Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
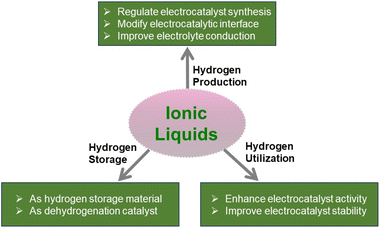
Ionic liquids as a new cornerstone to support hydrogen energy
Yanrong
Liu
abc,
Jiayao
Cui
ad,
Hao
Wang
 abc,
Ke
Wang
abc,
Ke
Wang
 abc,
Yuan
Tian
ae,
Xiaoyi
Xue
b,
Yueyang
Qiao
b,
Xiaoyan
Ji
f and
Suojiang
Zhang
abc,
Yuan
Tian
ae,
Xiaoyi
Xue
b,
Yueyang
Qiao
b,
Xiaoyan
Ji
f and
Suojiang
Zhang
 *abd
*abd
aBeijing Key Laboratory of Ionic Liquids Clean Process, CAS Key Laboratory of Green Process and Engineering, State Key Laboratory of Multiphase Complex Systems, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yrliu@ipe.ac.cn
bLongzihu New Energy Laboratory, Zhengzhou Institute of Emerging Industrial Technology, Henan University, Zhengzhou 450000, P. R. China
cHuizhou Institute of Green Energy and Advanced Materials, Huizhou, Guangdong 516081, China
dSchool of Chemical Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
eTianjin Key Laboratory of Molecular Optoelectronic Sciences, Department of Chemistry, School of Science, Tianjin University, Tianjin 300072, China
fEnergy Engineering, Division of Energy Science, Luleå University of Technology, 97187 Luleå, Sweden
First published on 29th May 2023
Abstract
As a fuel or energy carrier, hydrogen has been identified as a key way to decarbonize electricity, industry, transportation, and heating sectors. Hydrogen can be produced by a variety of methods, among which water electrolysis driven by renewable energy is sustainable and nearly carbon-free. To use hydrogen widely, storage and transportation over long distances are another key issue. Apart from storage at high pressure and low temperature, hydrogen can be stored in organic compounds via chemical bonding under relatively mild conditions. Efficient utilization of hydrogen includes hydrogen fuel cells as an alternative to internal combustion engines. From the above scenarios, catalysis and reaction media are the key factors for realizing hydrogen energy implementation. Ionic liquids (ILs) offer new opportunities due to their tunable functional groups, low vapor pressure, and stable structures as additives, solvents, and charge transfer materials. ILs are known to produce solid catalysts with controllable properties, decorate solid catalysts with modified electrons and geometric structures, and serve as electrolytes and hydrogen storage media. This review summarizes and recaps the recent progress in how ILs act as a cornerstone to support the production, storage, and utilization of hydrogen. Furthermore, critical challenges and future research directions of ILs in hydrogen energy applications are also outlined.
Introduction
As the world's population is growing and living standards are improving, the demand for energy is significantly growing. By far, the vast majority of the world's energy demand is still dependent on fossil sources (e.g., petroleum, natural gas, and coal), leading to energy crises, carbon dioxide (CO2) emissions, and even political tensions. Therefore, it is urgent and necessary to find new and clean energy sources that can reduce carbon emissions to accelerate the energy transition, especially in industry and transportation sectors.1 Hydrogen is the most abundant element on Earth and has been explored as a promising energy carrier due to its high energy density as well as its renewable and green nature.2–4 In particular, surplus power from renewable energy can be converted into hydrogen and stored as a back-up. During the peak load periods on the grid, power generated by hydrogen can be fed into the grid. Such a way significantly improves the utilization efficiency of renewable energy, reduces abandoned wind and solar energy, enhances grid dispatchability, and ensures grid security. Therefore, hydrogen energy is critical to the future construction of a multi-energy supply system, which may represent a longstanding clean energy solution. By 2050, hydrogen energy is expected to support 18% of global energy,5 and countries are taking ambitious action to seize this opportunity toward carbon neutrality.6,7Hydrogen does not purely exist in nature, and its production relies heavily on energy input. It can be manufactured from various sources, including both renewable and non-renewable sources. Hydrogen production from fossil fuels has reached the highest level of technical readiness, but the process is accompanied by large emissions of CO2. Further purification and carbon capture and storage techniques require additional costs. Hydrogen production through water electrolysis has the advantages of high purity and zero carbon emissions and has been identified as the most promising green hydrogen supply technology for the future.8 The challenge associated with water electrolysis is high power consumption, which originates from the low efficiency of the electrodes, requiring active, durable, and inexpensive electrocatalysts as well as low fluid-resistance electrolyzers.9
Another obstacle that hinders hydrogen energy implementation is the cost and immaturity of hydrogen storage under mild conditions. The compressed-gas and cryogenic-liquid hydrogen storage not only causes high energy penalties and rigorous requirements for containers but may also raise safety concerns. Hydrogen storage alloys have the advantages of high safety and high hydrogen purity but face problems of low gravimetric capacity and slow kinetics. New hydrogen storage media are desirable, such as liquid organics.10 In such media, dehydrogenation is a catalytic process using highly efficient catalytic agents.11 Recently, ammonia has also been realized as a hydrogen storage carrier. Although it requires large amounts of energy for cracking and the subsequent hydrogen compression at the end-user site, ammonia is easily liquefied for transportation.12 Ammonia produced by the traditional Haber–Bosch method results in giant carbon emissions. The new method through electrocatalysis using N2 and water to produce ammonia under ambient conditions is promising but requires electrocatalysts to activate inert N2 and suppress by-product formation.
The hydrogen fuel cell, in which hydrogen reacts with oxygen to produce electricity, is one of the most iconic technologies for the utilization of hydrogen. Hydrogen fuel cells are promising candidates to replace internal combustion engines without the limitations of the Carnot cycle and have been proven to be desirable for heavy-duty vehicles. The critical challenge is the sluggish kinetics of the oxygen reduction reaction (ORR) at the cathode. The current catalyst of choice is platinum (Pt), but its cost, mass activity, and durability are still unsatisfactory. Recent efforts have concentrated on reducing the Pt loading through alloying with Earth-abundant metals. In addition, new catalytic interfaces are also desirable to improve ion and charge transfer as well as mass transport, and to avoid corrosion.13
To ensure that hydrogen energy is more secure and affordable to release its potential, critical issues must be addressed. The abovementioned scenarios for hydrogen production, storage, and application share common problems of high cost, low efficiency, and poor stability of catalysts. Ionic liquids (ILs) could offer a solution to these problems. ILs are capable of facilitating electrocatalysis and thermocatalysis in the following ways: (1) ILs are known to act as surfactants or stabilizers to regulate the shape, particle size, and defect level of catalyst particles, thereby enhancing catalyst intrinsic activity and active site density;14 (2) ILs can be designed to contain metal and metal-free elements, which serve as sacrificial precursors to produce catalysts;15 (3) ILs can be immobilized on the surface of a solid catalyst to form a solid catalyst with an IL layer (SCILL) and regulate catalytic activity, durability, and selectivity;16 (4) ILs are favorable electrolytes or electrolyte additives due to their high ionic conductivity;17 and (5) ILs can serve as hydrogen storage media that can be hydrogenated based on imidazole or phenyl rings18 or can serve as additives to activate amineborane-based materials.19 Although ILs have the advantages described above, there are still concerns about their biological and environmental toxicity, which require careful handling during the process.20
Therefore, improving the intrinsic activity and active site density by rational design of ILs and IL–particle interactions is a new concept for enhancing the efficiency of electrocatalytic and thermocatalytic processes involved in hydrogen energy. In this respect, ILs will serve as a new cornerstone to support the implementation of hydrogen energy. ILs have been proven to be excellent electrolytes and catalysts and assist in materials synthesis.21–25 While some insightful reviews have summarized IL applications in several scenarios, such as the hydrogen evolution reaction (HER),15,26 hydrogen storage27 and fuel cells,28 the overall picture of how ILs support hydrogen energy is still missing. This review provides a summary of the recent progress in IL assistance in producing solid catalysts and IL–catalyst interactions, and their success in facilitating hydrogen production, storage, and application (Scheme 1). As we have recently carried out comprehensive reviews of the use of ILs in synthetic ammonia,29,30 this part will not be included in this review. Finally, critical challenges and perspectives on future research on IL applications in hydrogen energy are also highlighted.
Application of ionic liquids in water electrolysis
The advantages of ILs in facilitating water electrolysis are mainly reflected in the synthesis of highly efficient electrocatalysts where the particle size, active site exposure, and electronic structures can be controlled by the interfacial microenvironment. Additionally, ILs can act as binders and electrolytes to accelerate ion transfer and mass transport during water electrolysis. The research work from these aspects is reviewed in this section.Ionic liquids to regulate electrocatalyst synthesis
Due to steric hindrance, static electricity, viscosity stability, and solvation forces, ILs have been used in the synthesis of electrocatalysts to control their structure, morphology, and properties, and such successes have also been applied to water electrolysis, i.e., in the preparation of highly active and durable electrocatalysts for the cathodic HER and the anodic oxygen evolution reaction (OER). The HER performance of the ILs controlled electrocatalyst is summarized in Table 1.| ILs | Electrocatalyst | Electrolyte | Overpotential (mV vs. RHE)@10 mA cm−2 | Tafel slope (mV dec−1) | Entryref. |
|---|---|---|---|---|---|
[BMIM][TfO]: 1-butyl-3-methylimidazolium trifluoromethanesulfonate; [BMIM][BF4]: 1-butyl-3-methylimidazolium tetrafluoroborate; NG: nitrogen-doped graphene; [BPy][Br]: N-butyl pyridinium bromide; [C12MIM][Ac]: 1-dodecyl-3-methylimidazoliumacetate; Co0.6Ni0.4Se2-LN: loofah-like Co0.6Ni0.4Se2; [BMIM]2[MoO4]: 1-butyl-3-methylimidazole molybdate; [P4444][Cl]: tetrabutylphosphonium chloride; [P6,6,6,14]2[CoCl4]: trihexyl(tetradecyl)phosphoniumtetrachlorocobaltate; CNTs: carbon nanotubes; [OA][H2PO2]: N-octylammonium hypophosphite; [BMIM][Cl]: 1-butyl-3-methylimidazolium chloride; NHCS-W: nitrogen-doped hollow carbon spheres with nanoporous shells; Ni-MA: NiCl2·6H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) malonic acid 1 malonic acid 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5; GO: graphene oxide; PVEIB: poly(1-vinyl-3-ethylimidazoliumbromide); PPy: polypyrrole 5; GO: graphene oxide; PVEIB: poly(1-vinyl-3-ethylimidazoliumbromide); PPy: polypyrrole |
|||||
| [BMIM][TfO] | MoS2 | 0.5 M H2SO4 | — | 156 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| [BMIM][BF4] | MoS2/NG | 0.5 M H2SO4 | 150 | 48 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 32 |
| [BPy][Br] | MoS2 | 0.5 M H2SO4 | 259 | 59 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 33 |
| [C12MIM][Ac] | Co0.6Ni0.4Se2-LN | 0.5 M H2SO4 | 163 | 40 | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 34 |
| [BMIM]2[MoO4] | MoC | 0.5 M H2SO4 | 110 | — | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 35 |
| [P4444][Cl] | Ni2P | 0.5 M H2SO4 | 102 | 46 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
| [P4444][Cl] | Ni12P5 | 0.5 M H2SO4 | 182 | 80 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 36 |
| [P6,6,6,14]2[CoCl4] | Co2P/CNTs | 0.5 M H2SO4 | 150 | 47 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 37 |
| [OA][H2PO2] | Ni2P4O12 | 1.0 M KOH | 116 | 97 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 38 |
| [BMIM][Cl] | NHCS-W | 1.0 M KOH | 196 | 101 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 39 39 |
| Ni-MA | NiS2@GO | 1.0 M KOH | 57 | 47 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
| PVEIB | NiS2-MoS2/PVEIB/PPy/GO | 0.5 M H2SO4 | 205 | 49 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42 |
Lau et al. investigated the effects of aromatic and non-aromatic ILs on the synthesis of MoS2 as an HER electrocatalyst. It was found that the MoS2 regulated by [BMIM][TfO] and N-butylpyridinium trifluoromethanesulfonate ([BPy][TfO]) had higher crystallinity and possessed more unstacking/de-layering structures compared to those regulated by non-aromatic ILs,31 and such a high edge exposure was desirable for HER occurrence. The MoS2 synthesized in [BMIM][TfO] had the best HER performance, delivering 529 μA at −533 mV, which benefited from the effects of strong molecular interactions, H-bond acidity, and aromatic properties of ILs on the structure of MoS2. Liu et al. presented the mediation of [BMIM][BF4] to prepare MoS2/NG composites.32 Through electrostatic interactions, [BMIM][BF4] stabilized the edge sites of MoS2 and induced a delaminating morphology of the MoS2 crystals, thereby enhancing the HER electrocatalytic activity. In particular, with the addition of 1.0 mL [BMIM][BF4], the acquired MoS2/NG-IL10 displayed high HER performance and achieved 136.3 mA cm−2 at an overpotential of 300 mV in 0.5 M H2SO4, which is 7.4 times greater than that of MoS2 without [BMIM][BF4] mediation. Zhang et al. used [BPy][Br] as a structure directing agent to synthesize 1T/2H-MoS2.33 The result indicated that the proportion of the metal 1T phase in 1T/2H-MoS2 can be increased due to steric hindrance and the π-stacking interaction of [BPy][Br], thus promoting active site exposure and enhancing charge transfer. When the proportion of the 1T phase in 1T/2H-MoS2 reached 91.9%, the best HER performance was achieved with a Tafel slope of 59 mV dec−1. A facile synthesis strategy to directly grow a network of Co0.6Ni0.4Se2-LN on carbon cloth was investigated,34 where [C12MIM][Ac] played an important role in the construction of the 3D hierarchical architecture. The resulting Co0.6Ni0.4Se2-LN has the characteristic of a layered structure with sufficient active sites and channels for the transfer of electrolyte ions and electrons, leading to a low HER overpotential of 163 mV@10 mA cm−2 in 0.5 M H2SO4.
ILs can also serve as sacrificial precursors, such as metal sources, carbon sources, and heteroatom sources, to produce electrocatalysts through pyrolysis. To use ILs as metal sources, Zhang et al. prepared ordered mesoporous catalysts of MoC@C using [BMIM]2[MoO4].35 Uniform particle sizes (5 nm) of MoC with a highly crystalline structure were obtained due to the action of SBA-15 silica hard-template mediated pore channels, contributing to a low HER overpotential of 110 mV@10 mA cm−2 in 0.5 M H2SO4. Nanostructured Ni2P and Ni12P5 were developed using [P4444][Cl] as the reaction source by a microwave heating method,36 which dramatically reduced the reaction time. In 0.5 M H2SO4, Ni2P has a better HER overpotential of 102 mV than Ni12P5 of 182 mV at 10 mA cm−2. [P6,6,6,14]2[CoCl4] could serve as a source of Co and P for the preparation of Co2P/CNTs by means of a phosphidation step mixed with CNTs,37 and the resulting Co2P was uniformly distributed on CNTs to acquire only 150 mV at 10 mA cm−2 for the HER. Ying et al. used [OA][H2PO2] as a source of P to produce Ni2P4O12.38 The [OA][H2PO2] assisted synthesis not only speeds up the reaction process, but also offers a rich source of P for the manufacturing of metaphosphate, resulting in a low HER overpotential of 116 mV at 10 mA cm−2 in 1.0 M KOH. [BMIM][Cl] was applied as a source of both carbon and nitrogen to prepare NHCS-W.39 The dielectric constant of the solvent (e.g., water and ethanol) affects the dissociation/association of the IL precursor, thus affecting the morphologies and structures of the prepared carbon spheres. A solvent with a high dielectric constant is beneficial for the formation of hollow structures to enhance the water-splitting performance. The prepared NHCS-W was used as both the cathode and anode catalysts, which only required 1.61 V to achieve 10 mA cm−2. An eutectic IL of Ni-MA composed of NiCl2·6H2O and malonic acid with a mole ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 was pyrolyzed to produce NiS2@GO.40 The unique structure of the encapsulated NiS2 in the GO shell improves the kinetics and the exposure of the active sites. Consequently, the NiS2@GO electrocatalyst shows both a reduced HER overpotential of 57 mV and an OER overpotential of 294 mV at 10 mA cm−2 in 1.0 M KOH. The strategy of using 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]) as a dopant to introduce oxygen vacancies in N, P, and F tri-doped MoS2/NPF-CoFe2O4 was studied by Sun et al.,41 showing that [Bmim][PF6] not only modulated the electronic structure of MoS2/NPF-CoFe2O4, but also constructed an amorphous structure with abundant oxygen vacancies, contributing to a low OER overpotential of 250 mV at 10 mA cm−2 in 0.1 M KOH (Fig. 1).
5 was pyrolyzed to produce NiS2@GO.40 The unique structure of the encapsulated NiS2 in the GO shell improves the kinetics and the exposure of the active sites. Consequently, the NiS2@GO electrocatalyst shows both a reduced HER overpotential of 57 mV and an OER overpotential of 294 mV at 10 mA cm−2 in 1.0 M KOH. The strategy of using 1-butyl-3-methylimidazolium hexafluorophosphate ([BMIM][PF6]) as a dopant to introduce oxygen vacancies in N, P, and F tri-doped MoS2/NPF-CoFe2O4 was studied by Sun et al.,41 showing that [Bmim][PF6] not only modulated the electronic structure of MoS2/NPF-CoFe2O4, but also constructed an amorphous structure with abundant oxygen vacancies, contributing to a low OER overpotential of 250 mV at 10 mA cm−2 in 0.1 M KOH (Fig. 1).
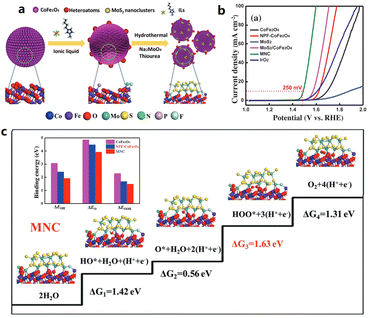 | ||
| Fig. 1 (a) Schematic illustration of the synthetic strategy for the MNC hybrid, and (b) OER polarization curves without iR-compensation. (c) The elementary steps and binding energies of the intermediates during the OER. Reproduced with permission.41 Copyright 2018, Wiley-VCH. | ||
Poly(ionic liquid)s (PILs) are a special type of IL that have both ionic properties and long chains, and also have the potential in the preparation of water-splitting catalysts. The PIL of PVEIB was applied to prepare an HER catalyst composed of NiS2-MoS2/PVEIB/PPy/GO.42 PVEIB played a crucial role in combining NiS2-MoS2 and PPy/GO with good stability and induced the formation of the 1T phase in MoS2, leading to a small HER overpotential of 205 mV@10 mA cm−2 in 0.5 M H2SO4. Different morphologies of bismuth sulfides (Bi2S3) were synthesized by Gao et al. using poly(1-methyl-3-(4-vinylbenzyl)-imidazolium chloride) (PIL-1), poly(diallyldimethylammonium bis(trifuoromethanesulfonyl)imide) (PIL-2), and poly(3-ethyl-1-vinylimidazolium bromide) (PIL-3) as additives.43 Notably, owing to the different properties of cations and anions, the backbone architectures of the PILs could be selectively coupled to specific crystal faces, modulated and even templated for sequential growth, thus significantly enhancing their effectiveness in regulating the nucleation and growth of inorganic materials (Fig. 2a–c). As shown in Fig. 2a, nanowires, hexagonal plates, and mesostructured plates of Bi2S3 were obtained by adjusting PIL-1, PIL-2, and PIL3, respectively. The obtained Bi2S3-nanowires showed the best OER performance, close to that of RuO2 at 10 mA cm−2 in 0.1 M KOH. Ding et al. used an IL as the precursor and CoCO3 to form a novel IL containing Co species (Fig. 2d and e),44 The prepared novel IL was further reacted with CNTs by polymerization to form a PIL-Co/CNT electrocatalyst with PIL and Co species well dispersed on the CNT surfaces. CoSSPIL/CNT afforded much better OER activity (1.64 V vs. RHE at 10 mA cm−2) compared to Co3O4/CNT and CoCO3.
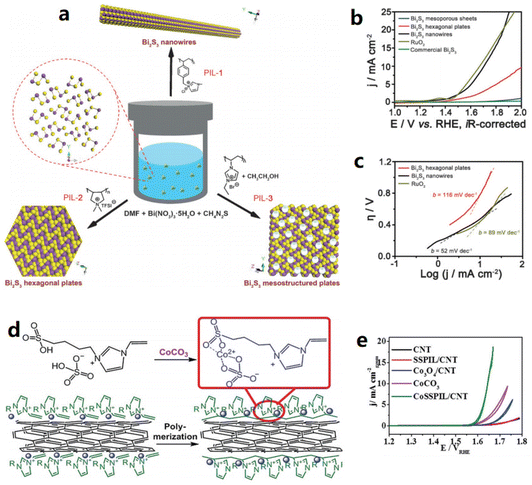 | ||
| Fig. 2 (a) PILs controlled the structures of Bi2S3, (b) OER linear sweep voltammetry (LSV) curves of Bi2S3 and RuO2, (c) Tafel slope of Bi2S3 and RuO2. Reproduced with permission.43 Copyright 2016, Wiley-VCH; (d) preparation procedure of CoSSPIL/CNT, and (e) cyclic voltammogram (CV) of CoSSPIL/CNT compared to different samples of CNT, SSPIL/CNT, Co3O4/CNT, and CoCO3. Reproduced with permission.44 Copyright 2018, Wiley-VCH. | ||
Ionic liquids to modify electrocatalytic interfaces
The electrochemical activity can be improved by modifying the electrode surface and constructing a well-defined solid/liquid or solid/liquid/gas three-phase junction, impacting the charge/ion transfer, mass transport, and hydrophilic/hydrophobic properties. Gidi et al. prepared and characterized hybrid electrocatalysts using N-octylpyridinium hexafluorophosphate ([OPy][PF6]) and mineral oil as the binder on multi-walled carbon nanotubes (MWCNTs).45 The prepared MWCNT/[OPy][PF6] presented high HER electrocatalytic activity and stability in 0.1 M H3PO4 solution (pH = 7), as [OPy][PF6] significantly improved the conductive performance and increased the electroactive area of the electrodes. The faradaic efficiency of the MWCNT/OPyPF6 system for hydrogen production was determined to be 60% at −0.30 vs. Ag/AgCl, and the sample obtained an extremely high TOF of 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274. Ji et al. used a hydrophobic IL, 1-methyl-2,3,4,6,7,8-hexahydro-1H-pyrimidol[1,2-a]pyrimidinium bis[(trifluoromethyl)sulfonyl]imide ([MTBD][NTf2]), to modify CoS2 microspheres where different amounts of IL were used during the evaporation process.46 All the CoS2-IL composites exhibited improved OER activity compared to CoS2 microspheres, and the resulting catalyst could require a low OER overpotential of 310 mV at 10 mA cm−2. The Tafel slope and impedance show improved ionic conductivity and the IL-induced hydrophobic environment at the CoS2/electrolyte interface provides a favorable microenvironment for triggering the OER process (Fig. 3).
274. Ji et al. used a hydrophobic IL, 1-methyl-2,3,4,6,7,8-hexahydro-1H-pyrimidol[1,2-a]pyrimidinium bis[(trifluoromethyl)sulfonyl]imide ([MTBD][NTf2]), to modify CoS2 microspheres where different amounts of IL were used during the evaporation process.46 All the CoS2-IL composites exhibited improved OER activity compared to CoS2 microspheres, and the resulting catalyst could require a low OER overpotential of 310 mV at 10 mA cm−2. The Tafel slope and impedance show improved ionic conductivity and the IL-induced hydrophobic environment at the CoS2/electrolyte interface provides a favorable microenvironment for triggering the OER process (Fig. 3).
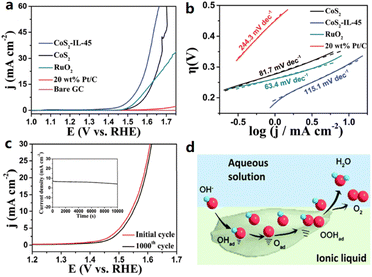 | ||
| Fig. 3 (a) OER LSV curves of the bare GC electrode, commercial 20 wt% Pt/C, CoS2 and IL-modified CoS2 and (b) the derived Tafel plots. (c) OER LSV curves of IL-modified CoS2 before and after 1000 CV tests. (d) Schematic illustration of the oxidation of water. Reproduced with permission.46 Copyright 2018, the Royal Society of Chemistry. | ||
PILs can also be used as surface modifiers. Pham Truong et al. demonstrated that the prepared poly(vinyl-imidazolium-methyl), i.e., poly(VImM), enhanced electrocatalytic activity by modifying the Pt catalyst.47 The hierarchical polymer brush structure accelerated the electron transfer and also the accessibility of water and oxygen. Compared to the Pt/C electrocatalyst, Pt/C/poly(VImM) exhibits a higher current density and lower OER overpotential, benefiting from the chemical composition and unique structure of poly(VImM).
Pandey et al. theoretically studied the geometric and electronic properties of a hybrid catalyst composed of [BMIM][TfO] and (TiO2)n (n = 2–12) nanoclusters, and the HER catalytic activity was predicted by the density functional theory (DFT).48 The calculation results show that the system ΔG of the hydrogen adsorption for the [BMIM][TfO]/(TiO2)5 is close to the ideal value (0 eV), suggesting a superior HER performance than that of the conventional Pt-based catalyst (Fig. 4).
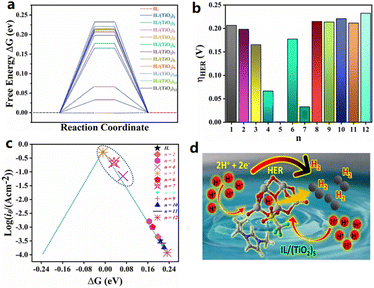 | ||
Fig. 4 (a) ΔG for hydrogen evolution at equilibrium potential of the IL and IL/(TiO2)n systems, (b) summary of calculated overpotentials for the IL and IL/(TiO2)n systems, (c) HER activity volcano plot of exchange current [log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (i0)] plotted against ΔG of hydrogen adsorption in an acidic medium, and (d) schematic illustration of HER on the IL/(TiO2)5 system. Reproduced with permission.48 Copyright 2021, American Chemical Society. (i0)] plotted against ΔG of hydrogen adsorption in an acidic medium, and (d) schematic illustration of HER on the IL/(TiO2)5 system. Reproduced with permission.48 Copyright 2021, American Chemical Society. | ||
Ionic liquids to improve electrolyte conduction
The electrolyte is also regarded as a key factor for determining the efficiency of an electrolysis system. The addition of an IL to aqueous electrolysis systems is promising to improve ion conductivity, reduce the activation energy, and modify the intrinsic structure of the electrode–electrolyte interfaces. The HER performance of various transition metal electrocatalysts (Ni, FeCr4, FeCr8, FeMn12, Ni77, and Mo) was tested using a 10 volume% aqueous solution of [BMIM][BF4] as the electrolyte.49,50 These six electrodes showed high efficiencies in the range of 94.6%–99.2%, indicating that almost all the consumed charges were involved in hydrogen production. A particularly high electrochemical performance of 77.5 mA cm−2 at −1.7 V using a Pt quasi reference electrode (PtQRE) was achieved on the Mo electrodes, which can be explained by the enhanced proton donating properties of water on the pre-adsorbed imidazolium surface with reduced activation energy. Fiegenbaum et al. investigated the water electrolysis performance in an electrolyte of 0.1 M triethylammonium-propanesulfonic acid tetrafluoroborate ([TEA-PS][BF4]).51–53 The electrical conductivity is 132.5 mS cm−1 for [TEA-PS][BF4], which is higher than those of [BMIM][BF4] (25.8 mS cm−1), KOH (96.5 mS cm−1), and KCl (52.3 mS cm−1). In 0.1 M [TEA-PS][BF4], the HER current density of a PtNi/C catalyst increased significantly from 97 to 149 mA cm−2 at −2.0 V vs. PtQRE as the reaction temperature increasing from 25 to 80 °C, following a Volmer–Heyrovsky mechanism (Fig. 5). Subsequently, 0.1 M [TEA-PS][BF4] was applied for the HER study using vitreous carbon (VC), pyrolytic carbon (PC) and moulded graphite (MG) as the cathodic materials,54 among which the VC and PC electrodes in 0.1 M [TEA-PS][BF4] presented higher performance compared to the Pt cathode, showing lower activation energy, higher cathodic exchange current, and lower charge transfer resistance. However, the use of MG for hydrogen production in this system is not recommended due to the deactivation of MG in 0.1 M [TEA-PS][BF4]. Amaral et al. evaluated the effect of adding 1–2 vol% 1-ethyl-3-methylimidazolium acetate ([EMIM][Ac]), 1-ethyl-3-methylimidazolium ethyl sulfate ([EMIM][EtSO4]) and 1-ethyl-3-methylimidazolium methanesulfonate ([EMIM][MeSO3]) to an 8 M KOH electrolyte on the HER performance.55 The best performance was obtained with the addition of 2 vol% [EMIM][MeSO3]. The HER performance in IL-added KOH has a higher Tafel slope, lower overall impedance coefficient, and higher exchange current density than that in IL-free KOH, evidencing the importance of ILs as electrolyte additives. Amaral et al. studied 1-butyl-3-ethylimidazolium bromide [BEIM][Br] and 1,3-diethylimidazolium bromide [EEIM][Br] as the electrolyte additives with a content of 1–2 vol% in 8 M KOH for HER evaluation.56 In general, the bromine based ILs, with the exception of 2 vol% [EEIM][Br], which resulted in slight increases in Tafel slopes, could improve the electrolyte properties. Thimmappa et al. chose 10% diethylmethylammonium trifluoromethanesulfonate ([Dema-H][TfO]) aqueous solution as the electrolyte to study its effect on the HER and OER performance.57 The study revealed that the IL–water system enabled two types of proton conduction, including H3O+ and [Dema-H]+. The HER electrocatalysis was accelerated by the reduction of [Dema-H]+ to [Dema], and [Dema] could be reprotonated at the anode by accepting protons from the OER.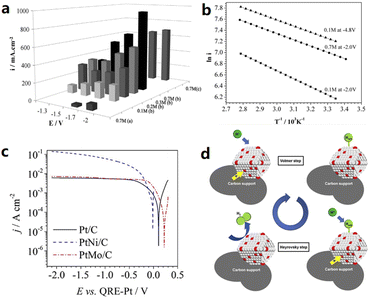 | ||
| Fig. 5 (a) Effects of the potential on the current density with different electrolytes for water electrolysis: (a) [BMIM][BF4], (b) [TEA-PS][BF4], and (c) KOH, and (b) effects of temperature on the current density for 0.1 M [TEA-PS][BF4] at −2.0 V and −4.8 V. Current density (i) is expressed in mA cm−2. Reproduced with permission.50 Copyright 2013, Elsevier. (c) Tafel plots for the HER on Pt/C, PtNi/C and PtMo/C in 0.1 M [TEA-PS][BF4], and the (d) schematic representation of the Volmer–Heyrovsky mechanism for the HER. Reproduced with permission.52 Copyright 2017, Elsevier. | ||
In addition to adding ILs to aqueous electrolytes, ILs can also serve as polymer electrolyte additives for membrane electrode assemblies (MEAs). The protic IL [Dema-H][TfO] possessed good thermal stability, and high ionic conductivity at intermediate temperatures, and was used by Thimmappa et al. to fabricate a polymer electrolyte membrane based on a polytetrafluoroethylene (PTFE).58 It was found that [Dema-H][TfO] almost completely filled the pores of the PTFE membrane. The low gas permeability was also confirmed by an H2 crossover test, in which H2 flowed to one side of the membrane (1 atm) and nitrogen flowed to the other side (50 cm3 min−1). As the temperature increased, the HER overpotential associated with the reduction of [Dema-H]+ decreased by approximately 200 mV at 100 °C. The decrease in the HER overpotential with increasing temperature can be explained by the enhancement in kinetics and decrease in pKa of [Dema-H]+ with increasing temperature. A single cell water electrolyser with the [Dema-H][TfO] impregnated membrane was successfully operated at 100 °C and 50% RH. This shows good water uptake of protic IL from the vapor phase, which is promising for intermediate-temperature water electrolysis (Fig. 6).
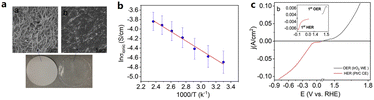 | ||
| Fig. 6 (a) SEM surface morphology of (a) the unfilled PTFE membrane, (b) [Dema][TfO] impregnated PTFE membrane and below images of both membranes, (b) ionic conductivity of [Dema-H]+[TfO]− filled in the PTFE membrane filter, and (c) OER and HER in IrO2 and Pt/C MEA with humidified (100% RH) N2 at 5 mV s−1 (inset figure for the lower potential region) at 20 °C. Reproduced with permission.58 Copyright 2020, Elsevier. | ||
Ionic liquid application in hydrogen storage
Due to their physicochemical properties, ILs have different roles to play in the field of hydrogen storage.59 For example, light-weight and hydrogen-rich ILs can be used directly as hydrogen storage materials. Additionally, ILs can also be used as catalysts for dehydrogenation.ILs as hydrogen storage materials
The ILs with efficient hydrogen storage capacities were for the first time discovered by Doroodian et al.60 Guanidinium-based borohydride ILs of (N3H6)CBH4 and (N3H8)CBH4 were obtained in tetrahydrofuran (THF) with guanidine chloride and sodium borohydride at 0 °C, as well as by mixing methylguanidine hydrochloride and sodium borohydride in the THF slurry at room temperature, respectively. The results indicated that (N3H6)CBH4 had a good hydrogen storage capacity, i.e., up to 10.7 wt% hydrogen, while for (N3H8C)CBH4, 9.0 wt% hydrogen could be released under thermal and catalytic conditions. Their high capacity to release pure hydrogen rendered these ILs as promising hydrogen storage materials. Rekken et al.61 developed a series of task-specific ILs: the N-substituted amine-borane ILs (N-ABILs), combining three different cations (imidazolium+, pyrrolidinium+, and pyrazolium+) with three different anions (Cl−, OTf−, and Tf2N−), and their hydrogen storage capacity was investigated. It was found that the Cl-based IL had a higher capacity than OTf- and Tf2N- based ILs. The optimized MeImEtABCl had a hydrogen storage capacity of 2.3 wt%. In addition, ILs can be mixed with other compounds to form liquid materials. For example, N-ABILs were mixed with amine-borane (AB) in different equivalents, resulting in improved hydrogen storage performance compared to pure N-ABILs. Using this strategy, EtImPrABTf2N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AB with a ratio of 1
AB with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 achieved a hydrogen storage capacity 4.9 wt%.
5 achieved a hydrogen storage capacity 4.9 wt%.
In contrast to the well-studied borohydride ILs, also known as tetrahydroborates (containing BH4), octahydrotriborate ILs (containing B3H8−) have also attracted a great deal of interest as intermediates in the thermal decomposition of borohydride ILs. For example, Chen et al.62 prepared guanidine octahydrotriborate ((N3H6)C(B3H8)) from NaB3H8 and guanidine chloride by ball milling or reaction in THF. Theoretically, ((N3H6)C(B3H8)) possesses a hydrogen storage capacity of 13.8 wt% due to the feature of 6H in the cation and 8H in the anion. The corresponding dehydrogenation characteristics were investigated. The dehydrogenation occurred in multiple steps when the solution was treated at 100 °C. After 95 hours, approximately 6.5 equivalents or 12.9 wt% of hydrogen was released, with approximately 4 equivalents of hydrogen being released in the first hour.
In addition to the hydrogen storage properties of ammonia borane and borohydride ILs, five-membered B/N anionic chain complexes and liquid organic hydrogen carrier ILs also showed excellent hydrogen storage properties. For example, the synthesis and dehydrogenation of four kinds of five-membered B/N anionic chain ILs, [Bu4N][BH3(NH2BH2)2H], [Et4N][BH3(NH2BH2)2H], [C(N3H6)][BH3(NH2BH2)2H], and [C(N3H5CH3)][BH3(NH2BH2)2H], were systematically investigated by Chen et al.63Fig. 7 shows the release of hydrogen from these four prepared IL aqueous solutions under the action of catalysts. Among the catalysts investigated, RuCl3 showed excellent activity, catalyzing the release of hydrogen from all four ILs within 2 min (about 33–34 mL, 8 equivalents of hydrogen). The catalytic activities of NiCl2 and Pt/C were similar, but lower than that of RuCl3, while no catalytic activity was observed for FeCl3. This work provides insight into the ILs with the five-membered B/N anionic chain and further development of the chemistry of boron and nitrogen.
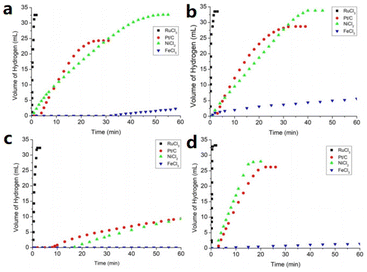 | ||
| Fig. 7 Metal-catalyzed hydrolytic H2 release from the aqueous solution of (a) [Bu4N][BH3(NH2BH2)2H], (b) [Et4N][BH3(NH2BH2)2H], (c) [C(N3H6)][BH3(NH2BH2)2H], and (d) [C(N3H5CH3)][BH3(NH2BH2)2H] at room temperature.63 Copyright 2021, Wiley-VCH. | ||
Hydrogenation of ILs was performed by Stracke et al.18 and no hydrogenation of the imidazole ring was found, which means that in principle not any imidazole-based IL could be used as the liquid-organic hydrogen carrier (LOHC) system. Subsequently, Deyko et al.64 developed ILs containing carbazole molecules as hydrogen-carrying sites using bis(trifluoromethylsulfonyl)imide imidazolium-based ILs (Fig. 8b), and compared the imidazole cation with carbazole. The results suggest that it was better to use the –(CH2)3– linkers to connect N and Si atoms. N-(3-Trimethylsilylpropyl)carbazole was successfully hydrogenated with high selectivity and no by-product with a low boiling point was formed. When the Pt/C was introduced as a hydrogenation and dehydrogenation catalyst, the theoretical total gravimetric hydrogen capacities of the synthesized ILs were 2.05 and 1.58 wt%, respectively.
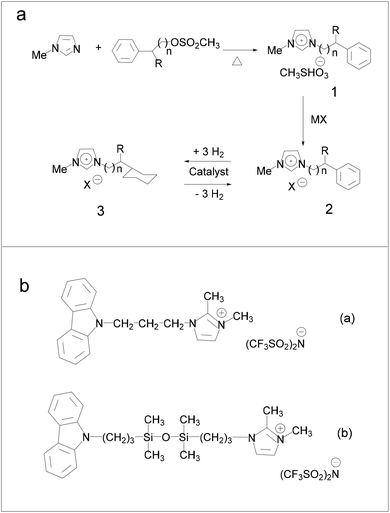 | ||
| Fig. 8 (a) Reaction paths involved in the preparation of imidazolium ionic liquids containing cyclohexane moieties. Reproduced with permission.18 Copyright 2007, American Chemical Society. (b) New ionic liquids for hydrogen storage. Reproduced with permission.64 Copyright 2020, Elsevier. | ||
Ionic liquids as dehydrogenation catalysts
ILs can serve as excellent catalysts for ammonia borane dehydrogenation.65,66 For example, Sahler et al. tested the catalytic activities of various ILs towards the dehydrogenation of ethylene diamine bisborane (EDB).67 It was found that the addition of certain ILs to EDB favors the dehydrogenation reaction. However, the adverse effects on the hydrogen yield (Fig. 9) also existed. The properties of imidazolium ILs are polarity-dependent, in which polar ILs are more favorable for the reaction than non-polar ones. A more in-depth study of 1-butyl-2,3-dimethylimidazolium chloride ([BMMIM][Cl]) showed that the [BMMIM]Cl/EDB mixture was capable of producing approximately 6.5 wt% hydrogen at 140 °C. The hydrogen content of morpholine borane is lower than that of ammonia borane, but the hydrogen production from morpholine borane is less expensive than from ammonia borane. Mishra et al. investigated the thermal dehydrogenation of morpholine borane in the presence of ILs,68 reporting the release of 1.46 equivalents of hydrogen at 60 °C and 1.75 equivalents of hydrogen at 80 °C. The amount of hydrogen released was significantly higher than that released by the dehydrogenation of hydride solid without ILs, which was equivalent to 0.62 equivalents of hydrogen.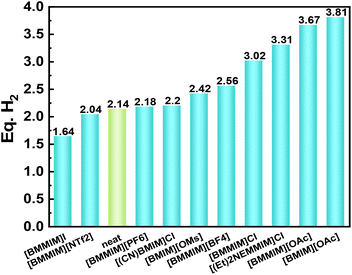 | ||
| Fig. 9 Dehydrogenation yields of EDB supported by imidazolium-based ILs. Reproduced with permission.67 Copyright 2013, Elsevier. | ||
ILs are also excellent catalysts for LOHC. Søgaard et al. used a cationic iridium (Ir) complex immobilized in [PPh4][NTf2] as a catalyst to study the dehydrogenation process (Fig. 10).69 They combined a molten salt catalyst immobilisation strategy with a homogeneous catalyst to achieve efficient low-temperature hydrogen storage/release of LOHC. Furthermore, the method allowed easy separation of catalyst and LOHC, enabling the release of large amounts of hydrogen and very small amounts of dissolved noble metal complexes.
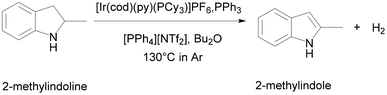 | ||
| Fig. 10 Catalytic dehydrogenation of 2-methylindoline to 2-methylindole in the liquid–liquid biphasic catalyst system using Crabtree's catalyst. Reproduced with permission.69 Copyright 2019, The Royal Society of Chemistry. | ||
Application of ionic liquids in hydrogen fuel cells
In recent decades, the development of new devices that can convert chemical energy to electricity has attracted extensive attention due to the gradually achieved global warming consensus.70 In particular, the proton exchange membrane fuel cells (PEMFCs) that can directly convert hydrogen to electrical energy in an efficient way, is the star among all the candidates. Compared with internal combustion engines using fossil fuels, the PEMFCs not only decrease the energy demand but also eliminate carbon emission with the sole product of H2O. However, the sluggish cathodic reaction (oxygen reduction reaction, ORR) kinetics and mass transfer are still the bottleneck for the large-scale application of PEMFCs.71 Generally, the ORR in fuel cells occurs at the triple-phase boundary (TPB) (electrocatalyst/electrolyte/oxygen), where electron transfer mainly occurs in the cathode catalyst layer, proton transfer in the ionomer, and gas transport through the gas diffusion layer. The limited TPB area is the main factor restricting the overall ORR performance due to the low utilization of cathode catalysts and poor proton conductivity.Ionic liquids to enhance electrocatalyst activity
ILs, especially the protic ILs, have been deemed as effective additives for promoting the ORR performances71,72 due to their high proton conductivity, great O2 diffusion coefficiency, excellent electrochemical stability, and tunable hydrophobicity.25,73 The concept of the “solid catalyst with IL layer” (SCILL) was firstly proposed by U. Kernchen and colleagues in 2007,16 coating a thin IL film on a solid heterogeneous electrocatalyst. Since then, many SCILL systems have been studied, exhibiting better activities in various reactions.74,75 In 2010, Erlebacher et al.76,77 for the first time implemented the hydrophobic SCILL concept in electrocatalysis. The constructed nano-PtNi-[MTBD][beti] ([MTBD][beti]: 1-methyl-2,3,4,6,7,8-hexahydro-1H-pyrimidol[1,2-a]pyrimidinium bis(perfluoroethylsulfonyl)imide) composite catalysts exhibited 2–3 times higher intrinsic activity than their IL-free counterpart, and remarkably 10 times higher than Pt/C. The [MTBD]-based ILs were also reported to be effective at enhancing the ORR performances of Pt3Ni nanoframes.78 The capillary force of Pt3Ni frames pulled the [MTBD][NTf2] into the pores, which retained the IL on the surface of the catalyst. The promoted ORR performances were attributed to the synergistic effects of material design and IL modifications. Similarly, a GN-Pt-IL composite catalyst prepared by impregnating [MTBD][NTf2] into the pores of graphene supported Pt nanoparticles showed improved ORR activity.79 In addition to the benefits derived from the hydrophobicity and great proton diffusion, the unique less methanol-philic feature of [MTBD][NTf2] ensured a high methanol tolerance of the electrocatalysts. Besides [MTBD]+-based ILs, a variety of other ILs are also reported to greatly boost the ORR. For example, Luo et al.80 prepared a well-connected and stable proton path for high-temperature PEMFCs by adding 1-hexyl-3-methylimidazolium trifluoromethansulfonate ([C6MIM][CF3SO3]) to the Pt electrocatalyst. Benefiting from the efficient proton delivery and IL-protector layer, the modified Pt catalyst exhibited a maximum power density (Pmax) of 512 mW cm−2 and 10% performance loss after 100 k cycles of stability test, which was superior to the durability performance of commercial Pt/C. Doblinger et al.81 compared the promotion effects of a broad range of IL cations and anion combinations (imidazolium, ammonium, phosphonium, pyrrolidinium anions and [BF4]−, [PF6]−, [NTf2]−, [FAP]− cations) at different humidity levels. The cations were found to affect the ORR current density within a wide humidity range; the anions were proven to influence the performance at a humidity level higher than 65% RH. This work pointed out that the optimal choice of the IL promoter needed to consider the humidity in the PEMFC cell. The protic ILs were also reported to be capable of replacing ionomers in the electrode for the ORR. Li et al.82 investigated the ORR performances of Pt/C under the conditions with sulfonated PIL ionomers only and a mixture of PIL ionomer/Nafion. The results suggested that the Pt/C with only the sulfonated PILs outperformed by 2 times due to the suppressed Pt oxidation, while the advantages on kinetics severely dropped at lower humidity levels. By appropriately combining PILs with Nafion, the high ORR activity was maintained across a wide humidity range. Yan et al.73 completely replaced Nafion with poly(DMVBAn-TfO-co-Stm) in the Pt/C electrocatalysts and achieved a 3.06 times higher performance than their Nafion-Pt/C counterpart. Besides the general explanations of the improved ORR activity, the PIL ionomers were believed to also suppress the nonreactive oxygenate adsorption on the Pt active sites.Many efforts in the literature are devoted to disclosing the mechanisms underlying the positive effects of ILs. In the case of enhanced proton conductivity, recent work by Wang et al.83 proved proton shuffling promotional effects of ILs on the ORR needed fine tuning. The authors screened a series of ILs as additives to Pt/C for the ORR and found a volcano correlation between ORR activities and pKa values. [MTBD][NTf2] was proven to be an optimal promoter with a pKa of approximately 15, which was close to the pKa of the product of the ORR rate-limiting step: H2O (pKa = 15.7). The “perfect match” of the pKa value and facilitated ORR performance were attributed to the hydrogen-bonding structure and the tunneling transfer of the proton. The larger product of Boltzmann probability and the proton vibration wavefunction overlap the integral of [MTBD]N-H+⋯OHPt, PμSμv2. With matched pKa, the largest PμSμv2 indicates a much higher hydrogen tunneling rate/probability for the ORR rate-determining step. The correlation between IL acidities and promotion effects on ORR activity was also investigated by Wippermann et al.84 In addition, Zhang et al.85 found that the Pt/C ORR performance had a strong dependence on the cationic side-chain length of imidazolyl ILs. If the alkyl chains were too short (e.g., 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([EMIM][NTf2])), the IL could not sufficiently enhance ORR activity because of their limited hydrophobicity as well as the low capability of suppressing the formation of nonreactive oxygenated species on Pt.
By contrast, the IL with too long alkyl chains (e.g., 1-hexyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C6MIM][NTf2]) and 1-decyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([C10MIM][NTf2])) would lead to a significant loss of the electrochemical active surface area (ECSA). The optimal chain length was needed to achieve a balance between effective inhibition of the formation of oxygenated species on the Pt surface and less passivation of active sites. Avid et al.86 further emphasized the roles of IL in the porous structure of the Pt/C in improving the ORR performance. The alkyl imidazolium ILs with different alkyl chain lengths were loaded into the Pt/C and the impacts of the IL chemical structure on the ORR performance were investigated. As shown in Fig. 11a and b, the ILs that could penetrate into the pores of carbon black would significantly enhance the proton and O2 transfer inside the pores. Additionally, the impacts of ILs on the ORR activity were conformational structure-dependent. Fig. 11c and d show that the combination of butyl and methyl functional groups promoted the ORR activity of Pt/C the most. By contrast, the butyl/methyl and butyl/bimethyl functioned imidazolium-based ILs were less beneficial or even detrimental. The authors highlighted the trade-off between proton conductivity and O2 transfer resistance, and pointed out the important roles of electrochemical ECSA change, pore-filling degree, hydrophobicity, and attraction/repulsion with Nafion in the rational design of SCILL towards the ORR.
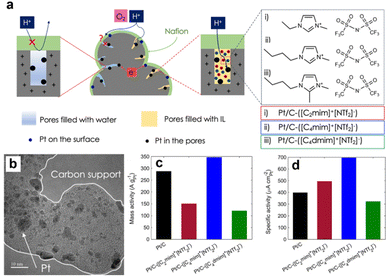 | ||
| Fig. 11 (a) The scheme of the ILs filled in the pores of carbon black supports. (b) The TEM images of Pt/C-IL catalysts. The gravimetric performance (c) and geometric performance (d) comparisons of catalysts modified with different ILs. Reproduced with permission from ref. 86. Copyright 2022, Springer Nature. | ||
Although the IL promotional effects are conventionally ascribed to the enhanced O2 transfer, quantitative investigations on the transfer resistances are relatively rare in the literature. Recently, Huang et al.87 have used the 1-methyl-2,3,4,6,7,8-hexahydro-1H-pyrimido[1,2-a]pyrimidinium 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate ([MTBD][C4F9SO3]) IL and boosted the ORR performance of PtCo/C by 48%. By conducting the limiting current measurements at different O2 partial pressures, the O2 transfer resistances were quantitatively determined. The results suggested that the IL resulted in an approximately 15% drop in oxygen diffusion resistances compared to its counterpart in the same electrode structure. In addition, the PtCo/C + IL showed a more durable O2 diffusion resistance during the 30 k cycles of stability tests, which was significantly better than the pristine PtCo/C. Another nontrivial mechanism of the IL-enhanced ORR is that the ILs alleviated the specific adsorption of the sulfonate functional group of Nafion on the Pt surface, which was detrimental to the ORR activity88,89 according to the ATR-SEIRAS method used to quantitatively investigated the specific adsorption of sulfonate anions. In the absence of ILs, the –SO3− specific adsorption was visible at 1125 cm−1. By contrast, after introducing [MTBD][beti] ILs, the vibrational absorption of –SO3− in the IR spectra diminished within the investigated polarization potentials.
Ionic liquids to improve electrocatalyst stability
Moreover, the electrochemical stability of Pt electrocatalysts could also be improved by introducing ILs via preventing Pt nanoparticles agglomeration, surface oxidation, and carbon corrosion mechanisms. In the pioneering works of SCILLs,90–93 the ILs including [MTBD]+, [BMIM]+ cations, etc., were proven to be beneficial for the durability of ORR electrocatalysts. More recently, Li et al.94 have synthesized an ultrathin PtFeNi nanowire loaded with the 1-(3-aminopropyl)-3-methylimidazolium bromide IL forming a conjugated structure (Fig. 12a). As shown in Fig. 12c, the PtFeNi nanowire/IL exhibited a high ORR activity at 2.83 A mgPt−1, which improved by 172% compared with the pristine PtFeNi. After 30 k cycles, the morphology and performance were largely maintained, and the durability was remarkably higher than the control group sample without IL modification (Fig. 12b and c). Li et al.95 reported a process to load the IL within the 3D catalyst layer forming an optimal conformational structure of the electrode. As shown in Fig. 12d, the porous catalyst layer was charged positively and dipped into the IL solution. The IL anions were uniformly distributed in the porous catalyst layer driven by the electrostatic force. The subsequent ion-exchange with IL cations resulted in a well-constructed protic IL film across the electrode hierarchical structure. Following this procedure, the authors prepared the Pt/C electrocatalyst with a ∼2 nm [MTBD][beti] IL coating. The Pt/C-[MTBD][beti] electrocatalysts showed an improvement of ORR activity by a factor of ∼2 compared to Pt/C (Fig. 12e). The durability was also improved after loading the ILs. As presented in Fig. 12f, the Pt/C + [MTBD][beti] exhibited remarkable advantages in durability with less than 30% ORR performance loss compared with Pt/C after 10 k cycles at the upper potential limit. The improved durability was attributed to the capability of maintaining the ECSA and much less Pt loss (Fig. 12g and h). The IL layer was believed to reduce the H*, destabilize OOH* and interrupt the hydrogen-bonding water network. Avid et al.86 further suggested that both the chemical structure of ILs and the conformational structure of the whole electrode were crucial to the ORR durability improvements. Since the performance of Pt degraded faster in contact with water than ionomer,96 the IL molecules with an appropriate alkyl chain length and interaction with Pt benefited durability the most. Because most of the Pt nanoparticles were within the pores of activated carbon, the capability of ILs to access the pores would improve the durability, which will also reduce the possibility of ILs to wash off during ORR tests. It is also important to note that ILs are not always beneficial for durability. For example, George et al.97 conducted an in situ ICP-MS investigation on the PtNiMo/C electrocatalyst degradation mechanism with and without IL modification. It was found that [MTBD][Beti] IL selectively increased the leaching of Mo elements in the PtNiMo/C electrocatalyst, though the mass activity was promoted by 70%.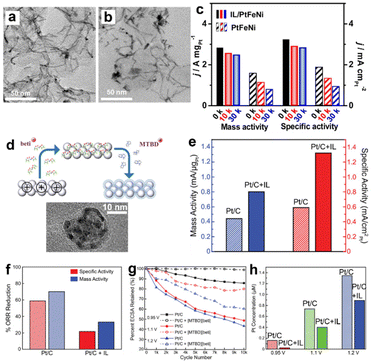 | ||
| Fig. 12 The TEM bright-field images of PtFeNi nanowires + ILs (a) before and (b) after 30 k cycles of durability tests. The ORR activities of PtFeNi nanowires + ILs and control group samples (c) before and after the stability tests. Reproduced with permission from ref. 94. Copyright 2019, Elsevier. (d) The scheme of sequential procedure loading [MTBD][beti] onto Pt/C electrocatalysts and the corresponding TEM image of Pt/C + IL. The (e) ORR activities of fresh Pt/C + [MTBD][beti] and Pt/C. The (f) ORR activities loss, (g) ECSA retaining ratios and (h) Pt concentrations in the electrolyte of Pt/C + [MTBD][beti] and Pt/C after the accelerated durability tests. Reproduced with permission from ref. 95. Copyright 2019, Copyright 2019, American Chemical Society. | ||
In summary, ILs have been proven effective in promoting the performances as well as enhancing the durability of electrocatalytic ORR via regulating the hydrogen bonding, hydrophobicity, proton/O2 transfer resistance, oxygenated species suppression, etc. Comparing the prosperous new catalyst developments, ILs are still limited to relatively few candidates. Although the general mechanisms underpinning the IL promotional effects have been proposed, quantitative investigations to prove a solid correlation between IL chemical–physical properties and specifically enhanced ORR kinetics are still lacking. The accurate description of the conformational structures of the IL-modified catalysts layer is still ambiguous. As a prosperous research topic of PEMFCs, new ILs with a clear understanding of the correlation between their structure and promotional effects are expected to further advance both the catalytic activities and durability of ORR electrocatalysis.
Summary and perspective
Hydrogen energy is deemed the most promising carbon-free secondary energy source and will fundamentally reshape both the industry and our modern way of life. Before a future powered by hydrogen energy becomes a reality, there are still important scientific challenges to be addressed within the scope of its production, transportation, and utilization. As summarized in this review, ILs, a class of green, highly designable organic compounds, have been proven to be capable of acting as a new cornerstone in all these aspects mentioned above, including but not limited to the roles of catalysts, catalyst supports and promoters, catalyst preparation regulators and hydrogen carriers. We should also be aware of the potential drawbacks of certain ILs, such as susceptibility to moisture in the air, potential environmental toxicity, and high cost, as well as the difficulty of recycling them in hydrogen energy applications, such as for catalyst synthesis and interface microenvironment enhancement. To further advance this emerging research area, the essential scientific questions for future in-depth investigations are proposed subsequently.In the case of ILs acting as catalyst supports and promoters, the understanding of the spatial and electronic relationship between the catalysts and the ILs is still limited. For catalysts with porous structures, the configurations of the ILs filled inside the pores and the effects of the confined structure on the functions of the ILs are less investigated. On the other hand, the PILs can also provide meso/microporous structures for catalysts. The confinement effects of PILs, including electronic, chemical, and steric effects, on the catalytic performances are still ambiguous. Another key issue is the stability of the IL catalyst composite structure. Considering that the IL-promoted catalytic reactions usually take place in a liquid environment with vigorous stirring, the leaching of ILs from the IL catalyst composites needs careful consideration. Finally, with the involvement of ILs in a catalytic reaction makes the mechanisms quite complex. In situ spectrokinetic characterization with high spatial/time resolutions and theoretical calculations on multi-scales are proposed to unravel the accurate and comprehensive reaction networks.
ILs acting as catalyst preparation regulators can enable the production of catalysts with different morphologies, sizes, and structures compared to conventional surfactants. However, the origin of these unique structures and the interaction of ILs with catalysts during the preparation process is less well understood. Time-resolved investigations on the morphology/crystalline structure evolution of catalysts during the IL-assisted preparation are highly recommended. Spectroscopic and theoretical investigations on the interaction between ILs and crystallites are required to disclose the mechanisms underlying the regulation effects.
Compared with conventional hydrogen storage organics, ILs show some unique advantages, including extremely low vapor pressure, high H2 storage density, self-catalytic effects, etc. For their practical application, the weight, cost penalty, and potential biological and environmental toxicity of certain ILs added to the hydrogen storage system would need to be addressed. The reversibility of ILs during H2 storage/release requires further evaluation.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2022YFA1505300), the National Natural Science Foundation of China (No. 22278402 and 22208348), the Henan Provincial Science and Technology R&D Program Joint Fund (Advantageous Discipline Cultivation, No. 222301420045), the Young Elite Scientists Sponsorship Program by CAST (No. 2021QNRC001), and the Innovation Academy for Green Manufacture, Chinese Academy of Sciences (No. IAGM2022D12).References
- X. Yang, C. P. Nielsen, S. J. Song and M. B. McElroy, Nat. Energy, 2022, 7, 955–965 CrossRef CAS.
- X. Hou, L. Yang, K. Hou, H. Shi, L. Feng, G. Suo, X. Ye, L. Zhang and Y. Yang, Green Energy Environ., 2021, 6, 124–137 CrossRef CAS.
- G. Yang, S. Guan, S. Mehdi, Y. Fan, B. Liu and B. Li, Green Energy Environ., 2021, 6, 236–243 CrossRef CAS.
- R. Kun-udom, S. Jantarang, Z. Du, B. Kitiyanan, T. Rirksomboon and V. Meeyoo, Green Chem. Eng., 2022, 3, 44–54 CrossRef.
- J. Rafiee, X. Mi, H. Gullapalli, A. V. Thomas, F. Yavari, Y. Shi, P. M. Ajayan and N. A. Koratkar, Nat. Mater., 2012, 11, 217–222 CrossRef CAS.
- R. Hanna and D. G. Victor, Nat. Energy, 2021, 6, 568–571 CrossRef.
- B. Pivovar, N. Rustagi and S. Satyapal, Electrochem. Soc. Interface, 2018, 27, 47–52 CrossRef CAS.
- S. Zhang, X. Zhang, Y. Rui, R. Wang and X. Li, Green Energy Environ., 2021, 6, 458–478 CrossRef CAS.
- M. F. Lagadec and A. Grimaud, Nat. Mater., 2020, 19, 1140–1150 CrossRef CAS PubMed.
- C. Tarhan and M. A. Çil, J. Energy Storage, 2021, 40, 102676 CrossRef.
- R. Singh, M. Singh and S. Gautam, Mater. Today: Proc., 2021, 46, 5420–5427 CAS.
- S. Chatterjee, R. K. Parsapur and K.-W. Huang, ACS Energy Lett., 2021, 6, 4390–4394 CrossRef CAS.
- S. Favero, I. E. L. Stephens and M. M. Titirici, Adv. Energy Sustainability Res., 2021, 2, 2000062 CrossRef CAS.
- C. Verma, E. E. Ebenso and M. A. Quraishi, J. Mol. Liq., 2019, 276, 826–849 CrossRef CAS.
- K. Chen, B. Xu, L. Y. Shen, D. H. Shen, M. J. Li and L. H. Guo, RSC Adv., 2022, 12, 19452–19469 RSC.
- U. Kernchen, B. Etzold, W. Korth and A. Jess, Chem. Eng. Technol., 2007, 30, 985–994 CrossRef CAS.
- X. Tan, X. Sun and B. Han, Natl. Sci. Rev., 2022, 9, nwab022 CrossRef.
- M. P. Stracke, G. Ebeling, R. Cataluña and J. Dupont, Energy Fuels, 2007, 21, 1695–1698 CrossRef CAS.
- M. E. Bluhm, M. G. Bradley, R. Butterick, U. Kusari and L. G. Sneddon, J. Am. Chem. Soc., 2006, 128, 7748–7749 CrossRef CAS PubMed.
- V. Thamke, P. Singh, S. Pal, M. Chaudhary, K. Kumari, I. Bahadur and R. S. Varma, J. Environ. Chem. Eng., 2022, 10, 107303 CrossRef CAS.
- R. L. Vekariya, J. Mol. Liq., 2017, 227, 44–60 CrossRef CAS.
- G. Kaur, H. Kumar and M. Singla, J. Mol. Liq., 2022, 351, 118556 CrossRef CAS.
- F. Li, F. Mocci, X. Zhang, X. Ji and A. Laaksonen, Chin. J. Chem. Eng., 2021, 31, 75–93 CrossRef CAS.
- O. S. Hammond and A. V. Mudring, Chem. Commun., 2022, 58, 3865–3892 RSC.
- H. A. Elwan, M. Mamlouk and K. Scott, J. Power Sources, 2021, 484, 229197 CrossRef CAS.
- A. Nasri, B. Jaleh, E. Shabanlou, M. Nasrollahzadeh, H. A. Khonakdar and B. Kruppke, J. Mol. Liq., 2022, 365, 120142 CrossRef CAS.
- S. Sahler and M. H. G. Prechtl, in Hydrogen Storage, ed. L. Jianjun, IntechOpen, Rijeka, 2012, ch. 6, pp. 147–164, DOI:10.5772/50154.
- A. Alashkar, A. Al-Othman, M. Tawalbeh and M. Qasim, Membranes, 2022, 12, 178 CrossRef CAS PubMed.
- Y. Tian, Y. R. Liu, H. Wang, L. Liu and W. P. Hu, ACS Sustainable Chem. Eng., 2022, 10, 4345–4358 CrossRef CAS.
- B. Yang, W. L. Ding, H. H. Zhang and S. J. Zhang, Energy Environ. Sci., 2021, 14, 672–687 RSC.
- V. W. H. Lau, A. F. Masters, A. M. Bond and T. Maschmeyer, Chem. – Eur. J., 2012, 18, 8230–8239 CrossRef CAS PubMed.
- J. Ye, Z. Yu, W. Chen, Q. Chen, S. Xu and R. Liu, Carbon, 2016, 107, 711–722 CrossRef CAS.
- X. Zhang, H. Li, H. Yang, F. Xie, Z. H. Yuan, L. Zajickova and W. J. Li, ChemElectroChem, 2020, 7, 3347–3352 CrossRef CAS.
- Y. X. Liu, Z. M. Tian, Q. C. Xu, Y. X. Yang, Y. T. Zheng, H. G. Pan, J. Chen, Z. Wang and W. J. Zheng, ACS Appl. Mater. Interfaces, 2022, 14, 8963–8973 CrossRef CAS.
- Y. Zhang, C. Li, Z. Chen, Y. Ni, F. Kong, A. Kong and Y. Shan, Catal. Lett., 2017, 147, 253–260 CrossRef CAS.
- C. Y. Zhang, B. W. Xin, Z. C. Xi, B. H. Zhang, Z. Y. Li, H. Zhang, Z. H. Li and J. C. Hao, ACS Sustainable Chem. Eng., 2018, 6, 1468–1477 CrossRef CAS.
- D. M. Tang, T. H. Li and C. M. Li, Int. J. Hydrogen Energy, 2019, 44, 1720–1726 CrossRef CAS.
- H. Ying, C. Zhang, T. Chen, X. Zhao, Z. Li and J. Hao, Nanotechnology, 2020, 31, 505402 CrossRef CAS.
- J. H. Tong, W. M. Ma, L. L. Bo, T. Li, W. Y. Li, Y. L. Li and Q. Zhang, J. Power Sources, 2019, 441, 227166 CrossRef CAS.
- D. L. Zhang, H. Y. Mou, L. Chen, D. B. Wang and C. X. Song, Appl. Surf. Sci., 2020, 510, 145483 CrossRef CAS.
- J. Sun, N. K. Guo, Z. Y. Shao, K. K. Huang, Y. W. Li, F. He and Q. Wang, Adv. Energy Mater., 2018, 8, 1800980 CrossRef.
- H. Mao, X. Guo, Q. Fan, Y. Fu, H. Yang, D. Liu, S. Wu, Q. Wu and X.-M. Song, Chem. Eng. J., 2021, 404, 126253 CrossRef CAS.
- M. R. Gao, S. H. Yu, J. Y. Yuan, W. Y. Zhang and M. Antonietti, Angew. Chem., Int. Ed., 2016, 55, 12812–12816 CrossRef CAS PubMed.
- Y. X. Ding, A. Klyushin, X. Huang, T. Jones, D. Teschner, F. Girgsdies, T. Rodenas, R. Schlogl and S. Heumann, Angew. Chem., Int. Ed., 2018, 57, 3514–3518 CrossRef CAS.
- L. Gidi, R. Arce, J. Ibarra, M. Isaacs, M. J. Aguirre and G. Ramirez, Electrochim. Acta, 2021, 372, 137859 CrossRef CAS.
- S. Ji, T. Li, Z.-D. Gao, Y.-Y. Song and J.-J. Xu, Chem. Commun., 2018, 54, 8765–8768 RSC.
- T. N. P. Truong, H. Randriamahazaka and J. Ghilane, ACS Catal., 2018, 8, 869–875 CrossRef CAS.
- D. K. Pandey, H. L. Kagdada, A. Materny and D. K. Singh, J. Phys. Chem. A, 2021, 125, 2653–2665 CrossRef CAS PubMed.
- G. Loget, J. C. Padilha, E. A. Martini, M. O. de Souza and R. F. de Souza, Int. J. Hydrogen Energy, 2009, 34, 84–90 CrossRef CAS.
- R. F. de Souza, G. Loget, J. C. Padilha, E. M. A. Martini and M. O. de Souza, Electrochem. Commun., 2008, 10, 1673–1675 CrossRef CAS.
- F. Fiegenbaum, E. M. Martini, M. O. de Souza, M. R. Becker and R. F. de Souza, J. Power Sources, 2013, 243, 822–825 CrossRef CAS.
- D. W. Lima, F. Fiegenbaum, F. Trombetta, M. O. de Souza and E. M. A. Martini, Int. J. Hydrogen Energy, 2017, 42, 5676–5683 CrossRef CAS.
- F. Fiegenbaum, M. O. de Souza, M. R. Becker, E. M. A. Martini and R. F. de Souza, J. Power Sources, 2015, 280, 12–17 CrossRef CAS.
- D. W. Lima, F. Fiegenbaum, F. Trombetta, M. O. de Souza and E. M. A. Martini, Int. J. Hydrogen Energy, 2018, 43, 1239–1250 CrossRef CAS.
- L. Amaral, D. S. P. Cardoso, B. Šljukić, D. M. F. Santos and C. A. C. Sequeira, Mater. Res. Bull., 2019, 112, 407–412 CrossRef CAS.
- L. Amaral, J. Minkiewicz, B. Sljukic, D. M. F. Santos, C. A. C. Sequeira, M. Vranes and S. Gadžurić, ECS Trans., 2018, 86, 711–717 CrossRef CAS.
- R. Thimmappa, D. Walsh, K. Scott and M. Mamlouk, J. Power Sources, 2020, 449, 227602 CrossRef CAS.
- R. Thimmappa, K. Scott and M. Mamlouk, Int. J. Hydrogen Energy, 2020, 45, 28303–28312 CrossRef CAS.
- M. H. G. Prechtl and S. Sahler, Curr. Org. Chem., 2013, 17, 220–228 CrossRef CAS.
- A. Doroodian, J. E. Dengler, A. Genest, N. Rösch and B. Rieger, Angew. Chem., Int. Ed., 2010, 49, 1871–1873 CrossRef CAS PubMed.
- B. D. Rekken, A. E. Carre-Burritt, B. L. Scott and B. L. Davis, J. Mater. Chem. A, 2014, 2, 16507–16515 RSC.
- W. D. Chen, Z. G. Huang, G. T. Wu, T. He, Z. Li, J. E. Chen, Z. P. Guo, H. K. Liu and P. Chen, J. Mater. Chem. A, 2015, 3, 11411–11416 RSC.
- X. M. Chen, X. Jiang, Y. Jing and X. N. Chen, Chem. – Asian J., 2021, 16, 2475–2480 CrossRef CAS PubMed.
- G. S. Deyko, L. M. Glukhov and L. M. Kustov, Int. J. Hydrogen Energy, 2020, 45, 33807–33817 CrossRef CAS.
- S. Sahler, S. Sturm, M. T. Kessler and M. H. G. Prechtl, Chem. – Eur. J., 2014, 20, 8934–8941 CAS.
- D. W. Himmelberger, L. R. Alden, M. E. Bluhm and L. G. Sneddon, Inorg. Chem., 2009, 48, 9883–9889 CrossRef CAS.
- S. Sahler, H. Konnerth, N. Knoblauch and M. H. G. Prechtl, Int. J. Hydrogen Energy, 2013, 38, 3283–3290 CrossRef CAS.
- D. K. Mishra, R. Hussain, G. Pugazhenthi and T. Banerjee, ACS Sustainable Chem. Eng., 2022, 10, 6157–6164 CrossRef CAS.
- A. Søgaard, M. Scheuermeyer, A. Bösmann, P. Wasserscheid and A. Riisager, Chem. Commun., 2019, 55, 2046–2049 RSC.
- J. Y. Cui, Q. J. Chen, X. J. Li and S. J. Zhang, Green Chem., 2021, 23, 6898–6925 RSC.
- S. Hu, J. Wang, J. Zhang, J. Lim, Y. Gao and S. Zhang, Appl. Catal., B, 2021, 282, 119593 CrossRef CAS.
- D. E. Smith and D. A. Walsh, Adv. Energy Mater., 2019, 9, 1900744 CrossRef.
- X. Yan, F. Zhang, H. Zhang, H. Tang, M. Pan and P. Fang, ACS Appl. Mater. Interfaces, 2019, 11, 6111–6117 CrossRef CAS PubMed.
- H. P. Steinruck, J. Libuda, P. Wasserscheid, T. Cremer, C. Kolbeck, M. Laurin, F. Maier, M. Sobota, P. S. Schulz and M. Stark, Adv. Mater., 2011, 23, 2571–2587 CrossRef PubMed.
- J. Arras, M. Steffan, Y. Shayeghi, D. Ruppert and P. Claus, Green Chem., 2009, 11, 716–723 RSC.
- J. Snyder, T. Fujita, M. W. Chen and J. Erlebacher, Nat. Mater., 2010, 9, 904–907 CrossRef CAS PubMed.
- J. Snyder, K. Livi and J. Erlebacher, Adv. Funct. Mater., 2013, 23, 5494–5501 CrossRef CAS.
- C. Chen, Y. Kang, Z. Huo, Z. Zhu, W. Huang, H. L. Xin, J. D. Snyder, D. Li, J. A. Herron, M. Mavrikakis, M. Chi, K. L. More, Y. Li, N. M. Markovic, G. A. Somorjai, P. Yang and V. R. Stamenkovic, Science, 2014, 343, 1339–1343 CrossRef CAS PubMed.
- Y. Tan, C. Xu, G. Chen, N. Zheng and Q. Xie, Energy Environ. Sci., 2012, 5, 6923–6927 RSC.
- F. Luo, Q. Zhang, Z. Yang, L. Guo, X. Yu, K. Qu, Y. Ling, J. Yang and W. Cai, ChemCatChem, 2018, 10, 5314–5322 CrossRef CAS.
- S. Doblinger, J. Lee and D. S. Silvester, J. Phys. Chem. C, 2019, 123, 10727–10737 CrossRef CAS.
- Y. Li, T. Van Cleve, R. Sun, R. Gawas, G. Wang, M. Tang, Y. A. Elabd, J. Snyder and K. C. Neyerlin, ACS Energy Lett., 2020, 5, 1726–1731 CrossRef CAS.
- T. Wang, Y. Zhang, B. Huang, B. Cai, R. R. Rao, L. Giordano, S.-G. Sun and Y. Shao-Horn, Nat. Catal., 2021, 4, 753–762 CrossRef CAS.
- K. Wippermann, Y. Suo and C. Korte, J. Phys. Chem. C, 2021, 125, 4423–4435 CrossRef CAS.
- G. R. Zhang, T. Wolker, D. J. S. Sandbeck, M. Munoz, K. J. J. Mayrhofer, S. Cherevko and B. J. M. Etzold, ACS Catal., 2018, 8, 8244–8254 CrossRef CAS PubMed.
- A. Avid, J. L. Ochoa, Y. Huang, Y. Liu, P. Atanassov and I. V. Zenyuk, Nat. Commun., 2022, 13, 6349 CrossRef CAS PubMed.
- K. Huang, H. Lin, L. Zhou, L. Wang and H. Jia, J. Electrochem. Soc., 2022, 169, 044516 CrossRef CAS.
- S. Holdcroft, Chem. Mater., 2014, 26, 381–393 CrossRef CAS.
- J. Tymoczko, F. Calle-Vallejo, V. Colic, M. T. M. Koper, W. Schuhmann and A. S. Bandarenka, ACS Catal., 2014, 4, 3772–3778 CrossRef CAS.
- G. R. Zhang, M. Munoz and B. J. M. Etzold, ACS Appl. Mater. Interfaces, 2015, 7, 3562–3570 CrossRef CAS.
- G. R. Zhang, M. Munoz and B. J. M. Etzold, Angew. Chem., Int. Ed., 2016, 55, 2257–2261 CrossRef CAS PubMed.
- K. Huang, T. Q. M. Song, O. Morales-Collazo, H. F. Jia and J. F. Brennecke, J. Electrochem. Soc., 2017, 164, F1448–F1459 CrossRef CAS.
- Q. C. Tran, V.-D. Dao, H. Y. Kim, K.-D. Jung and H.-S. Choi, Appl. Catal., B, 2017, 204, 365–373 CrossRef CAS.
- C. J. Li, B. L. Huang, M. C. Luo, Y. N. Qin, Y. J. Sun, Y. J. Li, Y. Yang, D. Wu, M. G. Li and S. J. Guo, Appl. Catal., B, 2019, 256, 117828 CrossRef CAS.
- Y. W. Li, J. Hart, L. Profit, S. Intikhab, S. Chatterjee, M. Taheri and J. Snyder, ACS Catal., 2019, 9, 9311–9316 CrossRef CAS.
- A. Perego, A. Avid, D. N. Mamania, Y. Chen, P. Atanassov, H. Yildirim, M. Odgaard and I. V. Zenyuk, Appl. Catal., B, 2022, 301, 120810 CrossRef CAS.
- M. George, G. R. Zhang, N. Schmitt, K. Brunnengräber, D. J. S. Sandbeck, K. J. J. Mayrhofer, S. Cherevko and B. J. M. Etzold, ACS Catal., 2019, 9, 8682–8692 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |