Open Access Article
Open Access ArticleIonothermal carbonization of sugarcane bagasse in imidazolium tetrachloroferrate ionic liquids: effect of the cation on textural and morphological properties†
Soha
Aldroubi
a,
Mohamed
El-Sakhawy
b,
Samir
Kamel
b,
Peter
Hesemann
 a,
Ahmad
Mehdi
a,
Ahmad
Mehdi
 *a and
Nicolas
Brun
*a and
Nicolas
Brun
 *a
*a
aICGM, Univ. Montpellier, CNRS, ENSCM, Montpellier, France. E-mail: nicolas.brun@enscm.fr; ahmad.mehdi@umontpellier.fr
bCellulose and Paper Department, National Research Centre, Giza, Egypt
First published on 6th April 2023
Abstract
The valorization of abundant agrowastes into chemicals and advanced materials is of key importance in the context of the circular bioeconomy and biorefinery. Herein, we report the ionothermal carbonization (ITC) of sugarcane bagasse, a fibrous residue made of lignocellulose, to engineer advanced carbonaceous materials, namely ionochars. We prepared a series of imidazolium tetrachloroferrate ionic liquids (IL) bearing modular substituents, e.g., alkyl chains with different lengths or benzyl groups. The effect of the cation on both textural and morphological properties is highlighted. Ionochars with high specific surface area (up to 800 m2 g−1), tuneable pore volume (up to 0.8 cm3 g−1), singular nanostructures and adjustable CO2 uptake/retention were obtained.
1. Introduction
Lignocellulosic agricultural wastes are abundant and renewable resources that can be used as feedstocks for the production of fuels, platform molecules and materials.1 Sugarcane bagasse is one of those agricultural residues produced in large quantities in sugar factories by grinding sugarcane. This fibrous agrowaste is mainly constituted of cellulose, hemicellulose and lignin. Bagasse comprises low-density fibers with a very wide particle size distribution.2 Burning bagasse to meet the internal energy needs of sugar mills remains wasteful and inefficient.3 A part of the surplus is used in the production of animal feed and in the manufacture of paper and board.3 Besides, the valuation of bagasse can be carried out by carbonization4 or activation5 to convert it into gas, bio-oils, biochars or other advanced carbonaceous materials.Amongst carbonization processes, the hydrothermal carbonization (HTC) has been reported as a profitable, atom efficient and environmentally safe process.6 HTC has been widely studied and several pilot and industrial-scale plants have been developed.7 HTC consists in converting organic matter, typically agrowaste or sewage sludge, into carbonaceous materials at mild processing temperature (<250 °C), in water and under autogenous pressure (<20 bars).8–10 When applied to lignocellulosic agrowastes, very complex cascade reactions of hydrolysis, dehydration, decarboxylation, polymerization, and aromatization take place.11 Firstly, holocellulose (i.e., hemicellulose and cellulose) hydrolyzes yielding monosaccharides, mainly xylose and glucose. This first step usually occurs at high temperatures, above 200 °C, and is limited at the lignocellulose–water interface. Secondly, glucose and xylose dehydrate to 5-hydroxymethylfurfural (HMF) and furfural, respectively. Further degradation of the furfural derivatives can generate organic acids, resulting in a decrease of the pH, and thus accelerating dehydration reactions. Finally, the furfural intermediates eventually condense to form furan-rich materials, namely hydrochars. At higher temperature (>220 °C), the aromatic character increases, with a low fraction of carboxyl and carbonyl groups. Due to weak dissolution in water, the lignocellulose–water interface is limited. As a direct consequence, HTC mainly occurs through intramolecular reactions, yielding non-porous hydrochars with poor textural and morphological control. Thus, HTC usually involves the use of sacrificial additives and templates, making the whole process costly and inefficient.12
Ionic liquids (ILs) are of great importance in modern chemistry,13 especially in the field of biomass processing and biorefinery.14 ILs are defined as salts that are liquid at a temperature below 100 °C and that usually consist of an organic cation in interaction with an inorganic anion. ILs are considered as green solvents15 due to singular properties such as: (i) almost zero vapor pressure which prohibits any evaporation in the atmosphere; (ii) non-flammability; and (iii) in many cases, high thermal stability, which offers potential recyclability and reusability.16 ILs demonstrated good ability for the dissolution and/or selective depolymerization of cellulose,17–19 lignin20,21 and even lignocellulosic agrowastes, including sugarcane residues.22 The dissolution of lignocellulose has been demonstrated in particular with ILs bearing a cation with short alkyl chains23 and an anion with strong hydrogen-bond acceptance;24 1-butyl-3-methylimidazolium chloride ([C4mim]Cl) being the most widely reported in the literature.17 Beyond dissolution, depolymerization and derivatization, ILs were used as recyclable solvents for the carbonization of simple carbohydrates,25–27 polysaccharides28 and raw lignocellulosic agrowastes,27,29–31 including sugarcane bagasse.31 This thermochemical process, known as ionothermal carbonization (ITC), must be considered as a relative of the HTC process and allows engineering advanced carbonaceous materials, namely ionochars. In particular, 1-butyl-3-methylimidazolium tetrachloroferrate ([C4mim]FeCl4) demonstrated advantageous features in ITC.29,30 Besides strong magnetic field response,32,33 and local ordering of its magnetic anion,34 [C4mim]FeCl4 displays an intermediate Lewis acidity35,36 and a relatively high thermal stability compared to [C4mim]Cl. It was demonstrated that [C4mim]FeCl4 catalyses both (i) the depolymerization of lignin through γ-O ester bond cleavage;20 and (ii) the dehydration and polycondensation reactions involved during solvothermal carbonization, yielding ionochars with a more pronounced aromatic aspect with respect to analogous hydrochars.25 In a previous study, our group demonstrated the beneficial inputs of [C4mim]FeCl4 in the ITC of a wet agrowaste, i.e., cocoa bean shells, yielding highly porous ionochars with high mass and carbon yields.30 While the role of the anion (i.e., Cl−, FeCl4−, BF4− and Tf2N−) associated with [C4mim]+ was clearly evidenced in the literature,30,31 the way the structure of the imidazolium cation affects the ITC of lignocellulose has not been explored yet and is still open to discussion. Herein, we focus on the systematic evaluation of the cationic structure to assess its effect on the ITC of sugarcane bagasse.
2. Experimental section
2.1. Materials
Sigmacell cellulose (type 50, 50 μm), 1-ethyl-3-methylimidazolium chloride (99%), 1-butyl-3-methylimidazolium chloride (99%), 1-hexyl-3-methylimidazolium chloride, and 1-octyl-3-methylimidazolium chloride were purchased from IoLiTec (Germany). Sodium chloride (NaCl, 99%), potassium chloride (KCl, 99%), iron(III) chloride hexahydrate (FeCl3·6H2O, 97%), 1-methylimidazole (C4H6N2, 99%), 1-butylimidazole (C7H12N2, 99%), benzyl chloride (C6H5CH2Cl, 99%), imidazole (C3H4N2, 99%) and sodium hydride (NaH, 60% dispersion in mineral oil) were purchased from Sigma Aldrich. Depithed and washed sugarcane bagasse (Bg) was donated by Quena Company for Paper Industry (Egypt). Sugarcane was previously pressed to extract the juice, ground and sieved to separate the pith. After depithing, Bg was washed three times with water (Bg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water mass ratio of 1
water mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to separate residual sugar and impurities. After each washing, Bg was dewatered to a dry content of 20–25 wt%. Finally, Bg was dried in a convection oven at 100 °C for 8 h, ground and sifted (>100 μm). An ash content below 2 wt% was determined by thermogravimetric analysis in air up to 800 °C. All the other reagents were used without further purification.
10) to separate residual sugar and impurities. After each washing, Bg was dewatered to a dry content of 20–25 wt%. Finally, Bg was dried in a convection oven at 100 °C for 8 h, ground and sifted (>100 μm). An ash content below 2 wt% was determined by thermogravimetric analysis in air up to 800 °C. All the other reagents were used without further purification.
2.2. Preparation of the tetrachloroferrate ionic liquids and salts
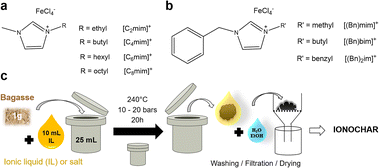
We prepared a series of imidazolium tetrachloroferrate ILs bearing modular side groups, e.g., alkyl chains with different lengths (from C2 to C8; Fig. 1a) and/or benzyl groups (Bn; Fig. 1b). The four 1-alkyl-3-methylimidazolium tetrachloroferrate ionic liquids (ILs) of the [Cxmim]FeCl4 series (with x = 2, 4, 6 or 8) were synthesized by adapting a procedure reported in the literature.37 In a typical synthesis, the analogous 1-alkyl-3-methylimidazolium chloride [Cxmim]Cl was mixed in an equimolar amount with iron(III) chloride hexahydrate. The mixture was heated at 80 °C under stirring for 6 hours. At the end of the reaction, two phases were obtained: a lower phase comprising the expected 1-alkyl-3-methylimidazolium tetrachloroferrate IL and an aqueous upper phase. Water was removed via rotary evaporation at 60 °C and 10 mbar for 4 hours and subsequent lyophilization for 12 hours. [(Bn)mim]FeCl4, [(Bn)bim]FeCl4 and [(Bn)2im]FeCl4 were prepared following the same procedure, using [(Bn)mim]Cl, [(Bn)bim]Cl and [(Bn)2im]Cl (synthesis details described in ESI†) as precursors. NaFeCl4 and KFeCl4 were prepared based on the procedures described in the literature.38 NaCl and KCl anhydrous salts were mixed with FeCl3·6H2O in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, stirred for 5 hours and heated to 165 and 240 °C respectively. Water was removed via rotary evaporation at 60 °C and 10 mbar for 4 hours followed by freeze-drying for 12 hours. Melting points of NaFeCl4 (163 °C) and KFeCl4 (240 °C) were determined with a Büchi B-540 melting point apparatus.
1 molar ratio, stirred for 5 hours and heated to 165 and 240 °C respectively. Water was removed via rotary evaporation at 60 °C and 10 mbar for 4 hours followed by freeze-drying for 12 hours. Melting points of NaFeCl4 (163 °C) and KFeCl4 (240 °C) were determined with a Büchi B-540 melting point apparatus.
2.3. Carbonization of sugarcane bagasse and cellulose
Ionothermal carbonization (ITC) of sugarcane bagasse was performed using the same protocol as described by Cibien, Parot et al.30 Typically, 1.0 g of ground and sifted bagasse (>100 μm) was mixed with 40.2 mmol of IL or salt, sealed in a Teflon lined autoclave (23 mL) and heated at 240 °C for 20 h under autogenous pressure. After quenching in cold water and cooling down to room temperature, samples were washed once with ultrapure water (150 mL), once with a water/ethanol mixture (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) (150 mL) and once with pure ethanol (150 mL), filtered, and dried at 80 °C overnight in a vacuum drying oven (Fig. 1c). The ionochars prepared in the [Cxmim]FeCl4 series were named Cxm-Bg. The ionochars prepared in [(Bn)mim]FeCl4, [(Bn)bim]FeCl4 and [(Bn)2im]FeCl4 were named (Bn)m-Bg, (Bn)b-Bg and (Bn)2-Bg, respectively. The ionochars prepared in NaFeCl4 and KFeCl4 were named Na-Bg and K-Bg, respectively. Similar ionothermal carbonization experiments were carried out with commercial cellulose; the ionochars were named Cxm-C.
50, v/v) (150 mL) and once with pure ethanol (150 mL), filtered, and dried at 80 °C overnight in a vacuum drying oven (Fig. 1c). The ionochars prepared in the [Cxmim]FeCl4 series were named Cxm-Bg. The ionochars prepared in [(Bn)mim]FeCl4, [(Bn)bim]FeCl4 and [(Bn)2im]FeCl4 were named (Bn)m-Bg, (Bn)b-Bg and (Bn)2-Bg, respectively. The ionochars prepared in NaFeCl4 and KFeCl4 were named Na-Bg and K-Bg, respectively. Similar ionothermal carbonization experiments were carried out with commercial cellulose; the ionochars were named Cxm-C.
Hydrothermal carbonization (HTC) in 10 mL of ultrapure water was also carried out on bagasse (1 g) under similar conditions, yielding the hydrochar named HTC-Bg. After washing and oven drying, blackish powders were collected and thoroughly characterized. Pyrochars were prepared by pyrolysis of ground and sifted bagasse (>100 μm) in a horizontal tube furnace under argon flow (50 mL min−1). Three different thermal treatments were carried out at 350, 550 and 750 °C for 4 h with a heating rate of 1 °C min−1, yielding P350-Bg, P550-Bg and P750-Bg, respectively. Blackish powders were directly collected and thoroughly characterized without any additional pre-treatment. All experiments were performed in duplicate without noting any significant difference regarding textural and morphological properties.
2.4. Characterization
The mass yield (eqn (1)) and the corrected mass yield (eqn (2)) were calculated as follows: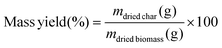 | (1) |
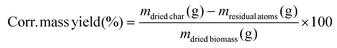 | (2) |
The carbon yield (eqn (3)) was calculated as follows:
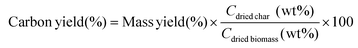 | (3) |
Combustional elemental analyses (C, H, N, O) were performed on a vario MICRO cube analyzer from Elementar. FTIR spectroscopy was performed in attenuated total reflectance (ATR) mode on a PerkinElmer Spectrum Two spectrometer. 13C solid-state NMR was made on a VARIAN VNMRS 300 MHz spectrometer at 75.44 MHz. A VARIAN T3 MAS probe was used with 3.2 mm ZrO2 rotors. Experiments were done using cross polarization magic angle (CP-MAS) with a contact time of 1 ms and a recycle delay of 5 s and a rotor speed of 15 kHz. GC-MS was performed on a Shimadzu QP2010SE gas chromatograph-mass spectrometer with a Phenomenex Zebron ZB-5 ms column (30 m × 0.18 mm × 0.18 μm). Injection: split 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30@250 °C. Oven program: 50 °C (hold 2 min) 50–280 °C at 22 °C min−1 (hold 2 min). Raman spectroscopy was carried out on a Renishaw inVia Raman microscope with a 532 nm laser excitation. Scanning electron microscopy (SEM) analyses were performed on a Hitachi S-4800 electron microscope. SEM-EDX (Energy Dispersive X-ray spectroscopy) analyses were performed on a Zeiss EVO HD15 electron microscope. Transmission electron microscopy (TEM) analyses were performed on a JEOL 1200 microscope from the MEA platform, Université de Montpellier. N2 (at 77 K) and CO2 (at 273, 298 and 323 K) physisorption experiments were carried out on a Micromeritics 3Flex apparatus. Ionochars and hydrochars were degassed at 150 °C for 6 h under high vacuum (ca. 0.1 Pa) before physisorption measurements. Pyrochars were degassed at 300 °C for 24 hours under high vacuum (ca. 0.1 Pa) before physisorption measurements. To determine the isosteric heats of adsorption, we applied the Clausius–Clapeyron equation (see eqn (4) below) to CO2 adsorption isotherms obtained at the three different temperatures.
30@250 °C. Oven program: 50 °C (hold 2 min) 50–280 °C at 22 °C min−1 (hold 2 min). Raman spectroscopy was carried out on a Renishaw inVia Raman microscope with a 532 nm laser excitation. Scanning electron microscopy (SEM) analyses were performed on a Hitachi S-4800 electron microscope. SEM-EDX (Energy Dispersive X-ray spectroscopy) analyses were performed on a Zeiss EVO HD15 electron microscope. Transmission electron microscopy (TEM) analyses were performed on a JEOL 1200 microscope from the MEA platform, Université de Montpellier. N2 (at 77 K) and CO2 (at 273, 298 and 323 K) physisorption experiments were carried out on a Micromeritics 3Flex apparatus. Ionochars and hydrochars were degassed at 150 °C for 6 h under high vacuum (ca. 0.1 Pa) before physisorption measurements. Pyrochars were degassed at 300 °C for 24 hours under high vacuum (ca. 0.1 Pa) before physisorption measurements. To determine the isosteric heats of adsorption, we applied the Clausius–Clapeyron equation (see eqn (4) below) to CO2 adsorption isotherms obtained at the three different temperatures.
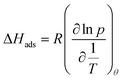 | (4) |
3. Results and discussion
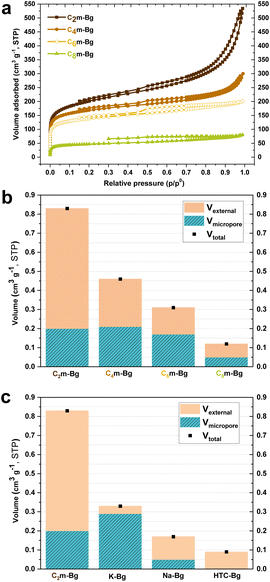
The ITC of sugarcane bagasse was first performed in four 1-alkyl-3-methylimidazolium tetrachloroferrate ILs with various alkyl chain lengths (Fig. 1a).37,39 The as-obtained ionochars were named C2m-Bg, C4m-Bg, C6m-Bg and C8m-Bg, respectively. The nitrogen sorption isotherms obtained at 77 K for these four ionochars display type I/II isotherms with a H4-type hysteresis loop (Fig. 2a), characteristic of porous materials with micro-, meso- and macropores. The shape of the isotherms changes significantly from C8m-Bg to C2m-Bg, with a higher volume of nitrogen adsorbed at high relative pressure. This feature reflects an increase in the contribution of the external pore volume (Fig. 2b), that is the interparticle void spaces that can be seen as a continuum of meso- and macropores. When decreasing the length of the alkyl chain, the BET-equivalent specific surface area (SSABET) gradually increases from 173 m2 g−1 for C8m-Bg to 734 m2 g−1 for C2m-Bg (Table S1† and Table 1). A similar trend is observed regarding pore volume that gradually increases from 0.12 cm3 g−1 for C8m-Bg to 0.84 cm3 g−1 for C2m-Bg (Fig. 2b and Table 1).| Chars | Y mc (wt%) | Y c (%) | Cc (wt%) | O/Cd | N/Cd | Fee (wt%) | Cle (wt%) | SSABET (m2 g−1) | SSAmicrof (m2 g−1) | V total (cm3 g−1) | V ext (cm3 g−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| a Y mc for corrected mass yield. b Y c for carbon yield. c Weight percentages obtained from elemental analyses. d Molar ratios obtained from elemental analyses. e SEM-EDX analyses. f Determined from nitrogen sorption isotherms at 77 K using the t-plot method. g Determined from the amount of nitrogen adsorbed at a relative pressure of 0.99. h Equivalent micropore surface area and micropore volume determined from carbon dioxide sorption isotherms at 273 K using the Dubinin–Astakhov equation. | |||||||||||
| C2m-Bg | 50.8 | 78.1 | 67.4 | 0.29 | 0.0026 | 2.3 | 1.7 | 734 | 467 | 0.83 | 0.63 |
| C2m-Bg-r1 | 51.3 | 80.7 | 69.6 | 0.23 | 0.0186 | 0.8 | 1.7 | 696 | 437 | 0.82 | 0.63 |
| C2m-Bg-r2 | 50.8 | 79.2 | 69.0 | 0.23 | 0.0261 | 1.0 | 1.4 | 701 | 390 | 1.05 | 0.88 |
| C4m-Bg | 52.2 | 81.9 | 69.0 | 0.28 | 0.0044 | 1.2 | 2.4 | 628 | 495 | 0.46 | 0.25 |
| C6m-Bg | 51.7 | 79.2 | 65.4 | 0.27 | 0.0040 | 3.9 | 2.7 | 518 | 396 | 0.31 | 0.14 |
| C6m-Bg-r1 | 48.2 | 75.4 | 70.4 | 0.23 | 0.0281 | 0.4 | 1.0 | 444 | 375 | 0.25 | 0.10 |
| C6m-Bg-r2 | 50.3 | 79.9 | 71.1 | 0.21 | 0.0363 | 0.5 | 1.3 | 452 | 333 | 0.45 | 0.31 |
| C8m-Bg | 52.6 | 80.3 | 66.0 | 0.26 | 0.0065 | 3.3 | 2.0 | 173 | 123 | 0.12 | 0.07 |
| C2m-C | 49.0 | 79.4 | 72.9 | 0.23 | 0.0012 | 0.3 | 1.9 | 842 | 514 | 0.81 | 0.59 |
| C4m-C | 47.9 | 76.9 | 72.1 | 0.23 | 0.0024 | 0.5 | 1.5 | 747 | 559 | 0.51 | 0.27 |
| C6m-C | 44.9 | 75.0 | 74.3 | 0.20 | 0.0011 | 0.5 | 2.5 | 611 | 451 | 0.51 | 0.32 |
| C8m-C | 42.6 | 70.9 | 67.6 | 0.15 | 0.0152 | 3.6 | 7.6 | 266 | 149 | 0.28 | 0.21 |
| (Bn)m-Bg | 66.5 | 110.3 | 70.0 | 0.19 | 0.0082 | 4.7 | 2.9 | 294 | 118 | 0.49 | 0.44 |
| (Bn)m-Bg-S | 66.1 | 105.2 | 70.7 | 0.22 | 0.0071 | 2.5 | 0.3 | 433 | 241 | 0.68 | 0.35 |
| (Bn)b-Bg | 72.0 | 121.6 | 72.0 | 0.17 | 0.0045 | 3.3 | 3.4 | 6 | 4 | n/a | n/a |
| (Bn)b-Bg-S | 66.1 | 107.6 | 72.8 | 0.24 | 0.0006 | 1.5 | 0.4 | 14 | 6 | 0.02 | 0.02 |
| (Bn)2-Bg | 127.6 | 256.2 | 71.5 | 0.06 | 0.0295 | 10.8 | 9.0 | 7 | 1 | 0.02 | 0.02 |
| (Bn)2-Bg-S | 114.4 | 208.1 | 78.1 | 0.07 | 0.0179 | 3.3 | 2.4 | 25 | 4 | 0.07 | 0.07 |
| HTC-Bg | 39.1 | 60.3 | 70.4 | 0.26 | 0.0019 | 0.0 | 0.0 | 24 | 7 | 0.00 | 0.09 |
| Na-Bg | 46.2 | 63.2 | 56.1 | 0.43 | 0.0032 | 6.1 | 3.9 | 168 | 123 | 0.05 | 0.12 |
| K-Bg | 42.9 | 63.9 | 66.7 | 0.38 | 0.0003 | 1.3 | 0.7 | 733 | 697 | 0.29 | 0.04 |
| P350-Bg | 22.9 | 34.3 | 68.4 | 0.17 | 0.0037 | n/a | n/a | 16 | 15 (315)h | 0.01 (0.12)h | 0.00 |
| P550-Bg | 17.5 | 31.0 | 80.9 | 0.08 | 0.0041 | n/a | n/a | 384 | 384 (470)h | 0.16 (0.18)h | 0.00 |
| P750-Bg | 16.7 | 30.3 | 82.8 | 0.09 | 0.0018 | n/a | n/a | 392 | 389 (588)h | 0.17 (0.22)h | 0.01 |
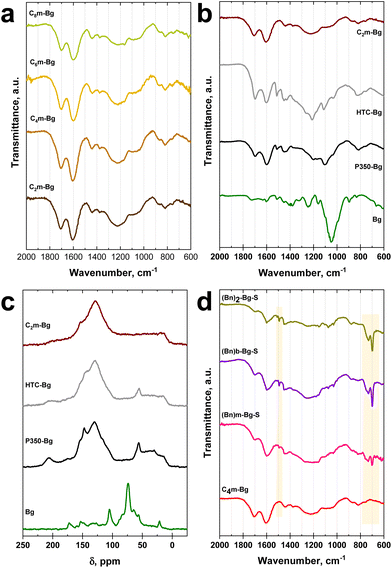
The length of the alkyl chain has, however, no effect on both mass (between 50.8 wt% and 52.6 wt%) and carbon yields (between 78.1 wt% and 81.9 wt%) which are very similar in the Cxm-Bg (with x = 2, 4, 6, or 8) series (Table S2† and Table 1). The four ionochars of the Cxm-Bg series exhibit similar infrared spectra regardless of the length of the alkyl chain (Fig. 3a). The four spectra show an intense band at 1700 cm−1, which is characteristic of the stretching vibrations of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds of aromatic carbonyl groups (νC
O bonds of aromatic carbonyl groups (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). Two bands centered at 1600 cm−1 and 1440 cm−1 correspond to the C
O). Two bands centered at 1600 cm−1 and 1440 cm−1 correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond of aromatic groups (νC
C bond of aromatic groups (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). A broad band between 1220 and 1280 cm−1 results from the stretching of the C–O bonds of aromatic ether and ester groups (νC–O). In addition, a weak band centered at 1374 cm−1, corresponding to the deformation δCH3, suggests the presence of aromatic methyl groups.
C). A broad band between 1220 and 1280 cm−1 results from the stretching of the C–O bonds of aromatic ether and ester groups (νC–O). In addition, a weak band centered at 1374 cm−1, corresponding to the deformation δCH3, suggests the presence of aromatic methyl groups.
We also studied (i) the HTC of sugarcane bagasse in ultrapure water, yielding the hydrochar named HTC-Bg, and (ii) the conventional pyrolysis of sugarcane bagasse under argon flow at three temperatures, i.e., 350, 550 or 750 °C, yielding pyrochars named P350-Bg, P550-Bg and P750-Bg, respectively. On the one hand, the hydrochar displays a type III isotherm typical of nonporous (or macroporous) materials (Fig. S1, Table S3† and Table 1). On the other hand, the two pyrochars P550-Bg and P750-Bg display type I isotherms typical of microporous materials (Fig. S2†). Note that the pyrochar P350-Bg displays a type III isotherm (Fig. S2†), supporting the absence of micropores or the presence of narrow micropores with poor accessibility. To confirm these features, we performed carbon dioxide sorption isotherms at 273 K on the three pyrochars (Fig. S2† and Table 1). The specific surface areas determined by carbon dioxide sorption at 273 K are systematically higher than the ones determined by nitrogen sorption at 77 K. This feature supports the presence of ultra-micropores, i.e., narrow micropores with an effective pore width below 0.7 nm (Fig. S2c and d†), which are more readily accessible due to kinetics that is more favorable at 273 K than at 77 K and the kinetic diameter of carbon dioxide molecules that is slightly smaller as compared with nitrogen. This result is in good agreement with the literature. Usually, sugarcane bagasse-derived pyrochars treated at moderate temperatures (below 500 °C) display limited specific surface areas and low pore volumes mainly attributed to narrow micropores.4 These features highlight the singularity of ITC compared to HTC and conventional pyrolysis. Achieving textural properties comparable to those obtained herein with ionochars (above 700 m2 g−1 and 0.8 cm3 g−1) would require harsher conditions together with the generation of a large amount of waste to be treated. Indeed, it usually involves liquid impregnation with activating agents such as H3PO4 or NaOH, oven-drying for several hours above 80 °C, subsequent thermal treatment above 750 °C for at least 1 hour, and intensive washing with water before final oven-drying for several hours above 80 °C.5 Thus, ITC can be positioned as a potentially sustainable approach in terms of energy consumption, environmental factor and atom economy – provided that the synthesis of the aforementioned ILs also respects the precepts of green chemistry and that the ILs are reusable as shown in a previous study.30 It is important to note that both elemental analyses performed on ionochars (Table S2† and Table 1) and GC-MS and FTIR (Fig. S3†) carried out on recovered ILs after ITC confirmed the good stability of the four imidazolium tetrachloroferrate ILs of the [Cxmim]FeCl4 series, which display a decomposition temperature above 280 °C.37 The yield of recovered ILs after ITC was about 96 wt% both for C2m-Bg and C6m-Bg, which is in good agreement with the ones determined in a previous study for the ITC of cocoa bean shells in [Bmim]FeCl4 at 240 °C for 20 h (ca. 95 wt%).30 The IL loss was mainly due to the tetrachloroferrate anion, with residual iron and chlorine in the ionochars ranging from 4 to 7 wt% (Table 1). To assess their reusability, two ILs of the [Cxmim]FeCl4 series, i.e., [C2mim]FeCl4 and [C6mim]FeCl4, were recycled twice, yielding Cxm-Bg-r1 and Cxm-Bg-r2. After each ITC cycle, the yield of recovered IL was maintained between 92 and 98 wt%. Moreover, both the mass and carbon yields reached for Cxm-Bg-r1 and Cxm-Bg-r2 remained relatively high, above 48 and 75 wt% respectively (Table 1). Despite a 5 to 10% decrease in the micropore contribution after each cycle, the textural properties obtained for Cxm-Bg-r1 and Cxm-Bg-r2 are really close to the ones obtained for the ionochars after the first ITC cycle (Table 1 and Fig. S4†). However, one may notice a 25 to 50% increase in pore volume (ca. 0.2 cm3 g−1) from Cxm-Bg-r1 to Cxm-Bg-r2. We believe that these features are due to iron loss with each ITC cycle, which might change the nature of the chloroferrate anions. In this respect, we are currently conducting a study to better understand the role of the anion speciation in the ITC of sugarcane bagasse in imidazolium chloroferrate ILs.
Moreover, pyrolysis and, to a lesser extent, HTC gave lower carbonization yields as compared with ITC (Table 1). This behavior is particularly relevant for the pyrochar prepared at 350 °C, namely P350-Bg, which displays an elemental composition similar to the analogous hydro- and ionochars (i.e., with a carbon content of 68.4 wt% and O/C molar ratio of 0.17; Table 1). The mass and carbon yields reached to prepare P350-Bg are half as efficient as the ones reached by ITC. This feature confirms the atom-economical character of ITC compared to more conventional carbonization approaches. At this stage, it is important to note that the relevance of the ITC approach depends closely on the selected IL. In a previous study Wang et al.31 performed the ITC of sugarcane bagasse in 1-(3-cyanopropyl)-3-methylimidazolium chloride, [BCNmim]Cl, at 200 °C for 24 hours. The authors obtained a ionochar with significantly lower BET-equivalent specific surface area (35 m2 g−1), pore volume (0.15 cm3 g−1) and mass yield (37 wt%) as compared with the ones reported herein. Besides, [BCNmim]Cl is unstable and could not be reused due to partial degradation, which resulted in nitrogen-doping of the ionochar with a nitrogen content of 6.6 wt%. This feature further emphasizes the applicative interest of short-chain imidazolium tetrachloroferrate ILs for the ITC of lignocellulosic biomass and agrowastes.
Besides textural properties and carbonization yields, significant differences were observed in terms of molecular structures between pyrochars, hydrochar and ionochars. The FTIR spectrum of HTC-Bg shows a clear dissimilitude compared to the ones of the four ionochars of the Cxm-Bg series (Fig. 3b). Three new bands were observed for HTC-Bg. The two sharp bands centered at 1504 and 1110 cm−1 correspond to the aromatic νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations of lignin and the νC–O–C stretching vibrations of ether groups of holocellulose (i.e., the carbohydrate fraction of sugarcane bagasse), respectively. The weak shoulder centered at 1040 cm−1 can correspond to the in plane deformation of the C–H bonds (δCH) in the aromatic rings of lignin. Moreover, the bands centered at 1440 cm−1 and between 1220 and 1280 cm−1 are more intense than for the four ionochars of the Cxm-Bg series. These features confirm that, whatever the alkyl chain length, ITC in 1-alkyl-3-methylimidazolium tetrachloroferrate ILs favors the deconstruction/carbonization of lignin. Interestingly, the FTIR spectrum of P350-Bg (Fig. 3b), which is the pyrochar with the elemental composition that is the closest to the analogous hydro- and ionochars, is very similar to the one obtained for HTC-Bg. This feature was also confirmed by Raman spectroscopy (Fig. S5†) and 13C solid-state NMR (Fig. 3c). The 13C solid-state NMR spectrum of raw sugarcane bagasse (Bg) displays the signature of lignocellulose with the characteristic peaks of cellulose centered at 65 (C6), 72–75 (C2, C3, C5), 84–89 (C4) and 105 ppm (C6) and hemicellulose centered at 20–25 and 70–75 ppm. After carbonization, the characteristic peaks of holocellulose disappeared. Two major domains can be clearly identified: the aliphatic region between 0 and 65 ppm and the aromatic region centered at 129 ppm. The 13C solid-state NMR spectra of HTC-Bg and P350-Bg show clear differences compared to the ones of the four ionochars of the Cxm-Bg series: two peaks centered at 55–56 ppm and 147–148 ppm can be observed. As reported by Baccile et al.,40 the peak centered at 55–56 ppm can be attributed to residual methoxy groups of lignin, while the peak centered at 147–148 ppm can be attributed to aromatic carbons in ipso position to methoxy groups of lignin. Note that the shoulder centered at 151 ppm can be attributed to the presence of polyfuranic aromatic structures coming from the thermochemical degradation of cellulose. We also performed Raman spectroscopy to provide additional information on the molecular structure of the materials. The Raman spectra of the four ionochars of the Cxm-Bg series are similar to that of P350-Bg (Fig. S5a†) and are typical of amorphous carbonaceous structures with a significant fraction of sp3-hybridized carbon atoms. Interestingly, the Raman spectrum of HTC-Bg shows stronger fluorescence with a significant deviation of the baseline (Fig. S5b†). This behavior may be attributed to the highest presence of auxochromes in the polyfuranic aromatic structures of HTC-Bg, due to a lower carbonization rate compared to the ionochars and P350-Bg. To conclude on this part, FTIR, 13C solid-state NMR and Raman spectroscopy confirm the beneficial character of ITC for the efficient degradation of lignin and carbonization of lignocellulose. This feature is probably due to the catalytic role of the tetrachloroferrate anions as shown in recent studies.20,30
C stretching vibrations of lignin and the νC–O–C stretching vibrations of ether groups of holocellulose (i.e., the carbohydrate fraction of sugarcane bagasse), respectively. The weak shoulder centered at 1040 cm−1 can correspond to the in plane deformation of the C–H bonds (δCH) in the aromatic rings of lignin. Moreover, the bands centered at 1440 cm−1 and between 1220 and 1280 cm−1 are more intense than for the four ionochars of the Cxm-Bg series. These features confirm that, whatever the alkyl chain length, ITC in 1-alkyl-3-methylimidazolium tetrachloroferrate ILs favors the deconstruction/carbonization of lignin. Interestingly, the FTIR spectrum of P350-Bg (Fig. 3b), which is the pyrochar with the elemental composition that is the closest to the analogous hydro- and ionochars, is very similar to the one obtained for HTC-Bg. This feature was also confirmed by Raman spectroscopy (Fig. S5†) and 13C solid-state NMR (Fig. 3c). The 13C solid-state NMR spectrum of raw sugarcane bagasse (Bg) displays the signature of lignocellulose with the characteristic peaks of cellulose centered at 65 (C6), 72–75 (C2, C3, C5), 84–89 (C4) and 105 ppm (C6) and hemicellulose centered at 20–25 and 70–75 ppm. After carbonization, the characteristic peaks of holocellulose disappeared. Two major domains can be clearly identified: the aliphatic region between 0 and 65 ppm and the aromatic region centered at 129 ppm. The 13C solid-state NMR spectra of HTC-Bg and P350-Bg show clear differences compared to the ones of the four ionochars of the Cxm-Bg series: two peaks centered at 55–56 ppm and 147–148 ppm can be observed. As reported by Baccile et al.,40 the peak centered at 55–56 ppm can be attributed to residual methoxy groups of lignin, while the peak centered at 147–148 ppm can be attributed to aromatic carbons in ipso position to methoxy groups of lignin. Note that the shoulder centered at 151 ppm can be attributed to the presence of polyfuranic aromatic structures coming from the thermochemical degradation of cellulose. We also performed Raman spectroscopy to provide additional information on the molecular structure of the materials. The Raman spectra of the four ionochars of the Cxm-Bg series are similar to that of P350-Bg (Fig. S5a†) and are typical of amorphous carbonaceous structures with a significant fraction of sp3-hybridized carbon atoms. Interestingly, the Raman spectrum of HTC-Bg shows stronger fluorescence with a significant deviation of the baseline (Fig. S5b†). This behavior may be attributed to the highest presence of auxochromes in the polyfuranic aromatic structures of HTC-Bg, due to a lower carbonization rate compared to the ionochars and P350-Bg. To conclude on this part, FTIR, 13C solid-state NMR and Raman spectroscopy confirm the beneficial character of ITC for the efficient degradation of lignin and carbonization of lignocellulose. This feature is probably due to the catalytic role of the tetrachloroferrate anions as shown in recent studies.20,30
To confirm the effect of the alkyl chain length in 1-alkyl-3-methylimidazolium tetrachloroferrate ILs on textural properties, a similar study was carried out with commercial cellulose, which is the main constituent of bagasse. Comparable trends were observed (Fig. S6 and Table S4†). The SSABET and the pore volume gradually increase from 266 m2 g−1 and 0.28 cm3 g−1 for C8m-C to 842 m2 g−1 and 0.81 cm3 g−1 for C2m-C. In the same way as for bagasse, no significant variation was observed for both mass (between 42.6 and 49.0 wt%) and carbon yields (between 70.9 and 79.4 wt%) (Fig. S6d and Table S5†). These features strongly suggest that the structure of the imidazolium cation plays a major role in the way (ligno)cellulose and IL interact and assemble during ITC. In the literature, the role of the cation in the dissolution of cellulose in IL was first neglected.24,41,42 The major driving force for cellulose dissolution can be attributed to the formation of hydrogen bonds between the anion of the IL and the hydroxyl groups of cellulose. Although still open to debate, the interactions between cellulose and the imidazolium cation are assumed to involve van der Waals forces rather than hydrogen-bonding.43–46 Chu et al.47 showed that the protons of the imidazolium cations strongly interact with side-chain and linker oxygen atoms of cellulose. Zhang et al. showed that the presence of long alkyl chains in [Cxmim]Cl has a negative impact on the dissolution of cellulose, mainly due to steric hindrance.23 The longer the alkyl chain length, the fewer the anions surrounding the cellulose and the weaker the intermolecular interactions between the anion and the cellulose. As the length of the alkyl chain increases, the ability of [Cxmim]Cl to dissolve cellulose becomes weaker23 and aggregates will tend to form in the medium for x > 8.48,49 Besides, as the length of the alkyl chain in [Cxmim]FeCl4 increases, both the fluidity and the Walden product of the IL decrease, due to pronounced van der Waals attraction between the alkyl chains.37 One may assume that the cation undoubtedly has a role in (ligno)cellulose dissolution.17,44,50,51 In our work, the results observed with the [Cxmim]FeCl4 (with x = 2, 4, 6, or 8) series for the ITC of both cellulose and bagasse seem to follow the same trend as the [Cxmim]Cl series for the dissolution of cellulose. Thus, the fact that [C2mim]FeCl4 leads to ionochars with the highest SSABET and pore volumes might be due to stronger interactions between (ligno)cellulose and IL.
To further support the role of the cation, ITC of bagasse was also carried out in imidazolium tetrachloroferrate ILs carrying one or two benzyl groups (Fig. 1a), i.e., [(Bn)mim]FeCl4, [(Bn)bim]FeCl4 and [(Bn)2im]FeCl4. The as-obtained ionochars were named (Bn)m-Bg, (Bn)b-Bg and (Bn)2-Bg, respectively. These ILs are rather unstable during ITC, especially [(Bn)2im]FeCl4, as the benzyl side groups are subject to Hofmann eliminations. This feature has been evidenced by elemental analyses, FTIR and EDX performed on ionochars (Fig. 3d and Table S6†) and GC-MS and FTIR carried out on recovered ILs after ITC (Fig. S7 and S8†). In particular, the FTIR spectra of the three ionochars obtained after Soxhlet extraction, samples named (Bn)m-Bg-S, (Bn)b-Bg-S and (Bn)2-Bg-S, display two intense bands between 690 and 730 cm−1, which are characteristic of the deformation of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H bonds (δ
C–H bonds (δ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H), together with a weaker band centered at 1500 cm−1, characteristic of the stretching vibration of C
C–H), together with a weaker band centered at 1500 cm−1, characteristic of the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (νC
C bonds (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). Thus, the presence of residual benzyl (Fig. 3d and Fig. S7†) and imidazole (Table S6 and Fig. S7†) moieties in these ionochars was clearly evidenced, even after Soxhlet extraction. Moreover, GC-MS of [(Bn)2im]FeCl4 recovered after ionothermal carbonization of bagasse at 240 °C for 20 h revealed the presence of 1-benzylimidazole (Fig. S7†). Our previous statement, positioning ITC as a potentially sustainable approach in terms of environmental factor and atom economy, is therefore directly dependent on the IL used and cannot be generalized to 1-benzylimidazolium-based ILs.
C). Thus, the presence of residual benzyl (Fig. 3d and Fig. S7†) and imidazole (Table S6 and Fig. S7†) moieties in these ionochars was clearly evidenced, even after Soxhlet extraction. Moreover, GC-MS of [(Bn)2im]FeCl4 recovered after ionothermal carbonization of bagasse at 240 °C for 20 h revealed the presence of 1-benzylimidazole (Fig. S7†). Our previous statement, positioning ITC as a potentially sustainable approach in terms of environmental factor and atom economy, is therefore directly dependent on the IL used and cannot be generalized to 1-benzylimidazolium-based ILs.
Besides chemical instability considerations, we showed that the structure of the 1-benzylimidazolium-based cation has a direct impact on the textural properties of the ionochar. In the same way as for the Cxm-Bg series, the cation with the shorter alkyl chain length leads to the ionochar ((Bn)m-Bg) with the highest SSABET (433 m2 g−1) and pore volume (0.68 cm3 g−1; Fig. S9 and Table S7†). Note that, due to partial degradation of these three ILs during ITC, both mass and carbon yields are significantly overestimated, in particular for (Bn)2-Bg and (Bn)2-Bg-S (above 100%). The grafting of benzyl groups onto ionochars cannot be excluded. Thus, we decided not to discuss the mass and carbon yields obtained for the three corresponding ionochars (Table S6†).
To provide better insight into the role of the cationic structure, ITC in imidazolium tetrachloroferrate ILs was also compared with ITC in tetrachloroferrate salts bearing cheap and abundant alkali metals, i.e., NaFeCl4 and KFeCl4. The as-obtained ionochars were named Na-Bg and K-Bg. On the one hand, Na-Bg displays a type I/II isotherm similar to the ones obtained for the Cxm-Bg series (Fig. S10a†). Both SSABET (168 m2 g−1) and pore volume (0.17 cm3 g−1) are close to the ones obtained for C8m-Bg (Table 1, Fig. S10b, c and Table S8†). On the other hand, K-Bg displays a type I(a) isotherm typical of microporous materials with narrow micropores (Fig. S10a†), yielding a high SSABET of 733 m2 g−1 and a pore volume of 0.33 cm3 g−1 (Table 1 and Table S8†). An increase in the cation size from Na to K leads to a significant increase in microporosity. However, the contribution of the external pore volume is limited as compared with the Cxm-Bg series (Fig. 2c). The mass (42.9 wt% for K-Bg; 46.2 wt% for Na-Bg) and carbon yields (63.9 wt% for K-Bg; 63.2 wt% for Na-Bg) for these two ionochars are slightly higher than those of HTC-Bg (39.1 wt% and 60.3 wt% respectively) (Table 1, Fig. S10d and Table S9†). They are, however, significantly lower than those obtained by ITC in the [Cxmim]FeCl4 series, confirming the benefit of cation engineering of the IL for ITC of (ligno)cellulose.
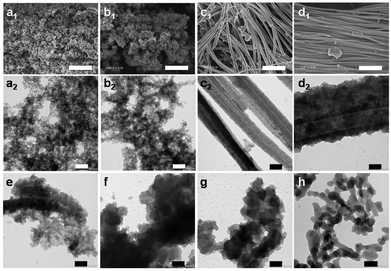
So far, we demonstrated that the nature and the constitution of the cation can be favorable to design advanced porous ionochars in terms of textural properties (high SSABET and pore volumes) and atom-economy (high mass and carbon yields). Interestingly, we also showed a fine control in terms of morphological properties (Fig. 3a and b). Firstly, significant differences were observed in the Cxm-Bg series. The two ionochars prepared in ILs with short alkyl chain lengths, i.e., C2m-Bg and C4m-Bg, are made of aggregates of small nanoparticles. SEM and TEM images reveal interparticle void spaces forming an interconnected pore network made of large mesopores and narrow macropores. This feature is in good agreement with the data extracted from the nitrogen sorption analyses (Fig. 2 and Table 1). As discussed earlier, the strong intermolecular interactions between ILs bearing short alkyl chains and lignocellulose favor lignin depolymerization, most probably through the cleavage of the γ-O ester and β-O-4 ether bonds.52–54 Then, cellulose is readily accessible for further dissolution, deconstruction and homogeneous solvothermal carbonization, yielding highly porous ionochars made of small nanoparticles. When increasing the alkyl chain length, very different morphologies were obtained (Fig. 4c and d). Thus, for C6m-Bg and C8m-Bg, bundles of large amorphous nanotubes with an external diameter of ca. 100 to 300 nm are observed. Note that the inner diameter of the tubes was clearly identified by TEM, confirming the hollow nature of the particles (Fig. 3c2–d2). Unlike bagasse, no tubular nanostructures were observed with cellulose (Fig. S11†). In the light of the previous discussion regarding lignocellulose dissolution in ILs, one may assume that the laborious dissolution/deconstruction of lignin in [C6mim]FeCl4 and [C8mim]FeCl4 offers a limited lignocellulose–solvent interface preventing complete depolymerisation. As a direct consequence, it is likely that the carbonization occurred in a heterogeneous solid–liquid biphasic medium through intramolecular reactions, thus preserving the fibrous structure of fibrils in lignocellulose and yielding a carbonaceous replica made of bundles of large nanotubes.
In view of the results obtained with cellulose, it is unlikely that such tubular structures result from a self-assembly of the IL itself. Surprisingly, no tubular nanostructures were observed in C6m-Bg-r1 and C6m-Bg-r2. As mentioned previously, we believe that these features are due to iron loss with each ITC cycle, which might change the nature of the chloroferrate anions in the IL and its intermolecular interactions with lignocellulose. Interestingly, TEM performed on (Bn)m-Bg, (Bn)b-Bg and (Bn)2-Bg did not show any tubular structures despite substantial steric hindrance on the cation, and the anticipation of a limited ability to dissolve lignocellulose. Instead, stacked nanoplatelets were observed, particularly for the two ionochars obtained in the bulkiest cations, i.e., (Bn)b-Bg and (Bn)2-Bg (Fig. 4e–g). One may assume that the presence of benzyl group(s) onto the cation promotes π–π type interactions with lignin and with the subsequent ionochars, which might favor the formation of two-dimensional stacked nanoplatelets. Despite the limited stability of the (Bn)-containing ILs, the textural and morphological properties of the resulting ionochars give interesting insights into their specific interactions with bagasse during ITC. We are currently studying other polyphenolic biomolecules and natural extracts to further support this hypothesis. Note that the micrographs of HTC-Bg reveal the aggregation of nonporous large nanoparticles of ca. 100 nm in diameter (Fig. 4h). No exotic morphologies, neither nanotubes nor nanoplatelets, were observed.
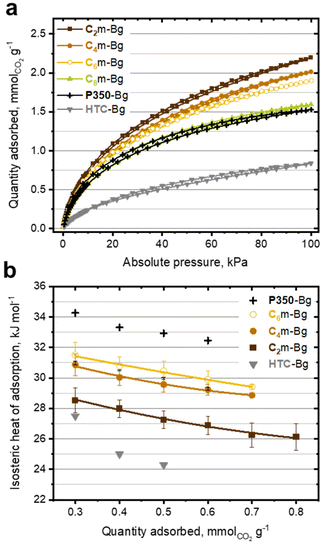
Due to advantageous textural and morphological properties, the ionochars reported herein can find applications in various fields, including catalysis, wastewater treatment,55 energy storage29 and environmental remediation.31 In our previous study, ITC and as-obtained ionochars demonstrated beneficial inputs for gas sorption, especially for post combustion carbon dioxide (CO2) capture.31 Herein, we confirm that the four ionochars of the Cxm-Bg series outperform the analogous hydro- and pyrochars, i.e., HTC-Bg and P350-Bg (Fig. 5a). Interestingly, we show that cation engineering can significantly improve the CO2 uptake, i.e., the quantity of CO2 adsorbed per gram of ionochar (Fig. 5a). At 273 K and 100 kPa, the CO2 uptake increases from 1.6 mmol of CO2 per gram for C8m-Bg to 2.2 mmol of CO2 per gram for C2m-Bg (which corresponds to a 37% increase). Similar features were observed at 298 and 323 K (Fig. S12†). Interestingly, we show that cation engineering may also offer the possibility to control the specific affinity of the ionochar for CO2 adsorption; in other words, its ability not only to capture, but also to retain CO2. Thus, the isosteric heat of adsorption of each ionochar was calculated for the Cxm-Bg series applying the Clausius–Clapeyron equation to CO2 adsorption isotherms obtained at three different temperatures (Fig. S13†). It appears that the isosteric heat of adsorption increases by ca. 10% (ca. 3 kJ mol−1) from C2m-Bg to C6m-Bg (Fig. 5b). While C2m-Bg has the higher CO2 uptake, it displays the lower affinity for CO2; thus, lower energy would be required to fully desorb CO2. Moreover, the isosteric heats of adsorption calculated for the ionochars of the Cxm-Bg series are intermediate between the analogous hydro- and pyrochars (Fig. 5b), supporting significant differences in terms of micropore size distribution and surface chemistry. These features confirm the high versatility and singularity of the ITC process, especially through cation engineering, to design advanced carbonaceous materials with unusual properties.
4. Conclusions
In summary, we demonstrate the possibility to control both textural and morphological properties of ionochars through cation engineering. We prepared a series of imidazolium tetrachloroferrate ILs bearing modular side groups, e.g., alkyl chains with different lengths and/or benzyl groups. We applied ITC to a lignocellulosic agrowaste (i.e., sugarcane bagasse) and commercial cellulose. We showed the reusability of two different 1-alkyl-3-methylimidazolium tetrachloroferrate ILs over three ITC cycles. When decreasing the alkyl chain length from octyl to ethyl in 1-alkyl-3-methylimidazolium tetrachloroferrate ILs, both BET-equivalent specific surface area (SSABET) and total pore volume gradually increased up to 800 m2 g−1 and 0.8 cm3 g−1, respectively. When increasing the alkyl chain length, lower SSABET and total pore volume were obtained, but singular tubular nanostructures with an external diameter of ca. 100 to 300 nm were observed. Besides, the formation of two-dimensional stacked nanoplatelets was observed for the ionochars prepared in imidazolium tetrachloroferrate ILs carrying one or two benzyl groups. While still open to discussion, the cation undoubtedly has a role in (ligno)cellulose dissolution, deconstruction and ionothermal carbonization. Finally, we show that cation engineering also offers the possibility to control both the CO2 uptake and retention of ionochars. Due to advantageous and adjustable properties offered by cation engineering, we expect that our study will open new opportunities for the ITC of (ligno)cellulose. We are currently studying potential applications in various fields, including catalysis, wastewater treatment and energy storage.Author contributions
S. A.: Investigation, writing – original draft. M. E.-S., S. K. and P. H.: Validation, writing – review & editing. N. B. and A. M.: Conceptualization, funding acquisition, supervision, validation, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
SA is grateful to Lebanese University for financial support. The authors are grateful to the Ministry for Europe and Foreign Affairs and the Ministry of Higher Education and Research (Government of the French Republic) for financial support through the Hubert Curien Partnership programme (PHC IMHOTEP 2019, no. 41859SB). Several characterizations were performed with the support of the “Balard Plateforme d'Analyses et de Caractérisation (PAC Balard)”. The authors would like to thank Emmanuel Fernandez for 13C solid-state NMR, Didier Cot for SEM analyses, Bertrand Rebière for SEM-EDX analyses, Franck Godiard (MEA platform, Université de Montpellier) for TEM analyses and Pascale Guiffrey for GC-MS analyses.References
- X.-F. Tan, Y.-G. Liu, Y.-L. Gu, Y. Xu, G.-M. Zeng, X.-J. Hu, S.-B. Liu, X. Wang, S.-M. Liu and J. Li, Bioresour. Technol., 2016, 212, 318–333 CrossRef CAS PubMed.
- D. A. Iryani, S. Kumagai, M. Nonaka, K. Sasaki and T. Hirajima, Waste Biomass Valorization, 2017, 8, 1941–1951 CrossRef CAS.
- S. Katyal, K. Thambimuthu and M. Valix, Renewable Eng., 2003, 28, 713–725 CrossRef CAS.
- A. K. Varma and P. Mondal, Ind. Crops Prod., 2017, 95, 704–717 CrossRef CAS.
- Y. Guo, C. Tan, J. Sun, W. Li, J. Zhang and C. Zhao, Chem. Eng. J., 2020, 381, 122736 CrossRef CAS.
- S. A. Nicolae, H. Au, P. Modugno, H. Luo, A. E. Szego, M. Qiao, L. Li, W. Yin, H. J. Heeres and N. Berge, Green Chem., 2020, 22, 4747–4800 RSC.
- G. Ischia and L. Fiori, Waste Biomass Valorization, 2021, 12, 2797–2824 CrossRef CAS.
- M.-M. Titirici and M. Antonietti, Chem. Soc. Rev., 2010, 39, 103–116 RSC.
- M. Sevilla, A. B. Fuertes and R. Mokaya, Energy Environ. Sci., 2011, 4, 1400–1410 RSC.
- T. P. Fellinger, R. J. White, M. M. Titirici and M. Antonietti, Adv. Funct. Mater., 2012, 22, 3254–3260 CrossRef CAS.
- M. Volpe, A. Messineo, M. Mäkelä, M. R. Barr, R. Volpe, C. Corrado and L. Fiori, Fuel Process. Technol., 2020, 206, 106456 CrossRef CAS.
- P. Zhang, Y. Gong, H. Li, Z. Chen and Y. Wang, Nat. Commun., 2013, 4, 1–11 CrossRef CAS.
- Z. G. Lei, B. H. Chen, Y. M. Koo and D. R. MacFarlane, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- H. Wang, G. Gurau and R. D. Rogers, Chem. Soc. Rev., 2012, 41, 1519–1537 RSC.
- C. Chiappe and D. Pieraccini, J. Phys. Org. Chem., 2005, 18, 275–297 CrossRef CAS.
- S. Aldroubi, N. Brun, I. B. Malham and A. Mehdi, Nanoscale, 2021, 13, 2750–2779 RSC.
- R. P. Swatloski, S. K. Spear, J. D. Holbrey and R. D. Rogers, J. Am. Chem. Soc., 2002, 124, 4974–4975 CrossRef CAS PubMed.
- A. Salama and P. Hesemann, ACS Sustainable Chem. Eng., 2020, 8, 17893–17907 CrossRef CAS.
- Y. Zhou, X. Zhang, D. Yin, J. Zhang, Q. Mi, H. Lu, D. Liang and J. Zhang, Green Chem., 2022, 24, 3824–3833 RSC.
- Z. Li, Z. Cai, Q. Zeng, T. Zhang, L. J. France, C. Song, Y. Zhang, H. He, L. Jiang and J. Long, Green Chem., 2018, 20, 3743–3752 RSC.
- M. J. Mehta, A. Kulshrestha, S. Sharma and A. Kumar, Green Chem., 2021, 23, 1240–1247 RSC.
- F. Ferrari, G. Nogueira, T. Franco, M. Dias, C. Cavaliero, G. J. Witkamp, L. Van Der Wielen and M. B. S. Forte, Green Chem., 2021, 23, 9126–9139 RSC.
- Y. Zhao, X. Liu, J. Wang and S. Zhang, ChemPhysChem, 2012, 13, 3126–3133 CrossRef CAS PubMed.
- R. C. Remsing, R. P. Swatloski, R. D. Rogers and G. Moyna, Chem. Commun., 2006, 1271–1273 RSC.
- Z.-L. Xie, R. J. White, J. Weber, A. Taubert and M. M. Titirici, J. Mater. Chem., 2011, 21, 7434–7442 RSC.
- X.-X. Lin, B. Tan, L. Peng, Z.-F. Wu and Z.-L. Xie, J. Mater. Chem. A, 2016, 4, 4497–4505 RSC.
- J. S. Lee, R. T. Mayes, H. Luo and S. Dai, Carbon, 2010, 48, 3364–3368 CrossRef CAS.
- M. Baccour, N. Louvain, J. G. Alauzun, L. Stievano, P. H. Mutin, B. Boury, L. Monconduit and N. Brun, J. Power Sources, 2020, 474, 228575 CrossRef CAS.
- Y. Liu, B. Huang, X. Lin and Z. Xie, J. Mater. Chem. A, 2017, 5, 13009–13018 RSC.
- L. Cibien, M. Parot, P. N. Fotsing, P. Gaveau, E. D. Woumfo, J. Vieillard, A. Napoli and N. Brun, Green Chem., 2020, 22, 5423–5436 RSC.
- P. Zhang, Y. Gong, Z. Wei, J. Wang, Z. Zhang, H. Li, S. Dai and Y. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 12515–12522 CrossRef CAS PubMed.
- S. Hayashi, S. Saha and H.-O. Hamaguchi, IEEE Trans. Magn., 2005, 42, 12–14 Search PubMed.
- J.-Y. Kim, J.-T. Kim, E.-A. Song, Y.-K. Min and H.-O. Hamaguchi, Macromolecules, 2008, 41, 2886–2889 CrossRef CAS.
- S. Hayashi and H.-O. Hamaguchi, Chem. Lett., 2004, 33, 1590–1591 CrossRef CAS.
- D. Yin, C. Li, L. Tao, N. Yu, S. Hu and D. Yin, J. Mol. Catal. A: Chem., 2006, 245, 260–265 CrossRef CAS.
- K. Bica and P. Gaertner, Org. Lett., 2006, 8, 733–735 CrossRef CAS PubMed.
- Y. Yoshida and G. Saito, J. Mater. Chem., 2006, 16, 1254–1262 RSC.
- W. E. Dunn, Jr., United States of America Pat, US3729543A, 1973 Search PubMed.
- T. Bäcker, O. Breunig, M. Valldor, K. Merz, V. Vasylyeva and A.-V. Mudring, Cryst. Growth Des., 2011, 11, 2564–2571 CrossRef.
- C. Falco, N. Baccile and M.-M. Titirici, Green Chem., 2011, 13, 3273–3281 RSC.
- R. C. Remsing, G. Hernandez, R. P. Swatloski, W. W. Massefski, R. D. Rogers and G. Moyna, J. Phys. Chem. B, 2008, 112, 11071–11078 CrossRef CAS PubMed.
- J. S. Moulthrop, R. P. Swatloski, G. Moyna and R. D. Rogers, Chem. Commun., 2005, 1557–1559 RSC.
- J. Zhang, H. Zhang, J. Wu, J. Zhang, J. He and J. Xiang, Phys. Chem. Chem. Phys., 2010, 12, 1941–1947 RSC.
- T. Youngs, C. Hardacre and J. Holbrey, J. Phys. Chem. B, 2007, 111, 13765–13774 CrossRef CAS PubMed.
- M. J. Earle, J. M. Esperança, M. A. Gilea, J. N. Canongia Lopes, L. P. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS PubMed.
- R. C. Remsing, I. D. Petrik, Z. Liu and G. Moyna, Phys. Chem. Chem. Phys., 2010, 12, 14827–14828 RSC.
- H. M. Cho, A. S. Gross and J.-W. Chu, J. Am. Chem. Soc., 2011, 133, 14033–14041 CrossRef CAS PubMed.
- J. Wang, H. Wang, S. Zhang, H. Zhang and Y. Zhao, J. Phys. Chem. B, 2007, 111, 6181–6188 CrossRef CAS PubMed.
- M. Blesic, M. H. Marques, N. V. Plechkova, K. R. Seddon, L. P. N. Rebelo and A. Lopes, Green Chem., 2007, 9, 481–490 RSC.
- Y. Fukaya, K. Hayashi, M. Wada and H. Ohno, Green Chem., 2008, 10, 44–46 RSC.
- A. Xu, J. Wang and H. Wang, Green Chem., 2010, 12, 268–275 RSC.
- T. Y. Kim, H. W. Lee, M. Stoller, D. R. Dreyer, C. W. Bielawski, R. S. Ruoff and K. S. Suh, ACS Nano, 2011, 5, 436–442 CrossRef CAS PubMed.
- T. Zhang, Y. Zhang, Y. Wang, F. Huo, Z. Li, Q. Zeng, H. He and X. Li, Front. Chem., 2019, 446 CrossRef PubMed.
- F. S. Chakar and A. J. Ragauskas, Ind. Crops Prod., 2004, 20, 131–141 CrossRef CAS.
- Z.-L. Xie, X. Huang, M.-M. Titirici and A. Taubert, RSC Adv., 2014, 4, 37423–37430 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3gc00300k |
| This journal is © The Royal Society of Chemistry 2023 |