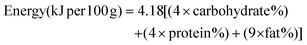Open Access Article
Open Access ArticleDesigning sustainable weaning foods for developing countries: not only a matter of nutrients†
Luigi
Moriconi
a,
Elena
Vittadini
a,
Anita R.
Linnemann
 b,
Vincenzo
Fogliano
b,
Vincenzo
Fogliano
 b and
Ruth T.
Ngadze
b and
Ruth T.
Ngadze
 *b
*b
aSchool of Biosciences and Veterinary Medicine, Università degli Studi di Camerino, Camerino (MC), Italy
bFood Quality and Design group, Wageningen University and Research, Wageningen, The Netherlands. E-mail: ruth.ngadze@wur.nl
First published on 25th September 2023
Abstract
Blended complementary foods from cereals and high-protein sources are used worldwide to cope with infants’ malnutrition. However, the usefulness of the food matrix during traditional processes reaches suboptimal effectiveness due to cereal gelatinization and viscosity, which reduce consumption. The interplay between nutritional and physical qualities needed for weaning children presents further significant constraints. A combination of processing methods can improve and optimize the overall product quality. This paper investigated the nutritional, functional, and anti-nutritional factors of a complementary infant porridge made by combining fermented sorghum flour with germinated bottle gourd seed flour. Overall, the combination improved the functional and physical properties of the porridge suitable for children of 10 months and over. A serving of 100 g would contribute 115–145% and 23–31% of the recommended nutritional intake of protein and energy, respectively, for low breast milk energy between 6–24 months. The results demonstrate that a combination of strategies and technologies are needed to balance nutritional and physical quality.
1 Introduction
Climate resilient crops (CRCs) in the small grains family, such as sorghum, millets, and amaranth, are often used to make weaning and complementary foods in Sub-Saharan Africa (SSA). These crops deserve special research attention in view of the current climate change. Within the group of CRCs, sorghum is particularly promising due to its nutritional superiority and functional qualities, which include bioactive compounds, dietary fibre, vitamin B, and minerals (potassium, iron, zinc).1,2 Sorghum is an energy-dense cereal but is limited in essential amino acids and protein digestibility. Hence, its monotonous consumption has been associated with protein-energy (PEM) malnutrition in SSA.3 In PEM, consumption of both energy and protein is insufficient leading to a form of hidden hunger and stunted growth in other cases kwashiorkor or marasmus.4The supply of animal protein is insufficient to meet the protein needs in resource-limited communities, stimulating the research to efficiently combat PEM via cost-effective strategies. Using protein from locally available underutilized high-protein oilseeds and legumes is the most pragmatic choice. Food-to-food fortification (FtFF) by blending nutrient-limited cereal-based traditional complementary foods (TCFs) with nutrient-dense ingredients, has been advocated as such a cost-effective strategy.5 In this respect,6 suggested adding oil to cereal-based complementary foods to improve energy and nutrient density. Furthermore, oil reduces the high glutinous nature of the porridges.7 So far, many reports investigated the effect of legume incorporation in complementary food but studies on promising oil-seed protein have received little attention, especially regarding the ubiquitous cucurbits, belonging to the Cucurbitaceae family and have good potential for food blending. To illustrate, seeds of the bottle gourd (Lagenaria siceraria), also known as calabash, bottle or white-flowered gourd, contain essential amino acids, i.e., threonine, valine, leucine, lysine, methionine and tyrosine, at levels higher than the Food and Agriculture Organization recommendations.8 Additionally, palmitic acid, linoleic acid, oleic acid and stearic acid have been identified in bottle gourd seeds.9 Besides the protein value, adding bottle gourd seed flour to porridge is a practical option for increasing its fat content. Repurposing bottle gourd seeds by integrating them into nutrient-limiting complementary foods has the potential to enhance product functionality and nutritional value.
A weaning cereal porridge with 20% dry matter can supply the Recommended Daily Intake (RDI) of 3000 kJ of energy in four feedings of 250 ml each.10 Unfortunately, concentrated weaning cereal porridges become highly viscous, making them difficult to swallow and digest for infants aged 6 to 12 months.11 To enable consumption, water is added to the viscous product, resulting in over-dilution and hence loss in energy-protein density causing subsequent malnutrition.12
Other cost-effective strategies to efficiently combat malnutrition are based on food processing methods. Traditional food bioprocessing methods such as steeping, germination, malting and fermentation can improve the nutritional value, bio-accessibility of nutrients, and reduce viscosity and antinutritional compound content.13,14 The use of optimized methods has the potential to further enhance nutrition and physical product quality and could be effective in the processing of complementary infant foods. Especially germination and fermentation are widely used in sorghum-producing regions in processing of weaning foods.15 Combinations of fermentation and germination, when applied to different ingredients of the same product, are expected to improve product quality even more.
To date, a global framework of the pathway of optimization of cereal-legume complementary food exists and the nutritional, functional and microbial characteristics of sorghum porridges have been widely investigated.15,16 Still unexplored is how a fermentation and germination combination on more than one ingredient impacts the nutritional density and the corresponding functionality of a traditionally processed complementary food. Therefore, this research seeks to answer these questions: (i) what is the synergistic effect of processing technologies (i.e., fermentation and germination) on nutrient density and physical properties of traditional complementary foods, and (ii) can complementary foods formulated using treated oilseed ingredients and state-of-the-art food processing methods meet the complementary food age recommendation?
2 Methodology
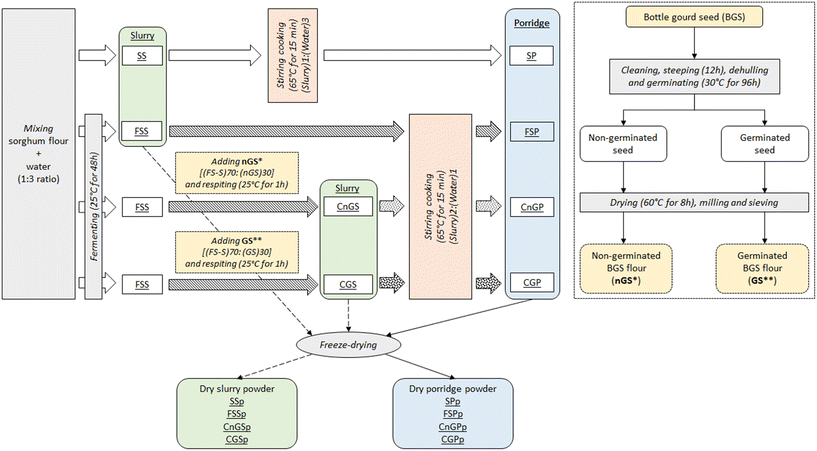
2.1 Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio using a regular cooking spoon until uniform consistency. Next, the slurry was incubated at 25 °C for 48 h in the dark for spontaneous fermentation.
3 ratio using a regular cooking spoon until uniform consistency. Next, the slurry was incubated at 25 °C for 48 h in the dark for spontaneous fermentation.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio (16.7% solids) to obtain a homogenous, lump-free slurry. The slurry was then either immediately cooked (unfermented porridge) or subjected to spontaneous fermentation (as described in section 2.1.2) and then cooked. To obtain blended samples, either non-germinated or germinated bottle gourd seed (BGS) flour was added to the fermented slurry. The four slurries (i.e., unfermented, fermented, fermented with non-germinated BGS, and fermented with germinated BGS) were heated to 65 °C on a heating element at 300 °C while stirring using a magnetic and an automatic stirrer (IKA eurostar 20 digital) rotating at 200 and 65 rpm, respectively, to ensure proper mixing of the whole porridge. Part of the cooked porridge was then cooled in a water bath to 40 °C and directly analysed for viscosity and nutritional properties, while the remainder was instantly freeze-dried for further analysis of functional properties (2 samples per analysis with 3 replicates per sample).
3 ratio (16.7% solids) to obtain a homogenous, lump-free slurry. The slurry was then either immediately cooked (unfermented porridge) or subjected to spontaneous fermentation (as described in section 2.1.2) and then cooked. To obtain blended samples, either non-germinated or germinated bottle gourd seed (BGS) flour was added to the fermented slurry. The four slurries (i.e., unfermented, fermented, fermented with non-germinated BGS, and fermented with germinated BGS) were heated to 65 °C on a heating element at 300 °C while stirring using a magnetic and an automatic stirrer (IKA eurostar 20 digital) rotating at 200 and 65 rpm, respectively, to ensure proper mixing of the whole porridge. Part of the cooked porridge was then cooled in a water bath to 40 °C and directly analysed for viscosity and nutritional properties, while the remainder was instantly freeze-dried for further analysis of functional properties (2 samples per analysis with 3 replicates per sample).
2.2 Characterization of freeze-dried sorghum porridge
2.3 Porridge biochemical analysis
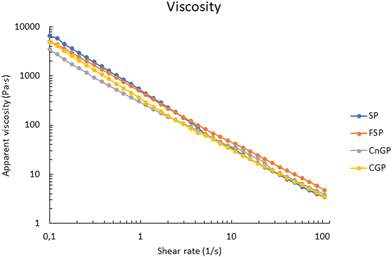
2.4 Viscosity
Viscosity was measured using a GmbH viscometer (Anton Paar, GmbH, Austria) equipped with a 50 mm measuring plate and a 1 × 0.5, Groove probe (A-8054 Graz, Anton Paar, GmbH, Austria). Approximately 10 g of porridge was placed on the rheometer plate, gently squeezed to 1 mm thickness and subjected at variable rates increasing from 0.1 to 1000 s−1 at a controlled temperature of 40 °C using a Peltier plate. The test, shear rate range, plate and probe settings were chosen following previous methods.122.5 Simulated in vitro gastrointestinal digestion
Simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) were used to carry out the in vitro digestion according to previous methods.19 Aliquots were taken at different time points of gastric and intestinal digestion, i.e., SGP: 0, 2 h and SIP at 15, 30, 60 and 120 min. Enzyme inactivation was performed by the addition of absolute ethanol for starch digestion then rested for 30 min at room temperature before centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. Enzyme inactivation for protein digestion was performed by the addition of 25 μL pefabloc© (0.1 M). Samples were left for 30 min and at room temperature, after which 0.83 mL 5% TCA was added, and then centrifuged at 10
000g for 10 min. Enzyme inactivation for protein digestion was performed by the addition of 25 μL pefabloc© (0.1 M). Samples were left for 30 min and at room temperature, after which 0.83 mL 5% TCA was added, and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. Supernatants were collected and stored at (−20° C) for further analysis. As a blank, 5 ml MilliQ water was digested.
000g for 10 min. Supernatants were collected and stored at (−20° C) for further analysis. As a blank, 5 ml MilliQ water was digested.
2.6 Statistical analysis
All results are given as means ± standard deviation of three independent determinations of each parameter. One-way analysis of variance (ANOVA) with the Tukey test was used to compare significant differences between means of triplicate readings. Pearson correlation coefficients were used to determine relationships among variables, differences were considered to be significant at p < 0.05. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS v23.0) software.3 Results and discussion
3.1 Effect of process combinations on functional properties of freeze-dried porridges
| Parameters | WAC (g g−1) | WSI (g g−1) | OAC (g g−1) | Bulk density (g ml−1) | pH | α-Amylase activity |
|---|---|---|---|---|---|---|
| Different superscripts in the same column denote a statistically significant difference (P < 0.05). SF: sorghum flour, FSF: fermented sorghum flour, CnG: blended with non-germinated seed, CG: blended with germinated seed. | ||||||
| SF | 5.38 ± 0.75b | 3.01 ± 0.20a | 1.70 ± 0.04a | 0.86 ± 0.03b | 6.33 ± 0.03c | 0.31 ± 0.15a |
| FSF | 5.02 ± 0.17a,b | 4.74 ± 1.83b | 1.92 ± 0.11a | 0.71 ± 0.02a | 3.84 ± 0.07a | 3.37 ± 1.33b |
| CnG | 4.40 ± 0.33a | 5.57 ± 0.82b,c | 1.72 ± 0.04a | 0.74 ± 0.06a | 4.42 ± 0.01b | 3.12 ± 0.44b |
| CG | 4.94 ± 0.26a,b | 6.94 ± 0.40c | 1.84 ± 0.08a | 0.71 ± 0.03a | 4.51 ± 0.05b | 2.84 ± 0.42b |
Oil absorption capacity (OAC) is the binding of fat by the nonpolar side chain of proteins that bind the oil hydrocarbon chains.22 OAC was not significantly different among samples. The results contrast with findings from other research.20 The rate of oil absorption was expected to be higher for the combined processed samples since the samples had higher protein contents. A possible explanation is that the protein's conformational characteristics, polarity, and degree of interaction with oil affected the absorption capacities. Due to specific fermentation and germination conditions, changes in the chemical composition may affect the spatial arrangement of molecules and therefore the functional properties of flours. A higher OAC would be useful in infant food formulation where an oil-holding property is an important consideration because it is an indication of flavor retention and increase of the mouth feel.
3.2 Effect of flour blends on biochemical properties
The proximate composition and estimated protein and energy intakes of infants (6–24 months) of blended fermented sorghum porridges are shown in Table 2.| Porridge type | Nutritional composition (g per 100 g) | kJ per 100 g | Energy intake (kJ day−1) | Protein intake (g day−1) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture | Fibre | Ash | Protein | Fat | Carbohydrate | Energy | 6–8 months | 9–11 months | 12–24 months | 6–8 months | 9–11 months | 12–24 months | |
| Different superscript in the same column denotes a statistically significant difference (P < 0.05). nGS: non-germinated seed flour, GS: germinated seed flour, SP: sorghum porridge FSP: fermented sorghum porridge (48 h fermentation), CnGP: blended sorghum porridge (48 h fermentation + non-germinated seed flour), CGP: blended sorghum porridge (48 h fermentation + germinated seed flour), BME: breast milk energy, RDI: recommended daily intake. Estimated protein and energy intakes were calculated using 16.67 g solid contents per 100 g porridge at 3 meals per day for functional gastric capacities of 249 g, 285 g and 345 g per meal for aged 6–8, 9–11 and 12–24 months children respectively.39 | |||||||||||||
| SP | 9.43 ± 1.06c | 1.92 ± 0.36a | 0.25 ± 0.13a | 10.63 ± 0.4a | 2.04 ± 0.12a | 74.37 ± 1.06c | 1497.8 ± 20.3a | 1865.1 | 2134.8 | 2584.2 | 13.24 | 15.15 | 18.35 |
| FSP | 6.84 ± 1.52b | 1.91 ± 0.12a | 0.83 ± 0.99a | 9.84 ± 2.2a | 2.81 ± 0.85a | 76.40 ± 2.15c | 1547.6 ± 40.7b | 1927.2 | 2205.8 | 2670.2 | 12.25 | 14.02 | 16.97 |
| CnGP | 4.18 ± 1.40a | 1.78 ± 0.19a | 1.08 ± 0.19a | 17.94 ± 0.96b | 14.86 ± 1.01c | 58.66 ± 1.35a | 1839.6 ± 44.4d | 2290.8 | 2622.0 | 3173.9 | 22.34 | 25.57 | 30.96 |
| CGP | 3.69 ± 0.19a | 2.08 ± 0.75a | 1.61 ± 1.30a | 18.25 ± 0.82b | 10.68 ± 1.21b | 62.50 ± 0.74b | 1751.7 ± 28.3c | 2181.4 | 2496.7 | 3022.4 | 22.72 | 26.01 | 31.48 |
| nGS | ND | 10.53 ± 1.54b | ND | 35.63 ± 0.82c | 48.55 ± 2.05d | ND | ND | ND | ND | ND | ND | ND | ND |
| GS | ND | 14.8 ± 2.55c | ND | 35.18 ± 2.15c | 36.69 ± 0.82e | ND | ND | ND | ND | ND | ND | ND | ND |
| RDI Low BME | 2310.0 | 2933.0 | 4301.0 | 5.20 | 6.70 | 9.10 | |||||||
| RDI Average BME | 1490.0 | 2004.0 | 3230.0 | 2.00 | 3.10 | 5.00 | |||||||
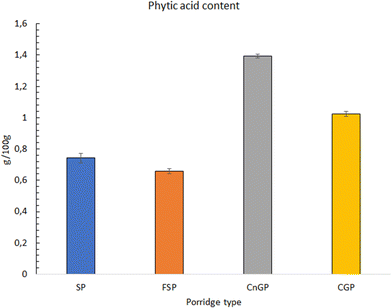
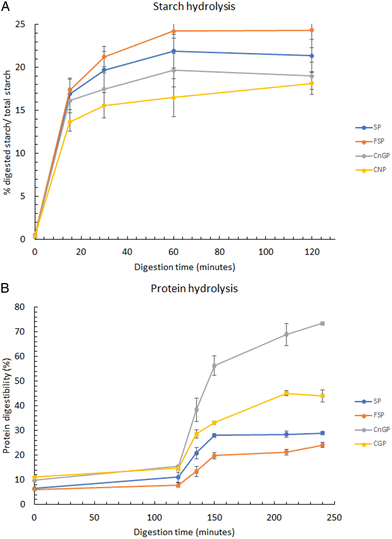
3.3 In vitro digestibility of porridges
4 Conclusion and recommendations
The combination of the traditional processing technologies of fermentation and germination improved the functional properties of the flour blend and subsequent nutritional and physical properties, cementing their applicability in infant complementary foods production. Neither fermentation nor germination alone is optimal in improving the functionalities of sorghum and bottle gourd seed flour blends. The combined effect of the “processing methods” and the “co-ingredient macronutrients” is a useful tool for further exploration of the design of affordable and diversified new complementary infant foods. Before undertaking in vivo studies, we recommend careful consideration of (i) interrelationships of processes when using highly nutritious underutilized crops from different regions, (ii) functionalities of various ingredients and target compounds – protein, fat and minerals (notably Fe and Zn) and (iii) designing a traditional complementary food using combinations of processing and raw materials to suit infants below the age of 10 months.Author contributions
Luigi Moriconi: formal analysis, investigation, data curation, writing – review & editing. Elena Vittadini: funding acquisition, supervision, writing – review & editing. Anita R. Linnemann: conceptualization, supervision, visualization, writing – review & editing. Vincenzo Fogliano: funding acquisition, resources, supervision, writing – review & editing. Ruth T. Ngadze: project administration, conceptualization, methodology, supervision, visualization, writing – original draft, review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors would like to thank Nawa Penparkgoon for the help with the experiments.References
- O. Ibidapo, F. Henshaw, T. Shittu and W. Afolabi, Bioactive components of malted millet (Pennisetum glaucum), Soy Residue “okara” and wheat flour and their antioxidant properties, Int. J. Food Prop., 2019, 22, 1886–1898 CrossRef CAS
.
- K. N. Rai, C. L. L. Gowda, B. V. S. Reddy and S. Sehgal, Adaptation and potential uses of sorghum and pearl millet in alternative and health foods, Compr. Rev. Food Sci. Food Saf., 2008, 7, 320–396 CrossRef
.
- M. A. Al-Gashanin and E. Y. Ghazwani, Knowledge, Attitude, and Practice of Weaning among Mothers in Najran Region, Saudi Arabia, 2021, J. Nutr. Metab., 2022, 6073878 Search PubMed
.
- F. K. A. Nightingale, S. Sarathi, V. Hemavathy, J. A. Monisha and B. Pandian, Protein Energy Malnutrition-an Overview, Cardiometry, 2023, 585–588 Search PubMed
.
- J. Kruger, J. R. N. Taylor, M. G. Ferruzzi and H. Debelo, What is food–to–food fortification? A working definition and framework for evaluation of efficiency and implementation of best practices, Compr. Rev. Food Sci. Food Saf., 2020, 19, 3618–3658 CrossRef
.
- M. Arimond, S. Abbeddou, C. Kumwenda, H. Okronipa, J. Hemsworth, E. Y. Jimenez, E. Ocansey, A. Lartey, U. Ashorn, S. Adu-Afarwuah, S. A. Vosti, S. Y. Hess and K. G. Dewey, Impact of small quantity lipid-based nutrient supplements on infant and young child feeding practices at 18 months of age: results from four randomized controlled trials in Africa, Matern. Child Nutr., 2017, 13, 1–11 Search PubMed
.
-
FAO/WHO/UNU, Energy and protein requirements. World Health Organization Technical Report Series 724, Geneva, Switzerland, 1991 Search PubMed
.
- L. Hassan, N. Sani, S. Dangoggo and M. Ladan, Nutritional value OF Bottle Gourd (Lagenaria siceraria) Seeds, Global J. Pure Appl. Sci., 2008, 14(3), 301–306 CAS
.
- C. Raza, A. Dildar, L. Inam, D. Parsa and S. Muhammad, Study of bioactivities of lipid content of fresh Lagenaria siceraria seeds pulp and identification of its chemical constituents, J. Med. Plants Res., 2014, 8, 1014–1020 CrossRef
.
- M. J. R. Nout and P. O. Ngoddy, Technological aspects of preparing affordable fermented complementary foods, Food Control, 1997, 8, 279–287 CrossRef
.
- D. A. Oladiran and N. M. Emmambux, Locally available african complementary foods: nutritional limitations and processing technologies to improve nutritional quality—a review, Food Rev. Int., 2022, 38, 1033–1063 CrossRef CAS
.
- J. Makame, H. De Kock and N. M. Emmambux, Nutrient density of common African indigenous/local complementary porridge samples, LWT, 2020, 133, 1–11 CrossRef
.
- O. A. Adebo, African sorghum-based fermented foods: past, current and future prospects, Nutrients, 2020, 12, 1111 CrossRef CAS
.
- F. W.-B. Tapsoba, S. Samandoulougou, H. Compaore, A. Ouedraogo, T. J. N. Yenouba, E. W. W. Bieogo, M. H. Dicko and H. Sawadogo-Lingani, Characterization of technological properties of sorghum and millet malt produced under control condition in Burkina Faso, Int. J. Biol. Chem. Sci., 2021, 15, 2261–2271 CrossRef
.
- A. E. O. Elkhalifa and R. Bernhardt, Combination Effect of Germination and Fermentation on Functional Properties of Sorghum Flour, Curr. J. Appl. Sci. Technol., 2018, 30, 1–12 CrossRef
.
- M. Gabaza, M. Joossens, M. Cnockaert, M. Muchuweti, K. Raes and P. Vandamme, Lactococci dominate the bacterial communities of fermented maize, sorghum and millet slurries in Zimbabwe, Int. J. Food Microbiol., 2019, 289, 77–87 CrossRef PubMed
.
- K. Kaur and N. Singh, Amylose-lipid complex formation during cooking of rice flour, Food Chem., 2000, 71, 511–517 CrossRef CAS
.
- R. Bazaz, W. N. Baba and F. A. Masoodi, Development and quality evaluation of hypoallergic complementary foods from rice incorporated with sprouted green gram flour, Cogent Food Agric., 2016, 2, 1154714, DOI:10.1080/23311932.2016.1154714
.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Ménard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, A standardised static in vitro digestion method suitable for food – an international consensus, Food Funct., 2014, 5, 1113–1124 RSC
.
- A. E. O. Elkhalifa, B. Schiffler and R. Bernhardt, Effect of fermentation on the functional properties of sorghum flour, Food Chem., 2005, 92, 1–5 CrossRef CAS
.
- A. E. O. Elkhalifa, B. Schiffler and R. Bernhardt, Effect of fermentation on the functional properties of sorghum flour, Food Chem., 2005, 92, 1–5 CrossRef CAS
.
- C. G. Awuchi, V. S. Igwe and C. K. Echeta, The functional properties of foods and flours, Int. J. Adv. Acad. Res., 2019, 5, 139–160 Search PubMed
.
- S. Chandra and S. Samsher, Assessment of functional properties of different flours, Afr. J. Agric. Res., 2013, 8, 4849–4852 Search PubMed
.
- C. Chaves-López, C. Rossi, F. Maggio, A. Paparella and A. Serio, Changes Occurring in Spontaneous Maize Fermentation: An Overview, Fermentation, 2020, 6, 36 CrossRef
.
- C. Mouquet-Rivier, C. Icard-Vernière, J.-P. Guyot, E. Hassane Tou, I. Rochette and S. Trèche, Consumption pattern, biochemical composition and nutritional value of fermented pearl millet gruels in Burkina Faso, Int. J. Food Sci. Nutr., 2008, 59, 716–729 CrossRef CAS PubMed
.
- C. L. Ramos and R. F. Schwan, Technological and nutritional aspects of indigenous Latin America fermented foods, Curr. Opin. Food Sci., 2017, 13, 97–102 CrossRef
.
- P. Mensah, B. S. Drasan, T. J. Harrison and A. M. Tomkins, Fermented Cereal Gruels: Towards a Solution of the Weanling's Dilemma, Food Nutr. Bull., 1991, 13, 1–8 Search PubMed
.
- A. Morris, A. Barnett and O. Burrows, Effect of processing on nutrient content of foods, Cajanus, 2004, 37, 160–164 Search PubMed
.
- I. O. Steve and O. I. Babatunde, Chemical Compositions and Nutritional Properties of Popcorn-Based Complementary Foods Supplemented With Moringa oleifera Leaves Flour, J. Food Res., 2013, 2, 117 CrossRef CAS
.
- C. A. Edwards and A. M. Parrett, Dietary fibre in infancy and childhood, Proc. Nutr. Soc., 2003, 62, 17–23 CrossRef CAS
.
- M. A. Asma, E. B. El Fadil and A. H. El Tinay, Development of Weaning Food from Sorghum Supplemented with Legumes and Oil Seeds, Food Nutr. Bull., 2006, 27, 26–34 CrossRef PubMed
.
- I. Hojsak, M. A. Benninga, B. Hauser, A. Kansu, V. B. Kelly, A. M. Stephen, A. Morais Lopez, J. Slavin and K. Tuohy, Benefits of dietary fibre for children in health and disease, Arch. Dis. Child., 2022, 107, 973–979 CrossRef PubMed
.
- S. Mariam, Nutritive Value Of Three Potential Complementary Foods Based On Cereals And Legumes, Afr. J. Food, Agric., Nutr. Dev., 2005, 5, 01–14 Search PubMed
.
- B. Kouakou, K. K. S. Alexis, D. Adjéhi, D. K. Marcelin and G. Dago, Biochemical Changes Occurring During Germination and Fermentation of Millet and Effect of Technological Processes on Starch Hydrolysis by the Crude Enzymatic Extract of Millet, J. Appl. Sci. Res., 2008, 4, 1502–1510 CAS
.
- J. E. Simwaka, M. V. M. Chamba, Z. Huiming, K. G. Masamba and Y. Luo, Effect of fermentation on physicochemical and antinutritional factors
of complementary foods from millet, sorghum, pumpkin and amaranth seed flours, Int. Food Res. J., 2017, 24, 1869–1879 CAS
.
- A. A. El-Rahman, A. Z. A. Mahmoud, A. A. Sayed and M. A. Abd El Latif, Physiochemical properties and phytochemical characteristics of bottle gourd (Lagenaria siceraria) seed oil, Egypt. J. Chem., 2022, 65(4), 269–277 Search PubMed
.
- C. E. Chinma, O. Adewuyi and J. O. Abu, Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus), Food Res. Int., 2009, 42, 1004–1009 CrossRef CAS
.
- M. A. Abeshu, A. Lelisa and B. Geleta, Complementary Feeding: Review of Recommendations, Feeding Practices, and Adequacy of Homemade Complementary Food Preparations in Developing Countries – Lessons from Ethiopia, Front. Nutr., 2016, 3(41), 1–9 Search PubMed
.
- K. G. Dewey and K. H. Brown, Update on Technical issues concerning Complementary Feeding of Young Children in Developing Countries and Implications for Intervention Programs, Food Nutr. Bull., 2003, 24, 5–28 CrossRef
.
- A. F. Akinsola, I. Osasona, E. T. Akintayo, T. O. Siyanbola and S. O. Omosebi, Nutritional Evaluation of Calabash Gourd (Lagenaria Siceraria) Seeds and Oil, J. Culin. Sci. Technol., 2022, 1–22 Search PubMed
.
- M. Rodríguez-España, C. Y. Figueroa-Hernández, J. de D. Figueroa-Cárdenas, P. Rayas-Duarte and Z. J. Hernández-Estrada, Effects of germination and lactic acid fermentation on nutritional and rheological properties of sorghum: A graphical review, Curr. Res. Food Sci., 2022, 5, 807–812 CrossRef PubMed
.
- N. M. Lowe, The global challenge of hidden hunger: perspectives from the field, Proc. Nutr. Soc., 2021, 80, 283–289 CrossRef
.
- P. Siddhuraju and K. Becker, Effect of Various Domestic Processing Methods on Antinutrients and in Vitro Protein and Starch Digestibility of Two Indigenous Varieties of Indian Tribal Pulse, Mucuna pruriens Var. utilis, J. Agric. Food Chem., 2001, 49, 3058–3067 CrossRef CAS
.
- M. Moussa, X. Qin, L.-F. Chen, O. H. Campanella and B. R. Hamaker, High-quality instant sorghum porridge flours for the West African market using continuous processor cooking, Int. J. Food Sci. Technol., 2011, 46, 2344–2350 CrossRef CAS
.
- C. M. S. Trèche, Viscosity of gruels for infants: a comparison of measurement procedures, Int. J. Food Sci. Nutr., 2001, 52, 389–400 CrossRef
.
- T. Gopaldas, S. Deshpande and C. John, Studies on a wheat-based amylase-rich food, Food Nutr. Bull., 1988, 10, 55–59 Search PubMed
.
- M. Dimopoulou, K. Alba, I. M. Sims and V. Kontogiorgos, Structure and rheology of pectic polysaccharides from baobab fruit and leaves, Carbohydr. Polym., 2021, 273, 118540 CrossRef CAS PubMed
.
- P. Myllärinen, A. Buleon, R. Lahtinen and P. Forssell, The crystallinity of amylose and amylopectin films, Carbohydr. Polym., 2002, 48, 41–48 CrossRef
.
-
S. Serna-Saldivar and L. W. Rooney, in Sorghum and Millets: Chemistry and Technology, ed. D. A. V. Dendy, American Association of Cereal Chemistry, St. Paul, MN, 1995, pp. 69–124 Search PubMed
.
- H. Teng and L. Chen, α-Glucosidase and α-amylase inhibitors from seed oil: A review of liposoluble substance to treat diabetes, Crit. Rev. Food Sci. Nutr., 2017, 57, 3438–3448 CrossRef CAS
.
- A. M. Rovalino-Córdova, V. Fogliano and E. Capuano, The effect of cell wall encapsulation on macronutrients digestion: A case study in kidney beans, Food Chem., 2019, 286, 557–566 CrossRef
.
- S. R. Devi, T. Kumari and S. C. Deka, Extraction of dietary fiber and phytochemicals from bottle gourd seeds (Lagenaria siceraria), its physicochemical properties and application in food model, Food Chem. Adv., 2023, 2, 100252 CrossRef
.
- Z. Karim, M. Holmes and C. Orfila, Inhibitory effect of chlorogenic acid on digestion of potato starch, Food Chem., 2017, 217, 498–504 CrossRef CAS
.
- L. de Morais Cardoso, S. S. Pinheiro, H. S. D. Martino and H. M. Pinheiro-Sant'Ana, Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health, Crit. Rev. Food Sci. Nutr., 2017, 57, 372–390 CrossRef CAS PubMed
.
- L. I. Ezeogu, K. G. Duodu and J. R. N. Taylor, Effects of endosperm texture and cooking conditions on the in vitro starch digestibility of sorghum and maize flours, J. Cereal Sci., 2005, 42, 33–44 CrossRef CAS
.
- A. Khoddami, V. Messina, K. Vadabalija Venkata, A. Farahnaky, C. L. Blanchard and T. H. Roberts, Sorghum in foods: Functionality and potential in innovative products, Crit. Rev. Food Sci. Nutr., 2023, 63, 1170–1186 CrossRef CAS PubMed
.
- M. Rodríguez-España, C. Y. Figueroa-Hernández, J. de D. Figueroa-Cárdenas, P. Rayas-Duarte and Z. J. Hernández-Estrada, Effects of germination and lactic acid fermentation on nutritional and rheological properties of sorghum: A graphical review, Curr. Res. Food Sci., 2022, 5, 807–812 CrossRef PubMed
.
- K. G. Duodu, J. R. N. Taylor, P. S. Belton and B. R. Hamaker, Factors affecting sorghum protein digestibility, J. Cereal Sci., 2003, 38, 117–131 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo02832a |
| This journal is © The Royal Society of Chemistry 2023 |