Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Parallel detection of chemical reactions in a microfluidic platform using hyperpolarized nuclear magnetic resonance†
Jose
Yeste‡
 a,
Marc
Azagra‡
a,
Maria A.
Ortega
a,
Alejandro
Portela
a,
Gergő
Matajsz
a,
Alba
Herrero-Gómez
a,
Marc
Azagra‡
a,
Maria A.
Ortega
a,
Alejandro
Portela
a,
Gergő
Matajsz
a,
Alba
Herrero-Gómez
 a,
Yaewon
Kim
b,
Renuka
Sriram
b,
John
Kurhanewicz
bc,
Daniel B.
Vigneron
bc and
Irene
Marco-Rius
a,
Yaewon
Kim
b,
Renuka
Sriram
b,
John
Kurhanewicz
bc,
Daniel B.
Vigneron
bc and
Irene
Marco-Rius
 *a
*a
aInstitute for Bioengineering of Catalonia, The Barcelona Institute of Science and Technology, Barcelona, Spain. E-mail: imarco@ibecbarcelona.eu
bDepartment of Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, California, USA
cGraduate program in Bioengineering, University of California, Berkeley and University of California, San Francisco, California, USA
First published on 19th October 2023
Abstract
The sensitivity of NMR may be enhanced by more than four orders of magnitude via dissolution dynamic nuclear polarization (dDNP), potentially allowing real-time, in situ analysis of chemical reactions. However, there has been no widespread use of the technique for this application and the major limitation has been the low experimental throughput caused by the time-consuming polarization build-up process at cryogenic temperatures and fast decay of the hyper-intense signal post dissolution. To overcome this limitation, we have developed a microfluidic device compatible with dDNP-MR spectroscopic imaging methods for detection of reactants and products in chemical reactions in which up to 8 reactions can be measured simultaneously using a single dDNP sample. Multiple MR spectroscopic data sets can be generated under the same exact conditions of hyperpolarized solute polarization, concentration, pH, and temperature. A proof-of-concept for the technology is demonstrated by identifying the reactants in the decarboxylation of pyruvate via hydrogen peroxide (e.g. 2-hydroperoxy-2-hydroxypropanoate, peroxymonocarbonate and CO2). dDNP-MR allows tracing of fast chemical reactions that would be barely detectable at thermal equilibrium by MR. We envisage that dDNP-MR spectroscopic imaging combined with microfluidics will provide a new high-throughput method for dDNP enhanced MR analysis of multiple components in chemical reactions and for non-destructive in situ metabolic analysis of hyperpolarized substrates in biological samples for laboratory and preclinical research.
Introduction
Dissolution dynamic nuclear polarization (dDNP) is a hyperpolarization (HP) method that enhances the sensitivity of magnetic resonance (MR) at thermal equilibrium by more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times1 and enables real-time, in situ monitoring of chemical reactions2 with a time resolution of seconds and nanomolar sensitivity.3 There is a large variety of NMR techniques to study chemical reaction kinetics. For instance, time-resolved measurements can be made by taking a sequence of spectra when the chemical dynamics are slower than the acquisition time of the NMR free induction decay (FID) — or by time-of-flight in-flow measurements in the opposite situation when chemical dynamics are relatively fast.4 Metabolic reactions that typically occur on the same time scale as the relaxation time of HP 13C spins fall into the former category.
000 times1 and enables real-time, in situ monitoring of chemical reactions2 with a time resolution of seconds and nanomolar sensitivity.3 There is a large variety of NMR techniques to study chemical reaction kinetics. For instance, time-resolved measurements can be made by taking a sequence of spectra when the chemical dynamics are slower than the acquisition time of the NMR free induction decay (FID) — or by time-of-flight in-flow measurements in the opposite situation when chemical dynamics are relatively fast.4 Metabolic reactions that typically occur on the same time scale as the relaxation time of HP 13C spins fall into the former category.
Unlike other HP techniques such as parahydrogen induced polarization5,6 or chemically induced dynamic nuclear polarization,7 the strength of dDNP lies in the wide variety of biocompatible hyperpolarized substrates that reveal key metabolic pathways untraceable by any other technique.8,9 Moreover, dDNP is the only HP-MR technique currently undergoing clinical investigation for safety and for diagnosis and post-treatment assessment.10,11
The dDNP process involves polarization transfer from free electrons to the sample nuclear spins, increasing the nuclear spin population in the lower energetic state, and therefore the corresponding NMR signal. To achieve this, the sample must be cooled to liquid helium temperatures (∼1.4 K) and placed in a strong magnetic field (>3 T). Microwaves are then used to excite the free electrons and transfer their polarization to the nuclear spins of interest.
Advances in dDNP-MR have improved polarization efficiency,12,13 chemical formulations to avoid persistent radicals in the sample14–17 and lifetime of spin hyperpolarization in the solid state for sample transportation.3,18,19 However, the application potential of dDNP-MR is still limited by the time-consuming polarization build-up process and fast relaxation of the nuclear magnetization post dissolution. For instance, polarization time of [1-13C]pyruvate is usually on the order of tens of minutes, while T1 relaxation is on the order of tens of seconds at room temperature.20
Reactants and products in chemical reactions can be detected with a time resolution of seconds and nanomolar sensitivity using dissolution dDNP-MR.15 A major drawback of the technique, however, is that the preparation of hyperpolarized substrates by dDNP is time-consuming and costly, which limits its ability to generate data. A minimum amount of hyperpolarized sample has to be produced as an equipment limitation, and usually not more than 3–10% of the sample is used in the experiment.21 The development of more efficient methods to fully utilize the polarized samples, like the one proposed here, will shorten experimental time, increase throughput, decrease its associated costs, improve control over experimental variables and reproducibility and, as a result, contribute to expand the applications of dDNP-MR.
Commercial MR instrumentation only allows single sample acquisitions or a handful of consecutive samples as the polarization decays. Repeated MR readings have been possible with a single dDNP sample by continuous filling of a volume microcoil8 or sequential acquisitions by removing, reloading, and introducing a new sample again in a MR imaging (MRI) scanner and a high field NMR spectrometer.3,22–24 However, consecutive acquisitions are not conducted under the same hyperpolarized substrate conditions (i.e. polarization level, substrate concentration and pH may vary between DNP dissolutions) and may be affected by B0 distortions (shim maladjustments), which affect data repeatability and make comparison between sample groups impractical. An alternative strategy to effectively shorten the polarization build-up cycle is simultaneous polarization of multiple samples. Currently in vivo dDNP-MR experiments are typically limited to four samples, which are dissolved one by one on demand, for [1-13C]pyruvate injections at 5–20 min intervals.14,25 However, despite these advances, the throughput of dDNP remains long and inefficient. A relatively large volume of hyperpolarized sample (∼4.5 mL) is produced in each polarization process, while only a few hundred microliters are usually consumed, and the remaining sample is discarded.
This project was designed to address the unmet need for technology which enables parallel dDNP-MR experiments using a single spectrometer and demonstrate that microfluidics can effectively enhance the throughput of a single dDNP shot and provide many replicates for comparing experimental parameters. An MRI-compatible microfluidic structure was produced containing eight sample wells, each independently and uniformly infused with spin-hyperpolarized solution and vital media. All 8 chambers can be probed with a single dDNP sample using the same MR acquisition, with consistent and uniform polarization conditions between sample chambers. As a demonstration of the technology, dDNP-MR spectroscopic images (dDNP-MRSI; i.e. MRI encoding of space with a chemical shift dimension) were acquired using commercially available RF coils in a preclinical 3 T MRI scanner, where we detected reactants and products generated from a pyruvate decarboxylation via hydrogen peroxide (H2O2).26 Three reaction replicates and five control samples were tested simultaneously in the device.
Methods
Fabrication of the microfluidic platform for DNP-MR applications
Molds with microfluidic channels and features were fabricated by standard photolithography methods.27 Silicon wafers (4′′ n-type <111>, MicroChemicals GmbH) were dehydrated using a hot plate (200 °C for 30 min) and treated with O2 plasma (PDC-002, Harrick Plasma) at 22.5 mL min−1 and 30 W for 20 min. Photoresist (SU-8 2100, KAYAKU Advanced Materials, Inc.) was spin-coated in two steps (500 rpm for 10 s with acceleration of 100 rpm s−1 followed by 1600 rpm for 30 s with 300 rpm s−1) to form a SU-8 layer of 200 μm thickness. The wafer was then soft-baked in two steps, firstly at 65 °C for 5 min, and secondly at 95 °C for 40 min. Photoresist was finally exposed to UV-light at 320 mJ cm−2 (17.5 mW cm−2 for 18.2 s) using a mask aligner machine (MJB4, Süss MicroTec) and post-baked at 65 °C for 5 min followed by 95 °C for 14 min. SU-8 structures were developed in SU-8 developer (MicroChemicals GmbH) for 16 min, rinsed with isopropyl alcohol, and dried with a N2 gun. The wafers were finally hard-baked at 95 °C for 30 min and silanized by vapor exposure of 1H,1H,2H,2H-perfluorooctyl-trichlorosilane (PFOTS, 97%, Merck) in a vacuum desiccator for 1 h.PDMS layers of the microfluidic device were fabricated by soft lithography protocols using the patterned SU-8 molds. PDMS prepolymer (Sylgard 184, Ellsworth Adhesives) was prepared in a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (elastomer base: curing agent, w/w) and degassed in a vacuum desiccator. The prepolymer was then cast on a Petri dish containing the SU-8 mold, baked at 65 °C for 4 h, and left overnight at room temperature. PDMS layers obtained included the microfluidic channels for 1) distributing the hyperpolarized sample (upper 4 mm thick layer), 2) suctioning fluid from the chambers (intermediate 1 mm thick layer), and 3) infusing fluid into the chambers (lower 5 mm thick layer). Chambers and ports (inlets and outlets) were punched in the corresponding PDMS layers, using 6 mm and 1.25 mm biopsy punches respectively. The three layer and a glass slide (75 × 38 mm Corning) were activated using O2 plasma (PDC-002, Harrick Plasma) and bonded together, resulting in a device with final dimensions of 11 mm thickness, 75 mm length, and 38 mm width.
1 (elastomer base: curing agent, w/w) and degassed in a vacuum desiccator. The prepolymer was then cast on a Petri dish containing the SU-8 mold, baked at 65 °C for 4 h, and left overnight at room temperature. PDMS layers obtained included the microfluidic channels for 1) distributing the hyperpolarized sample (upper 4 mm thick layer), 2) suctioning fluid from the chambers (intermediate 1 mm thick layer), and 3) infusing fluid into the chambers (lower 5 mm thick layer). Chambers and ports (inlets and outlets) were punched in the corresponding PDMS layers, using 6 mm and 1.25 mm biopsy punches respectively. The three layer and a glass slide (75 × 38 mm Corning) were activated using O2 plasma (PDC-002, Harrick Plasma) and bonded together, resulting in a device with final dimensions of 11 mm thickness, 75 mm length, and 38 mm width.
To operate the microfluidic device from outside the MRI scanner, the inlet port of the microfluidic network for injecting the hyperpolarized sample, was connected to a female Luer-to-barb fitting using 114 cm of polytetrafluoroethylene (PTFE) tubing (1/16′′ outer diameter × 1/32′′ internal diameter Darwin microfluidics). This allowed the fast administration of hyperpolarized solutions by means of a syringe. Remaining ports (i.e., infusion and withdrawal ports) were connected with the same tubing and closed using tubing clamps.
Hyperpolarization of [1-13C]pyruvate
A volume of 24 μL of [1-13C]pyruvic acid (Sigma Aldrich, Munich, Germany) containing 15 mM trityl radical OX063 (GE healthcare) and 1.5 mM Dotarem (Guerbet, Villepinte, France) were inserted into a commercial dissolution-DNP polarizer (HyperSense, Oxford Instruments Ltd.). This sample was polarized for approximately 55 min using 100 mW microwaves at 94.095 GHz in a magnetic field of 3.35 T, reaching a polarization level of around 15–20%. The hyperpolarized sample was dissolved in 4.5 mL of heated phosphate buffered saline supplemented with 1% HEPES, 0.01% EDTA, 0.1% NaCl, and 0.2% NaOH (pH 12), yielding 80 mM [1-13C]pyruvate and 80 μM trityl radical at pH 7.MRI data acquisition and hyperpolarized 13C-MRS
MR spectra were obtained from each individual chamber in the microfluidic platform by MR spectroscopic imaging (MRSI) techniques using a dual-tuned 1H–13C volume coil (42 mm inner diameter, Bruker) inside a horizontal 3 T MRI scanner (BioSpec 105 mm bore diameter, Bruker®). Magnetic field inhomogeneities were corrected with a shimming process applied to a water-filled device prior to the DNP-MRSI assays.Hyperpolarized sample, 1.6 mL, was injected into the microfluidic platform, which resulted in 125 ± 5 μL delivered in each chamber over a period of 3 seconds. Hyperpolarized 13C MR data was acquired with a chemical shift imaging (CSI) sequence (8 × 8 matrix, field of view of 40 mm × 40 mm, area of 5 mm × 5 mm per voxel, slice thickness of 12 mm, 15° flip angle, echo time of 1.49 ms, Tacq of 51.2 ms, and RT of 66.907 ms). Each point of the data set was acquired every 4 seconds. MR T2-weighted images were acquired using 4 scans. The whole matrix of voxels (i.e., 64 spectra) were acquired every 4 s with the first scan at the end of the hyperpolarized solution injection, i.e. at time 0. The transfer time between dissolution and injection was around 12 s.
The thermal equilibrium acquisition was performed on the microfluidic device with 100 μL solution of 8 M [13C]urea (Sigma Aldrich, Munich, Germany) and 10 mM Dota-Gd in two chambers located in the corners of the chip (Fig. 3A, highlighted in yellow). Those chambers were used to center the offset frequency, perform B1 calibration, shimming, and signal to noise ratio (SNR). Three chambers were filled with an aqueous solution containing 8.2 M H2O2 (Sigma Aldrich, Munich, Germany) and 37 mM NaOH (Fig. 3A, highlighted in red). The three remaining chambers were filled with 35 μL deionized water and 6.4 μL of 0.25 M NaOH solution (Fig. 3A, highlighted in blue). The MR localizer was performed with axial, sagittal, and coronal T2-weighted images using a spin echo T2 TurboRare sequence to assess signal location and accurately program the CSI voxels matrix.
MRI data processing
Resulting CSI data were reconstructed and visualized in SIVIC.28To determine the MR signal from each chamber, spectra from the voxels containing regions of the same chamber were summed. The T2 weighted image was used to select those voxels, and a total of 4 voxels were summed per each chamber.
Results & discussion
Microfluidic multiwell platform for parallel detection of chemical reactions by magnetic resonance
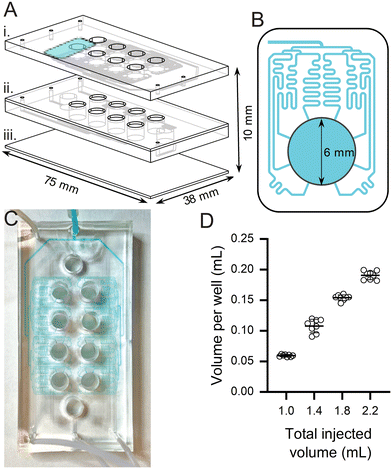
The microfluidic platform was designed using a CAD software 1) to be MR-compatible (i.e. made of non-magnetic materials), 2) to withstand a wide range of chemical reagents, solvents, and pHs, and 3) to be used in commercially available MRI hardware (i.e. to fit inside a 42 mm inner diameter volumetric RF coil and remain within the boundaries of the homogeneous B0 region of a preclinical 3 T MRI scanner) for improved usability and ease of implementation.The platform was engineered using microfabrication (photolithography and soft lithography) processes and consisted of a stack of three polydimethylsiloxane (PDMS) layers on a glass substrate (Fig. 1A).29 The PDMS layers were developed by replica molding using a photoresist-patterned mold and were dedicated to the microfluidic parts: the sample-containing 4 × 2 array of 6 mm-diameter chambers (of 15 mm center to center spacing, 10 mm height, ∼280 μL total volume) and the microfluidic channels to distribute the hyperpolarized solution among the chambers. A network of sinusoidal channels (Fig. 1B), acting as fluidic resistances, in the upper PDMS layer split the sample throughout the device and made it possible to deliver the hyperpolarized solution rapidly and simultaneously to all the chambers. Eight distributed microfluidic channels leading to each chamber were designed to deliver the hyperpolarized sample from the top of the chamber to the testing sample, facilitating a rapid delivery of the fluid under 4 s (video in ESI† material).
Two further PDMS layers with microfluidic channels were integrated between the above layers to continuously infuse and withdraw solutions into and from the chambers. These channels were grouped in 2 independent sets of 4 chambers that shared inlet and outlet ports (enabling 2 different conditions and 4 replicates when these channels are open). These allow for a myriad of experimental setups in future applications. For example, they can be used for continuous renewal of the solution in the chambers while the device is located inside the MRI scanner. In this work, all experiments were performed with the withdrawal circuits closed in the device shown in Fig. 1C.
To confirm uniform distribution of samples across the chambers, we injected different volumes of water throughout the inlet port and collected and weighed the liquid received in each chamber. For total injected volumes of 1 mL, 1.4 mL, 1.8 mL, and 2.2 mL, the volumes per chamber were 59 ± 2 μL, 108 ± 11 μL, 154 ± 5 μL, and 191 ± 7 μL, respectively (Fig. 1D). Applying linear regression, the data fit (R-squared of 0.96) the following equation:
 | (1) |
In order to increase the throughput of HP experiments by a factor of 8 using our microfluidic device, we exploited the potential of MR spectroscopic imaging (MRSI), by which spatial and spectral information can be obtained simultaneously (Fig. 2A). To test the device for HP-MRSI, a solution of 80 mM hyperpolarized [1-13C]pyruvate was injected through the fluidic channels. The matrix (8 × 8) and voxel size (5 mm × 5 mm) was selected to cover all the chambers while maintaining shimming performance (Fig. 2B). Although the voxel size was smaller than the chamber size, we found that multiple voxels per chamber simplified the alignment of the device inside the RF coil. Since no voxel included more than one chamber, analysis was performed by adding the signals from voxels encompassing the same chamber.
Fig. 2C shows a representative spectrum from one voxel that contains part of a chamber. The apparent relaxation time constant of [1-13C]pyruvate was 3.7 ± 0.7 s, as calculated from the exponential decay of the C1 pyruvate resonance at 176 ppm chemical shift. Although the T1 of [1-13C]pyruvate after dDNP is expected to be around 60 s,30 the repeated RF pulses used to obtain the spectroscopic images (i.e., 64 pulses per each scan) caused a much faster 13C polarization loss. The spectral linewidth of the 13C1 pyruvate peak was 1.8 ppm (53 Hz) at full width at half maximum height.
The axial, sagittal, and coronal proton T2 weighted MR images acquired after the HP 13C-MRSI experiment confirmed that the HP solution had been delivered to all the chambers as expected (Fig. 2B).
Contrary to previous studies that increased the throughput either by sequential measurements with the same dissolution sample3,8 or by parallel polarization of 4 samples,14,25 our MRSI approach improves repeatability as all tested samples are detected under the same hyperpolarized substrate and shim conditions (FWHM = 53 ± 5 Hz).
Simultaneous detection of eight chemical reaction processes: decarboxylation of hyperpolarized [1-13C]pyruvate and controls
[1-13C]pyruvate, a key metabolite in glycolysis31 and a cellular antioxidant agent capable of neutralizing peroxides by decarboxylation,32,33 is extensively used in dDNP-MR experiments due to its long T1 relaxation time constant (in excess of 1 minute)30 and high polarization (up to 36% with a SpinLab™ polarizer®),34 which provide MR signals of both pyruvate and its products.As a proof of concept for detecting fast reactions using our parallelized microfluidic approach, we traced the 13C resonance shifts of the biologically relevant chemical reaction between [1-13C]pyruvate and H2O2 by acquiring spatially-localized 13C-MR spectra of the whole device every 4 seconds. Using the entire chamber capacity of the platform, two chambers of the platform were filled with a [13C]urea solution for chamber arrangement verification, three with a H2O2 solution to initiate the chemical reaction, and three with a sodium hydroxide solution as a negative control of the reaction (Fig. 3A).
The placement of the microfluidic device and acquisition matrix was validated before to the HP experiment by a proton T2-weighted image (Fig. 3B) and again after by detecting the thermal signal of the [13C]urea solution from two opposite corners of the device (Fig. 3C). After injection of the hyperpolarized pyruvate solution, the spectra of the chamber regions without H2O2 displayed only the pyruvate peak since no reaction had occurred (Fig. 3D). In chambers where the reaction took place, the bubbles generated by CO2 as a side of the reaction were easily visible, and spectral acquisitions displayed the characteristic multi-peak spectrum of pyruvate decarboxylation18 (Fig. 3E and F), where the following peaks were identified: [1-13C]pyruvate (176 ppm), [1-13C]pyruvate hydrate (184 ppm), [1-13C]2-hydroperoxy-2-hydroxypropanoate (181 ppm), [1-13C]peroxymonocarbonate (161 ppm), and 13CO2 (125 ppm). Parallel MRSI in the preclinical MRI scanner achieves high throughput at the expense of some spectral resolution, in comparison to say, a dedicated NMR spectrometer. The trade-off in resolution is, however, more than acceptable for hyperpolarized 13C MRSI where the resonances of metabolic products are dispersed over a wide chemical shift range. Additionally, the temporal evolution of the [1-13C]pyruvate signal was compared between different conditions by averaging the signal intensities from replicated chambers (Fig. 4). Dispersion among the three chambers per condition for the pyruvate peak were 23% and 18% at the first acquisition for chambers with and without the chemical reaction, respectively. At the time of the first acquisition (i.e., 25 s from dissolution), the pyruvate peak intensity from chambers with H2O2 was 2.5–3 lower than the signal from chambers containing non-oxidant solutions because of pyruvate's rapid consumption during the reaction (Fig. 4A). The pyruvate signal decay was dominated by the RF pulse polarization consumption, with an apparent decay time constant of 3.03 s in chambers with H2O2 and 2.96 s in chambers without reactant.
The observation that the relaxation time constant of [1-13C]pyruvate was similar (∼3 s) among conditions (i.e., chambers with and without H2O2) indicates that the resulting decay was exclusively due to the signal exponential decay by T1 and signal depletion by RF pulsing 64 times/acquisition. This also suggests that practically all the pyruvate had already been converted into the intermediate at the time of the first acquisition. In the chambers with H2O2, the signals from reaction subproducts decayed as a result of both the generation of the reactant and the T1 relaxation time constant of the hyperpolarized carbon nuclei (Fig. 4B). While over-crowded peaks in the downfield region (pyruvate, its hydrate and 2-hydroperoxy-2-hydroxypropanoate) could be detected above the noise level (i.e., SNR > 5) for up to 33 s after the injection of hyperpolarized pyruvate, the upfield peaks of the two final products (13CO2 and [1-13C]peroxymonocarbonate) were detectable for over a minute. The longer observation window for the products is probably attributed to the oxidation reaction, as the ongoing production of the final molecules counteracts the signal decay by T1. This could account for the slower decay rate of the two generated products (i.e., [1-13C]peroxymonocarbonate and 13CO2) respect to the consumed reactant and intermediate compound (i.e., [1-13C]pyruvate and [1-13C]2-hydroperoxy-2-hydroxypropanoate). Apparent decay rates were 0.28 ± 0.03 s−1 for [1-13C]pyruvate hydrate, 0.50 ± 0.05 s−1 for [1-13C]2-hydroperoxy-2-hydroxypropanoate, 0.15 ± 0.01 s−1 for [1-13C]peroxymonocarbonate, and 0.09 ± 0.01 s−1 for 13CO2. The time-normalized mass sensitivity is defined as
 | (2) |
The concentrations of all the species in the reaction were quantified considering a uniform 13C polarization decay (i.e., decay of the pyruvate signal in the well without H2O2) among all the species. Although the T1 values of the products are likely to be slightly different, this simplification enables to determine, approximately, their concentration when T1 is unknown. Within the time period where the SNR of the pyruvate was high (first 12s), we determined a plateau of the [1-13C]pyruvate concentration, a decrease of the intermediate product ([1-13C]2-hydroperoxy-2-hydroxypropanoate), and an increase of the final products ([1-13C]peroxymonocarbonate, and 13CO2).
To monitor fast reactions by HP-MR, the HP solution must mix with the reactant under study within seconds of reaching the science chambers. Our microfluidic device addressed this constraint by delivering the HP solution to each chamber in a cascade mode through eight channels (Fig. 1). For experiments where samples may need a convective flow for a proper mixing, we speculate that alternate infusion and withdrawal of the testing sample in the chambers using the additional channels included in our device would further enhance the mixing with the polarized sample.
Hyperpolarization-enhanced MR techniques are rapidly evolving and are expected to transform current analytical tools. Increasing their throughput will help overcome the barrier that prevents these techniques from being more accessible. This work showed that combining dDNP-MRSI and microfluidics can increase the capacity of identify products from chemical reactions and demonstrated that multiple samples can be simultaneously and non-invasively interrogated. Reaction kinetics and in situ metabolomic studies are potential applications of our platform beyond the proof-of-principle in this work.
Author contributions
J. Y. designed the microfluidic device and wrote the manuscript. M. A. designed and performed the experiments, analyzed the data, and wrote the manuscript. M. A. O., A. P., and G. M. designed, fabricated, and tested the microfluidic device. A. H. G. performed experiments and analyzed the data. Y. K., R. S., J. K., and D. B. V. conducted the MRI experiments. I. M. R. designed the experiments, raised funding, supervised the work, wrote, and reviewed the manuscript.Conflicts of interest
I. M. R. and M. A. O. are co-founders of Vitala Technologies S. L. IBEC has filed a patent application related to this work. M. A., J. Y., M. A. O., A. H. G. and I. M. R. are co-inventors of this patent application. The authors declare that they have no other competing interests.Acknowledgements
We thank the MicroFabSpace and Microscopy Characterization Facility, Unit 7 of ICTS “NANBIOSIS” from CIBER-BBN at IBEC. We also thank Dr. Xiao Ji (UCSF) and Christoffer Laustsen (Aarhus University, Denmark), Lotte Bonde Bertelsen (Aarhus University, Denmark), Nichlas Vous Christensen (Aarhus University, Denmark) for experimental assistance. This work has received funding from: The European Union's Horizon 2020 research and innovation program (GA-863037); the Spanish grants with reference PID2020-117859RA-I00 funded by MCIN/AEI/10.13039/501100011033 (NARMYD), RYC2020-029099-I funded by MCIN/AEI10.13039/501100011033 and by “ESF Investing in your future”, PLEC2022-009256 funded by MCIN/AEI/10.13039/501100011033 and by the European Union NextGenerationEU/PRTR, a grant within the framework Biotechnology Plan Applied to Health funded by MCIN/AEI10.13039/501100011033 and by European Union NextGenerationEU (PRTR-C17.I1); The BIST (Barcelona Institute of Science and Technology)-“la Caixa” Banking Foundation Chemical Biology programme; and the National Institutes of Health (NIH) (P41EB013598). A. H. G. received financial support through the FI Fellowship Programme from AGAUR (Ref. 2021 FI_B_01039).Notes and references
- J. H. Ardenkjær-Larsen, B. Fridlund and A. Gram, et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(18), 10158–10163 CrossRef PubMed.
- H. Zeng, Y. Lee and C. Hilty, Quantitative rate determination by dynamic nuclear polarization enhanced NMR of a diels-alder reaction, Anal. Chem., 2010, 82(21), 8897–8902 CrossRef CAS PubMed.
- H. Lees, M. Millan and F. Ahamed, et al. Multi-sample measurement of hyperpolarized pyruvate-to-lactate flux in melanoma cells, NMR Biomed., 2021, 34(3), e4447 CrossRef CAS PubMed.
- B. Patra, M. Sharma, W. Hale and M. Utz, Time-resolved non-invasive metabolomic monitoring of a single cancer spheroid by microfluidic NMR, Sci. Rep., 2021, 11(1), 1–11 CrossRef PubMed.
- G. Buntkowsky, F. Theiss and J. Lins, et al. Recent advances in the application of parahydrogen in catalysis and biochemistry, RSC Adv., 2022, 12(20), 12477–12506 RSC.
- J. Eills, E. Cavallari, C. Carrera, D. Budker, S. Aime and F. Reineri, Real-Time Nuclear Magnetic Resonance Detection of Fumarase Activity Using Parahydrogen-Hyperpolarized [1-13C]Fumarate, J. Am. Chem. Soc., 2019, 141(51), 20209–20214 CrossRef CAS PubMed.
- K. F. Sheberstov, L. Chuchkova and Y. Hu, et al. Photochemically Induced Dynamic Nuclear Polarization of Heteronuclear Singlet Order, J. Phys. Chem. Lett., 2021, 12(19), 4686–4691 CrossRef CAS PubMed.
- S. Jeong, R. Eskandari and S. M. Park, et al. Real-time quantitative analysis of metabolic flux in live cells using a hyperpolarized micromagnetic resonance spectrometer, Sci. Adv., 2017, 3(6), e1700341 CrossRef PubMed.
- K. R. Keshari and D. M. Wilson, Chemistry and biochemistry of 13C hyperpolarized magnetic resonance using dynamic nuclear polarization, Chem. Soc. Rev., 2014, 43(5), 1627–1659 RSC.
- Home – ClinicalTrials.gov, Accessed January 9, 2023, https://clinicaltrials.gov/.
- M. Vaeggemose, R. F. Schulte and C. Laustsen, Comprehensive Literature Review of Hyperpolarized Carbon-13 MRI: The Road to Clinical Application, Metabolites, 2021, 11(4), 219 CrossRef CAS PubMed.
- S. R. Chaudhari, D. Wisser and A. C. Pinon, et al. Dynamic Nuclear Polarization Efficiency Increased by Very Fast Magic Angle Spinning, J. Am. Chem. Soc., 2017, 139(31), 10609–10612 CrossRef CAS PubMed.
- S. J. Elliott, O. Cala and Q. Stern, et al. Boosting dissolution-dynamic nuclear polarization by multiple-step dipolar order mediated 1H→13C cross-polarization, J. Magn. Reson. Open, 2021, 8–9, 100018 CrossRef.
- M. Krajewski, P. Wespi and J. Busch, et al. A multisample dissolution dynamic nuclear polarization system for serial injections in small animals, Magn. Reson. Med., 2017, 77(2), 904–910 CrossRef PubMed.
- Y. Su, L. Andreas and R. G. Griffin, Magic Angle Spinning NMR of Proteins: High-Frequency Dynamic Nuclear Polarization and 1H Detection, Annu. Rev. Biochem., 2015, 84, 465–497 CrossRef CAS PubMed.
- I. Marco-Rius, T. Cheng and A. P. Gaunt, et al. Photogenerated Radical in Phenylglyoxylic Acid for in Vivo Hyperpolarized 13C MR with Photosensitive Metabolic Substrates, J. Am. Chem. Soc., 2018, 140(43), 14455–14463 CrossRef CAS PubMed.
- A. P. Gaunt, J. S. Lewis and F. Hesse, et al. Labile Photo-Induced Free Radical in α-Ketoglutaric Acid: a Universal Endogenous Polarizing Agent for In Vivo Hyperpolarized 13C Magnetic Resonance, Angew. Chem., 2022, 61(2), e202112982 CrossRef CAS PubMed.
- A. Capozzi, Design and performance of a small bath cryostat with NMR capability for transport of hyperpolarized samples, Sci. Rep., 2022, 12(1), 1–16 CrossRef PubMed.
- T. El Daraï, S. F. Cousin and Q. Stern, et al. Porous functionalized polymers enable generating and transporting hyperpolarized mixtures of metabolites, Nat. Commun., 2021, 12(1), 1–9 CrossRef PubMed.
- I. Marco-Rius, M. C. D. Tayler and M. I. Kettunen, et al. Hyperpolarized singlet lifetimes of pyruvate in human blood and in the mouse, NMR Biomed., 2013, 26(12), 1696–1704 CrossRef CAS PubMed.
- S. Ros, A. J. Wright, A. Bruna, C. Caldas and K. M. Brindle, Metabolic imaging with hyperpolarized [1-13C]pyruvate in patient-derived preclinical mouse models of breast cancer, STAR Protoc., 2021, 2(3), 100608 CrossRef CAS PubMed.
- J. Eills, W. Hale, M. Sharma, M. Rossetto, M. H. Levitt and M. Utz, High-Resolution Nuclear Magnetic Resonance Spectroscopy with Picomole Sensitivity by Hyperpolarization on a Chip, J. Am. Chem. Soc., 2019, 141(25), 9955–9963 CrossRef CAS PubMed.
- J. Lee, M. S. Ramirez and C. M. Walker, et al. High-throughput hyperpolarized 13C metabolic investigations using a multi-channel acquisition system, J. Magn. Reson., 2015, 260, 20–27 CrossRef CAS PubMed.
- Y. Kim, M. Liu and C. Hilty, Determination of binding affinities using hyperpolarized NMR with simultaneous 4-channel detection, J. Magn. Reson., 2018, 295, 80–86 CrossRef CAS PubMed.
- S. Hu, P. E. Z. Larson and M. VanCriekinge, et al. Rapid sequential injections of hyperpolarized [1-13C]pyruvate in vivo using a sub-kelvin, multi-sample DNP polarizer, Magn. Reson. Imaging, 2013, 31(4), 490–496 CrossRef PubMed.
- N. Drachman, S. Kadlecek, I. Duncan and R. Rizi, Quantifying reaction kinetics of the non-enzymatic decarboxylation of pyruvate and production of peroxymonocarbonate with hyperpolarized 13C-NMR, Phys. Chem. Chem. Phys., 2017, 19(29), 19316–19325 RSC.
- D. Qin, Y. Xia and G. M. Whitesides, Soft lithography for micro- and nanoscale patterning, Nat. Protoc., 2010, 5(3), 491–502 CrossRef CAS PubMed.
- J. C. Crane, M. P. Olson and S. J. Nelson, SIVIC: Open-Source, Standards-Based Software for DICOM MR Spectroscopy Workflows, Int. J. Biomed. Imaging, 2013, 2013, e169526 Search PubMed.
- J. Friend and L. Yeo, Fabrication of microfluidic devices using polydimethylsiloxane, Biomicrofluidics, 2010, 4(2), 026502 CrossRef PubMed.
- N. Chattergoon, F. Martínez-Santiesteban, W. B. Handler, J. H. Ardenkjær-Larsen and T. J. Scholl, Field dependence of T1 for hyperpolarized [1-13C]pyruvate, Contrast Media Mol. Imaging, 2013, 8(1), 57–62 CrossRef CAS PubMed.
- V. A. Guarino, W. M. Oldham, J. Loscalzo and Y. Y. Zhang, Reaction rate of pyruvate and hydrogen peroxide: assessing antioxidant capacity of pyruvate under biological conditions, Sci. Rep., 2019, 9(1), 1–9 CrossRef PubMed.
- R. T. Mallet, J. E. Squires, S. Bhatia and J. Sun, Pyruvate restores contractile function and antioxidant defenses of hydrogen peroxide-challenged myocardium, J. Mol. Cell. Cardiol., 2002, 34(9), 1173–1184 CrossRef CAS PubMed.
- S. Desagher, J. Glowinski and J. Prémont, Pyruvate Protects Neurons against Hydrogen Peroxide-Induced Toxicity, J. Neurosci., 1997, 17(23), 9060–9067 CrossRef CAS PubMed.
- J. H. Ardenkjaer-Larsen, A. M. Leach, N. Clarke, J. Urbahn, D. Anderson and T. W. Skloss, Dynamic nuclear polarization polarizer for sterile use intent, NMR Biomed., 2011, 24(8), 927–932 CrossRef CAS PubMed.
- M. E. Lacey, R. Subramanian, D. L. Olson, A. G. Webb and J. V. Sweedler, High-Resolution NMR Spectroscopy of Sample Volumes from 1 nL to 10 μL, Chem. Rev., 1999, 99(10), 3133–3152 CrossRef CAS PubMed.
- D. L. Olson, T. L. Peck, A. G. Webb, R. L. Magin and J. V. Sweedler, High-Resolution Microcoil 1H-NMR for Mass-Limited, Nanoliter-Volume Samples, Science, 1995, 270(5244), 1967–1970 CrossRef CAS.
- M. Mompeán, R. M. Sánchez-Donoso, A. De La Hoz, V. Saggiomo, A. H. Velders and M. V. Gomez, Pushing nuclear magnetic resonance sensitivity limits with microfluidics and photo-chemically induced dynamic nuclear polarization, Nat. Commun., 2018, 9(1), 1–8 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3lc00474k |
| ‡ Authors contributed equally to this work |
| This journal is © The Royal Society of Chemistry 2023 |