Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ex-ante life cycle assessment of polyols using carbon captured from industrial process gas†
Natalya
Tsoy
 *,
Bernhard
Steubing
*,
Bernhard
Steubing
 and
Jeroen B.
Guinée
and
Jeroen B.
Guinée

Institute of Environmental Sciences (CML), Leiden University, Einsteinweg 2, 2333 CC Leiden, the Netherlands. E-mail: n.tsoy@cml.leidenuniv.nl
First published on 23rd June 2023
Abstract
The steel industry needs to significantly reduce greenhouse gas (GHG) emissions as it is considered as one of the major industrial contributors to global GHG emissions. Since CO and CO2 occur in high concentrations in steel mill gases, one of the possible options to do this is utilizing CO and CO2 for the production of value added chemicals. With this goal, a carbon capture and utilization (CCU) technology was developed for transforming CO and CO2 from the blast furnace gas (BFG) of a steel mill to building blocks for polyols. These polyols were then used to produce polyurethane (PUR) for the manufacturing of coatings and rigid foam for insulation boards. For assessing and comparing the life cycle environmental impacts of this novel CCU system with those of the incumbent steel and polyol system, ex-ante life cycle assessment (LCA) was carried out. Three possible scenarios of the CCU system were compared with the incumbent steel and polyol systems, assessed by performing LCAs and identifying hotspots. All three scenarios of the CCU technology showed improved environmental performance compared to the incumbent technology although limited to a maximum of about 10% reduction in carbon footprint. Energy and chemicals used to produce CCU polyols were identified as the main hotspots of the life cycle impacts of all three scenarios.
1 Introduction
Global warming caused by greenhouse gas (GHG) emissions has been recognized as a severe problem on a global scale that needs to be tackled. The European Union (EU) set up an objective to reduce GHG emissions by 80%–95% by 2050 compared to those in 1990.1 The steel industry is one of the largest industrial emitters of CO2, being responsible for 7%–9% of direct CO2 emissions from the global use of fossil fuels.2 Carbon Capture Utilization (CCU) using CO2 for chemical synthesis is currently seen as one of the possibly promising approaches to mitigate carbon emissions.3 CCU allows transformation of carbon emissions CO/CO2, e.g., from steel mills, into value-added chemical products.The Carbon4PUR project, funded by the European Union's Horizon 2020 research and innovation program, aimed at transforming carbon emissions from the blast furnace gas (BFG) of a steel mill to valuable intermediates (chemical building blocks) for the production of polyols in the polyurethane industry.4 Polyurethanes (PUR) are a large group of polymers used in a broad range of various applications. They can be used in the manufacture of coatings, adhesives, sealants, rigid foam for thermal and sound insulators, flexible foam for furniture, elastomers, etc.5 The Carbon4PUR project developed polyols for PUR that can be used in the production of coatings and rigid foam for insulation boards. In this study, the technology developed within the Carbon4PUR project is from here on referred to as the “CCU technology”.
Life Cycle Assessment (LCA) is a method to assess the environmental impacts related to products and services. It is, along with other assessment methods, such as e.g., techno-economic assessment, often used to support the development of new technologies. Generally, LCA is used to assess existing technologies operating at the industrial scale, for which data at the industrial scale are available. The application of LCA to the CCU technology is more challenging as it has so far only been developed at the laboratory and pilot scales. Consequently, this technology is lacking industrial scale data, which are essential for regular LCA studies.
Ex-ante LCA can be defined as LCA studies that (a) scale up an emerging technology using likely scenarios of future performance at the full operational scale and (b) compare the emerged technology at scale with the evolved incumbent technology,6 or as “performing an environmental life cycle assessment of a new technology before it is commercially implemented in order to guide R&D decisions to make this new technology environmentally competitive as compared to the incumbent technology mix.”7 Some LCA practitioners use the term “prospective LCA” rather than “ex-ante LCA” for this type of assessment.8 In this work, we will refer to the LCA of emerging technology as “ex-ante LCA”.
Several LCA studies have been published on CCU technologies used for the production of chemicals,9,10 most of which were focused on the production of polyols from CO2.11–14 However, to the best of our knowledge, there have been no LCA studies carried out for the joint production of both CO-based polyols and CO2-based polyols from the CO and CO2 fractions of BFG.
In this study, we perform an ex-ante LCA of a CCU technology that converts CO and CO2 gases from BFG to valuable intermediates for the production of polyether-ester polyol (CO-based polyol) and polyether-carbonate polyol (CO2-based polyol). The environmental performance of three scenarios of the CCU technology were compared with the existing commercial technology (baseline system), and the main contributors (hotspots) to the impacts were identified.
2 Methodology
2.1 Goal definition
The CCU technology assessed in this LCA study converts carbon emissions from steel production to intermediates to produce polyols. These polyols then are used to produce polyurethane for the manufacturing of coatings and rigid foam for insulation boards. The purpose of the LCA study was to assess life cycle impacts of the CCU technology (using a part of the BFGs for the production of polyols), to compare the CCU technology to the baseline system (incumbent way of using the BFGs by the steel industry itself and of producing polyols). The details of our approach are provided in section 2.2. The approach we developed aimed at determining whether the integrated new symbiotic system of the CCU technology performed environmentally better than the two independent incumbent systems using BFGs for the production of energy for steel production and the production of polyols from fossil fuels.Three research questions were formulated:
1. What is the overall environmental performance of the CCU technology system compared to the baseline system as described above?
2. How can differences in environmental performances be explained in terms of the main contributors (hotspots) and components differing between the CCU technology and the baseline system?
3. Do the identified hotspots in the CCU technology system offer options for further improvement?
2.2 Scope definition
The geographical coverage for this LCA study was Europe. A cradle-to-gate approach was applied meaning that the LCA included the processes starting from raw material extraction and ending with the production of polyols. The background processes were modelled using the ecoinvent v3.4 (cut-off version) database.15 The foreground processes were based on data provided by the project partners. The LCA followed the ISO 14044 framework.16 The AB (Activity Browser)17,18 and the CMLCA software (version 6.1)19 were used for the LCA calculations, and calculation results were mutually validated between these two software programs.For the comparison of the baseline system to the CCU technology system, we excluded those parts of steel production that were qualitatively and quantitatively the same for both systems (Fig. 1). The use of a portion of the BFGs produced from pig iron production was the only part of steel production that was different between the baseline and the CCU technology systems. In the baseline system, BFGs, produced from the pig iron production process, were incinerated to produce heat (5%) and electricity (75%) to be recycled back for use by the steel production processes, and a part was flared as waste (20%).20 In the CCU technology system, a part of the BFGs produced from the pig iron production was used to produce polyols. As a result, this part of the BFGs could not be used to produce heat and electricity, and thus, less heat and electricity were recycled back to the steel production processes. Therefore, the amount of heat and electricity that could be produced from BFGs needed to be substituted in the steel production processes by heat from an industrial boiler and electricity from the grid.
 | ||
| Fig. 1 The comparison adopted in this study. The CCU polyol production is an aggregate representation of several processes. | ||
In the CCU technology system, not all BFG components were used in the polyol production process. During this process, a waste BFG was produced that was returned to the steel mill. This waste BFG had a different chemical composition compared to the BFG generated from the pig iron production process. The BFG waste was composed of unreacted vapor components: CO, CO2, N2, H2, THF, ethanal, acrylic acid, dioxan, etc. The waste BFG had a lower carbon content and calorific value than the BFGs. However, the quantity of the waste BFG was so small compared to the total BFG flow that we assumed that it could be mixed with the BFGs at the steel mill, so that the carbon and calorific content of the total BFG flow would not be affected. Thus, the assumption was made that the waste BFG returned to the steel mill had the same carbon content and calorific value as the BFGs produced from pig iron production and was used at the steel mill in the same way for the production of electricity (75%) and heat (5%), as well as in flaring (20%). By doing this, we could calculate an amount of the BFGs used for polyol production by subtracting the returned waste BFG to the steel mill from the BFGs delivered by the steel mill.
The CCU technology reflects a technology readiness level (TRL) between TRL 2 and TRL 6 (different parts of the CCU technology including gas conditioning and production of polyols are at different TRL levels from TRL 2 to TRL 6). The data derived from these laboratory scale and pilot scale implementations cannot be applied in the environmental assessment of a future technology operated at full scale since these data are far from the industrial scale data (TRL 9). Thus, the laboratory scale and the pilot scale data were upscaled to the projected industrial scale following the upscaling framework presented by Tsoy et al. (2020)21 to get an estimate of its potential full-fledged future performance. According to this framework, the upscaling of an emerging technology in ex-ante LCA is composed of three steps: (1) projected technology scenario definition, (2) preparation of a projected LCA flowchart, and (3) projected data estimation.
It should be noted that different kinds of expertise are required to upscale a new technology in ex-ante LCA, and thus it is recommended that experts from different fields be involved in the upscaling, e.g., technology developers, engineers, and LCA practitioners.21 In the case of upscaling of the CCU technology in this study, steps 1 to 3 of the upscaling were performed by the technology developers of the CCU technology with support from the LCA experts in steps 2 and 3. Next, LCA calculations were performed using the results of the upscaling steps as the input.
The next sections of this paper (sections 2.2.1–2.3.2) provide a more detailed description of the three upscaling steps performed: (1) projected technology scenario definition, (2) preparation of a projected LCA flowchart, and (3) projected data estimation.
2.2.1.1 Scenario 1: BFGs to CO-based polyols. Selective catalytic combustion was analysed as a gas conditioning method in scenario 1. Selective catalytic combustion is a process where pure O2 is used to decrease the amount of H2 in the BFGs via a selective oxidation reaction.23 With regards to polyol production, CO-based polyols were assessed, while CO2-based polyols were not considered. Scenario 1 avoids the dependency between the two products observed in scenarios 2 and 3.
2.2.1.2 Scenario 2: sequential process – BFGs to CO2-based polyols and CO-based polyols. Scenario 2 presents the sequential production of CO2-based polyols and CO-based polyols. Similar to scenario 1, selective catalytic combustion was used to condition the BFGs. Conditioned BFGs containing CO/CO2 and inert N2 are used to produce CO2-based polyols. Next, the CO2 depleted stream is used in the production of succinic anhydride which is an intermediate to produce CO-based polyols. After the CO-based polyols are produced, the used gas stream is disposed of as waste.
2.2.1.3 Scenario 3: parallel process – BFGs to CO2-based polyols and CO-based polyols. Pressure swing chemical looping24 and a CO2 removal process (the DMX™ process)25,26 were used as a gas conditioning method in scenario 3. Pressure swing chemical looping produces a CO/CO2 stream almost free of H2 and inert gas N2. After that, a CO2 removal process is used to remove most of the CO2 from this CO/CO2 stream. As a result, two gas streams are produced: a CO-rich stream and a pure CO2 gas stream that can be used in the production of CO-based polyols and CO2-based polyols separately in a parallel process. Since the gas conditioning method in this scenario produces more pure gas streams for polyol production, the overall polyol production processes are more efficient: less gas volume is needed, thus the reactors are much smaller, and less compression is required. In this study, scenario 3 was only investigated as a holistic scenario (scenario, where both CO-based polyols and CO2-based polyols are produced) to compare to scenario 2.
The function, functional unit, and reference flows were defined for each scenario. As explained before, the function of the compared systems was defined as the production of polyols and the provision/compensation of heat and electricity for steel production. The functional unit was different for each scenario as the quantity of the BFGs used for polyol production in each scenario was different, affecting the amount of heat and electricity to be delivered for steel production. Table 1 shows the function, functional unit, and reference flows for each scenario.
| Function | Scenario | Functional unit | Reference flows | |
|---|---|---|---|---|
| Baseline system | CCU technology | |||
| Production of polyols and the provision/compensation of heat and electricity for steel production | Scenario 1: BFGs to CO-based polyols | Polyol production: 1 kg CO-based polyols | 1 kg of phthalic acid polyester polyol | 1 kg of CO-based polyols |
| Energy for steel production: 0.035 megajoules (MJ) of heat and 0.223 MJ of electricity | 0.035 MJ of heat and 0.223 MJ of electricity from BFG | 0.035 MJ of heat by an industrial boiler and 0.223 MJ of electricity by the average European grid | ||
| Scenario 2: sequential process – BFGs to CO2-based polyols and CO-based polyols | Polyol production: 1 kg of CO-based polyols and 1.99 kg of CO2-based polyols | 1 kg of phthalic acid polyester polyol and 1.99 kg of a polyether polyol | 1 kg of CO-based polyols and 1.99 kg of CO2-based polyols | |
| Energy for steel production: 0.083 MJ of heat and 0.526 MJ of electricity | 0.083 MJ of heat and 0.526 MJ of electricity from BFG | 0.083 MJ of heat by an industrial boiler and 0.526 MJ of electricity by the average European grid | ||
| Scenario 3: parallel process – BFGs to CO2-based polyols and CO-based polyols | Polyol production: 1 kg of CO-based polyols and 2.23 kg of CO2-based polyols | 1 kg of phthalic acid polyester polyol and 2.23 kg of a polyether polyol | 1 kg of CO-based polyols and 2.23 kg of CO2-based polyols | |
| Energy for steel production: 0.227 MJ of heat and 1.444 MJ of electricity | 0.227 MJ of heat and 1.444 MJ of electricity from BFG | 0.227 MJ of heat by an industrial boiler and 1.444 MJ of electricity by the average European grid | ||
2.3 Inventory analysis
2.3.1.1 Supply of the BFGs. The ecoinvent v3.4 database15 was used to model the production of steel including processes such as coke production, pig iron production, steel production and steel rolling. The alternative use of a portion of the BFGs from the pig iron production process was modelled partly as a source of heat and electricity and partly flared in the baseline system or as a carbon source for polyol production in the CCU technology system. All other parts of the steel mill model were excluded in the LCA, as those parts were qualitatively and quantitatively the same in both the baseline and the CCU technology systems. The data on the energy production and consumption in a steel mill and the thermal and electrical efficiencies in the production of heat and electricity from BFGs were provided by a steel producer.
2.3.1.2 Gas conditioning. BFGs consist of 49% N2, 22% CO2, 22% CO, 3.6% H2, 3.2% H2O and further impurities.22 The H2 gas in BFGs can interfere with the reaction of polyol production.22,23 Thus, BFGs should be conditioned prior to the production of polyols to decrease the H2 content in BFGs. For this, a selective catalytic combustion method for conditioning BFGs was developed.23 In this method, the H2 content is decreased in BFGs via the selective oxidation of H2 over a Ni-based catalyst. Currently, selective catalytic combustion reflects a TRL 5.
Pressure swing chemical looping is another method developed to condition BFGs.24 This method uses solid chemical intermediates (CO2 sorbents and oxygen storage materials) in reaction-regeneration cycles and produces CO/CO2 stream almost free of H2 and N2. In addition, a CO2 removal step is used to remove CO2 from the produced CO/CO2 stream using the DMX™ (demixing solvent) process.25,26 In the CO2 removal step, about 90% of the CO2 content is removed. In the current CCU technology system, the pressure swing chemical looping reflects a TRL 2. Selective catalytic combustion was modelled as a gas conditioning method in scenarios 1 and 2, while pressure swing chemical looping and CO2 removal were assessed in scenario 3 (Fig. 3).
2.3.1.3 Polyol production.
2.3.1.3.1 Production of CO-based polyols. The developed production process of the intermediate and CO-based polyols22 was assessed in all three scenarios (Fig. 3). In this process, CO from the BFGs is used in a double carbonylation reaction with ethylene oxide (EO) to produce succinic anhydride (an intermediate). This reaction is carried out using a catalyst in tetrahydrofuran (THF). In the first carbonylation step, CO reacts with EO to produce β-propiolactone, and in the second carbonylation step, CO reacts with the produced β-propiolactone to form succinic anhydride. Then the co-polymerisation of the formed succinic anhydride and EO in the presence of the catalyst results in the formation of polyether-ester polyol (CO-based polyol). In the current CCU technology system, the intermediate production was developed up to a TRL 5 and the CO-based polyol production to a TRL 6.
2.3.1.3.2 Production of CO2-based polyols. Production of CO2-based polyols was analysed in scenarios 2 and 3 (Fig. 3). For the development of the CO2-based polyol production process, a slightly adapted version of the existing CO2-based polyol production technology27 was taken. The production of CO2-based polyol in the CCU technology assessed in this LCA study is similar to that in the existing CO2-based polyol production technology: CO2 and propylene oxide (PO) are converted to polyether-carbonate polyol (CO2-based polyol) in the presence of a starter and a double metal cyanide (DMC) catalyst.22 However, in the case of the CCU technology assessed in this study, a mixed CO/CO2-containing BFG gas stream is used in polyol production, while in the existing CO2-based polyol production technology, pure CO2 is used.
In scenarios 2 and 3, two types of polyols are co-produced: CO2-based polyols and CO-based polyols. Mass allocation was applied to allocate environmental burdens between these polyols.
2.4 Impact assessment – characterization
The potential impact of the three scenarios was assessed applying following the characterization methods recommended by the International Reference Life Cycle Data System (ILCD) 2.0, 2018.29 Thirteen impact categories were included: (1) climate change (CC), (2) ecosystem quality, freshwater and terrestrial acidification (FTA), (3) ecosystem quality, freshwater ecotoxicity (FEC), (4) ecosystem quality, freshwater eutrophication (FEU), (5) ecosystem quality, marine eutrophication (MEU), (6) ecosystem quality, terrestrial eutrophication (TEU), (7) human health, carcinogenic effects (HHC), (8) human health, ionizing radiation (HIR), (9) human health, non-carcinogenic effects (HHNC), (10) human health, ozone layer depletion (OLD), (11) human health, photochemical ozone creation (POC), (12) resources, fossil resources (FR), and (13) resources, minerals and metals (MM).3 Results
3.1 Characterization results
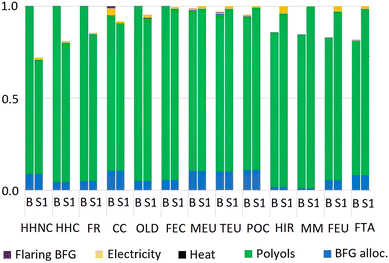
Both the baseline and the CCU technology systems show the same (mass-) allocated footprints as BFGs with 0.34 kg CO2-equivalents. The footprint results of heat from an industrial boiler, electricity from the grid and the CO-based polyols in the CCU technology system are slightly better than the ones for heat and electricity produced from the BFGs, and phthalic acid polyester polyol in the baseline system, respectively. The impact results for other impact categories show a diverse pattern (Fig. 4). The CCU technology system shows slightly lower impact results for human health (non-carcinogenic and carcinogenic effects), fossil resources, climate change, ozone layer depletion and freshwater ecotoxicity (1–25%), while the baseline system shows slightly better results for all other impact categories.
3.2 Interpretation
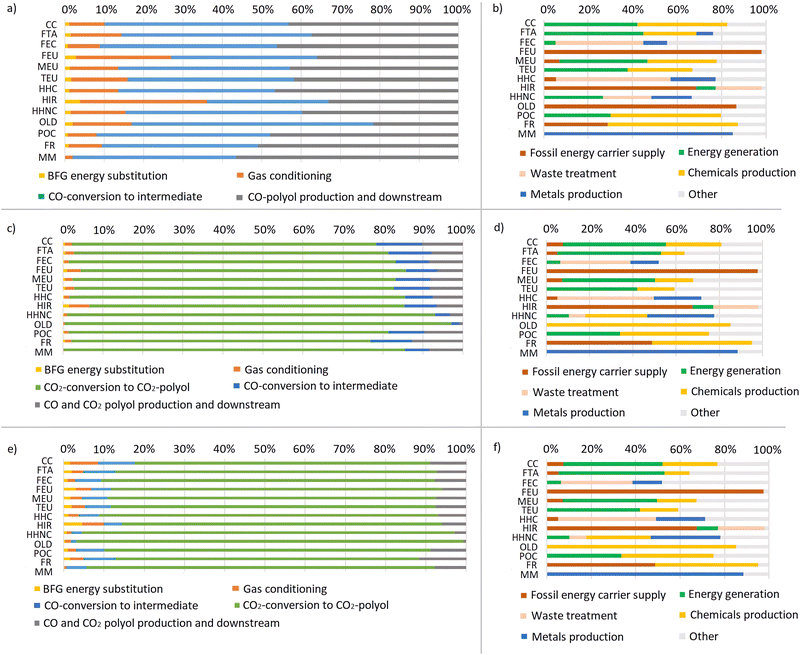
This section presents the contribution analysis results for the three CCU technology scenarios, focusing on the main contributors to the total impacts of the CCU technology. Firstly, for each scenario an analysis of the contributions of the individual processing steps of the CCU technology to the total environmental impacts was conducted, i.e. at the aggregate level it corresponds to the LCA flowcharts in Fig. 3: BFG energy substitution, gas conditioning, CO-conversion to intermediate, CO-polyol production and downstream, CO2-conversion to CO2 polyol (in scenarios 2 and 3), and CO2-polyol production and downstream (in scenarios 2 and 3).Secondly, an analysis of sectors (by means of grouping processes within the same sector) to the total environmental impacts over the life cycle of producing the polyols was performed. Six sectors were defined: fossil energy carrier supply, energy generation, waste treatment, chemical production, metal production, and other. The sectors “fossil energy carrier supply” and “energy generation” in Fig. 7(b, d and f) relate to energy. It should be noted that the results for sectors refer to not only the CCU technology, but to the entire product system, thus including supply chains as modelled in the ecoinvent database. The sectors aggregate one or several processes. For example, the sector “energy generation” sums up the impact across the life cycle of polyol production related to the production of electricity and heat. A cut-off value of 5% was used at the product level. This means that if a product related to a process contributes by more than 5%, this process is associated with one of the sectors (or “other” if no sector fits). If the product contribution is less than 5%, the process related to this product is accounted for in the category “other”.
3.2.1.1 Scenario 1: BFGs to CO-based polyols. Fig. 7(a) shows the results of the contribution analysis for the production of CO-based polyol in scenario 1 from the processing steps. CO-conversion to intermediates and polyol production have the highest impact on almost all impact categories. BFG energy substitution and gas conditioning account for a smaller share of impacts.
Fig. 7(b) presents the results of the contribution analysis for the CO-based polyol production in scenario 1 by sectors. Energy (that includes “fossil energy carrier supply” and “energy generation”) has the highest contribution to most impact categories. Also, chemicals are responsible for a large part of the production of polyols. To be specific, EO and the precursor ethylene used in the production of chemicals for polyol production have high contributions to the impact categories namely climate change, acidification, eutrophication (marine and terrestrial), ozone creation and resource depletion. The production of metals is the largest contributing factor to the impact category resources, minerals and metals and plays only a subordinate role in acidification, ecotoxicity, and human health effects (carcinogenic and non-carcinogenic).
3.2.1.2 Scenario 2: sequential process – BFGs to CO2-based polyols and CO-based polyols. CO2-conversion to CO2-based polyol has the highest contribution to all impact categories in scenario 2 (Fig. 7(c)). The other processes play only a minor role.
In scenario 2, CO2-conversion to CO2-polyol is a multifunctional process, and the impacts of this process are allocated over the production of CO2-based polyols and the production of CO-based polyols. Thus, the production of CO-based polyols has a share of the impacts of the process CO2-conversion to CO2-polyols, although the production of CO-based polyols does not include the CO2-conversion step (Fig. ESI 1(a)†). The process of CO2-based polyol downstream does not contain any direct emissions or environmentally relevant inputs, and thus, does not have any contribution to impact categories (Fig. ESI 1(b)†).
The results for contribution analysis for scenario 2 by sectors (Fig. 7(d) and Fig. ESI 3(a and b)†) are similar to the results in scenario 1. The environmental impacts across almost all impact categories are driven by energy (that includes “fossil energy carrier supply” and “energy generation”) and the production of chemicals. The sector “chemicals production” is mostly dominated by precursors ethylene and propylene, contributing 8% and 18% to the climate change, respectively. Sodium hydroxide and gaseous chlorine which are the precursors for PO have the highest contribution to non-carcinogenic effects and ozone layer depletion. The production of metals has the highest impact on resources, minerals and metals and a small contribution to ecotoxicity and human health effects (carcinogenic and non-carcinogenic).
3.2.1.3 Scenario 3: parallel process – BFGs to CO2-based polyols and CO-based polyols. The results in scenario 3 (Fig. 7(e)) show the same pattern as in scenario 2: all impact categories are dominated by the process of CO2-conversion to CO2-based polyols. However, unlike in scenario 2, gas conditioning separates conditioned CO2- and CO-rich streams before the CO2 to CO2-based polyol conversion step, and the production of CO-based polyols is not linked to the CO2 to CO2-based polyol conversion step. Thus, in the production of CO-based polyols in scenario 3 (Fig. ESI 2(a)†), the contribution analysis results show the same pattern as in scenario 1: the main contributors to all impact categories are CO-conversion to intermediates and the production of CO-based polyols and downstream processes. Similar to scenario 2, the production of CO2-based polyol and downstream processes do not show up in the results for the preparation of CO2-based polyols (Fig. ESI 2(b)†).
The contribution analysis results for scenario 3 by sectors (Fig. 7(f) and Fig. ESI 4(a and b)†) show similar results to those in scenario 1 and scenario 2. Overall, the main contributors to almost all impact categories are energy (that includes “fossil energy carrier supply” and “energy generation”) and chemicals. However, one difference to scenario 2 is that the process of the production of CO-based polyols is not linked to the CO2 to CO2-based polyol (using PO) conversion process. Thus, in the production of CO-based polyols in scenario 3, the sector “chemicals production” is dominated by ethylene (contributing approximately one third of the impacts to climate change, acidification, eutrophication (marine and terrestrial), 46% for ozone creation and 62% for fossil resources) (Fig. ESI 4(a)†). Also, scenario 3 differs from scenarios 1 and 2 by the fact that the unconverted BFGs are not delivered back to the steel mill and are incinerated onsite by thermal oxidation (without energy recovery), contributing approximately 15% of the impacts for climate change in the production of CO-based polyols.
 | ||
| Fig. 8 Indication of yearly carbon savings (in kt CO2-eq.) for a 50 kt a−1 plant CO-based polyol for each scenario; S1 – scenario 1, S2 – scenario 2, S3 – scenario 3. | ||
The results show that the environmental potency of scenario 3 is the most promising among all three scenarios. In scenario 3, the largest quantity of polyols is produced, and the highest GHG saving is attained of about 90 kt CO2-eq. per year.
All three CCU technology scenarios 1, 2 and 3 have lower carbon footprint compared to that of the baseline system. In scenario 1, the main reasons for having a lower footprint are changing the production of heat and electricity from the BFGs at the steel mill in the baseline scenario to the production of cleaner heat from an industrial boiler and electricity from the average European grid in the CCU technology scenario, and the reduction of the GHG emissions associated with polyol production. In scenarios 2 and 3, the GHG savings in the production of polyols are dominated by the substitution of PO by CO2 in the CO2-based polyol production process. However, in scenario 3 (unlike in scenarios 1 and 2), the unconverted BFGs are not delivered back to the steel mill but are incinerated onsite by thermal oxidation without energy recovery. As a result, the environmental impact caused by the emissions from thermal oxidation decrease partly the substitution effects mentioned above. Possibly, in a future technology implementation, it would be better to recover energy from the unconverted BFGs instead of incinerating them.
4 Discussion
Three scenarios of polyol production from BFGs from steel production (the CCU technology system) were compared to a baseline system, in which the BFGs were partly used to cover the steel mill's heat and electricity needs and partly flared. The comparison excluded those parts of the steel production system that were qualitatively and quantitatively the same in both systems. This approach was adopted as we aimed to determine whether an integrated CCU technology system performs environmentally better than the incumbent system, not focusing on how benefits may be divided between different products. Production of polyols, heat and electricity were all included in the functional units of the systems.Scenario 3 showed the most promising results. In comparison with the baseline system, this scenario is expected to have 4–15% lower impacts in most of the impact categories. The context of these results should be interpreted carefully, however. The CCU technology reflects TRLs from TRL 2 to TRL 6 (TRL 2 for pressure swing chemical looping, TRL 5 for selective catalytic combustion, the CO-based intermediate production is at TRL 5, and CO-polyol production and downstream processes are at TRL 6). Ex-ante LCA was carried out for the CCU technology that was upscaled using mainly process simulation to TRL 9. Since ex-ante LCA of the CCU technology was done at an early phase of development (TRL 2 to TRL 6), there is a possibility for the technology developers to implement improvements to future implementations of the CCU technology to further decrease its environmental impacts. One of the possibilities for improvement determined by this study for the foreground processes of the CCU technology is the use of energy in all three scenarios. Another improvement option could include the use particularly of EO for the production of CO-based polyols in all three scenarios and the use of PO for the production of CO2-based polyols in scenarios 2 and 3. Most impacts come from the background processes that the technology developers could not affect directly, for example, production of precursors EO and PO. However, a possibility could be to use precursors based on alternative, non-fossil feedstocks, e.g., chemicals from biobased materials or for which higher shares of renewables are applied in their manufacturing process. Furthermore, in the future, other options could be possibly explored to lower the amount of these precursors used in polyol production using a larger amount of CO/CO2 from the BFGs in polyols and related products. Based on current knowledge and data, scenario 3 shows ∼10% reduction in climate change. However, this footprint result is reached by using only up to ∼20% CO/CO2 in polyol production. This implies that a high improvement potential exists for the current CCU technology scenarios by applying larger amounts of CO/CO2 in the production of polyols by further optimizing the technology and addressing new application areas.
Another limitation of our study and of ex-ante LCAs, in general, is that our current (lower TRL) knowledge of the process data may only be a snapshot of what we may know in the end. For example, we currently have no knowledge and were not able to estimate possible emissions related to the novel foreground processes and thus assumed them to be negligible for the time being. Adding improvements related to energy and chemical usage (see above) may result in even better environmental performance of this system, however, filling emissions data gaps may decrease the performance. Thermal oxidation was the only foreground process in the CCU technology system for which the emissions were estimated applying stoichiometric calculations. To be more specific, these calculations allowed the estimation of carbon related emissions from incineration of waste gases containing organic compounds. However, these emissions may be only a part of the thermal oxidation emissions as other kinds of emissions may also occur depending on the quality of incineration.
Another limitation of this study is that the baseline polyols (phthalic acid polyester polyol and polyether polyol for the novel CO-based polyols and CO2-based polyols, respectively) were not included at the same level of detail as for the CCU polyols. This is due to confidentiality issues related to the datasets of these baseline polyols. Thus, there is a possibility that the results could as yet change with better data. Also, cyclic propylene carbonate (cPC), that is co-produced with CO2-based polyols, was excluded from the comparison. This was due to the absence of a baseline chemical for cPC available in the market. Thus, cPC was allocated away on a mass basis, however, this should not significantly affect the comparative results reported in this study since the quantity of co-produced cPC is very small.
Also, we assumed that the waste BFGs from the CCU polyol production returned to the steel mill had the same calorific value as the BFGs generated from the pig iron production process (see section 2.2). In practice, these waste BFGs may have 30%–50% less energy, and therefore, may not generate the same quantity of heat and electricity as the BFGs produced from pig iron production. However, the results in section 3.1 showed that the contribution of producing heat and electricity from the BFGs and flaring BFGs was very small. Based on these results, it is expected that this assumption would have a negligible effect on the results.
Also, datasets from the ecoinvent v3.4 database (cut-off version) were applied for modelling the background processes in the CCU technology system. Better datasets may become available or new assumptions should be made in the future when the CCU technology develops further. Thus, the current results are only valid for the current system, datasets, knowledge, and assumptions applied in this study.
Lastly, it was assumed that the end-of-life of the PUR products using the CCU technology polyols would remain the same as using the baseline polyols, which would imply incineration with associated emissions of CO2. A possible solution might be to use carbon capture and storage of CO2 emitted due to incineration of used PUR products or to adopt a more circular system of reusing the products.
5 Conclusion
Ex-ante LCA was carried out to assess the environmental performance of three scenarios of the production of polyols from the BFG from the production of steel (CCU technology system). These scenarios were compared with the baseline system, in which the same amount of the BFG was utilized to produce heat and electricity for use in the steel mill and flared. The comparison excluded those parts of the steel production system that were qualitatively and quantitatively the same in the CCU technology and the baseline systems. Three research questions were formulated at the beginning of the ex-ante LCA study. These research questions are answered below.(1) What is the overall (integrated system) environmental performance of the CCU technology system compared to the baseline (incumbent) system?
Overall, three scenarios of the CCU technology showed better environmental performance than that of the baseline system, ranging from approximately −20% to +25% for scenario 1, from 0.5% to 12.5% for the most impact categories for scenario 2 except for ionizing radiation (−4%), and from 4% to 15% for all impact categories for scenario 3. The scenario comparison showed that scenario 3 is the most promising among all three scenarios. In this scenario, the GHG saving of about 90 kt CO2-eq. per year may be achieved assuming yearly production levels of 50 kt a−1 of CO-based polyols and ∼110 kt a−1 of CO2-based polyols.
The current results of scenario 3 showed ∼10% reduction in carbon footprint. However, taking into consideration the low-medium TRL level of the CCU technology processes and the possibility of using more CO/CO2 in polyols in the future, it can be concluded that the current CCU technology scenarios have considerable potential for further improvement.
(2) How can differences in environmental performances be explained in terms of the main contributors (hotspots) and components differing between the CCU technology and the baseline system?
In the CCU technology system, thermal oxidation is the only foreground process that was determined as a direct contributor to the carbon footprint (e.g., up to approximately 15% for CO-based polyols in scenario 3). This is due to thermal oxidation currently being the only foreground process for which it was possible to estimate emissions.
Mostly, environmental impacts are due to chemicals and energy that were used to produce polyols in the CCU technology system.
The carbon footprints of the three scenarios were all identified to be lower than that of the baseline system. This is mainly due to the substitution of the production of heat and electricity from the BFG in the steel mill in the baseline system by cleaner heat from industrial boilers and electricity from the average European grid in the CCU technology system, and the reduction of the GHG emissions associated with the use of chemicals utilized in the production of polyols.
(3) Do the identified hotspots in the CCU technology system offer options for further improvement?
Contribution analysis showed that possibilities for the improvement of the CCU technology scenarios were mostly related to the production of energy and chemicals used to produce the CCU polyols. Using more renewable energy in the CCU technology processes may seem to be an obvious option, but this may help all systems equally not only the CCU technology scenarios but also the baseline system. However, using less energy in the CCU technology even after transition to renewable based energy would help in decreasing impacts compared to the baseline system. With regards to the improvement possibility for chemicals – EO (all scenarios) and PO (scenarios 2 and 3) used for the CO-based polyols and CO2-based polyols production, respectively – precursors could be used based on alternative, non-fossil feedstocks, e.g., chemicals from biobased materials or for which higher shares of renewables are applied in their production process. Also, in the future, other ways may be explored to reduce the amount of these chemicals used in the polyol production by using more CO/CO2 from the BFG in polyols and related products by optimizing the processes of the polyol production and addressing new application areas.
The conclusions are only valid for the current scenarios developed by the technology developers. The scenarios may show better environmental performance at higher TRLs; however, filling data gaps or including changes in the current LCA assumptions may decrease the performance. It should be noted that the conclusions above are “if…then…” conclusions. If the scenarios developed are implemented in practice according to their specifications, then the above conclusions can be drawn.
Finally, the purpose of ex-ante LCAs performed for technologies at lower TRLs is to determine possibilities for the environmental improvement and communicate them to the developers of those technologies rather than to estimate their exact environmental impact. This is also true for the CCU technology processes that reflect a TRL between TRL 2 and TRL 6. Therefore, the numerical results presented in this study should not be used in the comparative assertion or benchmarking exercise of product alternatives since the novel technologies assessed might yet change significantly at higher TRLs (so that in practice they may be different from the upscaled technologies using process simulation).
Abbreviations
| β-Propiolactone | Beta propiolactone |
| BFG | Blast furnace gas |
| CCU | Carbon capture and utilization |
| CO2 | Carbon dioxide |
| CO | Carbon monoxide |
| CC | Climate change |
| cPC | Cyclic propylene carbonate |
| DMX™ | Demixing solvent |
| DMC | Double metal cyanide |
| EO | Ethylene oxide |
| etc. | etcetera |
| EU | The European Union |
| e.g. | For example |
| FEC | Freshwater ecotoxicity |
| FR | Fossils resources |
| FEU | Freshwater eutrophication |
| FTA | Freshwater and terrestrial acidification |
| GHG | Greenhouse gas |
| HHC | Human health, carcinogenic effects |
| HHNC | Human health, non-carcinogenic effects |
| HIR | Human health, ionizing radiation |
| H2 | Hydrogen |
| ILCD | The international reference life cycle data system |
| kg | Kilogram |
| kt CO2-eq. | Kilotons of carbon dioxide equivalents |
| kt a−1 | Kilotons per annum |
| kW h | Kilowatt-hour |
| LCA | Life cycle assessment |
| MEU | Marine eutrophication |
| MJ | Megajoules |
| MM | Minerals and metals |
| N2 | Nitrogen |
| O2 | Oxygen |
| OLD | Ozone layer depletion |
| POC | Photochemical ozone creation |
| PUR | Polyurethane |
| PO | Propylene oxide |
| TRL | Technology readiness level |
| TEU | Terrestrial eutrophication |
| THF | Tetrahydrofuran |
| i.e. | That is |
Author contributions
Conceptualization: N. T., B. S., J. B. G.; methodology: N. T., B. S., J. B. G.; visualization: N. T., B. S., J. B. G.; data curation: N. T., B. S., J. B. G.; writing – original draft: N. T., B. S., J. B. G.; writing – review and editing: B. S., J. B. G.; funding acquisition: J. B. G.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was carried out within the project Carbon4PUR, which received funding from the European Union's Horizon 2020 research and innovation program under grant agreement No 768919. The authors thank all Carbon4PUR partners for providing data for LCA and helpful discussions. The European Commission is neither responsible nor liable for the content of this document.References
- European Commission, A Roadmap for moving to a competitive low carbon economy in 2050, Brussels, 2011 Search PubMed.
- World Steel Association, Climate change and the production of iron and steel, Brussels, 2021 Search PubMed.
- T. Ariyama, K. Takahashi, Y. Kawashiri and T. Nouchi, J. Sustain. Metall., 2019, 5, 276–294 CrossRef.
- Carbon4PUR, Carbon4PUR, https://www.carbon4pur.eu, (accessed 19 November 2022).
- J. O. Akindoyo, M. D. H. Beg, S. Ghazali, M. R. Islam, N. Jeyaratnam and A. R. Yuvaraj, RSC Adv., 2016, 6, 114453–114482 RSC.
- S. Cucurachi, C. Van Der Giesen and J. Guinée, Procedia CIRP, 2018, 69, 463–468 CrossRef.
- C. van der Giesen, S. Cucurachi, J. Guinée, G. J. Kramer and A. Tukker, J. Cleaner Prod., 2020, 259, 120904 CrossRef.
- R. Arvidsson, A. M. Tillman, B. A. Sandén, M. Janssen, A. Nordelöf, D. Kushnir and S. Molander, J. Ind. Ecol., 2018, 22, 1286–1294 CrossRef CAS.
- J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Chem. Rev., 2018, 118, 434–504 CrossRef CAS PubMed.
- M. A. N. Thonemann, Appl. Energy, 2020, 263, 114599 CrossRef CAS.
- N. Von Der Assen and A. Bardow, Green Chem., 2014, 16, 3272–3280 RSC.
- K. Carvalho, R. M. B. Alves and L. Kulay, Processes, 2021, 9, 1122 CrossRef CAS.
- R. Meys, A. Kätelhön and A. Bardow, Green Chem., 2019, 21, 3334–3342 RSC.
- C. Fernández-Dacosta, V. Stojcheva and A. Ramirez, J. CO2 Util., 2018, 23, 128–142 CrossRef.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- ISO International Standard 14044, Environmental management-Life cycle assessment-Requirements and guidelines, Geneva, 2006 Search PubMed.
- B. Steubing, M. Vos, A. Haas, C. Mutel and D. de Koning, LCA Activity Browser, https://github.com/LCA-ActivityBrowser/activity-browser, (accessed 19 November 2022).
- B. Steubing, D. de Koning, A. Haas and C. L. Mutel, Softw. Impacts, 2020, 3, 100012 CrossRef.
- R. Heijungs, CMLCA, https://www.cmlca.eu/, (accessed 19 November 2022).
- J. Collis, T. Strunge, B. Steubing, A. Zimmermann and R. Schomäcker, Front. Energy Res., 2021, 9, 642162 CrossRef.
- N. Tsoy, B. Steubing, C. van der Giesen and J. Guinée, Int. J. Life Cycle Assess., 2020, 25, 1680–1692 CrossRef.
- M. R. Machat, J. Marbach, H. Schumacher, S. Raju, M. Lansing, L. C. Over, L. Adler, J. Langanke, A. Wolf, W. Leitner and C. Gürtler, React. Chem. Eng., 2022, 7, 580–589 RSC.
- M. Aly, T. G. Gambu, Y. Zhang, V. V. Galvita and M. Saeys, ACS Catal., 2022, 12, 9011–9022 CrossRef CAS.
- M. Flores-Granobles and M. Saeys, Energy Convers. Manage., 2022, 258, 115515 CrossRef CAS.
- M. Dreillard, P. Broutin, P. Briot, T. Huard and A. Lettat, Energy Procedia, 2017, 114, 2573–2589 CrossRef CAS.
- M. Aleixo, M. Prigent, A. Gibert, F. Porcheron, I. Mokbel, J. Jose and M. Jacquin, Energy Procedia, 2011, 4, 148–155 CrossRef CAS.
- J. Langanke, A. Wolf, J. Hofmann, K. Böhm, M. A. Subhani, T. E. Müller, W. Leitner and C. Gürtler, Green Chem., 2014, 16, 1865–1870 RSC.
- Aspentech, Aspen Plus, https://www.aspentech.com, (accessed 20 November 2022).
- Institute for Environment and Sustainability, Characterisation factors of the ILCD Recommended Life Cycle Impact Assessment methods Database and supporting information. EUR 25167, Luxembourg, 1st edn, 2012 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The ex-ante LCA study. See DOI: https://doi.org/10.1039/d3gc00799e |
| This journal is © The Royal Society of Chemistry 2023 |