Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Feasibility of high-resolution continuum source molecular absorption spectrometry for vanadium determination†
Zofia
Kowalewska
 *a,
Carlos
Abad
*a,
Carlos
Abad
 b,
Michael
Okruss
c and
Sebastian
Recknagel
b,
Michael
Okruss
c and
Sebastian
Recknagel
 b
b
aFaculty of Civil Engineering, Mechanics and Petrochemistry, Warsaw University of Technology, Łukasiewicza 17, 09-400 Płock, Poland. E-mail: zofia.kowalewskah@gmail.com; zofia.kowalewska@pw.edu.pl; Tel: +48 604569041
bBundesanstalt für Materialforschung und -prüfung (BAM), Richard-Willstätter-Str. 11, Berlin, 12489, Germany
cAnalytik Jena GmbH, Konrad-Zuse-Str. 1, Jena, 07745, Germany
First published on 13th January 2023
Abstract
This work aimed to evaluate high-resolution continuum source molecular absorption spectrometry (HR-CS MAS), traditionally used to determine non-metals, for the determination of a new element, metal, vanadium. VO was selected as a target molecule because it is relatively stable and was expected to be spontaneously generated in a flame or a graphite furnace (GF). The high-resolution overview spectra of the molecule were obtained in a wide range of 480–630 nm, and absorption due to the X4Σ−–C4Σ− electronic transition was registered. A unique instrumental setup, comprising a prototype Modular Simultaneous Echelle Spectrograph (MOSES) and a commercial HR-CS MAS apparatus, was applied in the research. Finally, the spectral area centered at 550.6230 nm was selected for analysis. A method was developed to determine V in solutions of catalysts of heavy petroleum oil hydroprocessing using a commercial HR-CS spectrometer in a flame version. Although sensitivity was relatively poor (characteristic concentration 380 mg L−1), an extremely low noise enabled reaching a satisfactory detection limit (20 mg L−1 in solution, i.e. 0.1% m:m in the catalyst). For the first time vanadium was determined using ordinary air-acetylene flame. The obtained results were consistent with the results of atomic absorption spectrometry with N2O-C2H2 flame. Unfortunately, only a small population of VO molecules could have been generated in GF measurements. Furthermore, the observed VO molecules appeared only at unfavorably high temperatures. The work shows the potential of HR-CS MAS as a scientific tool for investigating the mechanism of processes occurring in the GF. This work can inspire other research of new analytes for HR-CS MAS.
1. Introduction
Since the commercial introduction of high-resolution continuum source absorption spectrometry in the first decade of the XXI century, numerous analytical procedures have been developed with its application.1–6 Usually, metals and metalloids have been determined using atomic absorption spectrometry (AAS) techniques. Most nonmetals cannot be determined using direct versions of AAS due to insufficient temperature of atomizers and inaccessibility of the required vacuum spectral range. Therefore, for the determination of nonmetals such as F, S, Cl, Br, I and N, molecular absorption spectrometry (MAS) techniques, such as high-resolution continuum source flame molecular absorption spectrometry (HR-CS FMAS) or high-resolution continuum source graphite furnace molecular absorption spectrometry (HR-CS GFMAS), have been engaged.1–6Nowadays, for some elements, two approaches are available as apart from the possibility of using AAS, HR-CS MAS methods were developed.7–20 Phosphorus was determined via PO7–13,15,16 or P2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 molecules, aluminum via AlF17 or AlH18 molecules and silicon via SiO molecules.19,20 In the determinations, the HR-CS FMAS7,8,11–13,19 or the HR-CS GFMAS9,10,14–18,20 techniques were applied.
14 molecules, aluminum via AlF17 or AlH18 molecules and silicon via SiO molecules.19,20 In the determinations, the HR-CS FMAS7,8,11–13,19 or the HR-CS GFMAS9,10,14–18,20 techniques were applied.
The benefit of MAS techniques can be significantly milder conditions of analysis. For example, Si was determined in xylene solutions using a safer and cheaper air-acetylene flame19 instead of a hotter nitrous oxide acetylene flame.21 Similarly, phosphorus was determined using an air-acetylene flame.7,8,11–13 In the case of the GF method, phosphorus can be determined via P2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 or via PO10 molecules using only 1700 °C or 1900 °C molecule formation temperature, respectively, compared to the 2650 °C temperature needed for P atomization.10
14 or via PO10 molecules using only 1700 °C or 1900 °C molecule formation temperature, respectively, compared to the 2650 °C temperature needed for P atomization.10
Besides, in the AAS case, a complex chemical modification was involved.10 In Al determination in whole blood, the HR-CS GFMAS technique enabled better sample matrix removal than in the case of HR-CS GFAAS.17 Simple external calibration could be achieved using the HR-CS GFMAS technique.15–17
Due to the application of HR-CS GFMAS, simultaneous determination of Al and three other metals was possible (an interesting case of simultaneous measurements of atomic and molecular absorption).18 Another benefit of MAS can be appropriate adjustment of sensitivity for the analysis of samples with relatively high analyte levels. For example, Si can be quickly determined at a percentage level via a direct HR-CS GF MAS procedure if solid samples, difficult to dissolve, are prepared and analyzed as suspension.20
Generally, if the same analytical case is considered, the sensitivity and detectability of MAS analysis are worse than those of AAS analysis. The detection limit of P determination using graphite furnace techniques was 5 mg kg−1 in AAS and 20 mg kg−1 in MAS.10 The detection limit of Si determination in the form of octamethylcyclotetrasiloxane was 0.05 mg L−1 in HR-CS FAAS21 and 0.92 mg L−1 in HR-CS FMAS.19 In other cases, detection limits are less different, for example, 1.5 and 1.8 μg L−1 for Al determination by HR-CS GFAAS and HR-CS GFMAS, respectively.17
Taking into account the literature data, a question may be posed, whether molecules of other elements can be analytically useful for determining the elements. A candidate analyte should form a sufficiently stable molecule during analysis, i.e. the molecule of the bond energy above 500 kJ mol−1.2 It would be preferable that the molecule be formed spontaneously, without using special reagents delivering complementary element. The new option of analysis should provide benefits relative to AAS methods.
In this work, for the first time, vanadium has been determined with molecular absorption spectrometry. The VO molecule of bond energy 627 kJ mol−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 has been selected as a target molecule. The refractory character of the molecule enforces the application of hot nitrous oxide-acetylene flame in conventional flame AAS23–27 and the application of a high temperature of 2550–2800 °C in graphite furnace AAS.23,24,26–32 The motivation of this work was to evaluate the possibility of V determination using air-acetylene flame in HR-CS FMAS and mild conditions in HR-CS GFMAS.
22 has been selected as a target molecule. The refractory character of the molecule enforces the application of hot nitrous oxide-acetylene flame in conventional flame AAS23–27 and the application of a high temperature of 2550–2800 °C in graphite furnace AAS.23,24,26–32 The motivation of this work was to evaluate the possibility of V determination using air-acetylene flame in HR-CS FMAS and mild conditions in HR-CS GFMAS.
It is expected that V would be present in air-acetylene flame in the form of VO molecules, as: (1) it does not occur as an atom, and (2) other theoretically possible molecules are much less stable (the bond energies of VC, VH, VN and VV are 427, 209, 477 and 269 kJ mol−1, respectively22).
There are controversies concerning vanadium forms and their transformations during graphite furnace analysis. The mechanism of V atomization, studied mainly in the 80s,32–39 has not been unambiguously explained up to now. Generally, the investigation of processes in a graphite furnace is extremely challenging due to low levels of analytes (usually tens and hundreds of pg up to ng), heterogeneity of the system (gas phase, solid phase and possibly liquid phase), porosity and reactivity of graphite, high temperature (up to approximately 3000 °C) and very fast-changing conditions (e.g. temperature rise >2000 °C s−1).
The following routes of V atomization were considered: reduction of solid V oxides by C with the final V transfer to the gas phase,34 dissociation of a V oxide in the solid, liquid or gas phase,33,35 dissociation of V carbide36–39 or even dissociation of a V dimer.33 All the listed final steps could be preceded by many steps involving various V species at different oxidation states. Various vanadium oxides were taken into account (V2O5, V2O4, V2O3, VO2, and the target molecule of this work, VO), as well as some carbides (V2C and VC). In the traditional approach to V atomization using a graphite furnace, the formation of solid carbides was highlighted.26,27,30,32 It was considered responsible for the high temperature needed for V atomization and severe tailing of the V signal. Nevertheless, the VO molecule was detected in experiments where an electrothermal atomizer (made of Ta or of vitreous carbon) was connected to a mass spectrometer.34
Matousek and Powell38,39 evaluated that the main V species transformation route is a sequence of reactions comprising, sequentially, V2O5(s), V2O3(s), VO(s) and VC(s), and atomization of V occurring via dissociation of VC(s). A minor route was also proposed via the formation of VO(g) at temperatures below 2087 °C (the VO(g) molecule further dissociates to release V atoms).38 It was considered that the transient existence of VO in the vapour phase might be a result of the evaporation of VO(s) deposited at colder positions of the graphite furnace (remote from the injection hole).38
Manning and Slavin considered the losses of V at the beginning of the atomization phase due to the evaporation of an oxide V form when the temperature is too low for its decomposition.32 Hulanicki et al.35 found opposite effects of the addition of air (a decrease of the V signal) and methane (an increase of the V signal) to the gas phase. It was supposed that methane, reacting with oxygen, promotes the dissociation of VO(g) to V(g), while the addition of air reverses the reaction. The literature mentioned above brings some hope for the possible utilization of absorption by the VO molecule for V determination.
Vanadium is recognized as a metal specific for petroleum as it is the metal most abundant in most crude oils.26–28,40,41 Unfortunately, V causes severe technological problems due to catalyst poisoning and equipment corrosion. During crude oil distillation, V concentrates in heavier oil fractions and residual oil.27,28 Vanadium can be removed from oils in catalytic hydroprocessing (hydrodemetallization and hydrodesulfurization).40–43 As a result of hydrodemetallization, the product of the process contains much less V than the feed (e.g. 5.9 and 73 mg kg−1, respectively41), while the removed vanadium deposits on the catalyst.
During the “life” of such a catalyst, V concentration can increase from below 0.04% w/w (a fresh catalyst) up to about 11% w/w (an extremely spent catalyst).42–45 There is a necessity to control the V content in such materials. The information on the V content enables effective management of the work of the hydrodemetallization/hydrodesulfurization unit. Moreover, V is a valuable metal that can be recovered from spent catalysts.42–49 Otherwise, the catalysts are hazardous waste.42–49 The important determination of V in hydroprocessing catalysts has been selected as an application case for this work.
2. Experimental
2.1. Instrumentation and its operation
A unique system was used in this work to record broad-range spectra in the wavelength range 480–630 nm. The source of radiation in the system was a laser-driven xenon lamp emitting high-intensity continuum radiation. The monochromator role was played by a unique modular simultaneous echelle spectrograph, MOSES, originally built in ISAS (Leibniz-Institute fur Analytische Wissenschaften, Berlin, Germany).14,50,51 For detection, a CCD detector was used. The system was connected with a commercially available spectrometer contrAA 800 (Analytik Jena GmbH, Jena, Germany), which worked in a graphite furnace solid sampling mode and delivered vapours for measurements with MOSES. The graphite furnace was transversely heated.The contrAA 800 spectrometer was also used in routine high-resolution continuum source graphite furnace absorption modes, and a commercially available contrAA 700 spectrometer (Analytik Jena GmbH, Jena, Germany) was used in high-resolution continuum source flame absorption modes. Each of the listed above contrAA spectrometers was equipped with a high-intensity xenon lamp as a radiation source, a double high-resolution monochromator (comprising a prism and an echelle grating) and a linear CCD-array detector. The spectrometers have a spectral resolution of λ/Δλ = 175![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The resolution corresponded to approximately 2.8 pm per pixel at 550.6 nm.
000. The resolution corresponded to approximately 2.8 pm per pixel at 550.6 nm.
The contrAA 700 worked mainly in HR-CS FMAS mode with air-acetylene flame generated using a burner with a 100 mm slit. If it is not indicated otherwise, the acetylene flow rate was 95 L h−1 (rich flame), and the observation height above the burner slit was 6 mm. For comparison, a standard HR-CS FAAS mode was also applied with acetylene-nitrous oxide flame generated using a burner with a 50 mm slit (the use of a 100 mm burner was not technically possible with this flame), 240 L h−1 acetylene flow rate and 8 mm over the burner slit observation height. The atomic absorption of V was measured at the sensitive 318.3982 nm line (catalyst solutions diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v). In measurements using flame techniques, a 5 s delay was applied. Each measurement lasted 3 s and was repeated three times (n = 3). The solution flow rate was 5–6 mL min−1.
50 v/v). In measurements using flame techniques, a 5 s delay was applied. Each measurement lasted 3 s and was repeated three times (n = 3). The solution flow rate was 5–6 mL min−1.
In all measurements using graphite furnace modes, a pyrolytically coated graphite furnace without a dosing hole and a pyrolytically coated platform were used. The investigated solutions were injected on the platform, and the solid material was weighed on the platform using a separate microbalance. In most measurements, Zr was applied as a permanent modifier. Zirconium in the form of nanoparticles was injected over the platform, and the platform was pretreated using a heating program presented elsewhere.52 The program of heating of the graphite furnace for measurements of absorption by the VO molecule was optimized, and the options used are listed in Table 1. In some experiments, air was applied as an alternative gas (as indicated in Table 1) and/or a Pd modifier was used. The Pd modifier was injected manually over a sample. For comparison, V atomic absorption was measured using HR-CS GFAAS mode with 2550 °C atomization temperature.
| Step | Temperature, °C | Heating rate, °C s−1 | Hold time, s−1 | Internal gas |
|---|---|---|---|---|
| a In some experiments, the “gas stop” option in step 6 was executed. | ||||
| (1) Drying | 80 | 6 | 10 | Argon |
| (2) Drying | 90 | 3 | 10 | Argon |
| (3) Drying | 110 | 5 | 10 | Argon |
| (4) Pyrolysis | 200 | 50 | 20 | Argon |
| (5) Pyrolysis | 500 | 100 | 20 | Air |
| (6) Pyrolysis | 500 | 0 | 30 | Argona |
| (7) Gas adaptation | 500 | 0 | 5 | Stop |
| (8) VO molecule formation | 2500 | 1500 | 8 | Stop |
| (9) Cleaning | 2600 | 500 | 4 | Argon |
A Soxhlet apparatus (Buchi, Switzerland), a muffle furnace Carbolite CWF 1200 (Sheffield, Great Britain), a Pulverisette 7 mill with an agate dish and balls, a heating plate Jolly 2 (Falc Instruments, Treviglio, Italy), and platinum crucibles were used for catalyst dissolution.
2.2. Reagents, samples and solutions
The following vanadium compounds were investigated: metallic vanadium, vanadium pentoxide and ammonium metavanadate, all from Johnson and Matthey Ltd. (London, Great Britain). The following substances were applied as a source of metals (potential interferents): aluminium chloride hexahydrate, ammonium molybdate tetrachloride, nickel(II) nitrate (V) hexahydrate and iron(III) chloride hexahydrate. The substances and caesium chloride (Cs was deionisation buffer in V determination using N2O-C2H2 flame in the FAAS technique) were obtained from Chempur (Piekary Śląskie, Poland).Hydrochloric acid (d = 1.19 kg L−1), nitric (V) acid (d = 1.42 kg L−1), perchloric (VII) acid (d = 1.60 kg L−1) and boric acid, all of analytical grade, were from POCH (Gliwice, Poland). Deionised water of specific conductivity below 0.1 mS cm−1 was used.
Metallic vanadium was dissolved in nitric acid, and the final solution contained 5000 mg L−1 of V and HNO3 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v.
10 v/v.
For chemical modification, zirconium dioxide, ZrO2, in the form of nanoparticles (45–55 nm) in aqueous suspension (20% m:m) was used, obtained from US Research Nanomaterials, Inc., Durham, USA. The material was diluted before application (100 μl of the material was mixed with 500 μl of methanol and 400 μl of water).
The palladium chemical modifier (1000 mg L−1 Pd as Pd nitrate in HNO3) was from Merck (Darmstadt, Germany).
The investigated fresh as well as spent catalysts were of NiMo type based on alumina and derived from units of hydroprocessing of heavy petroleum oil. The content of metals in the catalysts was (in % m/m): 2–5, 2–6, <0.01–17, 0.05–0.6 and above 80% in the case of Ni, Mo, V, Fe and Al, respectively.
The catalysts were washed in a Soxhlet apparatus to remove oil residue, ground in an agate mill and burned in a muffle furnace. Afterwards, the obtained ash was weighed into platinum crucibles and dissolved, using a 3-step procedure of heating with acids (1: HClO4 + HF, 2: H3BO3, and 3: HCl) on a heating plate. The procedure is precisely described elsewhere.53 The obtained solutions were homogeneous and contained HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v. Approximately 0.3–0.4 g of catalyst was transferred to a volume of 25 mL of the final solution.
5 v/v. Approximately 0.3–0.4 g of catalyst was transferred to a volume of 25 mL of the final solution.
3. Results and discussion
3.1. Selection of the wavelength for VO molecule absorption measurements
The disadvantage of molecular absorption spectrometry is its relatively low sensitivity, especially with the flame technique. Therefore, the main criterion of this work for the development of a new method was to achieve the best possible sensitivity and detectability. One of the steps to achieve this goal was the selection of the appropriate wavelength range of absorption by the VO molecule. Vanadium has not yet been determined with any version of high-temperature molecular absorption spectrometry, neither with traditional medium-resolution studies nor with new high-resolution continuum source spectrometers, nor with graphite furnace or flame techniques. However, the VO molecule spectra have been known since the XIX century (the spectra were observed using spark excitation already in 1895).54 Sometimes, there are difficulties in interpreting spectra obtained in different systems, whether emission or absorption, especially when using low-resolution instruments. In addition, commercially available HR-CS absorption spectrometers offer an excellent resolution, but the width of a measured spectrum is in a narrow range of about 0.2–1 nm.A unique opportunity for this work was a measurement of vapours generated in a commercial contrAA system in a broad wavelength range using a prototype MOSES spectrograph.50 A vanadium compound was introduced on a platform, preliminary coated using zirconium, and a program of graphite furnace heating was carried out (detailed explanation in Section 3.4). The obtained broad-range overview spectrum is presented in Fig. 1a. First of all, the spectrum shows groups of lines corresponding to rotations of the VO molecule, which belong to various vibrational sequences of the X4Σ−–C4Σ− electronic transition. The most interesting are the lines of the most intensive absorption belonging to the vibrational sequences in the vicinity of 550 nm and 573 nm. The areas are better visible on an extended wavelength scale in Fig. 1b–d. Some intensive and “promising” signals do not occur due to the VO molecule but were identified as atomic lines.55 Thus, many intensive lines observed in the 480–490 nm range were identified as V atomic lines. The Na doublet occurs at 588.9953 and 589.5924 nm, and a V atomic line occurs at 624.3107 nm.55
 | ||
| Fig. 1 Spectra acquired using a MOSES spectrometer in the wavelength range: 480–630 nm (a), 572.4–588.6 nm (b), 543.9–572.0 nm (c) and 549.7–552.6 nm (d). | ||
Four wavelength ranges were selected for FMAS work: the one centred at 546.9331 nm, the one centred at 573.7642 nm and two regions near 550.6 nm, comprising three or four high intensity rotational lines (indicated in Fig. 1d). The ranges are centred at 550.6230 nm or 550.5593 nm, respectively. The four regions were compared in flame measurements using a contrAA 700 spectrometer (optimization of the flame conditions is presented in the following Section 3.2), and respective spectra are presented in Fig. 2.
An important point is the selection of pixels from the available range of 200 pixels to evaluate the analyte signal. The selected pixels should cover the wavelength range of intensive absorption by the analyte. Otherwise, less intensive absorption will contribute to some increase in absorption, but unfortunately, there may also be an increase in non-specific absorption, which is associated with an increase in noise and deterioration in detectability. Various sets of pixels were taken into account to evaluate sensitivity and detectability. The selected pixels are marked as vertical bars in Fig. 2. For illustration, Fig. 1S† was also prepared, presenting a spectrum centred at 550.5593 nm (the most complicated spectrum). The sets of measured pixels, with a total number of pixels from 15 (Fig. 1Sa†) up to 50 (Fig. 1Sd†), are shown in particular subfigures of the figure. All considered spectral cases are characterized in Table 1S.† Measurements of a solution containing 2000 mg L−1 of V, 0.5% m/v Al and HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 were executed to evaluate sensitivity, while a similar solution, but without V, was ten times measured to evaluate the detection and determination limits (three and ten times, respectively, the standard deviation of the measurements). The obtained characteristic concentration and detection limit values are compared in Fig. 2S.†
5 were executed to evaluate sensitivity, while a similar solution, but without V, was ten times measured to evaluate the detection and determination limits (three and ten times, respectively, the standard deviation of the measurements). The obtained characteristic concentration and detection limit values are compared in Fig. 2S.†
The best detection limit, 15 mg L−1, has been obtained in the case of measurements with the central pixel at 550.6230 nm and a set of 28 pixels belonging to the eight most intensive lines used for the VO molecule signal evaluation. A similar detection limit, 17 mg L−1 of V, has been obtained for the spectrum centred at a bit lower wavelength, 550.5593 nm, while a set of 34 pixels belonging to nine most intensive rotational lines was evaluated. The characteristic concentrations in the two cases were 380 and 270 mg L−1, respectively. Further increase in the number of measured pixels does not improve detectability. For further analysis, the conditions of the best detectability were selected.
As expected, the detectability of HR-CS FMAS is worse than that of flame atomic absorption spectrometry (detection limit 0.1 mg L−1).
3.2. Optimization of flame conditions for measurements of absorption of VO molecules in air-acetylene flame
In order to obtain the highest possible V signal, the distribution of VO molecules in air-acetylene flame was studied, as well as the dependence of the VO molecule absorption on the acetylene flow rate. Three solutions were measured: a solution of metallic V dissolved in nitric acid (5000 mg L−1 of V), a solution containing V as NH4VO3 (2000 mg L−1) in HCl 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v and a similar solution but with added aluminium. The appearance of the solutions indicated the V oxidation state. The solution of initially metallic V in HNO3 was blue-green, and the analyte was in the form of [V(H2O)6]3+ ions in the +3 oxidation state or [VO(H2O)5]2+ ions in the +4 oxidation state. Two other yellow solutions contained pervanadyl ions, [VO2(H2O)4]+, with vanadium in the oxidation state +5.
5 v/v and a similar solution but with added aluminium. The appearance of the solutions indicated the V oxidation state. The solution of initially metallic V in HNO3 was blue-green, and the analyte was in the form of [V(H2O)6]3+ ions in the +3 oxidation state or [VO(H2O)5]2+ ions in the +4 oxidation state. Two other yellow solutions contained pervanadyl ions, [VO2(H2O)4]+, with vanadium in the oxidation state +5.
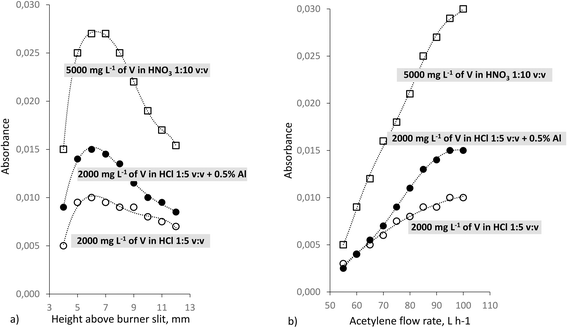
As Fig. 3a shows, the profiles of the vertical distribution of the VO molecule in the air-acetylene flame are very similar, independent of the vanadium oxidation state and added acid. Moreover, in all three cases, a rich flame is needed to effectively form the VO molecule via interaction with metastable or radical carbon species (Fig. 3b).
The magnitude of the signal does not depend on the V oxidation state and the acid used, as absorbances for the solution of metallic V dissolved in HNO3 and the solution of NH4VO3 in HCl are appropriately proportional (i.e. taking into account the V concentration).
Interestingly, the Al presence in the V solution significantly increases the V signal (Fig. 3a and b). A similar effect occurring in N2O-C2H2 flame is well known and widely used to increase the V signal in FAAS.23 The effect was explained as an influence of Al on the lateral distribution of V (and some other metals) in the determination using N2O-C2H2 flame.56 Due to the beneficial influence of Al on the VO signal (1) and the presence of Al in the catalysts' solutions (2), it was decided to add Al to the calibration solutions.
An exemplary calibration curve obtained for the V concentration range 100–2000 mg L−1 is shown in Fig. 3S.† Ammonium metavanadate was used as the V standard, which is easier to dissolve than other V compounds. Despite relatively low values of absorbance, excellent stability of measurements was achieved. The measured spectral range near 550 nm is in the spectral area known for its freedom from spectral interferences and exceptional stability.5,57,58 The determination coefficient (R2) of the curve is satisfactory (0.9991). Fig. 4S† shows good stability of measurements performed over two hours at relatively low absorbance values.
3.3. Determination of V in catalysts of hydroprocessing of heavy petroleum oil using the HR-CS FMAS technique
The possibility of V determination via the VO molecule absorption has been explored for the analysis of catalysts of hydroprocessing of heavy petroleum oil. Since the catalysts contain a large amount of Mo and Ni (active metals) and can contain a large amount of Fe (deposited from oil), the influence of these three metals and of Al (the base of the catalysts is alumina) on the measurement of VO molecule absorption was investigated. A solution of dissolved catalyst can contain 200–600 mg L−1 of Ni, 200–700 mg L−1 of Mo, <1–100 mg L−1 of Fe and 5000–6000 mg L−1 of Al. The content of V can reach from below 1 mg L−1 (fresh catalyst) up to more than 2000 mg L−1 (very contaminated, spent catalyst).In order to check the existence of any undesired spectral effects, the following solutions were measured in the spectral ranges shown in Fig. 2: 1% m/v Ni, 1% Mo, 100 mg L−1 of Fe and 2% of Al. Additionally, a mixed solution resembling a solution of a spent catalyst was measured, which contained 500 mg L−1 of Ni, 500 mg L−1 of Mo, 100 mg L−1 of Fe, 5000 mg L−1 of Al and 2000 mg L−1 of V. The spectra of pure metals obtained were similar to the spectrum of deionised water, with one single exception. A weak atomic Mo line has been found, which absorbs radiation at 550.6481 nm,55 quite closely to 550.6230 nm, i.e., one of the more intensive rotational lines of the VO molecule. The Mo atomic absorption can be seen in Fig. 4b and d. The spectra for the mixed solution are similar to the spectra of pure vanadium, except for the Mo atomic line, which can be seen in Fig. 4a and c. As shown in Fig. 4, the Mo line is distinctly separated from the VO molecule rotational lines. Also, the absorbance of the solution with 1% m/v of Mo was negligible. Therefore, it can be concluded that Mo does not disturb the determination of V. The most favourable (because of the low detection limit) wavelength range centred at 550.6230 nm was finally selected for the determination of V.
Apart from the above-considered metals, catalysts can contain other, not specified components. Thus, a valuable way of checking the accuracy of the proposed method was a comparison with an accepted procedure. A series of catalysts of various “lifetimes” was analysed using the proposed HR-CS FMAS method, with air-acetylene flame, and a common HR-CS FAAS method, with N2O-C2H2 flame. In the last case, all solutions of catalysts had to be diluted, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v. The results presented in Table 2 show satisfactory agreement between the proposed HR-CS FMAS and the reference HR-CS FAAS method as the texp value in a t-paired comparison59 was equal to 1.524, i.e. was lower than the tcrit value, 3.182 (95% confidence level). It should be added that spectra recorded for the catalyst solutions showed no additional and undesirable spectral events. Only signals of the target VO molecule and the above-considered Mo atomic line were observed.
50 v/v. The results presented in Table 2 show satisfactory agreement between the proposed HR-CS FMAS and the reference HR-CS FAAS method as the texp value in a t-paired comparison59 was equal to 1.524, i.e. was lower than the tcrit value, 3.182 (95% confidence level). It should be added that spectra recorded for the catalyst solutions showed no additional and undesirable spectral events. Only signals of the target VO molecule and the above-considered Mo atomic line were observed.
| Catalyst | Results of V determination ± result range, % m/ma (n = 3) | |
|---|---|---|
| HR-CS FAAS | HR-CS FMAS | |
| a Approximately 0.3 g of catalyst was transferred to 25 mL of solution; spent catalyst solutions were diluted before the HR-CS FAAS analysis. b The lower limit of the HR-CS FAMS method. | ||
| 1 (fresh) | <0.01 | <0.8b |
| 2 | 4.3 ± 0.2 | 3.9 ± 0.2 |
| 3 (fortified) | 7.7 ± 0.1 | 7.8 ± 0.4 |
| 4 | 12.1 ± 0.2 | 12.0 ± 0.2 |
| 5 | 15.2 ± 0.3 | 14.4 ± 0.5 |
Additionally, a recovery experiment was carried out. A catalyst sample containing 6.1 ± 0.2% m/m V, according to FAAS, was mixed with V2O5 powder and treated as other catalyst samples. The calculated V concentration in the fortified catalyst was 8.0% (m/m). In comparison, the determined concentration was 7.7% according to HR-CS FAAS and 7.8% according to HR-CS FMAS (sample 3 in Table 2). The V recovery is good, 97% and 98%, respectively, which confirms the equivalence of FAAS and FMAS and the accuracy of the whole analytical procedure, including sample dissolution.
The detection and determination limits of the HR-CS FMAS method calculated for an initial catalyst sample are 0.1 and 0.4% m/m of V, respectively. These parameters are much worse than in the case of the HR-CS FAAS method, with detection and determination limits in the initial catalyst sample 0.001 and 0.004% m/m, respectively. However, the detectability of the HR-CS FAAS method is even too high and sample solutions were diluted before measurement. The detectability of the HR-CS FMAS method is satisfactory from the point of view of catalyst analyses. Moreover, because there is no interest in the analysis of less spent catalysts with a low V content and there is a lack of such catalysts for validation, the lower limit of the proposed method has been established as 0.8% m/m. This value corresponds to the standard solution with the lowest V content (Fig. 3S†).
The results obtained in parallel analyses of the same sample deviate by less than 5% of their average. The precision achieved is satisfactory.
3.4. Evaluation of feasibility of vanadium determination via the VO molecule formed in the graphite furnace
Numerous papers on V determination using GF AAS were published. For chemical modification, mainly magnesium nitrate, noble metals, and metals qualified as the forming carbides of metal-like type were applied. No ideal modifier was found, and various discrepancies were identified. For example, similar behaviour of Mg nitrate and Mg acetylacetonate was expected.60 However, the first one improved analysis,32 while the second one acted as an interferent.60 Other controversies concern platform application. Manning and Slavin32 strongly recommended wall atomization for V determination, but nowadays, platform atomization is sometimes used,27,30 and it has to be used in the solid sampling option.31In preliminary experiments done in this work, no chemical modification was carried on. Unfortunately, the attempts to obtain any VO molecule signal were unsuccessful, even for solutions containing thousands of mg L−1 of V.
Zirconium is one of the most widely used permanent modifiers. Generally, it was thought that Zr forms metal-like carbides in a GF61–64 and the carbides protect the analyte from coming into direct contact with active carbon, preventing the formation of analyte carbides and enhancing the signal in GF AAS. However, it was also recognized that in the case of vanadium as an analyte, mixed carbides of the Zr–V–C type could be formed.32,35,64 This would not be desirable because V would be more strongly anchored in the solid phase, and evaporation of vanadium in any form would be more difficult and delayed.
Later studies of the furnace surface have shown that Zr used as a permanent modifier is not in the carbide form in the graphite furnace.65–67 Still, it occurs as zirconium oxide or in the metallic form. Niobium, a modifier of similar behaviour to Zr, was applied in the determination of V.29 The X-ray diffraction studies of the GF pre-treated with ammonium metavanadate showed the presence of vanadium carbides if no modifier was used and the presence of vanadium oxides when a Nb modifier was applied.
The zirconium permanent modifier was chosen for this work to promote the formation of VO molecules. Platforms pre-treated with the Zr modifier (as described in Section 3.1) were applied in the following experiments. Zirconium was used in the nanoparticle form to enhance its action.67
Initially, vanadium solutions were measured (metallic vanadium dissolved in HNO3). However, even for solutions with a very high content of vanadium (5000 mg L−1), very blurring and tailing signals, close to the baseline, were obtained. The signals had a shape of a poorly developed single peak.
In the further course of the study, measurements were carried out with a sample in the solid phase, i.e. vanadium pentoxide powder. The signals were high enough for optimization and had the shape of double peaks with a certain tailing part. The results of the experiments are presented in Fig. 5 and 6, and the data on the experiments are collected in Table 2S.† The following parameters were optimized to obtain the highest possible VO molecule signal: the temperature of the molecule formation phase and the heating rate to this phase, the application of air in the thermal decomposition step, the lack of argon purging after air application, and the use of a Pd modifier. The data are presented as time-resolved absorption (Fig. 5 and 6). The spectra come directly from contrAA's software. Therefore, time is expressed as a number of a spectrum appearing since the beginning of the measurement phase. Exemplarily, the spectra of a number 25, 50, 75, 100 and 125 correspond to 1.8, 3.6, 5.5, 7.4 and 9.1 seconds, respectively. The wavelength-resolved signals (not presented here) showed a pattern typical for the VO molecule and the used wavelength range.
The comparison of Fig. 5a (2200 °C, lower signal, appearing later) and 5b (2500 °C, higher signal, appearing earlier) confirms that higher temperature favours the occurrence of the VO molecule in the GF.
The possibility that the VO molecules exist at a temperature lower than 2200 °C during the molecule formation step was also considered. Two situations were compared when the GF is heated from the 500 °C temperature of the thermal decomposition step to the target 2500 °C temperature of the molecule formation step. A routine fast heating, 1500 °C s−1, and a slow heating, 500 °C s−1, were applied. As can be seen in Fig. 5b and e, the slow heating leads to no overall signal increase and no occurrence of additional early events. The obvious effect is some signal delay, as the temperature necessary to obtain the VO molecule in the gas phase is reached later.
The comparison of Fig. 5e and f shows the importance of oxygen application. Oxygen introduced with air into the GF during the pyrolysis phase at 500 °C (i.e. step 5 – Table 1) was probably chemisorbed in the graphite and released into the gas phase when the temperature of the molecule formation step was sufficiently high. Then, the VO molecule signal significantly increased (much higher in Fig. 5e compared to Fig. 5f). It is likely that the released oxygen reacted with atomised vanadium to form the VO molecule. Another option could be just improvements in the release of VO molecules from the solid phase by the chemisorbed oxygen.
Fig. 5c shows a significant increase in the second peak of the VO molecule. The most crucial difference between the experiments illustrated in Fig. 5b and c is the application (Fig. 5b) or the lack (Fig. 5c) of argon purging in step 6 (Table 1) after air application in the thermal decomposition step 5. Thus, in such a case (Fig. 5c), a lot of oxygen is present in the gaseous phase at the beginning of the molecule formation step (i.e. step 8). The V atoms, released from the initially taken V2O5 powder, can react with oxygen to form the VO molecule. In this way, a maximum signal of the VO molecule could have been obtained (Fig. 5c).
It is also possible that, as supposed by Styris and Kaye,34 the VO molecule could have been formed in the reaction of VO2 with the appearing V atoms. Styris and Kaye investigated V2O5 decomposition from vitreous carbon using a unique system comprising mass spectrometry detection of the gas coming from the GF.34 The double peaks, they observed, were attributed to VO and VO2 ions, and the VO signal was amplified at the expense of the VO2 signal.34
The next attempt made in the present work to increase the VO molecule signal concerns the application of a palladium modifier. Although Pd is one of the most widely used chemical modifiers, its action has not been fully explained. Traditionally, it has been applied in GF AAS to stabilize volatile analytes thermally. Palladium was also used68,69 in the determination of refractory analytes represented by vanadium (e.g. it was believed to decrease atomisation temperature68). In the present study, it was expected that Pd would favourably change processes in the solid phase to increase the VO molecule signal. Palladium (10 μl of 1000 mg L−1 of Pd solution) was injected over a sample. The joint application of Pd and oxygen (i.e. air in step 5 – Table 1) resulted in some increase in the overall signal (comparison of Fig. 5b and 6b). Admittedly, the VO peaks were reduced, but the tailing part of the VO signal, which can be linked with the stepwise release of the VO molecule from the solid phase, was significantly increased. It seems that Pd, applied with oxygen, promotes the generation of VO molecules directly from the solid phase. If, on the other hand, only Pd was applied without oxygen, the signal was lower than in the case of applying oxygen but without Pd (Fig. 5b and 6a). The joint action of Pd and oxygen is beneficial from the point of view of the overall signal. However, the signal is still lower than in the case of Fig. 5c of increased oxygen amount due to the lack of the argon purging step. Unfortunately, the increased tailing part of the VO molecule signal (Fig. 6b) would enforce stringent conditions of the cleaning step.
Some tailing of the VO signal can be observed in all cases of V compound analysis and when an empty wear platform is examined (Fig. 5d).
The idea of the VO molecule formation via a reaction involving atomic V seems to be confirmed by Fig. 6c, showing the atomic absorption signal of V recorded for an empty platform (previously used in HR-CS GFMAS experiments). The atomic absorption by vapours of V begins at a similar time and temperature to the formation of the VO molecule. The temporary character of the VO peaks can result from the consumption of oxygen (i.e. lack of supply of oxygen).
As shown, the behaviour of V in the GF is complicated, can involve various V forms and can run along different routes. Even in the case of one, the VO molecule, three components of the signal in the time-resolved spectra are distinguished (i.e. two peaks and a tailing part). The VO molecule could be formed at least according to three routes: in a reaction of atomized vanadium with oxygen in the gas phase (i), in a reaction of atomized vanadium with a VO2 molecule in the gas phase (ii) and in a process of release from the solid phase. The gas phase reaction (i) is most probable in the case of the second peak, while the delayed appearance of the VO molecule as a tailing signal could be linked with the stepwise release of the VO molecules from the solid phase. In any case, strong bonding of V in the solid phase occurs. The palladium modifier increases the tailing signal and seems to favour the release of the VO molecule from the solid phase.
Unfortunately, the studies performed did not lead to a new analytical method. Even if there is a chance to measure the VO molecule absorption in the GF, the analysis is non-sensitive. The best characteristic mass of V was higher than 100 ng (conditions of Fig. 5c, and 28 pixels taken for VO molecule signal evaluation) and needed using high temperature, as in the case of AAS.
A detailed explanation of the behaviour of vanadium in the graphite furnace would require systematic and extensive research that is beyond the scope of this work. However, it is evaluated that the HR-CS GFMAS technique has the potential for investigating the mechanism of processes in the GF, which has not been explored until now. This approach has the great advantage that the same system can be used in different experiments, e.g. MAS and AAS, or MAS and electrothermal evaporation (ETV). In the opposite case, when a sample is transferred from one system to another, with an inevitable temperature change, doubts may arise. For example, it was questioned if oxides, not carbides of analytes, exist in the GF.63 It was suspected that the oxides observed on the graphite surface outside the GF AAS apparatus could be formed after GF experiments.
4. Conclusions
For the first time, the absorption of radiation by the VO molecule was studied in flame and graphite furnace HR-CS molecular absorption spectrometry. The following conclusions can be drawn from the studies:- HR-CS FMAS can be applied for vanadium determination.
- The great advantage of the method is the use of common, cheap and easy-to-use air-C2H2 flame instead of N2O-C2H2 flame, which is essential for V determination using the FAAS method.
- HR-CS FMAS is relatively insensitive, which can be an advantage when analysing samples with high vanadium concentration (such as catalysts of heavy petroleum oil hydroprocessing); therefore, an additional dilution of the analysed solutions is not necessary.
- The HR-CA FMAS method of V determination in catalysts is simple, fast and cheap in comparison with other analytical techniques, for example, ICP-OES (inductively coupled plasma optical emission spectrometry) or ICP-MS (inductively coupled plasma).
- The hope for a new method for V determination using a GF with expected milder measurement conditions than AAS was not fulfilled.
- The spectral range near 550 nm is extremely stable (it can be used in various future research); therefore, signals of low absorbance can be analytically useful.
- The sensitivity and detectability of the HR-CS MAS technique can be improved by summing the signals from pixels of many more lines if a wider wavelength range can be detected simultaneously (equipment modification).
Author contributions
ZK: conceptualization, investigation, methodology, validation, visualization, writing – original draft, review & editing, resources, and funding acquisition. CA: conceptualization, investigation, formal analysis, methodology, and visualization. MO: conceptualization, investigation, formal analysis, methodology, writing – review & editing, and funding acquisition. SR: conceptualization, visualization, writing – review & editing, resources, and funding acquisition.Conflicts of interest
There are no conflicts of interests to declare.Acknowledgements
The work was partially financed by the Warsaw University of Technology (grant of the Dean of the Faculty of Civil Engineering, Mechanics and Petrochemistry, no. 504/04489/7192/44.000000). Z. K. is also deeply grateful for the financial support from the budget of the City of Płock in the frame of the task “Cooperation with Universities” and the Competition of the Mayor of the City of Płock (no. of grant 502250200007).References
- B. Welz, H. Becker-Ross, S. Florek and U. Heitmann, High Resolution Continuum Source AAS, Wiley-VCH, Weinheim, 2005 Search PubMed.
- B. Welz, F. G. Lepri, R. G. O. Araujo, S. L. C. Ferreira, M. D. Huang, M. Okruss and H. Becker-Ross, Anal. Chim. Acta, 2009, 647, 137–148 CrossRef CAS PubMed.
- B. Welz, S. Mores, E. Carasek, M. G. R. Vale, M. Okruss and H. Becker-Ross, Appl. Spectrosc. Rev., 2010, 45, 327–354 CrossRef CAS.
- D. J. Butcher, Anal. Chim. Acta, 2013, 804, 1–15 CrossRef CAS PubMed.
- M. Resano, E. Garcia-Ruiz, M. Aramendia and M. A. Belarra, J. Anal. At. Spectrom., 2019, 34, 59–80 RSC.
- M. Resano, M. Aramendia, F. V. Nakadi, E. Garcia-Ruiz, C. Alvarez-Llamas, N. Bordel, J. Pisoneiro, E. Bolea-Fernandez, T. Liu and F. Vanhaecke, Trends Anal. Chem., 2020, 129, 115955–115977 CrossRef CAS.
- M. D. Huang, H. Becker-Ross, S. Florek, U. Heitmann and M. Okruss, J. Anal. At. Spectrom., 2006, 21, 338–345 RSC.
- M. D. Huang, H. Becker-Ross, S. Florek, U. Heitmann and M. Okruss, J. Anal. At. Spectrom., 2006, 21, 346–349 RSC.
- U. Heitmann, H. Becker-Ross, S. Florek, M. D. Huang and M. Okruss, J. Anal. At. Spectrom., 2006, 21, 1314–1320 RSC.
- M. Resano, J. Briceno and M. A. Belarra, J. Anal. At. Spectrom., 2009, 24, 1343–1354 RSC.
- R. B. Ferreira, S. R. Oliveira, V. P. Franzini, A. Virgilio, J. L. Raposo Junior and J. A. Gomez Neto, At. Spectrosc., 2011, 32, 56–61 CrossRef CAS.
- M. A. Bechlin, J. A. G. Neto and J. A. Nobrega, Microchem. J., 2013, 109, 134–138 CrossRef CAS.
- Y. Wang, Y. Zhong, T. Yang and Z. Y. Shi, Yejin Fenxi/Metalurgical Analysis, 2016, 36, 1–5 Search PubMed.
- M. D. Huang, H. Becker-Ross, M. Okruss, S. Geisler and S. Florek, Spectrochim. Acta, Part B, 2016, 115, 23–30 CrossRef CAS.
- L. N. Pires, G. C. Brandao and L. S. G. Teixeira, Food Chem., 2017, 225, 162–166 CrossRef CAS PubMed.
- L. C. Pomarolli, M. A. M. S. da Veiga, M. Resano and F. V. Nakadi, J. Anal. At. Spectrom., 2020, 35, 2305–2314 RSC.
- M. Aramendia, M. R. Florez, M. Piette, F. Vanhaecke and M. Resano, J. Anal. At. Spectrom., 2011, 26, 1964–1973 RSC.
- W. Boschetti, M. Orlando, M. Dullius, M. B. Dessuy, M. G. R. Vale, B. Welz and J. B. De Andrade, J. Anal. At. Spectrom., 2016, 31, 1269–1277 RSC.
- Z. Kowalewska, J. Anal. At. Spectrom., 2018, 33, 260–273 RSC.
- M. S. P. Enders, G. S. Coehlo Junior, L. Z. Pires and D. L. G. Borges, Spectrochim. Acta, Part B, 2021, 183, 45–56, DOI:10.1016/j.sab.2021.106266.
- Z. Kowalewska, J. Pilarczyk and Ł. Gościniak, Spectrochim. Acta, Part B, 2016, 120, 45–56 CrossRef CAS.
- https://www.webelements.com/vanadium/compound_properties.html, accessed in November 2022.
- B. Welz and M. Sperling, Atomic Absorption Spectrometry, Wiley-VCH Verlag, Weinheim, 1999 Search PubMed.
- Aspect CS, 2.1.1.0, software to contrAA 700, Analytic Jena, Jena, Germany Search PubMed.
- R. Gurkan, S. Korkmaz and N. Altunay, Talanta, 2016, 155, 38–46 CrossRef CAS PubMed.
- F. A. C. Amorim, B. Welz, A. C. S. Costa, F. G. Lepri, M. G. R. Vale and S. L. C. Ferreira, Talanta, 2007, 27, 349–359 CrossRef PubMed.
- V. S. de Souza, L. S. G. Teixeira, J. S. S. O. Lima, U. M. F. da Mata Cerqueira, O. M. C. de Oliveira, A. F. de Souza Queiroz and M. A. Bezerra, Appl. Spectrosc. Rev., 2020, 55, 128–157 CrossRef.
- Z. Kowalewska, Spectrochim. Acta, Part B, 2007, 62, 273–282 CrossRef.
- R. Dobrowolski, A. Adamczyk and M. Otto, Talanta, 2013, 113, 19–25 CrossRef CAS PubMed.
- B. Ambrozini, V. R. A. Filho, S. R. Oliveira, L. V. S. Sacramanto and J. A. G. Neto, Food Chem., 2009, 116, 1024–1028 CrossRef CAS.
- A. C. Valdivia, E. V. Alonso, M. M. L. Guerrero, J. Gonzalez-Rodriguez, J. M. C. Pavon and A. G. de Torres, Talanta, 2018, 179, 1–8 CrossRef PubMed.
- D. C. Manning and W. Slavin, Spectrochim. Acta, Part B, 1985, 40, 461–473 CrossRef.
- R. E. Sturgeon, C. L. Chakrabarti and C. H. Langford, Anal. Chem., 1976, 48, 1792–1807 CrossRef CAS.
- D. L. Styris and J. H. Kaye, Anal. Chem., 1982, 54, 1792–1807 CrossRef.
- A. Hulanicki, R. Karwowska and J. Stańczak, Talanta, 1980, 27, 2214–2216 CrossRef PubMed.
- W. Wendl and G. Muller-Vogt, Spectrochim. Acta, Part B, 1984, 39, 237–242 CrossRef.
- W. Wendl and G. Muller-Vogt, Spectrochim. Acta, Part B, 1985, 40, 527–531 CrossRef.
- J. P. Matousek and H. K. J. Powell, Spectrochim. Acta, Part B, 1988, 43, 167–172 CrossRef.
- J. P. Matousek and H. K. J. Powell, Appl. Spectrosc., 1988, 42, 166–168 CrossRef CAS.
- M. F. Ali and S. Abbas, Fuel Process. Technol., 2006, 87, 573–584 CrossRef CAS.
- V. Garcia-Montoto, S. Verdier, Z. Maroun, R. Egeberg, J. L. Tiedje, S. Sandersen, P. Zeuthen and B. Bouyssiere, Fuel Process. Technol., 2020, 201, 106341 CrossRef CAS.
- E. Furimsky, Catal. Today, 1996, 30, 223–286 CrossRef CAS.
- S. P. Barik, K. H. Park, P. K. Parhi, J. T. Park and C. W. Nam, Sep. Purif. Technol., 2012, 101, 85–90 CrossRef CAS.
- H. Al-Sheeha, M. Marafi, V. Raghavan and M. S. Rana, Ind. Eng. Chem. Res., 2013, 52, 12794–12801 CrossRef CAS.
- K. K. Sahu, A. Agrawal and D. Mishra, J. Environ. Manage., 2013, 125, 68–73 CrossRef CAS PubMed.
- E. Furimsky and F. E. Massoth, Catal. Today, 1999, 52, 381–495 CrossRef CAS.
- M. Marafi and A. Stanislaus, Resour., Conserv. Recycl., 2008, 52, 859–873 CrossRef.
- A. Akcil, F. Verglio, F. Ferella, M. D. Okudan and A. Tuncuk, J. Waste Manag., 2015, 45, 420–433 CrossRef CAS PubMed.
- Z. Li, M. Chen, Q. Zhang, X. Liu and F. Saito, J. Waste Manag., 2016, 60, 734–738 CrossRef PubMed.
- S. Geisler, M. Okruss, H. Becker-Ross, M. D. Huang, N. Esser and S. Florek, Spectrochim. Acta, Part B, 2015, 107, 11–16 CrossRef CAS.
- M. D. Huang, H. Becker-Ross, S. Florek, C. Abad and M. Okruss, Spectrochim. Acta, Part B, 2017, 135, 15–21 CrossRef CAS.
- M. D. Huang, H. Becker-Ross, M. Okruss, S. Geisler, S. Florek, S. Richter and A. Meckelburg, Spectrochim. Acta, Part B, 2014, 107, 34–38 CrossRef.
- Z. Kowalewska, A. Ruszczyńska, I. Jaroń and E. Bulska, At. Spectrosc., 2012, 33, 196–204 CrossRef CAS.
- P. C. Mahanti, Proc. Phys. Soc., London, 1935, 47(3), 433–445 CrossRef CAS.
- https://www.nist.gov/pml/atomic-spectra-database, assessed in November 2022.
- A. C. West, V. A. Fassel and R. N. Kniseley, Anal. Chem., 1973, 45, 1586–1594 CrossRef CAS.
- S. Mores, G. C. Monteiro, F. S. Santos, E. Carasek and B. Welz, Talanta, 2011, 85, 2681–2685 CrossRef CAS PubMed.
- Z. Kowalewska and K. Brzezińska, Fuel, 2022, 309, 122197 CrossRef CAS.
- T. J. Farrant, Practical Statists for the Analytical Scientist. A Bench Guide, Royal Society of Chemistry, Cambridge, 1997 Search PubMed.
- Z. Kowalewska, B. Welz, I. N. B. Castilho and E. Carasek, Talanta, 2013, 103, 66–74 CrossRef CAS PubMed.
- H. Ortner, E. Bulska, U. Rohr, G. Schlemmer, S. Weinbruch and B. Welz, Spectrochim. Acta, Part B, 2002, 57, 1835–1853 CrossRef.
- D. L. Tsalev and V. I. Slaveykova, Chemical modification in electrothermal atomic absorption spectrometry, in Advances in Atomic Spectroscopy, ed. J. Sneddon, JAI Press, Greenwich, CT, 1998, vol. 4, pp. 27–150 Search PubMed.
- A. Volynsky, Spectrochim. Acta, Part B, 1998, 53, 509–535 CrossRef.
- A. Volynsky, Spectrochim. Acta, Part B, 1998, 53, 1607–1645 CrossRef.
- E. De Giglio, L. Sabbatini, L. Lampugnani, V. I. Slaveykova and L. L. Tsalev, Surf. Interface Anal., 2000, 29, 747–753 CrossRef CAS.
- M. A. Castro, A. J. Aller, A. McCabe, W. e. Smith and D. Littlejohn, J. Anal. At. Spectrom., 2005, 20, 385–394 RSC.
- C. Abad, S. Florek, H. Becker-Ross, M. D. Huang, A. G. Buzanich, M. Radke, A. Lippitz, V. D. Hodoroaba, T. Schmid, H. J. Heinrich, S. Recknagel, N. Jakubowski and U. Panne, J. Anal. At. Spectrom., 2018, 33, 2034–2042 RSC.
- V. A. Granadillo, R. A. Romero and J. A. Navarro, Anal. Sci., 1991, 7, 1189–1192 CrossRef CAS.
- A. Lechotycki, J. Anal. At. Spectrom., 1990, 5, 25–28 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ja00281g |
| This journal is © The Royal Society of Chemistry 2023 |