Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Crystal regeneration – a unique growth phenomenon observed in organic crystals post breakage†
Isha
Bade
 a,
Vivek
Verma
a,
Vivek
Verma
 a,
Ian
Rosbottom
a and
Jerry Y. Y.
Heng
a,
Ian
Rosbottom
a and
Jerry Y. Y.
Heng
 *ab
*ab
aDepartment of Chemical Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, UK. E-mail: jerry.heng@imperial.ac.uk; Web: https://twitter.com/HengGroup Web: https://twitter.com/ImperialChemEng
bInstitute for Molecular Science and Engineering, Department of Chemical Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, UK
First published on 3rd February 2023
Abstract
Crystal regeneration has been observed in macroscopic paracetamol crystals post breakage along their cleavage plane. High resolution imaging confirmed regeneration rates to be 3-fold faster than growth prior to breakage. Further analysis of the solute-solvent interactions is required to elucidate the process which currently lacks linearity with traditional growth theories.
New conceptsAlthough mechanical breakage is a common occurrence in industrial crystallisers, there exists limited knowledge on the consequent growth of broken crystals. Owing to this, specific post-breakage growth kinetics are rarely accounted for during process modelling, resulting in unreliable predictions of crystal shape and size. This work, with the aid of macro photography for single crystal imaging, presents an unconventional ‘regeneration’ phenomenon of a paracetamol crystal post-breakage along its cleavage plane. The cleaved crystal was observed to regenerate the broken fraction and attain its pre-breakage shape before exhibiting overall growth – analogous to the process of lizards regrowing their tails after autotomy. Despite the well-studied nature of paracetamol, this is the first article reporting this regeneration phenomenon which contradicts some of the long-standing thermodynamic theories of crystal morphology prediction. The crystal shape evolution elucidating from this regrowth process can improve downstream process efficiency and accurate process modelling attempts. This peculiar phenomenon comes at a time when ‘dynamic’ organic crystals are gaining increasing traction due to their unique behavioural responses to external stimuli, previously only reported in polymers. The growth kinetics of crystals as a result of the regeneration process therefore provides a new direction to crystal shape engineering. |
Crystallisation is a ubiquitous process resulting in the self-assembly of molecules into highly ordered three-dimensional structures. Crystal attributes are highly dependent on the crystallisation conditions, frequently resulting in unexpected morphologies, shapes, and sizes which has resulted in continued quests by scientists to understand the nature and properties of crystals. The emerging field of crystal adaptronics views organic crystals as ‘dynamic’ entities and highlights their unconventional response to external stimuli such as heat, light, mechanical forces etc. via transitions in their molecular packing, predominantly seen in hard materials like polymers and elastomers in the past.1,2 In the pharmaceutical industry, unique behavioral response of crystals to external mechanical forces is particularly fascinating as it can explicate their mechanical properties and hence aid improve their performance not only in the crystalliser but also in downstream processes such as milling and tableting.3
A plethora of studies have been carried out on intrinsic ‘self-healing’ molecular crystals where entities have demonstrated autonomous repair of cracks and defects in the crystalline lattice induced via localised pressure.2,4–6 The dynamic bonding in these crystals allow bond reformation even after plastic deformation hence broadening the scope of their mechanical properties.4,5,7 Moreover, elongated organic crystals have shown elastic deformation, ‘bending’, upon application of point pressure, mainly attributed to the activation of slip planes and synthonic interactions within the crystalline lattice, particularly useful when predicting compaction behaviour of pharmaceutical compounds.8,9 Along the lines of abnormalities in the mechanical properties of crystals, this paper reports on a novel observation of organic crystal growth post breakage – crystal regeneration, an extrinsic self-healing behaviour.
Non-mechanistic (thermodynamic) theories that form the basis of equilibrium morphology prediction have developed drastically since the late 1800's from considering surface energetics, crystal geometry, through to lattice energies and synthonic (intermolecular) interactions.10,11 Although long-established, these theories are considered system specific and fail to take into consideration the crystallisation environment which can radically influence the crystal shape and morphology.12 As a result, mechanistic (kinetic) models such as the layered growth (spiral growth and Birth & Spread mechanism) and rough growth theories are used to demonstrate that the anisotropic growth of crystal facets depends on the solution properties, solute surface kink density, and the hydrodynamic environment.13 Consequently, the growth morphology of the crystal is dominated by slow growing facets as the fast-growing ones outrun the former and disappear.14
Even with the evolution of approaches to predict crystal morphology12,15,16 there remains only limited knowledge about the effect of breakage on final crystal properties. The generation of crystal fragments via attrition, fluid shear and abrasion are well studied as a means of secondary nucleation,17 but crystal fracture resulting in the ‘death’ of the large crystal and the consequent ‘birth’ of two almost equally sized crystals is obscure. Moreover, the subsequent growth of these newly formed daughter crystals as well as the original crystal (mother) is poorly understood. Generally, their shape development and kinetics are purely size dependent, isotropic, and assumed to continue as before breakage.18 It is overlooked that the growth kinetics of crystals may vary depending on the method they have been fractured, particularly if they have been broken along their cleavage plane.
A cleavage plane is identified as the facet with the highest interplanar spacing and with the lowest attachment energy, making it the weakest plane of the crystal habit.19,20 The orientation and the packing of these planes can determine the mechanical properties of a crystalline lattice including anisotropy in fracture toughness and ease of compaction.21 Considering that breakage along this plane is most probable when a crystal is under pressure,22 understanding the growth behavior is highly crucial to accurately predict the end crystal shape and size.
Breakage and growth investigations were carried out using macroscopic crystals of a model compound, paracetamol, a rudimentary analgesic and antipyretic drug used in modern medicine. Although high quality macroscopic crystals can be difficult to retrieve, they are still capable of elucidating a variety of anomalous crystal properties that can then be translated to microscopic crystals. Crystals with well-defined facets of the most stable form I (monoclinic), were grown using slow solvent evaporation; ethanol in this case (Fig. 1(a)). A peculiarity in its structure exists where the internal plane (010) (Fig. 1(b)) has been identified to be the preferred cleavage plane, therefore most prone to fracture.18,19,23 The Bravais–Freidel–Donnay–Harker (BFDH) method24–26 was used to predict the form I morphology at equilibrium conditions which showed good agreement with the experimentally grown crystals where facet (010) was absent from the external habit (Fig. 1(c)). When a crystal was cut along its cleavage plane (010) (Fig. 1(d)) and grown in a saturated ethanolic solution, it was observed to have ‘regenerated’ into its pre-cut shape (Fig. 2(d)) – analogous to the process by which a lizard regrows its tail after autotomy. Although a very well-studied compound, paracetamol, this external self-healing phenomenon has not been studied before for crystal growth of particles post breakage and is being reported here for the first time.
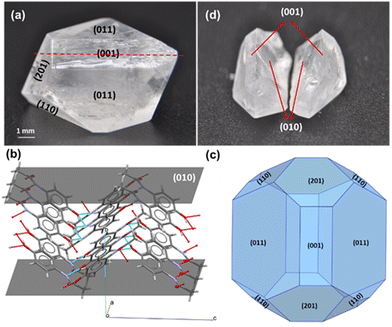 | ||
| Fig. 1 Macroscopic crystal of monoclinic paracetamol (a) whole crystal, (b) slice of facet (010) showing protruding methyl groups, (c) BFDH morphology predicted from Mercury, (d) cleaved crystal. | ||
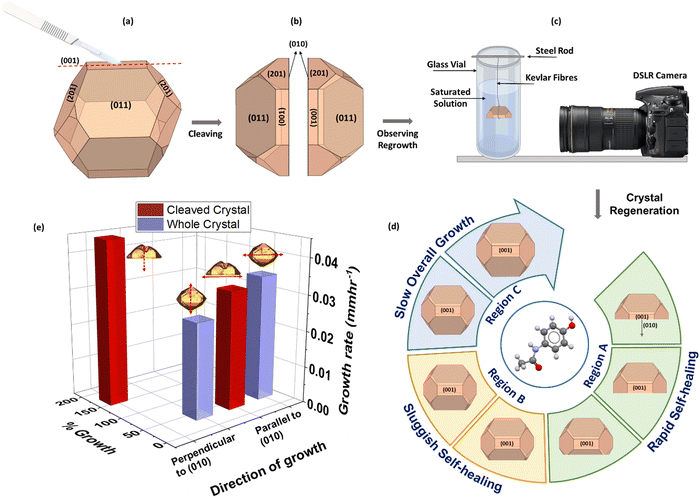
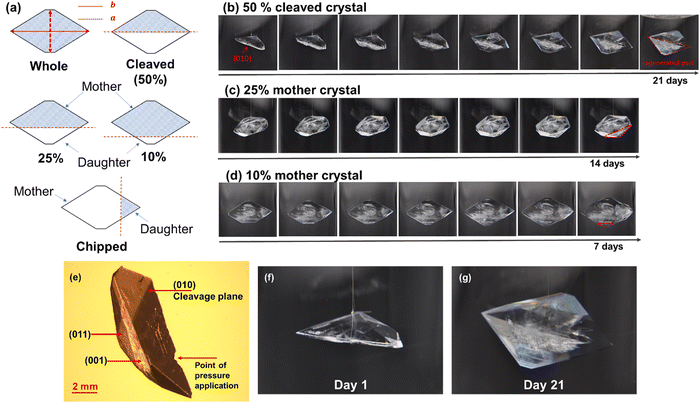
To understand the influence of cleavage planes in the regrowth process, crystals of two size groups, 4 mm ± 1 mm and 8 mm ± 1 mm were broken to different extents as well as along different orientations as summarized in Fig. 3(a). To cleave the crystal, a scalpel was used to put slight point pressure on the centre of the surface (001) – this produced an indentation resulting in a self-propagating crack that split the crystal into two clean halves and exposed the facet (010). Similarly, smaller parts were fragmented off the crystal by cutting parallel to the cleavage plane. In the final scenario, the crystal was ‘chipped’ i.e., cut perpendicular to the cleavage plane. Crystal growth was achieved via slow solvent evaporation of a saturated solution (0.173 gpara mlEtOh−1) at room temperature, 25 °C ± 1 °C, and observed over a period of 1–3 weeks. Growth of small crystals was recorded using optical microscopy while large crystals were monitored using a novel macro photography rig, developed in-house using DSLR cameras to achieve high resolution images (Fig. S1(B) and (C), ESI†). Fig. 2(a)–(c) summarise the experimental approach adapted for the work along with the key results of the growth behaviour of small crystals in Fig. 2(e).
It was observed that all three mother crystals, 50%, 25%, and 10% (Fig. 3(b)–(d)), regardless of their initial size, regenerated into their pre-breakage equilibrium shape. The kinetics of regeneration for small crystals is detailed below while the comparison with large crystals is summarized in Fig. S2 and Table S1 (ESI†). Crystals with a low degree of fracture exhibited faster repair owing to minimal solute demand. Similarly, the daughter crystals of both 10% and 25% also exhibit regeneration but instead of the large mother segment reappearing, it is energetically favourable for the crystal to grow into a smaller whole crystal and consequently grow bigger. The optical micrographs for the growth of a 25% daughter crystal can be found in Fig. S3(A) (ESI†).
However, the same is untrue when the crystal is cut perpendicular to its cleavage plane. It was postulated that by doing so the crystal did not expose any particular facet as multiple facets were being cut through at once. Therefore, regeneration does not occur, rather the crystal grows larger into a new shape with the exposed surface being a part of its exterior habit (Fig. S3(B), ESI†). This emphasises the concept of the dependence of breakage orientation on crystal shape evolution where an eventual undesired crystal habit can adversely affect further downstream processes. To understand the absence of regeneration in perpendicularly cut paracetamol crystals, extrinsic synthons of the exposed surface require studying. However, since the exposed surface is a non-native facet of the crystal, facet indexing, and surface characterisation is a cumbersome process and currently ongoing. Hence it has been excluded from the present work which only aims to explain via molecular packing the regeneration behaviour from cleavage planes. Overall, it has been observed that breaking the crystal along or parallel to its cleavage plane leads to regeneration of the crystal, irrespective of the crystal size. Powder x-Ray Diffraction (PXRD) studies on the regenerated crystals confirmed that the crystals retained their monoclinic form even after the regeneration process (Fig. S4, ESI†).
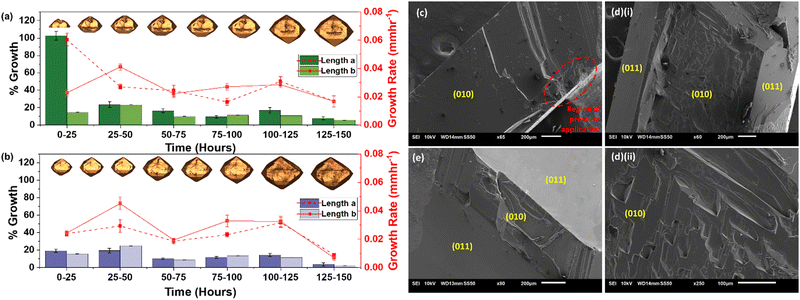
This regeneration behaviour occurs via rapid initial growth of the exposed (010) facets. This is unusual because, given the low attachment energy of the facet, it should display the slowest growth rate according to the Hartman–Perdock theorem27,28 – contradictory to the observation. To consolidate this, overall growth rates of two characteristic lengths, a and b13 of three small cleaved and whole crystals were measured over 150 hours using eqn S1 (ESI†). Length a was the crystal length normal to facet (010) whereas length b was the dimension parallel to facet (010) (Fig. 3(a)).
It can be seen from Fig. 4 that in the first 25 hours, the length a of the cleaved crystal (Fig. 4(a)) grew by an additional 80% compared to the whole crystal (Fig. 4(b)) at a rate approximately 3-fold faster. The growth rate of length b however, remains unchanged regardless of breakage, validating that the growth of (010) is dominating the crystal regrowth. Within this initial time frame, most of the regeneration process was completed (Fig. 4(a)). As regeneration progresses and new facets develop on the crystal, the number of growing facets competing for solute molecules increase and therefore decelerate the pace of regeneration. Hence, remainder of the regeneration took place over a period of next 100 hours where growth in both directions resonated with that of an unbroken whole crystal – relatively equivalent length propagation at a constant growth rate. During the last 25 hours the cleaved and whole crystal showed minimal overall growth with drastically low rates which was postulated to be due to the crystals reaching a stable equilibrium habit.
Hence, three distinct regions can be seen during post breakage growth: rapid regeneration, sluggish regeneration, and slow overall growth. Even though all experiments were carried out using the same mother liquor and under the same operating conditions the whole crystals did not show these three regions of growth. It was not possible to actively and rigorously control the rate of evaporation but since very slow yet continuous evaporation under stable ambient conditions was carried out over a period of multiple days, drastic changes in the concentration of the mother liquor were deemed unlikely. This was postulated to be due to a continuous creation and depletion of the supersaturation during the growth of crystals, resulting in a relatively constant concentration over the 150 hours. Hence the three distinct growth regions were associated with changes in the intrinsic growth behaviour of the crystal rather than due to variation in solution concentration. This can further be consolidated by comparing the growth of cleaved crystals to whole crystals which were carried out under the exact same conditions, but three distinct phases are not seen, instead growth is constant throughout the duration of the experiment. It is also appreciated that the rate of regeneration is not only depended on the extent of breakage but also on external factors such as the presence of impurities and the type of solvents used. Therefore, future work exploring these concepts is required to provide meaningful insight into the regeneration process.
Scanning electron microscopy (SEM) of facet (010) was performed at different time points during the regeneration process, 0 hours (Fig. 4(c)), 25 hours (Fig. 4(d)(i) and (ii)) and 50 hours (Fig. 4(e)). The obtained micrographs depict the change in surface topography of different facets over time which consolidate the hypothesised shape evolution of the cleaved crystal during regeneration. At 0 hours, prior to the onset of growth, (010) was observed to be relatively smooth apart from the region extending from the point of pressure application. After 25 hours, (010) can be seen comparatively rugged than at 0 hours pertaining to the growth on the facet. At this point, the smooth (011) facets can also be seen developing around the edges of (010). Finally at 50 hours, a very thin remainder of the growing (010) facet can be seen along with the almost entirely developed (011) facets.
Theoretically, sedated growth of (010) can also be consolidated by considering the synthonic interactions in form I paracetamol. The hydroxyl (OH) groups in a paracetamol molecule participate in hydrogen bonding by accepting a hydrogen bond from the amine (NH) group and donating it to the carbonyl (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) group of an adjacent molecule, forming criss-cross layers in the a and c crystallographic directions (Fig. 1(b)). The protruding methyl (–CH3) groups from the surface of (010) result in surface apolarity and therefore lower its hydrogen bonding potential with the incoming paracetamol molecules. Gibbs and Wulf postulated that a crystal at equilibrium will assume a form such that it minimises its total free energy and the distance of the facets from the growth origin of the crystal will be proportional to their surface energies.24,29 Surface energy studies on form I of paracetamol have also shown facet (010) to have the lowest total surface energy,30 but based on the experimental evidence presented in this article, it has been hypothesized that once in solution, facet (010) becomes extremely unstable as it is inherently not part of the external habit of an equilibrium crystal, prompting rapid facet advancement. This eventually leads to the cleaved crystal regenerating itself and minimising its overall surface free energy to attain a thermodynamically stable configuration.
O) group of an adjacent molecule, forming criss-cross layers in the a and c crystallographic directions (Fig. 1(b)). The protruding methyl (–CH3) groups from the surface of (010) result in surface apolarity and therefore lower its hydrogen bonding potential with the incoming paracetamol molecules. Gibbs and Wulf postulated that a crystal at equilibrium will assume a form such that it minimises its total free energy and the distance of the facets from the growth origin of the crystal will be proportional to their surface energies.24,29 Surface energy studies on form I of paracetamol have also shown facet (010) to have the lowest total surface energy,30 but based on the experimental evidence presented in this article, it has been hypothesized that once in solution, facet (010) becomes extremely unstable as it is inherently not part of the external habit of an equilibrium crystal, prompting rapid facet advancement. This eventually leads to the cleaved crystal regenerating itself and minimising its overall surface free energy to attain a thermodynamically stable configuration.
Even though surface energetics indicate as to why crystal regeneration occurs, the kinetic mechanism of this process still needs to be investigated. The attachment energy theory accounts for intrinsic synthons and presents a good starting point for studying cleavage planes but still fails to consider the external environment of the crystal. It is regarded as more accurate for crystals grown in near equilibrium conditions as these attachment energies are calculated in vacuum environments.10 Modified attachment energies could be used instead to attain a better understanding of the influence of the solvent environment during crystal growth. As they account for extrinsic synthons – ‘broken’ intermolecular interactions exposed at the crystal growth surface,31 the interactions between the crystal surface and the solute molecules in the presence of a solvent can therefore be deduced.32 Recent work has shown the development of models that incorporate mechanistic and non-mechanistic theories by combining molecular dynamic (MD) and kinetic Monte Carlo simulations for a more robust and generalized crystal shape prediction.14,33 It is suggested that further investigation using this approach could be undertaken to predict the detailed mechanism of crystal regeneration via cleavage planes.
Moreover, breakage and growth investigations using other small pharmaceutical compounds such as Aspirin and Carbamazepine (CBZ) are currently ongoing to consolidate the regeneration phenomenon. Although facet indexing of the macroscopic crystals is still under progress, growth from an arbitrary breakage plane in CBZ showed signs of regeneration of the crystal to its original shape during preliminary investigations (ESI† section 3). No conclusive statements can be made regarding the regeneration behavior in compounds other than paracetamol yet, however these results emphasise the need to further explore other pharmaceutical crystals and their various breakage planes to elucidate the mechanism of this external self-healing behaviour.
Conclusions
In summary, this paper introduces a novel concept of crystal regeneration as a means of crystal growth post breakage. It goes against some of the most widely used thermodynamically driven morphology prediction theories and highlights the need to explore crystal growth via a combination of hydrodynamics, solution chemistry, and solid properties. Crystal regeneration urges the reader to ponder over the recyclability of crystals by considering their life process from birth to growth to death as a cyclic process instead of an irreversible one-way mechanism. This knowledge can be of particular significance when determining the performance of seeded reactors where milled crystals are predominantly used as seeds as well as when considering impact breakage in crystallisers. When applied to process modelling, the crystal regeneration phenomenon can aid in accurate crystal shape and size prediction after crystal breakage and therefore alter the outlook on crystal engineering and control.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the UK's EPSRC (EP/N015916/1) for funding. The authors are also grateful for useful suggestions on crystal imaging from Dr Alex Moldovan and scientific discussions with Ms Allison Arber and Professor Nic Harrison.References
- P. Naumov, D. P. Karothu, E. Ahmed, L. Catalano, P. Commins, J. Mahmoud Halabi, M. B. Al-Handawi and L. Li, J. Am. Chem. Soc., 2020, 142, 13256–13272 CrossRef CAS PubMed.
- D. P. Karothu, J. Mahmoud Halabi, E. Ahmed, R. Ferreira, P. R. Spackman, M. A. Spackman and P. Naumov, Angew. Chem., Int. Ed., 2022, 61, e202113988 CrossRef CAS PubMed.
- P. Naumov, S. Chizhik, M. K. Panda, N. K. Nath and E. Boldyreva, Chem. Rev., 2015, 115, 12440–12490 CrossRef CAS PubMed.
- P. Commins, M. B. Al-Handawi, D. P. Karothu, G. Raj and P. Naumov, Chem. Sci., 2020, 11, 2606–2613 RSC.
- L. Zhang, J. B. Bailey, R. H. Subramanian, A. Groisman and F. A. Tezcan, Nature, 2018, 557, 86–91 CrossRef CAS PubMed.
- P. Commins, H. Hara and P. Naumov, Angew. Chem., Int. Ed., 2016, 55, 13028–13032 CrossRef CAS PubMed.
- F. Liu, D. E. Hooks, N. Li, N. A. Mara and J. A. Swift, Chem. Mater., 2018, 30, 3798–3805 CrossRef CAS.
- P. Gupta, S. A. Rather, B. K. Saha, T. Panda, D. P. Karothu and N. K. Nath, Cryst. Growth Des., 2020, 20, 2847–2852 CrossRef CAS.
- G. Vreeman, C. Wang, C. M. Reddy and C. C. Sun, Cryst. Growth Des., 2021, 21, 6655–6659 CrossRef CAS.
- I. Rosbottom and K. J. Roberts, in Engineering Crystallography: From Molecule to Crystal to Functional Form, ed. K. J. Roberts, R. Docherty and R. Tamura, Springer, Netherlands, Dordrecht, 2017, pp. 109–131 DOI:10.1007/978-94-024-1117-1_7.
- L. Song, F.-Q. Zhao, S.-Y. Xu, X.-H. Ju and C.-C. Ye, Sci. Rep., 2020, 10, 2317 CrossRef CAS PubMed.
- J. Li, C. J. Tilbury, S. H. Kim and M. F. Doherty, Prog. Mater. Sci., 2016, 82, 1–38 CrossRef CAS.
- T. T. H. Nguyen, R. B. Hammond, K. J. Roberts, I. Marziano and G. Nichols, CrystEngComm, 2014, 16, 4568–4586 RSC.
- S. Piana and J. D. Gale, J. Am. Chem. Soc., 2005, 127, 1975–1982 CrossRef CAS PubMed.
- J. Li and M. F. Doherty, Cryst. Growth Des., 2017, 17, 659–670 CrossRef CAS.
- R. C. Snyder and M. F. Doherty, Proc. R. Soc. A, 2009, 465, 1145–1171 CrossRef CAS.
- J. Anwar, S. Khan and L. Lindfors, Angew. Chem., Int. Ed., 2015, 54, 14681–14684 CrossRef CAS PubMed.
- L. Bosetti and M. Mazzotti, Cryst. Growth Des., 2020, 20, 307–319 CrossRef CAS.
- C. C. Sun and Y. H. Kiang, J. Pharm. Sci., 2008, 97, 3456–3461 CrossRef CAS PubMed.
- S. Kim, B. Lotz, M. Lindrud, K. Girard, T. Moore, K. Nagarajan, M. Alvarez, T. Lee, F. Nikfar, M. Davidovich, S. Srivastava and S. Kiang, Org. Process Res. Dev., 2005, 9, 894–901 CrossRef CAS.
- W. C. Duncan-Hewitt, D. L. Mount and A. Yu, Pharm. Res., 1994, 11, 616–623 CrossRef CAS PubMed.
- J. Y. Y. Heng, A. Bismarck and D. R. Williams, AAPS PharmSciTech, 2006, 7, E12–E20 CrossRef PubMed.
- K. V. R. Prasad, D. B. Sheen and J. N. Sherwood, Pharm. Res., 2001, 18, 867–872 CrossRef CAS PubMed.
- R. Docherty, G. Clydesdale, K. J. Roberts and P. Bennema, J. Phys. D: Appl. Phys., 1991, 24, 89–99 CrossRef CAS.
- D. S. Coombes, C. R. A. Catlow, J. D. Gale, M. J. Hardy and M. R. Saunders, J. Pharm. Sci., 2002, 91, 1652–1658 CrossRef CAS PubMed.
- J. D. H. Donnay and D. Harker, Am. Mineral., 1937, 22, 446–467 CAS.
- P. Hartman, J. Cryst. Growth, 1980, 49, 166–170 CrossRef CAS.
- P. Hartman and W. G. Perdok, Acta Crystallogr., 1955, 8, 49–52 CrossRef.
- R. Li, X. Zhang, H. Dong, Q. Li, Z. Shuai and W. Hu, Adv. Mater., 2016, 28, 1697–1702 CrossRef CAS PubMed.
- J. Y. Y. Heng, A. Bismarck, A. F. Lee, K. Wilson and D. R. Williams, Langmuir, 2006, 22, 2760–2769 CrossRef CAS PubMed.
- T. T. H. Nguyen, I. Rosbottom, I. Marziano, R. B. Hammond and K. J. Roberts, Cryst. Growth Des., 2017, 17, 3088–3099 CrossRef CAS.
- R. B. Hammond, K. Pencheva, V. Ramachandran and K. J. Roberts, Cryst. Growth Des., 2007, 7, 1571–1574 CrossRef CAS.
- M. Salvalaglio, T. Vetter, M. Mazzotti and M. Parrinello, Angew. Chem., Int. Ed., 2013, 52, 13369–13372 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2mh01180h |
| This journal is © The Royal Society of Chemistry 2023 |