Open Access Article
Open Access ArticleAssessment of human inter-individual variability of phloretin metabolites in urine after apple consumption. AppleCOR study†
Alba
Macià
 a,
María-Paz
Romero
a,
Anna
Pedret
a,
María-Paz
Romero
a,
Anna
Pedret
 b,
Rosa
Solà
b,
Michael N.
Clifford
b,
Rosa
Solà
b,
Michael N.
Clifford
 cd and
Laura
Rubió-Piqué
cd and
Laura
Rubió-Piqué
 *a
*a
aDepartment of Food Technology, Engineering and Science, University of Lleida, Agrotecnio-CERCA Center, Antioxidants Research Group, Av. Alcalde Rovira Roure 191, Lleida, 25198, Spain. E-mail: laura.rubio@udl.cat; Tel: +34 973 702554
bUniversitat Rovira i Virgili, Facultat de Medicina i Ciències de la Salut, Functional Nutrition, Oxidation, and Cardiovascular Diseases Group (NFOC-Salut), C/Sant Llorenç 21, 4320-Reus, Spain
cSchool of Bioscience and Medicine, Faculty of Health and Medical Sciences, University of Surrey, Guildford, UK
dDepartment of Nutrition, Dietetics, and Food, School of Clinical Sciences at Monash Health, Faculty of Medicine Nursing and Health Sciences, Monash University, Notting Hill, Victoria, Australia
First published on 3rd November 2023
Abstract
Purpose: This study aimed to assess the inter-individual variation in phloretin absorption and metabolism and to seek possible phloretin metabotypes following apple snack consumption. Methods: The excreted phloretin metabolites in 24 h urine samples were determined by UPLC-MS/MS in 62 volunteers after acute and sustained (6 weeks) interventions in a randomized and parallel study with a daily supplementation of 80 g of a low-phloretin (39.5 μmol) or a high-phloretin (103 μmol) freeze-dried apple snacks. Results: absorption estimated as phloridzin equivalents for 62 volunteers varied almost 70-fold ranging from 0.1% to 6.94% of phloretin glycoside intake. Volunteers were stratified into low, medium and high producers and by the balance between glucuronidation and sulphation. For 74% of the volunteers phloretin-O-glucuronide was the dominant urinary metabolite, especially at the higher phloretin glycoside intake and for higher producers. Sulphate conjugation assumed greater significance for the remaining volunteers especially for low producers. Females dominated glucuronide profile (64.1%) and males dominated the low excretion group. Analysis of plasma glucose and insulin at the start and end of the sustained study showed a trend towards modest reductions for high producers. Furthermore, plausible factors contributing to the inter-individual variation in phloretin uptake are discussed. Conclusions: extensive inter-individual variability exists in the excretion of phloretin phase-II conjugates following consumption of apple snacks, which could be related to oral microbiota phloridzin-hydrolysing activity, lactase non-persistence trait or the metabotype to which the subject belongs. There were inconsistent effects on post-prandial serum glucose concentrations but there was a tendency for decreases to be associated with higher excretion of phloretin phase-II conjugates. Trial registration: The acute and sustained studies were registered at ClinicalTrials.gov Identifier: NCT03795324.
1. Introduction
Apple (Malus domestica) is the most consumed fruit in Europe, and one of the most popular worldwide. It appears that apples may play a significant role in reducing the risk of a wide variety of chronic disease and maintaining a healthy lifestyle in general. Their consumption has been most consistently associated with reduced risk of cancer, heart disease, asthma and type II diabetes, and intervention correlated these health effects with apple (poly)phenolic content.1 Apple (poly)phenolics comprise several classes of (poly)phenols including hydroxycinnamic acids, flavan-3-ols, dihydrochalcones and flavonols.2 Among apple (poly)phenolic compounds, the dihydrochalcones phloretin-2′-O-glucoside (phloridzin) and phloretin-2′-O-(2′′-O-xylosyl)glucoside are rarely found in other dietary components, and when found, they are present at relatively low concentrations.3 In terms of bioactivity, growing evidence suggests that apple phloridzin interacts with glucose transporters in the small intestine and modulates glucose uptake after food or beverage consumption, demonstrating health-promoting effects on the control of postprandial hyperglycemia.4Concerning the absorption and metabolism, phloridzin is not transported by small intestinal mucosa but is a substrate for lactase-phloridzin hydrolase (LPH) (EC 3.2.1.108; 3.2.1.62).5,6 Lactose and phloridzin have separate substrate binding sites. The released aglycone, phloretin, is rapidly absorbed, probably by passive diffusion. After consuming a mixed fruit beverage incorporating apple juice delivering phloridzin (67.9 ± 1.7 μmol), the Cmax (204 nmol L−1) was less than 1 hour with a secondary Cmax (ca. 50 nmol L−1) at 4 hours,7 but in other similar studies with juice or cider there was a smoother decline8,9 and absorption is almost complete within 6 hours. After an apple snack intake, absorption and clearance were slightly delayed by the matrix effect with Cmax at 2 hours post-consumption.10 This early absorption peak is followed by a fast appearance of phloretin metabolites in urine samples, consistent with hydrolysis by LPH. After ileostomists consumed 500 ml cider delivering 46 μmol phloretin glycosides ileal fluid was found to contain 3.8 μmol of phloretin-xylosylglycosides, 11.4 μmol phloretin phase-II conjugates and 2.4 μmol phloretin indicating efficient hydrolysis of phloridzin, substantial but incomplete absorption of phloretin and significant efflux of phloretin phase-II conjugates direct from the enterocyte or in bile.8 Phloretin at up to 0.07 μmol per litre has been detected in fecal water of free-living volunteers.11
The bioactivity of the (poly)phenolic compounds consumed depends on their inherent bioactivity and the bioactivity of their metabolites and the concentration they reach in the organism.12 However, some heterogeneity in the pharmacokinetic response after (poly)phenol intake has been commonly observed in human clinical trials, and this fact can hinder the health beneficial effects in some sub-populations. Available evidence suggests that several determinants such as age, sex, gut microbiota composition and polymorphisms of phase-II enzymes and transporters may explain this inter-individual variability in absorption, metabolism and excretion of (poly)phenols.13 There are still very few studies suggesting that this variation could impact on the efficacy of (poly)phenolic compounds to modulate cardiometabolic outcomes.14 Thus, a preliminary step is to evaluate the inter-individual variation in the capacity of absorption and metabolism of the (poly)phenolic compounds to better understand their role on human health.15
Metabolomics represent crucial techniques to decipher inter-individual variability and to stratify individuals according to the gradient excretion (quantitative criterion) or according to metabotypes (qualitative criterion), which reflect the intrinsic capacity to absorb and metabolize (poly)phenolic compounds. Yet successful stratification examples are scarce and if available, validation in larger human studies is still required.13
Although phloretin phase-II conjugates have been reported as specific biomarkers of apple intake, and several studies have evaluated the bioavailability of apple phloretin after acute intake studies,16,17 investigation is lacking regarding the inter-individual variability after a repeated exposure. The aims of the present study were: (i) to quantify the inter-individual variation in phloretin absorption and metabolism following apple snack consumption in two diverse experimental settings (acute and sustained); (ii) to investigate the effect of dose by using two apple snacks with different phloretin concentration; and (iii) to seek possible phloretin metabotypes and determine whether the metabolite excretion profiles were temporally consistent, varied with phloretin dose, or varied after stratification of volunteers into high, medium and low excretion of phloretin phase-II metabolites.
2. Materials and methods
2.1. Chemicals and reagents
The standards used for the quantification of (poly)phenolic compounds in the low-phloretin apple (low-PhA) and high-phloretin apple (high-PhA) snacks were reported in our previous studies.18,21 Briefly, the standard used for the quantification of the phloretin compounds and their generated metabolites was phloretin-2′-O-glucoside (phloridzin), which was purchased from Extrasynthese (Genay, France).Methanol (HPLC grade), acetonitrile (HPLC grade), acetic acid, and hydrochloric acid (HCl) were purchased from Scharlab Chemie (Sentmenat, Catalonia, Spain). The water used was Milli-Q quality (Millipore Corp, Bedford, MA, USA).
A stock solution of phloretin-2′-O-glucoside was prepared by dissolving this standard compound in methanol at a concentration of 1000 mg L−1 and storing this in dark flasks at 4 °C. Stock dilutions were prepared daily.
2.2. Low- and high-phloretin apple snacks
In the present work, two different apple fruits were studied, the red-fleshed “Redlove” apple variety, a new genotype naturally biofortified in anthocyanins, and the common white-fleshed Granny Smith apple variety without anthocyanins, both provided by NUFRI SAT (Mollerussa, Lleida, Spain). The red-fleshed apple variety contained a high-phloretin concentration (high-PhA) and the Granny Smith contained a low-phloretin concentration (low-PhA). To preserve the (poly)phenolic compounds of the apples, a freeze-dried snack was prepared and provided to the volunteers. The detailed preparation process of the freeze-dried apple snacks is reported in our previous study.2 Briefly, the apple core was removed and the whole apple (with peel) was cut into 1 cm-sized cubes, which were immediately frozen in liquid nitrogen, and then lyophilized on a Lyobeta 15 TELSTAR Lyophilizer (Terrassa, Spain).2.3. Study design: acute and sustained apple snack intakes
The study consisted of a sub-sample of the AppleCOR study, a parallel and randomized trial conducted in hypercholesterolemic subjects which has been previously described.21 A CONSORT flow chart outlining the study schedule and number of participants in the AppleCOR study is displayed in our previous work, as well as the power calculation of the study population size.21In the present work, two different apple fruits were studied, and these contained different phloretin amounts: low-PhA and high-PhA. In a parallel design, two groups of participants (n = 33, low-PhA; n = 29, high-PhA) consumed 80 g day−1 of the corresponding apple snack for 6 weeks. Nested within the sustained study, a sub-sample of volunteers of each group (n = 7, low-PhA; n = 8, high-PhA) performed two acute intake studies. The first acute intake occurred at day 0 and the second acute study occurred at day 42 at the end of the sustained study, consuming all at once 80 g of low-PhA or high-PhA snacks. For the sustained intake study, 24 h urine was obtained at day 0 and day 42. For the acute study, 24 h urine sample was collected before and 24 h post-acute consumption. The study design is shown in Fig. 1. The total volume of urine of each volunteer in each interval time was measured and samples were stored at −80 °C in the central laboratory's Biobanc of HUSJ-Eurecat (biobanc.reus@iispv.cat) until required for batch analyses.
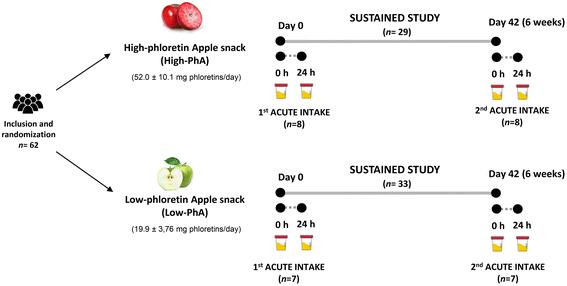 | ||
| Fig. 1 Study design of acute and sustained interventions with a low-phloretin or a high-phloretin freeze-dried apple snacks. | ||
Participants signed informed consent prior to their participation in the study, which was approved by the Clinical Research Ethics Committee of Institut d'Investigació Sanitària Pere Virgili (S033/04Nov2016), Reus, Spain. The protocol and trial were conducted in accordance with the Helsinki Declaration and Good Clinical Practice Guidelines of the International Conference of Harmonization (GCP ICH) and were reported as CONSORT criteria. The trial was registered at ClinicalTrials.gov Identifier: NCT03795324.
For the sustained study, blood samples were obtained by traditional venipuncture at fasting conditions, and serum glucose and insulin concentrations were measured by standardized methods in a Cobas Mira Plus autoanalyzer (Roche Diagnostics Systems, Madrid, Spain).
2.4. Analysis of phloretin metabolites in urine samples
Tandem MS analyses were carried out on a triple quadrupole detector (TQD) mass spectrometer (Waters) equipped with a Z-spray electrospray interface. The ionization source was electrospray ionization (ESI), in the negative ion mode. The data were acquired with the selected reaction monitoring mode (SRM). The MS/MS parameters were as follows: capillary voltage, 3 kV; source temperature, 150 °C; cone gas flow-rate, 90 L h−1 and desolvation gas flow-rate, 900 L h−1; desolvation temperature, 450 °C. Nitrogen (>99% purity) and argon (99% purity) were used as nebulizing and collision gases, respectively. The cone voltages and collision energies were optimized for each phloretin compound. Two transitions were studied for each compound, when possible, the most abundant being used for quantification, and the second one for confirmation purposes. The dwell time established for each transition was 30 ms. Data acquisition was carried out with the MassLynx v 4.1 Software. ESI Table S1† shows the SRM transition for quantification, as well as the cone voltage and collision energy for each phloretin compound and its generated metabolites. Due to the lack of commercial (poly)phenolic standards and their generated metabolites, all the phloretin glycosides and the generated metabolites were quantified by using the calibration curve of phloretin-2′-O-glucoside (phloridzin). Because it has been clearly demonstrated that during LC-MS the phase-II conjugates of any given aglycone may differ markedly in their ionisation, the relative yields of the individual conjugates must be treated as only approximate.22
2.5. Statistical analysis
The results are presented as mean values ± standard deviation (SD) for the phloretin compounds in low-PhA snack and high-PhA snack, and mean values ± standard error of the mean (SEM) for the generated phloretin metabolites in urine samples. Differences were considered significant at p ≤ 0.05 using t-test and two-way ANOVA analysis. The statistical analyses were performed with the Statgraphics plus version 5.1 software (Manugistics Inc., Rockville, MD, United States).3. Results and discussion
3.1. Phloretin composition in apple snacks
This study, which is part of the AppleCOR project, utilised two apple varieties: the common white-fleshed Granny Smith apple, and the red-fleshed “Redlove” apple. The total (poly)phenolic content in both apple snacks was very similar, 197 ± 17.5 mg per 80 g white-fleshed apple snack per day, and 193 ± 16.5 mg per 80 g red-fleshed apple snack per day, as previously reported,21 but there were significant differences in the contents of dihydrochalcone glycosides: phloretin-2′-O-glucoside, phloretin-O-xylosylglucoside, and hydroxyphloretin-O-xylosylglucoside. The white-fleshed apple (low-PhA) contained 19.9 ± 3.76 mg per 80 g (39.5 ± 8.03 μmol per 80 g), compared with red-fleshed apple (high-PhA), containing 52.0 ± 10.1 mg per 80 g (103 ± 20.0 μmol per 80 g) (ESI Table S2†). The chemical structures of apple phloretins are shown in ESI Fig. 1.† The concentration of both phloretin-2′-O-glucoside, and phloretin-O-xylosyl-glucoside was around 2.6-fold higher in high-PhA than in low-PhA snacks. Nevertheless, the concentration of hydroxyphloretin-O-xylosylglucoside, which was present at lower concentration levels than the other two phloretin glycosides, was only 1.55-fold higher in high-PhA than in low-PhA snacks. This hydroxyphloretin glycoside has been reported as an oxidation product of phloretin-2′-O-glucoside.23Regarding the nutritional composition of the apple snacks, both samples were matched as closely as possible to eliminate any confusing effect, observing no significant differences between them in any nutritional parameter, including total fiber and macronutrients (ESI Table S3†).
3.2. Identification of phloretin glycosides and phloretin phase-II metabolites in urine after apple consumption
Previous volunteer studies of phloridzin metabolism reported that urine contained three phloretin-O-glucuronides and one phloretin-O-sulphate-O-glucuronide. Ileal fluid contained two additional phloretin-O-sulphate-O-glucuronides and two phloretin-O-sulphates. The dominant urinary metabolite was identified as phloretin-2′-O-glucuronide7,8,21,24–26 but the other phloretin metabolites have not been characterised to regio-isomer level.In this study, a maximum of only three phloretin phase-II metabolites, a phloretin-O-glucuronide, a phloretin-O-sulphate, and a phloretin-O-sulphate-O-glucuronide, were identified in 24 h-urine samples using LC-MS/MS after the acute and sustained intakes of both apple snacks (low- and high-PhA). Marks et al.8 who reported three phloretin-O-glucuronides in the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1
0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.007 used cider delivering 46 μmol phloretin-O-glycosides compared with 39.5 μmol and 103 μmol in the two apple snacks, and the failure to detect the other two phloretin-O-glucuronides, especially after the larger intake with high-PhA snack, might be a consequence of the apple matrix impairing absorption of phloretin.
0.007 used cider delivering 46 μmol phloretin-O-glycosides compared with 39.5 μmol and 103 μmol in the two apple snacks, and the failure to detect the other two phloretin-O-glucuronides, especially after the larger intake with high-PhA snack, might be a consequence of the apple matrix impairing absorption of phloretin.
In the absence of standards, these phase-II metabolites were not characterised to regio-isomer level, but specimen structures are presented in ESI Fig. 1.† The urines from 10 of the 29 subjects (34.5%) who participated in the sustained intake of the high-PhA snack also contained the native phloretin-O-xylosylglucoside but phloridzin and hydroxyphloretin-O-xylosylglucoside were not detected in any urine sample.
Phloretins are not the most abundant apple polyphenols, but they are one of the more abundant phase-II urinary metabolites after apple consumption in animal20 and human21 studies. This denotes a greater bioavailability and suggests that phloretins could play a more significant role in the biological response to apple consumption. Of greater importance, as discussed above, they are derived primarily from phloridzin, one of very few dietary flavonoids for which there is longstanding and unequivocal evidence of biological activity.4
3.3. Stratification of individuals according to the urinary excretion of phloretin phase-II metabolites
Phloretin metabolites in urine samples have been evaluated in several studies as potential specific biomarkers for apple and apple product consumption.16,17 Because of the limited occurrence of phloretin-O-glycosides and then only in comparatively minor components of the diet (for example, strawberries and rose hips27,28), phloretin phase-II conjugates in urine samples can be viewed as a very good qualitative marker for that purpose. However, substantial inter-individual variation in the amounts excreted make them a less good quantitative biomarker, with for example, Marks et al.8 reporting standard deviations in the range 24 to 51% of the mean value, implying impossible negative values and suggesting that the distribution is skewed or even bimodal.22 These early studies did not provide data for individual volunteers and the nature of the variation remains unclear.In the present study the magnitude of this inter-individual variation is presented in Table 1 as the quotient (largest/smallest). Following sustained consumption of the low-PhA snack the quotients were >10.4 for phloretin-O-sulphate, >37.0 for phloretin-O-glucuronide-O-sulphate and >44.8 for phloretin-O-glucuronide, 10.5, >52.0, >23.2, respectively after consumption of the high-PhA snack, increasing to >12.0, >52.0 and >97.6, respectively, overall. These data are presented graphically in ESI Fig. 2 and 3.† These quotients are smaller than recorded by the same volunteers for several gut biota-generated metabolites such as 3′-methoxycinnamic acid-4′-O-sulphate, 3-(3′-methoxyphenyl)propanoic acid-4′-O-glucuronide, 3′-hydroxy-4′-methoxycinnamic acid, 3-(4′-methoxy-3′-hydroxyphenyl)propanoic acid and 3-(4′-methoxy-phenyl)propanoic acid-3′-O-glucuronide which ranged from 193 to 239.19
| Volunteer | Excretion | Stratification | Glucemic parameters | Sex | Age (years) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μmol per 24 h | Individual metabolites as percentage of total Phase-II metabolites | On excretion of phloretin-2′-O-glucuronide | By metabolite profile | By molar percentage glucuronide | Day 42 minus Day 0 | |||||||||
| PhXG | PhG | PhS | PhSG | PhG | PhS | PhSG | Glucose (mg dL−1) | Insulin (mcUI mL−1) | ||||||
| In addition to the sustained intake, the volunteers from L1 to L7 also participated in the two acute studies: days 0 and 42 before and after the sustained intake of low-PhA Snack, and H1 to H8 after the sustained intake of high-PhA snack. | ||||||||||||||
| Low-PhA snack | ||||||||||||||
| 1 | n.d. | n.d. | 0.17 | n.d. | 0.0 | 100.0 | 0.0 | Low | B* | 0 | 8 | 0.4 | M | 43 |
| 2 | n.d. | n.d. | 0.73 | 0.23 | 0.0 | 76.1 | 23.9 | Low | B | 19 | −2 | −4.1 | M | 49 |
| 11 | n.d. | n.d. | 0.08 | 0.08 | 0.0 | 50.1 | 49.9 | Low | B | 33 | 5 | 0.1 | M | 35 |
| 15 (L5) | n.d. | n.d. | 0.21 | n.d. | 0.0 | 100.0 | 0.0 | Low | B* | 0 | −5 | 1.3 | M | 61 |
| 21 | n.d. | 0.21 | 0.21 | 0.21 | 34.0 | 33.0 | 33.0 | Low | B | 50 | 0 | 0.7 | M | 39 |
| 22 | n.d. | n.d. | 0.23 | n.d. | 0.0 | 100.0 | 0.0 | Low | B* | 0 | 1 | 0.5 | M | 56 |
| 24 | n.d. | n.d. | 0.12 | n.d. | 0.0 | 100.0 | 0.0 | Low | B* | 0 | 11 | 2.1 | F | 46 |
| 25 | n.d. | 0.18 | 0.07 | n.d. | 72.3 | 27.7 | 0.0 | Low | A* | 72 | 4 | No value | M | 19 |
| 26 | n.d. | n.d. | 0.13 | n.d. | 0.0 | 100.0 | 0.0 | Low | B* | 0 | 1 | 0.9 | F | 70 |
| 29 | n.d. | 0.05 | 0.12 | 0.01 | 26.7 | 66.5 | 6.8 | Low | B | 32 | −4 | 1.2 | F | 59 |
| 32 | n.d. | n.d. | 0.15 | n.d. | 0.0 | 100.0 | 0.0 | Low | B* | 0 | −4 | −5.2 | F | 32 |
| Quotient (largest/smallest) | >4.20 | 10.4 | >23.0 | 1.36 ± 1.56 | −0.21 ± 0.76 | 46.3 ± 14.8 | ||||||||
| 6 | n.d. | 0.54 | 0.36 | n.d. | 60.3 | 39.7 | 0.0 | Medium | A* | 60 | 4 | 2.2 | F | 57 |
| 8 | n.d. | 0.86 | 0.27 | 0.22 | 63.6 | 19.9 | 16.5 | Medium | A | 69 | −4 | 3.5 | F | 48 |
| 10 (L4) | n.d. | 0.90 | 0.45 | 0.35 | 52.9 | 26.6 | 20.5 | Medium | A | 61 | −3 | No value | F | 60 |
| 13 | n.d. | 0.62 | 0.34 | 0.17 | 54.6 | 30.4 | 15.0 | Medium | A | 61 | 0 | 0.1 | F | 52 |
| 16 | n.d. | 0.39 | 0.08 | 0.16 | 61.7 | 12.8 | 25.4 | Medium | A | 70 | −2 | −0.6 | F | 56 |
| 23 | n.d. | 0.42 | 0.10 | n.d. | 81.7 | 18.2 | 0.0 | Medium | A* | 81 | 3 | 1.7 | M | 47 |
| 27 | n.d. | 0.26 | 0.25 | 0.02 | 49.4 | 47.6 | 3.0 | Medium | A | 51 | 20 | 0.8 | F | 69 |
| 28 | n.d. | 0.40 | 0.24 | 0.18 | 49.0 | 29.5 | 21.5 | Medium | A | 58 | 9 | −0.1 | F | 67 |
| 30 | n.d. | 0.69 | 0.38 | 0.08 | 59.8 | 33.3 | 6.8 | Medium | A | 63 | 1 | −0.3 | F | 61 |
| 31 | n.d. | 0.93 | 0.09 | 0.37 | 66.6 | 6.8 | 26.6 | Medium | A | 74 | 0 | −3.7 | F | 37 |
| 33 | n.d. | 0.22 | 0.17 | 0.07 | 47.0 | 37.6 | 15.4 | Medium | A | 55 | 2 | −3.0 | F | 51 |
| Quotient (largest/smallest) | 4.23 | 5.62 | >18.5 | 2.73 ± 2.04 | 0.06 ± 0.70 | 55.0 ± 9.30 | ||||||||
| 3 | n.d. | 1.24 | 0.13 | 0.17 | 80.4 | 8.6 | 11.0 | High | A | 82 | −5 | 1.5 | M | 60 |
| 4 (L1) | n.d. | 0.97 | n.d. | 0.18 | 84.1 | 0.0 | 15.9 | High | A | 86 | −5 | 0.8 | F | 55 |
| 5 (L2) | n.d. | 1.15 | 0.15 | 0.16 | 78.8 | 10.3 | 10.9 | High | A | 81 | −4 | −0.4 | F | 63 |
| 7 | n.d. | 1.62 | 0.16 | n.d. | 91.0 | 9.0 | 0.0 | High | A* | 91 | −3 | −2.3 | F | 37 |
| 9 (L3) | n.d. | 1.28 | 0.26 | 0.19 | 73.9 | 14.8 | 11.3 | High | A | 77 | −5 | 0.6 | M | 45 |
| 12 | n.d. | 2.24 | 0.25 | 0.25 | 82.0 | 9.0 | 9.0 | High | A | 83 | 3 | −1.3 | F | 51 |
| 14 | n.d. | 0.95 | n.d. | n.d. | 100.0 | 0.0 | 0.0 | High | A* | 100 | −6 | −5.1 | M | 55 |
| 17 (L6) | n.d. | 1.26 | 0.16 | n.d. | 88.8 | 11.2 | 0.0 | High | A* | 89 | −20 | 0.6 | F | 49 |
| 18 | n.d. | 1.45 | 0.24 | 0.19 | 77.1 | 13.0 | 9.9 | High | A | 79 | −6 | −2.0 | F | 61 |
| 19 | n.d. | 1.08 | n.d. | 0.14 | 88.4 | 0.0 | 11.6 | High | A | 90 | 1 | −1.3 | F | 61 |
| 20 (L7) | n.d. | 1.62 | 0.10 | 0.13 | 87.7 | 5.3 | 7.0 | High | A | 88 | −1 | 0.6 | F | 35 |
| Quotient (largest/smallest) | 2.36 | >2.60 | >1.93 | −4.64 ± 1.77 | −0.75 ± 0.58 | 52.0 ± 9.70 | ||||||||
| Overall Quotient Low-PhA (largest/smallest) | >44.8 | >10.4 | >37.0 | |||||||||||
| High-PhA snack | ||||||||||||||
| 39 (H4) | n.d. | n.d. | 0.25 | n.d. | 0.0 | 100 | 0.0 | Low | B* | 0 | 4 | −3.1 | F | 25 |
| 43 | n.d. | n.d. | 0.28 | n.d. | 0.0 | 100 | 0.0 | Low | B* | 0 | 3 | 0.6 | F | 54 |
| 45 | n.d. | n.d. | 0.11 | 0.05 | 0.0 | 67.9 | 32.1 | Low | B | 24 | 20 | No value | M | 50 |
| 48 | n.d. | n.d. | 0.10 | n.d. | 0.0 | 100 | 0.0 | Low | B* | 0 | 5 | 2.9 | M | 50 |
| 50 | n.d. | n.d. | 0.30 | n.d. | 0.0 | 100 | 0.0 | Low | B* | 0 | 0 | 3.2 | F | 29 |
| 55 | n.d. | 0.70 | 0.19 | n.d. | 78.6 | 21.4 | 0.0 | Low | A* | 79 | 3 | −1.4 | M | 45 |
| 58 | n.d. | 0.58 | 0.09 | 0.11 | 74.4 | 10.9 | 14.4 | Low | A | 78 | −6 | −0.4 | F | 44 |
| 61 | n.d. | n.d. | 0.18 | n.d. | 0.0 | 100 | 0.0 | Low | B* | 100 | 3 | −4.0 | M | 45 |
| 62 | n.d. | 0.21 | 0.08 | 0.07 | 57.6 | 23.5 | 19.0 | Low | A | 65 | 7 | −1.1 | M | 18 |
| Quotient (largest/smallest) | >3.33 | 3.75 | >2.20 | 4.33 ± 2.31 | −0.41 ± 0.86 | 40.0 ± 4.24 | ||||||||
| 36 | 0 | 0.86 | 0.15 | 0.20 | 71.1 | 12.5 | 16.4 | Medium | A | 75 | −6 | −1.2 | M | 50 |
| 37 (H2) | n.d. | 0.84 | 0.17 | 0.19 | 69.7 | 14.1 | 16.2 | Medium | A | 74 | −2 | 0.0 | F | 62 |
| 40 | n.d. | 1.60 | 0.13 | 0.01 | 92.2 | 7.3 | 0.6 | Medium | A | 92 | −2 | 0.5 | M | 51 |
| 44 | n.d. | 1.24 | 0.25 | 0.16 | 75.2 | 15.3 | 9.5 | Medium | A | 77 | −3 | 1.2 | F | 41 |
| 46 | 0.01 | 0.76 | 0.43 | 0.30 | 51.0 | 28.7 | 20.3 | Medium | A | 59 | −2 | −1.3 | M | 50 |
| 49 (H6) | n.d. | 0.83 | 0.12 | 0.11 | 77.9 | 11.7 | 10.4 | Medium | A | 80 | −3 | 3.7 | F | 66 |
| 51 | 0.01 | 2.15 | 0.39 | 0.30 | 75.8 | 13.8 | 10.4 | Medium | A | 78 | −5 | 0.2 | M | 33 |
| 52 | 0.01 | 1.52 | 0.50 | 0.29 | 65.8 | 21.6 | 12.6 | Medium | A | 70 | −5 | 0.1 | F | 41 |
| 56 | 0.02 | 1.86 | 0.21 | 0.25 | 80.0 | 9.1 | 10.9 | Medium | A | 82 | 0 | 1.9 | F | 66 |
| 59 | 0.02 | 2.16 | 0.24 | 0.20 | 82.9 | 9.3 | 7.9 | Medium | A | 84 | 1 | −1.9 | M | 55 |
| 60 | n.d. | 1.35 | 0.11 | 0.23 | 79.8 | 6.6 | 13.6 | Medium | A | 82 | −6 | 0.7 | M | 42 |
| Quotient (largest/smallest) | 2.84 | 4.55 | 30.0 | −3.00 ± 0.70 | 0.35 ± 0.48 | 50.6 ± 3.29 | ||||||||
| 34 (H1) | 0.02 | 4.88 | 0.84 | 0.38 | 79.9 | 13.8 | 6.3 | High | A | 81 | −5 | 1.8 | M | 59 |
| 35 | 0.01 | 2.27 | 0.53 | 0.52 | 68.3 | 16.1 | 15.6 | High | A | 73 | 3 | −2.5 | M | 51 |
| 38 (H3) | n.d. | 2.48 | 0.17 | 0.15 | 88.9 | 5.9 | 5.2 | High | A | 89 | −4 | −0.3 | M | 65 |
| 41 | n.d. | 3.22 | 0.24 | 0.16 | 89.2 | 6.5 | 4.3 | High | A | 89 | −3 | −0.9 | F | 45 |
| 42 (H5) | n.d. | 3.28 | 0.51 | 0.36 | 79.0 | 12.3 | 8.7 | High | A | 81 | −4 | 1.9 | F | 56 |
| 47 | n.d. | 2.47 | 0.52 | 0.14 | 79.0 | 16.7 | 4.3 | High | A | 80 | 2 | −1.8 | M | 44 |
| 53 | n.d. | 2.76 | 0.46 | 0.14 | 82.1 | 13.8 | 4.1 | High | A | 83 | −4 | −3.0 | M | 60 |
| 54 (H7) | 0.004 | 2.90 | 0.42 | 0.41 | 77.6 | 11.4 | 11.0 | High | A | 80 | −7 | −9.1 | F | 41 |
| 57 (H8) | 0.02 | 2.98 | 0.28 | 0.25 | 84.8 | 8.0 | 7.2 | High | A | 86 | 0 | No value | F | 33 |
| Quotient (largest/smallest) | 2.15 | 4.94 | 3.71 | −2.44 ± 1.12 | −1.74 ± 1.16 | 50.4 ± 3.48 | ||||||||
| Overall quotient high-PhA (largest/smallest) | >23.2 | 10.5 | >52.0 | |||||||||||
| Overall quotient both samples (largest/smallest) | >97.6 | >12.0 | >52.0 | |||||||||||
Such inter-individual variation can take three forms, and these are not mutually exclusive: (i) variation in the amount of phloretin absorbed, (ii) variation in its metabolism after absorption, and (iii) variation in either or both over time. All three forms have been investigated in this study and are discussed below.
We recognise that there are some limitations to the study, and the discussion which follows gives due regard to them. In particular, in the absence of authentic phase-II conjugates of phloretin for accurate quantification, phloridzin has been used as the calibrant, but this is no different to previous studies which have used either phloridzin or phloretin for the same reason. This method of calibration is acceptable for comparing the amounts of an individual metabolite in a range of samples from one study but because the MS response of the individual phase-II conjugates is almost certain to differ29 the value obtained by summing the content of the three phase-II metabolites in any given sample, is less accurate than the values for the individual metabolites. For example, a sample with a large content of a poorly ionising metabolite might appear to have smaller total metabolite content than a sample with a small amount of a strongly ionising metabolite, when in fact the reverse is true. This method of calibration also influences the LOD and LOQ, with a weakly ionising metabolite being more likely to be undetectable in a sample when only a small amount of phloretin had been absorbed, and limits the extent to which data from other studies using different calibrants and MS equipment can be reliably compared.
(i) Stratification on urinary excretion
Due to the high inter-individual variability, the subjects were stratified into “high”, “medium” and “low” producers for each phloretin phase-II metabolite after the sustained intake; and “high” and “low” after the acute intakes. Because of the greater imprecision, discussed above, associated with the total metabolite concentration the stratification reported here utilised the data for the phloretin-O-glucuronide, the dominant metabolite.
Where mathematically possible stratification utilised groups of equal size as previously reported by Nishioka et al.,30 but when this was not possible (numbers not multiple of 3), those extra volunteer(s) were placed in the middle group. Similarly, if a boundary would have separated two numerically identical values both were assigned to the middle group.
As example, ESI Table S4† shows the stratification of (a) phloretin-O-glucuronide, (b) phloretin-O-sulphate, and (c) phloretin-O-sulphate-O-glucuronide after the sustained intake of high-PhA snack, and ESI Table S5† the stratification of (a) phloretin-O-glucuronide, (b) phloretin-O-sulphate, and (c) phloretin-O-sulphate-O-glucuronide after the first acute intake of high-PhA snack.
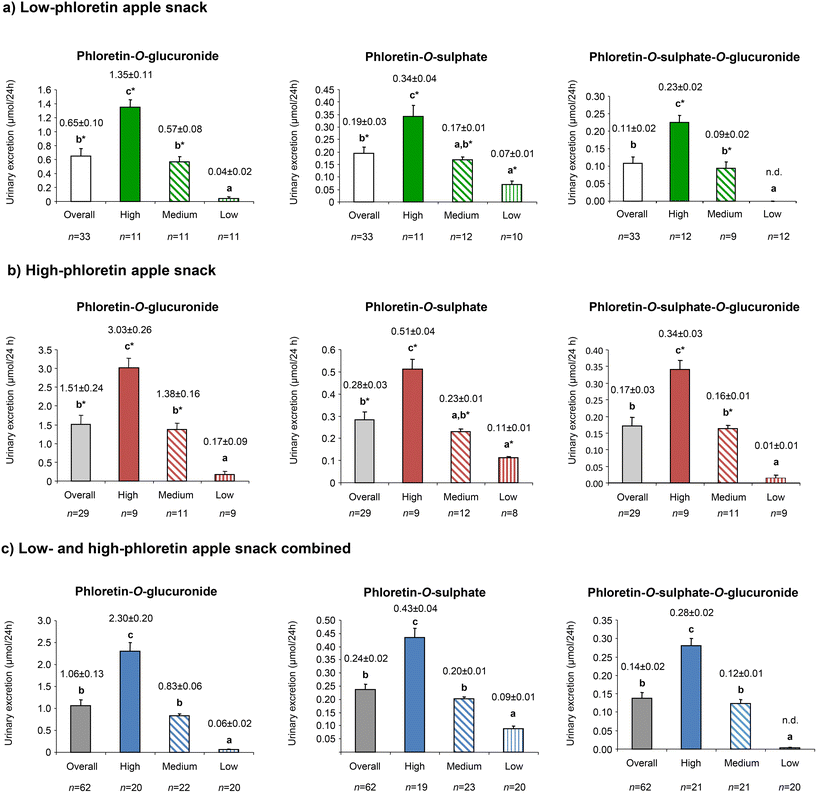
Subsequent analysis by t-test and two-way ANOVA failed to demonstrate any statistically significant difference between the middle and low phloretin-O-sulphate groups after the low-PhA and high-PhA snacks, but differences between all other groupings were confirmed (p ≤ 0.05) (Fig. 2). When the low-PhA and high-PhA snacks were combined (see Fig. 2C), significant differences were observed between the three producer groups (high, medium and low) and this was observed for the three phloretin metabolites.
When comparing the low-PhA and high-PhA snacks, excretion of phloretin-O-glucuronide and phloretin-O-sulphate-O-glucuronide by low producers and the mixed conjugate overall were not obviously increased but in all other comparisons a statistically significant increase was detected as the phloretin dose increases (marked by asterisk in Fig. 2). These increases were more pronounced for phloretin-O-glucuronide where the ratios for the increase in the high and medium groups (2.24 and 2.42 respectively) approached the ratio for the increased molar content of phloretin glycosides (2.49) and which was exceeded (4.25) in the low excretion group. For the other metabolites all groups returned ratios near 1.5.
In the absence of authentic standards for quantification it is not possible to give precise values for the percentage absorption but estimated as phloridzin equivalents absorption after the low-PhA snack (39.5 μmol phloretin glycosides) was calculated at 0.30–2.43%, 1.16–4.30% and 2.41–6.94% for low, medium and high producers. The corresponding values for the high-PhA snack (103 μmol phloretin glycosides) are 0.10–0.76%, 1.03–2.77% and 2.72–5.94%.
These values are similar to those recorded (5% and 5.5% quantified by LC-MS as phloridzin equivalents) when volunteers with an intact colon and ileostomists, respectively, consumed 500 ml cider delivering 46 μmol phloretin glycosides,8 but considerably lower than the 21 ± 5% (HPLC–UV quantified as phloretin equivalents after enzymatic hydrolysis) achieved after volunteers drank 1.1 litre of cider delivering 10 μmol phloretin glycosides.31 Because the molar extinction coefficients of conjugates of one flavonoid generally differ much less than their ionisation in the MS, this UV-quantified recovery might be more accurate than those obtained after MS quantification.
(ii) Stratification on urinary metabolite profile
In the absence of a vic-diol which might be a substrate for methylation, phase-II metabolism of phloretin is restricted to glucuronide and sulphate conjugation. Accordingly, stratification was achieved by expressing the molar contribution made by glucuronide as a percentage of the contribution made by glucuronide and sulphate and designating participants with >50–100% glucuronide as profile A and those with 0–50% glucuronide as profile B. Note that in this calculation the double conjugate contributes equally to glucuronide and sulphate. Because the double conjugate (phloretin-O-sulphate-O-glucuronide) was not detectable in 18 out of 62 samples (29.0%), sub-profiles of A* and B* have been introduced to highlight this (Table 1).
Following consumption of the low-PhA snack the 11 low producers consisted of 4B, 6B* and 1A* whereas medium and high producers consisted of 9A and 2A* and 8A and 3A*, respectively, clearly demonstrating that glucuronide conjugation becomes of greater importance in individuals absorbing more phloretin.
This inference is reinforced by the results for the high-PhA snack—the medium and high producer groups consisting of 11A and 9A, respectively, whereas the low producer group consisted of 2A, 1A*, 1B and 5B* making the A profiles more prominent even among low producers at the higher phloretin glycosides intake.
These observations are consistent with the understanding that the phase-II sulphation pathway has higher affinity than glucuronidation but lower capacity, so that when the ingested (poly)phenol dose is relatively low it can be quite well accommodated by sulphation, but as the (poly)phenol dose increases, a shift from sulphation toward glucuronidation may occur.32
Although phloretin aglycone was not included in the study (since it was not detected), metabolism of a range of flavonoids in cultured Caco-2 monolayers indicated that flavonoid-sulphates tend to be effluxed to the luminal side whereas flavonoid-glucuronides and flavonoid-sulphate-glucuronides pass predominantly to the basolateral side.33 The distribution of phloretin conjugates in ileal fluid and urine when ileostomists consumed cider8 is consistent with the in vitro data, suggesting that this generalisation does apply to phloretin metabolism in humans.
However, we found that the B* sulphate-only sub-profile dominated for the low producers on the low- (6 of 11) and high-PhA studies (5 of 9), and clearly at least for these individuals, efflux of phloretin-O-sulphate to the gut lumen was incomplete. The relevant efflux transporter has not been identified but Multidrug resistance protein 2 (MRP2/ABCC2) and Breast cancer resistance protein (BRCP/ABCG2) are possibilities34 and both have several single nucleotide polymorphisms35,36 which might contribute to this different behaviour.
Frequency plots of percentage glucuronidation presented in Fig. 3 are consistent with a bimodal distribution for phloretin metabolism although more detailed investigation on a larger number of volunteers is essential to evaluate this.
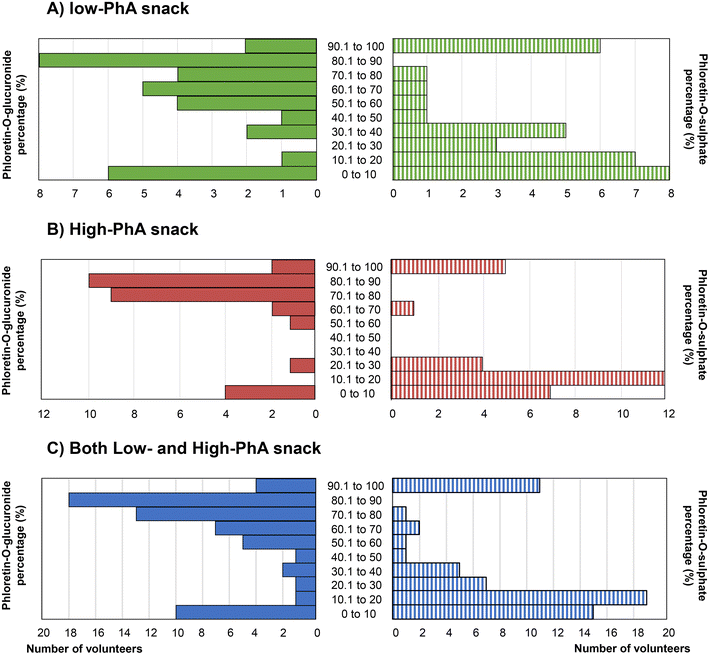 | ||
| Fig. 3 Bimodal distribution for phloretin-O-glucuronide and phloretin-O-sulphate after sustained intake of of low-phloretin apple snack (A), high-phloretin apple snack (B) or both combined (C). | ||
The glucuronide-conjugating UGT1A enzyme family has many polymorphisms,37 and phloretin is metabolised by hepatic UGT 1A1, 1A7, 1A8 and 1A9 in vitro25,38 but is a poor substrate for enteric UGT 1A10.39 The SULT enzyme responsible for sulphation of phloretin has not been identified but numerous SULT polymorphisms are known40 and these polymorphisms can be expected to contribute to these different patterns of urinary metabolites.
The balance between sulphation and glucuronidation is also affected by food deprivation, species, and sex41 of which only the latter is relevant in this study. It was observed that females dominate Profile A (64.1%) and males dominate the low excretion group (7 out of 11 low-PhA snack and 6 out of 9 high-PhA snack) after the sustained intake of apple snack, as it is shown in Table 2. There were no statistically significant differences in age range.
| % volunteers | Sex | Gradient excretion | ||||||
|---|---|---|---|---|---|---|---|---|
| Low-PhA snack | High-PhA snack | Total | % female | % male | % low | % medium | % high | |
| Profile A: participants with >50–100% of glucuronide conjugation. Profile B: participants with 0–50% of glucuronide conjugation (sulphate). In this calculation the double conjugate contributes equally to glucuronide and sulphate. Profile A*: subsample of participants with 100% phloretin-2′-O-glucuronide. Profile B*: subsample of participants with 100% phloretin-O-sulphate. | ||||||||
| Profile A | 51.5 | 75.9 | 62.9 | 64.1 | 35.9 | 10 | 90.9 | 85 |
| Profile A* | 18.2 | 3.40 | 11.3 | 42.9 | 57.1 | 10 | 9.10 | 15 |
| Profile B | 12.1 | 3.40 | 8.10 | 20.0 | 80.0 | 25 | 0 | 0 |
| Profile B* | 18.2 | 17.2 | 17.7 | 54.6 | 45.5 | 55 | 0 | 0 |
(iii) Temporal variation
Seven volunteers (L1–L7) participated in the low-PhA snack acute dose study and consumed the snack on two occasions six weeks apart. For volunteer L4 the value declined from 61% to 54% glucuronidation, but the metabolic profile was unchanged (see Table 3). For 6 out of 7, the percentage glucuronidation increased on the second occasion, changing the profile from B to A for three volunteers (L3, L5, L6).
| Volunteer | Sex | Molar 24-hour percentage glucuronide | Metabolic profile | ||||
|---|---|---|---|---|---|---|---|
| 1st acute | 2nd acute | Sustained | 1st acute | 2nd acute | Sustained | ||
| Low-PhA snack | |||||||
| L1 | F | 56 | 73 | 86 | A | A | A |
| L2 | F | 66 | 75 | 81 | A | A | A |
| L3 | M | 48 | 67 | 77 | B | A | A |
| L4 | F | 61 | 54 | 61 | A | A | A |
| L5 | M | 30 | 59 | 0 | B | A | B* |
| L6 | F | 33 | 56 | 89 | B | A | A* |
| L7 | F | 65 | 73 | 88 | A | A | A |
| High-PhA snack | |||||||
| H1 | M | 65 | 75 | 81 | A | A | A |
| H2 | M | 85 | 71 | 74 | A | A | A |
| H3 | F | 83 | 82 | 89 | A | A | A |
| H4 | M | 79 | 72 | 0 | A | A | B* |
| H5 | F | 67 | 70 | 81 | A | A | A |
| H6 | F | 76 | 77 | 80 | A | A | A |
| H7 | M | 75 | 78 | 80 | A | A | A |
| H8 | F | 86 | 84 | 86 | A | A | A |
Volunteer L3 at 48% glucuronidation had originally been only slightly below the A classification whereas L5 and L6 recorded much greater increases in glucuronidation (ca. 30 to >50%). Note, however, in the sustained study the high producer L6 recorded 89% glucuronidation and the low producer L5 recorded 100% sulphation (Table 1).
Eight volunteers (H1–H8, including one low, two medium and five high producers) similarly consumed the high-PhA snack. All recorded a profile A on both occasions with five volunteers (H3, H5, H6, H7, H8) showing marginal change in glucuronidation, volunteer H1 a clear increase and volunteers H2 and H4 clear decreases. For seven of the 8 volunteers, the glucuronidation percentage after the second acute intake was very close to that observed after the sustained intake. In contrast, volunteer H4, the only low producer to participate, recorded 79% and 72% in the acute study but zero glucuronidation in the sustained study.
Collectively, these observations demonstrate that the relative activity of phase-II conjugation pathways is not irrevocably fixed.
3.4. Comparison of plasma glucose and insulin values before and after sustained intake of the apple snacks
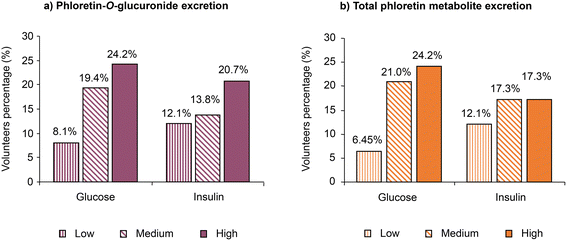
Pre- and post-prandial plasma glucose and insulin were determined for each volunteer at the start and end of the six-week sustained study for both the low-PhA and high-PhA snack groups. The data in Table 1 show a mixed response with reductions in plasma glucose (16 out of 33) and insulin (13 out of 31) for volunteers who consumed the low-PhA snack. For the high-PhA snack, the corresponding data for plasma glucose and insulin were 16 out of 29 and 14 out of 27, respectively. For the other volunteers there was either no change, or an increase in plasma glucose or insulin. Insulin data were not available for four volunteers. Note, however, that for 29 out of 62 volunteers only one parameter decreased and the other either increased or did not change indicating at most a weak biological effect.Nevertheless, the pooled data for both snack groups (Fig. 4A) indicate that the proportion of volunteers presenting a decreased plasma glucose or an insulin concentration at the end of the six-week sustained study increases in the sequence low, medium and high phloretin-O-glucuronide excretion. Similar results were also observed for the analysis of total phloretin metabolite excretion (Fig. 4B).
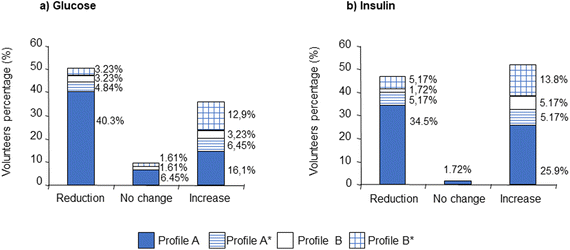
Fig. 5 presents data for changes in plasma glucose and insulin stratified for metabolite profile and shows clearly that there is little if any association between the profile and such reductions. This is consistent with reduction in plasma glucose being associated with interactions on the luminal surface of the duodenum (interaction with lactase–phloridzin hydrolase and blockade of SGLT1 and GLUT2) which occur prior to absorption of phloretin and phase-II conjugation.
It has been reported previously that when nine volunteers consumed on separate occasions 400 ml water, clear apple juice (lacking the fibrous components) and cloudy apple juice (retaining some of the apple pulp) delivering 25 g glucose, there were modest reductions in plasma glucose, plasma insulin, glucose-dependent insulinotropic polypeptide (GIP) and plasma glucagon-like peptide 1 (GLP1), which were tentatively attributed to greater contents of phloridzin (27 and 59 μmol), phloretin-xylosylglycoside (6.4 and 42 μmol), and caffeoylquinic acids (151 and 522 μmol) in the clear and cloudy apple juices, respectively. Collectively, these modest reductions were consistent with impairment of glucose absorption in the duodenum.42
The weaker response to the apple snacks reported here may reflect that in contrast to the apple juice study there was no control over the amount of glucose and glucose-yielding disaccharides consumed immediately before collection of the plasma samples, as would have been the case in a formal oral glucose tolerance test, and variation in the glucose load may have reduced the ability of the apple snack study to detect subtle effects associated with the excretion profile. It is also important to point out that the fibre contents of both apple snacks were very similar (ESI Table S3†) and such differences can be excluded as responsible for the effects seen.
4. Concluding remarks
This study demonstrates clearly a marked inter-individual variation in the excretion of phloretin phase-II conjugates following consumption of apple snacks prepared from Granny Smith and Redlove apples delivering 39.5 and 103 μmol phloretin glycosides, respectively. This inter-individual variation must arise from differences in the ability either to hydrolyse the phloretin glycosides or to absorb the released phloretin, or both. Overall, absorption estimated as phloridzin equivalents for 62 volunteers varied almost 70-fold ranging from 0.1% to 6.94% of phloretin glycoside intake. Inter-individual variation in the percentage absorption of phloretin was not surprising but at 70-fold the magnitude was unexpected. As discussed in the introduction, the first step is hydrolysis of phloretin glycosides by LPH and any impairment in that process will reduce absorption.Congenital lactase deficiency is known, although rare in Southern Europe. Lactase non-persistence is more common, with a recorded prevalence of 32.5% in Galicia, as quantified by a lactose breath hydrogen test conducted with 850 individuals. In the Galician population incidence increased with age up to 60 years (34.1% aged 19–24 and 38.7% aged 25–60).43 A lower incidence has been reported in the Barcelona population, 13–15%, being more representative of the volunteers participating in this study44 but lactase non-persistence is plausibly an important factor contributing to this substantial inter-individual variation in phloretin uptake. In European populations two single nucleotide polymorphisms (C/T-13910 and G/A-22108) have been characterised in the encoding gene and lactose non-persistence occurs if both C/C-13910 and G/G-22108 are present.44
Note also that it has been reported that phloridzin in apple juice is rapidly hydrolysed by bacteria present in crude human saliva and the authors drew attention to inter-individual variations in the extent of this hydrolysis.45 So, oral hydrolysis of phloridzin could also compromise the amount of phloridzin reaching the duodenum and available to blockade SGLT1 and variations in the load and competence of oral bacteria might be another factor contributing to the relatively weak and variable effects on plasma glucose and insulin recorded here.
Although a true dose–response study (using a single group of volunteers) was not performed, it was observed that the medium and high producers in the high-PhA snack group showed a greater absolute excretion (1.06–2.85 vs. 0.46–1.70 μmol and 2.80–6.12 vs. 1.15–2.74 μmol), whereas there was no difference for the low producers (0.1–0.89 vs. 0.12–0.96 μmol). When the excretion of phloretin phase-II conjugates is expressed as a percentage of the phloretin glycoside intake it is noticeable that there is a trend to slightly lower values in the high-PhA snack group (0.30–2.40% vs. 0.1–0.86%, 1.16–4.30% vs. 1.03–2.77% and 2.91–6.94% vs. 2.72–5.94%, respectively) implying that absorption might be approaching saturation at intakes much above 100 μmol phloretin glycosides (ESI Table S6†).
Volunteers were assigned to low, medium and high excreting groups and two groups depending on whether glucuronide conjugation (A) or sulphate conjugation (B) dominated in the urine plus subgroups A* and B* when the mixed conjugate was not produced. The stratification was independent of the sex of the volunteer; however, males were dominant in low producer groups.
For ca. 74% of the volunteers phloretin-O-glucuronide was the dominant urinary metabolite, especially at the higher phloretin glycoside intake and for higher producers independent of phloretin glycoside intake. Sulphate conjugation assumed greater significance for the remaining volunteers especially for low producers independent of intake. Indeed, for 6 of 11 volunteers at the lower intake and 5 of 9 at the higher intake a phloretin-O-sulphate was the only phase-II conjugate detected. It has not been characterised at regio-isomer level. Only one volunteer, a high producer who consumed the low-PhA snack, recorded 100% glucuronidation.
The data suggest possible low and high sulphate metabotypes but because the urinary metabolite profile may vary temporally it is not possible to decide unequivocally. The factor(s) responsible for this temporal variation are obscure but deserve further investigation.
Overall, there were inconsistent effects on post-prandial plasma glucose and insulin concentrations but there was a tendency for decreases in these parameters to be associated with higher excretion of phloretin phase-II conjugates. This observation in combination with the results of previous studies suggests that apples and apple products do have a modest potential for health protection at least for those individuals capable of greater phloretin absorption. Further investigations in larger cohorts are required to confirm these results and to identify the absorption-limiting mechanisms. It would be advantageous if future volunteers were phenotyped in respect of all relevant variables including phase II metabolism, and screened for lactose intolerance and the phloridzin-hydrolysing activity of their oral bacteria.
Author contributions
AM: conceptualization, formal analysis, investigation, methodology, supervision, and writing—original draft and review; MPR: funding acquisition, and project administration; AP: formal analysis, investigation, and methodology; RS: funding acquisition, and project administration; MNC: conceptualization, supervision, and writing—review; LR: investigation, supervision, and writing—review, and editing.All authors approved the final version of the manuscript to be submitted.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This study was supported by the Spanish Ministry of Industry, Economy and Competitiveness through the AGL2016-76943-C2-1-R and AGL2016-76943-C2-2-R projects (co-funded by the European Social Fund, European Union).References
- A. D. Hudson, A comprehensive review of apples and apple components and their relationship to human health, Adv. Nutr., 2011, 2, 408–420 CrossRef PubMed.
- D. Bars-Cortina, A. Macià, I. Iglesias, M. P. Romero and M. J. Motilva, Phytochemical profiles of new red-fleshed apple varieties compared with traditional and new white fleshed varieties, J. Agric. Food Chem., 2017, 65, 1685–1696 CrossRef PubMed.
- S. Behzad, A. Sureda, D. Barreca, S. F. Nabavi, L. Rastrelli and S. M. Nabavi, Health effects of phloretin: from chemistry to medicine, Phytochem. Rev., 2017, 16, 527–533 CrossRef CAS.
- G. Williamson, Effects of Polyphenols on Glucose-Induced Metabolic Changes in Healthy Human Subjects and on Glucose Transporters, Mol. Nutr. Food Res., 2022, e2101113 CrossRef PubMed.
- H. Deusser, D. Rogoll, W. Scheppach, A. Volk, R. Melcher and E. Richling, Gastrointestinal absorption and metabolism of apple polyphenols ex vivo by the pig intestinal mucosa in the Using chamber, Biotechnol. J., 2013, 8, 363–370 CrossRef CAS PubMed.
- V. Crespy, C. Morand, C. Besson, C. Manach, C. Demigne and C. Remesy, Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats, J. Nutr., 2001, 131, 2109–2114 CrossRef CAS PubMed.
- G. Borges, M. E. Lean, S. A. Roberts and A. Crozier, Bioavailability of dietary (poly)phenols: a study with ileostomists to discriminate between absorption in small and large intestine, Food Funct., 2013, 4, 754–762 RSC.
- S. C. Marks, W. Mullen, G. Borges and A. Crozier, Absorption, metabolism, and excretion of cider dihydrochalcones in healthy humans and subjects with an ileostomy, J. Agric. Food Chem., 2009, 57, 2009–2015 CrossRef CAS PubMed.
- K. Trošt, M. M. Ulaszewska, J. Stanstrup, D. Albanese, C. De Filippo, K. M. Tuohy, F. Natella, C. Scaccini and F. Mattivi, Host: Microbiome co -metabolic processing of dietary polyphenols – An acute, single blinded, cross-over study with different doses of apple polyphenols in healthy subjects, Food Res. Int., 2018, 112, 108–128 CrossRef PubMed.
- S. Yuste, A. Macià, I. A. Ludwig, M. P. Romero, S. Fernández-Castillejo, Ú. Catalán, M. J. Motilva and L. Rubió, Validation of dried blood spot cards to determine apple phenolic metabolites in human blood and plasma after an acute intake of red-fleshed apple snack, Mol. Nutr. Food Res., 2018, 62, 1800623 CrossRef PubMed.
- A. M. Jenner, J. Rafter and B. Halliwell, Human fecal water content of phenolics. The extent of colonic exposure to aromatic compounds, Free Radicals Biol. Med., 2005, 38, 763–772 CrossRef CAS PubMed.
- M. N. Clifford, I. B. Jagabath, I. A. Ludwig and A. Crozier, Chlorogenic acids and the acyl-quinic acids: Discovery, biosynthesis, bioavailability, and bioactivity, Nat. Prod. Rep., 2017, 34, 1391–1421 RSC.
- R. Landberg, C. Manach, F. M. Kerckhof, A. M. Minihane, R. N. Saleh, B. De Roos, F. Tomás-Barberán, C. Morand and T. Van de Wiele, Future prospects for dissecting inter-individual variability in the absorption, distribution and elimination of plant bioactives of relevance for cardiometabolic endpoints, Eur. J. Nutr., 2019, 58, 21–36 CrossRef PubMed.
- D. Milenkovic, C. Morand, A. Cassidy, A. Konic-Ristic, F. Tomás-Barberán, J. M. Ordovas, P. Kroon, R. De Catarina and A. Rodríguez-Mateos, Interindividual Variability in Biomarkers of Cardiometabolic Health after Consumption of Major Plant-Food Bioactive Compounds and the Determinants Involved, Adv. Nutr., 2017, 8, 558–570 CrossRef PubMed.
- C. Manach, D. Milenkovic, T. Van de Wiele, A. Rodríguez-Mateos, B. de Roos, M. T. Garcia-Conesa, R. Landberg, E. R. Gibney, M. Heinonen, F. Tomás-Barberán and C. Morand, Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction, Mol. Nutr. Food Res., 2017, 61, 1600557 CrossRef PubMed.
- T. Saenger, F. Hübner and H. U. Humpf, Short-term biomarkers of apple consumption, Mol. Nutr. Food Res., 2017, 61, 1600629 CrossRef PubMed.
- M. Ulaszewska, N. Vázquez-Manjarrez, M. Garcia-Aloy, R. Llorach, F. Mattivi, L. O. Dragsted, G. Praticò and C. Manach, Food intake biomarkers for apple, pear, and stone fruit, Genes Nutr., 2018, 13, 1–16 CrossRef PubMed.
- S. Yuste, I. A. Ludwig, L. Rubió, M. P. Romero, A. Pedret, R. M. Valls, R. Solà, M. J. Motilva and A. Macià, In vivo transformation of (poly)phenols and anthocyanins of red-fleshed apple and identification of intake biomarkers, J. Funct. Foods, 2019, 55, 146–155 CrossRef CAS.
- L. Rubió, M. P. Romero, R. Solà, M. J. Motilva, M. N. Clifford and A. Macià, Variation in the Methylation of Caffeoylquinic Acids and Urinary Excretion of 3′-methoxycinnamic acid-4′-Sulfate After Apple Consumption by Volunteers, Mol. Nutr. Food Res., 2021, 65, 2100471 CrossRef PubMed.
- S. Yuste, I. A. Ludwig, M. P. Romero, C. Piñol-Felis, Ú. Catalán, A. Pedret, R. M. Valls, S. Fernández-Castillejo, M. J. Motilva, A. Macià and L. Rubió, Metabolic fate and cardiometabolic effects of phenolic compounds from red-fleshed apple in hypercholesterolemic rats: A comparative study with common white-fleshed apple. The AppleCOR study, Mol. Nutr. Food Res., 2021, 65, 2001225 CrossRef CAS PubMed.
- A. Macià, M. P. Romero, S. Yuste, I. A. Ludwig, A. Pedret, R. M. Valls, P. Salamanca, R. Solà, M. J. Motilva and L. Rubió, Phenol metabolic fingerprint and selection of intake biomarkers after acute and sustained consumption of red-fleshed apple versus common apple in humans. The AppleCOR study, Food Chem., 2022, 384, 132612 CrossRef PubMed.
- M. N. Clifford and N. Kuhnert, LC–MS characterisation and quantification of known and unknown (poly)phenol metabolites—possible pitfalls and their avoidance, Mol. Nutr. Food Res., 2022, 66, e2101013 CrossRef PubMed.
- H. Bergmann, S. Triebel, K. Kahle and E. Richling, The Metabolic Fate of Apple Polyphenols in Humans, Curr. Nutr. Food Sci., 2010, 6, 19–35 CrossRef CAS.
- S. Burda, W. Oleszek and C. Y. Lee, Phenolic compounds and their changes in apples during maturation and cold storage, J. Agric. Food Chem., 1990, 38, 945–948 CrossRef CAS.
- K. Kahle, W. Huemmer, M. Kempf, W. Scheppach, T. Erk and E. Richling, Polyphenols are intensively metabolized in the human gastrointestinal tract after apple juice consumption, J. Agric. Food Chem., 2007, 55, 10605–10614 CrossRef CAS PubMed.
- K. Trošt, M. M. Ulaszewska, J. Stanstrup, D. Albanese, C. De Filippo, K. M. Tuohy, F. Natella, C. Scaccini and F. Mattivi, Host: Microbiome co -metabolic processing of dietary polyphenols – An acute, single blinded, cross-over study with different doses of apple polyphenols in healthy subjects, Food Res. Int., 2018, 112, 108–128 CrossRef PubMed.
- P. Hilt, A. Schieber, C. Yildirim, G. Arnold, I. Klaiber, J. Conrad, U. Beifuss and R. Carle, Detection of phloridzin in strawberries (Fragaria x ananassa Duch.) by HPLC–PDA–MS/MS and NMR spectroscopy, J. Agric. Food Chem., 2003, 51, 2896–2899 CrossRef CAS PubMed.
- E. Hvattum, Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionisation tandem mass spectrometry and diode-array detection, Rapid Commun. Mass Spectrom., 2002, 16, 655–662 CrossRef CAS PubMed.
- K. Redeuil, C. Smarrito-Menozzi, P. Guy, S. Rezzi, F. Dionisi, G. Williamson, K. Nagy and M. Renouf, Identification of novel circulating coffee metabolites in human plasma by liquid chromatography-mass spectrometry, J. Chromatogr. A, 2011, 1218, 4678–4688 CrossRef CAS PubMed.
- A. Nishioka, E. de Castro Tobaruela, L. N. Fraga, F. A. Tomás-Barberán, F. M. Lajolo and N. M. A. Hassimotto, Stratification of volunteers according to flavanone metabolite excretion and phase-II metabolism profile after single doses of “Pera” Orange and “Moro” blood orange juices, Nutrients, 2021, 13, 473–494 CrossRef CAS PubMed.
- M. S. DuPont, R. N. Bennett, F. A. Mellon and G. Williamson, Polyphenols from alcoholic apple cider are absorbed, metabolized and excreted by humans, J. Nutr., 2002, 132, 172–175 CrossRef CAS PubMed.
- H. Koster, I. Halsema, E. Scholtens, M. Knippers and G. J. Mulder, Dose-dependent shifts in the sulfation and glucuronidation of phenolic compounds in the rat in vivo and in isolated hepatocytes: The role of saturation of phenol sulfotransferase, Biochem. Pharmacol., 1981, 30, 2569–2575 CrossRef CAS PubMed.
- R. Barrington, G. Williamson, R. N. Bennett, B. D. Davis, J. S. Brodbelt and P. A. Kroon, Absorption, conjugation and efflux of the flavonoids, kaempferol and galangin, using the intestinal CaCo-2/TC7 cell model, J. Funct. Foods, 2009, 1, 74–87 CrossRef CAS PubMed.
- E. Järvinen, F. Deng, W. Kiander, A. Sinokki, H. Kidron and N. Sjöstedt, The Role of Uptake and Efflux Transporters in the Disposition of Glucuronide and Sulfate Conjugates, Front. Pharmacol., 2022, 1213, 802539 CrossRef PubMed.
- B. Poonkuzhali, J. Lamba, S. Strom, A. Sparreboom, K. Thummel, P. Watkins and E. Schuetz, Association of breast cancer resistance protein/ABCG2 phenotypes and novel promoter and intron 1 single nucleotide polymorphisms, Drug Metab. Dispos., 2008, 36, 780–795 CrossRef CAS PubMed.
- S. Sookoian, G. Castaño, T. F. Gianotti, C. Gemma and C. J. Pirola, Polymorphisms of MRP2 (ABCC2) are associated with susceptibility to nonalcoholic fatty liver disease, J. Nutr. Biochem., 2009, 20, 765–770 CrossRef CAS PubMed.
- D. G. Hu, P. I. Mackenzie, R. A. McKinnon and R. Meech, Genetic polymorphisms of human UDP-glucuronosyltransferase (UGT) genes and cancer risk, Drug Metab. Rev., 2016, 48, 47–69 CrossRef CAS PubMed.
- N. K. Basu, M. Ciotti, M. S. Hwang, L. Kole, P. S. Mitra, J. W. Cho and I. S. Owens, Differential and Special Properties of the Major Human UGT1-encoded Gastrointestinal UDP-glucuronosyltransferases Enhance Potential to Control Chemical Uptake, J. Biol. Chem., 2004, 279, 1429–14419 CrossRef CAS PubMed.
- P. I. Mackenzie, P. A. Gregory, R. H. Lewinsky, S. N. Yasmin, T. Height, R. A. McKinnon and D. A. Gardner-Stephen, Polymorphic variations in the expression of the chemical detoxifying UDP glucuronosyltransferases, Toxicol. Appl. Pharmacol., 2005, 207, 77–83 CrossRef CAS PubMed.
- K. Kurogi, M. I. Rasool, F. A. Alherz, A. A. El Daibani, A. F. Bairam, M. S. Abunnaja, S. Yasuda, L. J. Wilson, Y. Hui and M. C. Liu, SULT genetic polymorphisms: physiological, pharmacological and clinical implications, Expert Opin. Drug Metab. Toxicol., 2021, 17, 767–784 CrossRef CAS PubMed.
- C. Manach, A. Scalbert, C. Morand, C. Rémésy and L. Jiménez, Polyphenols: Food sources and bioavailability, Am. J. Clin. Nutr., 2004, 79, 727–747 CrossRef CAS PubMed.
- K. L. Johnston, M. N. Clifford and L. M. Morgan, Possible role for apple juice phenolic compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans, J. Sci. Food Agric., 2002, 82, 1800–1805 CrossRef CAS.
- R. Leis, R. Tojo, P. Pavon and A. Douwes, Prevalence of lactose malabsorption in Galicia, J. Pediatr. Gastroenterol. Nutr., 1997, 25, 296–300 CrossRef CAS PubMed.
- S. Ugidos-Rodríguez, M. C. Matallana-González and M. C. Sánchez-Mata, Lactose malabsorption and intolerance: A review, Food Funct., 2018, 9, 4056–4068 RSC.
- K. Kahle, M. Kempf, P. Schreier, W. Scheppach, D. Schrenk, T. Kautenburger, D. Hecker, W. Huemmer, M. Ackermann and E. Richling, Intestinal transit and systemic metabolism of apple polyphenols, Eur. J. Nutr., 2011, 50, 507–522 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo02985a |
| This journal is © The Royal Society of Chemistry 2023 |