Open Access Article
Open Access ArticleMatrix effects in simultaneous microwave induced plasma optical emission spectrometry: new perspectives on an old problem†
Franz
Hallwirth
a,
Matthias
Wolfgang
ab and
Helmar
Wiltsche
 *a
*a
aGraz University of Technology, Institute of Analytical Chemistry and Food Chemistry, Graz, Austria. E-mail: helmar.wiltsche@tugraz.at
bResearch Center Pharmaceutical Engineering GmbH, Graz, Austria
First published on 29th June 2023
Abstract
Easily ionizable elements (EIEs) such as alkaline or alkaline earth elements are well known to cause matrix effects on analytically used plasma sources in general and on microwave-induced plasmas (MIP) in particular. We investigated the matrix effects of 18 major matrix elements on 105 emission lines of 42 elements using an axially viewed microwave-sustained, inductively coupled, atmospheric-pressure plasma (MICAP) attached to a simultaneous spectrometer for optical emission spectrometry (MICAP-OES). In contrast to many previous studies, we did not adjust the nebulizer gas flow or axial observation spot for every emission line but used compromise conditions for simultaneously recording all investigated lines. The obtained data clearly show that inter-element matrix effects were encountered not only for g L−1 concentrations of matrix elements as reported in the literature but also as low as 20 mg L−1 with signal suppression of up to 25% on particularly affected emission lines. The magnitude of the matrix effects was not similar for all investigated matrix elements but particularly pronounced for the alkaline elements. The matrix effect of the alkaline elements was found to decrease in the order Li > Na > K > Cs for the same concentration (mg L−1). However, if equimolar amounts (mmol L−1) of alkaline elements were introduced into the MIP, the matrix effect on concomitant elements was comparable in magnitude. This indicates that the absolute number of atoms in the plasma is responsible for this effect rather than the ionization energy of the respective element. Neither optimizing microwave power (range 1.1 to 1.5 kW) nor nebulizer flow (0.5–1.1 L min−1) effectively diminished the encountered matrix effects in MICAP-OES.
Introduction
Line-dependent analyte signal suppression/enhancement by concomitant matrix elements is generally referred to as matrix effects. Flame atomic absorption spectrometry (FAAS) is more susceptible to such effects, though the magnitude varies from element to element.1 The inductively coupled plasma optical emission spectrometry (ICP-OES), on the other hand, is comparatively unaffected by matrix effects. Next to the considerably lower limits of detection (LODs), this might be one of the main reasons for its widespread use today. Still, under heavy loading of the ICP with a sample matrix containing easily ionizable elements (EIEs; elements with an ionization potential <5.5 eV)2–4 or carbon,5–8 an emission line-dependent signal suppression/enhancement in comparison with matrix-free solutions is encountered.The third plasma source used in routine laboratories for liquid analysis is the microwave-induced plasma (MIP). In terms of limits of detection, microwave-induced plasma-optical emission spectrometry (MIP-OES) is superior to FAAS for most elements but inferior to ICP-OES. This also holds true for matrix effects, where MIP-OES appears to be less affected than FAAS but not as resilient as ICP-OES.
In the past, several working groups investigated matrix effects in MIP-OES for low to medium-power MIPs. For a medium power (300–500 W) MIP-OES, Urh and Carnahan9 investigated the effect of Na, K, and Ca at concentrations ranging from 100 mg L−1 to 20 g L−1 on several atom lines. 100 mg L−1 of each matrix element caused minor matrix effects (<20% signal change). In contrast, high concentrations of matrix elements (20 g L−1) were found to enhance the signal of atom lines with an excitation energy <4.3 eV (Pb I 405.781 nm) and an ionization potential <7.5 eV of the corresponding element. On the other hand, signal suppression was encountered for elements with an excitation potential >3.9 eV and ionization energy of the corresponding element >7.5 eV.
Jankowski and Dreger10 investigated the matrix effects of EIEs, particularly Na, on the most sensitive emission line of 35 analyte elements using a low-power (130 W) MIP operated with Ar as the plasma gas. For most emission lines, signal enhancement caused by the matrix effect of 1 g L−1 Na was encountered, while only for atom lines of B, V, Mo and ion lines of Ti and Zr signal suppression was encountered. The authors conclude that the differences in the matrix-induced signal enhancement are rooted in several competing mechanisms, such as radiative energy transfer and changes in the ionization equilibrium.
Matousek, Orr and Selby11 investigated the effect of EIEs on analyte emission lines using a low power (75 W, Beenakker type) MIP with He as the plasma gas. The samples were introduced into the plasma discharge by means of electrothermal vaporization. The authors provide a detailed discussion on possible mechanisms of EIE interference, though their results relate to a dry plasma and a separation of volatilization of the matrix and analyte in time by the graphite furnace.
Today, high-power (>1 kW) MIP-OES instruments using nitrogen as the plasma gas are commercially available. Using an Okamoto-cavity based MIP-OES, Zhang and Wagatsuma12 were among the first to show what appears to be a general trend regarding matrix effects in high-power MIPs. They found that most atomic lines were enhanced, while ion lines showed signal suppression in the presence of 5 g L−1 Na or Ca. Atom lines that were not enhanced or suppressed had an excitation energy above about 5 eV. Similar observations were made by Serrano et al.13 using a Hammer-cavity MIP. These authors showed that matrix effects in MIP-OES were caused particularly by the alkaline and alkaline earth elements. Emission lines with excitation energies below about 3.3 eV experienced significant signal enhancement, while above about 3.8 eV total line energy (excitation energy plus ionization energy), a signal suppression was encountered.13,14
To mitigate matrix effects in MIPs, several authors investigated the line-specific adjustment of the nebulizer gas flow and changes to the axial observation zone (central channel vs. rim region of the axially viewed MIP).13–16 While this approach is acceptable for MIP-OES instruments with sequential spectrometers, such a procedure is not feasible for simultaneous multi-element analysis. Using Rh atom lines or molecular band heads of N2+ or OH as internal standards was also investigated and considered an approach to compensate for the matrix effect of EIEs on selected analyte emission lines.13 The OH molecular emission band at 308.970 nm and the N2+ at 391.439 nm are inherent species in the MIP that can be used to correct the analyte emission signal. This correction can be either multiplicative in the form A × X (A: analyte; X: N2+or OH) or as a divisor A/X for the analyte emission signal.17
Other, more apparent approaches reported in the literature were high sample dilution18 and matrix-matched calibration.13,19 However, high sample dilution will compromise the attainable limits of detection (LODs), while matrix matching requires prior knowledge of the approximate sample composition.
An interesting approach for defying matrix effects by EIEs is reported by Karlsson et al.20 for the analysis of plant material digests with high concentrations (about 100 mg L−1) of K and Ca. Also, these authors encountered systematic low values for K, Na, Ca and Mg, despite using internal standards (La, Lu, Y) and related these problems to ionization suppression. They investigated the addition of CsNO3 in the range of 1 to 2.5 g L−1 as an ionization buffer, much as in FAAS. Though this procedure caused signal suppression for some analytes, the overall effect improved the agreement between MIP-OES and the employed analytical reference technique, inductively coupled plasma mass spectrometry (ICP-MS).
The aim of this work is to investigate the matrix effects induced by main sample constituents of biological, geological or metallurgical samples on a series of analyte emission lines analyzed by MIP-OES. In this context, we also investigated matrix effects on emission lines of elements typically used as internal standards, such as Sc, Y, and Rh. As internal standards are commonly employed to compensate for instrument drift and dilution errors, any matrix effect on these elements will introduce further bias to the final result if the response to the sample matrix differs for the analyte and internal standard. In contrast to previous attempts in the literature, we do not aim at optimizing the plasma conditions, observation position or internal standards for each emission line individually. In stark contrast, we intentionally apply compromise conditions as is typical in today's routine analysis by simultaneous ICP-OES.
Experimental
Instrumentation
A microwave-sustained, inductively coupled, atmospheric-pressure plasma (MICAP) source21 was coupled to the simultaneous spectrometer of a commercial ICP-OES instrument (Ciros Vision EOP, Spectro, Germany), as described in our previous work.22,23 The microwave energy was provided by a 2M262A magnetron (1.5 kW at 2.45 GHz, Panasonic, Japan) powered by a Magdrive 2000 (Dipolar AB, Sweden) high-voltage power supply. A conventional one-piece ICP-OES torch with a wide-bore 2.5 mm injector (for Ciros Vision EOP, Spectro, Germany) was used throughout. The sample introduction system comprised of a standard baffled cyclonic spray chamber (Glass Expansion, Australia) and a concentric glass nebulizer (Type A, Meinhard, USA). Sample solutions were fed at a flow rate of 1 mL min−1 to the nebulizer using a peristaltic pump. If not stated differently, the standard operating conditions used throughout all experiments were 1500 W microwave power, 16 L min−1 outer gas flow, 0.6 L min−1 intermediate gas flow, 0.85 L min−1 nebulizer gas flow, 24 s detector integration time per replicate and five individual detector readings (replicates). The nebulizer gas flow was optimized based on the emission intensity of a 1 mg L−1 Mn solution for the Mn(II) 257.611 nm emission line. The maximum signal intensity was obtained for a nebulizer gas flow rate of 0.85 L min−1. Further details on optimizing the plasma conditions can be found in a previous publication.22Reagents
Purified water (18 MΩ cm, Barnstead Nanopure, Thermo Fisher Scientific, USA) and high-purity nitric acid (Fisher Scientific GmbH, Germany, purified by subboiling) were used throughout. Standard solutions were prepared using 1 g L−1 single-element stock solutions (As, B, Ba, Be, Bi, Ca, Cd, Ce, Co, Cr, Cu, Fe, Gd, Hf, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Ni, Pb, Pd, Pt, Rh, Sb, Sc, Se, Sr, Ta, Tb, Ti, Tm, V, W, Y, Zn, and Zr; Roth, Germany). Matrix effects were investigated using 10 g L−1 single-element stock solutions (Al, B, Ba, Ca, Co, Cr, Cs, Cu, Fe, K, Li, Mg, Mn, Na, Ni, Ti, V, Zn, Alfa Aesar, Germany) after dilution with 3% HNO3 (v/v).Nitrogen (5.0 quality, boil-off from a liquid nitrogen storage tank, Linde, Austria) was used as plasma gas.
Experimental procedure
The starting point of our investigation was the comparison of two multi-elemental calibrations covering the concentration range of 0–50 mg L−1 with eight standards each. Every solution contained several elements (Al, As, B, Bi, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Sb, Ti, Tl, V, and Zn) at the same concentration. However, one calibration series contained the alkaline elements Li, Na, and K, and the alkaline earth elements Mg, Ca, Sr, and Ba at the same concentration level as the other elements. Both calibration series contained Sc and Y as potential internal standards at a constant concentration of 1 mg L−1.To investigate the isolated matrix effects of Al, B, Co, Cr, Cu, Fe, Mn, Ni, Ti, V, Zn as well as the EIEs Li, Na, K, Cs, Mg, Ca, and Ba, a separate series of experiments was conducted. These “matrix elements” were selected based on the fact that they are present in high concentration in biological (Ca, Mg, Na, K), geological (Al, Ca, Mg) and metallurgical (Al, Co, Cu, Fe, Mn, Ni, Ti, V, Zn) samples. B was added to the matrix element list as boric acid is often used in large quantities in two-step microwave-assisted digestions using hydrofluoric acid as a reagent.24 Each matrix element's signal suppression/enhancement effect on 42 “analyte elements” was investigated for 105 emission lines. The analyte elements comprised of Al, As, B, Ba, Be, Bi, Ca, Cd, Ce, Co, Cr, Cu, Fe, Gd, Hf, K, La, Li, Lu, Mg, Mn, Mo, Na, Nb, Ni, Pb, Pd, Pt, Rh, Sb, Sc, Se, Sr, Ta, Tb, Ti, Tm, V, W, Y, Zn, and Zr. It should be noted that Sc and Y were selected as they are commonly used internal standards in ICP-OES. Rh was added to the list of potential internal standards based on the investigations of Serrano et al.13 and Ce, Gd, La, Pd, Pt, Tb, Tm and Lu, as they might be useful for this purpose, too. The following procedure for obtaining signal suppression/enhancement data was employed: firstly, a spectrum of a multi-element solution containing 1 mg L−1 of “analyte elements” in diluted nitric acid (3% v/v) was recorded. Then, a spectrum of a multi-element solution containing 1 mg L−1 of the analytes mentioned above and 50 mg L−1 of a single “matrix element” was acquired. Finally, spectra of 50 mg L−1 of each matrix element were recorded to correct for potential contamination of the matrix element stock solution with one of the analytes. Suppression/enhancement data were calculated by normalizing the background-corrected analyte emission signals for every matrix-containing multi-element solution to the analyte signals obtained in the matrix-free solution (diluted nitric acid). This resulted in 18 individual experiments.
Results and discussion
Inter-element matrix effects in multi-element calibration functions
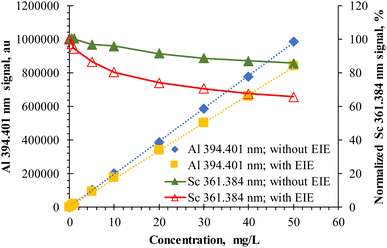
Routine ICP-OES analysis commonly uses multi-element stock solutions to prepare the calibration standards. As all modern instruments employ simultaneous spectrometers, the full spectral information can be utilized for sample analysis. Having a MICAP coupled to a simultaneous spectrometer, we took the same approach.The starting point of our investigation was a closer inspection of such multi-elemental calibration in the concentration range of 0–50 mg L−1. Fig. 1 exemplarily compares two aluminium calibration functions with the simultaneously recorded internal standard signal (Sc). In both cases, the calibration solutions contained several elements (Al, As, B, Bi, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Sb, Ti, Tl, V, and Zn) to mimic a commercial multi-element stock solution (e.g., Merck IV, #111355, Merck, Germany). The difference between these two calibration functions was that one calibration series also contained the alkaline elements Li, Na, K and the alkaline earth elements Mg, Ca, Sr, and Ba at the same concentration level as the other elements. The highest concentration of 50 mg L−1 was selected due to the higher LODs typical of MIP-OES compared to ICP-OES.22
Element and emission line-specific signal changes between the two calibration series were observed. At the highest calibration standard (50 mg L−1), the Al I 394.401 nm signal obtained from the calibration standard containing the EIEs was about 15% lower than the calibration standard without EIEs. Such suppression is surprising in two ways: firstly, the excitation energy of this Al emission line is only 3.1 eV. For this energy, Zhang and Wagatsuma12 encountered slight signal enhancement when Na and Ca were present as matrix elements, even though the concentration of Na or Ca was two orders of magnitude higher than in the present case. Secondly, matrix effects caused by EIEs are reported in the literature for concentrations in the g L−1 range and not as low as 50 mg L−1. It is important to note that the observed effect of EIEs on the Al I 394.401 nm line is a combined inter-element matrix effect of 50 mg L−1 of Li, Na, K, Mg, Ca, Sr, and Ba. Though not plotted for clarity in Fig. 1, calibration functions of all analytes (As, B, Bi, Cd, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, Sb, Ti, Tl, V, and Zn) were recorded together with Al in this calibration experiment. Element and emission line-dependent differences in the effect of EIEs on the slope of each analyte calibration function were encountered. These changes in the calibration function slope were reflected by the behavior of the highest calibration standard. Interestingly, the matrix effect caused by 50 mg L−1 Li on the analytes, listed in ESI Table S1† and discussed later, matches the suppression/enhancement of the highest calibration standard very well, which is within less than 8%. Li showed the largest matrix effect of all EIEs at the given concentration. However, the combined effect of 50 mg L−1 of all EIEs was larger than the sole effect of Li.
An even higher signal suppression was observed for the potential internal standard Sc and Y, present in all solutions at the same concentration of 1 mg L−1: in the presence of an additional 50 mg L−1 of each alkaline/alkaline earth element, the Sc(II) 361.384 nm signal was suppressed by 34% with respect to the blank. Interestingly, a similar trend has been observed for the Y ion lines Y(II) 360.073 nm and Y(II) 371.030 nm with a total line energy of 9.8 eV and 9.7 eV, respectively. In contrast, the Rh(I) 343.489 nm signal with an excitation energy of 3.6 eV showed no change in signal intensity. Based on these observations, it can be concluded that the presence of EIEs also induces considerable signal changes to the potential internal standards Sc and Y.
Furthermore, concomitant analytes in the calibration solution altered the signal of Sc and Y. When comparing the Sc signal of the blank solution with the Sc signal of the 50 mg L−1 calibration standard (without EIEs added), some 14% Sc signal suppression was encountered. This indicates that not only EIEs cause matrix effects on the ion lines of Sc and Y.
Three conclusions can be drawn from these results: firstly, the matrix effects of EIE start at significantly lower concentrations than anticipated by the literature. Secondly, these matrix effects seem to particularly affect Sc and Y ion lines. Though these elements are commonly used as internal standards in ICP-OES, their suitability as internal standards for MIP-OES requires a closer assessment. Thirdly, matrix effects in MIPs are not limited to alkaline and alkaline earth elements.
Effect of matrix elements on the analyte emission signal intensity
In the second step, matrix-induced signal suppression/enhancement of single matrix elements was investigated. A matrix effect is considered significant if a reduction or enhancement of the background corrected, blank subtracted emission signal of an analyte emission line by more than 10% is present when compared to diluted (3% v/v) nitric acid as a “matrix-free” sample solution.ESI Table S1† lists the effect of 18 matrix elements at a concentration of 50 mg L−1 on 105 analyte emission lines of 42 elements. Overall, 53% of all 105 investigated emission lines showed a signal suppression by more than 10% due to the presence of just 50 mg L−1 of at least one matrix element. Significant differences in the magnitude of the matrix effect of individual matrix elements were encountered, with the EIEs Li, Na, K, Cs, Mg, Ca, Ba and Al being dominantly responsible for signal suppression. Interestingly, only the emission intensity of K I 766.491 nm was enhanced by more than 10% by these EIEs. In contrast to the investigation of Serrano et al.,13,14 who encountered signal enhancement for emission lines with a total line energy <4 eV, no such dependence was found.
The difference between individual EIEs in terms of their impact on other elements' emission lines is striking: while Li suppressed the signal of 53% of all investigated emission lines, only 6% of these lines were affected by the same concentration of Cs. Interestingly, the magnitude of suppression decreases in the order Li > Na > K > Cs. No analogous behavior was observed for alkaline earth elements. For these elements, the suppression by Ca was strongest (7% of all investigated emission lines) and no trend concerning the atomic number or ionization energy was encountered when comparing the effect based on the same concentration in mg L−1. It is shown below in the section “Matrix effect of alkaline elements” that based on equimolar amounts of matrix elements, the suppression caused by the alkaline elements is similar for all alkaline elements. Referring to the alkaline earth elements, the lack of a trend of suppression as a function of the ionization energy of each element of this group might be linked to the fact that alkaline earth elements were investigated only for similar concentrations and not for equimolar amounts. In the latter case, the differences in ionization energy (9.3 eV for Be decreasing to 5.2 eV for Ba) might be relevant.
With an ionization energy that is even lower than that of Ca (5.9 eV and 6.1 eV, respectively), Al also belongs to the group of EIEs.25 Also, the matrix effect of Al on other elements and their emission lines in the microwave plasma is comparable to Ca: nearly 9% of all investigated emission lines were suppressed by 50 mg L−1 Al.
While elements with an ionization potential <6.1 eV (Ca) in general caused significant signal suppression of the investigated emission lines, matrix elements above this threshold affect the MIP to a minor extent. The latter group of matrix elements comprises B, Co, Cr, Cu, Fe, Mg, Mn, Ni, Ti, V, and Zn, with 1 to 4% of all investigated emission lines being suppressed by them.
Another aspect, which can be derived from ESI Table S1,† is that atom and ion lines of the same element behave similarly regarding suppression/enhancement with respect to the matrix elements. This statement holds true for Be, Cd, Co, Cr, Mg, Mn, and Zn. These observations deviate from literature reports such as from Karlsson et al.20 These authors explicitly recommend using atom lines over ion lines to minimize the impact of matrix effects on the measurement. The used experimental conditions might explain this discrepancy: while in our work, due to the simultaneous spectrometer, the same nebulizer gas flow and the same axial plasma observation position were used, Karlsson et al. optimized both parameters for every emission line as these authors used an instrument with a sequential spectrometer. While the optimization criterion used by Karlsson et al. is not explicitly stated (“the viewing angle and the nebulizer pressure were optimized before each analytical sequence by the software”), we assume, based on our experience with a similar instrument, that the instrument software optimizes for maximum signal to root background ratio as noted by Niedzielski et al.26 However, changing the nebulizer gas flow rate will affect the sample amount and the mass flow of the matrix introduced into the MIP. Thereby, individual emission lines will be recorded under different plasma conditions and are therefore not comparable. The same holds true for optimizing the plasma observation position. When moving the focus point of the spectrometer away from the center of the discharge towards the rim region, zones of different plasma temperatures and electron number density will be observed. This will make the comparison of matrix-induced effects on different emission lines problematic. In stark contrast, our data were indeed recorded under the same plasma conditions and using the same plasma observation spot for every line, allowing a direct comparison between atom and ion lines.
Optimizing the MIP for the lowest matrix effect for every emission line by line-specific settings for nebulizer gas flow, microwave power or axial observation spot might be possible. However, in this case, the optimization criterion should be the lowest signal change when switching from a matrix-free analyte solution to a matrix-bearing analyte solution rather than striving for the maximum signal intensity or signal-to-background ratio. However, by optimizing the measurement conditions this way, the advantages of a simultaneous spectrometer in terms of shorter analysis time for many analytes will be lost.
From ESI Table S1,† it is apparent that the matrix effect of Li on the analyte emission lines is the largest for all investigated matrix elements. An overview of lithium's matrix effect on all investigated emission lines is shown in Fig. 2. Once more, it becomes apparent that the matrix effect of Li is neither limited to nor particularly pronounced for ion lines but occurs irrespectively of the ionization state. While there is little correlation between the excitation energy of atom lines with the magnitude of lithium's matrix effect, the strongest signal suppression has been observed for low total line energy ion lines. Above about 14 eV total line energy, the signal suppression caused by Li was <5%. It is interesting to note that a comparable correlation of signal enhancement/suppression with the upper-level energy of the transitions was encountered. Using the comprehensive dataset published by Serrano et al.,27 a similar plot as shown in Fig. 2 was obtained.
 | ||
| Fig. 2 Effect of 50 mg L−1 Li on the normalized emission line intensity of 42 elements (105 emission lines) as a function of the total line energy (atom lines: excitation energy; ion lines: ionization energy + excitation energy). Please note that the underlying dataset plotted in this graph is listed in ESI Table S1.† Elements of particular interest have been highlighted with the element symbol, but the wavelength was omitted for clarity. RSD <2%; n = 5; the light green shaded rectangle illustrates the ±10% enhancement/suppression region. | ||
It is important to note that comparable results, as shown in Fig. 2, were obtained by Pelipasov and Polyakova15 for a dielectric resonator cavity MIP-OES and 1 g L−1 Na as a matrix element. These authors encountered either no change in the emission signal or suppression for all investigated emission lines at this concentration. This is in good agreement with our data. It should also be noted that Pelipasov and Polyakova did not investigate the effect of Na on K, whereby no information is available for the enhancement of K by other alkaline elements as we have encountered. Interestingly, for 5 g L−1 and 10 g L−1 Na, Pelipasov and Polyakova discovered significant enhancement of atom lines below about 4.5 eV and strong suppression of all ion lines. Clearly, the amount of matrix introduced into the MIP caused a change in the behavior of low energy atom lines: at lower matrix concentration (∼1 g L−1), these lines are suppressed. In contrast, above this concentration, signal enhancement occurs. Pelipasov and Polyakova concluded that Na causes an increase of the electron number density while simultaneously the excitation temperature decreases. Thereby, a shift in the atom–ion ionization equilibrium is caused. Thaler et al.28 investigated the matrix effect of Na on several elements using MICAP-OES in radial plasma viewing. For about 1.5 g L−1 Na, these authors encountered significant signal enhancements for all alkaline elements. Though this is also in agreement with our data, care should be exercised when comparing emission signals recorded in axial viewing with radially recorded data. Extrapolating from ICP-OES, data obtained in radial viewing can be expected to be less affected by matrix effects than those obtained in axial viewing.
Matrix effects on potential internal standards
The large variability in the line-dependent signal suppression, as shown in Fig. 2, implies that defying matrix effects by using internal standards is delicate, at best. This becomes obvious when considering the impact of Li on typical internal standards such as Rh, Sc and Y. ESI Table S1† shows that while the signal of the Rh atom line is not affected appreciably by either Li or any other investigated matrix element at a matrix concentration of 50 mg L−1, Li suppressed all Sc and Y ion lines by 24–29%.While the absence of a pronounced matrix effect for the Rh atom line has already been reported by Serrano et al.,13 a similar behavior was encountered for Pd and Pt atom lines. All three platinum group elements were only modestly (<6%) affected by all investigated matrix elements. To this group of “matrix tolerant” elements B, Be and Hf can be added. In the case of Be, not only did the atom line Be I 234.861 nm show little signal change due to the presence of matrix elements but also the Be II 313.042 nm ion line. This in turn emphasizes that atom lines are not necessarily less susceptible to matrix effects in an MIP than ion lines.
In contrast to Rh, all investigated rare earth elements (Ce, Gd, La, Lu, Tb, Tm) and Sc and Y were significantly affected by EIEs, particularly by Li. On average, these elements were suppressed by 20% due to the presence of just 50 mg L−1 Li as a matrix element.
It can be concluded that any attempt to compensate matrix effects in the MIP by internal standards will require meticulous line selection to match the matrix-induced suppression/enhancement behavior of each analyte emission line to an internal standard. Thereby, the application of internal standards in simultaneous multi-elemental analysis by MIP-OES to samples containing appreciable amounts of EIE can be expected to be very difficult at best.
Matrix effect of alkaline elements
As the investigation of matrix effects in the MICAP-OES using a set of 42 elements is rather time-consuming in the data processing step and expensive due to the consumption of large quantities of single-element solutions, the set of elements was reduced in further experiments. Due to the dominant matrix effect, Li was selected as the matrix element. Sc, Y and Rh were chosen as analytes due to their very different behavior regarding the sample matrix. These three elements represent the extreme values in ESI Table S1:† while the effect of Li on the emission intensity of Rh was small, strong suppression was encountered for Sc and Y.As noted, the enhancement/suppression data listed in ESI Table S1† were recorded for a constant matrix concentration of 50 mg L−1. When repeating these measurements with equimolar amounts of the alkaline elements (7.2 mM of each element resulting in 50 mg L−1 Li, 165 mg L−1 Na, 280 mg L−1 K, 960 mg L−1 Cs), a constant, matrix element independent behavior of the investigated emission lines was encountered. As listed in Table 1, the Rh atom line was again not affected by equimolar amounts of alkaline elements. However, the suppression of ion lines of Sc and Y were similar in magnitude irrespective of the matrix element. Though the ionization potential of Cs is significantly lower than that of Li (3.9 eV vs. 5.4 eV), the absolute number of alkaline atoms entering the plasma appears to be the dominant factor for the matrix effect on the Sc and Y ion lines. Due to the vastly lower atomic weight of Li compared to Cs, the same concentration of Li in mass per volume causes a considerably larger matrix effect than Cs.
| Emission line | 0.72 mM | 0.72 mM | 0.72 mM | 0.72 mM |
|---|---|---|---|---|
| Li | Na | K | Cs | |
| Rh I 343.489 nm | 96 | 97 | 97 | 100 |
| Sc II 361.384 nm | 77 | 78 | 78 | 79 |
| Sc II 424.683 nm | 76 | 77 | 76 | 76 |
| Y II 360.073 nm | 72 | 72 | 71 | 71 |
| Y II 371.030 nm | 70 | 69 | 68 | 68 |
| Y II 377.433 nm | 69 | 68 | 68 | 67 |
Karlsson et al.20 proposed, similar to flame atomic absorption, the use of CsNO3 at a concentration of about 1 g L−1 to intentionally cause an “overwhelming” matrix effect that vastly exceeds the matrix effect caused by actual sample constituents. When the standards used for external calibration and all samples contain the same concentration of CsNO3, the matrix effect is similar for calibration and samples, resulting in accurate results even in the presence of matrix effects. However, as nearly 20 times more Cs is necessary to reach the same signal suppression as with Li, Li would be better suited for this purpose, as high total dissolved solids can cause salt deposition on the injector and early devitrification of the plasma torch. Moreover, when adding CsNO3 to a final concentration of 1 g L−1, the purity requirements on this salt are very high to avoid contamination.
Effect of plasma power and nebulizer gas flow on the matrix effect of Li
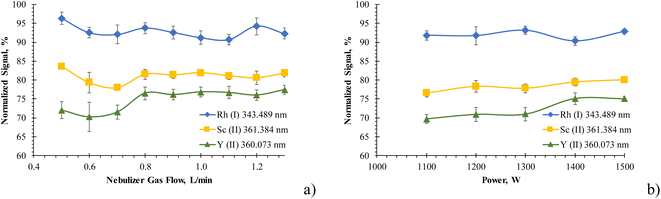
Several authors26,29–31 optimized the nebulizer gas flow for every emission line before the actual sample analysis with a commercial Hammer-cavity MIP-OES. Consequently, the effects of nebulizer gas flow and plasma power were investigated on the signal suppression caused by Li on Rh, Sc, and Y. Again, signal suppression/enhancement data were obtained by normalizing the emission signal of selected lines of Rh, Sc, and Y (1 mg L−1 each) recorded in the presence of 50 ml L−1 Li to the analyte signal obtained under the same plasma conditions in diluted (3% v/v) nitric acid.Somewhat surprisingly, altering the nebulizer gas flow (0.5–1.1 L min−1) or the plasma power (1.1–1.5 kW) changed the magnitude of lithium's matrix effect on Rh, Sc and Y only to a small extent, as shown in Fig. 3. When increasing the nebulizer gas flow from 0.6 L min−1 to the initially optimized flow of 0.85 L min−1, the absolute emission line intensity increased by about a factor of two. In contrast, the signal suppression by Li did not significantly change for the investigated Rh atom lines and the two Sc ion lines. Only for the two Y ion lines the suppression slightly reduced from 28 ± 2% at 0.6 L min−1 to 23 ± 2% at 0.85 L min−1. Increasing the nebulizer gas flow to 1.3 L min−1 resulted in no further Li-induced signal suppression change. Notably, the line emission intensity of each element changed significantly with the nebulizer gas flow, as previously reported,22 irrespective of whether Li as a matrix element was present or not.
The matrix effect of Li on Sc and Y was also only slightly reduced when increasing the MIP's plasma power. Similar to the impact of the nebulizer gas flow, the Y ion lines were affected most, showing a reduction of the signal suppression caused by Li from 30 ± 2% at 1100 W to 25 ± 1% at 1500 W. These findings are consistent with the report of Zhang and Wagatsuma12 even though the concentration of EIEs was lower in our work.
In conclusion, optimizing the plasma conditions was not found to reduce the matrix effect of Li to an acceptable level for routine analysis.
In ICP-OES, matrix effects by EIE can be reduced by applying conditions for a robust plasma. As noted by Mermet,32,33 the ratio of the Mg(II) 280.270 nm and Mg(I) 285.21 nm emission lines can be used as an indicator for this purpose. With an Mg line ratio above 10 for the ICP, the energy transfer between the plasma core and the analyte channel is sufficiently high, and the effects of EIEs on the emission signals of other elements are minimal. Generally speaking, in ICP-OES, the Mg-ratio can be maximized by increasing the plasma power and the injector tube inner diameter and reducing the nebulizer gas flow.34 All this does not hold true for MICAP-OES, as the plasma power and the nebulizer gas flow had only a small effect on the magnitude of lithium's matrix effect on ion lines of Sc and Y. It should also be noted in this context that the used torch already employs a wide bore (2.5 mm inner diameter) injector tube. Thereby, improvements regarding lithium's matrix effects by increasing the injector tube diameter from 2.5 mm to 3.0 mm seemed not promising and were consequently not ventured. Moreover, the MICAP differs significantly in excitation temperature and electron number density from the ICP.35 Considering this, a plasma robustness of 1.6 is expected for the MICAP23,28,36 and not 10, as with a robust ICP. In the absence of EIEs, the plasma robustness of a solution containing just 1 mg L−1 Sc, Y, Rh, and Mg was determined to be 1.6. As listed in ESI Table S1,† the effect of different matrix elements on the plasma robustness was marginal. Again, Li showed the most significant effect on the plasma robustness of all investigated matrix elements. However, 50 mg L−1 Li caused a relatively small reduction of the plasma robustness from 1.6 to 1.4 (14% difference), while for most other investigated matrix elements, an Mg-ratio of 1.5 was encountered. More significant changes in the plasma robustness were observed for changes in MICAP-OES power: at 1.1 kW, the plasma robustness in the absence of 50 mg L−1 Li was 1.1, linearly increasing to 1.6 at 1.5 kW. The presence of 50 mg L−1 Li also caused a nearly constant increase of the plasma robustness with power by about 8% from 1.0 at 1.1 kW, increasing linearly to 1.4 at 1.5 kW. Interestingly, a comparable increase in plasma robustness with power is also observed in ICP-OES.
The changes in plasma robustness with nebulizer gas flow also followed the general trend observed in ICP-OES: higher values were observed at low nebulizer gas flow rates declining with increasing gas flow. The highest value of the Mg ratio of 1.7 was observed at 0.7 L min−1 nebulizer gas flow without Li in the matrix. In the presence of 50 mg L−1 Li, the plasma robustness decreased slightly to 1.6 for the same nebulizer gas flow.
In this context, it is interesting to note that Thaler et al.28 also encountered changes in the plasma robustness only at relatively high concentrations of Na: for a radially viewed MICAP-OES, the plasma robustness decreased from about 1.77 in the absence of a Na matrix to 1.45 for 0.58 g L−1 Na. Using the formula provided by Thaler et al. for calculating the plasma robustness as a function of Na-concentration, 50 mg L−1 Na will reduce the plasma robustness from 1.77 to 1.74. This change can be regarded as insignificant.
From these observations, it must be concluded that line-specific optimization of the plasma condition cannot be expected to effectively mitigate EIE-induced matrix effects in MICAP-OES.
Conclusion
Matrix effects were encountered in MIP-OES at low concentrations of 20 mg L−1, as evident from Fig. 1. Of the investigated matrix elements Al, B, Co, Cr, Cu, Fe, Mn, Ni, Ti, V, Zn, as well as the EIEs Li, Na, K, Cs, Mg, Ca, and Ba, particularly the alkaline elements Li, Na and K, were found to cause significant signal suppression on some of the 42 analyte elements studied in this work. However, the magnitude of this suppression was strongly dependent on the matrix element, the analyte, and its emission line. No clear trend with the total line energy (excitation energy/excitation + ionization energy) or the energy of the electronic upper level involved in the electronic transition was found for the 105 lines studied. In contrast to previous literature reports, atom lines were affected by the matrix effect similarly to ion lines of the same element.It could also be shown that neither optimizing microwave power (range 1.1 to 1.5 kW) nor nebulizer flow (0.5–1.1 L min−1) rate effectively diminished these matrix effects.
Of the elements causing the matrix effects, the alkaline elements – particularly Li – resulted in the strongest analyte signal suppression when compared at the same concentration level of 50 mg L−1. These differences within the group of alkaline elements diminished for equimolar concentrations indicating that the actual number of matrix atoms introduced into the MICAP is of greater importance for matrix effects rather than the ionization energy of the alkaline element.
While the plasma robustness expressed as the Mg ion-to-atom ratio also showed only minor changes concerning the introduction of matrix elements, ion lines of Sc and Y were predominantly suppressed even by low concentrations of EIEs. Though the latter two elements are commonly used as internal standards in ICP-OES, their use in MIP-OES appears ineffective.
Conflicts of interest
There are no conflicts of interest to declare.References
- B. Welz and M. Sperling, Atomic Absorption Spectrometry, Wily VCH, Weinheim, Germany, 3rd edn, 1999 Search PubMed.
- I. B. Brenner, M. Zischka, B. Maichin and G. Knapp, Ca and Na interference effects in an axially viewed ICP using low and high aerosol loadings, J. Anal. At. Spectrom., 1998, 13, 1257–1264 RSC.
- M. Stepan, P. Musil, E. Poussel and J. M. Mermet, Matrix-induced shift effects in axially viewed inductively coupled plasma atomic emission spectrometry, Spectrochim. Acta, Part B, 2001, 56, 443–453 CrossRef.
- M. Wu and G. Hieftje, The effect of easily ionized elements on analyte emission efficiency in inductively coupled plasma spectrometry, Spectrochim. Acta, Part B, 1994, 49, 149–161 CrossRef.
- G. Grindlay, L. Gras, J. Mora and M. T. C. de Loos-Vollebregt, Carbon-, sulfur-, and phosphorus-based charge transfer reactions in inductively coupled plasma-atomic emission spectrometry, Spectrochim. Acta, Part B, 2016, 115, 8–15 CrossRef CAS.
- H. Wiltsche, M. Winkler and P. Tirk, Matrix effects of carbon and bromine in inductively coupled plasma optical emission spectrometry, J. Anal. At. Spectrom., 2015, 30, 2223–2234 RSC.
- J. Machát, V. Kanický and V. Otruba, Determination of selenium in blood serum by inductively coupled plasma atomic emission spectrometry with pneumatic nebulization, Anal. Bioanal. Chem., 2002, 372, 576–581 CrossRef.
- J. Machát, V. Otruba and V. Kanický, Spectral and non-spectral interferences in the determination of selenium by inductively coupled plasma atomic emission spectrometry, J. Anal. At. Spectrom., 2002, 17, 1096–1102 RSC.
- J. J. Urh and J. W. Carnahan, Analytical Figures of Merit and Interelement Effects with Air and Nitrogen Microwave-Induced Plasmas, Appl. Spectrosc., 1986, 40, 877–883 CrossRef CAS.
- K. Jankowski and M. Dreger, Study of an effect of easily ionizable elements on the excitation of 35 elements in an Ar-MIP system coupled with solution nebulization, J. Anal. At. Spectrom., 2000, 15, 269–274 RSC.
- J. P. Matousek, B. J. Orr and M. Selby, Interferences due to easily ionised elements in a microwave-induced plasma system with graphite-furnace sample introduction, Spectrochim. Acta, Part B, 1986, 41, 415–429 CrossRef.
- Z. Zhang and K. Wagatsuma, Matrix effects of easily ionizable elements and nitric acid in high-power microwave-induced nitrogen plasma atomic emission spectrometry, Spectrochim. Acta, Part B, 2002, 57, 1247–1257 CrossRef.
- R. Serrano, E. Anticó, G. Grindlay, L. Gras and C. Fontàs, Determination of elemental bioavailability in soils and sediments by microwave induced plasma optical emission spectrometry (MIP-OES): matrix effects and calibration strategies, Talanta, 2022, 240, 123166 CrossRef CAS.
- R. Serrano, G. Grindlay, L. Gras and J. Mora, Evaluation of calcium-, carbon- and sulfur-based non-spectral interferences in high-power MIP-OES: comparison with ICP-OES, J. Anal. At. Spectrom., 2019, 34, 1611–1617 RSC.
- O. V. Pelipasov and E. V. Polyakova, Matrix effects in atmospheric pressure nitrogen microwave induced plasma optical emission spectrometry, J. Anal. At. Spectrom., 2020, 35, 1389–1394 RSC.
- D. A. Goncalves, T. McSweeney and G. L. Donati, Characteristics of a resonant iris microwave-induced nitrogen plasma, J. Anal. At. Spectrom., 2016, 31, 1097–1104 RSC.
- K. L. Lowery, T. McSweeney, S. P. Adhikari, A. Lachgar and G. L. Donati, Signal correction using molecular species to improve biodiesel analysis by microwave-induced plasma optical emission spectrometry, Microchem. J., 2016, 129, 58–62 CrossRef CAS.
- W. Li, P. Simmons, D. Shrader, T. J. Herrman and S. Y. Dai, Microwave plasma-atomic emission spectroscopy as a tool for the determination of copper, iron, manganese and zinc in animal feed and fertilizer, Talanta, 2013, 112, 43–48 CrossRef CAS.
- E. V. Polyakova, Y. N. Nomerotskaya and A. I. Saprykin, Effect of Matrix Element and Acid on Analytical Signals in Nitrogen Microwave-Plasma Atomic Emission Spectrometry, J. Anal. Chem., 2020, 75, 474–478 CrossRef CAS.
- S. Karlsson, V. Sjöberg and A. Ogar, Comparison of MP AES and ICP-MS for analysis of principal and selected trace elements in nitric acid digests of sunflower (Helianthus annuus), Talanta, 2015, 135, 124–132 CrossRef CAS.
- A. J. Schwartz, Y. Cheung, J. Jevtic, V. Pikelja, A. Menon, S. J. Ray and G. M. Hieftje, New inductively coupled plasma for atomic spectrometry: the microwave-sustained, inductively coupled, atmospheric-pressure plasma (MICAP), J. Anal. At. Spectrom., 2016, 31, 440–449 RSC.
- H. Wiltsche and M. Wolfgang, Merits of microwave plasmas for optical emission spectrometry – characterization of an axially viewed microwave-sustained, inductively coupled, atmospheric-pressure plasma (MICAP), J. Anal. At. Spectrom., 2020, 35, 2369–2377 RSC.
- H. Wiltsche, M. Wolfgang and F. Hallwirth, Effects of argon on the analytical properties of a microwave-sustained, inductively coupled, atmospheric-pressure plasma, J. Anal. At. Spectrom., 2022, 37, 1298–1308 RSC.
- Anton Paar ASC Application Team, Digestion in Multiwave 5000 Using HF Complexation of Fluorides, Anton Paar GmbH, Graz, 2021 Search PubMed.
- G. F. Larson and V. A. Fassel, Comparison of interelement effects in a microwave single electrode plasma and in a radiofrequency inductively coupled plasma, Anal. Chem., 1976, 48, 1161–1166 CrossRef CAS.
- P. Niedzielski, L. Kozak, M. Wachelka, K. Jakubowski and J. Wybieralska, The microwave induced plasma with optical emission spectrometry (MIP-OES) in 23 elements determination in geological samples, Talanta, 2015, 132, 591–599 CrossRef CAS.
- R. Serrano, G. Grindlay, L. Gras and J. Mora, Insight into the origin of carbon matrix effects on the emission signal of atomic lines in inductively coupled plasma optical emission spectrometry, Spectrochim. Acta, Part B, 2021, 177, 106070 CrossRef CAS.
- K. M. Thaler, A. J. Schwartz, C. Haisch, R. Niessner and G. M. Hieftje, Preliminary survey of matrix effects in the Microwave-sustained, Inductively Coupled Atmospheric-pressure Plasma (MICAP), Talanta, 2018, 180, 25–31 CrossRef CAS PubMed.
- E. Helmeczi, Y. Wang and I. D. Brindle, A novel methodology for rapid digestion of rare earth element ores and determination by microwave plasma-atomic emission spectrometry and dynamic reaction cell-inductively coupled plasma-mass spectrometry, Talanta, 2016, 160, 521–527 CrossRef CAS.
- Z. Sajtos, P. Herman, S. Harangi and E. Baranyai, Elemental analysis of Hungarian honey samples and bee products by MP-AES method, Microchem. J., 2019, 149, 103968 CrossRef CAS.
- Ş. Sungur and F. Gülmez, Determination of Metal Contents of Various Fibers Used in Textile Industry by MP-AES, J. Spectrosc., 2015, 2015, 640271 Search PubMed.
- J. M. Mermet, Ionic to atomic line intensity ratio and residence time in inductively coupled plasma-atomic emission spectrometry, Spectrochim. Acta, Part B, 1989, 44, 1109–1116 CrossRef.
- J. M. Mermet, The use of Mg as a test element for ICP-AES diagnostics, Anal. Chim. Acta, 1991, 250, 85–94 CrossRef CAS.
- J. L. Todolí, L. Gras, V. Hernandis and J. Mora, Elemental matrix effects in ICP-AES, J. Anal. At. Spectrom., 2002, 17, 142–169 RSC.
- N. Chalyavi, P. S. Doidge, R. J. S. Morrison and G. B. Partridge, Fundamental studies of an atmospheric-pressure microwave plasma sustained in nitrogen for atomic emission spectrometry, J. Anal. At. Spectrom., 2017, 32, 1988–2002 RSC.
- H. Wiltsche, M. Wolfgang and F. Hallwirth, Effects of argon on the analytical properties of a microwave-sustained, inductively coupled, atmospheric-pressure plasma, J. Anal. At. Spectrom., 2022, 37, 1298–1308 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ja00061c |
| This journal is © The Royal Society of Chemistry 2023 |