Open Access Article
Open Access ArticleOrigin of the switchable photocurrent direction in BiFeO3 thin films†
Yaqiong
Wang
abc,
Matyas
Daboczi
 de,
Man
Zhang
b,
Joe
Briscoe
de,
Man
Zhang
b,
Joe
Briscoe
 b,
Ji-Seon
Kim
b,
Ji-Seon
Kim
 e,
Haixue
Yan
e,
Haixue
Yan
 *b and
Steve
Dunn
*c
*b and
Steve
Dunn
*c
aInstitute of Medical Engineering, Department of Biophysics, School of Basic Medical Sciences, Health Science Centre, Xi’an Jiaotong University, Xi’an, 710061, China
bSchool of Engineering and Materials Science, Queen Mary University of London, Mile End Road, London, E1 4NS, UK. E-mail: h.x.yan@qmul.ac.uk
cSchool of Engineering, London South Bank University, 103 Borough Road, London, SE1 0AA, UK. E-mail: dunns4@lsbu.ac.uk
dDepartment of Chemical Engineering and Centre for Processable Electronics, Imperial College London, South Kensington, London, SW7 2AZ, UK
eDepartment of Physics and Centre for Processable Electronics, Imperial College London, South Kensington, London, SW7 2AZ, UK
First published on 12th October 2023
Abstract
We report external bias driven switchable photocurrent (anodic and cathodic) in 2.3 eV indirect band gap perovskite (BiFeO3) photoactive thin films. Depending on the applied bias our BiFeO3 films exhibit photocurrents more usually found in p- or n-type semiconductor photoelectrodes. In order to understand the anomalous behaviour ambient photoemission spectroscopy and Kelvin-probe techniques have been used to determine the band structure of the BiFeO3. We found that the Fermi level (Ef) is at −4.96 eV (vs. vacuum) with a mid-gap at −4.93 eV (vs. vacuum). Our photochemically determined flat band potential (Efb) was found to be 0.3 V vs. NHE (−4.8 V vs. vacuum). These band positions indicate that Ef is close to mid-gap, and Efb is close to the equilibrium with the electrolyte enabling either cathodic or anodic band bending. We show an ability to control switching from n- to p-type behaviour through the application of external bias to the BiFeO3 thin film. This ability to control majority carrier dynamics at low applied bias opens a number of applications in novel optoelectronic switches, logic and energy conversion devices.
New conceptsIn this work, we produce a film of BiFeO3 that demonstrates switchable photocurrents which enable visible light driven anodic and cathodic photochemistry at the functional film and solution interface. Most reports on switchable photocurrent behaviour are associated with production of multi-material systems and it is very rare for simple metal oxide photoelectrodes to show both p- and n-type photocurrent behaviour. By investigating the photochemical and physical properties of the as-obtained BiFeO3 thin film, we show that it is the band structure and alignment which is responsible for the switchable photocurrents. Our key finding is that BiFeO3 can be used as a pro-forma system to predict ambipolar behaviour in other materials. This leads to making a contribution to a wide range of applications such as novel optoelectronic switches, optical logic, and energy conversion. |
1. Introduction
Switchable photocurrent polarity, such as cathodic to anodic at different applied external bias, is an important subject for the development of advanced optoelectronic systems, logic devices and selective energy conversion approaches.1–4 This property can be found in molecular, hybrid, and inorganic nanoparticle material systems2,3,5,6 and is quite an unusual phenomenon in single phase metal oxide materials. Demonstrations that molecular photodiodes can switch the direction of a photocurrent under different irradiating light wavelengths3 give an indication as to the importance of materials that exhibit both p and n type properties. For example, composites of titania nanosheets with polyaniline layers exhibited an n- to p-type switchable photoelectrode behaviour through a range of applied potentials.5 Similar behaviour was reported in a thin film photoelectrode that consisted of a blend containing n-type Fe2O3 and p-type CuFeO2. In this case the metal oxides were separated into microdomains and demonstrated tunable photo responses under varying applied bias.2To-date, most reports on bipolar photocurrent behaviour are associated with production of multi-material systems. It is very rare for simple metal oxide photoelectrodes to show both p- and n-type photocurrent behaviour. Typical semiconductor photoanodes such those reported in the studies of TiO2,7 BiVO4,8 Fe2O3,9etc., or representative photocathodes of Cu-based compounds such as CuBi2O410 and CuFeO211 act as an electrochemical diode allowing only one direction of current to flow. Photoelectrochemical (PEC) study has been long used to investigate the photodiode behaviour of a semiconductor. The derived band bending theory well explained the photoanodic behaviour of n-type semiconductor and photocathode behaviour of p-type semiconductor. Normally, no cathodic photocurrents are found in an n-type semiconductor as for a typical n-type semiconductor, the position of Fermi level is close to the conduction band with an energy difference of about ∼ 0.1 to 0.2 eV.12,13 At potentials negative relative to flat band potential there will be excess of majority charge carriers injected to the space charge region. This leads to an accumulation region forming instead of the depletion region. A ‘degenerate surface’ forms and the semiconductor electrode will behave like a metal electrode with significant carrier transfer in the dark or under illumination and there is no effective photocurrent generated.12 The inverse but analogous situation occurs in a p-type semiconductor. A very limited number of observations on bipolar photo response from simple metal oxide such as Fe2O314 and TiO215 have been reported previously. Cathodic photocurrents generation in these photoanode materials was either not explained14 or attributed to extrinsic contributions such as the co-existence of alkaline electrolyte and the dissolved oxygen in it, which acts as an electron accepter allowing electron transfer from the photoelectrode surface.15
A single-phase semiconductor material exhibiting intrinsic switchable photocurrents is of additional interest compared to conventional semiconductor photoelectrodes, especially in the application of energy conversion, e.g., via the selective PEC oxidation or reduction of water into solar fuels. The realization of practical PEC-based switching devices requires the semiconductor material having suitable band positions, e.g., the conduction band minimum and the valence band maximum straddle the water redox potentials. Meanwhile a material with a narrow band gap which allows visible light absorption is preferable. Other aspects, such as ease of fabrication using earth abundant materials is also important.
BiFeO3 has been extensively studied as it demonstrates room-temperature multiferroic, ferroelectric and magnetic properties and the material is well understood in terms of its physical and chemical properties.16–18 More recently there has been growing interest in ferroelectric materials as photocatalysts where the spontaneous dipole leads to enhanced carrier separation19–22 with associated increased chemical reaction rates. BiFeO3 has been extensively studied as it demonstrates room-temperature multiferroic, ferroelectric and magnetic, properties and the material is well understood in terms of its physical and chemical properties.16–18 More recently there has been growing interest in ferroelectric materials as photocatalysts where the spontaneous dipole leads to enhanced carrier separation19–22 with associated increased chemical reaction rates. BiFeO3 has been shown to demonstrate an anomalous photovoltage in photovoltaic devices, where the developed photovoltage exceeds the value of the band gap.23 Strained BiFeO3 films also exhibit a lot of special physical properties such as achieving of abnormal photocurrent through domain wall-defect interaction,24 short-circuit photocurrent reversing by the polarization switch25 and light-induced reversible local ferroelectric polarization.26 The impact of the inherent electric field of a ferroelectric sample associated with the spontaneous polarization has been demonstrated to influence the photochemical behavior of BiFeO3 in a number of previous studies.27,28,29 Common to most of these is the poling of a ferroelectric BiFeO3 sample to produce samples with a homogeneous surface domain structure. This previous work has observed that photocurrents changed direction depending on the state of the dipole associated with the poling of the BiFeO3 sample. The photocurrent effectively changed from positive to negative depending on the poling of the surface. This result was attributed to the ferroelectric effect and the generation of a depletion region caused by internal screening within the ferroelectric sample. Such behavior has been observed in a variety of ferroelectric samples where the dipole associated with the polarization of the ferroelectric can drive carrier separation.22,30
Herein, we have prepared visible light (Eg ∼ 2.3 eV) active ferroelectric BiFeO3 thin film photoelectrodes that exhibit switchable photocurrents around the flat band potential of −4.8 V vs. vacuum through a simple sol–gel method. The BiFeO3 thin film is un-poled with domain polarization direction randomly distributed. Thus ferroelectric effect on the photocurrents direction is limited with a summative compromise among different domains. Origin of the switchable photocurrents has been investigated by PEC methods, well supported by the band structure characterisation results. The specific band structure of the as-prepared BiFeO3 thin film is considered to be the reason of the switchable photocurrents, with a Fermi level that sits near the mid-gap position and both upward and downward band bending allowed under certain applied bias.
2. Results and discussion
Fig. 1a shows the X-ray diffraction (XRD) pattern of the thin film indicating the as-prepared film exhibits high phase purity. The surface morphology of the BiFeO3 electrode, as investigated by scanning electron microscopy (SEM), is shown in Fig. 1b. The micrographs show that the grains range from 50–200 nm in size forming a homogeneous and compact layer on the substrate. The ferroelectric nature of the film was demonstrated by piezo-electric force microscopy (PFM), shown in Fig. S1 (ESI†). The phase image in the PFM indicates that the BiFeO3 film is polycrystalline with different domains randomly oriented with no preferred polarization direction. Ferroelectric switching had been observed using PFM characterization, as shown in Fig. S2 (ESI†), while no switching observed in a further D–E and I–E measurement (Fig. S3, ESI†). The results indicate that the as-prepared BiFeO3 film can be polarized locally, though it is leaking in bulk scale. Our XPS results show that oxygen vacancies existed in the as-prepared BiFeO3 thin films, as well as the co-existence of Fe3+ and Fe2+ (Fig. S4, ESI†). It is common for BiFeO3 films to have oxygen vacancies and Fe2+, which leading to its leaking nature.31–33 | ||
| Fig. 1 (a) XRD characterization of the BiFeO3 thin film. (b) SEM image of the BiFeO3 thin film. (c) UV-vis absorption spectrum of the BiFeO3 thin film (d) Derived Tauc plots. | ||
The UV-vis absorption spectrum of the BiFeO3 thin film is shown in Fig. 1c. The absorbance goes through a slow increase from a wavelength of ∼540 nm followed by a sharp increase around 460 nm. The linear regions in the derived Tauc plots (Fig. 1d) indicate that there are indirect (2.3 eV) and direct band gaps (2.7 eV). BiFeO3 can be viewed as a charge-transfer insulator, with the bandgap controlled by the orbital overlap between the O 2p and the Fe 3d levels.16 According to previous studies the valence band of BiFeO3 has been considered to be flat.34 Whereas, the conduction band minimum can vary due to momentum shifts induced by hybridization between the orbital states and the valence state of Fe. This hybridization is dependent on oxygen vacancies and stoichiometry.34,35 This has led to a wide range of band gaps, from 2.1 eV to 2.8 eV being reported for BiFeO3 thin films. Both indirect/direct band gap characteristics have also been suggested.34–37 R. Palai et. al have shown that at room temperature (RT) BiFeO3 has an indirect bandgap with a direct gap that lies ∼0.05 eV above it. In that study the gap becomes more direct with increasing T.35 The existing body of literature around BiFeO3 indicates that there exist both direct and indirect photoexcitation of electrons at room temperature in BiFeO3. However, the indirect charge transfer mechanism is accepted to dominate at low room temperature. Our measured values of Eg for both the indirect and direct transition are consistent with current understanding. Therefore, the observed indirect optical bandgap of 2.3 eV will be applied for our discussion regarding the influence of band gap on the photochemical performance of the thin films.
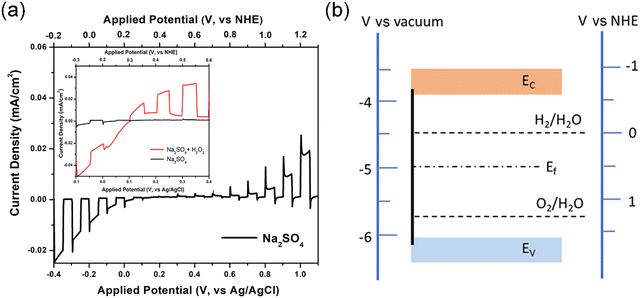
We present current density vs. applied potential plots under chopped simulated solar (AM 1.5) light for BiFeO3 electrode under Na2SO4 and Na2SO4–H2O2 electrolytes in Fig. 2a. H2O2 is well known as a good hole scavenger presenting a higher rate constant for oxidation compared to water. It also has a relatively negative reduction potential (E0 = +0.68 V vs. NHE for the O2/H2O2 couple) cf. water (E0 = +1.23 V vs. NHE for the O2/H2O couple).38 By increasing the rate of collecting available photoinduced holes at the electrode/electrolyte interface the H2O2 containing electrolyte gives a more accurate insight into the on-set potential for photocurrents. This value can be influenced due to the slow carrier transfer kinetics in an aqueous electrolyte solution and causes a shift to be reported. This shift in onset is apparent in our results when the H2O2 containing sample is compared against the sample without H2O2, Fig. 2a (inset). The on-set potential for both the anodic and cathodic photocurrents can be seen clearly from the plot containing H2O2 at around 0.3 V (vs. NHE). The on-set potential of a photocurrent is related to the flat band potential of the photoelectrode and indicates the potential at which band bending will start. An accurate electrochemical determination of flat band potential will place it at the same point as the Fermi level determined via photophysical techniques.
APS and Kelvin-probe measurements were performed to determine the energy levels of the as-prepared BiFeO3 films. The valence band maximum was measured through photoemission procedure. The sample was illuminated with monochromatic UV light and the cube root curve of the photoemission obtained and plotted. The valence band maximum (VBM) was determined by APS to be at −6.08 eV, as shown in Fig. S5 (ESI†). Using the indirect band gap value of 2.3 eV, obtained from Tauc analysis, the conduction band minimum was calculated at −3.78 eV. We have measured Ef to be at −4.96 eV (v's vacuum) (shown in Fig. S6, ESI†) and the Eg mid-point at −4.93 eV (v's vacuum). The NHE is regarded to sit at −4.5 eV (v's vacuum) leading to a flat band position of ∼ −4.8 eV (v's vacuum). A schematic band diagram for the BiFeO3 tested is shown in Fig. 2b. The electrochemically measured flat band potential and photophyscially measured value of Ef are within differences allowable by altered pH at the interface. The conduction band and valence band positions straddle the water redox levels.
It is possible to consider BiFeO3 as a simple semi-conductor if neglects the influence of the ferroelectric nature on the photocurrent produced by BiFeO3. In the thin films presented here each domain exposed to the electrolyte will have an inherent dipole associated with the spontaneous polarization (Fig. S7a and b, ESI†). The interface between the BiFeO3 and electrolyte further influences the band bending due to the movement of carriers across the barrier (Fig. S7c–e, ESI†). When the electrode comes into contact with the electrolyte the bands will align so that Ef at the electrode electrolyte interface is the same as the redox potential of the electrolyte (at 0.615 V v's NHE). In our case this results in an upward band bending of 0.095 eV. This band bending is common across all domains and is summative. In the case of regions of the BiFeO3 associated with C− domains the band bending will be increased (Fig. S7c, ESI†). For C+ domains there will be decreased band bending (Fig. S7e, ESI†). For domains with direction parallel to the interface, the spontaneous polarization does not contribute to the interface band bending, only the 0.095 eV upward band bending happens (Fig. S7d, ESI†). In a summary, the C+ domain favors holes transferring out while the C− domain favors electrons transferring out and they in total give a limited effect on the photocurrents due to the summative compromise among different domains.
The BiFeO3 photoelectrodes investigated here exhibited cathodic and anodic photocurrents at −ve or +ve applied potentials v's the Efb. This observation is indicative of a material that develops depletion regions associated with p- and n-type semiconductors under the appropriate applied potentials. In our sample we measure the Ef very close to the middle of the band gap (with only 30 meV difference). In a typical n-type semiconductor, the donor concentration dominates and Ef lies close to the conduction band, typically within 0.5 eV.13 The opposite is found for a p-type semiconductor where the acceptor dominates behavior. In a compensated semiconductor such as BiFeO3 the position of Ef will vary depending on the concentration of the cation vacancy (Bi or Fe vacancy) acting as an acceptor and the oxygen vacancy acting as a donor. BiFeO3 is well known for its complexity arising from the ease of defect formation.39–43 P-type conductivity is usually observed under normal atmospheric processing conditions with Bi and Fe vacancies dominating, and n-type conductivity is usually observed under oxygen-poor processing conditions with oxygen vacancy dominating.39,40,44 The BiFeO3 here was prepared by annealing in air and has resulted in Ef and Eg midpoint aligning very closely. This means our samples are self-compensated for defects and exhibit neither strong n- nor p-type characteristics.
The characteristics of band structure, location of Ef and Eg mid-point mean that the BiFeO3 photoelectrode produced here can present both upward and downward band bending at the interface with the electrolyte. Under potentials positive to that of the flat band potential Ef shifts more positively and the upward band bending increases (Fig. 3(iii)). As a result, the depletion region increases and photoexcited charge carriers are separated more efficiently with increased photocurrents being achieved. Under potentials negative to that of the flat potential a depletion region can also be formed following a downward band bending (Fig. 3(iv)). This means that these samples are able to produce both anodic and cathodic currents under the appropriate external bias.
By performing a full investigation of the band structure, in relation not only to the semiconductor but also ferroelectric properties of BiFeO3 photoelectrode films we can explain the observed ambipolar photoresponse of our material. This response comes from a combination of the ferroelectric nature of BiFeO3 and the band structure. However, it seems unlikely that the polar nature of the material has a strong influence on the ambipolar photocurrents. The sample presents randomly oriented domains at the interface between ferroelectric and electrolyte and these findings demonstrate that the ambipolar photocurrents in BiFeO3 photoelectrode are not necessarily controlled by the ferroelectric polarization of the sample. These findings can be used to inform the design of semiconductors with suitable doping levels to achieve a range of tailored photocurrents to meet the requirements of photovoltaic devices and photoelectrochemical cells.
Conclusions
In this study, a switchable photocurrent in BiFeO3 photoelectrode has been studied. The origin of the ambipolar behaviour has been investigated and explained from the perspective of the energy band levels. The specific bandgap structure of the BiFeO3 photoelectrode provides it with the advantage of generating either anodic or cathodic photocurrents as a function of applied bias. Since the conduction band minimum and valence band maximum of BiFeO3 straddle the water redox potential, it is possible to promote either the hydrogen or the oxygen evolution at one electrode just by tuning the applied potentials and thus allow for selective PEC oxidation or reduction, arousing particular interest for its future application in solar energy conversion. Furthermore, this finding can be adapted to understand and predict other semiconductor photoelectrodes with analogous ambipolar behaviour. It also gives a hint of the way to prepare switchable photoelectrode materials in the future, especially for the compensated semiconductors.Author contributions
Y. Wang: conceptualization, data curation, formal Analysis, investigation, methodology, validation, visualization, and writing – original draft; M. Daboczi: data curation, formal analysis, methodology, and writing – review & editing; M. Zhang: data curation, formal analysis, and writing – review & editing; J. Briscoe: data curation, formal analysis, and writing – review & editing; J. Kim: data curation, formal analysis, and writing – review & editing; H. Yan: conceptualization, formal analysis, investigation, methodology, supervision and writing – review & editing; S. Dunn: conceptualization, formal analysis, investigation, methodology, funding acquisition, supervision, and writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors would like to acknowledge the Chinese Scholarship Council for supporting this work. Financial supports from LSBU are acknowledged.References
- Y. L. Kim, H. Y. Jung, S. Park, B. Li, F. Liu, J. Hao, Y.-K. Kwon, Y. J. Jung and S. Kar, Nat. Photonics, 2014, 8, 239 CrossRef CAS.
- R. Beranek and H. Kisch, Angew. Chem., Int. Ed., 2008, 47, 1320–1322 CrossRef CAS PubMed.
- S. Yasutomi, T. Morita, Y. Imanishi and S. Kimura, Science, 2004, 304, 1944–1947 CrossRef CAS PubMed.
- K. Szaciłowski, W. Macyk and G. Stochel, J. Am. Chem. Soc., 2006, 128, 4550–4551 CrossRef.
- B. Seger, J. McCray, A. Mukherji, X. Zong, Z. Xing and L. Wang, Angew. Chem., Int. Ed., 2013, 52, 6400–6403 CrossRef CAS.
- A. Efrati, O. Yehezkeli, R. Tel-Vered, D. Michaeli, R. Nechushtai and I. Willner, ACS Nano, 2012, 6, 9258–9266 CrossRef CAS PubMed.
- S. Kment, F. Riboni, S. Pausova, L. Wang, L. Wang, H. Han, Z. Hubicka, J. Krysa, P. Schmuki and R. Zboril, Chem. Soc. Rev., 2017, 46, 3716–3769 RSC.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- B. Iandolo, B. Wickman, I. Zoric and A. Hellman, J. Mater. Chem. A, 2015, 3, 16896–16912 RSC.
- S. P. Berglund, F. F. Abdi, P. Bogdanoff, A. Chemseddine, D. Friedrich and R. van de Krol, Chem. Mater., 2016, 28, 4231–4242 CrossRef CAS.
- M. S. Prévot, N. Guijarro and K. Sivula, ChemSusChem, 2015, 8, 1359–1367 CrossRef PubMed.
- S. R. Morrison, Electrochemistry at semiconductor and oxidized metal electrodes, Plenum Press, New York and London, 1980 Search PubMed.
- D. A. Neamen, Semiconductor Physics and Devices: Basic Principles. 3rd edn, McGraw-Hill, New York, 2003 Search PubMed.
- M. P. Dare-Edwards, J. B. Goodenough, A. Hamnett and P. R. Trevellick, J. Chem. Soc., Faraday Trans. 1, 1983, 79, 2027–2041 RSC.
- A. Tsujiko, H. Itoh, T. Kisumi, A. Shiga, K. Murakoshi and Y. Nakato, J. Phys. Chem. B, 2002, 106, 5878–5885 CrossRef CAS.
- G. Catalan and J. F. Scott, Adv. Mater., 2009, 21, 2463–2485 CrossRef CAS.
- R. K. Vasudevan, Y.-C. Chen, H.-H. Tai, N. Balke, P. Wu, S. Bhattacharya, L. Q. Chen, Y.-H. Chu, I. N. Lin, S. V. Kalinin and V. Nagarajan, ACS Nano, 2011, 5, 879–887 CrossRef CAS PubMed.
- Q. Zhang, D. Sando and V. Nagarajan, J. Mater. Chem. C, 2016, 4, 4092–4124 RSC.
- Y. Cui, J. Briscoe and S. Dunn, Chem. Mater., 2013, 25, 4215–4223 CrossRef CAS.
- M. R. Morris, S. R. Pendlebury, J. Hong, S. Dunn and J. R. Durrant, Adv. Mater., 2016, 28, 7123–7128 CrossRef CAS PubMed.
- Y. Wang, M. Zhang, J. Liu, H. Zhang, F. Li, C.-W. Tseng, B. Yang, G. Smith, J. Zhai, Z. Zhang, S. Dunn and H. Yan, Adv. Energy Mater., 2020, 10, 2001802 CrossRef CAS.
- Y. Liu, S. Ye, H. Xie, J. Zhu, Q. Shi, N. Ta, R. Chen, Y. Gao, H. An, W. Nie, H. Jing, F. Fan and C. Li, Adv. Mater., 2020, 32, 1906513 CAS.
- T. Choi, S. Lee, Y. J. Choi, V. Kiryukhin and S.-W. Cheong, Science, 2009, 324, 63–66 CrossRef CAS PubMed.
- H.-B. Zhang, M.-M. Yang and M. Alexe, Adv. Photonics Res., 2023, 4, 2200189 CrossRef CAS.
- Y. Yang, W. Xu, X. Xu, Y. Wang, G. Yuan, Y. Wang and Z.-G. Liu, J. Appl. Phys., 2016, 119, 044102 CrossRef.
- M.-M. Yang and M. Alexe, Adv. Mater., 2018, 30, 1704908 CrossRef PubMed.
- Q. Liu, Y. Zhou, L. You, J. Wang, M. Shen and L. Fang, Appl. Phys. Lett., 2016, 108, 022902 Search PubMed.
- D. Cao, Z. Wang, N. Nasori, L. Wen, Y. Mi and Y. Lei, Angew. Chem., 2014, 126, 11207–11211 CrossRef.
- J. H. Shah, H. Ye, Y. Liu, A. M. Idris, A. S. Malik, Y. Zhang, H. Han and C. Li, J. Mater. Chem. A, 2020, 8, 6863–6873 RSC.
- G. Wan, L. Yin, X. Chen, X. Xu, J. Huang, C. Zhen, H. Zhu, B. Huang, W. Hu, Z. Ren, H. Tian, L. Wang, G. Liu and H. M. Cheng, J. Am. Chem. Soc., 2022, 144, 20342–20350 CAS.
- Y. Shuai, S. Zhou, S. Streit, H. Reuther, D. Bürger, S. Slesazeck, T. Mikolajick, M. Helm and H. Schmidt, Appl. Phys. Lett., 2011, 98, 232901 Search PubMed.
- Z. Zhang, P. Wu, L. Chen and J. L. Wang, Appl. Phys. Lett., 2010, 96 CAS.
- M. Makarovic, N. Kanas, A. Zorko, K. Ziberna, H. Ursic, D. R. Smabraten, S. M. Selbach and T. Rojac, J. Eur. Ceram. Soc., 2020, 40, 5483–5493 CAS.
- S. J. Clark and J. Robertson, Appl. Phys. Lett., 2007, 90, 132903 CrossRef.
- R. Palai, R. Katiyar, H. Schmid, P. Tissot, S. Clark, J. Robertson, S. Redfern, G. Catalan and J. Scott, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 014110 CrossRef.
- J. Ihlefeld, N. Podraza, Z. Liu, R. Rai, X. Xu, T. Heeg, Y. Chen, J. Li, R. Collins and J. Musfeldt, Appl. Phys. Lett., 2008, 92, 142908 Search PubMed.
- Y. Xu and M. Shen, Mater. Lett., 2008, 62, 3600–3602 CrossRef CAS.
- H. Dotan, K. Sivula, M. Gratzel, A. Rothschild and S. C. Warren, Energy Environ. Sci., 2011, 4, 958–964 CAS.
- Q. Xu, M. Sobhan, Q. Yang, F. Anariba, K. P. Ong and P. Wu, Dalton Trans., 2014, 43, 10787–10793 CAS.
- T. R. Paudel, S. S. Jaswal and E. Y. Tsymbal, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 104409 Search PubMed.
- M. Schrade, N. Masó, A. Perejón, L. A. Pérez-Maqueda and A. R. West, J. Mater. Chem. C, 2017, 5, 10077–10086 RSC.
- S. Clark and J. Robertson, Appl. Phys. Lett., 2009, 94, 022902 Search PubMed.
- X. Qi, J. Dho, R. Tomov, M. G. Blamire and J. L. MacManus-Driscoll, Appl. Phys. Lett., 2005, 86, 062903 CrossRef.
- Z. Zhang, P. Wu, L. Chen and J. Wang, Appl. Phys. Lett., 2010, 96, 232906 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01510f |
| This journal is © The Royal Society of Chemistry 2023 |