One of the crucial challenges in designing the molecular machine is energy supply as the sustainable input, which operates the device, and this can be possible be exergonic.201 Other chemical reactions, liberating energy when cold, can be used instead. We learn from nature's creation, and our collective knowledge drives us to recreate nature's functions while maintaining thermodynamic nonequilibrium. However, at some point, if the motors fail to drive away from the thermodynamic equilibrium, we face the hurdle of bringing them back to perform their desired functions. Thus, a random synthetic approach could sometimes result in a very unrealistic outcome that often turns into failure. Therefore, the molecular modeling approach is helpful for the preliminary investigation. We can suppose that the desired dynamics in a molecular system is based on theoretical simulation data and design them accordingly. The most challenging part of developing a molecular machine is to bring a proper dynamic motion and the corresponding control on the directionality. Generally, the molecular motors follow the Brownian ratchet mechanism by exploiting random thermal motion to move; however, the motors need external input factors for their control. Therefore, a continuous power source is required for the motor to maintain operational continuity. The movement depends on the correctness of the molecular design, and the ratchet function is a critical factor for the control. Unlike macroscale machines, a molecular machine faces the interatomic forces of intramolecular attraction-repulsion within submolecular spaces. These narrow spaces consider only quantum mechanical phenomena; thus, the direct miniaturization of the classical conceptual design may not work. However, the scientist took inspiration from the natural biomachine blueprint by considering the subatomic parameters as molecular-size machines work under low Reynolds numbers and the prevailing Brownian motion conditions. Thus, one must contemplate these factors while designing the molecular machine, and the ratchet mechanism is quite helpful. Once the molecular machines' design and synthesis are over, implementation becomes vital to extract practical work from them. These systems need to be incorporated into suitable metallic or semiconducting surfaces, nanocrystals, nanoparticles, DNA scaffolds, MOFs, etc., for extracting collective output to furnish the desired job.6,8,41,202–204 The properties of molecular machines are widely exploited in the solution phase; now, the incorporation and control of their dynamics in the solid state are necessary to extract macroscopic output to develop a solid-state device. In recent times, scientists have integrated potential nanomachines on the surface materials to improve the efficiency of the collective motion of molecular machines. This was possible due to the well-defined topology of the solid materials that allow the molecule's motion to synchronize precisely. For instance, DNA scaffolds and MOFs are receiving greater attention as their free molecular spaces enable the rotary motors to rotate freely, thereby overcoming the disadvantage of the liquid crystals. The last two decades have seen a vast development in molecular machines, from surface-rolling nanocars to MOFs incorporated with molecular machines. The change in the motor's geometry could lead to a change in the geometry of the associated surface, which may benefit some systems. Nevertheless, the geometrical change in MOF due to the integrated rotor molecules might hamper the system. LC polymers are also one of the relevant surfaces for rotary motors to amplify large-amplitude rotational motion. One of the fundamental challenges in a surface grafting machine is that all the motors need to rotate freely in the desired direction without the function hindrance of adjacent molecules. In other words, there should be little or no noise; otherwise, the motor's efficiency suffers, and collective output creation fails. Further examinations of various surfaces may aid in overcoming this. The potential macroscopic applications in energy storage and release, nanorobotics, soft actuators, and the biomedical field are already visible. Although researchers have come a long way from designing a molecular machine to generating macroscopic output, several issues still need to be addressed. Properly engineering these surfaces will resolve the issues that pave the way for developing effective solutions and solid-state devices for practical applications.5. Conclusion and perspectives
The artificial molecular machines developed in the last few decades manifest the multidisciplinary science approaches in this research field. The evolution of molecular motors has shown how these systems are introduced into materials science and chemical biology to a large extent. This review outlines different ratcheting strategies for building rotary nanomotors, their functional mechanism, and actuating properties with examples. Also, we have discussed the design of architectures that improves the performance of molecular machines at the macroscopic level to a specific scale. Moreover, we have discussed more detailed proof-of-concept to real-world implementations of diverse molecular motors, offering insights into their potential applications in various realms for future perspectives. Notably, the examples of the motors show their potential to perform or one could envision to implement in more than one application. It is captivating how far this field has come in the last two decades, from nanoscale motion to controlled cargo delivery or the movement of a droplet. The journey from the molecular level to the macroscopic scale suggests that integrating motors into different surfaces/interfaces, such as polymers, LCs, supramolecules, DNA scaffolds, and carbonaceous materials, could extract macroscopic work. Both theoretical and experimental investigations would be advantageous for designing such prototype systems.The examples discussed here show that NIR153 or visible light154,174 serves as excellent stimuli. Further, the development of 2PA motors156 or motorized nanobots58 (e.g., RF-driven) will undoubtedly be going to solve the issue related to UV-driven molecular machines. Other stimuli, such as electrical, redox, or chemical fuel, would be appropriate for the desired work. To achieve this, besides advancement in structures and architectures, a vigorous exploration of new classes of molecular motors is needed, and the library of molecular motors should increase, providing more options to engineers to develop more efficient works. In chemical synthesis, the motors may act as a robot that controls the production of different products in a programmed way.175 Reports have been published on switching chirality; yet, issues associated with the systems, such as the recyclability of a motor or switching activity, need to be addressed.
Thereby, many reactions will be benefited from the forthcoming rotary motor-based robotic systems. Nevertheless, manipulating other systems by molecular machines is established. This could be one of the potential areas where other classes of molecular systems should be employed to achieve nature's complexity and efficiency. A regular expression of misfolding and aggregation-prone proteins cause several diseases in human; polyglutamine expansion in different unrelated proteins are found to be responsible for many human neurodegenerative diseases. The development of dual-light active rotary motors for massive reversible elastic advantage is notable. This work may be further extrapolated to solve many such disputed biological events. For example, in prion protein disease, where protein misfolding causes severe neuro diseases, such motorized action may be helpful to repair protein misfolding, and redox-active molecular motors can inhibit the undesired polyglutamine expansion.
The development of different soft materials based on molecular motors with associated macroscopic output shows that the gap between macroscopic and molecular levels is resolving. These dynamics systems are covalently linked to the mesogen or as a chiral dopant to generate stimuli-responsive LC-based soft materials. These soft materials are quite promising as they have broad applications; yet, improvement could generate better and continuous actuation in any environment. The macroscopic actuators would be advanced by arranging hierarchical motor assemblies with supramolecular matrix using top-down and bottom-up methods such as 3D/4D printing, bundling, and layering. Flexible materials are essential for building new flexible devices; therefore, nanocage-based polymer materials having inbuilt motors may be highly futuristic to develop soft-touch materials, foldable devices, etc.205 These works have perfectly shown the thoughtful design and use of molecular machine's active and passive mechanical parts. Thus, the progress accelerated toward the complexity of design to achieve more practical advantages. We have seen that silicon chip processors are expensive and high power consuming. There is great scope to design multigated rotary motors for developing high computing power processors with low energy consumption. Scientists are working on a biocomputer development where the molecular motors of biological origin are the core unit.206 Rotary motors, if interfaced with supramolecular nanoplatform, deliver a powerful nanorobotic device that can solve very complex biocomputation and even target cancer cells with high precision, can disintegrate human Alzheimer's beta-plaque.
Another issue in the energy harvesting context is the engineering approach to device development using rotary motors to extract maximum power on a large scale. To produce maximum work, the collective output of molecular motors by cooperative nature is vital, which minimizes noise. Therefore, the assembly of molecular motors should work in unison. Thus, the different surfaces/interfaces should be put in trial and error to achieve this. To the best of our knowledge, to date, molecular rotary motors are not used in supercapacitor applications or as piezoelectricity materials. Hence, suitable functionalization with the rotary motors or exploration of rotary motors (or with different interlocked systems) in various platforms such as nanoparticles, MOFs, LCs, biomolecular scaffolds, and CQDs will undoubtedly lead to discoveries in new scientific applications.207 The light-gated remote-functionalized photoswitching can address other motor-based photosensing devices. These remotely functionalized optical single rotary motor systems may be further extended to control multiple rotary motors with appropriate design. It is fascinating that motorized nanocars are controlled as a single molecular system and could be envisioned for high-density data processing and storage. The proper immobilization on the surface and technologies to analyze the operation of these systems at the molecular level are complex and need to be resolved. Although scientists have dominated in achieving directional motion control and converting these motions to integrated materials, many choke points to practical applications exist. The output induced by the molecular motor's collective motion would encourage scientists to construct more responsive and standalone materials. So far, several macroscopic works have been reported; the question may arise about how far this amplification of the collective motion would go by means of actuation, workload, and robustness.
Finally, as natural biomolecular motors could perform various tasks in a chaotic environment, ratcheting and engineering to develop such machines are highly desirable and challenging. One step in this direction would be the development of low-frequency active macromolecular ratchets or platforms, which can remove noises in the working frame to deliver damping-free operation. While nature has attained its complex and multifunctional machines through billions of years of evolution, forthcoming artificial molecular machinery is bright, even though its journey has not been long. Although developing a molecular motor with prototype applications is still in progress, since 1993, scientists have gradually learned to design, synthesize, and control various artificial and biomimetic molecular machines and now amplify them into collective motion. Thus, we could anticipate prominent output from molecular machinery shortly. We hope that this review will show the potential to gain knowledge and ideas to build molecular machinery (mainly rotary motors) for real-world applications.
Conflicts of interest
The authors declare there are no conflicts of interest.Acknowledgements
The authors thank the Indian Government's DST-SERB for Grant No. ECR/2016/001534 (2017–2020). Sudeshna Kalita and Prerna Chettri are appreciative of their PhD grants from the CSIR and UGC of India. The utilities and assistance were provided by CSIR-NEIST, for which the authors are grateful. The authors appreciate the contributions of Dr Prathik Sahoo and Dr Anirban Bandyopadhyay.Notes and references
- M. Piccolino, Nat. Rev., 2000, 1, 149–152 CrossRef CAS PubMed.
- M. Schliwa and G. Woehlke, Nature, 2003, 422, 759–765 CrossRef CAS PubMed.
- C. Mavroidis, A. Dubey and M. L. Yarmush, Annu. Rev. Biomed. Eng., 2004, 6, 363–395 CrossRef CAS PubMed.
- K. Kinosita, R. Yasuda, H. Noji, S. Ishiwata and M. Yoshida, Cell, 1998, 93, 21–24 CrossRef CAS PubMed.
- R. Iino, K. Kinbara and Z. Bryant, Chem. Rev., 2020, 120, 1–4 CrossRef CAS PubMed.
- S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS PubMed.
- S. Corra, M. Curcio, M. Baroncini, S. Silvi and A. Credi, Adv. Mater., 2020, 32, 1906064 CrossRef CAS PubMed.
- M. Baroncini, S. Silvi and A. Credi, Chem. Rev., 2020, 120, 200–268 CrossRef CAS PubMed.
- A. W. Heard and S. M. Goldup, ACS Cent. Sci., 2020, 6, 117–128 CrossRef CAS PubMed.
- B. L. Feringa, Angew. Chem., Int. Ed., 2017, 56, 11060–11078 CrossRef CAS PubMed.
- B. L. Feringa, K. Nagatoshi, R. W. J. Zijlstra, R. A. Delden and N. Harada, Nature, 1999, 401, 152 CrossRef PubMed.
- M. Akter, J. J. Keya, K. Kayanoa, A. M. R. Kabir, D. Inoue, H. Hess, K. Sada, A. Kuzuya, H. Asanuma and A. Kakugo, Sci. Robot., 2022, 7, eabm0667 Search PubMed.
- J. J. Keya, R. Suzuki, A. M. R. Kabir, D. Inoue, H. Asanuma, K. Sada, H. Hess, A. Kuzuya and A. Kakugo, Nat. Commun., 2018, 9, 453 CrossRef PubMed.
- E. Wasserman, J. Am. Chem. Soc., 1960, 82, 4433–4434 CrossRef CAS.
- C. O. Dietrich-Buchecker and J. P. Sauvage, Tetrahedron Lett., 1983, 24, 5095–5098 CrossRef CAS.
- P. L. Anelli, N. J. Spencer and J. F. Stoddart, J. Am. Chem. Soc., 1991, 113, 5131–5133 CrossRef CAS PubMed.
- R. A. Bissell, E. Cordova, A. E. Kaifer and J. F. Stoddart, Nature, 1994, 369, 133–137 CrossRef CAS.
- C. P. Collier, G. Mattersteig, E. W. Wong, Y. Luo, K. Beverly, J. Sampaio, F. M. Raymo, J. F. Stoddart and J. R. Heath, Science, 2000, 289, 1172–1175 CrossRef CAS PubMed.
- E. R. Kay, D. A. Leigh and F. Zerbetto, Angew. Chem., Int. Ed., 2007, 46, 72–191 CrossRef CAS PubMed.
- R. Brown, Philos. Mag., 1828, 4, 161–173 CrossRef.
- R. Brown, New Philos., 1828, 5, 358–371 Search PubMed.
- A. Einstein, Ann. Phys., 1905, 17, 549–560 CrossRef CAS.
- J. Perrin, translated by D. L. Hammick, Constable, London, 1916.
- D. Shi, K. Komatsu, M. Hirao, Y. Toyooka, H. Koyama, F. Tissir, A. M. Goffinet, T. Uemura and T. Fujimori, Development, 2014, 141, 4558–4568 CrossRef CAS PubMed.
- S. M. King, Dyneins: Structure, Biology and Disease, Academic Press, 2018, pp. 162–201 Search PubMed.
- H. Ishikawa and W. F. Marshall, Cold Spring Harbor Perspect. Biol., 2017, 1(9), a021998 CrossRef PubMed.
- I. Rayment, W. Rypniewski, K. Schmidt-Base, R. Smith, D. Tomchick, M. Benning and H. Holden, Science, 1993, 261, 50 CrossRef CAS PubMed.
- N. Hirokawa, Y. Noda, Y. Tanaka and S. Niwa, Nat. Rev. Mol. Cell Biol., 2009, 10, 682 CrossRef CAS PubMed.
- S. L. Reck-Peterson, W. B. Redwine, R. D. Vale and A. P. Carter, Nat. Rev. Mol. Cell Biol., 2018, 19, 382–398 CrossRef CAS PubMed.
- H. Lodish, A. Berk, S. L. Zipursky, P. Matsudaira, D. Baltimore and J. Darnell, Photosynthetic Stages and Light-Absorbing Pigments, Molecular Cell Biology, W. H. Freeman, New York, 4th edn, 2000 Search PubMed.
- J. R. Sellers, Biochim. Biophys. Acta, Mol. Cell Res., 2000, 1496, 3–22 CrossRef CAS PubMed.
- S. Fischer, D. Horak, K. C. Holmes and J. C. Smith, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 6873–6878 CrossRef CAS PubMed.
- S. Sengupta, M. M. Spiering, K. K. Dey, W. Duan, D. Patra, P. J. Butler, R. D. Astumian, S. J. Benkovic and A. Sen, ACS Nano, 2014, 8, 2410–2418 CrossRef CAS PubMed.
- A. J. Berdis, Chem. Rev., 2009, 109, 2862–2879 CrossRef CAS PubMed.
- V. Ramakrishnan, Cell, 2002, 108, 557–572 CrossRef CAS PubMed.
- H. Yu, K. Jo, K. L. Kounovsky, J. J. de Pablo and D. C. Schwartz, J. Am. Chem. Soc., 2009, 131, 5722–5723 CrossRef CAS PubMed.
- C. Wang, M. P. O'Hagan, Z. Li, J. Zhang, X. Ma, H. Tian and I. Willner, Chem. Soc. Rev., 2022, 51, 720–760 RSC.
- D. Y. Tam, X. Zhuang, S. W. Wong and P. K. Lo, Small, 2019, 15, 1805481 CrossRef PubMed.
- S. Mohapatra, C.-T. Lin, X. A. Feng, A. Basu and T. Ha, Chem. Rev., 2019, 120, 36–78 CrossRef PubMed.
- A. Gennerich, Nat. Nanotechnol., 2014, 9, 11–12 CrossRef CAS PubMed.
- E. Moulin, L. Faour, C. C. Carmona-Vargas and N. Giuseppone, Adv. Mater., 2020, 32, 1906036 CrossRef CAS PubMed.
- A. Perrot, E. Moulin and N. Giuseppone, Trends Chem., 2021, 3, 926–942 CrossRef CAS.
- X. Yao, T. Li, J. Wang, X. Ma and H. Tian, Adv. Opt. Mater., 2016, 4, 1322–1349 CrossRef CAS.
- W. R. Bauer and W. Nadler, J. Chem. Phys., 2008, 129, 12B611 CrossRef PubMed.
- R. Ait-Haddou and W. Herzog, Cell Biochem. Biophys., 2003, 38, 191–213 CrossRef CAS PubMed.
- R. D. Astumian and I. Derényi, Biophys. J., 1999, 77, 993–1002 CrossRef CAS PubMed.
- O. Kulish, A. Wright and E. Terentjev, Sci. Rep., 2016, 6, 28180 CrossRef CAS PubMed.
- R. D. Astumian, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 19715–19718 CrossRef CAS.
- F. Moss and K. Wiesenfeld, Sci. Am., 1995, 273, 66–69 CrossRef.
- D. Keller and C. Bustamante, Biophys. J., 2000, 78, 541–556 CrossRef CAS PubMed.
- R. Ait-Haddou and W. Herzog, Cell Biochem. Biophys., 2003, 38, 191–213 CrossRef CAS PubMed.
- T. Strick, J.-F. Allemand, V. Croquette and D. B ensimon, Phys. Today, 2001, 54, 46–51 CrossRef CAS.
- R. D. Astumian, Nat. Commun., 2019, 10, 3837 CrossRef PubMed.
- H. Wang and G. Oster, Appl. Phys. A, 2002, 75, 315–323 CrossRef CAS.
- W. Hwang and M. Karplus, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 19777–19785 CrossRef CAS PubMed.
- R. M. Berry, Philos. Trans. R. Soc. London, Ser. B, 2000, 355, 503–509 CrossRef CAS PubMed.
- J. A. Wagoner and K. A. Dill, J. Phys. Chem. B, 2016, 120, 6327–6336 CrossRef CAS PubMed.
- A. Singhania, I. Ghosh, P. Sahoo, D. Fujita, S. Ghosh and A. Bandyopadhyay, Nano Lett., 2020, 20, 6891–6898 CrossRef CAS PubMed.
- T. R. Kelly, H. De Silva and R. A. Silva, Nature, 1999, 401, 150–152 CrossRef CAS PubMed.
- J. Sheng, W. Danowski, S. Crespi, A. Guinart, X. Chen, C. Stähler and B. L. Feringa, Chem. Sci., 2023, 14, 4328–4336 RSC.
- D. Roke, S. J. Wezenberg and B. L. Feringa, Molecular rotary motors, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 9423–9431 CrossRef CAS PubMed.
- N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152 CrossRef CAS PubMed.
- N. Koumura, E. M. Geertsema, M. B. van Gelder, A. Meetsma and B. L. Feringa, J. Am. Chem. Soc., 2002, 124, 5037 CrossRef CAS PubMed.
- J. C. Kistemaker, P. Štacko, D. Roke, A. T. Wolters, G. H. Heideman, M. C. Chang, P. Van Der Meulen, J. Visser, E. Otten and B. L. Feringa, J. Am. Chem. Soc., 2017, 139, 9650–9661 CrossRef CAS PubMed.
- T. van Leeuwen, A. S. Lubbe, P. Stacko, S. J. Wezenberg and B. L. Feringa, Nat. Rev. Chem., 2017, 1, 96 CrossRef CAS.
- D. Roke, M. Sen, W. Danowski, S. L. Wezenberg and B. L. Feringa, J. Am. Chem. Soc., 2019, 141, 7622–7627 CrossRef CAS PubMed.
- D. R. S. Pooler, R. Pierron, S. Crespi, R. Costil, L. Pfeifer, J. Leonard, M. M. Olivucci and B. L. Feringa, Chem. Sci., 2021, 12, 7486–7497 RSC.
- G. B. Boursalian, E. R. Nijboer, R. Dorel, L. Pfeifer, O. Markovitch, A. Blokhuis and B. L. Feringa, J. Am. Chem. Soc., 2020, 142, 16868–16876 CrossRef CAS PubMed.
- A. Padwa, Chem. Rev., 1977, 77, 37–68 CrossRef CAS.
- J.-M. Lehn, Chem. – Eur. J., 2006, 12, 5910–5915 CrossRef CAS PubMed.
- L. Greb, A. Eichhöfer and J.-M. Lehn, Angew. Chem., Int. Ed., 2015, 54, 14345–14348 CrossRef CAS PubMed.
- L. Greb and J.-M. Lehn, J. Am. Chem. Soc., 2014, 136, 13114–13117 CrossRef CAS PubMed.
- M. Guentner, M. Schildhauer, S. Thumser, P. Mayer, D. Stephenson, P. J. Mayer and H. Dube, Nat. Commun., 2015, 6, 8406 CrossRef CAS PubMed.
- L. A. Huber, K. Hoffmann, S. Thumser, N. Böcher, P. Mayer and H. Dube, Angew. Chem., Int. Ed., 2017, 56, 14536–14539 CrossRef CAS PubMed.
- R. Wilcken, M. Schildhauer, F. Rott, L. A. Huber, M. Guentner, S. Thumser, K. Hoffmann, S. Oesterling, R. de Vivie-Riedle, E. Riedle and H. Dube, J. Am. Chem. Soc., 2018, 140, 5311–5318 CrossRef CAS PubMed.
- A. Gerwien, P. Mayer and H. Dube, J. Am. Chem. Soc., 2018, 140, 16442–16445 CrossRef CAS PubMed.
- S. P. Fletcher, F. Dumur, M. M. Pollard and B. L. Feringa, Science, 2005, 310, 80–82 CrossRef CAS PubMed.
- B. S. Collins, J. C. Kistemaker, E. Otten and B. L. Feringa, Nat. Chem., 2016, 8, 860–866 CrossRef CAS.
- K. Mo, Y. Zhang, Z. Dong, Y. Yang, X. Ma, B. L. Feringa and D. Zhao, Nature, 2022, 609, 293–298 CrossRef CAS PubMed.
- S. Borsley, E. Kreidt, D. A. Leigh and B. M. W. Roberts, Nature, 2022, 604, 80–85 CrossRef CAS PubMed.
- C. García-Iriepa, M. Marazzi, F. Zapata, A. Valentini, D. Sampedro and L. M. Frutos, J. Phys. Chem. Lett., 2013, 4, 1389–1396 CrossRef PubMed.
- G. Haberhauer, Angew. Chem., Int. Ed., 2011, 50, 6415–6418 CrossRef CAS PubMed.
- M. Filatov, M. Paolino, S. K. Min and C. H. Choi, Chem. Commun., 2019, 55, 5247–5250 RSC.
- J. M. Abendroth, O. S. Bushuyev, P. S. Weiss and C. J. Barrett, ACS Nano, 2015, 9, 7746–7768 CrossRef CAS PubMed.
- A. Faulkner, T. van Leeuwen, B. L. Feringa and S. J. Wezenberg, J. Am. Chem. Soc., 2016, 138, 13597–13603 CrossRef CAS PubMed.
- S. Saha, A. Ghosh, T. Paululat and M. Schmittel, Dalton Trans., 2020, 49, 8693–8700 RSC.
- Y. Wu, G. Wang, Q. Li, J. Xiang, H. Jiang and Y. Wang, Nat. Commun., 2018, 9, 1953 CrossRef PubMed.
- T. van Leeuwen, W. Danowski, S. F. Pizzolato, P. Štacko, S. J. Wezenberg and B. L. Feringa, Chem. – Eur. J., 2018, 24, 81–84 CrossRef CAS PubMed.
- A. Singhania, S. Chatterjee, S. Kalita, S. Saha, P. Chettri, F. R. Gayen, B. Saha, P. Sahoo, A. Bandyopadhyay and S. Ghosh, ACS Appl. Mater. Interfaces, 2023, 15, 15595–15604 CrossRef CAS PubMed.
- D. Roke, C. Stuckhardt, W. Danowki, S. J. Wezenberg and B. L. Feringa, Angew. Chem., Int. Ed., 2018, 57, 10515 CrossRef CAS PubMed.
- L. Pfeifer, S. Crespi, P. van der Meulen, J. Kemmink, R. M. Scheek, M. F. Hilbers, W. J. Buma and B. L. Feringa, Nat. Commun., 2022, 13, 2124 CrossRef CAS PubMed.
- M. Li, S. Li, K. Zhang, X. Chi, H. Zhou, H.-B. Xu, Y. Zhang, Q. Li, D. Wang and M.-H. Zeng, Nanoscale, 2021, 13, 16748–16754 RSC.
- A. R. Hughes, N. J. Brownbill, R. C. Lalek, M. E. Briggs, A. G. Slater, A. I. Cooper and F. Blanc, Chem.–Eur. J., 2017, 23, 17217–17221 CrossRef CAS PubMed.
- G. Prando, J. Perego, M. Negroni, M. Riccò, S. Bracco, A. Comotti, P. Sozzani and P. Carretta, Nano Lett., 2020, 20(10), 7613–7618 CrossRef CAS PubMed.
- A. Comotti, S. Bracco, P. Valsesia, M. Beretta and P. Sozzani, Angew. Chem., Int. Ed., 2010, 49, 1760–1764 CrossRef CAS PubMed.
- A. Comotti, S. Bracco, T. Ben, S. Qiu and P. Sozzani, Angew. Chem., Int. Ed., 2014, 53, 1043–1047 CrossRef CAS PubMed.
- A. Comotti, S. Bracco, A. Yamamoto, M. Beretta, T. Hirukawa, N. Tohnai, M. Miyata and P. Sozzani, J. Am. Chem. Soc., 2014, 136, 618–621 CrossRef CAS PubMed.
- J. Perego, S. Bracco, M. Negroni, C. X. Bezuidenhout, G. Prando, P. Carretta, A. Comotti and P. Sozzani, Nat. Chem., 2020, 12, 845–851 CrossRef CAS PubMed.
- M. Inukai, T. Fukushima, Y. Hijikata, N. Ogiwara, S. Horike and S. Kitagawa, J. Am. Chem. Soc., 2015, 137, 12183–12186 CrossRef CAS PubMed.
- V. Balzani, A. Credi and M. Venturi, Nano Today, 2007, 2, 18–25 CrossRef.
- K. Ariga, Chem. Nanomater., 2020, 6, 870–880 CAS.
- P. Ceroni, A. Credi and M. Venturi, Chem. Soc. Rev., 2014, 43, 4068–4083 RSC.
- Here articles/newsheading related to the size of semicounductior chip: Monica Chen, Jessie Shen Hsinchu, TSMC ramping up 7 nm chip production, https://www.digitimes.com/news/a20220323PD215.html, June, 2018.
- A. Gopani, The Race to Reduce Nanometers in Chips, https://analyticsindiamag.com/the-race-to-reduce-nanometers-in-chips/, January, 2022 Search PubMed.
- D. Burg and J. H. Ausubel, PLoS ONE, 2021, 16, e0256245 CrossRef CAS PubMed.
- H. Yu, Y. Luo, K. Beverly, J. F. Stoddart, H. R. Tseng and J. R. Heath, Angew. Chem., Int. Ed., 2003, 42, 5706–5711 CrossRef CAS PubMed.
- M. M. Waldrop, Nature, 2016, 530, 144 CrossRef CAS PubMed.
- N. Xin, J. Guan, C. Zhou, X. Chen, C. Gu, Y. Li, M. A. Ratner, A. Nitzan, J. F. Stoddart and X. Guo, Nat. Rev. Phys., 2019, 1, 211–230 CrossRef.
- C. W. Fuller, P. S. Padayatti, H. Abderrahim, L. Adamiak, N. Alagar, N. Ananthapadmanabhan, J. Baek, S. Chinni, C. Choi, K. J. Delaney, R. Dubielzig, J. Frkanec, C. Garcia, C. Gardner, D. Gebhardt, T. Geiser, Z. Gutierrez, D. A. Hall, A. P. Hodges, G. Hou, S. Jain, T. Jones, R. Lobaton, Z. Majzik, A. Marte, P. Mohan, P. Mola II, P. Mudondo, J. Mullinix, T. Nguyen, F. Ollinger, S. Orr, Y. Ouyang, P. Pan, N. Park, D. Porras, K. Prabhu, C. Reese, T. Ruel, T. Sauerbrey, J. R. Sawyer, P. Sinha, J. Tu, A. G. Venkatesh, S. V. Kumar, L. Zheng, S. Jin, J. M. Tour, G. M. Church, P. W. Mola and B. Merriman, Appl. Biol. Sci., 2022, 119, e2112812119 CAS.
- W. Danowski, F. Castiglioni, A. S. Sardjan, S. Krause, L. Pfeifer, D. Roke, A. Comotti, W. R. Browne and B. L. Feringa, J. Am. Chem. Soc., 2020, 142, 9048–9056 CrossRef CAS PubMed.
- A. K. Thakur, M. Majumder, S. P. Patole, K. Zaghib and M. V. Reddy, Mater. Adv., 2021, 2, 2457–2482 RSC.
- N. I. Georgiev, N. V. Marinova and V. B. Bojinov, J. Photochem. Photobiol., A, 2020, 401, 112733 CrossRef CAS.
- S. Wiedbrauk and H. Dube, Tetrahedron Lett., 2015, 56, 4266–4274 CrossRef CAS.
- M. Guentner, M. Schildhauer, S. Thumser, P. Mayer, D. Stephenson, P. J. Mayer and H. Dube, Nat. Commun., 2015, 6, 8406 CrossRef CAS PubMed.
- R. Wilcken, M. Schildhauer, F. Rott, L. A. Huber, M. Guentner, S. Thumser, K. Hoffmann, S. Oesterling, R. de Vivie-Riedle, E. Riedle and D. Henry, J. Am. Chem. Soc., 2018, 140, 5311–5318 CrossRef CAS PubMed.
- A. Gerwien, P. Mayer and H. Dube, J. Am. Chem. Soc., 2018, 140, 16442–16445 CrossRef CAS PubMed.
- Z.-Y. Zhang, Y. He, Z. Wang, J. Xu, M. Xie, P. Tao, D. Ji, K. Moth-Poulsen and T. Li, J. Am. Chem. Soc., 2020, 142, 12256 CrossRef CAS PubMed.
- L. Andreoni, M. Baroncini, J. Groppi, S. Silvi, C. Taticchi and A. Credi, Energy Fuels, 2021, 35, 18900–18914 CrossRef CAS PubMed.
- Q. Li, G. Fuks, E. Moulin, M. Maaloum, M. Rawiso, I. Kulic, J. T. Foy and N. Giuseppone, Nat. Nanotechnol., 2015, 10, 161–165 CrossRef CAS PubMed.
- J. T. Foy, Q. Li, A. Goujon, J.-R. Colard-Itté, G. Fuks, E. Moulin, O. Schiffmann, D. Dattler, D. P. Funeriu and N. Giuseppone, Nat. Nanotechnol., 2017, 12, 540–545 CrossRef CAS PubMed.
- Z.-Y. Zhang, Y. He, Z. Wang, J. Xu, M. Xie, P. Tao, D. Ji, K. Moth-Poulsen and T. Li, J. Am. Chem. Soc., 2020, 142, 12256–12264 CrossRef CAS PubMed.
- G. Vives and J. M. Tour, Acc. Chem. Res., 2009, 42, 473–487 CrossRef CAS PubMed.
- J.-F. Morin, Y. Shirai and J. M. Tour, Org. Lett., 2006, 8, 1713–1716 CrossRef CAS PubMed.
- P.-T. Chiang, J. Mielke, J. Godoy, J. M. Guerrero, L. B. Alemany, C. J. Villagómez, A. Saywell, L. Grill and J. M. Tour, ACS Nano, 2012, 6, 592–597 CrossRef CAS PubMed.
- A. Saywell, A. Bakker, J. Mielke, T. Kumagai, M. Wolf, V. García-López, P.-T. Chiang, J. M. Tour and L. Grill, ACS Nano, 2016, 10, 10945–10952 CrossRef CAS PubMed.
- T. Jin, V. García-López, S. Kuwahara, P.-T. Chiang, J. M. Tour and G. Wang, J. Phys. Chem. C, 2018, 122, 19025–19036 CrossRef CAS.
- V. García-López, P.-L. E. Chu, P.-T. Chiang, J. Sun, A. A. Martí and J. M. Tour, Asian J. Org. Chem., 2015, 4, 1308 CrossRef.
- V. García-Lópeza, L. B. Alemanyad, P.-T. Chianga, J. Suna, P.-L. Chua, A. A. Martíac and J. M. Tour, Tetrahedron, 2017, 73, 4864–4873 CrossRef.
- T. Jin, V. García-López, P.-T. Chiang, S. Kuwahara, J. M. Tour and G. Wang, J. Phys. Chem. C, 2019, 123, 3011–3018 CrossRef CAS.
- G. J. Simpson, V. García-López, A. D. Boese, J. M. Tour and L. Grill, Nat. Commun., 2019, 10, 463 CrossRef PubMed.
- A. van Venrooy, V. García-López, J. T. Li, J. M. Tour and A. V. Dubrovskiy, J. Org. Chem., 2020, 85, 13644–13654 CrossRef CAS PubMed.
- G. J. Simpson, V. García-López, P. Petermeier, L. Grill and J. M. Tour, Nat. Nanotechnol., 2017, 12, 604–606 CrossRef CAS PubMed.
- P. Jacobson, D. Prezzi, D. Liu, M. Schied, J. M. Tour, S. Corni, A. Calzolari, E. Molinari and L. Grill, J. Phys. Chem. C, 2020, 124, 24776–24785 CrossRef CAS.
- H. Ube, Y. Yasuda, H. Sato and M. Shionoya, Nat. Commun., 2017, 8, 14296 CrossRef CAS PubMed.
- H. Ube, R. Yamada, J. Ishida, H. Sato, M. Shiro and M. Shionoya, J. Am. Chem. Soc., 2017, 139, 16470–16473 CrossRef CAS PubMed.
- R. Asato, C. J. Martin, S. Abid, Y. Gisbert, F. Asanoma, T. Nakashima, C. Kammerer, T. Kawai and G. Rapenne, Inorg. Chem., 2021, 60, 3492–3501 CrossRef CAS PubMed.
- A. Gerwien, F. Gnannt, P. Mayer and H. Dube, Nat. Chem., 2022, 14, 670–676 CrossRef CAS PubMed.
- R. Zhao, F. Qi, Y.-L. Zhao, K. E. Hermann, R.-Q. Zhang and M. A. Van Hove, J. Phys. Chem. Lett., 2018, 9, 2611–2619 CrossRef CAS PubMed.
- R. Zhao, Y.-L. Zhao, F. Qi, K. E. Hermann, R.-Q. Zhang and M. A. Van Hove, ACS Nano, 2018, 12, 3020–3029 CrossRef CAS PubMed.
- M. Baba, K. Iwamoto, R. Iino, H. Ueno, M. Hara, A. Nakanishi, J. Kishikawa, H. Noji and K. Yokoyama, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11214 CrossRef CAS PubMed.
- Y. Zhang, J. P. Calupitan, T. Rojas, R. Tumbleson, G. Erbland, C. Kammerer, T. M. Ajayi, S. Wang, L. A. Curtiss, A. T. Ngo, S. E. Ulloa, G. Rapenne and S. W. Hla, Nat. Commun., 2019, 10, 3742 CrossRef PubMed.
- J. J. Yu, Z. Q. Cao, Q. Zhang, S. Yang, D. H. Qu and H. Tian, Chem. Commun., 2016, 52, 12056–12059 RSC.
- A. C. Coleman, J. M. Beierle, M. C. A. Stuart, B. Maciá, G. Caroli, J. T. Mika, D. J. van Dijken, J. Chen, W. R. Browne and B. L. Feringa, Nat. Nanotechnol., 2011, 6, 547–552 CrossRef CAS PubMed.
- S. Chen, F. K.-C. Leung, M. C. A. Stuart, C. Wang and B. L. Feringa, J. Am. Chem. Soc., 2020, 142, 10163–10172 CrossRef CAS PubMed.
- G. Xie, P. Li, Z. Zhao, X.-Y. Kong, Z. Zhang, K. Xiao, H. Wang, L. Wen and L. Jiang, Angew. Chem., Int. Ed., 2018, 57, 16708 CrossRef CAS PubMed.
- W. Z. Wang, L. B. Huang, S. P. Zheng, E. Moulin, O. Gavat, M. Barboiu and N. Giuseppone, J. Am. Chem. Soc., 2021, 143(38), 15653–15660 CrossRef CAS PubMed.
- J. Hou, G. Long, W. Zhao, G. Zhou, D. Liu, D. J. Broer, B. L. Feringa and J. Chen, J. Am. Chem. Soc., 2022, 144, 6851–6860 CrossRef CAS PubMed.
- A. S. Amrutha, K. R. S. Kumar, T. Kikukawa and N. Tamaoki, ACS Nano, 2017, 11, 12292–12301 CrossRef CAS PubMed.
- S. Ghosh, S. Chatterjee, A. Roy, K. Ray, S. Swarnakar, D. Fujita and A. Bandyopadhyay, Curr. Top. Med. Chem., 2015, 15, 534 CrossRef CAS PubMed.
- S. Ghosh, A. Roy, A. Singhania, S. Chatterjee, S. Swarnakar, D. Fujit and A. Bandyopadhyay, Toxicol. Rep., 2018, 22, 1044–1052 CrossRef PubMed.
- A. Singhania, P. Sahoo, K. Ray, A. Bandyopadhyay and S. Ghosh, Proceedings of International Conference on Data Science and Applications, 2020, vol. 148, p. 281 Search PubMed.
- V. García-López, F. Chen, L. G. Nilewski, G. Duret, A. Aliyan, A. B. Kolomeisky, J. T. Robinson, G. Wang, R. Pal and J. M. Tour, Nature, 2017, 548, 567–572 CrossRef PubMed.
- D. Liu, V. García-López, R. S. Gunasekera, L. G. Nilewski, L. B. Alemany, A. Aliyan, T. Jin, G. Wang, J. M. Tour and R. Pal, ACS Nano, 2019, 13, 6813–6823 CrossRef CAS PubMed.
- C. A. Orozco, D. Liu, Y. Li, L. B. Alemany, R. Pal, S. Krishnan and J. M. Tour, ACS Appl. Mater. Interfaces, 2019, 12, 410–417 CrossRef PubMed.
- R. S. Gunasekera, T. Galbadage, C. Ayala-Orozco, D. Liu, V. García-López, B. E. Troutman, J. J. Tour, R. Pal, S. Krishnan, J. D. Cirillo and J. M. Tour, ACS Appl. Mater. Interfaces, 2020, 12, 13657–13670 CrossRef CAS PubMed.
- Y. Zheng, M. K. L Han, R. Zhao, J. Blass, J. Zhang, D. W. Zhou, J.-R. Colard-Itté, D. Dattler, A. Çolak, M. Hoth, A. J. García, B. Qu, R. Bennewitz, N. Giuseppone and A. del Campo, Nat. Commun., 2021, 12, 3580 CrossRef CAS PubMed.
- L. Pfeifer, N. V. Hoang, M. Scherübl, M. S. Pshenichnikov and B. L. Feringa, Sci. Adv., 2020, 6, eabb6165 CrossRef CAS PubMed.
- S. J. Wezenberg, L.-J. Chen, J. E. Bos, B. L. Feringa, E. N. W. Howe, X. Wu, M. A. Siegler and P. A. Gale, J. Am. Chem. Soc., 2022, 144, 331–338 CrossRef CAS PubMed.
- S. J. Wezenberg and B. L Feringa, Nat. Commun., 2018, 9, 1984 CrossRef PubMed.
- S. J. Wezenberg and B. L. Feringa, Org. Lett., 2017, 19, 324–327 CrossRef CAS PubMed.
- R. Dorel and B. L. Feringa, Chem. Commun., 2019, 55, 6477–6486 RSC.
- L. van Dijk, M. J. Tilby, R. Szpera, O. A. Smith, H. A. P. Bunce and S. P. Fletcher, Nat. Rev. Chem., 2018, 2, 0117 CrossRef CAS.
- P. J. Gilissen, P. B. White, J. A. Berrocal, N. Vanthuyne, F. P. J. T. Rutjes, B. L. Feringa, J. A. A. W. Elemans and R. J. M. Nolte, Nat. Commun., 2020, 11, 5291 CrossRef CAS PubMed.
- J. Wang and B. L. Feringa, Science, 2011, 331, 1429–1432 CrossRef CAS PubMed.
- M. Vlatković, L. Bernardi, E. Otten and B. L. Feringa, Chem. Commun., 2014, 50, 7773–7775 RSC.
- M. Vlatković, J. Volarić, B. S. L. Collins, L. Bernardi and B. L. Feringa, Org. Biomol. Chem., 2017, 15, 8285–8294 RSC.
- V. Blanco, D. A. Leigh and V. Marcos, Chem. Soc. Rev., 2015, 44, 5341–5370 RSC.
- M. Vlatković, B. S. L. Collins and B. L. Feringa, Chem. – Eur. J., 2016, 22, 17080–17111 CrossRef PubMed.
- G. Romanazzi, L. Degennaro, P. Mastrorilli and R. Luisi, ACS Catal., 2017, 7, 4100–4114 CrossRef CAS.
- D. Zhao, T. van Leeuwen, J. Cheng and B. L. Feringa, Nat. Chem., 2017, 9, 250–256 CrossRef CAS PubMed.
- S. F. Pizzolato, P. Štacko, J. C. M. Kistemaker, T. van Leeuwen, E. Otten and B. L. Feringa, J. Am. Chem. Soc., 2018, 140, 17278–17289 CrossRef CAS PubMed.
- R. Dorel and B. L. Feringa, Angew. Chem., Int. Ed., 2020, 59, 785–789 CrossRef CAS PubMed.
- H. Sell, A. Gehl, D. Plaul, F. D. Sönnichsen, C. Schütt, F. Köhler, K. Steinborn and R. Herges, Commun. Chem., 2019, 2, 62 CrossRef.
- K. Grill and H. Dube, J. Am. Chem. Soc., 2020, 142, 19300–19307 CrossRef CAS PubMed.
- S. Kassem, A. T. L. Lee, D. A. Leigh, V. Marcos, L. Palmer and S. Pisano, Nature, 2017, 549, 374–378 CrossRef CAS PubMed.
- T. D. Bennett, F.-X. Coudert, S. L. James and A. I. Cooper, Nat. Mater., 2021, 20, 1179–1187 CrossRef CAS PubMed.
- P. Martinez-Bulit, A. J. Stirk and S. J. Loeb, Trends Chem., 2019, 1, 588–600 CrossRef CAS.
- A. Comotti, S. Bracco and P. Sozzani, Acc. Chem. Res., 2016, 49, 1701–1710 CrossRef CAS PubMed.
- J. Perego, C. X. Bezuidenhout, S. Bracco, G. Prando, L. Marchiò, M. Negroni, P. Carretta, P. Sozzani and A. Comotti, J. Am. Chem. Soc., 2021, 143, 13082–13090 CrossRef CAS PubMed.
- F. Castiglioni, W. Danowski, J. Perego, F. K. C. Leung, P. Sozzani, S. Bracco, S. J. Wezenberg, A. Comotti and B. L. Feringa, Nat. Chem., 2020, 12, 595–602 CrossRef CAS PubMed.
- W. Danowski, T. van Leeuwen, S. Abdolahzadeh, D. Roke, W. R. Browne, S. J. Wezenberg and B. L. Feringa, Nat. Nanotechnol., 2019, 14, 488–494 CrossRef CAS PubMed.
- Z. –T. Shi, Y.-X. Hu, Z. Hu, Q. Zhang, S.-Y. Chen, M. Chen, J.-J. Yu, G.-Q. Yin, H. Sun, L. Xu, X. Li, B. L. Feringa, H.-B. Yang, H. Tian and D. -H. Qu, J. Am. Chem. Soc., 2021, 143, 442–452 CrossRef CAS PubMed.
- I. Liepuoniute, M. J. Jellen and M. A. Garcia-Garibay, Chem. Sci., 2020, 11, 12994–13007 RSC.
- M. Jin, R. Ando, M. J Jellen, M. A. Garcia-Garibay and H. Ito, J. Am. Chem. Soc., 2021, 143, 1144–1153 CrossRef CAS PubMed.
- S. Bracco, A. Comotti and P. Sozzani, Acc. Chem. Res., 2016, 49, 1701–1710 CrossRef PubMed.
- P. Naumov, D. P. Karothu, E. Ahmed, L. Catalano, P. Commins, J. M. Halabi, M. B. Al-Handawi and L. Liang, J. Am. Chem. Soc., 2020, 142, 13256–13272 CrossRef CAS PubMed.
- J. Chen, F. K.-C. Leung, M. C. A. Stuart, T. Kajitani, T. Fukushima, E. van der Giessen and B. L. Feringa, Nat. Chem., 2018, 10, 132–138 CrossRef CAS PubMed.
- J. Chen, S. J. Wezenberg and B. L. Feringa, Chem. Commun., 2016, 52, 6765–6768 RSC.
- T. van Leeuwen, G. H. Heideman, D. Zhao, S. J. Wezenberg and B. L. Feringa, Chem. Commun., 2017, 53, 6393–6396 RSC.
- C. Gao, A. V. Jentzsch, E. Moulin and N. Giuseppone, J. Am. Chem. Soc., 2022, 144, 9845–9852 CrossRef CAS PubMed.
- J.-R. Colard-Itté, Q. Li, D. Collin, G. Mariani, G. Fuks, E. Moulin, E. Buhler and N. Giuseppone, Nanoscale, 2019, 11, 5197–5202 RSC.
- A. Colin-Molina, D. P. Karothu, M. J. Jellen, R. A. Toscano, M. A. Garcia-Garibay, P. Naumov and B. Rodríguez-Molina, Matter, 2019, 1, 1033–1046 CrossRef.
- F. Long, Y. Cheng, Y. Ren, J. Wang, Z. Li, A. Sun and G. Xu, Adv. Eng. Mater., 2022, 24, 2100863 CrossRef CAS.
- Y. Zheng, X. Jia, K. Li, J. Xu and X. H. Bu, Adv. Energy Mater., 2022, 12, 2100324 CrossRef CAS.
- W. Feng, D. Liu and D. J. Broer, Small Struct., 2021, 2, 2000107 CrossRef CAS.
- D. Martella, P. Paoli, J. M. Pioner, L. Sacconi, R. Coppini, L. Santini, M. Lulli, E. Cerbai, D. S. Wiersma, C. Poggesi, C. Ferrantini and C. Parmeggiani, Small, 2017, 13, 1702677 CrossRef PubMed.
- T. Orlova, F. Lancia, C. Loussert, S. Iamsaard, N. Katsonis and E. Brasselet, Nat. Nanotechnol., 2018, 13, 304–308 CrossRef CAS PubMed.
- A. Ryabchun, F. Lancia, J. Chen, D. Morozov, B. L. Feringa and N. Katsonis, Adv. Mater., 2020, 32, 2004420 CrossRef PubMed.
- J. Hou, A. Mondal, G. Long, L. de Haan, W. Zhao, G. Zhou, D. Liu, D. J. Broer, J. Chen and B. L. Feringa, Angew. Chem., Int. Ed., 2021, 60, 8251–8257 CrossRef CAS PubMed.
- J. Hou, G. Long, W. Zhao, G. Zhou, D. Liu, D. J. Broer, B. L. Feringa and J. Chen, J. Am. Chem. Soc., 2022, 144, 6851–6860 CrossRef CAS PubMed.
- A. Credi, J. Phys.: Condens. Matter, 2006, 18, S1779 CrossRef CAS.
- C. Wang, M. P. O’ Hagan, Z. Li, J. Zhang, X. Ma, H. Tian and I. Willner, Chem. Soc. Rev., 2022, 51, 720–760 RSC.
- A. S. Lubbe, Q. Liu, S. J. Smith, J. Willem de Vries, J. C. M. Kistemaker, A. H. de Vries, I. Faustino, Z. Meng, W. Szymanski, A. Herrmann and B. L. Feringa, J. Am. Chem. Soc., 2018, 140, 5069–5076 CrossRef CAS PubMed.
- E. Kolodzeiski and S. Amirjalayer, Phys. Chem. Chem. Phys., 2021, 23, 4728–4735 RSC.
- A. Dhamija, C. K. Das, Y. H. Ko, Y. Kim, R. D. Mukhopadhyay, A. Gunnam, X. Yu, I. C. Hwang, L. V. Schäfer and K. Kim, Chem, 2022, 8, 543–556 CAS.
- D. V. Nicolau Jr, M. Lard, T. Korten, F. C. Van Delft, M. Persson, E. Bengtsson, A. Månsson, S. Diez, H. Linke and D. V. Nicolau, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 2591–2596 CrossRef PubMed.
- Z. Liu, H. I. Wang, A. Narita, Q. Chen, Z. Mics, D. Turchinovich, M. Kläui, M. Bonn and K. Müllen, J. Am. Chem. Soc., 2017, 139, 9443–9446 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |