 Open Access Article
Open Access ArticleEcotoxicity of isosorbide acrylate and methacrylate monomers and corresponding polymers†
Alina
Ismagilova
 a,
Livia
Matt
a,
Livia
Matt
 a,
Patric
Jannasch
a,
Patric
Jannasch
 ab,
Veljo
Kisand
*a and
Lauri
Vares
ab,
Veljo
Kisand
*a and
Lauri
Vares
 *a
*a
aInstitute of Technology, University of Tartu, Nooruse 1, Tartu 50411, Estonia. E-mail: veljo.kisand@ut.ee; lauri.vares@ut.ee
bDepartment of Chemistry, Lund University, Box 124, Lund 221 00, Sweden
First published on 1st February 2023
Abstract
Isosorbide is a well-investigated and accessible biomass-derived compound that has found wide use in medicine, cosmetics, and materials science. The efforts to employ this rigid bicyclic diol as a sustainable building block in high-performance biobased plastics for, e.g., the engineering, coating, and packaging sectors have grown sharply in recent years. Due to the biomass origin, there is an implicit assumption of a low toxicity and an environmentally benign nature of isosorbide-derived plastics. In the present work, the ecotoxicity of isosorbide acrylate and methacrylate monomers and the corresponding poly(meth)acrylates, as well as industrially produced latexes from these monomers, were evaluated towards bacteria (Escherichia coli, Aliivibrio fischeri), vascular plants (Spirodela polyrhiza) and invertebrates (Thamnocephalus platyurus) using widely acknowledged test assays. The measured half maximal effective concentration (EC50) values indicate that the chemically reactive isosorbide acrylate monomers are toxic towards higher multicellular organisms (S. polyrhiza and T. platyurus, EC50 ∼ 9 mg L−1) and moderately toxic towards bacteria (E. coli), whereas the corresponding methacrylate monomers can be considered as practically harmless or harmless on the same test assays. Corresponding isosorbide polyacrylate and polymethacrylate polymers are harmless towards the tested organisms (EC50 > 1000 mg L−1), except towards E. coli, where two polymers are classified as practically harmless (EC50 = 374 and 514 mg L−1). Moreover, industrially produced isosorbide methacrylate derived latexes can be classified as harmless based on this study.
Introduction
The development of novel sustainable chemicals and polymers from renewable resources in response to the growing environmental concerns has been intense during the past decades, both in academia and industry. Currently, only around 2% of the plastics world-wide are produced from renewable raw materials, but this is expected to grow in the near future.1 The most common bio-derived plastics produced on the commercial-scale today are poly(lactic acid) (PLA), poly(butylene adipate-co-terephthalate) (PBAT) and various starch blends.2 In addition, a number of novel biobased plastics are in a late stages of development, or have recently entered the market, e.g., poly(ethylene 2,5-furanedicarboxylate) (PEF),3 developed as a biobased alternative to poly(ethylene terephthalate) (PET), and isosorbide polycarbonate,4 a bisphenol A free non-aromatic polycarbonate for various high-performance applications.However, it is challenging to develop plastics from bio-resources that not only fulfill material processability and strict property requirements, but are also either efficiently recyclable, or biodegradable with a negligible impact on the natural environment. Although the physical and chemical properties of novel materials are usually thoroughly tested and evaluated at the early stages of development, the toxic effects on living organisms other than humans are rarely properly evaluated.5 Early testing, on the other hand, enables the direct identification of potentially hazardous compounds, hence avoiding the unnecessary cost of further developing potentially harmful chemicals and materials. Furthermore, non-toxicity is one of the cornerstones of green chemistry,6 but which is seldom thoroughly investigated and verified. A straightforward way is to use standard ecotoxicity tests that identify physiological and environmentally significant responses such as mortality, growth and reproduction disturbances within different groups of organisms.7 Ecotoxicology tests are also required by the REACH legislation within the EU.8
Isosorbide (Scheme 1), a bicyclic diol manufactured from D-glucose in an industrial scale,9 has been identified as one of the platform chemicals that could potentially replace fossil-based counterparts in various applications.10 The compound is known at least since 1927,11 and its derivatives were initially used mostly in the pharmaceutical sector. For example, isosorbide mono- and dinitro derivatives are today used as vasodilators12 and thus thoroughly evaluated towards humans and other mammals.13–16 On the other hand, data on isosorbide derivatives towards aquatic species are scarce. The Dutch National Institute for Public Health and the Environment has tested isosorbide dimethyl ether, a potential aprotic solvent used in cosmetic and pharmaceutical products,17 and found that the EC50 values towards aquatic species such as algae and Daphnia exceed >100 mg L−1 and the compound was described as not readily biodegradable.18
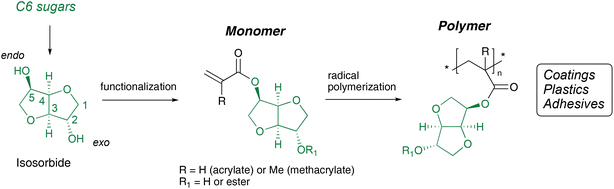 | ||
| Scheme 1 Conversion of isosorbide into mono-(meth)acrylate monomers and subsequent radical polymerization. | ||
More recently, the use of isosorbide as a building block or a plasticizer in polymer and material science has grown sharply.19,20 For example, the French company Roquette has introduced POLYSORB® ID, an isosorbide diester, to be used as a phthalate-free plasticizer that is compatible with polyvinyl chloride (PVC) resin.21 Furthermore, the incorporation of structurally rigid isosorbide units into polymer structures affords materials with relatively high glass transition temperatures (Tgs). Such bioderived high-Tg plastics are in large demand for a wide range of product areas, including coatings, automotive components, engineering plastics and packaging materials.22 The most investigated isosorbide-based condensation polymers are polycarbonates (PC), polyesters (PE) and polyurethanes (PU), some of which are commercially available. For example, the UV and scratch resistant isosorbide-containing polycarbonate DURABIO™ is offered by the Mitsubishi Chemical Corporation and used in the car industry, for sunglasses production and other purposes.23 Isosorbide-based polyesters (PEIT) and polyurethanes are currently developed by Roquette.24
In addition to condensation polymers such as PE, PC and PU, isosorbide is also interesting for monomers for chain polymerizations. We25,26 and other research groups27–31 have recently developed isosorbide mono-acrylates and -methacrylates, where the polymerizable group is only attached to either endo or exo hydroxyl group. Such monomers can undergo radical homo- or co-polymerization and afford corresponding poly(meth)acrylates where the isosorbide units form pendant side groups attached to all-carbon backbones (Scheme 1). Depending on the specific characteristics, these thermoplastic isosorbide poly(meth)acrylates offer viable biobased alternatives as a potential replacements of fossil counterparts in coatings, adhesives, and plastics. In particular, they have the potential to replace conventional fossil-derived poly(methyl methacrylate) (PMMA) and polystyrene (PS) in several plastics and coating products.20,25,27,32
Whereas the toxicity of PS and several types of other conventional plastics such as polyethylene, PET, PU, PVC, have been evaluated in bioassays,33 the data on isosorbide-based monomers and polymers is scarce. However, prior to larger-scale industrial development, the ecotoxicity and other environmental aspects of these new materials should be carefully evaluated. The introduction of various reactive functional groups needed for polymerization can affect the properties of the compound in different ways and even minor changes in the chemical structure may have large impact on its biological activity.34,35
Derivatives with acrylate- and methacrylate functional groups have been reported to possess moderate to high toxicity towards algae and other organisms.36–39 In general, acrylates have somewhat higher toxicity, compared to corresponding methacrylates, and the toxicity decreases when the compounds become more lipophilic. For example, whereas the acrylic acid has shown high toxicity towards algae, methyl methacrylate showed moderate toxicity in similar tests.36 Thus, due to potential toxicity-related issues with acrylates and methacrylates, any such new derivate of potential industrial use needs to be thoroughly assessed.
In the present work, we evaluated the ecotoxic effect of isosorbide mono-acrylates and mono-methacrylates, respectively, and the corresponding polymer materials prepared by radical polymerization. The ecotoxicity was evaluated on several aquatic organisms with various biological complexity, including bacteria, vascular plants, and invertebrates.
Experimental
Test compounds
We chose four isosorbide-based monomers and the four corresponding polymers for the study (Fig. 1). The selection of the samples was based on two main criteria: (a) the potential applicability of these compounds has been demonstrated in several applications, and thus, they have the relevance and wide industrial interest; (b) they are soluble in water or water/DMSO mixtures (using up to max 30% of DMSO in water). The monomers and homopolymers were synthesized according to the previously published methods (see ESI for details†).25,26,28,30 | ||
| Fig. 1 Overview of the chemical structure, abbreviation, name, and molecular weight of the compounds studied in the present work. | ||
The number average molecular weight (Mn) of the polymers tested varied from 4.8 to 69.3 kg mol−1. The Mn values were determined by size-exclusion chromatography (SEC) using either THF or DMF as eluents (Fig. S9–S12†). Isosorbide poly(meth)acrylates are rigid plastics with relatively high Tg, ranging from roughly 95 °C in the case of PIAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 to 170 °C in case of PIM.25
28 to 170 °C in case of PIM.25
Isosorbide methacrylate-containing emulsion polymers IMA-latex, IMP-latex, IMB-latex, as well as a corresponding fossil-based food-contact approved industrial standard styrene-acrylate (SA) latex binder (CHP BAR 1400, for Technical Data Sheet, see ESI†)40 listed in Table 2, were received as a gift from CH-Polymers (Finland). Compared to the industrial SA latex (CHP BAR 1400), the polymerization recipes and processes of IMA-latex, IMP-latex, IMB-latex were analogous, except that 50% of the styrene monomer was replaced by isosorbide 5-methacrylates, either by the 2-acetate (IMA), 2-propionate (IMP), or 2-butyrate (IMB) derivative. Semi-continuous emulsion polymerizations were carried out in glass reactors in laboratory setting. Aqueous solutions of poly(methacrylic acid) (PMAA) and poly(acrylic acid, sodium salt) (PAA), both in the sodium salt form, were purchased from SigmaAldrich.
Ecotoxicity testing
For the bacterial test on E. coli, the range of concentration of each sample was: [12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–781 mg L−1] for IM, [40
500–781 mg L−1] for IM, [40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2 500 mg L−1] for IMA, [1 000–62.5 mg L−1] for IAA, [80–4.8 mg L−1] for IA, [2 500–312.5 mg L−1] for PIM, [3 125–391 mg L−1] for PIMA, [20
000–2 500 mg L−1] for IMA, [1 000–62.5 mg L−1] for IAA, [80–4.8 mg L−1] for IA, [2 500–312.5 mg L−1] for PIM, [3 125–391 mg L−1] for PIMA, [20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–2500 mg L−1] for PIA, [1000–125 mg L−1] for PIAA, [132
000–2500 mg L−1] for PIA, [1000–125 mg L−1] for PIAA, [132![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–8281 mg L−1] for PAA, [195
500–8281 mg L−1] for PAA, [195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–12
000–12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 187 mg L−1] for PMAA. Sample concentrations for testing on A. fischeri were based on the results from the tests on E. coli. For tests on plants and invertebrates, the concentrations of the test substances were used according to the operational procedure prescribed for the kits: 0.1 mg L−1, 1.0 mg L−1, 10 mg L−1, 100 mg L−1 and 1000 mg L−1, respectively.
187 mg L−1] for PMAA. Sample concentrations for testing on A. fischeri were based on the results from the tests on E. coli. For tests on plants and invertebrates, the concentrations of the test substances were used according to the operational procedure prescribed for the kits: 0.1 mg L−1, 1.0 mg L−1, 10 mg L−1, 100 mg L−1 and 1000 mg L−1, respectively.
The level of toxicity of a substance was determined by establishing the effective concentration, EC50, i.e., the concentration of substances in the environment that will affect 50% of the organisms in the test population under specified conditions. The EC50 values for monomers and polymers were defined as mg L−1 and the evaluation of toxicity followed the toxicological categories adopted by the European Commission.41 According to this classification, the categories of aquatic toxicity are the following: very toxic: EC50 < 1 mg L−1, toxic: EC50 = 1–10 mg L−1, moderately toxic: EC50 = 10–100 mg L−1, practically harmless: EC50 = 100–1000 mg L−1 and harmless compounds with EC50 > 1000 mg L−1 in this study.42 The EC50 values for industrial latexes were defined as grams of total solids of latex in 1 L of latex. All samples were tested in triplicate for each assay to ensure test reproducibility, the EC50 data were represented by mean values and confidence intervals (95% CI) were calculated for each concentration.
Toxi-chromo test™: bacterial chromo inhibition test using Escherichia coli43. This analysis is based on the test compound's ability to inhibit the de novo synthesis of inducible β-galactosidase in a highly permeable mutant of Escherichia coli. Tests are carried out in 96-wells plates in the kit's standard diluent. Mercury chloride [4–0.6 μg mL−1] is used as positive highly toxic control. Samples with the bacterial mixture are incubated at 37 °C for 90 minutes. During the first incubation, the bacteria consume the sample components and attempt to induce the production and excretion of β-galactosidase. Next, a chromogen solution is added, lysing the cells and forming a blue color due to the presence of β-galactosidase. The color intensity, a measure of the toxicity, was recorded at 600 (±20) nm by a spectrophotometer. The obtained data (Table S6†) was used to calculate the EC50 values.
WaterTOX™ STD: bacterial luminescence inhibition test using Aliivibrio fischeri. Toxicity of samples towards the bioluminescent marine bacterium A. fischeri is measured by comparing initial and final light emission after 15 min according to the ISO standard 11348-3: 2007.44 The toxic effect caused by a decrease in cellular metabolism is expressed as a decrease of the luminescence intensity. Tests were carried out at 15 °C in the kit's standard diluent. Potassium dichromate [100–12.5 mg L−1] was used as a reference positive control. A series of dilutions were prepared for each sample according to the manufacturer's instructions. The measured data (Table S7†) was used to calculate the EC50 concentrations.
DuckWeed Toxkit F: growth inhibition test with Spirodela polyrhiza. The Spirodela duckweed microbiotest is based on the measurement of growth retardation of the germinated dormant vegetative buds (turions) after 3 days of exposure to samples according to the ISP standard.45 Before testing, S. polyrhiza turions are germinated in the Petri dish with Streinburg's medium for 3 days at 25 °C with continuous “top” illumination (at least 6000 lux). The tests were carried out on a 48-wells plate containing a dilution series of the monomers or polymers at 25 °C in the plant growth chamber, using an illumination system enabling at least 6000 lux. Potassium chloride [18
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–1800 mg L−1] was used as a positive control. A digital photo of the multiwell plate was taken at the start of the test and after the incubation to measure the growth inhibition by Image Analysis (Image J, National Institute of Mental Health, Bethesda, Maryland, USA, software for image processing and analysis) to determine the size of the vegetative buds (turions) before and after incubation. Next, by comparing the data obtained from the test plate, the growth of the duckweeds was calculated by subtracting the mean of “initial” size of the first frond from the mean “final” size, in the control and at various concentrations of diluted samples. The 72 h EC50 concentration of the compound was obtained from the percentage of growth inhibition of the duckweed as described in ESI (Table S8†).
000–1800 mg L−1] was used as a positive control. A digital photo of the multiwell plate was taken at the start of the test and after the incubation to measure the growth inhibition by Image Analysis (Image J, National Institute of Mental Health, Bethesda, Maryland, USA, software for image processing and analysis) to determine the size of the vegetative buds (turions) before and after incubation. Next, by comparing the data obtained from the test plate, the growth of the duckweeds was calculated by subtracting the mean of “initial” size of the first frond from the mean “final” size, in the control and at various concentrations of diluted samples. The 72 h EC50 concentration of the compound was obtained from the percentage of growth inhibition of the duckweed as described in ESI (Table S8†).
Thamnotoxkit F: crustacean toxicity screening test for freshwater using Thamnocephalus platyurus. This test determines the lethal effects of toxicants on the T. platyurus after 24 h exposure. The 24 h immobilization test was performed in a multiwell test plate using the fairy shrimp T. platyurus hatched from cysts based on ISO standard.46 Cyst hatching was initiated before the start of the toxicity test in Petri dish with Standard Freshwater medium at 25 °C for 20–22 h, under continuous illumination (at least 3000 lux). The test incubation was carried out on a 24-well plate containing a dilution series of the monomers or polymers in darkness for 24 hours. The number of immobilized (dead) organisms was counted after 24 h under microscope (magnification 10–12×). Potassium dichromate [0.32–0.032 mg L−1] was used as a positive control. The obtained data (Table S9†) was used to determine the EC50 values.
Results and discussion
Toxicity evaluation of monomers
The results of the toxicity measurements on the monomers and polymers are presented in Fig. 2. First, we evaluated the toxicity of the isosorbide methacrylate- and acrylate monomers (IM, IMA, IA, and IAA). The methacrylate monomers IM and IMA exhibited a non-toxic behavior towards bacteria (E. coli and A. fischeri, Fig. 2a and b, respectively), vascular plant (S. polyrhiza, Fig. 2c), and invertebrate (T. platyarus, Fig. 2d). Certain effect of the acetate group in IMA was observed, most notably on S. polyrhiza turions where the EC50 value of 139 mg L−1 (95% CI: 115.8; 162.9) was below of threshold of the practically harmful range. Interestingly, monomer IM, lacking the acetate group, showed an EC50 value above 1000 mg L−1 in the same test. We speculate, that the somewhat higher toxicity of the acetate derivative IMA could be due to the possible formation of free acetic acid, which has been reported to inhibit the growth of duckweed.47 The acetic acid might form as a result of de-esterification of the acetate, catalyzed by carboxylesterases present in the plants.48 | ||
| Fig. 2 Values of the mean effective concentration (EC50, mg L−1) of the tested monomers (light-blue columns) and polymers (dark-blue columns). The compounds are ordered according to the level of toxicity: values in green represent the harmless (EC50 values > 1000 mg L−1) and the practically harmless (EC50 values 100–1000 mg L−1) compounds; values in yellow denote moderately toxic (EC50 values 10–100 mg L−1) compounds and values in orange designate toxic compounds (EC50 values 1–10 mg L−1). For numerical 95% CI values, see Table S4.† For statistically significant differences between EC50 values, see Table S5.† | ||
Replacing the methacrylate group by the acrylate group, altered the results considerably. The isosorbide acrylate with free –OH group (IA) was clearly toxic to S. polyrhiza [EC50 = 9 (95% CI: 5.8; 12.2) mg L−1] and T. platyarus [EC50 = 8.7 (95% CI: 6.5; 11.1) mg L−1]. The corresponding acrylate with acetate capped –OH (IAA), showed the same level of toxicity towards S. polyrhiza [EC50 = 9 (95% CI: 6.8; 11.3) mg L−1], but was slightly less toxic towards T. platyarus [EC50 = 15.6 (95% CI: 8.46; 22.88) mg L−1], thus, being harmful.
In the bacterial tests, however, IA showed moderate toxicity towards E. Coli [EC50 = 16 (95% CI: 8.8; 24) mg L−1, Fig. 2a], but is practically harmless towards A. fischeri [EC50 = 456 (95% CI: 418; 511) mg L−1, Fig. 2b]. Acetate capped IAA, on the other hand, showed a non-toxic behavior towards both E. coli [EC50 = 124 (95% CI: 114; 158) mg L−1] and A. fischeri [EC50 = 585 (95% CI: 420; 749) mg L−1].
The aquatic toxicity of acrylic and methacrylic acids, as well as the alkyl esters, has been studied previously.36–39,49 These studies support our findings, that methacrylates are in general less harmful compared to the corresponding acrylate derivatives. For example, in the test using freshwater invertebrate Daphnia magna, the EC50 value for methacrylic acid was >130 mg L−1, while for acrylic acid the EC50 value was somewhat lower (95 mg L−1).38
It has been shown previously that isosorbide itself is a non-toxic compound towards mammals including humans.13 Thus, the toxicity of studied compounds seems to be related to different functional groups, that are attached to isosorbide. Both acrylate and methacrylate groups possess an electron-deficient double bond that can undergo different chemical transformations. For example, various carbon or heteroatom nucleophiles can be added to acrylates and methacrylates via Michael addition reaction forming a new carbon–carbon or carbon–heteroatom single bond.50 In the biological systems, this kind of nucleophilic Michael addition reaction is a possible origin for the cellular toxicity of these compounds targeting amino acids.51,52 For example, a reaction with the –SH functional group present in glutathione has been documented.53 In the case of methacrylates, the additional methyl group increases the electron density around the double bond, thus making it less prone to nucleophilic attack. This could be the reason for the lower toxicity of methacrylates compared to acrylic acid derivatives. Another detoxification mechanism for acrylic and methacrylic monomers could be carboxylesterase-mediated hydrolysis, since the carboxylic acid formed is not electrophilic under physiological conditions.54 For methacrylates, the hydrolysis could be the main metabolic pathway.55
In addition, the aquatic toxicity of monomers was modeled using Ecological Structure Activity Relationships (ECOSAR) predictive model.56 ECOSAR predictions were roughly in line with our experimental results – for acrylic monomers (IA and IAA) the model predicted slightly lower toxicities, but in case of methacrylates (IM and IMA), the experimental and predicted values were in the same range (practically harmless or harmless, Table S3†).
Toxicity evaluation of polymers
In the next phase, we studied polymers PIM, PIMA, PIA, and PIAA. These polymers were prepared by free-radical polymerization of the corresponding monomers as described in the Experimental section. The molecular weight (Mn) and polydispersity index (Đ) of the studied polymers are listed in Fig. 1. Although radical polymerization is a highly efficient polymerization method with good control over polymer structure and molecular weight, small amounts of unreacted monomers may remain in the material due to incomplete conversion.57 Moreover, the polymerization–depolymerization equilibrium can, especially in cases with relatively low ceiling temperature, result in trace monomer contents at ambient temperatures.58 For example, a ceiling temperature of poly(methyl methacrylate) is 220 °C, resulting in equilibrium monomer concentrations of 0.14 M and 0.001 M at 110 and 25 °C, respectively.58 Consequently, acrylate and methacrylate monomers may leach out from polymer materials and thus their potential impact on the toxicity must be considered. In our present study, the polymers were purified by precipitation, as described in the Experimental section, ensuring the removal of any residual monomer in the tested polymers beyond that determined by the polymerization–depolymerization equilibrium.All the isosorbide-based polymers showed harmless or practically harmless effects on all the tested organisms (Fig. 2a–d). Thus, the toxicity levels observed with the acrylic monomers were not carried over to the corresponding polymers. Tests on vascular plants (S. polyrhiza) and invertebrates (T. platyurus) showed that isosorbide-based polymers are harmless, with the EC50 values over 1000 mg L−1, which was the highest tested concentration. In the bacterial tests, the specific EC50 values could be determined for all tested polymers, except for PIAA and PIM(b) on A. fischeri, which had EC50 values over 4000 mg L−1 and 2000 mg L−1, respectively. Due to the limited solubility, these were the highest tested concentrations for PIAA and PIM(b).
We did not find any obvious correlation depending on the structure of polymer main chain (polymethacrylate vs. polyacrylate) and the measured EC50 values. Likewise, the effect of the isosorbide side chain (2-OH vs. 2-OAc) on E. coli did not seem to follow a clear trend. The EC50 values for the polymers with acetyl substituents (PIMA and PIAA) were 1081 (95% CI: 800; 1364) mg L−1 and 514 (95% CI: 433; 595) mg L−1, while the corresponding non-acetylated polymers PIM and PIA(a) exhibited EC50 values of 374 (95% CI: 280; 468) and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (95% CI: 5757; 18
100 (95% CI: 5757; 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443) mg L−1, respectively.
443) mg L−1, respectively.
The results on the polymers indicated that closely related structures can exhibit rather different levels of toxicity, and that the toxicity may vary significantly depending on the organism used. Thus, it is of great importance to study organisms across biological complexity and trophic levels such as e.g., bacteria, plants, and invertebrates, and to systematically test closely related compounds one by one. We investigated the effect of polymer molecular weight (Mn) on EC50. Polymers PIM, PIA, and PIMA were synthesized with different molecular weights and evaluated in the Toxi-Chromo inhibition test with E. coli (Table 1). In all cases, the EC50 values increased slightly with increasing Mn. Still, the effect remained relatively small. The effect was most noticeable in the case of PIMA where a roughly 5-fold increase of Mn (from 12.5 to 64.2 kg mol−1) increased the EC50 value from 1083 mg L−1 [PIMA(a)] to 1895 mg L−1 [PIMA(c)]. This trend is probably caused by the generally lower bioavailability of higher molecular weight polymers.59,60
| Entry | Polymer name | M n, kg mol−1 | Đ | EC50, mg L−1 (95% CI) |
|---|---|---|---|---|
| 1 | PIM(a) | 36.1 | 2.03 | 319.5 (153; 486) |
| 2 | PIM(b) | 69.3 | 1.3 | 374 (280; 468) |
| 3 | PIA(a) | 4.8 | 2.36 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (5757; 18 100 (5757; 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 443) 443) |
| 4 | PIA(b) | 42.9 | 2.2 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 410 (12 410 (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026; 20 026; 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794) 794) |
| 5 | PIA(c) | 55.1 | 1.8 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 973 (15 973 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 507; 22 507; 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 438) 438) |
| 6 | PIMA(a) | 12.5 | 2.35 | 1083 (800; 1364) |
| 7 | PIMA(b) | 45.7 | 1.95 | 1215 (917; 1514) |
| 8 | PIMA(c) | 64.2 | 1.9 | 1895 (1260; 2530) |
In the studied polymers, isosorbide unit was attached to the polymer backbone via an ester bond. The hydrolytic cleavage of this ester was a possible degradation pathway in these polymers, which would afford polyacrylic (PAA) or methacrylic acid (PMAA), or a salt thereof, and the corresponding isosorbide unit. Neutralized PAA is widely used in superabsorbents and detergents,61 and the salt of PMAA is found in, e.g., hydrogels and biomedical applications.62 For this reason, we evaluated the effect of PMAA and PAA sodium salts in bacterial tests with A. fischeri and E. coli, and found these polymers to be harmless (Fig. 2). This is in accordance with previously reported data.63
Evaluation of industrially prepared latexes
Finally, we tested the industrially produced isosorbide methacrylate-containing polymer dispersion samples IMA-latex, IMP-latex and IMB-latex, which are presently under development as novel “greener” coatings, and compared the results to the standard styrene-acrylate-based latex CHP BAR 1400 (Table 2). In the former emulsions, 50% of the styrene monomer was replaced by isosorbide 5-methacrylates with either 2-acetate (IMA), 2-propionate (IMP), or 2-butyrate (IMB) side chains, respectively. When these isosorbide-based barrier dispersions were coated on a paperboard, they demonstrate a uniform film formation, and their barrier properties in general match, or even exceed that of reference CHP BAR 1400 coating.64 Hence, it is important to measure and assess the ecotoxicological effects of these materials prior to the further industrial development of these materials.| Entry | Sample name | Sample description | Process type | WaterTOX™ A. fischeri | |
|---|---|---|---|---|---|
| Toxicitya | EC50, g L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (95% CI) b (95% CI) |
||||
| a H – harmless (EC50 values > 1000 mg L−1). b Weight corresponds to total solids of latex in 1 L of latex. c EC50 value could be observed even at the undiluted samples (i.e., at maximum viable concentration). | |||||
| 1 | IMA-latex | SA-latex, 50% of styrene replaced by IMA | lab synthesis | Harmless | 158.2 (141.3; 175) |
| 2 | IMP-latex | SA-latex, 50% of styrene replaced by IMP | lab synthesis | Harmless | 245.5 (157.2; 333.8) |
| 3 | IMB-latex | SA-latex, 50% of styrene replaced by IMB | lab synthesis | Harmless | 451.5 (372.1; 575.8) |
| 4 | CHP BAR 1400 | Conventional SA-latex binder | production grade | Harmless | >500c |
The ecotoxicity of the dispersions was evaluated using the marine bacteria A. fischeri (WaterTOX), because the tests on the other aquatic organisms are not fully compatible with the turbid dispersion. Our results indicated that the isosorbide containing latexes only had measurable EC50 values at very high concentrations (Table 2, entries 1–3), and that no EC50 value could be measured for the commercial reference SA-latex (entry 4) due to the lack of an effect even at the highest concentration tested. We speculate that the difference between the reference (CHP BAR 1400) and isosorbide-based samples may originate from the small amounts of unreacted isosorbide-methacrylate monomers that may remain in the emulsion. The length of the side chain on isosorbide monomers also had a slight effect on A. fischeri in this test. The EC50 concentration increased as the length of the isosorbide alkanoate side chain increased from acetate (C2) to propionate (C3) and butyrate (C4), i.e., as the corresponding monomer became less hydrophilic. A similar trend has also been observed with acrylic acid esters, where the aquatic toxicity decreased in the following order: acrylic acid > methyl acrylate > ethyl acrylate > butyl acrylate.33 However, the high concentration levels of isosorbide-containing latexes that are required to achieve a measurable EC50 value with A. fischeri are not practically reached in the aqueous environment, and thus these latexes can be considered as harmless according to the WaterTOX test.
Conclusion
The results obtained in the present study indicate that isosorbide acrylates monomers were toxic or moderately toxic towards vascular plants and invertebrates, but the effect was smaller on bacteria (moderately toxic or practically harmless). In contrast, the corresponding isosorbide methacrylate monomers gave significantly higher EC50 values, and could be classified as harmless, or practically harmless towards bacteria, vascular plants, and invertebrates.The monomer toxicity towards bacteria (both E. coli and A. fischeri) was correlated to the hydrophilicity, i.e., more hydrophilic monomers showed a higher toxicity. Hence, the toxicity was found to decrease in the order: isosorbide acrylate > isosorbide acrylate-acetate > isosorbide methacrylate > isosorbide methacrylate-acetate. When it came to vascular plants and invertebrates, both acrylate monomers IA and IAA had EC50 values in the same range (EC50 from 8.7 to 15.6 mg L−1, toxic to moderately toxic), while the corresponding methacrylate monomers were harmless (EC50 > 1000 mg L−1), with the exception IMA, which was practically harmless towards duckweed (EC50 = 139 mg L−1). The latter might be caused by the presence of acetic acid, which may form through de-esterification of the acetate group in IMA, catalyzed by carboxylesterases present in the vascular plants.
All isosorbide polyacrylate and polymethacrylate polymers were found to be harmless towards all tested organisms (EC50 > 1000 mg L−1), except PIAA, which was found to be practically harmless towards E. coli (EC50 = 514 mg L−1). The molecular weight of the polymers had only a small effect on the toxicity, and low molecular weight polymers had slightly lower EC50 values in the MicroTox test (E. coli), probably due to somewhat better bioavailability. The three industrially produced isosorbide methacrylate-containing latexes can be considered as completely harmless towards E. coli, as only very highly concentrated samples (>155 g L−1) gave measurable EC50 values.
Overall, our results demonstrate that bioderived isosorbide polyacrylates and polymethacrylates can be considered as an ecotoxicologically viable alternative to conventional fossil-based polymers, provided that no significant amounts of the acrylate monomers remain in the materials.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the EEA Grants through the Baltic Research Programme (grant EMP426), by the European Union and implemented under the European Neighbourhood Instrument (Project “BioStyrene” ER30), and by the European Regional Development Fund and the Estonian Research Council via MOBTT21 and ResTA7 projects. Veljo Kisand was supported by the Institute of Technology, University of Tartu basic funding grant. We are grateful to Joel Köykkä, Teemu Piesanen and Mia Ahokas (CH-Polymers, Finland) for providing the industrial latexes.References
- R. Chinthapalli, P. Skoczinski, M. Carus, W. Baltus, D. de Guzman, H. Käb, A. Raschka and J. Ravenstijn, Ind. Biotechnol., 2019, 15, 237–241 CrossRef.
- European Bioplastics, Bioplastics market development update 2021, https://docs.european-bioplastics.org/publications/market_data/Report_Bioplastics_Market_Data_2021_short_version.pdf, (accessed 17.10.2022).
- K.-R. Hwang, W. Jeon, S. Y. Lee, M.-S. Kim and Y.-K. Park, Chem. Eng. J., 2020, 390, 124636 CrossRef CAS.
- O. Gómez-de-Miranda-Jiménez-de-Aberasturi, A. Centeno-Pedrazo, S. Prieto Fernández, R. Rodriguez Alonso, S. Medel, J. María Cuevas, L. G. Monsegue, S. De Wildeman, E. Benedetti, D. Klein, H. Henneken and J. R. Ochoa-Gómez, Green Chem. Lett. Rev., 2021, 14, 534–544 CrossRef.
- S. P. M. Ventura, P. de Morais, J. A. S. Coelho, T. Sintra, J. A. P. Coutinho and C. A. M. Afonso, Green Chem., 2016, 18, 4733–4742 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- E. Rudnik, in Compostable Polymer Materials (Second Edition), ed. E. Rudnik, Elsevier, Boston, 2019, pp. 293–313, DOI:10.1016/B978-0-08-099438-3.00009-4.
- Regulation (EC) No 1907/2006 of the European Parliament and of the Council, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02006R1907-20220501, (accessed 17.10.2022).
- Additives for Polymers, 2015, 2015, 8–9, DOI:10.1016/s0306-3747(15)30073-7.
- M. Rose and R. Palkovits, ChemSusChem, 2012, 5, 167–176 CrossRef CAS PubMed.
- U. Hoffmann and J. Müller, Process for the production of valuable products from sorbitol. German Patent, DE488602C, 1927 Search PubMed.
- J. D. Parker and J. O. Parker, N. Engl. J. Med., 1998, 338, 520–531 CrossRef CAS PubMed.
- S. Seko, Oyo Yakuri Pharmacometrics, 1969, 3, 15–18 Search PubMed.
- O. Lavon, Clin. Toxicol., 2015, 53, 22–27 CrossRef CAS PubMed.
- K. C. Ferdinand, Expert Rev. Cardiovasc. Ther., 2005, 3, 993–1001 CrossRef CAS PubMed.
- S. A. Jamal, S. R. Cummings and G. A. Hawker, J. Bone Miner. Res., 2004, 19, 1512–1517 CrossRef CAS PubMed.
- P. Tundo, F. Aricò, G. Gauthier, L. Rossi, A. E. Rosamilia, H. S. Bevinakatti, R. L. Sievert and C. P. Newman, ChemSusChem, 2010, 3, 566–570 CrossRef CAS PubMed.
- RIVM, Toxicity screening of potential bio-based Polar Aprotic Solvents, https://www.rivm.nl/documenten/toxicity-screening-of-potential-bio-based-polar-aprotic-solvents, (accessed 17.10.2022).
- F. Fenouillot, A. Rousseau, G. Colomines, R. Saint-Loup and J. P. Pascault, Prog. Polym. Sci., 2010, 35, 578–622 CrossRef CAS.
- D. J. Saxon, A. M. Luke, H. Sajjad, W. B. Tolman and T. M. Reineke, Prog. Polym. Sci., 2020, 101, 101196 CrossRef CAS.
- Roquette, plasticizers, https://www.roquette.com/industries/performance-materials/plasticizers, (accessed 17.10.2022).
- H. T. H. Nguyen, P. Qi, M. Rostagno, A. Feteha and S. A. Miller, J. Mater. Chem. A, 2018, 6, 9298–9331 RSC.
- Mitsubishi Chemical Corporation, https://www.m-chemical.co.jp/en/products/departments/mcc/pc/product/1201026_9368.html, (accessed 17.10.2022).
- Roquette, polyesters, https://www.roquette.com/industries/performance-materials/polyesters, (accessed 17.10.2022).
- L. Matt, J. Parve, O. Parve, T. Pehk, T. H. Pham, I. Liblikas, L. Vares and P. Jannasch, ACS Sustainable Chem. Eng., 2018, 6, 17382–17390 CrossRef CAS.
- S. Laanesoo, O. Bonjour, J. Parve, O. Parve, L. Matt, L. Vares and P. Jannasch, Biomacromolecules, 2021, 22, 640–648 CrossRef CAS PubMed.
- J. J. Gallagher, M. A. Hillmyer and T. M. Reineke, ACS Sustainable Chem. Eng., 2015, 3, 662–667 CrossRef CAS.
- J. J. Gallagher, M. A. Hillmyer and T. M. Reineke, ACS Sustainable Chem. Eng., 2016, 4, 3379–3387 CrossRef CAS.
- A. Moreno, N. Bensabeh, J. Parve, J. C. Ronda, V. Cádiz, M. Galià, L. Vares, G. Lligadas and V. Percec, Biomacromolecules, 2019, 20, 1816–1827 CrossRef CAS PubMed.
- F. Nonque, A. Sahut, N. Jacquel, R. Saint-Loup, P. Woisel and J. Potier, Polym. Chem., 2020, 11, 6903–6909 RSC.
- F. Nonque, A. Benlahoues, J. Audourenc, A. Sahut, R. Saint-Loup, P. Woisel and J. Potier, Eur. Polym. J., 2021, 160, 110799 CrossRef CAS.
- N. Triantafillopoulos and A. A. Koukoulas, BioRes., 2020, 15, 7260–7287 Search PubMed.
- L. Zimmermann, G. Dierkes, T. A. Ternes, C. Völker and M. Wagner, Environ. Sci. Technol., 2019, 53, 11467–11477 CrossRef CAS PubMed.
- J. M. van Rossum, J. Pharm. Pharmacol., 1963, 15, 285–316 CrossRef CAS PubMed.
- W. Lewandowski, H. Lewandowska, A. Golonko, G. Świderski, R. Świsłocka and M. Kalinowska, PLoS One, 2020, 15, e0229477 CrossRef PubMed.
- H. Greim, J. Ahlers, R. Bias, B. Broecker, H. Hollander, H. P. Gelbke, S. Jacobi, H. J. Klimisch, I. Mangelsdorf, W. Mayr, N. Schön, G. Stropp, P. Stahnecker, R. Vogel, C. Weber, K. Ziegler-Skylakakis and E. Bayer, Chemosphere, 1995, 31, 2637–2659 CrossRef CAS PubMed.
- C. A. Staples, S. R. Murphy, J. E. McLaughlin, H. W. Leung, T. C. Cascieri and C. H. Farr, Chemosphere, 2000, 40, 29–38 CrossRef CAS PubMed.
- L. E. Sverdrup, T. Källqvist, A. E. Kelley, C. S. Fürst and S. B. Hagen, Chemosphere, 2001, 45, 653–658 CrossRef CAS PubMed.
- C. A. Staples, C. Farr, E. K. Hunt, J. E. McLaughlin, H. Müllerschön and M. A. Pemberton, Hum. Ecol. Risk Assess., 2009, 15, 503–525 CrossRef CAS.
- Product of CH-Polymers (Finland), https://ch-polymers.com/barrier-coatings/#barrier-coating-products, (accessed 17.10.2022).
- EU classification categories for hazardous to the aquatic environment, https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:083:0001:0053:en:PDF, (accessed 17.10.2022).
- EU Environmental Hazards, 10 March 2011, https://www.unece.org/fileadmin/DAM/trans/danger/publi/ghs/ghs_rev01/English/04e_part4.pdf, (accessed 9.08.2022).
- K. K. Kwan, Environ. Toxicol. Water Qual., 1993, 8, 223–230 CrossRef CAS.
- ISO 11348-3:2007. Determination of the Inhibitory Effect of Water Samples on the Light Emission of V. fischeri (Luminescent bacteria test).
- ISO 20079:2005. Water quality—Determination of the toxic effect of water constituents and waste water on duckweed (Lemna minor)—Duckweed growth inhibition test.
- ISO 14380:2011. Water quality—Determination of the acute toxicity to Thamnocephalus platyurus (Crustacea, Anostraca).
- B.-V. Boros, N. I. Grau and V. Ostafe, Res. J. Agric. Sci., 2019, 51, 21–29 Search PubMed.
- M. C. Gershater and R. Edwards, Plant Sci., 2007, 173, 579–588 CrossRef CAS.
- P. Radix, M. Léonard, C. Papantoniou, G. Roman, E. Saouter, S. Gallotti-Schmitt, H. Thiébaud and P. Vasseur, Ecotoxicol. Environ. Saf., 2000, 47, 186–194 CrossRef CAS PubMed.
- B. D. Mather, K. Viswanathan, K. M. Miller and T. E. Long, Prog. Polym. Sci., 2006, 31, 487–531 CrossRef CAS.
- R. Osman, K. Namboodiri, H. Weinstein and J. R. Rabinowitz, J. Am. Chem. Soc., 1988, 110, 1701–1707 CrossRef CAS.
- U. Blaschke, K. Eismann, A. Böhme, A. Paschke and G. Schüürmann, Chem. Res. Toxicol., 2012, 25, 170–180 Search PubMed.
- V. Ansteinsson, H. B. Kopperud, E. Morisbak and J. T. Samuelsen, J. Biomed. Mater. Res., Part A, 2013, 101, 3504–3510 CrossRef CAS PubMed.
- T. J. McCarthy and G. Witz, Toxicology, 1997, 116, 153–158 CrossRef CAS PubMed.
- R. J. Albertini, Regul. Toxicol. Pharmacol., 2017, 84, 77–93 CrossRef CAS PubMed.
- ECOSAR v2.2. https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosar-predictive-model (accessed 3.01.2023).
- K. Matyjaszewski, in Controlled Radical Polymerization: Mechanisms, American Chemical Society, 2015, ch. 1, vol. 1187, pp. 1–17 Search PubMed.
- G. Odian, in Principles of Polymerization, John Wiley & Sons, Inc., 4th edn, 2004, pp. 279–281, DOI:10.1002/047147875X.ch3.
- B. Gewert, M. M. Plassmann and M. MacLeod, Environ. Sci.: Processes Impacts, 2015, 17, 1513–1521 RSC.
- N. Mohanan, Z. Montazer, P. K. Sharma and D. B. Levin, Front. Microbiol., 2020, 11, 580709 CrossRef PubMed.
- M. Frank, in Ullmann's Encyclopedia of Industrial Chemistry, 2003, pp. 213–232, DOI:10.1002/14356007.f25_f01.
- N. A. Pattanashetti, G. B. Heggannavar and M. Y. Kariduraganavar, Procedia Manuf., 2017, 12, 263–279 CrossRef.
- G. Herth, G. Schornick and F. L. Buchholz, in Ullmann's Encyclopedia of Industrial Chemistry, 2015, pp. 1–16, DOI:10.1002/14356007.a21_143.pub2.
- For Technical Data of CHP BAR 1400, see ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc04178b |
| This journal is © The Royal Society of Chemistry 2023 |
