Open Access Article
Open Access ArticleProduction of propane and propene via carbon capture utilisation: comparison of its environmental and economic performance against conventional production methods†
Alexander
Payne
,
Guillermo
Garcia-Garcia
 and
Peter
Styring
and
Peter
Styring
 *
*
UK Centre for CO2 Utilization, Department of Chemical and Biological Engineering, The University of Sheffield, Sir Robert Hadfield Building, Sheffield, S1 3JD UK. E-mail: p.styring@sheffield.ac.uk
First published on 3rd May 2023
Abstract
With an unabated global petrochemical growth, more sustainable production methods for production of materials and fuels are essential in a decarbonised future. Although Carbon Capture Utilisation (CCU) is generally considered a sustainable production route, it is imperative to compare its environmental and economic performance with that of current methods. This article reviews the environmental impact and economics surrounding conventional production of propane and propene via natural gas liquid fractionation and crude oil refining for propane. In addition, fluid catalytic cracking and steam cracking were explored for propene production. A CCU process has been modelled using Aspen Plus and analysed through Life-Cycle Assessment and Techno-Economic Analysis. Processes simulated include carbon capture using piperazine, dry methane reforming, direct syngas to propane and methanol to propene. The results obtained show a significant reduction in environmental impacts across multiple impact categories for both products when compared to conventional production. In addition, the price of propene from CCU was competitive with conventional. However, the price of propane was significantly higher. Sensitivity analysis of hydrogen production technology and electricity grid emission intensity identified them both as key determinants of economic and environmental performance.
1 Introduction
The burning of fossil fuels and industrialisation that have facilitated economic and population growth has been increasing the concentration of anthropogenic greenhouse gases such as carbon dioxide, methane, and nitrous oxides in the atmosphere.1 Total global fossil CO2 emissions were estimated to be around 36.6 billion tonnes 2022.2 CO2 levels have been increasing rapidly and show no signs of slowing. Before the industrial revolution, our atmosphere contained 280 ppm of carbon dioxide, however, since 2015 this figure has stood at over 400 ppm,3 and is continuously increasing.Decarbonisation pathways are ways in which emissions reductions can be achieved through phasing in/out of technologies, introducing new laws around emission criteria or implementing carbon taxes that incentivise sectors to reduce emissions from their direct operations and entire supply chain. Collectively, these mitigation pathways aim to limit warming to below 2 °C relative to pre-industrial levels.4 However, without additional mitigation steps beyond those present today, there is a high risk of severe, widespread, and irreversible impacts globally.5
Carbon Capture Utilisation and Storage (CCUS) consists of capturing CO2 and either storing it or using it as a raw material. As such, CCUS forms part of several decarbonisation strategies worldwide. For example, in the UK, the “Zero Carbon Humber” project aims to capture CO2 from the UK's most carbon intensive industrial cluster.6 However, Carbon Capture Storage (CCS) has several challenges such as the slow pace of assessing and exploiting storage resources, large economic costs, lack of consistent legislation, and low public awareness.7 Alternatively, the captured CO2, a waste product, can be converted into several value-added products via Carbon Capture Utilisation (CCU). Examples include propane and propene. Conventionally, propane is produced from petroleum refining or natural gas processing8 and propene from steam cracking (SC) and refinery operations. A detailed analysis of the conventional methods to produce propane and propene can be found in sections 1–2 of the ESI.† CCU can provide an alternative production pathway that both reduces greenhouse gas emissions and fossil resource depletion by producing chemicals not from fossil fuels, but from captured CO2. The state of the art of CCU, particularly regarding its use to produce propane and propene, can be found in section 3 of the ESI.†
The aim of this article is to look at the viability of CCU production methods of propane and propene by assessing them economically and environmentally. Both products are firstly explored in terms of their environmental and economic impact of their conventional production pathways. Next, a CCU alternative process is designed and modelled using Aspen Plus. Life-Cycle Assessment (LCA) is then applied to evaluate the environmental performance of such CCU production method. Techno-Economic Analysis (TEA) is finally used to combine process modelling with economic evaluation to provide a thorough understanding of the economic cost of the CCU process proposed.
2 Environmental impacts of the conventional production methods
This section analyses the environmental impact caused by conventional methods to produce propane and propene presented in sections 1–2 of the ESI.† There is a lack of studies that report on environmental impacts caused by the novel CCU methods discussed in section 3 of the ESI.†2.1 Propane
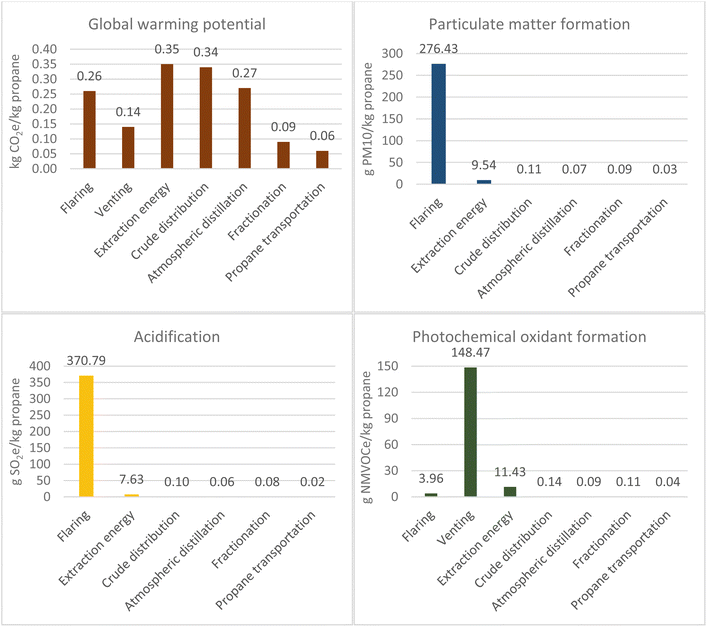
There are two conventional routes for propane production: from natural gas extraction and from refining crude oil (section 1 of the ESI†). Conventional crude oil is extracted from underground reservoirs using traditional drilling and pumping methods.9 Natural gas can also be present in the reservoir and be extracted. This results in the increased risk of explosions and build-up of pressure on the platform, so venting and flaring allow for a safer operation.10In 2018, the United States was the largest natural gas producer in the world, followed by Russia.11 Conventional natural gas is stored in a naturally porous reservoir with impermeable rock strata.12 However, shale gas is unconventional as the shale rock is not naturally porous, so requires hydraulic fracking to allow the gas to migrate from pockets within the rock formation. Fracking uses a mixture of water, sand and proprietary chemicals that is pumped underground at high pressures to create a fracture network. The supply of natural gas is predominantly via fracking and in 2019 it accounted for 87% of total U.S. production.13
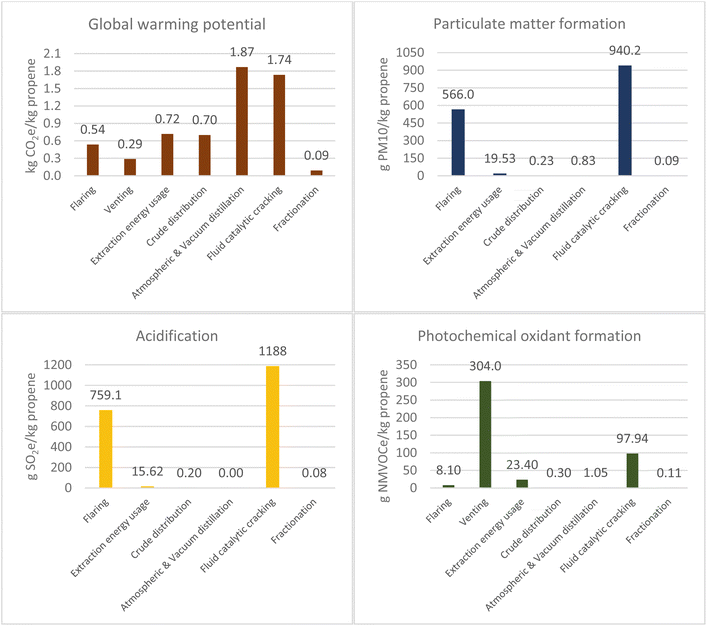
To calculate the environmental impacts reported in this section, emission intensities were found for each process and aggregated into each of the impact categories. Calculation and sources for this section are in the sections 4–16 of the ESI.† Results are shown in Fig. 1 and 2.
The natural gas route has a significantly higher global warming potential (GWP) for extraction compared to crude oil, as seen in Fig. 1 and 2. The completion of a well by fracking requires the “flowback” of drilling and reservoir fluids to open pits, which results in significant venting of natural gas, where the length of the period depends on the permeability of the reservoir.14 Furthermore, the figure for natural gas extraction is likely to be underestimated as a further review of three sources15–17 (section 12 of the ESI†) found that for shale gas the extraction emission intensity was 14.2 g CO2e per MJ natural gas. The extraction emission intensity used is 5.6 kg CO2e per kg propane (Fig. 2), but using the new value it would be 12.8 kg CO2e per kg propane. Nevertheless, it must be noted that diverse sources estimate different GWP for such processes, mostly due to different feedstock, technological and geographical considerations. For example, the US GREET model reported a carbon intensity 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 075 g per mmbtu (0.5 kg CO2e per kg propane)18 for propane production, while the Canadian Propane Association19 estimated a carbon intensity of 74 g CO2e per MJ (3.6 kg CO2e per kg propane), including combustion.
075 g per mmbtu (0.5 kg CO2e per kg propane)18 for propane production, while the Canadian Propane Association19 estimated a carbon intensity of 74 g CO2e per MJ (3.6 kg CO2e per kg propane), including combustion.
Particulate matter formation for the crude route is significantly worse due to flaring of natural gas. In oil exploration, natural gas is less valuable, and offtake requires transportation infrastructure to deliver it to consumers which is both challenging logistically and costlier than the value of the gas.20 Therefore, fracking for natural gas exploration contributes more to environmental impacts from venting during flowback and fugitive emissions than flaring of the gas. Hence, their reduced particulate contribution.
The flowback of fracking fluids contains excess salts, high levels of trace elements and radioactive materials that can pollute groundwater.13 However, the flowback of fracking fluids is mostly recycled to frack additional wells and the remainder trucked to wastewater treatment facilities and deep injection wells. Therefore, while surface water pollution is a serious problem, most U.S. regions have significant available capacity of deep injection wells for liquid waste disposal.21
In addition, one impact associated with natural gas extraction not quantitatively considered here are the small-to-moderate magnitude seismic activity linked to hydraulic fracturing of wells and in some cases microearthquakes.13
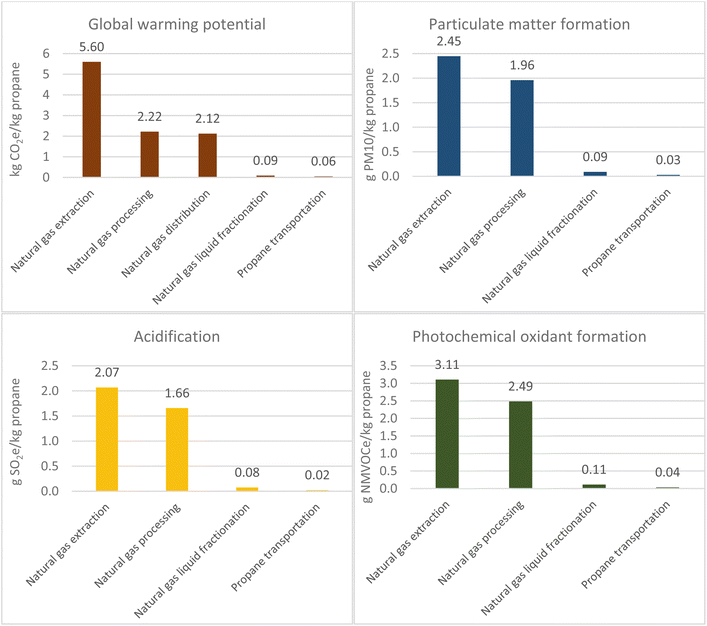
Specific processing steps for raw natural gas include amine gas treating and dehydration. Both these processes combined totalled 0.27 g CO2 per MJ natural gas and 0.028 g CH4 per MJ natural gas. However, as data could not be found for all processes, the average for processing overall from six sources in the U.S. was used. Further environmental impacts of processing include the evaporative losses and venting of degraded products of the amine solution such as nitrosamines and nitramines which are possible carcinogens and would contribute to human toxicity impacts.22 Surveys of gas processing plants using amine solvent report average losses of 0.2 g amine per Nm3 natural gas processed.23 Similarly, in dehydration, hazardous pollutants such as benzene, toluene, ethylbenzene and xylenes (BTEX) that have an affinity for the glycol solution are vented in the regeneration step in the stripper.24 Methane also has an affinity for the amine solution and approximately 0.971 g of methane per kg of natural gas treated is vented to atmosphere from the stripper.25
Natural gas distribution has a higher GWP (2.12 kg CO2e per kg propane) than for crude oil (0.34 kg CO2e per kg propane). This is due to fugitive emissions, particularly methane from sources such as compressor stations and valves.
Atmospheric distillation contributed mostly to GWP with a value of 0.27 kg CO2e per kg propane. In a petroleum refinery, propane is contained within light gases or gaseous refinery streams from atmospheric distillation. Two specific petroleum products containing propane are LPG and fuel gas. The energy use for each refinery product has been allocated based on energy content in certain sources which varies minimally from a mass-based allocation.26 Energy is consumed in the form of electricity, heat and steam where natural gas and refinery fuel gas are used to meet heating and steam demand.27 In 2012, 37% of processing energy at U.S. refineries was refinery fuel gas and 25% was natural gas.28
Life-cycle data for specific technologies, such as the cryogenic expansion process to recover NGLs from natural gas, are limited, so the fractionation figure was based on one U.S. source.25 However, the total greenhouse gases reported in the U.S. for natural gas processing in 2019 was 57.5 Mt CO2e.17 When combined with the total dry natural gas and NGL production (Table 1), the emission intensity can be approximated on an energy basis to 0.076 g CO2e per MJ. The value from this approximation of 0.068 kg CO2 per kg propane is minimally different to the source used:25 0.064 kg CO2 per kg.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29
29
| Energy carrier | Volume | Energy content | Allocation (%) | ||
|---|---|---|---|---|---|
| Value | Unit | Value | Unit | ||
| Dry natural gas | 962![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 773 773 |
Million m3 | 36.62554 | MJ m−3 | 83.58 |
| Ethane | 667![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 609 609 |
Thousand barrels | 3.249572 | GJ per barrel | 5.14 |
| Propane | 579![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 878 878 |
Thousand barrels | 4.051414 | GJ per barrel | 5.57 |
| Normal butane | 157![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 628 628 |
Thousand barrels | 4.568392 | GJ per barrel | 1.71 |
| Isobutane | 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 579 579 |
Thousand barrels | 4.568392 | GJ per barrel | 1.65 |
| Pentanes plus | 203![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 251 251 |
Thousand barrels | 4.874358 | GJ per barrel | 2.35 |
Water usage for the natural gas route totalled 14.474 kg per kg propane, where extraction contributed to 83% of this figure. The values for extraction were based on two studies of U.S. and Canadian shale (section 11 of the ESI†), while for processing this was based on a Chinese source (section 13 of the ESI†). As processing requires the gas to meet a standardised pipeline specification this value should be independent of location unlike extraction. Clark et al.30 found that shale gas consumes 13 to 37 l per GJ over its life cycle, or between 12 and 33 kg per kg propane. For the crude oil route, the total water consumption amounted to 2.75 kg per kg propane, sourced from a global weighted average for extraction and the average across three U.S. refinery configurations (cracking, light and heavy cracking). Atmospheric distillation consumed 0.975 kg water per kg propane. The major contributors to water use are for cooling due to evaporative losses in cooling towers and boiler feed water for steam generation.31 However, a study of crude oil production from five North American locations by Ali and Kumar32 found that a barrel of conventional oil cycle consumes 1.71 to 8.25 barrels of fresh water (33.4–157.41 kg per kg propane) over its life and 2.4 to 9.51 barrels of fresh water are withdrawn (46.11–181.26 kg per kg propane). Water usage is a significant problem as in the U.S. the newest oil and gas developments are in drought-affected and arid regions such as the Colorado River Basin.33 Furthermore, 72% of all water used in the U.S. comes from fresh surface water sources such as rivers and lakes and 10% comes from ground water (aquifers).31
Both routes use water for preparation of the drilling fluid, which has the function of cooling the drill bit, removing drilled rocks, and providing hydrostatic pressure to prevent well collapse.34 Water consumption for oil production is used mainly for enhanced oil recovery. Typically, drilling waste and produced water is discharged to sea for offshore oil exploration if it meets environmental requirements.35 This is devastating in terms of marine ecotoxicity, as the average oil concentration of discharged produced water is 3.9 mg l−1,36 resulting in 67.4 mg kg−1 of propane. The presence of aromatic hydrocarbons, alkylphenols, heavy metals and naturally occurring radioactive material causes the most environmental concern.37
Human toxicity of the crude route totalled 3.77 × 10−8 kg 1,4-dichlorobenzene equivalents (1,4-DCBe) per of kg propane. This is due to mercury presence in the raw natural gas that is emitted during venting and flaring. Fossil resource depletion for the crude route was 717.4 kg antimony equivalents (Sbe) per kg of propane, and 588.189 kg Sbe per kg propane for natural gas.
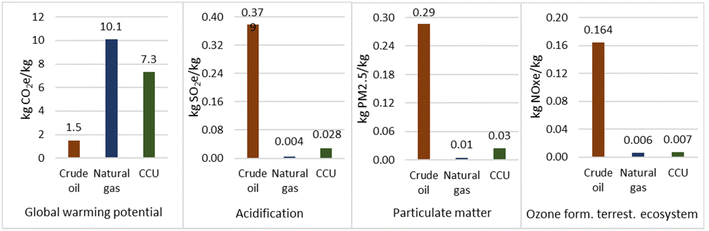
Ozone formation and acidification potential was significantly higher for the crude oil route totalling 164.24 g non-methane volatile organic compounds equivalents (NMVOCe) per kg and 378.68 g SO2e per kg propane respectively. The natural gas route resulted in values of 5.75 g NMVOCe per kg propane and 3.83 g SO2e per kg propane respectively. For both routes, extraction was the highest contributor.
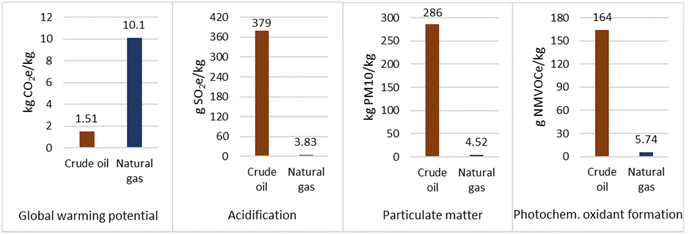
Overall, as seen in Fig. 3, production of propane from crude oil refining generated 1.51 kg CO2e per kg propane, whereas for natural gas this was 10.1 kg CO2e per kg propane. Therefore, based on GWP the natural gas route is the most environmentally damaging. However, for all other impact categories the crude oil was the most damaging. Therefore, propane production from CCU represents a good opportunity to prevent the significant environmental impacts associated with natural gas and crude oil extraction, processing, and distribution.
2.2 Propene
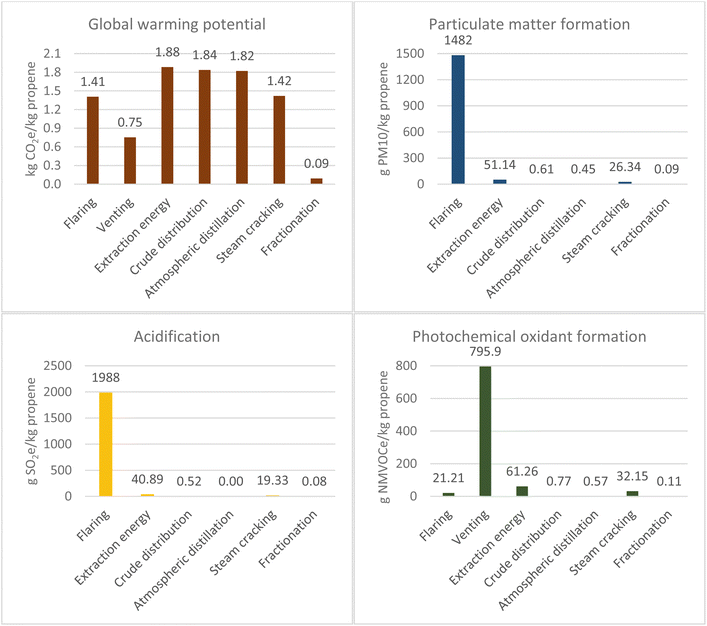
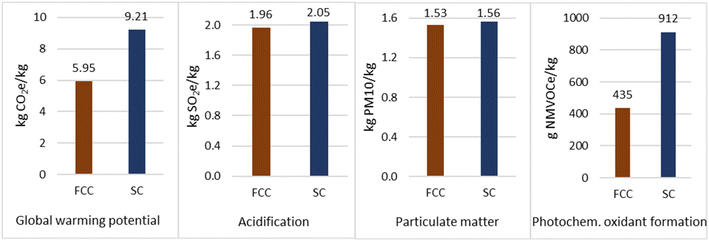
The production of propene via SC and via Fluid Catalytic Cracking (FCC) uses crude oil as the primary feedstock (section 2 of the ESI†). However, the feedstock for the steam cracker is predominantly naphtha, whereas for FCC this is vacuum gas oil and atmospheric residue. The yield of naphtha from crude oil after atmospheric distillation is 8 wt%,38 however, for gas oil and residue this totals 56 wt%.39 Therefore, significantly more crude oil is required to produce propene from SC. This is demonstrated by the resource depletion values: SC shows a value of 3846.2 kg Sbe per kg propene, while for FCC it is 1468.8 kg Sbe per kg propene. Therefore, the GWP for extraction is higher for the SC route (4.04 kg CO2e per kg propene vs. 1.54 kg CO2e per kg propene respectively) due to higher flaring and venting of natural gas. This is also evident in the higher values for acidification and particulate matter formation due to the contribution of flaring. Venting via SC also results in a higher value for photochemical oxidant depletion. Similarly, the impact associated to crude distribution is higher across all emission-related impact categories as more crude oil is required to produce the same quantity of propene.To prevent thermal cracking of the heavy fractions from atmospheric distillation,40 a further step of vacuum distillation is required to generate the feedstock for FCC. Therefore, the additional processing and associated energy use has contributed to a higher value across all impact categories.
To calculate the environmental impacts reported in this section, emission intensities were found for each process and aggregated into each of the impact categories. Calculation and sources for this section are in sections 17–21 of the ESI.† Results are shown in Fig. 4 and 5.
The FCC unit is the single biggest source of atmospheric pollution in an oil refinery due to sulphur oxides and particulates41 and of carbon dioxide emissions, accounting for approximately 30% of the total emitted from a refinery.42 Emissions from FCC include the combustion products from process heaters and the catalyst regenerator. FCC units are considered “self-contained” in terms of their energy sourcing and Jia et al.43 found that 82.9% of energy required for the process in China was from petroleum coke combustion. Contaminants present in the feedstock, which include metals such as nickel, vanadium and copper but also heteroatoms such as sulphur and nitrogen contaminants, end up in the coke, which is burned in the regenerator. The results show significantly higher contributions to acidification (1188.3 and 19.33 g SO2e per kg propene respectively) and particulate matter formation (940.2 and 26.3 g PM 10 per kg propene respectively) for the FCC process than for the SC process, where the greatest contributor was sulphur oxide.
The yield of propene from FCC is heavily dependent on the catalyst used, reactor configuration and operating conditions such as catalyst to oil ratio, residence time and reaction temperature.44 The results presented here were calculated using a base case yield across three studies that found that propene yield was 5.35 wt%. However, higher yields in excess of 20 wt% can be achieved when maximising propene production is the objective function of the refinery.44
Another contributor to particulate emissions and resource depletion often overlooked is the use of zeolite catalysts in FCC, which must be replaced constantly due to catalyst attrition and irreversible contamination. Cyclones and electrostatic precipitators are used to separate catalyst particles. However, a fraction becomes entrained in the exhaust gas.
Specific data for fractionation of the products of FCC and SC could not be found. However, the fractionation process is similar to NGL fractionation, so these data was used for analysis.
Water usage for the SC route amounted to 10 kg per kg propene and for the FCC route 4.1 kg per kg propene. Similar results were obtained by Yang and You,45 who found propene production from SC of naphtha in a mass-based allocation amounted to 11.25 kg per kg propene.
Although human toxicity for both routes were mostly caused by flaring and venting in crude oil extraction, the FCC units also emitted lead and arsenic. Overall, the value was 6.42 × 10−5 kg 1,4-DCBe per kg propene for the FCC route and 2.02 × 10−7 kg 1,4-DCBe per kg propene for SC.
Overall, the total GWP was higher for the SC route and totalled 9.21 kg CO2e per kg propene, whereas for FCC the total was 5.95 kg CO2e per kg propene. As seen in Fig. 6, there is less disparity in values of impact categories when compared to propane production. However, SC across all categories was more environmentally detrimental. Furthermore, the feed for FCC is abundant in a refinery compared to naphtha, so FCC can be considered more cost effective and less environmentally impactful.46 However, the FCC unit still represents a significant opportunity as a point source for carbon capture in CCU routes and would vastly reduce the environmental impact of a refinery.
3 Economic costs of the production methods
This section analyses the economic costs of conventional (sections 1–2 of the ESI†) and novel methods (section 3 of the ESI†) to produce propane and propene.3.1 Propene via steam cracking
Yang and You45 carried out an economic analysis of propene manufacturing by SC of naphtha. The plant was modelled in Aspen to produce 51.3 t h−1 of propene. The system boundary starts with naphtha as an incoming feedstock and therefore ignores crude oil extraction and processing. The total production cost was $1489.1 M and total capital cost was $1042.8 M, equating to $471 per t allocated by mass for the total product yield and 90% uptime.Xiang et al.47 carried out a TEA of a 1.5 Mt per a crude oil to olefins plant in China. The technology used was naphtha SC and the product cost found was $1480 per t (9340 RMB per t). The study found that 88% of the product cost was from raw materials. The assumption used was based on 2012 figures of $110 per bbl crude oil.
3.2 Propene via methanol to olefins (MTO)/methanol to propene (MTP)
Zhang et al.48 performed a TEA of a gas-to-liquids plant simulated in Aspen that used DRM and SMR to form syngas followed by the Fischer–Tropsch (FT) process. The total product cost was $708 per t ($90 per bbl approximately using 800 kg m−3 FT product49), which used the average natural gas price in the U.S. in 2013. The plant scale was 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 915 barrels per day and the capital cost of the most relevant units were $109.27M for the FT reactor and $56.76 M for the reformer.
915 barrels per day and the capital cost of the most relevant units were $109.27M for the FT reactor and $56.76 M for the reformer.
Zhao et al.50 carried out an economic analysis of twenty light olefin pathways. The benchmark was SC of naphtha which had a production cost of $949 per t olefins where the price of naphtha was $868 per t. One pathway utilised natural gas to form methanol followed by MTO, which had a production cost of $769 per t olefins, whereas the MTP pathway had a production cost of $850 per t olefins. Furthermore, the use of natural gas to form syngas and subsequent conversion into olefins by FT had a production cost of $2356 per t olefins due to the low selectivity for light olefins. The price of natural gas used was $405 per t, and for all cases, raw material cost dominated the production cost. However, in this study all processes excluding SC used air separation to produce syngas rather than CO2 utilisation.
Chen et al.51 found that when simulating a CO2-rich natural gas from China in a dry reforming process propene cost was $1029 per t and propane cost was $536 per t.
3.3 Propane via FT
Jaramillo et al.52 found a levelized cost for a natural gas to liquid plant (including FT process) of $149 per t and $189 per t ($19 per bbl and $24 per bbl approximated using 800 kg m−3 FT product49) when incorporated with CCS. The propane yield for the plant was 2.82% on an energy basis with the remainder gasoline and diesel.Ghorbani et al.53 modelled a natural gas to liquid plant (excluding CCU) and found that the cost of production for liquid fuels from FT synthesis was $89 per t ($71.39 per m3 approximated using 800 kg m−3 FT product49).
3.4 Propane from natural gas (NG)
Getu et al.54 studied natural gas liquids (NGL) recovery when eight different NG feeds and recovery processes were used, including a turbo expander process and variations of fractionation. The operating cost varied between $0.04 per t – $0.26 per t NGL produced ($0.002–0.012 kg mol−1, average 47 g mol−1 (ref. 55)).Park et al.56 performed a TEA on nine different configurations of offshore NGL recovery processes. The natural gas flowrate modelled for all configurations was 472.44 t h−1 at atmospheric temperature. The operating cost varied between $7.5 M and $13.5 M ($2–3.6 per t natural gas feed at 90% uptime), while the capital cost ranged from $0.8 M to $9.7 M. The study found that compressor duty dominated the operating cost. Similarly, AlNouss et al.57 modelled 6 different NGL recovery processes for an 84 t h−1 plant. The operating cost varied between $12 M and $18 M ($18–27 per t natural gas feed at 90% uptime), while the capital cost ranged from $16 M to $25 M.
Economic analysis of crude oil refining to produce propane or FCC to produce propene are limited. However, a study found that 86% of production costs in crude oil refining depend on raw material cost.58 Therefore, data would not provide extensive insight due to price volatility.
4 Proposition of a novel CCU process to produce propane and propene
We propose the production of propane and propene from syngas and the capture of CO2 from a fossil-fuel based stationary source. This production process has been simulated in Aspen Plus. SimaPro software was used to perform an environmental LCA. Furthermore, a TEA was performed to include raw material use, utility consumption and the sizing of equipment. Subsequent sensitivity analysis has been performed on the effects of factors such as carbon price, feedstock price and profitability optimisation.Modelling described within this section has used U.S. sources where applicable to allow comparison to conventional production methods in previous sections. In Aspen Plus, the property methods chosen were ELEC-NRTL for carbon capture and Peng-Robinson for the propane and propene production route as seen in literature.59 Within the Aspen simulation, heat and energy integration was carried out to achieve a more efficient energy network.
4.1 Process simulation
The following sections cover the operating conditions, modelling approach and assumptions used in creating the model. Full process flow diagrams are in section 23 of the ESI.†4.1.1.1 Absorber. An absorption column (ABSORBER) was used to simulate the contact of 32.5 wt% (to minimise corrosion) PZ absorbent (PZ-IN) in a counter-current flow with a flue gas stream composing 7.6 wt% CO2. Therefore, the gas outlet stream (GASOUT) had a lower mass fraction of CO2. The flue gas flowrate was 250 kg s−1 with temperature and pressure 313.15 K and 101
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 Pa, respectively. Chemical absorption is best achieved at low temperature and low CO2 partial pressure, unlike physical absorption that uses high pressure due to Henry's Law. The absorber used a rate-based modelling approach where rate-controlled reactions dictated the mass transfer from the gas phase to the liquid phase (section 24 of the ESI†).
325 Pa, respectively. Chemical absorption is best achieved at low temperature and low CO2 partial pressure, unlike physical absorption that uses high pressure due to Henry's Law. The absorber used a rate-based modelling approach where rate-controlled reactions dictated the mass transfer from the gas phase to the liquid phase (section 24 of the ESI†).
4.1.1.2 Stripper. The outlet stream from the absorber (RICH-PZ) containing dissolved CO2 was increased in pressure and temperature to 388.15 K and 170
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa utilising units (RCHPUMP, HEX1, HEX2) to further facilitate conditions required for phase transfer of the solute. The resultant stream (RICH-IN) entered the stripping column which regenerated the solvent by removing the absorbed solute through overcoming the regeneration energy as chemical absorption forms a reversible compound. The heat of desorption is the energy required to breakdown the carbamates, bicarbonates and carbonates formed between the solvent and solute.60 As such, the reboiler duty required by the stripper was 49.51 MW and a molar reflux ratio of 1.3712.61 This significant energy penalty of the process can contribute 70–80% of operating cost for a carbon capture plant, hence the choice of PZ over MEA.60 Both the stripper and absorber were set up as packed columns to enhance mass transfer, and were modelled with Radfrac blocks.
000 Pa utilising units (RCHPUMP, HEX1, HEX2) to further facilitate conditions required for phase transfer of the solute. The resultant stream (RICH-IN) entered the stripping column which regenerated the solvent by removing the absorbed solute through overcoming the regeneration energy as chemical absorption forms a reversible compound. The heat of desorption is the energy required to breakdown the carbamates, bicarbonates and carbonates formed between the solvent and solute.60 As such, the reboiler duty required by the stripper was 49.51 MW and a molar reflux ratio of 1.3712.61 This significant energy penalty of the process can contribute 70–80% of operating cost for a carbon capture plant, hence the choice of PZ over MEA.60 Both the stripper and absorber were set up as packed columns to enhance mass transfer, and were modelled with Radfrac blocks.
The regenerated solvent stream (LEANOUT) formed a closed loop as it was recycled back to the absorber. Heat integration was used to recover heat energy of the stream, aiding both process economics and environmental performance. In the absorber, some PZ was lost due to entrainment in the GASOUT stream (>0.2 wt%), thus the addition of a makeup stream (PZ-MK). Similarly, evaporative losses of water were present in both the stripper and absorber, hence an additional makeup stream (H2O-PZ).
4.1.1.3 Separation. The gaseous outlet of the stripper (CO2OUT) contained 27.5 wt% water which was removed by condensation in a flash separator (WATERSEP). The resultant gaseous stream (CO22) was >99.5 wt% CO2 and formed the feedstock for subsequent syngas production.
4.1.2.1 Dry methane reformer. A mixer (FEEDMIX) is used to combine a pure methane (CH4FEED) and CO2 stream (CO2FEED) along with recycled syngas from downstream processes (RECYCH4, RECYH21, RECYH22). The combined stream (FEED1) passes through an expansion valve (PRSRED1) and a series of heat exchangers utilising recovered waste heat from the product stream (SYNGAS) of the reactor (DRM). The optimal operating temperature and pressure are 950 °C and 101
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 325 Pa respectively. The reactor was modelled as RGibbs and achieved a conversion of 99% CH4. The highly endothermic reaction is catalysed with a nickel-based catalyst, which are preferred over noble metal alternatives due to cost, despite their lower susceptibility to deactivation from carbon deposits.63 A DRM reactor would usually produce a molar H2
325 Pa respectively. The reactor was modelled as RGibbs and achieved a conversion of 99% CH4. The highly endothermic reaction is catalysed with a nickel-based catalyst, which are preferred over noble metal alternatives due to cost, despite their lower susceptibility to deactivation from carbon deposits.63 A DRM reactor would usually produce a molar H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio of 1 due to reaction stoichiometry (eqn (1)), however, due to recycled streams the ratio in SYNGAS is approximately 1.4. The syngas stream is split equally (SYNSPLIT) to be used for propane and propene production, respectively.
CO ratio of 1 due to reaction stoichiometry (eqn (1)), however, due to recycled streams the ratio in SYNGAS is approximately 1.4. The syngas stream is split equally (SYNSPLIT) to be used for propane and propene production, respectively.| CH4 + CO2 → 2CO + 2H2 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO required by the methanol synthesis and propane reactors are higher than the outlet of the DRM, thus additional hydrogen is required. For this model, the source of hydrogen is from electrolysis, i.e. the generation of hydrogen and oxygen formed from the dissociation of water in the presence of a direct electric current. Electrolysis can achieve high-purity hydrogen exceeding 99.99 vol% once the outlet stream has been dried and oxygen impurities removed. Furthermore, Ursúa et al.64 found that the electric energy consumption is significantly lower at temperatures close to 1000 °C. The specific technology chosen is alkaline bipolar technology, which is most common commercially. Specific energy consumption of the entire process (not electrolysis exclusively) varied between 5 to 7 kW h Nm−3.64 The value chosen was 6 kW h Nm−3 producing hydrogen at 25 bar, hence, using the ideal gas law is equivalent to 66.7 kW h kg−1 hydrogen.
CO required by the methanol synthesis and propane reactors are higher than the outlet of the DRM, thus additional hydrogen is required. For this model, the source of hydrogen is from electrolysis, i.e. the generation of hydrogen and oxygen formed from the dissociation of water in the presence of a direct electric current. Electrolysis can achieve high-purity hydrogen exceeding 99.99 vol% once the outlet stream has been dried and oxygen impurities removed. Furthermore, Ursúa et al.64 found that the electric energy consumption is significantly lower at temperatures close to 1000 °C. The specific technology chosen is alkaline bipolar technology, which is most common commercially. Specific energy consumption of the entire process (not electrolysis exclusively) varied between 5 to 7 kW h Nm−3.64 The value chosen was 6 kW h Nm−3 producing hydrogen at 25 bar, hence, using the ideal gas law is equivalent to 66.7 kW h kg−1 hydrogen.
For the propane production route, hydrogen is used directly (H2ELPRO). For methanol synthesis, a compressor (CMPH21) was used to pressurise the gas to the required 49.95 bar.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio of 2 for optimality.59 Syngas passes through compressors CMPMTH1 and CMPMTH2 with interstage cooling (COOLMTH1) to reduce compressor duty. In addition, CMPMTH1 utilises energy recovered from a turbine (CMPMTH3) downstream. A limitation of the model is that the initial syngas pressure is 1 atm and increasing the pressure to 49.3 atm would require a 3-stage compressor according to the compression rate formula.65 Such a limitation would affect CAPEX, OPEX and environmental credentials; however, this was out of scope for this model. To meet the desired H2
CO ratio of 2 for optimality.59 Syngas passes through compressors CMPMTH1 and CMPMTH2 with interstage cooling (COOLMTH1) to reduce compressor duty. In addition, CMPMTH1 utilises energy recovered from a turbine (CMPMTH3) downstream. A limitation of the model is that the initial syngas pressure is 1 atm and increasing the pressure to 49.3 atm would require a 3-stage compressor according to the compression rate formula.65 Such a limitation would affect CAPEX, OPEX and environmental credentials; however, this was out of scope for this model. To meet the desired H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio, hydrogen supplied from the electrolyser at 25 bar, 15 °C is compressed (CMPH21) to 49.3 atm and mixed (SYNH2MX) with the syngas. Due to the Joule-Thomson effect of pressurising a gas, the compressed mixture is cooled (COOLMTH2) to 220 °C and heat recovered is used downstream (HTRMTH1).
CO ratio, hydrogen supplied from the electrolyser at 25 bar, 15 °C is compressed (CMPH21) to 49.3 atm and mixed (SYNH2MX) with the syngas. Due to the Joule-Thomson effect of pressurising a gas, the compressed mixture is cooled (COOLMTH2) to 220 °C and heat recovered is used downstream (HTRMTH1).
4.1.4.1 Methanol reactor. Methanol synthesis is limited by equilibrium, hence an equilibrium reactor (MTHREAC) was used in the model. eqn (2) and (3) are the reactions for methanol production while eqn (4) is the RWGS reaction:59
| 2H2 + CO ↔ CH3OH ΔH = −91 kJ mol−1 | (2) |
| CO2 + 3H2 ↔ CH3OH + H2O ΔH = 50 kJ mol−1 | (3) |
| CO + H2O ↔ CO2 + H2 ΔH = −41 kJ mol−1 | (4) |
Maximising methanol production requires reducing yields of methyl-formate and higher alcohols from side reactions. The reactions occurring are catalysed by a copper and zinc-based catalyst and are exothermic. Therefore, to maintain the reactor temperature, cooling water is used as a utility. The reactor outlet (MTH1) is 73 wt% methanol, while 26 wt% in descending order is made up of carbon monoxide, carbon dioxide and hydrogen. In Aspen, sensitivity analysis found the optimal conditions for the two-phase separation of the unreacted components to be 15 °C and 1 atm. The unreacted components were returned to the initial feedstock mixer (FEEDMIX) as a recycle stream to improve conversion. The liquid phase outlet (MTH5) of the flash separator (SEPMTH) is 99.82 wt% methanol which is first heated (HTRMTH1, HTRMTH2) and then compressed (CMPMTH4) to 300 °C and 16.5 bar.
4.1.4.2 Dimethyl ether reactor. The production of propene from methanol and DME was first developed by Lurgi Company, which used a multiple-stage fixed-bed adiabatic reactor set up. Multiple reactors were used so that both undesired higher olefins (butene to heptene) are recycled to improve propene selectivity and to allow for catalyst regeneration whilst maintaining continuous operation. Catalyst choice is what separates the MTO to the MTP process. The former uses SAPO-34 catalyst; however, the use of a zeolite-based catalyst such as ZSM-5 results in propene as the dominant product.66
The MTP process is generally accepted to involve the primary step of methanol to DME dehydration, followed by the conversion of DME to light olefins. Therefore, an intermediate reactor (DMEREAC) was set up to maximise production of DME before transfer into the propene reactor (PRPEREAC). The importance of using a zeolite catalyst and specific operating conditions in the propene reactor is because of increased selectivity for propene and to prevent further reaction of the light olefins to paraffins, aromatics and higher olefins by hydrogen transfer, alkylation and polycondensation.67–69 A recommendation for further research would be to study the direct conversion of syngas to DME to avoid the methanol production step in the production of propene through the direct reaction of DME to olefins (DTO process).
From literature, industrial operation of DME reactors are at 300 °C and 16.5 bar as a 90% methanol equilibrium conversion (eqn (5)) can be achieved.70 To maintain the thermodynamically favourable conditions of the reactor (DMEREAC) and due to its exothermicity, cooling water was chosen as a utility. The reactor outlet consisting of 62 wt% DME (DME1) with the remainder water and unreacted methanol is heated (HTRDME1) to 425 °C and 1.5 bar.
| 2CH3OH ↔ CH3OCH3 + H2O | (5) |
The production of DME in a separate reactor further diversifies the plant as DME is a viable alternative to diesel, producing less NOx, SOx and PM.71 Therefore, in the event of challenging propane market conditions this could be sold.
4.1.4.3 Propene reactor. The modelling approach for the reactor (PRPEREAC) presented difficulty for preliminary design as most literature reported only the mole fractions of hydrocarbons or complex kinetic models. However, Onel et al.72 detailed that, at 425 °C and 1.5 bar, ZSM-5 catalyst in a fixed-bed reactor could achieve a yield of 44 wt% hydrocarbons with the remainder being water. The product distribution of hydrocarbons included paraffins and olefins with propene at 71.37 wt% which was used in this model. As the outlet mass yields could be calculated, an RYield block was used. A limitation of this part of the model is that the propene reactor (PRPREAC) was modelled solely as one reactor, where in reality this would utilise multiple fixed-bed reactors. The use of an RYield block is restrictive as it uses a fixed user-defined outlet distribution, so cannot model the benefit of a recycle loop. The impact of such limitation would affect CAPEX, OPEX and environmental credentials.
The reactor outlet (CRDPRPE1), due to the high content of water, is cooled (COOLPRP1) to 20 °C and ambient pressure before entering a flash separator. Water is an unwanted by-product and would impact the duty of downstream distillation. The water content in the crude propene stream post separation (CRDPRPE3) is 3.2 wt%.
4.1.4.4 De-propaniser. The crude propene stream, due to the high selectivity achieved, is almost exclusively propene and gasoline fractions. Therefore, only a de-propaniser was used in the model. An MTO based setup produces higher fractions of paraffins such as propane which would require extractive over simple distillation to separate from propene.72
The distillation column (DE-PRP) was set up using a DSTWU block, operated at 16.9 bar with light (propene) and heavy key (pentane) recoveries of 0.99 and 0.01 respectively.61 The bottom product (DEPRPBT) is dominated by butane and pentane composing 9.99 and 86.5 wt% respectively. The top product (PROPENE) is composed of 91.1 wt% propene. To meet polymer grade propene purity >99.5% further work would be required such as optimisation of reflux ratio to increase the purity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO, the yield rarely exceeds 20 wt%.73 This distribution is a result of the surface polymerisation mechanism on the metal catalyst.74 Therefore, FT is not conducive with a high propane yield and such an indirect route would result in large separation and recycle costs, so was not chosen for this model.
CO, the yield rarely exceeds 20 wt%.73 This distribution is a result of the surface polymerisation mechanism on the metal catalyst.74 Therefore, FT is not conducive with a high propane yield and such an indirect route would result in large separation and recycle costs, so was not chosen for this model.
Zhang et al.75 reported that LPG could be produced from syngas using a hybrid, zeolite-methanol synthesis catalyst. The consecutive catalysis is efficiently carried out using a spherical zeolite shell and a metal-based catalyst core. The reaction mechanism involves four major steps: methanol synthesis within the core, dehydration of methanol to DME and olefins, selective hydrogenation to paraffins (C3–C4) and RWGS.76
The propane reactor (PROREAC) was based on the experimental results by Ge et al.77 on the use of a palladium-based methanol synthesis catalyst (Cu–ZnO/Pd-β) with a beta-zeolite shell. The process showed optimal performance at 350 °C, 21 bar and H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO of 2.71 achieving a 44.4% hydrocarbon yield, 72.9% CO conversion and 51.5% selectivity to propane. The use of a palladium-supported catalyst is important for commercial application as standard Cu–Zn methanol synthesis catalyst experiences significant deactivation from water vapour produced by the RWGS.78 Water becomes strongly adsorbed to zeolite active sites and increases the selectivity of DME from CO2 hydrogenation.79
CO of 2.71 achieving a 44.4% hydrocarbon yield, 72.9% CO conversion and 51.5% selectivity to propane. The use of a palladium-supported catalyst is important for commercial application as standard Cu–Zn methanol synthesis catalyst experiences significant deactivation from water vapour produced by the RWGS.78 Water becomes strongly adsorbed to zeolite active sites and increases the selectivity of DME from CO2 hydrogenation.79
Operating pressure had little effect on hydrocarbon distribution but increased CO conversion. However, H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio was found to significantly increase propane selectivity as when the ratio was dropped from an optimal 2.5–2.7 to 1 CO conversion and propane selectivity dropped to 36% and 30% respectively while propene selectivity increased to 31%.74 High temperatures of 350 to 380 °C were found to be optimal for both CO conversion and propane selectivity.78 Conventionally, this reaction is carried out in a fixed-bed reactor. However, for commercial application this is limited by deactivation of the catalyst from sintering due to inefficient heat removal. Therefore, Zhang et al.80 investigated the use of a slurry reactor: the suspension of the bifunctional catalyst in an inert hydrocarbon liquid. Results found significant improvement of catalyst stability, increased propane selectivity and reduction in CO2 yield. Further benefits of such design include lower cost from simpler construction, increased mass and heat transfer and increased longevity of the catalyst. While this was not modelled, the results show significant relevance to both commercial viability and application.
CO ratio was found to significantly increase propane selectivity as when the ratio was dropped from an optimal 2.5–2.7 to 1 CO conversion and propane selectivity dropped to 36% and 30% respectively while propene selectivity increased to 31%.74 High temperatures of 350 to 380 °C were found to be optimal for both CO conversion and propane selectivity.78 Conventionally, this reaction is carried out in a fixed-bed reactor. However, for commercial application this is limited by deactivation of the catalyst from sintering due to inefficient heat removal. Therefore, Zhang et al.80 investigated the use of a slurry reactor: the suspension of the bifunctional catalyst in an inert hydrocarbon liquid. Results found significant improvement of catalyst stability, increased propane selectivity and reduction in CO2 yield. Further benefits of such design include lower cost from simpler construction, increased mass and heat transfer and increased longevity of the catalyst. While this was not modelled, the results show significant relevance to both commercial viability and application.
Similar difficulty was experienced to model the propane reactor as for the propene reactor. As such, an Excel Solver was used to calculate the complete outlet yield distribution using the limited experimental results as constraints, an overall net equation (eqn (6)) occurring obtained from Zhang et al.75 and Aspen stream results.
| 2nCO + (n + 1)H2 ↔ CnH2n+2 + nCO2 | (6) |
The crude propane product (CRDPRO1) is cooled (COOLPRO2) before entry into a flash separator (SEPPRO1, SEPPRO2). The optimum temperature for the flash separator was found to be −72 °C through sensitivity analysis in Aspen, as it achieved the highest separation of hydrogen and methane to be recycled (RECYH21, RECYH22).
4.1.5.1 De-methaniser. The de-methaniser (DE-MTH) column was set up using a DSTWU block, operated at 21 bar with light (methane) and heavy key (ethane) recoveries of 0.99 and 0.01 respectively (Wang, 2021).50 The top product (DE-MTH1) consists of methane (48.3 wt%), carbon dioxide (38.9 wt%) and carbon monoxide (11.3 wt%). Due to the high impurities this would require further processing to be fed into the gas grid or sold. Alternatively, it could be recycled as feedstock or used as refinery gas.
4.1.5.2 De-ethaniser. The bottom product of the de-methaniser (CRDPRO6) is pressurised to 27.9 bar using a centrifugal pump (PUMP). Most of the stream is composed of propane (53 wt%), butane (27.6 wt%) and ethane (6.2 wt%). The de-ethaniser (DE-ETH) column was set up using a DSTWU block, operated at 27.9 bar with light key (ethane) and heavy key (propane) recoveries of 0.99 and 0.01 respectively (Wang, 2021).50 The top product (DE-ETH1) consists majorly of ethane (41.7 wt%) and carbon dioxide (54.5 wt%). Ethane is a valuable feedstock for ethene production, but it would require further downstream purification due to high impurities. Alternatively, it could be recycled as feedstock to be pyrolyzed into syngas in the reformer (DRM).
4.1.5.3 De-propaniser. The bottom product of the de-ethaniser (CRDPRO8) is depressurised to 16.9 bar using a pressure-reducing valve (PRSRED3). The stream is composed mostly of propane (61.5 wt%) and butane (32.4 wt%). The de-propaniser (DE-PRO) column was set up using a DSTWU block, operated at 16.9 bar with light (propane) and heavy key (butane) recoveries of 0.99 and 0.01 respectively.61 The top product (PROPANE) consists of nearly pure propane at 99.3 wt%. The bottom product (DEPROBT) consists of butane (82.9 wt%) and pentane (12.2 wt%) with impurities of propane and water. This stream can be sold for combination with propane to be sold as LPG, as a refrigerant or as a propellant in aerosols.
4.2 Life-cycle assessment (LCA)
LCA is a methodology to evaluate the environmental impact of a product or system through its entire life cycle. By identifying and quantifying all the inputs and outputs, their environmental impact can be evaluated cumulatively and inform which path or process has the highest impact on the environment. To ensure that an LCA is credible and comparable, the ISO standards 14040 and 14044 have been developed. SimaPro software was used to input relevant data from Aspen Plus and generate results for the different impact categories. LCA consists of four stages: goal and scope, inventory analysis, impact assessment and data interpretation. The data used and results obtained in these four stages are described next.• Determine the overall environmental impact and resource consumption of a CCU production method for propane and propene.
• Determine which are the most relevant impact categories.
• Determine which parts of the production process contribute the most to environmental impact and explore alternative technologies to mitigate.
• Compare the production of propane and propene via CCU methods against their conventional counterpart.
The functional unit of the LCA is the production of 1 kg of propane and propene. CO2 is captured from a medium-sized FCC unit via chemical absorption using PZ. Propane and propene are produced via direct conversion of syngas and the MTP process, respectively. The system boundary includes the emission of CO2 from an FCC unit up to and including the production of both propane and propene (gate). End use of the product and distribution are excluded. Background processes considered include electricity generation and sourcing of natural gas and water. The chemical plant is modelled in the U.S., so data libraries selected were USLCI and ecoinvent 3.5. Data were obtained from simulation results in Aspen Plus and heuristic-based calculations where appropriate. Operational data to input into Aspen Plus was obtained through a literature review of both commercially available technologies, published studies and experimental data discussed in previous sections.
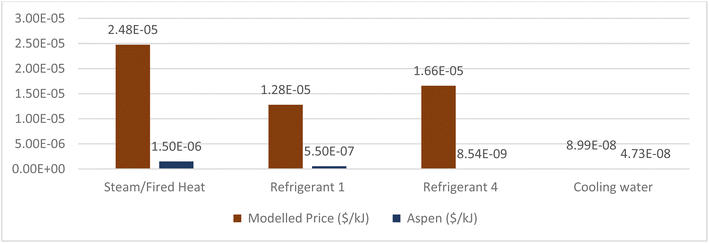
4.2.2.1 Refrigeration. The first duty is for cooling syngas post-compression from 270 °C to 15 °C using “Refrigerant 1”. Cooling water cannot be used when desired outlet temperatures decrease below 20 °C. In total, there are eight cooling duties that require refrigeration cycles based on the desired output. The required refrigerants, depending on the outlet temperature, were either “Refrigerant 1” or “Refrigerant 4” in Aspen. Typically, in conventional olefin plants, propene refrigeration cycles (assumed as “Refrigerant 1”) are used to cool streams to −35 °C and ethene refrigeration (assumed as “Refrigerant 4”) is utilised to cool to around −100 °C.81 Therefore, literature was sought to find both the energy source and intensity of such refrigeration cycles.
A key component in a refrigeration cycle is the compressor. This provides the work required to pressurise the saturated vapour from the evaporator, which is essential in facilitating heat transfer in the condenser from the higher temperature difference. As such, compressor duty was calculated from heuristics82 where the source of the mechanical work was provided from a gas turbine utilising natural gas, as this is the industry standard.
The duty required in Aspen was converted into compressor duty in W using 3.41 (BTU/HR) per W and 747.7 W per hp. To calculate the natural-gas consumption in the gas turbine to input into SimaPro, the calorific value of natural gas of 38.3 MJ m−3 was used and a mechanical efficiency of 0.4. Data from natural gas combusted in a U.S. industrial boiler was used. It was assumed that there are no fugitive emissions of refrigerant from the refrigeration cycles. Omission of such data results in an underestimation of majorly ozone depletion and GWP. Industrial estimated annual leakage rate can range between 7 to 25% of refrigeration volume, which is significant.83 However, the volume/inventory of refrigerants used was not determined and therefore the associated fugitive emission. Further research should investigate these effects in more detail.
Future work could also investigate the use of electric motors to mitigate against gas-turbine emissions. Only the environmental impact surrounding the compressor duty was included in this model and therefore the cooling duty required in the condenser (e.g. use of cooling water) was considered out of scope but should be included in further research. This omission results in water usage intensity and electrical consumption (fan utility in wet cooling tower) being underestimated. However, this impact is considered minor in this model.
4.2.2.2 Cooling tower. COOLSYN1 is a heat exchanger required to cool syngas from 950 °C to 220 °C, where cooling water was the heat transfer fluid. However, as before, Aspen Plus was only able to provide the cooling duty in W required. Therefore, modelling of the tower was important for the life cycle as the unit consumes freshwater that must be topped up due to evaporative losses, and also consumes power, mostly in the form of pump and fan duty. The cooling water duties were modelled as counterflow induced-draft cooling towers as it is the most common in the U.S. and counterflow is the most thermodynamically efficient.84
First, the cooling water concentration must be determined which is central to the design as it dictates the flow, contact and quantity of water required to achieve the desired performance. To calculate the cooling water concentration, the following assumptions were made: hot-water temperature, 39 °C; cold-water temperature, 26 °C; wet-bulb temperature, 31 °C.
Water concentration was estimated with the sizing chart to calculate cooling water concentration for counterflow induced-draft cooling tower.84 Furthermore, the cooling water flowrate was calculated from the duty, heat capacity of water (4.18 J (g K)−1) and temperature change. The required area of cooling tower was calculated by dividing the cooling water flowrate by the cooling water concentration. The horsepower per area of cooling tower was calculated using the chart horsepower per tower area.84 Fan efficiency varies depending on the power consumption, so the correct efficiency was selected from size of the motor and belt required85 to calculate the total electrical power to input into SimaPro.
Another relevant environmental impact is the resource depletion from use of cooling water. Losses occur due to evaporative loss, drift loss (water entrainment in vapour) and blowdown (purge of water to maintain system solid concentration). Calculations and equations regarding these amounts are summarised in section 26 of the ESI.† The makeup water total equated to approximately 1.62% of cooling water flowrate and was inputted as an emission to air in SimaPro.
4.2.2.3 Electrical duty. Electrical energy consumption in the model was due to compressor, pumps and cooling tower fan use and was modelled using ecoinvent “medium voltage (U.S.) cut-off, S” (2015 data).
4.2.2.4 Heating duty. Heating duty was required in heat exchangers and reactors and was modelled using an industrial U.S. boiler fed with natural gas. To calculate the natural gas consumption, the calorific value of natural gas was used (38.3 MJ m−3) alongside the energy efficiency of U.S. industrial boilers of 0.75 and duty required.86
4.2.2.5 Assumptions. In SimaPro there are extensive databases for the fuels, electricity and chemicals used. Decisions were made where possible to best reflect the scope of the model as discussed below. The model focused on the use of USLCI (U.S. Life Cycle Inventory) databases, and ecoinvent 3.5 for any missing values.
Natural gas was represented by “natural gas, high pressure (US), petroleum and gas production, on-shore, cut-off, S”. This assumption included energy use, infrastructure and associated emissions for onshore production in the Niger Delta (U.S.). This was justified as the model is based in the U.S. where gas supply is dominated by domestic sources.
Water for electrolysis was represented by “water, deionised, from tap water, at user (RoW) production, cut-off, S”. The dataset included the energy for operation, chemicals used for regeneration, emissions from regeneration chemicals, infrastructure of the plant and replacement of spent exchange resin. Electrolysis requires pure water (i.e. deionised) to prevent damage to the electrodes due to corrosion. Rest of the world (RoW) was chosen as no other option more relevant was available (e.g. U.S. or global (GLO)).
“Propylene (RoW), production, cut-off, S” was used to compare results to those generated by the model was chosen. This process used steam cracking of naphtha as the producing technology. RoW was chosen as above. As identified in the literature review, 47% of propene is sourced from steam cracking of naphtha. Therefore, this assumption would capture the relevant impacts.
Similarly, “propane (CA-AB) natural gas production, cut off, S” was used to compare results to those generated by the model. This includes exploration, drilling and ends at the gate of the processing plant. In addition, it includes all the fuels and emissions related to well testing, exploration, extraction and treatment (sweetening and drying): fugitive emissions, flaring, venting, and use of gas in turbines. As identified in the literature review, 60% of propane is produced from natural gas liquid fractionation. A limitation of such assumption is that the data are based on Alberta, Canada (CA-AB); however, as 98% of natural gas imports in the U.S. are from Canada, this was taken as a reasonable assumption.87 The most pressing limitation is that natural gas sourced from Canada, particularly Alberta, is where 85% of Canada's sour gas is produced. Therefore, in terms of environmental impacts with fugitive emissions, treatment, energy and material used to mitigate this is higher.88 Thus, overestimation is likely, compared to a U.S.-sourced scenario. The only other options in the databases were RoW and RER (Europe), which would not be appropriate to compare to conventional methods that are based on U.S. data.
Butane, pentane and ethane were produced in the model and were accounted for as avoided products. Therefore, the environmental impact of producing the same quantity via fossil-fuel derived sources was deducted from the total environmental impact of propane and propene. The assumptions for these were all “(CA-AB) natural gas production, cut off, S”, where the justification is the same as for propane above.
Water for cooling towers was represented by “process water, ion exchange, production mix, at plant, from surface water, RER S”. This assumption was not best represented, although there were no other alternatives for process water (industrial) within the databases. This is using data from Europe (RER), which is a limitation, however it considered that it is from a surface water source. Most water used by refineries comes from fresh water sources such as surface water.31
Finally, the allocation of emissions used an economic approach over the mass-based alternative. Use of a mass-based approach would have reduced the environmental burden of both products. For example, in the de-Propaniser (DE-PRO) along the propane production pathway, the top product (PROPANE) (99 wt% propane) mass flow was 5.96 kg s−1, and the bottom product (DEPROBT) was 3.76 kg s−1 (83 wt% butane). Therefore, based on a mass allocation, PROPANE would have been allocated circa 60%, compared to the economic approach of 85% of the total emissions. The advantage of economic allocation is that it allocates larger impacts to the products that the industry would favour their production (because of their higher prices). However, the drawback of economic allocation is the inherent instability as it is based on prices for products that vary based on market conditions.89 Therefore, future comparisons should consider the future prices with those used in this model (shown in Table 10).
Totals for propane and propene in Tables 2–5 have been specified (pre-avoided products) due to the production of valuable by-products such as ethane, butane and pentane and avoidance of carbon dioxide as an emission to air in carbon capture.
| Impact category | Unit | Propane (pre-avoided products) | Propane (total) | Contributor 1 | % Total | Contributor 2 | % Total | Contributor 3 | % Total |
|---|---|---|---|---|---|---|---|---|---|
| Global warming potential | kg CO2e | 7.4086 | 7.3300 | Electricity Med Voltage US | 84.23% | Natural gas combustion in boiler | 19.30% | Natural gas extraction | 3.04% |
| Terrestrial acidification | kg SO2e | 0.0298 | 0.0278 | Electricity Med Voltage US | 54.41% | Natural gas processing | 43.99% | Natural gas combustion in boiler | 1.47% |
| Particulate matter formation | kg PM2.5 | 0.0257 | 0.0251 | Electricity Med Voltage US | 84.79% | Natural gas processing | 14.78% | Natural gas combustion in boiler | 0.52% |
| Ozone formation terrestrial ecosystem | kg NOXe | 0.0080 | 0.0065 | Electricity Med Voltage US | 84.41% | Natural gas combustion in boiler | 14.86% | Natural gas, high pressure (US) production | 1.07% |
| Impact category | Value (pre-avoided products) | Value | Unit | Contributor 1 | % Total | Contributor 2 | % Total | Contributor 3 | % Total |
|---|---|---|---|---|---|---|---|---|---|
| Marine ecotoxicity | 0.2942 | 0.2910 | kg 1,4-DCB | Electricity, medium voltage | 97.90% | Natural gas, at extraction | 1.99% | Natural gas, high pressure (US), production | 0.05% |
| Freshwater ecotoxicity | 0.2171 | 0.2150 | kg 1,4-DCB | Electricity, medium voltage | 98.09% | Natural gas, at extraction | 2.14% | Crude oil, at production/RNA | 0.05% |
| Human carcinogenic toxicity | 0.3620 | 0.3560 | kg 1,4-DCB | Electricity, medium voltage | 99.99% | Natural gas, high pressure (US), production | 0.10% | Water, deionised, from tap water, at user (RoW) | 0.06% |
| Human non-carcinogenic toxicity | 5.3844 | 5.3300 | kg 1,4-DCB | Electricity, medium voltage | 97.50% | Natural gas, at extraction | 2.56% | Crude oil, at production/RNA | 0.09% |
| Impact category | Unit | Propene (pre-avoided products) | Propene (total) | Contributor 1 | % Total | Contributor 2 | % Total | Contributor 3 | % Total |
|---|---|---|---|---|---|---|---|---|---|
| Global warming potential | kg CO2e | 3.4323 | 3.2500 | Electricity Med Voltage US | 78.37% | Natural gas combustion in boiler | 31.47% | Natural gas extraction | 4.95% |
| Terrestrial acidification | kg SO2e | 0.0175 | 0.0130 | Natural gas, processed, at plant | 56.61% | Electricity Med Voltage US | 39.90% | Natural gas combustion in boiler | 1.90% |
| Particulate matter formation | kg PM2.5 | 0.0125 | 0.0110 | Electricity Med Voltage US | 75.26% | Natural gas, processed, at plant | 23.05% | Natural gas combustion in industrial boiler | 0.81% |
| Ozone formation terrestrial ecosystem | kg NOXe | 0.0040 | 0.0006 | Electricity Med Voltage US | 72.08% | Natural gas combustion in boiler | 22.32% | Natural gas, high pressure, production | 2.01% |
| Impact category | Value (pre-avoided products) | Value | Unit | Contributor 1 | % Total | Contributor 2 | % Total | Contributor 3 | % Total |
|---|---|---|---|---|---|---|---|---|---|
| Marine ecotoxicity | 0.1291 | 0.1220 | kg 1,4-DCB | Electricity, medium voltage | 96.06% | Natural gas, at extraction site | 3.42% | Natural gas, high pressure, production | 0.11% |
| Freshwater ecotoxicity | 0.0954 | 0.0906 | kg 1,4-DCB | Electricity, medium voltage | 96.04% | Natural gas, at extraction | 3.68% | Natural gas, high pressure, production | 0.10% |
| Human carcinogenic toxicity | 0.1560 | 0.1420 | kg 1,4-DCB | Electricity, medium voltage | 99.98% | Natural gas, high pressure, production | 0.23% | Water, deionised, from tap water, at user RoW | 0.05% |
| Human non-carcinogenic toxicity | 2.3721 | 2.2400 | kg 1,4-DCB | Electricity, medium voltage | 95.27% | Natural gas, at extraction | 4.38% | Crude oil, at production/RNA | 0.16% |
4.2.3.1 Propane. Table 2 shows the overall values of the environmental impact categories per kg of propane production alongside their top three contributors. The total GWP of propane is 7.33 kg CO2e per kg or 7.41 kg CO2e per kg without the inclusion of avoided products. Total water consumption is 0.0321 m3 kg−1 propane, where the highest contributor (85%) was the use of grid electricity. This might be surprising, as electrolysis consumes 9 kg of water per kg of hydrogen produced. The main contributor for all the impact factors is the use of grid electricity.
Normalisation of the impact factors was used to identify those that are of the most concern based on a comparison with a baseline. Table 3 shows the top four impact categories in descending order of importance. These impact categories represented 96.73% of the overall impact. The impact category of highest concern is marine ecotoxicity, where its main contributor (>97%) is the use of grid electricity.
4.2.3.2 Propene. Table 4 shows the overall values of the environmental impact categories per kg of propene production alongside their top three contributors. The total GWP of propene is 3.25 kg CO2e per kg or 3.43 kg CO2e per kg without the inclusion of avoided products. The main contributor for all the impact factors is the use of grid electricity. However, for terrestrial acidification, 56.61% was contributed from natural gas processing, likely due to nitrogen and sulphur compounds found as contaminants in the gas that are removed during processing.
Total water consumption is 0.0153 m3 kg−1 propene where the highest contributor (76%) was the use of grid electricity. However, the value is 48% the intensity for propane. When electrolysis is removed from the model, i.e. the electricity requirement of 66.741 kW h kg−1 hydrogen and 9 kg H2O kg−1 hydrogen, the water use intensity of propane reduces from 0.0321 to 0.00631 m3 kg−1 propane. Therefore, electrolysis contributed 80% to the total water use intensity. The propane reactor requires a H2/CO ratio of 2.7 compared to 2 for methanol synthesis, hence requiring significantly more hydrogen and explaining the difference in water use intensity.
The top four factors after normalisation represented 97.31% of the impact (Table 5). As with propane, the highest impact factor was marine ecotoxicity due to grid electricity use. As previously stated, U.S. medium voltage electricity was based on 2015 data. U.S. electricity generation data91 was found for 2015 which shows coal generation contributed 33% and natural gas 33%. The main components of coal are carbon, sulphur, oxygen and hydrogen with traces of heavy metals. During combustion, their respective oxides and particulate matter are formed, which can explain the high impact factor results.92 CO2 is formed during coal combustion; however, 60% of non-coal combustion emissions come from flue gas clean up, specifically limestone use.93 Overall, CO2 from coal combustion in conventional power stations (which are more prevalent than their modern integrated gasification combined cycle (IGCC) alternative) produce 50 to 60% more CO2 than natural gas in a new, efficient power plant.94 This explains the significant contribution of grid electricity use to global warming.
While stack emissions for natural gas may be cleaner than coal, the fugitive emissions, venting and flaring of natural gas during production pose a significant environmental burden. Thus, 33% electricity generation from natural gas significantly contributes to the environmental impact.
One limitation of the U.S. electricity generation data is that it illustrates the rapidly changing energy landscape, particularly in terms of coal contribution dropping to 19% in 2020. Therefore, as electrical duty is so influential in the model to emissions, future work should update databases in SimaPro for current grid energy mix. Based on this finding, the results of this model are likely to be overestimated across multiple impact factors compared to the present day. The effect of changing grid mix is discussed further in section 4.2.3.6.
The identification of marine and freshwater ecotoxicity as the top two factors in Table 5 and the significance of grid electricity can be explained through the presence of high quantities of arsenic, copper, selenium, lead and mercury in coal ash. These toxic components contaminate surface and groundwater, resulting in bioaccumulation.92
4.2.3.3 Process contribution. Exploration of the most significant contributors to the overall impact can help identifying where improvements to the model is more relevant. The column propane (total) or propene (total) in Table 6 represent the unmodified base case and the other columns show when each unit/process is removed (e.g. – electrolysis) from the model and reported as a percentage of the total original value, still including all the other upstream and downstream emissions. The use of grid electricity was the main contributor across all impact factors. Therefore, utility data was analysed to see which units or processes consumed the most electricity, starting with electrolysis. The impact was found by removing the electrical duty for both product routes in SimaPro and comparing the impact results, as shown in Table 6.
| Impact category | Unit | Propane (total) | –Electrolysis | Propene (total) | –Electrolysis |
|---|---|---|---|---|---|
| Global warming potential | kg CO2e | 7.33 | 23.06% | 3.25 | 31.69% |
| Terrestrial acidification | kg SO2e | 0.0278 | 47.48% | 0.013 | 55.54% |
| Particulate matter formation | kg PM2.5 | 0.0251 | 21.51% | 0.011 | 29.55% |
| Ozone formation terrestrial ecosystem | kg NOXe | 0.00651 | 6.05% | 0.000591 | −307.95% |
GWP and particulate matter formation reduced by 77% and 78% respectively and ozone formation by 94% for propane. The value for ozone formation terrestrial ecosystem is also reduced to reach a negative value, due to the inclusion of avoided products. As discussed with the differences in water use intensity, the same explanation applies to why the overall carbon intensity among other impact categories was higher for propane than propene. The propane pathway had a 35% higher H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio, in turn increasing electrical demand. Furthermore, as the propane reactor was less selective, higher quantities of by-products were produced. Therefore, resulting in higher overall duties from purification such as the front end de-methaniser fractionation (DE-MTH, DE-ETH, DE-PRO) compared to propene (DE-PRP).
CO ratio, in turn increasing electrical demand. Furthermore, as the propane reactor was less selective, higher quantities of by-products were produced. Therefore, resulting in higher overall duties from purification such as the front end de-methaniser fractionation (DE-MTH, DE-ETH, DE-PRO) compared to propene (DE-PRP).
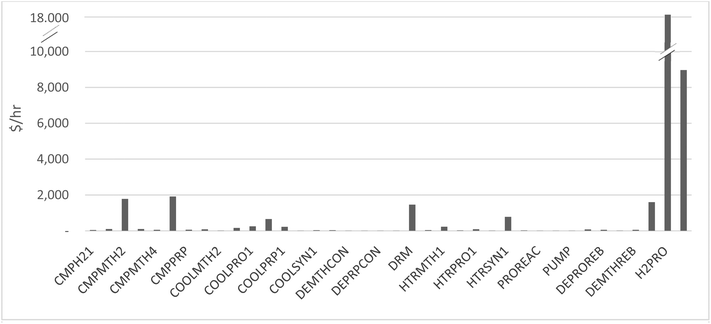
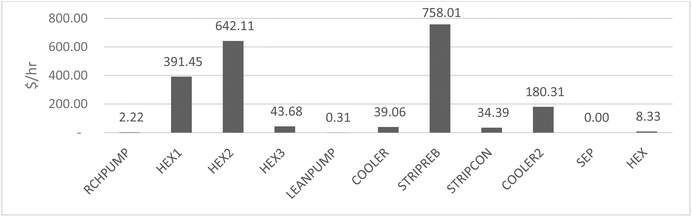
The process was repeated along the propane production pathway in Table 7 for the units with the highest duties. This included CMPPRO, the second highest duty (electrical) after electrolysis which contributed 0.58 kg CO2e per kg propane. COOLPRO2 was the highest cooling duty (ethylene refrigeration cycle) and contributed 0.13 kgCO2e per kg propane.
| Impact category | Unit | Propane (total) | –CMPPRO | –DRM | –COOLPRO2 |
|---|---|---|---|---|---|
| Global warming potential | kg CO2e | 7.33 | 92.09% | 94.13% | 98.23% |
| Terrestrial acidification | kg SO2e | 0.0278 | 94.60% | 87.77% | 96.40% |
| Particulate matter formation | kg PM2.5 | 0.0251 | 91.63% | 96.02% | 98.80% |
| Ozone formation terrestrial ecosystem | kg NOXe | 0.00651 | 90.32% | 94.78% | 98.46% |
Table 8 shows the impact of removing certain units along the propene production pathway. Notably, removal of CMPMTH2 had the greatest impact, closely followed by the dry methane reformer (DRM), which consumed large quantities of natural gas due to endothermicity.
| Impact category | Unit | Propene (total) | –CMPMTH2 | –DRM | –COOLPRP1 |
|---|---|---|---|---|---|
| Global warming potential | kg CO2e | 3.25 | 88.92% | 89.23% | 96.00% |
| Terrestrial acidification | kg SO2e | 0.013 | 92.31% | 78.46% | 91.54% |
| Particulate matter formation | kg PM2.5 | 0.011 | 88.55% | 92.73% | 97.27% |
| Ozone formation terrestrial ecosystem | kg NOXe | 0.000591 | 33.67% | 52.79% | 82.06% |
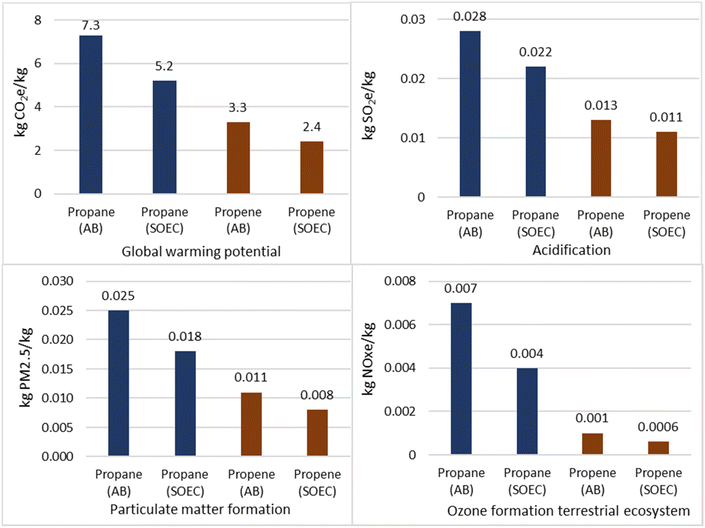
Since the electrical duty influences the results for some environmental impact categories significantly, alternative electricity grid mix would also influence the results greatly. For instance, if all electricity was provided by wind turbines or photovoltaic panels, the overall environmental impact would be much lower. Section 4.2.3.6 compares the results obtained with those when using an alternative energy mix.
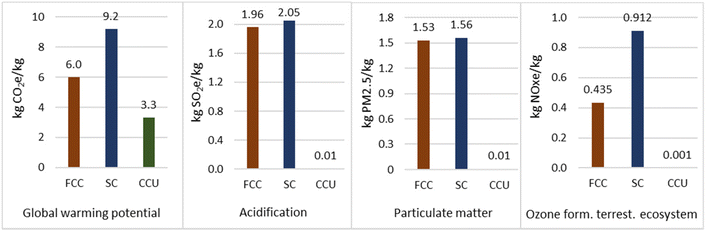
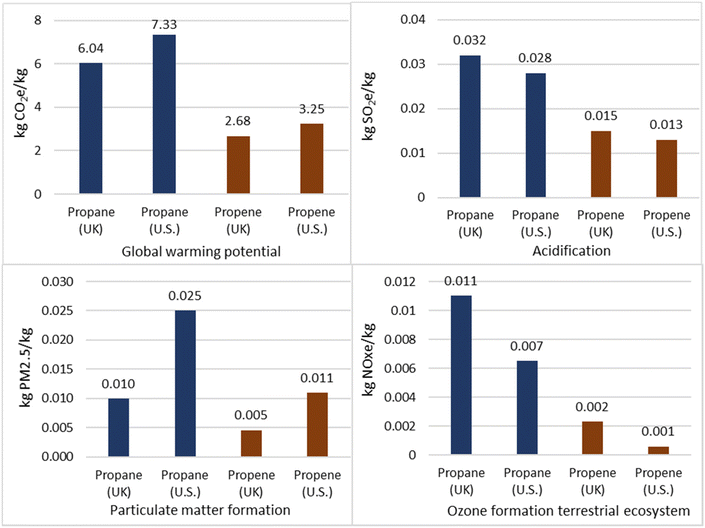
4.2.3.4 Comparison to conventional methods. Propane production via the novel CCU method offered a saving of 2.8 kg CO2 per kg propane for GWP with respect to production from natural gas fractionation, as shown in Fig. 7. However, across the other three impact categories, the CCU method had a higher environmental impact. This is attributed to coal and natural gas composing 66% of the electrical energy mix used in the model, which dominated the impact factors. The CCU method had a higher GWP than propane production from crude oil refining by 5.8 kg CO2 per kg propane. However, across all other impact factors the CCU method was significantly better, likely due to significant flaring associated with crude oil exploration.
Fig. 8 shows that, overall, the novel CCU method for propene production offered a significant saving across all impact factors. The greatest savings were compared to SC, which represents 47% of global production. Impact on terrestrial acidification for the CCU route amounted to just 0.5% when compared to FCC. FCC is the single biggest source of atmospheric pollution in a refinery and production of sulphur oxides and particulates would have accounted for a large proportion of this difference and for particulate matter formation (0.65% of FCC). Therefore, despite the high electrical intensity of electrolysis and the use of an electricity grid mix with high environmental impact, the novel method still offered substantial emission savings.
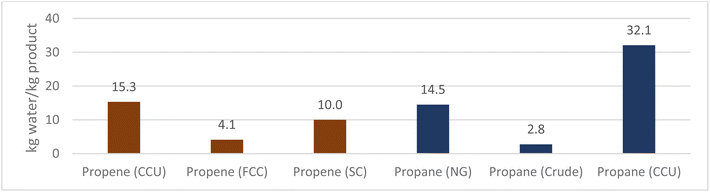
The overall water use intensity (Fig. 9) for the CCU route required 32.1 kg per kg propane, whereas for natural gas and crude oil routes was 14.5 and 2.75 kg per kg propane respectively. Electrolysis accounted for 80% of the water consumption of the propane production process. Similarly, the use of fracking for the conventional natural gas route contributed 83% to the intensity figure. While the use of water for electrolysis will not result in the same consequences as fracking, future work should investigate sustainable sources of water, particularly with regard to plant design and location due to the vast quantities required.
Water use for SC and FCC conventional routes amounted to 10 and 4.1 kg per kg propene respectively, compared to 15.3 kg per kg propene for the CCU route. Therefore, while water intensity of the CCU route is 50% higher compared to the dominant technology (SC), if water can be sustainably sourced, the overall environmental impact would greatly improve on conventional methods.
4.2.3.5 Comparison to ecoinvent 3 database. Results in Table 9 show that the modelled values greatly exceeded the values of conventional production from ecoinvent 3. However, the values were also significantly lower across all impact factors for those found for conventional production in the literature review. Therefore, the comparison offered little insight as the values are likely underestimated in ecoinvent 3 and further work should investigate the source of the information used within the databases, the assumptions used, accuracy and clarification on the boundaries of the system. The impact of this within the model is related to identifying propane and propene as avoided products. Their production as by-products is subtracted from the overall product environmental burden, however, if this value is underestimated then their true benefit is not realised.
| Impact category | Unit | Propane (total) | Propane (ecoinvent 3) | % Change | Propene (total) | Propene (ecoinvent 3) | % Change |
|---|---|---|---|---|---|---|---|
| Global warming potential | kg CO2e | 7.33 | 0.112 | 98.47% | 3.25 | 1.56 | 52.00% |
| Terrestrial acidification | kg SO2e | 0.0278 | 0.00274 | 90.14% | 0.013 | 0.00317 | 75.62% |
| Particulate matter formation | kg PM2.5 | 0.0251 | 0.000868 | 96.54% | 0.011 | 0.00104 | 90.55% |
| Ozone formation terrestrial ecosystem | kg NOXe | 0.00651 | 0.00212 | 67.43% | 0.000591 | 0.0028 | −373.77% |
4.2.3.6 Sensitivity analysis: geographical location. To provide an insight into the environmental impacts of a location within Europe, the model location was changed to the UK. The UK electricity grid was selected (2014 basis), natural gas supply was changed to high pressure (GB) petroleum and gas production, offshore cut off, S. Furthermore, the water for electrolysis was changed to be supplied from Europe. Everything else remained constant due to lack of options within the databases. Considering that the main contributor to all impact factors was the use of grid electricity, it still provides a valuable insight. One main limitation is the burning of natural gas in a U.S. boiler to provide duties such as heating and compression as there are likely substantial differences in emissions regulations between the U.S. and UK. In addition, avoided products for ethane, butane, pentane, propane all remained in Alberta, Canada. The only option was a GLO (global) ethane extraction from natural gas liquids, but the boundary started and ended in the fractionation train only, so disregarded extraction and processing emissions.
Results revealed that despite changing the location and associated databases, grid electricity use accounted for on average 80% of propane and 70% of propene contributions to the impact factors. Fig. 10 shows that the UK produced reductions for both products in GWP (18%) and particulate matter formation (60%). The UK grid compared to the U.S. in 2015 had a lower natural gas (31%) and coal use (30%).95 Furthermore, renewable penetration in was around 12% for the U.S. and 20% for the UK. Nevertheless, impacts for terrestrial acidification and ozone formation were higher. In 2014, coal imports made up 78% total supply in the UK, where 85% of the total imports was from Russia.96 76% of Russia's coal export came from the Kuzbass region, where the average life expectancy is 3 to 4 times lower than the Russian average and 93.8% of drinking water sources fail to meet sanitary chemical and microbiologic standards.97 Therefore, poor environmental standards for the extraction of coal and transportation to the UK are attributed to the higher terrestrial acidification and ozone formation increase compared to the U.S.
4.2.3.7 Sensitivity analysis: technology for hydrogen production. The choice of electrolysis technology for the CCU method is central to intensity of emissions and contribution to impact factors, as shown in Fig. 11, which compares a solid oxide electrolyser (SOEC) against alkaline bipolar (AB). The electrical intensity of SOEC (41.75 kW h kg−1) was 37% lower than AB (66.741 kW h kg−1). With respect to GWP, the use of SOEC resulted in a ∼30% reduction for both products. Terrestrial acidification reduced by 15–20% for both, while particulate matter reduced by nearly 30%. However, the highest reduction was ozone formation which reduced by over 40%. Thus, using SOEC further increases the environmental benefit of CCU over conventional methods for both products and further work should focus on hydrogen production and supply from a renewable electricity source.
4.3 Techno-economic analysis (TEA)
TEA is a methodology that provides information of technical and economic performance of technologies. Each stage of the product or system life cycle from raw materials to the final product needs to have all inputs and outputs identified and quantified. It is needed to identify all the material and energy flows, the equipment sizing, and then quantify the cost of the streams, utilities and equipment. Costs are generally separated into investment and operational cost, which include piping, engineering, legal expenses and the cost of raw materials and utilities. Economic revenue generated by the final product must also be considered. Finally, data are evaluated cumulatively to decide which process design is most economically and technically feasible.98The plant modelled produces propane and propene from CO2 captured from a medium-sized FCC unit. The FCC unit is the most emission intensive in a refinery and being a stationary source can be retrofitted with a post-combustion carbon capture technology such as the piperazine system. Case studies have proven its technical feasibility.99 The model was based on a unit producing 0.5 million tons of CO2 based on a feed rate of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 barrels per day.
000 barrels per day.
The economic analysis package within Aspen Plus was used to determine the capital cost, operation and maintenance cost (O&M) associated with the model at the desired flowrates and operating conditions. To improve the accuracy of the operational cost, literature was used to determine the price of certain utilities, feedstock and product pricing within the market. Table 10 details the assumptions used in the model. As Aspen calculates stream price in total $ per kg, the mass fractions of the stream were used to get an overall price using pure prices. For example, PROPENE has a total mass flow of around 3 kg s−1 where pure propene is 2.7 kg s−1. Therefore, the overall price of the stream was circa $3.3 per kg.
| Component | Price | Unit | Comment | Ref. |
|---|---|---|---|---|
| Piperazine | 9 | $ per kg | Market price | 100 |
| Natural gas | 0.1046 | $ per kg | U.S December 2020 | 101 |
| Propane | 1.076 | $ per kg | U.S. residential March 2021 | 102 |
| Propene | 1.157 | $ per kg | U.S. Polymer grade | 103 |
| Ethane | 0.1471 | $ per kg | U.S. December 2020 | 101 |
| Butane | 0.28621 | $ per kg | U.S. October 2020 | 101 |
| Pentane and above (gasoline) | 0.3721 | $ per kg | U.S. October 2020 | 101 |
| Electricity | 0.0635 | $ per kW h | U.S. Industrial January 2021 | 102 |
| Metric | Price | Unit |
|---|---|---|
| Investment cost: propane/propene plant | 40.3 | Million USD |
| Investment cost: carbon capture facility | 17.3 | Million USD |
| Total investment cost | 57.6 | Million USD |
| Total sales revenue | 328.7 | Million USD p.a. |
| Operating cost | 370.2 | Million USD p.a. |
| Raw material cost | 229.7 | Million USD p.a. |
| Utility cost | 82.2 | Million USD p.a. |
| Net present value (NPV) | −695.6 | Million USD |
| Gross profit (GP) | 13.4 | Million USD |
| Payback period | −0.77 | Years |
| Propane cost | 1.14 | $ per kg |
| Propene cost | 1.39 | $ per kg |
In comparison to conventional methods, the cost of propene via SC varied between $0.5 per kg45 and $1.48 per kg.47 However, the latter figure is from 2012 when crude oil was priced $110 per bbl. Current prices are around $60 per bbl, which if used in the model, would reduce the cost considerably. Therefore, the price of this technology is competitive ($1.39 per kg). However, as the price of crude oil varies significantly and can contribute up to 86% of production cost,58 comparisons must be made cautiously.
Alternatively, for propene production via MTP, cost varied in literature from $0.7 per kg (ref. 48) to $0.85 per kg. However, the use of natural gas to form syngas and subsequent conversion into olefins by FT had a production cost of $2.4 per kg.50 Furthermore, a dry reforming process using a CO2-rich natural gas from China resulted in a propene cost of $1 per kg.51
Propane cost in literature is considerably lower than the model output ($1.14 per kg). However, the majority of production requires only NGL recovery with no reactors or extensive operations. Therefore, the price will largely depend on the cost of extraction. Operational cost from NGL recovery excluding raw material cost varied between $0.04 per t to $0.26 per t.54 Propane production cost from FT synthesis varied in literature from $0.09 per kg (ref. 53) to $0.15 per kg.52
Another limitation of such a comparison is that a production cost for propene or propane that incorporated the cost of capture of CO2 was rare. However, carbon capture operational cost only contributed 4 and 8% of cost for propane and propene respectively.
The following subsections analyse each of the metrics in Table 11 more closely and show sensitivity analyses.
4.3.1.1 Gross profit (GP). GP is calculated by subtracting the cost of goods sold from the total revenue generated in the year. GP does not include fixed costs, so is a measure of the chemical plants efficiency in using materials, utilities and cost O&M to produce propane and propene.
To calculate, Fortran code was used within Aspen to multiply the quantity of utilities used by the cost, calculate feedstock stream cost and product sales revenue based on mass flows (eqn (7)).
| GP = Sales revenue − Costs − OPMT | (7) |
“OPMT” is the O&M cost, “Costs” is the cost of raw materials and utilities over the year where 330 operational days have been assumed (90% uptime).
As seen in Table 12, the value of GP is $13.4 M. Therefore, the total sales revenue generated exceeded the sum of the price of utilities, raw materials and the operation and maintenance cost. The values used in eqn (7) are summarised in Table 12.
| Component | Value | Unit |
|---|---|---|
| Sales revenue | 41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
$ per hour |
| Raw cost | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
$ per hour |
| Utility cost | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
$ per hour |
| OPMT | 3.5 | Million USD |
| GP | 13.4 | Million USD |
4.3.1.2 Net present value (NPV). An important metric in determining if a project is economically attractive is calculating the present value of all the future cash flows and deducting the total capital investment. The cash flows are the sales revenue minus the operating cost at the end of every year. Calculating the present value of future cash flows is important as it places greater emphasis on the earlier years of operation of a project rather than later years where prediction of cash flows is less reliable. To do so, a discount rate is applied (i) which accounts for the time value of money. The higher the discount rate the lower the value of future cash flows. High discount rates in industry are in the region of 10 to 15% and are used if market trends or company performance is not anticipated to be successful. However, based on the literature review, the demand for propane and propene is growing significantly, as too is the demand for such materials to have low carbon footprints. Thus, the value of discount rate applied in this model is 5%. Finally, the assumed lifetime of the plant is 30 years (t). If the value of NPV in eqn (8) is positive, the discounted value of such cash flows is greater than the capital investment outlay and the risk of the investment is lower as it is likely to turn a profit.
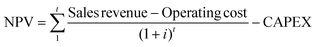 | (8) |
The value of operating cost used in the NPV formula varies slightly as it considers items such as operating labour cost, plant overhead cost, general and admin cost which were all assumed to be a factor of raw material and utility cost.84 Such assumptions are found in section 27 of the ESI† which were included in the Fortran code.
The value of NPV is −$695.6 M (Table 11), indicating a project that will return a net loss. While the GP was positive, this metric did not consider the time value of money, nor did it include the total capital investment or other contributions to operating cost discussed above. Therefore, based on the current assumptions the chemical plant is not be a profitable venture.
4.3.1.3 Payback period. Investors also determine if a project is economically attractive through payback period (PBP). This shows the time taken to recover the total capital investment and can be calculated with eqn (9).
 | (9) |
The value of PBP is −0.77 (Table 11), i.e. the project will never payback the initial capital investment. This is because total sales revenue is $328.7 M per year, but total operating cost is $370.2 M per year.
4.3.1.4 Operating cost & revenue contribution. Both NPV and PBP calculated are negative, indicating that the chemical plant under the current assumptions returns a net loss. Therefore, it is important to analyse which operating costs and revenues contribute greatly and therefore need to be pinpointed for further focus.
Fig. 12 shows the raw material and utility cost of different units in the plant. Hydrogen generates the highest cost due to significant electricity consumption, in turn increasing operational cost. The following section performs a sensitivity analysis to assess the difference in electricity required by different hydrogen production methods. Furthermore, the cost of natural gas (CH4FEED), while small in comparison to hydrogen feedstock cost, is high in comparison to other duties, thus sensitivity analysis is also performed for this.
One of the highest utilities is CMPPRO, with a duty of 30.14 MW. However, one limitation is that it does not consider the utility or environmental aspect of cooling for this compressor, which in this case would be interstage cooling. Such cooling would involve the use of refrigerants or cooling water, hence their associated duties and material usage. Evaporative/fugitive losses and cost are not examined within this model; hence make-up costs are omitted. In addition, there is a cost limitation as to pressurise the syngas from atmospheric to 21 bar would require a staged approach with multiple compressors which have not been costed individually but as one unit.
Similarly, CMPMTH2 is the second highest utility, which compresses syngas from 1.3 bar to 49 bar to be fed into the methanol reactor. Following the methanol reactor, the pressure of the system is brought to ambient conditions, so a turbine (CMPMTH3) is used to recover energy from the process that initially compresses the syngas in CMPMTH1. CMPMTH3 recovers 1.7 MW which is just over 7% of the duty for CMPMTH2, therefore, offering a saving of 0.46 kW h per second or $0.03 per s. Thus, further optimisation of the process can help to further reduce operational cost through energy and heat integration.
Propane was found to generate more revenue than propene. This is interesting as the price of propane and propene are similar ($1.076 per kg and $1.157 per kg, respectively). This is explained by the amount of product produced. While the MTP reactor had a higher selectivity for propene, there was a significant production of water that accounted for a significant loss of mass. On the contrary, the production of propane was slightly less selective (51.5% compared to 71.37%), but the impurities produced were CO and hydrogen, which were recycled.
Fig. 13 confirms literature surrounding carbon capture in that the greatest duty is that of the reboiler in the stripper (STRIPREB) due to overcoming the regeneration energy.
4.3.1.5 Sensitivity analysis: technology for hydrogen production. The price of hydrogen production contributed greatly to costs and therefore the different economic metrics would be most susceptible to change by altering assumptions regarding hydrogen production. The original assumption of the model used an AB electrolysis cell to produce hydrogen to meet the H2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO requirements of the reactors. However, this resulted in the highest operational cost and therefore contributed significantly to the negative payback and NPV, which would have made the plant uneconomically feasible. To mitigate against such a pinch point, alternative production methods of hydrogen were investigated. Such include the SOEC and the proton exchange membrane (PEM). One of the major advantages of the alternative technologies is their significantly reduced electrical intensities104 as seen in Table 13.
CO requirements of the reactors. However, this resulted in the highest operational cost and therefore contributed significantly to the negative payback and NPV, which would have made the plant uneconomically feasible. To mitigate against such a pinch point, alternative production methods of hydrogen were investigated. Such include the SOEC and the proton exchange membrane (PEM). One of the major advantages of the alternative technologies is their significantly reduced electrical intensities104 as seen in Table 13.
| Technology | Electricity consumption (kW h kg−1) | GP (Million USD) | NPV (Million USD) | Payback (Years) |
|---|---|---|---|---|
| AB | 66.7 | 13.4 | −695.6 | −0.77 |
| PEM | 55 | 51.4 | −0.78 | −1.98 |
| SOEC | 41.75 | 94.6 | 786 | 2.62 |
The use of SOEC resulted is the sole technology to provide a positive NPV (786 million USD) and a payback within 3 years. Furthermore, research surrounding SOEC has uncovered the potential of CCU to produce syngas. In addition, cost of goods sold for both products dropped by 22% (propene) and 28% (propane), further increasing price competitiveness. Kamlungsua et al.105 stated that with operation of SOEC at high temperatures, H2O and CO2 can undergo electrochemical conversion into syngas. As such, this would pose a significant recommendation for further research as not only could it prove more efficient than a dry methane reformer, but it would also reduce the need for a source of methane and therefore all the associated emission impacts with extraction, processing and transportation.
4.3.1.6 Sensitivity analysis: natural gas price. Natural gas is one of the highest contributors to operating cost. Varying the price of natural gas from $0.025 per kg to $0.15 per kg ($0.1046 base-case), equivalent to around $0.5 per MMBTU and $2.9 per MMBTU respectively, was relatively insignificant to values of sales revenue, considering SOEC (Fig. 14). However, as seen in Table 14, the base-case NPV (SOEC and $0.1046 per kg NG) was $786 M, but if the natural gas price drops by 30%, NPV increases by $65 M.
| Price ($ per kg) | GP (Million USD) | NPV (Million USD) | Payback (Years) |
|---|---|---|---|
| 0.025 | 104 | 961 | 1.72 |
| 0.05 | 101 | 906 | 1.93 |
| 0.075 | 98 | 851 | 2.19 |
| 0.1046 | 94 | 786 | 2.62 |
| 0.125 | 92 | 741 | 3.02 |
| 0.15 | 89 | 686 | 3.72 |
4.3.1.7 Sensitivity analysis: carbon tax. Section 45Q is a tax credit paid for each metric ton of carbon dioxide that is captured and either stored or utilised for a certain purpose in the U.S. In 2018, the section 45Q was amended. For a project such as the one modelled, a section 45Q credit today would be worth approximately $22.7 per t and would continue to increase linearly in value to $35 per t by 2026. The credit is available for 12 continuous years from the date of registration.106
Excel Solver was used to determine that a breakeven price on NPV requires the price of a carbon incentive to be $99.87 per t (section 28 of the ESI†). Therefore, values must exceed $100 per t for the project to be economically feasible using an AB electrolysis technology. However, when SOEC is combined with a carbon tax of $25 per t similar to the section 45Q, GP increases by almost $11.5 M. Furthermore, NPV increases by 20% to $960 M and payback reduces from 2.62 years to 1.72 years. At a price of $25 per t, this represents less than 5% of sales revenue, however, the impact on overall plant economics is clear. Therefore, acquiring such an incentive would significantly improve investment prospects.
4.3.1.8 Sensitivity analysis: utility costs. When carrying out economic analyses in Aspen, standard utility costs are attributed to streams based on in-built databases. However, to improve the real-world application of the results, the cost of natural gas in Table 10 was used to back-calculate the cost of utility streams. For example, to calculate the price of propene refrigeration (refrigerant 1 in Aspen), the natural gas consumption and price were used to calculate a $ per kJ. Fig. 15 illustrates that the Aspen pricing structure was significantly lower. When these newly modelled prices were used for the SOEC scenario the NPV was –$1.77 M and GP was –$45.64 M.
4.3.1.9 Capital cost contribution. The total capital cost for both the carbon capture plant and the main chemical plant was $57.6 M. The most expensive units included CMPH21 at $1.69 M, CMPPRP at $1.44 M, ABSORBER tower at $5.16 M and the STRIPPER tower at $1.34 M. One limitation of the model is that the use of stainless-steel piping and reactors was not modelled. Aspen Plus assumes the use of carbon steel as it is cheaper, however, as the chemical plant processes CO2 that is not completely “dry” (WATERSEP does not achieve 100% separation of water from CO2 exiting STRIPPER), this results in carbonic acid formation, which is very corrosive.
A further limitation of this model is that the price of the electrolyser AB or SOEC was not included in the model. However, capital cost can be expected of between $1100–1400 per kW for AB and greater than $2200 per kW for SOEC.107
4.3.1.10 Supply chain analysis. This subsection explores ideas concerned with the actual construction of the chemical plant modelled in the U.S.
The propane reactor utilises a bi-functional catalyst with Cu–ZnO/Pd core and beta-zeolite shell. While the Cu–ZnO catalyst is common for methanol synthesis applications, the modification to be palladium supported and contained within a zeolite shell presents a difficulty in commercial availability. Such a catalyst configuration is not widely used in industry as it remains a novel process/niche application. At an industrial scale, the catalyst requires frequent replacement due to irreversible deactivation such as sintering, which could result in production downtime due to lack of supply-chain security.
Furthermore, while the MTP process has been proven commercially, the syngas-to-propane reactor only exists in experimental studies. The technology is not mature and would be classified at a technology readiness level (TRL) of 3–4. Furthermore, although slurry reactors were suggested in literature, a pilot-scale plant for either configuration has not been developed. Such a setup would identify critical issues and lessons learned that could be eliminated when scaling up to an industrial plant, thus presenting a risk.
A key feedstock for the chemical plant is natural gas. The security of supply of natural gas in the U.S. is low risk due to the highly integrated and extensive pipeline network present and the abundance of domestic supply from shale resources. However, for a location such as the UK, it would present a reduced resilience in terms of energy security. As the UK moves towards a greater dependence on gas imports, such a plant would be exposed to market price volatility which results in a substantial change in plant economics.
5 Recommendations
The areas that require further research to optimise environmental impact or economic performance in the modelled plant or to obtain more reliable results can be found below:• Further optimisation of the process through heat and energy integration, such as organic Rankine cycle.
• Sourcing of a sustainable water supply for electrolysis.
• Use of electric motors instead of mechanical work supplied by gas turbine for compressor duty.
• Investigate the amount of refrigerant leakage from the refrigeration cycles and cooling required and its associated environmental and operational cost burden.
• Optimisation of DE-PRP such as of reflux ratio or further separation to achieve polymer-grade propene purity of >99.5%. Such a task would illustrate the economic trade-off between increased separation costs against the increased revenue from polymer-grade propene.
• Modelling the propane reactor as a slurry reactor.
• SOEC for hydrogen production and a source of sustainable heat for the process.
• Carbon dioxide utilisation through production of syngas using SOEC instead of a dry methane reformer.
• Direct synthesis of propene from syngas, similar to the propane reactor by utilising an alternative catalyst such as SAPO-34.
• Direct conversion of syngas to DME to avoid the methanol production step in the production of propene.
• To avoid solvent degradation in carbon capture, research should investigate if flue gas from an FCC stack has SO2 and NOx below 100 ppmv. If not, suitable pre-treatment technology should be added to the front end of the process.
• Undertake an LCA and TEA looking at the supply of renewable electricity to the plant, e.g. wind energy.
• Update databases for a present-day energy mix.
• Assumptions, accuracy and clarification on the boundaries of the system for propane and propene production within databases in SimaPro.
6 Conclusions
The CCU model for propane/propene production shows that the main contributor for all impact categories for propane was the use of grid electricity, mostly due to hydrogen production, followed by natural gas combustion. Normalisation revealed that impact categories associated with toxicity were the most significant, the highest being marine ecotoxicity. The production of propene compared to propane produced less than 50% of a contribution to all impact factors, which is attributed to the higher H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO ratio required along the pathway. For this same reason, water use intensity for propene was also significantly lower.
CO ratio required along the pathway. For this same reason, water use intensity for propene was also significantly lower.
Grid electricity was found to be very influential in the model as it was one of the highest duties and that the data in SimaPro are based on a U.S. grid where coal and natural gas combustion contributed two-thirds to total electricity generation. Other units that contributed majorly to the impact factors included the compression of syngas along the propene route (CMPMTH2) and the dry methane reformer (DRM) due to its endothermicity.
Production of propane via CCU with AB and U.S. electricity mix scenario resulted in a saving of 2.8 kg CO2 per kg propane compared to natural gas fractionation. With SOEC and a lower carbon intensity grid mix, the saving would be even higher. Similarly, water-use intensity compared to natural gas fractionation was 17.6 kg per kg propane higher. However, as electrolysis accounted for 80% of the water consumption, use of SOEC and cleaner electricity generation would further reduce this difference.
For propene, the novel CCU method also showed promising results with significant savings across all impact factors. The greatest were with respect to steam cracking of naphtha, which represents 47% of global production. The only drawback was a 50% higher water use intensity compared to steam cracking. However, if water were sustainably sourced, the environmental credentials would be much greater.
The choice of hydrogen technology was the real determinant of both economic and environmental performance across the whole model. The use of SOEC with a 37% lower electrical intensity greatly impacted profits and impact categories positively. Removal of electrolysis from the model reduced GWP by 77% and 68% for propane and propene respectively. Thus, further work should include SOEC technology and look to further optimise its performance. Furthermore, when the model utilised AB technology, it returned a negative NPV and therefore was incapable of paying back the capital investment. However, the use of SOEC produced a positive NPV of $786 M and a payback of 2.62 years. In addition, lower natural gas price and the incorporation of a carbon tax incentive produced significant and positive impacts on plant economics.
The cost of goods sold for propene was competitive with conventional production at $1.39 per kg. However, for propane the cost at $1.14 per kg was significantly higher, owing to the cheap cost of production of NGLs.
The use of the MTP in the model poses little deployment risk as it has been proven at scale. However, as the syngas-to-propane technology is at a low technology readiness level, further work must be done to prove that experimental results are achievable at a greater scale, e.g. in a pilot study.
As the world focuses on decarbonisation pathways to curb anthropogenic carbon emissions and halt the warming of the atmosphere, new, more sustainable production methods of fuels and materials are at centre stage. However, their successful implementation is based on two main criteria: economically feasible production and an environmentally superior performance compared to conventional production. This article has achieved this for propane and propene, two critical and demand-evolving products, by proving that CCU methods of production can be both environmentally and economically superior. Furthermore, not only has the model provided another example of feasibility with respect to carbon capture, but also emphasised the significant opportunity that syngas production offers in the utilisation of CO2 and extensive possibilities of transformation into valuable materials. Particularly, for hard-to-abate sectors or where electrification of heat for a process is not feasible.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been carried out with the financial support of the European Union through the Horizon 2020 research and innovation program under the grant agreement 837733 (COZMOS) and by a UKRI-EPSRC research grant (EP/V011863/1) for the UKRI Interdisciplinary Centre for Circular Chemical Economy (CircularChem).References
- NASA, Causes | Facts – Climate Change: Vital Signs of the Planet, 2020 [accessed 2023 Apr 24], available from: https://climate.nasa.gov/causes/.
- P. Friedlingstein, M. O'sullivan, M. W. Jones, R. M. Andrew, L. Gregor and J. Hauck, et al., Global Carbon Budget 2022, Earth Syst. Sci. Data, 2022, 14(11), 4811–4900 CrossRef.
- NASA, Carbon Dioxide | Vital Signs – Climate Change: Vital Signs of the Planet, 2020 [accessed 2023 Apr 24], available from: https://climate.nasa.gov/vital-signs/carbon-dioxide/.
- United Nations, Paris Agreement, 2015 [accessed 2023 Apr 24], available from: https://unfccc.int/sites/default/files/english_paris_agreement.pdf.
- IPCC, Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, 2022 Search PubMed.
- Zero Carbon Humber, Zero Carbon Humber: Supporting the ambitions of the East Coast Cluster, Zero Carbon Humber is a partnership which aims to build the world's first net zero industrial region, 2023 [accessed 2023 Apr 24], available from: https://www.zerocarbonhumber.co.uk/.
- E. Martin-Roberts, V. Scott, S. Flude, G. Johnson, R. S. Haszeldine and S. Gilfillan, Carbon capture and storage at the end of a lost decade, One Earth, 2021, 4(11), 1569–1584 CrossRef.
- WLPGA, Production & Distribution, 2020, available from: https://www.wlpga.org/about-lpg/production-distribution.
- CAPP, Oil Extraction, [accessed 2023 Apr 24], available from: https://www.capp.ca/oil/extraction.
- Generon, What is Gas Flaring? – Why is It Done & Viable Alternatives | GENERON. 2019 [accessed 2023 Apr 24], available from: https://www.generon.com/what-is-gas-flaring-why-is-it-done-alternatives/.
- Statista, Leading countries based on natural gas production in 2020, 2023 [accessed 2023 Apr 24], available from: https://www.statista.com/statistics/264771/top-countries-based-on-natural-gas-production/.
- Environment Protection Authority, Conventional and unconventional gas, 2015 [accessed 2023 Apr 24], available from: https://www.epa.nsw.gov.au/licensing-and-regulation/gas-industry/-/media/40b251dec4b44d378cc4ec56b7116602.ashx.
- University of Michigan – Center for Sustainable Systems, Unconventional Fossil Fuels Factsheet, 2020 [accessed 2023 Apr 24], available from: https://css.umich.edu/publications/factsheets/energy/unconventional-fossil-fuels-factsheet.
- Ipieca, Green Completions, 2014 [accessed 2023 Apr 24], available from: https://www.ipieca.org/resources/energy-efficiency-solutions/green-completions-2014.
- C. L. Weber and C. Clavin, Life cycle carbon footprint of shale gas: Review of evidence and implications, Environ. Sci. Technol., 2012, 46(11), 5688–5695 CrossRef CAS PubMed.
- California Air Resources Board, CA-Greet 3.0 Supplemental Document and Tables of Changes, 2018 Aug [accessed 2023 Apr 24], available from: https://ww2.arb.ca.gov/sites/default/files/classic/fuels/lcfs/ca-greet/cagreet_supp_doc_clean.pdf.
- US EPA, GHGRP Petroleum and Natural Gas Systems, [accessed 2023 Apr 24], available from: https://www.epa.gov/ghgreporting/ghgrp-petroleum-and-natural-gas-systems.
- Argonne, GREET® Model – The Greenhouse gases, Regulated Emissions, and Energy use in Technologies Model, 2022 [accessed 2023 Apr 24], available from: https://greet.es.anl.gov/index.php.
- Canadian Propane Association, GHG emissions intensity of Canadian propane, 2022 [accessed 2023 Apr 24], available from: https://propane.ca/wp-content/uploads/2022/08/GHG-emissions-intensity-of-Canadian-propane-EN-4-1.pdf.
- R. Collins and R. Adams-Heard, Flaring, or Why So Much Gas Is Going Up in Flames. Bloomberg, [accessed 2023 Apr 24], available from: https://www.bloomberg.com/news/articles/2019-08-30/flaring-or-why-so-much-gas-is-going-up-in-flames-quicktake.
- Y. Kuwayama, S. Olmstead and A. Krupnick, Water Quality and Quantity Impacts of Hydraulic Fracturing, Curr. Sustain. Energy Rep., 2015, 2(1), 17–24 CrossRef.
- SEPA, Review of amine emissions from carbon capture systems, Version 2.01, 2015 Search PubMed.
- E. Teletzke and B. Madhyani, Minimise amine losses in gas and liquid treating, Digital Refining, 2018 [accessed 2023 Apr 24], available from: https://www.digitalrefining.com/article/1001504/minimise-amine-losses-in-gas-and-liquid-treating.
- HY-BON, Air Pollution and Glycol Dehydration, 2014, available from: https://hy-bon.com/blog/air-pollution-and-glycol-dehydration/.
- Energy Sector Planning and Analysis (ESPA), Life Cycle Analysis of Natural Gas Extraction and Power Generation, DOE/NETL-2014/1646, 2014.
- M. Wang, H. Lee and J. Molburg, Allocation of Energy Use in Petroleum Refineries to Petroleum Products: Implications for Life-Cycle Energy Use and Emission Inventory of Petroleum Transportation Fuels, Int. J. Life Cycle Assess., 2004, 9(1), 34–44 CrossRef.
- M. I. H. Soiket, A. O. Oni, E. D. Gemechu and A. Kumar, Life cycle assessment of greenhouse gas emissions of upgrading and refining bitumen from the solvent extraction process, Appl. Energy, 2019, 240, 236–250 CrossRef CAS.
- A. Elgowainy, J. Han, H. Cai, M. Wang, G. S. Forman and V. B. Divita, Energy efficiency and greenhouse gas emission intensity of petroleum products at U.S. Refineries, Environ. Sci. Technol., 2014, 48(13), 7612–7624 CrossRef CAS PubMed.
- EIA, U.S. Natural Gas Plant Field Production, 2020 [accessed 2023 Apr 24], available from: https://www.eia.gov/dnav/pet/pet_pnp_gp_dc_nus_mbbl_a.htm.
- C. E. Clark, R. M. Horner and C. B. Harto, Life cycle water consumption for shale gas and conventional natural gas, Environ. Sci. Technol., 2013, 47(20), 11829–11836 CrossRef CAS PubMed.
- P. Sun, A. Elgowainy, M. Wang, J. Han and R. J. Henderson, Estimation of U.S. refinery water consumption and allocation to refinery products, Fuel, 2018, 221, 542–557 CrossRef CAS.
- B. Ali and A. Kumar, Life cycle water demand coefficients for crude oil production from five North American locations, Water Res., 2017, 123, 290–300 CrossRef CAS PubMed.
- B. Magill, Thirst for Oil Straining International Water Supplies, Climate Central, available from: https://www.climatecentral.org/news/us-oil-straining-international-water-supplies-19689.
- N. Zabbey, G. Olsson, N. Zabbey and G. Olsson, Conflicts – Oil Exploration and Water, Global Challenges, 2017, 1(5), 1600015 CrossRef PubMed.
- J. Seyedmohammadi, The effects of drilling fluids and environment protection from pollutants using some models, Model Earth Syst. Environ., 2017, 3(1), 1–14 CrossRef.
- IOGP Publications library, Environmental performance indicators – 2017 data, 2017 [accessed 2023 Apr 24], available from: https://www.iogp.org/bookstore/product/2017e-environmental-performance-indicators-2017-data/.
- T. Bakke, J. Klungsøyr and S. Sanni, Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry, Mar. Environ. Res., 2013, 92, 154–169 CrossRef CAS PubMed.
- T. Ren, M. K. Patel and K. Blok, Steam cracking and methane to olefins: Energy use, CO2 emissions and production costs, Energy, 2008, 33(5), 817–833 CAS.
- D. Babusiaux and A. Pierru, Modelling and allocation of CO2 emissions in a multiproduct industry: The case of oil refining, Appl. Energy, 2007, 84(7–8), 828–841 CrossRef CAS.
- A. Corma, E. Corresa, Y. Mathieu, L. Sauvanaud, S. Al-Bogami and M. S. Al-Ghrami, et al., Crude oil to chemicals: light olefins from crude oil, Catal. Sci. Technol., 2017, 7(1), 12–46 RSC . available from: https://pubs.rsc.org/en/content/articlehtml/2017/cy/c6cy01886f.
- R. G. Kunz, Environmental calculations: a multimedia approach, Wiley, 2009 [accessed 2023 Apr 24]. 703 p, available from: https://www.wiley.com/en-us/Environmental+Calculations%3A+A+Multimedia+Approach-p-9780470139851.
- J. Yakubu, R. Patel and I. M. Mujtaba, Optimization of Fluidized Catalytic Cracking Unit Regenerator to Minimize CO2 Emissions, Chem. Eng. Trans., 2017, 57, 1531–1536 Search PubMed.
- F. R. Jia, W. T. Jing, G. X. Liu, Q. Yue, H. M. Wang and L. Shi, Paraffin-based crude oil refining process unit-level energy consumption and CO2 emissions in China, J. Cleaner Prod., 2020, 255, 120347 CrossRef CAS.
- A. Akah and M. Al-Ghrami, Maximizing propylene production via FCC technology, Appl. Petrochem. Res., 2015, 5(4), 377–392 CrossRef CAS.
- M. Yang and F. You, Comparative Techno-Economic and Environmental Analysis of Ethylene and Propylene Manufacturing from Wet Shale Gas and Naphtha, Ind. Eng. Chem. Res., 2017, 56(14), 4038–4051 CrossRef CAS.
- Y. M. John, R. Patel and I. M. Mujtaba, Maximization of propylene in an industrial FCC unit, Appl. Petrochem. Res., 2018, 8(2), 79–95 CrossRef CAS.
- D. Xiang, Y. Qian, Y. Man and S. Yang, Techno-economic analysis of the coal-to-olefins process in comparison with the oil-to-olefins process, Appl. Energy, 2014, 113, 639–647 CrossRef CAS.
- C. Zhang, K. W. Jun, R. Gao, Y. J. Lee and S. C. Kang, Efficient utilization of carbon dioxide in gas-to-liquids process: Process simulation and techno-economic analysis, Fuel, 2015, 157, 285–291 CrossRef CAS.
- R. Guettel, U. Kunz and T. Turek, Reactors for Fischer-Tropsch Synthesis, Chem. Eng. Technol., 2008, 31(5), 746–754 CrossRef CAS.
- Z. Zhao, J. Jiang and F. Wang, An economic analysis of twenty light olefin production pathways, J. Energy Chem., 2021, 56, 193–202 CrossRef CAS.
- Q. Chen, D. Wang, Y. Gu, S. Yang, Z. Tang and Y. Sun, et al., Techno-economic evaluation of CO2-rich natural gas dry reforming for linear alpha olefins production, Energy Convers. Manage., 2020, 205, 112348 CrossRef CAS.
- P. Jaramillo, W. M. Griffin and H. S. Matthews, Comparative analysis of the production costs and life-cycle GHG emissions of FT liquid fuels from coal and natural gas, Environ. Sci. Technol., 2008, 42(20), 7559–7565 CrossRef CAS PubMed.
- B. Ghorbani, A. Ebrahimi, S. Rooholamini and M. Ziabasharhagh, Integrated Fischer-Tropsch synthesis process with hydrogen liquefaction cycle, J. Cleaner Prod., 2021, 283, 124592 CrossRef CAS.
- M. Getu, S. Mahadzir, N. V. D. Long and M. Lee, Techno-economic analysis of potential natural gas liquid (NGL) recovery processes under variations of feed compositions, Chem. Eng. Res. Des., 2013, 91(7), 1272–1283 CrossRef.
- J. Speight, 4.3.5 Environmental Issues, in Handbook of Industrial Hydrocarbon Processes, Gulf Professional Publishing, 2011 Search PubMed.
- J. H. Park, M. S. Khan, R. Andika, M. Getu, A. Bahadori and M. Lee, Techno-economic evaluation of a novel NGL recovery scheme with nine patented schemes for offshore applications, J. Nat. Gas Sci. Eng., 2015, 27, 2–17 CrossRef.
- A. AlNouss, M. Ibrahim and S. A. Al-Sobhi, Potential energy savings and greenhouse gases (GHGs) emissions reduction strategy for natural gas liquid (NGL) recovery: Process simulation and economic evaluation, J. Cleaner Prod., 2018, 194, 525–539 CrossRef.
- Q. Yang, Q. Yang, Y. Man, D. Zhang and H. Zhou, Technoeconomic and environmental evaluation of oil shale to liquid fuels process in comparison with conventional oil refining process, J. Cleaner Prod., 2020, 255, 120198 CrossRef CAS.
- Y. Zhang, J. Cruz, S. Zhang, H. H. Lou and T. J. Benson, Process simulation and optimization of methanol production coupled to tri-reforming process, Int. J. Hydrogen Energy, 2013, 38(31), 13617–13630 CrossRef CAS.
- C. Nwaoha, R. Idem, T. Supap, C. Saiwan, P. Tontiwachwuthikul and W. Rongwong, et al., Heat duty, heat of absorption, sensible heat and heat of vaporization of 2−Amino–2−Methyl–1−Propanol (AMP), Piperazine (PZ) and Monoethanolamine (MEA) tri–solvent blend for carbon dioxide (CO2) capture, Chem. Eng. Sci., 2017, 170, 26–35 CrossRef CAS.
- M. Wang, Lab Notes, CPE450 Advanced Process Modelling, Simulation and Optimisation, 2021 Search PubMed.
- Y. Lim, C. J. Lee, Y. S. Jeong, I. H. Song, C. J. Lee and C. Han, Optimal Design and Decision for Combined Steam Reforming Process with Dry Methane Reforming to Reuse CO2 as a Raw Material, Ind. Eng. Chem. Res., 2012, 51(13), 4982–4989 CrossRef CAS.
- S. G. Gopaul and A. Dutta, Dry reforming of multiple biogas types for syngas production simulated using Aspen Plus: The use of partial oxidation and hydrogen combustion to achieve thermo-neutrality, Int. J. Hydrogen Energy, 2015, 40(19), 6307–6318 CrossRef CAS.
- A. Ursúa, L. M. Gandía and P. Sanchis, Hydrogen production from water electrolysis: Current status and future trends, Proc. IEEE, 2012, 100(2), 410–426 Search PubMed.
- INTECH GmbH, Example problems for the calculation and selection of compressors, [accessed 2023 Apr 24], available from: https://intech-gmbh.com/compr_calc_and_selec_examples/.
- X. Huang, H. Li, H. Li and W. D. Xiao, A computationally efficient multi-scale simulation of a multi-stage fixed-bed reactor for methanol to propylene reactions, Fuel Process. Technol., 2016, 150, 104–116 CrossRef CAS.
- T. S. Zhao, T. Takemoto and N. Tsubaki, Direct synthesis of propylene and light olefins from dimethyl ether catalyzed by modified H-ZSM-5, Catal. Commun., 2006, 7(9), 647–650 CrossRef CAS.
- S. Park, Y. Watanabe, Y. Nishita, T. Fukuoka, S. Inagaki and Y. Kubota, Catalytic conversion of dimethyl ether into propylene over MCM-68 zeolite, J. Catal., 2014, 319, 265–273 CrossRef CAS.
- F. Sadeghian Jahromi and M. Beheshti, An extended energy saving method for modification of MTP process heat exchanger network, Energy, 2017, 140, 1059–1073 CrossRef.
- D. F. Rodríguez-Vallejo, G. Guillén-Gosálbez and B. Chachuat, What Is the True Cost of Producing Propylene from Methanol? The Role of Externalities, ACS Sustainable Chem. Eng., 2020, 8(8), 3072–3081 CrossRef.
- T. A. Semelsberger, R. L. Borup and H. L. Greene, Dimethyl ether (DME) as an alternative fuel, J. Power Sources, 2006, 156(2), 497–511 CrossRef CAS.
- O. Onel, A. M. Niziolek and C. A. Floudas, Optimal Production of Light Olefins from Natural Gas via the Methanol Intermediate, Ind. Eng. Chem. Res., 2016, 55(11), 3043–3063 CrossRef CAS.
- Y. Liu, H. Kamata, H. Ohara, Y. Izumi, D. S. W. Ong and J. Chang, et al., Low-Olefin Production Process Based on Fischer-Tropsch Synthesis: Process Synthesis, Optimization, and Techno-Economic Analysis, Ind. Eng. Chem. Res., 2020, 59(18), 8728–8739 CrossRef CAS.
- G. Li, F. Jiao, D. Miao, Y. Wang, X. Pan and T. Yokoi, et al., Selective conversion of syngas to propane over ZnCrOx-SSZ-39 OX-ZEO catalysts, J. Energy Chem., 2019, 36, 141–147 CrossRef.
- Q. Zhang, X. Li, K. Asami, S. Asaoka and K. Fujimoto, Synthesis of LPG from synthesis gas, Fuel Process. Technol., 2004, 85(8–10), 1139–1150 CrossRef CAS.
- P. Lu, J. Sun, D. Shen, R. Yang, C. Xing and C. Lu, et al., Direct syngas conversion to liquefied petroleum gas: Importance of a multifunctional metal-zeolite interface, Appl. Energy, 2018, 209, 1–7 CrossRef CAS.
- Q. Ge, Y. Lian, X. Yuan, X. Li and K. Fujimoto, High Performance Cu-ZnO/Pd-β Catalysts for Syngas to LPG, Catal. Commun., 2008, 9(2), 256–261 CrossRef CAS.
- Q. Zhang, X. Li, K. Asami, S. Asaoka and K. Fujimoto, Direct synthesis of LPG fuel from syngas with the hybrid catalyst based on modified Pd/SiO2 and zeolite, Catal. Today, 2005, 104(1), 30–36 CrossRef CAS.
- C. Li and K. Fujimoto, Efficient conversion of carbon dioxide to non-methane light hydrocarbons—Two stage process with intercooler, Fuel Process. Technol., 2015, 136, 50–55 CrossRef CAS.
- Q. Zhang, T. Ma, M. Zhao, T. Tomonobu and X. Li, Direct synthesis of LPG from syngas in a slurry phase, Catal. Sci. Technol., 2016, 6(5), 1523–1529 RSC . Available from: https://pubs.rsc.org/en/content/articlehtml/2016/cy/c5cy01266j.
- M. Mafi, M. Amidpour and S. M. Mousavi Naeynian, Development in Mixed Refrigerant Cycles Used in Olefin Plants, Proc. 1st Annu. Gas Process Symp., 2009 Search PubMed, pp. 154–161.
- C. R. Branan, Rules of Thumb for Chemical Engineers. A manual of quick, accurate solutions to everyday process engineering problems. 4th ed. Rules of Thumb for Chemical Engineers, Elsevier Inc., 2005 Search PubMed.
- Accuvio, Annual leakage rate (%) for the refrigeration/air-con/HVAC, 2017 [accessed 2023 Apr 25], available from: https://support.accuvio.com/support/solutions/articles/4000040366-annual-leakage-rate-for-the-refrigeration-air-con-hvac.
- D. W. Green and R. H. Perry, Perry's Chemical Engineers’ Handbook, Eighth Edition. Perry's Chemical Engineers’ Handbook, McGraw-Hill Education, 2008, p. 2400 Search PubMed.
- The Engineering Toolbox, Fans – Efficiency and Power Consumption. 2003 [accessed 2023 Apr 25], available from: https://www.engineeringtoolbox.com/fans-efficiency-power-consumption-d_197.html.
- IEA-ETSAP, Industrial Combustion Boilers, 2010, available from: https://iea-etsap.org/E-TechDS/PDF/I01-ind_boilers-GS-AD-gct.pdf.
- US EIA, Natural gas imports and exports, 2022 [accessed 2023 Apr 25], available from: https://www.eia.gov/energyexplained/natural-gas/imports-and-exports.php.
- Government of Alberta, Natural gas overview. [accessed 2023 Apr 25], available from: https://www.alberta.ca/natural-gas-overview.aspx.
- F. Dolezal, H. Mötzl and C. Spitzbart, Survey of Allocation Methods in Life Cycle Assessments of Wood Based Products, World SB14, Barcelona, 2014 Search PubMed.
- RIVM, LCIA: the ReCiPe model, 2018 [accessed 2023 Apr 25], available from: https://www.rivm.nl/en/life-cycle-assessment-lca/recipe.
- US EIA, Electric Power Monthly, 2021 [accessed 2023 Apr 25], available from: https://www.eia.gov/electricity/monthly/epm_table_grapher.php?t=epmt_5_6_a.
- M. E. Munawer, Human health and environmental impacts of coal combustion and post-combustion wastes, J. Sustain. Min., 2018, 17(2), 87–96 CrossRef.
- P. L. Spath, M. K. Mann and D. R. Kerr, Life Cycle Assessment of Coal-fired Power Production, 1999 [accessed 2023 Apr 25], available from: https://www.nrel.gov/docs/fy99osti/25119.pdf.
- NETL, Cost and Performance Baseline for Fossil Energy Plants, Volume 1: Bituminous Coal and Natural Gas to Electricity. Revision 2, DOE/NETL-2010/1397, Washington, D.C., 2010 Search PubMed.
- DECC, UK Energy Statistics, 2014 & Q4 2014, 2015 [accessed 2022 May 3], available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/416310/PN_March_15.pdf.
- DECC, Coal in 2014, [accessed 2022 May 3], available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/462360/Coal_2014.pdf.
- N. Paramonova, The Cost of Coal. Impact of Russian coal mining on the environment, local communities and indigenous peoples, 2015 [accessed 2023 Apr 25], available from: https://ecdru.files.wordpress.com/2015/12/russian-coal.pdf.
- A. Gholami, F. Pourfayaz and A. Maleki, Techno-economic assessment of biodiesel production from canola oil through ultrasonic cavitation, Energy Rep., 2021, 7, 266–277 CrossRef.
- F. Güleç, W. Meredith and C. E. Snape, Progress in the CO2 Capture Technologies for Fluid Catalytic Cracking (FCC) Units—A Review, Front. Energy Res., 2020, 8, 62 CrossRef.
- PharmaCompass, Piperazine | Price | per kg | USD, 2021 [accessed 2023 Apr 25], available from: https://www.pharmacompass.com/price/piperazine.
- US EIA, Prices for hydrocarbon gas liquids, 2020 [accessed 2023 Apr 25], available from: https://www.eia.gov/energyexplained/hydrocarbon-gas-liquids/prices-for-hydrocarbon-gas-liquids.php.
- US EIA, Petroleum & Other Liquids, 2021 [accessed 2022 Feb 27], available from: https://www.eia.gov/dnav/pet/hist/LeafHandler.ashx?n=PET&s=W_EPLLPA_PRS_NUS_DPG&f=W.
- J. Giordano, US propylene prices continue to surge, 2020 [accessed 2023 Apr 25], available from: https://chemweek.com/CW/Document/116083/US-propylene-prices-continue-to-surge.
- E. Tang, T. Wood, C. Brown, M. Casteel, M. Pastula, M. Richards, R. Petri, F. Energy, D. Manager and D. Peterson, Solid Oxide Based Electrolysis and Stack Technology with Ultra-High Electrolysis Current Density (>3A/Cm 2) and Efficiency. DE-EE0006961 Final Report, 2018 Search PubMed.
- K. Kamlungsua, P. C. Su and S. H. Chan, Hydrogen Generation Using Solid Oxide Electrolysis Cells, Fuel Cells, 2020, 20(6), 644–649 CrossRef CAS.
- M. Rodgers and B. Dubov, US tax credit encourages investment in carbon capture and storage, 2021 [accessed 2023 Apr 25], available from: https://www.whitecase.com/insight-our-thinking/us-tax-credit-encourages-investment-carbon-capture-and-storage.
- O. Schmidt, A. Gambhir, I. Staffell, A. Hawkes, J. Nelson and S. Few, Future cost and performance of water electrolysis: An expert elicitation study, Int. J. Hydrogen Energy, 2017, 42(52), 30470–30492 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc04721g |
| This journal is © The Royal Society of Chemistry 2023 |