Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Fully biomass-derived vitrimeric material with water-mediated recyclability and monomer recovery
Zhuang Mao
Png
 *ab,
Jie
Zheng
*ab,
Jie
Zheng
 ab,
Sirin
Kamarulzaman
ab,
Sirin
Kamarulzaman
 a,
Sheng
Wang
a,
Sheng
Wang
 ab,
Zibiao
Li
ab,
Zibiao
Li
 *abc and
Shermin S.
Goh
*abc and
Shermin S.
Goh
 *a
*a
aInstitute of Materials Research and Engineering (IMRE), A*STAR (Agency for Science, Technology and Research), 2 Fusionopolis Way, Innovis #08-03, 138634, Singapore. E-mail: lizb@imre.a-star.edu.sg; gohsms@imre.a-star.edu.sg
bInstitute of Sustainability for Chemicals, Energy and Environment (ISCE2), A*STAR, 2 Fusionopolis Way, Innovis #08-03, Singapore. E-mail: png_zhuang_mao@isce2.a-star.edu.sg
cDepartment of Materials Science and Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore
First published on 22nd December 2022
Abstract
Correction for ‘Fully biomass-derived vitrimeric material with water-mediated recyclability and monomer recovery’ by Zhuang Mao Png et al., Green Chem., 2022, 24, 5978–5986, https://doi.org/10.1039/D2GC01556K.
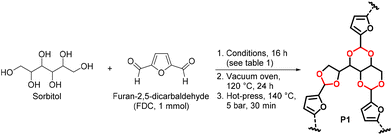
The authors regret that there was a mistake present in Table 2 and the caption of Table 2 in which the concentrations of furan-2,5-dicarbaldehyde and sorbitol were incorrectly displayed the wrong way around. There were also errors in the main text when discussing Table 2 on page 5981.
The corrected Table 2 (and caption) and revised text are shown below:
| No. | DMSO | T/°C | TsOHa/mol% | Sorbitol eq. | Pre-curing observation | Tensile strength post hot-pressd |
|---|---|---|---|---|---|---|
| Reaction conditions: (1) furan-2,5-dicarbaldehyde (FDC) (1 mmol) and indicated amount of sorbitol and catalyst were dissolved in DMSO (0.5 mL, where indicated) and stirred for 16 h under a constant stream of N2; (2) vacuum oven, 24 h, 120 °C; (3) hot-press, 140 °C, 5 bar, 30 min.a TsOH was added as catalyst.b In a sealed tube instead of under N2 stream.c N.R. = No reaction.d Only the gel-like polymers in entries 7, 9 and 10 were hot-pressed.e N.T. = cannot be tested as samples easily broke upon handling. | ||||||
| 1 | N | 120 | 0 | 0.8 | N.R.c | — |
| 2 | N | 130 | 0 | 0.8 | Oligomers | — |
| 3 | N | 140 | 0 | 0.8 | Oligomers | — |
| 4 | Y | 130 | 0 | 0.8 | Oligomers | — |
| 5b | Y | 130 | 0 | 0.8 | N.R.c | — |
| 6 | Y | 130 | 0.5 | 0.8 | Gel-like polymer | — |
| 7 | Y | 100 | 0.5 | 0.8 | Gel-like polymer | N.T.e |
| 8 | N | 100 | 0.5 | 0.8 | Decomposed | — |
| 9 | Y | 100 | 0.5 | 1.0 | Gel-like polymer | N.T.e |
| 10 | Y | 100 | 0.5 | 0.6 | Gel-like polymer | 13 MPa |
Revised discussion on page 5981:
We thus proceeded with the reaction conducted in DMSO, with 0.5 mol% of TsOH, under stream of nitrogen. The reaction was scalable to 50 mmol (∼10 g) through careful adjustment of the stream of nitrogen to ensure that most of the DMSO is removed at the end of the reaction. The gel-like solid was then fully dried in a vacuum oven at 120 °C for 24 hours to obtain a hard brown solid. The obtained polymer could then be directly hot-pressed at 140 °C, then cut into strips, or blended into fine powder before being hot-pressed in a mold. However, after hot-pressing, the polymer was found to be brittle and easily broke upon handling. Thus, the ratio of sorbitol and furan-2,5-dicarbaldehyde was adjusted. Increasing the amount of sorbitol to 1 equivalent (eq.) still resulted in a very brittle material (entry 9). However, when the amount of sorbitol was decreased to 0.6 eq., the polymer (P1) was observed to be significantly less brittle (entry 10). Polymer P1 was found to be hard and rigid at room temperature, but flexible and malleable at elevated temperatures. This was attributed to the high density of rings (furans and acetals) in the network, which has been shown to improve mechanical strength and rigidity due to inhibition of rotation of the polymer chain.57,58
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2023 |