Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthetic amorphous silica: environmental impacts of current industry and the benefit of biomass-derived silica†
Ethan
Errington
 a,
Miao
Guo
a,
Miao
Guo
 *b and
Jerry Y. Y.
Heng
*b and
Jerry Y. Y.
Heng
 *a
*a
aDepartment of Chemical Engineering, Imperial College London, London, SW7 2AZ, UK. E-mail: jerry.heng@imperial.ac.uk
bDepartment of Engineering, King's College London, London, WC2R 2LS, UK. E-mail: miao.guo@kcl.ac.uk
First published on 17th August 2022
Abstract
The production of Synthetic Amorphous Silica (SAS) is a billion-dollar industry. However, very little is shared publicly on the environmental impact of SAS production. This work provides the first complete treatment for the environmental impacts of SAS produced via the existing ‘dry’ and ‘wet’ industrial methods using Life Cycle Assessment (LCA). To provide a more robust method, this includes an evaluation of 8 environmental impact indicators and consideration for uncertainty during process comparison. Predictions are then used to compare the impact of the existing dry and wet methods as well as theoretical methods in which rice husk (RH) is used as a biomass-derived feedstock alternative. Results highlight cases in which using RH as an alternative feedstock is likely to be beneficial. However, it is demonstrated that these benefits are highly dependent on specifics of the process, region, and feedstock characteristics rather than the inherent “green-ness” of RH alone. Findings are therefore of significance to those interested in the existing SAS industry and the sustainable development of SAS. Moreover, findings also have potential implications for wider policy.
From glass and cement to tyres and textiles, an astounding amount of everyday life is built on ‘Synthetic Amorphous Silica’ (SAS). However, little information on the environmental impact of SAS is available publicly. This study aims to address this by evaluating the environmental impacts of the two most industrially relevant SAS production processes.
Industrial methods of SAS production can be grouped based on two broad categories, ‘wet’ or ‘dry’, depending on the environment in which silica is formed. Dry processing of silica is achieved by the process of flame pyrolysis in which a silane, such as silicon tetrachloride (SiCl4), is hydrolysed at high temperature (i.e. >1000 K).1 Alternately, wet processing relies on the use of an aqueous catalyst to hydrolyse silica precursors such as sodium silicate (Na2O·SiO) at lower temperatures (i.e. <373 K).1 Typical reaction stoichiometries of dry and wet syntheses are shown below:
| SiCl4 + 2H2O → SiO2 + 4HCl |
| Na2O·SiO2 + H2SO4 → SiO2 + Na2SO4 + H2O |
In both cases, the silica precursors used (SiCl4, Na2O·SiO) are derived from mineral feedstocks such as quartz sand.2,3 However, differences in each method lead to unique practical challenges for each approach and create ambiguity as to which process may be more environmentally impactful. While the wet process avoids energy penalties associated the high temperature synthesis of the dry process, it incurs energy penalties elsewhere due to the need for solid–liquid separation post-synthesis.4 Differences in reagent chemistry also lead to the production of by-products with environmental impacts that are hard to compare directly; for example, the emissions to air from the dry process are not equivalent to the emissions to water of the wet process.5 A method which quantifies the overall environmental impact of each process in terms of a common factor is therefore required.
Life Cycle Assessment (LCA) offers a systems approach to quantifying the environmental impacts of manufacturing processes. The principle of LCA is to model the overall impact of a process as the cumulation of impacts arising throughout the manufacturing life cycle from raw material acquisition, considering multiple environmental metrics. Importantly, the LCA methodology framework has been formalised by international standards6–9 and significant amounts of work have gone into the development of the metrics and methods used for calculating impacts by the scientific community. Finally, LCA also allows for sources of uncertainty (e.g. measurement error and data quality) to be incorporated directly into impact calculations – a topic reviewed at length in previous works.10–15
Yet despite the development of LCA, gaps have emerged in the literature with regards to the LCA of SAS production methods. At the time of writing the author could find only two independent publications in which the environmental impact of mineral-derived SAS (M-SAS) is evaluated via LCA.16,17 However, to the best of the authors knowledge, only one publication investigates both wet and dry SAS production methods.17 Published in 2010, the work17 details a cradle-to-gate LCA model based on material and energy inventories reported from average consumption of the European Union-15, (EU-15) industry.5 However, the results of the study consider only one impact metric, the global warming potential (GWP), for which it finds the dry method to have the highest impact. Furthermore, we believe the inventory for the wet process developed by Roes et al.17 overestimates use of sodium silicate due to an ambiguity of the source text (see ESI S1†). Consequently, the literature could benefit from a re-evaluation of the impacts of industrial SAS production methods as well as a more holistic understanding for the environmental footprint of each method by considering multiple impact factors.5
Contrasting the state of SAS-LCA literature (above), experimental research into the development of rice husk-derived (RH-SAS) silica is thriving. For example, their have been studies relevant for understanding the recovery of silica from rice husk (RH) published within literature since at least the 1970s.18 RH is the protective covering of rice which arises naturally during crop growth; it accounts for approximately 20% of the paddy harvested (annual mass basis) in the rice cultivation life cycle19 and is available in the scale of 100 s of Mtonnes annually.20 Major methods for the recovery of silica from RH biomass focus on the separation of inorganic materials from RH (step 1), which is then followed by the separation of silica from remaining inorganic materials (step 2). These steps are known as ‘thermochemical processing’ and ‘hydrothermal processing’ respectively, and have been summarised in the following two paragraphs.
Step one, the thermochemical processing of RH biomass, is characterised by separating the organic fraction (fixed carbon and volatile matter) from biomass.21 In its most direct form, this occurs by the firing of solid biomass, which is beneficial in enabling the recovery of biochemical energy as heat or electricity while leaving inorganic material as a “bio-ash” residual. Contrarily, indirect methods also exist which focus on recovering the organic fraction as simpler hydrocarbon fuels, which can be used later in more efficient combustion engines.22 However, this typically leaves behind a complex fixed carbon (e.g. asphaltenes) and inorganic structure known as biochar, rather than bio-ash, which makes subsequent silica recovery harder.21 A summary of common indirect reduction methods has been provided previously by Demirbas.23
Step two, hydrothermal processing, is characterised by the recovery of silica from the inorganic products of thermochemical processing (bio-ash or biochar). Whether bio-ash or biochar is used as a feedstock is dependent on the thermochemical process used (see above). However, in both cases, the recovery of silica is based on acid washing processes in which the silica present reacts to form silicate compounds such as sodium silicate.24–28 Importantly, stronger acids – particularly hydrofluoric acid29 – are used for the hydrothermal processing of bio-chars when compared to bio-ash due to the greater chemical complexity of biochar (see above).
In summary, the current SAS economy is energy intensive and relies heavily on the use of non-renewable mineral feedstocks.1,30,31 It is therefore unsurprising that the idea of bio-derived SAS seems a welcome “green” alternative, especially when sourced from agricultural wastes such as RH, given that:
• the use of a biomass feedstock removes the need for mineral excavation;
• the use of agricultural wastes incorporates a circular economic aspect;
• under certain conditions, the thermochemical processing of biomass is considered to have a net-neutral carbon flux;32,33
• the thermochemical processing of biomass provides opportunity for co-recovery and utilisation of bio-energy;
• the thermochemical processing of biomass may incentivise the processing of agricultural waste in a centralised way;
• the centralised processing of agricultural waste may mitigate pollution issues currently related to open burning of agricultural wastes.34,35
However, as with existing industrial methods, little information is publicly available to compare the environmental impacts that bio-derived SAS may have. In fact, relevant literature is largely split in two, focusing on either: (a) the process of biomass burning;36 or (b) the recovery of silica from biomass in very specific use-cases (i.e. “as a material itself rather than a potential source of silica”37).
The status-quo therefore fails to quantify the benefit of having a bio-derived SAS life cycle despite the volume of experimental investigations into this area. A more holistic investigation is therefore required. Consequently, this works aims to address research gaps associated with existing SAS and bio-derived SAS by:
1. Providing an understanding for the environmental impacts associated with SAS production (M-SAS and RH-SAS).
2. Using probabilistic LCA to establish the discernability of environmental impacts evaluated for each SAS production process.
3. Advancing the understanding of the carbon reduction potential/benefit of RH-SAS associated with by transitioning from use of mineral-derived (M-SAS) to RH-derived (RH-SAS) production methods.
1 Methods
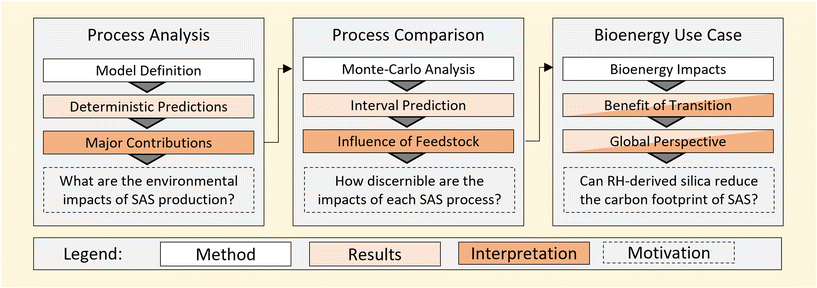
Life cycle assessment has been coupled with statistical methods to underpin the methodology in this work. As shown in Fig. 1, this includes both the deterministic prediction of environmental impacts as well as consideration of the sensitivity of results to model uncertainty. A detailed method description is provided in the following subsections.1.1 Life cycle assessment models
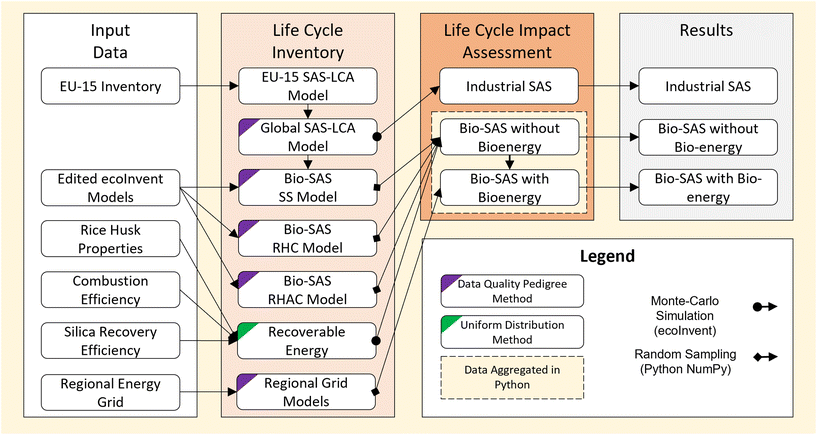
An attributional LCA was adopted to derive quantitative insights into the environmental impact of SAS derived from different processes. Detailed descriptions of the LCA goal, scope, functional unit, inventories and impact assessment are provided below.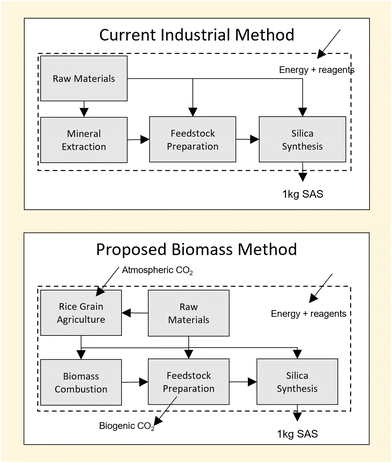 | ||
| Fig. 2 Summary of scope considered for life cycle assessment of mineral (top) and rice husk (bottom) derived SAS production processes. Dotted lines represent the system boundaries. | ||
Importantly, for the biomass processes, a multi-product system occurs across the rice supply chain (i.e. cultivation, collection and processing of rice) as both rice grain (primary product) and RH (co-product) are produced.38 An economic allocation approach has been adopted to assign the environmental impacts arising from rice grain agriculture – within which RH is an unavoidable by-product38 and therefore carries zero economic value.
| Flow type | Flow | Amount (wet process) | Amount (dry process) | Unit |
|---|---|---|---|---|
| a Values reported as less than or equal to this value in the source text,5 therefore max value used to provide worst case scenario. b Value taken based on midpoint of range reported in the source text.5 | ||||
| Reagent | Sulfuric acid | 0.6336 | — | kg |
| Sodium silicate | 1.4040 | — | kg | |
| Water | 42.5224 | — | kg | |
| Silicon tetrachloride | — | 2.7b | kg | |
| Hydrogen | — | 0.082 | kg | |
| Energy | Electricity | 19.5b | 16.5b | MJ |
| Air emission | Carbon monoxide | 0.000825a | — | kg |
| Carbon dioxide | — | 0.64a | kg | |
| Nitrogen oxides | 0.000723a | 0.0001a | kg | |
| Chlorine | — | 0.00005a | kg | |
| Hydrogen chloride | — | 0.0001a | kg | |
| Volatile organic compounds | — | 0.0003a | kg | |
| Particulate matter/dust | 0.0013 | 0.0003a | kg | |
| Water emission | Sulfate | 0.588a | — | kg |
| Chemical oxygen demand | 0.01200a | — | kg | |
| Dissolved solids | 0.0066a | — | kg | |
| Waste water | 0.035 | — | kg | |
| Waste | Non-hazardous waste | 0.029a | 0.01a | kg |
| Hazardous waste | — | 0.002a | kg | |
Where the source text5 reported the expected value of a flow to within a range (e.g. see silicon tetrachloride in Table 1), the mid-point of that range was used.
Supporting models for each mass and energy input were taken from entries in the ecoInvent database39 and are detailed in the ESI S2.†
All energy use was modelled as heat from natural gas. This was based on: (1) the energy in both wet and dry methods is used primarily for heating;5 (2) the use of majority heat from natural gas in equivalent wet and dry titaniumdioxide production processes present in the ecoInvent database;40 (3) the LCI presented by Roes et al.,17 which was informed by private communications with industry.
| Process stage | Inter-model flow | Region | Basis | Ref. |
|---|---|---|---|---|
| RHC | RH → RHA | GLO | State-of-the-art wood combustion. References to wood removed | 41 |
| RHAC (wet) | RHA → sodium silicate | RER | Assumed for wet process use-case. References to mineral feedstock removed | 42 |
| SS (wet) | Sodium silicate → SAS | GLO | Based on this work. References to mineral feedstock removed | N/a |
| RHAC (dry) | RHA → SiCl4 | GLO | Assumed for dry process use-case. References to mineral feedstock removed | 2 |
| SS (dry) | SiCl4 → SAS | GLO | Based on this work. References to mineral feedstock removed | N/a |
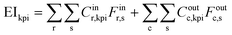 | (1) |
The 8 KPIs considered within this work (summarised in Table 3) are Global Warming Potential (GWP), Land Use Potential (LUP), Mineral Resource Scarcity (MRS), Marine Eutrophication Potential (MEP), Stratospheric Ozone Depletion Potential (ODP), Terrestrial Acidification Potential (TAP), Terrestrial Ecotoxicity (TEP) and Water Consumption Potential (WCP). These factors were selected to provide reflection of impacts on-land, in-water and in-air; importantly, they provide specific attention to trans-boundary pollution problems (GWP, ODP, TAP) as well as factors relevant to the agricultural (LUP, TEP and WCP) and mineral (MRS) theme of resources considered in this work. To achieve a trade-off between solution accuracy and computational time, a simulation calculation cut-off of 0.1% was applied.
| Indicator | Definition | Impact metric per functional unit |
|---|---|---|
| a Specifically stratospheric ozone depletion. | ||
| WCP | Water consumption | m3 |
| TEP | Terrestrial ecotoxicity | kg 1,4-DCB |
| TAP | Terrestrial acidification | kg SO2-Eq |
| ODP | Ozone depletiona | kg CFC-11-Eq |
| MRS | Mineral resources | kg Cu-Eq |
| MEP | Marine eutrophication | kg N-Eq |
| LUP | Land use | m2 crop-eq |
| GWP | Global warming | kg CO2-equivalent |
In the case of biomass processes, EIRH−SASkpi, overall process impacts were calculated based on the summation of impacts occurring at independent stages (RHC, RHAC and SS) along the life cycle as shown in eqn (2).
EIRH-SASkpi = EIRHCkpi![[Q with combining circumflex]](https://www.rsc.org/images/entities/i_char_0051_0302.gif) b,p + EIRHACkpi b,p + EIRHACkpi![[m with combining circumflex]](https://www.rsc.org/images/entities/i_char_006d_0302.gif) p + EISSkpi(2) p + EISSkpi(2) | (2) |
![[Q with combining circumflex]](https://www.rsc.org/images/entities/i_char_0051_0302.gif) b,p is the electrical energy recovered from a biomass, b, based on an process design, p, of the RHC stage (kWh kgSAS−1); EIRHACkpi is the environmental impact of the RHAC stage (KPI/kgfeedstock);
b,p is the electrical energy recovered from a biomass, b, based on an process design, p, of the RHC stage (kWh kgSAS−1); EIRHACkpi is the environmental impact of the RHAC stage (KPI/kgfeedstock); ![[m with combining circumflex]](https://www.rsc.org/images/entities/i_char_006d_0302.gif) p is the mass of biomass feedstock required to produce one functional unit of SAS (kgfeedstock/kgSAS); EISSkpi is the environmental impact of the RHC stage (KPI/kgSAS).
p is the mass of biomass feedstock required to produce one functional unit of SAS (kgfeedstock/kgSAS); EISSkpi is the environmental impact of the RHC stage (KPI/kgSAS).
The value of ![[m with combining circumflex]](https://www.rsc.org/images/entities/i_char_006d_0302.gif) p was taken directly from that used in the equivalent M-SAS model as described in Table 1. The value of
p was taken directly from that used in the equivalent M-SAS model as described in Table 1. The value of ![[Q with combining circumflex]](https://www.rsc.org/images/entities/i_char_0051_0302.gif) b,p was calculated based on eqn (3).44
b,p was calculated based on eqn (3).44
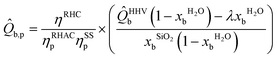 | (3) |
![[Q with combining circumflex]](https://www.rsc.org/images/entities/i_char_0051_0302.gif) LHVb is the lower heating value (kWh kgRHA−1) of dry biomass, b; λ is the latent heat of vaporisation of water (assumed 2.25 MJ kg−1); xbH2O is the moisture content of a wet biomass, b, (wt%); xbSiO2 is the silica content of a dry biomass, b, (wt%).
LHVb is the lower heating value (kWh kgRHA−1) of dry biomass, b; λ is the latent heat of vaporisation of water (assumed 2.25 MJ kg−1); xbH2O is the moisture content of a wet biomass, b, (wt%); xbSiO2 is the silica content of a dry biomass, b, (wt%).
The fraction outside of brackets may be considered as the contribution of technological inefficiency to overall bioenergy recovery potential. Contrarily, values within the brackets of eqn (3) describe limitations to the theoretical energy recoverable due to biomass feedstock quality.
Notably, the combustion model used considers the energy required for drying of wood biomass. The moisture content of wood is typically larger than that of RH. This is due to fact that rice kernels are dried to a moisture content of 14% prior to milling and separation of RH from rice grain within the rice grain value chain.38 Additionally, inventory models do not include the transport of RH or RHA between RGA, RHAC and SS sites; this is due to the fact that such information is case-specific and would therefore require further work considered outside of the scope of this work given the lack of information already available on the environmental impacts of RH-SAS, and the aim of this work to provide a baseline model.
Finally, the carbon released from the combustion of biomass was assumed to be net neutral with regards to the GWP KPI. This was justified as the crop rotation period of rice is short enough for the GWP time horizon being considered to make the assumption satisfactory.45
For a more thorough investigation into method surrounding calculation of EIRH−SASkpi and the sensitivity of recoverable bioenergy, ![[Q with combining circumflex]](https://www.rsc.org/images/entities/i_char_0051_0302.gif) b,p, and biomass demand,
b,p, and biomass demand, ![[m with combining circumflex]](https://www.rsc.org/images/entities/i_char_006d_0302.gif) p, to uncertainty in process efficiencies and feedstock properties, the reader is referred to our recent work.46
p, to uncertainty in process efficiencies and feedstock properties, the reader is referred to our recent work.46
1.2 Bio-energy impacts
Bnew,jGWP = EIRH-SASGWP− ![[Q with combining circumflex]](https://www.rsc.org/images/entities/i_char_0051_0302.gif) b,p EIgridGWP,l b,p EIgridGWP,l | (4) |
| Bexisting,jGWP = Bnew,jGWP− EIM-SAS,jGWP | (5) |
China (CN), Europe (RER), Japan (JP), South Korea (KR) and the United States of America (US) were considered as regions due to their large annual production amounts of SAS;47 India (IN) was also considered due to its large annual rice production.48
Bmarket,lGWP = BexistingGWP × ![[m with combining circumflex]](https://www.rsc.org/images/entities/i_char_006d_0302.gif) market,l market,l | (6) |
![[m with combining circumflex]](https://www.rsc.org/images/entities/i_char_006d_0302.gif) market is the annual mass production rate of SAS in a market region (kgSAS per year) and the subscript l represents the region location (no units). Calculations were carried out within python as described in section 1.3.2.
market is the annual mass production rate of SAS in a market region (kgSAS per year) and the subscript l represents the region location (no units). Calculations were carried out within python as described in section 1.3.2.
1.3 Uncertainty analysis
Analyses have been performed to account for: (a) uncertainty in the quality of LCI data used for each LCA model (sections 1.1.4 and 1.15), (b) uncertainty in the amount of energy recoverable from RH based on material and process variability (eqn (3)), and c) uncertainty in the benefit of transitioning to an RH-SAS industry with bioenergy co-recovery (section 1.2). Practically, uncertainties (a) and (b) were addressed at the LCI stage, while (c) was addressed at the life cycle inventory assessment (LCIA) stage. The workflow of information used during uncertainty analysis is illustrated in Fig. 3.1.3.1.1 Data quality of life cycle inventories. The data quality pedigree method was used to account for uncertainty present in model LCIs based on the quality of data used. This was done by following the method of scoring each LCI flow against five quality criteria: reliability, completeness, temporal, geographic and technological representativeness.49 Quality scores of each flow were then used to model overall uncertainty of a flow amount, F, as a lognormal distribution with geometric mean of, μ*, and geometric variance, σ*2 such that:
| F = lognormal(μ*, σ*2) | (7) |
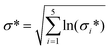 | (8) |
Score weights used were taken from those of Frischknecht and Jungbluth.50 Added measurement uncertainty of μ* was assumed to be negligible. The geographic score of all LCI data was maximised to reflect uncertainty in the global impact of SAS. A summary of all data quality scores used is provided in ESI S2.†
1.3.1.2 Variability of bio-energy recovered from rice husk. Uncertainty associated with energy recoverable from RHC, Qb,p, was simulated using a random sampling approach based on algorithms from the numpy package (v 1.20.1) in python (v3.7). Values used to calculate Qb,p were sampled for the variables in eqn (3) from uniform distributions based on range of values reported previously in peer literature – see Table 4.
| Variable | Value range (method) | Ref. |
|---|---|---|
| a Calculation basis provided in ESI S3.† | ||
| Electric efficiency, ηRHC (%) | 15–30 | 51–55 |
| RHAC stage efficiency, ηRHAC (%) | 55–95 (dry) | 18 |
| 73–90 (wet) | 24 and 56–58 | |
| SS stage efficiency, ηSS (%) | 98–100a (dry) | 5 |
| 95 (wet) | 5 | |
| Higher heating value (MJ kg−1) | 13–16 | 59 |
| Moisture content, xH2O (wt%) | 10–14 | 60–62 |
| Silica content, xSiO2 t (wt%) | 15–20 | 63 |
In doing this, Monte-Carlo simulation was used to generate empirical estimates for the combined effect of LCI uncertainty (section 1.3.1) of independent independent process models. This was done exclusively using the OpenLCA 1.10.184 software. Environmental impact of independent models were then aggregated following eqn (2) and (4)–(6) using the numpy package (v 1.20.1) in python (v3.7). Simulations were run a minimum of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times to provide reliable estimates (see ESI S4†).
000 times to provide reliable estimates (see ESI S4†).
1.4 Interpretation of uncertainty
In all cases, the uncertainty of variables have been represented using prediction intervals (PIs) for 50%, 95% and 99% of probability (two-tailed hypothesis).For pairwise process comparisons, the “relative difference” of impacts, Δk,jkpi, has been used:
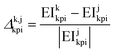 | (9) |
Note that the prediction intervals provided in this work should not interpreted as confidence intervals used in wider statistical inference.64 For this reason, p-values for hypothesis testing are not considered.
2 Environmental impact predictions
2.1 Existing mineral-derived processes
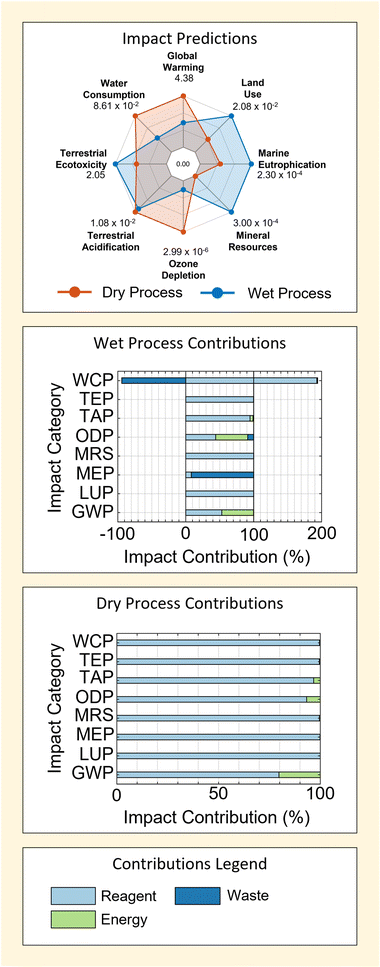 | ||
| Fig. 4 Environmental impact predictions and key contribution factors associated with wet and dry mineral-derived SAS methods. A summary of all deterministic impact predictions used in the plot are tabulated in ESI S5.† | ||
Results in Fig. 4 show that the dry process is predicted to have a larger environmental impact in 4 of 8 the categories considered – GWP, ODP, TAP and WCP. This is mainly driven by contributions from the reagents used, which account for ≥80% predicted impacts in all cases for the dry method. On the contrary, contributions from reagent use in the wet method are slightly more variable – being ≥50% for all impacts but Marine Eutrophication (MEP, in which emissions play a major role − ≥90%).
Following reagents, energy acts as the second most consistently important contributor to process impacts. This can be seen particularly in the GWP and TAP categories for wet and dry M-SAS processes. However, the importance of energy use is far more sensitive to the impact factor being considered – particularly due to the large contributions of reagents to process impacts (as discussed above). This diminished importance of energy (relative to reagents) is the most extreme in the case of the dry process, wherein energy use contributes no more than 20% to the total process impact across all categories considered.
Given the higher energy requirement of the wet process (Table 1), it would seem logical that energy provides greater contributions to the impacts of the wet method – which is observed. Moreover, the fact that energy contributions also occur in categories such as GWP, ODP and TAP is also intuitive because these categories are strongly associated with the production of fossil-fuel energy.
Contrarily, it is clear that the higher energy requirement of the wet process is not reflected in the relative size of energy use contributions when comparing results for GWP, ODP and TAP between the wet and dry M-SAS results. This indicates that even in cases where energy use is important the total process impact is still heavily governed by reagents. It will be shown that this is largely attributed to the importance of the silica precursor used by each method in the following section (section 2.1.2). Consequently, the findings demonstrate that process energy use cannot be used alone as a good gauge for process impact or process comparison. This is in agreement with findings for the dry and wet methods of titanium dioxide and zirconium dioxide reported previously;4 however, it also demonstrates the presence of a trade-off in process design given that energy requirement can be an important economic factor.4
Finally, the negative contribution of waste to the WCP of wet M-SAS stands out as a unique feature in Fig. 4. These contribution is associated with the inclusion of wastewater treatment in the life-cycle, which provides a reduction in the WCP equal to 94% of the reported impact. Therefore future work may benefit from refinement of the wastewater process model used to ensure that impact predictions accurately reflect the real wastewater treatment process used in industry.
| Impact category | SiCL4 in total impact (%) | Chlorine in SiCL4 (%) | Carbon black in SiCL4 (%) | Silica sand in SiCL4 (%) |
|---|---|---|---|---|
| GWP | 70.14 | 63.04 | 21.13 | 1.49 |
| LUP | 99.86 | 0.08 | −0.23 | 141.40 |
| MEP | 98.54 | 94.52 | 0.55 | 0.16 |
| MRS | 21.85 | 92.61 | 0.02 | 0.01 |
| ODP | 92.36 | 78.35 | 16.92 | 0.34 |
| TAP | 94.06 | 61.99 | 29.71 | 2.27 |
| TEP | 99.03 | 85.76 | 5.20 | 2.86 |
| WCP | 96.72 | 67.72 | 0.36 | 0.41 |
| Impact category | Sod. Sil. in total impact (%) | Heat in Sod. Sil. (%) | Soda ash in Sod. Sil. (%) | Silica sand in Sod. Sil (%) |
|---|---|---|---|---|
| GWP | 51.41 | 19.62 | 16.30 | 3.85 |
| LUP | 100.00 | 32.35 | 17.14 | 46.46 |
| MEP | 8.07 | 19.88 | 71.63 | 1.62 |
| MRS | 100.00 | 0.16 | 99.67 | <0.00 |
| ODP | 41.75 | 48.31 | 15.09 | 3.84 |
| TAP | 40.65 | 33.93 | 45.50 | 5.25 |
| TEP | 56.84 | 54.89 | 19.49 | 2.36 |
| WCP | 36.44 | 14.09 | 68.74 | 2.71 |
For dry M-SAS, the contributions of silicon tetrachloride to the total process impact is governed by two main factors – chlorine and carbon black.
Chlorine, which is required for the tetrachloride (i.e. Cl4) chemistry, is derived from the electrolysis of basic chemicals in wet environments. Consequently, it contributes significantly across all impacts largely due to its energy use as well as due to the aquatic impact of the basic chemicals used (i.e. for MEP).65 Meanwhile Carbon black is derived from petrochemical sources,66 which causes it to provide significant contributions to impacts on land and in air, such as GWP, ODP and TAP.
For wet M-SAS, the contributions of sodium silicate is also explained by two main factors – energy/heat and soda ash. Energy (split across electricity, heat from natural gas and heat from other sources) – is required for the furnace process used in sodium silicate production. Consequently, it contributes 20% to the GWP, 50% to the ODP and 55% to the TEP of sodium silicate. Soda ash, which acts as a source of sodium for sodium silicate, accounts for 46% TAP, 72% of MEP and 100% of the MRS of sodium silicate. This is because soda ash is recovered from lime rock via the solvay process,3,40 which is both energy intensive, and requires aqueous alkaline solutions – linking it to aquatic impact indicators (as similarly discussed for the electrolysis of chlorine in basic solutions for the production of SiCL4 above).
It should be noted that the generally lower magnitude of sodium silicate contributions to wet process impacts (when compared with the typically ≥90% contribution of SiCL4 to the dry process impacts) is associated with the importance of sulfuric acid use in the wet method. Similarly, the low contribution of silicon tetrachloride to MRS (Table 5) is associated with important contributions from hydrogen use (see Table 1) for that impact factor.
Differences in the magnitude of contribution of each silica precursor to overall impact can also be partly understood in terms of the material efficiency of each process. Particularly, the atom economy of silicon tetrachloride use in the dry process (29%) is lower than that of the sodium silicate wet process (42–59%, see ESI S1†). Given the similar conversion efficiencies of each process (see Table 4), this increases the amount of silicon tetrachloride required per functional unit of SAS produced when compared to sodium silicate. Consequently, the absolute impact associated with use of silicon tetrachloride is amplified by the inefficiency of the chemistry on which the process relies (silicon tetrachloride, SiCl4). This is in agreement with findings for the wet and dry methods used to produce titanium dioxide and zirconium dioxide.4
Finally, the dominance of silicon tetrachloride in the impact of dry SAS (also recently noted by Resalati et al.16) also has wider implications on the environmental impact that can be expected for SAS produced by the Stöber method67 – which is used prolifically in academia. This is because the Stöber method uses organo-silane precursors, which are derived from silicon tetrachloride.37 Further consideration of the Stöber method is considered outside of the scope of this work.
| Impact category | Wet process (%) | Dry process (%) |
|---|---|---|
| GWP | 1.98 | 1.05 |
| LUP | 46.46 | 141.21 |
| MEP | 0.13 | 0.16 |
| MRS | <0.00 | <0.00 |
| ODP | 1.60 | 0.31 |
| TAP | 2.13 | 2.14 |
| TEP | 1.34 | 2.83 |
| WCP | 0.99 | 0.39 |
2.2 Theoretical rice husk-derived processes
Predicted impacts of wet and dry RH-SAS processes and contributions by process stage are shown in Fig. 5.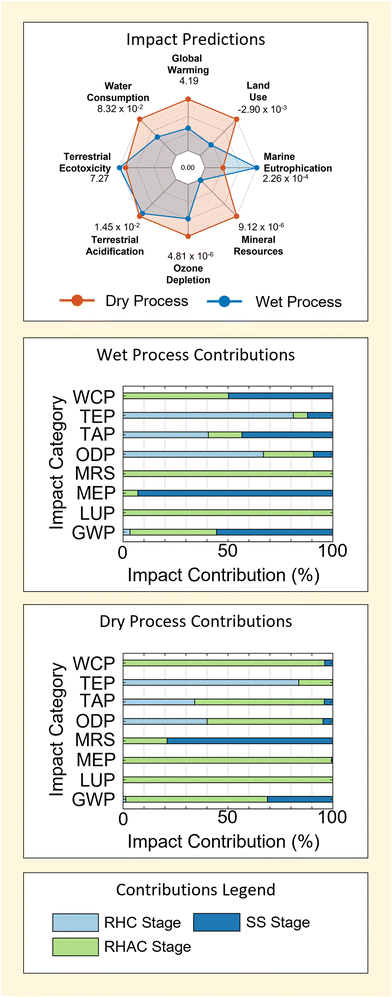 | ||
| Fig. 5 Environmental impact predictions and key contribution factors associated with wet and dry rice husk-derived SAS. A summary of all deterministic impact predictions used in the plot are tabulated in ESI S5.† | ||
Results in Fig. 5 show that the wet process is predicted to have the highest environmental impact in 2 of the 8 indicators considered (MEP and TEP). This is different to the results for processes in existing industry (Fig. 4), where the MRS of wet M-SAS (3 × 10−4 kgCu-eq) was larger than the dry M-SAS MRS (9 × 10−9 kgCu-eq), and also 30 times greater than the largest MRS reported for RH-SAS processes here (dry process, 9.1 × 10−6 kgCu-eq). Given the high contribution of the SS stage to the dry process MRS and the low contribution of mineral feedstock (Table 7) identified for M-SAS predictions (section 2.1), this difference is related to the use of a hydrothermal method42 to model impacts of the RHAC stage in the wet RH-SAS life cycle instead of the furnace method used for the wet M-SAS process (see ESI S2†). Specifically, the reduction observed with this change in process can be related to the use of sodium hydroxide in the hydrothermal method (as an alternative to soda ash used by the Furnace method, see section 2.1) for sodium silicate production.3,42
Contrarily, an importance in the difference of process at the RHAC stage is not observed for the TAP and MEP impact indicators – where the RHC stage contributes to impacts significantly (i.e. >30% for both wet and dry RH-SAS processes). In both cases, the large contributions of the RHC stage to TAP may be unsurprising as the production of sulphur oxides is a well-known problem associated with the organic chemistry of biomass fuels.68,69 Therefore the findings highlight the importance of considering multiple impact categories when comparing the environmental footprint of processes.
By comparing contributions for wet and dry RH-SAS methods, it is also possible to see that differences exist in the magnitude of relative contribution from the RHC stage. This is interesting given that the absolute impacts of the RHC stage are similar in their contribution to the impacts of both wet and dry RH-SAS. Consequently, the difference in relative contribution can be thought of as a direct indication of higher absolute impact associated with the RHAC and SS stage of both processes. This further reflects the high dominance of silica precursors (silicon tetrachloride and sodium silicate) in process footprints – as identified for existing industrial methods in section 2.1.
Importantly, to the best of the author's knowledge these are the first predictions for the impact of rice-husk derived silica. Consequently, no data exists for direct comparison within the literature. However, it is worth noting that differences in the economic allocation for rice grain cultivation may become an important point of uncertainty when comparing results here to any similar work produced in the future.
3 Comparison of process performance
Fig. 6 illustrates prediction intervals for the relative difference, Δk,jkpi of three cases of pairwise comparison. These are: wet M-SAS and dry M-SAS (Fig. 6a), dry M-SAS and dry RH-SAS (Fig. 6b), and wet M-SAS and wet RH-SAS (Fig. 6c). Interpretations of each comparison are provided in the following subsections.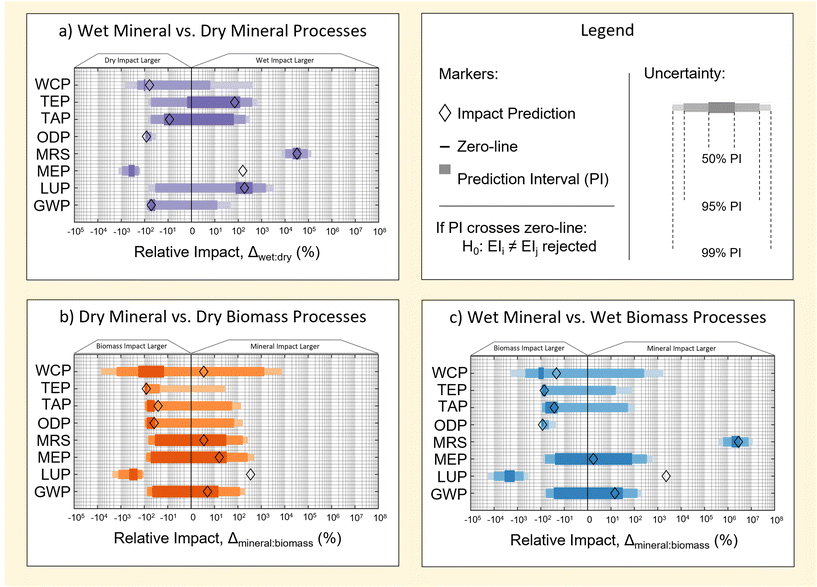 | ||
| Fig. 6 Prediction intervals for uncertainty in the difference between mineral processes (a), wet processes (b) and dry processes (c). A summary of all deterministic impact predictions used in the plot are tabulated in ESI S5.† | ||
3.1 Existing industrial methods (wet M-SAS vs. dry M-SAS)
Prediction intervals for the relative difference, Δwet,drykpi, of M-SAS production methods are shown in Fig. 6a. Of the 8 indicators considered, TAP and TEP show no discernability (for the prediction intervals considered). Contrarily, GWP, LUP and WCP show discernability to the 50% PI and MEP, MRS and ODP show discernability above the 99% PI.Interestingly, results suggest that of the 4 impact categories in which the wet process is highlighted as having impacts larger than the dry process by deterministic predictions (LUP, MEP, MRS and TEP and GWP), only the MRS impact can be considered to be significantly different to the highest level of certainty considered. Similar outcomes can also be seen for cases in which the dry process was predicted to have a larger impact by deterministic predictions. These findings thereby demonstrate the importance of accounting for uncertainty when making pairwise comparisons.
3.2 Dry method feedstock choice (M-SAS vs. RH-SAS)
Prediction intervals for the relative difference, Δmineral,biomasskpi, in impact of M-SAS and RH-SAS processes according to dry method are provided in Fig. 6b.Results in Fig. 6b show that, for the prediction intervals considered, only 5 of the 8 indicators show some level of discernible comparison. Specifically, GWP, MRS and MEP show no discernability (for the prediction intervals considered). Contrarily, ODP, TAP and WCP show discernability at the 50% PI, while TEP shows discernability at the 95% PI and LUP is discernible at the 99% PI.
The lower discernability of the dry methods (compared to Fig. 6a and c) may be thought of as the product of two factors: (a) near-equivalence of methods (notwithstanding a change in feedstock and inclusion of the RHC stage for the RH-SAS method), and (b) the low contribution of feedstock to impact of indicators considered (as discussed in section 2.1.3). Consequently, any difference in discernability for the dry methods may be interpreted largely as arising from contributions of the RHC stage in the RH-SAS process. Nonetheless, multiple impact factors display strong skews toward RH-SAS having a larger impact when compared to M-SAS, which suggests contributions from the RHC stage are important. Finally, the high discernability of LUP can be linked to the transition away from amineral derived feedstock – which provides a large contribution to the LUP of the dry M-SAS process. Finally, the lack of agreement between deterministic and probabilistic predictions for LUP in Fig. 6 reflect sensitivities of the data quality method (particularly the use of lognormal distributions to represent uncertainty) to uncertainties across the M-SAS and RH-SAS life cycles – for which more investigation could be done in future work.
3.3 Wet method feedstock choice (M-SAS vs. RH-SAS)
Prediction intervals for the relative difference, Δmineral,biomasskpi, in impact of M-SAS and RH-SAS processes according to wet methods are provided in Fig. 6c.Results in Fig. 6c show that, for the prediction intervals considered, 6 of the 8 indicators are discernible to some prediction interval. GWP and MEP show no discernability (for the prediction intervals considered). Contrarily, TAP, TEP and WCP show discernability to the 50% PI, while ODP is discernible to the 95% PI, and LUP, MRS and ODP are discernible to the 99% PI. Also, ODP, TAP and TEP seem biased towards values of Δmineral,biomasskpi < 0 (i.e. RH-SAS having a larger impact than M-SAS), which is understandable from the higher contribution of RHC to these categories (see section 2.2).
As previously discussed in section 2.2, the discernability of wet methods may be considered as being affected by one additional factor when compared to the dry M-SAS and RH-SAS methods – the difference in process assumed for the recovery of sodium silicate from RHA (i.e. the RHAC stage, which uses a hydrothermal method42 instead of the furnace method3 used in existing M-SAS industry). Consequently, differences may be attributed to a combination of the RHC stage and technological differences in hydrothermal RHAC stage in the RH-SAS process.
The high discernability of MRS (Fig. 6c) is due to differences in reagent use in hydrothermal and furnace methods for sodium silicate production. In particular, the hydrothermal method relies on the use of sodium hydroxide as a sodium source as opposed to soda ash in the case of the furnace process;3,42 the contributions of sodium hydroxide to the MRS are significantly lower than that of soda ash in the case of mineral processes (see Table 6).
Finally, the high discernability of LUP can be linked to the transition away from a mineral derived feedstock – which provided large contribution to the LUP of the dry M-SAS process.
3.4 Discussion
In all cases, findings demonstrate that the effect of feedstock choice on environmental impact of SAS is most commonly not discernible to a high extent of certainty when comparing M-SAS and RH-SAS on the basis of feedstock importance. A major outlier to this observation is the case of LUP, which demonstrates a benefit of transition to RH-SAS that is discernible to the 99% PI for both dry and wet methods. Moreover, by comparing results in Fig. 6, a change in silica recovery to a hydrothermal method (associated with the wet method) has a relatively little effect on the discernability of relative impact in most categories – MRS being an exception. This further demonstrates that the importance of feedstock is relatively low in comparison to the contribution of intermediate processing steps, which cannot be avoided due to silica precursor chemistry (see section 2.2). However, it should be considered that the extent of discernability may change in cases where the co-recovery and utilisation of bio-energy released during RH combustion is considered. This is explored further in section 4.1.Additionally it is striking that the visual trend of data in sub Fig. 6b and c, shows a tendency for greater certainty in the impact of RH-SAS being larger the M-SAS according to probabilistic methods (across the 8 impact factors considered). This therefore provides a basis for suggesting that the “green-ness” of a “green silica” feedstock may not be as important as qualitatively seems. However, to the best of the authors knowledge, results in this section are the first of their kind, therefore no comparison can be made with previous literature. Consequently care should be taken not to make strong conclusions based on the results of this work alone. Particularly, future work would benefit from refining the uncertainty method used with regards to the scoring of data pedigree matrix (section 1.3.1.1) and a further investigation of the source of uncertainty associated with PIs reported in sub Fig. 6b and c. Additionally, further work could be done on incorporating effect of rice grain agriculture in specific cases where the economic allocation used in this work is not appropriate.
4 Benefits of bio-energy co-recovery
Results in Fig. 7 illustrate uncertainty in the benefit of producing RH-SAS when the co-recovery and utilisation of bioenergy is considered. Predictions are provided for cases where SAS meets new market demand, Bnew,jGWP, or replaces existing market demand, Bexisting,jGWP, in subsections 4.1 and 4.2 (see eqn (4) and (5)). A case study is then provided for the expected benefit of a global transition to RH-SAS methods on the annual GWP of SAS industry in subsection 4.3.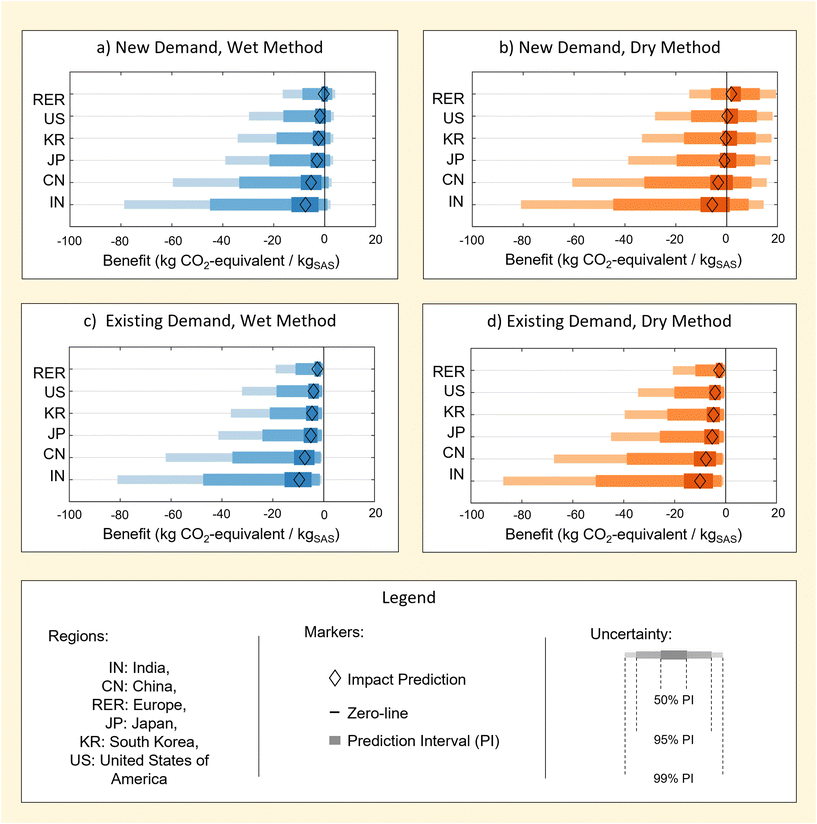 | ||
| Fig. 7 Prediction intervals for the benefit of rice husk-derived SAS derived from the wet (a, c) and dry (b, d) processing methods in combination with co-recovery of bio-energy. Results are provided for separate scenarios in which rice husk-derived SAS is produced to meet new market demand (a, b) and existing market demand (c, d) A summary of all deterministic impact predictions used in the plot are tabulated in ESI S5.† | ||
4.1 Benefit of meeting new market demand
Results in Fig. 7a and b demonstrate that the benefit of RH-SAS production with bioenergy co-recovery lead to net-negative GWP values in some cases. This net-negativity represents a net reduction in the GWP compared to a case in which RH-SAS is not produced. Therefore it should be interpreted as GWP avoidance rather than carbon sequestration.Interestingly, the net-negativity of benefit predictions is sensitive to the region considered. Specifically, a consistent hierarchy exists in which India and China posses the greatest (i.e. most negative) benefit and higher certainty of benefit (i.e. of the value being net-negative). Contrarily, the United States and Europe posses the least (i.e. lest negative) benefit and a lower certainty of benefit.
The presence of a hierarchy in the benefits of bio-energy co-recovery can be understood based on differences in regional grid electricity. Particularly, it may be shown that the benefit predicted for each region is heavily correlated with the use of coal as a regional energy source (ESI S5†). Consequently, it could be expected that the environmental benefit of RH-SAS processes employed with bio-energy co-recovery to meet new market demand may reduce if regional grids are de-carbonised – which is probable for many countries given current trends in international climate change policy.
4.2 Benefit of meeting existing market demand
Fig. 7c and d illustrate benefits associated with use of RH-SAS to meet existing market demand. Unsurprisingly, meeting existing market demand is associated with a greater benefit and increased certainty of benefit for all regions when compared to meeting new market demand (section 4.1). This is unsurprising given that meeting existing demand includes the benefit of meeting new market demand as well as the benefit of avoiding any the impacts that would otherwise be created from the production of M-SAS by existing industrial methods.For wet RH-SAS (Fig. 7c), results show that the added benefit of meeting meeting existing market demand is large enough to provide prediction intervals of 99% for all regions considered. This is significant in including relatively high certainty in benefit for RH-SAS even in regions with relatively low coal reliance, which was not the case for RH-SAS meeting new demand (section 4.1).
Similarly, results for dry RH-SAS (Fig. 7d) show that the added benefit of meeting existing market demand also improves certainty in benefit of RH transition – though albeit to a lower extent (50% PI) for all regions. This is interesting in that it demonstrates again that greater certainty is predicted of the benefit of wet method despite that SAS produced by dry M-SAS methods have a higher GWP. This is reflective of the importance of a change in process in the wet RH-SAS method (section 2.2) on the impacts of RH-SAS as a product, as well as differences in bio-energy recoverable by wet and dry methods.
4.3 Global benefit of an industry transition
The implication of expected benefit of RH-SAS in meeting existing demand based on historical regional production volumes (eqn (6)) is summarised in Table 8. Results in Table 8 suggest that 3.8 Mtonnes of carbon dioxide equivalent (100-year time horizon) could be avoided by transitioning existing M-SAS industry to a RH-SAS paradigm in which bio-energy is co-recovered and utilised. This is equivalent to a reduction of 161% of the GWP impact of the industry as whole, or 50 and 214% for dry and wet industries independently. For wider context, this total benefit is equivalent to roughly 0.01% of global annual equivalent carbon dioxide emissions (36.3 Gt70).| Region | Method | Annual productiona (kTSAS per year) | Expected benefit (kTCO2 per kTSAS) | Transition benefit (kT COCO2 per year) |
|---|---|---|---|---|
| N.G.: Data not given within reference used.a Annual production based on values reported by Wadell.71 | ||||
| China, CN | Dry | N.G. | −7.8 | — |
| Wet | 250 | −7.4 | −1850.0 | |
| Europe, RER | Dry | 61 | −2.6 | −158.6 |
| Wet | 280 | −2.5 | −700.0 | |
| Japan, JP | Dry | 21 | −5.3 | −111.3 |
| Wet | 45 | −5.1 | −229.5 | |
| South Korea, KR | Dry | N.G. | −4.7 | — |
| Wet | 45 | −4.6 | 207.0 | |
| United State, US | Dry | 37 | −4.1 | −151.7 |
| Wet | 191 | −4.0 | −764.0 | |
| World | Dry | 191 | — | −421.6 |
| Wet | 839 | — | −3750.5 | |
The fact that reductions are greater in the wet industry is heavily driven by transition in the wet method industry, which accounts for ∼90% of the predicted benefit despite accounting for ~80% of SAS production by mass. This is rationalised by the fact that the information available for regional production (CN, RER, JP, SK and US) fall slightly short of the reported world production values. Therefore values in Table 8 provide a slight systematic underestimation for the total world benefit. Care should be taken in interpretation of this result as making a case for a transition toward RH utilisation. Particularly, further studies are required to understand the additional costs (both environmental and economic) associated with physically implementing changes associated with the suggested feedstock transition.
Finally, deterministic predictions for the amount of RH requirement to produce RH-SAS are 8.4 and 7.6 kgRH per 1 kg of RH-SAS for wet and dry processes respectively – which is in agreement with estimates for wet RH-SAS reported in our previous work.44 Differences in the predictions for wet and dry RH-SAS can be attributed to slight differences in process efficiencies detailed in Table 4. This would suggest that a total of 7.0 and 1.5 MTonnes of RH required annually to supply the global demand provided in Table 8. Importantly, both of these values are significantly lower than the 100 s MTonnes of RH produced globally each year.20
5 Conclusions
In conclusion, this work has aimed to develop an understanding for the environmental impact of industrial SAS production methods and their biomass-derived equivalents. This has been done in three stages: (1) improvement of existing SAS-LCA models to evaluate the environmental footprint of existing industrial M-SAS production and potential RH-SAS production (section 2), (2) consideration for the effect of uncertainty on the discernability of the environmental impacts of M-SAS and RH-SAS (section 3), and (3) scenario-based analysis of the benefit associated with the co-recovery of bio-energy within the RH-SAS life cycle (section 4).Findings from section 2 reaffirm previous literature that the impact of the dry process is higher than the wet process. However, estimates for the wet process were found to be lower than previously thought due to a misinterpretation of source text in prior works (see ESI S1†). For both production methods, the silica precursor (silicon tetrachloride or sodium silicate) acts as a major hotspot for the environmental impact of SAS regardless of production method – though in some cases energy use is important too. Through further analysis, it is also shown that the footprint of silica precursors are unavoidable due to the processing demands (energy demand, material chemistry and atom economy) associated with achieving their chemistries, rather than the derivation of either silica precursor from a specific feedstock (mineral or biomass).
In section 3, the importance of chemistry (rather than feedstock source) is then demonstrated in a further two ways. Firstly, it is found that the discernability of current industrial techniques are much higher in relative comparison to biomass processes, which have been found to remain largely indiscernible for all but one impact factor. This case is attributed to the effect of feedstock change on process use rather than sustainability of the RH feedstock itself. Secondly, it is shown, that uncertainty favours the existing mineral derived processes having a lower impact (than the equivalent RH-SAS process) in most cases. This can be related to the impact of incorporating an RHC stage being higher than the impact avoided by avoiding mineral feedstocks (as mineral feedstock contributions are typically small, except for in the case of LUP). However, these findings are limited only to the scenario in which the co-recovery and use of bio-energy during RH-SAS production is not considered.
Finally, in Section 4 the effect of co-recovering bioenergy for use in offsetting grid demand is considered for two scenarios. Results show that it may be possible to reduce the global warming potential of existing SAS industry by using RH-SAS methods. However, this is heavily dependent on the amount of bio-energy recoverable, and sensitive to the GWP of regional electricity grids. For the scenario in which RH-SAS is produced to meet new SAS market demand, this causes regions such as China and India to have a greater certainty of a benefit from producing RH-SAS (which also coincides with regions of higher RH production), but also indicates that the benefit of RH-SAS with bio-energy recovery will be reduced by future energy grid de-carbonisation. Contrarily, a high level of certainty is found in the benefit of a scenario where RH-SAS is produced (with co-recovery of bioenergy) to replace M-SAS meeting existing market demand for all regions - suggesting a far greater robustness in the benefit of using RH-SAS to meet existing market demand when compared to meeting new market demand.
This work therefore highlights three key points to consider within the future development of sustainable SAS: (a) the consideration of uncertainty may significantly affect the degree to which the impact of wet and dry methods are considered discernable (relative to when using only deterministic prediction methods); (b) there is little certainty in the inherent merit of the “green-ness” of using RH or other biomasses as a silica feedstock across many of the impact categories considered, and thus RH-SAS should only be considered beneficial under very clear circumstances; and (c) the conditions required for RH-SAS silica to be beneficial to a high level of certainty (with regards to GWP) include proper management and utilisation of bio-energy released during the initial combustion of biomass feedstocks.
Findings of this work are novel given the lack of information currently available on the environmental impacts of both M-SAS and RH-SAS. Consequently, it is hoped that the findings may act to provide a baseline from which further and more refined LCAs of both M-SAS and RH-SAS processes can be made in the future. Particularly, results for the impact of RH-SAS are the first of their kind and may act as a reference point which future works can use to investigate more specific case studies such as the optimum allocation of RH – including re-purposing of RH already being utilised in other industries – from an environmental impact perspective.
Finally, as findings demonstrate the importance of process rather than material feedstock in the impact of SAS, readers should be cautious in equating a study limited to combustion of biomass as a recovery method to other methods of recovering silica and energy from biomass.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to acknowledge the funding support provided to this article from Scottish Water and the UK EPSRC through the Science and Solutions for a Changing Planet Doctoral Training Programme (SSCP-DTP). Finally, our thanks are extended to Alex Bowles for his insight on processes for the recovery of resources from waste materials.References
- O. W. Flörke, H. A. Graetsch, F. Brunk, L. Benda, S. Paschen, H. E. Bergna, W. O. Roberts, W. A. Welsh, C. Libanati, M. Ettlinger, D. Kerner, M. Maier, W. Meon, R. Schmoll, H. Gies and D. Schiffmann, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2008 Search PubMed.
- T. Levova, Silicon tetrachloride production, GLO, Allocation at the Point of Substitution, ecoinvent database version 3.6, 2012 Search PubMed.
- D. FitzGerald, Market for sodium silicate, without water, in 37% solution state, RER, Allocation at the Point of Substitution, ecoinvent database version 3.6, 2018 Search PubMed.
- N. Osterwalder, C. Capello, K. Hungerbühler and W. J. Stark, J. Nanopart. Res., 2006, 8, 1–9 CrossRef CAS.
- European Commission, Integrated Pollution Prevention and Control (IPCC): Reference Document on Best Available Techniques for the Manufacture of Large Volume Inorganic Chemicals-Solids and Others industry, 2007 Search PubMed.
- International Organization for Standardisation, ISO 14040:2006 Environmental management—Life cycle assessment—Principles and framework, 2006 Search PubMed.
- International Organization for Standardisation, ISO 14040:1998 Environmental management—Life cycle assessment—Goal and scope definition and inventory analysis, 1998 Search PubMed.
- International Organization for Standardisation, ISO 14042:2000 Environmental management—Life cycle assessment—Life cycle impact assessment, 2000 Search PubMed.
- International Organization for Standardisation, ISO 14044:2006 Environmental management—Life cycle assessment—Requirements and guidelines, 2006 Search PubMed.
- A. E. Björklund, Int. J. Life Cycle Assess., 2002, 7, 64–72 CrossRef.
- S. Ross, D. Evans and M. Webber, Int. J. Life Cycle Assess., 2002, 7, 47–52 CrossRef.
- S. M. Lloyd and R. Ries, J. Ind. Ecol., 2007, 11, 161–179 CrossRef.
- A. Mendoza Beltran, V. Prado, D. Font Vivanco, P. J. Henriksson, J. B. Guinée and R. Heijungs, Environ. Sci. Technol., 2018, 52, 2152–2161 CrossRef CAS PubMed.
- Y. Qin, S. Cucurachi and S. Suh, Int. J. Life Cycle Assess., 2020, 25, 1846–1858 CrossRef.
- M. Guo and R. J. Murphy, Sci. Total Environ., 2012, 435–436, 230–243 CrossRef CAS PubMed.
- S. Resalati, T. Okoroafor, P. Henshall, N. Simões, M. Gonçalves and M. Alam, Build. Environ., 2021, 188, 107501 CrossRef.
- A. L. Roes, L. B. Tabak, L. Shen, E. Nieuwlaar and M. K. Patel, J. Nanopart. Res., 2010, 12, 2011–2028 CrossRef CAS.
- P. K. Basu, C. J. King and S. Lynn, AIChE J., 1973, 19, 439–445 CrossRef CAS.
- J. S. Lim, Z. Abdul Manan, S. R. Wan Alwi and H. Hashim, Renewable Sustainable Energy Rev., 2012, 16, 3084–3094 CrossRef CAS.
- A. Abbas and S. Ansumali, BioEnergy Res., 2010, 3, 328–334 CrossRef.
- J. Lehmann and S. Joseph, Biochar for Environmental Management: Science, Technology and Implementation, Routledge, London, 2nd edn, 2015, pp. 1–976 Search PubMed.
- P. Basu, Biomass gasification and pyrolysis: practical design and theory, 2010 Search PubMed.
- A. Demirbas, Prog. Energy Combust. Sci., 2005, 31, 171–192 CrossRef CAS.
- U. Kalapathy, A. Proctor and J. Shultz, Bioresour. Technol., 2000, 73, 257–262 CrossRef CAS.
- E. L. Foletto, E. Gratieri, L. H. de Oliveira and S. L. Jahn, Mater. Res., 2006, 9, 335–338 CrossRef CAS.
- N. A. Sánchez-Flores, G. Pacheco-Malagón, P. Pérez-Romo, H. Armendariz, M. d. L. Guzmán-Castillo, J. M. Saniger and J. J. Fripiat, J. Chem. Technol. Biotechnol., 2007, 82, 614–619 CrossRef.
- R. Kumar, M. Vinjamur and M. Mukhopadhyay, Int. J. Chem. Eng. Appl., 2013, 4, 321–325 CAS.
- S.-J. Kang, Method for extracting silica from herbaceous plants, United States Patent and Trademark Office 6843974B2, CPCIC, 01 Search PubMed B 33/126, 2005.
- D. Bossert, D. A. Urban, M. Maceroni, L. Ackermann-Hirschi, L. Haeni, P. Yajan, M. Spuch-Calvar, B. Rothen-Rutishauser, L. Rodriguez-Lorenzo, A. Petri-Fink and F. Schwab, Sci. Rep., 2019, 9, 1–12 CrossRef CAS.
- W. Simmler, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2000 Search PubMed.
- W. Zulehner, B. Neuer and G. Rau, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000 Search PubMed.
- P. Colnes, A. Gunn, J. Kittler, B. Perschel, R. Recchia, C. Saah and D. Walker, Biomass sustainability and carbon policy study, 2010 Search PubMed.
- Organisation for Economic Co-operation and Development, Estimation of greenhouse gas emissions and sinks, Organisation for economic co-operation and development technical report, 1991 Search PubMed.
- L. Zhang, Y. Liu and L. Hao, Environ. Res. Lett., 2016, 11, 014014 CrossRef.
- B. Gadde, S. Bonnet, C. Menke and S. Garivait, Environ. Pollut., 2009, 157, 1554–1558 CrossRef CAS.
- T. Chungsangunsit, S. H. Gheewala and S. Patumsawad, Environmental Assessment of Electricity Production from Rice Husk: A Case Study in Thailand, 2005, vol. 1 Search PubMed.
- N. Baccile, F. Babonneau, B. Thomas and T. Coradin, J. Mater. Chem., 2009, 19, 8537–8559 RSC.
- N. Van Hung, M. V. Migo, R. Quilloy, P. Chivenge and M. Gummert, Sustainable Rice Straw Management, Springer International Publishing, 2020, pp. 161–174 Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- H. Althaus, M. Chudacoff, R. Hischier, N. Jungbluth, M. Osses, A. Primas and S. Hellweg, Life Cycle Inventories of Chemicals, 2007, ch. 8 Search PubMed.
- C. Bauer, Electricity production, wood, future, GLO, Allocation at the Point of Substitution, ecoinvent database version 3.6, 2007 Search PubMed.
- R. Hirschier, Sodium silicate production, hydrothermal liquor, product in 48% solution state, RER, Allocation at the Point of Substitution, ecoinvent database version 3.6, 2010 Search PubMed.
- M. A. Huijbregts, Z. J. Steinmann, P. M. Elshout, G. Stam, F. Verones, M. Vieira, M. Zijp, A. Hollander and R. van Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- E. Errington, M. Guo and J. Heng, Proceedings of the 32nd European Symposium on Computer Aided Process Engineering, Toulouse, 2022, pp. 1615–1620 Search PubMed.
- F. Cherubini, G. P. Peters, T. Berntsen, A. H. Strømman and E. Hertwich, GCB Bioenergy, 2011, 3, 413–426 CrossRef CAS.
- E. Errington, J. Y. Y. Heng and M. Guo, Computer Aided Chemical Engineering, Elsevier, 2022, vol. 32nd European Symposium on Computer Aided Process Engineering (in print) Search PubMed.
- W. H. Waddell and L. R. Evans, Kirk-Othmer Encyclopedia of Chemical Technology, American Cancer Society, 2000 Search PubMed.
- Food and Agriculture Organization of the United Nations, Food and Agriculture Data: Crops and Livestock Products - Rice, Paddy, 2020 Search PubMed.
- D. A. Ciroth, C. D. Noi, T. Lohse and M. Srocka, openLCA 1.10: Comprehensive User Manual, Green delta technical report, 2019 Search PubMed.
- R. Frischknecht, N. Jungbluth, H.-J. Althaus, G. Doka, R. Dones, T. Heck, S. Hellweg, R. Hischier, T. Nemecek, G. Rebitzer and M. Spielmann, Int. J. Life Cycle Assess., 2004, 10, 3–9 CrossRef.
- R. L. Bain, Bioresour. Technol., 1993, 46, 86–93 CrossRef CAS.
- Y. Kawahara, Coatings, 2016, 6, 34 CrossRef.
- J. Portugal-Pereira and L. Lee, J. Cleaner Prod., 2016, 112, 4419–4429 CrossRef CAS.
- T. Chungsangunsit, S. Gheewala and S. Patumsawad, International Journal of Mechanical, Aerospace, Industrial, Mechatronic and Manufacturing Engineering, 2009, vol. 53, p. 1070 Search PubMed.
- A. Dyrelund, H. Lund, B. Möller, B. Mathiesen, K. Fafner, B. L. S. Knudsen, F. Ulbjerg, T. Laustsen, J. Larsen and P. Holm, Heatplan Denmark, Danish district heating association, technical report, 2008 Search PubMed.
- N. Pijarn, A. Jaroenworaluck, W. Sunsaneeyametha and R. Stevens, Powder Technol., 2010, 203, 462–468 CrossRef CAS.
- U. Kalapathy, A. Proctor and J. Shultz, Bioresour. Technol., 2002, 85, 285–289 CrossRef CAS PubMed.
- V. Vaibhav, U. Vijayalakshmi and S. M. Roopan, Spectrochim. Acta, Part A, 2015, 139, 515–520 CrossRef CAS PubMed.
- International Rice Research Institute, IRRI Rice Knowledge Bank - Using rice husk for energy production, 2022, https://knowledgebank.irri.org/step-by-step-production/postharvest/rice-by-products/rice-husk/using-rice-husk-for-energy-production Search PubMed.
- B. Webb, Proceedings of UNEP/ESCAP/FAO Workshop on Agricultural and Agroindustrial Residue Utilization in Asia and Pacific Region, 1979 Search PubMed.
- O. P. Vimal and P. D. Tyagi, Energy from biomass, Agricole Publishing Academy, New Dehli, India, 1984 Search PubMed.
- S. Bhattacharya, Workshop on Global Warming Issues in Asia, Asian Institute of Technology, Bangkok, 1993 Search PubMed.
- R. Siddique and P. Cachim, Waste and Supplementary Cementitious Materials in Concrete: Characterisation, Properties and Applications, Elsevier, Woodhead Publishing, 1st edn, 2018 Search PubMed.
- C. von Brömssen and E. Röös, Int. J. Life Cycle Assess., 2020, 25, 2101–2105 CrossRef.
- D. FitzGerald, market for chlorine, liquid, RER, Allocation at the Point of Substitution, ecoinvent database version 3.6, 2018 Search PubMed.
- M. Voll and P. Kleinschmit, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, 2010 Search PubMed.
- W. Stöber, A. Fink and E. Bohn, J. Colloid Interface Sci., 1968, 26, 62–69 CrossRef.
- A. Williams, J. M. Jones, L. Ma and M. Pourkashanian, Prog. Energy Combust. Sci., 2012, 38, 113–137 CrossRef CAS.
- B. M. Jenkins, L. L. Baxter, T. R. Miles and T. R. Miles, Fuel Process. Technol., 1998, 54, 17–46 CrossRef CAS.
- International Energy Agency, Global Energy Review: CO2 Emissions in 2021, International energy agency technical report, 2021 Search PubMed.
- W. H. Waddell, Kirk-Othmer Encyclopedia of Chemical Technology, John Wiley & Sons, Ltd, 2006 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc01433e |
| This journal is © The Royal Society of Chemistry 2023 |