DOI:
10.1039/D2NA00394E
(Paper)
Nanoscale Adv., 2023,
5, 153-159
Retracted Article: Decoration of graphene oxide nanosheets with carboxymethylcellulose hydrogel, silk fibroin and magnetic nanoparticles for biomedical and hyperthermia applications
Received
19th June 2022
, Accepted 13th November 2022
First published on 14th November 2022
Abstract
In this study, an efficient nanobiocomposite based on graphene oxide (GO), carboxymethylcellulose (CMC) hydrogel, silk fibroin (SF), and Fe3O4 nanoparticles was synthesized. For this purpose and in order to provide a suitable scaffold for the nanobiocomposite, GO was functionalized with a CMC hydrogel via covalent bonding. In the next step, SF was added to the synthesized structure to increase biocompatibility and biodegradability. Fe3O4 was added into the structure by an in situ process and the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite was synthesized. The synthesized structure was evaluated in terms of toxicity and hemocompatibility and finally, it was used in the hyperthermia technique. This nanocomposite did not destroy healthy HEK293T cells after 48 h and 72 h, while it did annihilate BT549 cancer cells. The GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite has EC50 values of 0.01466 and 0.1415 against HEK293T normal cells and BT549 cancer cells, respectively (after 72 h). The nanocomposite has good potential in hyperthermia applications and at a concentration and a frequency of 1 mg mL−1 and 400 kHz it has a SAR of 67.7 W g−1.
Introduction
GO is the oxidized form of graphene that contains hydroxyl, epoxy, carboxy, carbonyl, phenol, lactone, and quinone groups.1,2 Due to this unique structure, GO can be functionalized with different molecules to improve its properties.3 The features of GO have led to its use in a variety of biological applications, including drug delivery,4 dental implants,5 cancer therapy,6 wound healing,7 bioimaging,8 and tissue engineering.9 Recently, GO has been used in nanocomposites and nanobiocomposites due to its synergistic effect and combined with various natural and synthetic materials. In this regard, CMC hydrogels, SF and Fe3O4 have been considered. Hydrogels are cross-linked polymeric networks that can hold large volumes of water due to the hydrophilic groups in their structure.10 Hydrogels can be classified according to physical characteristics, the preparation method, the origin of components, ionic charge, rate of biodegradation, and type of crosslinking.11 Various natural and synthetic polymers have been used to make hydrogels, but the compatibility of natural polymers with biological systems has made them more popular in the biological field. Hyaluronate, alginate, starch, gelatin, cellulose, chitosan, pectin, agarose, and their derivatives were used in this field due to their biocompatibility, biodegradability, and hydrophilicity.12 Cellulose is the most abundant natural substance on earth and it is significantly present in plants. Various derivatives such as hydroxypropyl methylcellulose, hydroxyethyl cellulose, carboxymethyl cellulose, and hydroxypropyl cellulose have been prepared to modify cellulose properties. CMC is a hydrophilic derivative of cellulose and is prepared by replacing 2, 3, and 6 hydroxyl groups in the cellulose structure with carboxymethyl groups.13 This material has eye-catching applications in enzyme immobilization, wound-healing, drug delivery, adsorbents, antibacterial activity, and tissue engineering.13 However, sometimes the various applications of CMC are limited due to its poor mechanical strength. Various methods such as formation of double-networks, composites, hybrids, and fiber-reinforced hydrogels are used to increase the mechanical strength of these hydrogels.14–18 SF is a natural polypeptide with a molecular weight of 200–350 kDa and it is used to modify the mechanical strength of hydrogels as a reinforcing material.19,20 The SF biopolymer with features such as noncytotoxicity, noncarcinogenicity, low immunogenicity, and hemostatic qualities has been used in tissue regeneration, drug delivery, and wound dressings.21–23 The use of SF, CMC, and GO as part of anticancer composites has been reported in many studies.24–26 Cancer is usually treated via chemotherapy, surgery, radiation therapy, and targeted therapy. But, the risks and negative effects of these methods have led to the use of new procedures such as hyperthermia. In this method, after injecting magnetic nanoparticles to the patient and applying AMF, magnetic nanoparticle vibration induced Brownian and Néel relaxation. During the vibration of metal nanoparticles, the temperature of cancer cells raises (42–46 °C) and tumors are destroyed.27–29 Various magnetic nanoparticles such as ZnFe2O4,30 NiFe2O4,31 CoFe2O4,32 Fe3O4,33 CuFe2O4,33 and MnFe2O434 have been studied for hyperthermia application. In this study, a novel and efficient nanobiocomposite based on GO, CMC hydrogel, SF, and Fe3O4 nanoparticles was synthesized for hyperthermia cancer treatment.
Experimental
General
All materials except silkworm cocoons were sourced from companies such as Merck, Sigma-Aldrich and Fluka. Fourier-transform infrared spectroscopy (FT-IR) analysis was performed to identify the functional groups in the nanobiocomposite and it was performed using a Thermo's Avatar device. Thermogravimetric analysis (TGA) and vibrating-sample magnetometry (VSM) were performed to evaluate the structure in terms of thermal stability and magnetic strength, respectively. TGA was accomplished using a STA504 analyzer in a temperature range of 25 °C to 1200 °C at a rate of 10 °C min−1. 5 mg of sample was used in the crucible for the TGA analysis. Also, a Kavir's LBKFB device was used for VSM analysis. Field emission scanning electron microscopy (FE-SEM) imaging and energy dispersive X-ray spectroscopy (EDS) analysis were done to evaluate the morphology and structural elements of the structure using an EMK000 KYKY device.
Preparation of GO
Modified Hummer's method was used to synthesize GO.27 1 g of graphite, 1 g of sodium nitrate, and 23 mL of sulfuric acid (98%) were mixed at 66 °C and then placed in an ultrasonic bath at room temperature for 30 minutes. In the next step, the obtained solution was sonicated in an ice bath and 3 g of KMnO4 was added to it slowly. The resulting suspension was kept in an ultrasonic bath for another hour. The suspension temperature reached 98 °C and 50 mL of water was added to the container. Then, 700 mL of warm distilled water and 12 mL of H2O2 were added to the container, and the color of the solution changed from brown to yellow. Eventually, 2 mL of HCl and 98 mL of distilled water were added to the mixture and the container remained stationary for 24 hours until the precipitate settled well. After the mentioned time, the solution (above the precipitate) was removed using a syringe, and it was replaced by 700 mL of distilled water. This process was continued for 3 days and eventually GO was dried at 60 °C.
Preparation of CMC hydrogel
7 mL of NaOH and 12 mL of urea were mixed in 81 mL of water, and then 4 g of CMC was added to the mixture and stirred at ambient temperature for 15 minutes. The resulting mixture was placed at −12 °C for 15 hours. Then, the mixture was placed at room temperature and after melting, it was stirred for 30 minutes and a clear solution was obtained. In the next step, 10 wt% of epyclorohydrine was added to the mixture as a cross-linker and stirred for 30 minutes. The solution was placed at 50 °C for 4 hours and then poured into a non-stick microplate and kept at −70 °C for 1 day. The synthesized hydrogel was stored in a freezer for subsequent use.
Extraction of SF
Three silkworm cocoons were cut into small pieces and boiled for 2 hours in a sodium carbonate solution (500 mL, 0.21% w/v). In the next step, the silk fibers were washed and dried at room temperature for 12 hours. The silk fibers were dissolved in a 9.3 M aqueous solution of LiBr and stirred for 2 hours at 60 °C (10 times of dry silk fiber weight was considered as the volume of LiBr solution). Then, the remaining LiBr was removed using a 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 daltons dialysis tube cellulose membrane within 3 days at room temperature. Finally, the synthesized SF was stored at 4 °C.
000 daltons dialysis tube cellulose membrane within 3 days at room temperature. Finally, the synthesized SF was stored at 4 °C.
Modification of GO with CMC hydrogel
0.5 g of GO and 40 mL of ethanol were mixed and placed in an ultrasonic bath for 30 minutes. Then 2 g of N,N′-dicyclohexylcarbodiimide (DCC) and 1.2 g of 4-dimethylaminopyridine (DMAP) were added to the mixture of the previous step and sonicated for 30 minutes. In the next step, 4 mL of CMC hydrogel was added to the mixture and refluxed for 24 hours. The product was separated using a centrifuge (5000 rpm) and washed 3 times with ethanol. Finally, the product was dried in a 60 °C oven for 12 hours.
Preparation of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite
Initially, 1 g of GO–CMC hydrogel was dispersed in 40 mL of ethanol and subsequently, 10 mL of SF solution was added to them. The resulting mixture was stirred at 70 °C for 3 hours. Then, 200 mL of distilled water, 0.97 g FeCl3·6H2O and 0.43 g FeCl2·4H2O were added to the GO–CMC hydrogel/SF container and stirred for 30 minutes under a N2 atmosphere (50 °C). 10 mL of 25% aqueous ammonia was slowly added to the reaction container and the mixture was stirred for 3 hours at 80 °C. Finally, the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite was separated using an external magnet and washed with distilled water to reach natural pH (Fig. 1).
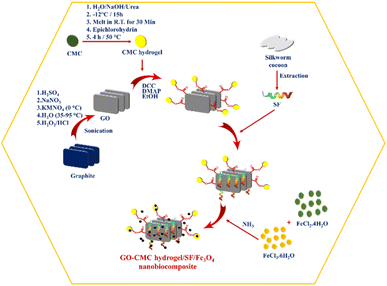 |
| | Fig. 1 Graphical illustration of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite preparation procedure. | |
Hemolysis assay
The red blood cell (RBC) hemolytic assay was used to determine the hemocompatibility of the synthesized GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite on human erythrocytes. Primarily, RBCs were washed and diluted with physiological serum (pH 7.0) in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. 100 microliters of the prepared solution were transferred to a 96-well microplate with a v-shaped bottom (Citotest, China). 100 μL of dispersed nanobiocomposite in physiological serum with different concentrations (0.25, 0.5, 0.75, 1 and 2 mg mL−1) was added to each well, and deionized water and physiological serum were applied as hemolysis positive and negative controls, respectively. The plate was incubated for 2 h at 37 °C and then centrifuged at 2000 rpm for 10 min. The supernatant of each well was transferred to the flat bottom plate and OD was quantified using an ELISA reader (Biohit, Finland) at 405 nm.35
100. 100 microliters of the prepared solution were transferred to a 96-well microplate with a v-shaped bottom (Citotest, China). 100 μL of dispersed nanobiocomposite in physiological serum with different concentrations (0.25, 0.5, 0.75, 1 and 2 mg mL−1) was added to each well, and deionized water and physiological serum were applied as hemolysis positive and negative controls, respectively. The plate was incubated for 2 h at 37 °C and then centrifuged at 2000 rpm for 10 min. The supernatant of each well was transferred to the flat bottom plate and OD was quantified using an ELISA reader (Biohit, Finland) at 405 nm.35
MTT assay
The cytotoxicity of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite was measured by using BT549 cells (breast cancer cell line) and HEK293T cells (human embryonic kidney cell line) which were prepared by the Pasteur Institute of Iran, in comparison with cisplatin anti-cancer drug as a positive control (Sigma-Aldrich, MO, United States). Briefly the cells were cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Then, 5 × 103 cells per well were seeded in 96-well plates and serial dilutions (0.0156, 0.312, 0.625, 0.125, 0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75 mg mL−1) of nanobiocomposites were added to the wells and incubated for 48 h and 72 h. Culture medium alone served as a negative control. The cells were treated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma, USA) and incubated for further 4 h at 37 °C. 1% SDS was added to the wells and incubated for 16 h at 37 °C. Finally, optical densities were measured at 550 nm using a microplate reader spectrophotometer (BioTeK, USA).36 All tests were done in duplicate. EC50 values, the concentration of the substrate (here mg mL−1) at which 50% of maximum proliferation was achieved, were calculated using Prism software (v. 8.0).
Results and discussion
FT-IR analysis
As shown in the FT-IR spectra of GO in Fig. 2a, the broad peak in the range of 3200 cm−1 to 3600 cm−1 is related to the stretching vibration of the hydroxyl group in the GO.27 Also, stretching vibrations of carbonyl (1722 cm−1), aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1624 cm−1), carboxy C–O (1415 cm−1), epoxy C–O (1224 cm−1), and C–O (1051 cm−1) are observed.37 Observed peaks in the range of 2800–2950 cm−1 indicate the stretching vibration of C–H.38Fig. 2b shows the FT-IR spectrum of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. The stretching vibration of the pyranose ring, C–O–C, C–C, and O–C in the structure of CMC hydrogel is determined according to the peaks at 1041 cm−1 and 1157 cm−1 (ref. 19). The presence of carboxyl peaks in the area of 1446 cm−1 and 1660 cm−1 confirms the cross-linked structure of the cellulose with epichlorohydrin.19 According to the literature, FT-IR spectra of SF contain peaks in the areas of 1620–1650 cm−1, 1520–1540 cm−1, and 1200–1350 cm−1, which are related to type 1, 2, and 3 amides, respectively.39 The FT-IR spectra of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite demonstrated peaks in the areas of 1240 cm−1 and 1626 cm−1. So, the presence of SF is confirmed. Fe3O4 as a metal nanoparticle exhibits peaks in the region of 590 cm−1 due to the stretching vibration of Fe–O. The peak in 1734 cm−1 is related to the stretching vibration of carbonyl in the ester via the covalent bonding of CMC hydrogel and GO.
C (1624 cm−1), carboxy C–O (1415 cm−1), epoxy C–O (1224 cm−1), and C–O (1051 cm−1) are observed.37 Observed peaks in the range of 2800–2950 cm−1 indicate the stretching vibration of C–H.38Fig. 2b shows the FT-IR spectrum of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. The stretching vibration of the pyranose ring, C–O–C, C–C, and O–C in the structure of CMC hydrogel is determined according to the peaks at 1041 cm−1 and 1157 cm−1 (ref. 19). The presence of carboxyl peaks in the area of 1446 cm−1 and 1660 cm−1 confirms the cross-linked structure of the cellulose with epichlorohydrin.19 According to the literature, FT-IR spectra of SF contain peaks in the areas of 1620–1650 cm−1, 1520–1540 cm−1, and 1200–1350 cm−1, which are related to type 1, 2, and 3 amides, respectively.39 The FT-IR spectra of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite demonstrated peaks in the areas of 1240 cm−1 and 1626 cm−1. So, the presence of SF is confirmed. Fe3O4 as a metal nanoparticle exhibits peaks in the region of 590 cm−1 due to the stretching vibration of Fe–O. The peak in 1734 cm−1 is related to the stretching vibration of carbonyl in the ester via the covalent bonding of CMC hydrogel and GO.
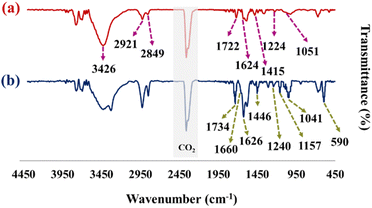 |
| | Fig. 2 FT-IR spectrum of GO (a) and the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite (b). | |
VSM analysis
VSM analysis has been used to evaluate the magnetic properties of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite (Fig. 3a). According to previous studies, if magnetic nanoparticles are placed in a composite structure, their value of saturation magnetization will be reduced.27,40 By comparing the saturation magnetization of pure Fe3O4 (which is about 75 emu g−1)27 and the nanobiocomposite (which is about 8.47 emu g−1), it can be concluded that magnetic nanoparticles are well placed in the structure of the composite.
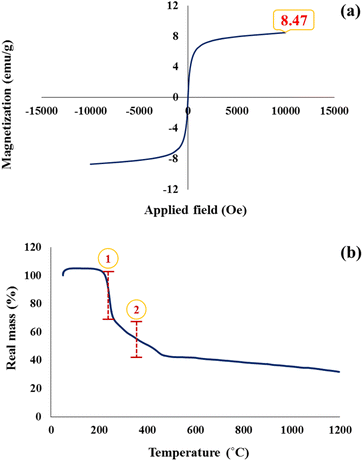 |
| | Fig. 3 Hysteresis loop curve (a) and TGA spectrum (b) of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. | |
TG analysis
Thermal analysis was performed to evaluate the thermal stability and thermogravimetric behavior of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite (Fig. 3b). According to the obtained results from the TGA, the synthesized structure is stable up to 200 °C and has no mass reduction. The first mass reduction is in the range of 200 to 300 °C, which is related to the pyrolysis of oxygenated groups (carboxyl, epoxide, and hydroxyl) in the structure of GO.41 The second decrease in mass is observed in the range of about 300 to 500 °C. This reduction in mass could be related to the degradation of the cellulose backbone in the CMC structure as well as the cleavage of peptides in SF.41,42 After 500 °C the mass of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite is reduced at a very low speed and eventually, about 30% of the mass remains at 1200 °C due to the inorganic parts.
FE-SEM imaging
FE-SEM imaging was performed to evaluate the morphology of the GO, GO–CMC hydrogel/SF and GO–CMC hydrogel/SF/Fe3O4. As shown in Fig. 4a, the GO sheets are completely visible and they were properly synthesized. Fig. 4b demonstrates a mixture of GO, CMC hydrogel and SF without a specific morphology. Eventually, by comparing the FE-SEM images of GO–CMC hydrogel/SF and GO–CMC hydrogel/SF/Fe3O4 in Fig. 4b and c, it is clear that Fe3O4 metal nanoparticles are dispersed in the other components of the nanobiocomposite.
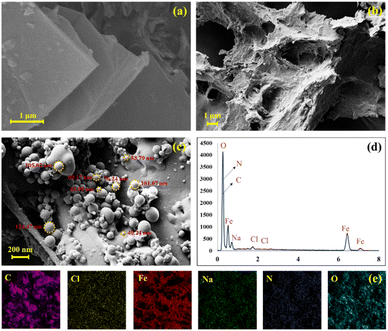 |
| | Fig. 4 FE-SEM images of GO (a), GO–CMC hydrogel/SF (b), and the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite (c). EDS analysis (d) and elemental mapping images (e) of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. | |
EDS analysis
EDS analysis was performed to evaluate the elements present in the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. As shown in Fig. 4c and d, N, O, Na, Cl and Fe are present in the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. Carbon and oxygen are related to GO, CMC hydrogel, and SF. Nitrogen represents the amino acid structure of SF. Also, sodium and chlorine indicate the presence of CMC hydrogel. Finally, oxygen and iron peaks are related to the Fe3O4 magnetic nanoparticles. Elemental mapping was performed on the final structure and, as expected, a good dispersion of the elements was observed (Fig. 4e).
RBCs lysis inhibition assay
The deionized water that was used as a positive control showed the highest rate of hemolysis, lysing almost all RBCs. Our nanobiocomposite-treated erythrocytes did not show significant differences in RBC hemolysis compared with physiological serum as a negative control. At some concentrations, this amount was even less than the negative control (Fig. 5). Therefore, the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite is fully compatible with blood.
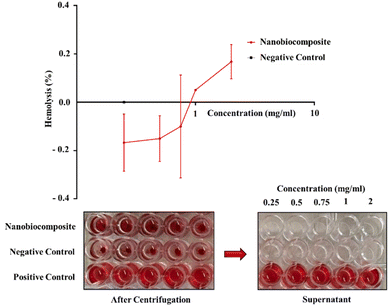 |
| | Fig. 5 Hemolysis line chart of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite, comes with a 96-well plate image. | |
Cell proliferation assay
The results showed that the viability percentage of HEK293T normal cells did not change significantly after 48 h and 72 h, and therefore the synthesized GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite is not toxic to this cell line. At the same time, the proliferation rate and viability percentage of BT549 cancer cells exposed to the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite decreased (Fig. 6a–d). Therefore, it can be said that this nanobiocomposite has anti-cancer properties against the breast cancer cell line. The survival rate of both cell lines after treatment with cisplatin (as a positive control) can also be seen in Fig. 6. EC50 values for HEK293T and BT549 cells after 48 h and 72 h also can be seen in Table 1.
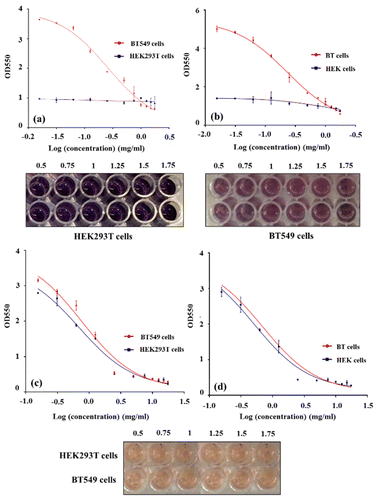 |
| | Fig. 6 This illustration shows the viability percentage of HEK293T and BT549 cells after treatment with the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite after 48 h (a) and 72 h (b), and the survival rate of HEK293T and BT549 cells after treatment with cisplatin (positive control) at days 2 (c) and 3 (d); comes with 96-well plate images (after 72 h treatment). | |
Table 1 EC50 values of HEK293T and BT549 cells after treatment with the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite after 48 h and 72 h
| EC50 of HEK293T normal cells |
| 48 h |
72 h |
| 0.1508 |
0.01466 |
| EC50 of BT549 cancer cells |
| 48 h |
72 h |
| 0.2567 |
0.1415 |
Evaluating the heating capacities of GO–CMC hydrogel/SF/Fe3O4 nanobiocomposites
Magnetic nanoparticles generate heat in the presence of an alternating magnetic field due to hysteresis losses and Néel and Brownian relaxations. This feature is of high importance in a therapeutic method called magnetic nanoparticle hyperthermia which is used to treat cancer by increasing the temperature of the tumor to the range of 41–45 °C for a predetermined period of time. There are several factors such as magnetic properties and morphology of the nanoparticles and strength and frequency of the magnetic field involved in the amount of the heat generated by magnetic nanoparticles. However, in experiments, power dissipation of the MNPs is measured by a parameter called the specific absorption rate (SAR) which is defined as eqn (1)| |  | (1) |
where m is the concentration of the MNPs in the carrier fluid (water in this study), C and T are the specific heat capacity and the temperature of the nanofluid, respectively, and t is time. In fact, higher values of SAR imply higher capability of the MNPs for increasing the temperature of the tumor.
Here, the heating efficiency of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite was investigated at various concentrations of 1, 2, and 5 mg mL−1 under different magnetic field frequencies of 100, 200, 300, and 400 kHz. All the experiments were performed for 10 minutes and the temperature of the samples was checked every 5 minutes. Values of SAR in this study varied from 5.6 W g−1 to 67.7 W g−1 which was observed at the lowest concentration (1 mg mL−1) and at the highest frequency (400 kHz). Fig. 7a shows a decrease of SAR with concentration under all magnetic field frequencies which is attributed to the dipole–dipole interaction of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. However, as shown in Fig. 7b, increasing the concentration of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite in the nanofluid from 1 to 2 mg mL−1 led to the highest changes in the SAR by up to 68%, while SAR decreased by up to 46% when the concentration increased from 2 to 5 mg mL−1. Moreover, although no clear pattern was observed between SAR and the frequency of the magnetic field, all the samples showed the highest values of SAR under the highest value of frequency (400 kHz) (Fig. 7c). In addition to this, from Fig. 7d, it is clear that lower concentrations of the nanofluid responded more considerably to the changes in the frequency of the magnetic field especially when increasing it from 300 to 400 kHz. All these results can be used to determine the appropriate concentration of the sample and the frequency of the magnetic field based on the desired temperature and the location of the tumor. For example, it is interesting to note that despite the need for increasing the temperature of the tumor for destroying it, higher temperatures are not always preferable as there is the risk of damaging the surrounding healthy tissue. In addition to this, choosing the right magnitude of the frequency is of utmost importance since killing deep-seated tumors needs lower frequencies with longer wavelengths while higher frequencies with shorter wavelengths are more suitable for the treatment of superficial tumors.
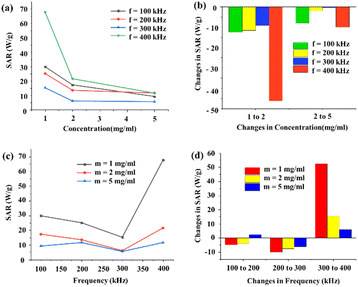 |
| | Fig. 7 Concentration dependence of SAR at frequencies of 100, 200, 300, and 400 kHz (a). Changes in SAR versus changes in the concentration of the sample at frequencies of 100, 200, 300, and 400 kHz (b). Frequency dependence of SAR at the concentrations of 1, 2, and 5 mg mL−1 (c). Changes in SAR versus changes in the frequency of the magnetic field at the concentrations of 1, 2, and 5 mg mL−1 (d). | |
Conclusions
A GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite was synthesized as a biocompatible and hemocompatible candidate for hyperthermia applications. To synthesize the nanobiocomposite, the CMC hydrogel and the GO are covalently bonded together. Then, SF and Fe3O4 are added to the GO–CMC hydrogel scaffold to form a biocompatible structure. This structure with a saturation magnetization of 8.47 emu g−1, had a good ability for hyperthermia application and 1 mg mL−1 of nanobiocomposite at a frequency of 400 kHz had a SAR equal to 67.7 W g−1. According to the obtained results, the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite as an anticancer agent can destroy cancer cells (BT549 cells), while it does not harm healthy cells (HEK293T cells). As a result, components of this structure via the synergetic effect can destroy cancer cells directly as an anticancer material as well as through the hyperthermia process.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology (IUST).
Notes and references
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132–145 CrossRef CAS PubMed.
- D. Chen, H. Feng and J. Li, Chem. Rev., 2012, 112, 6027–6053 CrossRef CAS PubMed.
- B. Shen, W. Zhai, M. Tao, D. Lu and W. Zheng, Compos. Sci. Technol., 2013, 77, 87–94 CrossRef CAS.
- J. Liu, L. Cui and D. Losic, Acta Biomater., 2013, 9, 9243–9257 CrossRef CAS PubMed.
- M. Z. I. Nizami, S. Takashiba and Y. Nishina, Appl. Mater. Today, 2020, 19, 100576 CrossRef.
- C. Martin, A. Ruiz, S. Keshavan, G. Reina, D. Murera, Y. Nishina, B. Fadeel and A. Bianco, Adv. Funct. Mater., 2019, 29, 1901761 CrossRef.
- Y. Li, X. Liu, L. Tan, Z. Cui, X. Yang, Y. Zheng, K. W. Yeung, P. K. Chu and S. Wu, Adv. Funct. Mater., 2018, 28, 1800299 CrossRef.
- Z. Zang, X. Zeng, M. Wang, W. Hu, C. Liu and X. Tang, Sens. Actuators, B, 2017, 252, 1179–1186 CrossRef CAS.
-
L. Shang, Y. Qi, H. Lu, H. Pei, Y. Li, L. Qu, Z. Wu and W. Zhang, in Theranostic Bionanomaterials, Elsevier, 2019, pp. 165–185 Search PubMed.
- M. F. Akhtar, M. Hanif and N. M. Ranjha, Saudi Pharm. J., 2016, 24, 554–559 CrossRef PubMed.
- F. Ullah, M. B. H. Othman, F. Javed, Z. Ahmad and H. M. Akil, Mater. Sci. Eng., C, 2015, 57, 414–433 CrossRef CAS PubMed.
- C. Chang and L. Zhang, Carbohydr. Polym., 2011, 84, 40–53 CrossRef CAS.
- S. H. Zainal, N. H. Mohd, N. Suhaili, F. H. Anuar, A. M. Lazim and R. Othaman, J. Mater. Res. Technol., 2021, 10, 935–952 CrossRef CAS.
- H. Shin, B. D. Olsen and A. Khademhosseini, Biomaterials, 2012, 33, 3143–3152 CrossRef CAS PubMed.
- C. B. Hutson, J. W. Nichol, H. Aubin, H. Bae, S. Yamanlar, S. Al-Haque, S. T. Koshy and A. Khademhosseini, Tissue Eng., Part A, 2011, 17, 1713–1723 CrossRef CAS PubMed.
- P. Hassanzadeh, M. Kazemzadeh-Narbat, R. Rosenzweig, X. Zhang, A. Khademhosseini, N. Annabi and M. Rolandi, J. Mater. Chem. B, 2016, 4, 2539–2543 RSC.
- S. R. Shin, B. Aghaei-Ghareh-Bolagh, T. T. Dang, S. N. Topkaya, X. Gao, S. Y. Yang, S. M. Jung, J. H. Oh, M. R. Dokmeci, X. Tang and A. Khademhosseini, Adv. Mater., 2013, 25, 6385–6391 CrossRef CAS PubMed.
- M. Eslami, N. E. Vrana, P. Zorlutuna, S. Sant, S. Jung, N. Masoumi, R. A. Khavari-Nejad, G. Javadi and A. Khademhosseini, J. Biomater. Appl., 2014, 29, 399–410 CrossRef CAS PubMed.
- R. Eivazzadeh-Keihan, R. Taheri-Ledari, M. S. Mehrabad, S. Dalvand, H. Sohrabi, A. Maleki, S. M. Mousavi-Khoshdel and A. E. Shalan, Energy Fuels, 2021, 35, 10869–10877 CrossRef CAS.
- T. P. Nguyen, Q. V. Nguyen, V.-H. Nguyen, T. H. Le, V. Q. N. Huynh, D. V. N. Vo, Q. T. Trinh, S. Y. Kim and Q. V. Le, Polymers, 2019, 11, 1933 CrossRef CAS PubMed.
- B. Kundu, R. Rajkhowa, S. C. Kundu and X. Wang, Adv. Drug Delivery Rev., 2013, 65, 457–470 CrossRef CAS PubMed.
- F. Mottaghitalab, M. Farokhi, M. A. Shokrgozar, F. Atyabi and H. Hosseinkhani, J. Controlled Release, 2015, 206, 161–176 CrossRef CAS PubMed.
- M. Farokhi, F. Mottaghitalab, Y. Fatahi, A. Khademhosseini and D. Kaplan, Trends Biotechnol., 2018, 36, 907–922 CrossRef CAS PubMed.
- M. Rasoulzadeh and H. Namazi, Carbohydr. Polym., 2017, 168, 320–326 CrossRef CAS PubMed.
- Z. Jiao, B. Zhang, C. Li, W. Kuang, J. Zhang, Y. Xiong, S. Tan, X. Cai and L. Huang, Nanotechnol. Rev., 2018, 7, 291–301 CrossRef CAS.
- N. S. Capanema, I. C. Carvalho, A. A. Mansur, S. M. Carvalho, A. P. Lage and H. Mansur, ACS Appl. Nano Mater., 2019, 2, 7393–7408 CrossRef CAS.
- R. Eivazzadeh-Keihan, S. Asgharnasl, H. A. M. Aliabadi, B. Tahmasebi, F. Radinekiyan, A. Maleki, H. Bahreinizad, M. Mahdavi, M. S. Alavijeh, R. Saber and S. Lanceros-Méndez, RSC Adv., 2022, 12, 3593–3601 RSC.
- A. Dahaghin, S. Emadiyanrazavi, M. Haghpanahi, M. Salimibani, H. Bahreinizad, R. Eivazzadeh-Keihan and A. Maleki, J. Drug Delivery Sci. Technol., 2021, 63, 102542 CrossRef CAS.
- A. Dahaghin, S. Emadiyanrazavi, M. Salimibani, H. Bahreinizad, M. Haghpanahi, R. Eivazzadeh-Keihan and A. Maleki, Probl. Biocybern. Biomed. Eng., 2021, 41, 516–526 CrossRef.
- A. Manohar, C. Krishnamoorthi, K. Naidu and C. Pavithra, Appl. Phys. A, 2019, 125, 1–10 CrossRef.
- A. Manohar, V. Vijayakanth and R. Hong, J. Mater. Sci.: Mater. Electron., 2020, 31, 799–806 CrossRef CAS.
- D. H. Kim, D. E. Nikles, D. T. Johnson and C. S. Brazel, J. Magn. Magn. Mater., 2008, 320, 2390–2396 CrossRef CAS.
- S. M. Fotukian, A. Barati, M. Soleymani and A. M. Alizadeh, J. Alloys Compd., 2020, 816, 152548 CrossRef CAS.
- S. R. Patade, D. D. Andhare, S. B. Somvanshi, S. A. Jadhav, M. V. Khedkar and K. Jadhav, Ceram. Int., 2020, 46, 25576–25583 CrossRef CAS.
- S. Komijani, E. Bayat, E. Rismani, S. Hosseini, R. Moazzami, L. Nematollahi, S. Sardari, Y. Talebkhan, F. Davami, F. Barkhordari and F. Hosseini, Sci. Rep., 2021, 11, 1–14 CrossRef PubMed.
- F. Y. Nikravesh, S. Shirkhani, E. Bayat, Y. Talebkhan, E. Mirabzadeh, M. Sabzalinejad, H. A. M. Aliabadi, L. Nematollahi, Y. H. Ardakani and S. Sardari, Sci. Rep., 2022, 12, 1–13 CrossRef PubMed.
- A. Gholampour, M. Valizadeh Kiamahalleh, D. N. Tran, T. Ozbakkaloglu and D. Losic, ACS Appl. Mater. Interfaces, 2017, 9, 43275–43286 CrossRef CAS PubMed.
- N. Kumar, S. Das, C. Bernhard and G. Varma, Supercond. Sci. Technol., 2013, 26, 095008 CrossRef.
- R. Yadav and R. Purwar, J. Mater. Sci.: Mater. Electron., 2020, 31, 17784–17797 CrossRef.
- R. Eivazzadeh-Keihan, M. Ghafori Gorab, H. Aghamirza Moghim Aliabadi, A. R. Akbarzadeh, A. Maleki and H. Ghafuri, Sci. Rep., 2021, 11, 1–13 CrossRef PubMed.
- R. Eivazzadeh-Keihan, F. Radinekiyan, H. Madanchi, H. A. M. Aliabadi and A. Maleki, Carbohydr. Polym., 2020, 248, 116802 CrossRef CAS PubMed.
- T. Mohamood, N. Fattima'Al-Zahara, A. H. Abdul Halim and N. Zainuddin, Polymers, 2021, 13, 4056 CrossRef PubMed.
|
| This journal is © The Royal Society of Chemistry 2023 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article b,
Amir
Kashtiaray
a,
Mohammad
Mahdavi
b,
Amir
Kashtiaray
a,
Mohammad
Mahdavi
 c,
Milad Salimi
Bani
d,
Andisheh
Etminan
e,
Nabi
Salehpour
f,
Reza
Eivazzadeh-Keihan
*a and
Ali
Maleki
c,
Milad Salimi
Bani
d,
Andisheh
Etminan
e,
Nabi
Salehpour
f,
Reza
Eivazzadeh-Keihan
*a and
Ali
Maleki
 *a
*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 daltons dialysis tube cellulose membrane within 3 days at room temperature. Finally, the synthesized SF was stored at 4 °C.
000 daltons dialysis tube cellulose membrane within 3 days at room temperature. Finally, the synthesized SF was stored at 4 °C.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. 100 microliters of the prepared solution were transferred to a 96-well microplate with a v-shaped bottom (Citotest, China). 100 μL of dispersed nanobiocomposite in physiological serum with different concentrations (0.25, 0.5, 0.75, 1 and 2 mg mL−1) was added to each well, and deionized water and physiological serum were applied as hemolysis positive and negative controls, respectively. The plate was incubated for 2 h at 37 °C and then centrifuged at 2000 rpm for 10 min. The supernatant of each well was transferred to the flat bottom plate and OD was quantified using an ELISA reader (Biohit, Finland) at 405 nm.35
100. 100 microliters of the prepared solution were transferred to a 96-well microplate with a v-shaped bottom (Citotest, China). 100 μL of dispersed nanobiocomposite in physiological serum with different concentrations (0.25, 0.5, 0.75, 1 and 2 mg mL−1) was added to each well, and deionized water and physiological serum were applied as hemolysis positive and negative controls, respectively. The plate was incubated for 2 h at 37 °C and then centrifuged at 2000 rpm for 10 min. The supernatant of each well was transferred to the flat bottom plate and OD was quantified using an ELISA reader (Biohit, Finland) at 405 nm.35
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1624 cm−1), carboxy C–O (1415 cm−1), epoxy C–O (1224 cm−1), and C–O (1051 cm−1) are observed.37 Observed peaks in the range of 2800–2950 cm−1 indicate the stretching vibration of C–H.38Fig. 2b shows the FT-IR spectrum of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. The stretching vibration of the pyranose ring, C–O–C, C–C, and O–C in the structure of CMC hydrogel is determined according to the peaks at 1041 cm−1 and 1157 cm−1 (ref. 19). The presence of carboxyl peaks in the area of 1446 cm−1 and 1660 cm−1 confirms the cross-linked structure of the cellulose with epichlorohydrin.19 According to the literature, FT-IR spectra of SF contain peaks in the areas of 1620–1650 cm−1, 1520–1540 cm−1, and 1200–1350 cm−1, which are related to type 1, 2, and 3 amides, respectively.39 The FT-IR spectra of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite demonstrated peaks in the areas of 1240 cm−1 and 1626 cm−1. So, the presence of SF is confirmed. Fe3O4 as a metal nanoparticle exhibits peaks in the region of 590 cm−1 due to the stretching vibration of Fe–O. The peak in 1734 cm−1 is related to the stretching vibration of carbonyl in the ester via the covalent bonding of CMC hydrogel and GO.
C (1624 cm−1), carboxy C–O (1415 cm−1), epoxy C–O (1224 cm−1), and C–O (1051 cm−1) are observed.37 Observed peaks in the range of 2800–2950 cm−1 indicate the stretching vibration of C–H.38Fig. 2b shows the FT-IR spectrum of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite. The stretching vibration of the pyranose ring, C–O–C, C–C, and O–C in the structure of CMC hydrogel is determined according to the peaks at 1041 cm−1 and 1157 cm−1 (ref. 19). The presence of carboxyl peaks in the area of 1446 cm−1 and 1660 cm−1 confirms the cross-linked structure of the cellulose with epichlorohydrin.19 According to the literature, FT-IR spectra of SF contain peaks in the areas of 1620–1650 cm−1, 1520–1540 cm−1, and 1200–1350 cm−1, which are related to type 1, 2, and 3 amides, respectively.39 The FT-IR spectra of the GO–CMC hydrogel/SF/Fe3O4 nanobiocomposite demonstrated peaks in the areas of 1240 cm−1 and 1626 cm−1. So, the presence of SF is confirmed. Fe3O4 as a metal nanoparticle exhibits peaks in the region of 590 cm−1 due to the stretching vibration of Fe–O. The peak in 1734 cm−1 is related to the stretching vibration of carbonyl in the ester via the covalent bonding of CMC hydrogel and GO.







