 Open Access Article
Open Access ArticleEnantiopure natural deep eutectic solvents for metal–organic framework chiral induction†
Renata A.
Maia
 a,
Audrey
Fluck
a,
Audrey
Fluck
 a,
Catalin
Maxim
a,
Catalin
Maxim
 b,
Benoît
Louis
b,
Benoît
Louis
 *c and
Stéphane A.
Baudron
*c and
Stéphane A.
Baudron
 *a
*a
aCNRS, CMC UMR 7140, Université de Strasbourg, 4 rue Blaise Pascal, F-67000 Strasbourg, France. E-mail: sbaudron@unistra.fr
bFaculty of Chemistry, University of Bucharest, 4-12 Regina Elisabeta Boulevard, 030018, sector 3, Bucharest, Romania
cCNRS, ICPEES UMR 7515, Université de Strasbourg, 25 rue Becquerel, F-67087 Strasbourg, France. E-mail: blouis@unistra.fr
First published on 18th October 2023
Abstract
A green alternative approach to the chiral induction of a metal–organic framework (MOF) has been explored using the natural deep eutectic solvents (DES) proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1
thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) and menthol
7) and menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1
thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). These solvents have been successfully employed for the preparation of the MIL-103 MOF and lead to an enantioenrichment of the porous material when used in enantiopure form, as demonstrated by circular dichroism.
1). These solvents have been successfully employed for the preparation of the MIL-103 MOF and lead to an enantioenrichment of the porous material when used in enantiopure form, as demonstrated by circular dichroism.
Chiral metal–organic frameworks (CMOFs) represent an interesting class of crystalline porous materials with a broad range of applications in catalysis, optics and separation.1 The most common approach to the elaboration of CMOFs consists in employing enantiopure building blocks.2 Albeit successful, this strategy requires the prior preparation of such enantiopure species, either metal nodes or most often organic ligands. This can represent a costly, energy-demanding and time-consuming process, potentially involving multiple steps (protection/deprotection, coupling reaction using metal-based catalysts, for example), the use of chiral reagents and purification using organic solvents. Aiming at reducing the number of steps, the energy consumption and the use of toxic/flammable reagents and solvents, it seems appealing to envision preparing CMOFs from achiral precursors via chiral induction based on an enantiopure additive or guest.3 For this purpose, a variety of chiral additives have been screened and employed in the literature for CMOF chiral induction (Table 1).4 Hence using representative compounds such as cinchonidine, leucine, proline, carvone, 2-amino-1-butanol, camphoric acid and camphor sulfonate, CMOFs and enantioenriched MOFs have been reported.4 However, it is worth noting that, while this process represents a great advancement, it still relies on the solvothermal synthesis of MOFs in toxic and flammable solvents like DMF, DEF or NMP, in the vast majority of these reports (Table 1). An alternative to chiral additive is the development of enantiopure solvents, as such chiral media straightforwardly provide an enantiopure environment (Table 1).5 In this respect, it has been demonstrated that chiral ionic liquids may be well suited since the large coulombic interactions between the enantiopure components yield a structured organization of the medium reflecting the asymmetry.5b,c Despite this successful proof-of-concept, ionic liquids have the drawback of sometimes requiring a purification step in their synthesis and may, themselves, show toxicity.6 Another class of media, cousin of ionic liquids, that may be considered for this aim is deep eutectic solvents (DESs).7 DESs are formed by mixing, in an appropriate molar ratio, two or more species that are usually solids at room temperature and prone to hydrogen bonding. The resulting fluids have a freezing temperature below that of either component, low vapor pressure, relatively wide liquid range, non-flammability and the ability to dissolve polar species.8 The preparation of these media consisting in the simple mixing of their components, with sometimes an additional heating/cooling step, can be considered green, as it limits the generation of waste and does not require a purification step, in contrast to ionic liquids. Furthermore, DESs may be composed of naturally occurring compounds,9 hence of readily accessible enantiopure species affording straightforward access to a variety of green chiral solvents, with potentially lower toxicity and flammability than solvents traditionally employed for solvothermal synthesis. Interestingly, DESs have demonstrated their adequacy for MOF synthesis,10 allowing the formation of prototypical materials, impacting their morphology and properties as well as their stability.11 However, a particular emphasis has been put on the use of DES based on choline chloride and urea derivatives,12 and, to the best of our knowledge, natural enantiopure DESs have not been explored as solvents for MOF chiral induction (Fig. 1). Such an approach would represent a green advancement in the field of CMOF preparation, as it constitutes a one-pot strategy without the prerequisite synthesis of chiral building blocks, hence showing a favorable profile in terms of cost, energy and time consumption as well as a limited number of involved chemicals and reagents. In addition, these media may be considered as potential substitutes for flammable solvents commonly employed for solvothermal synthesis, and, depending on the nature of their components, may show limited toxicity, the latter aspect being a matter of current active research.13
| Entry | Solvent | Temperature | Reaction time | Chiral inductor | Ref. |
|---|---|---|---|---|---|
| 1 | DMA | 140 °C | 2 days | L-Leucine | 4a |
| 2 | DMF/DEF | 120 °C | 3 days | (−)-Cinchonidine | 4b |
| (+)-Cinchonine | |||||
| 3 | Ethylene–urea | 140 °C | 4 days | (−)-Carvone | 4c |
| (+)-Carvone | |||||
| 4 | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1 MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
RT | 1 week | (−)-Camphoric acid | 4d |
| (+)-Camphoric acid | |||||
| 5 | NMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4 H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
120 °C | 2 days | L-Proline | 4e |
| D-Proline | |||||
| 6 | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (5 H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60 °C | 7 days | (R)-(−)-2-Amino-1-butanol | 4f |
| (S)-(+)-2-Amino-1-butanol | |||||
| 7 | DMSO/MeOH | 4 °C | 24 h | L-Proline | 4g |
| D-Proline | |||||
| L-Menthol | |||||
| D-Menthol | |||||
| 8 | 1-Butyl-3-methylimidazolium L-aspartate | 110 °C | 12 days | L-Aspartate | 5b |
| 9 | 1-Ethyl-3-methylimidazolium L-lactate | 140 °C | 5 days | L-Lactate | 5c |
| 10 | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
RT | 5 days | Low molecular weight gelators | 5e |
| 11 | (i) D- or L-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1 thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
120 °C | 3 days | L-Proline | This work |
| D-Proline | |||||
(ii) D- or L-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1 thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
L-Menthol | ||||
| D-Menthol |
In this work, the use of such chiral DES as green natural media for the preparation and enantioenrichment of a MOF has been investigated. To that aim, the MIL-103 material, Ln(btb) (Ln = Lanthanide, btb = 1,3,5-benzenetrisbenzoate), was chosen as it crystallizes in the R32 space group as a conglomerate with each individual crystal comprising either enantiomer resulting from the propeller-like twisted conformation of the btb ligand.14 Furthermore, a recent report has shown the two-step enantioenrichment of this MOF using first the formation of a complex with lanthanide cations with either L- or D-phenylalanine, followed by a solvothermal reaction with the tricarboxylic acid ligand in a mixture of DMF, methanol and water.15
In light of this literature precedent, the use of a carboxylic acid-based chiral DES was envisioned for a one-pot chiral induction process and the proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1
thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) DES was thus selected.16 Heating a 1
7) DES was thus selected.16 Heating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8 mixture of TbCl3·6H2O, btbH3 and NaOH (aq. solution) in D-, L-, or DL-proline
1.8 mixture of TbCl3·6H2O, btbH3 and NaOH (aq. solution) in D-, L-, or DL-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1
thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) at 120 °C for 3 days afforded a solid that was washed with water, DMF and EtOH. The material was then further activated by immersion in EtOH for one week with the solution being changed every two days, and then drying at 80 °C in an oven overnight. The solids were characterized by elemental analysis, powder X-ray diffraction, infrared spectroscopy, TGA and N2 adsorption–desorption isotherms at 77 K. The powder X-ray diffraction patterns (Fig. 2 and Fig. ESI1†) and the FT-IR spectra (Fig. ESI2†) of the three obtained materials are in agreement with what is expected for this MOF and match those of the reference material prepared under the reported14 water
7) at 120 °C for 3 days afforded a solid that was washed with water, DMF and EtOH. The material was then further activated by immersion in EtOH for one week with the solution being changed every two days, and then drying at 80 °C in an oven overnight. The solids were characterized by elemental analysis, powder X-ray diffraction, infrared spectroscopy, TGA and N2 adsorption–desorption isotherms at 77 K. The powder X-ray diffraction patterns (Fig. 2 and Fig. ESI1†) and the FT-IR spectra (Fig. ESI2†) of the three obtained materials are in agreement with what is expected for this MOF and match those of the reference material prepared under the reported14 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol (1
cyclohexanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) conditions. Elemental analysis confirmed the expected Tb(btb)(H2O)x formula, highlighting the efficiency of the work-up procedure to remove potential impurities such as ligands, salts or components of the DES. Only traces of remaining proline were observed for the D-pro-MIL-103(Tb) and DL-pro-MIL-103(Tb) materials.
1) conditions. Elemental analysis confirmed the expected Tb(btb)(H2O)x formula, highlighting the efficiency of the work-up procedure to remove potential impurities such as ligands, salts or components of the DES. Only traces of remaining proline were observed for the D-pro-MIL-103(Tb) and DL-pro-MIL-103(Tb) materials.
TGA analysis under nitrogen demonstrated analogous thermal behavior and stability for the MOFs synthesized from the three DES and water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol (1
cyclohexanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) MIL-103(Tb) prepared here as a reference (Table ESI1, Fig. ESI3–6†). A plateau with no major weight loss followed by decomposition at around 578–588 °C was observed for all four materials. The obtained decomposition temperature range was higher than the reported value of ca. 400 °C. This difference was expected and can be explained by the fact that TGA analyses were herein performed in the absence of oxygen, unlike the experimental conditions employed for the reported material. Oxygen-free analysis avoids chemical decomposition by oxidation, thus providing higher thermal stability, and consequently, a higher decomposition temperature. Consistently, a plateau until decomposition at around 600 °C has been determined in the literature for the TGA study of MIL-103(La) under N2.17
1) MIL-103(Tb) prepared here as a reference (Table ESI1, Fig. ESI3–6†). A plateau with no major weight loss followed by decomposition at around 578–588 °C was observed for all four materials. The obtained decomposition temperature range was higher than the reported value of ca. 400 °C. This difference was expected and can be explained by the fact that TGA analyses were herein performed in the absence of oxygen, unlike the experimental conditions employed for the reported material. Oxygen-free analysis avoids chemical decomposition by oxidation, thus providing higher thermal stability, and consequently, a higher decomposition temperature. Consistently, a plateau until decomposition at around 600 °C has been determined in the literature for the TGA study of MIL-103(La) under N2.17
The SEM micrographs for the MIL-103(Tb) MOFs prepared from DESs (Fig. ESI28–30†) show a rod morphology that is consistent with what has been reported for MIL-103(La)17a and MIL-103(Eu).17b Interestingly, block-shaped crystals were observed for the reference MOF prepared from water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol (1
cyclohexanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. ESI27†). The difference in morphology highlights the impact of the solvents, in particular DES, as previously illustrated for the benchmark materials HKUST-1 and Mg-MOF-74.11
1) (Fig. ESI27†). The difference in morphology highlights the impact of the solvents, in particular DES, as previously illustrated for the benchmark materials HKUST-1 and Mg-MOF-74.11
The specific surface area of the MOFs was determined from N2 adsorption–desorption isotherms at 77 K and calculated by the Brunauer–Emmett–Teller (BET) method (Fig. 3 and Table ESI1†). The microporous DES-synthesized MOFs presented a BET-specific surface area (SBET) range (592–787 m2 g−1) that was in accordance with the one reported in the literature, albeit slightly lower (730–930 m2 g−1).14 Notably, the water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol (1
cyclohexanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) MIL-103(Tb) MOF, prepared here as a reference, presented a higher SBET value of 1087 m2 g−1. This may be correlated to the different crystalline morphology. It is worth noting that a similar SBET value has been reported for MIL-103(La) prepared from DMF/MeOH/H2O (3
1) MIL-103(Tb) MOF, prepared here as a reference, presented a higher SBET value of 1087 m2 g−1. This may be correlated to the different crystalline morphology. It is worth noting that a similar SBET value has been reported for MIL-103(La) prepared from DMF/MeOH/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5),18 once again highlighting the effect of the preparation method on the textural properties. Interestingly, for all materials, a consistent pore distribution was observed with pores of around ca. 15 Å (Fig. ESI20–26†). Not only did these results confirm that the porosity of the MOFs prepared in DES is maintained but they also further support the absence of residues or impurities within the pores.
0.5),18 once again highlighting the effect of the preparation method on the textural properties. Interestingly, for all materials, a consistent pore distribution was observed with pores of around ca. 15 Å (Fig. ESI20–26†). Not only did these results confirm that the porosity of the MOFs prepared in DES is maintained but they also further support the absence of residues or impurities within the pores.
 | ||
| Fig. 3 N2 uptake vs. relative pressure plot of the MIL-103(Tb) MOFs. SBET values are shown in bold, respectively, for each material, in the legend. | ||
The study by diffuse-reflectance circular dichroism showed signals of opposite magnitudes for the D- and L-MIL-103(Tb) MOFs (Fig. 4). The CD spectra are the same, albeit weaker in intensity, as the ones reported when using a two-step process based on D-/L-phenylalanine.15a In their study, Yamada, Narushima and coworkers unambiguously demonstrated the absence of the remaining chiral inductor in the final material and attributed the CD signal around 310 nm to the signature of the chirality of the propeller-like twisted conformation of the btb ligand, hence of the MOF.15a Furthermore, it can be noted that the circular dichroism of free proline is expected at a different spectral range (Fig. ESI7†), strongly supporting the absence of a free residual chiral inductor in the pores of the MOF, in agreement with the above-mentioned results from elemental analysis and TGA. This unequivocally indicates an enantioenrichment of the material itself, dependent on the chirality of the DES component employed for the MOF synthesis. Such an effect was not observed for DL-MIL-103(Tb) prepared in a racemic solvent and for the reference material synthesized in water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol (1
cyclohexanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), as expected for a conglomerate. Interestingly, the activation process involving the immersion of the MOF in EtOH for a week followed by drying at 80 °C had limited impact on the CD signal, showing the stability of the MOF.
1), as expected for a conglomerate. Interestingly, the activation process involving the immersion of the MOF in EtOH for a week followed by drying at 80 °C had limited impact on the CD signal, showing the stability of the MOF.
 | ||
Fig. 4 Circular dichroism spectra of MIL-103 MOF(Tb) prepared in different solvents: D-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1 thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7), L-proline 7), L-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1 thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7), and DL-proline 7), and DL-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1 thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7), before and after activation. 7), before and after activation. | ||
In order to assess whether this approach can be extended to other lanthanides, MIL-103(Nd) and MIL-103(Eu) were prepared under the same experimental conditions. The materials were characterized by X-ray powder diffraction (Fig. ESI8–12†), confirming their identity, and then by diffuse-reflectance circular dichroism (Fig. 5). As for MIL-103(Tb), opposite signals were observed depending on the chirality of the proline composing the DES used for synthesis. For the three MOFs, a negative Cotton effect was consistently observed when using L-proline, whereas it was positive in the case of the MOF prepared from the D-proline-based DES. This consistency highlights the impact of the chirality of the DES on the enantioenrichment of the MOF. The difference in the signal intensity depending on the nature of the lanthanide can be related to the different sizes of the Ln3+ due to the lanthanide contraction phenomenon.19
 | ||
Fig. 5 Circular dichroism spectra of the MIL-103 MOF prepared in D-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol or L-proline thymol or L-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol, and DL-proline thymol, and DL-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol with different lanthanide cations: Tb, Eu, and Nd. thymol with different lanthanide cations: Tb, Eu, and Nd. | ||
In light of the literature precedent employing a two-step approach based on the preliminary formation of complexes with D-/L-phenylalanine, one can envision that the mechanism involved in the enantioenrichment at stake here using our one-pot strategy involves the in situ formation of chiral assemblies of Ln(III) cations with the enantiopure proline derivative that subsequently react with the btb ligand. It appeared of interest to employ another solvent, not comprising a carboxylic acid unit, to further extend our chiral induction strategy based on the use of enantiopure DES. In this context, the menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1
thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)20 DES was explored. Using this solvent and under the same conditions as detailed for the proline
1)20 DES was explored. Using this solvent and under the same conditions as detailed for the proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1
thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) DES, the synthesis of MIL-103(Tb) was explored. As confirmed by X-ray powder diffraction (Fig. 2 and Fig. ESI14†), the desired crystalline phase was obtained. The identity of MIL-103 was further supported by the FT-IR spectra corresponding to the ones obtained for the reference material as well as the MOFs obtained from the proline-based DES (Fig. ESI15 and ESI19†). It also shows the absence of residual menthol and/or thymol (Fig. ESI15†). TGA analysis under N2 demonstrated the same thermal stability of these materials as for the ones presented above (Table ESI1 and Fig. ESI16–18†). SEM micrographs of the materials prepared from the menthol–thymol (1
7) DES, the synthesis of MIL-103(Tb) was explored. As confirmed by X-ray powder diffraction (Fig. 2 and Fig. ESI14†), the desired crystalline phase was obtained. The identity of MIL-103 was further supported by the FT-IR spectra corresponding to the ones obtained for the reference material as well as the MOFs obtained from the proline-based DES (Fig. ESI15 and ESI19†). It also shows the absence of residual menthol and/or thymol (Fig. ESI15†). TGA analysis under N2 demonstrated the same thermal stability of these materials as for the ones presented above (Table ESI1 and Fig. ESI16–18†). SEM micrographs of the materials prepared from the menthol–thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) DES (Fig. ESI31–33†) also show a rod morphology, as when prepared from proline
1) DES (Fig. ESI31–33†) also show a rod morphology, as when prepared from proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1
thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). The specific surface area calculated using the BET method based on N2 adsorption–desorption isotherms at 77 K (Table ESI1†) is in the same range (589–758 m2 g−1) as the ones for the MOFs prepared in proline–thymol (1
7). The specific surface area calculated using the BET method based on N2 adsorption–desorption isotherms at 77 K (Table ESI1†) is in the same range (589–758 m2 g−1) as the ones for the MOFs prepared in proline–thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) (Fig. 3).
7) (Fig. 3).
Enantioenrichment was unambiguously demonstrated by diffuse-reflectance circular dichroism (Fig. 6) showing spectra with a signal at around 320 nm analogous, although weaker in intensity, to the ones collected for the MOFs prepared from the proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol DES and the one reported for MOF enantioenriched using a two-step approach based on phenylalanine.15a This confirms that the emergence of the signal is derived from the chirality in the MOF and not any residual chiral component from the DES. However, it can be noted that the activation process seems to impact the chirality more than that in the previous case. As expected, the material obtained from the racemic DL-menthol–thymol (1
thymol DES and the one reported for MOF enantioenriched using a two-step approach based on phenylalanine.15a This confirms that the emergence of the signal is derived from the chirality in the MOF and not any residual chiral component from the DES. However, it can be noted that the activation process seems to impact the chirality more than that in the previous case. As expected, the material obtained from the racemic DL-menthol–thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) DES did not lead to the appearance of a Cotton effect.
1) DES did not lead to the appearance of a Cotton effect.
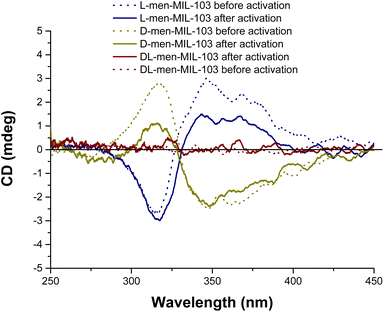 | ||
Fig. 6 Circular dichroism spectra of the MIL-103 MOF(Tb) prepared in D-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1 thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), L-menthol 1), L-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1 thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and DL-menthol 1), and DL-menthol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1 thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), before and after activation. 1), before and after activation. | ||
Conclusion
In conclusion, the foregoing results demonstrate that enantiopure natural DESs can be employed as green media for the one-pot synthesis of enantioenriched MIL-103. The identity and purity of the MOFs were characterized using elemental analysis, FT-IR, TGA, X-ray powder diffraction and N2 sorption-desorption isotherms. Chiral induction was assessed by diffuse-reflectance circular dichroism showing a Cotton effect for materials prepared from different enantiopure DESs, D- or L-proline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thymol (1
thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) and D- or L-menthol–thymol (1
7) and D- or L-menthol–thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). When using the racemic version of these solvents, such an effect could not be observed, consistent with the formation of a conglomerate, as for the reported reference material prepared from water
1). When using the racemic version of these solvents, such an effect could not be observed, consistent with the formation of a conglomerate, as for the reported reference material prepared from water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cyclohexanol (1
cyclohexanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Since proline presents a structural analogy with phenylalanine previously reported to lead to the two-step enantioenrichment of this MOF,15a the chiral induction was first demonstrated using this solvent for the preparation of not only MIL-103(Tb) but also the Eu and Nd analogues, showing consistent Cotton effects for the three MOFs, depending on the chirality of the DES. In order to extend this strategy, a preliminary investigation was focused on the menthol–thymol (1
1). Since proline presents a structural analogy with phenylalanine previously reported to lead to the two-step enantioenrichment of this MOF,15a the chiral induction was first demonstrated using this solvent for the preparation of not only MIL-103(Tb) but also the Eu and Nd analogues, showing consistent Cotton effects for the three MOFs, depending on the chirality of the DES. In order to extend this strategy, a preliminary investigation was focused on the menthol–thymol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) DES. While this solvent also led to the emergence of a Cotton effect, the process seems to be less efficient and straightforward using this DES and needs to be further optimised. Given the different nature of the chiral inductor (menthol vs. proline), a different mechanism, that remains to be determined, may be at stake for the enantioenrichment and responsible for this difference. As the CD spectrum of enantiopure MIL-103 could not be obtained, a quantitative estimation of the degree of enantioenrichment could not be assessed with this approach, preventing the determination of an enantiomeric ratio within the crystalline batches. However, it can be noted that mirror circular dichroism signals of opposite magnitude were observed depending on the chirality of the DES component. Future work will focus on the study of the impact of the chiral induction on the stereoselective activity of the MOFs such as for the cyanosilylation of aldehydes21 or on their photophysical properties.
1) DES. While this solvent also led to the emergence of a Cotton effect, the process seems to be less efficient and straightforward using this DES and needs to be further optimised. Given the different nature of the chiral inductor (menthol vs. proline), a different mechanism, that remains to be determined, may be at stake for the enantioenrichment and responsible for this difference. As the CD spectrum of enantiopure MIL-103 could not be obtained, a quantitative estimation of the degree of enantioenrichment could not be assessed with this approach, preventing the determination of an enantiomeric ratio within the crystalline batches. However, it can be noted that mirror circular dichroism signals of opposite magnitude were observed depending on the chirality of the DES component. Future work will focus on the study of the impact of the chiral induction on the stereoselective activity of the MOFs such as for the cyanosilylation of aldehydes21 or on their photophysical properties.
The one-pot strategy proposed herein may be considered as a green alternative to the conventional synthesis of CMOFs based on the preparation of chiral building units, an energy and time-demanding process and often requiring multiple steps and the use of diverse chemicals and reagents. Furthermore, in contrast with ionic liquids, previously reported as potential enantiopure solvents for such applications, DESs can be readily prepared from their natural affordable components, limiting waste generation and the need for purification steps. In addition to these advantages, the enantiopure natural DESs employed in this work can be regarded as alternatives to solvents commonly used for solvothermal synthesis, such as DMF, DEF or NMP. The extension of this one-pot approach using enantiopure green solvents for the preparation of other MOFs as well as other types of porous materials such as COFs or zeolites is also envisioned to fully demonstrate its potential.
Author contributions
Funding acquisition: S. A. B. and B. L.; experiments and data curation: R. A. M., A. F. and C. M.; investigation and interpretation of the results: R. A. M., C. M., S. A. B. and B. L; writing of the original draft: R. A. M. and S. B.; revision of the manuscript and additional contributions: R. A. M. C. M., S. A. B. and B. L.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has benefited from support provided by the University of Strasbourg Institute of Advanced Study (USIAS), within the French national programme “Investment for the future” (IdEx-Unistra). We also thank the Université de Strasbourg, the C.N.R.S., and the Ministère de l'Enseignement Supérieur, de la Recherche et de l'Innovation for financial support. We thank Thierry Romero for assistance in the SEM investigation.References
- For a recent review, see: W. Gong, Z. Chen, J. Dong, Y. Liu and Y. Cui, Chem. Rev., 2022, 9, 9078 CrossRef PubMed.
- (a) H. M. Tay, N. Kyratzis, S. Thoonen, S. A. Boer, D. R. Turner and C. Hua, Coord. Chem. Rev., 2021, 435, 213763 CrossRef CAS; (b) Z. Sharifzadeh, K. Berijani and A. Morsali, Coord. Chem. Rev., 2021, 445, 214083 CrossRef CAS.
- (a) R. E. Morris and X. Bu, Nat. Chem., 2010, 2, 353 CrossRef CAS PubMed; (b) T. Buhse, J.-M. Cruz, M. E. Noble-Terán, D. Hochberg, J. M. Ribó, J. Crusats and J.-C. Micheau, Chem. Rev., 2021, 121, 2147 CrossRef CAS PubMed.
- (a) X.-R. Hao, X.-L. Wang, C. Qin, Z.-M. Su, E.-B. Wang, Y. Q. Lan and K.-Z. Shao, Chem. Commun., 2007, 4620 RSC; (b) J. Zhang, S. Chen, T. Wu, P. Feng and X. Bu, J. Am. Chem. Soc., 2008, 130, 12882 CrossRef CAS PubMed; (c) Y. Kang, S. Chen, F. Wang, J. Zhang and X. Bu, Chem. Commun., 2011, 47, 4950 RSC; (d) K. K. Bisht and E. Suresh, J. Am. Chem. Soc., 2013, 135, 15690 CrossRef CAS PubMed; (e) S.-Y. Zhang, D. Li, D. Guo, H. Zhang, W. Shi, P. Cheng, L. Wojtas and M. J. Zaworotko, J. Am. Chem. Soc., 2015, 137, 15406 CrossRef CAS PubMed; (f) S. Das, S. Xu, T. Ben and S. Qiu, Angew. Chem., Int. Ed., 2018, 57, 8629 CrossRef CAS PubMed; (g) T. Zuo, D. Luo, Y.-L. Huang, Y. Y. Li, X.-P. Zhou and D. Li, Chem. – Eur. J., 2020, 26, 1936 CrossRef CAS PubMed.
- (a) L. Pérez-García and D. B. Amabilino, Chem. Soc. Rev., 2002, 31, 343 RSC; (b) Z. Lin, A. Slawin and R. E. Morris, J. Am. Chem. Soc., 2007, 129, 4880 CrossRef CAS PubMed; (c) Q.-Y. Liu, W.-L. Wong, C.-M. Liu, Y.-L. Wang, J.-J. Wei, Z.-J. Xiahou and L.-H. Xiong, Inorg. Chem., 2013, 53, 6773 CrossRef PubMed; (d) S. Xue, P. Xing, J. Zhang, Y. Zeng and Y. Zhao, Chem. – Eur. J., 2019, 25, 7426 CrossRef CAS PubMed; (e) D. Ghosh, M. Górecki, G. Pesticelli and K. K. Damodaran, Angew. Chem., Int. Ed., 2021, 60, 24406 CrossRef CAS PubMed.
- V. Hessel, N. N. Tran, M. R. Asrami, Q. D. Tran, N. V. D. Long, M. Escribà-Gelonch, J. Osorio Tejada, S. Linke and K. Sundmacher, Green Chem., 2022, 24, 410 RSC.
- B. B. Hansen, St. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich, B. W. Doherty, B. Gurkan, E. J. Maginn, A. Ragauskas, M. Dadmun, T. A. Zawodzinski, G. A. Baker, M. E. Tuckerman, R. F. Savinell and J. R. Sangoro, Chem. Rev., 2021, 121, 1232 CrossRef CAS PubMed.
- M. A. R. Martins, S. P. Pinho and J. A. P. Coutinho, J. Solution Chem., 2019, 48, 962 CrossRef CAS.
- (a) Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Anal. Chem. Acta, 2013, 766, 61 CrossRef CAS PubMed; (b) A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, ACS Sustainable Chem. Eng., 2014, 2, 1063 CrossRef CAS; (c) Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, J. Nat. Prod., 2018, 81, 679 CrossRef CAS PubMed.
- (a) R. E. Morris, Chem. Commun., 2009, 2990 RSC; (b) P. Li, F.-F. Cheng, W.-W. Xiong and Q. Zhang, Inorg. Chem. Front., 2018, 5, 2693 RSC; (c) R. A. Maia, B. Louis and S. A. Baudron, CrystEngComm, 2021, 23, 5016 RSC.
- (a) J. Zhang, T. Wu, S. Chen, P. Feng and X. Bu, Angew. Chem., Int. Ed., 2009, 48, 3486 CrossRef CAS PubMed; (b) R. A. Maia, B. Louis and S. A. Baudron, Dalton Trans., 2021, 50, 4145 RSC; (c) M. Teixeira, R. A. Maia, B. Louis and S. A. Baudron, CrystEngComm, 2022, 24, 601 RSC; (d) M. Teixeira, R. A. Maia, S. Shanmugam, B. Louis and S. A. Baudron, Microporous Mesoporous Mater., 2022, 343, 112148 CrossRef CAS.
- A. P. Abbott, G. Capper, D. L. Davies, R. K. Rasheed and V. Tambyrajah, Chem. Commun., 2003, 70 RSC.
- (a) M. Hayyan, M. A. Hashim, A. Hayyan, M. A. Al-Saadi, I. M. AlNashef, M. E. S. Mirghani and O. K. Saheed, Chemosphere, 2013, 90, 2193 CrossRef CAS PubMed; (b) M. Hayyan, Y. P. Mbous, C. Y. Looi, W. F. Wong, A. Hayyan, Z. Salleh and O. Mohd-Ali, SpringerPlus, 2016, 5, 913 CrossRef PubMed; (c) M. Marchel, H. Cieśliński and G. Boczkaj, J. Hazard. Mater., 2022, 425, 127963 CrossRef CAS PubMed.
- (a) T. Devic, C. Serre, N. Audebrand, J. Marrot and G. Férey, J. Am. Chem. Soc., 2005, 127, 12788 CrossRef CAS PubMed; (b) T. Devic, V. Wagner, N. Guillou, A. Vimont, M. Haouas, M. Pascolini, C. Serre, J. Marrot, M. Daturi, F. Taulelle and G. Férey, Microporous Mesoporous Mater., 2011, 140, 25 CrossRef CAS.
- (a) T. Yamada, T. Eguchi, T. Wakiyama, T. Narushima, H. Okamoto and N. Kimizuka, Chem. – Eur. J., 2019, 25, 6698 CrossRef PubMed; (b) T. Kitao, Y. Nagasaka, M. Karasawa, T. Eguchi, N. Kimizuka, K. Ishii, T. Yamada and T. Uemura, J. Am. Chem. Soc., 2019, 141, 19565 CrossRef CAS PubMed.
- M. Tiecco, D. A. Alonso, D. Ros-Ñíguez, G. Ciancaleoni, G. Guillena, D. J. Ramón, A. Apio Bonillo and R. Germani, J. Mol. Liq., 2020, 313, 113573 CrossRef CAS.
- (a) T. Uemura, N. Uchida, A. Asano, A. Saeki, S. Seki, M. Tsujimoto, S. Isoda and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 8360 CrossRef CAS PubMed; (b) Q. Liu, J.-M. Yang, F. Guo, L.-N. Jin and W.-Y. Sun, Dalton Trans., 2016, 45, 5841 RSC.
- J. Duan, M. Higuchi, S. Horike, M. L. Foo, K. P. Rao, Y. Imbushi, T. Fukushima and S. Kitagawa, Adv. Funct. Mater., 2013, 23, 3525–3530 CrossRef CAS.
- M. Górecki, L. Carpita, L. Arrico, F. Zinna and L. Di Bari, Dalton Trans., 2018, 47, 7166 RSC.
- (a) D. J. G. P. van Osch, C. H. J. T. Dietz, J. van Spronsen, M. C. Kroon, F. Gallucci, M. van Sint Annaland and R. Tuinier, ACS Sustainable Chem. Eng., 2019, 7, 2933 CrossRef CAS; (b) D. O. Abranches, M. A. R. Martins, L. P. Silva, N. Schaeffer, S. P. Pinho and J. A. P. Coutinho, Chem. Commun., 2019, 55, 10253 RSC.
- T. Kajiwara, M. Higuchi, A. Yuasa, H. Higashimura and S. Kitagawa, Chem. Commun., 2013, 49, 10459 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, X-ray powder patterns, TGA plots, SEM micrographs and IR spectra. See DOI: https://doi.org/10.1039/d3gc02993j |
| This journal is © The Royal Society of Chemistry 2023 |


