 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Seasonal dynamics in structural characteristics within bark stems of cultivated willow (Salix sp.) by NMR and time-gated Raman spectroscopy†
Jinze
Dou
 *a,
Martin
Kögler
b,
Kavindra Kumar
Kesari
a,
Leena
Pitkänen
a and
Tapani
Vuorinen
a
*a,
Martin
Kögler
b,
Kavindra Kumar
Kesari
a,
Leena
Pitkänen
a and
Tapani
Vuorinen
a
aDepartment of Bioproducts and Biosystems, Aalto University, 02150 Espoo, Finland. E-mail: jinze.dou@aalto.fi; Tel: +358 413115001
bVTT Technical Research Centre of Finland, 90570 Oulu, Finland
First published on 26th January 2023
Abstract
The present study measures the seasonal dynamics of the chemical characteristics of pectin, hemicellulose, starch, protein, and soluble carbohydrates in the tissues of Scandinavian willow bark, which were examined during a complete annual cycle. Wet-chemical purification and non-destructive time-gated (TG) Raman spectroscopy were applied to illustrate the chemical characteristics as a response to the seasonal effect. Starch was found in a more complex linkage connection with hemicellulose and pectin in non-winter seasons, which is also supported by TG Raman spectroscopy. Interestingly, TG Raman spectroscopy also revealed that protein is more prominent in winter-harvested willow bark. The most striking seasonal effect investigated was in the structural characteristics of pectin. The pectin of winter-harvested willow bark has a larger proportion of the highly branched rhamnogalacturonan I (RG-I) domain compared to non-winter-harvested pectin. The study aims to build on this structural knowledge to provide seasonal-tailored enzymes and treat willow bark biologically so that sclerenchyma fibre bundles can be isolated. We demonstrate here for the first time that a combination of non-destructive TG Raman and wet-chemical purification identification methods provides more knowledge about the compositional changes throughout the seasonal variation of compounds in willow bark. The correlation between the seasonal dynamics and the structure of pectin from willow bark is discussed for the first time, which provides the essential knowledge required for optimizing the microbial consortium in valorizing willow bark that can possibly be harvested from multiple seasons.
Introduction
In the production of the pulp and paper industry, for example in mechanical pulp mills, bark represents roughly 10 wt% of tree logs.1 Apart from the major constituents of wood (cellulose, hemicellulose, and lignin), the bark contains a significant number of compounds such as pectin, starch, and structural proteins.2 The presence of high amounts of pectin and proanthocyanidin-enriched extractives in the bark may consume chemicals used in cooking and bleaching. Thus, they are considered as the least desirable fractions to be chemically consumed in a typical pulp and paper mill. Traditionally, the first step in pulp, paper, and timber production has been bark removal. Biological pretreatment3 has been suggested as an ideal method so that the pretreated-wood can be more effectively chemically cooked to produce pulp fibers. In certain fast-growing trees (like willow,4,5 eucalyptus,6 and poplar7), lignified sclerenchyma fiber bundles are organized in a continuous distribution and are surrounded by non-lignified tissue (mostly pectin).A tailor-made enzyme (pectinase and hemicellulase) consortium, which has been built up upon the structural characteristics of the substrate, succeeded in segregating sclerenchyma fiber bundles from willow bark.4 The bark is a very complex tissue with many layers and seasonal/annual variations in biosynthesis, which is related to the formation of different tissues and cell types. One of the major obstacles in optimizing this “tailor-made enzyme consortium based on the structural features of the substrate” strategy4 is simply the lack of a holistic approach to understanding the seasonal effects on structural features of major constituents in the bark cell walls, namely pectin and hemicellulose. Pectinases and hemicellulases could be tailored more precisely to liberate the sclerenchyma fiber bundle from the wood bark. For example, pectin synthesis is related to cell wall biosynthesis8,9 and heat stress.10 Although structural features of pectin have been reported to be related to the seasonal cycle of fruit plants (such as citrus11) and wood (such as poplar12 and spruce13), this seasonal variation has very rarely been reported in wood bark.
Understanding the chemical structure of pectin and hemicellulose is essential for designing a customized biological treatment to smartly recover the sclerenchyma fiber bundles from wood bark. Pectin consists mainly of a “smooth” region of homogalacturonan (HG) and rhamnogalacturonan I (RG-I) that are substituted at O-4 through side chains of arabinan, galactan, and arabinogalactan. The skeleton of RG-I has been considered the “hairy region” of pectin, which consists of 1,4-linked galacturonic acid (GalA) and 1,2-linked rhamnose units. Compared with strong mineral acids, extraction with citric acid is known to retain the pectin structure to its maximum extent.14 Additionally, hemicelluloses, the second most abundant group of polysaccharides, play an important role in strengthening the structural properties of the cell wall. Also, glucuronoxylan and xylan are the most prominent hemicelluloses.15 Glucuronoxylan, as the primary hemicellulose in hardwood, contains xylose and glucuronic acid as the main constituents. It is characterized by a linear β-(1,4)-linked β-D-xylopyranosyl units and substituted by 4-O-methyl-D-glucuronic acid (–MG) and acetyl groups. Alkaline extraction16 and peracetic acid delignification followed by DMSO extraction17 are conventional methods to extract hemicellulose from wood. However, hemicellulose extraction from tree bark has seldom been reported.
Raman spectroscopy is recognized as a non-deconstructive technique for the characterization of complex biochemical structures, and in comparison to other vibrational spectroscopic methods, such as near-infrared spectroscopy, there is very little interference from water. However, one limiting factor is the strong fluorescence background that may be superimposed on the weak signal from Raman scattering of the sample when using traditional continuous wave (CW) excitation Raman.18 In particular, lignin-containing compounds often suffer from high fluorescence. An advanced novel approach to overcome this is time-gated (TG) Raman spectroscopy that makes use of a pico-second pulsed laser synchronized with the detector to be able to “gate-out” the disturbing background signals, such as fluorescence, before they have a chance to spoil the actual Raman signal of the sample.
Herein, we followed classical protocols to extract and further identify differences in structural features of multiple fractions (i.e. extracts, pectin, and hemicellulose) from willow bark that was harvested at five intervals throughout one calendar year. Newer pretreatments have been developed to recover the hemicellulose fraction, particularly from bark. Furthermore, time-gated Raman spectroscopy was implemented as a non-destructive novel technique to reveal the structural profile from the original form of willow bark with a drastically reduced fluorescence background. Both destructive and non-destructive techniques were employed to explore the correlation between the chemical composition of polysaccharides (particularly of pectin, hemicellulose, starch, and pectin) and seasonal variations.
Material and methods
Materials and chemicals
Willow hybrid Klara was harvested at five intervals (Fig. 1) throughout the year 2020 from Carbons Finland Oy (Kouvola, Finland). The willow bark was manually peeled and Wiley-milled (<1 mm mesh) and stored at −20 °C before further use. Acetone, arabinose, citric acid, dichloromethane, dimethyl sulfoxide (DMSO), DMSO-d6, ethanol, ferulic acid, formic acid, fructose, galactose, glucose, hydrochloric acid, mannose, methanol, pectin from citrus peel (galacturonic acid > 74%), pepsin, peracetic acid, pyridine-d5, rhamnose, sodium hydroxide, starch (practical grade from corn), and xylose were purchased from Sigma-Aldrich, Finland. Kraft lignin (BiPiva™ 100) was purchased from UPM-Kymmene Oyj.Experimental flow
The milled original form (O) of willow bark was extracted under a Soxhlet unit (Electromantle: ColeParmer Extractors: Lenz). Three different solvents (i.e., water, dichloromethane, and acetone) were utilized for 4 h/solvent to remove both lipophilic and hydrophilic extracts. Extracts only soluble in water were recovered using a freeze-dryer and the lyophilized water-soluble extracts were kept in a desiccator for further characterization. The experimental flow comprised two additional major steps: pectin recovery using citric acid and hemicellulose recovery, as presented in Fig. 1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, w/v, pH 2) for 60 min at a temperature of 90 °C to chelate pectin polysaccharides. The soluble liquid fractions were recovered through a membrane (diameter size of 15–20 μm) and the citric-acid-treated solid residue (Citric) was preserved for further steps (Fig. 1). Ethanol was added to the filtered liquid with a final ethanol concentration of 75 v/v% and the liquid mixture was stored in cold conditions (+5 °C) for pectin precipitation. Centrifugation (8000 rpm, Eppendorf 5804R) and freeze drying were implemented to obtain lyophilized crude citric acid pectin (CAP). The CAP was further purified using dialysis membranes (Spectra/Por, MWCO 6–8 kDa, 96 h) to remove small molecular weight compounds. Finally, the dialyzed citric acid pectin (DCAP) was further centrifuged and freeze-dried for structural characterization.
30, w/v, pH 2) for 60 min at a temperature of 90 °C to chelate pectin polysaccharides. The soluble liquid fractions were recovered through a membrane (diameter size of 15–20 μm) and the citric-acid-treated solid residue (Citric) was preserved for further steps (Fig. 1). Ethanol was added to the filtered liquid with a final ethanol concentration of 75 v/v% and the liquid mixture was stored in cold conditions (+5 °C) for pectin precipitation. Centrifugation (8000 rpm, Eppendorf 5804R) and freeze drying were implemented to obtain lyophilized crude citric acid pectin (CAP). The CAP was further purified using dialysis membranes (Spectra/Por, MWCO 6–8 kDa, 96 h) to remove small molecular weight compounds. Finally, the dialyzed citric acid pectin (DCAP) was further centrifuged and freeze-dried for structural characterization.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 37 °C for 16 h in an incubation shaker at a speed of 300 rpm (CERTOMAT, Sartorius Biotech Inc.). The protein-free solid residue was further rinsed with hot water until the washing liquid became neutral. Most of the tannin-type compounds were removed with further treatment using 0.1 M NaOH (1/50, w/v) under nitrogen flow at 100 °C for 1 h and the solid residue was washed with water. Approximately 5 g of 0.1 M NaOH treated solid residue was processed further with delignification (1/30, w/v) using 10% peracetic acid at 85 °C at pH 4.0 for 1 h.17 The solid residues of holocellulose (P) were further extracted twice with DMSO (1/30, w/v) at 50–60 °C for 12 h. The united DMSO extracts (pH 3) were precipitated in 1 L of EtOH
1) at 37 °C for 16 h in an incubation shaker at a speed of 300 rpm (CERTOMAT, Sartorius Biotech Inc.). The protein-free solid residue was further rinsed with hot water until the washing liquid became neutral. Most of the tannin-type compounds were removed with further treatment using 0.1 M NaOH (1/50, w/v) under nitrogen flow at 100 °C for 1 h and the solid residue was washed with water. Approximately 5 g of 0.1 M NaOH treated solid residue was processed further with delignification (1/30, w/v) using 10% peracetic acid at 85 °C at pH 4.0 for 1 h.17 The solid residues of holocellulose (P) were further extracted twice with DMSO (1/30, w/v) at 50–60 °C for 12 h. The united DMSO extracts (pH 3) were precipitated in 1 L of EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH mixture (7/3, v/v), and the hemicellulose was recovered after centrifugation. Once the residual solvent was eliminated from the hemicellulose under a fume hood, freeze-drying was introduced to obtain the lyophilized hemicellulose (H). The solid residue (C) after DMSO extraction was rich in cellulose and collected for further analysis.
MeOH mixture (7/3, v/v), and the hemicellulose was recovered after centrifugation. Once the residual solvent was eliminated from the hemicellulose under a fume hood, freeze-drying was introduced to obtain the lyophilized hemicellulose (H). The solid residue (C) after DMSO extraction was rich in cellulose and collected for further analysis.
Analytics
Several chromatographic (HPAEC-PAD, HPLC, and SEC) and spectroscopic (Raman and NMR) techniques were employed for chemical characterization. Viscosity measurement was implemented only for the purified cellulose.Chromatographic techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Đ = 1.04) (dissolved in the same solvent of LiCl/DMAc) was used to determine the constants for both MALS and DRI detectors. A ∂n/∂c value of 0.136 ml g−1 was used for cellulose in 0.9% LiCl/DMAc. The viscosities of the cellulose were also determined with cupriethylenediamine (CED) according to the standard SCAN-CM 15:99.
000 g mol−1, Đ = 1.04) (dissolved in the same solvent of LiCl/DMAc) was used to determine the constants for both MALS and DRI detectors. A ∂n/∂c value of 0.136 ml g−1 was used for cellulose in 0.9% LiCl/DMAc. The viscosities of the cellulose were also determined with cupriethylenediamine (CED) according to the standard SCAN-CM 15:99.
Spectroscopic techniques
Nuclear magnetic resonance (NMR) spectroscopy was applied for analyses of the linkage structures of pectin and hemicellulose, respectively. Spectra were processed using a Topspin 4.0 (Bruker) and colorized using Adobe Illustrator 2023 (Adobe Inc.). The detailed experimental parameters are summarized below.Results and discussion
Mass balance and seasonal effect on recovered extracts and cellulose
The chemical composition of willow bark is measured as hexoses after acid hydrolysis of di- and oligosaccharides. In general, the seasonal dynamics of the hydrolyzed monosaccharides were significantly pronounced in the investigated samples, as shown in Fig. 2. The overall carbohydrate content reached its maximum in summer-season-harvested willow bark (WB) (i.e. 07-13), which is roughly 20%, 15%, and 10% higher than the winter-harvested (i.e. 02-17 and 12-04), spring-harvested (04-24), and late-autumn-harvested WB (09-23), respectively. However the recovered cellulose (with a repeating unit of β-glucose) content (Table S1†) differed from the overall trend of the carbohydrates (Fig. 2a), suggesting that the determined glucose (Fig. 2b) was possibly partly derived from starch. The exact conversion mechanism between starch and sugar is not within the scope of this study. Glucose was the main monosaccharide found in all samples, whereas xylose and mannose were the dominant non-cellulosic sugars besides pectin-characteristic monosaccharides. A comparison of the xylose/mannose ratio indicates that xylan was the main hemicellulose component in all investigated willow bark. Pectin characteristics (arabinose, rhamnose, galactose, galacturonic acid) were equally present in all the bark (Fig. 2b). The acid-insoluble “lignin” content of WB (O) (Fig. 2a) was roughly 15–20% higher than that of 0.1 M NaOH treated solid residues (N) (Table S1 and Fig. S1†), suggesting the overestimation of the acid-insoluble “lignin” was probably caused by the heterogenous chemicals that originated from the extractives, pectin, protein, and proanthocyanidins. Overall, the seasonal variation in the carbohydrate content of willow bark is clear and suggests that the bark tissues have a potential physiological role in synthesizing carbohydrates for over-wintering purposes. A similar observation about seasonal dynamics has previously been reported for the woody stems of Cornus sericea L.21 and Salix caprea L.22 as well as oak and beech,23 however, it is seldom reported for the bark.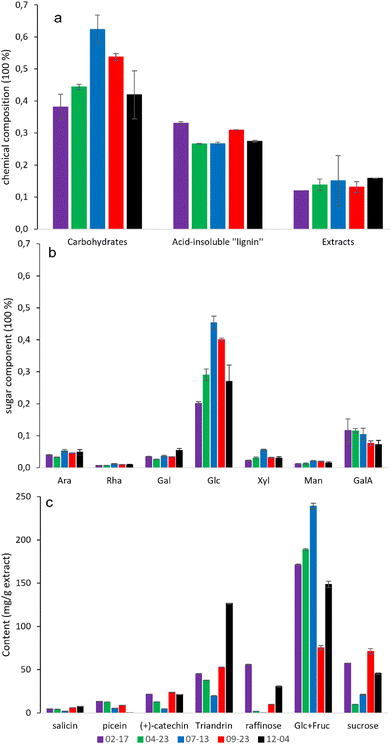 | ||
| Fig. 2 Chemical compositional analysis of the original form (O) of willow bark (02-17; 04-23; 07-13; 09-23; 12-04). (a) An overall chemical composition (% of the accepted dry mass) after acid hydrolysis of di- and oligosaccharides. (b) Carbohydrate composition (% of anhydro sugars in the monosaccharide) after acid hydrolysis of di- and oligosaccharides. (c) Chemical composition of the water-soluble extracts (without hydrolysis). For a detailed chemical profile of the sample after each pretreatment of citric acid extraction plus 0.1 M NaOH (N), peracetic acid delignification (P), recovered hemicelluloses (H), and the recovered cellulose (C), see Fig. S1.† Abbreviations: arabinose (Ara), rhamnose (Rha), galactose (Gal), glucose (Glc), xylose (Xyl), mannose (Man), galacturonic acid (GalA), fructose (Fruc). | ||
Similar seasonal variation also seems to be pronounced when looking at the sucrose and raffinose from the extractive composition, which was more abundantly present in water extract samples that are harvested during winter. A similar observation has been reported for the willow bark of other Salix hybrids (Tordis, Schwerenee, Winter, and Tora).24 Raffinose, known as a cryoprotectant oligosaccharide, has a high response to the cold hardening of Salix.21,25
Seasonal effect on pectin
To liberate the acidic polysaccharide pectin from willow bark, pH 2 citric acid was introduced as a chelating agent, and samples were correspondingly named CA-P. The crude pectin yield of winter-harvested samples (12-04 and 02-17) was slightly smaller than that of quantities purified from other seasons, such as early spring (04-23), the middle of summer (07-13), and the middle of autumn (09-23) (Table 1). In particular, the gravimetric yield of purified pectin has an almost equal presence in all seasons of willow bark. The dialysis treatment removed almost half to two-thirds of the small molecular weight fractions from crude pectin. Although dialysis of CA-P had a minimal effect on neutral monosaccharide composition (Table 1 and Fig. S2†), the treatment resulted in a roughly two to three-fold increase in GalA in all pectin samples. The relative amount of glucose in pectin harvested in winter was much smaller than in other seasons, suggesting that glucose may be derived in part from starch, and the mobilization of starch-to-sugar is well known as a self-regulatory mechanism for responding to cold acclimation. This starch breakdown mechanism during temperature stress26 has also been reported for tree species of willow22 and poplar.27 The ratio of Rha/GalA is generally low for pectin that is recovered from all samples, indicating there are higher HG fragments in the bark that are harvested from all seasons. This observation is also supported by the significant presence of GalA in recovered pectin. Interestingly, the (Gal + Ara)/Rha ratio was highest in early winter (10.6, 12-04) and slowly dropped until midsummer (5.9, 07-13), indicating that pectin harvested in winter had roughly twice as many branched RG-I domains as summer-harvested pectin (07-13). The degree of methylation (DM) and acetylation (DA), and Mw of recovered pectin from late winter (02-17) were the lowest among all pectin samples (Table 1).| 02-17-CA-P | 02-17-DCA-P | 04-23-CA-P | 04-23-DCA-P | 07-13-CA-P | 07-13-DCA-P | 09-23-CA-P | 09-23-DCA-P | 12-04-CA-P | 12-04-DCA-P | |
|---|---|---|---|---|---|---|---|---|---|---|
| HG and RG-I contents (mol%) were calculated from the monosaccharide composition. Standard deviations are shown in parentheses based on two independent measurements. For further clarification of the abbreviations, see Fig. 1 and 2. | ||||||||||
| Pectin yield (% WB) | 3.3 | 2.1 | 4.0 | 1.5 | 3.9 | 1.7 | 4.3 | 1.5 | 2.9 | 1.3 |
| M w (kDa) | 135 | 300 | 237 | 234 | 207 | |||||
| M w/Mn | 1.4 | 5.3 | 5.2 | 5.4 | 4.6 | |||||
| Monosaccharides (mg g −1 ) | ||||||||||
| Ara | 80 (1) | 49 (0) | 76 (2) | 115 (0) | 67 (2) | 104 (0) | 52 (1) | 83 (0) | 95 (2) | 122 (0) |
| Rha | 23 (0) | 16 (0) | 24 (1) | 29 (0) | 21 (1) | 29 (0) | 16 (0) | 23 (0) | 26 (1) | 29 (0) |
| Gal | 126 (1) | 77 (1) | 108 (3) | 107 (0) | 52 (1) | 75 (0) | 59 (1) | 74 (0) | 191 (4) | 205 (0) |
| Glc | 47 (2) | 29 (1) | 352 (10) | 202 (0) | 280 (8) | 168 (0) | 687 (12) | 412 (3) | 22 (0) | 18 (1) |
| Xyl | 4 (6) | 2 (3) | 3 (0) | 4 (0) | 4 (0) | 3 (0) | 1 (1) | 3 (0) | 3 (0) | 4 (1) |
| Man | 4 (6) | 2 (3) | 5 (0) | 4 (1) | 3 (0) | 1 (1) | 1 (2) | 6 (0) | 5 (0) | 7 (0) |
| GalA | 178 (0) | 450 (0) | 138 (0) | 446 (0) | 138 (0) | 340 (0) | 90 (0) | 307 (0) | 168 (0) | 446 (0) |
| Overall | 463 | 624 | 707 | 907 | 564 | 721 | 906 | 908 | 511 | 832 |
| Molar composition | ||||||||||
| Rha/GalA | 0.2 (0.002) | 0.0 (0.001) | 0.2 (0.009) | 0.1 (0) | 0.2 (0.006) | 0.1 (0) | 0.2 (0.005) | 0.1 (0) | 0.2 (0.005) | 0.1 (0.001) |
| (Gal + Ara)/Rha | 8.9 (0.03) | 7.7 (0.1) | 7.5 (0.1) | 7.7 (0.02) | 5.9 (0.05) | 6.2 (0.02) | 6.9 (0.1) | 6.8 (0.05) | 10.6 (0.1) | 11.0 (0.2) |
| HG (mol%) | 29.9 (0.5) | 66.3 (0.3) | 14.2 (0.5) | 42.4 (0.004) | 18.5 (0.6) | 39.3 (0.1) | 7.2 (0.2) | 28.6 (0.1) | 24.4 (0.5) | 46.1 (0.2) |
| RG-I (mol%) | 58.2 (1.6) | 28.2 (0.5) | 35.4 (0.3) | 34.3 (0.04) | 31.1 (0.2) | 36.8 (0.1) | 17.2 (0.04) | 24.8 (0.1) | 69.6 (0.5) | 50.2 (0) |
| DM (%) | 11 (5) | 55 (0) | 53 (0) | 92 (0) | 87 (0) | n.d. | 40 (2) | n.d. | 66 (18) | 71 (1) |
| DA (%) | 5 (1) | 20 (0) | 17 (0) | 28 (0) | 23 (0) | 28 (1) | 19 (2) | 36 (0) | 20 (1) | 27 (2) |
Solution-state 2D HSQC NMR (Fig. 3) revealed typical interunit linkages of pectin, and its spectra were assigned based on the literature4,28,29 and authentic starch (Fig. S3†). Three clear signals at δC/δH of 56.0/3.81, 23.4/2.09, and 19.5/1.26 ppm indicate the presence of methyl groups of 1,4-α-D-GalpA (OMe), 1,4-α-D-GalpA (OAc), and rhamnose, respectively. The non-anomeric methylene of GalA (δC/δH of 71.5/3.92, 71.5/4.01, 73.5/5.09, and 83.6/4.29 ppm) was also confirmed. Interestingly, strong starch (1,4-α-D-Glcp) signals were identified mostly in non-winter-harvested pectin at C1/H1 (102.8/5.40), C2/H2 (74.8/3.64), C3/H3 (76.4/3.96), C4/H4 (79.8/3.67), C5/H5 (74.35/3.83), and C6/H6 (63.9/3.83 ppm), confirming that starch linked with pectin can possibly act as energy storage in response to the chilling season. Specific non-anomeric and anomeric methine signals revealed the presence of arabinofuranosyl groups (terminal and 1,5-, 2,5-, and 2,3,5-linked) from the pectin that are recovered from all seasons. However, an almost negligible amount of galactopyranosyl groups was detected from the pectin of non-winter-harvested seasons (04-23, 07-13, and 09-23) in comparison to the pectin recovered from winter seasons (12-04 and 02-17). Overall, the temperature affects the biosynthesis of pectin. Starch was abundantly linked with the pectin that is harvested during non-winter seasons (04-23, 07-13, and 09-23). The presence of high amounts of galactose indicated that the pectin recovered from winter-season WB was more highly branched than in other seasons, which is also in line with the value of (Gal + Ara)/Rha (Table 1).
 | ||
| Fig. 3 2D heteronuclear single quantum coherence (HSQC) NMR spectrum of dialyzed citric acid extracted pectin (DCA-P) from five seasons of willow bark: 02-17; 04-23; 07-13; 09-23; 12-04. (a) Methyl (δC/δH, 0–59.84/0–3.943 ppm). (b) Non-anomeric methylene and methine (δC/δH, 58.82–94.67/3.155–4.548 ppm). (c) Anomeric methine group regions (δC/δH, 95.78–115/4.2–5.696 ppm). For abbreviations, see Fig. 1 and 2. | ||
Pectin is chemically heterogenous from winter-harvested pectin (02-17 and 12-04) to non-winter-harvested pectin (04-23, 07-13, and 09-23) (Table 1). Therefore the enzyme consortium4 also needs to be tailored based on the substrate structure and specific characteristics of the enzymes. For pectin, the ratio and composition of the HG and RG-I regions affect its degradation by pectinases. The pectin of winter-harvested WB (i.e. 12-04 and 02-17) showed a high proportion of highly branched RG-I and was rich in non-branched galactan sidechains, indicating the necessity of selecting galactanase as part of the consortium besides arabinose. In contrast, when the content of RG-I in pectin is low (i.e. 09-23), only the enzymes targeting the HG region need to be considered. The DM also influences the selection of HG-degrading enzymes. Pectate lyase and polygalacturonase show higher activity on low esterified pectin (i.e. 02-17), while pectin lyase and polymethyl galacturonase prefer highly esterified pectin (i.e. 07-13) with a high degree of esterification.4,30,31 In addition, acetylated GalpA residues can be removed by pectin/rhamnogalacturonan acetyl esterase. The correlation between the seasonal dynamics and the structure of pectin is reported for the first time, which provides the essential knowledge required for optimizing the microbial consortium in valorizing willow bark4 that is harvested in multiple seasons.
Seasonal effect on hemicellulose
Pretreatment using citric acid is important not only for pectin chelation but also for removing metallic inorganic components, which is crucial for minimizing the reactivity of peracetic acid. The recovered hemicellulose yield is within the range of 1–3 wt% willow bark; nevertheless, this fraction of recovered hemicellulose may not represent all hemicelluloses. The molar mass distribution of cellulose shifted to a shoulder peak at lower molar mass (Fig. S4†), which indicates that the shoulder peaks can be attributed to unrecovered hemicelluloses.32 Hemicelluloses from summer-harvested (07-13) pectin (>44 kDa) reached the maximum molecular mass that is recovered from other seasons (Table 2).| 02-17 | 04-23 | 07-13 | 09-23 | 12-04 | |
|---|---|---|---|---|---|
| Standard deviations are shown in parentheses based on two independent measurements. For further clarification of the abbreviations, see Fig. 1.a Percentage ratio between volumes of xylan-MG and xylan (Xy-1 and Xy-4 for xylan; Xy-MG-1 and Xy-MG-4 for Xy-MG).b Percentage ratio between volumes of xylan-acetyl and xylan (Xy-5a for xylan; Xy-acetyl for acetylated xylan).c Percentage ratio between volumes of starch and xylan (Xy-4 for xylan; St-4 for starch). | |||||
| Yield (w/w% WB) | 1 (0) | 3 (0) | 2 (0) | 3 (0) | 2 (0) |
| M w (kDa) | 29 | 35 | 44 | 32 | 33 |
| M w/Mn | 1.4 | 1.5 | 1.6 | 1.5 | 1.5 |
| Monosaccharides (mg g −1 ) | |||||
| Gal | 48 (0) | 29 (1) | 28 (1) | 28 (1) | 37 (1) |
| Xyl | 417 (2) | 461 (6) | 366 (1) | 350 (0.4) | 478 (0.4) |
| Characteristics | |||||
| Xylan-MG/xylana | 0.04 (0.01) | 0.03 (0.01) | 0.04 (0.02) | 0.03 (0.02) | 0.07 (0.002) |
| Degree of acetylationb | 0.008 | 0.0001 | 0.006 | 0.004 | 0.005 |
| Starch/xylanc | n.d. | 0.006 | 0.02 | 0.01 | n.d. |
2D HSQC NMR (Fig. 4) has been applied to elucidate the linkage feature of hemicelluloses. The assignments were based on the references.16,33,34 Willow bark's hemicellulose is not the same as xylan from a typical wood section of hardwood species, which contains very little (3–7%, Table 2) of the substituted –MG group. Specifically, C1/H1–C5/H5 of terminal xylose were identified for their characteristic peaks of 97.6/5.24, 72.7/3.19, 73.9/3.39, 75.5/3.62, and 63.2/3.25 (5a) and 3.96 (5b) ppm, respectively. The identified peak at δC/δH 20.4/1.97 ppm could be assigned to the acetyl group of xylan, although the degree of acetylation is less than 1% (Table 1). The peaks at δC/δH 81.7/3.23 (Xy-MG-4), δC/δH 72.1/3.74 (Xy-MG-3), and 97.6/5.24 ppm (Xy-MG-1) represent the C1/H1–C6/H6 correlations of the branched linkages between the 4-O-α-D-glucuronic acid (MeGlcA) and (1→4)-β-D-Xylp ((1→4)-β-D-Xylp-2-O-(4-OMe-D-GlcpA)), which were identified in all hemicellulose fractions (Fig. 4). δC/δH 55.9/3.46 (Xy-MG-OCH3) is only identified for hemicellulose fractions of WB that is harvested at 04-23 and 09-23. Furthermore, a signal for starch was detected only in the hemicelluloses fractions (at 0.6–2% on the basis of xylan) from WB that is harvested from non-winter seasons (04-23, 07-13, and 09-23), indicating that DMSO is possibly capable of partially dissolving α-glucan starch besides hemicellulose.35,36
 | ||
| Fig. 4 2D HSQC NMR spectrum of hemicellulose fractions that are recovered from willow bark of different seasons: 02-17; 04-23; 07-13; 09-23; 12-04. (a) Methyl (δC/δH, 15.47–51.17/1.7–3.1 ppm). (b) Non-anomeric methylene and methine (δC/δH, 52.86–87.58/2.005–4.888 ppm). (c) Anomeric methine group regions (δC/δH, 93.88–106.96/3.943–5.538 ppm). For abbreviations, see Fig. 1. | ||
Seasonal effect on protein and starch through TG Raman spectroscopy
TG Raman spectroscopy has been implemented to analyze the bark non-destructively without any pretreatment and with a low fluorescence background. Therefore, prior to TG Raman acquisition (Fig. 5), individual spectra of the main components, including ferulic acid, starch, pectin, and lignin, were recorded with both TG and CW Raman (Fig. S5†). Generally, the typical fingerprint of polysaccharide spectra is represented by pectin, glucan, starch, protein, and glucuronoxylan (hemicellulose) that exhibit bands in these three domains based on the literature.18,37–41 They could be assigned as C–O–C, C–O, and C–C stretching (1000–1200 cm−1); CH/CH2 deformation and C–O–H bending modes (1200–1500 cm−1); and skeletal and torsional vibration modes (below 800 cm−1). Interestingly, the peak intensity at 1440 cm−1 is assigned to a symmetric vibration of CH2 stretching of lignin. The vibrational intensity of 1440 cm−1 has higher intensities for WB harvested in the winter season (12-04 and 02-17) than for WB that is harvested in other seasons, which is in line with the determined acid-insoluble lignin that is shown in Table S1.† | ||
| Fig. 5 Time-gated Raman spectroscopic analysis of willow bark that was harvested from 02-17 to 12-04 (Fig. 1). For assignments see Table 3. | ||
The vibration of starch was displayed at 478 cm−1 (skeletal vibration of the glucopyranose unit), 850/920 cm−1 (C–O–C), 1100 cm−1 (glycosidic link C–O–C), and 1372 cm−1 (C–O–H symmetric stretching of starch), as summarized in Table 3.42 In particular, starch displayed a stronger band at 478 and 1378 cm−1 for bark that was harvested during non-winter seasons (04-23, 07-13, and 09-23), which supports the previous identifications that starch has a higher abundance in the recovered pectin and hemicellulose fractions. Moreover, the spectra show the characteristic signature of protein given by the amide I band (around 1650 cm−1) that was assigned to the vibration of the trans peptide group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ONH. Also, the amide III band (β-sheet) that arises from the combination of N–H bending and C–N stretching of the peptide group (1233 cm−1), the sharp band of the phenylalanine ring vibration (1005 cm−1), S–S carbohydrates (530–560 cm−1), and aromatic amino acids (632 cm−1). There are highly significant characteristic carbohydrate and protein peaks at 530–560 cm−1 as well as 632 and 1005 cm−1 for WB of 02-17 (late winter) and 12-04 (early winter) in comparison to other seasons. The parenchyma tissue from the inner bark might act as temporary storage of reduced nitrogen as a protein reserve in overwintering,43 where the protein has previously been identified in the fraction of enzymatically purified lignin of willow bark.44
ONH. Also, the amide III band (β-sheet) that arises from the combination of N–H bending and C–N stretching of the peptide group (1233 cm−1), the sharp band of the phenylalanine ring vibration (1005 cm−1), S–S carbohydrates (530–560 cm−1), and aromatic amino acids (632 cm−1). There are highly significant characteristic carbohydrate and protein peaks at 530–560 cm−1 as well as 632 and 1005 cm−1 for WB of 02-17 (late winter) and 12-04 (early winter) in comparison to other seasons. The parenchyma tissue from the inner bark might act as temporary storage of reduced nitrogen as a protein reserve in overwintering,43 where the protein has previously been identified in the fraction of enzymatically purified lignin of willow bark.44
| Peaks [cm−1] | Bond type/band assignment | Ref.a |
|---|---|---|
| a The Raman spectrum of the authentic ferulic acid, starch, pectin, and lignin are summarized in Fig. S5.† | ||
| 333–394 | Ferulic acid, pectin | 45 |
| 478 | S–S stretch containing starch | 42 |
| 440 | Pectin | |
| 530–560 | S–S stretch, carbohydrates, proteins | 18 and 37 |
| 632 | C–C twist, aromatic amino acids | 46 |
| 722 | CH2 rocking, nucleic acids (adenine) | 47 |
| 823 | C–O–C glycosidic link in RNA backbone | 48 |
| 844/856 | Pectin | 49 |
| 868/920 | Starch | 42 |
| 915 | Pectin, cellulose | 50 |
| 938 | Starch | 42 |
| 980 | Lignin | 51 |
| 1005 | CH3 rocking of protein (phenylalanine) | 47 and 52 |
| 1100 | Carotenoids | 52 |
| 1130 | C–N stretch (starch) | 52 |
| 1165 | Phenylalanine, tryptophan | 38 |
| 1233 | Amide III (β-sheet) | 47 |
| 1320 | C–C–C symmetric stretching, cellulose | 52 |
| 1378 | C–O–H symmetric stretching of starch | 42 |
| 1405 | Cellulose | 51 |
| 1420–1450 | Lignin υ(CH2) | 51 |
| 1520 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the plane of carotenoids C in the plane of carotenoids |
52 |
| 1631 | Lignin υ(CH2) shoulder of 1600 | 51 |
| 1650–1672 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch (amide I), protein O stretch (amide I), protein |
47 |
Conclusions
In this work, the chemical composition of willow bark has been demonstrated for its seasonal response to the harvesting seasons. Thus this information is interpreted in both a destructive manner (wet-chemical purification and characterization using NMR spectroscopy) and a non-destructive manner (novel time-gate Raman spectroscopy). The most striking seasonal effect involved the structural characteristics of pectin. Specifically, pectin showed clear structural differences as a response to the season, including the ratio and composition of HG and RG-I domains, and the branching profile of the RG-I sidechain. The correlation between seasonal dynamics and pectin structure is discussed for the first time, which provides the basic knowledge needed for optimizing the microbial consortium in valorizing willow bark that can possibly be harvested from multiple seasons. One of the major obstacles in further optimizing this “tailor-made enzyme consortium based on the structural features of the substrate” strategy4 and “controlled valorization of willow bark as a source of biochemicals and fiber”53 is simply the lack of a holistic approach to understand the seasonal effects on structural features of major constituents in the bark cell walls, namely pectin and hemicellulose. Pectinases and hemicellulases can then be tailored more precisely to liberate the sclerenchyma fiber bundle from the willow bark.The identified starch in the recovered fractions of both pectin and hemicellulose was found to be more abundantly present in non-winter seasons (04-23, 07-13, and 09-23) than in winter seasons (02-17 and 12-04). This is also supported by the relatively higher abundance of starch in the native form of willow bark that is harvested in non-winter seasons, as revealed by Raman spectroscopy. Polarized Raman spectroscopy also revealed that protein is more pronounced at 02-17 (late winter) and 12-04 (early winter) in comparison to other seasons. In addition to the pectinases and hemicellulases, the addition of starch- and protein-degrading enzymes to the enzyme cocktail consortium might also be carefully tailored according to its season of harvesting. The synergetic (or competing) effects between different enzymes acting on substrates still need to be further optimized by response surface methodology (RSM) experiments and this will be systematically investigated in another study. TG Raman spectroscopy has not been implemented previously as a facile and non-destructive technique to reveal a structural profile from the original form of willow bark with a drastically reduced fluorescence background.
These findings remind us that we must carefully consider seasonal dynamics in tailoring the enzyme consortium to recover sclerenchyma fiber bundles from willow bark, which play a crucial role in further optimizing the “tailor-made enzyme consortium based on the structural features of the substrate” strategy.4 It is also essential for designing controlled and tailored valorization strategies to utilize willow bark as a potential source of fiber bundles and extracts.53 A similar strategy can be tailored for the valorization of bark from other fast-growing trees, such as eucalyptus and poplar. However, it is still far from an understanding of the specific mechanism involved in the regulation of all these structural fractions as a response to the season. The combination of classical wet-chemical characterization and non-destructive TG Raman paves the way for a more intuitive understanding of seasonal effects.
Conflicts of interest
The authors declare that they have no known competing financial interests.Acknowledgements
This work also made use of the RawMatTERS Finland infrastructure (RAMI) facilities based at Aalto University. This work was a part of the Academy of Finland's Flagship Programme under project no. 318890 and no. 318891 (Competence Center for Materials Bioeconomy, FinnCERES). VTT Finland was supported by the Academy of Finland, provided through an Academy of Finland Flagship Programme (PREIN) on Photonics Research and Innovation, PREIN, no. 320165. The authors thank the Sensing Solutions research area of VTT for their support with the spectroscopic equipment and measurement time.References
- M. K. Hassan, A. Villa, S. Kuittinen, J. Jänis and A. Pappinen, An assessment of side-stream generation from Finnish forest industry, J. Mater. Cycles Waste Manage., 2019, 21, 265–280, DOI:10.1007/s10163-018-0787-5
.
-
E. Sjöstrom, Wood Chemistry: Fundamentals and Applications, Academic Press, New York, USA, 1981 Search PubMed
.
- M. Lehr, M. Miltner and A. Friedl, Removal of wood extractives as pulp (pre-)treatment: a technological review, SN Appl. Sci., 2021, 3, 886, DOI:10.1007/s42452-021-04873-1
.
- J. Dou, J. Wang, J. Zhao and T. Vuorinen, Tailor-made enzyme consortium segregating sclerenchyma fibre bundles from willow bark, Green Chem., 2022, 24, 2576–2587, 10.1039/D2GC00188H
.
- J. Dou, M. Rissanen, P. Ilina, H. Mäkkylä, P. Tammela, S. Haslinger and T. Vuorinen, Separation of fiber bundles from willow bark using sodium bicarbonate and their novel use in yarns for superior UV protection and antibacterial performance, Ind. Crops Prod., 2021, 164, 113387, DOI:10.1016/j.indcrop.2021.113387
.
- J. Dou, A. Karakoç, L. S. Johansson, S. Hietala, D. Evtyugin and T. Vuorinen, Mild alkaline separation of fiber bundles from eucalyptus bark and their composites with cellulose acetate butyrate, Ind. Crops Prod., 2021, 165, 113436, DOI:10.1016/j.indcrop.2021.113436
.
- S. Ö. Keleş, Variation in morphological and wood cell traits in coppice stems of Populus nigra L. and Salix alba L., J. Forensic Sci., 2021, 67, 396–407, DOI:10.17221/208/2020-JFS
.
- D. Mohnen, Pectin structure and biosynthesis, Curr. Opin. Plant Biol., 2008, 11, 266–277, DOI:10.1016/j.pbi.2008.03.006
.
- J. Harholt, A. Suttangkakul and H. V. Scheller, Biosynthesis of Pectin, Plant Physiol., 2010, 153, 384–395, DOI:10.1104/pp.110.156588
.
- T. Qu, R. Liu, W. Wang, L. An, T. Chen, G. Liu and Z. Zhao, Brassinosteroids regulate pectin methylesterase activity and AtPME41 expression in Arabidopsis under chilling stress, Cryobiology, 2011, 63, 111–117, DOI:10.1016/j.cryobiol.2011.07.003
.
- Y. Liu, H. Ahmad, Y. Luo, D. T. Gardiner, R. S. Gunasekera, W. L. McKeehan and B. S. Patil, Influence of harvest time on citrus pectin and its in vitro inhibition of fibroblast growth factor signal transduction, J. Sci. Food Agric., 2002, 82, 469–477, DOI:10.1002/jsfa.1037
.
- M. Baïer, R. Goldberg, A. M. Catesson, M. Liberman, N. Bouchemal, V. Michon and C. H. du Penhoat, Pectin changes in samples containing poplar cambium and inner bark in relation to the seasonal cycle, Planta, 1994, 193, 446–454, DOI:10.1007/BF00201825
.
- E. N. Makarova, E. G. Shakhmatov and V. A. Belyy, Seasonal dynamics of polysaccharides in Norway spruce (Picea abies), Carbohydr. Polym., 2017, 157, 686–694, DOI:10.1016/j.carbpol.2016.10.035
.
- F. Dranca and M. Oroian, Extraction, purification and characterization of pectin from alternative sources with potential technological applications, Food Res. Int., 2018, 113, 327–350, DOI:10.1016/j.foodres.2018.06.065
.
- J. Berglund, D. Mikkelsen, B. M. Flanagan, S. Dhital, S. Gaunitz, G. Henriksson, M. E. Lindström, G. E. Yakubov, M. J. Gidley and F. Vilaplana, Wood hemicelluloses exert distinct biomechanical contributions to cellulose fibrillar networks, Nat. Commun., 2020, 11, 4692, DOI:10.1038/s41467-020-18390-z
.
- S. Sun, X. Cao, H. Li, F. Xu and R. Sun, Structural characterization of residual hemicelluloses from hydrothermal pretreated Eucalyptus fiber, Int. J. Biol. Macromol., 2014, 69, 158–164, DOI:10.1016/j.ijbiomac.2014.05.037
.
- D. V. Evtuguin, J. L. Tomás, A. M. S. Silva and C. P. Neto, Characterization of an acetylated heteroxylan from Eucalyptus globulus Labill, Carbohydr. Res., 2003, 338, 597–604, DOI:10.1016/S0008-6215(02)00529-3
.
- M. Kögler and B. Heilala, Time-gated Raman spectroscopy–a review, Meas. Sci. Technol., 2020, 32, 012002, DOI:10.1088/1361-6501/abb044
.
- A. Potthast, S. Radosta, B. Saake, S. Lebioda, T. Heinze, U. Henniges, A. Isogai, A. Koschella, P. Kosma, T. Rosenau, S. Schiehser, H. Sixta, M. Strlič, G. Strobin, W. Vorwerg and H. Wetzel, Comparison testing of methods for gel permeation chromatography of cellulose: coming closer to a standard protocol, Cellulose, 2015, 22, 1591–1613, DOI:10.1007/s10570-015-0586-2
.
- J. Müller-Maatsch, A. Caligiani, T. Tedeschi, K. Elst and S. Sforza, Simple and Validated Quantitative 1H NMR Method for the Determination of Methylation, Acetylation, and Feruloylation Degree of Pectin, J. Agric. Food Chem., 2014, 62, 9081–9087, DOI:10.1021/jf502679s
.
- E. N. Ashworth, V. E. Stirm and J. J. Volenec, Seasonal variations in soluble sugars and starch within woody stems of Cornus sericea L., Tree Physiol., 1993, 13, 379–388, DOI:10.1093/treephys/13.4.379
.
- J. J. Sauter and S. Wellenkamp, Seasonal Changes in Content of Starch, Protein and Sugars in the Twig Wood of Salix caprea L., Holzforschung, 1998, 52, 255–262, DOI:10.1515/hfsg.1998.52.3.255
.
- C. Barbaroux and N. Bréda, Contrasting distribution and seasonal dynamics of carbohydrate reserves in stem wood of adult ring-porous sessile oak and diffuse-porous beech trees, Tree Physiol., 2002, 22, 1201–1210, DOI:10.1093/treephys/22.17.1201
.
- J. Dou, P. Ilina, J. Hemming, K. Malinen, H. Mäkkylä, N. O. de Farias, P. Tammela, G. D. A. Umbuzeiro, R. Räisänen and T. Vuorinen, Effect of Hybrid Type and Harvesting Season on Phytochemistry and Antibacterial Activity of Extracted Metabolites from Salix Bark, J. Agric. Food Chem., 2022, 70, 2948–2956, DOI:10.1021/acs.jafc.1c08161
.
- A. Janská, P. Maršík, S. Zelenková and J. Ovesná, Cold stress and acclimation – what is important for metabolic adjustment?, Plant Biol., 2010, 12, 395–405, DOI:10.1111/j.1438-8677.2009.00299.x
.
- F. Kaplan, D. Y. Sung and C. L. Guy, Roles of β-amylase and starch breakdown during temperatures stress, Physiol. Plant., 2006, 126, 120–128, DOI:10.1111/j.1399-3054.2006.00604.x
.
- J. J. Sauter and B. van Cleve, Storage, mobilization and interrelations of starch, sugars, protein and fat in the ray storage tissue of poplar trees, Trees, 1994, 8, 297–304, DOI:10.1007/BF00202674
.
- E. N. Makarova and E. G. Shakhmatov, Structural characteristics of oxalate-soluble polysaccharides from Norway spruce (Picea abies) foliage, Carbohydr. Polym., 2020, 246, 116544, DOI:10.1016/j.carbpol.2020.116544
.
- E. G. Shakhmatov, P. V. Toukach, E. A. Michailowa and E. N. Makarova, Structural studies of arabinan-rich pectic polysaccharides from Abies sibirica L. Biological activity of pectins of A. sibirica, Carbohydr. Polym., 2014, 113, 515–524, DOI:10.1016/j.carbpol.2014.07.037
.
- M. Zou, X. Li, J. Zhao and Y. Qu, Characteristics of Polygalacturonate Lyase C from Bacillus subtilis 7-3-3 and Its Synergistic Action with PelA in Enzymatic Degumming, PLoS One, 2013, 8, e79357, DOI:10.1371/journal.pone.0079357
.
- M. Zou, X. Li, W. Shi, F. Guo, J. Zhao and Y. Qu, Improved production of alkaline polygalacturonate lyase by homologous overexpression pelA in Bacillus subtilis, Process Biochem., 2013, 48, 1143–1150, DOI:10.1016/j.procbio.2013.05.023
.
- T. Schult, T. Hjerde, O. I. Optun, P. J. Kleppe and S. Moe, Characterization of cellulose by SEC-MALLS, Cellulose, 2002, 9, 149–158, DOI:10.1023/A:1020139409903
.
- J. Ding, C. G. Yoo, Y. Pu, X. Meng, S. Bhagia, C. Yu and A. J. Ragauskas, Cellulolytic enzyme-aided extraction of hemicellulose from switchgrass and its characteristics, Green Chem., 2019, 21, 3902–3910, 10.1039/C9GC00252A
.
- T. Yuan, F. Xu, J. He and R. Sun, Structural and physico-chemical characterization of hemicelluloses from ultrasound-assisted extractions of partially delignified fast-growing poplar wood through organic solvent and alkaline solutions, Biotechnol. Adv., 2010, 28, 583–593, DOI:10.1016/j.biotechadv.2010.05.016
.
- S. Santacruz, K. Koch, R. Andersson and P. Åman, Characterization of Potato Leaf Starch, J. Agric. Food Chem., 2004, 52, 1985–1989, DOI:10.1021/jf030601k
.
- D. S. Jackson, Solubility Behavior of Granular Corn Starches in Methyl Sulfoxide (DMSO) as Measured by High Performance Size Exclusion Chromatography, Starch, 1991, 43, 422–427, DOI:10.1002/star.19910431103
.
- A. Rygula, K. Majzner, K. M. Marzec, A. Kaczor, M. Pilarczyk and M. Baranska, Raman spectroscopy of proteins: a review, J. Raman Spectrosc., 2013, 44, 1061–1076, DOI:10.1002/jrs.4335
.
- J. De Gelder, K. De Gussem, P. Vandenabeele and L. Moens, Reference database of Raman spectra of biological molecules, J. Raman Spectrosc., 2007, 38, 1133–1147, DOI:10.1002/jrs.1734
.
- T. Rojalin, L. Kurki, T. Laaksonen, T. Viitala, J. Kostamovaara, K. C. Gordon, L. Galvis, S. Wachsmann-Hogiu, C. J. Strachan and M. Yliperttula, Fluorescence-suppressed time-resolved Raman spectroscopy of pharmaceuticals using complementary metal-oxide semiconductor (CMOS) single-photon avalanche diode (SPAD) detector, Anal. Bioanal. Chem., 2016, 408, 761–774, DOI:10.1007/s00216-015-9156-6
.
- L. Galvis, C. G. Bertinetto, J. L. Putaux, N. Montesanti and T. Vuorinen, Crystallite orientation maps in starch granules from polarized Raman spectroscopy (PRS) data, Carbohydr. Polym., 2016, 154, 70–76, DOI:10.1016/j.carbpol.2016.08.032
.
- J. Dou, W. Xu, J. J. Koivisto, J. K. Mobley, D. Padmakshan, M. Kögler, C. Xu, S. Willför, J. Ralph and T. Vuorinen, Characteristics of Hot Water Extracts from the Bark of Cultivated Willow (Salix sp.), ACS Sustainable Chem. Eng., 2018, 6, 5566–5573, DOI:10.1021/acssuschemeng.8b00498
.
- Y. Liu, Y. Xu, Y. Yan, D. Hu, L. Yang and R. Shen, Application of Raman spectroscopy in structure analysis and crystallinity calculation of corn starch, Starch, 2015, 67, 612–619, DOI:10.1002/star.201400246
.
- J. S. Greenwood, C. Demmers and S. Wetzel, Seasonally dependent formation of protein-storage vacuoles in the inner bark tissues of tissues of Salix microstachya, Can. J. Bot., 1990, 68, 1747–1755, DOI:10.1139/b90-225
.
- J. Dou, H. Kim, Y. Li, D. Padmakshan, F. Yue, J. Ralph and T. Vuorinen, Structural Characterization of Lignins from Willow Bark and Wood, J. Agric. Food Chem., 2018, 66, 7294–7300, DOI:10.1021/acs.jafc.8b02014
.
- I. Aguilar-Hernández, N. K. Afseth, T. López-Luke, F. F. Contreras-Torres, J. P. Wold and N. Ornelas-Soto, Surface enhanced Raman spectroscopy of phenolic antioxidants: A systematic evaluation of ferulic acid, p-coumaric acid, caffeic acid and sinapic acid, Vib. Spectrosc., 2017, 89, 113–122, DOI:10.1016/j.vibspec.2017.02.002
.
- E. Witkowska, T. Jagielski and A. Kamińska, Genus-and species-level identification of dermatophyte fungi by surface-enhanced Raman spectroscopy, Spectrochim. Acta, Part A, 2018, 192, 285–290, DOI:10.1016/j.saa.2017.11.008
.
- M. Kögler, J. Itkonen, T. Viitala and M. G. Casteleijn, Assessment of recombinant protein production in E. coli with Time-Gated Surface Enhanced Raman Spectroscopy (TG-SERS), Sci. Rep., 2020, 10, 2472, DOI:10.1038/s41598-020-59091-3
.
- N. P. Ivleva, R. Niessner and U. Panne, Characterization and discrimination of pollen by Raman microscopy, Anal. Bioanal. Chem., 2005, 381, 261–267, DOI:10.1007/s00216-004-2942-1
.
- H. Wu, H. Xu, H. Li, D. Wei, J. Lin and X. Li, Seasonal development of cambial activity in relation to xylem formation in Chinese fir, J. Plant Physiol., 2016, 195, 23–30, DOI:10.1016/j.jplph.2015.12.013
.
-
T. Dieing, O. Hollricher and J. Toporski, Confocal Raman Microscopy, Springer Berlin, Heidelberg, Germany, 2011. DOI:10.1007/978-3-642-12522-5
.
- U. P. Agarwal, Analysis of cellulose and lignocellulose materials by Raman spectroscopy: a review of the current status, Molecules, 2019, 24, 1659, DOI:10.3390/molecules24091659
.
- W. Z. Payne and D. Kurouski, Raman spectroscopy enables phenotyping and assessment of nutrition values of plants: a review, Plant Methods, 2021, 17, 78, DOI:10.1186/s13007-021-00781-y
.
- J. Dou, L. Galvis, U. Holopainen-Mantila, M. Reza, T. Tamminen and T. Vuorinen, Morphology and Overall Chemical Characterization of Willow (Salix sp.) Inner Bark and Wood: Toward Controlled Deconstruction of Willow Biomass, ACS Sustainable Chem. Eng., 2016, 4, 3871–3876, DOI:10.1021/acssuschemeng.6b00641
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc04476e |
| This journal is © The Royal Society of Chemistry 2023 |

