Bis-ferrocenyl-hydrazide metal complexes: studying electronic functional groups as newly potent homogeneous photocatalysts for C(sp3)–H and C(sp2)–H bond oxidation utilizing visible light condition†
Mohammad
Bashiri
 a,
Mona
Hosseini-Sarvari
a,
Mona
Hosseini-Sarvari
 *a and
Sara
Fakhraee
b
*a and
Sara
Fakhraee
b
aNanophotocatalysis Lab., Department of Chemistry, Shiraz University, Shiraz 7194684795, I.R. Iran. E-mail: hossaini@shirazu.ac.ir
bDepartment of Chemistry, Payame Noor University, 19395-3697, Tehran, Iran
First published on 30th October 2023
Abstract
A crucial challenge in using organo-metal complexes for photocatalytic organic reactions is the need to develop applications of homogeneous photocatalysts that can effectively function under visible light conditions. For the first time, the use of binuclear complexes containing ferrocenyl-hydrazides as a ligand and nickel or copper as central metals as homogeneous photocatalysts in the oxidation of organic compounds is presented. The new organometal photocatalysts were prepared and identified using techniques, such as FT-IR spectroscopy, NMR spectroscopy, XRD, XRF, XPS, SEM, TGA, EDX, UV-visible, and photocurrent measurements. The oxidation of benzylic C(sp3)–H bonds to produce oxygenated molecules and the selective conversion of C–C double bonds to benzaldehyde can be achieved using bis-ferrocenyl hydrazide complexes with electron-withdrawing or electron-donating groups on the hydrazide moiety under visible-light irradiation in an air atmosphere, at ambient temperature and without the need for external oxidants. The synthesized complexes also can be used to oxygenate 1H-indole to 1H-indole-2,3-dione. The investigation of the role of donating and withdrawing functional groups in the synthesized complexes for selected oxidation reactions is a significant benefit of this report. It was found that only the [(FcHz)2Ni] and [(FcHz)2Cu] complexes without functional groups were able to provide a suitable response in the oxidation of the compounds. Additionally, the theoretical DFT and TD-DFT methodologies enabled us to describe the photocatalytic oxidation behavior of these metal complexes. The calculations showed conformational changes in the structure of metal complexes after oxidation. The molecular orbital and natural transition orbital analyses revealed the nature of electronic transitions in the UV-visible absorption bands.
Design, System, ApplicationToday, as the promotion of organic reactions under visible light irradiation is of great importance, it is necessary to design and synthesize compounds with unique optical properties that have the efficiency and capabilities of the first-generation homogeneous photocatalysts. Accordingly, the development of homogeneous non-noble metal-based catalysts is of great economic and application importance. The merging of the organometallic chemistry of nickel and copper with ferrocene has been introduced by utilizing ferrocene-based hydrazide Schiff bases as new ligands for the first time. The noteworthy point of this study is the synthesis of complexes containing two metals, all of which include non-noble-metals. One of the great important issues is the use of ferrocene as an organometallic compound in the synthesis of metal complexes, where the Schiff base containing ferrocene is employed as a ligand. The culmination of the investigations is that electron donor and withdrawing groups are used on these ligands, and their structure and applications in selective reactions are fully examined. Finally, to investigate the performance of the complexes, we investigated the selective oxidation reactions of toluene, styrene, and indole. |
Introduction
Merging groups that have desirable electronic activity in the presence of light irradiation have been considered in the field of photocatalysts over the past decade.1 Recently, a variety of photocatalysts have been introduced, including semiconductors, organometallic compounds, and organic dyes that are able to perform organic reactions by absorbing light irradiation and electron excitation.2–4 Each of these types of photocatalysts with unique mechanisms can be used to carry out responses, such as electron–hole or transferring a single electron from metals by changing the oxidation state.5,6 Under light irradiation, particularly in the visible region, organic dyes and organometallic compounds can be converted into electronically excited compounds, which can undergo photoinduced electron transfer to initiate a chemical reaction.7 In the illustration of organometallic compounds for photocatalytic activity, the oxidation state of the central metal is important and its adjustment is not easy.8 Although iridium and ruthenium complexes with bipyridyl ligands were initially used as homogeneous photoredox catalysts, new photocatalysts have since been developed by changing both the ligands and the central metals.9 Diverse organic ligands with different metals, such as Ni, Cu, Zn, Fe, and Co, were studied as photocatalysts (some examples are shown in Fig. 1).10–18 Recent years have seen significant advancements in the use of nickel and copper complexes as photocatalysts under visible light sources owing to their numerous advantages over the first generation of organometallic photocatalysts (Ir, Ru). Copper, as one of the abundant elements of the Earth's crust, has been welcomed instead of plasmonic metals in the scaffold of photocatalysts. Due to the unique optical and electrochemical properties of copper compounds, a great opportunity has been created to design redox catalysts under visible light irradiation.19 Copper(II) complexes have been confirmed to be highly effective as photocatalysts in a wide range of organic reactions. Their ability to absorb visible light, unique electronic properties, and structural versatility make them highly attractive for use in both academic research and industrial applications. With continued research and development, it is likely that copper(II) complexes will play an increasingly important role in the future of photocatalysis.20–22 Nickel is capable of forming intermediate metal complexes with ligands of varying oxidation numbers, resulting in a diverse range of geometric structures. These structures can include squares with four coordinating atoms, triangular or pyramidal squares with five coordinating atoms, and octahedrons with six coordinating atoms.23 Over the years, nickel complexes have also been used as dual photocatalysts beside other organic compounds to complete the mechanism of these new types of catalysts by the Macmillan and Molander research groups.16,24,25 New designs of nickel complexes in covalent organic framework (COF) scaffolds and organic semiconductors such as graphitic carbon nitride (g-C3N4) for photocatalytic applications have also been introduced.26,27One of the most common ligands used by organometallic chemists to design efficient optical complexes is Schiff bases. Schiff bases have been studied as a ligand for the synthesis of transition metal complexes in coordination chemistry from the past to the present, and have many applications as catalysts and drug molecules.28–30 Schiff bases possess nitrogen atoms in their azomethine bonds, which enable them to attach to different metals.31 The addition of a carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) to the hydrazide Schiff base further increases its ability to coordinate with metals in these compounds.23
O) to the hydrazide Schiff base further increases its ability to coordinate with metals in these compounds.23
Interest in the research field of redox-switchable catalysts has expanded. In recent years, many binuclear and multinuclear complexes have been reported as redox-switchable metallo-ligand compounds.32 Due to its unique electron properties, ferrocene acts as a substantial electron donor when attached to the electron-withdrawing group and forms numerous pull–push compounds.33 Ferrocene-based compounds can absorb light in the visible, near-infrared (NIR), and even infrared (IR) regions, which modified the oxidation state of one of the two groups participating in the pull–push structure.34 In this issue, the ferrocene moiety constitutes a candidate for the election due to its easy oxidation and the reversibility of the oxidation process.35 Until now, dyes with excellent electrochemical and photo-electrochemical properties based on ferrocene have been published in the literature (Fig. 2).36–40
Finally, as reported in the literature, numerous advantages have been mentioned for homogeneous photocatalysts over heterogeneous ones, including potent light absorption behaviors that can absorb irradiation in the visible region of the electromagnetic spectrum, low optical scattering coefficient, which is significant for impressive photocatalytic reactions, performance more versatile in terms of reaction conditions, operating under ambient conditions or at relatively mild temperatures and pressures, and can be easily synthesized and characterized using standard analytical techniques.41,42 Scientists have recently focused on the homogeneous photocatalysts in green chemistry and environmental issues.
In recent years, studies on the oxidation of toluene, styrene, and indole with heterogeneous photocatalysts have been reported.43–47 After considering all of the aspects mentioned above, the objective of our current research study is to develop novel homogeneous complexes of Ni and Cu using ferrocenyl-hydrazide Schiff base ligands. These complexes have been chosen owing to their characteristic optical and electrochemical behaviors that make them appropriate for efficient light absorption in the visible region. The photocatalytic properties of the synthesized [(FcHz)2Ni] and [(FcHz)2Cu] complexes have been investigated, and they have been found to exhibit promising performance as catalysts in oxidation reactions (as shown in Fig. 3). These reactions involve the excitation of electrons within the complexes, followed by a single electron transfer (SET) process. Since the charge of the complex is neutral, the cyclic voltammetry studies suggest that only the iron component of ferrocene is involved. Therefore, it can be deduced that the dynamic equilibrium of iron (Fe2+/Fe3+) within the complex is responsible for its impressive performance in selective oxidation reactions. Various identification analyses were performed to determine the structure and compare the photocatalytic activity, as well as physical chemistry calculations to present the better properties of these complexes.
 | ||
| Fig. 3 The oxidation process of aromatic alkane and alkene to their corresponding carbonyl products. | ||
Results and discussion
According to our design for the synthesis of a high-performance photocatalytic system, the first step was the preparation of the ferrocenyl-hydrazide Schiff bases [FcHz-X] (3a–c). This step was performed via the condensation between commercially-available ferrocene carboxaldehyde (1) and benzohydrazides (2a–c) by adding two drops of glacial acetic acid in anhydrous methanol under reflux conditions for 9 hours. [FcHz-X] (3a–c) were synthesized with excellent yield. Then, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 molar mixture of [FcHz-X] (3a–c), Ni or Cu acetate, and sodium acetate in dry methanol was mixed for 8 hours at 70 °C to result in [(FcHz-X)2Ni] and [(FcHz-X)2Cu] (4a–c and 5a–c), respectively. These red organometal complexes were filtered and purified with water and hot methanol.48 We performed this strategy for the synthesis of our new [(FcHz-X)2Ni] and [(FcHz-X)2Cu] complexes, as outlined in Scheme 1. After the successful preparation of the [(FcHz-X)2Ni] and [(FcHz-X)2Cu] complexes, they were identified with various spectroscopy and non-spectroscopy methods, including FT-IR, NMR (1H), XRD, XRF, XPS, SEM, TGA, EDX, photocurrent, cyclic voltammetry, and UV-visible spectroscopy.
4 molar mixture of [FcHz-X] (3a–c), Ni or Cu acetate, and sodium acetate in dry methanol was mixed for 8 hours at 70 °C to result in [(FcHz-X)2Ni] and [(FcHz-X)2Cu] (4a–c and 5a–c), respectively. These red organometal complexes were filtered and purified with water and hot methanol.48 We performed this strategy for the synthesis of our new [(FcHz-X)2Ni] and [(FcHz-X)2Cu] complexes, as outlined in Scheme 1. After the successful preparation of the [(FcHz-X)2Ni] and [(FcHz-X)2Cu] complexes, they were identified with various spectroscopy and non-spectroscopy methods, including FT-IR, NMR (1H), XRD, XRF, XPS, SEM, TGA, EDX, photocurrent, cyclic voltammetry, and UV-visible spectroscopy.
 | ||
| Scheme 1 Preparation of bis ferrocenyl-hydrazide complexes with various electronic functional groups 4a–c and 5a–c. | ||
Photocatalysts characterization
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of azomethine, and the absorption band at 1645 cm−1 was confirmed as the N–C
N bond of azomethine, and the absorption band at 1645 cm−1 was confirmed as the N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O carbonyl stretching mode.49 FcHz–OH showed a strong absorption band at 1605 cm−1 with a shoulder at 1647 cm−1, which corresponds to the stretching vibration of the C
O carbonyl stretching mode.49 FcHz–OH showed a strong absorption band at 1605 cm−1 with a shoulder at 1647 cm−1, which corresponds to the stretching vibration of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and the C
N bond and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the Schiff base ligand, respectively.50 In FcHz–NO2, due to the presence of an electron-withdrawing group (NO2) on the phenylhydrazine, the amide-C
O in the Schiff base ligand, respectively.50 In FcHz–NO2, due to the presence of an electron-withdrawing group (NO2) on the phenylhydrazine, the amide-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching band appeared at 1667 cm−1 and the azomethine C
O stretching band appeared at 1667 cm−1 and the azomethine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N band emerged at 1598 cm−1.51 After coordination of Ni to the FcHz ligand for the [(FcHz)2Ni] complex, the peak at 1645 cm−1 disappeared and two new absorption peaks at 1584 cm−1 and 1612 cm−1 emerged, corresponding to the carbonyl of the amido N–C–O and C
N band emerged at 1598 cm−1.51 After coordination of Ni to the FcHz ligand for the [(FcHz)2Ni] complex, the peak at 1645 cm−1 disappeared and two new absorption peaks at 1584 cm−1 and 1612 cm−1 emerged, corresponding to the carbonyl of the amido N–C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N azomethine stretching, respectively.52–54 The absorption band of the C
N azomethine stretching, respectively.52–54 The absorption band of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N band in the [(FcHz)2Cu] complex appeared at 1612 cm−1 and that of carbonyl of the N–C–O group was observed at 1588 cm−1, respectively. For [(FcHz–OH)2Ni], a peak was observed at 1597 cm−1 with a shoulder at 1614 cm−1 assigned to amidate-CO and the C
N band in the [(FcHz)2Cu] complex appeared at 1612 cm−1 and that of carbonyl of the N–C–O group was observed at 1588 cm−1, respectively. For [(FcHz–OH)2Ni], a peak was observed at 1597 cm−1 with a shoulder at 1614 cm−1 assigned to amidate-CO and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-azomethine moieties, respectively.55 A similar pattern was found for [(FcHz–OH)2Cu] (1598 and 1617 cm−1).56 The [(FcHz–NO2)2Ni] complex displayed two distinct and intense bands at 1600 and 1530 cm−1, which are attributed to the amidate-CO and C
N-azomethine moieties, respectively.55 A similar pattern was found for [(FcHz–OH)2Cu] (1598 and 1617 cm−1).56 The [(FcHz–NO2)2Ni] complex displayed two distinct and intense bands at 1600 and 1530 cm−1, which are attributed to the amidate-CO and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N azomethine groups, respectively. The FT-IR spectrum of the [(FcHz–NO2)2Cu] complex exhibited two significant signals at 1600 and 1530 cm−1, which are indicative of the presence of the amidated-CO and C
N azomethine groups, respectively. The FT-IR spectrum of the [(FcHz–NO2)2Cu] complex exhibited two significant signals at 1600 and 1530 cm−1, which are indicative of the presence of the amidated-CO and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N azomethine moieties,57 respectively. A series of signals in the region of 400–800 cm−1 correspond to the stretching and bending vibrations of the metal–ligand bonds.58 The reduction of the absorption band for the amido-carbonyl group in all complexes is due to the resonance between the negative charge of nitrogen and the double C
N azomethine moieties,57 respectively. A series of signals in the region of 400–800 cm−1 correspond to the stretching and bending vibrations of the metal–ligand bonds.58 The reduction of the absorption band for the amido-carbonyl group in all complexes is due to the resonance between the negative charge of nitrogen and the double C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond. The absorption band is around 1000–900 cm−1, which corresponds to the stretching vibration of the Fe–C bond.59 The strong absorption band is around 500 cm−1, which corresponds to the bending vibration of the Fe–C bond.60 As further evidence to confirm the structure of the synthesized complexes, the FT-IR spectra of the [(FcHz)2Ni] and [(FcHz)2Cu] complexes (Fig. 4) were compared with the theoretical IR spectra obtained from DFT calculations. The comparison of the spectra exhibited their great similarity and confirmed the accuracy of the proposed computational structures for the synthesized complexes (Fig. S41†).
O bond. The absorption band is around 1000–900 cm−1, which corresponds to the stretching vibration of the Fe–C bond.59 The strong absorption band is around 500 cm−1, which corresponds to the bending vibration of the Fe–C bond.60 As further evidence to confirm the structure of the synthesized complexes, the FT-IR spectra of the [(FcHz)2Ni] and [(FcHz)2Cu] complexes (Fig. 4) were compared with the theoretical IR spectra obtained from DFT calculations. The comparison of the spectra exhibited their great similarity and confirmed the accuracy of the proposed computational structures for the synthesized complexes (Fig. S41†).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), as depicted in Fig. S10.† Based on previous extensive literature reports, we attribute this isomer to the E-isomer.61–64 The protons of the two cyclopentadienyls of ferrocene appeared separately in the 4.0–4.5 ppm region.65,66 Also, the aromatic protons of benzohydrazide were observed at the appropriate positions. A broadened signal appeared at 11.42 ppm, which was attributed to the –NH of the amido group. A broad signal at 11.30 ppm, attributed to the –NH of the amido group, was also observed. In the 4.06–4.57 ppm region, the protons of ferrocene emerged separately, while the aromatic protons of benzohydrazide were detected at their expected positions. Validation of the generation of FcHz–NO2 was obtained by the detection of a singlet signal at 8.20 ppm for the azomethine proton (HC
N), as depicted in Fig. S10.† Based on previous extensive literature reports, we attribute this isomer to the E-isomer.61–64 The protons of the two cyclopentadienyls of ferrocene appeared separately in the 4.0–4.5 ppm region.65,66 Also, the aromatic protons of benzohydrazide were observed at the appropriate positions. A broadened signal appeared at 11.42 ppm, which was attributed to the –NH of the amido group. A broad signal at 11.30 ppm, attributed to the –NH of the amido group, was also observed. In the 4.06–4.57 ppm region, the protons of ferrocene emerged separately, while the aromatic protons of benzohydrazide were detected at their expected positions. Validation of the generation of FcHz–NO2 was obtained by the detection of a singlet signal at 8.20 ppm for the azomethine proton (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), as illustrated in Fig. S11.† A wide peak located at 11.43 ppm was identified as the –NH signal of the amido group. A peak corresponding to the hydroxyl group was observed in the 10.24 ppm region. The protons of the ferrocene appeared individually in the range of 4.20–4.64 ppm, while the aromatic protons of benzohydrazide were detected at their anticipated positions. To confirm the formation of FcHz–OH, a singlet signal was detected at 8.24 ppm for the azomethine proton (HC
N), as illustrated in Fig. S11.† A wide peak located at 11.43 ppm was identified as the –NH signal of the amido group. A peak corresponding to the hydroxyl group was observed in the 10.24 ppm region. The protons of the ferrocene appeared individually in the range of 4.20–4.64 ppm, while the aromatic protons of benzohydrazide were detected at their anticipated positions. To confirm the formation of FcHz–OH, a singlet signal was detected at 8.24 ppm for the azomethine proton (HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), as shown in Fig. S12.† As shown in Fig. S13-S14,† the chemical shifts for the protons of [(FcHz)2Ni] and [(FcHz)2Cu] were observed as distinct peaks across the entire spectrum, indicating the presence of the two expected ligands attached to Ni or Cu as the central metals. Although assigning each peak to the corresponding protons in the compound is challenging due to the complexity of the compounds, it can be confidently concluded that the synthesis of the compounds was successful based on the number of observed protons.
N), as shown in Fig. S12.† As shown in Fig. S13-S14,† the chemical shifts for the protons of [(FcHz)2Ni] and [(FcHz)2Cu] were observed as distinct peaks across the entire spectrum, indicating the presence of the two expected ligands attached to Ni or Cu as the central metals. Although assigning each peak to the corresponding protons in the compound is challenging due to the complexity of the compounds, it can be confidently concluded that the synthesis of the compounds was successful based on the number of observed protons.
| Compound | Elements | Concentration | Intensity |
|---|---|---|---|
| [(FcHz)2Ni] | Fe | 53.30 | 1336.81 |
| Ni | 46.70 | 613.89 | |
| [(FcHz)2Cu] | Fe | 52.04 | 1436.26 |
| Cu | 47.96 | 803.12 | |
| [(FcHz–OH)2Ni] | Fe | 54.59 | 1110.23 |
| Ni | 45.41 | 479.27 | |
| [(FcHz–OH)2Cu] | Fe | 55.97 | 1343.59 |
| Cu | 44.03 | 801.04 | |
| [(FcHz–NO2)2Ni] | Fe | 54.39 | 1284.37 |
| Ni | 45.60 | 598.90 | |
| [(FcHz–NO2)2Cu] | Fe | 51.43 | 1485.50 |
| Cu | 48.57 | 898.74 |
 | ||
| Fig. 5 XRD patterns of (a): FcHz, [(FcHz)2Ni] and [(FcHz)2Cu], (b): FcHz–OH, [(FcHz–OH)2Ni] and [(FcHz–OH)2Cu], (c): FcHz–NO2, [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu]. | ||
The attractive issue about FcHz–OH is that it has a crystalline structure, unlike FcHz and FcHz–NO2. The XRD pattern of FcHz–OH showed high-intensity diffraction signals at 2θ values of 5.2, 10.5, 15.8, and 21.2°. The XRD patterns for the [(FcHz–OH)2Ni] and [(FcHz–OH)2Cu] complexes did not show any discernible crystal structure, likely due to their amorphous state. The XRD patterns of the [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu] complexes showed a marked difference upon coordination of the FcHz–NO2 complex to Ni and Cu. The presence of weak peaks in the 2θ = 10–20° region indicated that the metal complexes were in the crystalline phase. In contrast, the Schiff base exhibited a less defined pattern. Both Ni and Cu complexes exhibited high-intensity diffraction signals at 2θ = 9.1, 16.7, 17.8, 18.91°, and 2θ = 6.81°, respectively, with a few low-intensity signals. These observations suggest that the Ni and Cu complexes possess a defined crystalline structure. Based on the XRD study, the [(FcHz)2Ni] complex demonstrated a stronger crystalline phase in comparison to the Cu one.
Molecular geometries of photocatalysts
All quantum chemical calculations for the neutral [(FcHz)2M] and oxidized [(FcHz)2M]2+ complexes (M = Ni and Cu) were performed by the Gaussian16 software suite68 using the ωB97 GGA exchange–correlation density functional method (DFT)69 combined with the valence triple-zeta polarization Def2TZVP basis set.68 The calculations were done in the dimethylformamide (DMF) solution using the integral equation formalism variant polarizable continuum model (IEFPCM).70,71 The geometry optimizations were carried out without symmetry restrictions, and followed by vibrational frequency calculations to ensure that all the structures were at true minima. To determine the reliable molecular structures, we tried other functionals and basis sets, such as CAM-B3LYP/TZVP and B3LYP/6-311g(d,p). The long-range corrected functional ωB97 along with the Def2TZVP basis set provides the most similar IR (Fig. S41†) and UV-visible spectra (Fig. 7a) to the experimental FT-IR and UV-visible spectra. The TD-DFT calculations of the UV-visible spectra, which will be described in detail in the next section, demonstrated that the long-range corrected functional ωB97 along with the Def2TZVP basis set results in the most similar spectra and excitations to the experimental UV-visible spectra (Fig. 7a). Therefore, ωB97/Def2TZVP was chosen to study the geometric and electronic structures of all complexes in the ground and excited state in the DFT/TD-DFT calculations. The optimized structures for neutral and oxidized complexes are shown in Fig. 6, and some important geometrical parameters are summarized in Table S2.† | ||
| Fig. 6 Optimized structures of the [(FcHz)2M] and [(FcHz)2M]2+ complexes (M = Ni and Cu) at ωB97/Def2TZVP level of theory. | ||
The molecular structures in Fig. 6 demonstrate how the change in the oxidation state of [(FcHz)2Ni] and [(FcHz)2Cu] affects the geometric environment of the Ni and Cu atoms, and transforms the coordination geometry of these two central metals from see-saw in neutral complexes to square-planar in oxidized complexes (Table S2†). Accordingly, the whole structure of the complexes changes conformationally. Two phenyl hydrazide groups in the neutral [(FcHz)2Ni] and [(FcHz)2Cu] complexes have torsional angles of 95.82° and 114.87°, respectively. Conversely, in the [(FcHz)2Ni]2+ and [(FcHz)2Cu]2+ complexes, the torsional angles approach zero, and these complexes display planar structures. Furthermore, the dihedral angles between the two ferrocene groups (C56–C3–C26–C66) greatly increase from ≈60° in neutral complexes to ≈120° in oxidized complexes. Moreover, Fig. 6 shows that the ferrocenes have eclipsed conformations in both neutral and oxidized structures. Interestingly, the bond lengths of M75–O11 and M75–O34 are greatly reduced from ≈2.60 to 1.86 Å in [(FcHz)2Ni]2+, and from ≈2.34 to 1.95 Å in [(FcHz)2Cu]2+.
Theoretical UV-visible spectra
The time-dependent density functional theory (TD-DFT) was employed to evaluate the UV-visible absorption spectra with ten selected singlet excitations. The TD-DFT calculations were performed at the ωB97/Def2TZVP level of theory in the presence of DMF solvent. The [(FcHz)2Ni] and [(FcHz)2Cu] complexes as the photocatalysts were considered in their +2-oxidation state (being oxidized).Fig. 8 displays the calculated UV-visible absorption spectra for the neutral [(FcHz)2Ni] and [(FcHz)2Cu] complexes. To characterize the excitations, the iso-surfaces of the main natural transition orbitals (NTOs) for the more significant excitation bands are presented in Fig. 8. The NTOs are the linear combination of molecular orbitals involved in the electronic transition.72 Compared to canonical molecular orbitals, the NTOs provide a more compact orbital representation of the transition density matrix to analyze the excited states and charge transfers.73 Each electronically excited state is described in terms of the occupied (holes) and unoccupied (particle) NTO pairs. For the [(FcHz)2Ni] and [(FcHz)2Cu] complexes, the hole and particle NTO pairs that contribute more than 50% to each excited state are included in Fig. 8. The UV-visible absorption spectrum for neutral [(FcHz)2Ni] demonstrates three strong absorptions at λ = 483, 501, and 564 nm and a weak absorption band at 614 nm (Fig. 8A). For excitations at 501, 564, and 614 nm, the hole and particle NTO pairs are clearly located on different parts of the [(FcHz)2Ni] molecule. For these three excitations, the charge moves mainly from nickel to the phenyl hydrazide and slightly to the ferrocene unit, indicating a metal–ligand charge transfer (MLCT). For λ = 483 nm, both NTOs of the particle and hole are located on the same parts of the molecule, which indicates a local excitation (LE). For this transition, the particle and hole NTOs are mainly delocalized over the ferrocene unit, and partly extended on the Ni central transition metal and hydrazide group.
The UV-visible absorption spectrum for [(FcHz)2Cu] in Fig. 8B exhibits two excitations at λ = 538 and 671 nm. The high-intensity absorption bond at 538 nm corresponds to the singlet α–α transition. In this case, the hole NTO is mainly delocalized between the copper atom, hydrazide group, and ferrocene unit. Meanwhile, in the particle NTO the electron charge is transferred from copper to the entire phenyl ring. Therefore, this excitation possesses an MLCT. The low-intensity absorption peak for [(FcHz)2Cu] at the 671 nm state is represented by two transitions involving two NTO pairs with different spins. The α–α transition is a local excitation on the ferrocene unit, while the β–β transition occurs from ferrocene to the hydrazide ligand, which has a ligand–ligand charge transfer (LLCT) character. The electron excitations in Fig. 8 confirm that in these intramolecular CT complexes, the Ni and Cu transition metals act as electron donors, and the phenyl hydrazide groups act as electron acceptors.
To better understand the characteristics of the absorption peaks of UV-visible spectra in Fig. 8, the wavelength, oscillator strength, percentage of contribution for each transition (weight factor), and the corresponding molecular orbital plots for neutral [(FcHz)2Ni] and [(FcHz)2Cu] are summarized in Tables 2 and 3, respectively. The excitations with oscillator strength >0.01 and the weight factors of transitions >20% have been reported in Tables 3 and 4, respectively. According to the TD-DFT calculations for [(FcHz)2Ni], the active orbitals in transitions are localized as follows: the occupied orbitals 178–181 are on the ferrocene moiety and 182–186 on the d orbitals of nickel, the unoccupied orbitals 187–192 and 193–196 are on the phenyl hydrazide and ferrocene moieties, respectively. For the [(FcHz)2Ni] complex, the calculated energy gap between the highest occupied molecular orbital 186 (HOMO) and the lowest unoccupied molecular orbital 187 (LUMO) is 0.237 a.u. or 6.45 eV. The absorption peak for the S0 → S1 transition in the range of 614 nm is attributed to the HOMO → LUMO transition, which corresponds to the MLCT. The HOMO is a nickel metal-centered combination orbital of s and dx2−y2. The LUMO is a π* molecular orbital mainly delocalized over the phenyl hydrazide group with a minor contribution to the ferrocene unit. The absorption S0 → S3 at λmax = 564 nm, like λ = 614, is also an MLCT from HOMO on nickel to LUMO + 1 on the phenyl hydrazide and parts of the ferrocene groups. The excitation S0 → S6 at λ = 501 nm (from MO 183 to 187) is also assigned to an MLCT transition from the dxz orbitals of Ni to LUMO. These three absorption bands demonstrate that the [(FcHz)2Ni] complex possesses the MLCT excited states, and are more delocalized on the hydrazide ligands. The excited complex [(FcHz)2Ni]* exhibits a strong redox potential and electron transfer ability, with respect to [(FcHz)2Ni], based on the results obtained in Table 6 of the present article. In addition, the calculated UV-visible spectrum in the range of 483 nm shows an S0 → S7 absorption band corresponding to local excitation in the ferrocene moiety. This excitation occurs from the occupied molecular orbital 180 (a combination of the dx2−y2 and dxz orbitals of Fe in interaction with the cyclopentadienyl π-orbitals) to the unoccupied molecular orbital 194 (an anti-bonding combination of the dyz orbitals of the Fe with cyclopentadienyl π*-orbitals) (Table 2).
| Entry | Photocatalysts | Solvent | Light | Base | Atmosphere | Yieldb (%) of benzaldehyde | Yieldb (%) of benzoic acid |
|---|---|---|---|---|---|---|---|
| Reaction conditions: photocatalysts 5 mg, p-nitro toluene 1 mmol, base 0.1 g, 25–28 °C, 48 h.a M = Ni and Cu.b The yields of products were determined with HPLC analysis. | |||||||
| 1 | [(FcHz-X)2M]a | MeCN | Blue | Cs2CO3 | Air | Trace | 0 |
| 2 | [(FcHz-X)2M] | EtOH | Blue | Cs2CO3 | Air | 0 | 0 |
| 3 | [(FcHz-X)2M] | MeOH | Blue | Cs2CO3 | Air | 0 | 0 |
| 4 | [(FcHz-X)2M] | 1,4 dioxane | Blue | Cs2CO3 | Air | 0 | 0 |
| 5 | [(FcHz-X)2M] | CH2Cl2 | Blue | Cs2CO3 | Air | 0 | 0 |
| 6 | [(FcHz-X)2M] | DMSO | Blue | Cs2CO3 | Air | 0 | 0 |
| 7 | [(FcHz-X)2M] | DMF | Blue | Cs2CO3 | Air | 0 | 0 |
| 8 | [(FcHz-X)2M] | H2O | Blue | Cs2CO3 | Air | 0 | 0 |
| 9 | [(FcHz–OH)2Ni] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 10 | [(FcHz–OH)2Cu] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 11 | [(FcHz–NO2)2Ni] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 12 | [(FcHz–NO2)2Cu] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 13 | [(FcHz) 2 Ni] |
MeCN/H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
Blue | Cs 2 CO 3 | Air | 81.3 | 13.3 |
| 14 | [(FcHz) 2 Cu] |
MeCN/H
2
O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) 1)
|
Blue | Cs 2 CO 3 | Air | 97.0 | 0 |
| 15 | FcHz | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 16 | FcHz–OH | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 17 | FcHz–NO2 | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 18 | Ferrocene | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 19 | [(FcHz)2M] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | K2CO3 | Air | 0 | 0 |
| 20 | [(FcHz)2M] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | K3PO4 | Air | 0 | 0 |
| 21 | [(FcHz)2M] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Na2CO3 | Air | 0 | 0 |
| 22 | [(FcHz)2M] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Et3N | Air | 0 | 0 |
| 23 | [(PhHz)2M] | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 24 | Ni(OAc)2·4H2O | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
| 25 | Cu(OAc)2·1H2O | MeCN/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Blue | Cs2CO3 | Air | 0 | 0 |
The TD-DFT results for the excitation states and the active molecular orbitals involved in transitions for [(FcHz)2Cu] are as follows: the occupied orbitals 173, 174, 177–179, 186α and 185β are localized on the d orbitals of the copper atom, the occupied orbitals 180–184, 185α and 186β are fully localized on the ferrocene units, and the unoccupied orbitals 188–198 are distributed on the phenyl hydrazide and/or ferrocene moieties. The singly occupied molecular orbital 187α (SOMO) is mainly delocalized over the hydrazide and ferrocene groups with a minor contribution of the dxz orbital of the Cu atom, and the unoccupied orbitals 188α or 187β (as LUMO) with the same energy levels and equal orbital isosurface plots are delocalized over the phenyl hydrazide and ferrocene groups. The calculated SOMO–LUMO energy gap for the [(FcHz)2Cu] complex is 0.184 a.u. or 5.00 eV. A detailed analysis of the electronic transitions in the UV-visible spectra in Table 3 reveals that for the [(FcHz)2Cu] complex, the excitation S0 → S9 at λ = 538 nm has an intense α–α transition from 187 (HOMO) to 189 (LUMO + 1), which is assigned to the LLCT from the ferrocene and hydrazide groups to the phenyl ring. The excitation S0 → S4 at λ = 671 for [(FcHz)2Cu] involves two considerable transitions. The first transition occurs from molecular orbital 185α (a combination of dx2−y2 and dz2 orbitals of Fe, π-orbital on hydrazide with a minor contribution of dxz of Cu) to the MO 196α (composition of dxy molecular orbital of the Fe with cyclopentadienyl π*-orbitals). The latter occurs from 184β (dxz molecular orbital of the Fe with cyclopentadienyl π*-orbitals) to the 196β molecular orbital (anti-bonding combination of the dx2−y2 and dz2 orbitals of Fe with cyclopentadienyl π*-orbitals). These two transitions are local charge transfers in ferrocene units. In electron excitations in the [(FcHz)2Cu] complex, there is a small amount of electrons, and the charge is also transferred from the d orbitals of the Cu atom to the phenyl hydrazide or ferrocene ligands. A comparison between the results of Tables 3 and 4 and the molecular orbital analysis for [(FcHz)2Ni] and [(FcHz)2Cu] demonstrate that electron transfer from Ni to ligands or MLCT plays an essential role in electron excitations of [(FcHz)2Ni], while in the [(FcHz)2Cu] complex, LLCT is more important.
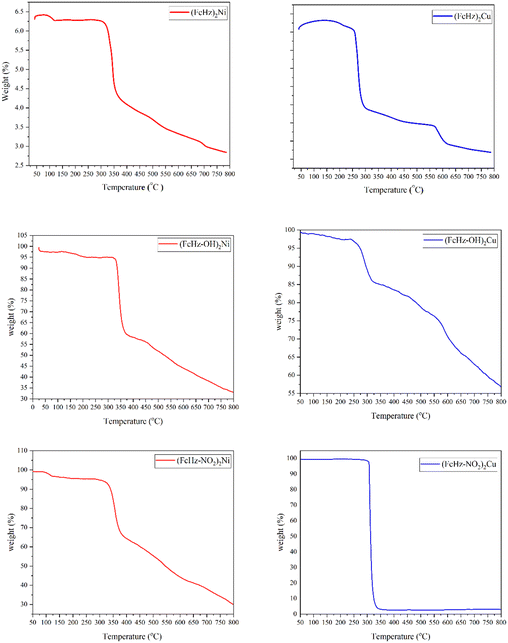 | ||
| Fig. 9 TGA graphs of FcHz, [(FcHz)2Ni], [(FcHz)2Cu], FcHz–OH, [(FcHz–OH)2Ni] and [(FcHz–OH)2Cu], FcHz–NO2, [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu]. | ||
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds in the cyclopentadienyl rings coordinated to iron.76 Carbon–oxygen bonds (C–O) are commonly found in organometal compounds containing ether functional groups or resonance of C
C bonds in the cyclopentadienyl rings coordinated to iron.76 Carbon–oxygen bonds (C–O) are commonly found in organometal compounds containing ether functional groups or resonance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O with other functional groups. These bonds typically appear in the XPS spectrum at a higher binding energy than C
O with other functional groups. These bonds typically appear in the XPS spectrum at a higher binding energy than C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C or C–C bonds, at around 286 eV.77 Carbon–nitrogen double bonds (C
C or C–C bonds, at around 286 eV.77 Carbon–nitrogen double bonds (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) are found in organic compounds containing imine groups. These bonds typically appear in the XPS spectrum at a higher binding energy at around 287 eV.78 The C 1s core XPS spectra of all synthesized complexes are shown in Fig. 12. In XPS analysis, the signal of N–C and N
N) are found in organic compounds containing imine groups. These bonds typically appear in the XPS spectrum at a higher binding energy at around 287 eV.78 The C 1s core XPS spectra of all synthesized complexes are shown in Fig. 12. In XPS analysis, the signal of N–C and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds can be observed in the nitrogen N 1s region (Fig. 13). The N 1s core level energy is affected by the surrounding chemical environment, and the presence of different chemical bonds results in distinct energy shifts. N–C bonds typically exhibit an N 1s binding energy of around 398 eV, while N
C bonds can be observed in the nitrogen N 1s region (Fig. 13). The N 1s core level energy is affected by the surrounding chemical environment, and the presence of different chemical bonds results in distinct energy shifts. N–C bonds typically exhibit an N 1s binding energy of around 398 eV, while N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds have a higher binding energy of around 400 eV.79 One notable observation that can result from the XPS spectra of N 1s is the distinctive peak at approximately 405 eV, which indicates the presence of a nitro group (–NO2) in the structure of both complexes [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu].80 The O–C bonds typically display a binding energy of approximately 532 eV for the oxygen 1s orbital in X-ray photoelectron spectroscopy (XPS).81 The presence of O–C bonds in the compounds can be validated based on the signal detected in this region (Fig. 14). Fig. 15 shows that the maximum binding energy for the Fe 2p3/2 photoelectron lines in all of the synthesized complexes is approximately 707 eV. Additionally, there is a noticeable signal on the higher energy side of the main photoelectron line, which corresponded to Fe 2p1/2.82 The organometallic structure contains two oxidation states of iron: Fe2+ and Fe3+. The peak with the lowest binding energy at 707.6 eV is assigned to the Fe2+ of the bis ferrocene groups within the structure. This finding is consistent with previous literature on compounds containing ferrocene in their structure.83 The observed graphs suggest that the majority of the Fe ions in the ferrocene material were in the (+2) oxidation state, as evidenced by the binding energy of 709.7 eV. The peak at 722.7 eV is identified as corresponding to the Fe3+ state of iron. Fig. 15 displays the Fe 2p3/2 and 2p1/2 spectra for all of the ferrocenyl hydrazide complexes. Cu 2p XPS spectra are commonly used to analyze copper complexes due to their sensitivity to even minor changes in the electronic structure of the complexes. The Cu 2p spectra of the complexes (Fig. 16) exhibit typical characteristics of Cu2+ compounds, including prominent satellites on the high-energy side of the main lines alongside the Cu 2p3/2 line. The core XPS spectra of transition metals are effectively described by the charge transfer (CT) model. As per this model, the main lines in the Cu 2p spectra are attributed to the |2p5 3d10 L − 1 > state that arises from CT between ligand atoms and the copper ion with a hole in the 2p core level. Additionally, the satellites in the Cu 2p spectra correspond to the transition of the copper ion during photoemission into the |2p5 3d9 > state. Identifying the oxidation state of copper through XPS is made simpler by the absence of satellites in the Cu 2p spectra of diamagnetic Cu1+ compounds, as opposed to paramagnetic Cu2+ compounds.84 The XPS spectrum of Ni2+ in organometallic compounds typically shows two major peaks: the Ni 2p3/2 and Ni 2p1/2 peaks. These peaks appear at binding energies of approximately 855 and 873 eV, respectively.85,86 The presence of nickel centers can be clearly observed in the complexes containing nickel. The XPS spectra of the Cu and Ni elements of complexes are displayed in Fig. 16.
C bonds have a higher binding energy of around 400 eV.79 One notable observation that can result from the XPS spectra of N 1s is the distinctive peak at approximately 405 eV, which indicates the presence of a nitro group (–NO2) in the structure of both complexes [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu].80 The O–C bonds typically display a binding energy of approximately 532 eV for the oxygen 1s orbital in X-ray photoelectron spectroscopy (XPS).81 The presence of O–C bonds in the compounds can be validated based on the signal detected in this region (Fig. 14). Fig. 15 shows that the maximum binding energy for the Fe 2p3/2 photoelectron lines in all of the synthesized complexes is approximately 707 eV. Additionally, there is a noticeable signal on the higher energy side of the main photoelectron line, which corresponded to Fe 2p1/2.82 The organometallic structure contains two oxidation states of iron: Fe2+ and Fe3+. The peak with the lowest binding energy at 707.6 eV is assigned to the Fe2+ of the bis ferrocene groups within the structure. This finding is consistent with previous literature on compounds containing ferrocene in their structure.83 The observed graphs suggest that the majority of the Fe ions in the ferrocene material were in the (+2) oxidation state, as evidenced by the binding energy of 709.7 eV. The peak at 722.7 eV is identified as corresponding to the Fe3+ state of iron. Fig. 15 displays the Fe 2p3/2 and 2p1/2 spectra for all of the ferrocenyl hydrazide complexes. Cu 2p XPS spectra are commonly used to analyze copper complexes due to their sensitivity to even minor changes in the electronic structure of the complexes. The Cu 2p spectra of the complexes (Fig. 16) exhibit typical characteristics of Cu2+ compounds, including prominent satellites on the high-energy side of the main lines alongside the Cu 2p3/2 line. The core XPS spectra of transition metals are effectively described by the charge transfer (CT) model. As per this model, the main lines in the Cu 2p spectra are attributed to the |2p5 3d10 L − 1 > state that arises from CT between ligand atoms and the copper ion with a hole in the 2p core level. Additionally, the satellites in the Cu 2p spectra correspond to the transition of the copper ion during photoemission into the |2p5 3d9 > state. Identifying the oxidation state of copper through XPS is made simpler by the absence of satellites in the Cu 2p spectra of diamagnetic Cu1+ compounds, as opposed to paramagnetic Cu2+ compounds.84 The XPS spectrum of Ni2+ in organometallic compounds typically shows two major peaks: the Ni 2p3/2 and Ni 2p1/2 peaks. These peaks appear at binding energies of approximately 855 and 873 eV, respectively.85,86 The presence of nickel centers can be clearly observed in the complexes containing nickel. The XPS spectra of the Cu and Ni elements of complexes are displayed in Fig. 16.
 | ||
| Fig. 12 XPS spectra of the C 1s core of FcHz, [(FcHz)2Ni], [(FcHz)2Cu], FcHz–OH, [(FcHz–OH)2Ni] and [(FcHz–OH)2Cu], FcHz–NO2, [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu]. | ||
 | ||
| Fig. 13 XPS spectra of the N 1s core of FcHz, [(FcHz)2Ni], [(FcHz)2Cu], FcHz–OH, [(FcHz–OH)2Ni] and [(FcHz–OH)2Cu], FcHz–NO2, [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu]. | ||
 | ||
| Fig. 14 XPS spectra of the O 1s core of FcHz, [(FcHz)2Ni], [(FcHz)2Cu], FcHz–OH, [(FcHz–OH)2Ni] and [(FcHz–OH)2Cu], FcHz–NO2, [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu]. | ||
 | ||
| Fig. 15 XPS spectra of the Fe 2p core of FcHz, [(FcHz)2Ni], [(FcHz)2Cu], FcHz–OH, [(FcHz–OH)2Ni] and [(FcHz–OH)2Cu], FcHz–NO2, [(FcHz–NO2)2Ni] and [(FcHz–NO2)2Cu]. | ||
Photocatalytic activities of [(FcHz-X)2Ni] and [(FcHz-X)2Cu] complexes under visible light condition
It is necessary to emphasize that the selective preparation of benzaldehyde is a very demanding function. This is due to its more facile oxidation susceptibility in aerobic states compared to that of toluene.87 Numerous attempts have been made to explore appropriate photocatalytic approaches for the selective toluene oxidation to benzaldehyde, with particular consideration to the stability.88 In typical cases, homogeneous catalysts or deep eutectic solvents (DES) as special reaction conditions, photocatalytic oxidation systems, electrochemical processes, and heterogeneous catalysts for toluene oxidation have been reported.89–92 Many charge transfer complexes and metal-bromide catalysts have been studied for toluene oxidation.93,94 Unluckily, the principal problems for this oxidation process are the rare production rate and the absence of selectivity.95 In fact, the production rate enhancement was carried out with a reduction in selectivity towards benzaldehyde because of the formation of benzoic aci.96 Moreover, the oxidation process conditions have been demonstrated as basically green by researchers. Generally, the catalyst formation needs several steps or contains perilous solvents and reagents, which completely increase the environmental effect of the total reaction.95 Therefore, in order to overcome the problems and defects that catalysts have for toluene oxidation, we designed homogeneous catalysts based on ferrocenyl-hydrazide as a ligand and the metals including nickel and copper as a central metal, which have a high conversion rate under visible light conditions. By comparing the activity, we found that they have high selectivity in oxidation. So, the photocatalytic activities of these new complexes [(FcHz-X)2Ni] and [(FcHz-X)2Cu] were investigated in the oxidation reactions. At first, p-nitro toluene (6a) was selected as the model compound for the study of oxidation of the benzylic C(sp3)–H bond (Scheme 2). To find the best reaction conditions, various parameters were evaluated. One of these parameters is the solvent. To evaluate the effect of the solvent, a wide range of polar and non-polar solvents were studied. Solvents such as MeOH, EtOH, H2O, DMF, DMSO, THF, CH2Cl2, 1,4-dioxane, and CH3CN as well as a mixture of organic solvents with water were chosen. However, none of them showed a good effect on the oxidation reaction, except a mixture of water/acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Table 4). Afterward, the effect of different bases was investigated and Cs2CO3 was shown to be the best result (Table 4). Next, the amount and kinds of photocatalysts were investigated. Varying amounts from 3 to 10 mg of different photocatalysts Fc, FcHz-X, [(FcHz-X)2Ni], [(FcHz-X)2Cu], [(PhHz)2Ni], [(PhHz)2Cu], Ni(OAc)2, and Cu(OAc)2 were checked. The oxidation reaction was affected in the presence of 5 mg of the [(FcHz)2Ni] and [(FcHz)2Cu]. However, Fc or other ferrocenyl-hydrazide Schiff bases and metal complexes could not oxidize the model compound 6a to the corresponding carbonyl molecules 6b and 6c. The oxidation reaction with less than 5 mg of the photocatalysts achieved low yield, and no products were produced in the absence of [(FcHz)2Ni] and [(FcHz)2Cu] complexes, respectively (Table 5). Comparison of the photo-activity of these complexes [(FcHz)2Ni] and [(FcHz)2Cu] under the same reaction conditions showed that the [(FcHz)2Ni] oxidized p-nitro toluene to p-nitro benzaldehyde and p-nitro benzoic acid after 48 h, but the [(FcHz)2Cu] complex performed this reaction to produce only p-nitro benzaldehyde after 48 h. Based on the better properties observed in the optical and electrochemical analysis of the [(FcHz)2Ni] complex, we expected to have more activity for this complex. The high activity of [(FcHz)2Ni] caused the oxidation of p-nitro toluene to proceed to the formation of p-nitro benzoic acid and the selectivity of this photocatalyst decreased. However, the lower activity of [(FcHz)2Cu] has increased the selectivity of this catalyst. Therefore, the [(FcHz)2Cu] complex has more selectivity than the Ni one (Table 6). A comparison of different light sources with various wavelengths confirmed that the blue lamp has the highest efficiency (Table 6). As shown in Fig. 7a, the absorption spectrum (UV-visible) of the catalysts showed the maximum absorption range of [(FcHz)2Ni] in the 448–600 nm (λmax = 480 nm) and [(FcHz)2Cu] in the range of 440–577 (λmax = 465 nm), respectively. Therefore, it is justifiable for the blue light to give the best result. The role of the atmosphere (O2, and Ar) in promoting the reaction was the other factor that was examined (Table 7). The reaction slightly progressed in the presence of argon, but was highly efficient in the presence of oxygen. It can be concluded that oxygen is effective. Almost similar results were observed under atmospheric oxygen. So, we chose the air atmosphere as a facile condition for the oxidation of benzylic C(sp3)–H bonds.
1) (Table 4). Afterward, the effect of different bases was investigated and Cs2CO3 was shown to be the best result (Table 4). Next, the amount and kinds of photocatalysts were investigated. Varying amounts from 3 to 10 mg of different photocatalysts Fc, FcHz-X, [(FcHz-X)2Ni], [(FcHz-X)2Cu], [(PhHz)2Ni], [(PhHz)2Cu], Ni(OAc)2, and Cu(OAc)2 were checked. The oxidation reaction was affected in the presence of 5 mg of the [(FcHz)2Ni] and [(FcHz)2Cu]. However, Fc or other ferrocenyl-hydrazide Schiff bases and metal complexes could not oxidize the model compound 6a to the corresponding carbonyl molecules 6b and 6c. The oxidation reaction with less than 5 mg of the photocatalysts achieved low yield, and no products were produced in the absence of [(FcHz)2Ni] and [(FcHz)2Cu] complexes, respectively (Table 5). Comparison of the photo-activity of these complexes [(FcHz)2Ni] and [(FcHz)2Cu] under the same reaction conditions showed that the [(FcHz)2Ni] oxidized p-nitro toluene to p-nitro benzaldehyde and p-nitro benzoic acid after 48 h, but the [(FcHz)2Cu] complex performed this reaction to produce only p-nitro benzaldehyde after 48 h. Based on the better properties observed in the optical and electrochemical analysis of the [(FcHz)2Ni] complex, we expected to have more activity for this complex. The high activity of [(FcHz)2Ni] caused the oxidation of p-nitro toluene to proceed to the formation of p-nitro benzoic acid and the selectivity of this photocatalyst decreased. However, the lower activity of [(FcHz)2Cu] has increased the selectivity of this catalyst. Therefore, the [(FcHz)2Cu] complex has more selectivity than the Ni one (Table 6). A comparison of different light sources with various wavelengths confirmed that the blue lamp has the highest efficiency (Table 6). As shown in Fig. 7a, the absorption spectrum (UV-visible) of the catalysts showed the maximum absorption range of [(FcHz)2Ni] in the 448–600 nm (λmax = 480 nm) and [(FcHz)2Cu] in the range of 440–577 (λmax = 465 nm), respectively. Therefore, it is justifiable for the blue light to give the best result. The role of the atmosphere (O2, and Ar) in promoting the reaction was the other factor that was examined (Table 7). The reaction slightly progressed in the presence of argon, but was highly efficient in the presence of oxygen. It can be concluded that oxygen is effective. Almost similar results were observed under atmospheric oxygen. So, we chose the air atmosphere as a facile condition for the oxidation of benzylic C(sp3)–H bonds.
 | ||
| Scheme 2 Photocatalytic activity of [(FcHz)2Ni] and [(FcHz)2Cu] complexes in p-nitro toluene oxidation. | ||
| Entry | Photocatalysts | Amount of Photocatalyst (g) | Solvent | Light | Yielda (%) of benzaldehyde | Yield (%) of benzoic acid |
|---|---|---|---|---|---|---|
| Reaction conditions: p-nitro toluene 1 mmol, Cs2CO3 0.1 g, 25–28 °C, 48 h, presence air atmosphere.a The yields of products were determined with HPLC analysis. | ||||||
| 1 | [(FcHz)2Ni] | 0.003 | MeCN/H2O | Blue | 55 | 5 |
| 2 | [(FcHz)2Cu] | 0.003 | MeCN/H2O | Blue | 65 | 0 |
| 3 | [(FcHz)2Ni] | 0.01 | MeCN/H2O | Blue | 80 | 15 |
| 4 | [(FcHz)2Cu] | 0.01 | MeCN/H2O | Blue | 97 | 0 |
| 5 | FcHz | 0.003 | MeCN/H2O | Blue | 0 | 0 |
| 6 | Ferrocene | 0.003 | MeCN/H2O | Blue | 0 | 0 |
| 7 | FcHz | 0.01 | MeCN/H2O | Blue | 0 | 0 |
| 8 | Ferrocene | 0.01 | MeCN/H2O | Blue | 0 | 0 |
| 9 | Ni(OAc)2·4H2O | 0.003 | MeCN/H2O | Blue | 0 | 0 |
| 10 | Cu(OAc)2·1H2O | 0.003 | MeCN/H2O | Blue | 0 | 0 |
| 11 | Ni(OAc)2·4H2O | 0.01 | MeCN/H2O | Blue | 0 | 0 |
| 12 | Cu(OAc)2·1H2O | 0.01 | MeCN/H2O | Blue | 0 | 0 |
| Entry | Photocatalysts | Light | Solvent | Atmosphere | Yielda (%) of benzaldehyde | Yielda (%) of benzoic acid |
|---|---|---|---|---|---|---|
| Reaction conditions: photocatalysts 5 mg, p-nitro toluene 1 mmol, Cs2CO3 0.1 g, 25–28 °C, 48 h, presence air atmosphere.a The yields of products were determined with HPLC analysis. | ||||||
| 1 | [(FcHz)2Ni] | Blue | MeCN/H2O | Air | 81.3 | 13.3 |
| 2 | [(FcHz)2Ni] | Violet | MeCN/H2O | Air | 71.2 | 24.1 |
| 3 | [(FcHz)2Ni] | White | MeCN/H2O | Air | 52.6 | 15.2 |
| 4 | [(FcHz)2Ni] | Green | MeCN/H2O | Air | 75.7 | 0 |
| 5 | [(FcHz)2Ni] | Red | MeCN/H2O | Air | 66.0 | 0 |
| 6 | [(FcHz)2Ni] | Dark | MeCN/H2O | Air | 0 | 0 |
| 7 | [(FcHz)2Cu] | Blue | MeCN/H2O | Air | 97 | 0 |
| 8 | [(FcHz)2Cu] | Violet | MeCN/H2O | Air | 61.8 | 0 |
| 9 | [(FcHz)2Cu] | White | MeCN/H2O | Air | 31.1 | 0 |
| 10 | [(FcHz)2Cu] | Green | MeCN/H2O | Air | 72.6 | 0 |
| 11 | [(FcHz)2Cu] | Red | MeCN/H2O | Air | 65.3 | 0 |
| 12 | [(FcHz)2Cu] | Dark | MeCN/H2O | Air | 0 | 0 |
| Entry | Photocatalysts | Light | Solvent | Atmosphere | Yielda (%) of benzaldehyde | Yielda (%) of benzoic acid |
|---|---|---|---|---|---|---|
| Reaction conditions: photocatalysts 5 mg, p-nitro toluene 1 mmol, Cs2CO3 0.1 g, 25–28 °C, 48 h.a The yields of products were determined with HPLC analysis. | ||||||
| 1 | [(FcHz)2Ni] | Blue | MeCN/H2O | O2 | 83 | 15 |
| 2 | [(FcHz)2Ni] | Blue | MeCN/H2O | Argon | Trace | 0 |
| 3 | [(FcHz)2Cu] | Blue | MeCN/H2O | O2 | 95 | 0 |
| 4 | [(FcHz)2Cu] | Blue | MeCN/H2O | Argon | 0 | 0 |
Furthermore, in order to examine the impact of various electron groups on toluene oxidation, the para-methoxy electron-donating group was selected for investigation. The findings reveal that the oxidation of p-methoxy toluene exhibited the highest efficiency (57.6% p-methoxy benzaldehyde and 30.4% p-methoxy benzoic acid) when [(FcHz)2Ni] was employed as the catalyst and blue light was utilized. The experimental conditions were monitored using HPLC, and the corresponding graphs can be found in ESI† Fig. S41–S48.
After toluene was successfully oxidized, we decided to investigate the ability of these new photocatalysts to oxidize the C(sp2)–H bond as well. So, styrene was chosen and the oxidation process conditions (such as photocatalyst quantities, solvents, visible-light sources, atmosphere, as well as different photocatalysts) were studied. After checking a series of oxidation reaction factors, it has been found that [(FcHz-X)2M] (5 mg) as photocatalysts, blue LED light, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water/acetonitrile as the solvent, air atmosphere, and ambient temperature were the most ideal options for benzaldehyde formation (Table 8). It can be seen that the results are the same as those for toluene oxidation (Scheme 3). The conversion of p-nitro toluene to p-nitro benzaldehyde and p-nitro benzoic acid, as well as the oxidation of styrene to benzaldehyde, was monitored by HPLC. All HPLC diagrams for the oxidation process in different visible lights can be seen in ESI.†
1 water/acetonitrile as the solvent, air atmosphere, and ambient temperature were the most ideal options for benzaldehyde formation (Table 8). It can be seen that the results are the same as those for toluene oxidation (Scheme 3). The conversion of p-nitro toluene to p-nitro benzaldehyde and p-nitro benzoic acid, as well as the oxidation of styrene to benzaldehyde, was monitored by HPLC. All HPLC diagrams for the oxidation process in different visible lights can be seen in ESI.†
| Entry | Photocatalysts | Light | Solvent | Atmosphere | Yielda (%) of benzaldehyde |
|---|---|---|---|---|---|
| Reaction conditions: photocatalysts 5 mg, styrene 1 mmol, Cs2CO3 0.1 g, 25–28 °C, 24 h.a The yields of the products were determined with HPLC analysis. | |||||
| 1 | [(FcHz)2Ni] | Blue | MeCN/H2O | Air | 95.3 |
| 2 | [(FcHz)2Ni] | Violet | MeCN/H2O | Air | 85.4 |
| 3 | [(FcHz)2Ni] | White | MeCN/H2O | Air | 74.6 |
| 4 | [(FcHz)2Ni] | Green | MeCN/H2O | Air | 78.8 |
| 5 | [(FcHz)2Ni] | Red | MeCN/H2O | Air | 76.2 |
| 6 | [(FcHz)2Ni] | Dark | MeCN/H2O | Air | 0 |
| 7 | [(FcHz)2Cu] | Blue | MeCN/H2O | Air | 95.0 |
| 8 | [(FcHz)2Cu] | Violet | MeCN/H2O | Air | 87.0 |
| 9 | [(FcHz)2Cu] | White | MeCN/H2O | Air | 83.3 |
| 10 | [(FcHz)2Cu] | Green | MeCN/H2O | Air | 84.1 |
| 11 | [(FcHz)2Cu] | Red | MeCN/H2O | Air | 81.0 |
| 12 | [(FcHz)2Cu] | Dark | MeCN/H2O | Air | 0 |
In order to delve deeper into the oxidation of styrene, we conducted experiments using p-nitrostyrene. The findings unequivocally demonstrate that the presence of the nitro group influences the oxidation process, serving as evidence for the suggested mechanism involving the formation of a destabilized cation-radical, which subsequently diminishes the product efficiency. Notably, the highest efficiency in the oxidation of p-nitrostyrene was achieved when [(FcHz)2Ni] was employed as the catalyst and blue light was utilized (73.9% p-nitro benzaldehyde). Additional results can be found in ESI† in Fig. S48–S56.
Mechanistic studies: a proposed mechanism
The synthesized complexes offer a notable advancement in the realm of homogeneous photocatalysts. Their distinct characteristics and properties set them apart from other photocatalysts, making them a subject of great interest and significance. Compared to other homogeneous photocatalysts, the synthesized complexes exhibit enhanced performance in various photocatalytic reactions.41 Their unique structural features and optimized electronic properties contribute to their superior efficiency and effectiveness. Additionally, the synthesized complexes demonstrate excellent stability under harsh reaction conditions, ensuring prolonged catalytic activity and minimizing catalyst degradation. Furthermore, the synthesized complexes offer a promising avenue for sustainable photocatalysis. By utilizing earth-abundant metals and incorporating environmentally friendly ligands, these complexes address the limitations associated with noble metal photocatalysts.97 This not only reduces the cost of production, but also promotes the use of more accessible and sustainable materials. Utilizing ferrocene in the design of complexes for photocatalytic activity provides additional benefits. Ferrocene's redox properties enable efficient electron transfer processes, while its stability ensures sustained photocatalytic activity over extended periods.98 Overall, non-noble metal complexes, particularly those incorporating ferrocene, show promise as alternatives to noble metal photocatalysts. On the contrary, it is important to highlight that there is a dearth of prior research on the oxidation of toluene and styrene using organometallic compounds under visible light. The prevailing focus in existing studies lies in thermal reactions that require external oxidants. What distinguishes our study is the exclusive use of organometallic complexes, eliminating the necessity for external oxidants, while simultaneously being exposed to visible light irradiation (Fig. 17).47,99,100 | ||
| Fig. 17 Comparison of the oxidation of selected compounds in the presence of different organometallic catalysts. | ||
After analyzing the electrochemical behaviors of the metals in the [(FcHz)2Ni] and [(FcHz)2Cu] complexes using cyclic voltammetry (CV) (Fig. S3†), it is apparent that Ni and Cu do not exhibit any electronic activities and remain inactive in their respective complexes. However, FeII in the ferrocene moieties demonstrates significant electrochemical activities, as it can be oxidized and reduced. Thus, the electron transfer responsibility lies with FeII in the ferrocene moieties. Additionally, the UV-visible spectra revealed that the [(FcHz)2Ni] and [(FcHz)2Cu] complexes have strong absorption in the visible region. The absorption diagram highlights the significant role that nickel and copper play in the absorption of the visible region. It is evident that when exposed to light, nickel or copper complexes undergo oxidation and transform into the excited state. This process cannot be ignored, as it directly influences the visible absorption spectra of the complexes. Accordingly, detecting a detailed discovery of the oxidation reaction mechanism(s) is challenging. Indeed, from the previous reports96,101 and investigation of several control reactions, spectroscopy analysis of a mechanism can be proposed, as shown in Fig. 18. The process commences with the excitation of the photocatalyst, which triggers a single-electron transfer, resulting in the creation of a radical-cation. Ultimately, the photocatalyst is quenched, bringing the mechanism to a close.
 | ||
| Fig. 18 Possible mechanism of the photocatalytic oxidation of p-nitro toluene with [(FcHz)2Ni] and [(FcHz)2Cu] complexes. | ||
Through an electrochemical study (CV) of the [(FcHz)2Ni] and [(FcHz)2Cu] complexes, it has been observed that ferrocenyl-hydrazide complexes play a crucial role in electron transfer. As a result, the ferrocene moiety can function as the SET cycle. Initially, the [(FcHz)2M] complexes were excited via light irradiation to generate [(FcHz)2M]*. Subsequently, the superoxide radical-anion (O2˙−) is formed when oxygen captures the single excited electron from the complex, resulting in the photocatalyst becoming a radical-cation (PC˙+). The photocatalyst can be quenched by toluene, which donates an electron to the radical-cation of photocatalyst (PC˙+), transforming it into a benzyl radical-cation I (benzyl˙+). The benzyl radical-cation (benzyl˙+) reacted with the superoxide radical-anion (O2˙−), resulting in the production of PhHOOH II. It is speculated that this behavior is due to the electron donor-acceptor properties of the ferrocene moiety upon visible light irradiation. Thus, the homogeneous dispersion and distinct electronic activity of bis-ferrocene scaffolds in metal complexes result in the in situ generation of reactive oxygen species and the benzylic cation.102,103 Finally, intermediate II leads to the formation of an oxygenated compound (benzaldehyde).104
For the styrene oxidation reaction, the most probable mechanism is shown in Fig. 19. The photocatalyst undergoes a similar oxidation mechanism to toluene when oxygen is transformed into superoxide radical-anion species (O2˙−), resulting in the photocatalyst being converted into the radical-cation species (PC˙+). When styrene donates electrons, it can quench the photocatalyst and produce a styrene radical-cation (styrene˙+).46,105,106 The intermediate III is formed through the attack of the superoxide radical-anion (O2˙−) to the radical-cation of styrene undergoing intramolecular cyclization to produce intermediate (III). The desired carbonyl compounds were obtained via [2 + 2] cleavage of the four-membered ring in (IV).107
 | ||
| Fig. 19 Possible mechanism of the photocatalytic oxidation of styrene with [(FcHz)2Ni] and [(FcHz)2Cu]. | ||
Given the desirable results of the oxidation of p-nitro toluene and styrene in the presence of [(FcHz)2Ni] and [(FcHz)2Cu] complexes as photocatalysts, we decided to distribute the oxidation to the C–C double bond of indoles (Scheme 4). Based on all of the data, we developed a transition metal methodology for the oxidation of indoles to isatin in a mild reaction condition with the help of a visible light source. Hence, 1H-indole was applied as the pattern molecule to optimize the reaction conditions under an oxygen atmosphere. As shown in (Table 9), different ferrocene-catalysts, such as commercially available ferrocene, synthesized FcHz and [(FcHz-X)2Ni] and [(FcHz-X)2Cu] complexes, were screened with several polar and non-polar solvents and a mixture of organic solvents with water under the irradiation of blue, white, green, red and violet LEDs for 48 h. This reaction was very hard and, in many cases, ineffective. In the presence of any solvents, there was no response, except for a blend of MeCN and H2O with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In addition, based on the blend of the reaction, it would be ineffective and the reaction was stopped. Among visible lights with different wavelengths, only the blue LED was effective. More favorable results were obtained under oxygen balloon conditions. The progress of the reactions was controlled by TLC. Finally, the isatin as the desired product was separated from the unreacted indole by plate chromatography (petroleum ether/ethyl acetate 1
1. In addition, based on the blend of the reaction, it would be ineffective and the reaction was stopped. Among visible lights with different wavelengths, only the blue LED was effective. More favorable results were obtained under oxygen balloon conditions. The progress of the reactions was controlled by TLC. Finally, the isatin as the desired product was separated from the unreacted indole by plate chromatography (petroleum ether/ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2).
2).
| Entry | Photocatalysts | Light | Solvent | Atmosphere | Yielda (%) of benzaldehyde |
|---|---|---|---|---|---|
| Reaction conditions: photocatalysts 5 mg, indole 1 mmol, 25–28 °C, 48 h.a Isolated yield, separation with plate chromatography. | |||||
| 1 | [(FcHz)2Ni] | Blue | MeCN/H2O | Air | 30 |
| 2 | [(FcHz)2Ni] | Blue | MeCN/H2O | O2 | 45.0 |
| 3 | [(FcHz)2Cu] | Blue | MeCN/H2O | Air | 0 |
| 4 | [(FcHz)2Cu] | Blue | MeCN/H2O | O2 | 0 |
According to a previous similar mechanism,47 to perform the indole oxidation reaction, the photocatalyst was first excited by a blue lamp. This excitation caused the photocatalyst to enter an excited state. In this state, it transferred an electron to generate a radical-cation of the photocatalyst. The C–C double bond of indole 8a as the same styrene was transformed to radical-cation (IV) by giving an electron to the excited photocatalyst. The formed superoxide radical anion (O2˙−) then attacked the radical-cation intermediate (IV) to produce the related peroxo species, which was then further oxidized to (V). After the formation of (V), the resulting peroxo-indole moiety (V) again transferred a radical to an excited photocatalyst in another SET cycle, generating the corresponding radical cation (VI). Meanwhile, the radical-anion superoxide was converted to hydrogen peroxide, and isatin 8b was subsequently produced. Fig. 20 illustrates the possible mechanism of the photocatalyst oxidation of indole with [(FcHz)2Ni].47
Generally, the coordination of the ferrocenyl hydrazide ligands to copper and nickel enhances the photoelectronic and electronic characteristics, such as UV-visible, photoluminescence, cyclic voltammetry, and electrochemical impedance spectroscopy in the system, thereby facilitating oxidation reactions when exposed to light irradiation. Indeed, it can be stated that the ferrocene component within the ferrocenyl hydrazide ligand bears the responsibility for executing the oxidation of the chosen organic compounds, while nickel and copper contribute to enhancing the properties of this ligand.
We studied a series of control reactions to prove that the oxidation reaction process proceeds by a radical pattern. Besides argon and oxygen atmosphere, TEMPO (2,2,6,6-tetramethylpiperidin-1-oxyl) was used for Fe trapping. By adding it to the reaction, the production of carbonyl compounds reached trace levels (Scheme 5).
Experimental section
A common method for the preparation of ferrocenyl-hydrazide Schiff bases (FcHz-X) 3a–c
Treatment of commercially-available ferrocene carboxaldehyde 1 (1 mmol) with benzoic hydrazides (with hydroxy and nitro at the para position) 2a–c (1 mmol) in methanol (30 mL) as a solvent and two or three drops of glacial acetic acid as catalyst was refluxed for 12 h to result in FcHz-X (3a–c). The evidence of synthesis of the ferrocenyl Schiff base ligand was indicated with TLC (petroleum ether/ethylacetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). At the end of the reaction, the mixture was chilled to 25 °C. The solvent was vaporized under reduced pressure to give the crude FcHz-X.48
2). At the end of the reaction, the mixture was chilled to 25 °C. The solvent was vaporized under reduced pressure to give the crude FcHz-X.48
A common method for the preparation of ferrocenyl-hydrazide attached Ni and Cu complexes ([(FcHz)2M]) 4a–c and 5a–c
Sodium acetate (6 mmol, 0.84 mL) was launched into a mixture of FcHz (3a–c) (1 mmol) and Ni(II) acetate·4 H2O (0.5 mmol, 0.124 g) or Cu(II) acetate·1 H2O (0.5 mmol, 0.099 g) in methanol (30 mL). The mixture was stirred for 9 h at 70 °C under reflux. The end of the coordination process was checked via thin-layer chromatography (petroleum ether/ethylacetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). When the process was augmented, the red precipitates were separated and washed with hot methanol and water several times.48
2). When the process was augmented, the red precipitates were separated and washed with hot methanol and water several times.48
A common method for C–H (sp3) oxidation processes
A blend of p-nitro toluene (1 mmol) and cesium carbonate (0.3 mmol, ≃0.1 g) in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1
H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (2 mL) was placed in the test tube (16 mm). ([(FcHz)2Ni]) and ([(FcHz)2Cu]) (5 mg) were added and the tubes of the processes were illuminated by 12 W LED lamps at the previously described homemade photoreactor system for the appropriate time (48 h). Normally, optimized conditions were kept (air atmosphere, cesium carbonate as base, and room temperature). The end of the process was checked by thin-layer chromatography. After completion, for working up the reaction, the mixture of the reactions was put in the separatory funnel, then water and EtOAc were added, and the organic phase was separated and dried over anhydrous Na2SO4 and concentrated under vacuum pressure. The percentage of the oxidation products was screened through high-performance liquid chromatography (HPLC).
1 (2 mL) was placed in the test tube (16 mm). ([(FcHz)2Ni]) and ([(FcHz)2Cu]) (5 mg) were added and the tubes of the processes were illuminated by 12 W LED lamps at the previously described homemade photoreactor system for the appropriate time (48 h). Normally, optimized conditions were kept (air atmosphere, cesium carbonate as base, and room temperature). The end of the process was checked by thin-layer chromatography. After completion, for working up the reaction, the mixture of the reactions was put in the separatory funnel, then water and EtOAc were added, and the organic phase was separated and dried over anhydrous Na2SO4 and concentrated under vacuum pressure. The percentage of the oxidation products was screened through high-performance liquid chromatography (HPLC).
A common method for C–H (sp2) oxidation reactions
A blend of styrene (1 mmol), cesium carbonate (0.3 mmol, ≃0.1 g) in MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1
H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (2 mL), then ([(FcHz)2Ni]) and ([(FcHz)2Cu]) (5 mg) were combined, and the tube of the reaction was irradiated by 12 W LED lamps at the homemade photoreactor system for 24 h. Optimized conditions were normally maintained (air atmosphere, Cs2CO3 as base, and room temperature). The end of the process was controlled by thin-layer chromatography (TLC). After completion, for working up the reaction, the mixture of the reactions was put in the separatory funnel, then water and ethyl acetate were added, and the organic phase was separated and dried over anhydrous Na2SO4 and concentrated under vacuum pressure. The efficiency of the oxidation products was screened through high-performance liquid chromatography (HPLC).
1 (2 mL), then ([(FcHz)2Ni]) and ([(FcHz)2Cu]) (5 mg) were combined, and the tube of the reaction was irradiated by 12 W LED lamps at the homemade photoreactor system for 24 h. Optimized conditions were normally maintained (air atmosphere, Cs2CO3 as base, and room temperature). The end of the process was controlled by thin-layer chromatography (TLC). After completion, for working up the reaction, the mixture of the reactions was put in the separatory funnel, then water and ethyl acetate were added, and the organic phase was separated and dried over anhydrous Na2SO4 and concentrated under vacuum pressure. The efficiency of the oxidation products was screened through high-performance liquid chromatography (HPLC).
A common method for indole oxidation to isatin reaction
To a test tube (16 mm) with a stirring bar, indole (1 mmol), ([(FcHz)2Ni]) (5 mg), MeCN (1 mL), and H2O (1 mL) were added. Then, the O2 gas was inserted via an O2-filled balloon. The obtained blend was stirred for 48 h under a blue LED lamp (the oxidation reaction can be checked via TLC). Then, the obtained blend was worked up with an aqueous medium (utilizing deionized H2O) in the separatory funnel, and was extracted several times with EtOAc. The organic layer was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The products were purified using silica gel chromatography with EtOAc and petroleum ether as solvents (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate
1 ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether).
petroleum ether).
Conclusion
Six novel ferrocene-based homogeneous photocatalysts with different central metals have been synthesized, and their electrochemical and spectroscopic properties were investigated with support from physicochemical modeling. The synthesized compounds were fully identified, and oxidation of C(sp3)–H and C(sp2)–H bonds were selected to check their efficiency. By comparing the activity of the complexes [(FcHz)2Ni] and [(FcHz)2Cu], we found that they were highly efficient in the oxidation of toluene derivatives. However, the [(FcHz)2Cu] complex had a high selectivity and the oxidation product was only benzaldehyde. The [(FcHz)2Ni] complex also produced benzoic acid. Regarding the selective cleavage of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of styrene, both complexes performed similarly and showed high efficiency. Finally, the [(FcHz)2Ni] complex was able to convert indole to isatin under mild conditions in the existence of an oxygen balloon. Mechanistic details for the reactions were presented, and DFT and TD-DFT theoretical analyses were performed for further investigations on the geometrical and electronic structure of the [(FcHz)2Ni] and [(FcHz)2Cu] photocatalysts.
C bond of styrene, both complexes performed similarly and showed high efficiency. Finally, the [(FcHz)2Ni] complex was able to convert indole to isatin under mild conditions in the existence of an oxygen balloon. Mechanistic details for the reactions were presented, and DFT and TD-DFT theoretical analyses were performed for further investigations on the geometrical and electronic structure of the [(FcHz)2Ni] and [(FcHz)2Cu] photocatalysts.
Author contributions
Mohammad Bashiri conceived and planned the experiments, carried out the experiments, and wrote the manuscript. Mona Hosseini-Sarvari designed, directed the project, aided in interpreting the results, and edited the manuscript. Sara Fakhraee performed the theoretical and DFT studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Shiraz University council for their support, and are grateful for financial support from the Iran National Science Foundation (grant no. 4015011).Notes and references
- A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li and Y. Liang, Metallaphotoredox: the merger of photoredox and transition metal catalysis, Chem. Rev., 2021, 122, 1485–1542 CrossRef.
- L. Hao, H. Huang, Y. Zhang and T. Ma, Oxygen vacant semiconductor photocatalysts, Adv. Funct. Mater., 2021, 31, 2100919 CrossRef CAS.
- H.-J. Son, C. Pac and S. O. Kang, Inorganometallic Photocatalyst for CO2 Reduction, Acc. Chem. Res., 2021, 54, 4530–4544 CrossRef CAS PubMed.
- A. K. Bagdi and P. Sikdar, Rhodamines as Photocatalyst in Organic Synthesis, Curr. Green Chem., 2021, 8, 46–61 CrossRef CAS.
- Z. Wei, W. Wang, W. Li, X. Bai, J. Zhao, E. C. Tse, D. L. Phillips and Y. Zhu, Steering electron–hole migration pathways using oxygen vacancies in tungsten oxides to enhance their photocatalytic oxygen evolution performance, Angew. Chem., Int. Ed., 2021, 60, 8236–8242 CrossRef CAS PubMed.
- Q. Z. Li, R. Zeng, B. Han and J. L. Li, Single-Electron Transfer Reactions Enabled by N-Heterocyclic Carbene Organocatalysis, Chem. – Eur. J., 2021, 27, 3238–3250 CrossRef CAS.
- C. K. Prier, D. A. Rankic and D. W. MacMillan, Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS.
- I. Kalvet, Q. Guo, G. J. Tizzard and F. Schoenebeck, When Weaker Can Be Tougher: The Role of Oxidation State (I) in P-vs N-Ligand-Derived Ni-Catalyzed Trifluoromethylthiolation of Aryl Halides, ACS Catal., 2017, 7, 2126–2132 CrossRef CAS.
- D. M. Schultz and T. P. Yoon, Solar synthesis: prospects in visible light photocatalysis, Science, 2014, 343, 1239176 CrossRef PubMed.
- S. Panagiotakis, G. Landrou, V. Nikolaou, A. Putri, R. Hardré, J. Massin, G. Charalambidis, A. G. Coutsolelos and M. Orio, Efficient light-driven hydrogen evolution using a thiosemicarbazone-nickel (II) complex, Front. Chem., 2019, 7, 405 CrossRef CAS.
- Y. Gao, L. Ye, H. Chen and L. Sun, Highly efficient photocatalytic reduction of CO2 and H2O to CO and H2 with a cobalt bipyridyl complex, J. Energy Chem., 2018, 27, 502–506 CrossRef.
- Y. Qin, L. Chen, G. Chen, Z. Guo, L. Wang, H. Fan, M. Robert and T.-C. Lau, A highly active and robust iron quinquepyridine complex for photocatalytic CO 2 reduction in aqueous acetonitrile solution, Chem. Commun., 2020, 56, 6249–6252 RSC.
- B. Han, Y. Li, Y. Yu and L. Gong, Photocatalytic enantioselective α-aminoalkylation of acyclic imine derivatives by a chiral copper catalyst, Nat. Commun., 2019, 10, 1–9 CrossRef.
- V. C. Polites, S. O. Badir, S. Keess, A. Jolit and G. A. Molander, Nickel-catalyzed decarboxylative cross-coupling of bicyclo [1.1. 1] pentyl radicals enabled by electron donor–acceptor complex photoactivation, Org. Lett., 2021, 23, 4828–4833 CrossRef CAS PubMed.
- T. Q. Chen, P. S. Pedersen, N. W. Dow, R. Fayad, C. E. Hauke, M. C. Rosko, E. O. Danilov, D. C. Blakemore, A.-M. Dechert-Schmitt and T. Knauber, Ligand-to-Copper Charge Transfer: A General Catalytic Approach to Aromatic Decarboxylative Functionalization, ChemRxiv, 2021, preprint, DOI:10.26434/chemrxiv.14451117.v1.
- N. A. Till, S. Oh, D. W. MacMillan and M. J. Bird, The Application of Pulse Radiolysis to the Study of Ni (I) Intermediates in Ni-Catalyzed Cross-Coupling Reactions, J. Am. Chem. Soc., 2021, 143, 9332–9337 CrossRef CAS.
- Y. Liang, X. Zhang and D. W. MacMillan, Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis, Nature, 2018, 559, 83–88 CrossRef CAS.
- C. Cavedon, S. Gisbertz, S. Reischauer, S. Vogl, E. Sperlich, J. H. Burke, R. F. Wallick, S. Schrottke, W. H. Hsu and L. Anghileri, Intraligand Charge Transfer Enables Visible-Light-Mediated Nickel-Catalyzed Cross-Coupling Reactions, Angew. Chem., Int. Ed., 2022, 61, e202211433 CrossRef CAS.
- J. Beaudelot, S. Oger, S. Peruško, T.-A. Phan, T. Teunens, C. Moucheron and G. Evano, Photoactive Copper Complexes: Properties and Applications, Chem. Rev., 2022, 122, 16365–16609 CrossRef CAS PubMed.
- K. Wu, C. Liu, Y. Chen, H. Jiang, Q. Peng, Y. Chen, D. Fang, B. Shen, Q. Wu and L. Zhan, Constructing asymmetric unsaturated copper coordination in Zinc (II)/Copper (I, II)-based metal-organic framework toward productive CO2-to-methanol photocatalytic conversion from CO2-capturing solution, Appl. Catal., A, 2023, 650, 118970 CrossRef CAS.
- Y. Li, K. Zhou, Z. Wen, S. Cao, X. Shen, M. Lei and L. Gong, Copper (II)-catalyzed asymmetric photoredox reactions: enantioselective alkylation of imines driven by visible light, J. Am. Chem. Soc., 2018, 140, 15850–15858 CrossRef CAS.
- D. Beydoun, H. Tse, R. Amal, G. Low and S. McEvoy, Effect of copper (II) on the photocatalytic degradation of sucrose, J. Mol. Catal. A: Chem., 2002, 177, 265–272 CrossRef CAS.
- I. Al-Qadsy, A.-B. Al-Odayni, W. S. Saeed, A. Alrabie, A. Al-Adhreai, L. A. S. Al-Faqeeh, P. Lama, A. A. Alghamdi and M. Farooqui, Synthesis, Characterization, Single-Crystal X-ray Structure and Biological Activities of [(Z)-N′-(4-Methoxybenzylidene) benzohydrazide–Nickel (II)] Complex, Crystals, 2021, 11, 110 CrossRef CAS.
- M. W. Campbell, M. Yuan, V. C. Polites, O. Gutierrez and G. A. Molander, Photochemical C–H activation enables nickel-catalyzed olefin dicarbofunctionalization, J. Am. Chem. Soc., 2021, 143, 3901–3910 CrossRef CAS PubMed.
- M. Yuan, Z. Song, S. O. Badir, G. A. Molander and O. Gutierrez, On the nature of C (sp3)–C (sp2) bond formation in nickel-catalyzed tertiary radical cross-couplings: a case study of Ni/photoredox catalytic cross-coupling of alkyl radicals and aryl halides, J. Am. Chem. Soc., 2020, 142, 7225–7234 CrossRef CAS.
- Y. Fan, D. W. Kang, S. Labalme, J. Li and W. Lin, Enhanced Energy Transfer in A π-Conjugated Covalent Organic Framework Facilitates Excited-State Nickel Catalysis, Angew. Chem., 2023, 135, e202218908 CrossRef.
- A. Vijeta, C. Casadevall and E. Reisner, An Integrated Carbon Nitride-Nickel Photocatalyst for the Amination of Aryl Halides Using Sodium Azide, Angew. Chem., 2022, 134, e202203176 CrossRef.
- A. M. Abu-Dief and I. Mohamed, A review on application of transition metal complexes incorporating Schiff bases, Beni-Suef Univ. J. Basic Appl. Sci., 2015, 4(2), 119–133 Search PubMed.
- M. Bashiri, A. Jarrahpour, S. M. Nabavizadeh, S. Karimian, B. Rastegari, E. Haddadi and E. Turos, Potent antiproliferative active agents: novel bis Schiff bases and bis spiro β-lactams bearing isatin tethered with butylene and phenylene as spacer and DNA/BSA binding behavior as well as studying molecular docking, Med. Chem. Res., 2021, 30, 258–284 CrossRef CAS.
- F. R. Gayen, A. A. Ali, D. Bora, S. Roy, S. Saha, L. Saikia, R. L. Goswamee and B. Saha, A ferrocene functionalized Schiff base containing Cu (II) complex: synthesis, characterization and parts-per-million level catalysis for azide alkyne cycloaddition, Dalton Trans., 2020, 49, 6578–6586 RSC.
- Â. de Fátima, C. de Paula Pereira, C. R. S. D. G. Olímpio, B. G. de Freitas Oliveira, L. L. Franco and P. H. C. da Silva, Schiff bases and their metal complexes as urease inhibitors–a brief review, J. Adv. Res., 2018, 13, 113–126 CrossRef.
- S. Kaler and M. D. Jones, Recent advances in externally controlled ring-opening polymerisations, Dalton Trans., 2022, 51, 1241–1256 RSC.
- D. Brunel, G. Noirbent and F. Dumur, Ferrocene: An unrivaled electroactive building block for the design of push-pull dyes with near-infrared and infrared absorptions, Dyes Pigm., 2019, 170, 107611 CrossRef CAS.
- G. Noirbent, D. Brunel, T.-T. Bui, S. Péralta, P.-H. Aubert, D. Gigmes and F. Dumur, D–A dyads and A–D–A triads based on ferrocene: push–pull dyes with unusual behaviours in solution, New J. Chem., 2021, 45, 13475–13498 RSC.
- N. Neghmouche, A. Khelef and T. Lanez, Electrochemistry characterization of ferrocene/ferricenium redox couple at glassycarbon electrode, J. Fundam. Appl. Sci., 2009, 1, 23–30 CrossRef.
- W. Han, Y. Shi, T. Xue and T. Wang, Synthesis and electrochemical, linear and third-order nonlinear optical properties of ferrocene-based D-π-A dyes as novel photoredox catalysts in photopolymerization under visible LED irradiations, Dyes Pigm., 2019, 166, 140–148 CrossRef CAS.
- P. Veit, C. Volkert, C. Förster, V. Ksenofontov, S. Schlicher, M. Bauer and K. Heinze, Gold (ii) in redox-switchable gold (i) catalysis, Chem. Commun., 2019, 55, 4615–4618 RSC.
- S. R. Gupta, P. Mourya, M. Singh and V. P. Singh, Synthesis, structural, electrochemical and corrosion inhibition properties of two new ferrocene Schiff bases derived from hydrazides, J. Organomet. Chem., 2014, 767, 136–143 CrossRef CAS.
- A. A. Kalinin, M. A. Smirnov, L. N. Islamova, G. M. Fazleeva, T. A. Vakhonina, A. I. Levitskaya, O. D. Fominykh, N. V. Ivanova, A. R. Khamatgalimov and I. R. Nizameev, Synthesis and characterization of new second-order NLO chromophores containing the isomeric indolizine moiety for electro-optical materials, Dyes Pigm., 2017, 147, 444–454 CrossRef CAS.
- R. J. Durand, S. Achelle, S. Gauthier, N. Cabon, M. Ducamp, S. Kahlal, J.-Y. Saillard, A. Barsella and F. Robin-Le Guen, Incorporation of a ferrocene unit in the π-conjugated structure of donor-linker-acceptor (D-π-A) chromophores for nonlinear optics (NLO), Dyes Pigm., 2018, 155, 68–74 CrossRef CAS.
- C. H. Mak, X. Han, M. Du, J.-J. Kai, K. F. Tsang, G. Jia, K.-C. Cheng, H.-H. Shen and H.-Y. Hsu, Heterogenization of homogeneous photocatalysts utilizing synthetic and natural support materials, J. Mater. Chem. A, 2021, 9, 4454–4504 RSC.
- H. Hennig, Homogeneous photo catalysis by transition metal complexes, Coord. Chem. Rev., 1999, 182, 101–123 CrossRef.
- M. Hosseini-Sarvari and Z. Akrami, Visible-light assisted of nano Ni/g-C3N4 with efficient photocatalytic activity and stability for selective aerobic C− H activation and epoxidation, J. Organomet. Chem., 2020, 928, 121549 CrossRef CAS.
- Y.-X. Tan, Z.-M. Chai, B.-H. Wang, S. Tian, X.-X. Deng, Z.-J. Bai, L. Chen, S. Shen, J.-K. Guo and M.-Q. Cai, Boosted photocatalytic oxidation of toluene into benzaldehyde on CdIn2S4-CdS: Synergetic effect of compact heterojunction and S-vacancy, ACS Catal., 2021, 11, 2492–2503 CrossRef CAS.
- Y. Fu, L. Xu, H. Shen, H. Yang, F. Zhang, W. Zhu and M. Fan, Tunable catalytic properties of multi-metal–organic frameworks for aerobic styrene oxidation, Chem. Eng. J., 2016, 299, 135–141 CrossRef CAS.
- C. Cheng, B. Zhu, B. Cheng, W. Macyk, L. Wang and J. Yu, Catalytic Conversion of Styrene to Benzaldehyde over S-Scheme Photocatalysts by Singlet Oxygen, ACS Catal., 2022, 13, 459–468 CrossRef.
- W. Schilling, Y. Zhang, D. Riemer and S. Das, Visible-Light-Mediated Dearomatisation of Indoles and Pyrroles to Pharmaceuticals and Pesticides, Chem. – Eur. J., 2020, 26, 390–395 CrossRef CAS.
- G. N. Babu and S. Pal, N-(acyl)-N′-(ferrocenylidene) hydrazines and their nickel (II) complexes: Syntheses, structures and physical properties, J. Chem. Sci., 2017, 129, 1105–1113 CrossRef CAS.
- J. N. Asegbeloyin, O. T. Ujam, E. C. Okafor, I. Babahan, E. P. Coban, A. Özmen and H. Biyik, Synthesis, Characterization, and Biological Activity of N′-[(Z)-(3-Methyl-5-oxo-1-phenyl-1, 5-dihydro-4H-pyrazol-4-ylidene)(phenyl) methyl] benzohydrazide and Its Co (II), Ni (II), and Cu (II) Complexes, Bioinorg. Chem. Appl., 2014, 2014, 718175 Search PubMed.
- C. Gokce and R. Gup, Synthesis and characterisation of Cu (II), Ni (II), and Zn (II) complexes of furfural derived from aroylhydrazones bearing aliphatic groups and their interactions with DNA, Chem. Pap., 2013, 67, 1293–1303 CAS.
- C. W. Dikio, I. P. Ejidike, F. M. Mtunzi, M. J. Klink and E. D. Dikio, Hydrazide Schiff bases of acetylacetonate metal complexes: Synthesis, spectroscopic and biological studies, Int. J. Pharm. Pharm. Sci., 2017, 9, 257–267 CrossRef CAS.
- H. Bera, S. Kumar and S. Maiti, Facile synthesis and characterization of tailor-made pectin-gellan gum-bionanofiller composites as intragastric drug delivery shuttles, Int. J. Biol. Macromol., 2018, 118, 149–159 CrossRef CAS PubMed.
- F. A. Mautner, R. C. Fischer, K. Reichmann, E. Gullett, K. Ashkar and S. S. Massoud, Synthesis and characterization of 1D and 2D cadmium (II)-2, 2′-bipyridine-N, N′-dioxide coordination polymers bridged by pseudohalides, J. Mol. Struct., 2019, 1175, 797–803 CrossRef CAS.
- A. B. Deilami, M. Salehi, A. Amiri and A. Arab, New copper (II) and vanadium (IV) complexes based on allylamine-derived Schiff base ligand; synthesis, crystal structure, electrochemical properties and DFT calculations, J. Mol. Struct., 2019, 1181, 190–196 CrossRef CAS.
- N. Noorussabah, M. Choudhary, N. Das, B. Mohan, K. Singh, R. K. Singh, K. Ahmad, S. Muhammad and S. Kumar, Copper (II) and nickel (II) complexes of tridentate hydrazide and schiff base ligands containing phenyl and naphthalyl groups: synthesis, structural, molecular docking and density functional study, J. Inorg. Organomet. Polym. Mater., 2020, 30, 4426–4440 CrossRef CAS.
- J.-H. Jiang, Y.-H. Lei, X. Li, Y. Pi, H. Zhu, Q.-G. Li and C.-H. Li, New cobalt (II) Schiff base complex: Synthesis, characterization, DFT calculation and antimicrobial activity, Inorg. Chem. Commun., 2021, 127, 108350 CrossRef CAS.
- M. Manimohan, S. Pugalmani, K. Ravichandran and M. A. Sithique, Synthesis and characterisation of novel Cu (II)-anchored biopolymer complexes as reusable materials for the photocatalytic degradation of methylene blue, RSC Adv., 2020, 10, 18259–18279 RSC.
- J. Anacona and J. Santaella, Synthesis, magnetic and spectroscopic studies of a Schiff base derived from cephaclor and 1, 2-diaminobenzene and its transition metal complexes, Spectrochim. Acta, Part A, 2013, 115, 800–804 CrossRef CAS PubMed.
- E. Andris, R. Navrátil, J. Jasik, T. Terencio, M. Srnec, M. Costas and J. Roithová, Chasing the Evasive FeO Stretch and the Spin State of the Iron (IV)–Oxo Complexes by Photodissociation Spectroscopy, J. Am. Chem. Soc., 2017, 139, 2757–2765 CrossRef CAS.
- M. Tsubaki, R. B. Srivastava and N. T. Yu, Resonance Raman investigation of carbon monoxide bonding in (carbon monoxy) hemoglobin and-myoglobin: detection of iron-carbon monoxide stretching and iron-carbon-oxygen bending vibrations and influence of the quaternary structure change, Biochemistry, 1982, 21, 1132–1140 CrossRef CAS PubMed.
- S. S. Machakanur, B. R. Patil, D. S. Badiger, R. P. Bakale, K. B. Gudasi and S. A. Bligh, Synthesis, characterization and anticancer evaluation of novel tri-arm star shaped 1, 3, 5-triazine hydrazones, J. Mol. Struct., 2012, 1011, 121–127 CrossRef CAS.
- G.-M. Chen and H. C. Brown, An efficient synthesis of n-unsubstituted imines as organoborane adducts stable at room temperature: New promising intermediates for synthesis, J. Am. Chem. Soc., 2000, 122, 4217–4218 CrossRef CAS.
- L. Simón and J. M. Goodman, A model for the enantioselectivity of imine reactions catalyzed by BINOL− phosphoric acid catalysts, J. Org. Chem., 2011, 76, 1775–1788 CrossRef.
- M. Bashiri, A. Jarrahpour, B. Rastegari, A. Iraji, C. Irajie, Z. Amirghofran, S. Malek-Hosseini, M. Motamedifar, M. Haddadi and K. Zomorodian, Synthesis and evaluation of biological activities of tripodal imines and β-lactams attached to the 1, 3, 5-triazine nucleus, Monatsh. Chem., 2020, 151, 821–835 CrossRef CAS.
- S.-H. Lee, A. G. Larsen, K. Ohkubo, Z.-L. Cai, J. R. Reimers, S. Fukuzumi and M. J. Crossley, Long-lived long-distance photochemically induced spin-polarized charge separation in β, β'-pyrrolic fused ferrocene-porphyrin-fullerene systems, Chem. Sci., 2012, 3, 257–269 RSC.
- R. Ayranci, D. O. Demirkol, M. Ak and S. Timur, Ferrocene-Functionalized 4-(2, 5-Di (Thiophen-2-yl)-1 H-Pyrrol-1-yl) Aniline: A Novel Design in Conducting Polymer-Based Electrochemical Biosensors, Sensors, 2015, 15, 1389–1403 CrossRef CAS.
- I. Sianoudis, E. Drakaki and A. Hein, Educational x-ray experiments and XRF measurements with a portable setup adapted for the characterization of cultural heritage objects, Eur. J. Phys., 2010, 31, 419 CrossRef.
- J.-D. Chai and M. Head-Gordon, Systematic optimization of long-range corrected hybrid density functionals, J. Chem. Phys., 2008, 128, 084106 CrossRef.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- J. Tomasi, B. Mennucci and E. Cancès, The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level, J. Mol. Struct.: THEOCHEM, 1999, 464, 211–226 CrossRef CAS.
- J. Tomasi and M. Persico, Molecular interactions in solution: an overview of methods based on continuous distributions of the solvent, Chem. Rev., 1994, 94, 2027–2094 CrossRef CAS.
- T. H. Peterson Jr and D. Woon, Encyclopedia of computational chemistry, ed. P. R. Schleyer, 1998, pp. 399–402 Search PubMed.
- B. S. Brunschwig, J. Logan, M. D. Newton and N. Sutin, A semiclassical treatment of electron-exchange reactions. Application to the hexaaquoiron (II)-hexaaquoiron (III) system, J. Am. Chem. Soc., 1980, 102, 5798–5809 CrossRef CAS.
- K. Du, G. Liu, X. Chen and K. Wang, Fast charge separation and photocurrent enhancement on black TiO2 nanotubes co-sensitized with Au nanoparticles and PbS quantum dots, Electrochim. Acta, 2018, 277, 244–254 CrossRef CAS.
- M. Smith, L. Scudiero, J. Espinal, J.-S. McEwen and M. Garcia-Perez, Improving the deconvolution and interpretation of XPS spectra from chars by ab initio calculations, Carbon, 2016, 110, 155–171 CrossRef CAS.
- M. Zhang, X. Wang, H. Sun, N. Wang, Q. Lv, W. Cui, Y. Long and C. Huang, Enhanced paramagnetism of mesoscopic graphdiyne by doping with nitrogen, Sci. Rep., 2017, 7, 11535 CrossRef PubMed.
- Z. Wang, F. Jie, W. Li, Z. Zhao, F. Niu, J. Zhu, W. Qin and K. Zhou, Investigation of the occurrence characteristics of organic components in high-sulfur waste residues (HSWR), Front. Environ. Sci., 2022, 10, 978559 CrossRef.
- M. Afshari, M. Dinari, H. Farrokhpour and F. Zamora, Imine-linked covalent organic framework with a naphthalene moiety as a sensitive phosphate ion sensing, ACS Appl. Mater. Interfaces, 2022, 14, 22398–22406 CrossRef CAS PubMed.
- G. Xu, N. Guo, Q. Zhang, T. Wang, P. Song and L. Xia, An ultrasensitive surface-enhanced Raman scattering sensor for the detection of hydrazine via the Schiff base reaction, J. Hazard. Mater., 2022, 424, 127303 CrossRef CAS PubMed.
- W. Bo and F. Dianzhong, EPR, XPS and Spectra Studies of Some 3D Complexes with Salicylidene-Benzol Hydrazide, Bull. Soc. Chim. Belg., 1993, 102, 75–77 CrossRef.
- B. Sivaranjini, R. Mangaiyarkarasi, V. Ganesh and S. Umadevi, Vertical alignment of liquid crystals over a functionalized flexible substrate, Sci. Rep., 2018, 8, 8891 CrossRef CAS PubMed.
- L. V. Erkhova, I. A. Presniakov, M. I. Afanasov, D. A. Lemenovskiy, H. Yu, L. Wang, M. Danilson and M. Koel, Ferrocene introduced into 5-methylresorcinol-based organic aerogels, Polymer, 2020, 12, 1582 CAS.
- S. Kusuma, K. N. Patil, P. M. Srinivasappa, N. Chaudhari, A. Soni, W. Nabgan and A. H. Jadhav, Ferrocene anchored activated carbon as a versatile catalyst for the synthesis of 1, 5-benzodiazepines via one-pot environmentally benign conditions, RSC Adv., 2022, 12, 14740–14756 RSC.
- T. Ivanova, K. Maslakov, A. Sidorov, M. Kiskin, R. Linko, S. Savilov, V. Lunin and I. Eremenko, XPS detection of unusual Cu (II) to Cu (I) transition on the surface of complexes with redox-active ligands, J. Electron Spectrosc. Relat. Phenom., 2020, 238, 146878 CrossRef CAS.
- A. Silva, M. Martins, M. Freitas, A. Valente, C. Freire, B. De Castro and J. Figueiredo, Immobilisation of amine-functionalised nickel (II) Schiff base complexes onto activated carbon treated with thionyl chloride, Microporous Mesoporous Mater., 2002, 55, 275–284 CrossRef CAS.
- H. Huang, Y. Zhao, Y. Bai, F. Li, Y. Zhang and Y. Chen, Conductive metal–organic frameworks with extra metallic sites as an efficient electrocatalyst for the hydrogen evolution reaction, Adv. Sci., 2020, 7, 2000012 CrossRef CAS PubMed.
- W. Partenheimer, The high yield synthesis of benzaldehydes from benzylic alcohols using homogeneously catalyzed aerobic oxidation in acetic acid, Adv. Synth. Catal., 2006, 348, 559–568 CrossRef CAS.
- F. Valentini, G. Ferracci, P. Galloni, G. Pomarico, V. Conte and F. Sabuzi, Sustainable Highly Selective Toluene Oxidation to Benzaldehyde, Catalysts, 2021, 11, 262 CrossRef CAS.
- B. Lu, N. Cai, J. Sun, X. Wang, X. Li, J. Zhao and Q. Cai, Solvent-free oxidation of toluene in an ionic liquid with H2O2 as oxidant, Chem. Eng. J., 2013, 225, 266–270 CrossRef CAS.
- N. Guajardo, C. Carlesi and Á. Aracena, Toluene oxidation by hydrogen peroxide in deep eutectic solvents, ChemCatChem, 2015, 7, 2451–2454 CrossRef CAS.
- A. M. Pembere, C. Cui, R. Anumula, H. Wu, P. An, T. Liang and Z. Luo, A hexagonal Ni 6 cluster protected by 2-phenylethanethiol for catalytic conversion of toluene to benzaldehyde, Phys. Chem. Chem. Phys., 2019, 21, 17933–17938 RSC.
- Y. Zhu, Y. Zhu, H. Zeng, Z. Chen and R. D. Little, A promising electro-oxidation of methyl-substituted aromatic compounds to aldehydes in aqueous imidazole ionic liquid solutions, J. Electroanal. Chem., 2015, 751, 105–110 CrossRef CAS.
- M. R. Maurya, B. Sarkar, A. Kumar, N. Ribeiro, A. Miliute and J. C. Pessoa, New thiosemicarbazide and dithiocarbazate based oxidovanadium (iv) and dioxidovanadium (v) complexes. Reactivity and catalytic potential, New J. Chem., 2019, 43, 17620–17635 RSC.
- X. Wu, Z. Deng, J. Yan, F. Zhang and Z. Zhang, Effect of acetic anhydride on the oxidation of toluene to benzaldehyde with metal/bromide catalysts, Ind. Eng. Chem. Res., 2014, 53, 14601–14606 CrossRef CAS.
- J. B. Feng and X. F. Wu, Transition metal-catalyzed oxidative transformations of methylarenes, Appl. Organomet. Chem., 2015, 29, 63–86 CrossRef CAS.
- G. Shi, S. Xu, Y. Bao, J. Xu and Y. Liang, Selective aerobic oxidation of toluene to benzaldehyde on immobilized CoOx on SiO2 catalyst in the presence of N-hydroxyphthalimide and hexafluoropropan-2-ol, Catal. Commun., 2019, 123, 73–78 CrossRef CAS.
- M. Grübel, I. Bosque, P. J. Altmann, T. Bach and C. R. Hess, Redox and photocatalytic properties of a NiII complex with a macrocyclic biquinazoline (Mabiq) ligand, Chem. Sci., 2018, 9, 3313–3317 RSC.
- S. Gillhuber, J. O. Holloway, H. Frisch, F. Feist, F. Weigend, C. Barner-Kowollik and P. W. Roesky, Ferrocene-driven single-chain polymer compaction, Chem. Commun., 2023, 59, 4672–4675 RSC.
- C.-B. Bo, Q. Bu, X. Li, G. Ma, D. Wei, C. Guo, B. Dai and N. Liu, Highly Active and Robust Ruthenium Complexes Based on Hemilability of Hybrid Ligands for C–H Oxidation, J. Org. Chem., 2020, 85, 4324–4334 CrossRef CAS PubMed.
- I. Mahmudov, A. V. Gurbanov, L. M. Martins, Y. Abdullayev, A. Sujayev, K. T. Mahmudov and A. J. Pombeiro, Co (ii/iii), Ni (ii) and Cu (ii) complexes with a pyrazole-functionalized 1, 3, 5-triazopentadiene: synthesis, structure and application in the oxidation of styrene to benzaldehyde, New J. Chem., 2023, 47, 10826–10833 RSC.
- K.-G. Liu, F. Rouhani, X.-M. Gao, M. Abbasi-Azad, J.-Z. Li, X.-D. Hu, W. Wang, M.-L. Hu and A. Morsali, Bilateral photocatalytic mechanism of dye degradation by a designed ferrocene-functionalized cluster under natural sunlight, Catal. Sci. Technol., 2020, 10, 757–767 RSC.
- P. M. Pérez-García, A. Darù, A. R. Scheerder, M. Lutz, J. N. Harvey and M.-E. Moret, Oxidative Addition of Aryl Halides to a Triphosphine Ni (0) Center to Form pentacoordinate Ni (II) Aryl Species, Organometallics, 2020, 39, 1139–1144 CrossRef PubMed.
- S. Haaf, E. Kaifer, H. Wadepohl and H. J. Himmel, Use of Crown Ether Functions as Secondary Coordination Spheres for the Manipulation of Ligand–Metal Intramolecular Electron Transfer in Copper–Guanidine Complexes, Chem. – Eur. J., 2021, 27, 959–970 CrossRef CAS PubMed.
- H. Wang, C. Cao, D. Li, Y. Ge, R. Chen, R. Song, W. Gao, X. Wang, X. Deng and H. Zhang, Achieving High Selectivity in Photocatalytic Oxidation of Toluene on Amorphous BiOCl Nanosheets Coupled with TiO2, J. Am. Chem. Soc., 2023, 145, 16852–16861 CrossRef CAS PubMed.
- S. Ohmura, K. Katagiri, H. Kato, T. Horibe, S. Miyakawa, J.-Y. Hasegawa and K. Ishihara, Highly Enantioselective Radical Cation [2+ 2] and [4+ 2] Cycloadditions by Chiral Iron (III) Photoredox Catalysis, J. Am. Chem. Soc., 2023, 145, 15054–15060 CrossRef CAS PubMed.
- S. Firoozi and M. Hosseini-Sarvari, Photo-Difunctionalization and Photo-Oxidative Cleavage of the C–C Double Bond of Styrenes in the Presence of Nanosized Cadmium Sulfide (CdS) as a Highly Efficient Photo-Induced Reusable Nanocatalyst, Eur. J. Org. Chem., 2020, 2020, 3834–3843 CrossRef CAS.
- C. Sun and H. Liu, Highly Selective Oxidation of Styrene to Styrene Oxide over a Tetraphenylporphyrin-Bridged Silsesquioxane-Based Hybrid Porous Polymer, ACS Appl. Polym. Mater., 2022, 4, 5471–5481 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3me00133d |
| This journal is © The Royal Society of Chemistry 2024 |



















