Open Access Article
Open Access ArticleChallenges and recent advances in bio-based isocyanate production
Joanna
Niesiobędzka
 and
Janusz
Datta
and
Janusz
Datta
 *
*
Department of Polymer Technology, Faculty of Chemistry, Gdansk University of Technology, G. Narutowicza St. 11/12, 80-233 Gdańsk, Poland. E-mail: jandatta@pg.edu.pl
First published on 28th February 2023
Abstract
Polyurethanes (PUs) are key players in the plastics industry. According to the Global Polyurethane Market (2019–2023), their value in the global market reached $95.13 billion in 2019 and is expected to grow at a compound annual growth rate of 12%. This is in line with the increase in the number of research papers and patents on the synthesis and application of petroleum-based PUs (P-PUs) and biomass or green PUs (G-PUs) (from 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 657 to 79
657 to 79![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 468, and from 198
468, and from 198![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 810 to 226
810 to 226![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 164 for papers and patents, respectively, just over the past decade). Environmental concerns about fossil-based isocyanates are the reason why investigations on their green counterparts have got receiving more attention. According to statistics, almost 8% of research papers published on PUs between 2015 and the present used green-based resources. However, no comprehensive report or review dealt with such an important field in the chemistry of polyurethanes. This review highlights and reviews the production of isocyanate compounds directly from biomass. Moreover, various methods applied in the synthesis of bio-derived isocyanates are discussed. Technically, we discuss the main difficulties in the synthesis of biomass-based PUs. To address sustainability concerns, we overview the whole range of isocyanate compounds economically viable for biomass-based synthesis. We believe that the elimination of phosgene is a key to the production of green PUs. However, there is still a long way to go to develop green PUs with properties and performance comparable to fossil-based ones.
164 for papers and patents, respectively, just over the past decade). Environmental concerns about fossil-based isocyanates are the reason why investigations on their green counterparts have got receiving more attention. According to statistics, almost 8% of research papers published on PUs between 2015 and the present used green-based resources. However, no comprehensive report or review dealt with such an important field in the chemistry of polyurethanes. This review highlights and reviews the production of isocyanate compounds directly from biomass. Moreover, various methods applied in the synthesis of bio-derived isocyanates are discussed. Technically, we discuss the main difficulties in the synthesis of biomass-based PUs. To address sustainability concerns, we overview the whole range of isocyanate compounds economically viable for biomass-based synthesis. We believe that the elimination of phosgene is a key to the production of green PUs. However, there is still a long way to go to develop green PUs with properties and performance comparable to fossil-based ones.
1. Introduction
Polyurethanes (PUs) are versatile polymers widely used in a broad range of applications, such as protective coatings, adhesives and insulation, in automotive and construction industries. PUs can be found in a variety of forms,1–3 such as solid products, foams, paints, varnishes, adhesives, and impregnations.4 They are best known for their excellent mechanical, chemical, and physical properties, mainly abrasion resistance, durability, and tensile strength.5,6 Thanks to promising features, the PU market has always experienced growth. Fig. 1 gives, by product, a useful view of the PU market in the United States. Accordingly, the global PU market is expected to grow at a compound annual growth rate (CAGR) of 3.8% between 2021 and 2028.7 Estimations also suggest that urbanization and migration may result in an increase in PU consumption in the construction and transport industries. Correspondingly, the demand for PUs should taken an upward trend.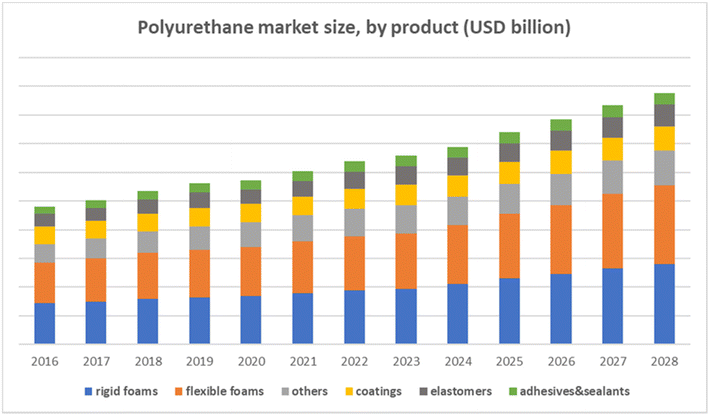 | ||
| Fig. 1 Polyurethane market size based on data from the Market Analysis Report.8 | ||
The use of fossil-based feedstocks has been seriously limited over the last few decades to avoid greenhouse gas emissions. Moreover, not surprisingly, huge amounts of plastic waste are dumped in landfills, ending up in the seas and oceans, posing a serious global crisis.9,10 Therefore, researchers have been working to obtain greener and cleaner products. As European standard EN16575 defines, plastics derived fully or partially from biomass (e.g. vegetable oils, sugar, starch) are known as green materials.11 Thus, in line with a green planet, hydrogen production, circular economy, CO2 capture, smart building constructions, etc., concerns about petroleum-based PUs (P-PUs) have increased. Fig. 2 shows the number of publications on non-isocyanate polyurethanes (NIPUs) and biomass or green PUs (G-PUs).12,13 As can be seen, interest in this topic among researchers has been growing since 1978, but the real growth in the number of articles in this field began in the early 2000s. Since then, new articles about “greener” polyurethanes are published every year. A notable increase in interest on G-PU topics began in 2008, and on NIPU topics in 2013. Nowadays, more than 200 articles related to G-PUs and about 60 related to NIPUs are published annually. Of course, the data presented are only simplified statistics to observe a trend in the field of polyurethanes. Research papers related to the production of G-PUs or NIPUs are still relatively rare in the total number of publications on polyurethanes, therefore further development of this topic is very important.
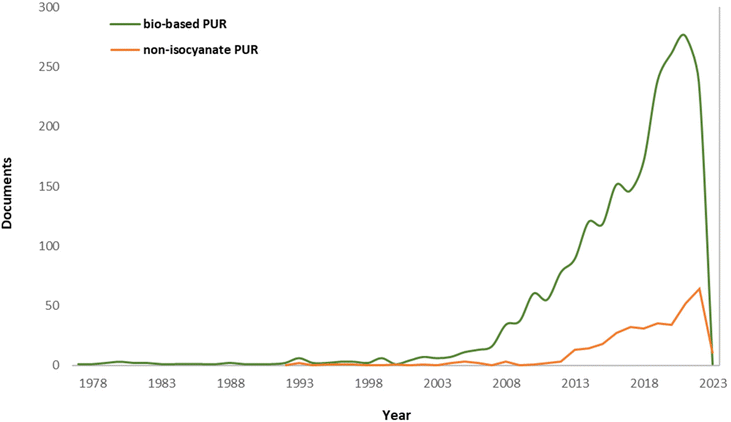 | ||
| Fig. 2 The number of articles over the years.12,13 | ||
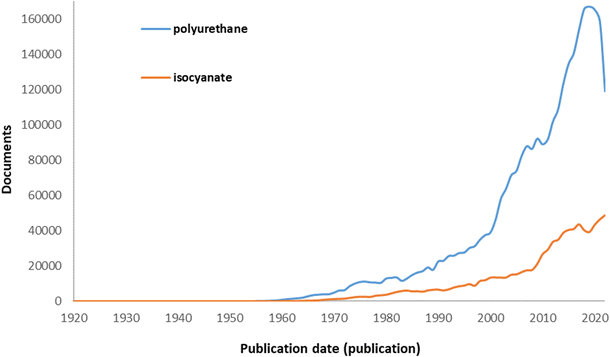
Furthermore, a patent analysis has been conducted to confirm technological trends in the production of isocyanates. At the outset, the quantitative features of patents related to polyurethanes are examined. Fig. 3 shows the general growing trend in the field of polyurethanes and monomers for their production. The analysis was based on two keywords “polyurethane” and “isocyanate”. Between 1920 and 2022, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 442
442![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 374 patent applications for polyurethanes were registered worldwide. The increase in patent filings occurred in the 1970s. Thereafter, interest in the subject has steadily increased. Another marked increase was observed in the 2000s, and from that time the development of this topic really began. There was a slight decline in 2010, compared to 2009, which may have been caused by delays between the filing and publication of patents, as is also seen in other cases. Based on the International Patent Classification, the majority of patent applications searched in the field of “polyurethane” are layered products containing a synthetic resin (B32B27), macromolecular compounds obtained otherwise than by reactions only involving unsaturated carbon-to-carbon bonds (C08G), coating compositions, e.g. paints, varnishes or lacquers (C09D5), and the use of organic (C08K5) and inorganic ingredients as components of mixtures (C08K3). However, isocyanates are mainly registered in two groups: polymeric products of isocyanates or isothiocyanates (C08G18) and layered products comprising a synthetic resin (B32B27).
374 patent applications for polyurethanes were registered worldwide. The increase in patent filings occurred in the 1970s. Thereafter, interest in the subject has steadily increased. Another marked increase was observed in the 2000s, and from that time the development of this topic really began. There was a slight decline in 2010, compared to 2009, which may have been caused by delays between the filing and publication of patents, as is also seen in other cases. Based on the International Patent Classification, the majority of patent applications searched in the field of “polyurethane” are layered products containing a synthetic resin (B32B27), macromolecular compounds obtained otherwise than by reactions only involving unsaturated carbon-to-carbon bonds (C08G), coating compositions, e.g. paints, varnishes or lacquers (C09D5), and the use of organic (C08K5) and inorganic ingredients as components of mixtures (C08K3). However, isocyanates are mainly registered in two groups: polymeric products of isocyanates or isothiocyanates (C08G18) and layered products comprising a synthetic resin (B32B27).
Typically, PUs are obtained via the polyaddition reaction of di- or poly-isocyanates and polyols.14 The mechanism of the polyaddition reaction between isocyanates and polyhydroxy compounds was first introduced by Otto Bayer and coworkers in 1937.15 This reaction can be simply explained as shown in Fig. 4.
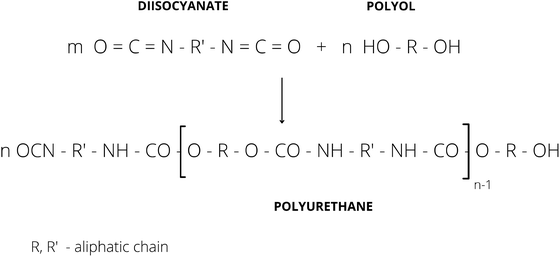 | ||
| Fig. 4 Reaction scheme of diisocyanate with polyol.16 | ||
The production of PUs is predominantly based on petroleum-based feedstocks, with benzene, toluene, ethylene, and propylene as the most common chemicals. On the other hand, raw materials based on natural products (oils, sugars, and bio-based glycerol) are used quite limitedly. Over the past two decades, the plastics industry has sought to replace P-PUs with G-PUs from renewable resources to acknowledge sustainability concerns about fossil-based resources.15 Bio-polyols are widely known for the production of G-PUs, among them are polyols produced from rapeseed oil, soybean oil, castor oil, lignin, or cardanol. Polyurethanes are often derived from chemically modified triglycerides and their fatty acids.17 Isocyanates, on the other hand, are mainly obtained by reacting an amine with the toxic phosgene. Currently, there is a lack of comprehensive knowledge on the preparation of isocyanates from renewable raw materials. Their main drawback in addition to the toxicity of phosgene consists of limited fossil resources used to produce isocyanates.6 Therefore, it is necessary to develop an alternative, more environmentally friendly way to produce isocyanates.18
In recent years, several researchers have reported the synthesis of G-PUs and measured the properties and performance of bio-based products. In this regard, some of them have also classified and reviewed the syntheses of G-PUs and green isocyanates. In recent years, several researchers have reported the synthesis of G-PUs and measured the properties and performance of bio-based products. Therefore, some of them have classified and reviewed the syntheses of G-PUs and green isocyanates. We have information on the production of both polyols and isocyanates from vegetable oils, sugars or lignin. The researchers outlined their progress in the field of bio-based isocyanates over the last few years. As a result, the potential to synthesize isocyanates also from algae or CNSL (cashew nut shell liquid) is known, and will be described later in this paper. Nevertheless, published review papers have presented some parts of this broad research field. In addition, no report or review on patents covering G-PUs has been published. Overall, three review papers published recently can be mentioned. Paraskar et al. also presented perspectives on the polyurethane coating industry with a focus on the use of bio-based monomers. The synthesis of polyols from vegetable oils by transesterification, epoxidation and thiol–ene coupling reactions was described in detail. However, in the review there is only a mention of the toxicity of phosgene in the production of isocyanates. The possibility of using fatty acids to synthesize bio-based isocyanates was briefly reported, and several commercially available bio-based isocyanates were mentioned. Unfortunately, this paper is weak in its detailed overview of possible routes for the synthesis of bio-based isocyanates.19 The second paper is a summary of recent developments in the production of polyols and isocyanates based on renewable raw materials for the production of PUs. The paper presents commercially available petrochemical isocyanates, but does not deal with their toxicity and effects on human health. Moreover, this work mainly focuses on obtaining non-isocyanate polyurethanes (NIPUs), and only mentions the possibility of producing diisocyanates, for example, from dimerized fatty acids, which come from renewable resources.19 Very recently, Ma et al. reported on recent advances in the synthesis of biomass-based polyols and their application in polyurethane materials. The possibilities of using such raw materials as turpentine, vegetable oils, lignin or cellulose were presented. Low price, high efficiency and reduction of pollution were pointed out as advantages of the above-mentioned materials. The paper underlines the urgency of the replacement of petrochemical polyols with bio-based polyols and reports on the research direction of bio-based PUs in the future.20
Therefore, a comprehensive review of the toxicity of commercial isocyanates as well as the classification of G-PUs seems necessary. Moreover, a patent analysis of the development of the G-PU market could shed more light on the present and future research on G-PU formulations. We comprehensively reviewed the current state of knowledge in the field of isocyanates based on renewable raw materials. In addition, the environmental impacts of fossil-based isocyanates and isocyanates derived from renewable raw materials are discussed. Furthermore, the toxicity of petrochemical-based isocyanates is systematically addressed.
The article is organized as follows: the next section provides a brief overview of polyurethanes and their trends in the global market, as well as the challenges ahead for the chemical market. The next sections include an overview of isocyanates currently used, as well as the impact of isocyanates on human health, with a focus on the toxicity of phosgene, which is used extensively in isocyanate production. Section 4 focuses on isocyanates derived from bio-based feedstocks. Specifically, it describes the possibilities of synthesizing such isocyanates, the raw materials for their production, and potential applications. It also provides a detailed patent analysis to show trends in the industry, as patents give important information about the technological trends and new technologies. Finally, future research directions and a summary of the review is included in the final section.
2. Characteristics of polyurethanes
The overall properties of polyurethanes are related to their chemical composition, structure, and morphology. Polyurethane, which consists of alternating hard (HS) and soft (SS) segments, is a block copolymer. A general structure of polyurethanes is shown in Scheme 1. The content of hard and soft segments affects the structure, mechanical properties, thermal stability, and low temperature capability.21 In the temperature range from 30 to 150 °C, the hard segments are glassy (or even semi-crystalline) polymers, while the soft segments are already above the glass transition temperature. By varying the relative amounts of hard and soft phases, the physical and mechanical properties of polyurethanes can be adjusted. Increasing the content of hard segments should lead to increased modulus, strength, and brittleness of the material.22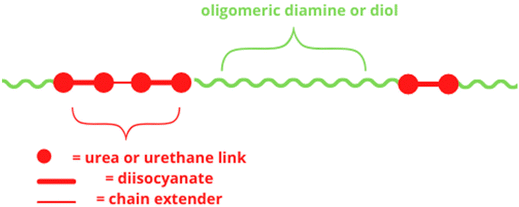 | ||
| Scheme 1 The structure of polyurethane.22 | ||
Because of differences in the polarity and chemical nature of monomers between segments, domain structure formation and phase separation occur. The hard segment domains and the soft segment domains can be distinguished, as shown in Scheme 2.23 Phase separation and domain formation depend on many factors, such as the nature of the monomers, the type and size of HS and SS, the reaction conditions, and the average molecular weight of the polyol. For example, in thermoplastic polyurethanes, the proportion of soft segments is much higher than the proportion of hard segments.24 Consequently, these materials exhibit a complex morphology in which flexible segments form a continuous matrix in which rigid segments are dispersed.
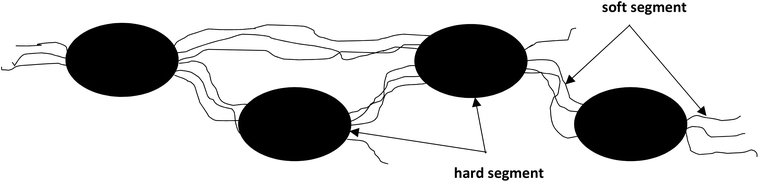 | ||
| Scheme 2 The phase structures of segmented polyurethanes.25 | ||
The microphase separation between the segments results in thermodynamic incompatibility. This incompatibility depends on several factors such as the length of the segments, the structure of the reactants, and the degree of crystallinity. Because hydrogen bonds are formed between the N–H bond of one segment and the carbonyl group of the other segment, the rigid domains act as cross-links. Therefore, the hard domains are responsible for high modulus reinforcement, while the soft segments affect plasticity and extensibility.26
Moreover chemistry and formulation of PUs largely affect the performance of the final product. For example, thermoplastic PUs are high-strength, lightweight materials and have relatively low processing costs. In addition, thermoplastic components are relatively easy to manufacture with high volume and precision.27 The properties of thermoplastics can be formed by an adequate ratio of hard segments to soft segments. For example, the increase of the hard segments ratio in the molecular structure results in a better combination of matrices, which leads to higher tensile strength, better chemical resistance and higher hardness.28 On the other hand, thermoset PUs retain their strength and shape even after heating. This makes them well suited for manufacturing durable parts and large, solid shapes.29 For example, different diisocyanates affect the degree of swelling and thermomechanical properties of thermoset PUs. For systems based on TDI (toluene diisocyanate) and HDI (hexamethylene diisocyanate), PUs showed higher Young's modulus and lower elongation at break than for systems based on IPDI (isophorone diisocyanate). However, the type of isocyanate did not affect the thermal stability of the materials, which was good for all systems.30
3. Fossil-based isocyanates
Commercially available isocyanates usually contain one or two isocyanate groups attached to an aromatic or aliphatic molecule. Aromatic isocyanates have some improved mechanical properties and higher reactivity, while aliphatic isocyanates do not turn yellow when exposed to light, which is a definite advantage, unfortunately they are more expensive than aromatic isocyanates.31 In industrially produced P-PUs, two isocyanates are used in 90% of the applications: TDI (toluene diisocyanate) and MDI (diphenylmethane diisocyanate).32 These isocyanates are produced on a large scale. TDI was the pioneer and is currently used mainly for low-density flexible foams.33 TDI exists in two isomeric varieties; we distinguish 2,4-diisocyanatoluene and 2,6-diisocyanatoluene. TDI is obtained by hydrogenating dinitrotoluene and then reacting the product (toluenediamine) with phosgene. For commercial use, mainly a mixture containing 20% of the 2,6-isomer and 80% of the 2,4-isomer is used.34 Unfortunately, the disadvantage of this product is the chemical inequality of the –NCO groups. The isocyanate group in position 4 may be up to 6 times more reactive than that in position 2.35 Another important isocyanate is MDI, which is also obtained by reacting with phosgene. MDI is generally a mixture of three isomers: 4,4′-methylene diphenyl diisocyanate (97%), 2,4′-methylene diphenyl diisocyanate (1.5 ÷ 2.5%) and 2,2′-methylene diphenyl diisocyanate (0.5%).36Table 1 provides a summary of commonly used isocyanates and their properties.| Compound | Structure | Molecular weight (g mol−1) | Melting point [°C] | Solubility | Commercial use | Ref. |
|---|---|---|---|---|---|---|
| MDI |
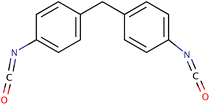
|
250.25 | 38 | Benzene nitrobenzene, acetone, kerosene | P-PUs, PIR | 37 and 38 |
| 2,4-TDI |
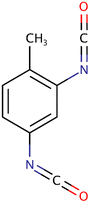
|
174.16 | 20 | Acetone, benzene, ethyl ether | P-PUs, PUF | 39 |
| 2,6-TDI |
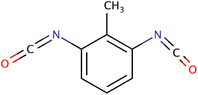
|
174.16 | 18 | Acetone, benzene | P-PUs, PUF | 40 |
| NDI |
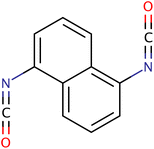
|
210.19 | 130 | Water | P-PUs, curing agent | 41 |
| HDI |
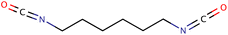
|
168.19 | −55 | Water | Coatings | 42 and 43 |
| IPDI |
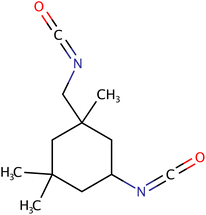
|
222.28 | −60 | Esters, ketones, ethers, aromatic and aliphatic hydrocarbons | Coatings, cross-linking, agent | 37 and 44 |
Fig. 5 shows the isocyanate market forecast from 2021 to 2025, the estimated growth of the sales market is 5%.45 The increase in demand in the global market is driven by the high demand for building materials (e.g. adhesives and foams). Furthermore, the growth of production in the automotive industry also contributes to the increase in isocyanate sales. Due to the fact that isocyanates are an intermediate product in many applications, the demand for isocyanates is expected to expand in the next few years.46
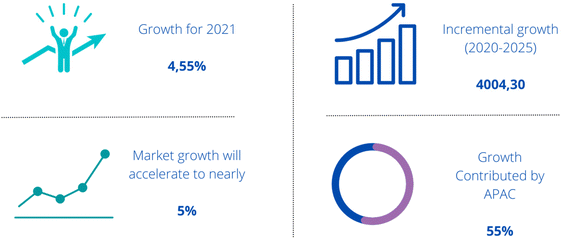 | ||
| Fig. 5 Global isocyanate market 2021–2025.45 | ||
The prominent players in the isocyanate market are listed below:
• Asahi Kasei Corporation,
• BASF SE,
• Bayer Material Science,
• Chemtura Corporation,
• Covestro AG,
• Dow Inc.,
• Evonik Industries AG,
• Gujarat N.V. Fertilizers,
• Huntsman Corporation,
• Kumho Mitsui Chemicals,
• Lanxess AG,
• Mitsui Chemicals Inc.,
• Tosoh Corporation,
• Wanhua Chemical Group,
• Vencorex.45
However, volatile raw material prices and their availability are constraints for the isocyanate market. Over and above this, the toxicity of isocyanates and their negative impact on the environment is an extremely important issue. The most economical (and therefore most widely used) way to produce isocyanates is through the reaction of phosgene with amines. Since phosgene is a highly toxic gas, this reaction is harmful to human and animal health.47 The global phosgene market was worth $3460.35 million in 2015 to $4672.05 million by 2021, with isocyanate production consuming around 77% of global phosgene production.48 The most significant route of exposure to phosgene is inhalation. The best way to prevent poisoning is to control exposure to hazardous levels. Unfortunately, this is difficult in the case of isocyanates because of the very low concentrations required to induce bronchospasm after sensitization. The odor threshold starts at 0.5 ppm, but toxic effects have been observed at concentrations below the threshold.49 Animal studies suggest that exposure levels range from ≤5 ppb to 5–10 ppb.50 In the respiratory tract, phosgene interacts with amino, hydroxyl, and sulfhydryl groups, causing the destruction of proteins and disruption of cellular functions. This results in a breakdown of the blood–air barrier and subsequent death.51 Industries that use phosgene must have a rigorous Safety Management System. This requires companies to invest a significant amount of capital, for example, in developing hazard identification and assessment procedures and an accident prevention system. Furthermore, phosgene installation must comply with national and international agreements and regulations. The associated regulatory requirements bring additional costs and obstacles to the use of phosgene.52 Moreover, household isocyanate contamination can also be an important problem due to the presence of isocyanates in various consumer products such as mattresses, pillows and soles. Furthermore, semi-volatile organic compounds such as isocyanates can be adsorbed by dust indoors, exposing household members to long-term exposure. Unfortunately, this problem has not been solved yet studied in depth, but there are concerns that continuous exposure to even low levels of isocyanates may influence the risk of allergy diseases.53
In addition, commonly used isocyanates, such as TDI or MDI, are highly toxic not only due to their method of production but also during decomposition because they release carcinogenic diamines. The most harmful route to human exposure is the inhalation of isocyanate vapors, which can cause respiratory diseases.54 Lower molecular weight isocyanates are more volatile at room temperature, increasing the risk of inhaling their vapors. In contrast, higher molecular weight isocyanates pose a hazard when aerosolized or heated in a workplace environment. Due to the exothermic nature of the underlying reactions, inhalation risks are very common while working with isocyanates.55 Dermal contact with isocyanates has also been shown to have negative effects on human health.56 Their toxicity is directly related to the high reactivity of isocyanates. Exposure to isocyanates is one of the most common causes of occupational asthma in industrialized countries.57,58 Furthermore, even low levels of isocyanates in the air can be highly toxic during inhalation. Samples of isocyanates have even been identified in wastewater after building material fires.59 The production of isocyanates and products containing them is regulated from a health and safety perspective (Mishra et al., 2009).60Table 2 shows the toxic degradation products of commercially used isocyanates. The above factors influence the development of bio-based isocyanates. The growth in the production of bio-based isocyanates is expected to further stimulate the demand in the market as the prices of crude oil, which is used in the production of isocyanates, are highly variable.
| Compound | Toxicity | Ref. |
|---|---|---|
| Butane diisocyanate (BDI) | The degradation product is putrescine, which is a chemical compound that belongs to biogenic amines. It is formed by the breakdown of proteins. In large doses, it is toxic | 62 |
| Hexamethylene diisocyanate (HDI) | Diamines released in degradation are toxic, although less toxic than aromatic diamines from MDI or TDI | 63 |
| Isophorone diisocyanate (IPDI) | Degradation product less toxic than aromatic isocyanates | 64 |
| Lysine diisocyanate (LDI) | During degradation, LDI degrades to lysine, a nontoxic by-product | 65 |
| Methylenebis (phenyl isocyanate) (MDI) | Toxic aromatic diamine degradation products | 66 |
| Toluene diisocyanate (TDI) | Very toxic by inhalation | 67 |
From research, we already know that polyurethanes based on bio-based polyols can exhibit properties similar to PUR based on petrochemical polyols. For example, Zhao et al. proved that TPUs based on a PLA derivative showed balanced mechanical properties, and better damping properties after multiple reprocessing.68 Meanwhile, replacing petrochemical isocyanates with their bio-based alternatives is not so obvious, but it is feasible, this process can be implemented in various ways. One method is to synthesize PUs entirely from bio-based isocyanates, but this is rarely applied; more often a mixture of bio- and petrochemical isocyanate is used. Both solutions are economically and environmentally beneficial. When using a petrochemical isocyanate, the cost of the mixture will certainly be reduced, while using bioisocyanate, entirely or partially, will have a beneficial effect on environmental concerns. Unfortunately, bio-based isocyanates have low reactivity, which is due to their aliphatic structure.69 However, it does not mean that their application is unfeasible. Hojabri et al. obtained diisocyanate from oleic acid by the Curtius rearrangement and proved its feasibility for polyurethane production by reacting with both bio- and fossil-based polyols. In their study, they found that polyurethanes based on bio-HPMDI (1,7-heptamethylene diisocyanate) had similar mechanical properties and thermal stability to HDI-based samples.70 The same research group obtained fully bio-based PUR on HDEDI (1,16-diisocyanatohexadec-8-ene), the materials obtained had better tensile strength, but had lower Young's modulus and higher elongation than HPMDI-based PUR.71 A group of researchers from the Netherlands also obtained PUR based on bio-isocyanate. Two isocyanates were used in this process: L-lysine diisocyanate ethyl ester (LLDI) and L-lysine tri-isocyanate ethyl ester (LLTI). The materials obtained were slightly yellow, as LLDI and LLTI were colored. Polyurethanes based on LLDI were more flexible than samples based on petrochemical isocyanate (IPDI). Unfortunately, PUs based on bio-based isocyanates swell in organic solvents, which does not happen with petrochemical PUs. In this research, PUR based on a mixture of bio-based and petrochemical isocyanates was also obtained. It has been found that the mechanical properties depend on the ratio of substrates used. The samples in which the IPDI content predominated were brittle and hard. The addition of bio-isocyanate caused the material to soften and become more fragile. On the other hand, PUs based on the LLDI/LLTI mixture showed the highest deformation at the maximum load. Meanwhile, Głowińska et al. studied the properties of PURs obtained from a mixture of isocyanates (Tolonate X FLO 100 (for more on this product see section 4.1), MDI and HDI).69 The analysis showed that the type of diisocyanate mixture affects the thermomechanical properties of partially bio-TPUs. Polyurethanes based on a mixture of HDI and Tolonate X FLO 100 have higher thermal stability and show higher tensile strength than those based on a mixture of MDI.
In summary, the properties of G-PUs require further improvement. Obtaining fully bio-based PUs is expensive and more challenging than producing P-PUs. An interesting alternative is the partial replacement of petrochemical hard segments, with bio-monomers. That would certainly reduce production costs, but, unfortunately will have less environmental benefit than a full G-PU. Therefore, undertaking large-scale production of bio-based isocyanates is important from an environmental point of view. The production of fully G-PUs is expected to be an important scientific challenge at that point.
4. Bio-based isocyanates and their synthesis
In light of the restrictions on diisocyanates adopted by the European Union authorities under REACH regulation, it is extremely important to look for alternative ways to produce isocyanates or to modify the synthesis of polyurethanes.72 The results of the patent analysis indicate a growing trend in the field of polyurethanes, while publication analysis shows increasing interest in the field of bio-isocyanates/bio-polyurethanes. The scientific results can contribute to the changes in the policy and use of isocyanates, providing information on the risks posed by various substances. A brief summary of patents related to the production of bio-based isocyanates is presented below. Patent analysis can readily identify current research gaps and can provide an important source of information for researchers. Moreover, it is a kind of science-industry connection. Later in this paper, the authors describe commercially available bio-based isocyanates and refer to papers outlining new ways to synthesize bio-isocyanates.Table 3 shows selected patents in the field of bio-based isocyanates. Patents US9950996B2, US4749806A and CN102659631B show the synthesis of isocyanates without the use of toxic phosgene, which reduces the harmfulness of the production process itself.73–75 Patent WO2014147142A1 discloses an allophanate composition for coating production.76 Examples of implementation disclose an allophanate-type bio-based isocyanate prepared from hexamethylene diisocyanate. The invention EP3819259A1 relates to a process for the low-carbon synthesis of isocyanates using the RWGS reaction, providing hydrogen from water electrolysis to produce chlorine, as well as using oxygen from water electrolysis to burn polyurethane materials to form carbon dioxide and, in some cases, burning pyrolysis residues obtained from polyurethane-containing materials and using the resulting carbon dioxide as a feedstock for the RWGS reaction.77 The carbon monoxide, advantageously produced from the recycling of the waste polyurethane material, reacts with chlorine to form phosgene, which in turn reacts with amines to form isocyanates. From isocyanates, polyurethane materials can be re-produced by reacting with polyether polyol or polyester polyol. If CO2 and electricity from renewable energy sources are used to electrolyze water, a polyurethane material can be produced with improved sustainability. Even though the invention uses phosgene, it is an example of producing polyurethanes with a significant reduction in fossil carbon, which is in line with the sustainability policy. The last invention presented, CN113461894A, provides a sponge obtained from a bio-based isocyanate.78 The bio-based TDI is prepared by reacting toluene extracted from organisms with nitric acid, hydrogen, carbon monoxide and oxygen. The listed applications represent only part of the state of the art in the field of sustainable isocyanate application. It can be observed that there is a correlation between current research trends and patent applications based on the results of our patent analysis.
| Patent number | Title | Brief description |
|---|---|---|
| US9950996B2 | Bio-based aromatic diisocyanates for the preparation of polyurethanes74 | The present invention provides bio-based aromatic diisocyanate of formula (I) (scheme included in the patent description), wherein X is OCH3, Y is selected from —H or OCH3, and m = 0–12. The present invention further provides a method for the preparation of aromatic diisocyanate of formula (I) useful for the preparation of polyurethane |
| US4749806A | Process for the synthesis of isocyanates and of isocyanate derivatives75 | The present invention relates to a process for the synthesis of isocyanates and isocyanate derivatives. Isocyanates are obtained by reacting an organic halide with a metal cyanate in an organic medium in the presence of a catalyst consisting of a complex of nickel with at least one organic ligand, in which complex the nickel is in the zero oxidation state. A carbamate or a urea, respectively, are obtained by a subsequent reaction with a hydroxy compound or a primary or secondary amine. Isocyanates and their derivatives are used especially either as refined synthesis agents for the production of pesticides and medications, or as monomers or comonomers for the preparation of many macromolecular compounds |
| CN102659631B | One-step synthesis of ethyl isocyanate73 | The invention relates to one-step synthesis of ethyl isocyanate. The method abandons a process of phosgene feeding in routine techniques, and uses xylene as a solvent 150–200 kg ethylamine hydrochloride is dissolved in 400–600 L xylene; the xylene solution containing 200–300 kg trichloromethyl carbonate is added into a reaction vessel in a dropwise manner in the presence of 5–10 kg of a catalyst; and the following steps are carried out: preparation of the catalyst, synthesis of the target product, separation of the target product, synthesis of ethyl isocyanate, and preparation of the product after separation. The method effectively solves the problems of high risk, severe pollution, high cost and poor quality and the like in the process of phosgene feeding in the production process in the routine techniques that use phosgene |
| WO2014147142A1 | Allophanate composition76 | The invention relates to a composition for preparing a coating comprising (a) at least one compound comprising at least two secondary amine functions and (b) an isocyanate component comprising at least one allophanate and at least one polyfunctional isocyanate, and optionally a solvent |
| EP3819259A1 | Method for the production of isocyanates and polyurethanes with improved sustainability77 | The invention relates to a method for producing isocyanates and optionally polyurethanes by the following method: synthesising phosgene from carbon monoxide and chlorine; reacting phosgene with diamines to form diisocyanates and hydrogen chloride; providing carbon dioxide gas flow; and cleaning the carbon dioxide gas flow of additional components, wherein carbon dioxide is converted by means of an RWGS reaction to form carbon monoxide and hydrogen, which are used as raw materials for polyurethane production, as well as optionally reacting the diisocyanates with polyether polyol and/or polyester polyol to form polyurethanes |
| CN113461894A | Sponge synthesized from bio-based isocyanate and mattress prepared from sponge78 | The invention relates to the technical field of mattresses, in particular to a sponge synthesized from bio-based toluene diisocyanate and a mattress prepared from the sponge. Preferably, the bio-based TDI is prepared by reacting toluene extracted from organisms with nitric acid, hydrogen, carbon monoxide and oxygen |
4.1. Feedstock for the synthesis of bio-based isocyanates
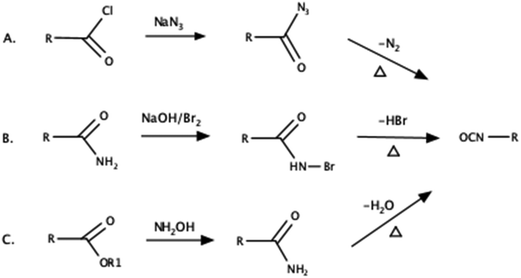
Since the early days of the polyurethane industry, biomass-derived substrates have been used. Initially, rigid polyurethane foams were obtained using castor oil.33 The development of synthetic alternatives led to the complete elimination of precursors from bio-based sources. Work on the synthesis of polyurethanes from renewable raw materials has now resumed due to predictions that fossil fuels will be depleted. Commercially produced bio-based isocyanates are already available, but their accessibility is limited. For example, Vencorex Chemicals (Saint-Priest, France) has launched an aliphatic diisocyanate called Tolonate™ X FLO 100 derived from palm oil. This isocyanate is characterized by low viscosity and 32% green carbon content.79 This isocyanate is typically used as a precursor for the synthesis of epoxy resin for coating applications. However, to the best of our knowledge, there are no examples related to the preparation of flexible polyurethane foams using Tolonate™. Moreover, Covestro offers a PDI trimer under the trade name Desmodur® eco N 7300.80 It is the world's first bio-based curing compound for lightweight polyurethane coating systems. It is characterized by being biobased (70%) and having a reduced carbon footprint (by 30%) compared to fossil-based products. It is used, for example, in coatings for the automotive or plastics industries. It can be used in both solvent-based and solvent-free systems. In terms of properties, it is similar to its petrochemical counterparts and has similar qualities. The last commercially available isocyanate is STABIO™ PDI, manufactured by Mitsui Chemicals.81 It is an aliphatic polyisocyanurate based on biobased 1,5-pentamethylene diisocyanate. It is characterized by a biomass content of more than 60% and a high NCO content. It shows appropriate resistance to solvents and weathering. It is applicable e.g. in paints. Unfortunately, the companies listed do not disclose their formulas in patent applications, presumably they use so-called trade secrets due to the unique product. Table 4 presents a comparison of the commercially available isocyanates.Bio-based isocyanate synthesis can be carried out by the Curtius, Hoffman or Lossen rearrangement illustrated in Fig. 6. Unfortunately, these methods have some disadvantages. The Curtius process uses highly toxic and explosive azides, while in the Hoffman and Lossen rearrangement, only aliphatic isocyanates can be obtained.83
In addition to different methods of synthesis, isocyanates can also originate from different biomass components. This section provides a description of the raw materials for the synthesis of bio-based isocyanates.
Hojabri et al.85 in their work proposed a method for obtaining aliphatic 1,7-heptamethylene diisocyanate (HPMDI) from oleic acid by the Curtius rearrangement (Scheme 3). The reaction involved the thermal decomposition of acyl azide, which was an intermediate in the preparation of isocyanates. The resulting product was then converted in situ to polyurethane. The problem of hydrolysis of acyl azide and isocyanate was eliminated by the use of anhydrous reagents. The obtained diisocyanate was successfully used in the next step for the synthesis of polyurethanes. The materials obtained exhibited physical properties comparable to those of similar materials based on petrochemical feedstocks. One drawback of the described diisocyanate production process is the requirement for dry reaction conditions. Moreover, the azides used are explosive and require special conditions to work at lower temperatures. Furthermore, the described method uses the solvent THF, which is a hazardous substance, so it would require further studies with the use of milder solvents. Therefore, producing isocyanates based on the described method would be difficult to implement on a large scale, and would require the development of a process and a lot of money. In a subsequent work by Hojabri et al.86 the synthesized poly(ester-urethanes) obtained from polyester diols and aliphatic diisocyanates were from natural raw materials. The results obtained confirmed the structure and properties of materials based on natural products.
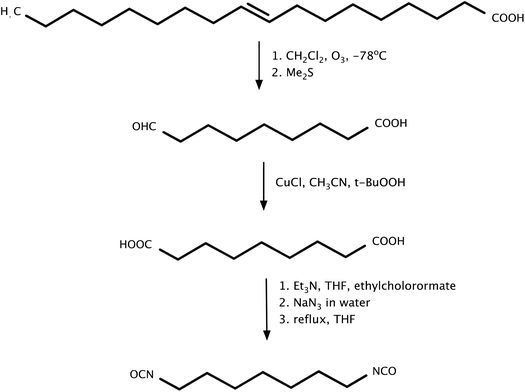 | ||
| Scheme 3 Synthesis of 1,7-heptamethylene diisocyanate (HPMDI) from oleic acid by the Curtius rearrangement. | ||
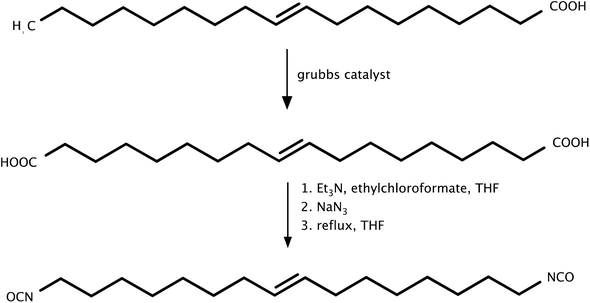
In another work, Hojrabi et al.71 synthesized 1,16-diisocyanatohexadec-8-ene (HDEDI) from oleic acid by the Curtius rearrangement (Scheme 4). First, oleic acid was reacted with the Grubbs catalyst to give unsaturated dicarboxylic acid. In the second step, the Curtius rearrangements were carried out using ethyl chloroformate, triethylamine, and dehydrated tetrahydrofuran. Sodium azide was then added dropwise under a nitrogen atmosphere to obtain a light yellow oil, which was used to synthesize fully bio-based polyurethanes. From the study, it was found that for HDEDI-based polyurethanes, the hydrogen bond strength was higher than that of polyurethanes produced from HPMDI, which influenced their higher tensile strength at break in comparison to that of TPUs synthesized from HPMDI. In addition, polyurethanes based on HDEDI showed a lower Young's modulus and a higher elongation. Unfortunately, the melting point of HDEDI-based PUs (80 °C) and HPMDI (73 °C) was much lower than that of HDI-based polyurethanes (140 °C). Moreover, the separation of the diol from the polyol mixture required a large amount of solvent and time, so large-scale production would be impossible. The last key factor limiting large-scale production of the aforementioned isocyanates (HPMDI and HDEDI) is their reaction yields; at the laboratory scale, they were obtained with 68% and 57% yields, respectively.
Cifarelli et al. obtained bio-polyols from epoxidized soybean oil and linseed oil.87 A solvent-free method using caprylic acid or 3-phenyl butyric acid with triethylamine as the catalyst was used. Then, as shown in Scheme 5, water blown polyurethane foams were prepared from the obtained bio-polyol and partially bio-based isocyanate (Tolonate X FLO 100). The G-PU foams were characterized by an open cell structure with a well-developed pore network. The polyurethane foams obtained were characterized by slightly lower values of Young's modulus and compressive strain in comparison to those of the reference foam. Unfortunately, vegetable oil-based polyols are more flammable than petrochemical polyols.88 As a result, the obtained foams may not be suitable for commercial applications. There are several possibilities to make such foams less flammable, e.g. by incorporating elements such as phosphorus or nitrogen into their backbone, however, it will cause the process to be more complicated and more expensive.
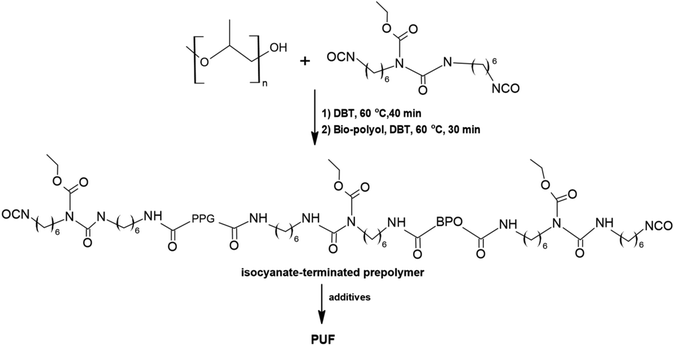 | ||
| Scheme 5 Scheme of the synthesis of polyurethane foam from bio-polyol and partially bio-based isocyanate.87 | ||
Another paper presents the synthesis of unsaturated triglycerides of vegetable oils with isocyanate groups.89 The synthesis had several steps; in the first step, methyl oleate was brominated in the allylic position using N-bromosuccinimide. The crude product was purified by column chromatography on silica gel with n-pentane/CHCl3 as the eluent. In the next step, the obtained product was dissolved in THF and portions of AgNCO were added. In both the first and the second stages, the mixture was protected from sunlight. The methyl oleate isocyanate thus obtained was mixed with methanol and refluxed overnight. Dry conditions were maintained during the synthesis. Allyl isocyanates were obtained in 60–70% yield. When dried soybean oil was used instead of methyl oleate, the by-products obtained were alkyl halides, but they reacted readily with AgNCO. Polyurethanes and polyurea were characterized by low mechanical strength and high elongation. All polyurethanes were characterized by a high swelling ratio. These polyurethanes can be used in applications where high mechanical properties are not required. Therefore, the main drawback of this synthesis was the use of an expensive reagent such as AgNCO. Due to its unique nature, it is not possible to replace it with a cheaper component. Moreover, this synthesis implies the use of solvents such as THF, therefore this synthesis has only a limited environmental benefit.
For the synthesis of diisocyanates also oleic and erucic acid esters were used. Following nucleophilic substitution, novel linear isocyanates were obtained: 1,19-diisocyanatononadecane and 1,23-diisocyanatotricosane.90 The process consisted of four steps; in the first step, dimethyl nonadecanedioate and dimethyl tricosandioate were obtained by isomerizing alkoxycarbonylation (Scheme 6). The obtained products were then reduced to nonadecanediol and tricosanediol, which were then converted to the corresponding bromides (the Appel II reaction). In the final step, nucleophilic substitution was carried out to obtain nonadecane diisocyanate and tricosane diisocyanate. The yield of the process was up to 40%. Despite the low efficiency, this type of reaction does not cause hydrocarbon chain shortening. The diisocyanates thus synthesized were then used for the polyaddition reaction with diols. The research group successfully obtained transparent polyurethane-polyether bio-copolymers in this method. These polymers had lower melting points and reduced ductility and elasticity. To bring this method to a large scale, it would be necessary to increase the efficiency and eliminate the use of toxic solvents.
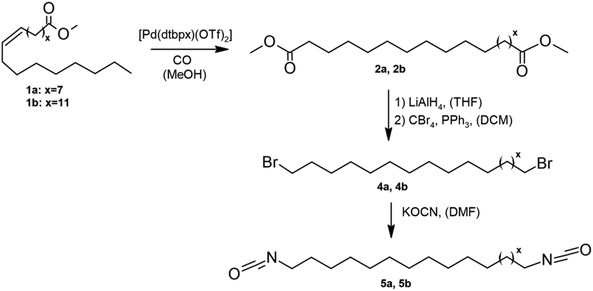 | ||
| Scheme 6 Synthesis of fatty acid based 1,19-diisocyanatononadecane and 1,23-diisocyanatotricosane.90 | ||
More et al. developed an environmentally friendly method for the preparation of isocyanates by the phosgene-free route presented in Scheme 7.91 The substrates in the process were castor oil derivatives – sebacic acid and undecylenic acid. Both acids reacted with hydrated hydrazine, and after filtration, the solid was refluxed in ethanol and filtered to obtain a white solid. Concentrated hydrochloric acid, acetic acid, and dichloromethane were added to the prepared suspension, and then aqueous solution of sodium nitrite was dropped into the mixture. This solution in anhydrous tetrahydrofuran was then refluxed under a nitrogen atmosphere. The compound thus prepared had a yield of about 70%. DITD diisocyanate had non-equivalent NCO groups and was unstable in nature, while isocyanate with equivalent units was stable and easy to work with. Diisocyanates were used to synthesize polyurethanes with different diols. The type of monomers affected the properties of the obtained PUs, and both amorphous and semi-crystalline morphologies were obtained. PUs based on DITD/propanediol were amorphous, while when the number of carbon atoms in the diol structure was increased, semi-crystallinity was induced. PUs based on DIO and linear aliphatic diol were semi-crystalline. An increase in melting point was observed when the number of methylene units increased from 3 to 4, while a decrease in melting point was caused by a higher number of methylene spacers.
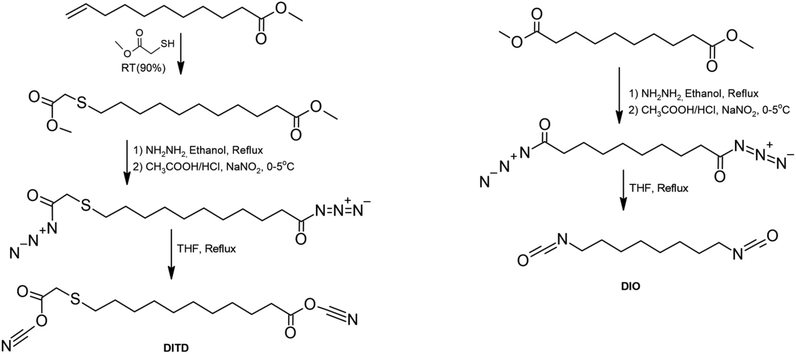 | ||
| Scheme 7 Synthesis of diisocyanates based on castor oil derivatives.91 | ||
 | ||
| Scheme 8 Reaction scheme for obtaining isocyanate from carboxylic acid.96 | ||
Kuhire et al. obtained in their work aromatic diisocyanates: bis(4-isocyanato-2-methoxyphenoxy)alkane and bis(4-isocyanato-2,6-dimethoxyphenoxy)alkane, synthesized by the Curtius rearrangement (Scheme 9). Vanillic acid and syringic acid, derived from lignin, were used for the synthesis. Subsequently, the obtained diisocyanates were used to synthesize poly(etherurethanes) with aliphatic bioderived diol(1,10-decanediol and 1,12-dodecanediol).97,98
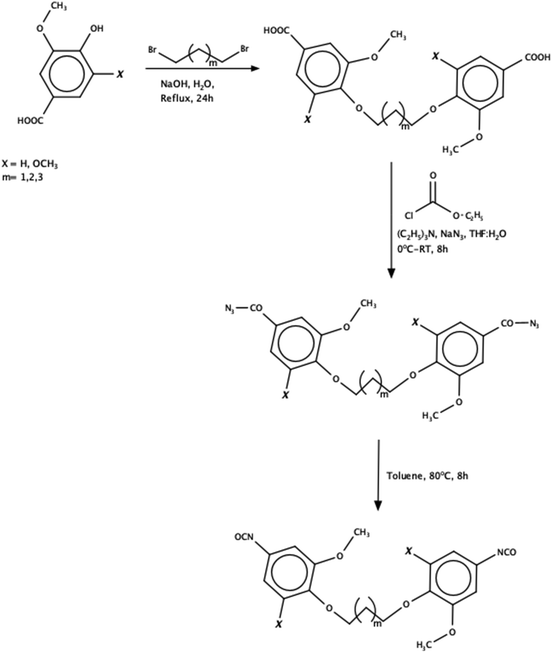 | ||
| Scheme 9 Synthesis of aromatic diisocyanates.97 | ||
The direct route of synthesis by the McMurry coupling method has also been communicated. In this method, vanillin was reacted with TiCl4/Mg in THF (Scheme 10). The stilbene obtained in this process then reacted with CNBr and NEt3 to give cyanate esters. Esters were isolated by filtration and water washing. The products were obtained in appropriate yields, and their structure was studied by single crystal X-ray diffraction.92 The prepared (bis)cyanate esters were thermally curable under mild conditions to obtain a thermosetting material, which was comparable to petroleum-derived epoxy resins.
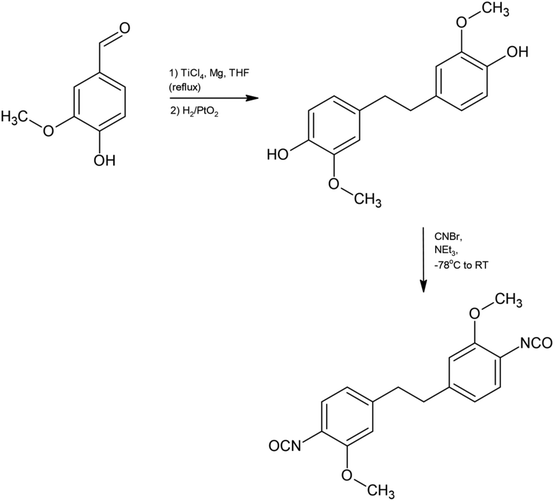 | ||
| Scheme 10 Synthesis of bisphenols and cyanate esters from vanillin.92 | ||
Considering the good properties of lignin, its availability, low cost and biodegradability, many attempts are currently undertaken to use it as one of the components of polymer matrices and other industrial applications.99 However, the structure and functionality of lignin depend on its source and processing conditions. These fluctuations can affect the properties of the final product causing structure inhomogeneity. Such variations are unacceptable for commercial applications that require repeatable properties and high purity of the material.100
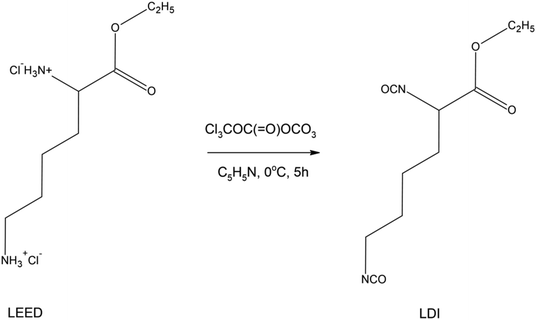 | ||
| Scheme 11 Synthesis of L-lysine ethyl ester diisocyanate (LDI).102 | ||
Analysis of mechanical properties showed that the maximum tensile strength was 23 MPa and the maximum elongation was 1700%. Since PCL is a biodegradable polyester, it impacts the biodegradable properties of polyurethane. Degradation products of the obtained polyurethanes are, for e.g., non-toxic L-lysine amino acids and α-hydroxylcaproic acid. However, in this method the products were obtained with a yield of 50%. Moreover, the study used pyridine, which was responsible for absorbing the chloride formed in the reaction. Before the proposed method finds industrial application, the efficiency of the reaction should be improved and the use of toxic substances like pyridine must be eliminated. It was concluded that this type of degradable polyurethane has potential for use in porous tubular scaffolds. In another work, a biodegradable poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA-PEG-PLA) triblock oligomer was synthesized and then, using the same stoichiometric ratio of PLA-PEG-PLA, L-lysine ethyl ester diisocyanate (LDI) and 1,4-butanediol (BDO), biodegradable polyurethanes were prepared.104 The obtained polyurethanes exhibited rapid degradation rates and may find applications in drug delivery systems and contrast agents for magnetic resonance imaging. Also, the paper ‘Non-toxic polyester urethanes based on poly(lactic acid), poly(ethylene glycol) and lysine diisocyanate’ presents the synthesis of polyurethanes for medical applications.105 The authors prepared copolymers of poly(lactic acid) and poly(ethylene glycol), followed by a chain-extension reaction with L-lysine ethyl ester diisocyanate. The polyurethanes obtained had acceptable properties.
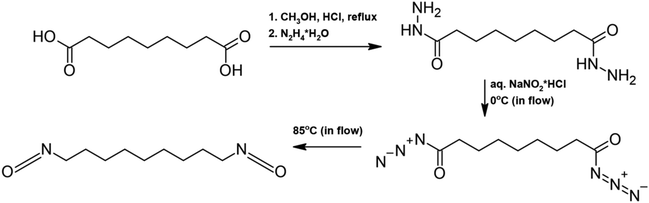 | ||
| Scheme 12 Synthesis of heptamethylene diisocyanate from algae-based azelaic acid using flow chemistry.106 | ||
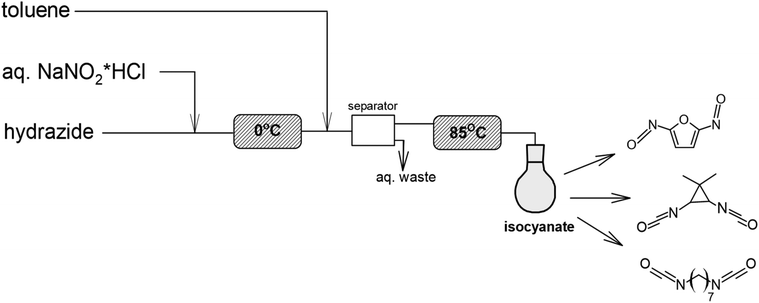 | ||
| Scheme 13 Synthesis of heptamethylene diisocyanate from algae-based azelaic acid using flow chemistry.106 | ||
To the authors’ best knowledge, the presented papers are the only reports on the synthesis of isocyanates from algae. These methods may be a promising way to synthesize bio-based isocyanates in an environmentally and economically friendly way. Among the major challenges of algal production is identifying strains with high lipid content, growth rates and high lipid yields for large-scale production.
Bifunctional dianhydrohexytols can be obtained from polysaccharides. They are obtained from renewable resources, such as by double dehydration of D-glucose.110,111 Bachmann et al. used dianhydrohexitols with three configurations (D-gluco, L-ido, D-manno) to prepare diisocyanates (Scheme 14).112 Two methods, cold and hot phosgenation, were used to convert the substrates into diisocyanates. The yield of the process depended on the stereochemistry of the dianhydrohexitols. Phosgene addition was more efficient when the amino groups were orientated out of the molecular plane. Therefore, the synthesis of diisocyanate with an L-ido configuration gave the highest yield. The obtained diisocyanates were then used in a polyaddition reaction to obtain new polyurethanes. The advantage of such an isocyanate will be the use of natural raw materials as substrates, however, the authors should consider using a synthesis method other than phosgenation.
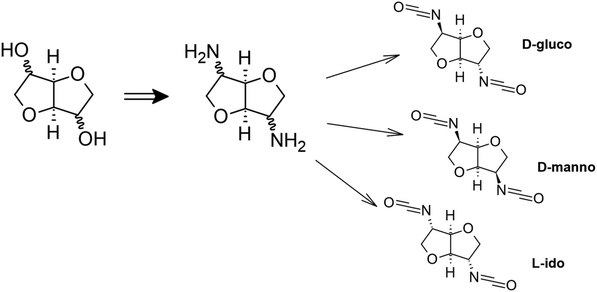 | ||
| Scheme 14 Monomer synthesis.112 | ||
An interesting substitute for petroleum-based substances is isosorbide, due to its rigidity and thermal stability. Zenner et al. obtained diisocyanates based on isosorbide. In the synthesis, succinic anhydride was also used because of the competitive price of production of succinic acid and the ability to eliminate petrochemical reagents.113 As shown in Scheme 15 in the first step of the reaction isosorbide and succinic anhydride were reacted by double esterification under solvent-free conditions. Then the Curtius rearrangement was used to obtain the product, thionyl chloride and sodium azide were used for this step. Unfortunately, aromatic solvents such as toluene were used in this synthesis. The yield of the process to obtain diisocyanate from isosorbide was 60% in total, it is possible to increase the yield to 70% by obtaining isocyanate directly from diacyl azide. The diisocyanates thus obtained were successfully applied in the synthesis of thermoplastic polyurethanes.
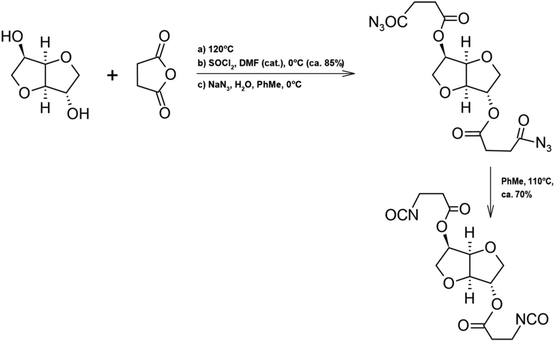 | ||
| Scheme 15 Synthesis of diisocyanate.113 | ||
Starch derived from field corn (non-edible) was used by Covestro to develop aliphatic pentamethylene isocyanate (PDI). It is the first commercial diisocyanate that contains 70% renewable carbon content. Scheme 16 illustrates the production process of this isocyanate.114
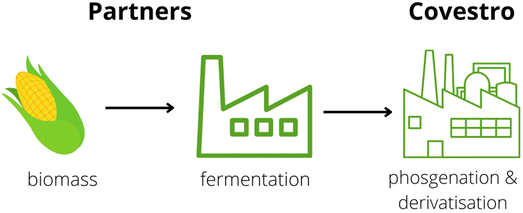 | ||
| Scheme 16 Industrial procedure for Desmodur eco N7300 from Covestro.114 | ||
Novolac resins that contain cardanol can be converted to the corresponding cyanate esters. Nair et al. in their work investigated the synthesis of cardanol-modified novolac resins and the effect of cardanol on their thermal stability (Scheme 17). The cyanate esters formed thermally stable phenol-triazine networks after curing.117 However, increasing the cardanol content decreased the thermal stability of the cured polymers. While Kulkarni et al. synthesized cyanate ester monomers containing pentadecyl-substituted cyclohexyl moieties.118 The obtained compounds, 1,1-bis(4-cyanatophenyl) 3-pentadecylcyclohexane, as well as 1,1-bis(4-cyanatophenyl) cyclohexane were characterized by Fourier-transform infrared spectroscopy and proton-nuclear magnetic resonance, among others. These compounds exhibited better processability, lower melting point and lower onset of cure compared to commercially available cyanate esters.
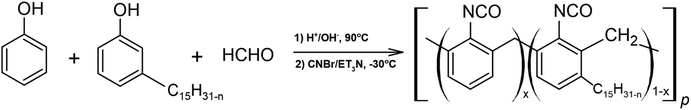 | ||
| Scheme 17 Synthesis of cardanol-based cyanate ester resins.116 | ||
5. Conclusions and future perspectives
This article presents up-to-date information about biomass-derived raw materials for isocyanate production. In recent years, increased interest in the topic of bio-based feedstocks has been reported. Bio-based raw materials used in the synthesis can include vegetable oils, lignin or amino acids. The isocyanates produced from biomass are characterized by a high green carbon content and low CO2 emissions. Moreover, the use of biomass provides an alternative to depleting fossil fuel resources. Monomers of biological origin are currently not used on a large scale, mainly for financial reasons, but, as can be seen, the environmental costs are much lower than those of petrochemical raw materials. In addition, the toxicity of isocyanates of petrochemical origin and the phosgene used in isocyanate production were discussed. Many review papers have considered the synthesis of polyols from feedstock of natural origin, but limited information is present for the synthesis of bio-based isocyanates. This review analyzes both patent applications and papers related to sustainability in isocyanate production. Among the factors slowing the development of bio-based polyurethanes are high production costs and limited production of commercially available monomers. The authors believe that long-term efforts are required to achieve a sustainable and environmentally friendly way of producing polyurethanes. These include improving technology to produce biobased monomers on a large scale at low cost, and developing new biobased monomers. Researchers should also emphasize on accelerating the commercialization of existing bio-based monomers. Ongoing development of sustainability policies and consumer awareness is also extremely important. Therefore, this paper presents the advantages and disadvantages of currently known methods of isocyanate production. Each of them has its own critical points that should be considered before large-scale production. Several of the outlined methods seem to be interesting alternatives to petrochemical isocyanates. Certainly, the transition from petrochemical substrates to renewable feedstocks will be a major success for the environment and industry. Perhaps a good alternative in the beginning would be to produce PUR from a mixture of isocyanates to reduce the cost while on the other hand increasing the green carbon content of the product, without sacrificing properties. It is believed that this review will be beneficial for researchers, academics or any other industry professionals seeking alternatives to commercially used isocyanates of petrochemical origin. The authors express hope for further development in this direction.Conflicts of interest
There are no conflicts to declare.References
- J. John, M. Bhattacharya and R. B. Turner, J. Appl. Polym. Sci., 2002, 86, 3097–3107 CrossRef CAS.
- C. Zhang, S. Bhoyate, M. Ionescu, P. K. Kahol and R. K. Gupta, Polym. Eng. Sci., 2018, 58, 2078–2087 CrossRef CAS.
- C. S. Carriço, T. Fraga, V. E. Carvalho and V. M. D. Pasa, Molecules, 2017, 22, 1–2 CrossRef PubMed.
- I. Singh, S. K. Samal, S. Mohanty and S. K. Nayak, Eur. J. Lipid Sci. Technol., 2020, 122, 1 CrossRef.
- C. Fu, Z. Zheng, Z. Yang, Y. Chen and L. Shen, Prog. Org. Coat., 2014, 77, 53–60 CrossRef CAS.
- K. Błażek and J. Datta, Crit. Rev. Environ. Sci. Technol., 2019, 49, 173–211 CrossRef.
- The Business Research Company, Polyurethane Global Market, 2021 Search PubMed.
- Grand View Research, Polyurethane (PU) Foam Market Share, Size & Trend Analysis Report By Product (Rigid Foam, Flexible Foam), By Application And Segment Forecasts To 2024, 2021.
- M. Macleod, H. Peter, H. Arp, M. B. Tekman and A. Jahnke, The Global Threat from Plastic Pollution, Science, 2021, 373, 61–65 CrossRef CAS PubMed.
- E. Petsko, https://Oceana.Org/Blog/Global-Plastic-Pollution-Crisis-Approaching-Irreversible-Tipping-Point/, 2021.
- European standard EN 16575:2014, Biobased products - Vocabulary.
- Scopus, https://www.scopus.com/results/results.uri?sort=plf-f&src=s&st1=polyurethanes&sid=0fd7baaa37aab8683c35eba41aaa7225&sot=b&sdt=b&sl=28&s=TITLE-ABS-KEY%28polyurethanes%29&origin=searchbasic&editSaveSearch=&yearFrom=Before+1960&yearTo=Present, 2022.
- Scopus, https://www.scopus.com/results/results.uri?sort=plf-f&src=s&st1=bio+polyurethanes&sid=f1f0a2bb7876bb4a0d64277b0ef55c02&sot=b&sdt=b&sl=32&s=TITLE-ABS-KEY%28bio+polyurethanes%29&origin=searchbasic&editSaveSearch=&yearFrom=Before+1960&yearTo=Present, 2022.
- A. Laue, D. Uhlig, B. Fiedler, J. Friedrich, C. Mende, L. Kroll and S. Spange, J. Polym. Sci., Part A: Polym. Chem., 2018, 56, 977–985 CrossRef CAS.
- G. Avar, U. Meier-Westhues, H. Casselmann and D. Achten, Polymer Science: A Comprehensive Reference, 10 Volume Set, Elsevier, 2012, pp. 411–441 Search PubMed.
- B. R. Donovan and D. L. Patton, Encyclopedia of Polymeric Nanomaterials, Springer, Berlin, Heidelberg, 2015 Search PubMed.
- C. Fu, Z. Zheng, Z. Yang, Y. Chen and L. Shen, Prog. Org. Coat., 2014, 77, 53–60 CrossRef CAS.
- L. M. Chiacchiarelli, Biomass, Biopolymer-Based Materials, and Bioenergy, Elsevier, 2019, pp. 135–160 Search PubMed.
- P. M. Paraskar, M. S. Prabhudesai, V. M. Hatkar and R. D. Kulkarni, Prog. Org. Coat., 2021, 156, 106267 CrossRef CAS.
- Y. Ma, Y. Xiao, Y. Zhao, Y. Bei, L. Hu, Y. Zhou and P. Jia, React. Funct. Polym., 2022, 175, 105285 CrossRef CAS.
- V. v. Ginzburg, J. Bicerano, C. P. Christenson, A. K. Schrock and A. Z. Patashinski, Nano- and Micromechanics of Polymer Blends and Composites, Carl Hanser Verlag GmbH & Co. KG, 2009, pp. 59–89 Search PubMed.
- L. Cong, G. Guo, F. Yang and M. Ren, Int. J. Transp. Sci. Technol., 2020, 10, 254–265 CrossRef.
- I. Javni, O. Bilić, N. Bilić, Z. S. Petrović, E. A. Eastwood, F. Zhang and J. Ilavský, Polym. Int., 2015, 64, 1607–1616 CrossRef CAS.
- L. Ugarte, B. Fernández-D'Arlas, A. Valea, M. L. Gonzaĺez, M. A. Corcuera and A. Eceiza, Polym. Eng. Sci., 2014, 54, 2282–2291 CrossRef CAS.
- B. Fernández-D'Arlas, L. Rueda, R. Fernández, U. Khan, J. N. Coleman, I. Mondragon and A. Eceiza, Soft Mater., 2011, 9, 79–93 CrossRef.
- A. Niemczyk, A. Piegat, Á. Sonseca Olalla and M. El Fray, Eur. Polym. J., 2017, 93, 182–191 CrossRef CAS.
- Y. Luo, Y. Xie, W. Geng, J. Chu, H. Wu, D. Xie, X. Sheng and Y. Mei, J. Mater. Sci. Technol., 2022, 129, 27–39 CrossRef.
- X. L. Xiang, S. Mi Hyun and C. Ur Ryong, Elastomers Compos., 2019, 54, 225–231 Search PubMed.
- Thomasnet, https://www.thomasnet.com/articles/plastics-rubber/thermoset-vs-thermoplastics/, 2022.
- E. Hablot, D. Zheng, M. Bouquey and L. Avérous, Macromol. Mater. Eng., 2008, 293, 922–929 CrossRef CAS.
- M. J. Mullins, D. Liu and H.-J. Sue, Thermosets, Elsevier, 2012, pp. 28–61 Search PubMed.
- Y. Pagnis, Chem. Eng. World, 1990, 25, 89–99 Search PubMed.
- L. M. Chiacchiarelli, Biomass, Biopolymer-Based Materials, and Bioenergy, Elsevier, 2019, pp. 135–160 Search PubMed.
- J. K. Fink, Reactive Polymers Fundamentals and Applications, Elsevier, 2013, pp. 49–93 Search PubMed.
- P. Król, Polimery/Polymers, 2009, 54, 489–500 Search PubMed.
- A. Kilanowicz, Podstawy i Metody Oceny Środowiska Pracy, 2009, 4(62), 59–88 Search PubMed.
- L. Bengtström, M. Salden and A. A. Stec, Fire Sci. Rev., 2016, 5, 2 CrossRef.
- National Center for Biotechnology Information, PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/4_4_-Diphenylmethane-diisocyanate, 2021.
- National Center for Biotechnology Information, PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/toluene-2%2C4-diisocyanate, 2021.
- National Library of Medicine, PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/2_6-Diisocyanatotoluene, 2021.
- Chemical book, Chemical Book, https://www.chemicalbook.com/ChemicalProductProperty_EN_CB1715235.htm, 2021.
- https://pubchem.ncbi.nlm.nih.gov/compound/Hexamethylene-diisocyanate#section=Spectral-Information, 2021.
- https://www.chembk.com/en/chem/HDI, 2021.
- Chemical book, Chemical Book, https://www.chemicalbook.com/ChemicalProductProperty_EN_CB4130253.htm, 2021.
- Attractive Opportunities in Isocyanate Market by Product, Application, End-User, and Geography - Forecast and Analysis 2021–2025, Canada, https://finance.yahoo.com/news/isocyanate-market-size-grow-4-023000388.html, 2021.
- Market Research Report - Isocyanates Market Forecast, https://www.grandviewresearch.com/industry-analysis/isocyanates-market/request/rs15, 2019 Search PubMed.
- T. A. Ryan, C. Ryan, E. A. Seddon and K. R. Seddon, in 1996, pp. 167–221.
- Global Phosgene Outlook 2016–2021, Gen Consulting Company, https://www.marketresearch.com/Heavy-Industry-c1595/Materials-Chemicals-c91/, 2016 Search PubMed.
- C. B. Bast and D. F. Glass-Mattie, Handbook of Toxicology of Chemical Warfare Agents, Elsevier, 2015, pp. 327–335 Search PubMed.
- S. M. Lee and D. Koh, Singapore Med J., 2008, 49(5), 372–5 CAS.
- M. Gutch, N. Jain, M. Singh, A. Agrawal, A. Vaish, S. Consul and S. Chaudhary, J. Emerg. Trauma Shock, 2013, 6, 271 CrossRef PubMed.
- Phosgene Handling, Indian Phosgene Council, 2017 Search PubMed.
- K. Bekki, S. Uchiyama and N. Kunugita, Anal. Bioanal. Chem., 2018, 410, 4247–4251 CrossRef CAS PubMed.
- R. J. Dearman and I. Kimber, Toxicology of Chemical Respiratory Hypersensitivity, CRC Press, London, 1st Edition, 2002 Search PubMed.
- Beth Donovan Reh, A Summary of Health Hazard Evaluations, Department of Health and Human Services, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, 2004 Search PubMed.
- A. Pronk, Occup. Environ. Med., 2006, 63, 624–631 CrossRef CAS PubMed.
- C. A. Redlich and M. H. Karol, Int. Immunopharmacol., 2002, 2, 213–224 CrossRef CAS PubMed.
- J. E. Lockey, C. A. Redlich, R. Streicher, A. Pfahles-Hutchens, P. (Bert) J. Hakkinen, G. L. Ellison, P. Harber, M. Utell, J. Holland, A. Comai and M. White, J. Occup. Environ. Med., 2015, 57, 44–51 CrossRef CAS PubMed.
- P. Blomqvist, T. Hertzberg, M. Dalene and G. Skarping, Fire Mater., 2003, 27, 275–294 CrossRef CAS.
- P. K. Mishra, R. M. Samarth, N. Pathak, S. K. Jain, S. Banerjee and K. K. Maudar, Int. J. Occup. Med. Environ. Health, 2009, 22, 193–202 Search PubMed.
- X. Zhang, K. G. Battiston, J. E. McBane, L. A. Matheson, R. S. Labow and J. Paul Santerre, Adv. Polyurethane Biomater., 2016, 75–114 Search PubMed.
- J. Guan, J. J. Stankus and W. R. Wagner, Cell Transplant., 2006, 15, 17–27 Search PubMed.
- M. Spagnuolo and L. Liu, ISRN Nanotechnol., 2012, 2012, 1–11 CrossRef.
- G. Morral-Ruíz, C. Solans, M. L. García and M. J. García-Celma, Langmuir, 2012, 28, 6256–6264 CrossRef PubMed.
- J.-Y. Zhang, E. J. Beckman, J. Hu, G.-G. Yang, S. Agarwal and J. O. Hollinger, Tissue Eng., 2002, 8, 771–785 CrossRef CAS PubMed.
- L. P. Gabriel, C. A. C. Zavaglia, A. L. Jardini, C. G. B. T. Dias and R. M. Filho, Chem. Eng. Trans., 2014, 38, 253–258 Search PubMed.
- Properties, Hazards and Safety Information for TDI, Belgium, n.d.
- X. Zhao, T. Shou, R. Liang, S. Hu, P. Yu and L. Zhang, Ind. Crops Prod., 2020, 154, 112619 CrossRef CAS.
- E. Głowińska, W. Wolak and J. Datta, J. Polym. Environ., 2021, 29, 2140–2149 CrossRef PubMed.
- L. Hojabri, X. Kong and S. S. Narine, Biomacromolecules, 2009, pp. 884–891 Search PubMed.
- L. Hojabri, X. Kong and S. S. Narine, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3302–3310 CrossRef CAS.
- https://worldwide.espacenet.com/patent/search?q=polyurethane, 2022.
- W. U. Shuyong, One-Step Synthesis of Ethyl Isocyanate, CN102659631B, 2011.
- P. P. Wadgaonkar and S. S. Kuhire, Bio-Based Aromatic Diisocyanates for Preparation of Polyurethanes, US9950996B2, 2015.
- T. Igor, J. Rabih, B. Michel, D. Gordon and L. Serge, Process for the Synthesis of Isocyanates and of Isocyanate Derivatives, US4749806A, 1987.
- R. Klucker and J.-M. Bernard, Allophanate Composition, WO2014147142A1, 2014.
- A. G. Covestro Deutschland, Method for the Production of Isocyanates and Polyurethanes with Improved Sustainability, EP3819259A1, 2019.
- X Linli, Sponge Synthesized from Bio-Based Isocyanate and Mattress Prepared from Sponge, CN113461894A, 2021.
- Vencorex, https://www.vencorex.com/product/tolonate-x-flo-100/, 2021.
- https://solutions.covestro.com/en/products/desmodur/desmodur-eco-n-7300_84603813-18871289?SelectedCountry=PL, 2019.
- Mitsui Chemicals, https://cms.ice.be/files/249/tds-stabio-presentation-.pdf, 2021.
- Mitsui Chemicals Inc., Mitsui Chemicals, Inc., https://jp.mitsuichemicals.com/en/service/product/stabio.htm, 2015.
- P. Kasprzyk and J. Datta, Elastomery, 2018, 22, 200–213 CAS.
- B. Nohra, L. Candy, J. F. Blanco, C. Guerin, Y. Raoul and Z. Mouloungui, Macromolecules, 2013, 46, 3771–3792 CrossRef CAS.
- L. Hojabri, X. Kong and S. S. Narine, Biomacromolecules, 2009, 10, 884–891 CrossRef CAS PubMed.
- L. Hojabri, J. Jose, A. L. Leao, L. Bouzidi and S. S. Narine, Polymer, 2012, 53, 3762–3771 CrossRef CAS.
- A. Cifarelli, L. Boggioni, A. Vignali, I. Tritto, F. Bertini and S. Losio, Polymers, 2021, 13, 1–21 CrossRef PubMed.
- Y. Y. Chan and B. Schartel, Polymers, 2022, 14, 2562 CrossRef CAS PubMed.
- G. Çayli and S. Küsefoǧlu, J. Appl. Polym. Sci., 2008, 109, 2948–2955 CrossRef.
- B. Schemmer, C. Kronenbitter and S. Mecking, Macromol. Mater. Eng., 2018, 303, 1700416 CrossRef.
- A. S. More, T. Lebarbé, L. Maisonneuve, B. Gadenne, C. Alfos and H. Cramail, Eur. Polym. J., 2013, 49, 823–833 CrossRef CAS.
- B. G. Harvey, A. J. Guenthner, H. A. Meylemans, S. R. L. Haines, K. R. Lamison, T. J. Groshens, L. R. Cambrea, M. C. Davis and W. W. Lai, Green Chem., 2015, 17, 1249–1258 RSC.
- R. R. Sederoff, J. J. MacKay, J. Ralph and R. D. Hatfield, Curr. Opin. Plant Biol., 1999, 2, 145–152 CrossRef CAS PubMed.
- J. Huang, H. Wang, W. Liu, J. Huang, D. Yang, X. Qiu, L. Zhao, F. Hu and Y. Feng, Int. J. Biol. Macromol., 2023, 225, 1505–1516 CrossRef CAS PubMed.
- S. Laurichesse and L. Avérous, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef CAS.
- W. G. Glasser, O. H.-H. Hsu, D. L. Reed, R. C. Forte and L. C.-F. Wu, Urethane Chemistry and Applications, 1981, pp. 311–338 Search PubMed.
- S. S. Kuhire, S. S. Nagane and P. P. Wadgaonkar, Polym. Int., 2017, 66, 892–899 CrossRef CAS.
- S. Kuhire and P. Wadgaonkar, Bio-Based Aromatic Diisocyanates for Preparation of Polyurethanes, WO2016103283, 2016.
- V. K. Thakur, M. K. Thakur, P. Raghavan and M. R. Kessler, ACS Sustainable Chem. Eng., 2014, 2, 1072–1092 CrossRef CAS.
- C. Zhang, H. Wu and M. R. Kessler, Polymer, 2015, 69, 52–57 CrossRef CAS.
- J. Konieczny and K. Loos, Polymers, 2019, 11, 1–8 CrossRef PubMed.
- J. Han, B. Chen, L. Ye, A. Y. Zhang, J. Zhang and Z. G. Feng, Front. Mater. Sci. China, 2009, 3, 25–32 CrossRef.
- J. S. Nowick, N. A. Powell, T. M. Nguyen and G. Noronha, J. Org. Chem., 1992, 57, 7364–7366 CrossRef CAS.
- Z. Wang, L. Yu, M. Ding, H. Tan, J. Li and Q. Fu, Polym. Chem., 2011, 2, 601–607 RSC.
- A. Pavelková, P. Kucharczyk, Z. Kuceková, J. Zedník and V. Sedlařík, J. Bioact. Compat. Polym., 2017, 32, 225–241 CrossRef.
- T. A. Phung Hai, L. J. S. de Backer, N. D. P. Cosford and M. D. Burkart, Org. Process Res. Dev., 2020, 24, 2342–2346 CrossRef CAS.
- X. Feng, A. J. East, W. Hammond and M. Jaffe, Contemporary Science of Polymeric Materials, 2010, pp. 3–27 Search PubMed.
- J. A. Galbis, M. d. G. García-Martín, M. V. de Paz and E. Galbis, Chem. Rev., 2016, 116, 1600–1636 CrossRef CAS PubMed.
- Y. Zhang, J. W. Chan, A. Moretti and K. E. Uhrich, J. Controlled Release, 2015, 219, 355–368 CrossRef CAS PubMed.
- J. Thiem and H. Lüders, Makromol. Chem., 1986, 187, 2775–2785 CrossRef CAS.
- E. Wolff, D. Bogdal, A. Loupy and M. Bortolussi, Proceedings of The 9th International Electronic Conference on Synthetic Organic Chemistry, MDPI, Basel, Switzerland, 2005, p. 1644 Search PubMed.
- F. Bachmann, J. Reimer, M. Ruppenstein and J. Thiem, Macromol. Chem. Phys., 2001, 202, 3410–3419 CrossRef CAS.
- M. D. Zenner, Y. Xia, J. S. Chen and M. R. Kessler, ChemSusChem, 2013, 6, 1182–1185 CrossRef CAS PubMed.
- B. v. Sanchez, G. Behnken and A. Hecking, NVVT Symp, 2016, pp. 1–27 Search PubMed.
- D. Chatterjee, N. v. Sadavarte, R. D. Shingte, A. S. More, B. v. Tawade, A. D. Kulkarni, A. B. Ichake, C. v. Avadhani and P. P. Wadgaonkar, Cashew Nut Shell Liquid, Springer International Publishing, Cham, 2017, pp. 163–214 Search PubMed.
- C. Voirin, S. Caillol, N. v. Sadavarte, B. v. Tawade, B. Boutevin and P. P. Wadgaonkar, Polym. Chem., 2014, 5, 3142–3162 RSC.
- C. P. R. Nair, R. L. Bindu and V. C. Joseph, J. Polym. Sci., Part A: Polym. Chem., 1995, 33, 621–627 CrossRef CAS.
- A. D. Kulkarni, B. v. Tawade and P. P. Wadgaonkar, High Perform. Polym., 2013, 25, 278–286 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |