Open Access Article
Open Access ArticleDynamic polymer nanocomposites towards strain sensors and customizable resistors†
Obed J.
Dodo
 a,
Ibrahim O.
Raji
a,
Ian J.
Arny
a,
Camryn P.
Myers
a,
Leilah
Petit
a,
Kumari
Walpita
a,
Derrick
Dunn
a,
Carl J.
Thrasher
b and
Dominik
Konkolewicz
a,
Ibrahim O.
Raji
a,
Ian J.
Arny
a,
Camryn P.
Myers
a,
Leilah
Petit
a,
Kumari
Walpita
a,
Derrick
Dunn
a,
Carl J.
Thrasher
b and
Dominik
Konkolewicz
 *a
*a
aDepartment of Chemistry and Biochemistry, Miami University, 651 East High Street, Oxford, OH 45056, USA. E-mail: d.konkolewicz@miamioh.edu
bDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, Cambridge, MA 02142, USA
First published on 20th July 2023
Abstract
In the future, well-engineered and optimized flexible electronic devices will be woven into everyday accessories such as clothes, furniture, and healthcare monitoring devices. Herein, a series of multifunctional, flexible, conductive, and self-healing polymer nanocomposites that contribute to multiple electronic applications are reported. RAFT polymerization is employed in a modular approach to synthesize dynamic polymer nanocomposites (DPNs) using different architectures including interpenetrating (IPN) and block copolymer (BCN) networks through dynamic Diels–Alder and hydrogen bond cross-links. Structure–property relationships highlighting the impact of network architecture, chain-length, cross-link density, and carbon nanotubes loading are explored. Controlled addition of multiwalled carbon nanotubes (CNTs) as nano-reinforcements produces electrically conductive and mechanically enhanced DPNs with demonstrated application in the regulation of current flow towards a dimmable light emitting diode (LED). Further application of DPNs as strain sensors and customizable/tunable electrical resistors is demonstrated. Overall, this report furnishes new insights into designing next-generation custom resistors and materials for smart LED lighting.
Introduction
Dynamic polymer nanocomposites (DPNs) have potential applications in electronics and energy devices such as electronic skins, soft robots, flexible circuits, sensors, and energy harvesting devices due to their flexibility, low cost, facile processing, chemical resistance, and adjustable electrical properties.1–8 Conventional electronic devices based on metal–oxide-semiconductor systems have limited mechanical flexibility, which presents challenges in this booming era of flexible and wearable electronics.9–11 Owing to the inherent advantage of being light, healable, flexible, stretchable (>50% strain), DPNs have received attention, especially with demonstrated applications in soft robotics, human motion detection, personalized healthcare monitoring, and human machine interfaces.12–15Introducing healability into polymeric electronic devices is appealing because functional self-healing materials enhance the durability, toughness, of the system and reduce its maintanance.8,16–18 Nevertheless, the dynamic chemistries that enable self-healing polymers19,20 have received significantly more attention than intrinsically self-healing electronic and energy devices which restore device performance after damage.21,22 Intrinsic self-healing in polymeric materials is enabled by supramolecular/noncovalent interactions or dynamic covalent bonds. Integrating these dynamic chemistries into polymers leads to materials with ability to undergo multiple reprocessing/repair cycles. In contrast, extrinsic self-healing polymers rely on interconnected vesicles or microcapsules of healing agents, giving a single-time healing upon damage to the material.23 While progress has been made in extrinsically self-healing conductive composites, the single healing cycle limits their applications,24,25 increasing the need for intrinsically dynamic conducting materials.
Exchange reactions involving reversible covalent and non-covalent bonds are advantageous in dynamic polymers and impart excellent bond strength and versatility. Dynamic covalent reactions include disulfide exchange, Diels–Alder (DA) cycloaddition, siloxane exchange, transesterification, thiol-Michael adduct exchange, [2 + 2] coumarin cycloaddition, and [4 + 4] anthracene cycloaddition.20 Among non-covalent systems hydrogen (H) bonding is the most common non-covalent supramolecular interaction, resulting in highly dynamic polymers, since the weak interactions lead to viscoelastic materials with creep susceptibility but rapid self-healing.26 In contrast, dynamic covalent linkers are typically static, until an external stimulus is applied. Unique materials are possible by combining dynamic covalent and supramolecular linkers with dramatically different lifetimes.26–28 These dynamic polymers have exciting properties such as self-healing and adaptability, however, they often have lower strength, and opto-electronic functionality compared to traditional electronic materials.29
To obtain simultaneous self-healing, enhanced mechanical properties, and electrical functionalities, conductive self-healing polymer composites have been developed by introducing conductive networks of nanomaterials into dynamic polymers.29,30 Combining dynamic polymers with conductive nanomaterials (e.g., carbon nanomaterials and metallic fillers) results in a new class of bulk polymeric materials called DPNs. In DPNs, the nano-filler provides the electrical properties and mechanical reinforcement, and the polymer matrix harnesses dynamic exchange reactions to reconstruct damaged DPNs.31
For DPNs to function as electronic devices, the conductive nanofiller must surpass the percolation threshold, hence nanotubes or nanowires are preferred conductive fillers due to their rod like structure leading to interconnected conductive pathways.22 Multiwalled carbon nanotubes (hereafter denoted as CNTs) or buckytubes are rolled up sheets of graphene forming nanoscale tubes featuring high aspect ratios greater than 103. These CNTs have extraordinary electrical conductivity, high mechanical strength, thermal conductivity, and high specific surface area (1.32 × 103 m2 g−1), making them excellent candidates in nanotechnology and sensing applications.32,33 Since multiwalled CNTs provide the advantage of lower cost compared to the single walled alternatives, they are more suitable for cost-effective electronic devices and have received significant attention in literature.31,34,35 CNT-enhanced DPNs have demonstrated healability, recyclability, conductivity, and thermoresponsive properties leading to applications in electromagnetic shielding, strain sensors, and electronic packaging materials.36,37
However, there remain key design and functionality challenges associated with DPNs including: [i] inefficient load transfer from the dynamic polymer matrix to the conductive filler, [ii] the trade-off between enhanced mechanical strength, dynamic properties, and material stretchability,3,31,37 and [iii] demonstrating new electronic component applications. Challenge [i], the inefficient load transfer between CNT and the dynamic matrix is due to a lack of chemical interaction between conductive fillers and the polymer matrices. Poor transfer of load can lead to materials with inefficient reinforcement, or lower toughness. Prior attempts to solve this challenge pre-functionalized CNTs through harsh reactions.38,39 Challenge [ii], the potential trade-offs between strength, dynamic properties, and stretchability is partly due to limited exploration of controlled and tailored polymer architecture explored for DPNs. Complex polymer architectures are needed for DPNs to demonstrate flexibility and stretchability even with the inclusion of a conductive nanofiller.35 Challenge [iii], developing new functionalities beyond sensing, dielectric actuators, field-effect transistors, and stretchable conductors is still challenging, in part because of challenges in material design.22 Overall, this work provides enhanced structure property relationships between dynamic polymer networks and CNT-DPN mechanical and electrical performance, resulting in progress towards solving challenges [i], [ii], and [iii].
When designing DPNs, the choice of polymer architecture contributes significantly to the overall thermomechanical properties of materials.40 Key polymer architectural features such as cross-link density, chain length, cross-link distribution, topology, and orientation of polymer networks impact properties such as material strength, phase transition temperatures, stress relaxation, creep, and elongation at break. The relationship between polymer architecture and the properties of DPNs is underexplored,41,42 especially in dynamic polymer composite systems. Due to the limited studies correlating network architecture to materials properties, and inefficient load transfer between reinforcement and matrix, it is critical to develop reliable approaches towards DPN composites which can be tuned by polymer and network architecture. Determining how network architecture impacts DPN properties will provide new insights into designing highly functional materials with advanced properties.42
In a previous study, we demonstrated a method for macromolecular engineered nano reinforcement in linear and branched polymers using CNT as a nanofiller to achieve bulk DPNs. This system used the bonding of furan to the CNT surface through Diels–Alder chemistry to transfer load between reinforcement and matrix,31 however the DPNs had limited functionality. This led to a subsequent development of DPNs containing three types of dynamic bonds with distinct roles, resulting in recyclable DPNs with better elongation and higher electrical conductivity.35 Results from that study not only confirmed the synergy of dynamic bonds, but also highlighted the potential interplay of dynamic bonds and primary chain structure on advanced materials properties. However, there is still no comprehensive study that connects the network architecture of polymers to their corresponding composite material's electrical and mechanical properties, significantly limiting the functionalities and high-performance applications of DPNs.42
Here, we present an advanced category of DPNs with inherent ability to modify the surface of CNTs under mild conditions, without requiring pre-functionalization.43 The approach results in effective reinforcement of mechanical strength without stretchability trade-off, and meaningful electrical conductivity. Findings in this contribution enabled application of CNT composites as customizable resistors and a strain sensing device. This work presents a detailed structure–property study of the impact of network architecture, cross-link density, cross-link distribution, chain length, and polymer microstructure on the mechanical and electrical performance of CNT composite DPNs. While there are numerous reports on healable DPNs for strain sensing applications, to the best of our knowledge, this is the first demonstration of DPNs (with inherent conductivity) as customizable/tunable resistors enabled through self-healing and an electrically conductive nanofiller. More broadly, the extensive structure–property study, and simple approach to allow load transfer from the filler to reinforcement, can guide future DPN composites towards new applications in challenging environments.
Results and discussion
CNT reinforced DPN enabled by macromolecular engineering
Reversible addition fragmentation chain transfer (RAFT)44 polymerization was used as a reversible deactivation radical polymerization (RDRP)45 technique to synthesize well-defined acrylate-based polymers with the main polymer chain comprising of ethyl acrylate (EA). Scheme 1 gives the polymer architectures and dynamic properties of the materials. Scheme 1A shows the structure of dynamic UPy and FMA linkers and the ranges of dynamic polymer architectures. Scheme 1B gives the dynamic crosslinks between UPy units, furan–maleimide bonds, and dynamic interactions between the CNT surface and the furan unit. Scheme 1C and D show the BCN synthesis and dynamic exchange for IPN and BCN type materials respectively. Scheme 1E shows the non-dynamic GMA linker after ring opening of the epoxide with ethylene diamine and its non-dynamic nature. The GMA based control materials is non-dynamic and also unable to bind to the CNTs. This way the GMA based materials can identifying the impact of Diels–Alder chemistry on the load transfer between the reinforcement and matrix and dynamic exchange within the network.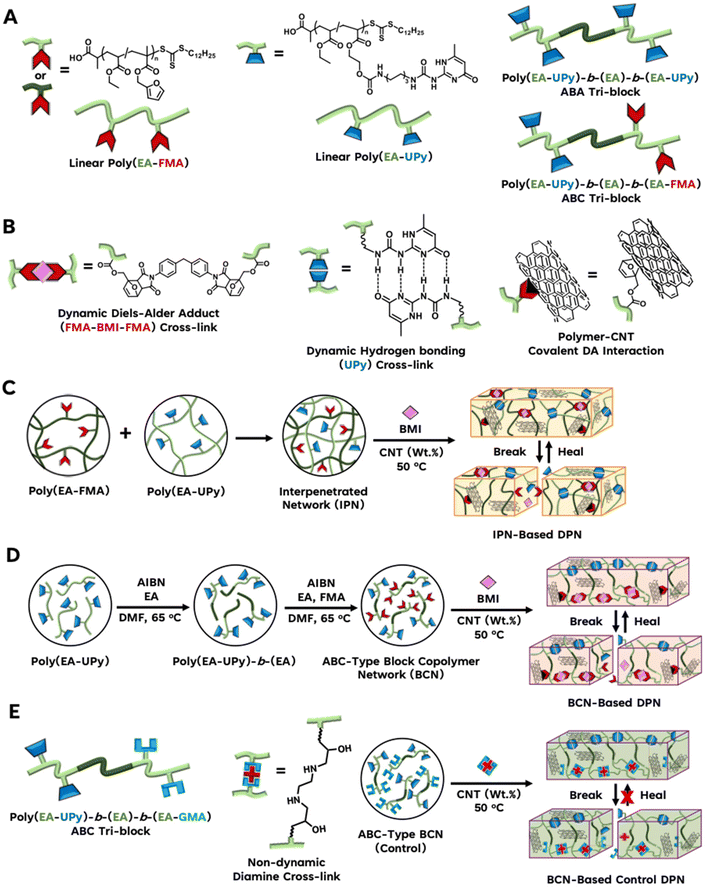 | ||
| Scheme 1 DPN synthesis enabled by macromolecular engineering of dynamic polymer networks with CNTs. (A) Legends describing the dynamic polymer network architecture. (B) dynamic non-covalent hydrogen bonding and covalent Diels–Alder interaction between polymer chains and/or CNT nanofiller. (C) Synthesis of IPN-based DPN materials through the combination of interpenetrated linear poly(EA-FMA) and linear poly(EA-UPy). (D) Synthesis of BCN-based DPNs by chain extension of linear poly(EA-UPy) to obtain poly(EA-UPy)-b-(EA) which was further chain extended to obtain poly(EA-UPy)-b-(EA)-b-(EA-FMA) tri-block DPN. (E) Poly(EA-UPy)-b-(EA)-b-(EA-GMA) control polymer and its synthesis into material without dynamic furan-CNT interactions. All DPN polymer compositions are outlined in Table 1. | ||
Various formulations of dynamic polymers (Table 1) confirmed by 1H NMR and GPC (Table S1†) were used to develop a novel class of DPNs. In general, the synthesized polymers have well controlled molecular weight distributions, with number averaged molecular weight (Mn) close to the theoretical value and dispersity (Đ) below 1.4. In the case of the block copolymers, the final block copolymer has a Mn comparable to the theoretical Mn, with positive shifts in molecular weight upon each chain extension. However, since the final block only constituted a small increase in total molecular weight, the shift upon the third chain extension was relatively small.
| Entry | Polymers | CNT wt% | DPN designation | ε break [%] | σ peak [MPa] | Y [MPa] | κ S cm−1 |
|---|---|---|---|---|---|---|---|
| a Where — is given for conductivity (κ) no measurement could be made with the instrumentation. | |||||||
| 1 | Poly[EA100-UPy5] & poly[EA100-FMA5] | 0 | IPN-Lin100UPy5FMA50%CNT | 169 ± 05 | 0.237 ± 0.009 | 0.3 ± 0.1 | — |
| 2 | Poly[EA100-UPy5] & poly[EA100-FMA5] | 0.5 | IPN-Lin100UPy5FMA50.5%CNT | 164 ± 05 | 0.31 ± 0.03 | 0.45 ± 0.03 | — |
| 3 | Poly[EA100-UPy5] & poly[EA100-FMA5] | 1 | IPN-Lin100UPy5FMA51%CNT | 150 ± 20 | 0.61 ± 0.03 | 0.74 ± 0.04 | 0.038 ± 0.005 |
| 4 | Poly[EA100-UPy7.5] & poly[EA100-FMA7.]5 | 0 | IPN-Lin100UPy7.5FMA7.50%CNT | 110 ± 20 | 1.64 ± 0.04 | 3.3 ± 0.3 | — |
| 5 | Poly[EA100-UPy7.5] & poly[EA100-FMA7.5] | 1 | IPN-Lin100UPy7.5FMA7.51%CNT | 70 ± 10 | 3.2 ± 0.2 | 7.9 ± 0.5 | 0.134 ± 0.027 |
| 6 | Poly[EA100-UPy7.5] & poly[EA100-FMA7.5] | 2.5 | IPN-Lin100UPy7.5FMA7.52.5%CNT | 71 ± 0.1 | 5.8 ± 0.2 | 14.6 ± 0.2 | 0.400 ± 0.005 |
| 7 | Poly[EA100-UPy7.5] & double poly[EA100-FMA7.5] | 0 | IPN-Lin100UPy7.5FMA150%CNT | 110 ± 10 | 3.16 ± 0.03 | 5.3 ± 0.4 | — |
| 8 | Poly[EA100-UPy7.5] & double poly[EA100-FMA7.5] | 1 | IPN-Lin100UPy7.5FMA151%CNT | 90 ± 10 | 4 ± 1 | 8.1 ± 0.6 | 0.09 ± 0.01 |
| 9 | Double poly[EA100-UPy7.5] & poly[EA100-FMA7.5] | 0 | IPN-Lin100UPy15FMA7.50%CNT | 128 ± 02 | 2.46 ± 0.07 | 3.3 ± 0.1 | — |
| 10 | Double poly[EA100-UPy7.5] & poly[EA100-FMA7.5] | 1 | IPN-Lin100UPy15FMA7.51%CNT | 99 ± 05 | 2.87 ± 0.07 | 5.9 ± 0.2 | 0.059 ± 0.004 |
| 11 | Poly[EA150-UPy11.25] & poly[EA150-FMA11.25] | 0 | IPN-Lin150UPy11.25FMA11.250%CNT | 50.5 ± 0.5 | 5.5 ± 0.3 | 22 ± 2 | — |
| 12 | Poly[EA150-UPy11.25] & poly[EA150-FMA11.25] | 1 | IPN-Lin150UPy11.25FMA11.251%CNT | 50 ± 1 | 8.0 ± 0.9 | 31.5 ± 2 | 0.125 ± 0.007 |
| 13 | Poly[(EA20-UPy3.75)-b-(EA60)-b-(EA20-UPy3.75)] | 0 | BCN-Blk100UPy7.50%CNT | 100 ± 10 | 2.28 ± 0.02 | 4.7 ± 0.2 | — |
| 14 | Poly[(EA20-UPy3.75)-b-(EA60)-b-(EA20-UPy3.75)] | 1 | BCN-Blk100UPy7.51%CNT | 83 ± 03 | 2.69 ± 0.03 | 5.3 ± 0.8 | 0.027 ± 0.001 |
| 15 | Poly[(EA20-UPy3.75)-b-(EA60)-b-(EA20-FMA3.75)] | 0 | BCN-Blk100UPy3.75FMA3.750%CNT | 85.6 ± 0.5 | 3.9 ± 0.2 | 5.9 ± 0.2 | — |
| 16 | Poly[(EA20-UPy3.75)-b-(EA60)-b-(EA20-FMA3.75)] | 1 | BCN-Blk100UPy3.75FMA3.751%CNT | 74 ± 03 | 5.94 ± 0.04 | 12.1 ± 0.1 | 0.07 ± 0.01 |
| 17 | Poly[(EA20-UPy3.75)-b-(EA60)-b-(EA20-GMA3.75)] | 0 | BCN-Blk100UPy3.75GMA3.750%CNT | 84 ± 04 | 5.90 ± 0.03 | 9.6 ± 0.2 | — |
| 18 | Poly[(EA20-UPy3.75)-b-(EA60)-b-(EA20-GMA3.75)] | 1 | BCN-Blk100UPy3.75GMA3.751%CNT | 86 ± 02 | 6.6 ± 0.3 | 12.2 ± 0.2 | 0.039 ± 0.001 |
Subsequently, a facile approach was used to prepare DPNs through solution processing of dynamic polymers with CNTs using ultrasonication. Covalent bonding between polymer chains and CNTs was achieved through the intrinsic Diels–Alder chemistry,31,43 without the need for pre-functionalization or post-polymerization modification. Integration of 2-ureido-4[1H]-pyrimidinone (UPy) and furfuryl-methacrylate (FMA) motifs enabled dynamic supramolecular H-bonding through dimerized quadrupole H-bond interactions and dynamic covalent DA cross-links respectively (Scheme 1). Multiple dynamic polymer structures were explored to study the impact of network architecture in nanoreinforced conductive composites. Specific thermomechanical properties studied include Tg, strain at break (εbreak), peak stress (σpeak), and Young's modulus (Y) as outlined in Table 1 and Table S2.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 Pendant furan groups on FMA were leveraged as a diene on polymer side chains and cross-linked using 1,1′-(methylene-di-4,1-phenyl-ene)bismaleimide (BMI) through a reversible [4 + 2] DA cycloaddition reaction. About ½ mole equivalent of BMI was used to cross-link 1 mole equivalent of FMA motifs through dynamic covalent DA chemistry. Since commercial BMI is ∼95% pure,46 consistent with findings reported by Bai et al.,47 previous reports showed that the small amount of unreacted FMA motifs have the ability to engage in DA chemistry through FMA furan groups on polymer chains with π-bonds on CNTs acting as dienophiles.31,35
42 Pendant furan groups on FMA were leveraged as a diene on polymer side chains and cross-linked using 1,1′-(methylene-di-4,1-phenyl-ene)bismaleimide (BMI) through a reversible [4 + 2] DA cycloaddition reaction. About ½ mole equivalent of BMI was used to cross-link 1 mole equivalent of FMA motifs through dynamic covalent DA chemistry. Since commercial BMI is ∼95% pure,46 consistent with findings reported by Bai et al.,47 previous reports showed that the small amount of unreacted FMA motifs have the ability to engage in DA chemistry through FMA furan groups on polymer chains with π-bonds on CNTs acting as dienophiles.31,35
To study the impact of network architecture on DPNs, interpenetrating polymer networks (IPNs) and tri-block copolymer networks (BCNs) were synthesized resulting in DPN entries 1–5 and 6–8 in Table 1 respectively. Designations IPN-LinwUPyxFMAy and BCN-BlkwUPyxFMAy are used to describe IPN and BCN-based DPNs in this study respectively. Linw represents a linear polymer chain with w number of EA units, BCN-Blkw represents a tri-block polymer chain with w number of EA units, while x and y represent the number of UPy and FMA units respectively. Eight different polymers were studied with CNT loadings ranging from 0, 0.5, 1, to 2.5 wt% leading to the comprehensive entries in Table 1. To confirm the significance of FMA bonding to the CNT surface, control materials were developed without furan group that can bind to the CNT surface through Diels–Alder chemistry. A BCN was developed where only the H-bonding UPy units were used, in entries 13 and 14 of Table 1. Similarly, the epoxide containing GMA replaced FMA in BCN 17 and 18 respectively, which serves as an important control, since the crosslinked epoxy chemistry derived from GMA cannot bind to the CNT surface through Diels–Alder chemistry.
Herein, we successfully validate the multifunctionality of DPNs in electrical circuits, demonstrating their ability to function as on-demand tunable electronic devices for custom resistors with ability to also act as semiconductors without the need for switching their electronic capacity unlike traditional memory resistors.48 This work hence contributes to the fast-growing field of healable electronic devices with new insight on tailoring the electronic properties of advanced conductive polymers.
Structure–property relationships in macromolecular engineered DPNs
Relatively low glass transition temperatures (Tg) below room temperature (−7.1 to +3.4 °C) were obtained for all DPNs. The low Tg arises from EA, with an uncrosslinked Tg of −24 °C,49 being the major component of each of the materials, facilitating dynamic behavior.50 Fig. S3(A)† gives the full IR spectrum of unreinforced bulk IPN-Lin100UPy7.5FMA7.50%CNT and reinforced IPN-Lin100UPy7.5FMA7.51%CNT with the proposed characterization outlined in Table S3† and a closer look at the spectra is provided in Fig. S4,† confirming the functional groups present. Additionally, a DSC trace used to estimate the Tg of materials is provided in Fig. S3(B)† using a BCN-Blk100UPy7.50%CNT sample as a typical example.
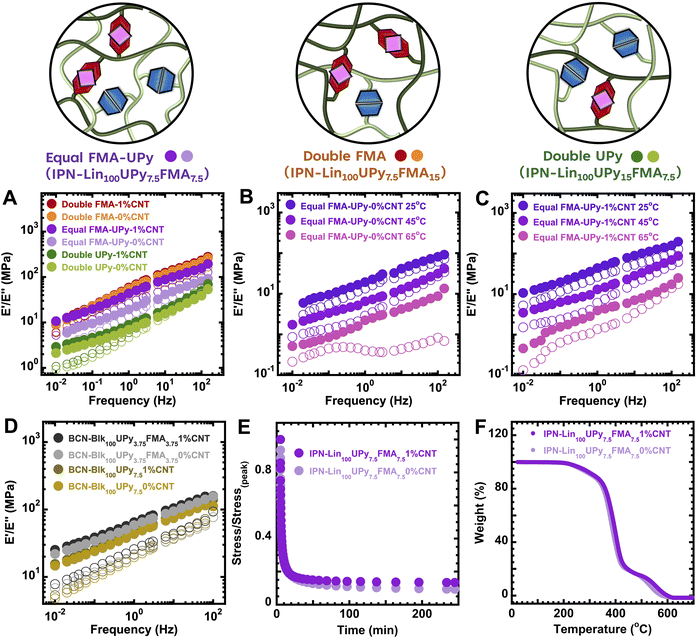
Presence of 1 wt% CNT in IPN-Lin100UPy7.5FMA7.5 gave a substantial increase in the storage modulus (E′) of IPN-Lin100UPy7.5FMA7.51%CNT compared to unreinforced IPN-Lin100UPy7.5FMA7.5 0%CNT (dark and light purple circles in Fig. 1A), this can be attributed to the presence of CNT as effective nanoreinforcements and an equal molar amount of DA and UPy cross-linked IPN networks. Doubling the amount of DA cross-linked networks as is the case with IPN-Lin100UPy7.5FMA15 resulted in a slightly higher E′ compared to the unreinforced IPN-Lin100UPy7.5FMA7.5, (Fig. 1A dark and light orange circles). This suggests that DA cross-links largely contributes to the modulus of the IPN-Lin100UPy7.5FMA15 materials such that addition of only 1 wt% CNT does not significantly change the E′. The smaller measured reinforcement in IPN-Lin100UPy7.5FMA15 compared to IPN-Lin100UPy7.5FMA7.5 could be due to a more uniform network in IPN-Lin100UPy7.5FMA7.5.
IPN-Lin100UPy7.5FMA15 materials had higher modulus compared to IPN-Lin100UPy15FMA7.5 (Fig. 1A). This has been observed in prior work,40 where higher loadings of UPy containing polymers, compared to dynamic covalent polymers, leads to overall lower moduli, presumably due to weaker linkages in the network. Additionally, due to the lower loading of FMA units, the IPN-Lin100UPy15FMA7.5 material as fewer opportunities for effective bonding between the matrix and CNTs.
Overall, IPN-Lin100UPy7.5FMA7.5 which contains an equal mol% of FMA and UPy cross-links stands out in Fig. 1A, with the most significant increase in E′ upon addition of 1% of CNTs. This could be in part due to the material with equimolar UPy and FMA polymer giving a more uniform network than when an excess of the FMA or UPy based linker is used. This indicates that true reinforcement without trade-offs in dynamic property of DPNs was achieved by using an equimolar amount of DA and UPy cross-linking, making IPN-Lin100UPy7.5FMA7.5 an excellent material for further studies.
Fig. 1(B and C) reveals the impact of temperature on frequency sweep traces of unreinforced IPN-Lin100UPy7.5FMA7.50%CNT and reinforced IPN-Lin100UPy7.5FMA7.51%CNT respectively. Higher temperatures decreased the E′ of IPN-Lin100UPy7.5FMA7.5, suggesting a pattern of thermoresponsive behavior due to partial dissociation of Diels–Alder units, which was present both with and without 1% of CNTs.35 Furthermore, triblock-based BCN-Blk100-UPy7.5 and BCN-Blk100UPy3.75FMA3.75 gave comparable E′, although BCN-Blk100UPy3.75FMA3.75 materials had higher E′ upon CNT reinforcement as shown in Fig. 1D. In the case of BCN-based DPN materials in Fig. 1D, DA containing BCN-Blk100UPy3.75FMA3.75 gave higher E′ (dark and light blue circles in Fig. 1D) compared to BCN-Blk100-UPy7.5 which only contains UPy cross-links as shown in Scheme S2(C).† This further supports the previous suggestion that DA cross-links contribute significantly to material's modulus, due to effectively permanent linkers at ambient temperature.
Importantly, BCN-Blk100-UPy7.5 materials can be considered as control systems because they do not contain any FMA-based DA cross-links and as such showed minimal difference between the frequency sweep of the reinforced and unreinforced variants. This is likely due to no possibility for DA bonding between CNT and BCN-Blk100-UPy7.5. BCN-Blk100UPy3.75FMA3.751%CNT had only a small increase in in E′ compared to the unreinforced variants BCN-Blk100UPy3.75FMA3.750%CNT (Fig. 1D), which again could be due to lower network uniformity in the system with blocky DA units. This further highlights IPN-Lin100UPy7.5FMA7.5 materials as candidates for further studies. Stress-relaxation experiments of IPN-Lin100UPy7.5FMA7.5 revealed ∼90% stress relaxation with time (Fig. 1E) indicating their potential for energy absorbing applications.51 Addition of 1 wt% CNT nanoreinforcement only caused a slight reduction in the stress relaxation capabilities of IPN-Lin100UPy7.5FMA7.51%CNT. Prior work showed that IPN dynamic materials crosslinked with FMA-BMI and UPy linkers maintain permanent shape fidelity from the essentially static Diels–Alder linkers, despite their excellent stress relaxation which occurs through exchange of UPy linkers.52
Degradation temperatures Td of IPN-Lin100UPy7.5FMA7.5 were in the range of 300–400 °C (Fig. 1F) similar to the Td of other poly(EA)-based materials reported in literature.31,35 Addition of CNT as nanofillers had no significant impact on the stress relaxation and Td of IPN-Lin100UPy7.5FMA7.5, presumably due to the small fraction of nanofiller added, and the thermoreversibility of the Diels–Alder bonds between the matrix and CNT.
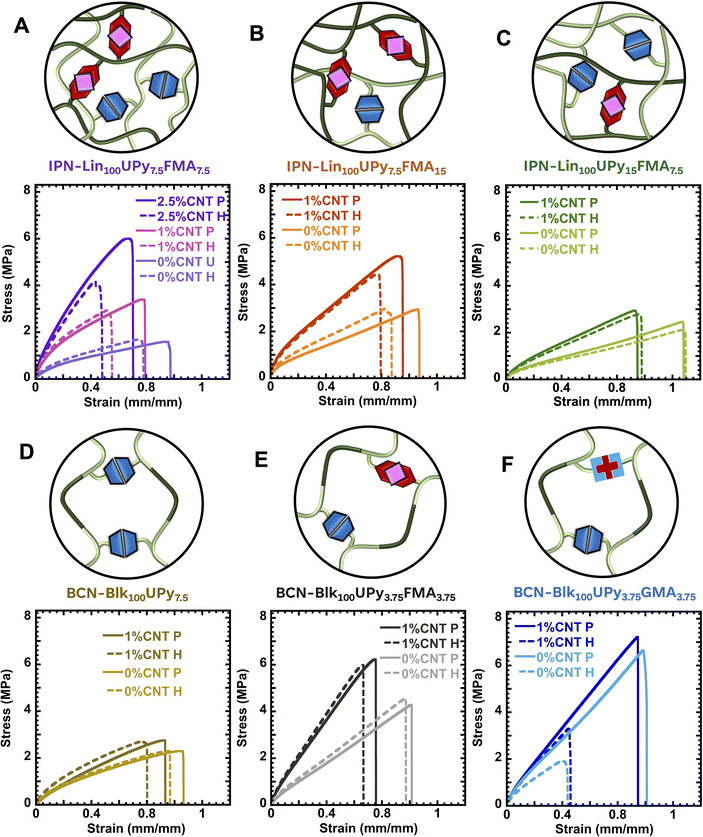
The impact of CNT loading is considered in Fig. 2A showing a progressive rise in peak stress (σpeak) as CNT loading increases in IPN-Lin100UPy7.5FMA7.5. σpeak increased by ∼100% upon the addition of 1% CNTs and by 250% upon the addition of 2.5% CNTs. Addition of CNT nanofillers led to a slight decrease in material strain; however, self-healing was achieved in all materials under thermal stimulus of 90 °C for up to 24 hours (Fig. 3B). A similar trend is also observed in Fig. S3(C)† with less cross-linked IPN-Lin100UPy5FMA5 containing 5 mol% cross-link density. Double DA cross-linked IPN-Lin100UPy7.5FMA151%CNT gave a 27% increase in σpeak compared to the unreinforced IPN-Lin100UPy7.5FMA7.50%CNT as shown in Fig. 2B and Table 1. This is most likely due to the presence of more covalent DA cross-links in IPN-Lin100UPy7.5FMA15, increasing the 0% CNT material's strength. This is consistent with Fig. 1 where DA cross-links were found to significantly contribute to material modulus, with a smaller impact of CNT reinforcement. Fig. 2C shows that doubling the H-bond cross-links compared to DA cross-links resulted in lower mechanical strength of IPN-Lin100UPy15FMA7.5 compared to IPN-Lin100UPy7.5FMA7.5 and IPN-Lin100UPy7.5FMA15 (Fig. 2A and B). However, the presence of more H-bonds gave better healing efficiency as shown in the time evolution healing of IPN-Lin100UPy15FMA7.5 where healing properties of the materials can be as shown in Fig. S3(D and E),† further confirming thermoresponsive behavior.35,53 Consistent with the DMA data in Fig. 1A, there is minimal reinforcement of IPN-Lin100UPy15FMA7.5 upon the addition of 1% CNTs.
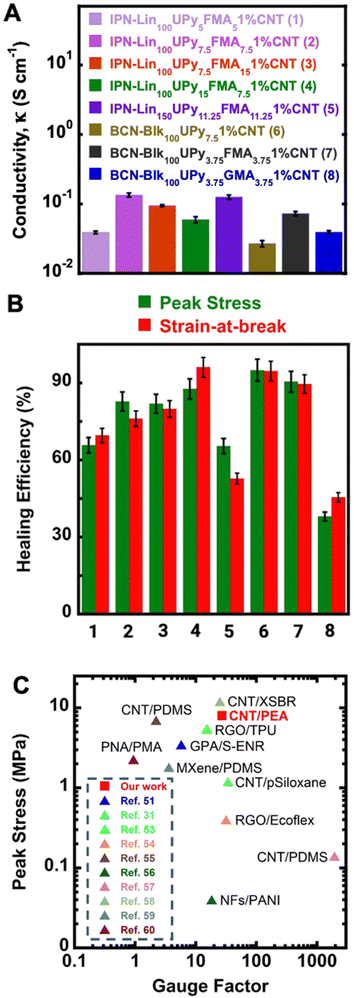 | ||
| Fig. 3 (A) Electrical conductivity of all 1 wt% CNT reinforced DPNs in this study. DPN designations (1–8) are used in (B). (B) Healing efficiency determined by the recovery of breaking (tensile) stress as well as breaking strain. (C) Ashby plot of σpeak and GF of IPN-Lin100UPy7.5FMA7.52.5%CNT compared to other reported conductive polymer composites in literature.33,54,56–63 | ||
To better understand the impact of architecture on the mechanical properties of DPNs, different crosslink densities and chain lengths were studied. The lower density of crosslinks in IPN-Lin100UPy5FMA51%CNT gave a σpeak of 0.61 ± 0.03 MPa resulted in ∼25% of the peak stress of 7.5 mol% cross-linked IPN-Lin100UPy7.5FMA7.51%CNT with a σpeak of 3.2 ± 0.2 MPa (Fig. S3(C)† and Fig. 2A). This aligns with previous reports that suggest enhanced mechanical performance increases with cross-link density.33,35 Longer-chain variant IPN-Lin150UPy11.25FMA11.251%CNT with a σpeak of 8 ± 0.9 MPa and 150 EA units resulted in an impressive ∼2.5-fold increase in stress compared to shorter-chain IPN-Lin100UPy7.5FMA7.51%CNT with only 3.2 ± 0.2 MPa σpeak and 100 EA units (Fig. S3(F)† and Fig. 2A). This is attributed to the longer chains having more elastically effective crosslinks, increased number of entanglements, and effective interactions with the CNT nanofiller.
Furthermore, to explore the impact of architecture on DPN performance, tri-block BCN-Blk100UPy7.5, BCN-Blk100UPy3.75FMA3.75, and BCN-Blk100UPy3.75GMA3.75 were studied. Integration of only UPy units into BCN-Blk100UPy7.5 resulted in relatively similar outcomes when compared to double UPy containing IPN-Lin100UPy15FMA7.5. This includes high self-healing efficiencies, ≤17% increase in σpeak on addition of 1 wt% CNT, and generally lower σpeak (Fig. 2D).
However, similar to IPN-Lin100UPy7.5FMA7.5, BCN-Blk100UPy3.75FMA3.75-1%CNT containing equimolar DA-UPy cross-links resulted in a σpeak of 5.9 ± 0.04 MPa compared to IPN-Lin100UPy7.5FMA7.51%CNT with σpeak of 3.2 ± 0.2 MPa. Hence tri-block BCN-Blk100UPy3.75FMA3.75 leads to not only higher self-healing efficiency (Fig. 2E), but also higher mechanical performance (Fig. 2E) compared to IPN-Lin100UPy7.5FMA7.5 (Fig. 2A). This suggests that at high strains, the block like structure, with domains of dense UPy and DA crosslinks leads to higher tolerance to stress, while enabling CNT reinforcement and load transfer.
A second control material was designed using GMA instead of FMA as a cross-linking motif leading to BCN-Blk100UPy3.75GMA3.75. Fig. 2F reveals that addition of 1 wt% CNT to BCN-Blk100UPy3.75GMA3.75 made no significant impact on material properties (healing, εbreak, and σpeak) of BCN-Blk100UPy3.75GMA3.751%CNT compared to BCN-Blk100UPy3.75GMA3.750%CNT. This indicates that poor healing and poor mechanical enhancement observed is due to weak interaction between the polymer chains and CNT nanofillers. Since the epoxy group on GMA of BCN-Blk100UPy3.75GMA3.75 lacks the ability to chemically bond polymer chains to CNT nanofillers, poor nanoreinforcement performance is observed. The poor dynamic performance (i.e., self-healing) observed is due to the non dynamic nature of GMA cross-linked with diamines (Scheme S3†) with all dynamic exchange coming from UPy units as observed in Fig. 3B. To further confirm this, BCN-Blk100UPy7.5 which is also a control material, gave >1 order magnitude higher healing efficiency compared to BCN-Blk100UPy3.75GMA3.75. Overall, the very poor reinforcement in control materials that do not contain FMA units, specifically BCN-Blk100UPy3.75GMA3.75 and BCN-Blk100UPy7.5, indicate that efficient load transfer from the matrix to the CNT reinforcement requires the furan units to bond to the CNT surface in these materials. This highlights the potential of the direct and mild modification of CNT surfaces using Diels–Alder chemistry from furan.
The surface morphology of IPN-Lin100UPy7.5FMA7.52.5%CNT and IPN-Lin100UPy7.5FMA7.50%CNT was analyzed using scanning electron microscopy as represented in Fig. S5.† No distinctive feature was observed in the micrographs of unreinforced IPN-Lin100UPy7.5FMA7.50%CNT. However, reinforced IPN-Lin100UPy7.5FMA7.52.5%CNT revealed distinct features both on the surface and cross-sectional micrographs that suggests the presence of a nanofiller as shown in Fig. S5(C and D).† The presence of two types of dynamic bonds contributed to the healing efficiency observed in all CNT reinforced DPNs represented in Fig. 3B. One is dynamic H-bond between polymer chains via UPy motifs which reversibly associate and dissociate under temperate room conditions, providing an attractive force for better damage repair and healability.35 The other is the dynamic covalent DA bonds between polymer chains via FMA furan groups and BMI cross-linker which was can reversibly dissociate and associate at elevated temperatures.31 Generally, IPN-based IPN-Lin100UPy15FMA7.5 and BCN-based BCN-Blk100UPy7.5 had the highest healing efficiencies and this most likely due to their possession of abundant UPy driven quadrupole H-bonding, which contributes significantly to healing properties.54,55 In particular, the generation of domains of high DA and UPy crosslink density appears to facilitate self-healing, consistent with prior studies on blocky multiply dynamic networks.40
Electrical properties and applications of DPNs
Fig. 3A gives the conductivity (κ) for all 1 wt% CNT reinforced DPNs. The highest κ values were observed in IPN-Lin100UPy7.5FMA7.51%CNT and the longer-chain variant IPN-Lin150UPy11.25FMA11.251%CNT with 0.099 ± 0.003 and 0.125 ± 0.007 S cm−1 respectively. IPN-Lin100UPy7.5FMA7.52.5%CNT with higher CNT loading gave a κ of 0.402 ± 0.005 S cm−1 which is higher than that of the 1 wt% CNT variant by over a factor of 4, thus confirming the contribution of CNT nanofiller to conductive properties of reinforced DPNs. Based on the conductivity profile of all DPNs reported in Fig. 3A, it is evident that equimolar DA and UPy cross-linked IPN-Lin100UPy7.5FMA7.5 and the longer chain length variant IPN-Lin150UPy11.25FMA11.25 had better κ values overall. This again confirms the better performance of IPN-Lin100UPy7.5FMA7.5 material both mechanically and electrically which suggests they have better compatibility with CNT nanofillers. Although BCN-Blk100UPy3.75FMA3.751%CNT exhibited a competitive mechanical performance, IPN-Lin100UPy7.5FMA7.51%CNT possessed better electrical conductivity (Fig. 3A), hence making IPN-Lin100UPy7.5FMA7.5 an all-around better material for further studies.
Given the electrical conductivity of DPNs in this study, a key parameter to be considered is the gauge factor (GF) which is defined as the ratio of fractional changes in electrical resistance, R, to the ratio of fractional change in material length (i.e., strain):66
| GF = (ΔR/R)/(ΔL/L) = (ΔR/R)/ε | (1) |
For perspective, the GF of metallic foils under deformation are typically 2–5 mostly owing to changes in the cross-sectional area and length of the metal instead of changes in resistivity as response to mechanical deformation.67 Elastomeric semiconducting materials however have better strain gauging for precision measurements because they can withstand severe bending (flexion) or stretching due to the presence of polymeric substrates.68,69 Hence to further investigate the electrical properties of DPNs, the relative change in resistance denoted by 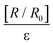 at only 60% strain was calculated and a GF of 27 ± 3 was obtained for IPN-Lin100UPy7.5FMA7.52.5%CNT. The GF value is often used to evaluate the sensitivity of a strain sensor where R, R0, ε represent the testing resistance, initial resistance, and applied strain respectively. Fig. 3C gives the comparison of IPN-Lin100UPy7.5FMA7.52.5%CNT GF value relative to some previously reported electrically conductive self-healing polymer nanocomposites. Additionally, comparison of σpeak gives insight into the mechanical performance of our IPN-Lin100UPy7.5FMA7.52.5%CNT material.
at only 60% strain was calculated and a GF of 27 ± 3 was obtained for IPN-Lin100UPy7.5FMA7.52.5%CNT. The GF value is often used to evaluate the sensitivity of a strain sensor where R, R0, ε represent the testing resistance, initial resistance, and applied strain respectively. Fig. 3C gives the comparison of IPN-Lin100UPy7.5FMA7.52.5%CNT GF value relative to some previously reported electrically conductive self-healing polymer nanocomposites. Additionally, comparison of σpeak gives insight into the mechanical performance of our IPN-Lin100UPy7.5FMA7.52.5%CNT material.
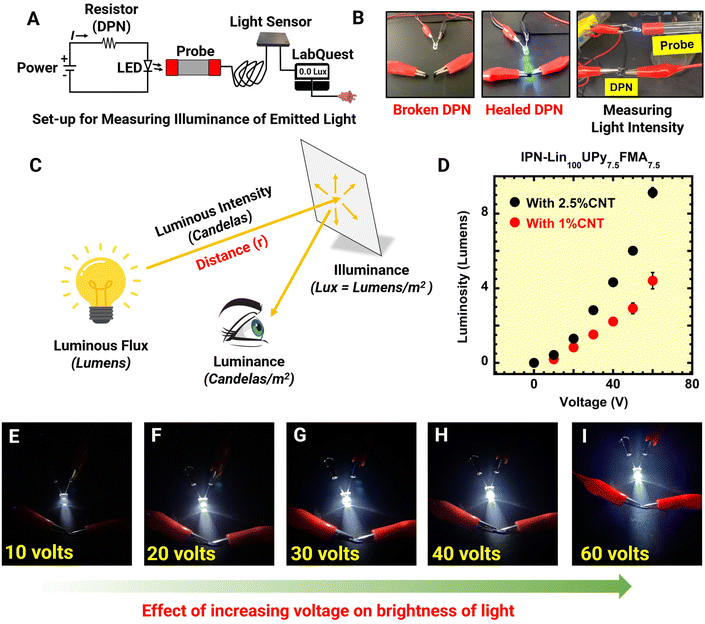
IPN-Lin100UPy7.5FMA7.52.5%CNT and IPN-Lin100UPy7.5FMA7.51%CNT were used as soft electronic materials for regulating the amount of current flowing through a circuit and consequently the luminous intensity of LED connected to the circuit (Fig. 3D and 4). Increasing the source potential difference (PD) of the circuit system from 10, 20, 30, 40, 50, and 60 V directly results in a progressive rise in luminosity as demonstrated in Fig. 4B. Although IPN-Lin100UPy7.5FMA7.52.5%CNT and IPN-Lin100UPy7.5FMA7.51%CNT showed similar trends, IPN-Lin100UPy7.5FMA7.52.5%CNT had a steeper rise in luminosity due to the higher CNT loading facilitating electrical percolation and current flow through the material compared to IPN-Lin100UPy7.5FMA7.51%CNT. Furthermore, Fig. 4(C–G) shows a continuous increase in illuminance/brightness of LED as PD applied to the circuit system increases. This highlights the potential of CNT reinforced IPN-Lin100UPy7.5FMA7.5 for next generation electronic materials for smart lighting devices where light intensity could be regulated via integrated conductive elastomeric materials that controls the current.
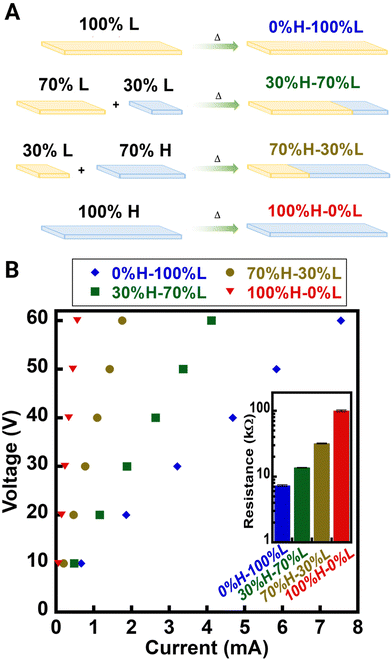
| Designation for custom resistors | DPN combinations | Resistance [kΩ] |
|---|---|---|
| 0%H–100%L | 100% of IPN-Lin100UPy7.5FMA7.52.5%CNT | 7.3 ± 0.2 |
| 30%H–70%L | (70% of IPN-Lin100UPy7.5FMA7.52.5%CNT) + (30% of IPN-Lin100UPy7.5FMA7.51%CNT) | 13.7 ± 0.1 |
| 70%H–30%L | (30% of IPN-Lin100UPy7.5FMA7.52.5%CNT) + (70% of IPN-Lin100UPy7.5FMA7.51%CNT) | 31.9 ± 0.6 |
| 100%H–0%L | 100% IPN-Lin100UPy7.5FMA7.51%CNT | 100 ± 3 |
Through self-healing ability in both materials, 70%H–30%L and 30%H–70%L were achieved with electrical conductivity that falls in-between 100%H–0%L and 0%H–100%L hence facilitating the modulation of electrical properties besides through changing CNT loadings (Table 2). Movies 1–3 shows the function of IPN-Lin100UPy7.5FMA7.52.5%CNT as a resistor in a circuit wherein absence of DPN causes an overflow of current towards the LED which consequently damages the diode whereas addition of DPN resistor regulates the amount of current that flows to the LED. We observed that after using a 9 V battery, only a PD of 2.6 V was measured across the LED suggesting that the PD across the IPN-Lin100UPy7.5FMA7.52.5%CNT was ∼6.4 V. Further investigation confirms that increasing CNT loadings decreases the electrical resistance in IPN-Lin100UPy7.5FMA7.5 materials (Fig. S7†).
To gain insight into the impact of prolonged exposure to electrical current, IPN-Lin100UPy7.5FMA7.52.5%CNT was integrated into a circuit system with a 9 V battery leading to illumination of LED in the circuit as shown in Fig. S8.† The system was left untampered for 12 days after which the battery voltage was depleted to <1 V (measured using a multimeter). However, when the depleted battery is replaced with a new 9 V battery, the LED bulb produces light and IPN-Lin100UPy7.5FMA7.52.5%CNT still acts as a resistor by limiting the flow of current towards the LED. This indicates that IPN-Lin100UPy7.5FMA7.52.5%CNT and LED were not affected by prolonged use in electronic applications, and suitable for long term electrical applications. To confirm the ability of IPN-Lin100UPy7.5FMA7.5 to act as resistors, a series of measurements were collected (Table S4†) enabled by circuit systems illustrated in Fig. S9.† IPN-Lin100UPy7.5FMA7.52.5%CNT consistently restricted the current flow from a PD of 10, 20, 30, 40, 50, and 60 V ensuring that only the necessary PD across the diode remained at the ∼2.5 V needed to power the LED, hence preventing it from damage.
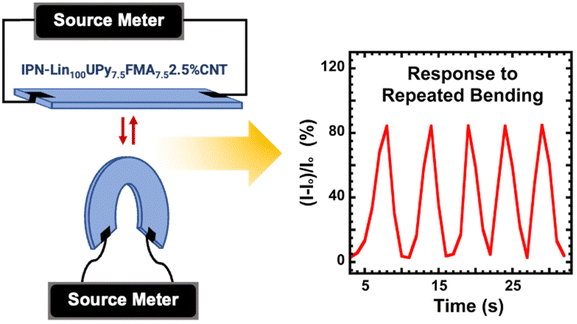
Additionally, the detection of bending and unbending (flexion) motions of IPN-Lin100UPy7.5FMA7.52.5%CNT was achieved, indicating piezoresistive behavior.33Fig. 6 shows changes in the relative current flowing through IPN-Lin100UPy7.5FMA7.52.5%CNT in response to multiple bending and unbending cycles, confirming the ability of the material to convert mechanical deformation into electrical signals, highlighting the potential for strain sensing functions for IPN-Lin100UPy7.5FMA7.52.5%CNT. This is consistent with the excellent gauge factor performance in Fig. 3C. Sensitivity of IPN-Lin100UPy7.5FMA7.52.5%CNT was further confirmed in a simple demonstration using a multimeter (Fig. S10†) which shows an increase in resistance under bending and decreased resistance when the material was straightened. This indicates that there is less resistance to the flow of current across IPN-Lin100UPy7.5FMA7.52.5%CNT under little or no mechanical deformation, consistent with other electrically conductive polymer nanocomposites.33,72
Conclusion
In summary, we present the facile synthesis of a new class of self-healable dynamic polymer nanocomposites (DPNs). The multifunctionality of the DPNs was demonstrated through applications in responsive lighting systems, customizable resistors, and their potential as strain sensing composite materials. Combining dynamic covalent Diels–Alder (DA) chemistry of FMA pendant furan groups with BMI cross-linkers and dynamic quadruple H-bonding of UPy cross-linking units afforded multiple DPN materials with distinct architectural designs. FMA enabled a mild in situ [4 + 2] DA reaction with π-bonds on the CNT surface, resulting in effective transfer of load/stress from polymer chains to CNT nanofillers without pre-functionalization. Well-designed interpenetrated (IPN) and block (BCN)-based DPNs were synthesized, each with different architectural features. Structure–property studies revealed that polymer architecture not only impacts mechanical performance but also the electrical properties of DPNs. IPN-Lin100UPy7.5FMA7.5 materials generally outperform other IPN and BCN-based DPN materials in this work. IPN-Lin100UPy7.5FMA7.5 materials had better mechanical enhancement upon addition of CNT nanofillers and higher electrical conductivity of CNT reinforced IPN-Lin100UPy7.5FMA7.5 materials, resulting in a gauge factor of 27 ± 3. This goes to show that equimolar use of Diels–Alder (DA) and UPy H-bonding cross-link units in IPN systems in combination with macromolecular engineered interaction with CNT nanofillers favored the thermomechanical, electrical, and dynamic properties of resulting in multifunctional DPN materials. The results suggest that using equal mole% equivalents of DA and UPy cross-links is an effective strategy for designing DPNs with CNT nanofillers compared to unequal distribution of DA and UPy cross-linking motifs. In addition to demonstrated application of IPN-Lin100UPy7.5FMA7.52.5%CNT and IPN-Lin100UPy7.5FMA7.51%CNT as electronic materials for regulating smart dimmable LED lighting systems, DPNs in this work also exhibited remarkable self-healing traits which enabled their application as customizable resistors. Electrical and mechanical properties combined with unique network structures of DPNs led to overall multifunctional applications including strain sensing. DPNs introduced in this work could lend themselves to other promising applications in multifunctional flexible electronics such as human vocalization, acoustic vibration, human motion detection, and portable smart lighting systems.Experimental section
Materials and reagents
All starting materials, reagents, and solvents were purchased from commercial sources and used directly without further purification unless specified otherwise. 2-(((6-(3-(6-Methyl-4-oxo-1,4-dihydropyrimidin-2-yl)ureido)hexyl)carbamoyl)-oxy)ethyl acrylate (UPy) and (2-propionic acid)yldodecyl trithiocarbonate (PADTC) were synthesized as outlined in literature.73Synthesis of a typical IPN-based DPN using poly[EA100-Upy7.5-FMA3.75]
Synthesis of BCN-based DPN using poly[EA20-UPy3.75]-b-[EA60]-b-[EA20-FMA3.75]
To a 50 mL round bottom flask equipped with magnetic stir bar, AIBN, (0.008 g, 0.045 mmol), PADTC [0.159 g, 0.45 mmol], UPy [0.7 g, 1.7 mmol], EA [0.91 g, 9.1 mmol] and 3.55 g of DMF were added. The reaction mixture was sealed and deoxygenate for 15 minutes. After which the reaction mixture was stirred at 65 °C for 24 h. The resulting poly[EA20-UPy3.75] polymer was confirmed by 1H-NMR indicating >95% conversion. To carry out the chain extension, AIBN [0.008 g, 0.045 mmol], EA [2.73 g, 27.3 mmol] and 2.80 g of DMF were added to the above reaction mixture. The reaction mixture was sealed and deoxygenate for 15 minutes. The AB block chain extension reaction was then carried out at 65 °C for 24 hours with continuous stirring. Monomer conversion of AB poly[EA20-UPy3.75]-b-[EA60] was confirmed to be >95% using 1H-NMR. For the final chain extension to obtain ABC-type polymer, AIBN [0.0080 g, 0.045 mmol], FMA [0.28 g, 1.7 mmol], EA [0.91 g, 9.1 mmol] and 3.10 g of DMF was added to the reaction mixture and deoxygenated for 15 minutes. The reaction was then carried out at 65 °C for 10 hours with continuous stirring. After 24 h, monomer conversion (78%) of poly[EA20-UPy3.75]-b-[EA60]-b-[EA20-FMA3.75] was confirmed using 1H-NMR. The compositions and SEC characterization data of all the BCN polymers are reported in Fig. S2 and Table S1.†Self-healing experiment
Materials subjected to self-healing were first sliced in half using a razor blade and the two separate ends of such material are then pressed back together at the sliced area using mild pressure from fingers. Afterwards all materials, both pristine (P) and healable (H) were placed in an oven at 90 °C for 24 hours to expose the materials to equivalent thermal conditions. Samples were then subjected to tensile tests. For the time evolution self-healing test, similar steps were followed except the materials were heated for 1, 7, 14, and 24 hours at 90 °C respectively as reported in Fig. S3(D and E).†Electrical conductivity [κ]
Resistance (R) was measured using a Keithley 2450 Source Measure Unit Instrument. The ohmmeter setting was used to determine specimen resistance. A 4-point probe system was used for all CNT reinforced samples. The resistivity [ρ] was obtained by dividing resistance R by the length [l] of materials and multiplying by cross-sectional area A.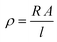 | (2) |
Electrical conductivity [κ] was determined using the inverse of resistivity.
| κ = ρ−1 | (3) |
Illumination measurement
A probe for light sensing was connected to a lab quest which was connected to a power source. The light probe was positioned at an approximate distance of 2.5 mm from a lighted diode connected to an electrical circuit where a DPN was employed as an organic resistor. Illumination in lux was obtained from the lab quest and used to determine luminosity in lumens while changing the potential difference (PD) applied to the circuit from 10, 20, 30, 40, 50, and 60 V. Luminosity [Φ] was determined by multiplying illumination [E] and surface area of the spherical diode [A] leading to a plot of Φ against voltage showing the impact of increasing PD on Φ and potential of DPNs as organic resistors in electrical circuits as demonstrated in Fig. 4. | (4) |
Custom resistor test
Experiment was carried out by setting-up a circuit and measuring the amount of current [I] traveling from an organic resistor to the diode. Impact of changing the PD applied to the circuit on the current is recorded for each resistor and added to a joint plot showing the result of customizing or tuning the properties of organic resistors.Strain sensing
IPN-Lin100UPy7.5FMA7.52.5%CNT was connected to a Keithley 2450 Source Meter which was set to Ammeter mode to measure current. A 2-point probe system was used to link the DPN to the meter for ease of bending. Repeated bending of IPN-Lin100UPy7.5FMA7.52.5%CNT at a combined 180° gave fluctuating wave response in current measurement over time. A plot of ΔI/I0 (%) on the y-axis against Time (s) on the x-axis shows response of the material to repeated bending in Fig. 6.Young's modulus [Y] calculation
Incompressible Ogden hyper-elastic constitutive law similar to previous reports in our group31,35,55 (eqn (5)) was used to model the tensile response of materials.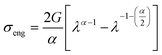 | (5) |
| Y = 2G[1 + ν] | (6) |
Strain calculation
Strain was evaluated from eqn (7).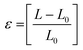 | (7) |
ε break is the strain at break.
Stress calculation
Stress was evaluated from eqn (8).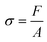 | (8) |
Author contributions
O. J. D. and D. K. conceptualized the materials studied with D. K. O. J. D and C. J. T. involved in conceptualization of applications. O. J. D., I. O. R., I. J. A., C. P. M., L. P., K. W., and D. D. synthesized the materials and characterized the structures. O. J. D. and D. K. analyzed the results. O. J. D. prepared the manuscript, D. K. contributed to manuscript development. All authors reviewed and edited the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was financially supported by the National Science Foundation (NSF) through Grant No. DMR-1749730. Partial support from NSF Grant No. CHE-1851795 (Supporting L. P. for REU in Chemistry at Miami University) for material synthesis is also acknowledged. 400 MHz NMR instrumentation at Miami University is supported through funding from the NSF under Grant No. CHE-1919850. D. K. acknowledges support from the Robert H. and Nancy J. Blayney Professorship.References
- B. C. K. Tee, C. Wang, R. Allen and Z. Bao, Nat. Nanotechnol., 2012, 7, 825–832 CrossRef CAS PubMed.
- R. F. Shepherd, A. A. Stokes, R. M. D. Nunes and G. M. Whitesides, Adv. Mater., 2013, 25, 6709–6713 CrossRef CAS PubMed.
- Y. Xin, Y. Lou, H. Liu, D. Wu and J. Zhang, Adv. Mater. Technol., 2021, 6, 1–9 Search PubMed.
- S. Park, G. Thangavel, K. Parida, S. Li and P. S. Lee, Adv. Mater., 2019, 31, 1805536 CrossRef PubMed.
- Z. Wang, Y. Liu, D. Zhang, K. Zhang, C. Gao and Y. Wu, Compos. Sci. Technol., 2021, 216, 109042 CrossRef CAS.
- D. Chen, D. Wang, Y. Yang, Q. Huang, S. Zhu and Z. Zheng, Adv. Energy Mater., 2017, 7 CAS.
- C. J. Thrasher, Z. J. Farrell, N. J. Morris, C. L. Willey and C. E. Tabor, Adv. Mater., 2019, 31, 1903864 CrossRef CAS PubMed.
- E. F. Gomez, S. V. Wanasinghe, A. E. Flynn, O. J. Dodo, J. L. Sparks, L. A. Baldwin, C. E. Tabor, M. F. Durstock, D. Konkolewicz and C. J. Thrasher, ACS Appl. Mater. Interfaces, 2021, 13, 28870–28877 CrossRef CAS PubMed.
- K. Myny, Nat. Electron., 2018, 1, 30–39 CrossRef CAS.
- A. M. Hussain and M. M. Hussain, Adv. Mater., 2016, 28, 4219–4249 CrossRef CAS PubMed.
- S. K. Arya, C. C. Wong, Y. J. Jeon, T. Bansal and M. K. Park, Chem. Rev., 2015, 115, 5116–5158 CrossRef CAS PubMed.
- Y. Cheng, R. Wang, J. Sun and L. Gao, Adv. Mater., 2015, 27, 7365–7371 CrossRef CAS PubMed.
- P. Lavrador, M. R. Esteves, V. M. Gaspar and J. F. Mano, Adv. Funct. Mater., 2021, 31 Search PubMed.
- L. Zhang, H. Li, X. Lai, T. Gao and X. Zeng, ACS Appl. Mater. Interfaces, 2020, 12, 44360–44370 CrossRef CAS PubMed.
- Z. Ma, H. Li, X. Jing, Y. Liu and H. Y. Mi, Sens. Actuators, A, 2021, 329, 112800 CrossRef CAS.
- C. S. Luo, P. Wan, H. Yang, S. A. A. Shah and X. Chen, Adv. Funct. Mater., 2017, 27 Search PubMed.
- T. P. Huynh, P. Sonar and H. Haick, Adv. Mater., 2017, 29, 1–14 CrossRef PubMed.
- Y. J. Tan, J. Wu, H. Li and B. C. K. Tee, ACS Appl. Mater. Interfaces, 2018, 10, 15331–15345 CrossRef CAS PubMed.
- S. Wang and M. W. Urban, Nat. Rev. Mater., 2020, 5, 562–583 CrossRef CAS.
- P. Chakma and D. Konkolewicz, Angew. Chem., Int. Ed., 2019, 58, 9682–9695 CrossRef CAS PubMed.
- Y. Gai, H. Li and Z. Li, Small, 2021, 17, 1–19 CrossRef PubMed.
- Y. Zhou, L. Li, Z. Han, Q. Li, J. He and Q. Wang, Chem. Rev., 2023, 123, 558–612 CrossRef CAS PubMed.
- S. Utrera-Barrios, R. Verdejo, M. A. López-Manchado and M. Hernández Santana, Mater. Horiz., 2020, 7, 2882–2902 RSC.
- S. Kang, A. R. Jones, J. S. Moore, S. R. White and N. R. Sottos, Adv. Funct. Mater., 2014, 24, 2947–2956 CrossRef CAS.
- B. J. Blaiszik, A. R. Jones, N. R. Sottos and S. R. White, J. Microencapsulation, 2014, 31, 350–354 CrossRef CAS PubMed.
- S. V. Wanasinghe, O. J. Dodo and D. Konkolewicz, Angew. Chem., Int. Ed., 2022, 61, e202206938 CrossRef CAS PubMed.
- L. Hammer, N. J. Van Zee and R. Nicolaÿ, Polymers, 2021, 13, 396 CrossRef CAS PubMed.
- B. Zhang, N. De Alwis Watuthanthrige, S. V. Wanasinghe, S. Averick and D. Konkolewicz, Adv. Funct. Mater., 2022, 32, 2108431 CrossRef CAS.
- X. Xiao, T. Xie and Y. T. Cheng, J. Mater. Chem., 2010, 20, 3508–3514 RSC.
- V. K. Thakur and M. R. Kessler, Polymer, 2015, 69, 369–383 CrossRef CAS.
- E. B. Stopler, O. J. Dodo, A. C. Hull, K. A. Weaver, P. Chakma, R. Edelmann, L. Ranly, M. B. Zanjani, Z. Ye and D. Konkolewicz, Mater. Adv., 2020, 1, 1071–1076 RSC.
- H. Dai, Surf. Sci., 2002, 500, 218–241 CrossRef CAS.
- D. Mai, J. Mo, S. Shan, Y. Lin and A. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 49266–49278 CrossRef CAS PubMed.
- A. Peigney, C. Laurent, E. Flahaut, R. R. Bacsa and A. Rousset, Carbon, 2001, 39, 507 CrossRef CAS.
- O. J. Dodo, L. Petit, D. Dunn, C. P. Myers and D. Konkolewicz, ACS Appl. Polym. Mater., 2022, 4, 6850–6862 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56–58 CrossRef CAS.
- D. Yuan, H. Guo, K. Ke and I. Manas-Zloczower, Composites, Part A, 2020, 132, 105837 CrossRef CAS.
- Q. T. Li, M. J. Jiang, G. Wu, L. Chen, S. C. Chen, Y. X. Cao and Y. Z. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 20797–20807 CrossRef CAS PubMed.
- P. Liu, Eur. Polym. J., 2005, 41, 2693–2703 CrossRef CAS.
- N. De Alwis Watuthanthrige, D. Dunn, M. Dolan, J. L. Sparks, Z. Ye, M. B. Zanjani and D. Konkolewicz, ACS Appl. Polym. Mater., 2022, 4, 1475–1486 CrossRef CAS.
- S. J. Garcia, Eur. Polym. J., 2014, 53, 118–125 CrossRef CAS.
- N. De Alwis Watuthanthrige, P. Chakma and D. Konkolewicz, Trends Chem., 2021, 3, 231–247 CrossRef CAS.
- C.-M. Chang and Y.-L. Liu, Carbon, 2009, 47, 3041–3049 CrossRef CAS.
- N. P. Truong, G. R. Jones, K. G. E. Bradford, D. Konkolewicz and A. Anastasaki, Nat. Rev. Chem., 2021, 5, 859–869 CrossRef CAS PubMed.
- G. R. Jones, A. Anastasaki, R. Whitfield, N. Engelis, E. Liarou and D. M. Haddleton, Angew. Chem., Int. Ed., 2018, 57, 10468–10482 CrossRef CAS PubMed.
- Millipore Sigma, 1,1′-(Methylenedi-4,1-phenylene) bis maleimide Product Specification: https://www.sigmaaldrich.com/US/en/product/aldrich/227463.
- J. Bai and Z. Shi, ACS Appl. Mater. Interfaces, 2017, 9, 27213–27222 CrossRef CAS PubMed.
- Y. Chen, G. Liu, C. Wang, W. Zhang, R. W. Li and L. Wang, Mater. Horiz., 2014, 1, 489–506 RSC.
- C. U. Pittman and G. A. Stahl, J. Appl. Polym. Sci., 1981, 26, 2403–2413 CrossRef CAS.
- A. P. King and H. Naidus, J. Polym. Sci., Part C: Polym. Symp., 1969, 27, 311–319 CrossRef.
- A. Schiavi and A. Prato, Polym. Test., 2017, 59, 220–229 CrossRef CAS.
- B. Zhang, J. Ke, J. R. Vakil, S. C. Cummings, Z. A. Digby, J. L. Sparks, Z. Ye, M. B. Zanjani and D. Konkolewicz, Polym. Chem., 2019, 10, 6290–6304 RSC.
- B. Zhang, Z. A. Digby, J. A. Flum, P. Chakma, J. M. Saul, J. L. Sparks and D. Konkolewicz, Macromolecules, 2016, 49, 6871–6878 CrossRef CAS.
- L. Shuai, Z. H. Guo, P. Zhang, J. Wan, X. Pu and Z. L. Wang, Nano Energy, 2020, 78, 105389 CrossRef CAS.
- S. C. Cummings, O. J. Dodo, A. C. Hull, B. Zhang, C. P. Myers, J. L. Sparks and D. Konkolewicz, ACS Appl. Polym. Mater., 2020, 2, 1108–1113 CrossRef CAS.
- Y. Ting, K. Dajiang, H. Weiyi, Y. Yunjie and W. Chaoxia, Colloids Surf., A, 2022, 130411 Search PubMed.
- Y. Wang, J. Hao, Z. Huang, G. Zheng, K. Dai, C. Liu and C. Shen, Carbon, 2018, 126, 360–371 CrossRef CAS.
- M. Xu, J. Qi, F. Li and Y. Zhang, Nanoscale, 2018, 10, 5264–5271 RSC.
- J. Sun, Y. Zhao, Z. Yang, J. Shen, E. Cabrera, M. J. Lertola, W. Yang, D. Zhang, A. Benatar, J. M. Castro, D. Wu and L. J. Lee, Nanotechnology, 2018, 29, 355304 CrossRef PubMed.
- G. Ge, Y. Lu, X. Qu, W. Zhao, Y. Ren, W. Wang, Q. Wang, W. Huang and X. Dong, ACS Nano, 2020, 14, 218–228 CrossRef CAS PubMed.
- B. Xu, F. Ye, R. Chen, X. Luo, G. Chang and R. Li, Ceram. Int., 2022, 48, 10220–10226 CrossRef CAS.
- M. Lin, Z. Zheng, L. Yang, M. Luo, L. Fu, B. Lin and C. Xu, Adv. Mater., 2022, 34, 1–13 Search PubMed.
- K. Zhang, J. Sun, J. Song, C. Gao, Z. Wang, C. Song, Y. Wu and Y. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 45306–45314 CrossRef CAS PubMed.
- M. Park, J. Park and U. Jeong, Nano Today, 2014, 9, 244–260 CrossRef CAS.
- J.-S. Noh, Polymers, 2016, 8, 123 CrossRef PubMed.
- National Instruments, Application Note 078, 1998, 1–12.
- N. Lu, X. Wang, Z. Suo and J. Vlassak, Appl. Phys. Lett., 2007, 91, 8–11 Search PubMed.
- S. Yang and N. Lu, Sensors, 2013, 13, 8577–8594 CrossRef CAS PubMed.
- J. A. Rogers, M. G. Lagally and R. G. Nuzzo, Nature, 2011, 477, 45–53 CrossRef CAS PubMed.
- Y. Gao, Y. Cheng, H. Zhang and N. Zou, Measurement, 2019, 139, 380–386 CrossRef.
- S. Jung, A. Sou, E. Gili and H. Sirringhaus, Org. Electron., 2013, 14, 699–702 CrossRef CAS.
- Z. Chen, W. Ren, L. Gao, B. Liu, S. Pei and H. M. Cheng, Nat. Mater., 2011, 10, 424–428 CrossRef CAS PubMed.
- B. Zhang, Z. A. Digby, J. A. Flum, E. M. Foster, J. L. Sparks and D. Konkolewicz, Polym. Chem., 2015, 6, 7368–7372 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Supplemental synthetic and characterization methods, SEC data of polymers synthesized, glass transition temperature data of materials, supplemental infrared, mechanical, and scanning electron microscopy data, circuit diagrams and images of circuits. See DOI: https://doi.org/10.1039/d3lp00012e |
| This journal is © The Royal Society of Chemistry 2023 |