Open Access Article
Open Access ArticlePolymeric and inorganic sorbents as a green option to recover critical raw materials at trace levels from sea saltwork bitterns†
V.
Vallès
 *ab,
J.
López
ab,
M.
Fernández de Labastida
ab,
O.
Gibert
ab,
A.
Leskinen
c,
R. T.
Koivula
*ab,
J.
López
ab,
M.
Fernández de Labastida
ab,
O.
Gibert
ab,
A.
Leskinen
c,
R. T.
Koivula
 d and
J. L.
Cortina
d and
J. L.
Cortina
 abe
abe
aChemical Engineering Department, Escola d'Enginyeria de Barcelona Est (EEBE), Universitat Politècnica de Catalunya (UPC)-BarcelonaTECH, C/Eduard Maristany 16, Campus Diagonal-Besòs, 08019 Barcelona, Spain. E-mail: victor.valles.nebot@upc.edu
bBarcelona Research Center for Multiscale Science and Engineering, C/Eduard Maristany 16, Campus Diagonal-Besòs, 08019 Barcelona, Spain
cNuclear Safety, VTT Technical Research Centre of Finland Ltd., P.O. Box 1000, FI-02150 Espoo, Finland
dDepartment of Chemistry-Radiochemistry, University of Helsinki, P.O. Box 55, FI-00014 Helsinki, Finland
eCETaqua, Carretera d'Esplugues 75, 08940 Cornellà de Llobregat, Spain
First published on 5th January 2023
Abstract
Seawater mining is certainly a green alternative source for obtaining minerals as seawater is a natural renewable and unlimited available resource. Based on the lack of ways to obtain certain raw materials, the European Union has created the Critical Raw Materials (CRM) list. Seawater contains almost all elements, including some of those present in the CRM list, but only a few are economically feasible to be extracted as most of them are considered Trace Elements (TEs) (μg L−1). Therefore, an improvement in TEs extraction must be carried out. Saltwork brines can be considered as they are naturally concentrated (20–40 times) compared to seawater, which makes the extraction and recovery of TEs easier. Selective polymeric and inorganic sorbents were evaluated for TEs recovery (Li, B, Co, Ga, Ge, Rb, Sr, and Cs) from synthetic brines mimicking sea saltwork bitterns. Distribution coefficients were determined to characterize selectivity patterns toward TEs. Although amine and sulphonic sorbents showed low sorption of TEs, carboxylic sorbents presented good sorption and recovery for Co and Ga. Among phosphonic/phosphinic sorbents, MTX8010 achieved >98% sorption and desorption of Ga. Aminophosphonic and iminodiacetic are the best sorbents for Sr, but its desorption was incomplete. B was only sorbed by N-Methylglucamine (>98%) and N-Methylpyridine sorbents (75%), and its desorption was 37–64% and 66−>99%, respectively. SbTreat presented good performance targeting Ga and Ge, and CsTreat demonstrated high Cs uptake, but its desorption was unachieved. The most highly selective sorbents could provide the possibility of building a green option to recover critical elements for societal development in the next decade.
Introduction
The economy of European Union (EU) has a wide industrial sector, which needs raw materials to prosper with their activities. Many of these raw materials are produced in countries outside the EU. This fact makes the EU dependent on international markets to ensure accessibility to these materials. To obtain raw materials on a continuous and secure basis, the EU has begun to implement circular economy policies. Considering their economic importance (i.e., the value that these materials add to the final products) and their supply risk, taking into account the location of primary sources, the European Commission created a list of Critical Raw Materials (CRMs) in 2011, which nowadays has been reviewed and expanded to include 30 elements.1 Many of them are metals or minerals, of which the EU has no primary sources. As an example, 98% of rare earth elements used in the EU are provided by China, whereas Turkey supplies 98% of borate minerals. Practically, the EU itself has reserves of only two of the CRMs, hafnium and strontium.2 Therefore, due to the fact that most CRMs are needed not only in process industries but also in technological sectors (e.g., communication, energy, and mobility), the EU is looking for secondary sources of these CRMs from the recovery and valorization of waste. One of the alternative sources is seawater mining.3Seawater, with a 3.3% (w/w) content of dissolved salts, contains almost all the elements from the periodic table, although most of them are at very low concentration levels.4,5 This makes seawater a potential alternative source for most CRMs.6 In fact, humanity has been extracting minerals from seas and oceans since ancient times; the most evident example is the common table salt NaCl. However, only the elements at higher concentration (major elements, i.e., Na, Mg, Ca, and K) are extracted from seawater with commercial purposes.7 Nevertheless, seawater extraction may represent an alternative source for minor elements considered as CRMs (e.g., Li, Co, or rare earth elements), without producing any damaging impact on marine ecosystems.8 Moreover, seawater presents the advantage of being homogeneous, which, in contrast to land mining, leads to the scenario in which there is no mineral grade difference.4
Although the concept of extracting minerals from seawater could be possible, the extremely low concentrations of some of the elements (i.e., Trace Elements (TEs); at μg L−1 levels or less) would make the process extremely complex and economically unviable. Therefore, the use of seawater brines, such as those from membrane-based desalination plants, with salt concentrations higher compared to seawater (ca., 6.6% (w/w) would favour the recovery of these metals or minerals.9 Another kind of seawater brine can be found in seawater solar saltworks, which are defined as a group of shallow ponds where the concentration of saline water and sodium chloride crystallization take place.10 In comparison to the desalination plants, their operation is based on solar energy, although most of them are constructed in such a way that they can also take advantage of the wind for evaporating the water.11,12 Even though their efficiency depends on their location and environmental conditions, all of them are constructed with a similar structure: supply, evaporation, and crystallization ponds.12 Firstly, seawater enters the supply ponds, which are the biggest and deepest ponds of the saltworks. Then, water flows into evaporation ponds, a group of basins covering 90% of the saltworks’ area.13 In these ponds, water is evaporated thanks to the sun radiation and the wind until NaCl concentration approaches its saturation point. Finally, the water enters into crystallization ponds, where NaCl achieves its solubility limit, precipitates, and accumulates at the bottom of the ponds.12 Along these long-term evaporation stages (e.g., typically one year), many minerals precipitate along the ponds, causing a fractionated precipitation of different inorganic Ca(II) salts. First, carbonates (mainly CaCO3(s)) precipitate when water reaches a total salt concentration of about 100–120 g L−1. Then, CaSO4·2H2O(s) precipitates when total dissolved salt concentration is about 180 g L−1. Finally, NaCl precipitates after reaching concentrations of 300–350 g L−1. This type of fractionated precipitation makes it possible to obtain a salt with a low content of impurities (<0.3% (w/w)).11 The remaining concentrated liquid is commonly called bittern, and it has high amounts of Mg(II), K(I), Cl(−I), and S(VI).14,15 Although this bittern is up to 40 times more concentrated than seawater, some CRMs that could be recovered are still at trace level concentrations (μg L−1 or ng L−1). Therefore, it is necessary to make efforts for improving the extraction procedures to achieve economic feasibility.16
Several processes can be used for mining these bitterns and recovering valuable minerals. These processes can be classified, for example, depending on the type of driving force used: pressure-driven (e.g., reverse osmosis), thermally driven (e.g., evaporation), electrochemical potential-driven (e.g., electrodialysis), and physico-chemically driven (e.g., sorption and ion exchange).9,17 In fact, although not many studies have been reported regarding the recovery of TEs from seawater or brines, the literature indicates sorption and ion exchange processes as the most suitable methods for the recovery of most of them (e.g., Li, Sr, Rb, and Cs) because of the high selectivity of the sorbents, which appears as an essential property when dealing with elements at trace levels (μg L−1 or ng L−1).4,9,18,19 In addition, sorption processes are not only selective but also simple, efficient, and low-cost methods to extract these TEs.4,17,20
Scarce works can be found in the literature regarding the extraction of valuable elements at low concentrations from brines. Arroyo et al. (2019),21 for example, evaluated three polymeric commercial ion exchange sorbents (K2629 – sulphonic sorbent – and TP207 and TP208 – both of them iminodiacetic sorbents) targeting Li recovery in brines. For solutions (50 mL) containing only 10 mg L−1 Li, they reported that more than 95% of Li was removed from the solution in 20 minutes using only 1 g of the sorbent. In addition, after achieving over 98% sorption, an elution test was performed with 50 mL HCl (3 or 4 M). When 3 M HCl was used, the desorption percentages of Li were between 74% and 87%, whereas using 4 M HCl these values increased and ranged between 80% and 90%. After the elution, the authors conditioned again the sorbents to the H+ and Na+ form and reused them for further Li extraction. On the other hand, in their Na+ form, Li removal remained at about 98%, and in their H+ form, removals using TP207 and TP208 decreased to 52% and 40%, respectively (for K2629 removal, it was still about 98%). Despite the high Li sorption achieved with synthetic solutions, when real Seawater Reverse Osmosis (SWRO) brines (15.3 g L−1 Na+, 28.7 g L−1 Cl−, 4.60 g L−1 SO42−, and 0.60 mg L−1 Li+) were tested, Li retention was in the range of 30–80% depending on the type and amount (10 or 20 g) of sorbent. Nur et al. (2017)22 synthesized a Resorcinol Formaldehyde (RF) sorbent and compared its performance with commercial ones (i.e., CsTreat, Ammonium MolybdoPhosphate (AMP), a natural zeolite, and Amberlite FPC 3500) targeting Sr removal. Among all sorbents tested for a solution containing only 10 mg L−1 Sr, the Na+ form of RF achieved the highest Sr removal (>95%) for doses of 1 g L−1 sorbent or higher. However, when synthetic seawater (20 g L−1 Na, 2 g L−1 Mg, 1 g L−1 Ca, 0.85 g L−1 K, and 0.01 g L−1 Sr) was tested, Sr sorption decreased to 40% due to competition between Ca and Mg ions. Desorption tests with 100 mL NaCl or NaOH (1 and 2 M) were performed for 1 g saturated RF, but Sr recoveries over 90% were only possible using NaCl. RF sorbent was also tested in column experiments with three inlet solutions with 10 mg L−1 Sr but differing in that one contained only Sr in water, a second one Sr in the seawater medium, and the third one Sr in the seawater medium but without Ca and Mg. Sr adsorption capacities were 22, 6, and 11 mg g−1 for the three solutions described, indicating that seawater medium influence the Sr adsorption and that part of this influence is caused by the presence of Ca and Mg. Gibert et al. (2010)23 performed column experiments to retain Rb and Cs with CsTreat sorbent (K2CoFe(CN)6) using a SWRO brine containing mainly 70.0 g L−1 NaCl, 6.4 g L−1 SO42−, and 2.5 g L−1 Mg2+, with other ions (i.e., CO32−, K+, and Ca2+) in the range from 130 to 669 mg L−1 and a few elements (i.e., Rb+ and Cs+) spiked to 20 mg L−1. They reported sorption capacities of 44 mg g−1 for Cs and 238 mg g−1 for Rb. Using the same sorbent and brine but enriching its Rb and Cs concentrations to range 5–80 mg L−1, Peterková et al. (2012)24 reported Langmuir maximum sorption capacities (Qmax) of 32 mg g−1 for Cs and 47 mg g−1 for Rb. Also regarding Rb sorption in the batch mode, Naidu et al. (2016)25 synthesized other hexacyanoferrate compounds and reported that KCuFC had the highest Rb sorption (Qmax = 144 mg g−1) as Qmax values for KNiFC, KCoFC, and KFeFC were under 122 mg g−1. Authors encapsulated KCuFC in PolyAcryloNitrile (PAN), which resulted in a new sorbent (KCuFC-PAN) with a slight lower Qmax value of 105 mg g−1. Under high salinity (0.2–2.5 M NaCl, simulating SWRO brine), the sorption capacity was reduced by only 12–30%, although the presence of K led to a reduction of 65–70%. A Rb sorption capacity of 86.32 mg g−1 was then reported for KCuFC-PAN when they operated it in the column mode, which was also able to desorb it by a 95% using 0.2 M KCl. After desorption, the sorption capacity was reduced by only 16% in a second cycle of sorption/desorption. These works revealed that it is possible to recover valuable elements at relatively low concentration values from seawater brines.
Within this context, several research projects aim to recover CRMs and other valuable elements (Sc, V, Mo, and In, among others) from seawater or brines,26,27 specifically targeting the recovery of Mg, Li, and some TEs (i.e., B, Co, Ga, Ge, Rb, Sr, and Cs) from saltworks bitterns.28 Moreover, considering their initial estimations, about 107 m3 per year of bitterns are produced in the Mediterranean area. Therefore, several TEs could be recovered from them, including about 360 ton per year (tn per year) Li, 2300 tn per year B, 0.43 tn per year Co, 0.47 tn per year Ga, 1.18 tn per year Ge, 75 tn per year Rb, 285 tn per year Sr, and 1.6 tn per year Cs. To demonstrate the potential of recovering these TEs from bitterns, 20 Mediterranean saltworks collaborate with this initiative. In addition, according to Sadoul et al. (1998),29 168 saltworks could be found in 18 countries around the Mediterranean basin.
The novel idea of reusing and recycling resources should be highlighted as it must be taken into account that bitterns are the “waste” stream produced in saltworks that, in most cases, is returned to the sea. Therefore, a green sustainable circular process (see Fig. 1) has been designed by the EU to transform the bittern into a valuable source of CRMs (from “waste” to raw materials). In addition, advantage is taken of the bitterns as salty streams and, by causing a gradient of salinity, part of the electricity required to operate the different technologies included in this proposal is produced using Reverse Electrodialysis.30 Electrodialysis with bipolar membranes is also used to generate the reagents needed along the whole process. In this way, some of the principles of green engineering31 were accomplished as, apart from reusing the bittern as a new source to obtain CRMs, the waste generated along this circular scheme is minimized and reused, as well as to obtain energy. Various aspects also allow this initiative to achieve some of the principles of green chemistry.32,33 For example, the fact that at the end of the process, the bittern remaining is at neutral pH and is used to produce energy allow to achieve principles 1 (prevent waste) and 6 (energy efficiency). In addition, as non-hazardous sorbents and membranes are principally used in the different technologies of this proposal, principle 5 (safer solvents and auxiliaries) is also obtained. Finally, considering that the main raw material of the whole process is the bittern produced by the solar evaporation of seawater, that could be considered an unlimited resource available for anyone, principle 7 (renewable feedstocks) is attained.
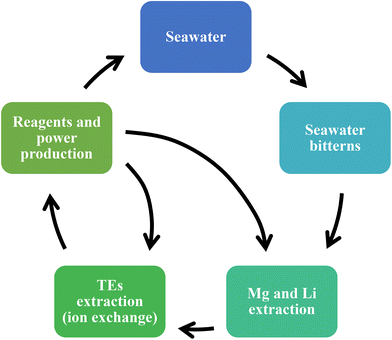 | ||
| Fig. 1 Green sustainable circular process for transforming bittern rejected streams into alternative sources to recover CRMs (adapted scheme).34 | ||
This paper compares the behavior of 30 commercial sorbents for the recovery of TEs (Li, B, Co, Ga, Ge, Rb, Sr, and Cs) from saltworks bitterns using sorption processes. The sorbents tested were either polymeric (containing, e.g., amine, carboxylic, sulphonic, and phosphinic groups) or inorganic (e.g., metal oxides and hexacyanoferrates). The screening of sorbents was performed by testing three different bittern compositions to ascertain the competition between the targeted valuable TEs and the major elements in solution for the sorption sites. In addition, potential sorption mechanisms are suggested for the main retained TEs. Subsequently, desorption was carried out using 0.1 M and 1 M acids (e.g., HCl and H2SO4) and bases (e.g., NaOH and NH3). Finally, a characterization of the best sorbents was performed in terms of distribution coefficients.
Materials and methods
Testing of the sorbents for the take-up and release of CRMs was divided into two groups: polymeric and inorganic sorbents. Due to their different nature both in physical and chemical properties, and more importantly in their level of selectivity, the division is kept also in the text presenting their properties and results.Sorbents and chemicals
Different polymeric and inorganic commercial sorbents were selected for targeting the TEs present in the bitterns. Polymeric sorbents evaluated are collected in Table 1 and classified according to their functional groups.| Functional group | Sorbent (abbreviation) | Supplier | Ionic form | Capacity (eq L−1) | Humidity content (%) | Particle size (mm) | Density (g L−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| F.B.: free base. n.a.: not available.a Capacity in meq g−1.b Water uptake in g H2O per g sorbent.c Filament diameter (as it is a fibrous sorbent) in μm. | ||||||||
| Tertiary amine | Amberlite IRA-94 S (IRA94) | Dupont | F.B. | 1.20 | 60 | 0.30–1.19 | 640 | 35 |
| Lewatit MP 62 (MP62) | Lanxess | F.B. | 1.70 | 50–55 | 0.32–1.25 | 620 | 36 | |
| Purolite A100 (A100) | Purolite | F.B. | 1.30 | 53–60 | 0.60–0.85 | 655–685 | 37 | |
| Quaternary amine | Purolite A500Plus (A500Plus) | Purolite | Cl− | 1.15 | 57–63 | 0.30–1.20 | 640–690 | 38 |
| Lewatit MonoPlus MP 500 (MP500) | Lanxess | Cl− | 1.10 | 60–65 | 0.57–0.67 | 640 | 39 | |
| Lewatit K 6362 (K6362) | Lanxess | Cl− | 1.30 | 48–55 | 0.57–0.67 | 690 | 40 | |
| Amberlite IRA-904 (IRA904) | Dupont | Cl− | 0.70 | 56–62 | n.a. | n.a. | 41 | |
| Tertiary and quaternary amine | Lewatit MP 64 (MP64) | Lanxess | Cl− | 1.30 | 61–66 | 0.54–0.64 | 620 | 42 |
| Carboxylic with amines | Fiban K-3 (K3) | Imatek&K | H+ | 4.00–5.50a | 0.4–1.5b | 25–40c | n.a. | 43 |
| Fiban K-5 (K5) | Imatek&K | H+ | 4.00–6.00a | 0.5–1.5b | 25–40c | n.a. | 43 | |
| Sulphonic | Purolite C150 (C150) | Purolite | Na+ | 1.80 | 48–53 | 0.30–1.20 | 785–825 | 44 |
| Purolite C160 (C160) | Purolite | Na+ | 2.30 | 35–40 | 0.30–1.20 | 820–860 | 45 | |
| Lewatit K 2629 (K2629) | Lanxess | H+ | 1.70 | 50–55 | 0.50–0.62 | 760 | 46 | |
| Lewatit S100 (S100) | Lanxess | Na+ | 2.00 | 42–48 | 0.55–0.65 | 830 | 47 | |
| Phosphonic and sulphonic | Purolite S957 (S957) | Purolite | H+ | 0.64–0.97 | 55–70 | 0.55–0.75 | 755–785 | 48 |
| Aminophosphonic | Purolite S940 (S940) | Purolite | Na+ | 1.00 | 55–65 | 0.43–0.85 | 710–760 | 49 |
| Ambersep IRC747 UPS (IRC747) | Dupont | Na+ | 1.75 | 64–69 | 0.50–0.60 | 755 | 50 | |
| Bis-(2,4,4-trimethylpentyl-) phosphinic | Puromet MTX8010 (MTX8010) | Purolite | H+ | 0.38 | 28–35 | 0.30–1.40 | 530–580 | 51 |
| Iminodiacetic | Ambersep IRC748 UPS (IRC748) | Dupont | Na+ | 1.35 | 60–69 | 0.50–0.65 | 750 | 52 |
| Lewatit MonoPlus TP 207 (TP207) | Lanxess | Na+ | 2.00 | 55–60 | 0.56–0.66 | 700 | 53 | |
| Purolite S9320 (S9320) | Purolite | Na+ | 1.57 | 54–62 | 0.57–0.67 | 730–780 | 54 | |
| Lewatit MDS TP 208 (TP208) | Lanxess | Na+ | 2.80 | 59–65 | 0.36–0.42 | 740 | 55 | |
| N-Methylglucamine | Purolite S108 (S108) | Purolite | F.B. | 0.60 | 61–67 | 0.43–0.63 | 670–730 | 56 |
| Diaion CRB03 (CRB03) | Mitsubishi Chemical | F.B. | 0.70 | 45–55 | 0.55 | 670 | 57 | |
| Diaion CRB05 (CRB05) | Mitsubishi Chemical | F.B. | 0.95 | 45–53 | 0.55 | 750 | 58 | |
| N-Methylpyridine | Sopolimer-8 | Smoly State Enterprise | Cl− | n.a. | 35–65 | 0.40–0.80 | 550–650 | 59 |
Table 2 presents the inorganic sorbents evaluated. In addition, their main properties (e.g., supplier, ionic form, capacity, humidity content, particle size, and density) are also shown.
| Functional group | Sorbent | Supplier | Ionic form | Capacity (eq L−1) | Humidity content (%) | Particle size (mm) | Density (g L−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| n.a.: not available | ||||||||
| Zirconium oxide | SbTreat | Fortum Power and Heat Oy | n.a. | 1.00 | n.a. | 0.25–0.85 | 670 | 60 |
| Sodium titanium oxide hydrate | CoTreat | Fortum Power and Heat Oy | n.a. | 5.00 | n.a. | 0.30–0.85 | 1000 | 60 |
| Potassium hexacyano cobalt(II) ferrate(II) | CsTreat | Fortum Power and Heat Oy | n.a. | 0.35 | n.a. | 0.25–0.85 | 670 | 60 |
| Sodium titanium oxide hydrate | SrTreat | Fortum Power and Heat Oy | n.a. | 5.00 | n.a. | 0.30–0.85 | 880 | 60 |
Screening was performed with synthetic solutions and all chemicals used were at least of analytical grade. The following ones were used for the testing of polymeric sorbents: NaCl (PanReac), KCl (PanReac), MgCl2·6H2O (Alfa Aesar), CaCl2·2H2O (PanReac), NaBr (PanReac), Na2SO4 anhydrous (Glentham), LiCl (PanReac), H3BO3 (PanReac), Rb2CO3 (Sigma-Aldrich), CoCl2·6H2O (Alfa Aesar), gallium plasma standard solution Specpure® 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1 (Alfa Aesar), germanium plasma standard solution Specpure® 10
000 μg mL−1 (Alfa Aesar), germanium plasma standard solution Specpure® 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1 (Alfa Aesar), CsCl (Glentham), NaOH (PanReac), 37% HCl (PanReac), and 69% HNO3 (PanReac). The following chemicals were used for the preparation of bitterns for the testing of inorganic sorbents: NaCl (Merck), KCl (Merck), MgCl2·6H2O (Merck), CaCl2·2H2O (Merck), NaBr (VWR), Na2SO4 anhydrous (Supelco), LiCl (Merck), H3BO3 (VWR), RbCl (Merck), CoCl2·6H2O (Merck), gallium plasma standard solution 10
000 μg mL−1 (Alfa Aesar), CsCl (Glentham), NaOH (PanReac), 37% HCl (PanReac), and 69% HNO3 (PanReac). The following chemicals were used for the preparation of bitterns for the testing of inorganic sorbents: NaCl (Merck), KCl (Merck), MgCl2·6H2O (Merck), CaCl2·2H2O (Merck), NaBr (VWR), Na2SO4 anhydrous (Supelco), LiCl (Merck), H3BO3 (VWR), RbCl (Merck), CoCl2·6H2O (Merck), gallium plasma standard solution 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg mL−1 (J. T. Baker), germanium standard for AAS 1005 mg mL−1 (Sigma-Aldrich), CsCl (Merck), and SrCl2·6H2O (J. T. Baker). Desorption solutions for the testing of inorganic resins were at least of analytical grade and prepared from NaOH (J. T. Baker) and 95–97% H2SO4 (Merck).
000 μg mL−1 (J. T. Baker), germanium standard for AAS 1005 mg mL−1 (Sigma-Aldrich), CsCl (Merck), and SrCl2·6H2O (J. T. Baker). Desorption solutions for the testing of inorganic resins were at least of analytical grade and prepared from NaOH (J. T. Baker) and 95–97% H2SO4 (Merck).
Experimental design
Three different synthetic bitterns were prepared to test the sorbents in different conditions, which are expected to take place at the TEs recovery unit. The exiting bittern from the crystallization ponds undergoes a pretreatment process, where most of Li, Ca, and Mg is removed before entering the TEs recovery unit. Consequently, the variability proposed accounts for the different removal or recovery ratios achieved in that pretreatment. Therefore, screening was performed with the following bitterns (Table 3): (i) bittern 1 simulates the expected composition at the TEs recovery unit, (ii) bittern 2 accounts for lower levels of Ca and Mg in the solution (considering higher removal of both elements in the pretreatment), and (iii) bittern 3 simulates a scenario with lower content of major elements (lower concentration factor in the pretreatment). To make the concentration of TEs measurable by the analytical techniques, it was decided to spike the concentration of Li, Co, Ga, Ge, and Cs to 500 μg L−1. Inorganic sorbents were only tested using bitterns 1 and 2.| Bittern 1 | Bittern 2 | Bittern 3 | ||
|---|---|---|---|---|
| With Ca and Mg | Without Ca and Mg | Low concentration | ||
| pH | 7.00 ± 0.02 | 7.00 ± 0.02 | 7.00 ± 0.02 | |
| g L−1 | Na(I) | 71.64 ± 0.76 | 72.16 ± 0.10 | 38.63 ± 0.29 |
| K(I) | 8.07 ± 0.07 | 8.07 ± 0.02 | 5.10 ± 0.04 | |
| Mg(II) | 0.39 ± 0.03 | <0.01 | <0.01 | |
| Ca(II) | 0.10 ± 0.00 | <0.01 | <0.01 | |
| Cl(−I) | 68.22 ± 0.71 | 66.58 ± 0.11 | 36.26 ± 0.42 | |
| Br(−I) | 1.67 ± 0.02 | 1.65 ± 0.01 | 0.85 ± 0.01 | |
| S(VI) | 63.28 ± 0.83 | 63.11 ± 0.02 | 29.75 ± 0.87 | |
| mg L−1 | Li(I) | 0.32 ± 0.01 | 0.19 ± 0.01 | 0.80 ± 0.01 |
| B(III) | 110.35 ± 0.83 | 113.88 ± 1.56 | 124.50 ± 0.88 | |
| Rb(I) | 3.49 ± 0.02 | 2.26 ± 0.02 | 10.28 ± 0.03 | |
| Sr(II) | 10.77 ± 0.07 | 8.79 ± 0.27 | 10.35 ± 0.01 | |
| μg L−1 | Co(II) | 407.35 ± 16.19 | 287.02 ± 12.98 | 403.67 ± 21.68 |
| Ga(III) | 411.27 ± 9.96 | 276.76 ± 0.48 | 420.95 ± 4.44 | |
| Ge(IV) | 478.20 ± 17.96 | 301.81 ± 5.32 | 475.84 ± 64.68 | |
| Cs(I) | 552.28 ± 4.78 | 888.29 ± 1.81 | 535.87 ± 12.64 | |
Screening of commercial sorbents: sorption and desorption
Sorption experiments for polymeric sorbents were performed by mixing two grams of dried sorbent (in the ionic form indicated in Table 1) and 20 mL of bittern. After 1 h under rotation (60 rpm, Heidolph mixer Reax 2), the supernatant was filtered through a 0.22 μm filter and analyzed, while the sorbent was recovered and put into contact with 10 mL of 1 M HCl for the recovery of the sorbed elements. After another 1 h of rotation, the supernatant was also analyzed. Kinetic experiments (data not shown) have demonstrated that equilibrium was attained within 45 min. Screening was carried out in duplicate for each sorbent for both sorption and desorption, and the pH measurements of the supernatant after the sorption step are collected in Table S1 of the ESI.†As indicated in Table 1, the preferred option for the application of the most of the ion exchange sorbents selected would be in the sodium form, as the bitterns to be treated are having pH values close to neutral values and because the TEs to be removed are at μg L−1 levels (μmol L−1) and, therefore, the expected changes in pH will be small.
Sorption experiments for inorganic sorbents were performed by mixing 20 mg dried sorbent and 10 mL bittern. Lower amounts of inorganic sorbents were needed compared to polymeric ones due to their higher selectivity. After 1 h overhead rotation (24 rpm, Heidolph overhead mixer), the samples were centrifuged (10 min, 2100 G) and the liquids were recovered by filtration. Desorption studies were carried out by washing the sorbent three times with 5 mL deionized water and centrifugation (10 min, 2100 G) first and then by adding 10 mL desorption solution (0.1 M NaOH for SbTreat and 0.1 M H2SO4 for CoTreat, CsTreat, and SrTreat). The experimental procedure was the same as with the sorption studies. The sorption and desorption studies were carried out with up to 3 replicates.
For each target element, sorption removal and desorption recovery efficiencies were calculated as a percentage according to eqn (1) and (2), respectively, based on the ones proposed by Ali et al. (2018).61 In addition, Na sorption removal is provided in Table S2 of the ESI.†
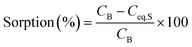 | (1) |
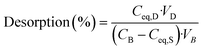 | (2) |
In addition, to compare the performance or selectivity of the sorbents toward the TEs, distribution coefficients (Kd (L kg−1)) have been calculated according to eqn (3).62
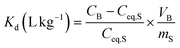 | (3) |
Analytical methodologies
Major elements were analyzed by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES; Agilent Technologies 5100 ICP-OES), while minor elements by Inductively Coupled Plasma Mass Spectrometry (ICP-MS; Agilent Technologies 7800 ICP-MS for samples from tests with polymeric sorbents; Thermo Scientific Element 2 ICP-MS for samples from tests with inorganic sorbents). All samples were filtered through 0.22 μm filters and diluted with 2% HNO3.Results and discussion
Speciation of trace elements in bitterns
Initially, a speciation analysis of the TEs in the bittern was carried out using Hydra/Medusa63 since it will determine the extraction mechanisms. From Fig. 2, it can be observed that under the composition of bittern 1 and at pH 7, the TEs present four different behaviors: (i) they are found completely as free ions (e.g., Rb+ and Cs+), (ii) they form complexes with sulphate (e.g., Li+/LiSO4−, and Sr2+/SrSO4(aq)), (iii) they form complexes with both chloride and sulphate (e.g., Co2+/CoSO4(aq)/CoCl+), or (iv) they behave as an oxyanion (e.g., H3BO3(aq)/H2BO3−, H3GaO3(aq)/H4GaO4−, and H4GeO4(aq)/H3GeO4−). No significant changes were found regarding speciation between the different bitterns evaluated.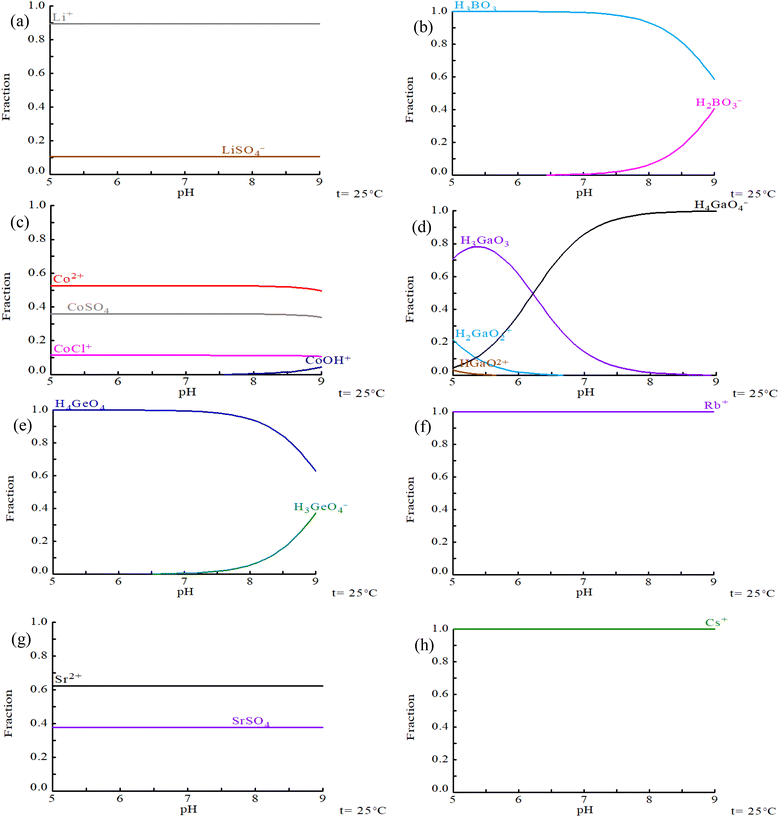 | ||
| Fig. 2 Speciation diagrams as a function of pH for the TEs in solution: (a) Li, (b) B, (c) Co, (d) Ga, (e) Ge, (f) Rb, (g) Sr, (h) Cs. Composition: bittern 1. Diagrams were built using Hydra/Medusa.63 | ||
Screening of polymeric sorbents for the recovery of trace elements: sorption and desorption
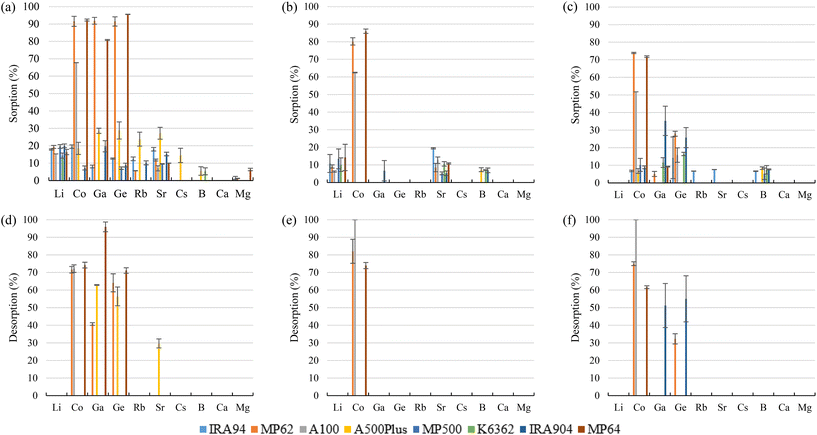
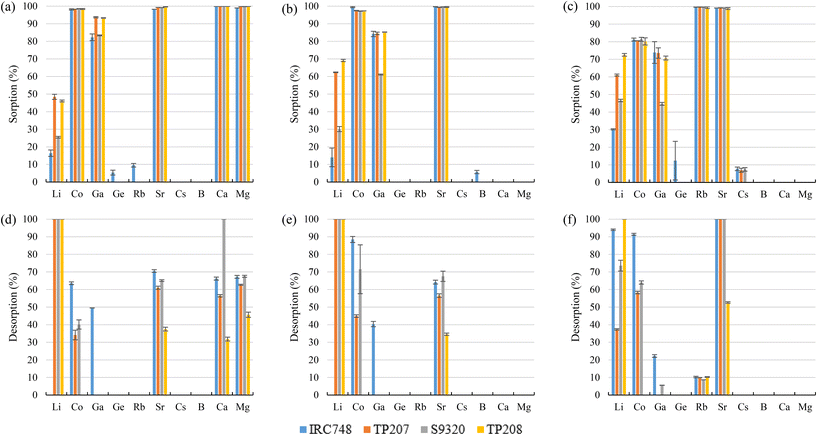
It is expected that due to the positive charge of amine sorbents, negatively-charged species (H4GaO4− for Ga and H3GeO4− for Ge) will be retained. From the experimental results (Fig. 3a–c), it was observed that quaternary amine sorbents (A500Plus, MP500, K6362, and IRA904) did not show affinity toward any of the target elements of interest for the project (removals <10%) and that only appreciable Ga and Ge sorption (25–30%) was achieved by A500Plus with bittern 1 and IRA904 with bittern 3 (25–35%). This would be in accordance with the explanation of Yi-Gong Chen et al. (2021),64 where it is said that strong basic anion exchangers with quaternary ammonium groups could have good kinetics and short rinses but, in contrast, not a very high exchange efficiency. Among tertiary amine sorbents, IRA94 did not show affinity toward any element, but A100 was able to sorb around 67%, 62%, and 52% Co for bitterns 1, 2, and 3, respectively. In addition, MP62 with a tertiary amine group and also MP64, which has both tertiary and quaternary amine functional groups, presented Co sorption that ranged between 72% and 92%. Both sorbents were also able to sorb about 86% Ga and 93% Ge when bittern 1 was evaluated. Yi-Gong Chen et al. (2021)64 reported that large operating capacity and regeneration efficiency are characteristics of tertiary amine sorbents, while Silva et al. (2018)65 described mixed anion exchangers (those combining, for example, quaternary ammonium groups with tertiary amines, such as MP64) as sorbents with good metal selectivity and loading capacity.
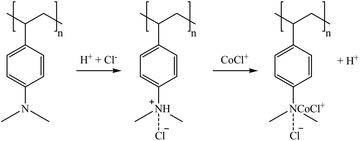
The sorption mechanisms suggested in reactions (4)–(8) are based on the structure of tertiary and quaternary amine sorbents.66 For tertiary amines, Co(II) (in solution as a mixture of Co2+/CoSO4(aq)/CoCl+) can be retained in the sorbent in its CoCl+ form by replacing the proton of the amine group (reaction (4)), as reported by Wołowicz and Hubicki (2018).67 However, as suggested by Chirskt et al. (2013),68 H4GaO4− would completely replace the chloride present in the tertiary amine (reaction (5)). In the case of Ge, it is proposed that H4GeO4 will lose a proton to form a bond with the free amine group, also replacing the chloride present in the tertiary amine (reaction (6)). In the case of quaternary amines, Höll (2011)69 reported that the chloride atom is replaced by other anion species. Therefore, reactions (7) and (8) are suggested as Ga and Ge sorption mechanisms.
 | (4) |
 | (5) |
 | (6) |
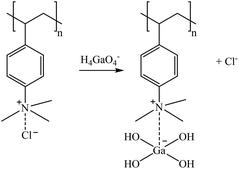 | (7) |
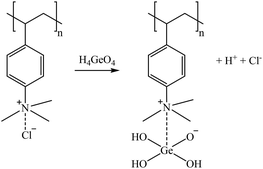 | (8) |
Regeneration of the sorbents was carried out using 1 M HCl (Fig. 3d–f). During regeneration, Co retained in A100 was recovered by more than 70% with bittern 1 and completely recovered (>99%) with bitterns 2 and 3. Regarding MP62 and MP64, recoveries over 60% were achieved for Co and, in the case of bittern 1, also for Ge. When testing bittern 1, Ga recovery was about 40% for MP62 and 96% for MP64. About 60% Ga and Ge was desorbed from A500Plus after using bittern 1, and about 53% from IRA904 after evaluating bittern 3. However, it must be borne in mind that these two sorbents presented sorption efficiencies below 30% for the other TEs. Accordingly, tertiary resins are preferred over quaternary ammonium resins from the perspective of being used to recover those elements in the form of oxyacids, as in the case of Ga and Ge.
Two suggested mechanisms of carboxylic sorbents for Co and Sr are represented as a general bivalent metal cation M2+, depending on if it is bonded to only one (reaction (9)) or two carboxylic groups (reaction (10)). In the case of Rb, which at the prevailing pH was present as a monovalent cation (Rb+), it will replace the hydrogen of the carboxylic group. Then, reactions (11)–(14) represent the suggested mechanisms on how the sorbent could retain Ga (reactions (11) and (12)) and Ge (reactions (13) and (14)), depending on if they are bonded to one or two carboxylic groups. These mechanisms are suggested based on the metal ion binding options for a carboxylate group presented by Lawrance (2010)72 and Zagorodni (2007).73
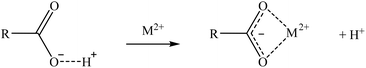 | (9) |
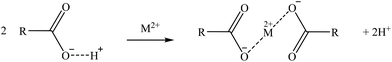 | (10) |
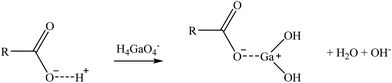 | (11) |
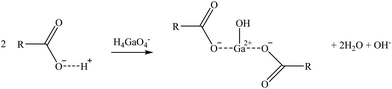 | (12) |
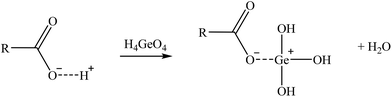 | (13) |
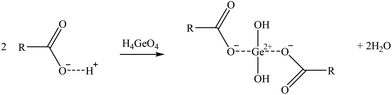 | (14) |
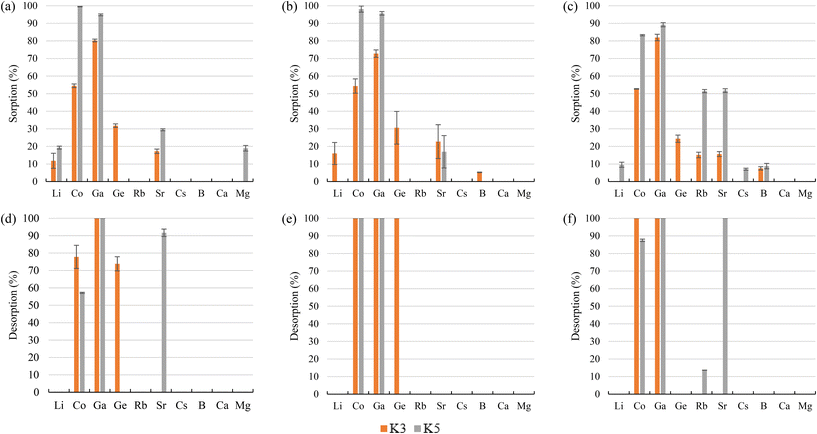
Regarding the desorption stage, it was possible to recover 78% Co, >99% Ga, and 74% Ge from K3 when bittern 1 was tested. When bitterns 2 and 3 were used, all elements sorbed were completely recovered (>99%). When K5 was evaluated, the desorption recoveries were over 87% for all elements excluding Co (57%) for bittern 1 and Rb (13%) for bittern 3.
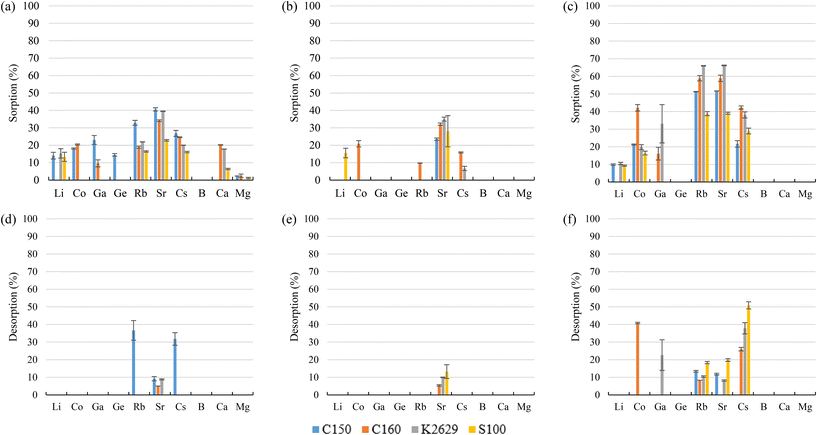
Considering the sulphonic sorbents structure and the mechanism suggested by Taha (2021),75 Sikora et al. (2021),76 and Zhang et al. (2022),77 the suggested mechanisms for Co and Sr sorption are represented in reactions (15) and (16) depending on whether they are bonded to one or two functional groups. A similar mechanism to the one described in reaction (15) is expected for Cs and Rb (as Cs+ and Rb+) by the substitution of the Na+ in the sorbent functional groups obtaining, in that case, a neutral molecule. Then, regarding Ga, similar mechanisms are suggested in reactions (17) and (18).
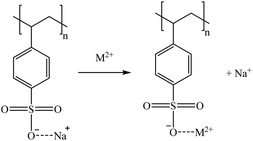 | (15) |
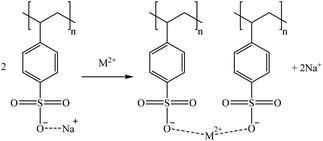 | (16) |
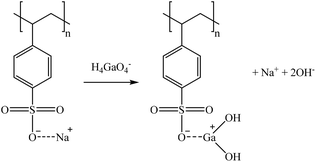 | (17) |
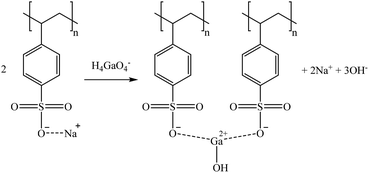 | (18) |
Most of the sulphonic sorbents used were in the sodium form. Therefore, that was the form applied in the suggested mechanisms. Nevertheless, it must be noticed that K2629 was used in its acidic form. Consequently, although the sorption reactions were expected to be similar, a slight sorption of Na (<8%) was observed, which could be explained by the substitution of H+ by the Na+ from the bitterns.
Regarding desorption, it was not possible with either bittern 1 or 2 to recover more than 37% of the elements sorbed. It was only possible to recover significant amounts of the TEs of interest after using bittern 3. For example, 40% Co and 50% Cs were desorbed from C160 and S100, respectively. The other sorbents presented lower desorption values for TEs (<40%). These low desorption results were not surprisingly as Silva et al. (2018)65 also reported that sulphonic sorbents presented difficulties to elute the adsorbed metals.
S957 has not only sulphonic but also phosphonic functional groups. Their sulphonic groups justify similar sorption values to the ones achieved with sulphonic sorbents for most elements of interest. However, although sulphonic sorbents did not have affinity for Ga, S957 phosphonic groups explain their good Ga sorption (>92%) for the three bitterns tested. Despite the extremely high Ga sorption, it was not desorbed. This behavior was the one expected, as Chiariza et al. (1997)78 had reported that phosphonic sorbents and those having both phosphonic and sulphonic groups had higher adsorption properties than sorbents having only sulphonic ligands. However, the strong interaction that metals could have with the phosphonic group causes some difficulties to elute them.79
Considering its structure and the evidences reported by Amphlett et al. (2020),80 a suggestion on how S957 could retain Sr and Ga are represented in reactions (19) and (20), respectively.
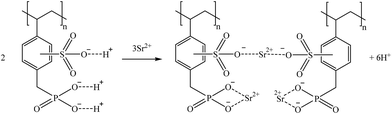 | (19) |
 | (20) |
In that case, a small amount of Na sorption was also observed, which could be probably related with the presence of sulphonic groups in the sorbent. However, it must be highlighted that Na removal (<6%) is noticeably lower than the other retained elements.
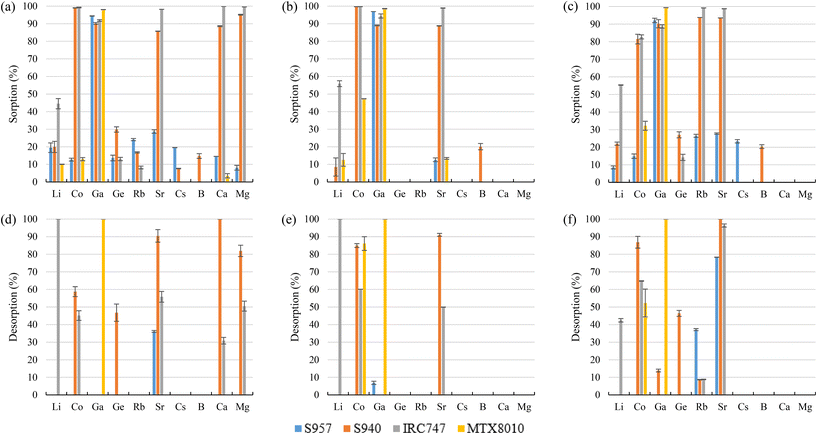
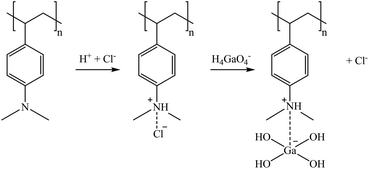
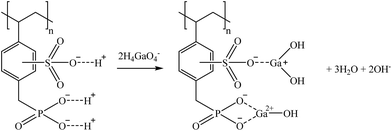
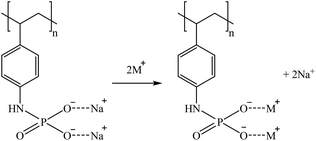
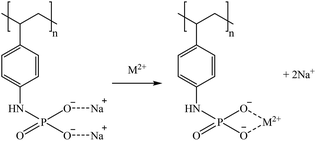
S940 and IRC747 have other similar functional groups, i.e., aminophosphonic groups. In this case, sorption over 80% was achieved for Co, Ga, and Sr for all bitterns tested. Both sorbents were also able to sorb more than 90% Rb only when bittern 3 was used, which can be related to the lower concentration of major elements (mainly Na and K). Finally, although both sorbents have an extremely similar behavior, they differ when considering Li. On one hand, S940 has no affinity toward it; on the other hand, IRC747 was able to sorb 45–55% of it from all bitterns. The results obtained were in concordance with the affinity series proposed by Hubicki and Kołodyńska (2012):74 Mg2+ > Sr2+ > Ca2+ > Co2+. In addition, they also presented a mechanism on how aminophosphonic sorbents could sorb bivalent metals. Therefore, similar ones are suggested to retain monovalent cations (i.e., Li+ and Rb+), bivalent cations (i.e., Co2+ and Sr2+), and Ga in reactions (21), (22), and (23), respectively.
 | (21) |
 | (22) |
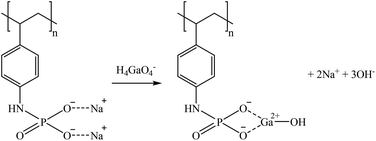 | (23) |
Another sorbent included in the study was MTX8010, which is an impregnated sorbent with bis-(2,4,4-trimethylpentyl-) phosphinic acid. MTX8010 was extremely selective toward Ga as it was the only element that was completely sorbed (>98%). Co sorption was below 15% when bittern 1 was used, but it increased to 47% and 32% for bitterns 2 and 3, respectively. The increase when testing bittern 3 would be explained by the lower quantity of major elements in solution. However, the increment with bittern 2 might be explained by a lower concentration of Ga (277 μg L−1), the main element sorbed, which would lead to a less competition, although Co was also lower (287 μg L−1) than in bittern 1. The results obtained by Horwitz et al. (1997)81 indicated that this type of impregnated sorbents had affinity toward actinides in tri-, tetra-, and hexavalent oxidation states. Therefore, although it is not an actinide, as Ga is present in the bittern in its trivalent oxidation state as an oxyanion, it may be the reason for its good sorption on the sorbent. Considering the structure of the sorbent and its reaction for Co sorption proposed by Vaughan et al. (2016),82 the following mechanisms are suggested in reactions (24) and (25) for Co and Ga retention.
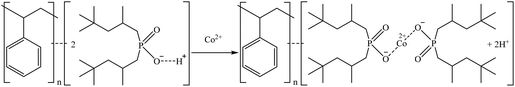 | (24) |
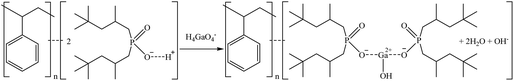 | (25) |
Regarding the desorption, for all the bitterns tested, S940 presented higher recovery efficiencies than IRC747 (excluding Li, for which S940 presented very low sorption). S940 achieved desorption values of about 85% for Co (excluding 59% for bittern 1) and over 90% for Sr, while IRC747 got values of about 62% for Co (45% for bittern 1) and 50% for Sr (excluding 96% for bittern 3). Neither of the two sorbents was able to desorb Ga or Rb. Li was completely recovered (>99%) from IRC747 when bitterns 1 and 2 were evaluated. When bittern 3 was used in the sorption stage, only 42% Li could then be desorbed. As mentioned before, the difficulty to desorb some of the elements may be related to the strong interaction between the metal and the phosphonic group.79 For MTX8010, Co desorption was about 86% and 52% for bittern 2 and 3, respectively, and, in addition, Ga was completely recovered for all the bitterns tested.
The results obtained were in accordance with the data provided in the data sheets of the sorbents, where their affinity series was presented as Co2+ > Ca2+ > Mg2+ > Sr2+, although in this case, Sr2+ was better retained than Co2+. In addition, Yuchi et al. (1997)83 reported that iminodiacetic sorbents could sorb and have high affinity for trivalent metal ions such as Ga3+ or Fe3+. This fact could be useful to confirm the evidence of this study as, although in the bitterns Ga tends to be as oxyanion, it maintains the trivalent oxidation state.
A general mechanism that could be suitable for Co and Sr and another one for Ga are suggested in reactions (26) and (27), respectively. Then, as some iminodiacetic sorbents were able to sorb Li, a suggested mechanism for it is shown in reaction (28). These three mechanisms are based on the ones presented by Zagorodni (2007).73
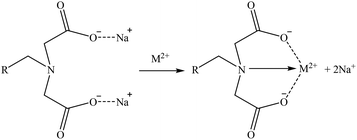 | (26) |
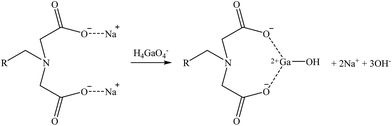 | (27) |
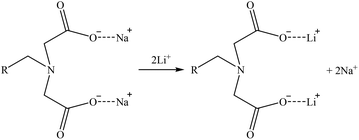 | (28) |
Despite the good Sr sorption among these sorbents, only 55–70% of it could be recovered from them during desorption after bitterns 1 and 2 were evaluated (only 35–37% for TP208). However, it could be possible to desorb it completely when bittern 3 was tested (only 53% for TP208). Desorption of Co ranged between 35% and 70% for S9320 and TP207, respectively. However, about 64% of Co for bittern 1 and 90% for bitterns 2 and 3 was possible to be recovered from IRC748. No Co desorption was observed for TP208. Only IRC748 was able to desorb Ga, which achieved desorption recoveries of 50%, 40%, and 22% for bitterns 1, 2, and 3, respectively. Li desorption ranged between 74% and >99% (37% for TP207 when bittern 3 was tested). No Li desorption was observed for IRC748 when bitterns 1 and 2 were analyzed (it must be considered that its sorption was only about 15%). Practically no desorption was observed for Rb (that was only sorbed when testing bittern 3) in any sorbent.
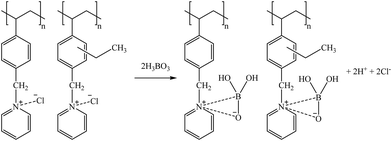
According to the literature,88 a bidentate extraction mechanism for H3BO3(aq) is postulated as shown in reaction (31).
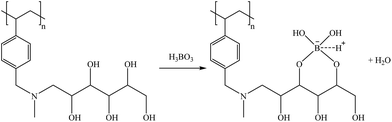 | (29) |
Taking into account that both Ge and Ga presented a similar structure as H3BO3(aq) (i.e., H4GaO4− for Ga and H4GeO4(aq) for Ge), similar sorption mechanisms are postulated, as shown in reactions (30) and (31), respectively.
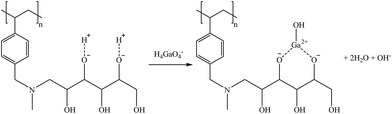 | (30) |
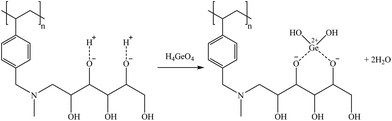 | (31) |
However, Co(II) is not found in the solution as the oxyanion; instead, it is mostly present as Co2+. Therefore, it is expected to be extracted, as shown in reaction (32).
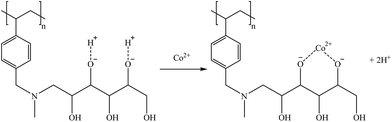 | (32) |
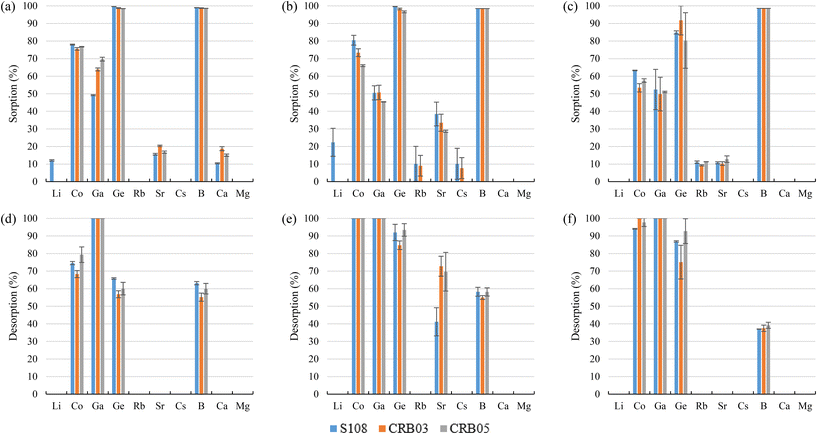
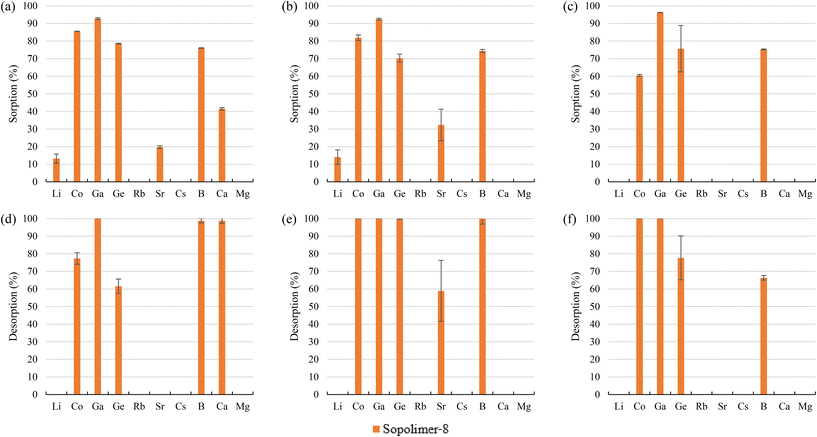
Using 1 M HCl, Co and Ga were almost completely recovered (>94%) from the sorbents, excluding 68–80% desorption of Co for bittern 1. Regarding Ge desorption, 56–66% for bittern 1 and 75–94% for bitterns 2 and 3 was achieved. However, B desorption was about 55–64% for bitterns 1 and 2 and 37–39% for bittern 3. Finally, although S108 only reached a value of 41%, Sr desorption of about 69–73% was achieved with CRB03 and CRB05 after using bittern 2, which was the only one in which Sr was appreciably (>25%) sorbed.
Suggested mechanisms for Co, Ga, Ge, and B sorption are presented in reactions (33), (34), (35), and (36), respectively.
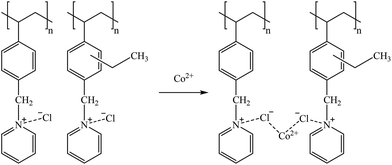 | (33) |
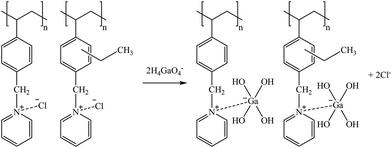 | (34) |
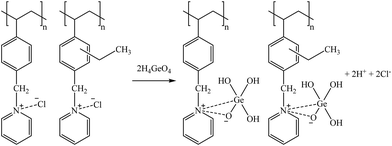 | (35) |
 | (36) |
Screening of inorganic sorbents for the recovery of trace elements: sorption and desorption
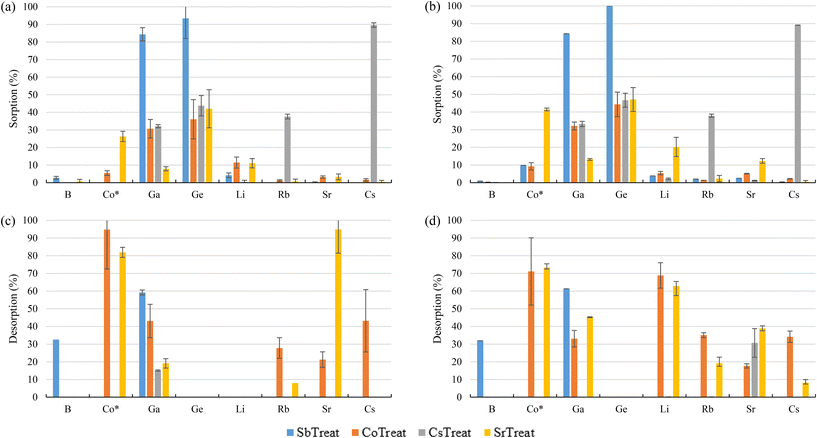
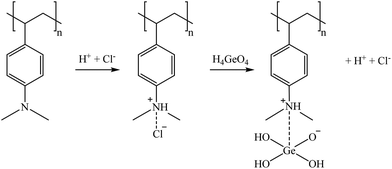
Fig. 10 shows both the sorption and desorption screening results of the inorganic sorbents, namely, SbTreat, CoTreat, CsTreat, and SrTreat. The SbTreat results showed that efficient sorption (>80%) was achieved for Ga and Ge from bittern 1. Similar behavior was seen also for bittern 2, indicating that the presence of Mg and Ca did not affect the sorption efficiency. This high uptake of Ga and Ge was expected because of their oxyanionic speciation and the anionic exchange character of zirconia-based material.89 Based on the results observed, the sorption mechanism is believed to be partly by anion uptake and partly by complexation. The desorption studies (carried out using 0.1 M NaOH) yielded 60% recovery for Ga, but no desorption of Ge was observed. However, 24 h contact time with other desorption solutions (i.e., 1 M and 0.1 M NH3, H2SO4, and HCl; data not shown) exhibit 60–70% desorption with acid (1 M and 0.1 M H2SO4) for Ge, whereas 40–60% desorption was achieved with both acid and base (0.1 M and 1 M H2SO4 and NaOH). A maximum desorption of 25% for Ge was achieved when HCl was used as the desorption solution, whereas Ga desorption was below 5%. However, the desorption for both Ga and Ge was below 5% when the desorption solution was NH3. The results show that even though Ga and Ge desorption mechanisms are different, both elements can be efficiently recovered from SbTreat using 1 M or 0.1 M H2SO4 and NaOH.Due to their similar chemical structure, both CoTreat and SrTreat were expected to show similar metal uptake with difference in their target elements Co and Sr. The highest metal uptake (36–47%) for these materials was observed for Ge, which was higher compared to Co or Sr for which these materials are targeted. Surprisingly, the absence of Ca and Mg in bittern 2 did not increase the Co or Sr uptake significantly, even though Ca is known to compete with Sr uptake. The difference in Co uptake can be explained by the materials affinity for H+ ions. In the case of CoTreat samples, the average pH was 6.8, whereas the average pH in the SrTreat ones was 7.8, exhibiting approximately 5 times higher sorption percentage for Co. The sorption percentages for Sr were similar for CoTreat and SrTreat (<5%). It is worth to remember that the original concentrations of Co and Sr in bitterns were significantly different, as the former was at μg L−1 whereas the latter was at mg L−1 levels. In addition, CoTreat sorbed about 30% Ga from both bitterns. Despite the low sorption for the targeted elements (<10% Co for CoTreat and <13% Sr for SrTreat), desorption ranged between 70% and 95% for CoTreat and it was about 95% with bittern 1 and 40% with bittern 2 when testing SrTreat. Lehto et al. (1999)90 observed that very low Sr sorption occurred at neutral pH when using SrTreat and that, in addition, whereas K+, Li+, and Mg2+ did not highly affect Sr uptake, Ca2+ strongly decreased its retention. Therefore, as the bitterns in this study were at pH 7, this may be the reason why Sr removal from them was so low and why it increased a bit when Ca2+ was not present (bittern 2), even when they were still at neutral pH. Regarding the CoTreat sorbent, Co, Ga, and Ge removal were slightly improved when changing from bittern 1 to bittern 2. In that case, Harjula et al. (2003)91 also reported that Ca presence produced a Co decreasing sorption as both of them compete in ion-exchange processes due to the similarity of their hydrated ionic radius.
The results for CsTreat showed sorptions of 33% Ga, 45% Ge, and 40% Rb, apart from an excellent sorption achieved for Cs (90%). The uptake mechanism of Cs on CsTreat (hexacyanoferrate-type material) has been explained by the tight fit of Cs ion inside the cavities of its structure, which does not allow its release in the desorption stage.92 In fact, the elements retained by the sorbent were not desorbed from it. In addition, the release of Co from the CsTreat structure occurred with the degradation of the sorbent. The desorption studies of CsTreat were continued with several acids and bases, but only maximum of 1% and 15% desorption was achieved for Cs and Rb, respectively. The retention of Cs could be done either by co-precipitation or ion-exchange, although the latter seems to be more efficient.93 Hexacyanoferrate(II) compounds, most of them with K+ in the cavities of their cubic structure, are the most suitable ones regarding Cs sorption due to their high selectivity toward that element, their low cost, and their thermal and radiation stability, apart from their fast kinetics.94,95 Then, taking into account the structure of the sorbent, the literature considers that the main mechanism for Cs sorption is the K+ replacement in the sorbent by the Cs+ present in the solution by ion-exchange.96,97
Comparison and characterization of the selected polymeric and inorganic sorbents
The screening results allowed selecting which polymeric and inorganic sorbents presented a good behavior regarding the sorption and desorption of TEs, taking into account a minimum sorption of 70% and/or having affinity toward a specific or single element. Therefore, the following polymeric sorbents were selected: (i) tertiary amine MP62; (ii) tertiary and quaternary amine MP64; (iii) aminophosphonic S940 and IRC747; (iv) the impregnated sorbent with bis-(2,4,4-trimethylpentyl-) phosphinic (MTX8010); and, (v) the N-Methylglucamine sorbents (CRB03, CRB05, and S108). The inorganic sorbents selected for further studies were SbTreat and SrTreat. CsTreat was shown to be an efficient sorbent for Rb and Cs, whereas poor desorption capabilities prohibited their use in this application. SrTreat was selected over CoTreat because, even though they are chemically similar, SrTreat exhibited higher sorption capabilities.Considering that the main sorption mechanisms for the TEs of interest were by ligands to a specific group in the case of polymeric sorbents and by inletting into the structure cavities in the case of inorganic ones, the distribution coefficients (Kd) for each of the selected sorbents is collected in Table 4. Apart from the TEs, Ca and Mg have been included, as they are the most competing elements in the sorption process. For example, MP62 and MP64 (both with amine functional groups) presented Kd values ranging between 42 and 213 L kg−1 for Co(II), Ga(III), and Ge(IV). IRC747 (aminophosphonic sorbent) has good affinity toward Co(II) (Kd ≥ 1343 L kg−1), Ga(III) (Kd ≥ 113 L kg−1), and Sr(II) (Kd ≥ 545 L kg−1), but also for Ca(II) and Mg(II), demonstrating that they are the main competing elements. The other aminophosphonic sorbent, S940, has similar Kd values for Co(II) and Ga(III) but lower ones for Ca(II) and Mg(II). Although the value for Sr(II) also decreased, it still was the second largest one regarding this element. MTX8010 has selectivity practically toward only Ga(III) (504 L kg−1). N-Methylglucamine sorbents (i.e., S108, CRB03, and CRB05) presented certain affinity (Kd in the range of 10–35 L kg−1) to Co(II) and Ga(III), but they are selective toward B(III) and Ge(IV) (Kd ≥ 637 L kg−1). In addition, inorganic sorbents are also extremely selective, as SrTreat reported Kd over 170 L kg−1 for Co(II) and Ge(IV) and SbTreat presented Kd over 2000 L kg−1 for Ga(III) and Ge(IV).
| B(III) | Ca(II) | Mg(II) | Li(I) | Co(II) | Ga(III) | Ge(IV) | Rb(I) | Sr(II) | Cs(I) | |
|---|---|---|---|---|---|---|---|---|---|---|
| MP62 | 0 | 0 | 0.1 | 2 | 115 | 116 | 113 | 0.6 | 1 | 0.2 |
| MP64 | 0 | 0 | 0.7 | 2 | 120 | 42 | 213 | 0.3 | 1 | 0 |
| S940 | 2 | 78 | 195 | 3 | 991 | 92 | 4 | 2 | 60 | 0.8 |
| IRC747 | 0.2 | 7691 | 2234 | 8 | 1343 | 113 | 2 | 0.9 | 545 | 0.1 |
| MTX8010 | 0 | 0.4 | 0 | 1 | 2 | 504 | 0 | 0 | 0.1 | 0 |
| S108 | 972 | 1 | 0 | 1 | 35 | 10 | 2644 | 0 | 2 | 0 |
| CRB03 | 778 | 2 | 0 | 0 | 31 | 18 | 795 | 0 | 3 | 0 |
| CRB05 | 711 | 2 | 0 | 0 | 33 | 23 | 637 | 0 | 2 | 0 |
| SbTreat | 14 | 0 | 0 | 22 | 0 | 2800 | 2033 | 0 | 3 | 0 |
| SrTreat | 6 | 16 | 9 | 62 | 178 | 43 | 382 | 11 | 17 | 7 |
It must be highlighted that the distribution coefficients were determined with the screening results of bittern 1 (the most unfavorable case). It is expected that these values increase as, for example, by achieving higher removal ratios of Ca and Mg. For example, Vassallo et al. (2021)98 reported the removal percentages of Ca and Mg of 98.5% and >99.9% from brines, respectively.
Conclusion
This study identified the most selective functional groups toward TEs (Li, B, Co, Ga, Ge, Rb, Sr, and Cs) present in the bitterns generated in solar sea saltworks by a screening (sorption and desorption) with synthetic bitterns with different conditions (i.e., levels of Ca and Mg, and major elements). For example, it was observed that the quaternary and/or tertiary amine functional groups as well as sulphonic sorbents showed low sorption of TEs. Sorbents containing carboxylic with amine groups showed a good performance for targeting Co and Ga (>90%, with bitterns 1 and 2). In relation to phosphonic sorbents, the best performance was achieved with the aminophosphonic ones, allowing to recover Co and Sr. In addition, the impregnated sorbent with the phosphinic acid (MTX8010) showed an extremely good affinity for Ga with both sorption and desorption over 98%. Iminodiacetic sorbents showed Sr sorption over 98% in all the bitterns, but its complete desorption was not achieved. In addition, the N-Methylglucamine and N-Methylpyridine sorbents presented good affinity toward B, Co, Ga, and Ge. No polymeric sorbents with high affinity toward Li, Cs, and Rb were found.It was observed that one critical parameter during the operation, which will affect the affinity and selectivity of the sorbents, are the levels of Ca and Mg in solution. In addition, the concentration of major elements can also have a noticeable impact on TEs extraction, as sorption removal efficiencies generally increased at lower concentration of major elements.
The experimental results allowed to select the polymeric sorbents that were the most promising ones for recovering the TEs: (i) tertiary amine MP62; (ii) tertiary and quaternary amine MP64; (iii) aminophosphonic S940 and IRC747; (iv) impregnated sorbent with bis-(2,4,4-trimethylpentyl-) phosphinic acid MTX8010; and, (v) the N-Methylglucamine sorbents (S108, CRB03, and CRB05). Distribution coefficients of some TEs have shown values up to 2644 L kg−1, showing the possibility of recovering them.
Regarding the inorganic sorbents, zirconium oxide (SbTreat) showed excellent (>80%) sorption for Ga and Ge. The desorption results suggested different uptake mechanisms for both of them since Ga was desorbed with both acid and base (i.e., H2SO4 and NaOH), whereas Ge was desorbed with only acid (e.g., H2SO4). The sodium titanium oxide sorbents showed good uptake for Co (about 25% with SrTreat) and Ge (about 40% for both SrTreat and CoTreat), whereas Sr sorption was low (<13%). However, the original Sr composition in bittern 1 was almost 30 times higher compared to Co, and Sr uptake for these materials is known to be interfered by Ca ions. The potassium hexacyanoferrate compound (CsTreat) showed excellent sorption for Cs and it could also sorb Rb, but its desorption was negligible.
A comparison of Kd values show that inorganic sorbents have as much capacity as polymeric ones to retain the targeted TEs as they are in the same orders of magnitude. However, desorption recoveries are generally much higher for polymeric sorbents than those for inorganics.
The selection of suitable sorbents for TEs recovery from saltworks bitterns will be based on: (i) a trade-off between sorption and desorption efficiencies; (ii) the need to use further separation stages as the selectivity factors are not high enough to achieve the individual separation of the selected group of TEs. Therefore, the selected sorbents will continue to be studied regarding equilibrium and kinetics parameters and column performance testing different bitterns.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under Grant Agreement No. 869467 (SEArcularMINE). This output reflects only the author's view. The European Health and Digital Executive Agency (HaDEA) and the European Commission cannot be held responsible for any use that may be made of the information contained therein.Authors acknowledge suppliers for providing the samples of the sorbents evaluated in this work: DuPont, Fortum Power and Heat Oy, Imatek&K, Lanxess, Mitsubishi Chemical, Purolite, Smoly State Enterprise.
References
- European Commission, Critical raw materials | Internal Market, Industry, Entrepreneurship and SMEs, https://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en, (accessed 5 June 2021).
- European Commission, Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability, Brussels, 2020 Search PubMed.
- A. Kumar, G. Naidu, H. Fukuda, F. Du, S. Vigneswaran, E. Drioli and J. H. Lienhard, ACS Sustainable Chem. Eng., 2021, 9, 7704–7712 CrossRef CAS.
- P. Loganathan, G. Naidu and S. Vigneswaran, Environ. Sci.: Water Res. Technol., 2017, 3, 37–53 RSC.
- M. S. Nahar and J. Zhang, ACS Sustainable Chem. Eng., 2013, 1, 488–495 CrossRef CAS.
- S. E. Can Sener, V. M. Thomas, D. E. Hogan, R. M. Maier, M. Carbajales-Dale, M. D. Barton, T. Karanfil, J. C. Crittenden and G. L. Amy, ACS Sustainable Chem. Eng., 2021, 9, 11616–11634 CrossRef CAS PubMed.
- U. Bardi, Sustainability, 2010, 2, 980–992 CrossRef CAS.
- M. S. Diallo, M. R. Kotte and M. Cho, Environ. Sci. Technol., 2015, 49, 9390–9399 CrossRef CAS PubMed.
- X. Zhang, W. Zhao, Y. Zhang and V. Jegatheesan, Rev. Environ. Sci. Biotechnol., 2021, 20, 333–361 CrossRef CAS.
- J. S. Davis, Global NEST J., 2018, 2, 217–226 Search PubMed.
- S. Gorjian, F. J. Jamshidian and B. Hosseinqolilou, in Solar Desalination Technology, Springer Nature, 2019, pp. 25–48 Search PubMed.
- C. M. Rodrigues, A. Bio, F. Amat and N. Vieira, Saline Syst., 2011, 7, 1–14 CrossRef PubMed.
- N. A. Korovessis and T. D. Lekkas, Global NEST J., 2009, 11, 49–57 Search PubMed.
- R. De Medeiros Rocha, D. F. Costa, M. A. Lucena-Filho, R. M. Bezerra, D. H. Medeiros, A. M. Azevedo-Silva, C. N. Araújo and L. Xavier-Filho, Aquat. Biosyst., 2012, 8, 8 CrossRef PubMed.
- A. Oren, Aquat. Microb. Ecol., 2009, 56, 193–204 CrossRef.
- A. Brewer, J. Florek and F. Kleitz, Green Chem., 2022, 24, 2752–2765 RSC.
- R. S. Al-Absi, M. Abu-Dieyeh and M. A. Al-Ghouti, Environ. Technol. Innovation, 2021, 22, 101541 CrossRef CAS.
- A. Shahmansouri, J. Min, L. Jin and C. Bellona, J. Cleaner Prod., 2015, 100, 4–16 CrossRef CAS.
- Y. Wang, H. Liu, J. Fan, X. Liu, Y. Hu, Y. Hu, Z. Zhou and Z. Ren, ACS Sustainable Chem. Eng., 2019, 7, 3062–3072 CrossRef CAS.
- F. Zhao, E. Repo, Y. Song, D. Yin, S. Ben Hammouda, L. Chen, S. Kalliola, J. Tang, K. C. Tam and M. Sillanpää, Green Chem., 2017, 19, 4816–4828 RSC.
- F. Arroyo, J. Morillo, J. Usero, D. Rosado and H. El Bakouri, Desalination, 2019, 468, 114073 CrossRef CAS.
- T. Nur, P. Loganathan, J. Kandasamy and S. Vigneswaran, Desalination, 2017, 420, 283–291 CrossRef CAS.
- O. Gibert, C. Valderrama, M. Peterková and J. L. Cortina, Solvent Extr. Ion Exch., 2010, 28, 543–562 CrossRef CAS.
- M. Peterková, C. Valderrama, O. Gibert and J. L. Cortina, Desalination, 2012, 286, 316–323 CrossRef.
- G. Naidu, P. Loganathan, S. Jeong, M. A. H. Johir, V. H. P. To, J. Kandasamy and S. Vigneswaran, Chem. Eng. J., 2016, 306, 31–42 CrossRef CAS.
- CORDIS, Development of radical innovations to recover minerals and metals from seawater desalination brines|Sea4Value Project, https://cordis.europa.eu/project/id/869703, (accessed 6 June 2021).
- Anson Resources, Paradox Basin Brine Project, https://www.ansonresources.com/paradoxbasin/, (accessed 9 November 2022).
- CORDIS, Circular Processing of Seawater Brines from Saltworks for Recovery of Valuable Raw Materials | SEArcularMINE Project, https://cordis.europa.eu/project/id/869467, (accessed 9 May 2021).
- N. Sadoul, J. G. Walmsley and B. Charpentier, Conservation of Mediterranean Wetlands, 1998, vol. 9 Search PubMed.
- M. Tedesco, C. Scalici, D. Vaccari, A. Cipollina, A. Tamburini and G. Micale, J. Membr. Sci., 2016, 500, 33–45 CrossRef CAS.
- P. T. Anastas and J. B. Zimmerman, Environ. Sci. Technol., 2003, 37, 94A–101A CrossRef PubMed.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, 1998 Search PubMed.
- J. C. Warner, A. S. Cannon and K. M. Dye, Environ. Impact Assess. Rev., 2004, 24, 775–799 CrossRef.
- SEArcularMINE, Circular Processing of Seawater Brines from Saltworks for Recovery of Valuable Raw Materials – SEArcularMINE, https://searcularmine.eu/, (accessed 9 May 2021).
- T. W. Chen and N. G. Pinto, Ind. Eng. Chem. Res., 1990, 29, 440–447 CrossRef CAS.
- Lanxess , MP62 Product Data Sheet.
- Purolite , A100 Product Data Sheet.
- Purolite , A500Plus Product Data Sheet.
- Lanxess , MP500 Product Data Sheet.
- Lanxess , K6362 Product Data Sheet.
- K. Sakanishi, H. Obata, I. Mochida and T. Sakaki, Ind. Eng. Chem. Res., 1996, 35, 335–337 CrossRef CAS.
- Lanxess , MP64 Product Data Sheet.
- V. S. Soldatov, A. A. Shunkevich, I. S. Elinson, J. Johann and H. Iraushek, Desalination, 1999, 124, 181–192 CrossRef CAS.
- Purolite , C150 Product Data Sheet.
- Purolite , C160 Product Data Sheet.
- Lanxess , K2629 Product Data Sheet.
- Lanxess , S100 Product Data Sheet.
- Purolite , S957 Product Data Sheet.
- Purolite , S940 Product Data Sheet.
- DuPont , IRC747 Product Data Sheet.
- Purolite , MTX8010 Product Data Sheet.
- DuPont , IRC748 Product Data Sheet.
- Lanxess , TP207 Product Data Sheet.
- Purolite , S9320 Product Data Sheet.
- Lanxess , TP208 Product Data Sheet.
- Purolite , S108 Product Data Sheet.
- Mitsubishi Chemical , CRB03 Product Data Sheet.
- Mitsubishi Chemical , CRB05 Product Data Sheet.
- Smoly State Enterprise , Sopolimer-8 Product Data Sheet.
- Fortum, NURES® Technology for Radionuclide Removal, https://www.fortum.com/products-and-services/power-plant-services/nuclear-services/decommissioning-and-waste-treatment/nures, (accessed 3 March 2021).
- I. Ali, C. Peng, T. Ye and I. Naz, RSC Adv., 2018, 8, 8878–8897 RSC.
- V. S. Yankovskaya, I. I. Dovhyi, N. A. Bezhin, V. V. Milyutin, N. A. Nekrasova, S. V. Kapranov and V. F. Shulgin, J. Radioanal. Nucl. Chem., 2018, 318, 1085–1097 CrossRef CAS.
- I. Puigdomenech, Hydra/Medusa software, 2015.
- Y.-G. Chen, W. Sofińska-Chmiel, G.-Y. Lv, D. Kołodyńska and S.-H. Chen, Materials, 2021, 14, 7067 CrossRef CAS PubMed.
- R. A. Silva, K. Hawboldt and Y. Zhang, Miner. Process. Extr. Metall. Rev., 2018, 39, 395–413 CrossRef CAS.
- E. Laforce, I. Stals, E. R. Cornelissen, P. Vermeir and J. De Clercq, J. Environ. Chem. Eng., 2022, 10, 108315 CrossRef CAS.
- A. Wołowicz and Z. Hubicki, Chem. Eng. Commun., 2018, 205, 1207–1225 CrossRef.
- D. E. Chirkst, E. A. Cheremisina, O. V. Cheremisina and M. A. Ponomareva, Russian J. of Non-Ferrous Met., 2013, 54, 201–208 CrossRef.
- W. Höll, in Ullmann's Encyclopedia of Industrial Chemistry, 2011, vol. 39, pp. 51–63 Search PubMed.
- M. Shimamura, K. Teramoto, T. Yoshioka and M. Tanaka, in Handbook of Fiber Science and Technology, 1989, vol. 3, pp. 209–252 Search PubMed.
- L. Y. Novoselova and E. E. Sirotkina, Chem. Sustainable Dev., 2006, 14, 199–213 Search PubMed.
- G. A. Lawrance, Introduction to Coordination Chemistry, John Wiley & Sons Ltd, Chichester, UK, 2010 Search PubMed.
- A. A. Zagorodni, in Ion Exchange Materials: Properties and Applications, Elesevier, Oxford, 2007, pp. 55–82 Search PubMed.
- Z. Hubicki and D. Kołodyńska, in Ion Exchange Technologies, 2012, pp. 193–240 Search PubMed.
- M. H. Taha, Environ. Sci. Pollut. Res., 2021, 28, 12475–12489 CrossRef CAS PubMed.
- E. Sikora, V. Hajdu, G. Muránszky, K. K. Katona, I. Kocserha, T. Kanazawa, B. Fiser, B. Viskolcz and L. Vanyorek, Chem. Pap., 2021, 75, 1187–1195 CrossRef CAS.
- Y. Zhang, S. Elfeghe and Z. Tang, J. Mol. Liq., 2022, 358, 119199 CrossRef CAS.
- R. Chiarizia, E. P. Horwitz, S. D. Alexandratos and M. J. Gula, Sep. Sci. Technol., 1997, 32, 1–35 CrossRef CAS.
- K. C. Sole, M. B. Mooiman and E. Hardwick, Sep. Purif. Rev., 2018, 47, 159–178 CrossRef CAS.
- J. T. M. Amphlett, S. Choi, S. A. Parry, E. M. Moon, C. A. Sharrad and M. D. Ogden, Chem. Eng. J., 2020, 392, 123712 CrossRef CAS , Contents.
- E. P. Horwitz, R. Chiarizia and M. L. Dietz, React. Funct. Polym., 1997, 33, 25–36 CrossRef CAS.
- J. Vaughan, C. Dieters, W. Fu and K. Byrne, Miner. Eng., 2016, 88, 2–8 CrossRef CAS.
- A. Yuchi, T. Sato, Y. Morimoto, H. Mizuno and H. Wada, Anal. Chem., 1997, 69, 2941–2944 CrossRef CAS PubMed.
- S. Nishihama, Y. Sumiyoshi, T. Ookubo and K. Yoshizuka, Desalination, 2013, 310, 81–86 CrossRef CAS.
- S. Virolainen, J. Heinonen and E. Paatero, Sep. Purif. Technol., 2013, 104, 193–199 CrossRef CAS.
- L. Melnyk, V. Goncharuk, I. Butnyk and E. Tsapiuk, Desalination, 2005, 185, 147–157 CrossRef CAS.
- U. Schilde and E. Uhlemann, React. Polym., 1993, 20, 181–188 CrossRef CAS.
- J. Wolska and M. Bryjak, Desalination, 2013, 310, 18–24 CrossRef CAS.
- R. Chitrakar, S. Tezuka, A. Sonoda, K. Sakane, K. Ooi and T. Hirotsu, J. Colloid Interface Sci., 2006, 297, 426–433 CrossRef CAS PubMed.
- J. Lehto, L. Brodkin, R. Harjula and E. Tusa, Nucl. Technol., 1999, 127, 81–87 CrossRef CAS.
- R. Harjula, A. Paajanen, J. Lehto, E. Tusa and P. Standring, in WM'03 Conference, Tucson, 2003, pp. 1–7.
- E. Puhakka, M. Ritala and J. Lehto, Radiochim. Acta, 2020, 108, 451–457 CrossRef CAS.
- D. Alby, C. Charnay, M. Heran, B. Prelot and J. Zajac, J. Hazard. Mater., 2018, 344, 511–530 CrossRef CAS PubMed.
- T. Sangvanich, V. Sukwarotwat, R. J. Wiacek, R. M. Grudzien, G. E. Fryxell, R. S. Addleman, C. Timchalk and W. Yantasee, J. Hazard. Mater., 2010, 182, 225–231 CrossRef CAS PubMed.
- H. Yang, L. Sun, J. Zhai, H. Li, Y. Zhao and H. Yu, J. Mater. Chem. A, 2014, 2, 326–332 RSC.
- C. Loos-Neskovic, S. Ayrault, V. Badillo, B. Jimenez, E. Garnier, M. Fedoroff, D. J. Jones and B. Merinov, J. Solid State Chem., 2004, 177, 1817–1828 CrossRef CAS.
- T. Vincent, C. Vincent and E. Guibal, Molecules, 2015, 20, 20582–20613 CrossRef CAS PubMed.
- F. Vassallo, D. La Corte, N. Cancilla, A. Tamburini, M. Bevacqua, A. Cipollina and G. Micale, Desalination, 2021, 517, 115231 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc02338e |
| This journal is © The Royal Society of Chemistry 2023 |