 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metal biosorption onto non-living algae: a critical review on metal recovery from wastewater†
Ana R. F.
Carreira
,
Helena
Passos
 * and
João A. P.
Coutinho
* and
João A. P.
Coutinho

CICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193 Aveiro, Portugal. E-mail: hpassos@ua.pt
First published on 17th July 2023
Abstract
The widespread occurrence of metals in water bodies has been fueling the development of platforms for removing and recovering these elements. Biosorption has emerged as a potential tool for metal removal from wastewater. Among the available biosorbents, algae have been highlighted as a sustainable and cost-effective sorbent. Despite the blooming interest in this field, most studies comprise transversal gaps that prevent it from progressing. Herein, the application of non-living algae for metal recovery from wastewater is discussed. Limitations such as rudimentary cultivation, water decontamination emphasis in detriment to metal recovery and lack of reports contemplating ion and metal competition are addressed. Due to the limited number of studies conducted in natural wastewater, a practical application of non-living Sargassum sp. into acid mine drainage is shown. The obtained sorption capacity values are compared with those from other wastewater to evaluate the potential of non-living algae for metal sorption in real matrices. A critical review on the cost performance of algae as opposed to commercial and waste-based sorbents is presented.
1. Introduction
Metals are valuable elements relevant to the digitalization drive and energy transition outlook. Their overexploitation due to anthropogenic activities is causing metal leakage into water bodies.1 The large-scale production of metal-rich effluents is raising concerns and shifting the status of metals from fundamental elements to persistent contaminants in the aquatic system. Downstream wastewater processing has to tolerate feed composition fluctuations while being cost-effective, reliable and simple. If the designed platform cannot adjust to oscillations in the effluent composition, an upstream pretreatment must be applied to minimize this effect.2 Several approaches have been explored to recover metals from wastewater, including electrodeposition, flocculation, chemical precipitation, coagulation, membrane filtration, ion-exchange, bioleaching, photocatalysis and (bio)sorption.3,4 Most of these processes rely on the exhaustive use of chemicals, are energy-intensive and can lead to the production of toxic sludges. Among the potential candidates, sorption has been proposed as an efficient, low-cost alternative that overcomes some of the aforementioned issues.5Sorption is a general term that refers to the passive binding of ions onto the surface of a solid material, often regarded as sorbent. Biosorption is a subset of sorption that relies on biological materials, including living and non-living biomass, to remove ions from a solution in an energy-independent way. The use of living biomass widens the metal removal mechanisms as ions can be both sorbed into their surface (biosorption) and actively internalized and accumulated in living organisms (bioaccumulation). The main differences between the use of non-living and living biomass are summarized in Table 1. Metal biosorption and bioaccumulation have advantages and drawbacks depending on the specific application and circumstances. Whilst biosorption is a widely studied technique for treating metal-contaminated aqueous solutions, metal bioaccumulation is a promising technique for in situ remediation of contaminated environments.6,7 Biomass can be a valuable tool for treating several types of metal-rich wastewater, including industrial, mining, agricultural, and municipal effluents, facilitating water decontamination and metal recovery.8–10 The application of living biomass for metal recovery is challenging since recovering the internalized metals requires cell disruption, preventing biomass regeneration and affording an intricate metal solution.11 Moreover, wastewater often contains high concentrations of metals, which can be detrimental to the survival of algae.12 Upon their demise, biomass tends to release the accumulated metals into the aqueous solution, rendering them unsuitable for metal preconcentration and recovery under these conditions. Unlike living biomass, non-living biomass is unaffected by metal toxicity and only interacts with metals at a surface level. The latter feature facilitates metal desorption and recovery, enabling biosorbent regeneration and reuse in subsequent cycles. Since this review intends to highlight the potential of biomass for metal recovery from wastewater, it will be mainly focused on non-living sorbents.
| Non-living biomass (biosorption) | Living biomass (bioaccumulation) | |
|---|---|---|
| Cost | Biosorbents can be by-products and wastes. | High. Living biomass requires maintenance. |
| pH | A wide range of pH conditions may be applied. | Living biomass is strongly affected by pH. Only mild pH ranges are feasible. |
| Maintenance/storage | Non-living biomass is easy to store. | Requires external metabolic energy. |
| Versatility | Good versatility. Binding sites may interact with several ions. | Poor versatility. Affected by high metal or salt concentrations. |
| Metal uptake | High. Can be affected by other contaminants. | Usually low. Living biomass has metal toxicity thresholds. |
| Uptake rate | Generally fast. | Slower than passive sorption. |
| Regeneration and reuse | Possible. | Unlikely. Metals are intracellularly accumulated. |
| Metal recovery | Acidic or alkaline eluents enable metal recovery. | If possible, biomass cannot be reused. |
Many sorbents have been explored for metal sorption, including peat,13 fruit,14 shells,15 agricultural waste,16 algae,17 activated carbon,18 metal–organic frameworks (MOF),19 fly ash20 and magnetic beads.21 Despite their metal removal effectiveness, some sorbents present challenges and limitations. For instance, active carbon has been widely used for sorption but its production demands high energy inputs; regardless of the popularity of metal–organic frameworks (MOFs), their synthesis often relies on critical and strategic raw materials.22,23 Some sorbents may also have durability and stability issues, causing their degradation and potential particle leaching into the environment.24,25 Waste-based and natural biosorbents have been gaining attention over synthetic and conventional sorbents due to their enhanced green character.26 Among these, algae have been widely applied as a natural metal sorbent.27–30 The application of non-living algal as a metal sorbent is thriving due to their easy handling, non-additional nutritional requirements, enhanced sorption kinetics, biocompatibility, structural variety and availability.31–33 Algae are readily available and can be used directly without complex and costly processing, saving resources and minimizing waste formation.33 Algae-based sorbents are also environmentally friendly as they eliminate harmful chemicals and solvents often used in the synthesis and preparation of sorbents. Their biodegradability ensures easy disposal once metals are desorbed. The large surface area of algae and unique structural properties often afford good metal sorption capacities while exhibiting high selectivity towards some metals.34,35 The contact area of algae is usually further enhanced by applying a drying and grinding step.36 However, the use of small particles hinders algae recovery upon contact with wastewater. Algae immobilization can overcome this issue, often improving metal sorption.37–39 Within this framework, studies of metal sorption onto algae encompass algae cultivation, harvest, pretreatment, immobilization, sorption, reuse, and product recovery in a simplistic overview. Parameters such as concentration, pH, time of contact, sorbent dosage and initial metal concentration are well-known to influence the sorption capacity of algae and have been thoroughly reviewed in previous works.40–44 Therefore, this review will not delve into a detailed discussion of these parameters.
While algae hold great promise for metal sorption, several shortcomings remain unscrutinized. This work addresses the main challenges of implementing non-living algae as metal sorbents while providing insight into their potential as metal pre-concentrators in real matrices. These challenges include algae cultivation scale-up, algae harvesting, emphasis on algae use for water decontamination, lack of consideration of ion and metal competition, insufficient experimental data on natural wastewater and the cost performance of algae.33,45–49 In metal recovery, wastewaters with higher metal concentrations are advantageous due to their greater inherent value. Acid mine drainage wastewater (AMD) is an environmental issue resulting from the oxidation of sulfide minerals in mining activities that usually contain a considerable metal content and an acidic pH.50 The composition of AMD varies depending on geological and environmental conditions, but it can be regarded as a potential source of valuable metals. This review introduces a proof of concept for employing non-living algae as a metal sorbent in AMD to bridge the research gap in algae usage in natural wastewater, thereby contributing to the foundational knowledge required for the practical implementation of the proposed technology into wastewater.
2. Using algae as a metal sorbent
Algae are ubiquitous photosynthetic organisms with rapid growth. Over the past decades, their natural ability to bind and uptake metals has generated increasing interest from the scientific community, as seen in Fig. 1.The blooming interest in algae as a biosorbent is mainly due to their widespread availability, renewability, sustainability, absence of arable land requirements and deemed good cost-effectiveness.27,51,52 Another well-established characteristic of algae is their wide structural diversity. It is estimated that European waters harbor around 1700 algae species.53 Studies on algae screening for metal sorption are prompted by their distinct characteristics, which ultimately lead to different metal sorption capacities.54–56 Despite the great algae variety, only a small fraction of these is produced on a large-scale.57 This represents a bottleneck in the industrial implementation of algae as a metal biosorbent. The adopted cultivation techniques need to be improved to warrant the industrial transition of algae. Around 68% of the European cultivation units harvest algae directly from wild stocks, with the remaining cultivation units focusing on aquaculture.58 Of the cultivation units handling wild stocks, around 85% harvest algae manually. This approach is time-consuming and reduces the efficiency of the process while increasing costs. Regarding microalgae, their cultivation is mainly performed in open ponds or closed photobioreactors, the latter having a greater economic impact.59,60 The footprint of microalgae cultivation is still under debate since it requires large water volumes and can be energetically demanding.59,61 The emerging algae applications are expected to stimulate aquaculture exploration, promote the optimization of the process and lower overall costs.62–64
Collecting invasive algae could bypass some current issues inherent to algae production. Some algae are hyperproliferative, causing the formation of algal blooms.65 Algal blooms can release toxins, harming aquatic and coastal ecosystems, damaging fisheries and compromising human health and welfare.66,67 Eutrophication poses a severe environmental and socio-economic threat, with economic losses ranging from 5.5 to 265 million euros per algal bloom.68 For these reasons, algal bloom management is fundamental. Algal bloom mitigation is usually done by killing or inhibiting algae growth.69,70 Alternatively, algal blooms could be overturned into a value-added opportunity, used for the production of biofuel, recovery of carotenoids, lipids, proteins and phenolics and metal sorption.71,72 Repurposing invasive algae is an interesting alternative to landfill or incineration. Although harvesting invasive algae does not mitigate eutrophication or its negative environmental impact, repurposing this biosource could aid in decreasing the economic losses inherent to algal blooms.72 The sporadic nature of algal blooms and the commitment to their eradication from the ecosystem constrains the continued application of this bioresource.
Aquaculture and unintentional ship-related dissemination correspond to over 85% of the acknowledged sources of alien algae species, with aquaculture accounting for 54% of the new introductions.73 Algae cultivation has to be thoroughly studied before implementation to avoid cross-contamination and ecological disasters. While algae cultivation and harvest require improvements, the application of algae as a metal sorbent has been widely studied in synthetic single-metal solutions.74,75 Nevertheless, the performed studies are somehow limited experimentally.
3. Bioremediation and metal recovery
The use of algae for metal sorption has been mainly performed from a bioremediation perspective.54,74,76,77 The main differences in using metal sorption within a metal recovery and bioremediation point of view are summarized in Fig. 2. | ||
| Fig. 2 Schematic representation of the differences in metal sorption for metal preconcentration and water decontamination. | ||
The bioremediation outlook is mostly environmental, aiming at water decontamination with no accent on metal recovery. Perceiving metals as contaminants rather than critical elements constrains the evaluated experimental conditions. Algae usage for metal bioremediation addresses metals with well-known toxicity or metals deemed as widespread pollutants.78 Consequently, the diversity of the evaluated metals is restricted. Focusing on legal metal concentration limits in the aquatic system is also common within this context.47,79 Narrowing down the usage of algae for water decontamination also means that the main goal is to completely remove metals from wastewater, even under impractical conditions. Several bioremediation studies have unreasonably high algae-to-metal ratios to achieve maximum metal removal percentages.74,80,81 This approach fails to reach biomass saturation, resulting in low sorption capacity values (q, mmol g−1). Many algae-binding sites remain unoccupied, leading to an underwhelming usage of the algae sorption potential.
Bioremediation studies often dismiss the obtained metal-loaded algae, offering no waste management insights or valorization prospects.54,74,76,80 Without a suitable management strategy for the metal-loaded sorbent, the resulting waste has to be disposed of as hazardous solid waste. Addressing the integration of the metal-loaded algae in a regenerative loop is fundamental to developing a sustainable process. Algae regeneration is usually accomplished by resorting to dilute acidic or alkaline solutions.81–83 Enclosing algae in a packed bed column reactor allows the development of a continuous process by alternating sorption and desorption cycles.84 Consecutive sorption–desorption cycles eventually result in sorbent weight loss and performance decline, forcing the addition of new sorbent.85 This methodology enables algae regeneration but produces metal-bearing eluate. Altogether, this procedure is unsustainable if metal recovery is excluded. Ultimately, bioremediation would lead to the production of metal-laden algae waste or metal-tainted eluate. These wastes need proper disposal to avoid returning to the bioremediation starting point – aquatic contamination. Although bioremediation is of utmost importance for public health, it marginalizes the use of algae as a metal pre-concentrator for recovery. These mindsets are not mutually exclusive and should be bridged to avoid further gaps in this field. Metal recovery and bioremediation have been historically linked. In 1987 Volesky86 stated that metal sorption could be used to decontaminate metal- or radioactive-bearing wastewater, recover metals from effluents, and strategically concentrate and recover scarce metals. It was acknowledged that metals should be targeted based on their toxicity and commercial value. This idea faded with time, resulting in numerous studies entirely focusing on water decontamination.
The growing dependence on metal-based commodities allied with the growth of anthropologic activities and industry intensification contributes to the formation of metal-bearing wastewater and metal depletion.87 Some wastewaters require a preconcentration step since they have a lower metal content. When aiming at metal recovery, metals are strategically selected based on their criticality, societal importance and market value.43,88–90 This approach aspires to achieve optimal sorption capacity values to ensure maximum metal preconcentration onto the sorbent resorting to minimum algae-to-metal ratios. Saturation of the algae functional groups is promoted while boosting their cost performance as metal pre-concentrators. While the metal recovery process design does not target water decontamination, the latter is a beneficial secondary aftermath. One of the advantages of using algae for metal sorption is their ability to bind metals selectively.17,81,91 This feature is particularly relevant for metal recovery since it allows to refine metal preconcentration.
Following metal preconcentration, the best desorption methodology has to be ascertained. Employing eluents for metal desorption dilutes metal again. The selection of this approach must consider that minimal solid:liquid ratios are required to obtain a highly concentrated metal solution without compromising the economic viability of metal recovery. Ultimately, metals can be recovered by applying conventional electrochemical techniques.92 Both sorbent and eluent should be regenerated and used in subsequent sorption–desorption cycles. Instead of using eluents, the metal-loaded algae can be converted into biochar to facilitate its direct application.93 Metal-laden biochar can be used as a supercapacitor or catalyst.94–96 A broad range of reactions can be catalyzed with metal-laden biochar, including transesterification for biodiesel production, biomass hydrogenation and biomass hydrolysis.96–99 Developing an integrative process entailing metal sorption from real matrices and applying the resultant biochar might be a sustainable metal recovery methodology.100,101 More studies are required to assess the feasibility of the process. Ideally, a metal sorption assay should be conducted in wastewater and the recovered metal-loaded algae should be treated and applied to investigate its potential as a supercapacitor or catalyst. Prior to advancing to these applications, it is necessary to understand the underlying mechanisms of metal sorption.
4. Metal sorption mechanisms
The metal sorption mechanism is complex and involves a combination of several independent mechanisms.6 Sorption mechanisms may include chemical sorption, physical sorption and ion-exchange. In chemisorption, complexation between the metal ions and the surface functional groups of the biomass may occur, leading to the removal of metals from the aqueous solution. Physical sorption is usually a fast and reversible process occurring via polar and coulombic attraction forces between the metal ions and the functional groups of the algae. Ion-exchange is a fundamental mechanism for metal sorption in algae. Since the overall charge of the biomass particle must remain neutral, ion-exchange is followed by either a stoichiometric release of other cations, such as Ca2+, Mg2+, K+ and Na+, or by binding anions.43,102 In some cases, it is possible to evaluate the release of these ions into the surrounding aqueous solution and correlate their release with metal sorption.34The interaction between metals and the binding sites of algae is influenced by factors such as the charge of the binding sites, the nature of the metal ions and the composition of the algae.6,34,103 The algae functional groups play a significant role in metal sorption and its extent. While synthetic sorbents often display limited surface diversity, algae contain several functional groups capable of acting as ligands for metal ions, including carboxyl, hydroxyl, amino, thiol and phosphate groups.41 Based on the pKa values of these groups, carboxyl, sulfonate and phosphate are particularly influential contributors to the overall sorption capacity. However, the abundance of these functional groups may not translate into a better sorption capacity as some functional groups do not contribute to metal binding due to steric, conformational, or other barriers.6 These hindrances are also applicable to other sorbents. Despite carboxyl being deemed the most relevant functional group for metal sorption, there seems to be no correlation between the oxygen content of different algae and their sorption capacity.34 On the other hand, there is a strong correlation between the carbon, nitrogen and hydrogen content and metal sorption capacity. The protonation of the algae functional groups is influenced by the pH of the surrounding aqueous solution. Carboxyl groups tend to deprotonate as pH increases, resulting in an overall negative net charge on the algae surface. This condition facilitates the sorption of cationic metal ions. Conversely, functional groups protonated at lower pH values have an enhanced interaction with metal oxyanions. In brown algae, most metals exhibit optimal or near-optimal metal sorption capacity at pH values close to the apparent dissociation constant of carboxylic acids (pKa ≈ 5).102 The influence of pH on the interaction between metals and algae functional groups is contingent upon the classification of the metals. Metals can be classified into three classes, as depicted in Table 2.
| Metal classes | Optimal pH sorption | Metals |
|---|---|---|
| I | 4–7 | Al(III), Be(III), Cu(II), Cr(III), Co(II), Fe(III), Ni(II), Pb(II), Zn(II), and UO22+ |
| II | <4 | PtCl42−, SeO42−, CrO42− |
| III | pH independent | Ag(I), Au(III) Hg(II) |
Class I metals display their highest sorption capacity within the pH range of 4 to 7 but are unable to bind to the functional groups of biomass at pH levels below 2.103 Conversely, class II metals exhibit strong interactions with the ligand at low values due to the overall positive net charge of the ligand.6 However, they are unable to interact with the functional groups above pH 5. Finally, class III metals display stronger interactions with the sorbent than other metals and are less affected by variations in pH.41 This data demonstrates that non-living algae can exhibit a certain degree of tolerance to pH fluctuations without experiencing a notable decline in their sorption capacity. While an optimal pH range exists, it does not necessarily imply that sorption will be significantly compromised at other pH values. A limitation in using algae, and many other biosorbents, arises from their susceptibility to extreme pH conditions.11 Besides damaging the ligand, extreme pH values can hinder metal bioavailability or increase proton competition. Aqueous media with higher pH values restrict the metal solubility, forming metal hydroxide precipitates and limiting their sorption. At low pH values, protons and metals compete for the available binding sites resulting in decreased metal sorption. The effect of ion competition in metal sorption will be further discussed in the next section.
5. Ion competition
Using single-metal synthetic solutions is the most common approach in metal sorption studies.74,75,83,104–107 This simplistic methodology was valuable for understanding the influence of several factors on metal sorption onto algae while unravelling the underlying mechanisms. However, this pathway is outdated as it no longer contributes to significant knowledge advancements. Regardless of the focus being metal recovery or bioremediation, the end goal is to apply the developed process to real wastewater. Transitioning from synthetic to real wastewater based on single-metal studies is challenging as they do not represent the complexity of wastewater. Multi-elemental systems resemble real wastewater more accurately since metal competition is considered. Metal competition has an antagonist effect on metal sorption, resulting in lower sorption capacity values.108,109 When several metal ions are in solution, some are preferentially sorbed over others. This selectivity is related to the complexation constant of each metal with relevant sorbent functional groups.The hard and soft acid and bases (HSAB) theory has been used to justify the selective metal sorption behavior in a competitive system.110 According to Pearson,111 metals and ligands are categorized as hard or soft depending on their size, charge and polarizability. Hard species tend to have a small ionic radius, high charge density and are not particularly polarizable. Soft species have large radii, low charge states and are more polarizable. Metals within a multi-metallic system are categorized as soft or hard acids, and sorbent ligands are soft or hard bases. In this situation, hard acids will tend to complex with hard bases and soft acids will be more prone to form complexes with soft bases. Therefore, depending on the metal ionic radius, charge density and available complexing agents, some metals will be preferentially sorbed over others. The Irving–Williams series has also been employed to support metal selectivity in multi-metallic solutions, especially for borderline metals and intermediate ligands.112,113 According to this series, the complex octahedral stability of first-row transition divalent metals is the following: Mn2+ < Fe2+ < Co2+ < Ni2+ < Cu2+ > Zn2+.114,115 This trend is independent of the ligand and refers to water replacement by other complexing agents while being overall supported by metal ionic radius and crystal field stabilization energy. Therefore, certain metals will exhibit a higher affinity towards the sorbent depending on the surface functional groups and metals in the solution. This extends to all sorbents, including carbon-based sorbents.116
Understanding the complexation tendencies in multi-component systems helps predict the sorbent behavior in more complex matrices, but it is still an incomplete depiction of wastewater. Metal-bearing effluents also have contaminants such as organic debris and light ions.117 Besides metal competition, it is expectable to experience interference from background ions, such as Ca2+, K+, Na+ and Mg2+.118–120 Metal sorption is affected differently by various co-ions. For instance, Ca2+ and Mg2+ have a lower impact on metal sorption than Al3+. In binary systems, Al3+ can compromise metal sorption by up to 89%, while Ca2+ and Mg2+ cause a decrease of about 30%.118,121,122 The negatively charged binding sites will interact with background ions at high ionic strength, hindering metal binding and decreasing the overall sorption capacity. The extent of metal sorption inhibition depends on the complexation constant of each ion with the biomass functional groups. For example, alkali metals such as Na+ and K+ bind more weakly than metals to biomass-relevant functional groups.123 Since Mg2+ and Ca2+ have complexation constants greater than Na+ and K+, the influence of the former is more important, especially in matrices bearing metals with weaker binding strength.114,115,121,123 Background anions are also present in wastewater. The anions present in the solution can form complexes with free metal ions. Experimental data shows that metal sorption is decreased by co-anions, especially ethylenediamine tetraacetic acid (EDTA) and sulfate.122,124,125 Either the metal–ligand complexes have a lower affinity to the sorbent than the free metal ion, or the anionic species interact with the sorbent, changing the binding site state and influencing sorption.124 From the evaluated anions, EDTA has the greatest impact on metal sorption due to its well-known ability to form strong complexes with metal ions. Nevertheless, EDTA is not expected to be widely distributed in wastewater. For over 20 years, it has been recognized that metals and other ions compete for the sorbent available binding sites.121–125 Despite this, metal sorption research retracted to single-element studies instead of engaging in more complex studies to ultimately enable the application of the developed methodology to real wastewater.
6. Algae application to wastewater for metal recovery
Wastewater usually contains light ions and several metals in solution.121 The pH, metal concentration, and impurities will vary depending on the effluent source. Ion competition will inevitably compromise metal sorption in wastewater. The scarcity of studies conducted in real wastewater samples is a significant gap in the field of metal sorption based on non-living algae. This is especially astounding since the motivation behind metal sorption research is to remove and, in some cases, to recover metals from real matrices. Some investigations conducted in real matrices use living algae, which is beyond the scope of this work.126–130 When non-living algae are applied to effluents, it is common to perform algae surface modifications, spiking wastewater with metals or using high algae-to-metal ratios.118,131–136 Onyancha et al.118 reported the use of pre-treated Spirogyra condensate as a sorbent for Cr3+ in treated tannery effluent (Cr3+ concentration of 8.26 ± 0.06 mg L−1) and tannery sludge (Cr3+ concentration of 3356.70 ± 0.25 mg L−1). While metal sorption in synthetic Cr3+ solutions was above 90%, the metal sorption percentage was under 55% in the treated tannery effluent and sludge. The modest sorption was attributed to ion competition and the potential existence of ligands with stronger Cr3+ binding affinities than algae. Another study used Turbinaria ornata as a Pb2+ sorbent from municipal wastewater.134 The collected municipal wastewater contained 0.013 mg L−1 of Pb2+, to which 15.2 g L−1 of dried algae was added. Although this study remarks that T. ornate is suitable for treating urban wastewater, this is unwary due to the hyperbolic algae-to-metal ratio.In a previous work, we optimized metal sorption from synthetic quaternary metal systems.137 Herein, we present a case study dealing with natural wastewater to better understand the potential of non-living algae as a metal sorbent. The reported optimal conditions in quaternary synthetic systems were adapted to fit the composition of the AMD collected at São Domingos mine, Portugal. The pH of AMD (1.7) was below the reported optimal pH range. The AMD pH was adjusted to 4 and 5, to mimic the reported optimal pH value in synthetic multi-elemental assays. The original data on AMD composition is presented in Table 3 and experimental details are given in the ESI.†
| Ion | AMD composition (mg L−1) | ||
|---|---|---|---|
| No pretreatment | pH = 4.0 | pH = 5.0 | |
| Ca2+ | 664 ± 49 | 659 ± 11 | 637 ± 15 |
| K+ | 7.7 ± 0.8 | 7.7 ± 0.3 | 14.4 ± 0.8 |
| Mn2+ | 142 ± 5 | 121.7 ± 0.3 | 114.4 ± 0.8 |
| Fe3+ | 704 ± 17 | 1.0 ± 0.1 | — |
| Co2+ | 4.95 ± 0.04 | 3.56 ± 0.03 | 3.40 ± 0.04 |
| Ni2+ | 2.36 ± 0.01 | 1.84 ± 0.01 | 1.72 ± 0.03 |
| Cu2+ | 57 ± 1 | 48.97 ± 0.07 | 18.00 ± 0.08 |
| Zn2+ | 134 ± 3 | 125.4 ± 0.9 | 117.8 ± 0.4 |
Precipitation of Fe3+ was facilitated by pH adjustment. Most Fe3+ was precipitated at pH 4 with minor losses of the remaining metals. Fe3+ was entirely precipitated at pH 5, although considerable precipitation of the remaining metals was also observed. AMD is expected to contain ions like Na+ and Mg2+ but their quantification by total reflection X-ray fluorescence spectrometer (TXRF) is not possible. After analyzing the AMD initial composition under different conditions, the sorption capacity of the non-living brown macroalgae Sargassum sp. was evaluated at AMD with no pretreatment, pH 4 and 5.
Concerning our data, tweaking the pH from 1.7 to 4.0 or 5.0 significantly affected metal sorption (Fig. 3A). At pH 1.7 there is a large amount of Fe3+ in AMD. At this condition, Sargassum sp. showed good Fe3+ sorption capacity. The total sorption capacity at this pH (qtotal = 0.67 ± 0.03 mmol g−1) was similar to that obtained in optimal conditions in the synthetic quaternary system (qtotal = 0.657 ± 0.004 mmol g−1).137 Adjusting the AMD pH enabled to precipitate most Fe3+ while improving Cu2+ sorption. However, the total sorption capacity at pH 4.0 and 5.0 was underwhelming, especially when considering that the optimal sorption pH of Sargassum sp. was reported to range between 4.0 and 5.0. Applying the optimized algae-to-metal ratio and optimal pH values in AMD resulted in poor sorption capacity values compared to multi-component synthetic assays. The obtained results still portray the selective feature of algae. At pH 1.7 a good selectivity for Fe3+ was observed. According to the Pearson hard–soft-acid-based theory, Fe3+ is considered a hard acid and exhibits greater affinity towards hard bases such as –OH and –COOH groups.111 Conversely, metals like Cu2+, Co2+ and Ni2+ are classified as borderline metals, implying they have intermediate properties between hard and soft acids. Consequently, their interactions with hard bases are generally weaker. Since –OH and –COOH groups are recognized as important algae functional groups for metal sorption, the presence of Fe3+ will lead to its preferential sorption onto the algae biomass due to its stronger interaction with these hard bases. At pH 4.0 and 5.0, the absence of Fe3+ enabled the selective sorption of Cu2+, although sorption occurred to a lower extent (qtotal < 0.14 mmol g−1). The preferable Cu2+ sorption was also observed in the synthetic multi-elemental assays and further supported by the Irving–Williams series.114,115,137 Herein, employing Sargassum sp. in AMD was not particularly attractive from a metal recovery point of view. Still, this macroalgae removed 43% of the Fe3+ present in AMD without wastewater pretreatment. Since AMD usually contains a high Fe3+ content, its sorption may be appealing for developing a hybrid wastewater treatment.138
 | ||
| Fig. 3 (A) Original data on the sorption capacity of Sargassum sp. for each metal at an algae-to-metal ratio of 0.83 and pH 1.7, 4.0 or 5.0 and respective Y-axis zoom. (B) Total metal sorption capacity of different non-living algae in real wastewater, including AMD, industrial wastewater (IWW), tannery sludge and treated IWW.118,131,132,136,139 Original data (pink bars) and previously reported data (blue bars) are presented. The numbers following the wastewater label refer to their respective pH value and the Wn numeration catalogues the wastewater number. | ||
Other studies have struggled to apply non-living algae for metal sorption in real wastewater, including in AMD, as depicted in Fig. 3B and detailed in Table S1.† For instance, Castro et al.139 employed an algae-based technique for metal sorption in São Domingos AMD (W4 and 5, Fig. 3B). Two glass columns filled with the non-living brown algae Fucus vesiculosus were used for continuous metal sorption. The sorption capacity in both columns at pH 5 was 0.066 mmol g−1 and 0.020 mmol g−1 for Zn2+ and Cu2+, respectively. Conversely, applying F. vesiculosus in galvanic wastewater afforded interesting sorption capacity values (>1 mmol g−1, W6 and 7). The variation in pH from 4 to 5 did not significantly impact the metal sorption capacity. Although the used AMD contained a 20-fold lower metal concentration than the galvanic wastewater, the algae dosage was the same for both wastewaters. The excess of algae likely contributed to the lower sorption capacity obtained in AMD as many binding sites remained vacant. Another study achieved a promising sorption capacity of Cr4+ from electroplating wastewater at pH 1.9 in a continuous configuration using a packed bed column (W8).131 The column was packed with 20 g of non-living algae and it was able to treat 2 L of electroplating wastewater containing 16 mmol L−1 of Cr4+. The algae and the effluents were not subjected to any pretreatment, other than the drying and grinding of the algae. The system displayed a noteworthy sorption capacity of 2.1 mmol g−1, indicating a promising outlook for the implementation of this technology. Conversely, using dried and ground algae for metal sorption in petrochemical and fertilizing wastewaters were not successful, as seen in W9 and 10.132,136 A common characteristic among reports showing low sorption capacity values (qtotal < 0.1 mmol g−1) is their focus on wastewater containing lower metal concentrations, with the total metal content ranging from 6 to 36 ppm.118,132,136,139 More promising results have been observed when dealing with natural wastewater containing metal concentrations ranging from 400 to 3356 ppm.118,131,139 Higher metal concentrations in wastewater increase the likelihood of saturating the binding sites of algae, leading to higher sorption capacities, as supported by Onyancha et al.118 Applying the same algae-based approach to sorb metals from treated tannery effluent (qtotal = 0.01 mmol g−1, W12) and tannery sludge (qtotal = 2.78 mmol g−1, W11) afforded completely different sorption capacities. Altogether, the composition and complexity of the wastewater significantly impact metal sorption.
As mentioned in section 5, background ions significantly impair metal sorption. Background ions affect metals having weak complexation constants with relevant functional groups more. To demonstrate the effect of ion competition, a synthetic quaternary solution of Co2+, Cu2+, Ni2+ and Zn2+ with a total concentration of 75 mg L−1 was spiked with Ca2+ ranging from 8 to 204 mg L−1 (Fig. 4). Experimental details are given in the ESI.†
 | ||
| Fig. 4 Impact of Ca2+ concentration on the total sorption capacity (qtotal, mmol g−1) of the non-living Sargassum sp. at pH 5 (original data). | ||
Increasing Ca2+ concentration from 0 to 204 mg L−1 reduced metal sorption by 44%. Considering that the AMD used in our proof-of-concept has around 650 mg L−1 of Ca2+, it is not surprising that metal sorption was compromised compared to the quaternary synthetic metal systems.137 The inhibition of metal sorption at higher ionic strength is in agreement with the literature.118 The suitability of non-living algae for metal sorption from wastewater and the limitations associated with their composition remain uncertain. The compiled data suggests a positive correlation between metal sorption capacity and metal content in natural wastewater.118,131,132,136,139 However, further investigation is required to determine the proficiency and constraints of using non-living algae for metal removal from wastewater.
The use of algae as a metal sorbent has been mainly advocated due to their alleged good cost-efficiency. Claiming that algae are cost-efficient is speculative since, to the best of our knowledge, there are no in-depth life cycle analysis reports of metal sorption onto non-living algae for metal recovery purposes. To better understand if non-living algae are more promising than other sorbents, the cost-efficiency of different sorbents in synthetic mono-metallic solutions is discussed in the following section.
7. Comparing algae to commercial and waste-based sorbents
The application of algae as metal sorbents has been endorsed as a cheaper alternative to commercial sorbents, with macroalgae costs allegedly ranging from 1 to 3 € per kg and production costs estimated to be on average 0.2 to 0.3 € per kg.108,140 Yet, according to Bak et al.141 the cultivation of the macroalgae Saccharina latissima can range from 9.3 to 36.7 € per kg. The production cost of macroalgae is not universally agreed upon.142 Including or omitting operational, harvesting and transportation costs contribute to these discrepancies. As for microalgae, their production is more expensive, with values ranging from 69 up to 573 € per kg.62,143 Despite the disagreement on cost estimates, we believe it is important to consider the algae production cost and the retail price of commercial sorbents to compare the cost performance of different sorbents. The economic viability of different sorbents was assessed by comparing the cost-effectiveness of Cu2+ sorption in single-metal solutions for different non-living algae, commercial sorbents and sorbent-based wastes (Fig. 5 and Table S2†). Some considerations need to be addressed: algae prices refer to the market value of dry samples; sorbent prices should be lower when purchased in bulk; the maximum sorption capacity is highly dependent on the evaluated conditions of each study; the sorption capacity in multi-metallic systems is expected to be lower than in mono-metallic assays; although the ability to regenerate and reuse the sorbents greatly impacts the cost performance of the process, this was not taken into account in this analysis. In any case, the reusability of commercial sorbents is expected to be better than biomass-based sorbents such as algae and some wastes.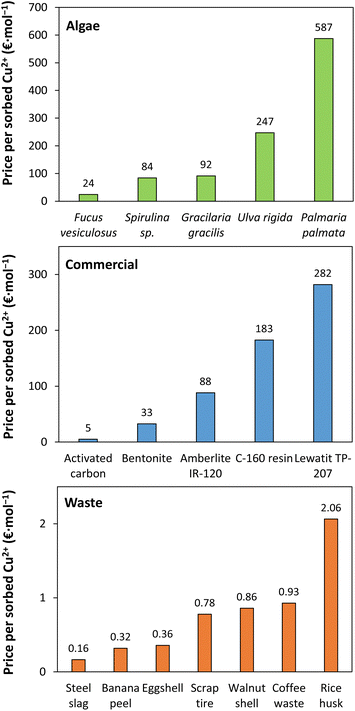 | ||
| Fig. 5 Ratio of the sorbent purchase price (€ per g) and their maximum Cu2+ sorption capacity (qmax, mol g−1) in single Cu2+ assays. Sorption capacity values were taken from the literature (more details in Table S2†).82,147–161 | ||
The brown algae Fucus vesiculosus is the most cost-effective among the evaluated algae. The good alginic acid content of brown algae promotes metal sorption via interaction with carboxyl and hydroxyl groups.102,104,144 This translates into a better sorption capacity than green (e.g. Ulva rigida) and red algae (e.g. Palmaria palmata and Gracilaria gracilis). The cost performance of algae is comparable to that of commercial sorbents, except for activated carbon. Although activated carbon is often described as expensive compared to other sorbents,145,146 this material is more cost-effective than the evaluated algae. Within the presented sorbents, waste-based sorbents are the most cost-effective. The cost-efficiency of waste sorbents is between one to two orders of magnitude better than algae, with pristine steel slag affording the most promising results. Even so, the presented cost-effectiveness of waste-based sorbents may be underrated due to cost overestimation. Waste management represents a significant economic burden to the industry. For example, the annual bill for eggshell discharge produced by an egg processing industry is estimated to be nearly 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 €.162,163 Delegating waste at no cost or for a small fee to other companies would allow reducing expenses while prompting waste valorization. This symbiotic partnership would eliminate waste management expenses and reduce the raw waste purchase cost leading to improved cost-effectiveness values. To assess the feasibility of each sorbent application, it is relevant to consider the current price of Cu2+ (0.53 € per mol).164 From all sorbents, only eggshell, banana peel and pristine steel slag would be profitable for Cu2+ sorption without considering downstream processing. Naturally, the process would be more economically viable if these materials were applied to wastewater composed of more valuable metals, such as Ni2+ (1.44 € per mol) and Co2+ (1.96 € per mol).165,166
000 €.162,163 Delegating waste at no cost or for a small fee to other companies would allow reducing expenses while prompting waste valorization. This symbiotic partnership would eliminate waste management expenses and reduce the raw waste purchase cost leading to improved cost-effectiveness values. To assess the feasibility of each sorbent application, it is relevant to consider the current price of Cu2+ (0.53 € per mol).164 From all sorbents, only eggshell, banana peel and pristine steel slag would be profitable for Cu2+ sorption without considering downstream processing. Naturally, the process would be more economically viable if these materials were applied to wastewater composed of more valuable metals, such as Ni2+ (1.44 € per mol) and Co2+ (1.96 € per mol).165,166
As previously mentioned, the sorption capacity depends on the conditions of each study, including pH, initial metal concentration, sorbent dosage and time of contact. For this reason, comparing cost-efficiency values within the same experimental conditions is a more unbiased assessment. Cochrane et al.149 reported Cu2+ removal efficiency by Fucus vesiculosus, peat, crab carapace, activated carbon and ion-exchange resin Dowex® 50WX4. At the same conditions (pH = 4.2, t = 12 h, 100 mg L−1 of Cu2+ and 5 g L−1 of sorbent dosage), the metal removal percents increased as follows: peat < macroalgae < activated carbon ≈ ion-exchange resin ≈ crab carapace. Crab carapace waste, ion-exchange resin and activated carbon achieved close to complete metal removal percent while macroalgae and peat had ≈80% and ≈50% of Cu2+ removal, respectively. Regarding the sorption capacity of each sorbent, only the calculated sorption capacity values for crab carapace, ion-exchange and macroalgae are provided: 1.24, 1.12 and 1.81 mmol g−1, respectively. Attending to the metal removal percents, the maximum sorption capacity of the macroalgae Fucus vesiculosus is likely to be overestimated. When comparing the ratio of the sorbent purchase price and their sorption capacity, shell carapace is the most cost-effective (1.9 € per mol), followed by macroalgae (26 € per mol) and the commercial ion-exchange resin (780 € per mol). This particular resin is expensive, hampering its cost performance. Even under the same conditions, waste-derived sorbents are the most cost-effective.
Besides cost-effectiveness, the selection of sorbents should also consider parameters such as environmental impact, food chain competition, and public and animal health guidelines.162,167 Algae are attractive from an environmental point of view since they contribute to CO2 capture. Yet, algae are susceptible to contamination, productivity variations, seasonality, geographic limitations and climate change.168,169 Since seaweed consumption in Asia is well-established and their incorporation in European gastronomy is expanding, using edible algae as a biosorbent would compete with the food industry.170 Commercial sorbents mitigate some of these concerns while having similar cost-effectiveness to algae. Waste-based sorbents outperform commercial sorbents and can be a viable and cost-effective alternative for metal sorption. Using wastes for metal sorption is also a sustainable way of managing them and contributing to a circular economy.171 A life cycle analysis found that using alginate extraction waste for metal sorption affords lower environmental impacts than activated carbon in several categories, including acidification, climate change, eutrophication, human toxicity, and photochemical oxidation.172 Moreover, using waste-based sorbents like calcinated eggshells can effectively increase the pH of the wastewater.173 The pH increase is attributed to the high content of calcium hydroxide in the eggshells, and it provides a solution to the inherent challenges posed by wastewater acidity. A hybrid eggshell and living microalgae system have been proposed for treating AMD.174 However, a life cycle analysis determined that employing living immobilized acid-adapted microalgae to treat AMD is more environmentally sustainable than using the conventional limestone or the previously mentioned hybrid microalgal treatment.175 No similar studies were found for non-living algae.
Scaling-up algae-derived applications demand significant cradle-to-grave processing developments. Algae have a lower or similar cost performance in single-metal solutions than commercial and waste-based sorbents. Although many studies conclude that non-living algae are adequate alternatives for metal removal in wastewater, these conclusions are drawn based on results obtained in synthetic solutions.47,148,176 More studies on the application of algae as a metal sorbent in wastewater are required to evaluate their potential to pre-concentrate and recover metal from secondary metal sources.
8. Challenges, knowledge gaps and future perspectives
The implementation of non-living algae as metal pre-concentrators needs to overcome several limitations and more studies are required to connect the dots. The large-scale production of algae faces various challenges that must be addressed. These include the development and implementation of integrated algae systems on a large volume and the efficient harvesting of algae.45 Algae production demands continuous attention to temperature, light exposure, culture integrity, carbon dioxide availability, reliable water source and supply of (micro)nutrients. These parameters are embedded in algae growth and productivity, but their complexity is regarded as a barrier to large-scale algae production. The cost-efficiency and footprint of algae cultivation should also be improved. Using wastewater as a microalgae culture medium can reduce the water footprint by around 35%, alleviate some of the costs associated with nutrient supplementation and decrease greenhouse gas emissions.60,61Despite the dual role of algae in metal preconcentration and water decontamination, most literature is focused on the use of algae for environmental decontamination.45,86,177 This is problematic from a metal recovery perspective since these studies tend to focus on simplistic scenarios involving low-concentration single metallic solutions and often overlook the potential of metal-laden algae.46,47 These studies do not represent the higher metal concentration and the diversity of ions dissolved in wastewater. Among all the Cu(II) sorption studies, only 2% targeted natural wastewater.33 Conducting more multi-elemental studies encompassing both synthetic and real wastewater is vital to gaining a more comprehensive understanding of the non-living algae potential for metal recovery from effluents. Broadening the scope of research to include metal recovery and considering the unique characteristics of wastewater will highlight the limitations and opportunities associated with using non-living algae for metal recovery from effluents. This knowledge will facilitate the development of more efficient metal removal and recovery strategies from wastewater.
Employing algae as a metal sorbent has been promoted due to their biocompatibility, renewability, wide distribution and perceived low cost.31,32 However, the presented cost performance comparison with commercial and waste-based sorbents reveals that the suitability of algae for metal recovery is not straightforward. The compiled cost performance data suggests that algae are not as competitive as waste-based sorbents that can be obtained at lower or no cost.173 Enhancing the selectivity and performance of algae for metal sorption can be achieved through various approaches, including surface chemical modification, development of nanoparticles based on algae and genetic engineering.178 These modifications could also improve the performance of algae at acidic pH values. Nevertheless, this fine-tuning will inevitably increase the cost of the sorbent, so a careful cost performance must be conducted. A life cycle analysis should be carried out to identify the environmental and economic aspects of employing non-living algae for metal sorption from wastewater, especially compared to other sorbents.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC). Ana R. F. Carreira acknowledges FCT for the Ph.D. grant SFRH/BD/143612/2019. Helena Passos acknowledges FCT – Fundação para a Ciência e a Tecnologia, I.P. for the researcher contract CEECIND/00831/2017 under the Scientific Employment Stimulus – Individual Call 2017. The authors thank Professor Dr Eduardo Ferreira da Silva (University of Aveiro – Department of Geosciences) for kindly providing the used sample of acid mine drainage wastewater.References
- D. Kapoor and M. P. Singh, in Heavy Metals in the Environment: Impact, Assessment, and Remediation, Elsevier, 2020, pp. 179–189 Search PubMed.
- H. Eccles, Trends Biotechnol., 1999, 17, 462–465 CrossRef CAS PubMed.
- H. Gogoi, T. Leiviskä, E. Heiderscheidt, H. Postila and J. Tanskanen, J. Environ. Manage., 2018, 209, 316–327 CrossRef CAS PubMed.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- G. Dodbiba, J. Ponou and T. Fujita, Microbiology for Minerals, Metals, Materials and the Environment, 2015, vol. 3, pp. 409–426 Search PubMed.
- K. A. Salam, Biofuel Res. J., 2019, 6, 948–961 CrossRef CAS.
- K. Vijayaraghavan and Y. S. Yun, Biotechnol. Adv., 2008, 26, 266–291 CrossRef CAS PubMed.
- A. Nobahar, A. B. Melka, A. Pusta, J. P. Lourenço, J. D. Carlier and M. C. Costa, Mine Water Environ., 2022, 41, 387–401 CrossRef CAS.
- R. Millán-Becerro, F. Macías, C. R. Cánovas, R. Pérez-López and J. M. Fuentes-López, Chemosphere, 2022, 295, 133876 CrossRef PubMed.
- D. Dutta, S. Arya and S. Kumar, Chemosphere, 2021, 285, 131245 CrossRef CAS PubMed.
- Z. Lin, J. Li, Y. Luan and W. Dai, Ecotoxicol. Environ. Saf., 2020, 190, 110089 CrossRef CAS PubMed.
- K. L. Wilde, J. L. Stauber, S. J. Markich, N. M. Franklin and P. L. Brown, Arch. Environ. Contam. Toxicol., 2006, 51, 174–185 CrossRef CAS PubMed.
- J. Z. Lima, E. Ferreira da Silva, C. Patinha and V. G. S. Rodrigues, J. Environ. Manage., 2022, 321, 115968 CrossRef CAS PubMed.
- Y. Zheng, Y. Li, J. Xu, G. Gao and F. Niu, RSC Adv., 2018, 8, 2844–2850 RSC.
- A. Jahanban-Esfahlan, R. Jahanban-Esfahlan, M. Tabibiazar, L. Roufegarinejad and R. Amarowicz, RSC Adv., 2020, 10, 7026–7047 RSC.
- P. M. Melia, R. Busquets, S. Ray and A. B. Cundy, RSC Adv., 2018, 8, 40378–40386 RSC.
- J. R. Guarín-Romero, P. Rodríguez-Estupiñán, L. Giraldo and J. C. Moreno-Piraján, ACS Omega, 2019, 4, 18147–18158 CrossRef PubMed.
- A. Rahmani-Sani, P. Singh, P. Raizada, E. Claudio Lima, I. Anastopoulos, D. A. Giannakoudakis, S. Sivamani, T. A. Dontsova and A. Hosseini-Bandegharaei, Bioresour. Technol., 2020, 297, 122452 CrossRef CAS PubMed.
- S. Lin, D. H. Kumar Reddy, J. K. Bediako, M. H. Song, W. Wei, J. A. Kim and Y. S. Yun, J. Mater. Chem. A, 2017, 5, 13557–13564 RSC.
- J. Wang, X. Teng, H. Wang and H. Ban, Environ. Sci. Technol., 2004, 38, 6710–6715 CrossRef CAS PubMed.
- X. Luo, X. Lei, N. Cai, X. Xie, Y. Xue and F. Yu, ACS Sustainable Chem. Eng., 2016, 4, 3960–3969 CrossRef CAS.
- Y. Kang, Y. Yu, B. Zhang, J. Fu, X. Jiang, B. Jia, X. Men and L. Li, Water, Air, Soil Pollut., 2023, 234, 1–16 CrossRef.
- Y. Qian, F. Zhang, D. J. Kang and H. Pang, Energy Environ. Mater., 2023, 6, e12414 CrossRef CAS.
- M. Bazargan, F. Ghaemi, A. Amiri and M. Mirzaei, Coord. Chem. Rev., 2021, 445, 214107 CrossRef CAS.
- R. Dandautiya, A. P. Singh and S. Kundu, Waste Manage. Res., 2018, 36, 624–634 CrossRef CAS PubMed.
- G. Crini, E. Lichtfouse, L. D. Wilson and N. Morin-Crini, Environ. Chem. Lett., 2019, 17, 195–213 CrossRef CAS.
- E. Sandau, P. Sandau, O. Pulz and M. Zimmermann, Acta Biotechnol., 1996, 16, 103–119 CrossRef CAS.
- Y. Suzuki, T. Kametani and T. Maruyama, Water Res., 2005, 39, 1803–1808 CrossRef CAS PubMed.
- Z. Mahmood, S. Zahra, M. Iqbal, M. A. Raza and S. Nasir, Appl. Water Sci., 2017, 7, 3469–3481 CrossRef CAS.
- H. Namkoong, E. Biehler, G. Namkoong and T. M. Abdel-Fattah, ACS Omega, 2022, 7, 39931–39937 CrossRef CAS PubMed.
- N. E. A. El-Naggar, R. A. Hamouda, I. E. Mousa, M. S. Abdel-Hamid and N. H. Rabei, Sci. Rep., 2018, 8, 13456 CrossRef PubMed.
- A. Kumar, S. Sidharth, B. Kandasubramanian, T. R. Cadaval and B. Kandasubramanian, Environ. Sci. Pollut. Res., 2023, 30, 39474–39493 CrossRef CAS PubMed.
- J. I. Ordóñez, S. Cortés, P. Maluenda and I. Soto, Sustainability, 2023, 15, 5521 CrossRef.
- A. R. F. Carreira, T. Veloso, I. P. E. Macário, J. L. Pereira, S. P. M. Ventura, H. Passos and J. A. P. Coutinho, Chemosphere, 2023, 314, 137675 CrossRef CAS PubMed.
- C. C. Femina, T. Kamalesh, P. Senthil Kumar and G. Rangasamy, Ind. Eng. Chem. Res., 2023, 62, 8575–8601 CrossRef.
- A. A. Mohammed, A. A. Najim, T. J. Al-Musawi and A. I. Alwared, J. Environ. Health Sci. Eng., 2019, 17, 529–538 CrossRef CAS PubMed.
- N. Akhtar, J. Iqbal and M. Iqbal, J. Hazard. Mater., 2004, 108, 85–94 CrossRef CAS PubMed.
- Suharso, Buhani and Sumadi, Desalination, 2010, 263, 64–69 CrossRef CAS.
- C. E. R. Barquilha, E. S. Cossich, C. R. G. Tavares and E. A. da Silva, J. Water Process Eng., 2019, 32, 100904 CrossRef.
- S. Saldarriaga-Hernandez, G. Hernandez-Vargas, H. M. N. Iqbal, D. Barceló and R. Parra-Saldívar, Sci. Total Environ., 2020, 715, 136978 CrossRef CAS PubMed.
- A. K. Zeraatkar, H. Ahmadzadeh, A. F. Talebi, N. R. Moheimani and M. P. McHenry, J. Environ. Manage., 2016, 181, 817–831 CrossRef CAS PubMed.
- S. Rangabhashiyam and P. Balasubramanian, Bioresour. Technol. Rep., 2019, 5, 261–279 CrossRef.
- Y. Cao, P. Shao, Y. Chen, X. Zhou, L. Yang, H. Shi, K. Yu, X. Luo and X. Luo, Resour., Conserv. Recycl., 2021, 169, 105519 CrossRef CAS.
- J. M. Jacob, C. Karthik, R. G. Saratale, S. S. Kumar, D. Prabakar, K. Kadirvelu and A. Pugazhendhi, J. Environ. Manage., 2018, 217, 56–70 CrossRef CAS PubMed.
- B. Ramesh, A. Saravanan, P. Senthil Kumar, P. R. Yaashikaa, P. Thamarai, A. Shaji and G. Rangasamy, Environ. Pollut., 2023, 327, 121572 CrossRef CAS PubMed.
- A. Abidli, Y. Huang, Z. Ben Rejeb, A. Zaoui and C. B. Park, Chemosphere, 2022, 292, 133102 CrossRef CAS PubMed.
- H. Znad, M. R. Awual and S. Martini, Molecules, 2022, 27, 1275 CrossRef CAS PubMed.
- Z. Hashmi, M. R. Bilad, Fahrurrozi, J. Zaini, J. W. Lim and Y. Wibisono, Fermentation, 2023, 9, 311 CrossRef CAS.
- L. J. da Silva and C. C. Figueredo, Biologia, 2022, 78, 1–14 CrossRef.
- E. León-Venegas, L. F. Vilches-Arenas, C. Fernández-Baco and F. Arroyo-Torralvo, Resour., Conserv. Recycl., 2023, 188, 106629 CrossRef.
- M. P. Sudhakar and S. Viswanaathan, in New and Future Developments in Microbial Biotechnology and Bioengineering, Elsevier, 2019, pp. 77–84 Search PubMed.
- M. Fabris, R. M. Abbriano, M. Pernice, D. L. Sutherland, A. S. Commault, C. C. Hall, L. Labeeuw, J. I. McCauley, U. Kuzhiuparambil, P. Ray, T. Kahlke and P. J. Ralph, Front. Plant Sci., 2020, 11, 279 CrossRef PubMed.
- M. J. Costello, P. Bouchet, C. S. Emblow and A. Legakis, Mar. Ecol.: Prog. Ser., 2006, 316, 257–268 CrossRef.
- R. Saavedra, R. Muñoz, M. E. Taboada, M. Vega and S. Bolado, Bioresour. Technol., 2018, 263, 49–57 CrossRef CAS PubMed.
- A. Chan, H. Salsali and E. McBean, ACS Sustainable Chem. Eng., 2014, 2, 130–137 CrossRef CAS.
- J. Hockaday, A. Harvey and S. Velasquez-Orta, Algal Res., 2022, 64, 102710 CrossRef.
- M. Mac Monagail and L. Morrison, J. Appl. Phycol., 2020, 32, 1287–1300 CrossRef.
- R. Araújo, F. Vázquez Calderón, J. Sánchez López, I. C. Azevedo, A. Bruhn, S. Fluch, M. Garcia Tasende, F. Ghaderiardakani, T. Ilmjärv, M. Laurans, M. Mac Monagail, S. Mangini, C. Peteiro, C. Rebours, T. Stefansson and J. Ullmann, Front. Mar. Sci., 2021, 7, 1247 Search PubMed.
- J. J. Mayers, A. Ekman Nilsson, E. Svensson and E. Albers, Ind. Biotechnol., 2016, 12, 219–234 CrossRef.
- W. Wu, L. Tan, H. Chang, C. Zhang, X. Tan, Q. Liao, N. Zhong, X. Zhang, Y. Zhang and S. H. Ho, Renewable Sustainable Energy Rev., 2023, 171, 112969 CrossRef CAS.
- J. Yang, M. Xu, X. Zhang, Q. Hu, M. Sommerfeld and Y. Chen, Bioresour. Technol., 2011, 102, 159–165 CrossRef CAS PubMed.
- F. G. Acién, J. M. Fernández, J. J. Magán and E. Molina, Biotechnol. Adv., 2012, 30, 1344–1353 CrossRef PubMed.
- A. D. Hughes, M. S. Kelly, K. D. Black and M. S. Stanley, Biotechnol. Biofuels, 2012, 5, 1–7 CrossRef PubMed.
- S. Chutia, L. Gohain, N. M. Kakoty and D. Deka, Aquac. Eng., 2022, 98, 102266 CrossRef.
- S. Sonak, K. Patil and P. Devi, Environ. Pollut. Prot., 2018, 3, 40–55 Search PubMed.
- E. Berdalet, L. E. Fleming, R. Gowen, K. Davidson, P. Hess, L. C. Backer, S. K. Moore, P. Hoagland and H. Enevoldsen, J. Mar. Biol. Assoc. U. K., 2016, 96, 61–91 CrossRef PubMed.
- P. Mishra, S. Naik, P. V. Babu, U. Pradhan, M. Begum, T. Kaviarasan, A. Vashi, D. Bandyopadhyay, P. Ezhilarasan, U. S. Panda and M. V. R. Murthy, Oceanologia, 2022, 64, 396–403 CrossRef.
- S. Sakamoto, W. A. Lim, D. Lu, X. Dai, T. Orlova and M. Iwataki, Harmful Algae, 2021, 102, 101787 CrossRef CAS PubMed.
- Y. Jia, Z. Yang, W. Su, D. Johnson and F. Kong, J. Freshwater Ecol., 2014, 29, 129–140 CrossRef CAS.
- Y. Li, D. Xu, H. Zheng, X. Liu, J. Zhao and B. Xing, Chem. Eng. J., 2021, 417, 128094 CrossRef CAS.
- J. J. Milledge, B. V. Nielsen and D. Bailey, Rev. Environ. Sci. Bio/Technol., 2015, 15, 67–88 CrossRef.
- J. K. Kim, S. Kottuparambil, S. H. Moh, T. K. Lee, Y. J. Kim, J. S. Rhee, E. M. Choi, B. H. Kim, Y. J. Yu, C. Yarish and T. Han, J. Appl. Phycol., 2015, 27, 1223–1234 CrossRef CAS.
- S. L. Williams and J. E. Smith, Annu. Rev. Ecol., Evol., Syst., 2007, 38, 327–359 CrossRef.
- W. M. Ibrahim, Y. S. Abdel Aziz, S. M. Hamdy and N. S. Gad, J. Biorem. Biodegrad., 2018, 09, 1–7 Search PubMed.
- E. Romera, F. González, A. Ballester, M. L. Blázquez and J. A. Muñoz, Bioresour. Technol., 2007, 98, 3344–3353 CrossRef CAS PubMed.
- Vidyalaxmi, G. Kaushik and K. Raza, Ecotoxicol. Environ. Saf., 2019, 180, 430–438 CrossRef CAS PubMed.
- J. He and J. P. Chen, Bioresour. Technol., 2014, 160, 67–78 CrossRef CAS PubMed.
- R. K. Goswami, K. Agrawal, M. P. Shah and P. Verma, Lett. Appl. Microbiol., 2022, 75, 701–717 CrossRef CAS PubMed.
- M. M. Hussain, J. Wang, I. Bibi, M. Shahid, N. K. Niazi, J. Iqbal, I. A. Mian, S. M. Shaheen, S. Bashir, N. S. Shah, K. Hina and J. Rinklebe, J. Hazard. Mater., 2021, 403, 124027 CrossRef CAS PubMed.
- C. H. A. I. Raju, J. Anitha, R. Mahalakshmi Kalyani, K. Satyanandam and P. Jagadeesh, Mater. Today: Proc., 2021, 44, 1816–1827 CrossRef CAS.
- L. Bulgariu and D. Bulgariu, J. Bioprocess. Biotech., 2014, 04 DOI:10.4172/2155-9821.1000146.
- Y. Li, B. Helmreich and H. Horn, Mater. Sci. Appl., 2011, 02, 70–80 CrossRef CAS PubMed.
- V. Jayakumar, S. Govindaradjane and M. Rajasimman, Surf. Interfaces, 2021, 22, 100798 CrossRef CAS.
- D. Kumar, L. K. Pandey and J. P. Gaur, Algal Res., 2016, 18, 95–109 CrossRef.
- B. Volesky, J. Weber and J. M. Park, Water Res., 2003, 37, 297–306 CrossRef CAS PubMed.
- B. Volesky, Trends Biotechnol., 1987, 5, 96–101 CrossRef CAS.
- N. A. A. Qasem, R. H. Mohammed and D. U. Lawal, npj Clean Water, 2021, 4, 1–15 CrossRef.
- J. R. Dodson, H. L. Parker, A. M. García, A. Hicken, K. Asemave, T. J. Farmer, H. He, J. H. Clark and A. J. Hunt, Green Chem., 2015, 17, 1951–1965 RSC.
- M. Heilmann, R. Breiter and A. M. Becker, Appl. Microbiol. Biotechnol., 2021, 105, 5229–5239 CrossRef CAS PubMed.
- D. Ballesteros-Plata, Y. Zhang, E. Rodríguez-Castellón, T. Vincent and E. Guibal, Adv. Sustainable Syst., 2023, 7, 2200420 CrossRef CAS.
- D. Nayak, S. Lahiri, A. Mukhopadhyay and R. Pal, Green Chem., 2002, 4, 581–583 RSC.
- C. Ascensão, L. Ciríaco, M. J. Pacheco and A. Lopes, Port. Electrochim. Acta, 2011, 29, 349–359 CrossRef.
- I. S. Bădescu, D. Bulgariu, I. Ahmad and L. Bulgariu, J. Environ. Manage., 2018, 224, 288–297 CrossRef PubMed.
- Y. Wang, Y. Zhang, L. Pei, D. Ying, X. Xu, L. Zhao, J. Jia and X. Cao, Sci. Rep., 2017, 7, 1–8 CrossRef PubMed.
- P. Johar, C. R. McElroy, E. L. Rylott, A. S. Matharu and J. H. Clark, Appl. Catal., B, 2022, 306, 121105 CrossRef CAS.
- R. Ramos, V. K. Abdelkader-fernández, R. Matos, A. F. Peixoto and D. M. Fernandes, Catalysts, 2022, 12, 207 CrossRef CAS.
- Q. yan Liu, F. Yang, Z. hua Liu and G. Li, J. Ind. Eng. Chem., 2015, 26, 46–54 CrossRef.
- F. Zhang, X. H. Wu, M. Yao, Z. Fang and Y. T. Wang, Green Chem., 2016, 18, 3302–3314 RSC.
- H. K. D. Nguyen, V. V. Pham and H. T. Do, Catal. Lett., 2016, 146, 2381–2391 CrossRef CAS.
- Y. Chai, M. Bai, A. Chen, X. Xu, Z. Tong, J. Yuan, L. Peng, J. Shao, J. Xiong and C. Peng, Chem. Eng. J., 2023, 453, 139814 CrossRef CAS.
- H. Wang, H. Wang, H. Zhao and Q. Yan, Chem. Eng. J., 2020, 379, 122372 CrossRef CAS.
- T. A. Davis, B. Volesky and A. Mucci, Water Res., 2003, 37, 4311–4330 CrossRef CAS PubMed.
- D. W. Darnall, B. Greene, M. T. Henzl, J. M. Hosea, R. A. McPherson, J. Sneddon and M. D. Alexander, Environ. Sci. Technol., 1986, 20, 206–208 CrossRef CAS PubMed.
- C. Pennesi, C. Totti, T. Romagnoli, B. Bianco, I. De Michelis and F. Beolchini, Water Environ. Res., 2012, 84, 9–16 CrossRef CAS PubMed.
- L. Brinza, K. Geraki, I. G. Breaban and M. Neamtu, J. Hazard. Mater., 2019, 365, 252–260 CrossRef CAS PubMed.
- Y. Prasanna Kumar, P. King and V. S. R. K. Prasad, Chem. Eng. J., 2007, 129, 161–166 CrossRef.
- M. Tuzen and A. Sari, Chem. Eng. J., 2010, 158, 200–206 CrossRef CAS.
- A. H. Sulaymon, A. A. Mohammed and T. J. Al-Musawi, Environ. Sci. Pollut. Res., 2013, 20, 3011–3023 CrossRef CAS PubMed.
- J. Monroy-Figueroa, D. I. Mendoza-Castillo, A. Bonilla-Petriciolet and M. A. Pérez-Cruz, Int. J. Environ. Sci. Technol., 2015, 12, 2867–2880 CrossRef CAS.
- M. Tsezos and E. Remoudaki, Res. Microbiol., 1997, 148, 515–517 CrossRef CAS PubMed.
- R. T. Myers, Inorg. Chem., 1974, 13, 2040–2041 CrossRef CAS.
- V. Strelko, D. J. Malik and M. Streat, Sep. Sci. Technol., 2004, 39, 1885–1905 CrossRef CAS.
- G. F. Morley and G. M. Gadd, Mycol. Res., 1995, 99, 1429–1438 CrossRef CAS.
- H. Irving and R. J. P. Williams, Nature, 1948, 162, 746–747 CrossRef CAS.
- H. Irving and R. J. P. Williams, J. Chem. Soc. (Resumed), 1953, 3192–3210 RSC.
- A. Kuroki, M. Hiroto, Y. Urushihara, T. Horikawa, K. I. Sotowa and J. R. Alcántara Avila, Adsorption, 2019, 25, 1251–1258 CrossRef CAS.
- S. K. Mehta and J. P. Gaur, Crit. Rev. Biotechnol., 2005, 25, 113–152 CrossRef CAS PubMed.
- D. Onyancha, W. Mavura, J. C. Ngila, P. Ongoma and J. Chacha, J. Hazard. Mater., 2008, 158, 605–614 CrossRef CAS PubMed.
- H. S. Lee and B. Volesky, Water Qual. Res. J. Can., 1999, 34, 519–533 CrossRef CAS.
- V. J. P. Vilar, C. M. S. Botelho and R. A. R. Boaventura, Bioresour. Technol., 2008, 99, 750–762 CrossRef CAS PubMed.
- H. S. Lee, J. H. Suh, I. B. Kim and T. Yoon, Miner. Eng., 2004, 17, 487–493 CrossRef CAS.
- V. K. Gupta and A. Rastogi, J. Hazard. Mater., 2008, 154, 347–354 CrossRef CAS PubMed.
- R. M. Smith and A. E. Martell, Critical Stability Constants: Inorganic Complexes, 1975, vol. 4 Search PubMed.
- J. M. Tobin, D. G. Cooper and R. J. Neufeld, Biotechnol. Bioeng., 1987, 30, 882–886 CrossRef CAS PubMed.
- P. Kaewsarn, Q. Yu and W. Ma, Environ. Eng. Sci., 2001, 18, 99–104 CrossRef CAS.
- J. Pinto, J. Colónia, T. Viana, N. Ferreira, D. Tavares, J. Jacinto, A. Abdolvasei, F. L. Monteiro, B. Henriques and E. Pereira, J. Cleaner Prod., 2022, 369, 133299 CrossRef CAS.
- Y. Sun, T. Lu, Y. Pan, M. Shi, D. Ding, Z. Ma, J. Liu, Y. Yuan, L. Fei and Y. Sun, Environ. Sci. Ecotechnology, 2022, 12, 100204 CrossRef CAS PubMed.
- J. Iqbal, A. Javed and M. A. Baig, Biorem. J., 2022, 26, 31–40 CrossRef CAS.
- M. M. El-Sheekh, A. A. Farghl, H. R. Galal and H. S. Bayoumi, Rend. Lincei, 2016, 27, 401–410 CrossRef.
- T. Du, A. Bogush, O. Mašek, S. Purton and L. C. Campos, Chemosphere, 2022, 304, 135284 CrossRef CAS PubMed.
- F. V. Hackbarth, D. Maass, A. A. U. de Souza, V. J. P. Vilar and S. M. A. G. U. de Souza, Chem. Eng. J., 2016, 290, 477–489 CrossRef CAS.
- J. Ye, H. Xiao, B. Xiao, W. Xu, L. Gao and G. Lin, Water Sci. Technol., 2015, 72, 1662–1666 CrossRef CAS PubMed.
- F. Almomani and R. R. Bohsale, Sci. Total Environ., 2021, 755, 142654 CrossRef CAS PubMed.
- N. A. Al-Dhabi and M. V. Arasu, Environ. Res., 2022, 204, 112115 CrossRef PubMed.
- D. L. Ramasamy, S. Porada and M. Sillanpää, Chem. Eng. J., 2019, 371, 759–768 CrossRef CAS.
- M. A. P. Cechinel, D. A. Mayer, T. A. Pozdniakova, L. P. Mazur, R. A. R. Boaventura, A. A. U. de Souza, S. M. A. G. U. de Souza and V. J. P. Vilar, Chem. Eng. J., 2016, 286, 1–15 CrossRef CAS.
- A. R. F. Carreira, N. Schaeffer, H. Passos and J. A. P. Coutinho, Chem. Eng. Res. Des., 2023, 192, 546–555 CrossRef CAS.
- I. Park, C. B. Tabelin, S. Jeon, X. Li, K. Seno, M. Ito and N. Hiroyoshi, Chemosphere, 2019, 219, 588–606 CrossRef CAS PubMed.
- L. Castro, L. A. Bonilla, F. González, A. Ballester, M. L. Blázquez and J. A. Muñoz, Environ. Earth Sci., 2017, 76, 1–12 CrossRef CAS.
- H. L. Kite-Powell, E. Ask, S. Augyte, D. Bailey, J. Decker, C. A. Goudey, G. Grebe, Y. Li, S. Lindell, D. Manganelli, M. Marty-Rivera, C. Ng, L. Roberson, M. Stekoll, S. Umanzor and C. Yarish, Appl. Phycol., 2022, 3, 435–445 CrossRef.
- U. G. Bak, A. Mols-Mortensen and O. Gregersen, Algal Res., 2018, 33, 36–47 CrossRef.
- S. W. K. van den Burg, A. P. van Duijn, H. Bartelings, M. M. van Krimpen and M. Poelman, Aquac. Econ. Manage., 2016, 20, 235–252 CrossRef.
- P. C. Oostlander, J. van Houcke, R. H. Wijffels and M. J. Barbosa, Aquaculture, 2020, 525, 112115 CrossRef.
- J. Wang and C. Chen, Biotechnol. Adv., 2009, 27, 195–226 CrossRef CAS PubMed.
- A. Erto, L. Giraldo, A. Lancia and J. C. Moreno-Piraján, Water, Air, Soil Pollut., 2013, 224, 1531 CrossRef.
- J. N. Nsami and J. K. Mbadcam, J. Chem., 2013, 2013, 469170 Search PubMed.
- P. X. Sheng, Y. P. Ting, J. P. Chen and L. Hong, J. Colloid Interface Sci., 2004, 275, 131–141 CrossRef CAS PubMed.
- M. M. Areco, S. Hanela, J. Duran and M. dos Santos Afonso, J. Hazard. Mater., 2012, 213–214, 123–132 CrossRef CAS PubMed.
- E. L. Cochrane, S. Lu, S. W. Gibb and I. Villaescusa, J. Hazard. Mater., 2006, 137, 198–206 CrossRef CAS PubMed.
- A. Çelekli, M. Yavuzatmaca and H. Bozkurt, J. Hazard. Mater., 2010, 173, 123–129 CrossRef PubMed.
- M. K. Jha, N. Van Nguyen, J. chun Lee, J. Jeong and J. M. Yoo, J. Hazard. Mater., 2009, 164, 948–953 CrossRef CAS PubMed.
- A. Bożęcka, M. Orlof-Naturalna and A. Korpalski, J. Ecol. Eng., 2020, 21, 84–90 CrossRef.
- M. H. Morcali, B. Zeytuncu, A. Baysal, S. Akman and O. Yucel, J. Environ. Chem. Eng., 2014, 2, 1655–1662 CrossRef CAS.
- S. Richards, J. Dawson and M. Stutter, J. Environ. Manage., 2019, 231, 275–281 CrossRef CAS PubMed.
- M. H. Al-Qunaibit, W. K. Mekhemer and A. A. Zaghloul, J. Colloid Interface Sci., 2005, 283, 316–321 CrossRef CAS PubMed.
- Y. S. Chang, P. I. Au, N. M. Mubarak, M. Khalid, P. Jagadish, R. Walvekar and E. C. Abdullah, Environ. Sci. Pollut. Res., 2020, 27, 33270–33296 CrossRef CAS PubMed.
- M. A. Hossain, H. H. Ngo, W. S. Guo and T. V. Nguyen, Int. J. GEOMATE, 2012, 2, 227–234 Search PubMed.
- N. A. Oladoja, A. E. Ofomaja, J. A. Idiaghe, A. K. Akinlabi and E. E. Egbon, Desalin. Water Treat., 2010, 16, 83–94 CrossRef CAS.
- G. Z. Kyzas, Materials, 2012, 5, 1826–1840 CrossRef CAS.
- M. Feizi and M. Jalali, J. Taiwan Inst. Chem. Eng., 2015, 54, 125–136 CrossRef CAS.
- I. Nikolić, D. Đurović, M. Tadić, V. V. Radmilović and V. R. Radmilović, Chem. Eng. Commun., 2020, 207, 1278–1297 CrossRef.
- M. J. Quina, M. A. R. Soares and R. Quinta-Ferreira, Resour., Conserv. Recycl., 2017, 123, 176–186 CrossRef.
- S. Mignardi, L. Archilletti, L. Medeghini and C. De Vito, Sci. Rep., 2020, 10, 1–10 CrossRef PubMed.
- LME Copper | London Metal Exchange, https://www.lme.com/en/Metals/Non-ferrous/LME-Copper#Trading+day+summary, (accessed 15 February 2023).
- LME Nickel | London Metal Exchange, https://www.lme.com/Metals/Non-ferrous/LME-Nickel, (accessed 15 February 2023).
- LME Cobalt | London Metal Exchange, https://www.lme.com/en/Metals/EV/LME-Cobalt#Trading+day+summary, (accessed 15 February 2023).
- Â. P. Matos, E. Novelli and G. Tribuzi, Front. Food Sci. Technol., 2022, 2, 23 Search PubMed.
- R. C. McBride, S. Lopez, C. Meenach, M. Burnett, P. A. Lee, F. Nohilly and C. Behnke, Ind. Biotechnol., 2014, 10, 221–227 CrossRef.
- B. R. Kumar, T. Mathimani, M. P. Sudhakar, K. Rajendran, A. S. Nizami, K. Brindhadevi and A. Pugazhendhi, Renewable Sustainable Energy Rev., 2021, 138, 110649 CrossRef CAS.
- J. Peng, S. Min, P. Qing and M. Yang, Foods, 2021, 10, 1373 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2023, 25, 1704–1728 RSC.
- E. Nishikawa, M. G. C. da Silva and M. G. A. Vieira, J. Cleaner Prod., 2018, 178, 166–175 CrossRef CAS.
- S. Charazińska, E. Burszta-Adamiak and P. Lochyński, Sci. Rep., 2022, 12, 17766 CrossRef PubMed.
- H. J. Choi and S. M. Lee, Environ. Sci. Pollut. Res., 2015, 22, 13404–13411 CrossRef CAS PubMed.
- S. Abinandan, K. Praveen, S. R. Subashchandrabose, K. Venkateswarlu and M. Megharaj, ACS Sustainable Chem. Eng., 2020, 8, 15670–15677 CrossRef CAS.
- S. Rangabhashiyam, E. Suganya, N. Selvaraju and L. A. Varghese, World J. Microbiol. Biotechnol., 2014, 30, 1669–1689 CrossRef CAS PubMed.
- Z. Chen, A. I. Osman, D. W. Rooney, W.-D. Oh, P.-S. Yap, Z. Chen, A. I. Osman, D. W. Rooney, W.-D. Oh and P.-S. Yap, Sustainability, 2023, 15, 5128 CrossRef CAS.
- S. Y. Cheng, P. L. Show, B. F. Lau, J. S. Chang and T. C. Ling, Trends Biotechnol., 2019, 37, 1255–1268 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc01993d |
| This journal is © The Royal Society of Chemistry 2023 |

