Siderophore conjugates to combat antibiotic-resistant bacteria
Beth
Rayner
 ab,
Anthony D.
Verderosa
ab,
Anthony D.
Verderosa
 ab,
Vito
Ferro
ab,
Vito
Ferro
 bc and
Mark A. T.
Blaskovich
bc and
Mark A. T.
Blaskovich
 *abc
*abc
aCentre for Superbug Solutions, Institute for Molecular Bioscience, University of Queensland, Brisbane, Queensland, Australia. E-mail: m.blaskovich@uq.edu.au
bAustralian Infectious Disease Research Centre, The University of Queensland, Brisbane, Queensland, Australia
cSchool of Chemistry and Molecular Biosciences, The University of Queensland, Australia
First published on 1st March 2023
Abstract
Antimicrobial resistance (AMR) is a global threat to society due to the increasing emergence of multi-drug resistant bacteria that are not susceptible to our last line of defence antibiotics. Exacerbating this issue is a severe gap in antibiotic development, with no new clinically relevant classes of antibiotics developed in the last two decades. The combination of the rapidly increasing emergence of resistance and scarcity of new antibiotics in the clinical pipeline means there is an urgent need for new efficacious treatment strategies. One promising solution, known as the ‘Trojan horse’ approach, hijacks the iron transport system of bacteria to deliver antibiotics directly into cells – effectively tricking bacteria into killing themselves. This transport system uses natively produced siderophores, which are small molecules with a high affinity for iron. By linking antibiotics to siderophores, to make siderophore antibiotic conjugates, the activity of existing antibiotics can potentially be reinvigorated. The success of this strategy was recently exemplified with the clinical release of cefiderocol, a cephalosporin-siderophore conjugate with potent antibacterial activity against carbapenem-resistant and multi-drug resistant Gram-negative bacilli. This review discusses the recent advancements in siderophore antibiotic conjugates and the challenges associated with the design of these compounds that need to be overcome to deliver more efficacious therapeutics. Potential strategies have also been suggested for new generations of siderophore–antibiotics with enhanced activity.
Introduction
Antimicrobial resistance
Antimicrobial resistance (AMR) is a rapidly developing global pandemic, with current estimates suggesting that by 2050 more than 10 million deaths per annum will be directly attributable to AMR.1 It is predicted that these deaths will be caused by several critical nosocomial pathogens, collectively, several of these have been termed the ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.).2 These pathogens develop resistance through three broad mechanisms – target site modification, drug inactivation (typically enzymatic modification), and/or diminished intracellular accumulation, due to efflux or reduced permeability.3Exacerbating the AMR crisis is a severe gap in antibiotic development, due to the exit of most major pharmaceutical companies from antibiotic R&D. The antibiotic pipeline is sparsely populated with fewer than 50 candidates in clinical testing, and startlingly few of these demonstrating activity against the difficult-to-treat Gram-negative pathogens.4,5
Scope
A promising strategy to combat antibiotic-resistant Gram-negative bacteria is to utilise siderophores to overcome the difficult-to-penetrate Gram-negative outer membrane and deliver antibiotics that can act on intracellular targets to their site of action. Siderophores are secondary metabolites that are secreted by bacteria to scavenge iron from the surrounding environment and deliver it to the bacterial cell via specific receptors.6 The aim of this review is to summarise recent progress in the development of novel antibacterial siderophore–antibiotic conjugates (SACs), the challenges associated with designing SACs, and future directions in the field. For more comprehensive overviews of the field the readers are directed to several recent reviews.7–11 Throughout this review, all synthetic compounds will be referred to as siderophore–antibiotic conjugates (SACs); however, this is synonymous with synthetic sideromycins. In addition, some of the iron-binding motifs reported as SACs are synthetic siderophore mimics and we have endeavoured to clarify this at the requisite points.Gram-negative pathogens
Infections caused by Gram-negative pathogens are difficult to treat, primarily due to the complexity of the cell envelope which differs significantly in structural composition compared with Gram-positive bacteria. Gram-positive bacteria are characterised by a cytoplasmic membrane consisting of a hydrophobic phospholipid bilayer which is covered by a thick peptidoglycan layer that provides structural rigidity.12,13 On the extracellular face of the peptidoglycan layer are wall teichoic acids, long anionic polysaccharide chains that interact with divalent cations resulting in the high hydrophilicity observed in this layer.7,13Gram-negative bacteria also contain a hydrophobic phospholipid bilayer cytoplasmic membrane; however, this is where the similarities end.7 Unlike Gram-positive bacteria, Gram-negative bacteria also possess a hydrophobic outer membrane that encloses a thin peptidoglycan structural layer and an interstitial space called the periplasm.7,14 The outer membrane lipid bilayer consists of phospholipids on the inner leaflet and glycolipids (primarily lipopolysaccharide) as the outer leaflet.14
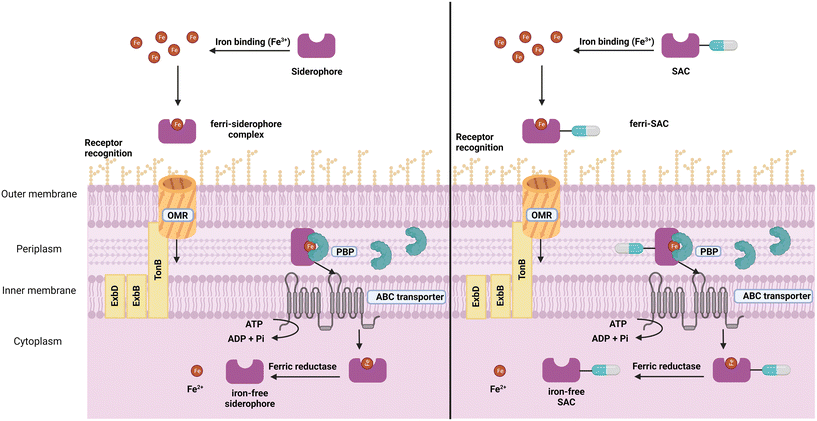
The presence of this difficult-to-penetrate outer membrane in Gram-negative bacteria alters the capacity for a variety of crucial cellular functions to occur, notably nutrient uptake, with several embedded translocation systems providing selective entry. Such systems can be classified as outer membrane receptors, porins, specific diffusion channels, or energy-dependent transmembrane transport systems like efflux pumps and TonB-dependent transporters. Of specific interest are the TonB-dependent transporters, which are outer membrane proteins specific to bacteria that bind and transport a variety of compounds including siderophores, vitamin B12 and carbohydrates.15
Siderophores
Siderophores are small molecule metal chelators (200–2000 Da), secreted by bacteria under depleted conditions.16,17 Of the over 500 different siderophores identified and characterised to date, five primary iron-binding motifs have been identified: catecholate, hydroxamate, phenolate, carboxylate, and, α-hydroxy carboxylate (Fig. 1).18,19 Siderophores with multiple types of iron-binding motifs, termed mixed-type, have also been observed.18,19 Bacteria (and fungi) typically produce one or two primary endogenous siderophores, however, some also have the uptake systems to utilise exogenous siderophores (those produced by neighbouring organisms) known as xenosiderophores.20 Bacteria utilise siderophores in iron depleted environments to sequester and transport iron into the cell for growth and metabolism as a means of overcoming the difficult-to-penetrate bacterial cell envelope.7 They also play a role in the transport of non-iron metals,21–23 signalling,24 protection from oxidative stress,25,26 immunomodulation,27 and biofilm formation.28–33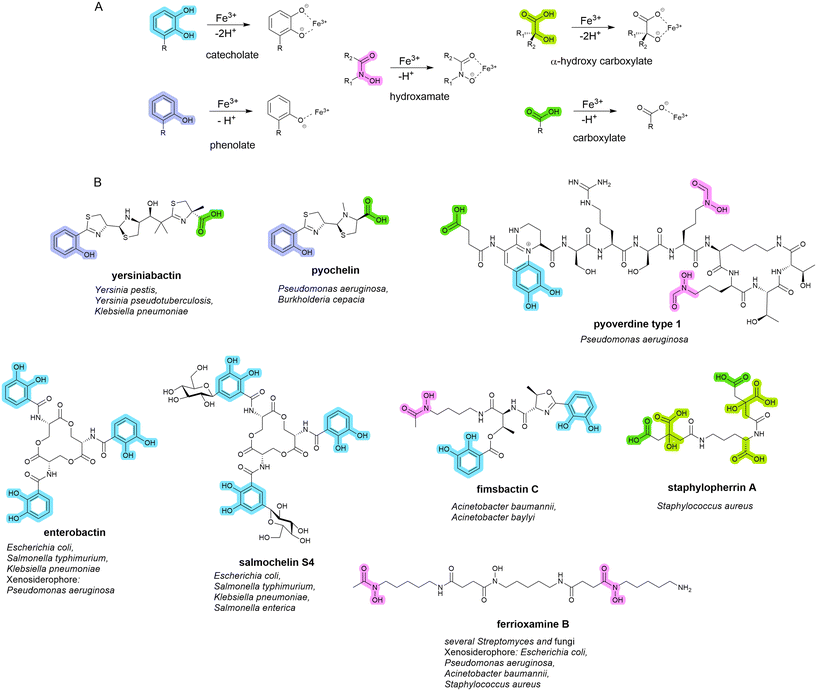 | ||
| Fig. 1 A. Five conserved iron-binding motifs in endogenously produced siderophores. B. A selection of common endogenous siderophores, respective host organism and xenosiderophore capacity. | ||
Targeting siderophores for new antimicrobials
The siderophore pathway presents several potential opportunities to develop novel antimicrobial agents with new mechanisms of action. Although not covered in this review, one interesting approach is the inhibition of siderophore biosynthesis, a promising target for new therapeutics. Some investigated inhibitors include siderophore mimics and repurposed drugs, among others.38 These inhibitors were thoroughly discussed in a 2015 review by Lamb.38 For a comprehensive report of the biosynthetic pathways of siderophores, readers are directed to the 2010 review by Hider et al.17A common obstacle when designing Gram-negative active antimicrobials that act on intracellular targets is delivering a sufficient quantity of a therapeutic through the outer membrane to the site of action. One way this can be overcome is by exploiting the TonB pathway, which is inherently upregulated during host microbial infection. However, the focus of this review is a second method that targets the siderophore pathway by suitably modifying an antibiotic and conjugating it to a siderophore, which enables the exploitation of this innate iron-acquisition system to deliver a fatal antibiotic payload into the bacterial cell. Commonly referred to as the Trojan Horse strategy (RHS, Fig. 2),39 these compounds are known as siderophore–antibiotic conjugates (SACs).
Nature's SACs
The concept of SACs is not one designed by scientists. Several bacteria endogenously produce SACs, known as sideromycins. Sideromycin-producing bacteria include both Gram-negative and Gram-positive species such as Streptomyces, Salmonella, Escherichia, and Klebsiella.7–9,18 The most notable sideromycins include albomycin (1) and salmycin (2) (Fig. 3). In addition to this, a new family of sideromycins called the amodesmycins were recently isolated and identified from Streptomyces albus J1074, however, these compounds displayed no significant activity against the test panel of bacteria so will not be discussed any further.40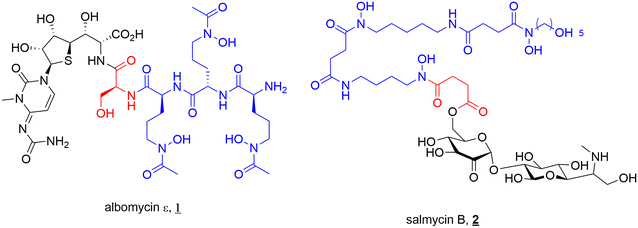 | ||
| Fig. 3 Sideromycins albomycin ε (1) and salmycin B (2). Siderophore motif highlighted in blue, antibacterial component in black and linker in red. | ||
Albomycin (1) (Fig. 3) (originally known as grisein) is a siderophore–antibiotic conjugate that was first isolated from Streptomyces griseus in the 1940s and is the most highly studied of the sideromycins.41 Structurally albomycin consists of a tri-hydroxamate iron chelating component, N-acetyl-N-hydroxy-L-ornithine, and an antibacterial thioribosyl pyrimidine moiety connected via a cleavable peptide linker.8,42 Albomycin (1) is thought to cross both the outer and inner membranes to reach its cytoplasmic target seryl-t-RNA synthetase. This is achieved by utilising a cleavable linker (vide infra) that is crucial to its activity.43 Potent activity against both Gram-negative (e.g., E. coli, MIC 5 ng mL−1) and Gram-positive (e.g., Streptomyces pneumoniae, MIC 10 ng mL−1) pathogens (including multi-drug resistant (MDR) strains) has been reported for albomycin.41 Significant research has been conducted into the activity and efficacy of the albomycins since their initial isolation. Some of the most notable studies include; demonstrated in vivo efficacy of albomycin (and salmycin) in murine models by Braun and colleagues,43 the first complete synthesis and subsequent full structure elucidation of albomycin in 1982 by Benz et al.,44 and mechanism of action and therapeutic studies by Gause.45 The potent antimicrobial activity of albomycin led to its use in the Soviet Union during the 1940s–1950s for the treatment of penicillin-resistant bacteria (including pneumococci).45
A second sideromycin, also isolated from a Streptomyces spp., is the aminoglycoside-danoxamine SAC salmycin (2) (Fig. 3). First isolated from Streptomyces violaceus in 1995, salmycin (2) consists of an aminoglycoside antibiotic linked to the tri-hydroxamate siderophore danoxamine via a succinoyl linker. Salmycin (2) exerts antimicrobial activity solely against Gram-positive bacteria including Staphylococci and Streptococci.9 The ester component of the succinoyl linker demonstrates high lability unless metal bound (Fe3+, Ga3+) or the hydroxamates are protected.42 This plays an important role in the activity of the compound, with cleavage of the antibiotic after iron release inside the cell allowing for effective action of the aminoglycosides.42
As is often the case in drug design and development, the elegance of nature can supply inspiration for new therapeutics, and these sideromycins are no different. As will be discussed in depth later in this review, designing a suitable linker to join the antibiotic of interest to a siderophore can be challenging. For antibiotics with cytoplasmic targets, it is conventionally believed that a cleavable linker is necessary to ensure the drug can reach the site of action. However, the design of a selectively cleavable linker can be challenging. Thus, the selectively cleavable peptidic linker in albomycin (1) provides a validated starting point for further development of cleavable linkers. In a similar vein, the ester linkage of salmycin (2) is believed to undergo intramolecular cyclisation after iron reduction leading to release of the antibiotic from the conjugate, again providing a highly specific cleavage mechanism.18,42 These elegantly designed components of natural SACs should be investigated to a greater extent in synthetic SACs.
Advanced conjugates
Over the last few decades, several synthetic SACs have progressed through drug discovery and into pre-clinical and clinical trials. Thus, for this review, we are defining an “advanced” conjugate as one that has progressed through early drug development into preclinical and/or clinical trials.Of particular note, is the recent US Food and Drug Administration (FDA) approved cephalosporin-catecholate, cefiderocol (Fetroja®) (3) by Shionogi & Company Ltd. (Fig. 4).9 This first-in-class compound, utilising a synthetic chlorine-substituted catecholate siderophore mimic, displays activity against a range of carbapenem-resistant Gram-negative pathogens.39 Cefiderocol (3) was approved by the FDA in 2019 for the treatment of complex urinary tract infections.46 Before approval, the Clinical & Laboratory Standards Institute (CLSI) approved provisional susceptible, intermediate, and resistant breakpoints of 4, 8, and 16 μg mL−1 against Enterobacteriaceae, P. aeruginosa, A. baumannii, and S. maltophilia.39 Multinational studies utilising clinical isolates have demonstrated MIC90 (the concentration of antibiotic that results in the growth inhibition of 90% of the tested bacterial isolates) values of 0.25–1 μg mL−1 for Enterobacteriaceae including E. coli. Klebsiella spp., Enterobacter spp., and Serratia spp. Even lower MIC90 values were reported for P. aeruginosa, B. cepacia, and S. maltophilia.39
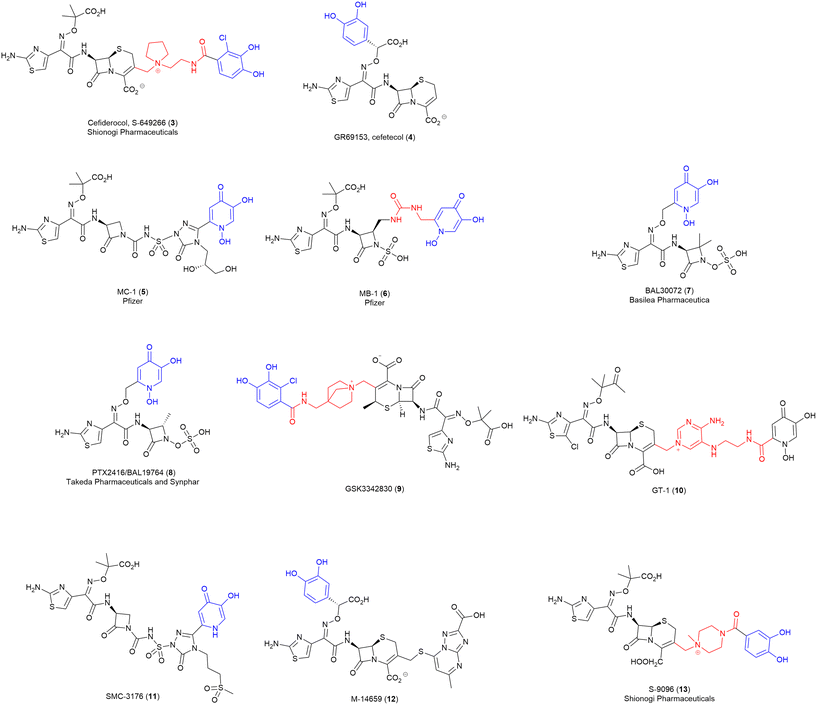 | ||
| Fig. 4 Advanced SACs. Siderophore motif highlighted in blue, antibiotic component in black, linker in red. | ||
Although cefiderocol (3) is the first approved SAC, significant work by several pharmaceutical companies predates this, and the results of these early studies can provide insight and critical information for future work. Notably, many of these use monodentate catechol siderophore mimics – while the small size of this motif presumably helps in maintaining ‘drug-like’ properties, it is unclear how effectively the conjugates coordinate iron and become actively transported.
GR69153 (cefetecol) (4) (Fig. 4), is a novel catechol-substituted cephalosporin reported by Glaxo Group Research Ltd. in 1990. This broad-spectrum cephalosporin with an integrated catechol-substituted 7-aminothiazolyl-oxime demonstrates specific chelation of iron and was designed to mimic a typical catecholate siderophore.47 To assess the extent of intrinsic resistance and the impact of alternative transport systems for GR69153 (4), wild-type (WT) and permeability mutant strains (E. coli DC2 and P. aeruginosa 799/169) were utilised for susceptibility testing.47 The antibiotic showed similar MICs for both E. coli strains indicating minimal impact of increased ‘leakiness’ on drug permeability, as was also seen in iron-depleted conditions of P. aeruginosa assays.47 It was concluded that active transport of GR69153 (4) via the siderophore-uptake pathway allowed for effective drug uptake into the periplasm.47 However, despite the potent antimicrobial activity, the compound did not progress past the early clinical stages.48
Another SAC with promising data is MC-1 (5) (Fig. 4), a monobactam conjugate with a hydroxypyridone siderophore mimic motif from Pfizer that displayed good activity against MDR P. aeruginosa and extended-spectrum β-lactamase producing Enterobacteriaceae spp.9,49 However, MC-1 (5) possessed several limitations including hydrolytic instability and high plasma protein affinity when tested in murine pulmonary infection models.9 MC-1 (5) did not progress to clinical trials.50
Pfizer also investigated a related monobactam MB-1 (6) (Fig. 4), again with a hydroxypridone siderophore mimic, that displayed broad-spectrum in vitro activity against MDR Gram-negative pathogens like P. aeruginosa.51 However, high variability in efficacy was observed in a neutropenic murine infection model. This is an excellent example of how efficacy in vivo is not directly predictable from traditional in vitro assays.51In vitro testing of MB-1 (6) saw MIC90 values against clinically relevant Gram-negative pathogens ranging from 0.12–8 μg mL−1, with highest activity against Proteus spp. (0.12 μg mL−1) and S. maltophilia (0.25 μg mL−1) and lowest against Enterobacter spp. (8 μg mL−1).52 As with MC-1 (5), MB-1 (6) did not progress past preclinical studies.50
Basilea pharmaceuticals also developed a hydroxypyridone-based SAC, BAL30072 (7) (Fig. 4), with the siderophore conjugated to a monosulfactam antibiotic (analogous to tigemonam).53 BAL30027 demonstrated potent in vitro antimicrobial activity against the majority (70%) of the carbapenem-resistant Enterobacteriaceae strains tested (including those expressing class A, B and D carbapenemases), however, reduced activity was observed in strains expressing high levels of class A and D extended spectrum β-lactamases and class C cephalosporinases.53 The promising in vitro data led BAL30072 to progress into clinical development as an inhaled antibiotic, it did not progress beyond phase I50 due to severe hepatotoxicity.54
Similarly, PTX-2416/BAL19764 (8) (Fig. 4) was developed by Takeda and Synphar, as a hydroxypyridone monosulfactam conjugate with broad-spectrum activity against several Gram-negative species, including Enterobacteriaceae (MIC ≤ 1 μg mL−1), and P. aeruginosa (MIC 2–4 μg mL−1). However, MICs for several Enterobacteriaceae strains were reported up to 8 μg mL−1, which is significantly higher than comparator antibiotic aztreonam alone (2 μg mL−1).55 Unfortunately this SAC was also found to be highly labile to class C β-lactamases, consequently, it was deemed unfit for monotherapy and was subsequently investigated as an adjuvant in combination therapy.55,56 No new results of this combination therapy have been released.
In 2016, GlaxoSmithKline (GSK) initiated a phase I clinical trial of a siderophore-β-lactam conjugate GSK3342830 (9) (Fig. 4),57 consisting of a highly functionalised β-lactam core with improved stability to β-lactamases, linked to a catecholate siderophore–mimic.57In vitro testing of GSK3342830 (9) showed promising data against carbapenem-resistant Acinetobacter baumannii with an MIC range of ≤ 0.03 to 4 μg mL−1.58 However, despite the good in vitro data, and preclinical data demonstrating a similar pharmacokinetic profile to cefiderocol, the drug failed to progress past phase I trials due to significant adverse effects.57
Some additional examples of structurally similar “advanced” conjugates include GT-1 (10),59 SMC-3176 (11), M-14659 (12),60 and S-9096 (13)61 (Fig. 4). These compounds are structurally similar to the advanced conjugates discussed above, so offer little additional insight into viable SAC combinations. However, they do demonstrate that, despite potent antimicrobial activity, unreported obstacles (potentially adverse effects) have ultimately prevented progression to clinical trials. Notably, these SACs all utilise β-lactam antibiotics, albeit with some variation between monobactams, monosulfactams, and cephalosporins. Presumably, pharmaceutical companies have opted to focus on β-lactam SACs as the use of this class of periplasmic targeting antibiotics circumvents the need for cleavable linkers.
When it comes to the siderophore component of the SACs, again limited variation is observed, with the two main iron-binding motifs being either pyridone-based or catechol-based chelators. Interestingly, all the siderophores used in these SACs are synthetically derived and contain only a single-iron binding subunit. This is in stark contrast to the majority of the endogenous siderophores which contain three bidentate ligands, often with mixed binding motifs. Hydroxypyridone siderophores, as seen in MC-1 (5), MB-1 (6), BAL30072 (7), SMC-3176 (11) and BAL19764 (8), are isosteres of catechols and can avoid the deleterious effects of the human methylating enzyme catechol-O-methyltransferase (COMT) (see below, challenges associated with developing SACs).62 The hydroxypyridone's relatively small size and ability to avoid COMT are presumably why this siderophore class is favoured in pharmaceutical settings. Similarly, several catechols with chlorine substituents adjacent to the phenolic groups are observed (e.g., cefiderocol (3) and GSK3342830 (9)). Chugai Pharmaceutical Co. have demonstrated that chlorine substitution on catechols can enhance stability to COMT.63 This poses an interesting question of how other substituents adjacent to the phenolic groups affects stability toward COMT.
Although few SACs have entered the clinic, the success of Shionogi's cefiderocol suggests that with further research efficacious SACs can be developed and brought to market. Ultimately this research should also include a critical analysis of why these other more advanced compounds failed or were abandoned. This would provide important lessons for those developing the next generation of SACs.
Although we have deemed these ten compounds as the advanced drug leads in the field, significant work in both academia and industry in earlier stage studies have provided invaluable insight into SAC developments.
Early-stage conjugates
In recent years several comprehensive reviews have been published that provide an in-depth discussion and analyses of the published SACs.7–11 Here, we have opted to summarise the field into a table and provide a commentary on the more recent and unique additions (Table 1).| Antibiotic | Siderophore | Linker | Active | Year Ref |
|---|---|---|---|---|
| Ampicillin | Pyoverdine (PaT II) | NC | Y | 1998 (ref. 64) |
| Ampicillin | Pyoverdine (suc-pyoverdin 9446) | NC | Y | 1998 (ref. 64) |
| Cephalexin | Pyoverdine D | NC | Y | 2008 (ref. 65) |
| Lorcarbef | Agrobactin | NC | N | 1992 (ref. 66) |
| Lorcarbef | N-Acetyl-N-hydroxyl-L-ornithine | NC | N | 1992 (ref. 66) |
| Ampicillin | Tris-catechol | NC | Y | 2012 (ref. 67) |
| Amoxicillin | Tris-catechol | NC | Y | 2012 (ref. 67) |
| Aztreonam | Bis-catechol | NC | Y | 2021 (ref. 68) |
| Lorcarbef | Bis-catechol-monohydroxamate mixed-ligand | NC | Y | 2013 (ref. 69) |
| Carbacephalosporin | N-Acetyl-N-hydroxyl-L-ornithine | NC | Y | 1990 (ref. 70) |
| Carbacephalosporin | Bis-catechol-spermidine derivative | NC | N | 1996 (ref. 71) |
| Aminopenicillins | Bis-catechol | NC | Y | 2009 (ref. 72) |
| Aminopenicillins | Tris-catechol | NC | Y | 2009 (ref. 72) |
| Aminopenicillins | Substituted hydroxamate | NC | Y | 2009 (ref. 72) |
| Ampicillin | Protochelin | NC | Y | 2002 (ref. 73) |
| Amoxicillin | Protochelin | NC | Y | 2002 (ref. 73) |
| Ampicillin | Bis-catechol | NC | Y | 2002 (ref. 74) |
| Amoxicillin | Bis-catechol | NC | Y | 2002 (ref. 74) |
| Cephalexin | Bis-catechol | NC | Y | 2002 (ref. 74) |
| Cefaclor | Bis-catechol | NC | Y | 2002 (ref. 74) |
| Ampicillin | Tris-catechol | NC | Y | 2002 (ref. 74) |
| Amoxicillin | Tris-catechol | NC | Y | 2002 (ref. 74) |
| Cephalexin | Tris-catechol | NC | Y | 2002 (ref. 74) |
| Cefaclor | Tris-catechol | NC | Y | 2002 (ref. 74) |
| Ampicillin | MECAM | NC | Y | 2021 (ref. 75) |
| Amoxicillin | MECAM | NC | Y | 2021 (ref. 75) |
| Ampicillin | MECAM | NC | Y | 2021 (ref. 75) |
| Loracarbef | Desferrioxamine B | NC | Y | 2012 (ref. 76) |
| Monobactam | Pyridone | NC | Y | 2013 (ref. 77) |
| Loracarbef | Tri-hydroxamate | NC | Y | 1992 (ref. 78) |
| Loracarbef | Bis-catechol | NC | Y | 1992 (ref. 78) |
| Loracarbef | Desferridanoxamine | NC | Y | 2013 (ref. 79) |
| Cefaclor | Fimsbactin | NC | Y | 2021 (ref. 80) |
| Ampicillin | Tris-catechol | NC | Y | 2003 (ref. 81) |
| Ampicillin | Tetrakis-catechol | NC | Y | 2003 (ref. 81) |
| Amoxicillin | Tris-catechol | NC | Y | 2003 (ref. 81) |
| Amoxicillin | Tetrakis-catechol | NC | Y | 2003 (ref. 81) |
| Bacampacillin | Tris-catechol | NC | Y | 2003 (ref. 81) |
| Bacampacillin | Tetrakis-catechol | NC | Y | 2003 (ref. 81) |
| Cefaclor | Tris-catechol | NC | Y | 2003 (ref. 81) |
| Cefaclor | Tetrakis-catechol | NC | Y | 2003 (ref. 81) |
| Ampicillin | Enterobactin | NC | NR | 2014 (ref. 82) |
| Amoxicillin | Enterobactin | NC | NR | 2014 (ref. 82) |
| Ampicillin | Salmochelin | NC | NR | 2015 (ref. 83) |
| Amoxicillin | Salmochelin | NC | NR | 2015 (ref. 83) |
| Lactivicin | Hydroxypyridone | NC | Y | 2014 (ref. 84) |
| Lactivicin | Hydroxypyridone | NL | Y | 2014 (ref. 84) |
| Lactivicin | Dihydroxyphthalimide | NL | Y | 2014 (ref. 84) |
| Pyrazolidinone | Dihydroxyphthalimide | NC | Y | 2020 (ref. 85) |
| Ciprofloxacin | Desferrioxamine B | NC | Y | 2012 (ref. 76) |
| Nadifloxacin | Desferrioxamine B | NC | Y | 2012 (ref. 76) |
| Ciprofloxacin | Desferrioxamine B | C | N | 2012 (ref. 86) |
| Ciprofloxacin | Desferrioxamine B | C | N | 2012 (ref. 86) |
| Ciprofloxacin | Desferrioxamine B | NC | N | 2012 (ref. 86) |
| Ciprofloxacin | Enterobactin | NC | N | 2012 (ref. 87) |
| Ciprofloxacin | Desferridanoxamine | NC | N | 2013 (ref. 79) |
| Ciprofloxacin | Pyochelin | NC | N | 2011 (ref. 88) |
| N-desmethyl ofloxacin | Pyochelin | NC | N | 2011 (ref. 88) |
| Norfloxacin | Pyochelin | NC | N | 2011 (ref. 88) |
| Ciprofloxacin | Pyochelin | C | N | 2011 (ref. 88) |
| Norfloxacin | Pyochelin | C | N | 2011 (ref. 88) |
| Norfloxacin | Pyochelin | C | NR | 2007 (ref. 89) |
| Norfloxacin | Pyoverdine | C | N | 2001 (ref. 90) |
| Norfloxacin | Pyoverdine | NC | N | 2001 (ref. 90) |
| Benzonaphthyridone | Pyoverdine | C | Y | 2001 (ref. 90) |
| Benzonaphthyridone | Pyoverdine | NC | Y | 2001 (ref. 90) |
| Ciprofloxacin | Tris-catechol | NC | N | 2014 (ref. 91) |
| Ciprofloxacin | Enterobactin | C | NR | 2015 (ref. 92) |
| Ciprofloxacin | N-Acetyl-N-hydroxyl-L-ornithine | NC | Y | 2021 (ref. 93) |
| Ciprofloxacin | Staphyloferrin A | NC | N | 2013 (ref. 94) |
| Norfloxacin | Staphyloferrin A | NC | N | 2013 (ref. 94) |
| Ciprofloxacin | Citrate | NC | N | 2009 (ref. 95) |
| Ciprofloxacin | Salmochelin derivative | NC | N | 2020 (ref. 96) |
| Ciprofloxacin | Bis-catechol-monohydroxamate mixed-ligand | NC | N | 2013 (ref. 69) |
| Ciprofloxacin | Hydroxypyridone | NC | N | 2023 (ref. 97) |
| Daptomycin | Fimsbactin analogue | NC | Y | 2017 (ref. 98) |
| Daptomycin | MECAM | NC | Y | 2021 (ref. 75) |
| Daptomycin | DOTAM | NC | N | 2022 (ref. 99) |
| Daptomycin | Bis-catechol | C | Y | 2020 (ref. 100) |
| Teicoplanin | Bis-catechol | NC | Y | 2020 (ref. 101) |
| Teicoplanin | Mixed-ligand Bis-catechol-hydroxamate | NC | Y | 2020 (ref. 101) |
| Vancomcyin | Enterobactin | NC | NR | 2012 (ref. 87) |
| Vancomycin | Agrobactin | NC | N | 1996 (ref. 102) |
| Vancomycin | Bis-catechol-spermidine derivative | NC | N | 1996 (ref. 102) |
| Eprezolid | Bis-catechol | C | Y | 2018 (ref. 103) |
| Eprezolid | Bis-catechol | C | Y | 2020 (ref. 100) |
| Linezolid | Aminochelin | NC | NR | 2017 (ref. 104) |
| Gallidermin | Pyochelin | NC | N | 2011 (ref. 105) |
| Gallidermin | Agrobactin | NC | N | 2011 (ref. 105) |
| Gallidermin | Desferrioxamine B | NC | N | 2011 (ref. 105) |
| Artemisinin | Mycobactin analogue | NC | Y | 2011 (ref. 106) |
| Methotrexate | N-Acetyl-N-hydroxyl-L-ornithine | NC | Y | 2022 (ref. 107) |
| Chlorpromazine | Azotochelin | NC | NR | 2018 (ref. 108) |
| Cis-platin | Enterobactin | NC | Y | 2022 (ref. 109) |
| Solithromycin | Bis-catechol | C | Y | 2020 (ref. 100) |
| Chlorpromazine | Azotochelin | NC | NR | 2018 (ref. 108) |
Pfizer's Lactivicin-based SACs
One interesting set of conjugates produced by Pfizer, explored a combination of monodentate iron binding ligand siderophores conjugated to the natural product non-β-lactam PBP inhibitor, lactivicin.84 Lactivicin and analogues typically contain linked cycloserine and γ-lactone motifs.84,110 This work used a lactivicin analogue produced by Takeda Pharmaceutical Company that was reported as having a balanced spectrum of activity towards Gram-negative bacteria and in vivo efficacy.84 However, Pfizer's evaluation of the lactivicin analogue against a contemporary panel of Gram-negative bacteria (P. aeruginosa PAO1 and 1091-05, K. pneumoniae KP-3700, and A. baumannii AB-3176) with clinical relevance showed variable antibacterial activity (MICs 4–32 μg mL−1).84 To improve activity conjugates were prepared using two siderophore motifs – a dihydroxypyridone (14 and 15) (Fig. 5) and a phthalimide-catechol (16) (Fig. 5). The dihydroxypyridone moiety was conjugated onto the antibiotic at two different sites: oxime-linked (14) and α-lactone linked (15). Of the two, only the oxime-linked (14) conjugate demonstrated significantly improved activity compared to the parent compound (e.g., MICs as low as 0.5 μg mL−1 against A. baumannii) compared to MIC 32 μg mL−1 for the parent compound. The authors took this positive result as an indication of successful uptake via the TonB pathway, however, no formal analysis was conducted.84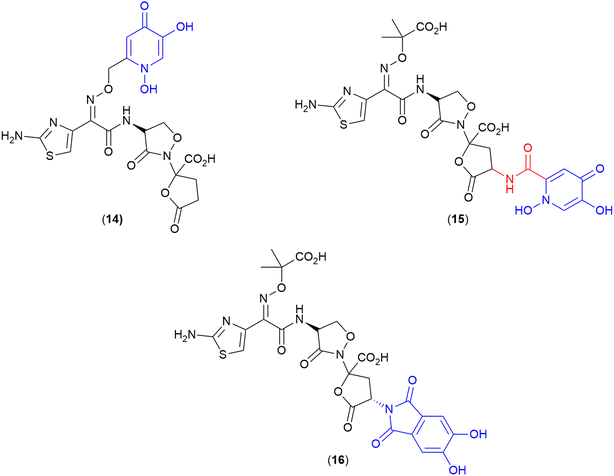 | ||
| Fig. 5 Lactivicin-based SACs produced by Pfizer. Siderophore motif highlighted in blue, antibiotic component in black, linker in red. | ||
To develop a conjugate with enhanced interactions with PBP3 enzymes, the potential to exploit aryl–aryl interactions between the linked siderophore and PBP3 aromatic residues (Tyr503, Tyr532, and Phe533) was also investigated.84 The resulting compound (16) with a phthalimide-catechol siderophore conjugated via the same α-lactone position as (15), showed the most potent MICs and PBP IC50 of the series, with MICs ranging from 0.06–0.5 μg mL−1 against E.coli, P. aeruginosa, K. pneumoniae, and A. baumannii.84 Due to the novelty of this phthalimide siderophore, whole-cell uptake studies were utilised in both Mueller-Hinton broth (MHB) and a modified iron-deplete version, in which the activity of the compound was compared against P. aeruginosa PAO1 and isogenic strains lacking one or more siderophore receptors.84 A significant increase in MIC (>4×) was observed in the receptor deficient strain compared to the parent strain indicating involvement of the receptor is key for activity. From these studies it was determined that PiuA, PirA, FpvA, FptA and other receptors are involved in the uptake of this compound.84
This is the first study utilising lactivicins as the antibiotic in a SAC, and also the first report of a phthalimide–catechol synthetic siderophore. Given the potent activity observed for this derivative, it will be of interest to see how the novel phthalimide-catechol siderophore performs when conjugated with other PBP3-targeting lactam antibiotics.
Methotrexate
A common paradigm in the development of SACs with a cytoplasmic target is the requirement for a cleavable linker for successful delivery of the antibiotic to the target site.100 However, He and co-workers recently demonstrated that a non-cleavable linker can be successfully employed to conjugate a siderophore to the drug methotrexate (17).107 Methotrexate, commonly known for its anticancer activity, also demonstrates potent antimicrobial activity, as a dihydrofolate reductase inhibitor, when assessed via enzyme inhibition assays. However, the drug displays poor bacterial membrane permeability and high cytotoxicity, preventing its use as an efficacious antibiotic.107 In this work, methotrexate was conjugated to the tri-hydroxamate siderophore motif, as observed in albomycin (1) (Fig. 3) via a series of linkers. These linkers included alkyl, glycol and polyglutamate functional groups with a variety of different lengths, as well as a linker containing the 4-nitro-benzo[1,2,5] oxadiazole (NBD) fluorophore.107 These conjugates demonstrated potent activity against the Gram-positive bacterium Streptococcus pneumoniae, with the polyglutamate-linked compound (17) (Fig. 6) displaying an MIC of 1.96 ng mL−1, and more importantly, an MIC of 7.80 ng mL−1 against the Gram-negative Yersinia enterocolitica in iron-depleted media.107 This conjugate was also shown to be >2000-fold less cytotoxic to human cells (HEK293T) than the parent drug.107 This work demonstrates that utilising the correct antimicrobial agent and siderophore combination can result in potent SACs with non-cleavable linkers.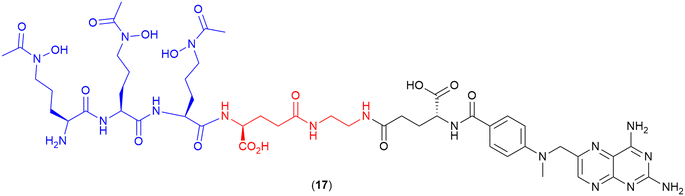 | ||
| Fig. 6 Methotrexate-tri-hydroxamate SAC with non-cleavable linker. Siderophore motif highlighted in blue, linker in red and antibiotic payload in black.107 | ||
Cisplatin
Recent work by Nolan and co-workers reported the repurposing of the anticancer agent cisplatin into a potent SAC (18) (Fig. 7).109 The parent drug (cisplatin) normally demonstrates low antibacterial activity and high toxicity; however, when conjugated to the siderophore enterobactin, a potent antibiotic with low toxicity was obtained.109 The siderophore enterobactin (Ent) (both L- and D- enantiomeric forms), was conjugated to a cisplatin pro-drug derivative via a polyethylene glycol (PEG)3 linker.109 The D-Ent conjugate demonstrated enhanced activity (compared to the L-enantiomer) against E. coli, and conjugation with either enterobactin enantiomer facilitated Pt uptake in bacteria whilst minimising uptake in human cells (HEK293T).109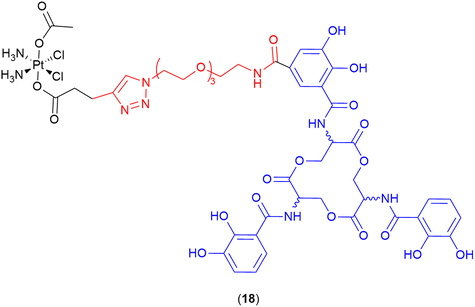 | ||
| Fig. 7 Cisplatin enterobactin conjugate. Both the L- and the D-enterobactin were utilised in this study. Siderophore motif highlighted in blue, linker in red and antibiotic payload in black. | ||
The success of this work by the Nolan and He groups should inspire further investigations on new SACs which incorporate therapeutics not used as antimicrobials due to toxicity, poor cell permeability or other non-drug like properties.
MECAM and DOTAM
In 2021, the Miller group published the first synthesis of a synthetic derivative of enterobactin, 1,3,5-N,N′,N′′-tris-(2,3-dihydroxybenzoyl)-triaminomethylbenzene (MECAM) (Fig. 8).75 In this work the synthetic siderophore was shown to form stable complexes with iron both computationally and experimentally.75 Furthermore, they demonstrated that periplasmic uptake of MECAM alone utilised the ferric enterobactin protein (FepA) outer membrane transporter, by testing in E. coli ΔentA and ΔentAΔfepA mutant strains. E. coli ΔentA is unable to produce the endogenous siderophore enterobactin, and thus requires xenosiderophore addition to promote growth under iron depleted conditions, and ΔentAΔfepA is deficient in both the production of enterobactin but also the outer membrane receptor FepA responsible for enterobactin uptake.75 To evaluate these compounds as SACs, both the acetylated and free phenol MECAM siderophores were conjugated to ampicillin, amoxicillin, and daptomycin via an alkyl or (PEG)4 linkers (Fig. 8).75 The ampicillin (19, 21) and amoxicillin (20) conjugates possessed 12–24-fold lower inhibitory concentrations than either of the respective parent antibiotics, against E. coli.75 Importantly, the addition of MECAM to daptomycin (22), an antibiotic previously only active against Gram-positive bacteria, conferred antimicrobial activity against the Gram-negative pathogen A. baumannii.75 Enabling daptomycin activity against Gram-negative pathogens is beneficial in the search for new therapeutics as there are currently no resistance mechanisms in Gram-negative pathogens to daptomycin. This work has also demonstrated that even a large lipopeptide drug is still able to be effectively transported via the TonB pathway despite the large size of the conjugate with a tris-catecholate siderophore such as MECAM (MW > 2400 Da).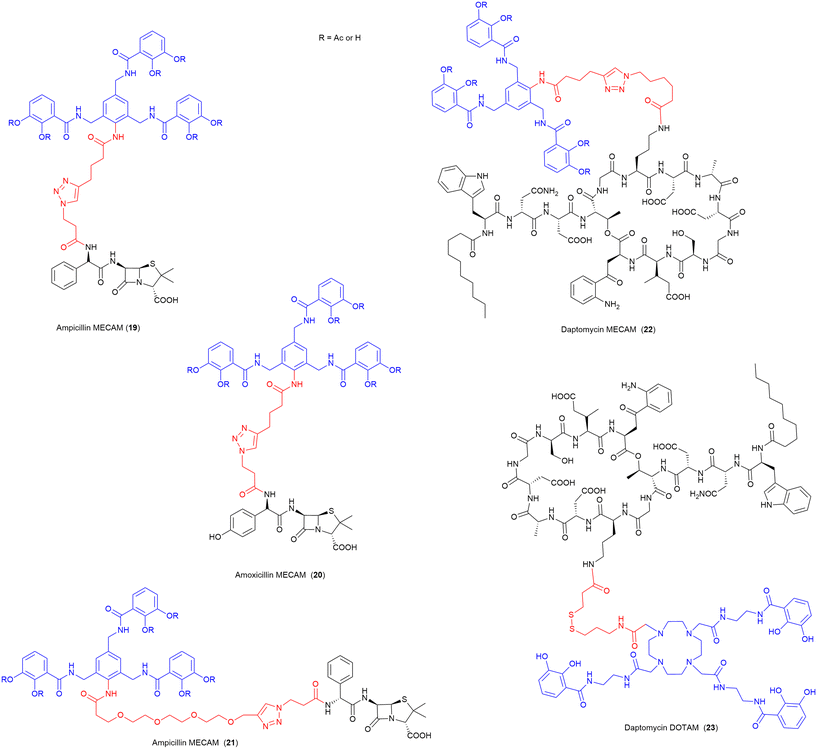 | ||
| Fig. 8 SACs utilising the synthetic Enterobactin derivatives MECAM and DOTAM.75 Siderophore motif highlighted in blue, antibiotic component in black, linker in red. R = Ac or H. | ||
In a follow-up study by the same group, these MECAM-based conjugates, as well as additional SACs using the artificial siderophore DOTAM (1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane), were evaluated in P. aeruginosa for their uptake mechanisms and regulatory responses.99 Although the daptomycin-DOTAM (23), and ampicillin-MECAM (19) conjugates showed no activity against P. aeruginosa, they were utilised to probe the iron-binding capacity and uptake of these novel synthetic siderophores. These studies determined that MECAM siderophores are transported into P. aeruginosa by the transporters PfeA and PirA, whereas DOTAM is solely transported via PirA.99 Both siderophores were less efficient than enterobactin at removing iron from the endogenous siderophore pyoverdine.99 It was concluded that MECAM could be a promising vector for further SACs targeting P. aeruginosa.99
Although we have opted to only discuss a few of the more recent early-stage conjugates that have been published, these SACs provide hope and evidence that siderophores can be utilised to repurpose both existing antimicrobial scaffolds, as well as other drug classes as potent antimicrobials to help alleviate the AMR crisis.
TonB inhibitors
In innovative work, Brönstrup, Schalk and colleagues have demonstrated the first use of a TonB box peptide conjugated to a siderophore to induce TonB inhibition and subsequent bacterial suicide.111 Their recent publication repurposed previously described synthetic siderophores MECAM and DOTAM (Fig. 8). The TonB box is a component of TonB dependent transporters (TBDTs) that extends off the periplasmic domain of the TonB protein into the periplasm and is a sequence of five to seven amino acids which show some conservation.111 This work investigated the potential for the disruption of the TBDT-TonB interaction as novel target in antimicrobial chemotherapy.111 To execute this, a series of synthetic TonB box peptides were designed as competitive inhibitors of protein–protein interactions with several P. aeruginosa TBDTs (FpvA, HasR and PfeA) and were conjugated to the aforementioned triscatecholate synthetic siderophores. Several of the resulting conjugates displayed potent antimicrobial activity (MICs ranging from 0.1–0.5 μM). Ultimately this work further demonstrated the ability of MECAM and DOTAM to transport a large cargo (kilodalton range) into bacterial cells.111 Further, this is the first example of antimicrobial agents that disrupt a key periplasmic protein–protein interaction in a “cellular-suicide” manner.111 This seminal work paves the way for the utilisation of siderophores in ‘out-of-the-box’ ways, as well as providing new antimicrobial targets which are urgently needed. We await further work in this field, potentially investigating peptidomimetics or even small-molecular inhibitors of this key protein–protein interaction.Dual-acting antibacterial and antibiofilm activity
One of the most recent pieces of work in the SAC field has utilised a 3-hydroxy-pyridin-4(1H)-one as a dual-acting iron-chelator and bacterial quorum-sensing inhibitor.97 Previous work by the authors demonstrated the ability of 3-hydroxy-pyridin-4(1H)-one derivatives to block quinolone biosynthesis in the Pseudomonas quorum-sensing system, which further enhanced the antimicrobial activity of ciprofloxacin in MDR P. aeruginosa.112 Subsequently, this compound was utilised as a dual-action iron-chelating and biofilm inhibiting agent in conjugation with ciprofloxacin. A series of compounds were synthesised and tested, with one compound demonstrating potent inhibitory activity and biofilm reduction. The lead compound in this work contained the 3-hydroxy-pyridin-4(1H)-one motif which was conjugated to ciprofloxacin via a non-cleavable linker (24, Fig. 9).97 This compound displayed potent activity against both planktonic and biofilm residing P. aeruginosa cells (MICs 0.43 μM and 78.3% biofilm reduction at ¼ MIC). These results provide a pathway to advance other siderophore-based therapeutics and SACs as novel biofilm-targeting antimicrobials.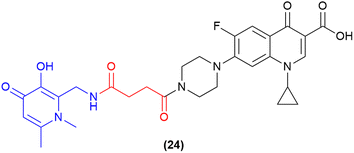 | ||
| Fig. 9 Dual-acting siderophore-antibiofilm-ciprofloxacin conjugate.97 | ||
Challenges associated with developing SACs
Although the “Trojan horse” concept of SACs, where a pathogen's own survival mechanism is hijacked to deliver an antibiotic payload, has proven to be effective and holds promise for future antimicrobial development, there are several challenges associated with the design process. Here we will discuss some of these challenges and the ways in which they have been addressed.Synthetic challenges
Non-cleavable linkers. As the name suggests, non-cleavable linkers are highly stable chemical structures that have no known chemical, biological or enzymatic trigger. Non-cleavable linkers are typically employed in situations where the antibiotic of choice has a periplasmic target (i.e., β-lactam, daptomycin, vancomycin). Due to their hyper stable nature, non-cleavable linkers are relatively simple to design and include a variety of different functional groups. However, optimisation of linker length, rigidity, and attachment points to both the siderophore and antibiotic components provide considerable scope for evaluation.
Over the last few decades of work in the SAC space, a range of non-cleavable linkers have been reported. These have ranged from simple alkyl chains,85 polyethylene glycol (PEG) chains87 and carbonyl-based linkers such as sebacic acid,64 succinic acid,69 and 1,3-diketones,65 to thiol-maleimides,76 dimethyl squarate,105 pyrazinyl succinate,113 and piperazine.91
Vederas and colleagues utilised a bifunctional non-cleavable linker to pursue stepwise addition of two unique molecules. This work conjugated the lantibiotic gallidermin with several different siderophores including agrobactin, pyochelin and desferrioxamine B.105 The initial work utilised 1,5-difluoro-2,4-dinitrobenzene (DFDNB) (Fig. 10) as the bifunctional linking motif, however, the high reactivity of DFNB prevented the desired regio-selective addition of the siderophore and antibiotic. Subsequent efforts employed dimethyl squarate (Fig. 10), where pH-controlled reactions allowed for stepwise addition of the desired siderophore followed by gallidermin. None of the conjugates produced demonstrated antibacterial activity (in iron-replete or iron-deplete media) up to 100 μM. Instead, they demonstrated growth promoting activity against P. aeruginosa when pre-complexed to Fe3+.105 Subsequently it was speculated that the ferri-siderophore conjugates were taken up via the TonB pathway, but only in small local concentrations, as evidenced by the slight growth promotion that was observed.105
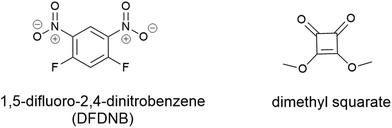 | ||
| Fig. 10 Non-cleavable linkers 1,5-difluoro-2,4-dinitrobenzene (DFDNB) and dimethyl squarate used by Verderas and colleagues in their gallidermin-siderophore conjugates.105 | ||
Cleavable linker types. Cleavable linkers are potentially the most useful in the design of novel antibiotic conjugates, especially SACs. As the name suggests, they are labile moieties that have known chemical or biological triggers. One important caveat is the pro-drug nature of the final compound increases the difficulty and cost associated with drug development. Pro-drugs require additional preclinical studies (e.g., pharmacokinetics, pharmacodynamics, and toxicity) to investigate both the intact drug and all the released components. The linker must also be carefully designed to avoid premature cleavage before the intact moiety reaches the desired target location, the bacterial periplasm.
As discussed earlier, cleavable linker motifs have been observed in nature. One example is the labile peptide linker in the sideromycin albomycin (1), where cleavage by peptidase N at the N-terminal serine-amide bond results in the release of the serine-bound inhibitor.100,114
Despite their potential utility, over the last few decades of development of SACs very few cleavable linkers have been reported. The following section of this review will discuss some of the more recently reported cleavable linkers, their cleavage mechanism and the advantages and disadvantages of these strategies.
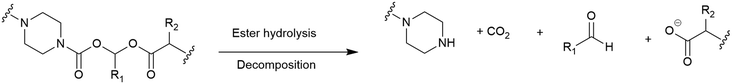
(Acyloxy)alkyl esters. One of the most consistently used moieties for intracellular release of antibiotics from different conjugates are esters, whereby intracellular esterase or the acidic conditions of the periplasm are utilised to facilitate ester hydrolysis.115 However, the hydrolytic lability of esters often leads to premature cleavage by plasma proteases, rendering them unsuitable.115 An alternative to the standard ester linkers was reported by Nolan and co-workers.92 Their use of an (acyloxy)alkyl ester aimed to improve the hydrolytic stability by replacing an (acyloxy)methyl ester with a bulkier substituent (Fig. 11).92 All of the conjugates synthesised in this study focussed on the release of the fluoroquinolone ciprofloxacin. It was concluded that introducing steric hindrance into the linker did indeed reduce hydrolytic lability, however, further work is required to evaluate the practical utility of the linker in SACs.92
Trimethyl lock. The trimethyl lock is an ortho-hydroxydihydrocinnamic acid derivative, which undergoes rapid lactonization due to unfavourable steric interactions between three pendant methyl groups, which is typically triggered by esterases and phosphatases.86 This lactonization forms a hydrocoumarin (Fig. 12) with the release of a leaving group.116 Miller and co-workers demonstrated that a quinone analogue of the trimethyl lock linker could be successfully utilised in the preparation of two siderophore–ciprofloxacin conjugates.86 They hypothesised that unlike the typical trimethyl lock that is susceptible to frequently occurring esterases and phosphatases, this quinone analogue would be specific to ferric reductases which are almost exclusively found in bacterial cells could be utilised for the development of cleavable linkers in SACs.86 In these conjugates, the trimethyl lock linker underwent lactonization and released the antibiotic from the conjugate upon entry into the periplasm. Conjugates which demonstrated successful cleavage from the siderophore exhibited improved antibacterial activity compared with the parent antibiotic.86
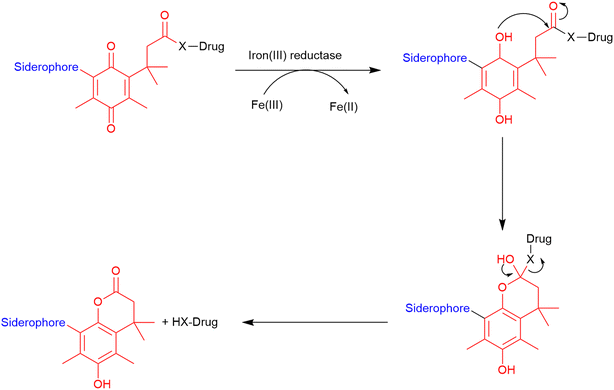 | ||
| Fig. 12 Cleavage of trimethyl lock linker. Scheme adapted from Levine et al.116 Siderophore motif highlighted in blue, antibiotic component in black, linker in red. | ||
Cephalosporin. The cephalosporin-core is commonly utilised as a highly selective cleavable linker. As cleavage occurs only in the presence of a bacterial β-lactamase, the non-selective issues of other cleavable linkers (e.g., premature cleavage) are circumvented. Miller and co-workers were the first to utilise a cephalosporin as the linker in a siderophore–oxazolidinone conjugate (24) (Fig. 13).103 In this work an analogue of the oxazolidinone eprezolid was conjugated to a bis-catechol siderophore utilising a cephalosporin linker.103 Enzymatic and antibacterial assays were utilised in conjunction with LC–MS analysis, and confirmed that in the presence of a purified ADC-type β-lactamase that rapid and complete hydrolysis occurred along with released of the oxazolidinone.103 The proposed cleavage mechanism for the cephalosporinase-triggered release of the oxazolidinone antibiotic is shown in Fig. 13.103
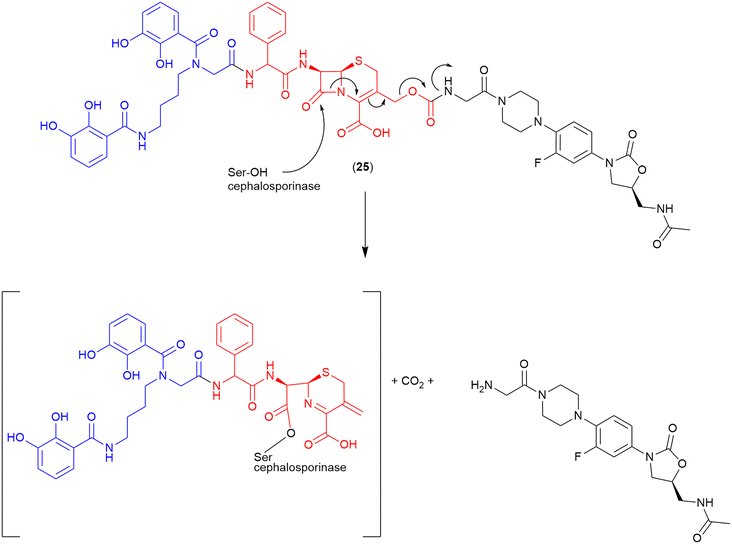 | ||
| Fig. 13 Proposed cleavage mechanism if cephalosporin linker in the oxazolidinone-bis-catechol conjugate.103 Siderophore motif highlighted in blue, antibiotic component in black, linker in red. | ||
This compound showed potent activity against a range of pathogens with MICs from <0.025 μM against E. coli to 12.5 μM against A. baumannii (ATCC BAA 1793).103 Activity was also observed against P. aeruginosa.
Similar β-lactamase triggered cleavages have been comprehensively reviewed by Aldrich and colleagues in a variety of settings including diagnostics, imaging, and antibacterial design.117 These may provide inspiration for further work on site-specific cleavable linkers in the SAC space.
Platform approach. Seiple and co-workers recently developed an “unbiased platform” for the discovery of cleavable linkers, with specific susceptibility to periplasmic proteases.100 This approach utilised a phage display library to screen for peptides that would be susceptible to bacterial periplasmic proteases present in an unfractionated periplasmic extract.100 Susceptible peptide sequences could then be utilised to develop SACs with periplasmic-specific cleavage. Additional studies will be required to optimise the current linkers for practical use, however, once optimised this should be a broadly applicable strategy to designing prodrugs based on protease cleavable peptide linkers.100
The development of cleavable linkers for utilisation in SACs with cytoplasmic targets has provided an invaluable contribution to the field. However, the recent demonstration of a non-cleavable linker being utilised in a SAC with a cytoplasmic provides a starting point for further research using non-cleavable linkers in cytoplasmic acting SACs.107 Further investigations should also build on the advances made in other fields employing cleavable linkers, such as antibody-drug conjugates.118
Evaluation of SAC efficacy
The evaluation of SAC efficacy is multifaceted, with additional requirements in comparison with other more traditional antimicrobial agents. Currently, a variety of different methods are utilised to test SAC efficacy, which makes comparison of these compounds challenging.Recently the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) released guidelines for the Antimicrobial Susceptibility Testing (AST) of cefiderocol. These guidelines can now be applied to pre-existing and newly developed SACs to allow the direct comparison of SACs.
The EUCAST guidelines for the MIC testing of cefiderocol (broth microdilution method) require the use of iron-depleted Mueller-Hinton broth, where iron has been removed via chelation. As iron depletion also removes other important cations, the media also needs to be supplemented with calcium (20–25 mg L−1), magnesium (10–12.5 mg L−1) and zinc (0.5–1.0 mg L−1). The CLSI guidelines for cefiderocol are almost identical to EUCAST; however, they specify the use of a cation-adjusted Mueller-Hinton broth (CAMHB) with Chelex100 as the iron-chelating resin. They also recommend using commercially available tests to ensure iron levels are below 0.03 mg L−1. Both CLSI and EUCAST reference Hackel et al. who provides detailed instructions for the preparation of this media.119
The above guidelines were only released in 2019 following the FDA approval of cefiderocol. Therefore, across the field various methods, assays and media preparations have been utilised to evaluate the activity of the conjugates produced. For example, Shionogi in their original publication on cefiderocol reported using CAMHB plus 20 μM apo-transferrin to mimic iron deplete conditions.120 Other conditions reported in the literature include the addition of 2,2′-bipyridyl as an iron chelating agent to a media of choice.76 However, following the CLSI guidelines will allow for standardised comparison of SACs across the field.
Innate host responses
The action of this enzyme can be detrimental when utilising catechol motifs in drug design as the methylation will usually render the drug inactive. Therefore, finding a way to overcome the deleterious effects of COMT against SACs is important.
Early in the design and synthesis of SACs, Chugai Pharmaceutical Co demonstrated that the addition of either one or two chlorines adjacent to the catechol groups significantly reduced susceptibility to COMT.63 Alternatively, the use of a hydroxypyridone motif avoids COMT, as the catechol isostere is not susceptible to modification by COMT.62
The ability to negate COMT is an important advancement. Although the use of isosteres is promising, further research should continue to explore catechols with reduced susceptibility. Thus, when designing new SACs using catechol-based siderophores, enzymatic assays should be employed to evaluate their susceptibility to COMT.
The siderophore-binding property of Lcn2 was discovered by Strong and co-workers – they observed a red complex formed between Lcn2 and ferric iron-bound enterobactin. To combat this host response, catecholate-type siderophore producing bacteria have adapted to also produce modified siderophores that are not susceptible to Lcn2; known as “stealth siderophores”.123,124 An excellent example of this can be seen in certain members of the Enterobacteriaceae family (E. coli, S. thyphimurium and K. pneumoniae) which not only produce the tris-catecholate siderophore enterobactin (or enterochelin) which is susceptible to Lcn2, but also its glycosylated sister compound salmochelin which shows no susceptibility to Lcn2 (Fig. 2).124
The isolation of these additional siderophores that are not susceptible to Lcn2, from bacteria that produce multiple siderophores suggests Lcn2 may cause widespread binding. Thus, it would be worthwhile considering the action of Lcn2 when designing SACs.
Future directions
SACs have been designed and developed as novel antimicrobial therapeutics for decades, and with the recent success of cefiderocol as a treatment for complex UTIs the field is gaining more traction. So where will we see these Trojan Horse antibiotics venturing over the next decade or two?Next generation SACs
As identified throughout this review, the scope of antibiotics that have been studied in SACs is quite limited. Thus, future work should see an expansion of research into both antibiotics with cytoplasmic targets and to drugs which normally display no antimicrobial activity. This could include Gram-positive only antibiotics (for development as Gram-negative antibiotics), as well as other anti-infectives/anti-cancer agents which previously were unfit for use as antibiotics.Cytoplasmic-targeting antibiotics have typically been avoided when designing new SACs as they must cross both the outer and inner membranes, which creates added difficulties. However, the recent success of the methotrexate–siderophore conjugate, which utilises a non-cleavable linker, creates hope that the traditional belief that cleavable linkers are required may not be true. It would be interesting to see antibiotics such as the fluoroquinolones, aminoglycosides, tetracyclines and even other DHFR inhibitors like trimethoprim investigated as potential antibiotics in the next generation of SACs.
As exemplified by the He group107 and the Nolan group,109 siderophores are an effective way of transforming clinically utilised drugs which on their own display no antimicrobial activity (and are potentially quite toxic), into potent antibiotics with reduced toxicity. Drug repurposing in this form is currently underexplored, which means that a new arsenal of drugs is just waiting to be developed.
This review also highlights variations in the types of siderophores utilised, from endogenous to semi-synthetic and synthetic derivatives. Notably, the more advanced SACs (mostly by pharmaceutical companies) typically contain siderophores with mono-iron-binding subunits. In contrast, early-stage (often academic) research typically employs more complex bis-binding or larger siderophores. Is siderophore size a relevant factor to consider when designing SACs? Could bis-binding units show enhanced efficacy? Should we utilise mixed ligand siderophores to a greater extent? These are critical questions that need to be answered to fill our knowledge gap in the field and progress towards more successful SACs that can be introduced to the clinic.
Finally, there is further potential to improve activity by pre-binding the siderophore motif of the SAC with metal ions with antimicrobial properties, as demonstrated in galbofloxacin – a ciprofloxacin- N-acetyl-N-hydroxyl-L-ornithine conjugate bound with gallium (25) (Fig. 14).93 This study demonstrated that galbofloxacin was more potent both in vivo and in vitro than the either the parent antibiotic, or the derivative without bound gallium.93 This opens up avenues for further investigation of how pre-complexation of the siderophore component of an SAC with zinc, copper or other antimicrobial metals affects activity.21–23 Others have already begun to explore the potential for other metal-based SAC approaches such as the use of a siderophore-linked ruthenium catalyst for targeted intracellular prodrug activation.126
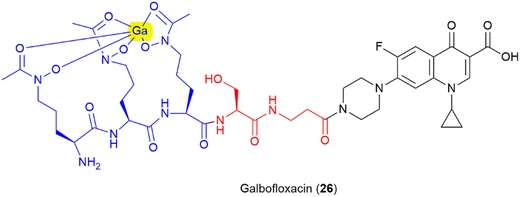 | ||
| Fig. 14 Gallium bound ciprofloxacin-N-acetyl-N-hydroxy ornithine conjugate galbofloxacin.93 Siderophore motif highlighted in blue, antibiotic component in black, linker in red, complexed Ga highlighted in yellow. | ||
Additional uses for siderophores
In this review, we have focused on the ways siderophores have been used for the development of SACs. However, the utility of siderophores goes far beyond just iron transport, and includes transport of non-iron metals,21–23 signalling,24 protection from oxidative stress,25,26 immunomodulation27 and biofilm formation.28–33 Of these uses, biofilms are known to play a pivotal role in the development of AMR. Thus, the deployment of siderophores and/or SACs into antibiofilm agents is an exciting prospect.Concluding remarks
Over the last few decades significant work has been produced in the SAC field, expanding our knowledge base and providing invaluable foundational information to build upon to further advance this new class of antimicrobial therapeutics.It is clear from the success of cefiderocol that there is significant potential for SACs to be utilised as front-line therapies in overcoming antibiotic-resistant infections. The interest over the last few decades from pharmaceutical companies also provides hope that with more research we could start to see more SACs come to market. To date, most advanced work on SACs has focused on utilising the β-lactam antibiotic class, but there is untapped potential in exploring other antibiotic and non-antibiotic active components. When combined with variations in linker strategies and types of siderophores, the breadth of variety embodied by this new class of antimicrobial therapeutics becomes apparent. In the future, the advancement of multiple SACs into clinical use may provide one critical piece of the puzzle in solving the antimicrobial resistance crisis.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
NHMRC Ideas Grant #2004367.References
- J. O'Neill, Tackling drug-resistant infections globally: final report and recommendations, Government of the United Kingdom, 2016 Search PubMed.
- E. Tacconelli, E. Carrara, A. Savoldi, S. Harbarth, M. Mendelson, D. L. Monnet, C. Pulcini, G. Kahlmeter, J. Kluytmans, Y. Carmeli, M. Ouellette, K. Outterson, J. Patel, M. Cavaleri, E. M. Cox, C. R. Houchens, M. L. Grayson, P. Hansen, N. Singh, U. Theuretzbacher and N. Magrini, Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis, Lancet Infect. Dis., 2018, 18, 318–327 CrossRef PubMed.
- M. S. Mulani, E. E. Kamble, S. N. Kumkar, M. S. Tawre and K. R. Pardesi, Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review, Front. Microbiol., 2019, 10, 539 CrossRef PubMed.
- S. Butler Mark, V. Gigante, H. Sati, S. Paulin, L. Al-Sulaiman, H. Rex John, P. Fernandes, A. Arias Cesar, M. Paul, E. Thwaites Guy, L. Czaplewski, A. Alm Richard, C. Lienhardt, M. Spigelman, L. Silver Lynn, N. Ohmagari, R. Kozlov, S. Harbarth and P. Beyer, Analysis of the Clinical Pipeline of Treatments for Drug-Resistant Bacterial Infections: Despite Progress, More Action Is Needed, Antimicrob. Agents Chemother., 2022, 66, e01991-01921 Search PubMed.
- M. S. Butler and D. L. Paterson, Antibiotics in the clinical pipeline in October 2019, J. Antibiot., 2020, 73, 329–364 CrossRef CAS PubMed.
- I. J. Schalk, Metal trafficking via siderophores in Gram-negative bacteria: Specificities and characteristics of the pyoverdine pathway, J. Inorg. Biochem., 2008, 102, 1159–1169 CrossRef CAS PubMed.
- P. Klahn and M. Brönstrup, Bifunctional antimicrobial conjugates and hybrid antimicrobials, Nat. Prod. Rep., 2017, 34, 832–885 RSC.
- K. H. Negash, J. K. S. Norris and J. T. Hodgkinson, Siderophore-Antibiotic Conjugate Design: New Drugs for Bad Bugs?, Molecules, 2019, 24, 3314 CrossRef CAS PubMed.
- G. Tonziello, E. Caraffa, B. Pinchera, G. Granata and N. Petrosillo, Present and future of siderophore-based therapeutic and diagnostic approaches in infectious diseases, Infect. Dis. Rep., 2019, 11, 8208 CrossRef PubMed.
- G. S. Tillotson, Trojan Horse Antibiotics-A Novel Way to Circumvent Gram-Negative Bacterial Resistance?, J. Infect. Dis., 2016, 9, 45–52 Search PubMed.
- M. Ribeiro and M. Simões, Advances in the antimicrobial and therapeutic potential of siderophores, Environ. Chem. Lett., 2019, 17, 1485–1494 CrossRef CAS.
- E. Breukink and B. de Kruijff, Lipid II as a target for antibiotics, Nat. Rev. Drug Discovery, 2006, 5, 321–323 CrossRef CAS PubMed.
- M. Rajagopal and S. Walker, Envelope Structures of Gram-Positive Bacteria, Curr. Top. Microbiol. Immunol., 2017, 404, 1–44 CAS.
- T. J. Silhavy, D. Kahne and S. Walker, The bacterial cell envelope, Cold Spring Harbor Perspect. Biol., 2010, 2, a000414 Search PubMed.
- N. Noinaj, M. Guillier, T. J. Barnard and S. K. Buchanan, TonB-dependent transporters: regulation, structure, and function, Annu. Rev. Microbiol., 2010, 64, 43–60 CrossRef CAS PubMed.
- I. J. Schalk, Siderophore–antibiotic conjugates: exploiting iron uptake to deliver drugs into bacteria, Clin. Microbiol. Infect., 2018, 24, 801–802 CrossRef CAS PubMed.
- R. C. Hider and X. Kong, Chemistry and biology of siderophores, Nat. Prod. Rep., 2010, 27, 637–657 RSC.
- H. Kong, W. Cheng, H. Wei, Y. Yuan, Z. Yang and X. Zhang, An overview of recent progress in siderophore-antibiotic conjugates, Eur. J. Med. Chem., 2019, 182, 111615 CrossRef CAS PubMed.
- A. Khan, P. Singh and A. Srivastava, Synthesis, nature and utility of universal iron chelator – Siderophore: A review, Microbiol. Res., 2018, 212-213, 103–111 CrossRef CAS PubMed.
- M. Miethke, T. Kraushaar and M. A. Marahiel, Uptake of xenosiderophores in Bacillus subtilis occurs with high affinity and enhances the folding stabilities of substrate binding proteins, FEBS Lett., 2013, 587, 206–213 CrossRef CAS PubMed.
- B. E. Allred, P. B. Rupert, S. S. Gauny, D. D. An, C. Y. Ralston, M. Sturzbecher-Hoehne, R. K. Strong and R. J. Abergel, Siderocalin-mediated recognition, sensitization, and cellular uptake of actinides, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 10342–10347 CrossRef CAS PubMed.
- K. S. Chaturvedi, C. S. Hung, J. R. Crowley, A. E. Stapleton and J. P. Henderson, The siderophore yersiniabactin binds copper to protect pathogens during infection, Nat. Chem. Biol., 2012, 8, 731–736 CrossRef CAS PubMed.
- T. C. Johnstone and E. M. Nolan, Beyond iron: non-classical biological functions of bacterial siderophores, Dalton Trans., 2015, 44, 6320–6339 RSC.
- I. L. Lamont, P. A. Beare, U. Ochsner, A. I. Vasil and M. L. Vasil, Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 7072–7077 CrossRef CAS PubMed.
- C. Adler, N. S. Corbalan, D. R. Peralta, M. F. Pomares, R. E. de Cristóbal and P. A. Vincent, The alternative role of enterobactin as an oxidative stress protector allows Escherichia coli colony development, PLoS One, 2014, 9, e84734 CrossRef PubMed.
- D. R. Peralta, C. Adler, N. S. Corbalán, E. C. Paz García, M. F. Pomares and P. A. Vincent, Enterobactin as Part of the Oxidative Stress Response Repertoire, PLoS One, 2016, 11, e0157799 CrossRef PubMed.
- M. A. Bachman, V. L. Miller and J. N. Weiser, Mucosal lipocalin 2 has pro-inflammatory and iron-sequestering effects in response to bacterial enterobactin, PLoS Pathog., 2009, 5, e1000622 CrossRef PubMed.
- P. K. Singh, M. R. Parsek, E. P. Greenberg and M. J. Welsh, A component of innate immunity prevents bacterial biofilm development, Nature, 2002, 417, 552–555 CrossRef CAS PubMed.
- P. K. Singh, Iron sequestration by human lactoferrin stimulates P. aeruginosa surface motility and blocks biofilm formation, BioMetals, 2004, 17, 267–270 CrossRef CAS PubMed.
- E. Banin, M. L. Vasil and E. P. Greenberg, Iron and Pseudomonas aeruginosa biofilm formation, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 11076–11081 CrossRef CAS PubMed.
- E. Banin, M. Brady Keith and E. P. Greenberg, Chelator-Induced Dispersal and Killing of Pseudomonas aeruginosa Cells in a Biofilm, Appl. Environ. Microbiol., 2006, 72, 2064–2069 CrossRef CAS PubMed.
- E. Banin, A. Lozinski, K. M. Brady, E. Berenshtein, P. W. Butterfield, M. Moshe, M. Chevion, E. P. Greenberg and E. Banin, The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 16761–16766 CrossRef CAS PubMed.
- I. Raad, I. Chatzinikolaou, G. Chaiban, H. Hanna, R. Hachem, T. Dvorak, G. Cook and W. Costerton, In Vitro and Ex Vivo Activities of Minocycline and EDTA against Microorganisms Embedded in Biofilm on Catheter Surfaces, Antimicrob. Agents Chemother., 2003, 47, 3580–3585 CrossRef CAS PubMed.
- I. J. Schalk, M. Hannauer and A. Braud, New roles for bacterial siderophores in metal transport and tolerance, Environ. Microbiol., 2011, 13, 2844–2854 CrossRef CAS PubMed.
- V. Braun and M. Braun, Iron transport and signaling in Escherichia coli, FEBS Lett., 2002, 529, 78–85 CrossRef CAS PubMed.
- E. Udho, K. S. Jakes, S. K. Buchanan, K. J. James, X. Jiang, P. E. Klebba and A. Finkelstein, Reconstitution of bacterial outer membrane TonB-dependent transporters in planar lipid bilayer membranes, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 21990–21995 CrossRef CAS PubMed.
- W. Köster, ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12, Res. Microbiol., 2001, 152, 291–301 CrossRef PubMed.
- A. L. Lamb, Breaking a pathogen's iron will: Inhibiting siderophore production as an antimicrobial strategy, Biochim. Biophys. Acta, 2015, 1854, 1054–1070 CrossRef CAS PubMed.
- Y. Yamano, In Vitro Activity of Cefiderocol Against a Broad Range of Clinically Important Gram-negative Bacteria, Clin. Infect. Dis., 2019, 69, S544–S551 CrossRef CAS PubMed.
- R. Huang, X. Meng, K. Tao, M. Cao, L. Nie, Y. Dong, Y. Lyu, S. Wang and Z. Feng, Discovery and Biosynthesis of the Amodesmycins, Aromatic Polyketide-Siderophore Hybrid Conjugates, Org. Lett., 2022, 24, 9408–9412 CrossRef CAS PubMed.
- Z. Lin, X. Xu, S. Zhao, X. Yang, J. Guo, Q. Zhang, C. Jing, S. Chen and Y. He, Total synthesis and antimicrobial evaluation of natural albomycins against clinical pathogens, Nat. Commun., 2018, 9, 3445 CrossRef PubMed.
- Y.-M. Lin, M. Ghosh, P. A. Miller, U. Möllmann and M. J. Miller, Synthetic sideromycins (skepticism and optimism): selective generation of either broad or narrow spectrum Gram-negative antibiotics, BioMetals, 2019, 32, 425–451 CrossRef CAS PubMed.
- V. Braun, A. Pramanik, T. Gwinner, M. Köberle and E. Bohn, Sideromycins: tools and antibiotics, BioMetals, 2009, 22, 3–13 CrossRef CAS PubMed.
- G. Benz, T. Schröder, J. Kurz, C. Wünsche, W. Karl, G. Steffens, J. Pfitzner and D. Schmidt, Constitution of the Deferriform of the Albomycins δ1, δ2 and ε, Angew. Chem., Int. Ed. Engl., 1982, 21, 527–528 CrossRef.
- G. F. Gause, Recent studies on albomycin, a new antibiotic, Br. Med. J., 1955, 2, 1177–1179 CrossRef CAS PubMed.
- Q. Perraud, P. Cantero, M. Munier, F. Hoegy, N. Zill, V. Gasser, G. L. A. Mislin, L. Ehret-Sabatier and I. J. Schalk, Phenotypic Adaptation of Pseudomonas aeruginosa in the Presence of Siderophore-Antibiotic Conjugates during Epithelial Cell Infection, Microorganisms, 2020, 8, 1820 CrossRef CAS PubMed.
- P. Silley, J. W. Griffiths, D. Monsey and A. M. Harris, Mode of action of GR69153, a novel catechol-substituted cephalosporin, and its interaction with the tonB-dependent iron transport system, Antimicrob. Agents Chemother., 1990, 34, 1806–1808 CrossRef CAS PubMed.
- M. Terreni, M. Taccani and M. Pregnolato, New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives, Molecules, 2021, 26, 2671 CrossRef CAS PubMed.
- C. J. McPherson, L. M. Aschenbrenner, B. M. Lacey, K. C. Fahnoe, M. M. Lemmon, S. M. Finegan, B. Tadakamalla, J. P. O'Donnell, J. P. Mueller and A. P. Tomaras, Clinically relevant Gram-negative resistance mechanisms have no effect on the efficacy of MC-1, a novel siderophore-conjugated monocarbam, Antimicrob. Agents Chemother., 2012, 56, 6334–6342 CrossRef CAS PubMed.
- M. G. P. Page, The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics, Clin. Infect. Dis., 2019, 69, S529–S537 CrossRef CAS PubMed.
- P. Tomaras Andrew, L. Crandon Jared, J. McPherson Craig and P. Nicolau David, Potentiation of Antibacterial Activity of the MB-1 Siderophore-Monobactam Conjugate Using an Efflux Pump Inhibitor, Antimicrob. Agents Chemother., 2015, 59, 2439–2442 CrossRef CAS PubMed.
- P. Tomaras Andrew, L. Crandon Jared, J. McPherson Craig, A. Banevicius Mary, M. Finegan Steven, L. Irvine Rebecca, F. Brown Matthew, P. O'Donnell John and P. Nicolau David, Adaptation-Based Resistance to Siderophore-Conjugated Antibacterial Agents by Pseudomonas aeruginosa, Antimicrob. Agents Chemother., 2013, 57, 4197–4207 CrossRef CAS PubMed.
- M. G. Page, C. Dantier and E. Desarbre, In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant gram-negative bacilli, Antimicrob. Agents Chemother., 2010, 54, 2291–2302 CrossRef CAS PubMed.
- F. Paech, S. Messner, J. Spickermann, M. Wind, A.-H. Schmitt-Hoffmann, A. T. Witschi, B. A. Howell, R. J. Church, J. Woodhead, M. Engelhardt, S. Krähenbühl and M. Maurer, Mechanisms of hepatotoxicity associated with the monocyclic β-lactam antibiotic BAL30072, Arch. Toxicol., 2017, 91, 3647–3662 CrossRef CAS PubMed.
- D. M. Livermore, S. Mushtaq and M. Warner, Activity of BAL30376 (monobactam BAL19764
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BAL29880
BAL29880![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) clavulanate) versus Gram-negative bacteria with characterized resistance mechanisms, J. Antimicrob. Chemother., 2010, 65, 2382–2395 CrossRef CAS PubMed.
clavulanate) versus Gram-negative bacteria with characterized resistance mechanisms, J. Antimicrob. Chemother., 2010, 65, 2382–2395 CrossRef CAS PubMed. - G. P. Page Malcolm, C. Dantier, E. Desarbre, B. Gaucher, K. Gebhardt and A. Schmitt-Hoffmann, In Vitro and In Vivo Properties of BAL30376, a β-Lactam and Dual β-Lactamase Inhibitor Combination with Enhanced Activity against Gram-Negative Bacilli That Express Multiple β-Lactamases, Antimicrob. Agents Chemother., 2011, 55, 1510–1519 CrossRef CAS PubMed.
- D. Tenero, N. Farinola, E. M. Berkowitz, C. A. Tiffany, Y. Qian, Z. Xue, A. Raychaudhuri and D. F. Gardiner, Pharmacokinetics, Safety, and Tolerability Evaluation of Single and Multiple Doses of GSK3342830 in Healthy Volunteers, Clin. Pharmacol. Drug Dev., 2019, 8, 754–764 CAS.
- B. Isler, Y. Doi, R. A. Bonomo and D. L. Paterson, New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections, Antimicrob. Agents Chemother., 2018, 63, e01110–e01118 Search PubMed.
- L. P. Nguyen, N. A. Pinto, T. N. Vu, H. Lee, Y. L. Cho, J. H. Byun, R. D'Souza and D. Yong, In Vitro Activity of a Novel Siderophore-Cephalosporin, GT-1 and Serine-Type β-Lactamase Inhibitor, GT-055, against Escherichia coli, Klebsiella pneumoniae and Acinetobacter spp. Panel Strains, Antibiotics, 2020, 9, 267 CrossRef CAS PubMed.
- H. Mochizuki, H. Yamada, Y. Oikawa, K. Murakami, J. Ishiguro, H. Kosuzume, N. Aizawa and E. Mochida, Bactericidal activity of M14659 enhanced in low-iron environments, Antimicrob. Agents Chemother., 1988, 32, 1648–1654 CrossRef CAS PubMed.
- Y. Yamano, T. Nishikawa and Y. Komatsu, Ferric iron transport system of Pseudomonas aeruginosa PAO1 that functions as the uptake pathway of a novel catechol-substituted cephalosporin, S-9096, Appl. Microbiol. Biotechnol., 1994, 40, 892–897 CrossRef CAS.
- C. Ji, R. E. Juárez-Hernández and M. J. Miller, Exploiting bacterial iron acquisition: siderophore conjugates, Future Med. Chem., 2012, 4, 297–313 CrossRef CAS PubMed.
- N. Ohi, B. Aoki, T. Kuroki, M. Matsumoto, K. Kojima and T. Nehashi, Semisynthetic beta-lactam antibiotics. III. Effect on antibacterial activity and comt-susceptibility of chlorine-introduction into the catechol nucleus of 6-[(R)-2-[3-(3,4-dihydroxybenzoyl)-3-(3-hydroxypropyl)-1-ureido]-2- phenylacetamido]penicillanic acid, J. Antibiot., 1987, 40, 22–28 CrossRef CAS PubMed.
- O. Kinzel, R. Tappe, I. Gerus and H. Budzikiewicz, The synthesis and antibacterial activity of two pyoverdin-ampicillin conjugates, entering Pseudomonas aeruginosa via the pyoverdin-mediated iron uptake pathway, J. Antibiot., 1998, 51, 499–507 CrossRef CAS PubMed.
- O. Kinzel and H. Budzikiewicz, Synthesis and biological evaluation of a pyoverdin-β-lactam conjugate: a new type of arginine-specific cross-linking in aqueous solution, J. Pept. Res., 1999, 53, 618–625 CrossRef CAS PubMed.
- A. Brochu, N. Brochu, T. I. Nicas, T. R. Parr, Jr., A. A. Minnick, Jr., E. K. Dolence, J. A. McKee, M. J. Miller, M. C. Lavoie and F. Malouin, Modes of action and inhibitory activities of new siderophore-beta-lactam conjugates that use specific iron uptake pathways for entry into bacteria, Antimicrob. Agents Chemother., 1992, 36, 2166–2175 CrossRef CAS PubMed.
- C. Ji, P. A. Miller and M. J. Miller, Iron Transport-Mediated Drug Delivery: Practical Syntheses and In Vitro Antibacterial Studies of Tris-Catecholate Siderophore–Aminopenicillin Conjugates Reveals Selectively Potent Antipseudomonal Activity, J. Am. Chem. Soc., 2012, 134, 9898–9901 CrossRef CAS PubMed.
- R. Liu, P. A. Miller and M. J. Miller, Conjugation of Aztreonam, a Synthetic Monocyclic β-Lactam Antibiotic, to a Siderophore Mimetic Significantly Expands Activity Against Gram-Negative Bacteria, ACS Infect. Dis., 2021, 7, 2979–2986 CrossRef CAS PubMed.
- T. A. Wencewicz and M. J. Miller, Biscatecholate–Monohydroxamate Mixed Ligand Siderophore–Carbacephalosporin Conjugates are Selective Sideromycin Antibiotics that Target Acinetobacter baumannii, J. Med. Chem., 2013, 56, 4044–4052 CrossRef CAS PubMed.
- E. K. Dolence, A. A. Minnick and M. J. Miller, N5-Acetyl-N5-hydroxy-L-ornithine-derived siderophore-carbacephalosporin .beta.-lactam conjugates: iron transport mediated drug delivery, J. Med. Chem., 1990, 33, 461–464 CrossRef CAS PubMed.
- A. Ghosh, M. Ghosh, C. Niu, F. Malouin, U. Moellmann and M. J. Miller, Iron transport-mediated drug delivery using mixed-ligand siderophore-β-lactam conjugates, Chem. Biol., 1996, 3, 1011–1019 CrossRef CAS PubMed.
- U. Möllmann, L. Heinisch, A. Bauernfeind, T. Köhler and D. Ankel-Fuchs, Siderophores as drug delivery agents: application of the “Trojan Horse” strategy, BioMetals, 2009, 22, 615–624 CrossRef PubMed.
- L. Heinisch, S. Wittmann, T. Stoiber, A. Berg, D. Ankel-Fuchs and U. Möllmann, Highly Antibacterial Active Aminoacyl Penicillin Conjugates with Acylated Bis-Catecholate Siderophores Based on Secondary Diamino Acids and Related Compounds, J. Med. Chem., 2002, 45, 3032–3040 CrossRef CAS PubMed.
- S. Wittmann, M. Schnabelrauch, I. Scherlitz-Hofmann, U. Möllmann, D. Ankel-Fuchs and L. Heinisch, New synthetic siderophores and Their β-Lactam conjugates based on diamino acids and dipeptides, Bioorg. Med. Chem., 2002, 10, 1659–1670 CrossRef CAS PubMed.
- L. Pinkert, Y.-H. Lai, C. Peukert, S.-K. Hotop, B. Karge, L. M. Schulze, J. Grunenberg and M. Brönstrup, Antibiotic Conjugates with an Artificial MECAM-Based Siderophore Are Potent Agents against Gram-Positive and Gram-Negative Bacterial Pathogens, J. Med. Chem., 2021, 64, 15440–15460 CrossRef CAS PubMed.
- R. E. Juárez-Hernández, P. A. Miller and M. J. Miller, Syntheses of Siderophore-Drug Conjugates Using a Convergent Thiol-Maleimide System, ACS Med. Chem. Lett., 2012, 3, 799–803 CrossRef PubMed.
- M. F. Brown, M. J. Mitton-Fry, J. T. Arcari, R. Barham, J. Casavant, B. S. Gerstenberger, S. Han, J. R. Hardink, T. M. Harris, T. Hoang, M. D. Huband, M. S. Lall, M. M. Lemmon, C. Li, J. Lin, S. P. McCurdy, E. McElroy, C. McPherson, E. S. Marr, J. P. Mueller, L. Mullins, A. A. Nikitenko, M. C. Noe, J. Penzien, M. S. Plummer, B. P. Schuff, V. Shanmugasundaram, J. T. Starr, J. Sun, A. Tomaras, J. A. Young and R. P. Zaniewski, Pyridone-Conjugated Monobactam Antibiotics with Gram-Negative Activity, J. Med. Chem., 2013, 56, 5541–5552 CrossRef CAS PubMed.
- A. A. Minnick, J. A. McKee, E. K. Dolence and M. J. Miller, Iron transport-mediated antibacterial activity of and development of resistance to hydroxamate and catechol siderophore-carbacephalosporin conjugates, Antimicrob. Agents Chemother., 1992, 36, 840–850 CrossRef CAS PubMed.
- T. A. Wencewicz, T. E. Long, U. Möllmann and M. J. Miller, Trihydroxamate siderophore-fluoroquinolone conjugates are selective sideromycin antibiotics that target Staphylococcus aureus, Bioconjugate Chem., 2013, 24, 473–486 CrossRef CAS PubMed.
- D. Y. Kim and H. J. Kim, Function of Fimsbactin B as an Acinetobacter-Selective Antibiotic Delivery Vehicle, Org. Lett., 2021, 23, 5256–5260 CrossRef CAS PubMed.
- L. Heinisch, S. Wittmann, T. Stoiber, I. Hofmann, D. Ankel-Fuchs and U. Möllmann, Synthesis and Biological Activity of Trisand Tetrakiscatecholate Siderophores Based on Poly-aza Alkanoic Acids or Alkylbenzoic Acids and their Conjugates with β-Lactam Antibiotics, Arzneim. Forsch., 2003, 53, 188–195 CAS.
- T. Zheng and E. M. Nolan, Enterobactin-Mediated Delivery of β-Lactam Antibiotics Enhances Antibacterial Activity against Pathogenic Escherichia coli, J. Am. Chem. Soc., 2014, 136, 9677–9691 CrossRef CAS PubMed.
- P. Chairatana, T. Zheng and E. M. Nolan, Targeting virulence: salmochelin modification tunes the antibacterial activity spectrum of β-lactams for pathogen-selective killing of Escherichia coli, Chem. Sci., 2015, 6, 4458–4471 RSC.
- J. Starr, M. F. Brown, L. Aschenbrenner, N. Caspers, Y. Che, B. S. Gerstenberger, M. Huband, J. D. Knafels, M. M. Lemmon, C. Li, S. P. McCurdy, E. McElroy, M. R. Rauckhorst, A. P. Tomaras, J. A. Young, R. P. Zaniewski, V. Shanmugasundaram and S. Han, Siderophore Receptor-Mediated Uptake of Lactivicin Analogues in Gram-Negative Bacteria, J. Med. Chem., 2014, 57, 3845–3855 CrossRef CAS PubMed.
- J. A. Goldberg, H. Nguyen, V. Kumar, E. J. Spencer, D. Hoyer, E. K. Marshall, A. Cmolik, M. O'Shea, S. H. Marshall, A. M. Hujer, K. M. Hujer, S. D. Rudin, T. N. Domitrovic, C. R. Bethel, K. M. Papp-Wallace, L. K. Logan, F. Perez, M. R. Jacobs, D. van Duin, B. M. Kreiswirth, R. A. Bonomo, M. S. Plummer and F. van den Akker, A γ-Lactam Siderophore Antibiotic Effective against Multidrug-Resistant Gram-Negative Bacilli, J. Med. Chem., 2020, 63, 5990–6002 CrossRef CAS PubMed.
- C. Ji and M. J. Miller, Siderophore-fluoroquinolone conjugates containing potential reduction-triggered linkers for drug release: synthesis and antibacterial activity, BioMetals, 2015, 28, 541–551 CrossRef CAS PubMed.
- T. Zheng, J. L. Bullock and E. M. Nolan, Siderophore-Mediated Cargo Delivery to the Cytoplasm of Escherichia coli and Pseudomonas aeruginosa: Syntheses of Monofunctionalized Enterobactin Scaffolds and Evaluation of Enterobactin–Cargo Conjugate Uptake, J. Am. Chem. Soc., 2012, 134, 18388–18400 CrossRef CAS PubMed.
- S. Noël, V. Gasser, B. Pesset, F. Hoegy, D. Rognan, I. J. Schalk and G. L. A. Mislin, Synthesis and biological properties of conjugates between fluoroquinolones and a N3′′-functionalized pyochelin, Org. Biomol. Chem., 2011, 9, 8288–8300 RSC.
- F. Rivault, C. Liébert, A. Burger, F. Hoegy, M. A. Abdallah, I. J. Schalk and G. L. A. Mislin, Synthesis of pyochelin–norfloxacin conjugates, Bioorg. Med. Chem. Lett., 2007, 17, 640–644 CrossRef CAS PubMed.
- C. Hennard, Q. C. Truong, J.-F. Desnottes, J.-M. Paris, N. J. Moreau and M. A. Abdallah, Synthesis and Activities of Pyoverdin−Quinolone Adducts: A Prospective Approach to a Specific Therapy Against Pseudomonas aeruginosa, J. Med. Chem., 2001, 44, 2139–2151 CrossRef CAS PubMed.
- S. Fardeau, A. Dassonville-Klimpt, N. Audic, A. Sasaki, M. Pillon, E. Baudrin, C. Mullié and P. Sonnet, Synthesis and antibacterial activity of catecholate–ciprofloxacin conjugates, Bioorg. Med. Chem., 2014, 22, 4049–4060 CrossRef CAS PubMed.
- T. Zheng and E. M. Nolan, Evaluation of (acyloxy)alkyl ester linkers for antibiotic release from siderophore–antibiotic conjugates, Bioorg. Med. Chem. Lett., 2015, 25, 4987–4991 CrossRef CAS PubMed.
- A. Pandey, D. Śmiłowicz and E. Boros, Galbofloxacin: a xenometal-antibiotic with potent in vitro and in vivo efficacy against S. aureus, Chem. Sci., 2021, 12, 14546–14556 RSC.
- S. J. Milner, A. Seve, A. M. Snelling, G. H. Thomas, K. G. Kerr, A. Routledge and A. K. Duhme-Klair, Staphyloferrin A as siderophore-component in fluoroquinolone-based Trojan horse antibiotics, Org. Biomol. Chem., 2013, 11, 3461–3468 RSC.
- S. R. Md-Saleh, E. C. Chilvers, K. G. Kerr, S. J. Milner, A. M. Snelling, J. P. Weber, G. H. Thomas, A.-K. Duhme-Klair and A. Routledge, Synthesis of citrate–ciprofloxacin conjugates, Bioorg. Med. Chem. Lett., 2009, 19, 1496–1498 CrossRef CAS PubMed.
- T. J. Sanderson, C. M. Black, J. W. Southwell, E. J. Wilde, A. Pandey, R. Herman, G. H. Thomas, E. Boros, A.-K. Duhme-Klair and A. Routledge, A Salmochelin S4-Inspired Ciprofloxacin Trojan Horse Conjugate, ACS Infect. Dis., 2020, 6, 2532–2541 CrossRef CAS PubMed.
- Y.-Y. Wang, X.-Y. Zhang, X.-L. Zhong, Y.-J. Huang, J. Lin and W.-M. Chen, Design and Synthesis of 3-Hydroxy-pyridin-4(1H)-ones–Ciprofloxacin Conjugates as Dual Antibacterial and Antibiofilm Agents against Pseudomonas aeruginosa, J. Med. Chem., 2023, 66, 2169–2193 CrossRef CAS PubMed.
- M. Ghosh, P. A. Miller, U. Möllmann, W. D. Claypool, V. A. Schroeder, W. R. Wolter, M. Suckow, H. Yu, S. Li, W. Huang, J. Zajicek and M. J. Miller, Targeted Antibiotic Delivery: Selective Siderophore Conjugation with Daptomycin Confers Potent Activity against Multidrug Resistant Acinetobacter baumannii Both in Vitro and in Vivo, J. Med. Chem., 2017, 60, 4577–4583 CrossRef CAS PubMed.
- S. Fritsch, V. Gasser, C. Peukert, L. Pinkert, L. Kuhn, Q. Perraud, V. Normant, M. Brönstrup and I. J. Schalk, Uptake Mechanisms and Regulatory Responses to MECAM- and DOTAM-Based Artificial Siderophores and Their Antibiotic Conjugates in Pseudomonas aeruginosa, ACS Infect. Dis., 2022, 8, 1134–1146 CrossRef CAS PubMed.
- J. H. Boyce, B. Dang, B. Ary, Q. Edmondson, C. S. Craik, W. F. DeGrado and I. B. Seiple, Platform to Discover Protease-Activated Antibiotics and Application to Siderophore–Antibiotic Conjugates, J. Am. Chem. Soc., 2020, 142, 21310–21321 CrossRef CAS PubMed.
- M. Ghosh, P. A. Miller and M. J. Miller, Antibiotic repurposing: bis-catechol- and mixed ligand (bis-catechol-mono-hydroxamate)-teicoplanin conjugates are active against multidrug resistant Acinetobacter baumannii, J. Antibiot., 2020, 73, 152–157 CrossRef CAS PubMed.
- M. Ghosh and M. J. Miller, Synthesis and in vitro antibacterial activity of spermidine-based mixed catechol- and hydroxamate-containing siderophore—Vancomycin conjugates, Bioorg. Med. Chem., 1996, 4, 43–48 CrossRef CAS PubMed.
- R. Liu, P. A. Miller, S. B. Vakulenko, N. K. Stewart, W. C. Boggess and M. J. Miller, A Synthetic Dual Drug Sideromycin Induces Gram-Negative Bacteria To Commit Suicide with a Gram-Positive Antibiotic, J. Med. Chem., 2018, 61, 3845–3854 CrossRef CAS PubMed.
- A. Paulen, F. Hoegy, B. Roche, I. J. Schalk and G. L. A. Mislin, Synthesis of conjugates between oxazolidinone antibiotics and a pyochelin analogue, Bioorg. Med. Chem. Lett., 2017, 27, 4867–4870 CrossRef CAS PubMed.
- S. Yoganathan, C. S. Sit and J. C. Vederas, Chemical synthesis and biological evaluation of gallidermin-siderophore conjugates, Org. Biomol. Chem., 2011, 9, 2133–2141 RSC.
- M. J. Miller, A. J. Walz, H. Zhu, C. Wu, G. Moraski, U. Möllmann, E. M. Tristani, A. L. Crumbliss, M. T. Ferdig, L. Checkley, R. L. Edwards and H. I. Boshoff, Design, Synthesis, and Study of a Mycobactin−Artemisinin Conjugate That Has Selective and Potent Activity against Tuberculosis and Malaria, J. Am. Chem. Soc., 2011, 133, 2076–2079 CrossRef CAS PubMed.
- S. Zhao, Z.-P. Wang, Z. Lin, G. Wei, X. Wen, S. Li, X. Yang, Q. Zhang, C. Jing, Y. Dai, J. Guo and Y. He, Drug Repurposing by Siderophore Conjugation: Synthesis and Biological Evaluation of Siderophore-Methotrexate Conjugates as Antibiotics, Angew. Chem., 2022, 61, e202204139 CAS.
- A. Tarapdar, J. K. S. Norris, O. Sampson, G. Mukamolova and J. T. Hodgkinson, The design and synthesis of an antibacterial phenothiazine–siderophore conjugate, Beilstein J. Org. Chem., 2018, 14, 2646–2650 CrossRef CAS PubMed.
- C. Guo and E. M. Nolan, Heavy-Metal Trojan Horse: Enterobactin-Directed Delivery of Platinum(IV) Prodrugs to Escherichia coli, J. Am. Chem. Soc., 2022, 144, 12756–12768 CrossRef CAS PubMed.
- T. Brown, P. Charlier, R. Herman, C. J. Schofield and E. Sauvage, Structural Basis for the Interaction of Lactivicins with Serine β-Lactamases, J. Med. Chem., 2010, 53, 5890–5894 CrossRef CAS PubMed.
- C. Peukert, V. Gasser, T. Orth, S. Fritsch, V. Normant, O. Cunrath, I. J. Schalk and M. Brönstrup, Trojan Horse Siderophore Conjugates Induce Pseudomonas aeruginosa Suicide and Qualify the TonB Protein as a Novel Antibiotic Target, J. Med. Chem., 2023, 66, 553–576 CrossRef CAS PubMed.
- J. Liu, J. S. Hou, Y. Q. Chang, L. J. Peng, X. Y. Zhang, Z. Y. Miao, P. H. Sun, J. Lin and W. M. Chen, New Pqs Quorum Sensing System Inhibitor as an Antibacterial Synergist against Multidrug-Resistant Pseudomonas aeruginosa, J. Med. Chem., 2022, 65, 688–709 CrossRef CAS PubMed.
- S. Noël, L. Guillon, I. J. Schalk and G. L. A. Mislin, Synthesis of Fluorescent Probes Based on the Pyochelin Siderophore Scaffold, Org. Lett., 2011, 13, 844–847 CrossRef PubMed.
- Y. Zeng, H. Roy, B. Patil Preeti, M. Ibba and S. Chen, Characterization of Two Seryl-tRNA Synthetases in Albomycin-Producing Streptomyces sp. Strain ATCC 700974, Antimicrob. Agents Chemother., 2009, 53, 4619–4627 CrossRef CAS PubMed.
- W. Neumann and E. M. Nolan, Evaluation of a reducible disulfide linker for siderophore-mediated delivery of antibiotics, J. Biol. Inorg. Chem., 2018, 23, 1025–1036 CrossRef CAS PubMed.
- M. N. Levine and R. T. Raines, Trimethyl lock: A trigger for molecular release in chemistry, biology, and pharmacology, Chem. Sci., 2012, 3, 2412–2420 RSC.
- M. S. Cole, P. V. Hegde and C. C. Aldrich, β-Lactamase-Mediated Fragmentation: Historical Perspectives and Recent Advances in Diagnostics, Imaging, and Antibacterial Design, ACS Infect. Dis., 2022, 8, 1992–2018 CrossRef CAS PubMed.
- J. D. Bargh, A. Isidro-Llobet, J. S. Parker and D. R. Spring, Cleavable linkers in antibody-drug conjugates, Chem. Soc. Rev., 2019, 48, 4361–4374 RSC.
- M. A. Hackel, M. Tsuji, Y. Yamano, R. Echols, J. A. Karlowsky and D. F. Sahm, Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth, Diagn. Microbiol. Infect. Dis., 2019, 94, 321–325 CrossRef CAS PubMed.
- T. Aoki, H. Yoshizawa, K. Yamawaki, K. Yokoo, J. Sato, S. Hisakawa, Y. Hasegawa, H. Kusano, M. Sano, H. Sugimoto, Y. Nishitani, T. Sato, M. Tsuji, R. Nakamura, T. Nishikawa and Y. Yamano, Cefiderocol (S-649266), A new siderophore cephalosporin exhibiting potent activities against Pseudomonas aeruginosa and other gram-negative pathogens including multi-drug resistant bacteria: Structure activity relationship, Eur. J. Med. Chem., 2018, 155, 847–868 CrossRef CAS PubMed.
- K. Kurogi, A. Alazizi, M. Y. Liu, Y. Sakakibara, M. Suiko, T. Sugahara and M. C. Liu, ACS Medicinal Chemistry Letters, Biochem. Pharmacol., 2012, 84, 1186–1195 CrossRef CAS PubMed.
- B. R. Wilson, A. R. Bogdan, M. Miyazawa, K. Hashimoto and Y. Tsuji, Siderophores in Iron Metabolism: From Mechanism to Therapy Potential, Trends Mol. Med., 2016, 22, 1077–1090 CrossRef CAS PubMed.
- R. Golonka, B. S. Yeoh and M. Vijay-Kumar, The Iron Tug-of-War between Bacterial Siderophores and Innate Immunity, J. Innate Immun., 2019, 11, 249–262 CrossRef CAS PubMed.
- J. Behnsen and M. Raffatellu, Siderophores: More than Stealing Iron, MBio, 2016, 7, e01906-01916 CrossRef PubMed.
- X. Xiao, B. S. Yeoh and M. Vijay-Kumar, Lipocalin 2: An Emerging Player in Iron Homeostasis and Inflammation, Annu. Rev. Nutr., 2017, 37, 103–130 CrossRef CAS PubMed.
- J. W. Southwell, R. Herman, D. J. Raines, J. E. Clarke, I. Böswald, T. Dreher, S. M. Gutenthaler, N. Schubert, J. Seefeldt, N. Metzler-Nolte, G. H. Thomas, K. S. Wilson and A.-K. Duhme-Klair, Siderophore-Linked Ruthenium Catalysts for Targeted Allyl Ester Prodrug Activation within Bacterial Cells, Chem. – Eur. J., 2023, 29, e202202536 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |