Open Access Article
Open Access ArticleExploiting α-benzylated 1,4-butanesultones to expedite the discovery of small-molecule, LCST-type sulfobetaine zwitterionic materials†
Yen-Ho
Chu
 *,
Pin-Hsuan
Chen
*,
Pin-Hsuan
Chen
 and
Hsin-Heng
Huang
and
Hsin-Heng
Huang
Department of Chemistry and Biochemistry, National Chung Cheng University, Chiayi, 62102, Taiwan, Republic of China. E-mail: cheyhc@ccu.edu.tw; Fax: +886 52721040; Tel: +886 52729139
First published on 10th March 2023
Abstract
Herein, we report both the concise synthesis and discovery of a small library of 16 sulfobetaine-like zwitterionic materials (ZMs) that exhibit LCST phase transitions in water and their structural optimization to further fine-tune the polarity of the benzyl substituent on the α-position of the sulfonate moiety. Ultimately, we achieved the preparation of novel ZMs carrying attractive Tc values, which demonstrated the real value of the structural tunability of these ZMs. Furthermore, we present a proof-of-concept application for the analysis of biomolecules. To the best of our knowledge, this is the first report on sulfobetaine-substituted ZMs as small-molecule smart materials exhibiting LCST property and their miscibility/immiscibility with water triggered by temperature are entirely switchable and reversible.
Introduction
Materials research is focused on understanding the properties, structural optimization, and application of developed materials. Thermoresponsive materials are smart materials that are designed and synthesized to flexibly respond to changes in the surrounding temperature.1–4 Lower and upper critical solution temperature (LCST and UCST, respectively) systems are the two typical phase behaviors of thermoresponsive materials with water.4 In an LCST system, the solubility of the material in water decreases with an increase in temperature and a homogeneous solution is formed below its critical temperature (Tc); that is, its components are miscible at all concentrations below the LCST. In contrast, for UCST systems, two heterogeneous mixtures become homogeneously mixed upon heating above Tc. To date, many polymeric materials1,2 have been found to exhibit LCST phase transitions in water, whereas there are scarce reports on small-molecule materials exhibiting LCST properties.3,4 Because of the recent advances and significant progress in zwitterionic materials (ZMs) by Ohno and co-workers,3 we envisioned that small molecules such as sulfobetaines5 are zwitterionic, their structures are chemically tuneable, and in particular the sulfonate motif can be readily modified. Consequently, these sulfobetaine-based ZMs should be new candidate smart materials, potentially exhibiting LCST thermoresponsive property (Fig. 1).Herein, we report the concise two-step synthesis of a library of small-molecule ZMs (ZM 1a–e, ZM 2a–e, and ZM 3a–e), exploring their ability to undergo an LCST-type phase transition with water, structurally engineering their Tc values, and lastly presenting a proof-of-concept preliminary application for the analysis of biomolecules. Sulfobetaine ZMs are highly charged organic salts, with their cation and anion covalently tethered, sustaining their overall electroneutrality (Fig. 1). These ZMs carry unique advantages, including the absence of undesirable ion exchange and negligible vapor pressure. Furthermore, many ZMs are known to be biocompatible, non-fouling and resistant nonspecific protein adsorption in solution and on surfaces, mainly owing to their ultra-high dipole moments (18–30 D and 35–41 D for sulfobetaines and zwitterionic imidazolium sulfonates, respectively, while 1.9 D for water) and superhydrophilicity.5–9 In addition to their strong attraction with water,10 ZMs readily associate with themselves via interactions between their cationic and anionic groups. Accordingly, the intermolecular strong hydration and self-association ability of ZMs make them susceptible to external stimuli and phase responsive to temperature changes in water.7,8 These ZMs exhibit exceptional properties compared to common ionic liquids and have potential as valuable materials for studies of biomacromolecules with high selectivity and efficiency.
Experimental
Synthetic procedures
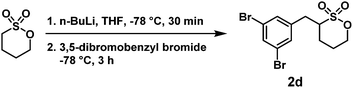
The detailed experimental procedures, 1H and 13C NMR spectra, and high-resolution mass spectrometry (HRMS) data of all 16 ZMs (ZM 1a–e, ZM 2a–e, ZM 3a–e, and ZM 4c) are summarized in the (ESI†).Briefly, n-BuLi (1.1 equiv., 2.5 M in hexane) was slowly added to a stirring solution of 1,4-butanesultone (0.35 mL, 1.0 equiv.) in THF (0.5 M) at −78 °C. This α-deprotonation reaction on 1,4-butanesultone was carried out at –78 °C for 30 min. Then, a solution of 3,5-dibromobenzyl bromide (1.2 equiv. in THF) was added at −78 °C. The reaction mixture was stirred for another 3 h, and finally quenched with water. The resulting mixture was extracted with ethyl acetate three times. The combined organic layers were concentrated and purified by column chromatography (silica gel; ethyl acetate/hexane = 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10–1
10–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) to give α-(3,5-dibromobenzyl)-1,4-butanesultone (2d) as a colorless liquid with 63% isolated yield. 1H NMR (400 MHz, CDCl3) δ 1.83–2.07 (m, SOCH2CH2 + SCHCH2CH2, 4H), 2.68–2.75 (m, SCHCH2Ar, 1H), 3.21–3.28 (m, SCH, 1H), 3.41–3.46 (m, SCHCH2Ar, 1H), 4.46–4.61 (m, SOCH2CH2, 2H), 7.30 (d, J = 1.6 Hz, aryl H, 1H), 7.30 (d, J = 1.6 Hz, aryl H, 1H), 7.59 (dd, J = 1.6, 1.6 Hz, aryl H, 1H); 13C NMR (100 MHz, CDCl3) δ 24.04, 27.77, 33.69, 60.18, 73.94, 123.44, 131.14, 133.21, 139.96; FAB-HRMS m/z [M + H]+ calculated for C11H13Br2O3S+ 382.8947, 384.8926, 386.8906, found 382.8953, 384.8943, 386.8925.
2, v/v) to give α-(3,5-dibromobenzyl)-1,4-butanesultone (2d) as a colorless liquid with 63% isolated yield. 1H NMR (400 MHz, CDCl3) δ 1.83–2.07 (m, SOCH2CH2 + SCHCH2CH2, 4H), 2.68–2.75 (m, SCHCH2Ar, 1H), 3.21–3.28 (m, SCH, 1H), 3.41–3.46 (m, SCHCH2Ar, 1H), 4.46–4.61 (m, SOCH2CH2, 2H), 7.30 (d, J = 1.6 Hz, aryl H, 1H), 7.30 (d, J = 1.6 Hz, aryl H, 1H), 7.59 (dd, J = 1.6, 1.6 Hz, aryl H, 1H); 13C NMR (100 MHz, CDCl3) δ 24.04, 27.77, 33.69, 60.18, 73.94, 123.44, 131.14, 133.21, 139.96; FAB-HRMS m/z [M + H]+ calculated for C11H13Br2O3S+ 382.8947, 384.8926, 386.8906, found 382.8953, 384.8943, 386.8925.
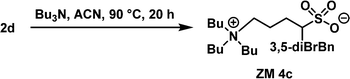
Tributylamine (1.5 equiv.) and acetonitrile (3.0 M) were added to a 4 mL sample bottle containing 2d (0.27 g, 1.0 equiv.), and the corresponding α-benzylation reaction was performed at 90 °C for 20 h. The reaction product was readily purified by column chromatography (silica gel; dichloromethane/methanol = 30
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–6
1–6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to afford the desired ZM 4c as a white solid (mp 191 °C) with 62% isolated yield. 1H NMR (400 MHz, CDCl3) δ 1.00 (t, J = 7.3 Hz, N(CH2)3CH3, 9H), 1.38–1.47 (m, N(CH2)2CH2CH3, 6H), 1.57–1.63 (m, NCH2CH2CH2CH3 + SCHCH2CH2, 7H), 1.79–1.85 (m, SCHCH2CH2, 1H), 1.97–1.99 (m, SCHCH2CH2, 2H), 2.63–2.69 (m, SCHCH2Ar, 1H), 2.90–3.34 (m, SCH + NCH2, 9H), 3.61–3.65 (m, SCHCH2Ar, 1H), 7.35 (s, aryl H, 1H), 7.50 (s, aryl H, 2H); 13C NMR (100 MHz, CDCl3) δ 13.78, 19.62, 19.90, 24.06, 26.54, 37.15, 59.03, 59.44, 59.82, 122.98, 131.25, 131.87, 144.89; ESI-HRMS m/z [M + H]+ calculated for C23H40Br2NO3S+ 568.1090, 570.1070, 572.1049, found 568.1082 ([M + H]+), 570.1062 ([M + H]+), 572.1040 ([M + H]+), 590.0901 ([M + Na]+), 592.0879 ([M + Na]+), 594.0859 ([M + Na]+).
1, v/v) to afford the desired ZM 4c as a white solid (mp 191 °C) with 62% isolated yield. 1H NMR (400 MHz, CDCl3) δ 1.00 (t, J = 7.3 Hz, N(CH2)3CH3, 9H), 1.38–1.47 (m, N(CH2)2CH2CH3, 6H), 1.57–1.63 (m, NCH2CH2CH2CH3 + SCHCH2CH2, 7H), 1.79–1.85 (m, SCHCH2CH2, 1H), 1.97–1.99 (m, SCHCH2CH2, 2H), 2.63–2.69 (m, SCHCH2Ar, 1H), 2.90–3.34 (m, SCH + NCH2, 9H), 3.61–3.65 (m, SCHCH2Ar, 1H), 7.35 (s, aryl H, 1H), 7.50 (s, aryl H, 2H); 13C NMR (100 MHz, CDCl3) δ 13.78, 19.62, 19.90, 24.06, 26.54, 37.15, 59.03, 59.44, 59.82, 122.98, 131.25, 131.87, 144.89; ESI-HRMS m/z [M + H]+ calculated for C23H40Br2NO3S+ 568.1090, 570.1070, 572.1049, found 568.1082 ([M + H]+), 570.1062 ([M + H]+), 572.1040 ([M + H]+), 590.0901 ([M + Na]+), 592.0879 ([M + Na]+), 594.0859 ([M + Na]+).
Results and discussion
Library synthesis of zwitterionic materials
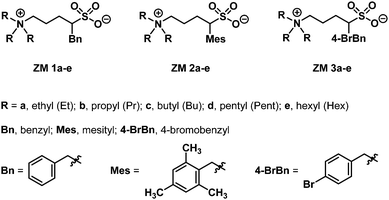
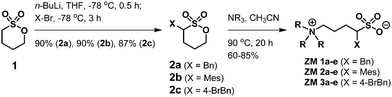
Using α-benzylated sulfonates as anions, we aimed at exploring their potential to participate in intermolecular π–π and hydrophobic interactions among ZMs; that is, for a ZM-in-H2O system exhibiting an LCST phase transition, a homogeneous solution appeared at low temperatures (due to strong and favorable interactions such as hydrogen bonding between the sufonyl groups in the ZM and water), and, upon heating above its Tc, two immiscible phases were formed, likely resulting from the rupture of the aforementioned hydrogen bonding and the enhancement of π–π interactions between ZMs. Accordingly, we synthesized a small-molecule ZM library with the aim to facilitate the discovery of LCST-type zwitterionic materials.Although there are two examples of substituted sulfobetaines incorporated in zwitterionic polymers in the literature,11,12 small-molecule sulfobetaine ZMs featuring embedded functional substituents carrying thermoresponsive properties remain totally unexplored. Sulfobetaines can be readily prepared from tertiary amine reactions with inexpensive 1,3-propanesultone or 1,4-butanesultone. Sultones and their derivatives are useful scaffolds of sulfonyl drugs in medicinal chemistry research and valuable heterocyclic intermediates in natural product synthesis.13 In this work, we describe a new strategy to synthesize sulfobetaine-based ZMs with various benzyl groups attached directly on the sultone moiety. Fig. 2 illustrates our library synthesis of ZM 1a–e, ZM 2a–e, and ZM 3a–e, where the key element 2a–c could be structurally engineered by initially metalating 1,4-butanesultone 1 using butyllithium,14 and in the same pot, mixing with the corresponding substituted benzyl bromides (benzyl bromide for 2a, mesityl bromide for 2b, and 4-bromobenzyl bromide for 2c), resulting in the formation of α-alkylated sultones 2a–c, followed by their ring opening with trialkylamines under heating conditions to finally afford the desired ZMs with 54–74% overall isolated yields in 2 steps. These syntheses proved straightforward on a multigram scale. The detailed experimental procedures, 1H and 13C NMR spectra, and high-resolution mass spectrometry (HRMS) data of all the ZMs (ZM 1a–e, ZM 2a–e, and ZM 3a–e) are summarized in the (ESI†).
Discovery of small-molecule zwitterionic materials exhibiting LCST thermoresponsive property
Our structural design of sulfobetaine-based ZMs was based on the chemical construction of a relatively hydrophilic, short-chain tetraalkylammonium cation with a substantially hydrophilic sulfonate anion carrying an engineered hydrophobic benzyl moiety. These benzylated sulfonates in ZMs should offer significant influence for phase separation with water, and structurally tune the ZMs to fine-balance their overall temperature-responsive property. Ultimately, the rational tuning of these ZMs enabled the discovery of thermoresponsive ZMs.
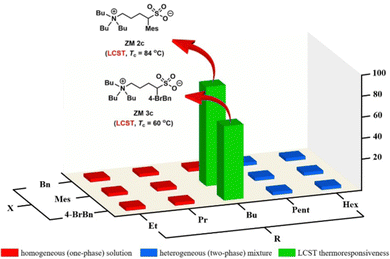
Fig. 3 shows a small library of 15 zwitterionic sulfobetaine salts (ZM 1a–e, ZM 2a–e, and ZM 3a–e) and their phase behaviors toward temperature changes in water. To discover ZMs carrying thermoresponsive property, each zwitterionic salt was mixed with water in a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
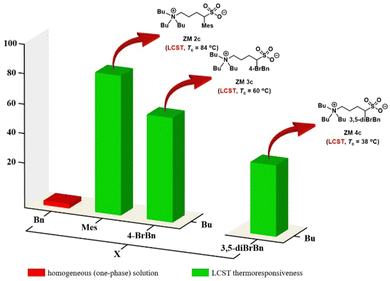
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (w/w), and then the mixture was placed in a water bath (4 °C) followed by gradual heating until it reached 90 °C. The phase transition temperature (Tc) for LCST was experimentally determined at a temperature point when the solution turned rigid during heating, as observed by the naked eye. To facilitate this determination, the organic dye Coomassie blue (0.006 wt% in water) was used to make the phase separation visible. Pleasantly, in the library of 15 ZMs investigated, two zwitterions ZM 2c and ZM 3c (labeled in green) were found to exhibit the LCST property with a Tc value of 84 °C and 60 °C, respectively. The library screening results for the ZMs labeled in red and blue indicate the formation of a homogeneous one-phase solution and a heterogeneous two-phase mixture between 0 °C and 90 °C, respectively (Fig. 3). In the case of the thermoresponsive ZMs discovered, because of the higher hydrophobicity on the benzyl group (log
3 (w/w), and then the mixture was placed in a water bath (4 °C) followed by gradual heating until it reached 90 °C. The phase transition temperature (Tc) for LCST was experimentally determined at a temperature point when the solution turned rigid during heating, as observed by the naked eye. To facilitate this determination, the organic dye Coomassie blue (0.006 wt% in water) was used to make the phase separation visible. Pleasantly, in the library of 15 ZMs investigated, two zwitterions ZM 2c and ZM 3c (labeled in green) were found to exhibit the LCST property with a Tc value of 84 °C and 60 °C, respectively. The library screening results for the ZMs labeled in red and blue indicate the formation of a homogeneous one-phase solution and a heterogeneous two-phase mixture between 0 °C and 90 °C, respectively (Fig. 3). In the case of the thermoresponsive ZMs discovered, because of the higher hydrophobicity on the benzyl group (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: Bn < Mes < 4-BrBn; computed by X
P: Bn < Mes < 4-BrBn; computed by X![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P 3 3.0),15 the structurally more hydrophobic ZM 3c (Tc = 60 °C) exhibited a lower Tc value than that of ZM 2c (Tc = 84 °C), as expected (Fig. 3). Furthermore, it has been reported that the phase property of a thermoresponsive material is a fine balance between the hydrophobicity and hydrophilicity of the ionic salts investigated,3,16 which is consistent with our results, as shown in Fig. 3, where both ZM 2c and ZM 3c (green) were identified to reside on the rim between being totally hydrophilic (red) and totally hydrophobic (blue).
P 3 3.0),15 the structurally more hydrophobic ZM 3c (Tc = 60 °C) exhibited a lower Tc value than that of ZM 2c (Tc = 84 °C), as expected (Fig. 3). Furthermore, it has been reported that the phase property of a thermoresponsive material is a fine balance between the hydrophobicity and hydrophilicity of the ionic salts investigated,3,16 which is consistent with our results, as shown in Fig. 3, where both ZM 2c and ZM 3c (green) were identified to reside on the rim between being totally hydrophilic (red) and totally hydrophobic (blue).
Structural optimization of zwitterionic sulfobetaine materials
Because the Tc values of ZM 2c and ZM 3c obtained were not close to the body temperature (37 °C), both ZMs are less attractive for the direct analysis of biomolecules. Accordingly, we predicted that further structural optimization by incorporating two substituents on the benzyl group, such as 3,5-dibromobenzyl, carrying greater hydrophobicity should, in principle, result in a lower Tc value in water (Fig. 4). Accordingly, we employed α-3,5-dibromobenzylated 1,4-butanesultone (2d) and proceeded to synthesize ZM 4c from tributylamine and 2d with the aim to discover new thermoresponsive ZMs carrying a lower Tc value. This newly engineered ZM was successfully prepared using the same synthetic route as depicted in Fig. 2. Finally, ZM 4c was afforded with 39% overall isolated yield in 2 steps (see Experimental section). The detailed experimental procedures, 1H and 13C NMR spectra, and HRMS data of ZM 4c are summarized in the Experimental section and ESI.†We were very pleased to discover that ZM 4c indeed exhibited LCST thermoresponsive property, carrying a lower Tc value (38 °C) (Fig. 5). This structural modification of the ZMs (i.e., from 4-BrBn in ZM 3c to 3,5-diBrBn in ZM 4c) and the successful engineering of ZM 4c clearly demonstrated the true value of the fine tunability of sulfobetaine zwitterion structures. The Tc value of this ZM 4c is at body temperature, and thus it was employed as our candidate ZM for the analysis of biomolecules.
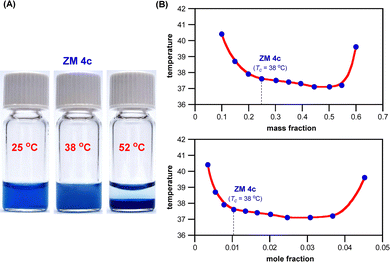
Fig. 6A shows that ZM 4c forms a one-phase homogeneous solution with water at ambient temperature (25 °C), and upon heating above 38 °C, a two-phase system started to develop with this ZM in the bottom layer, and ultimately a well separated two-layer solution mixture was formed at much higher temperatures (52 °C). The temperature-switchable mixing and demixing with water were entirely reversible, clearly illustrating that ZM 4c is a smart material exhibiting LCST phase transition with water.
For LCST systems, their phase transition temperature highly depends on the mass fraction and mole fraction of the thermoresponsive materials in water. Fig. 6B shows the phase diagrams of a mixture of water with ZM 4c, revealing the typical cup-shape concave curves with the lowest critical temperatures near its mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and mole ratio of 1
1 and mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 (ZM 4c/H2O).
32 (ZM 4c/H2O).
This successful development of ZM 4c as an LCST-type thermoresponsive zwitterionic material clearly highlights the structural tunability of sulfobetaines, which is a fruitful ZM example using the combinatorial discovery platform developed in this work.
Thermal stability of ZMs
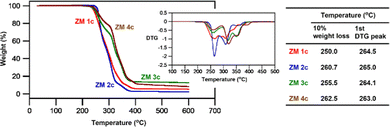
Sulfobetaines are among most widely used ZMs, and many of them are studied and used at elevated temperatures (e.g., withstanding steam sterilization of medical ZM devices and oilfield antifouling ZMs).17 Accordingly, thermal stability is a prerequisite for the application of ZMs. Fig. 7 depicts the thermogravimetric behaviors and summarizes the key thermal stability data of the representative ZM 1c, ZM 2c, ZM 3c, and ZM 4c. The thermogravimetric analysis (TGA) indicates that, similarly for all ZMs, the major weight loss was observed after 250 °C and all the ZMs displayed multi-stage degradation processes with their first derivative thermogravimetry (DTG) peaks at 265 °C, 265 °C, 264 °C, and 263 °C (their decomposition temperatures Td at 10% mass loss are 250 °C, 261 °C, 256 °C, and 263 °C) (table in Fig. 7), respectively. The results from the TGA thermograms (ZM 3c and ZM 4cvs.ZM 1c, ZM 2c) suggested that the bromo substituent enhanced the thermal stability of the ZMs. We also found that the greater the number of substituents on the benzyl group (ZM 1cvs.ZM 2c, ZM 3c, and ZM 4c; also, ZM 3cvs.ZM 2c and ZM 4c), the higher the degradation temperatures, resulting in more thermally stable ZMs. These thermal decomposition temperatures are similar to that of zwitterionic sulfonates reported in the literature.18–20A preliminary, proof-of-concept application
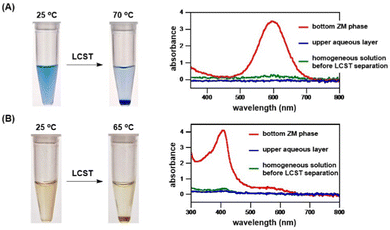
We developed novel zwitterionic materials considering their potential applications. Consequently, we successfully engineered α-benzylated sulfobetaines as thermoresponsive materials carrying LCST property in water, and then performed a preliminary study with the aim to prove that these ZMs are compatible with biomacromolecules for temperature-triggered phase separation; that is, the target biomolecules favorably partition in the ZM-rich layer, which will make future studies of biomolecular recognition and transformation possible.Today, the separation and purification of biomolecules are routinely carried out using various chromatographic procedures, which are employed in almost all bioengineering processes. Therefore, it is necessary to design new technology platforms for concentrating dilute solutions or aqueous solutions containing only minute amounts of precious biomolecules. In this work, we explored the potential of ZMs for use as an immediate application to enrich dilute biomolecule solutions. Here, ZM 4c (Tc = 38 °C) was used as a candidate zwitterionic material, simply because it exhibits an LCST phase transition around body temperature in water. As a proof-of-concept application, we chose a high molecular-weight Coomassie blue dye (MW 826) and human hemoglobin protein (MW 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) for ease of visualization by the naked eye to test if its concentration enrichment of a highly diluted solution could be realized by ZM 4c. Pleasingly, as shown in Fig. 8, biomolecules were greatly enriched upon adding ZM 4c and mixing with dilute dye and protein solutions, respectively. Consequently, the Coomassie blue dye and hemoglobin protein were readily enriched by 16- and 11-fold, respectively. According to the spectra shown in Fig. 8A, the upper aqueous phases (blue spectra) were essentially free of the blue dye, that is, most, if not all the Coomassie dye was preferentially partitioned and concentrated in the ZM-rich bottom phase. This ZM 4c also readily enriched human hemoglobin protein (Fig. 8B). In our hands, no precipitation of hemoglobin was observed (e.g., its Soret band was preserved) during temperature-switchable mixing and demixing.
500) for ease of visualization by the naked eye to test if its concentration enrichment of a highly diluted solution could be realized by ZM 4c. Pleasingly, as shown in Fig. 8, biomolecules were greatly enriched upon adding ZM 4c and mixing with dilute dye and protein solutions, respectively. Consequently, the Coomassie blue dye and hemoglobin protein were readily enriched by 16- and 11-fold, respectively. According to the spectra shown in Fig. 8A, the upper aqueous phases (blue spectra) were essentially free of the blue dye, that is, most, if not all the Coomassie dye was preferentially partitioned and concentrated in the ZM-rich bottom phase. This ZM 4c also readily enriched human hemoglobin protein (Fig. 8B). In our hands, no precipitation of hemoglobin was observed (e.g., its Soret band was preserved) during temperature-switchable mixing and demixing.
Conclusion
Ohno and co-workers previously reported the 3-step synthesis of a novel 3-(N,N-dihexyl-N-pentylammonio)propane-1-sulfonate zwitterionic salt (N665C3S), which exhibited an intriguing LCST thermoresponsive property in water.20 We reported in this work the 2-step synthesis of a small library of 16 ZMs, discovered 3 ZMs (ZM 2c, ZM 3c, and ZM 4c) carrying good thermal stability and LCST phase separation from water, and underlined the important role of ZMs for use in preliminary biomolecule analysis. Also, we demonstrated the successful structure optimization of ZM 4c to highlight its close relationship with thermoresponsiveness. Recently, our group also reported N-triflimide (NTf)-based zwitterionic ionic liquids (ZILs) that exhibit LCST phase transitions in water.21 To the best of our knowledge, this is the first report on α-benzylated sulfobetaine ZMs as small-molecule smart materials exhibiting LCST property and that their miscibility/immiscibility with water triggered by temperature are entirely switchable and reversible. However, although our combinatorial approach for the discovery of smart materials is highly promising and effective, it is still being developed and more successful ZM examples are needed.Author contributions
Yen-Ho Chu: conceived the idea, funding acquisition, designed experiments. Pin-Hsuan Chen, Hsin-Heng Huang: performed experiments. Yen-Ho Chu, Pin-Hsuan Chen: Interpreted the data. Yen-Ho Chu: wrote the manuscript and all authors discussed and approved the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants (MOST 109-2113-M-194-005-MY2 and NSTC 111-2113-M-194-010-MY2) from the Ministry of Science and Technology of Taiwan, Republic of China.References
- X. Xu, N. Bizmark, K. S. S. Christie, S. S. Datta, Z. J. Ren and R. D. Priestley, Thermoresponsive polymers for water treatment and collection, Macromolecules, 2022, 55, 1894–1909 CrossRef CAS.
- M. A. Ward and T. K. Georgiou, Thermoresponsive polymers for biomedical applications, Polymers, 2011, 3, 1215–1242 CrossRef CAS.
- H. Ohno, M. Yoshizawa-Fujita and Y. Kohno, Functional design of ionic liquids: Unprecedented liquids that contribute to energy technology, bioscience, and materials sciences, Bull. Chem. Soc. Jpn., 2019, 92, 852–868 CrossRef CAS.
- Y. Qiao, W. Ma, N. Theyssen, C. Chen and Z. Hou, Temperature-responsive ionic liquids: Fundamental behaviors and catalytic applications, Chem. Rev., 2017, 117, 6881–6928 CrossRef CAS PubMed.
- Q. Shao and S. Jiang, Molecular understanding and design of zwitterionic materials, Adv. Mater., 2015, 27, 15–26 CrossRef CAS PubMed.
- J. B. Schlenoff, Zwitteration: coating surfaces with zwitterionic functionality to reduce nonspecific adsorption, Langmuir, 2014, 30, 9625–9636 CrossRef CAS PubMed.
- M. Galin, A. Chapoton and J.-C. Galin, Dielectric increments, intercharge distances and conformation of quaternary ammonioalkylsulfonates and alkoxydicyanoethenolates in aqueous and trifluoroethanol solutions, J. Chem. Soc., Perkin Trans. 2, 1993, 545–553 RSC.
- T. Wu, F. L. Beyer, R. H. Brown, R. B. Moore and T. E. Long, Influence of zwitterions on thermomechanical properties and morphology of acrylic copolymers: implications for electroactive applications, Macromolecules, 2011, 44, 8056–8063 CrossRef CAS.
- W. Mei, A. J. Rothenberger, J. E. Bostwick, J. M. Rinehart, R. J. Hickey and R. H. Colby, Zwitterions raise the dielectric constant of soft materials, Phys. Rev. Lett., 2021, 127, 228001 CrossRef CAS PubMed.
- J. Wu, W. Lin, Z. Wang, S. Chen and Y. Chang, Investigation of the hydration of nonfouling material poly(sulfobetaine methacrylate) by low-field nuclear magnetic resonance, Langmuir, 2012, 28, 7436–7441 CrossRef CAS PubMed.
- C.-C. Chang, R. Letteri, R. C. Hayward and T. Emrick, Functional sulfobetaine polymers: Synthesis and salt-responsive stabilization of oil-in-water droplets, Macromolecules, 2015, 48, 7843–7850 CrossRef CAS.
- P. Mary and D. D. Bendejacq, Interactions between sulfobetaine-based polyzwitterions and polyelectrolytes, J. Phys. Chem. B, 2008, 112, 2299–2310 CrossRef CAS PubMed.
- S. Mondal, Recent developments in the synthesis and application of sultones, Chem. Rev., 2012, 112, 5339–5355 CrossRef CAS PubMed.
- M. B. Smith and J. Wolinsky, Lithium aluminum hydride-aluminum hydride reduction of sultones, J. Org. Chem., 1981, 46, 101–106 CrossRef CAS.
- T. Cheng, Y. Zhao, X. Li, F. Lin, Y. Xu, X. Zhang, Y. Li and R. Wang, Computation of octanol–water partition coefficients by guiding an additive model with knowledge, J. Chem. Inf. Model., 2007, 47, 2140–2148 CrossRef CAS PubMed.
- H. Ohno, M. Yoshizawa-Fujita and Y. Kohno, Design and properties of functional zwitterions derived from ionic liquids, Phys. Chem. Chem. Phys., 2018, 20, 10978–10991 RSC.
- C.-C. Yang, C.-T. Lo, Y.-L. Luo, A. Venault and Y. Chang, Thermally stable bioinert zwitterionic sulfobetaine interfaces tolerated in the medical sterilization process, ACS Biomater. Sci. Eng., 2021, 7, 1031–1045 CrossRef CAS PubMed.
- S. M. S. Hussain, M. S. Kamal and L. T. Fogang, Synthesis and physicochemical investigation of betaine type polyoxyethylene zwitterionic surfactants containing different ionic headgroups, J. Mol. Struct., 2019, 1178, 83–88 CrossRef CAS.
- E. Schönemann, J. Koc, J. F. Karthäuser, O. Özcan, D. Schanzenbach, L. Schardt, A. Rosenhahn and A. Laschewsky, Sulfobetaine methacrylate polymers of unconventional polyzwitterion architecture and their antifouling properties, Biomacromolecules, 2021, 22, 1494–1508 CrossRef PubMed.
- Y. Mieno, Y. Kohno, S. Saita and H. Ohno, Zwitterion/Bronsted acid mixtures showing controlled lower critical solution temperature-type phase changes with water, Chem. – Eur. J., 2016, 22, 12262–12265 CrossRef CAS PubMed.
- Y.-H. Chu and C.-Y. Chen, Structural fine-tuning of zwitterionic salts for the discovery of LCST-type thermoresponsive materials, Mater. Chem. Front., 2021, 5, 7286–7290 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, 1H and 13C NMR, and high-resolution mass spectrometry (HRMS) spectra and data of all 16 ZMs (ZM 1a–e, ZM 2a–e, ZM 3a–e, and ZM 4c). See DOI: https://doi.org/10.1039/d2ma01063a |
| This journal is © The Royal Society of Chemistry 2023 |