Open Access Article
Open Access ArticleCharacterizing a nitrogen microwave inductively coupled atmospheric-pressure plasma ion source for element mass spectrometry†
Monique
Kuonen
 ,
Guanghui
Niu
,
Guanghui
Niu
 ,
Bodo
Hattendorf
,
Bodo
Hattendorf
 * and
Detlef
Günther
* and
Detlef
Günther

Laboratory of Inorganic Chemistry, Department of Chemistry and Applied Biosciences, ETH Zurich, Vladimir-Prelog-Weg 1, Zurich 8093, Switzerland. E-mail: bodo@inorg.chem.ethz.ch
First published on 25th January 2023
Abstract
A high-power nitrogen-based microwave inductively coupled atmospheric-pressure plasma was coupled to a quadrupole mass spectrometer to investigate its characteristics as an ion source for element mass spectrometry. The influence of operating conditions on analyte sensitivity, plasma background, and polyatomic ion formation was investigated for conventional solution-based analysis. By varying the forward power and the nebulizer gas flow rate, the plasma background ions were found to decrease with increasing gas flow rates and decreasing operating power. Analyte ions showed different trends, which could be related to the physical–chemical properties of the elements. We could identify three groups based on the location of maximum intensity in the power vs. flow rate contour plot. Atomic ions of elements with low first ionization energy and low oxygen bond strength were found to maximize at a high nebulizer gas flow rate and lower microwave power. Elements with intermediate ionization energy and higher oxygen bond strength required higher power settings for optimum sensitivity, while elements with the highest ionization energies required the highest power and lowest gas flow rates for their optimization. The latter group showed a substantial suppression in sensitivity compared to elements of similar mass, which is considered to result from the high abundance of NO in the plasma source, whose ionization energy is close to that of these elements. Metal oxide ions were found at similar or higher abundances than in the conventional argon-based ICP and could be minimized only by using a low gas flow rate and high power settings. These general trends were also observed when the vacuum interface was modified. To change the dynamics of the supersonic expansion, different sampler cone orifice sizes and sampler–skimmer distances were investigated and the interface pressure was lowered through an additional pump. These modifications did not yield significant differences in ion transmission but lowering the interface pressure reduced the relative abundance of metal oxide ions. The limits of detection were evaluated for optimized plasma conditions and found comparable to those of an argon ICP source with the same mass spectrometer.
Introduction
Since its introduction1 inductively coupled plasma mass spectrometry (ICPMS) has evolved over the past four decades into one of the most widely used lab-based and routine techniques for the chemical analysis of the elemental or isotopic composition of almost any sample type.2 Its success is mostly due to its high sensitivity for most elements in the periodic table in particular though for metals, a wide linear dynamic range (up to 12 orders of magnitude),3 and straightforward coupling to various sample introduction systems such as solution nebulization, laser ablation4,5 and various separation techniques such as high performance liquid chromatography and capillary electrophoresis.6–8 While technological advancements were mainly focused on the mass spectrometer by optimizing ion transfer9,10 and employing reaction cells11–13 or different mass analysers,14–16 the fundamental setup of the ion source17,18 however has not undergone substantial changes within the last two decades. Even though different manufacturers of commercial instruments employ distinct configurations for the ICP, interface ion optics, and mass analysers, ICPMS has proven to be a reliable and robust analytical method.Still, current instruments suffer from multiple drawbacks, such as the need for a complex power generator design, the occurrence of spectral interferences from Ar containing molecular ions, and the substantial running cost due to the consumption of up to 1 m3 of Ar per hour in a common operating mode. Nitrogen-based plasma sources have been proposed as an alternative to the argon ICP decades ago.19,20 Such microwave induced plasmas have also been described with elemental MS,21–23 which however did not receive much attention. Most recently a new configuration of an inductively coupled microwave plasma source was introduced,24 which can be operated with nitrogen as plasma gas while being capable of delivering a power of up to 1.5 kW, which compares favourably with common Ar ICPs. This novel microwave inductively coupled atmospheric pressure plasma (MICAP) has already been successfully employed as a plasma source for ICP optical emission spectrometry25 and an initial study26 using a prototype time-of-flight MS has revealed that with solution nebulization, the sensitivities were lower by a factor of two than those of an Ar ICP coupled to the same MS. Desolvation of the aerosol was found to enhance the sensitivity significantly and most recently solution nebulization and laser ablation sampling were employed with an ion source coupled to a quadrupole MS (QMS), where similar figures of merit to those obtained with an Ar ICP were obtained.27,28 This study was aimed at investigating the characteristics of the new nitrogen-sustained plasma ion source coupled to a QMS with pneumatic nebulization. Therefore, the influence of forward power and the nebulizer gas flow rate was investigated to evaluate the analytical performance of the nitrogen microwave induced plasma (MIP). Since all the previous experiments used the vacuum interface and ion optic system designed for argon ICPMS instruments, various modifications of the interface arrangement were implemented to evaluate whether the gas dynamics at the vacuum interface or the ion optics could be optimized for the expansion dynamics of nitrogen versus argon plasmas.
Experimental
Instrumentation
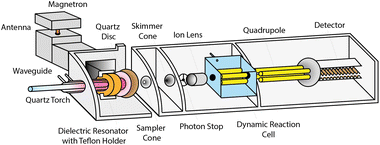
The instrument prototype (Fig. 1) is based on a commercial ELAN 6100PLUS ICPMS (PerkinElmer, Canada), for which the conventional ICP has been replaced by a MICAP (Radom Corp, USA).24–26 A cavity magnetron (1.5 kW Panasonic 2M262A-07AAM) generates a microwave field that is directed to a dielectric resonator ring and couples inductively to the plasma. The magnetron was powered by a Magdrive 2000 power supply (Dipolar AB, Sweden). A quartz disc between the MS vacuum interface and the torch protects the Teflon holder of the dielectric resonator from the heat of the plasma. A conventional Fassel-type quartz torch (Spectro Arcos SOP-Torch, 1.8 mm inner diameter injector, Spectro, Germany) was centred within the resonator ring with a 3D translation stage.The plasma ignition process as well as the operating parameters of the plasma were controlled through a MICAP control unit (Radom Corp., USA). During the start-up sequence, argon gas is introduced for 8 s together with nitrogen (99.996%, PanGas AG, Switzerland) simultaneously with a spark to facilitate the ignition. After the plasma ignition, no more argon is added, and the nitrogen-sustained plasma emits a pale-pink light.
The vacuum interface consisted of standard Elan-type sampler (1.1 mm orifice, platinum) and skimmer (0.8 mm, orifice platinum) cones, respectively. To investigate the supersonic expansion dynamics, the vacuum interface was modified. Different combinations of the sampler cone aperture, skimmer cone position, and interface pressure were studied: the sampler orifice diameter was decreased from 1.1 mm to 0.8 mm and the sampler–skimmer distance was shortened by introducing one or two 0.5 mm aluminium spacers at the base of the skimmer. Additionally, a second rotary vane pump (Leybold S25B) was employed to reduce the interface pressure. A pressure sensor (Pfeiffer Vacuum Single Gauge, Typ361) was connected to the vacuum line approximately 80 cm downstream of the interface to monitor changes in pressure for different configurations. The ion optics arrangement was not altered and a dynamic reaction cell was not employed in this study. The employed operating conditions of the instrument can be found in the ESI (Table S1†). The liquid sample was introduced with a glass concentric nebulizer (Micromist™ L90347) and a cyclonic spray chamber. The sample flow rate was controlled with a peristaltic pump, corresponding to 0.99 mL min−1. The sample uptake efficiency into the plasma was determined to be 3–5% of the sample flow rate, depending on the nebulizer gas flow rate.
Materials and methods
A multi-element solution was prepared as 1 μg kg−1 of each analyte by successive dilution with 1% nitric acid from single element stock solutions obtained from Merck (Na), Inorganic Ventures (Be, Mg, K, Ca, Co, Zn, Ge, As, Se, Rb, Sr, Cd, In, Cs, Te, Ba, Ce, Lu, Pb, Th, and U) and VWR Chemicals (Li) to investigate the influence of operating parameters and interface geometry on analyte sensitivity. The instrument was initially optimized for each interface configuration by adjusting the ion lens voltage and the torch position for maximum 238U+ intensity. For the optimization the plasma was operated at a power of 1350 W, with a cooling gas flow rate of 14 L min−1, a nebulizer gas flow rate of 1000 mL min−1, and an auxiliary gas flow rate of 750 mL min−1.The influence of the operating conditions on analyte sensitivity was investigated by varying the operating power from 1150 W to 1450 W in steps of 100 W, while the nebulizer gas flow rate was increased from 800 mL min−1 to 1100 mL min−1 in steps of 50 mL min−1. The cooling and auxiliary gas flow rates remained fixed. The different interface configurations with which the measurement series were performed and how the operating conditions were varied are given in Table 1. For two interface configurations, only the gas flow rate was changed while the power remained at 1450 W. After each change in the interface configuration or operating parameters, the ion lens voltages were re-calibrated. In addition to the analyte elements, the instrumental background at m/z 220 and the plasma background ions 15N+, 14N16O+, 14N3+, and 14N4+ were monitored and the metal oxides 140Ce16O+, 232Th16O+, and 238U16O+ and their corresponding nitrides 140Ce14N+, 232Th14N+, and 238U14N+ were acquired. Each isotope was measured with a dwell time of 500 ms in one sweep and with 5 replicates.
| Interface configuration | Sampler aperture [mm] | Spacers | Additional pump | Power variation [W] | Flow variation [mL min−1] |
|---|---|---|---|---|---|
| Standard interface | 1.1 | None | No | 1150–1450 | 800–1100 |
| Reduced sampler | 0.8 | None | No | 1150–1450 | 800–1100 |
| Standard interface & pump | 1.1 | None | Yes | 1150–1450 | 800–1100 |
| 0.5 mm skimmer shift | 1.1 | One | No | 1150–1450 | 800–1100 |
| 1.0 mm skimmer shift | 1.1 | Two | No | 1150–1450 | 800–1100 |
| 0.5 mm skimmer shift & pump | 1.1 | One | Yes | 1150–1450 | 800–1100 |
| 1.0 mm skimmer shift & pump | 1.1 | Two | Yes | 1150–1450 | 800–1100 |
| 1.0 mm skimmer shift & reduced sampler | 0.8 | Two | No | 1450 | 800–1100 |
| 1.0 mm skimmer shift & reduced sampler & pump | 0.8 | Two | Yes | 1450 | 800–1100 |
To determine the limits of detection (LODs), a 1% nitric acid solution containing between 1 and 100 μg kg−1 of the analytes in the Certipur® ICP multi-element standard solution VI from Merck was measured analogously after optimizing the instrument for maximum 238U+ signal intensity while keeping the 140Ce16O+ abundance (expressed as 140Ce16O+/(140Ce+ + 140Ce16O+)) below 3%. Additionally, the same solution was measured on a commercial Ar ELAN 6100PLUS.
Data evaluation
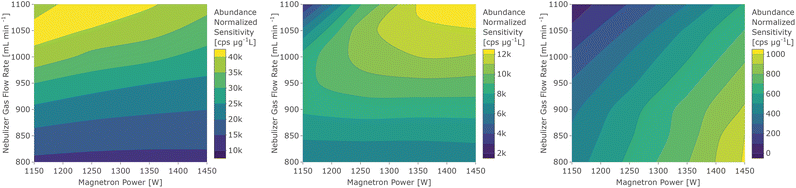
Abundance corrected sensitivities for the analyte isotopes were calculated after subtracting the instrumental background signal. For the plasma background and molecular ion signals, only background subtraction was performed. The sensitivity of different analyte elements was analysed in dependence of forward power and nebulizer gas flow rates and trends were identified (e.g. oxide formation, sensitivity etc.). For visualization of the influence of forward power and nebulizer gas flow rates, the sensitivities were depicted in a contour plot for each analyte. The different interface configurations were compared using their response curves as well as their nitride and oxide ratios. For each configuration the operating conditions yielding the maximum achievable sensitivities while maintaining a 140Ce16O+ abundance at or below 3% were determined. The LODs were determined from three times the standard deviation of the blank divided by the elements' blank-corrected sensitivities.Results and discussion
Influence of operating conditions
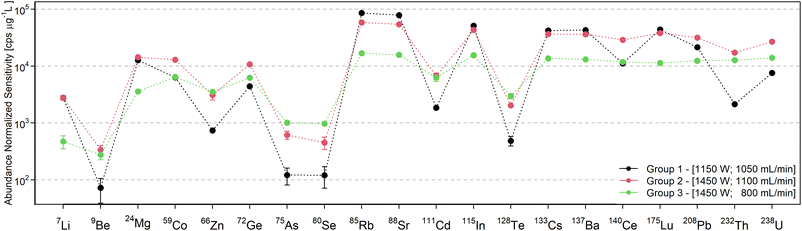
The following discussion about the influence of the operating conditions is visualized with data from the standard interface & pump configuration, which has been shown in preliminary experiments to be beneficial in terms of oxide abundance. However, the trends are representative for the other configurations, unless noted in the later section in which the interface modifications are discussed.For a better overview of these effects and the determination of operating conditions that yield the maximum sensitivity, the sensitivity dependence on forward power and nebulizer gas flow rate was depicted in a contour plot for every analyte. Three element groups can be identified based on the location of the maximum sensitivity, as seen in Fig. 2. The first group (7Li, 23Na, 24Mg, 39K, 85Rb, 88Sr, 115In, 133Cs, 137Ba, and 175Lu) is governed by elements with a low first IE, whose sensitivity increases with higher gas flow rates due to the higher sampling efficiency. Since many analytes in this group have a low mass, a higher operating power decreases the sensitivity through increased radial diffusion and possibly space charge effects at higher temperatures. The elements of the second group (9Be, 59Co, 72Ge, 111Cd, 140Ce, 208Pb, 232Th, and 238U) have intermediate first IEs or higher oxygen bond strengths33 which are possibly not efficiently dissociated or ionized at low temperatures. At higher forward power settings however they showed an increase in sensitivity due to more efficient sampling. Elements with high first IEs (66Zn, 75As, 80Se, and 128Te) form the third group, which shows the highest sensitivity for the highest power and lowest gas flow settings. Detection of these elements merely benefits from a higher temperature increasing the ion yield rather than less diffusion. An exception in this respect was 9Be+, which also has a high first IE, but due to its low mass it also benefits from higher sampling efficiency at a higher flow rate and thus resembled more the trends observed for group 2. Similarly, some contour plots show a trend that is in between group 2 and group 3, namely those of the elements 66Zn+, 111Cd+, and 232Th+, which have been assigned to the group they are closest to.
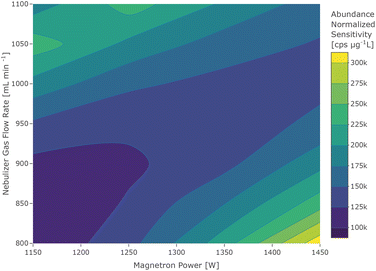
The contour plot of 40Ca+ showed a bimodal distribution which can be seen in Fig. 3. The locations of the two maxima correspond to an element of the first group (high flow and low power), which is considered to be due to 40Ca+ with its low first IE and an element of the third group, which is most likely caused by residual argon in the plasma gas. This is further supported by the occurrence of the other argon isotopes in a blank sample and the knowledge that standard-grade nitrogen from a conventional cryogenic air distillation may contain up to 0.5% of argon.34
Based on our experience, the response curve for group 3 most closely resembles the characteristics of the Ar ICP with the same MS. However, even for these settings, the sensitivity of elements with first IE ≥ 9 eV appeared to be suppressed to a greater extent in the MICAP source. Specifically 75As+ and 80Se+ were detected at more than one order of magnitude lower sensitivities than isotopes of similar mass and lower IEs, which was not the case in an Ar ICP. This is considered to be the result of the high abundance of NO in the plasma source, whose IE (9.264 eV) is close to that of these elements.33
Interface modifications
The evaluation of plasma operating conditions as described above was repeated for six additional interface configurations as listed in Table 1. The observed trends in the isotopes' responses were highly similar with the exception of the 40Ca+, 80Se+, and 14N4+ trends present in some configurations.Limits of detection
The determined LODs of the MICAP are compared with those from previous reports using nitrogen-based MIPs for aqueous solutions.21,22,35 Additionally, the LODs are compared to those from an Ar-based ICPMS, comprising the same vacuum interface, ion optics and mass analyser as in our laboratory (see Table 2). A comprehensive summary including the blank standard deviations and the analyte sensitivities can be found in the ESI (Table S2†).| Element | Isotope | MINDAP21 | MP MIP22 | HP MIP35 | MICAP | Ar ICP |
|---|---|---|---|---|---|---|
| a Measured 137Ba. | ||||||
| Li | 7 | — | 0.4 | 0.0004 | 0.03 | 0.004 |
| Mg | 24 | — | 0.4 | 0.002 | 0.002 | 0.013 |
| Ca | 40 | 22 | 0.24 | 0.014 | 0.05 | — |
| V | 51 | — | 0.3 | 0.001 | 0.001 | 0.003 |
| Cr | 52 | — | 0.4 | 0.001 | 0.001 | 0.02 |
| Mn | 55 | — | 0.5 | 0.001 | 0.001 | 0.02 |
| Fe | 56 | — | 7 | 0.008 | 0.017 | — |
| Co | 59 | 10 | 0.17 | 0.001 | 0.002 | 0.002 |
| As | 75 | — | 0.3 | 0.02 | 0.012 | 0.009 |
| Se | 80 | — | 1.4 | 0.04 | 0.08 | — |
| Sr | 88 | 3 | 0.4 | 0.002 | 0.002 | 0.002 |
| Ba | 138 | 11 | 0.3 | 0.004 | 0.001 | 0.003a |
| Pb | 208 | — | 6 | 0.008 | 0.001 | 0.002 |
While the LODs of the microwave induced nitrogen discharge at atmospheric pressure plasma (MINDAP) and of the moderate power MIP are in the ng mL−1 range, the detection limits of MICAP and other high-power MIPs are in the range of pg mL−1, making them comparable to the Ar-based ICPMS used here. The higher LOD for 7Li+ with the MICAP source may be due to the specific optimization of the instrument, while the detection of 40Ca+ is affected by substantial background signals from 40Ar+ in the MICAP case. 80Se+ on the other hand appears to be affected by a not identified molecular ion, causing an elevated background signal. Okamoto23 reported the preliminary detection limits of a nitrogen MIP for 39K+, 40Ca+, 52Cr+, and 56Fe+ to be below 5 pg mL−1 which are even lower than the ones reported here. The difference in LOD of 56Fe+ might be the result of a higher abundance of 14N4+. Furthermore, the LODs reported here are well comparable with those found in the other solution nebulization MICAP QMS study.27 Nonetheless, it could be shown that the MICAP has comparable limits of detection to those of an argon ICP coupled to the same mass spectrometer.
Conclusions
The investigation of the characteristics of the novel high-power nitrogen-sustained microwave inductively coupled atmospheric pressure plasma as the ion source for element mass spectrometry has revealed the following main findings:The change in the plasma gas temperature could be estimated through the interface pressure changes, showing a 2–4% lower plasma temperature with increasing the nebulizer gas flow rate by 100 mL min−1 or decreasing the microwave power by 100 W. The influence of the operating conditions on analyte sensitivity has been assessed by varying the forward power and the nebulizer gas flow rate, which indicate that the monitored elements could be divided into three groups based on the location of their maximum sensitivity, which can be related to their respective physical–chemical properties such as mass, first IE, and oxygen bond strength. The signals of the investigated plasma background ions were found to mimic the trends observed for analytes with a high IE, as the signal decreased with increasing gas flow rates and decreasing forward power. The metal oxide ions followed the trends observed in the conventional argon-based ICP, but their abundances were slightly higher, indicating an overall lower temperature in the nitrogen plasma. On the other hand, the observed nitride abundances were near 0.5% and seemed to be mostly independent from the operating conditions. Based on these observations and a comparison of response curves under different operating conditions, it can be recommended to operate the MICAP ion source at a high power and a low flow rate to maintain a low abundance of oxides while also reducing the relative suppression of high IE elements.
The influence of different interface variations including a smaller sampler aperture, shortening the sampler–skimmer distance, and the use of a second interface pump was evaluated. The use of a smaller sampler orifice appears to result in sampling from a colder plasma region since the oxide ratios were higher and the 40Ar ion signal was less abundant. Shifting the skimmer position further upstream led to a decrease in the analyte sensitivity and oxide ratios with decreasing sampler–skimmer distance. Additionally, the ion signal at m/z 80 and 82 increased when the skimmer was shifted further upstream and the plasma was operated at higher temperatures, which originates from a yet unknown interference. In any case, no substantial advantage in sensitivity was observed by shortening the sampler–skimmer distance or using a smaller sampler orifice. A slight increase in the response was obtained when using the standard interface with the second interface pump, which allowed a higher nebulizer gas flow rate to be used while maintaining a low metal oxide abundance level. Nonetheless, this study shows that the ion source can be operated under conditions that result in only slightly lower analyte sensitivities compared to an Ar-based ICP, a comparable abundance of metal oxides and negligible amounts of nitrides, even for the standard configuration, indicating that no major change in the interface geometry is needed when introducing wet aerosols.
The performance of the MICAP ion source was further evaluated by calculating the LODs, which were found to be comparable to those of other nitrogen sustained MIPs as well as to an argon-based ICP instrument, using the same ion optics and mass spectrometer. Considering all these findings and the operating cost of the MICAP source, it has proven to be a complementary or even competitive ion source for element mass spectrometry.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
Special thanks go to Ashok Menon and Jovan Jevtic of Radom Corporation and Philippe Trüssel from the DCHAB central workshop for their assistance and to the Swiss National Science Foundation for their financial support through project number 200021_197224.References
- R. S. Houk, Anal. Chem., 1980, 52, 2283–2289 CrossRef CAS.
- A. Montaser, Inductively Coupled Plasma Mass Spectrometry, Wiley-VCH, New York, 1998 Search PubMed.
- L. Hendriks, A. Gundlach-Graham, B. Hattendorf and D. Günther, J. Anal. At. Spectrom., 2017, 32, 548–561 RSC.
- A. L. Gray, Analyst, 1985, 110, 551–556 RSC.
- D. Günther and B. Hattendorf, TrAC, Trends Anal. Chem., 2005, 24, 255–265 CrossRef.
- A. Prange and D. Pröfrock, Anal. Bioanal. Chem., 2005, 383, 372–389 CrossRef CAS PubMed.
- M. Montes-Bayon, D. Pröfrock, A. Sanz-Medel and A. Prange, J. Chromatogr. A, 2006, 1114, 138–144 CrossRef CAS.
- E. Krupp, F. Seby, R. Rodríguez Martín-Doimeadios, A. Holliday, M. Moldován, G. Köllensperger, S. Hann and O. F. X. Donard, in Inductively Coupled Plasma Mass Spectrometry Handbook, Blackwell Publishing Ltd., 2009, pp. 259–335 Search PubMed.
- D. J. Douglas and J. B. French, J. Anal. At. Spectrom., 1988, 3, 743–748 RSC.
- S. Elliott, M. Knowles and I. Kalinitchenko, Spectroscopy, 2004, 19, 30–38 CAS.
- I. Feldmann, N. Jakubowski and D. Stuewer, Fresenius. J. Anal. Chem., 1999, 365, 415–421 CrossRef CAS.
- S. D. Tanner and V. I. Baranov, At. Spectrosc., 1999, 20, 45–52 CAS.
- B. Hattendorf and D. Günther, J. Anal. At. Spectrom., 2000, 15, 1125–1131 RSC.
- D. P. Myers and G. M. Hieftje, Microchem. J., 1993, 48, 259–277 CrossRef CAS.
- I. Feldmann, W. Tittes, N. Jakubowski, D. Stuewer and U. Giessmann, J. Anal. At. Spectrom., 1994, 9, 1007–1014 RSC.
- O. Borovinskaya, B. Hattendorf, M. Tanner, S. Gschwind and D. Günther, J. Anal. At. Spectrom., 2013, 28, 226–233 RSC.
- S. Greenfield, I. L. Jones, C. T. Berry and J. M. Mermet, J. Anal. At. Spectrom., 1964, 4, 559–560 Search PubMed.
- R. H. Wendt and V. A. Fassel, Anal. Chem., 1965, 37, 920–922 CrossRef CAS.
- C. I. M. Beenakker, Spectrochim. Acta, Part B, 1976, 31, 483–486 CrossRef.
- Y. Okamoto, M. Yasuda and S. Murayama, Jpn. J. Appl. Phys., Part 1, 1990, 29, L670–L672 CrossRef CAS.
- D. A. Wilson, G. M. Hieftje and G. H. Vickers, Anal. Chem., 1987, 59, 1664–1670 CrossRef CAS.
- W.-L. Shen, T. M. Davidson, J. T. Creed and J. A. Caruso, Appl. Spectrosc., 1990, 44, 1003–1010 CrossRef CAS.
- Y. Okamoto, J. Anal. At. Spectrom., 1994, 9, 745–749 RSC.
- J. Jevtic, A. Menon and V. Pikelja, U.S. Pat., WO 2014/159588 A1, 2014 Search PubMed.
- A. J. Schwartz, Y. Cheung, J. Jevtic, V. Pikelja, A. Menon, S. J. Ray and G. M. Hieftje, J. Anal. At. Spectrom., 2016, 31, 440–449 RSC.
- M. Schild, A. Gundlach-Graham, A. Menon, J. Jevtic, V. Pikelja, M. Tanner, B. Hattendorf and D. Günther, Anal. Chem., 2018, 90, 13443–13450 CrossRef CAS PubMed.
- Z. You, A. Akkuş, W. Weisheit, T. Giray, S. Penk, S. Buttler, S. Recknagel and C. Abad, J. Anal. At. Spectrom., 2022, 37, 2556–2562 RSC.
- C. Neff, P. Becker, B. Hattendorf and D. Günther, J. Anal. At. Spectrom., 2021, 36, 1750–1757 RSC.
- J. E. Fulford and D. J. Douglas, Appl. Spectrosc., 1986, 40, 971–974 CrossRef CAS.
- R. S. Houk, Anal. Chem., 1986, 58, 97A–105A CrossRef CAS.
- S. Gschwind, L. Flamigni, J. Koch, O. Borovinskaya, S. Groh, K. Niemax and D. Günther, J. Anal. At. Spectrom., 2011, 26, 1166–1174 RSC.
- L. Flamigni, J. Koch and D. Günther, J. Anal. At. Spectrom., 2014, 29, 280–286 RSC.
- CRC Handbook of Chemistry and Physics, ed. J. Rumble, CRC Press, Boca Raton, FL, 102nd edn, 2021 Search PubMed.
- R. Agrawal, Ind. Eng. Chem. Res., 1996, 35, 1059–1071 CrossRef CAS.
- K. Oishi, T. Okumoto, T. Iino, M. Koga, T. Shirasaki and N. Furuta, Spectrochim. Acta, Part B, 1994, 49, 901–914 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ja00369d |
| This journal is © The Royal Society of Chemistry 2023 |