Open Access Article
Open Access ArticlePhysico-chemical challenges on the self-assembly of natural and bio-based ingredients on hair surfaces: towards sustainable haircare formulations
Gustavo S.
Luengo
 *a,
Fabien
Leonforte
a,
Andrew
Greaves
a,
Ramon G.
Rubio
b and
Eduardo
Guzman
*a,
Fabien
Leonforte
a,
Andrew
Greaves
a,
Ramon G.
Rubio
b and
Eduardo
Guzman
 *bc
*bc
aL'Oréal Research and Innovation, Aulnay-Sous Bois, France. E-mail: gluengo@rd.loreal.com
bDepartment of Physical Chemistry, Universidad Complutense de Madrid, Spain. E-mail: eduardogs@quim.ucm.es
cPluridisciplinar Institute, Universidad Complutense de Madrid, Spain
First published on 15th September 2023
Abstract
Polymers and surfactants are used in many technological and industrial applications such as the manufacture of functional materials and coatings, personal care and pharmaceutical products, food science, paints, anti-icing fluids, tertiary oil recovery and the paper industry. Polymer–surfactant mixtures are particularly important in shampoos and conditioners. However, as in almost every other industry over the past five or more decades, the performance of hair care formulations has been significantly improved by the use of petrochemical-derived ingredients. As a result, cosmetic formulations, and hair care formulations in particular, have been based primarily on polymers and surfactants that are neither renewable, nor derived from environmentally friendly processes, nor have a positive environmental impact. This contrasts with the extensive use of natural and renewable products, mainly plant extracts, in cosmetics in ancient times. Therefore, the substitution of currently used ingredients with others of natural origin has been a top priority for the cosmetic industry over the last two decades, and in order to achieve greater consumer acceptance, it is crucial to maintain and, where possible, improve the technical performance of such products. This paper describes the complexities and challenges of developing greener shampoo and conditioner ingredients and formulations to meet current and future needs, and outlines a methodological approach based on model hair surfaces and a selection of appropriate experimental and numerical techniques to achieve our goals. Some encouraging technical routes using biosurfactants, biopolymers and bio-based polymers are presented, along with the significant opportunity to obtain a wide range of green ingredients through molecular design and well-controlled biotechnological processes. Similar concerns apply to other cosmetic products such as waxes, fragrances, bleaching agents, etc.
1. Introduction
Natural resource depletion, waste generation, climate change, and water and air pollution are some of the major challenges that modern society must address.1 This has driven very important changes in consumer as well as industrial behaviour particularly in the design of new, eco-friendly products.2 For instance, the cosmetics industry is interested in designing new ingredients and products of natural origin, and, if possible, biocompatible and/or biodegradable and with low aquatic toxicity to replace those that are neither renewable, obtained from processes that are not responsible towards the environment, nor have a favourable environmental impact (the 3 pillars of green science, fundamental and inseparable). This is nothing new, as the use of natural products in cosmetics has been a common practice since ancient times. In fact, there is a wealth of archaeological and documentary evidence of the historical use of renewable and natural plant extracts in vegetable oils for therapeutic and aesthetic purposes for thousands of years.3 Probably the most famous example of the use of natural products in cosmetics is Cleopatra's donkey milk-based skin treatment.4,5 In the last two decades, so-called fossil-based ingredients are being replaced by bio-based ingredients, and new sustainable production technologies are being introduced that also aim to reduce greenhouse gas emissions.6–10 In addition, with this in mind, significant efforts are also being made to redesign and develop packaging materials that are recyclable and biodegradable.11,12 In fact, one of the current trends of industry is to exploit green chemistry, biotechnology and green extraction to develop innovate processes and products that reduce industrial environmental footprint.This paper attempts to guide the reader through some of the main challenges faced by the cosmetic industry in replacing traditional petrochemical ingredients with new ingredients to ensure the manufacture of products with a lower environmental and health impact. In particular, this work aims to provide a comprehensive background on the substitution of two common ingredients in conditioning shampoos: cationic conditioning polyelectrolytes and anionic sulphate-based surfactants. It also discusses some experimental aspects related to the evaluation of the efficacy of these new ingredients, using specific examples for this purpose, and to the evaluation of the sustainability of the formulations.
2. Green Chemistry and sustainability foundations: impact in cosmetics industry
In the late 1990s, Paul Anastas and John C. Warner proposed the so-called Twelve Principles of Green Chemistry to guide chemists in promoting the use of substances obtained in a manner that is more respectful of the environment and human health.13Table 1 summarises these principles, which should be applied to the fabrication of any cosmetic product.| 1. | Prevention of waste production |
| 2. | Atom economy |
| 3. | Less hazardous chemical synthesis |
| 4. | Design benign chemicals |
| 5. | Benign solvents and auxiliaries |
| 6. | Design for energy efficiency |
| 7. | Use of renewable feedstocks |
| 8. | Reduce derivatives |
| 9. | Catalysis (vs. stoichiometric) |
| 10. | Design for degradation |
| 11. | Real-time analysis for pollution prevention |
| 12. | Inherently benign Chemistry for accident prevention |
A very brief description of the Twelve principles is given in the following. Prevention means that it is preferable to prevent waste than to treat or clean it up.14 Atom economy refers to the fact that any synthetic method used has to be designed for incorporating all the materials used in the process into the final product.15 Less hazardous chemical synthesis, implies that synthetic methods have to be designed to use and generate substances that are little or no toxic to health or environment. Designing safer chemicals means that chemical products must be designed to be efficient while reducing toxicity.16 Benign solvents and auxiliaries, indicates that auxiliary substances, e.g., solvents, should be unnecessary, whenever possible and innocuous when used. Design for energy efficiency means that energy requirements should be minimize for reducing the environmental and economic impacts, and the synthetic methods should be conducted at environmental temperature and pressure. Reduce derivative refers to the fact that derivatization of substances or processes should be minimized or avoided, because those steps usually require additional reagents and/or solvents and can generate waste. Whenever possible, catalysts must be used, because they can be chosen to be selective, and thus better than stoichiometric.17 Chemical products have to be designed for breaking down into innocuous degradation products and not persist in the environment. The development of real-time analytical processes have to be used or developed to control formation of hazardous substances.13 The introduction of green chemistry in the development of cosmetics industry is a key point towards reducing the environmental footprint of cosmetics products. In most of the cases, cosmetics ingredients have been chosen up to recent dates in such a way that maximizes their performance by minimizing costs. This approach does not consider the environmental impact of cosmetics industry and its byproducts and residues. Therefore, it is of a paramount importance to use green approaches on the production and selection of raw materials.18
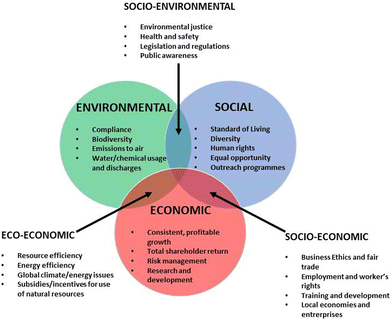
In a wider context, the concept of sustainability is based on three core principles. These principles are environmental, social, and economic sustainability, each focusing on a company's impact on those three aspects of the world. Sustainability is a broader concept than Green Chemistry, that extends the principles of Table 1 to include other more directly related to the production and social impact. Fig. 1 shows the three dimensions of sustainability, and Table 2 includes the so-called seven principles of sustainability.
| 1. | Sustainable design |
| 2. | Durability |
| 3. | Energy efficiency |
| 4. | Waste reduction |
| 5. | Indoor air quality |
| 6. | Water conservation |
| 7. | Sustainable building materials |
Nowadays, it is possible to find metrics that allows evaluating the sustainability profile of consumer products, including cosmetics.19–21 This metrics are aimed to provide unambiguous and objective scoring, which helps on the comparison of the potential environmental impacts for processes, projects, or industries.20 Among them, the environmental factor, E, is extensively used, providing an evaluation of the ratio between the tonnage of waste and that of the desired molecule. The usual scale for E is 0 to 50, with typical values for cosmetics and pharmaceuticals ranging from 5 to 50, but for petrochemical processes such as oil refining, E can be as low as 0.1.22 The definition of metrics is important because industry, in particular cosmetics one, has adopted practices aimed at improving the sustainability of their products by formulating them with natural ingredients. This approach enables the industry to meet consumer expectations for more environmentally friendly formulations while demonstrating corporate social responsibility. Cosmetic companies are committed to sustainable development and actively seeking raw materials of natural origin.23,24 The alignment of cosmetics industry with UN sustainable development goals (SDGs) requires a comprehensive approach that includes all stages of the product life cycle, including the ingredients used, and follows the principles of green chemistry.24–27 The relationships between the main development lines of cosmetics industry and the SDGs is summarized in Fig. 2.
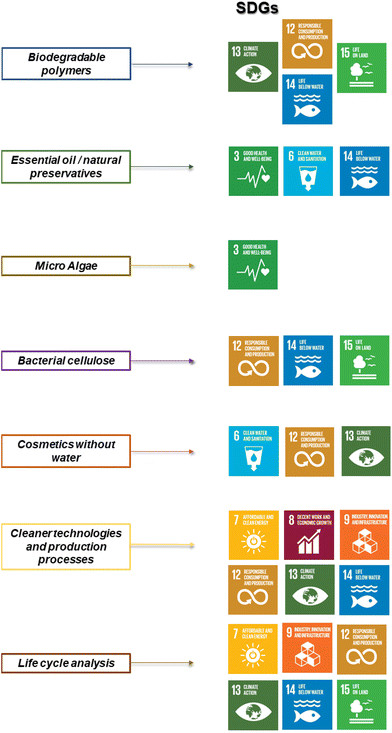 | ||
| Fig. 2 Relationships between cosmetics industry research and development lines and the SDGs. Adapted from Cubas et al.,27 with permission from MDPI under Open access CC by 4.0 license, Copyright (2022). | ||
Sustainability in the cosmetics industry begins at the development and design stage, where the product is carefully crafted, and extends through various stages, including ingredient selection, raw material sourcing, manufacturing, packaging, transportation and distribution, retail operations, consumer use and post-use management.28 It should be emphasized that even though there is a range of natural ingredients available, certain recommendations outline the selection of sustainable ingredients, which include: (i) the use of renewable resources; (ii) the use of environmentally friendly manufacturing processes, and (iii) minimising environmental impact.24,25 Therefore, it can be considered that modern cosmetic industry in its seeking of more sustainable and environmental friendly products tries to accommodate its products and processes to the SDGs.27
It is worth noting that sustainability is usually used as a synonym of green, organic or natural. However, it is not always true. In fact, organic, green or natural are terms that are generally associated with the origin and type of practices used to obtain the ingredients for the products, and these aspects are not necessarily linked to the sustainability. For instance, green cosmetics are those that contain organic and/or natural ingredients, avoiding synthetic ones. On the other side, sustainable cosmetics requires to fulfil the three dimensions of the sustainability within the whole product lifecycle.11
3. Assessment of sustainability in cosmetics industry
Sustainability assessment is a valuable tool aimed at recognizing, anticipating, and evaluating the potential environmental, social, and economic impacts of processes and products. Applying sustainability assessment from the outset is critical because it helps identify trade-offs and streamlines optimization throughout the planning and decision-making stages.29 In the cosmetics sector, the analysis of sustainability can be approached from various perspectives, encompassing consumers, supply chain, raw materials, and the assessment of product sustainability throughout its life cycle.30 However, up to date the cosmetics industry does not present standards or precise regulations to drive the transition towards more sustainable practices.30A wide range of sustainability assessment methodologies are available. However, they have different objectives, scope and data requirements, which raises many questions about their similarity and the validity of the conclusions drawn.11,31 From a general perspective, sustainability assessment can be carried out according to three different approaches: (i) indicators/indices; (ii) product-related assessment, and (iii) integrated assessment.32 The first approach provide an assessment of sustainability through an index that assigns a quantifiable value to qualitative dimensions.32 The flexibility and ease of interpretation of the data obtained has encouraged the development of this strategy for assessing sustainability. The use of a product-related assessment is commonly associated with the environmental aspect of sustainability and considers the life cycle of the product (LCA – Life Cycle Assessment), providing a quantitative assessment. However, the use of this approach requires a large amount of data and makes it difficult to include some important elements in the sustainability assessment. The last approach (Integrated Assessment) is more complex than the previous ones and includes a wide range of qualitative and quantitative parameters to assess sustainability.32
In the specific case of the cosmetics industry, the use of sustainability metrics has grown significantly in recent years.33 The starting point for any sustainability assessment that seeks to provide a perspective that includes the three fundamental dimensions of sustainable development requires the introduction of the concept of Life Cycle Thinking (LCT).34 This is an assessment that includes the impacts of a specific product along the supply chain and during its use and end-of-life management. This assessment seeks to reduce the negative impacts that may counteract any improvements made at another stage of the life cycle. On the other hand, the use of LCA considers the existence of an interdependence between all stages of the product life cycle, which is the basis for the development of LCT. This has led to the use of the LCT approach to identify and evaluate the environmental impacts of the cosmetics industry, giving priority to the adaptation of products, processes, and packaging to ensure the production of cosmetics with good environmental profiles and to evaluate the footprint (environmental, carbon and water) of the company. Based on the above considerations, LCA and LCT are mostly focused on the analysis of the environmental dimension, but they can be extended to consider the economic and social factors.11,32
It is worth noting that the assessment of the environmental dimension of sustainability can be supported by different tools. Environmental risk assessment (ERA) is probably the most widely used, while environmental management systems (EMS) are tools mainly used by producers.35,36 These tools allow the assessment of specific stages of the product life cycle. For example, ERA helps to assess the consumer and post-consumer phases, while EMS only provides information on the manufacturing phase.11
On the basis of the above discussion, it is important to emphasize that in most cases the evaluation of the sustainability of cosmetics products and processes is limited to the analysis of the environmental impact, while the social and economic dimensions are most neglected.30
4. Bioinspired research in cosmetics
Nature has led to many mechanisms for modifying the physico-chemical properties so that living species can adapt to their environment. Bioinspired and biomimetic approaches are being used in the cosmetic industry as inspiration for eco-design and in particular to modulate the physico-chemical properties of surfaces. Nowadays, there are different ways to exploit biologically inspired strategies, which have contributed to shape the so-called “green and sustainable science and technology”.37 The cosmetics industry has paid much attention to optimise the performance and sustainability of raw materials, the different manufacturing processes, and the methodologies for analysing the formulations.6 For instance, an extensive activity has been developed to improve the sustainability profile of different ingredients (polymers, surfactants, etc.) for obtaining commercial products with different functions.38 There are currently a broad range of ingredients that are obtained or produced through sustainable processes. This is important because the development of processes ensuring the production of eco-friendly and sustainable raw materials is only a part of the green challenge of the cosmetic industry, and the circular economy concepts should include individual ingredients, and also the whole formulation and the packaging.11,33,39The future of cosmetics formulations relies on a holistic approach to ingredient design and production which accounts for the environmental impacts of their whole lifecycle. When looking for new cosmetic products, it is important to differentiate between products of natural origin, biocompatible products, and biodegradable ones. The three aspects are not always fulfilled by a given compound, thus leading to rather different environmental effects. Moreover, we will discuss that in each commercial product a mixture of ingredients that can fulfil all, some, or none of the above conditions can be found. This is evident after looking to the ingredients of products that are advertised as containing only compounds of natural origin, excluding those by GMO (genetically modified organisms) species.
In choosing ingredients, we must be vigilant of the sources of raw materials, ensuring fair trade practices that lead to positive societal impacts.6,40 Moreover, the increasing environmental awareness of the cosmetics industry has contributed to the development of new ingredients compliant with Green Chemistry principles obtained from natural and sustainable resources. Depending on the specific cosmetic application, there is already a wide range of ingredients available to the formulator as suitable replacements for petrochemical-based ingredients. These offer many advantages, among them their improved safety profile, good biodegradability, and environmentally friendly character, which have become of paramount importance for cosmetics industry future developments.41,42 Even so, it is important to state that there are some potential drawbacks with some of them, such as stability and compatibility with existing ingredients. Moreover, they are sometimes complex mixtures of ingredients, as in the case of compounds obtained by fermentation, which can pose a problem in batch-to-batch production reproducibility. In fact, even though the differences in the chemical structures of the surfactants produced can be subtle, this can lead to notably different behaviors.43,44
Besides all the above considerations, one must keep in mind that any final cosmetics product has to ensure: (i) the correct blend of ingredients for treating the specific hair or skin condition or type; (ii) each formula must contain the right proportion of ingredients: antioxidants, anti-irritants, softeners, polyelectrolytes, surfactants, etc.; (iii) the different ingredients work synergistically; (iv) the concentrations of the ingredients present the optimal values; (v) the formula has the appropriate pH, ionic strength, etc., and (vi) only the best ingredients should be used, regardless of their costs. This is very important because cosmetics products contain a large mixture of chemical compounds that can be classified according to their function, as shown in Table 3.
| Type of ingredient | Function |
|---|---|
| Dyes or pigments | Colour cosmetics |
| Emollients | Prevent water loss |
| Emulsifiers | Stop ingredients from separation. |
| Fragrances | Improve the smell of cosmetic products. |
| pH stabilizers | Adjust the pH of cosmetics |
| Preservatives | Prevent the growth of microorganisms. |
| Solvents | Dissolve other ingredients |
| Thickeners | Increase the viscosity of the products. |
In the recent Cosmetics Global Meeting (Barcelona, March 28–30, 2023),45 some results of the increase of fulfilment of all the criteria above mentioned by cosmetic industries were discussed. For instance, Dow presented three new surfactants, obtained by fermentation, whose production leads to lower greenhouse gas emissions than the petrochemical-based ones to be replaced. Furthermore, the new Dexcare™ polysaccharide is intended to substitute the traditional silicone used as conditioner in hair cosmetics. Rhamnolipids have been shown to be useful for substituting thickeners in shampoos and conditioners.46 Another strategy, followed by Sharon Personal Care, is to reduce the loading levels of active and functional ingredients. An example of this strategy for skin care products is to make the actives to penetrate precisely to the skin layer where they must produce a beneficial effect. For instance, sunscreen protection ingredients must remain at the surface, while other, e.g., polyphenol quercetin must penetrate between the epidermis and the dermis layers. Companies such as Evonik, Dow and Solvay have produced several sophorolipid surfactants made by fermentation. Frequently, production of biosurfactants implies lower greenhouse gas emissions, although they use to be more expensive.
Other examples are the utilization of molecules, such as hydroxystearic acid, obtained using biotechnological process that meet the Green Chemistry principles.47 In fact, DSM has recently introduced hydroxystearic acid (and other hydroxy fatty acids) obtained by biotechnological processes as an adjuvant in their skin care products to counteract skin aging effects.48,49 On its side, L'Oréal has adopted the use of Green Pro-xylane,50 an anti-aging ingredient, using a natural sugar obtained from a renewable raw material. The traditional synthesis was substituted for a new one containing only two steps instead or eight and using water as the only solvent. For this process the E factor was 13 that is quite acceptable. This molecule has demonstrated to pass the standard ecotoxicity tests. Also, its water solubility ensures a very low solubility in fats, thus not leading to accumulation through the food chain. Finally, its storage is easy after dissolution in water, not needing any preservative. The company claimed that in 2019 up to 28% of their raw materials accomplished with the Green Chemistry principles. Recently another strategy is being explored by start-up companies such as Microphyt in partnership with L'Oréal in the use of microalgae, microscopic plants, that can be produced by low-carbon processes, and that can become actives in skin cosmetics.51 Similar concept is also exploited by other companies such as AlgaeSense.52 More recently Ulé (Shiseido group) has developed cosmetics products from plants obtained from the so-called vertical agriculture, free of pesticides, etc., and in which up to 95% of water is recycled, thus being environmentally friendly.53 It is therefore clear that there is an increasing need to produce even more natural ingredients while guaranteeing performance requirements to meet the current green goals of the cosmetics industry and reduce its environmental impact.
A very important aspect to consider in the design of new formulations with improved sustainability profiles is the stability against biological degradation mechanisms. In fact, the understanding of the microbial degradation processes of biologically derived ingredients plays a central role on the ingredient choice, and even in the design of new biodegradable compounds. Sugar-like ingredients are specially prone to such a degradation.54 Therefore, it is of a paramount importance to understand the degradation processes, and its consideration in the design of new ingredients.2,55,56
In summary, the choice of ingredients with suitable characteristics is a very complex process that requires some help of modelling approaches.57 Moreover, the transformation or chemical modification of renewable resources by using green chemistry concepts ensure the production of novel sustainable raw material which should ensure 3 main aspects: (i) the cosmetic performance; (ii) the environmental performance (reducing the environmental impact of the final formulation), and (iii) economic performance (offering affordable and sustainable products).25 Therefore, the eco-design of cosmetics products is challenging, and cosmetics industry is working extensively in the production of new products or the renovation of the currently commercialized paying special attention to the potential environmental impacts during their entire lifecycle. This requires seeking satisfactory solutions in relation to the consumer demands, technical feasibility, and environmental footprint.
5. The case of conditioning shampoo formulations
Nowadays, it is expected that cosmetics formulations for hair care, and in particular shampoos, present a dual role, i.e., allows one to accomplish two different challenges. On one side, shampoos must contribute to the removal of different endogenous compounds existing in sebum and sweat as well as of exogenous compounds, e.g., dirt or cosmetics treatments, from the surface of the hair fibres. Moreover, hair care products should contribute to the temporal reparation of the damaged and weakened hair, making hair shiny, manageable, and soft. This is only possible by modifying the chemistry and the physico-chemical properties of the surface of the hair fibres by the deposition of specific components of cosmetics formulations, e.g., polymers such as silicones, which alter adhesion and friction properties of the fibres in such a way that makes their combing easier, styling and manageability of hair; quaternary ammonium compounds for easier combing; proteins for thickening, or polyvinylpyrrolidone for shaping and styling. Unfortunately, so far it is not possible to make a complete restoration of the hair to its initial state due to the absence of biological mechanisms for such purpose.58,59 Therefore, the choice of the ingredients for cosmetic products, especially those oriented to the hair care, must consider that their activity is commonly determined by the interaction of the ingredients with the surface and its penetration.25 In particular, the performance of hair care formulations, e.g., conditioners and shampoos, depends on the adsorption of specific compounds on the surface of the hair fibres which is commonly damaged by different chemical and physical processes (see Fig. 3 for a scheme of the typical result of the degradation process of hair). It should be noted that hair damage results in the appearance of different functional groups (sulfonate, hydroxyl, ammonium, among others) at the surface of the hair fibres. These functional groups originate from delipidation and subsequent oxidation of the hair surface as well as chemical and physical damage to the outer layers of the cuticle proteins leading to rupture of disulphide (cystine) linkages and exposure, sometimes by delamination, of the different protein structures that form the multi-layered structure of the hair.59 This is the result of the combined action of environmental factors, e.g., UV light, chemical treatments, e.g., dyeing or aggregation and physical processes.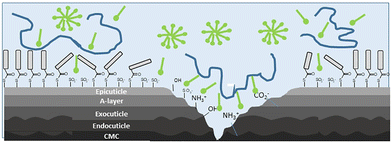 | ||
| Fig. 3 Sketch representing the typical result of the degradation process of hair, and how cosmetic ingredients interact with damaged hair fibres. Blue molecules represent cationic polyelectrolyte chains, and green ones the surfactant molecules. Adapted from Luengo et al.25 with permission from Elsevier, Copyright (2021). | ||
Nowadays, the hair care sector is seeking new alternatives based entirely in the use of new ingredients or the combination of conventional ones by using new strategies. In particular, the large diversity in the molecular structures of natural and bio-based ingredients may provide many opportunities to tune their interactions with cosmetics substrates, modulating their cosmetics functions.60 This is important because hair cleansing and conditioning formulations have a set of specific ingredients characterized by their higher environmental impact in relation to others. For instance, replacing cationic polyelectrolytes and silicones is one of the most important challenges to the success on the green cosmetic challenge.61 However, there are currently different alternatives that are promising for their replacement. For instance, it is common to find in the market silicone-free and sulphate-free products for cleansing and haircare, which are aligned to the reduction of the environmental impact of cosmetics industry. Unfortunately, up to date it is not possible to commercialize products enabling the conditioning of highly damaged hair fibres without using silicones and/or cationic polyelectrolytes.25 Thus, even most of the current formulation can contain around ∼60% of renewable ingredients, there is still need of all formulations to be respectful for the environment.62 Moreover, in some cases, bio-based ingredients are obtained through chemical processes that are far from being considered green chemistry, and it is therefore also necessary to replace them with others that are not only derived from fully renewable sources but also obtained through eco-responsible processes with minimal environmental impact.2,26,63–67 On the other side, it is necessary that the use of bio-based ingredients can ensure a formulation performance that is, at least, at the same level than that obtained by using petroleum-based ingredients. Therefore, an extensive research activity is mandatory for ensuring a rational design, synthesis, and evaluation of bio-based ingredients with suitable properties. This requires a strong investment in innovative green and sustainable chemistry processes enabling a broad range of chemical modifications ensuring the transformation of naturally sourced ingredients or novel bio-based ingredients in suitable ingredients for cosmetics.25 Among, the bio-based ingredients in hair care products are included different surfactants such as sodium methyl cocoyl taurate. (SMCT), coco betaine (CB) or cocoamidopropyl betaine (CAPB), or polymers such as cationic hydroxyethyl cellulose, the so-called JR400 or polyquaternium 10. However, in the specific area of shampoos and conditioners, a single formula may contain more between 10 to 30 ingredients. From surfactants to oils, disinfectants, fragrances, preservatives, etc. All individual ingredients are scrutinized in terms of impact into the environment, and it is not a particular family that is the target of replacement. Table 4 summarizes the list of components of three different commercial shampoos, highlighting in italic characters the bio-based ingredients contained in the specific products. It should be noted that bio-based character of the ingredients in Table 4 is defined in terms of different aspects: (i) natural occurrence of the ingredients; (ii) isolation from natural sources; (iii) inclusion of nature-derived components, or (iv) biomimetic character of some of components.68–70 At this point, it is necessary to distinguish between bio-based compounds, which are obtained from renewable raw materials through chemical or enzymatic processes without the involvement of living organisms, and nature-derived compounds, which are the result of the metabolic pathways of living organisms, usually microorganisms.71 The latter represent approximately 30% of the compounds used in the cosmetic industry.50 It should be noted that there are currently some discrepancies about the true classification of specific molecules, which in some cases are classified as bio-based but other times as synthetic ingredients. In addition, it also possible to find molecules with the same characteristics as natural occurring molecules but they have been completely synthetized in the laboratory.28
| Shampoo | Ingredients |
|---|---|
| Elvive total repair 5 | |
| (L'Oréal)68 | Water, sodium laureth sulphate, NaCl, Mica, CAPB, dimethicone, Guar hydroxypropyltrimonium chloride, fragrance, titanium dioxide, CB, sodium benzoate, sodium hydroxide, acetic acid, phenoxyethanol, steareth-6, trideceth-10 and -3, Peg-100 stearate, arginine, salicylic acid, limonene, fumaric acid, linalool, benzyl alcohol, amodimethicone, 2-oleamido-1,3-octadecanediol, α-isomethyl carbomer, serine, citric acid, citronellol, hexylene glycol, hexyl cinnamal, glycol distearate |
| Ultrasoft Detox (Instituto Español)69 | Water, sodium laureth sulphate, NaCl, Fruit extracts, CAPB, glycerin, sodium lactate, Cocamide mipa, laureth-4, Peg-7 glyceryl cocoate, PQ 7, sodium benzoate, potassium sorbate, glyceryl caprylate, fragrance, propanediol, Diisopropyl adipate, Lecithin, sulfonic acid copolymer, acrylic acid/acrylamidomethyl propane, Xanthan gum, dimethylmethoxy chromanol |
| Repair and protect (Pantene Pro V/Procter & Gamble)70 | Water, sodium lauryl sulphate, sodium laureth sulphate, CAPN, glycol distearate, dimethicone, sodium citrate, Cocamide MEA, citric acid, sodium xylenesulfonate, fragrance, NaCl, sodium benzoate, tetrasodium EDTA, Guar hydroxypropyltrimonium chloride, polyquaternium-6, Histidine, Panthenol, Panthenyl ethyl ether, methylisothiazolinone, methylchloroisothiazolinone. |
In the following, the present work intends to guide the reader through some of the main challenges of the cosmetics industry in the replacement of two of the most common ingredients of commercial shampoos, cationic polyelectrolytes and anionic sulphate-based surfactants, by introducing new environmentally friendly ingredients, and specifically about the physico-chemical parameters to consider when it is needed to replace a particular ingredient without compromising the conditioning effect for example. It is true that as was above mentioned shampoos are formed by many ingredients, and many of them requires a replacement to accomplish the current goals of cosmetics in relation to reduce their environmental impact without compromising the formulation performance.63,64 A detailed discussion about the importance of the substitution of different cosmetics ingredients can be found in the work by Martins and Marto.28
The substitution of cationic polyelectrolytes and synthetic anionic surfactants is of a paramount importance, because they contribute to the main performance of the product. In fact, the association of anionic surfactant and cationic polyelectrolytes and their deposition on hair fibres is on the bases of the conditioning process.72–76 In fact, conventional conditioning shampoo performance depends on the interaction of cationic polymers with anionic surfactants leading to the formation of phase separate coacervates during the rinse stage. In fact, under application conditions, shampoos undergo a phase separation process characterized by the appearance of phase separated polyelectrolyte–surfactant complexes enriched in the cationic polyelectrolyte (coacervate) which contribute to the conditioning process. This coacervate deposits on the hair, contributing simultaneously to the deposition of other beneficial ingredients, including silicones or other conditioning oils.77 In the case of anionic sulphate-based surfactants, even they are cost-effective cleansers and have excellent foaming properties, offering some viscosity-building characteristics. Their substitution is a consumer demand because this type of surfactants is perceived as harsh cleaners, contributing to the removal of soils but also stripping hair and skin of its natural oils which can be the origin of dermal irritation.78 Moreover, in sulphate-based anionic surfactants with petro-chemical origin, it is possible to find residues of the ethoxylation reactions, such as 1,4-dioxane and ethylene oxide, in the final products.28 It is important to note that most of the currently used sulphate-based surfactants, such as SLES, use bio-based long chain alcohols to prepare the surfactant and can be eco-respectful. One of the principal drivers to removing sulphate surfactants are their potential irritancy and skin drying properties.
The substitution of petrochemical ingredients from cosmetic formulations is highly challenging, mainly because of the huge influence of these ingredients in the effectiveness of the products. Consumers are unrelenting and demand high performance from their products, particularly sensory performance (touch, feel etc.) and these should not be altered by any ingredient substitution process. Fig. 4 illustrates some aspects to consider for ensuring a good sensory performance in the new formulations: a good combination of ingredients in a formula that being stable in solution can be deposited upon water rinsing, while maintaining a reasonable price.
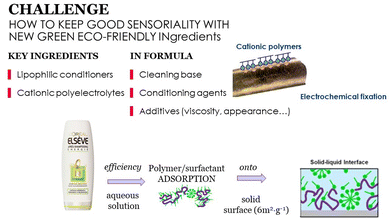 | ||
| Fig. 4 Important aspects to consider for guaranteeing good sensory performance in new cosmetic formulations. | ||
To conclude this section, it should be emphasised that the cosmetics industry is undergoing a green transformation that affects all stages of the life cycle of cosmetics products. Thus, at the design stage, the selection and sourcing of raw materials must increase the sustainability of the cosmetics industry by replacing conventional ingredients with sustainable alternatives. This requires the use of fair-trade ingredients, the synthesis of specific molecules through green chemistry processes, the up-cycling of agro-food by-products into value-added ingredients, and the development of waterless products to minimize the water footprint. At the manufacturing level, sustainability must be reflected in the implementation of innovative factories. Finally, education plays a very important role in promoting sustainable practices in cosmetics, especially from the consumer side. In addition, in the post-consumer stage, it is important to reduce wastes. In this context, the recycling, reuse or refilling of used packaging is a very important issue for the sustainability of the cosmetics industry. On the other side, cosmetics industry should evaluate and moderate the carbon footprint associated with the distribution chain.28
5.1. Towards the use of biopolymers in hair conditioning as substitutes of traditional cationic polymers
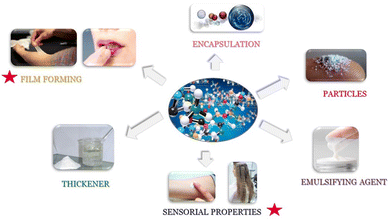
Polymers are a very important class of raw materials in the fabrication of cosmetics formulations, presenting a broad range of functions. The broad range of functions is due to the structural diversity of polymers, which enable the use of polymers as rheology modifiers, thickeners, foam stabilizers and destabilizers, emulsifiers, fixatives, conditioning, and film formers.79–81 Moreover, polymers can be also used for preparing nanoparticles for delivering of active ingredients.67Fig. 5 summarizes some of the most common functions of polymers in the cosmetic industry.In general, cationic polymers are used in a wide range of cosmetics formulations for their conditioning and film-forming ability on *cosmetics substrates such as hair or skin. This type of polymer is highly substantive and adsorbs to hair or skin, providing an increase in lubricity and moisturisation. In particular, when used in hair care products, they contribute to increasing the resistance of the cuticle scales, thus helping to keep the hair in good condition. Among the cationic polymers, the most used in conditioning shampoos are those classified as Polyquaternium polymers (PQ).82,83Table 5 reports the most commonly used PQ polymers in conditioning shampoos.
| Polymer | Chemical composition |
|---|---|
| PQ 4 | Copolymer of hydroxyethyl cellulose and diallyl dimethyl ammonium chloride |
| PQ 6 | Poly(diallyl dimethyl ammonium chloride) |
| PQ 7 | Copolymer of acrylamide and diallyl dimethyl ammonium chloride |
| PQ 10 | Cationic hydroxyethyl cellulose |
| PQ 11 | Copolymer of vinylpyrrolidone and quaternized dimethylaminoethyl methacrylate |
| PQ 22 | Copolymer of acrylic acid and dimethyl ammonium chloride |
| PQ 39 | Terpolymer of acrylic acid, acrylamide and diallyl dimethyl ammonium chloride |
| PQ44 | Poly(2-oxopyrrolidin-1-ylethylene, 3-methylimidazolium-1-ylethylene methyl sulfate) |
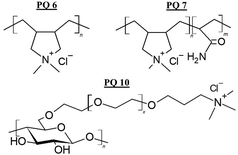
Polyquaternium, according to the International Nomenclature for Cosmetic Ingredients (INCI), defines a series of molecules that are characterised by the presence of cationic quaternary ammonium groups within their structure, which allow them to easily interact with damaged hair fibres. In general, Polyquaternium polymers are homopolymers containing a quaternary ammonium moiety in their monomeric units, or copolymers containing monomers with quaternary ammonium units and various acrylic and/or vinylic comonomers. In the first group, the most common is Polyquaternium 6 (poly(diallyl dimethyl ammonium chloride), PDADMAC or Merquat 100).84 Among the copolymers, the Polyquaternium 7 (copolymer of acrylamide and diallyl dimethyl ammonium chloride). It should be noted that among the Polyquaternium polymers it is also possible to find some bio-based compounds, such as the Polyquaternium 10 (cationic hydroxyethyl cellulose or JR400).84Fig. 6 shows the molecular formula of some Polyquaternium conditioning polymers.
The use of conventional synthetic cationic conditioning polymers is associated with cases of skin and eye irritation, as well as negative impacts on the natural environment. For instance, these compounds are toxic to aquatic organisms, resulting in long-term adverse effects.85 In addition, this type of polymers do not fulfil, in most of the cases, the conditions to ensure the safety, biodegradability and sustainability for new cosmetic formulations. As a result, there is an urgent need to develop hair conditioners that are both highly effective and have minimal impact on human health and the environment.86 This has stimulated the research on the use of biopolymers in hair care, and particularly in hair conditioning products, as a first step toward the substitution of synthetic cationic conditioners.87,88 The popularity of biopolymers has increased due to consumer demand and industry goals to develop biodegradable and environmentally friendly products. The biocompatibility of biopolymers is also a very important aspect in the increased interest for this type of raw material since certain petrochemical based raw materials can contribute to the harmful and irritant character of the formulations, particularly for skin and mucosa.89 For many years, specific shampoos and conditioners have been sold as hypoallergenic and “tear free” or “no tears”, particularly for the baby market. Today, there is a growing wealth of scientific data linking scalp health, including the microbiome, to hair fibre quality. Thus, it is of huge importance that the next generation of hair care products can be more respectful for the scalp, the scalp's microbiome as well as the hair fibre. Biopolymers will play a big role in this revolution for the reasons above mentioned. The first approach that has been adopted by the cosmetic industry is the introduction of cationically-modified biopolymers into hair care formulations to substitute one of the most extended family of conditioning agents, the cationic polyelectrolytes. Research into alternative approaches to replace and improving the performance of cationically-modified biopolymers with non-cationic biopolymers, or biobased polymers is to pick up speed as the cosmetic industry pushes forward with its drive to improve the sustainability and environmental impact of its ingredients.
Biopolymers, such as proteins (e.g., collagen and wheat proteins) and polysaccharides (e.g., cellulose derivatives, alginic acid, chitosan and hyaluronic acid), are widely exploited as cosmetic ingredients, and are used commonly to ameliorate the product properties (e.g., texture and consistency) or boost their action (e.g., hydration and wrinkles reduction). Fig. 7 displays the molecular formula of some of the most common polysaccharides with interest in the development of formulations for hair care. The importance of the research on biopolymers for hair care products is evidenced from the recent introduction of five different biopolymers as cosmetic ingredients by BASF. These biopolymers are obtained using renewable feedstocks such as rice or algae, having a true biodegradable character and good eco-toxicological profiles. In addition, the ingredients are non-GMO, contain no preservatives and are produced using low-emissions processes.90 More recently, IFF has created a biodegradable hair conditioning cationic biopolymer (AURIST AGC) obtained through an enzymatic polymerization of glucose derived from responsible sources.
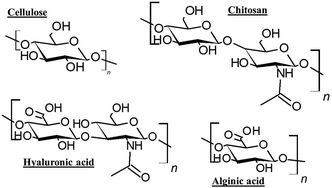 | ||
| Fig. 7 Molecular formula of some polysaccharides of interest for the development of cosmetics formulations for hair care and conditioning. | ||
Cellulose derivatives are included in the list of the most interesting biopolymers for the substitution of petrochemical compounds in hair care products. This is, in part, due to its natural character and abundancy (cellulose is the most abundant biopolymer), and their specific properties, e.g., extensive modification capability, and high thermal stability.91,92 Moreover, cellulose is mainly extracted from renewable and sustainable sources, commonly plants, e.g., wood, cotton, hemp, wheat, and straw,93 but also from bacteria, fungi, algae, and marine animals.94 Therefore, cellulose can be considered as a sustainable and non-petroleum based raw material with reduced environmental, health, and safety footprints.95 Independently of its origin, cellulose is obtained by the polymerization of β-D-glucopyranose through β-1,4-glycosidic bonds.96 Among the different types of available celluloses, those isolated from plants are accounted amongst the most widely exploited. However, in recent years, the use of bacterial cellulose has undergone a spectacular development.97,98 Despite the promising properties of cellulose, and its abundancy, cellulose cannot be exploited in its native form in cosmetics due to its limited solubility in water, and hence it is necessary to chemically modify it, commonly through substitution reactions. Modified celluloses can be and are exploited for different applications in the cosmetic industry, e.g., colloidal stabilizers and rheology modifiers. In hair care formulation. Among cellulose derivatives, it is frequent the use of Polyquaternium 10, which has good moisturizing and anti-static properties, contributing to hair conditioning and leaving the hair shiny and smooth. Moreover, modified celluloses can find applications in cosmetics that go beyond hair conditioning. For instance, they are used for enhancing the adherence and spreading of formulations, mainly in skin care products, and they can be used as thickeners for providing stability and enhancing the rheological properties of specific formulations.99,100 It has to be stressed that for a polymer to be eco-friendly not only its origin from natural resources is necessary, but also the chemical methods used for its modification have to be as well as the environmental profile of the modified biopolymer. Therefore, not all the cellulose derivatives can be strictly considered as natural ingredients. It is critical to adhere to the well-known 12 Green Chemistry principles when transforming any biopolymer or biobased polymer.
Chitosan, another bio-based polymer, is a very promising alternative for cosmetic industry, and, in particular, for the manufacturing of hair and skin care formulations, nail lacquers and lotions, or moisturized for lips and skin.101,102 Moreover, chitosan can be used in other types of products for different purposes, including for providing water-resistant character to sunscreens or antimicrobial properties to deodorants.67,103 Chitosan is a copolymer of 2-amino-2-deoxy-β-D-glucose units and a certain number of N-acetyl-2-amino-2-deoxy-β-D-glucose residues linked through β-1,4-glycosidic bonds. Chitosan itself is not a naturally occurring polymer, but is obtained from chitin upon a hydrolytic deacetylation process, which results in the creation of primary amino groups in the polymer chain (i.e., 2-amino-2-deoxy-β-D-glucose units). These are of paramount importance for tuning the solubility in water of chitosan. In fact, the higher the number of 2-amino-2-deoxy-β-D-glucose units in the polymer chain, i.e., the deacetylation degree (DD), the higher the solubility in aqueous medium under acidic conditions.104–106 In fact, chitosan is pH sensitive since it contains primary amino groups that are protonated at acid pH to form the so-called cationic polymer. Due to this fact the solubility of chitosan can be tuned depending on the pH, the molecular weight, the degree of deacetylation and the counterion.
Oligochitosans, with molecular weights lower than around 5 kDa, are readily soluble in aqueous solutions at all pH's whereas higher molecular weight chitosans are only soluble in acidic media. It must be underlined that after been dissolved at low pH, the polymer solution remains stable at almost neutral pH. Chitosan, as in the case of cellulose, can be isolated from different sources, with the exoskeleton of different invertebrates being the most common source, e.g., crustaceans, molluscs, and insects, but it is also possible to extract chitosan from the cell wall of different fungi and yeasts.107,108 Due to an increase in the population who are allergic reactions to shellfish, microbial sources of chitosans are generally preferred these days. On the contrary to cellulose, unmodified chitosan is an acceptable raw material, and nowadays chitosan with different chain lengths and characteristics are exploited in cosmetic industry. Moreover, there are several chitosan derivatives that have been progressively introduced in cosmetics for improving specific properties of the formulations. Some of the different chitosans found in cosmetics are chitosan acetate, carboxymethyl chitosan, oligochitosan, chitosan hydrochloride, chitosan lactate and their quaternized derivatives.109,110 This has led the appearance of a broad range of chitosan derived products directly addressed for their use in the cosmetic industry. Novel chitin derivatives, such as carboxymethyl chitin and chitin sulphate are also promising alternatives to water soluble chitosans. Table 6 summarizes some of the chitosan derived ingredients with interest for hair care and conditioning.
| Ingredient | Manufacturer |
|---|---|
| Hydamer™ | Chitinor AS (Tromsø, Norway) |
| Ritachitosan® | Rita Corporation (Crystal Lake, IL, USA) |
| Curasan™ | Chemisches Laboratorium Dr Kurt Richter GmbH (Berlin, Germany) |
| Zenvivo™ | Clariant (Muttenz, Switzerland) |
| KIOsmetine® | Kitozyme (Herstal, Belgium) |
| ChitoClear™ | Primex Manufacturing Inc. (Langley, BC, Canada) |
| Everquat™ Q50H | Sino Lion (Florham Park, NJ, USA) |
| Vinkocos p-6N | Vink Chemicals GmbH & Co. KG (Kakenstorf, Germany) |
| Jeen-Chitosan | Jeen International (Fairfield, NJ, USA) |
Cellulose and chitosan are only two examples of many sustainable polymers that can open new avenues in the transition towards “green sustainable” products for hair and skin care and conditioning. The ability to obtain cellulose and chitosan from non-animal sources is of paramount importance because it avoids problems associated with possible zoonosis transmission, and limits the possible ethical issues and concerns related to biodiversity and endangered species protection.111 Unfortunately, using biopolymers or biobased polymers brings a whole raft of unknowns and complications. In general, many biopolymers based on polysaccharide chains are favourable media for the growth of bacteria and fungi.112 This is unfavourable from a shelf stability point of view in comparison to than what happens when petrochemical polymers (such as PQ-6 and PQ-7) are considered. However, it is highly favourable from a biodegradation and environmental aspects. In fact, it is needed the biodegradation of the biologically derived ingredients is slow enough to ensure that their cosmetic activity is not compromised during the shelf storage.113
What is clear is that there is an important lack of knowledge about the compatibility of the next generation of biopolymers with other components in formulations, and how that compatibility modifies the effectiveness in shampoos and conditioners.114
5.2. Biosurfactants as alternative to traditional anionic surfactants in hair care cosmetics
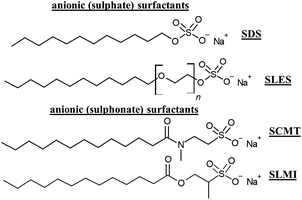
Surfactants are a class of ingredients with a paramount importance in hair care cosmetics, playing a broad range of roles, including, amongst other, detergency, emulsification, de-emulsification, wetting, foaming, dispersion, solubilization of hydrophobic substances or to modify surfaces.41,115Fig. 8 shows some anionic (sulphate-based) surfactants included currently in conditioning shampoos.Unfortunately, conventional surfactants derived from petrochemical sources present several drawbacks, including their high potential to induce the irritation of skin and mucosa, and, in rare cases, more significant immunological responses. Moreover, classical surfactants can contribute to the removal of the lipids from the epidermal surface and the hair epicuticule.116 Unfortunately, in most of the cases, there are no direct substitutes for traditional anionic surfactant (commonly sulphate-based surfactants), and it is needed to use a combination of different ingredients to obtain the same effects offered by traditional surfactant, which is a challenge for cosmetic formulators.78 Therefore, it is important to innovate with new alternatives enabling the reduction of the harmful effects associated with the use of surfactants whilst concomitantly improving their environmental footprint. This requires defining the sustainability profile of a surfactant, which is the result of a complex balance between four different aspects: (i) the application-specific performance of the surfactant; (ii) its potential impact on human health; (iii) its potential impact on the environment; and (iv) the cost–benefit ratio.117 The sustainability profile of surfactants is defined in terms of different indicators.118 The first one, known as the biorenewable carbon index, assesses the percentage of carbon atoms originating from renewable natural sources compared to non-renewable petrochemical sources. One advantage of this method is that it avoids distortion caused by high atomic mass atoms, like sulphur found in anionic surfactants. The second indicator, named the percentage renewable by molecular weight, determines the proportion of a surfactant molecular weight derived from renewable sources. However, this method is more complex in practice as each atom in the surfactant must be precisely categorized as coming from either a renewable or non-renewable source. This requires a thorough understanding of the chemical reactions used in the synthesis of the surfactant, and it often involves challenging judgments regarding the origin of each atom in the process.
Formulators face a crucial choice when selecting surfactants for hair care products. They must decide between opting for commonly used and cost-effective materials or choosing more specialized surfactants that offer distinct advantages. For instance, specialized surfactants can provide benefits like “no tears” for baby shampoos, deep-cleansing capabilities for “detox” shampoos, or alternative options suitable for “sulphate-free” formulations. In the process of surfactant selection, it becomes essential to take technical constraints into account. These constraints include the target cost of the final product, the specific product pH required for the preservation system, and any potential processing issues. Balancing these factors is critical to ensure that the chosen surfactants meet both the desired product performance and the overall formulation goals.118
A promising alternative to suphate-based surfactants are surfactant containing sulphonate in their polar head group. They offer a more chemically stable C–S bond between the sulphonate head-group and the alkyl chain compared to the ester bond found in sulphate surfactants. Various sub-classes of sulphonates exist, including taurates, sulphoacetates, sulphosuccinates, and isothionates (see Fig. 8 for specific examples). In general, sulphonates present good foaming properties and chemical stability over a wide pH range.118 On the other hand, alkyl polyglucosides (APGs) are nonionic surfactants that offer a favorable natural alternative to charged and synthetic nonionic surfactants. The key advantage lies in their composition, as they are derived entirely from renewable plant-based raw materials, making them highly eco-friendly and readily biodegradable. These APGs, including coco, lauryl, decyl, and caprylyl/capryl glucosides, are synthesized from fatty alcohols sourced from coconut and palm oils, along with glucose obtained from corn or potato. This natural origin contributes to their sustainability and reduces the environmental impact. Moreover, APGs demonstrate low toxicity, ensuring their safety for various applications. Additionally, they possess mild properties and exhibit excellent compatibility with the skin, making them an ideal choice for personal care products. Overall, the combination of being environmentally friendly, renewable, and skin-friendly underscores the advantages of using APGs in shampoos. An alternative to APGs is the use of biosurfactants.28 These are natural origin molecules produced by different microorganisms, including yeast, fungi and bacteria, and their classification can be done in terms of their chemical composition and molecular weights.119 Thus, it is possible to define different families of low molecular weight biosurfactants (glycolipids, lipopeptides, lipoproteins, fatty acids and phospholipids), and high molecular weight polymeric biosurfactants.41
Biosurfactants offer great potential as alternatives to traditional surfactants, due to their lower environmental impact, improved sustainability, and reduced impact on skin health. It should be noted that the sustainability of biosurfactants should be assessed in terms of several parameters, such as production of raw materials, transport and distribution, processing and conversion, transport, and distribution of the final product. In fact, biosurfactants are usually much more sustainable than conventional surfactants. However, it is necessary to study them in depth, especially to develop production strategies to ensure full acceptance of biosurfactants for their use in consumer products. It is also necessary to reduce their production costs in relation to those of traditional synthetic surfactants.120 In fact, biosurfactants are commonly more expensive than classical surfactants.120 However, with ever greater commercial interest and increasingly larger scale production it is believed that the cost of such ingredients will fall and increase competitiveness to traditional products. For instance, a recent partnership between Evonik and Unilever has brought rhamnolipids to the business-to-consumer (B2C) market.121 This has been by possible exploiting the advanced bioprocess engineering and strain improvement, the so-called white biotechnology,122 which has pushed the cosmetics research towards the production of biosurfactants with a broad range of functions, which has pushed to the cosmetic industry towards the introduction of several biotechnologically derived compounds in their formulations.123 Moreover, this introduction is supported by the higher biodegradability and eco-friendly character of biosurfactant than traditional petrochemical surfactants. Therefore, their use is aligned with the current aims of cosmetic industry towards the production of products with low impact into the environment.113,116 For instance, the biodegradation of biosurfactants appears faster than that of traditional surfactants as sodium dodecyl sulphate (SDS).124
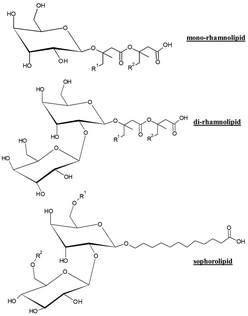
In recent years, cosmetic industry has paid an extensive interest to the potential uses of different glycolipids in different types of formulations.119 In particular, rhamnolipids (isolated from Pseudomonas strains) and sophorolipids (isolated from Candida strains) are currently considered as two of the most important families of ingredients for substituting traditional surfactants.125–127 In fact, there are several companies commercializing rhamnolipids and sophorolipids (see Table 7 for a summary).
| Glycolipid family | Company |
|---|---|
| Rhamnolipids | Urumqi Unite Bio-Technology Co., Ltd (Urumqi, Xinjiang, China) |
| AGAE Technologies LLC (Corvallis, OR, USA) | |
| BOC Science (Shirley, NY, USA) | |
| Logos Technology (Fairfax, VA, USA) | |
| Biosynth Limited (Compton, UK) | |
| TeeGene Biotech (Redcar, United Kingdom) | |
| Toronto Research Chemicals Inc. (North York, ON, Canada) | |
| Sophorolipids | Saraya Co. Ltd (Osaka, Japan) |
| MG Intobio Co., Ltd (Yonghyun-Dong, Namgu Incheon, South Korea) | |
| InvivoChem® (Libertyville, IL, USA) | |
| Synthezyme LLC (Rensselaer, NY, USA) | |
| Evonik (Slovenská Ľupča, Slovakia) | |
| Groupe Soliance (Pomacle, France) |
Fig. 9 shows the chemical formula of the most common glycolipids (rhamnolipids and sophorolipids) in this manuscript. It is clearly observed that both rhamnolipids and sophorolipids are really families of surfactants that include surfactants with different structures. The differences in the hydrophilic and hydrophobic moieties for the different surfactants lead to noticeable differences in their properties.43,44
The preference for the use of glycolipids in cosmetic industry is not arbitrary and is mainly related to their easy availability. From a chemical point of view, glycolipids consist of a hydrocarbon lipid-like tail covalently bonded to a carbohydrate residue, and they are usually classified as monosaccharides, disaccharides, and polysaccharides.128,129
It should be emphasised that the transition away from sulphate-based anionic surfactants presents several challenges that should be carefully addressed. Firstly, alternative surfactants are typically more expensive than standard sulphate surfactants. Secondly, ‘sulphate-free’ products often require higher levels of surfactant to achieve comparable foam, further increasing costs. Thirdly, while sulphate surfactants thicken with sodium chloride and betaines, many alternative surfactants lack this property. This necessitates the addition of polymeric thickeners, which increases formulation costs and can affect the deposition of cationic polymers and silicones, leading to compromised performance. Another obstacle is achieving equivalent product performance in ‘sulphate-free’ systems, which can be quite challenging for a number of reasons. For example, cationic polymer deposition is highly sensitive to the types of anionic surfactants used, the levels of co-surfactants and salt. Therefore, moving away from established standard sulphate surfactant blends requires extensive deposition testing. In addition, alternative surfactants can lead to unwanted structured phases that affect foaming and silicone deposition and make products more difficult to rinse. In addition, alternative surfactants may be less chemically stable at the low pH levels required for preservatives. This requires careful consideration and evaluation of the suitability of the surfactant to maintain product integrity.118
6. Methodological approaches for evaluating the potential effectiveness of new ingredients
The effectiveness of a specific shampoo is generally correlated to the mechanism underlying the deposition of specific ingredients on the surface of the hair fibres. In fact, conditioning molecules should adsorb on the negatively charged surface of the damaged hair fibers.118,130 However, in the case of conditioning shampoos, which are the pillar of high quality shampoos, the understanding of the adsorption process is far from being trivial, mainly due to the interaction of the conditioning agent (commonly a cationic polymer) with the cleansing based one (generally anionic surfactants). The combination of these types of ingredients results in the formation of colloidal aggregates that can remain solubilized in the aqueous medium or separate from the solution as solid (precipitates) or liquid phases (coacervates). The formation of such different phases is strongly dependent on the concentration of the components as well as on the ionic strength and pH of the formulations.131–134The most accepted mechanism describing the transition of the colloidal aggregates from the bulk solution to the hair fibre surface is the so-called deposition induced by dilution mechanism. This can be understood considering that the one-phase commercial product during its application under the shower is pushed, as result of the dilution process (10–20 times), towards a two-phase state phase, which allows the depletion of aggregates from the bulk phase to the surface of the damaged fibres.74,135 However, the link between the formation of deposits on the surface of the hair fibres and the consumer sensorial perception is not well understood yet, even though it is generally accepted that the nano-structuration of the deposit at the surface contributes to the tactile perception.136
The direct evaluation of the formation of conditioning deposits on the surface of hair fibres is not trivial, and hence it is necessary to design experimental strategies that provide information to understand such processes. This requires the use of well-established surface science methodologies in the preliminary assessment of the formulation's performance.58
The hair surface is highly complex, often heterogeneous, and thus it is not easy to attain reliable, high quality and reproducible tribological data. As an initial step for assessing the performance of cosmetic ingredients, it was necessary to define and create experimental models mimicking the hair surface. A reasonable choice is the use of conventional macroscopic surfaces with suitable surface modifications to mimic some specific physico-chemical aspects of the hair fibres, e.g., surface charge and wettability. In fact, the presence of specific chemical groups defines the chemical fingerprint of the hair fibre surface, and hence the replication of this is essential for any valuable model to study the interactions between hair care ingredients and the model hair surfaces. The hair surface varies depending on the age of the hair and its history (chemical treatments such as bleaching, grooming rituals, etc.). Damaged hair shows significant differences in the chemical fingerprint compared to natural hair. Typically damaged hair has a lower concentration of surface bound lipids and a higher concentration of oxidised amino acids, particularly cysteine which is oxidised to numerous oxidised sulphur species, including cysteine sulphonic acid (cysteic acid) and cysteine sulphinic acid, leading to a change from a highly hydrophobic surface to a hydrophilic one.25
There are different methodologies available to reproduce the negative charge of hair, and its overall hydrophobic/hydrophilic character, and thus produce interesting mimic the hair surface. The use of surfaces such as mica, gold or silicon dioxide have been commonly exploited as models. In most cases, these surfaces require a pre-treatment step before their use to ensure that their wettability and surface charge are as close as possible to that what are expected for (damaged) hair fibres. Often a surface functionalization with specific chemical groups is necessary. For instance, the use of thiol modified gold plates in which the thiol terminal end groups are sulfonic acids have been used for some time now. Fig. 10 schematizes the concept behind the use of self-assembled thiol monolayers terminating with sulfonic acid groups which mimic the characteristics of oxidatively damaged hair.137
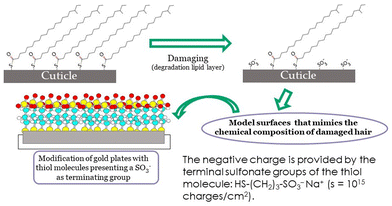 | ||
| Fig. 10 General concepts of the chemical modification of surfaces for mimicking the physico-chemical properties of oxidatively damaged hair. | ||
The above discussion makes it clear that the interaction of cosmetic ingredients with damaged hair fibres mainly occurs at the surface level. Therefore, it is essential to adapt experimental and computational strategies providing information about the adsorption process. Currently, there is a broad range of techniques available that are used for the evaluation of the effectiveness of the cosmetic ingredients upon their interaction with solid surfaces. Table 8 summarizes some of the available surface science techniques.43,138–147
| Technique | Information | Ref. |
|---|---|---|
| Quartz crystal microbalances with dissipation monitoring (QCM-D) | Adsorption kinetics | 43, 138–143 |
| Adsorbed mass (wet mass) | ||
| Mechanical properties of the layers | ||
| Ellipsometry | Adsorption kinetics | 43, 138–143 |
| Adsorbed mass (dry mass) | ||
| Combined with QCM-D experiments: water content | ||
| Surface force apparatus (SFA) | Friction | 138 and 144 |
| Forces | ||
| Rheology | ||
| Atomic force microscopy (AFM) | Layer topography | 43, 140 and 141 |
| Forces (Colloidal probe) | ||
| THEORETICAL STUDIES: self-consistent field (SCF) calculations and molecular dynamics simulation | Layer thickness | 43, 57, 138, 139, 145–147 |
| Density profiles of the adsorbed layers |
The Quartz Crystal Microbalance with dissipation monitoring (QCM-D) is a well-established surface science technique that can provide information on the adsorption kinetics, the wet adsorbed mass (adsorbed mass of the ingredients plus hydration water), and the shear viscoelastic properties of the layers obtained upon the deposition of cosmetic ingredients on surfaces148 The use of QCM-D relies of the evaluation of the changes on the electrical impedance spectrum of a quartz crystal during the experiments as result of the adsorption of material on its surface. In fact, the formation of a layer on the quartz crystal surface leads a decrease of the central frequency of the spectrum and its broadening. The analysis of their changes on the characteristics of the impedance spectrum of the quartz crystal, in terms of the Voigt-Voinova model, allows one to obtain information about the adsorbed mass and the shear modulus of the adsorbed layer.149 The combination of the QCM-D results with those obtained by ellipsometry, an optical technique relying on the evaluation of refractive index variations, provides information about the dry mass,150 making it possible to obtain information about the hydration degree of the deposited films according to the definition by Halthur et al.151 Similar information to that of ellipsometry can be obtained using surface plasmon resonance (SPR)152 or optical waveguide light-mode spectroscopy (OWLS).153
Sensorial perception is correlated to friction forces, and hence their evaluation plays a very important role in the optimization of cosmetic formulations.37,154 Sensory analysis is a scientific discipline used to measure, analyze and interpret the characteristics of a product as they are perceived by the five senses either in vitro or in vivo. Highly trained personnel are educated in each product category and develop specific skills to master the differences between products. They are highly knowledgeable in the state of the art of products as well as in consumer routines and behaviors particularly as routines and even formula vary immensely around the world such that individual and cultural differences require specific attention to create a successful product. They assess the physical and sensorial properties of any product and translate these impressions into objective data, regardless of whether they are evaluating new or old formulas or technologies. They contribute immensely to the innovation process giving feedback and guidance to formulators and research scientists. Neuroscience and psychophysical approaches of evaluation are becoming complementary methods to traditional methods of evaluation. To note, many guidelines exist for the evaluation of the efficacy of cosmetic products. Unfortunately, the use of in vivo sensorial studies is not always possible due to their high cost, the time consumption and different safety and ethical issues. Consequently, the use of instrumental measurements for obtaining insights into the sensorial/texture attributes of cosmetic formulations must be considered as very important tools.155 The Surface Force Apparatus (SFA) is a well-suited technique for evaluating the tribological properties of treated and untreated model surfaces.156 It is clear, according to the information reported in Table 9, that different features expected from hair conditioners are ascribed to the required tribological properties. However, a problem with this technique is that it is not commercial, thus this type of measurements is rather scarce. Although commercial microtribological techniques exist, and have frequently used to study the surfaces of polymeric materials, they are not easily adapted to the study of fibres.
| Conditioner feature | Tribological properties |
|---|---|
| Smooth feel in wet and dry environments | Low friction between hair and skin |
| Shaking and bouncing during daily activities | Low friction between hair fibres and groups of hair |
| Easy combing and styling | Low friction between hair and comb (plastic), and low adhesion |
SFA provides information of the mechanical properties of thin films sandwiched between two molecularly smooth surfaces (generally mica plates) under static and dynamic deformation conditions.157–159 SFA experiments allow researchers to study the adsorption of cosmetic ingredients on the mica surfaces, and how this contributes to modifying the profile of the forces between two surfaces as they approach one another. In the case of surfaces modified with cosmetic ingredients this allows researchers to estimate the forces between two hair fibres as they approach or retract from each other. The mica plates are supposed to mimic two hair fibres. The separation distance between the mica surfaces can be evaluated by using interferometry. In fact, the light reflects back and forth between the mica surfaces generating fringes separated by a distance which is equivalent to the separation between the surfaces.144,160
The Atomic Force Microscope (AFM) can be used for morphological characterization of surfaces on the nanoscale, as well as for determining their adhesion properties. Moreover, it is possible to exploit the AFM for evaluating the frictional properties of surfaces at nano- and micro-scale.161 This information is obtained by measuring the lateral forces in the scanning direction, which allows obtaining high resolution 3D images of the sample. It should be noted that AFM can be used directly to study hair under ambient conditions.162 Furthermore, it can also provide information about the electrical charge of the degraded hair fibres, that strongly depends on the sulfonate groups arising in the delipidation process.
The use of modern digital/numerical methods for obtaining information about properties and functions of specific ingredients included in complex cosmetic formulations may also help to design more effective formulations for cleansing and conditioning formulations, and to produce highly performant ingredients based in the principles of the green chemistry.25 In fact, the introduction of numerical and theoretical methods integrating the hair surface complexity can help in understanding the science behind hair conditioning and cleansing.
7. Introducing bio-based compounds into cosmetic formulations: studies based on model systems
The chemistry of the ingredients used in cosmetic formulations for hair care must be carefully examined to exploit their interactions with hair surface.25 This section summarizes some of the most recent efforts on the understanding of the performance of bio-based ingredients in cosmetic haircare formulations.Chitosan has emerged in recent years as a very promising ingredient to replace synthetic conditioning polymers of petrochemical origin.101,163 One of the first and important studies on the adsorption of chitosan on model hair surfaces was conducted by Hernández-Rivas et al.139 They combined different experimental methods (QCM-D and ellipsometry) and Self-Consistent Mean Field (SCF) numerical calculations to understand the performance of chitosan in terms of adsorption as compared with two polymers currently present in hair conditioners and shampoos: poly(diallyl-dimethyl-ammonium chloride), the so-called PDADMAC, Merquat 100 or polyquaternium 6, and hydroxy-ethyl-cellulose quaternized with 2,3-epoxypropyltrimethylammonium chloride, the so-called JR400 or Polyquaternium 10 (PQ 10). The results showed a good agreement as evidenced by the results shown in Fig. 11. This approach demonstrated the power of the digital method in predicting some aspects related to the performance of cosmetic ingredients for haircare and conditioning. Moreover, the results showed that the chitosan adsorption on the model surfaces is comparable to that found for PDADMAC, but worse than that of Polyquaternium 10. This suggests that chitosan may be a good alternative to substitute some of the currently used polymers in hair care products. These results are important since PDADMAC is the most widely used cationic polyelectrolyte in hair conditioning.84 Unfortunately, the density profile obtained using the SCF calculations predicts that the chitosan deposits for simple milieu will be less performant at reducing friction than Polyquaternium 10. However, a conditioning shampoo also contains a surfactant, and the introduction of the most common shampoo surfactant (sodium laurylether sulphate, SLES) the numerical model showed improved chitosan deposition and thus reduced friction. The authors’ experiences of such experiments are that the chitosan–SLES system is particularly efficient at improving chitosan deposition. Furthermore, the chemical specificity of chitosan and other cationic polymers in formulations becomes critical for controlling the deposition process. As mentioned previously, there are many variables that can be changed in the molecular structure of chitosan, and therefore, there is a vast room available to develop chitosans with optimised performance, both from the deposition viewpoint, as well as any other molecular feature that will play a role in the environmental profile. Using such methodologies will only help to understand the interaction between all the variables, and ultimately be integrated into the design of new more sustainable polymers. It is worth mentioning that chitosan continues to be explored for other hair applications such as styling gels due to its strong affinity for the hair surface. Hartson et al.164 showed that a composite based on chitosan and methylcellulose had excellent hair styling performance.
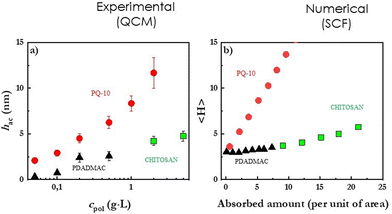 | ||
| Fig. 11 (a) Polymer concentration dependence on the acoustic thickness of polyelectrolyte layers (PDADMAC, PQ 10 and Chitosan) onto negatively charged model surfaces that mimic the physico-chemical properties of hair as were obtained by QCM-D (hac). (b) Dependence of the average layer thickness 〈H〉 on the adsorbed amount (i.e., concentration in the solution) as was obtained from SCF calculations for polyelectrolyte layers (PDADMAC, PQ-10 and Chitosan). Experiments and calculations were performed using solutions with a NaCl concentration of 100 mM. Adapted from Hernández-Rivas et al.,139 with permission from MDPI under Open access CC BY 4.0 license, Copyright (2020). | ||
As stated above, glycolipids are very promising biosurfactants for use in hair care products. This has led to a very strong interest in rhamnolipids as alternatives to conventional anionic surfactants. In this direction, Fernández-Peña et al.43 performed the first systematic study comparing the behavior of PDADMAC-surfactant mixtures containing glycolipid surfactants with different chemical structures with those containing SLES. For this purpose, they studied the adsorption process on model surfaces by using different surface techniques (QCM-D, ellipsometry and AFM) and SCF theoretical calculations. The adsorption of PDADMAC–rhamnolipid mixtures on real hair fibers was also evaluated by using Scanning Electron Microscopy (SEM). The results highlighted that specific physico-chemical properties of the chosen biosurfactants, e.g., charge density, number of sugar rings on the hydrophilic head, and length of the hydrocarbon tail, are of paramount importance for controlling the association process with the conditioning polymers as well as for the formation of the conditioning deposits, and hence the effect on the efficiency of the conditioning process. More specifically, the hydration levels of the conditioning deposits were found to be improved by the reduction in the length of the alkyl chains in the rhamnolipids. Fernández-Peña et al.,43 using model surfaces, also showed that alkyl polyglucosides are not suitable alternatives for substituting SLES in SLES-PDADMAC systems due to the limited adsorption of the PDADMAC–surfactant complexes. However, mixtures with rhamnolipids were found to be much more performant than PDADMAC-SLES ones. This better deposition can be ascribed to the higher dimensions of the polyelectrolyte–surfactant aggregates that are formed in presence of rhamnolipids, favoring their sedimentation on the hair fibers. Moreover, the high charge density of the aggregates may also favor their deposition. Mixtures of PDADMAC with rhamnolipids containing a single sugar ring on the hydrophilic head and decyl chains as hydrophobic tails presented a maximum adsorption that is more than twice that found for mixtures with SLES. Moreover, the use of rhamnolipids also increases the hydration level of the deposits compared to those created from sulphate-based surfactants. These results open important avenues for the potential substitution of SLES by rhamnolipids in commercial formulations. Fig. 12 shows the SEM images of the PDADMAC–rhamnolipids deposits. The coating of the hair fibers is evidenced for the disappearance of the signal corresponding to the sulphur in the energy-dispersive X-ray spectra. The hair surface is relatively homogeneously covered with the deposit which in term leads to improved conditioning of the hair and consumer perceived hair care. Nevertheless, it has to be remarked that some of the rhamnolipid solutions are favorable for the growth of fungi colonies, which compromises their shelf life.
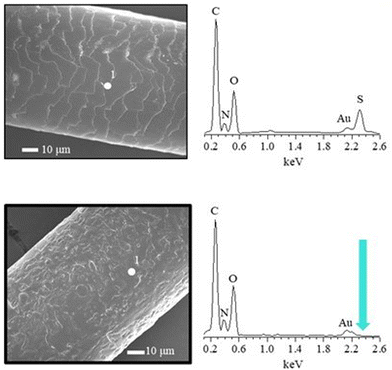 | ||
| Fig. 12 SEM images and their respective energy-dispersive X-Ray spectra for uncoated damaged hair fibres (top panel), and hair fibres coated upon deposition of a PDADMAC-rhamnolipid mixture (bottom panel). The arrow indicates the disappearance of the sulphur signal upon the deposition of the conditioning film. Reprinted from Fernández-Peña et al.43 with permission from Elsevier, Copyright (2020). | ||
Morita et al.165 extended our understanding of the power of glycolipids for hair care/repair. They used mannosylerythritol lipids and found that the application of formulations containing this type of biosurfactant on damaged hair fibres favours the formation of conditioning deposits that improve the recovery of damaged hair, i.e., repair the hair. Moreover, the adsorption of the formulations containing the biosurfactant contributes to enhance its the mechanical properties, increasing the tensile strength and the frictional properties, and decreasing the bending rigidity. Therefore, it may be considered that a rational choice of biosurfactants can contribute to hair cleansing, conditioning and repairing.
8. Conclusions
In recent years, the cosmetic industry has invested heavily in finding solutions to current environmental, social and economic challenges. In the case of haircare formulations this has been, and continues to be, through the eco-design of renewable ingredients and formulations made using processes that are responsible towards the environment and all with a favourable environmental impact. Numerous experimental and numerical methods are readily available to aid researchers in their pursuit to replace traditional ingredients with biobased ingredients such as biopolymers and biosurfactants whilst improving the technical performance including added benefits of shampoos and conditioners. Huge strides have been made by pioneers in this field. Many avenues and opportunities exist due to the molecular diversity of such ingredients and in the opinion of the authors, the future is bright. However, the transition towards a more sustainable cosmetics industry requires to return to the basics, needing a thorough examination of the key functions of the different functions of the ingredients of shampoos Addressing these essential functions while overcoming cost and performance challenges is crucial for successful sustainable cosmetic practices. In fact, the cost to benefit ratios, chemical stability, formulation compatibility, and efficiency, ease of rinsing and water reduction (if a rinsed product) amongst many other aspects should be analysed to ensure the success. Therefore, the eco-design is a challenge for the cosmetic industry because the new cosmetic products should present cosmetic properties and quality matching with the benchmark reference, which is not always easy. In fact, the green advent on the cosmetic industry should maximize the quality of the products in relation to three different aspects: efficiency, cosmeticity and sustainability.Author contributions
Conceptualization: G.S.L. and E.G.; methodology: E.G.; visualization: E.G.; writing – original draft: E.G.; writing – review & editing: G.S.L., F.L., A.G., R.G.R. and E.G.; funding acquisition: G.S.L.; R.G.R. and E.G.Conflicts of interest
G.S.L., A.G. and F.L. are employed by L'Oréal (France). The rest of the authors declare no conflict of interest.Acknowledgements
This work was funded by MICINN (Spain) under grant PID2019-106557GB-C21, and by E.U. on the framework of the European Innovative Training Network-Marie Sklodowska-Curie Action NanoPaInt (grant agreement 955612). Authors also acknowledge the financial support received from L'Oréal (France).References
- G. Liobikienė and J. Bernatonienė, J. Cleaner Prod., 2017, 162, 109–120 CrossRef.
- J. L'Haridon, P. Martz, J.-C. Chenéble, J.-F. Campion and L. Colombe, Int. J. Cosmet. Sci., 2018, 40, 165–177 CrossRef PubMed.
- I. Giannenas, E. Sidiropoulou, E. Bonos, E. Christaki and P. Florou-Paneri, in Feed Additives, ed. P. Florou-Paneri, E. Christaki and I. Giannenas, Academic Press, Cambridge, MA, USA, 2020, ch. 1, pp. 1–18, DOI:10.1016/B978-0-12-814700-9.00001-7.
- Y. D. Jagdale, S. V. Mahale, B. Zohra, G. A. Nayik, A. H. Dar, K. A. Khan, G. Abdi and I. K. Karabagias, Sustainability, 2021, 13, 9240 CrossRef CAS.
- N. F. Rahim, Ulum Islamiyyah, 2019, 26, 9–18 CrossRef.
- M. Philippe, B. Didillon and L. Gilbert, Green Chem., 2012, 14, 952–956 RSC.
- H. P. Fiedler and W. Umbach, in Surfactants in Consumer Products: Theory, Technology and Application, ed. J. Falbe, Springer Verlag, Heidelberg, Germany, 1987, pp. 350–398 Search PubMed.
- R. Höfer and J. Bigorra, Green Chem. Lett. Rev., 2008, 1, 79–97 CrossRef.
- Hair Care Ingredient Makers Get Creative https://cen.acs.org/articles/91/i19/Hair-Care-Ingredient-Makers-Creative.html, (accessed 12 July 2023).
- S. Bom, M. Fitas, A. M. Martins, P. Pinto, H. M. Ribeiro and J. Marto, Molecules, 2020, 25, 4887 CrossRef CAS PubMed.
- S. Bom, J. Jorge, H. M. Ribeiro and J. Marto, J. Cleaner Prod., 2019, 225, 270–290 CrossRef.
- P. Cinelli, M. B. Coltelli, F. Signori, P. Morganti and A. Lazzeri, Cosmetics, 2019, 6, 26 CrossRef CAS.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, NY, USA, 1998 Search PubMed.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- B. M. Trost, Science, 1991, 254, 1471–1477 CrossRef CAS PubMed.
- P. Anastas, in Green Techniques for Organic Synthesis and Medicinal Chemistry, ed. W. Zhang and B. W. Cue, Wiley, London, UK, 2012 Search PubMed.
- R. A. Sheldon, I. W. C. E. Arends and U. Hanefeld, Green Chemistry and Catalysis, Wiley-VCH, Weinheim, Germany, 2007 Search PubMed.
- K. Culliney, Green chemistry can ‘future proof’ cosmetics: Expert https://www.cosmeticsdesign-europe.com/Article/2021/10/08/Green-chemistry-innovation-in-cosmetics-preservation-can-future-proof-industry-says-expert, (accessed 18 July 2023).
- C. A. Moreno-Camacho, J. R. Montoya-Torres, A. Jaegler and N. Gondran, J. Cleaner Prod., 2019, 231, 600–618 CrossRef.
- H. B. Rose, B. Kosjek, B. M. Armstrong and S. A. Robaire, Curr. Res. Green Sustainable Chem., 2022, 5, 100324 CrossRef CAS.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- S. Fortunati, L. Martiniello and D. Morea, Sustainability, 2020, 12, 5120 CrossRef.
- M. Philippe, B. Didillon and L. Gilbert, Green Chem., 2012, 14, 952–956 RSC.
- G. S. Luengo, A.-L. Fameau, F. Léonforte and A. J. Greaves, Adv. Colloid Interface Sci., 2021, 290, 102383 CrossRef CAS PubMed.
- J. Hitce, J. Xu, M. Brossat, M.-C. Frantz, A.-C. Dublanchet, M. Philippe and M. Dalko-Csiba, Curr. Opin. Green Sustainable Chem., 2018, 13, 164–169 CrossRef.
- A. L. V. Cubas, R. T. Bianchet, I. M. A. S. d. Reis and I. C. Gouveia, Polymers, 2022, 14, 4576 CrossRef CAS PubMed.
- A. M. Martins and J. M. Marto, Sustainable Chem. Pharm., 2023, 35, 101178 CrossRef CAS.
- S. Bom, J. Jorge, H. M. Ribeiro and J. Marto, J. Cleaner Prod., 2019, 225, 270–290 CrossRef.
- R. Rocca, F. Acerbi, L. Fumagalli and M. Taisch, Cleaner Waste Syst., 2022, 3, 100057 CrossRef.
- M. Saurat, M. Ritthoff and L. Smith, Overview of existing sustainability assessment methods and tools, and of relevant standards: sustainability assessment methods and tools to support decision-making in the process industries; SAMT deliverable 1.1,Europ. Union, Brussels, 2015.
- B. Ness, E. Urbel-Piirsalu, S. Anderberg and L. Olsson, Ecol. Econ., 2007, 60, 498–508 CrossRef.
- M. Secchi, V. Castellani, E. Collina, N. Mirabella and S. Sala, J. Cleaner Prod., 2016, 129, 269–281 CrossRef CAS.
- C. Kolling, J. L. D. Ribeiro and J. F. de Medeiros, Sustain. Prod. Consum., 2022, 30, 171–185 CrossRef.
- W. H. Motta, L.-R. Issberner and P. Prado, J. Cleaner Prod., 2018, 187, 1103–1114 CrossRef.
- S. K. Haldar, in Mineral Exploration, ed. S. K. Haldar, Elsevier, Boston, MA, USA, 2013, ch. 14, pp. 267–285, DOI:10.1016/B978-0-12-416005-7.00014-3.
- B. Bhushan, J. Colloid Interface Sci., 2020, 577, 127–162 CrossRef CAS PubMed.
- B. Bhushan, Biomimetics: Bioinspired Hierarchical-Structured Surfaces for Green Science and Technology, Springer-Verlag, Berlin, Germany, 2012 Search PubMed.
- M. Vargas-Gonzalez, F. Witte, P. Martz, L. Gilbert, S. Humbert, O. Jolliet, R. van Zelm and J. L'Haridon, Ecol. Indic., 2019, 107, 105498 CrossRef.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- R. Marchant and I. M. Banat, Biotechnol. Lett., 2012, 34, 1597–1605 CrossRef CAS PubMed.
- N. Joondan, S. J. Laulloo and P. Caumul, J. Dispersion Sci. Technol., 2018, 39, 1550–1564 CrossRef CAS.
- L. Fernández-Peña, E. Guzmán, F. Leonforte, A. Serrano-Pueyo, K. Regulski, L. Tournier-Couturier, F. Ortega, R. G. Rubio and G. S. Luengo, Colloids Surf., B, 2020, 185, 110578 CrossRef PubMed.
- T. S. Banipal, H. Kaur, P. K. Banipal and A. K. Sood, J. Surfactants Deterg., 2014, 17, 1181–1191 CrossRef CAS.
- C. Bettenhausen, Chem. Eng. News, 2023, 101, 8 Search PubMed.
- R. Kumar, R. I. Barbhuiya, V. Bohra, J. W. C. Wong, A. Singh and G. Kaur, Microbiol. Res., 2023, 272, 127386 CrossRef CAS PubMed.
- R. Schütz, A. V. Rawlings, E. Wandeler, E. Jackson, S. Trevisan, J. M. Monneuse, I. Bendik, M. Massironi and D. Imfeld, Int. J. Cosmet. Sci., 2019, 41, 240–256 CrossRef PubMed.
- BEAUACTIVE®, https://www.dsm.com/personal-care/en_US/products/skin-bioactives/beauactive.html, (accessed 28th August 2023).
- DSM Nutritional Products, 2016059169, 2015.
- Green Sciences at the Heart of the Cosmetic Transition for L'Oréal for the Future, https://www.loreal.com/en/articles/science-and-technology/green-sciences-noveal/, (accessed 14 July 2023).
- L'Oréal and the French biotech, Microphyt , announce a strategic partnership L'Oréal and the French biotech, Microphyt, announce a strategic partnership 14 July 2023.
- Algaesense, https://algaesense.com/, (accessed 14 July 2023).
- New Skincare Brand “Ulé” to be Launched in France, https://corp.shiseido.com/en/news/detail.html?n=00000000003380, (accessed 14 July 2023).
- C. Carnero-Ruiz, Sugar-Based Surfactants Fundamentals and Applications, CRC Press, Boca Raton, FL, USA, 2009 Search PubMed.
- A. Ammala, Int. J. Cosmet. Sci., 2013, 35, 113–124 CrossRef CAS PubMed.
- E. Guzmán and A. Lucia, Cosmetics, 2021, 8, 114 CrossRef.
- B. J. Coscia, J. C. Shelley, A. R. Browning, J. M. Sanders, R. Chaudret, R. Rozot, F. Léonforte, M. D. Halls and G. S. Luengo, Phys. Chem. Chem. Phys., 2023, 25, 1768–1780 RSC.
- S. Llamas, E. Guzmán, F. Ortega, N. Baghdadli, C. Cazeneuve, R. G. Rubio and G. S. Luengo, Adv. Colloid Interface Sci., 2015, 222, 461–487 CrossRef CAS PubMed.
- L. Fernández-Peña and E. Guzmán, Cosmetics, 2020, 7, 26 CrossRef.
- C. Wang, P. Zhang, Z. Chen, Y. Liu, L. Zhao, N. Wang and B. Xu, J. Mol. Liq., 2021, 325, 114823 CrossRef CAS.
- J. Cumming, D. Hawker, H. Chapman and K. Nugent, Water, Air, Soil Pollut., 2011, 216, 441–450 CrossRef CAS.
- C. Esposito, L'Oréal's Committed to Sustainability, Green Chemistry & Circular Economy; https://www.happi.com/contents/view_online-exclusives/2021-03-05/loreals-committed-to-sustainability-green-chemistry-circular-economy/(accessed 14 July 2023).
- I. Dini and S. Laneri, Molecules, 2021, 26, 3921 CrossRef CAS PubMed.
- A. Alessandrini and B. M. Piraccini, Cosmetics, 2016, 3, 34 CrossRef.
- M. Philippe, B. Didillon and L. Gilbert, Ann. des Falsif. l'expertise Chim. Toxicol., 2016, 985, 36–43 Search PubMed.
- A. Benhur, S. Pingali and S. Amin, J. Cosmet. Sci., 2020, 71, 455–480 Search PubMed.
- T. F. R. Alves, M. Morsink, F. Batain, M. V. Chaud, T. Almeida, D. A. Fernandes, C. F. da Silva, E. B. Souto and P. Severino, Cosmetics, 2020, 7, 75 CrossRef CAS.
- Total Repair 5 Champú Reparador pelo dañado, https://www.loreal-paris.es/elvive/total-repair-5/champu-reparador-pelo-danado-370ml, (accessed 14 July 2023).
- Champú Extrasuave Detox, https://institutoespanol.com/producto/champu-extrasuave-detox/#1591723784510-d3f1e6b3-8a5f, (accessed 14 July 2023).
- Repair & Protect Shampoo, https://pantene.com/en-us/product/repair-protect-shampoo, (accessed 14 June 2023).
- C. E. Drakontis and S. Amin, Curr. Opin. Colloid Interface Sci., 2020, 48, 77–90 CrossRef CAS.
- S. Llamas, L. Fernández-Peña, A. Akanno, E. Guzmán, V. Ortega, F. Ortega, A. G. Csaky, R. A. Campbell and R. G. Rubio, Phys. Chem. Chem. Phys., 2018, 20, 1395–1407 RSC.
- S. Llamas, E. Guzmán, A. Akanno, L. Fernández-Peña, F. Ortega, R. A. Campbell, R. Miller and R. G. Rubio, J. Phys. Chem. C, 2018, 122, 4419–4427 CrossRef CAS.
- L. Fernández-Peña, E. Guzmán, C. Fernández-Pérez, I. Barba-Nieto, F. Ortega, F. Leonforte, R. G. Rubio and G. S. Luengo, Polymers, 2022, 14, 1335 CrossRef PubMed.
- E. Guzmán, A. Maestro, F. Ortega and R. G. Rubio, J. Phys.: Condens. Matter, 2023, 35, 323001, DOI:10.1088/1361-648X/acd041.
- L. Fernández-Peña, E. Guzmán, T. Oñate-Martínez, C. Fernández-Pérez, F. Ortega, R. G. Rubio and G. S. Luengo, Polymers, 2023, 15, 3011 CrossRef PubMed.
- R. Y. Lochhead, L. R. Huisinga and T. Waller, Cosmet. Toiletries, 2006, 121, 76–82 Search PubMed.
- D. Jamieson, Sulphates in the Personal Care industry - myth busting, https://www.crodapersonalcare.com/en-gb/blog/sulphates-in-the-personal-care-industry-myth-busting, (accessed 17 July 2023).
- J. Dias-Ferreira, A. R. Fernandes, J. L. Soriano, B. C. Naveros, P. Severino, C. F. da Silva and E. B. Souto, in Biopolymer Membranes and Films, ed. M. A. de Moraes, C. F. da Silva and R. S. Vieira, Elsevier, Amsterdam, The Netherlands, 2020, pp. 309–330, DOI:10.1016/B978-0-12-818134-8.00013-4.
- R. P. Gawade, S. L. Chinke and P. S. Alegaonkar, in Polymer Science and Innovative Applications, ed. M. A. A. AlMaadeed, D. Ponnamma and M. A. Carignano, Elsevier, Amsterdam, The Netherlands, 2020, pp. 545–565, DOI:10.1016/B978-0-12-816808-0.00017-2.
- P. Severino, J. F. Fangueiro, M. V. Chaud, J. Cordeiro, A. M. Silva and E. B. Souto, in Nanobiomaterials in Galenic Formulations and Cosmetics, ed. A. M. Grumezescu, William Andrew Publishing, Norwich, NY, USA, 2016, vol. 10, pp. 1–23 Search PubMed.
- Polyquaternium, https://www.atamanchemicals.com/polyquaternium_u24914/, (accessed 19 July 2023).
- Cosmética y detergencia, https://www.derypol.com/productos/personal-care-home-care/, (accessed 20 July 2023).
- E. Guzmán, F. Ortega, N. Baghdadli, G. S. Luengo and R. G. Rubio, Colloids Surf., A, 2011, 375, 209–218 CrossRef.
- T. Bujak, Z. Nizioł-Łukaszewska and A. Ziemlewska, Int. J. Biol. Macromol., 2020, 147, 973–979 CrossRef CAS PubMed.
- C. Fernandes, B. Medronho, L. Alves and M. G. Rasteiro, Polymers, 2023, 15, 608 CrossRef CAS PubMed.
- Conditioning Agents for Hair and Skin, ed. R. Schueller and P. Romanowski, CRC Press, Boca Ratón, FL, USA, 1999 Search PubMed.
- M. Philippe, J. Haridon, J. Portal, S. Chodorowski and G. Luengo, L'Actual. Chim., 2020, 456–458, 101–107 Search PubMed.
- S.-H. Jun, S.-G. Park and N.-G. Kang, Polymers, 2019, 11, 1044 CrossRef CAS PubMed.
- Biopolymers for cosmetics, https://www.cosmeticsdesign-europe.com/Headlines/Promotional-Features/Biopolymers-for-cosmetics, (accessed 20 July 2023).
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- A. Rana, A. Sudhaik, P. Raizada, A. A. P. Khan, Q. Van Le, A. Singh, R. Selvasembian, A. Nadda and P. Singh, Nanotechnol. Environ. Eng., 2021, 6, 1–38 CrossRef.
- D. Klemm, F. Kramer, S. Moritz, T. Lindström, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 5438–5466 CrossRef CAS PubMed.
- S. D. Rohan, A. Sanjit and A. Noureddine, in Advanced Sorption Process Applications, ed. E. Serpil, IntechOpen, Rijeka, Croatia, 2018, ch. 1, pp. 3–26, DOI:10.5772/intechopen.80898.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941–3994 RSC.
- P. Jacek, F. Dourado, M. Gama and S. Bielecki, Microb. Biotechnol., 2019, 12, 633–649 CrossRef PubMed.
- R. T. Bianchet, A. L. Vieira Cubas, M. M. Machado and E. H. Siegel Moecke, Biotechnol. Rep., 2020, 27, e00502 CrossRef PubMed.
- H. Ullah, H. A. Santos and T. Khan, Cellulose, 2016, 23, 2291–2314 CrossRef CAS.
- B. V. Mohite and S. V. Patil, Biotechnol. Appl. Biochem., 2014, 61, 101–110 CrossRef CAS PubMed.
- R. M. Savage, Food Hydrocolloids, 2000, 14, 209–215 CrossRef CAS.
- E. Guzmán, F. Ortega and R. G. Rubio, Cosmetics, 2022, 9, 99 CrossRef.
- C. Bogdan and M. L. Moldovan, in Biopolymeric Nanomaterials, ed. S. Kanwar, A. Kumar, T. A. Nguyen, S. Sharma and Y. Slimani, Elsevier, Amsterdam,The Netherlands, 2021, ch. 18, pp. 387–407, DOI:10.1016/B978-0-12-824364-0.00022-8.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603–632 CrossRef CAS.
- I. Aranaz, N. Acosta, C. Civera, B. Elorza, J. Mingo, C. Castro, M. D. l. L. Gandía and A. Heras Caballero, Polymers, 2018, 10, 213 CrossRef PubMed.
- E. Guzmán, R. Chuliá-Jordán, F. Ortega and R. G. Rubio, Phys. Chem. Chem. Phys., 2011, 13, 18200–18207 RSC.
- E. Guzmán, J. A. Cavallo, R. Chuliá-Jordán, C. Gómez, M. C. Strumia, F. Ortega and R. G. Rubio, Langmuir, 2011, 27, 6836–6845 CrossRef PubMed.
- T. Pusztahelyi, Mycology, 2018, 9, 189–201 CrossRef CAS PubMed.
- M. Triunfo, E. Tafi, A. Guarnieri, C. Scieuzo, T. Hahn, S. Zibek, R. Salvia and P. Falabella, Cosmetics, 2021, 8, 40 CrossRef CAS.
- N. Morin-Crini, E. Lichtfouse, G. Torri and G. Crini, Environ. Chem. Lett., 2019, 17, 1667–1692 CrossRef CAS.
- A. Jimtaisong and N. Saewan, Int. J. Cosmet. Sci., 2014, 36, 12–21 CrossRef CAS PubMed.
- N. Abedin, R. Bashar, A. N. Jimmy and N. A. Khan, Am. J. Mark. Res., 2020, 6, 19–27 Search PubMed.
- M. R. Yates and C. Y. Barlow, Resour., Conserv. Recycl., 2013, 78, 54–66 CrossRef.
- A. B. Moldes, L. Rodríguez-López, M. Rincón-Fontán, A. López-Prieto, X. Vecino and J. M. Cruz, Int. J. Mol. Sci., 2021, 22, 2371 CrossRef CAS PubMed.
- L. Cristiano and M. Guagni, Cosmetics, 2022, 9, 13 CrossRef.
- T. Bujak, T. Wasilewski and Z. Nizioł-Łukaszewska, Colloids Surf., B, 2015, 135, 497–503 CrossRef CAS PubMed.
- X. Vecino, J. M. Cruz, A. B. Moldes and L. R. Rodrigues, Crit. Rev. Biotechnol., 2017, 37, 911–923 CrossRef CAS PubMed.
- J. Ranke, S. Stolte, R. Störmann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183–2206 CrossRef CAS PubMed.
- P. A. Cornwell, Int. J. Cosmet. Sci., 2018, 40, 16–30 CrossRef CAS PubMed.
- L. R. Rodrigues, J. Colloid Interface Sci., 2015, 449, 304–316 CrossRef CAS PubMed.
- E. B. Manga, P. A. Celik, A. Cabuk and I. M. Banat, Curr. Opin. Colloid Interface Sci., 2021, 56, 101514 CrossRef CAS.
- N. Baccile, C. Seyrig, A. Poirier, S. Alonso-de Castro, S. L. K. W. Roelants and S. Abel, Green Chem., 2021, 23, 3842–3944 RSC.
- K. V. Sajna, R. Höfer, R. K. Sukumaran, L. D. Gottumukkala and A. Pandey, in Industrial Biorefineries & White Biotechnology, ed. A. Pandey, R. Höfer, M. Taherzadeh, K. M. Nampoothiri and C. Larroche, Elsevier, Amsterdam, The Netherlands, 2015, ch. 14, pp. 499–521, DOI:10.1016/B978-0-444-63453-5.00016-1.
- K. V. Sajna, L. D. Gottumukkala, R. K. Sukumaran and A. Pandey, in Industrial Biorefineries & White Biotechnology, ed. A. Pandey, R. Höfer, M. Taherzadeh, K. M. Nampoothiri and C. Larroche, Elsevier, Amsterdam, The Netherlands, 2015, ch. 18, pp. 607–652, DOI:10.1016/B978-0-444-63453-5.00020-3.
- T. M. S. Lima, L. C. Procópio, F. D. Brandão, A. M. X. Carvalho, M. R. Tótola and A. C. Borges, Biodegradation, 2011, 22, 585–592 CrossRef CAS PubMed.
- Z. Ben Belgacem, S. Bijttebier, C. Verreth, S. Voorspoels, I. Van de Voorde, G. Aerts, K. A. Willems, H. Jacquemyn, S. Ruyters and B. Lievens, J. Appl. Microbiol., 2015, 118, 1370–1384 CrossRef CAS PubMed.
- K. Kim, D. Yoo, Y. Kim, B. Lee, D. Shin and E.-K. Kim, J. Microbiol. Biotechnol., 2002, 12, 235–241 CAS.
- C. Hazra, D. Kundu, P. Ghosh, S. Joshi, N. Dandi and A. Chaudhari, J. Chem. Technol. Biotechol., 2011, 86, 185–198 CrossRef CAS.
- G. Bognolo, Colloids Surf., A, 1999, 152, 41–52 CrossRef CAS.
- C. E. Drakontis and S. Amin, Curr. Opin. Colloid Interface Sci., 2020, 48, 77–90 CrossRef CAS.
- A. Pfau, P. Hössel, S. Vogt, R. Sander and W. Schrepp, Macromol. Symp., 1998, 126, 241–252 CrossRef CAS.
- E. Guzmán, L. Fernández-Peña, F. Ortega and R. G. Rubio, Curr. Opin. Colloid Interface Sci., 2020, 48, 91–108 CrossRef.
- E. Guzmán, L. Fernández-Peña, A. Akanno, S. Llamas, F. Ortega and R. G. Rubio, Coatings, 2019, 9, 438 CrossRef.
- E. Guzmán, S. Llamas, A. Maestro, L. Fernández-Peña, A. Akanno, R. Miller, F. Ortega and R. G. Rubio, Adv. Colloid Interface Sci., 2016, 233, 38–64 CrossRef PubMed.
- I. Varga and R. A. Campbell, Langmuir, 2017, 33, 5915–5924 CrossRef CAS PubMed.
- M. Miyake, Adv. Colloid Interface Sci., 2017, 239, 146–157 CrossRef CAS PubMed.
- S. Zhang, X. Zeng, D. T. A. Matthews, A. Igartua, E. Rodriguez-Vidal, J. Contreras Fortes and E. Van Der Heide, Friction, 2017, 5, 207–218 CrossRef CAS.
- E. Guzmán, F. Ortega, N. Baghdadli, C. Cazeneuve, G. S. Luengo and R. G. Rubio, ACS Appl. Mater. Interfaces, 2011, 3, 3181–3188 CrossRef PubMed.
- L. Fernández-Peña, E. Guzmán, F. Ortega, L. Bureau, F. Leonforte, D. Velasco, R. G. Rubio and G. S. Luengo, Polymer, 2021, 217, 123442 CrossRef.
- M. Hernández-Rivas, E. Guzmán, L. Fernández-Peña, A. Akanno, A. Greaves, F. Léonforte, F. Ortega, R. G. Rubio and G. S. Luengo, Colloids Interfaces, 2020, 4, 33 CrossRef.
- E. Guzmán, S. Llamas, L. Fernández-Peña, F. Léonforte, N. Baghdadli, C. Cazeneuve, F. Ortega, R. G. Rubio and G. S. Luengo, Colloids Surf., A, 2020, 585, 124178 CrossRef.
- S. Llamas, E. Guzmán, N. Baghdadli, F. Ortega, C. Cazeneuve, R. G. Rubio and G. S. Luengo, Colloids Surf., A, 2016, 505, 150–157 CrossRef CAS.
- A. V. Svensson, L. Huang, E. S. Johnson, T. Nylander and L. Piculell, ACS Appl. Mater. Interfaces, 2009, 1, 2431–2442 CrossRef CAS PubMed.
- A. V. Svensson, E. S. Johnson, T. Nylander and L. Piculell, ACS Appl. Mater. Interfaces, 2010, 2, 143–156 CrossRef CAS.
- L. Qian, M. Charlot, E. Perez, G. Luengo, A. Potter and C. Cazeneuve, J. Phys. Chem. B, 2004, 108, 18608–18614 CrossRef CAS.
- S. Banerjee, C. Cazeneuve, N. Baghdadli, S. Ringeissen, F. Léonforte, F. A. M. Leermakers and G. S. Luengo, J. Phys. Chem. B, 2017, 121, 8638–8651 CrossRef CAS PubMed.
- F. A. M. Leermakers, G. S. Luengo, N. Baghdadli, C. Mazilier, A. Potter and F. Léonforte, Soft Matter, 2020, 16, 4823–4839 RSC.
- E. Guzmán, L. Fernández-Peña, G. S. Luengo, A. M. Rubio, A. Rey and F. Léonforte, Polymers, 2020, 12, 624 CrossRef PubMed.
- G. Sauerbrey, Z. Med. Phys., 1959, 155, 206–222 CAS.
- M. V. Voinova, M. Rodahl, M. Jonson and B. Kasemo, Phys. Scr., 1999, 59, 391 CrossRef CAS.
- R. M. A. Azzam and N. M. Bashara, Ellipsometry and polarized light, Elsevier, North-Holland, The Netherlands, 1987 Search PubMed.
- T. J. Halthur and U. M. Elofsson, Langmuir, 2004, 20, 1739–1745 CrossRef CAS PubMed.
- T. A. Perea Cubides and S. Amin, Cosmetics, 2022, 9, 105 CrossRef CAS.
- S. Pasche, S. M. De Paul, J. Vörös, N. D. Spencer and M. Textor, Langmuir, 2003, 19, 9216–9225 CrossRef CAS.
- L. Skedung, M. Arvidsson, J. Y. Chung, C. M. Stafford, B. Berglund and M. W. Rutland, Sci. Rep., 2013, 3, 2617 CrossRef PubMed.
- F. Eudier, D. Hirel, M. Grisel, C. Picard and G. Savary, Colloids Surf., B, 2019, 174, 181–188 CrossRef CAS PubMed.
- R. Jin, L. Skedung, C. Cazeneuve, J. C. Chang, M. W. Rutland, M. Ruths and G. S. Luengo, Biotribology, 2020, 24, 100151 CrossRef.
- D. Tabor and R. H. S. Winterton, Proc. R. Soc. A, 1969, 312, 435–450 CAS.
- J. N. Israelachvili and D. Tabor, Proc. R. Soc. A, 1972, 331, 19–38 CAS.
- J. N. Israelachvili, P. M. McGuiggan and A. M. Homola, Science, 1988, 240, 189–191 CrossRef CAS PubMed.
- A.-S. Bouchet, C. Cazeneuve, N. Baghdadli, G. S. Luengo and C. Drummond, Macromolecules, 2015, 48, 2244–2253 CrossRef CAS.
- B. Bhushan, J. N. Israelachvili and U. Landman, Nature, 1995, 374, 607–616 CrossRef CAS.
- J. R. Smith and J. A. Swift, J. Microsc., 2002, 206, 182–193 CrossRef CAS PubMed.
- J. Matusiak, E. Grządka, U. Maciołek, E. Godek and E. Guzmán, Chem. Eng. J., 2022, 450, 138145 CrossRef CAS.
- M. Hartson, C. Coyle and S. Amin, Cosmetics, 2022, 9, 69 CrossRef CAS.
- T. Morita, M. Kitagawa, S. Yamamoto, A. Sogabe, T. Imura, T. Fukuoka and D. Kitamoto, J. Oleo Sci., 2010, 59, 267–272 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |