Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nutrikinetics and urinary excretion of phenolic compounds after a 16-week supplementation with a flavanone-rich ingredient†
Jananee
Muralidharan
 a,
Cindy
Romain
a,
Letizia
Bresciani
a,
Cindy
Romain
a,
Letizia
Bresciani
 b,
Pedro
Mena
b,
Pedro
Mena
 b,
Donato
Angelino
b,
Donato
Angelino
 c,
Daniele
Del Rio
c,
Daniele
Del Rio
 b,
Linda H.
Chung
de,
Pedro E.
Alcaraz
de and
Julien
Cases
*a
b,
Linda H.
Chung
de,
Pedro E.
Alcaraz
de and
Julien
Cases
*a
aFytexia, ZAE via Europa – 3 rue d'Athènes, 34350 Vendres, France. E-mail: jcases@fytexia.com; Fax: +33 467 306 582; Tel: +33 467 219 098
bHuman Nutrition Unit, Department of Food & Drug, University of Parma, Via Volturno 39, 43125 Parma, Italy
cFaculty of Bioscience and Technology for Food, Agriculture and Environment, University of Teramo, Teramo, 64100, Italy
dResearch Center for High Performance Sport – UCAM Universidad Católica de Murcia, Murcia, Spain
eDepartment of Food and Nutrition Technology, Universidad Católica de Murcia, Murcia, Spain
First published on 9th November 2023
Abstract
Background: Polyphenols are a broad group of compounds with a complex metabolic fate. Flavanones and their metabolites provide cardiovascular protection and assistance in long-term body composition management. Objective: This study evaluates the nutrikinetics and the bioavailability of phenolic compounds after both acute and chronic supplementation with a flavanone-rich product, namely Sinetrol® Xpur, in healthy overweight and obese volunteers. Design: An open-label study including 20 volunteers was conducted for 16 weeks. Participants received Sinetrol® Xpur, either a low dose (900 mg per day) or a high dose (1800 mg per day), in capsules during breakfast and lunch. They were advised to follow an individualized isocaloric diet and avoid a list of polyphenol-rich foods 48 hours before and during the pharmacokinetic measurements. Results: Over 20 phase II and colonic metabolites were measured in the plasma. Two peaks were observed at 1 h and 7h–10 h after the first capsule ingestion. No significant differences in the AUC were observed in circulating metabolites between both doses. In urine excretion, 53 metabolites were monitored, including human phase II and colonic metabolites, at weeks 1 and 16. Cumulative urine excretion was higher after the high dose than after the low dose in both acute and chronic studies. Total urinary metabolites were significantly lower in week 16 compared to week 1. Conclusion: Although the urinary excreted metabolites reduced significantly over 16 weeks, the circulating metabolites did not decrease significantly. This study suggests that chronic intake might not offer the same bioavailability as in the acute study, and this effect does not seem to be dose-dependent. The clinical trial registry number is NCT03823196.
Introduction
In the past decade, obesity has become a major worldwide health issue. According to the World Health Organization (WHO), the obese population has tripled since the ‘80s. At this rate, half of the world's adult population is predicted to be overweight or obese by 2030.1 The main reasons for this phenomenon are a modern and sedentary lifestyle, linked with a high-fat, high-sugar diet.2 Overweight/obesity is the leading risk factor for type 2 diabetes development, among other non-communicable diseases. Accumulation of visceral adipose tissue during weight gain is one of the strongest predictors of insulin resistance.3 In particular, fat accumulation in the ectopic sites (liver, skeletal muscle, heart), independent of BMI, contributes to insulin resistance development. The use of natural bioactive compounds such as (poly)phenols has offered an interesting strategy to prevent and combat the obesity epidemic and reduce associated risk factors.(Poly)phenols, first described for their antioxidant effect, are now acclaimed for their other attributes, such as a beneficial regulation of metabolic activities.4 Flavonoids are an important class of (poly)phenols, which have shown protection in the development of risk factors of metabolic syndrome such as type 2 diabetes (T2D), hypertension, cholesterolemia, and abdominal obesity in various observational and clinical studies.5–7 They are a vast family of compounds, including flavanones, flavonols, flavones, flavan-3-ols, anthocyanins and isoflavones. Naringin and hesperidin are members of the flavanone class, which has been widely investigated for its anti-inflammatory and anti-adipogenic effects.8,9 These compounds are mainly present in citrus fruits (Citrus sinensis, Citrus grandis, Lippia graveolens and Citrus paradisi)10,11 and their chronic consumption has been shown to improve insulin sensitivity and lipid regulation.12–15
Pharmacokinetic studies suggest that hesperidin and naringin are almost exclusively found in their conjugated forms in circulation and urinary excretion.16 Metabolites arising from the conjugation of (poly)phenols in the liver, referred to as phase-II metabolites, have been accredited for the effects of (poly)phenols on health. For example, phase-II metabolites of naringin, namely naringenin-7-glucuronide and naringenin-4′-glucuronide, have shown anti-inflammatory effects in in vitro studies.17 Other than the phase-II metabolites, the products of gut microbial catabolism are also of interest as they have been reported to provide health effects.17–19 Animal studies have reported the presence of flavonoid metabolites in various tissues and organs, indicating the potential for action on specific organs and cells.20,21 Although naringin and hesperidin have shown potential for clinical applications, their bioavailability is limited and depends on various factors such as interaction with food components, host metabolizing enzymes, intestinal microbiota and BMI.7,22,23 Understanding the biological fate of the ingested compounds, their nutrikinetics and their bioavailability would allow for improving the knowledge of the potential health benefits and safety (poly)phenols.24
Sinetrol® Xpur is a food-based ingredient designed to provide various naturally bioactive components from Citrus fruits, namely grapefruit, pomelo and orange, and guarana.24 Sinetrol® Xpur is mainly composed of flavanones and minorly caffeine. In previous clinical trials, a significant weight loss, coupled with fat mass decrease, was shown, mainly in the abdomen area, through lipolysis enhancement.25–27 Another study investigated the mechanisms possibly associated with the observed effect, demonstrating the involvement of the c-AMP (cyclic adenosine monophosphate)-dependent UCP2 (uncoupled protein 2) pathway in lipolysis.28 Although the physiological benefits and part of its mechanism of action have been already described, the nutrikinetics and the bioavailability of this flavanone-based ingredient have never been studied.
Nutrikinetics and bioavailability are key aspects to understanding the (poly)phenol action mechanism. Although several studies are available on the pharmacokinetics of citrus polyphenols,29 to the best of our knowledge, no study has evaluated the nutrikinetics and urinary excretion of these compounds during chronic consumption. Indeed, a few chronic studies conducted on flavan-3-ols, resveratrol, and mango (poly)phenols have shown inconsistent results on the circulating and excretory metabolites compared to the acute studies, raising the question of the effect of repeated exposure of (poly)phenols and their metabolism.22,29,30
Based on the above considerations, in this randomized open-label clinical trial, a flavanone-rich ingredient's nutrikinetics and urinary excretion were evaluated after acute and chronic supplementation (16 weeks) in healthy overweight or obese subjects.
Subjects and methods
Supplement composition
A flavanone-rich ingredient was obtained by alcohol and/or water extraction from specific varieties of Citrus paradisi Macfad (grapefruit), Citrus sinensis L. Osbec (sweet orange), and Paullinia cupana Kunth (guarana). This contained 29–36% of flavanones, of which naringin and hesperidin were considered markers of grapefruit and both sweet and blood orange, respectively. Caffeine (2.1–2.9%) was provided through the guarana extract. Each pill contained 450 mg of flavanone-rich extract.Supplement's bioactive compound characterization
A supplement was prepared by accurately weighing 1000 mg and dissolving in 100 ml of DMSO. This solution was filtered through a 0.45 μm filter. The prepared supplement was analysed by a high-performance liquid chromatography (HPLC) technique. An Agilent HPLC 1260 apparatus (software Openlab CDS chemstation edition) coupled with a diode array detector was used. Separations were carried out by means of a Zorbax StableBond SB-C18 column (4.6 × 2 mm; 5 μm particle size). Mobile phases A, B and C were water, acetic acid and 100% acetonitrile, respectively. The linear gradient program was used as follows: (a) 0 to 5 min 94% A and 6% B; (b) 5 to 10 min 82.4% A, 5.6% B and 12% C; (c) 10 to 15 min 76.6% A, 5.4% B and 18% C; (d) 15 to 25 min 67.9% A, 5.1% B and 27% C; (e) 25 to 30 min 65% A, 5% B and 30% C; (f) 30 to 35 min 100% C; (g) 35 to 40 min 100% C; (h) 40 to 45 min 64% A and 6% B. Monitoring was performed at 280 nm at a flow rate of 1 mL min−1 and injection volume of 25 μL. Flavanones and caffeine were expressed as naringin, hesperidin and caffeine, respectively. Naringin, hesperidin and caffeine standards were purchased from Sigma-Aldrich Co. (St Louis, MO, USA) and a kuromanin chloride standard from Extrasynthese (Genay, France).Moreover, a detailed characterization of the product using uHPLC-ESI-MSn analysis was carried out. Detailed methods are provided in ESI 1.†
Study design and subjects
Twenty healthy overweight or obese men (n = 10) and women (n = 10) were supplemented with 900 mg per day (n = 10) or 1800 mg per day (n = 10) of the flavanone-based ingredient for 16 weeks. They were recruited by Universidad Catolica San Antonio (UCAM), Murcia, Spain. Subjects’ agreement was obtained by signing a written consent form. Ethics approval was obtained from the Ethical Committee of Universidad Catolica San Antonio de Murcia in compliance with the guidelines laid out in the Declaration of Helsinki31 and with Good Clinical Practices defined in the ICH Harmonized Tripartite Guideline, CE5551.32 This trial was registered at clinicaltrials.gov as NCT03823196. For the enrolment, a randomization number was generated using a simple block randomization of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with an additional stratification for sex (40% minimum and 60% for each sex).
1 with an additional stratification for sex (40% minimum and 60% for each sex).
Inclusion and exclusion criteria
Volunteers were selected between 20 and 50 years old, with a BMI ranging from 25 kg m−2 to 30.5 kg m−2. In order to ensure overweight volunteers’ recruitment with excess fat mass only (high lean mass can induce a high BMI), an additional inclusion criterion was set, selecting subjects having a total fat mass ≥ 25% for men and ≥32% for women – measured using a bioelectrical impedance analysis. Subjects under medication, suffering from metabolic and/or chronic diseases, such as diabetes, dyslipidaemia, infectious diseases or asthma, were excluded. Volunteers with food allergies to components of the ingredient, smokers, subjects who followed a chronic treatment or went through surgery to modify their body weight in the last 6 months, as well as pregnant, breastfeeding or menopausal women, were not included in the study.Diet and physical activity
Subjects were asked to follow an isocaloric diet (individually calculated and monitored) according to the revised Harris–Benedict equation,33 corresponding to 2200–2500 kcal per day. Each participant was provided with a list of forbidden foods (ESI 2†) on Friday and advised not to consume them from Saturday until Wednesday. On Monday morning, participants arrived in a fasted state at the study centre at 8 am. First, blood and urine samples were collected, after which participants were provided with the pill and followed with a standardized breakfast (low in polyphenols). At 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 pm, participants were provided with another pill, after which they consumed the standardized lunch. Subjects performed a 24-hour dietary recall interview twice during the week and once during the weekend to check their compliance with the recommended diet. These interviews were conducted at the baseline (W1) and at the end of the study period. A ±10% difference between the reported and recommended food intake was considered acceptable. Volunteers received the flavanone-rich ingredient at a total dose of 900 mg per day (two pills) or 1800 mg per day (4 pills), taken two or four times, at breakfast time and at lunchtime. Subjects were provided with a pedometer to assess their physical activity by counting the daily step number detected by the motion of the hips. Blood pressure was measured with an automated cuff (Visomat Comfort 20/40) after the volunteer sat quietly for 5 minutes. Measurements were performed on the same arm in the same position. Three measures were done and mean resting BP was calculated. Heart rate was also recorded using the same automated cuff.
00 pm, participants were provided with another pill, after which they consumed the standardized lunch. Subjects performed a 24-hour dietary recall interview twice during the week and once during the weekend to check their compliance with the recommended diet. These interviews were conducted at the baseline (W1) and at the end of the study period. A ±10% difference between the reported and recommended food intake was considered acceptable. Volunteers received the flavanone-rich ingredient at a total dose of 900 mg per day (two pills) or 1800 mg per day (4 pills), taken two or four times, at breakfast time and at lunchtime. Subjects were provided with a pedometer to assess their physical activity by counting the daily step number detected by the motion of the hips. Blood pressure was measured with an automated cuff (Visomat Comfort 20/40) after the volunteer sat quietly for 5 minutes. Measurements were performed on the same arm in the same position. Three measures were done and mean resting BP was calculated. Heart rate was also recorded using the same automated cuff.
Plasma and urine preparation
Blood sampling was collected by a certified nurse. The arm where blood extraction would be conducted was cleansed and sterilized with alcohol. An IV access was put in place in the arm to extract the blood at different time points (by inserting the tubes to the IV access). For plasma, blood was collected in EDTA tubes and centrifuged at 5000 rpm at 4 °C. Immediately afterwards, the plasma was pipetted into 3 Eppendorf tubes of 1 mL and then stored at −80 °C. At the end of the study, one of the 3 frozen aliquots was sent to the University of Parma for analysis. Plasma samples of all volunteers were extracted using a micro-solid phase extraction (μ-SPE) method as previously reported.34,35 Briefly, 350 μL of plasma were diluted (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with o-phosphoric acid 4% (v/v). After plate activation, 600 μL of sample were loaded on a 96 well μ-SPE HLB plate (Oasis® HLB μElution Plate, 30 μm, Waters), and washed with 200 μL of water and 200 μL of 0.2% (v/v) acetic acid and finally analytes were eluted with 60 μL of methanol.
1) with o-phosphoric acid 4% (v/v). After plate activation, 600 μL of sample were loaded on a 96 well μ-SPE HLB plate (Oasis® HLB μElution Plate, 30 μm, Waters), and washed with 200 μL of water and 200 μL of 0.2% (v/v) acetic acid and finally analytes were eluted with 60 μL of methanol.
Urine sampling was done from a collection bottle. For each time frame of the pharmokinetic visit, the urine was stored at room temperature. After each time frame, the volume of the urine was recorded, and the bottle was turned to mix the urine prior to collecting 3 aliquots of 1 mL and then stored at −80 °C. Urine samples were prepared, as previously reported by Brindani and colleagues.36 Briefly, urine samples were defrosted, vortexed, diluted in 0.1% formic acid in water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v), centrifuged at 14
3 v/v), centrifuged at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and finally filtered through a 0.45 μm nylon filter.
000 rpm for 10 min and finally filtered through a 0.45 μm nylon filter.
UHPLC-ESI-MS/MS analysis of biological sample metabolites
Plasma and urine samples were analyzed using a UHPLC DIONEX Ultimate 3000 equipped with a triple quadrupole TSQ Vantage (Thermo Fisher Scientific Inc., San Jose, CA, USA) fitted with a heated-ESI (H-ESI) (Thermo Fisher Scientific Inc.) probe. Separations were carried out by means of a Kinetex EVO C18 (100 × 2.1 mm), 2.6 μm particle size (Phenomenex, Torrance, CA, USA), installed with a precolumn cartridge (Phenomenex). For chromatographic separation, mobile phase A was 0.2% formic acid in water and mobile phase B was acetonitrile containing 0.2% formic acid. The gradient started with 5% B, and isocratic conditions were maintained for 0.5 min. Eluent B reached 75% after 7.5 min, and the gradient was maintained for 2 min prior to rapidly re-establishing the starting conditions, which were maintained for 3 min to re-equilibrate the column. The flow rate was 0.4 mL min−1, the injection volume was 5 μL, and the column temperature was set at 40 °C. The applied MS method consisted of the selective determination of each target precursor ion by the acquisition of characteristic product ions in selective reaction monitoring (SRM) mode (ESI Table 1†), applying the negative ionization mode. The spray voltage was set at 3 kV, the vaporizer temperature at 300 °C, and the capillary temperature at 270 °C. The sheath gas flow was 50 units, and the auxiliary gas pressure was set to 10 units. Ultrahigh-purity argon gas was used for collision-induced dissociation (CID). The S-lens values were defined for each compound based on infusion parameter optimization (ESI Table 1†). Conversely, S-lens values were set using the values obtained for the chemically closest available standards for compounds that were not available for infusion. Moreover, caffeine metabolites were analysed following the well-known metabolism reported for caffeine.37,38 The applied MS method consisted of the selective determination of each target precursor ion by the acquisition of characteristic product ions in the selective reaction monitoring (SRM) mode (ESI Table 1†), applying both negative and positive ionization. Quantification was performed with the calibration curves of the standards, when available or using the most structurally similar compound. Data processing was performed using Xcalibur software (Thermo Fisher Scientific Inc., Waltham, MA, USA). All data were expressed as mean values ± SEM.Safety parameters
Safety parameters were assessed before inclusion onto the study (W0) and at the end of the intervention period (W16). These included liver function parameters (alanine transaminase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT)), renal function parameters (urea, creatinine, sodium (Na), potassium (K)), blood parameters (haemoglobin, erythrocytes, leucocytes, lymphocytes) and heart rate. The above safety parameters were evaluated at Laboratory Munuera, Spain using all standard methods.Statistical analysis
Data are represented as mean ± SEM for blood and urinary metabolite measurements. Safety parameters and baseline characteristics are represented by mean ± SD. The PKsolver add-on program was used to perform pharmacokinetic analyses in Microsoft Excel,39 including area under the curve (AUC0–24), maximum (Cmax) and average (Cavg) plasma concentration (the latter calculated as AUC0–24/24 h) and time to reach the maximum (Tmax) plasma concentration. The effect of dose and time of chronic consumption was determined using a two-way ANOVA (dose, time effects), and a post-hoc Tukey HSD test was conducted to evaluate the within-group differences. The metabolites were log-transformed before the statistical evaluation. Throughout the study, a p-value of 0.05 was used as a cutoff to determine statistical significance.Results
Population characteristics
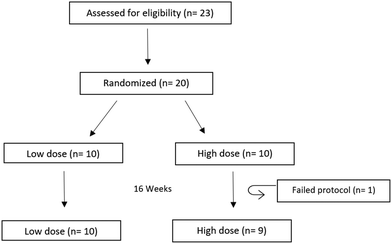
The supplement was given to a population of healthy overweight or obese subjects. The study design is described in Fig. 1. A total of 19 subjects concluded the chronic study, whereas one subject, receiving the high dose, developed diarrhoea and was excluded from the study, but no link has been established to supplement ingestion. Baseline parameters indicated no significant differences between the two groups (Table 1). Safety parameters (ESI Table 2†) indicated all measured safety parameters were within limits, and no significant differences were detected during the study duration. No adverse events linked to the supplement were reported during the study.| Characteristics of all volunteers | Low dose | High dose | p-Value |
|---|---|---|---|
| N (M/F) | 10 (4/6) | 9 (6/3) | NS |
| Age (years, mean ± SD) | 33 ± 10 | 29 ± 5 | NS |
| Height (cm, mean ± SD) | 1.70 ± 0.09 | 1.75 ± 0.07 | NS |
| Weight (kg, mean ± SD) | 83.1 ± 9.5 | 87.8 ± 6.1 | NS |
| BMI (kg m−2, mean ± SD) | 28.67 ± 0.86 | 28.83 ± 1.26 | NS |
Characterization of the supplement
The basic composition of the supplement is provided in Table 2, highlighting the 8 main compounds quantified through HPLC analysis. A more detailed (poly)phenolic composition analysis was performed to determine the precise nature of all the different compounds (ESI Table 3†). The analysis allowed the detection of 28 flavanones, 10 (poly)phenolic compounds and 3 unknown compounds, probably linked to the flavanone class. Total flavanones resulted in 334.79 mg per 900 mg of powder (739.27 μmol per 900 mg).| Compound | R t (min) | Λ max (nm) | Content (g per 100g) | Content (mg per 900 mg) | |
|---|---|---|---|---|---|
| Mean | SD | ||||
| Caffeine | 13 | 283 | 2.22 | 0.1 | 19.98 |
| Flavanone 1 | 16 | 284.330sh | 0.14 | 0.01 | 1.26 |
| Isonaringin | 18 | 283.330sh | 0.92 | 0.05 | 8.28 |
| Naringin | 19 | 284.330sh | 19.18 | 0.96 | 172.62 |
| Hesperidin | 16 | 284.330sh | 8.65 | 0.52 | 77.85 |
| Flavanone 2 | 26 | 285.330sh | 0.17 | 0.01 | 1.53 |
| Poncirin | 27 | 285.331sh | 0.17 | 0.01 | 1.53 |
| Naringenin | 29 | 287.333sh | 0.18 | 0.01 | 1.62 |
| Total | 284.67 | ||||
Circulating and excreted metabolites in the acute study (W1)
During W1, after subjects arrived in fasting conditions and after a (poly)phenol-controlled diet, flavanone-rich ingredient compounds were evaluated. Among 133 monitored flavanone and caffeine-derived possible metabolites29,37,38,40–42 a total of 20 phase II metabolites and catabolites were detected and quantified in plasma over 24 hours (Table 3). 4′- and 7′-glucuronide conjugates of naringenin were the predominant metabolites in circulation in both groups after the flavanone-rich ingredient consumption. The sum of glucuronide conjugates was higher than that of sulfate conjugates in both doses (data not shown). AUC0–24, Tmax and Cmax were generally not significantly different between low- and high-flavanone doses, except for 3-hydroxybenzoic acid-4-glucuronide, which displayed a higher AUC0–24 and Cmax in subjects consuming the low dose. Most metabolites had a Tmax of 4 hours. The supplementation of the flavanone-rich ingredient at breakfast and at lunch could show a cumulative effect on the observed higher Tmax values. There was a high inter-individual variability observed in the AUC0–24, with naringenin-7-glucuronide having the highest variability in both low (CV: 207.7%) and high doses (CV: 118.7%). Amongst the colonic metabolites, 3-(phenyl)propionic acid-3′-sulfate had the highest variability.| PK parameters | Week 1 – low | Week 16 – low | Week 1 – high | Week 16 – high | |||||
|---|---|---|---|---|---|---|---|---|---|
| Consumption | Consumption | Consumption | Consumption | ||||||
| Native compounds | Mean ± SEM | CV% | Mean ± SEM | CV% | Mean ± SEM | CV% | Mean ± SEM | CV% | |
| a Indicates significant differences between W1 and W16 (time effect). b Indicates significant differences between the doses at W1. c Indicates significant differences between W1 and W16 at a high dose. d Indicates the dose–time effect. | |||||||||
| Naringenin (5-, 7-, or 4′)-sulfate | C max (nmol L−1) | 13.8 ± 5.5 | 118.8 | 11.7 ± 3.4 | 85.4 | 19.2 ± 6.6 | 102 | 20.2 ± 8.8 | 129.5 |
| T max (h) | 7.9 ± 3.1 | 114.9 | 3.7 ± 2.4 | 189.8 | 6.8 ± 2.99 | 132 | 1.2 ± 0.5 | 132.5 | |
| AUC0–24 (nmol h L−1) | 147.4 ± 66.1 | 134.6 | 45.9 ± 8.9 | 58.2 | 197 ± 65.2 | 99.2 | 80.3 ± 22.3 | 83.3 | |
| C avg (nmol L−1) | 6.2 ± 2.8 | 134.4 | 1.91 ± 0.4 | 58.2 | 8.3 ± 2.8 | 99.1 | 3.4 ± 0.93a | 83.6 | |
| Naringenin-7-glucuronide | C max (nmol L−1) | 87.97 ± 56.9 | 194.1 | 67.6 ± 16.8 | 74.3 | 154 ± 52.9 | 103 | 109.3 ± 20.9 | 57.5 |
| T max (h) | 7.6 ± 0.9 | 34.6 | 6.6 ± 0.9 | 36.9 | 8.5 ± 0.5 | 17.7 | 7.8 ± 0.6 | 20.9 | |
| AUC0–24 (nmol h L−1) | 827.1 ± 572.5 | 207.7 | 612.3 ± 198.3 | 97.2 | 1261.8 ± 498.9 | 118.7 | 923.9 ± 241 | 78.3 | |
| C avg (nmol L−1) | 34.5 ± 23.9 | 207.7 | 25.6 ± 8.3 | 97.2 | 52.6 ± 20.8 | 118.7 | 38.5 ± 10.1 | 78.4 | |
| Naringenin-4′-glucuronide | C max (nmol L−1) | 181.5 ± 89.7 | 148.2 | 173.6 ± 40.7 | 70.3 | 278.2 ± 90.7 | 97.9 | 287.4 ± 62.6 | 65.4 |
| T max (h) | 6.5 ± 1.2 | 51.8 | 6.6 ± 0.9 | 36.9 | 8.3 ± 0.9 | 30 | 7.8 ± 0.6 | 20.9 | |
| AUC0–24 (nmol h L−1) | 1606.4 ± 891.7 | 166.6 | 1590.7 ± 545.8 | 103 | 2698.6 ± 912 | 101.4 | 2559.9 ± 744 | 87.2 | |
| C avg (nmol L−1) | 66.93 ± 37.2 | 166.6 | 66.3 ± 22.8 | 103 | 112.5 ± 38 | 101.4 | 106.7 ± 31 | 87.2 | |
| Naringenin (5-, 7-, or 4′)-diglucuronide | C max (nmol L−1) | 5.3 ± 0.9 | 47.3 | 13.9 ± 8.92 | 193.4 | 4.9 ± 0.8 | 47.5 | 7.1 ± 1.4 | 57.8 |
| T max (h) | 8.4 ± 2.1 | 75.7 | 3.8 ± 0.93 | 73.5 | 11.3 ± 3 | 79.7 | 7.5 ± 2.1a | 83.5 | |
| AUC0–24 (nmol h L−1) | 74.9 ± 10.6 | 42.4 | 173.3 ± 113 | 195.8 | 68.1 ± 9.4 | 41.1 | 79 ± 23.9 | 90.5 | |
| C avg (nmol L−1) | 3.2 ± 0.5 | 42.4 | 7.3 ± 4.8 | 195.8 | 2.9 ± 0.4 | 41.2 | 3.3 ± 0.99 | 90.3 | |
| Naringenin (5-, 7-, or 4′)-sulfo-glucuronide | C max (nmol L−1) | 1.3 ± 0.2 | 40.2 | 1.5 ± 0.3 | 63.9 | 1.8 ± 0.3 | 42.4 | 2.1 ± 0.3 | 42.7 |
| T max (h) | 11.3 ± 3.2 | 83.2 | 3.2 ± 1.2 | 104.1 | 13.1 ± 2.6 | 57.5 | 3.8 ± 1.2a,c | 89.7 | |
| AUC0–24 (nmol h L−1) | 11.9 ± 1.5 | 36.7 | 8.9 ± 1.6 | 52.9 | 20 ± 2.6 | 38.9 | 14.7 ± 3.8a | 77.7 | |
| C avg (nmol L−1) | 0.5 ± 0.1 | 36.8 | 0.4 ± 0.1 | 56.8 | 0.9 ± 0.2 | 39.8 | 0.7 ± 0.2 | 78.7 | |
| Isosakuranetin (5-, or 7)-sulfate | C max (nmol L−1) | 0.9 ± 0.4 | 128.1 | 0.9 ± 0.3 | 76.8 | 1.4 ± 0.5 | 99.3 | 1.9 ± 0.7a | 111.9 |
| T max (h) | 11 ± 3.3 | 87.9 | 4.1 ± 2.4 | 172 | 7.3 ± 3 | 120.9 | 1.5 ± 0.7 | 143.8 | |
| AUC0–24 (nmol h L−1) | 9.3 ± 5.4 | 171.9 | 2.5 ± 0.6 | 64.7 | 12.8 ± 4.2 | 98.6 | 5.7 ± 1.9 | 96.3 | |
| C avg (nmol L−1) | 0.4 ± 0.3 | 169.3 | 0.1 ± 0.1 | 60 | 0.6 ± 0.2 | 96.3 | 0.3 ± 0.1 | 100 | |
| Isosakuranetin (5-, or 7)-glucuronide | C max (nmol L−1) | 2.7 ± 0.9 | 92.8 | 2.6 ± 0.7 | 77.7 | 2.5 ± 0.6 | 62.5 | 3.2 ± 0.7 | 64.1 |
| T max (h) | 12.7 ± 2.8 | 65.2 | 4.5 ± 1.2 | 79.4 | 15.2 ± 2.5 | 48 | 6.3 ± 2.4a | 115.3 | |
| AUC0–24 (nmol h L−1) | 35.9 ± 11.7 | 98 | 24.8 ± 6.1 | 73.9 | 29.8 ± 5.9 | 59.4 | 30.7 ± 6.3 | 60.9 | |
| C avg (nmol L−1) | 1.5 ± 0.5 | 98.7 | 1.1 ± 0.3 | 72.9 | 1.3 ± 0.3 | 60.5 | 1.3 ± 0.3 | 61 | |
| Eriodictyol (5-, 7-, 3′-, or 4′)-sulfate | C max (nmol L−1) | 0.4 ± 0.2 | 115.4 | 0.3 ± 0.1 | 100 | 0.5 ± 0.2 | 110.9 | 0.3 ± 0.1 | 100 |
| T max (h) | 10.8 ± 3.2 | 88.8 | 3.8 ± 1.3 | 101.1 | 3.1 ± 1.1 | 98.8 | 2.6 ± 0.9 | 99.7 | |
| AUC0–24 (nmol h L−1) | 2.7 ± 0.9 | 91.7 | 1 ± 0.2 | 55.5 | 2.7 ± 0.9 | 92.4 | 1 ± 0.3 | 81 | |
| C avg (nmol L−1) | 0.2 ± 0.1 | 81.9 | 0.1 ± 0.1 | 75 | 0.2 ± 0.1 | 81.9 | 0.1 ± 0.1a | 75 | |
| Hesperetin (5-, 7-, or 3′)-sulfate | C max (nmol L−1) | 5.5 ± 3.1 | 166 | 5.8 ± 2.8 | 140.7 | 7.5 ± 3 | 119.6 | 5.4 ± 1.7 | 91.4 |
| T max (h) | 10.6 ± 1.8 | 48.6 | 7.3 ± 2.3 | 90.9 | 13.9 ± 2.3 | 48 | 2.7 ± 1.2a | 132.6 | |
| AUC0–24 (nmol h L−1) | 60.5 ± 33.8 | 167.6 | 33.1 ± 15.2 | 137.6 | 76.2 ± 28 | 110.1 | 42.2 ± 13.3 | 94.5 | |
| C avg (nmol L−1) | 2.6 ± 1.5 | 167.9 | 1.4 ± 0.7 | 137 | 3.2 ± 1.2 | 109.8 | 1.8 ± 0.6 | 93.8 | |
| Hesperetin-7-glucuronide | C max (nmol L−1) | 12.3 ± 5.3 | 129.6 | 11.4 ± 2.9 | 76 | 15.1 ± 4.3 | 83.7 | 16.9 ± 3.5 | 60.9 |
| T max (h) | 12.8 ± 2.1 | 47.9 | 6.7 ± 2.3 | 101.7 | 15 ± 2.5 | 49.6 | 10.4 ± 2.8 | 79 | |
| AUC0–24 (nmol h L−1) | 170.5 ± 82.9 | 145.9 | 121.2 ± 29.9 | 74.1 | 171.4 ± 42.9 | 75.2 | 200.8 ± 52.5 | 78.4 | |
| C avg (nmol L−1) | 7.1 ± 3.5 | 145.8 | 5.1 ± 1.3 | 74.3 | 7.2 ± 1.8 | 75.3 | 8.4 ± 2.2 | 78.5 | |
| Hesperetin-3′-glucuronide | C max (nmol L−1) | 18.2 ± 7.4 | 121.6 | 15.9 ± 3.4 | 64.5 | 24.5 ± 8.1 | 99.4 | 20.9 ± 4.6 | 65.6 |
| T max (h) | 10 ± 0 | 0 | 7.5 ± 2.2 | 85.2 | 15.6 ± 2.3 | 44.1 | 5.4 ± 1.3a | 71.5 | |
| AUC0–24 (nmol h L−1) | 256.2 ± 123.1 | 144.2 | 177.7 ± 40.7 | 68.6 | 291.2 ± 89.5 | 92.2 | 248.7 ± 61.1 | 73.8 | |
| C avg (nmol L−1) | 10.7 ± 5.2 | 144.3 | 7.4 ± 1.7 | 68.6 | 12.2 ± 3.8 | 92.3 | 10.4 ± 2.6 | 73.9 | |
| Colonic metabolites | |||||||||
| 3′-Methoxycinnamic acid-4′-sulfate | C max (nmol L−1) | 2.6 ± 0.9 | 104.7 | 2.2 ± 0.8 | 109.9 | 2.8 ± 0.7 | 69.1 | 1.7 ± 0.6 | 103.1 |
| T max (h) | 3.6 ± 1 | 77.6 | 2.7 ± 0.9 | 92.3 | 4.9 ± 0.9 | 52.7 | 2.9 ± 1.3 | 134 | |
| AUC0–24 (nmol h L−1) | 12.9 ± 4.2 | 97.2 | 6.4 ± 1.7 | 79 | 15.3 ± 3.2 | 61.1 | 6.4 ± 1.7a | 79 | |
| C avg (nmol L−1) | 0.6 ± 0.2 | 94.5 | 0.3 ± 0.1 | 77.8 | 0.7 ± 0.2 | 61 | 0.3 ± 0.1a | 77.8 | |
| 3-(3′-Methoxyphenyl)propionic acid-4′-sulfate | C max (nmol L−1) | 3 ± 1.1 | 113.5 | 2.3 ± 0.6 | 67.9 | 2.5 ± 0.4 | 49.4 | 1.8 ± 0.5 | 72 |
| T max (h) | 1.9 ± 0.8 | 122.3 | 4.1 ± 2.4 | 174.2 | 14 ± 2.9 | 62 | 1.9 ± 0.7a,c | 108 | |
| AUC0–24 (nmol h L−1) | 15.4 ± 6.6 | 128.8 | 7.7 ± 2.2 | 85.4 | 21.5 ± 4.4 | 60.5 | 8.9 ± 3.1 | 101.5 | |
| C avg (nmol L−1) | 0.7 ± 0.3 | 126.6 | 0.4 ± 0.1 | 84.4 | 0.9 ± 0.2 | 60.7 | 0.4 ± 0.2 | 105.5 | |
| 3-(4′-Methoxyphenyl)propionic acid-3′-sulfate | C max (nmol L−1) | 1.8 ± 0.3 | 50.3 | 3 ± 0.8 | 82.5 | 2.4 ± 0.6 | 64.9 | 3.1 ± 0.5 | 41.5 |
| T max (h) | 10.8 ± 3.3 | 91.3 | 4.1 ± 1.1 | 77.6 | 12.2 ± 3.5 | 84.1 | 5.9 ± 3.3 | 165.6 | |
| AUC0–24 (nmol h L−1) | 12.2 ± 3.2 | 77.8 | 11.2 ± 3.1 | 82 | 17.4 ± 4.7 | 80.6 | 13.2 ± 3.1 | 68.7 | |
| C avg (nmol L−1) | 0.6 ± 0.2 | 76.5 | 0.5 ± 0.2 | 83 | 0.8 ± 0.2 | 79.2 | 0.6 ± 0.2 | 71 | |
| 3-(4′-Methoxyphenyl)propionic acid-3′-glucuronide | C max (nmol L−1) | 17.2 ± 4.1 | 71 | 14.7 ± 3.2 | 64.6 | 23.3 ± 6.4 | 81.6 | 39.3 ± 12.4 | 94.2 |
| T max (h) | 12.2 ± 3.1 | 75.1 | 6 ± 2.3 | 113 | 20.3 ± 2.6 | 37.9 | 10.8 ± 3.4a,c,d | 92.4 | |
| AUC0–24 (nmol h L−1) | 202.6 ± 51.7 | 76.5 | 177.3 ± 47.7 | 80.6 | 233.8 ± 55.3 | 70.9 | 382.1 ± 142 | 111.7 | |
| C avg (nmol L−1) | 8.5 ± 2.2 | 76.5 | 7.4 ± 2 | 80.4 | 9.8 ± 2.3 | 70.9 | 16 ± 6 | 111.6 | |
| 3-(Phenyl)propionic acid-4′-sulfate | C max (nmol L−1) | 26.8 ± 13.9 | 155 | 44.7 ± 17.1 | 115 | 17.5 ± 5.6 | 96.3 | 24 ± 8.5 | 105.4 |
| T max (h) | 4.8 ± 2.4 | 146.3 | 1.6 ± 0.7 | 120 | 1.5 ± 0.2 | 34 | 4 ± 2.5 | 184.5 | |
| AUC0–24 (nmol h L−1) | 204.1 ± 119.7 | 176 | 141.4 ± 66.6 | 141.4 | 94.5 ± 28.6 | 90.8 | 111.4 ± 42.8 | 115.2 | |
| C avg (nmol L−1) | 8.5 ± 5 | 176.2 | 5.9 ± 2.8 | 141.1 | 4 ± 1.2 | 90.9 | 4.7 ± 1.8 | 115.1 | |
| 3-(Phenyl)propionic acid-3′-sulfate | C max (nmol L−1) | 51.8 ± 31.8 | 184 | 69.5 ± 34.8 | 150.1 | 20 ± 7.4 | 110.8 | 54.1 ± 20.9 | 115.8 |
| T max (h) | 8.4 ± 2.9 | 103.4 | 1.8 ± 0.7 | 108.4 | 8.3 ± 3.5 | 124 | 3.6 ± 2.5 | 209 | |
| AUC0–24 (nmol h L−1) | 516.8 ± 367.9 | 213.6 | 266.6 ± 142.6 | 160.5 | 192.8 ± 79.5 | 123.7 | 252.5 ± 103 | 122 | |
| C avg (nmol L−1) | 21.6 ± 15.4 | 213.7 | 11.1 ± 6 | 160.6 | 8.1 ± 3.4 | 123.7 | 10.6 ± 4.3 | 121.8 | |
| 3-(3′-Hydroxyphenyl)propionic acid-4′-sulfate | C max (nmol L−1) | 0.8 ± 0.2 | 48.8 | 1.3 ± 0.4 | 93.5 | 1.2 ± 0.4 | 89.5 | 1.2 ± 0.3 | 57.9 |
| T max (h) | 4.5 ± 2.4 | 162.2 | 7.2 ± 3 | 121.3 | 6.8 ± 3 | 128.9 | 2.2 ± 0.8 | 101 | |
| AUC0–24 (nmol h L−1) | 6.2 ± 1.5 | 68.9 | 5.2 ± 1.3 | 71.7 | 7.6 ± 1.3 | 48.6 | 5.6 ± 1.1 | 56.8 | |
| C avg (nmol L−1) | 0.3 ± 0.1 | 69.3 | 0.3 ± 0.1 | 68.2 | 0.4 ± 0.1 | 46.9 | 0.3 ± 0.1 | 52.2 | |
| 3′-Hydroxybenzoic acid-4′-glucuronide | C max (nmol L−1) | 21.1 ± 3.8b | 53.4 | 10.5 ± 1.5 | 43 | 10 ± 1.2 | 33.5 | 11.7 ± 2.1 | 51.9 |
| T max (h) | 11.3 ± 3.1 | 81.6 | 4 ± 1.3 | 96.8 | 8 ± 3.5 | 130.9 | 3.4 ± 1.2a | 106.4 | |
| AUC0–24 (nmol h L−1) | 183.8 ± 25.4b | 41.4 | 92.8 ± 11.6 | 37.4 | 113.9 ± 15.3 | 40.1 | 88.6 ± 12.1a | 40.9 | |
| C avg (nmol L−1) | 7.7 ± 1.1 | 41.6 | 3.9 ± 0.5 | 37.3 | 4.8 ± 0.7 | 39.8 | 3.7 ± 0.5a | 40.7 | |
| Benzoic acid-4′-sulfate | C max (nmol L−1) | 21.8 ± 4.4 | 60.3 | 24.3 ± 4.7 | 57.3 | 32.2 ± 7.7 | 71.6 | 36.2 ± 10.8 | 88.93 |
| T max (h) | 12.5 ± 3.5 | 84.5 | 3.5 ± 0.95 | 81.5 | 8.8 ± 2.8 | 94.1 | 4.2 ± 2.5a | 182.5 | |
| AUC0–24 (nmol h L−1) | 240.7 ± 38.9 | 48.5 | 179 ± 49.9 | 83.7 | 347.6 ± 61.1 | 52.8 | 219.8 ± 44 | 60 | |
| C avg (nmol L−1) | 10.1 ± 1.7 | 48.5 | 7.5 ± 2.1 | 83.7 | 14.5 ± 2.6 | 52.9 | 9.2 ± 1.9 | 59.94 | |
Based on the available data on the human and microbial metabolism of flavanones,29,40,41,43 about 54 metabolites were detected and quantified in urine samples (Tables 4 and 5), including both native phase II and low molecular weight conjugated metabolites. The main circulating metabolites, namely naringenin 4′ and 7′-glucuronides, were also the highest phase II metabolites excreted over 48 hours. Based on the urinary excretion of quantified metabolites after the low and the high dose of the flavanone-rich ingredient, no metabolites reached a statistically significant difference between the two doses in the acute study. The predominant metabolites had a coefficient of variation of greater than 100%. The average of the coefficient of phase II metabolites was greater than that of colonic metabolites in all the four groups (average data not shown). Native conjugated metabolite excretion was equal to 36 μmol in the low dose arm and 77.9 μmol in the high dose arm (Table 4), whereas the low molecular weight phenolic excretion was higher compared to native phase II metabolite excretion, in low and high dose consumption, respectively (Table 5). The bioavailability was calculated based on the 48-hour cumulative excretion of different flavanone-rich ingredient doses (Table 6): the highest dose resulted in a higher excretion of metabolites from the ingredient but showed a poorer bioavailability (27.1 ± 4.4%); on the other hand, the lower dose produced a lower excretion amount of metabolites but showed a higher bioavailability (44.9 ± 12.0%).
| Compound | Week 1 – low dose | Week 16 – low dose | Week 1 – high dose | Week 16 – high dose | ||||
|---|---|---|---|---|---|---|---|---|
| 48 h cumulative excretion (μmol) | CV (%) | 48 h cumulative excretion (μmol) | CV (%) | 48 h cumulative excretion (μmol) | CV (%) | 48 h cumulative excretion (μmol) | CV (%) | |
| a Indicates significant differences between W1 and W16 (time effect). | ||||||||
| Naringenin-sulfate | 3.3 ± 5.6 | 179.9 | 2.9 ± 1.3 | 134.1 | 7.1 ± 3.6 | 151.9 | 8.2 ± 3.1 | 114.1 |
| Naringenin-sulfate | 0.4 ± 0.2 | 170.2 | 0.3 ± 0.2 | 157.5 | 0.8 ± 0.5 | 177.5 | 0.5 ± 0.2 | 105.7 |
| Naringenin-7-glucuronide | 6.9 ± 3.9 | 170.3 | 4.0 ± 2.5 | 189.5 | 16.8 ± 8.4 | 150.5 | 7.7 ± 2.9 | 112.4 |
| Naringenin-4′-glucuronide | 17.7 ± 7.9 | 134.6 | 13.9 ± 9.0 | 193.3 | 45.1 ± 21.4 | 142.6 | 24.1 ± 8.2 | 102.0 |
| Naringenin-diglucuronide | 0.1 ± 0.0 | 130.2 | 0.0 ± 0.0 | 184.5 | 0.1 ± 0.0 | 77.2 | 0.1 ± 0.1a | 180.2 |
| Naringenin-diglucuronide | 0.4 ± 0.2 | 157.5 | 0.3 ± 0.2 | 216.0 | 1.2 ± 0.7 | 176.5 | 0.6 ± 0.3 | 178.1 |
| Naringenin-sulfo-glucuronide | 0.4 ± 0.2 | 125.2 | 0.2 ± 0.1 | 150.8 | 1.1 ± 0.6 | 173.5 | 0.5 ± 0.2 | 142.4 |
| Isosakuranetin-sulfate | 0.3 ± 0.2 | 205.2 | 0.2 ± 0.1 | 143.1 | 0.4 ± 0.2 | 134.9 | 0.4 ± 0.2 | 118.9 |
| Isosakuranetin-glucuronide | 0.3 ± 0.1 | 103.6 | 0.2 ± 0.1 | 121.7 | 0.5 ± 0.2 | 92.4 | 0.4 ± 0.1 | 50.7 |
| Eriodictyol-sulfate | 0.3 ± 0.2 | 175.6 | 0.1 ± 0.1 | 155.4 | 0.4 ± 0.2 | 147.7 | 0.5 ± 0.2 | 116.9 |
| Hesperetin-sulfate | 0.0 ± 0.0 | 108.7 | 0.1 ± 0.0 | 111.0 | 0.1 ± 0.1 | 139.3 | 0.1 ± 0.0 | 140.1 |
| Hesperetin-7-glucuronide | 1.2 ± 0.7 | 161.8 | 0.7 ± 0.3 | 113.3 | 1.7 ± 0.5 | 77.6 | 1.3 ± 0.2 | 54.0 |
| Hesperetin-3′-glucuronide | 1.3 ± 0.3 | 67.7 | 1.0 ± 0.2 | 60.9 | 1.0 ± 0.1 | 24.6 | 1.4 ± 0.5 | 101.9 |
| Hesperetin-sulfo-glucuronide | 1.2 ± 0.8 | 196.1 | 0.5 ± 0.3 | 167.1 | 1.6 ± 0.6 | 111.1 | 1.2 ± 0.3 | 76.3 |
| Total phase II metabolites | 36.0 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 μg) 790 μg) |
24.5 (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 845 μg) 845 μg) |
77.9 (37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 331 μg) 331 μg) |
46.9 (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172 μg) 172 μg) |
||||
| Compound | Week 1 – low dose | Week 16 – low dose | Week 1 – high dose | Week 16 – high dose | ||||
|---|---|---|---|---|---|---|---|---|
| 48 h cumulative excretion (μmol) | CV (%) | 48 h cumulative excretion (μmol) | CV (%) | 48 h cumulative excretion (μmol) | CV (%) | 48 h cumulative excretion (μmol) | CV (%) | |
| a Indicates significant differences between W1 and W16. | ||||||||
| 3′-Methoxycinnamic acid-4′-sulfate | 4.3 ± 1.3 | 89.6 | 6.9 ± 4.8 | 209.4 | 3.8 ± 0.6 | 45.4 | 3.6 ± 1.0 | 82.4 |
| 4′-Methoxycinnamic acid-3′-sulfate | 0.1 ± 0.0 | 114.7 | 0.1 ± 0.1 | 139.4 | 0.2 ± 0.0 | 50.0 | 0.2 ± 0.0 | 88.4 |
| 4′-Methoxycinnamic acid-3′-glucuronide | 1.8 ± 0.6 | 94.3 | 1.4 ± 0.5 | 117.1 | 2.0 ± 0.5 | 69.2 | 2.1 ± 0.7 | 104.5 |
| 3-(3′-Methoxyphenyl)propanoic acid-4′-sulfate | 1.3 ± 0.4 | 93.4 | 1.0 ± 0.3 | 86.1 | 1.0 ± 0.2 | 65.8 | 0.9 ± 0.2 | 62.4 |
| 3-(4′-Methoxyphenyl)propanoic acid-3′-sulfate | 1.3 ± 0.3 | 73.7 | 0.8 ± 0.3 | 97.8 | 1.7 ± 0.5 | 86.6 | 2.1 ± 0.9 | 128.5 |
| 3-(3′-Methoxyphenyl)propanoic acid-4′-glucuronide | 1.3 ± 0.6 | 129.6 | 1.0 ± 0.3 | 91.3 | 0.6 ± 0.1 | 58.6 | 0.6 ± 0.2 | 107.6 |
| 3-(4′-Methoxyphenyl)propanoic acid-3′-glucuronide | 4.1 ± 1.4 | 101.2 | 5.2 ± 2.2 | 130.3 | 8.1 ± 1.4 | 53.7 | 6.6 ± 2.3 | 102.9 |
| Hydroxycinnamic acid-sulfate | 1.1 ± 0.4 | 107.8 | 1.8 ± 0.8 | 134.4 | 1.9 ± 0.4 | 67.7 | 1.5 ± 0.5 | 108.9 |
| 3-(3′-Hydroxyphenyl)propanoic acid-4′-sulfate | 3.2 ± 0.5 | 51.9 | 2.2 ± 0.3 | 45.9 | 3.2 ± 0.6 | 52.3 | 4.4 ± 1.7 | 114.8 |
| 3-(4′-Hydroxyphenyl)propanoic acid-3′-sulfate | 1.8 ± 0.7 | 109.2 | 3.7 ± 1.3 | 108.8 | 3.5 ± 1.5 | 130.4 | 2.9 ± 0.8 | 82.8 |
| Hydroxyphenylacetic acid-sulfate | 0.3 ± 0.1 | 119.0 | 0.1 ± 0.0 | 95.6 | 0.4 ± 0.1 | 74.4 | 0.3 ± 0.1 | 69.9 |
| Hydroxyphenylacetic acid-glucuronide | 3.7 ± 1.7 | 139.6 | 2.5 ± 0.8 | 91.3 | 7.1 ± 4.6 | 194.7 | 4.9 ± 2.9 | 176.0 |
| Hydroxybenzoic acid-sulfate (coelution of 3-hydroxybenzoic acid-4-sulfate and 4-hydroxybenzoic acid-3-sulfate) | 1.6 ± 0.5 | 90.1 | 1.8 ± 0.4 | 64.3 | 2.1 ± 0.5 | 75.7 | 3.1 ± 1.3 | 127.1 |
| 3-Hydroxybenzoic acid-4-glucuronide | 0.4 ± 0.1 | 81.5 | 0.3 ± 0.1 | 122.7 | 1.0 ± 0.7 | 233.3 | 0.5 ± 0.4 | 200.2 |
| 4-Hydroxybenzoic acid-3-glucuronide | 0.6 ± 0.2 | 85.0 | 0.3 ± 0.1 | 67.0 | 0.4 ± 0.1 | 84.4 | 0.6 ± 0.2 | 112.3 |
| Methoxyphenylacetic acid-glucuronide | 0.6 ± 0.3 | 147.0 | 2.7 ± 2.5 | 277.1 | 0.6 ± 0.1 | 50.9 | 0.8 ± 0.3 | 115.4 |
| 3-Methoxybenzoic acid-4-sulfate | 7.8 ± 3.0 | 113.3 | 7.6 ± 3.6 | 141.5 | 21.7 ± 13.8 | 190.3 | 14.8 ± 9.4 | 190.3 |
| 4-Methoxybenzoic acid-3-sulfate | 0.4 ± 0.1 | 82.0 | 0.4 ± 0.1 | 111.2 | 0.5 ± 0.2 | 107.2 | 0.7 ± 0.5 | 204.1 |
| 3-Methoxybenzoic acid-4-glucuronide | 3.1 ± 1.2 | 113.0 | 3.0 ± 1.3 | 125.1 | 8.1 ± 5.7 | 212.6 | 5.8 ± 3.8 | 197.4 |
| 4-Methoxybenzoic acid-3-glucuronide | 0.4 ± 0.1 | 55.4 | 0.3 ± 0.1 | 81.3 | 0.8 ± 0.3 | 115.9 | 1.4 ± 0.8 | 163.5 |
| 3-Hydroxy-3-(phenyl)propanoic acid-3′-sulfate | 41.7 ± 14.6 | 104.8 | 18.6 ± 5.1 | 82.9 | 43.0 ± 5.0 | 34.9 | 27.8 ± 5.9a | 63.4 |
| Cinnamic acid-4′-sulfate | 0.1 ± 0.0 | 52.3 | 0.1 ± 0.0 | 80.9 | 0.2 ± 0.0 | 49.4 | 0.1 ± 0.0a | 107.6 |
| Cinnamic acid-3′-sulfate | 0.3 ± 0.1 | 90.9 | 0.2 ± 0.0 | 90.6 | 0.3 ± 0.1 | 92.5 | 0.3 ± 0.1 | 61.4 |
| 3-(Phenyl)propanoic acid-4′-sulfate | 0.2 ± 0.1 | 86.8 | 0.3 ± 0.1 | 66.9 | 0.4 ± 0.1 | 61.4 | 0.4 ± 0.1 | 78.7 |
| 3-(Phenyl)propanoic acid-3′-sulfate | 1.7 ± 1.0 | 176.9 | 1.3 ± 0.7 | 164.9 | 0.5 ± 0.2 | 101.9 | 1.4 ± 0.6 | 134.3 |
| 3-(Phenyl)propanoic acid-glucuronide | 113.3 ± 30.6 | 81.0 | 63.3 ± 18.1 | 85.9 | 102.0 ± 19.7 | 58.0 | 89.7 ± 23.8 | 79.6 |
| Phenylacetic acid-4′-sulfate | 8.1 ± 3.9 | 146.3 | 7.3 ± 3.3 | 136.7 | 8.1 ± 3.0 | 110.7 | 5.5 ± 1.5 | 82.0 |
| Phenylacetic acid-3′-sulfate | 5.2 ± 1.3 | 73.3 | 3.6 ± 1.9 | 157.1 | 16.9 ± 11.1 | 196.7 | 9.1 ± 3.6 | 119.8 |
| Phenylacetic acid-glucuronide | 1.4 ± 0.7 | 156.3 | 0.7 ± 0.3 | 124.3 | 1.1 ± 0.2 | 63.7 | 0.9 ± 0.2 | 80.2 |
| Benzoic acid-4′-sulfate | 243.1 ± 84.9 | 104.8 | 108.2 ± 29.9 | 82.9 | 250.8 ± 29.2 | 34.9 | 162.0 ± 34.2a | 63.4 |
| 4′-Hydroxyhippuric acid | 108.7 ± 26.8 | 74.0 | 55.5 ± 8.7 | 47.1 | 129.2 ± 17.6 | 40.9 | 130.9 ± 24.8 | 56.7 |
| 3′-Hydroxyhippuric acid | 60.7 ± 16.4 | 81.0 | 33.9 ± 9.7 | 85.9 | 54.7 ± 10.6 | 58.0 | 48.1 ± 12.8 | 79.6 |
| Hippuric acid | 390.9 ± 121.0 | 92.9 | 389.8 ± 108.5 | 83.5 | 556.8 ± 178.2 | 96.0 | 479.7 ± 113.1 | 70.7 |
| Dihydroxybenzene-sulfate (isomer 1) | 10.9 ± 3.3 | 90.6 | 8.2 ± 1.6 | 58.6 | 21.6 ± 4.1 | 57.1 | 14.9 ± 2.5a | 49.6 |
| Methoxyhydroxybenzene-sulfate | 24.3 ± 10.4 | 128.5 | 13.8 ± 4.0 | 87.2 | 17.3 ± 2.3 | 40.4 | 15.7 ± 2.8 | 52.8 |
| Hydroxybenzene-sulfate | 51.6 ± 16.0 | 92.9 | 51.5 ± 14.3 | 83.5 | 73.6 ± 23.5 | 96.0 | 63.4 ± 14.9 | 70.7 |
| Hydroxybenzene-glucuronide | 85.9 ± 23.2 | 81.0 | 48.0 ± 13.7 | 85.9 | 77.4 ± 15.0 | 58.0 | 68.1 ± 18.1 | 79.6 |
| Methoxybenzene-sulfate | 43.2 ± 10.3 | 71.5 | 15.5 ± 4.0 | 77.5 | 33.5 ± 5.5 | 49.0 | 33.7 ± 13.2 | 117.8 |
| 2-Hydroxy-2-(3′-hydroxy-methoxyphenyl)acetic acid | 0.5 ± 0.2 | 154.8 | 0.2 ± 0.1 | 127.9 | 0.5 ± 0.1 | 86.5 | 0.5 ± 0.1a | 73.8 |
| 2-Hydroxy-2-(phenyl)acetic acid-3′-sulfate | 10.0 ± 2.2 | 64.8 | 5.4 ± 1.2 | 63.8 | 18.4 ± 3.9 | 63.2 | 12.6 ± 3.0 | 71.5 |
| Total 48 h colonic metabolite excretion | 1241.2 (293![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 042 μg) 042 μg) |
868.5 (231![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 163 μg) 163 μg) |
1474.5 (404![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 403 μg) 403 μg) |
1222.2 (276![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 821 μg) 821 μg) |
||||
| Total metabolites | 1277.2 | 893.1 | 1552.4 | 1269.2 | ||||
| Week 1 – low dose | Week 16 – low dose | Week 1 – high dose | Week 16 – high dose | |
|---|---|---|---|---|
| Bioavailability 0–24 h (%) | 63.9 ± 8.9 | 45.2 ± 9.4 | 42.8 ± 7.6 | 37.9 ± 7.0 |
| Bioavailability 24–48 h (%) | 14.9 ± 10.6 | 15.3 ± 9.4 | 11.3 ± 6.5 | 5.7 ± 2.2 |
| Bioavailability 0–48 h (%) | 44.9 ± 12.0 | 30.2 ± 6.3 | 27.1 ± 4.4 | 21.8 ± 3.6 |
Considering the presence of caffeine in the tested supplement (ESI Table 1†), its metabolism was monitored in plasma and urine (ESI Tables 4 and 5†). Overall, besides a few exceptions, the nutrikinetic profiles of the metabolites were similar between the two doses in the acute study. Caffeine and three of its metabolites, including paraxanthine, 1-methylxanthine, and 1,7-dimethyluric acid, were quantified in plasma (ESI Table 4†). The complete set of circulating metabolites in each group is presented in Fig. 2.
 | ||
| Fig. 2 The total plasma concentration of the polyphenol metabolites. Values are expressed as mean ± SEM (n = 10 for the low dose (LD), n = 9 for the high dose (HD); W1: week 1, W16: week 16). | ||
Circulating and excreted metabolites in the chronic study (W16)
The metabolites identified and quantified at the circulatory and urinary levels after acute consumption were confirmed after 16 weeks (W16) of supplementation with the flavanone-rich ingredient. Contrary to the expectations, an overall reduction in total circulating metabolites was observed. Some individual metabolites, including naringenin (5-, 7-, or 4′)-sulfo-glucuronide, 3′-methoxycinnamic acid-4′-sulfate and 3-hydroxybenzoic acid-4-glucuronide, showed a decrease in their AUC0–24 significantly irrespective of the dosage, indicating a time effect on repeated exposure. The decrease in the AUC0–24 of naringenin (5-, 7-, or 4′)-sulfo-glucuronide and 3-hydroxybenzoic acid-4-glucuronide was accompanied by a significant decrease in Tmax, too. Interestingly, the Tmax of some metabolites, such as isosakuranetin (5-, or 7)-sulfate, isosakuranetin (5-, or 7)-glucuronide, hesperetin (5-, 7-, or 3′)-sulfate, hesperetin-3′-glucuronide and benzoic acid-4-sulfate, decreased over 16 weeks irrespective of the dose and without differences in Cmax or Cavg. Additionally, a dose-dependent decrease in the Tmax of naringenin (5-, 7-, or 4′)-sulfo-glucuronide, 3-(3′-methoxyphenyl)propanoic acid-4′-sulfate, and 3-(4′-methoxyphenyl)propanoic acid-3′-glucuronide was observed. Cmax of 3-hydroxybenzoic acid-4-glucuronide decreased significantly at a low dose and increased insignificantly at a high dose. Cavg of 4 metabolites, namely naringenin (5-, 7-, or 4′)-sulfate, eriodictyol (5-, 7-, 3′-, or 4′)-sulfate, 3′-methoxycinnamic acid-4′-sulfate and 3-hydroxybenzoic acid-4-glucuronide, decreased significantly over 16 weeks. Similar to week 1, the CV% was higher at week 16, although we observed an interesting trend for reduction in CV% for majority of the compounds over time.The mean 48 h cumulative metabolite excretion in urine of each metabolite confirmed the tendency highlighted for circulating metabolites, displaying higher amounts of excreted metabolites at W1 than at W16 (Fig. 3). The sum of all native phase II metabolites, the sum of low molecular weight catabolites and naringenin-diglucuronide reduced significantly over 16 weeks of flavanone-rich ingredient consumption, without differences between doses (Tables 4 and 5). Among low molecular weight phenolics, methoxybenzene-sulfate, benzoic acid-4-sulfate, 3-hydroxy-3-(phenyl)propanoic acid-3′-sulfate, cinnamic acid-4′-sulfate, and 2-hydroxy-2-(3′-hydroxy-methoxyphenyl)acetic acid reduced significantly over time. There were no dose-dependent changes in the urinary excretion of quantified metabolites after 16-week flavanone-rich ingredient supplementation and benzoic acid-4-sulfate was the predominant phenolic metabolite in urine.
 | ||
| Fig. 3 48 h cumulative excretion of polyphenol metabolites in urine. Values are expressed as mean ± SEM (n = 10 for the low dose (LD), n = 9 for the high dose (HD); W1: week 1, W16: week 16). | ||
After the daily chronic supplementation (W16), non-significant changes in the flavanone-rich ingredient bioavailability were observed, resulting in 30.2 ± 6.3% and 21.8 ± 3.6% at low and high doses, respectively.
The low and the high dose accounted for 739.27 μmol (334.8 mg) and 1478.54 μmol (669.6 mg) of flavanones per day, respectively. The consideration of the daily consumption allowed to calculate the daily bioavailability of flavanones. Considering the first visit (W1), the 0–24 h mean bioavailability resulted 62.3 ± 6.2% in the low dose arm and 45.7 ± 7.5% in the high dose arm (Table 6), indicating a bioavailability reduction of about 15%. Taking into account the 24–48 h excretion period, the mean bioavailability was twice lower compared to that calculated after the first day consumption, suggesting that a multiple and continual dose could influence the bioavailability of the flavanone-rich extract. This observation was confirmed when the bioavailability after the 16-week treatment was calculated. The 0–24 h mean bioavailability after the 16-week treatment was −20% in the low dose arm and −7% in the high dose arm (Table 6).
Finally, contrary to what has been observed for flavanone metabolites, the chronic consumption (W16) of the flavanone-rich ingredient resulted in lower circulating concentrations and excreted amounts of caffeine and its metabolites, compared to acute administration, both in the low and high dose arms (ESI Table 5†). The total excreted metabolites in each group are represented in Fig. 3.
Discussion
Sinetrol® Xpur is a (poly)phenol extract rich in flavanones, mainly containing hesperidin and naringin, and it has been developed to help body fat mass reduction, within a varied and balanced diet. This study reports the detailed characterization of the supplement and the metabolic fate of its potentially bioactive compounds in an acute (1 week) and chronic (16 weeks) setup. The characterization of the flavanone-rich ingredient identified naringenin-7-O-neohesperidoside and hesperidin-7-O-rutinoside as the main flavonoid compounds. In line with this (poly)phenolic composition, high concentrations of naringenin and hesperidin phase II conjugates were quantified in plasma, namely 4′, 7′ and 3′-glucuronide flavanones. The Tmax values of these conjugated metabolites, generally >4 h after supplement administration in both arms, indicated that these compounds underwent hydrolysis of their rhamnoglycosides by lactase phlorizin hydrolase and enzymes of the intestinal microbiota. Released aglycones are absorbed and undergo sulfation or glucuronidation after absorption. This hypothesis is in line with previous studies that reported the maximum concentration of these phase II metabolites 4–5 h after citrus fruit consumption.44,45 Another potential reason, which may justify the calculated Tmax for most of the detected metabolites, could be the study design: flavanone-rich supplements were administered on two different occasions (the first one at breakfast, the second one at lunch), for 24 hours. The accumulation of compounds in plasma could have led to a later observed Tmax. Finally, the calculated Tmax could represent hybrid values resulting from both the absorption at the small intestinal level of those metabolites produced after the consumption of the second dose at lunch, and those catabolites produced from the native compounds which reached undigested to the colon after the consumption of the first supplement dose at breakfast, as previously reported in a daily repeated-dose human intervention study with coffee.46Comparing the different tested doses, the high dose presented a higher excretion of total metabolites with respect to the low dose in both acute and chronic studies. In urine, several phenylpropanoic, phenylacetic and benzoic acid derivatives were detected, indicating the extensive enzymatic actions of gut microbiota. Besides hippuric acid, for which mammalian pathways from benzoic acid, phenylalanine and tyrosine have been reported,47–49 benzoic acid-4-sulfate was the metabolite excreted at the highest concentration, being this catabolite identified as a potential end-product of several metabolic pathways including naringenin, caffeic acid and hesperidin.50 Microorganisms of the gut, such as Bifidobacterium and Lactobacilli, have shown the ability to metabolize naringenin and hesperidin into various metabolites, such as 3-(3′-hydroxyphenyl)propanoic acid and 3-(phenyl)propanoic acid, which was confirmed in the present study.40 It is important to insist that the huge inter-individual variability in the gut microbial composition could also play an important role in the quantity and diversity of these metabolites, as recently suggested. We also observed a high inter-individual variability in the excreted metabolites, which interestingly showed a lowered variability at week 16 compared to week 1. Indeed, Fraga and colleagues, after administration of orange juice to subjects for 60 days, concluded that stratification of volunteers based on flavanone metabolite and catabolite production could be a strategy to explain the high variability in the responsiveness of orange juice consumption in body fat percentage and blood pressure reduction.51
Interestingly, the observations in the chronic study compared to the acute study were contradictory to the expectations. A reduction in circulating and excreted metabolites at W16 compared to W1 was observed. Although no previous studies have investigated the chronic effects of citrus (poly)phenol metabolism, a few studies dealt with the chronic and repeated consumption of mango and grape (poly)phenols.22,52 In the above-mentioned studies, the authors observed that the repeated exposure to (poly)phenols in a lean population differed from the obese population. In particular, in the obese population, the repeated exposure of (poly)phenols resulted in decreased circulating and excreted metabolites. Contrastingly, rodent models have shown that the obese/diabetic state can increase the mRNA expressions of phase II enzymes in comparison to healthy controls, which resulted in an increased AUC and Cmax of metabolites in obese/diabetic rodents.23 Similar to the phase II enzymes, the cellular efflux transporters play an important role in determining the cellular availability of these metabolites, and factors influencing these transporters can affect the bioavailability and rate of excretion of conjugated metabolites. An example is the transporter multidrug resistance-associated protein (MRP)-3 that has shown responsiveness to inflammatory stimuli, which could, in turn, affect the transport of various (poly)phenol metabolites.30 It could be hypothesized that during the 16-week consumption of the flavanone-rich ingredient, the changes in adipose tissue and/or body weight (data not yet published) could alter the metabolic state and, consequently, the catabolism and the uptake of flavanones contained in the supplement. Rangel-Huerta and colleagues showed that the consumption of orange juice protected against DNA damage and lipid peroxidation, modified several antioxidant enzymes, and reduced body weight in overweight or obese non-smoking adults regardless of the (poly)phenol content.53 Although the exact alterations in (poly)phenol metabolism during the obese state are unknown, it can be said that there are important considerations to make while interpreting pharmacokinetic studies depending on the metabolic state of the study population.
Kim and colleagues observed in a rodent model an “increase and then decrease” pattern for (epi)catechin derivatives, where the (epi)catechin metabolites in plasma increased from day 1 to 14 and then decreased to the starting values on day 28.30 Based on this evidence, it could be suggested that a possible similar pattern might have occurred in the present study, although no in-between W1 and W16 time points have been collected for (poly)phenol metabolite analysis. The results obtained for a 16-week supplementation with a flavanone-rich ingredient could indicate an “absorption saturation” condition involving both enzymes and efflux transporters, which needs to be confirmed by further studies aimed at looking at a multiple dose metabolic effect. As suggested by Kim and colleagues, it can be supposed that some flavanone-rich ingredient-derived metabolites and catabolites would not be identified, and some metabolites would be accumulated in tissues. Several studies showed an accumulation of (poly)phenolic compounds in the liver, lungs, brain, heart and adipose tissue.19,54–56 Therefore, exploring tissue accumulation of those metabolites derived from this flavanone-rich ingredient in future studies would be essential to investigate its possible mechanisms of action on adipose tissue and, consequently, on its potential role in weight control.
Despite a reduction in the calculated bioavailability, high dose supplementation of the flavanone-rich ingredient had a 20% and 44% higher excretion of metabolites compared to low dose supplementation in acute and chronic studies, respectively. This observation raises an important question: would quantifying urinary metabolites be sufficient to calculate the bioavailability of ingested (poly)phenols? The evaluation of individual (poly)phenol classes in a systematic review, taking into consideration only intervention studies, has shown that hesperidin and naringenin showed a weak recovery yield.57 The limitation of bioavailability evaluation also lies in the lack of adequate standard compounds, which could lead to an over- or under-estimation of metabolites. Thus, future discussions on bioavailability evaluation, considering not only urinary and plasma metabolites, but also factors which could affect bioefficacy, as well as the possible presence of metabolites in target cells, could be of interest. Although evaluating tissue metabolites and bioefficacy in in vitro and animal studies could require more resources, it could give detailed information on the fate of (poly)phenolic metabolites.49,50 The sample size of this study is a limitation to be mentioned; although we did not conduct power estimation for this study, we relied on previous studies conducted in the chronic setup for polyphenol pharmacokinetics evaluations. Even though participants were advised about restrictions on diet, measuring baseline polyphenol levels prior to start of the restriction diet in future studies would allow to remove the confounders. Other limitations of this study are mainly related to sample analysis, since neither faeces nor possible accumulation in tissues were analysed for their possible flavanone metabolite content. Moreover, the lack of proper available standard compounds may have led to a possible under- or over-estimation of the quantified metabolites and catabolites.58 Finally, recruited people were overweight/obese, and their physiological status may have influenced the ingredient flavanone metabolism, and consequently, the results of this study must be carefully evaluated against a healthy population.
In conclusion, the consumption of a high dose of a flavanone-rich ingredient, within a varied and balanced diet, in an acute setup, could increase circulating bioactive (poly)phenolic metabolites and catabolites compared to a low dose. Considering the currently available definition of bioavailability, flavanones consumed at a lower dose appear to be more bioavailable compared to a high dose, both in acute and chronic consumption. Future chronic studies with citrus (poly)phenols are required to understand their absorption bioefficacy and their possible presence in various tissues.
Data availability
The data presented in this study are available on request from the corresponding author, due to privacy restriction.Author contributions
Conceptualization, C. R. and J. C.; methodology, L. H. C., P. A., L. B., P. M., D. A. and D. D. R.; investigation, L. H. C., P. E. A., L. B. and P. M.; data analysis, L. B. and J. M.; writing—original draft preparation, J. M. and L. B.; writing—review and editing, J. M., L. B., C. R., P. M., D. D. R. and J. C. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
C. R., J. M. and J. C. are employed by Fytexia. Fytexia is involved in the research and development and marketing and sales of (poly)phenol extract-based ingredients for the food and nutraceutical industries and supported the study. Therefore, Fytexia has a commercial interest in this publication. Human Nutrition Unit, Department of Food & Drug, University of Parma was paid by Fytexia to perform and report the scientific work that formed the basis of this publication. Fytexia approved the final trial protocol prior to its implementation, but was not involved in the study implementation and data collection. L. B., P. M., L. H. C., P. E. A., D. A. and D. D. R. declare no conflict of interest.Acknowledgements
This study was financially supported by Fytexia SAS.References
- R. N. Haththotuwa, C. N. Wijeyaratne and U. Senarath, Worldwide epidemic of obesity, in Obesity and Obstetrics, ed. T. A. Mahmood and S. Arulkumaran, F. A. Chervenak, Elsevier, 2nd edn, 2020, ch. 1, pp. 3–8. Available from: https://www.sciencedirect.com/science/article/pii/B9780128179215000011 Search PubMed.
- WHO, Obesity and overweight Key facts, Fact sheet No. 311, 2015.
- M. Zhang, T. Hu, S. Zhang and L. Zhou, Associations of Different Adipose Tissue Depots with Insulin Resistance: A Systematic Review and Meta-analysis of Observational Studies, Sci. Rep., 2015, 5, 18495, DOI:10.1038/srep18495.
- H. Cory, S. Passarelli, J. Szeto, M. Tamez and J. Mattei, The Role of Polyphenols in Human Health and Food Systems: A Mini-Review, Front. Nutr., 2018, 87, DOI:10.3389/fnut.2018.00087.
- J. J. Peterson, J. T. Dwyer, P. F. Jacques and M. L. McCullough, Associations between flavonoids and cardiovascular disease incidence or mortality in European and Us populations, Nutr. Rev., 2012, 70, 491–508, DOI:10.1111/j.1753-4887.2012.00508.x.
- A. C. D. Lima, C. Cecatti, M. P. Fidélix, M. A. T. Adorno, I. K. Sakamoto, T. B. Cesar and K. Sivieri, Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials, J. Med. Food, 2019, 22, 202–210, DOI:10.1089/jmf.2018.0080.
- M. Zhang, S. Zhu, W. Yang, Q. Huang and C. T. Ho, The biological fate and bioefficacy of citrus flavonoids: bioavailability, biotransformation, and delivery systems, Food Funct., 2021, 12, 3307–3323 RSC . Available from: https://pubs.rsc.org/en/content/articlehtml/2021/fo/d0fo03403g.
- N. H. Zaidun, Z. C. Thent and A. A. Latiff, Combating oxidative stress disorders with citrus flavonoid: Naringenin, Life Sci., 2018, 111–122 CrossRef CAS PubMed.
- R. C. L. A. Coelho, H. H. M. Hermsdorff and J. Bressan, Anti-inflammatory Properties of Orange Juice: Possible Favorable Molecular and Metabolic Effects, Plant Foods Hum., 2013, 1–10 Search PubMed.
- S. A. Ross, D. S. Ziska, K. Zhao and M. A. Elsohly, Variance of common flavonoids by brand of grapefruit juice, Fitoterapia, 2000, 154–161 CrossRef CAS PubMed.
- P. Mouly, E. M. Gaydou and A. Auffray, Simultaneous separation of flavanone glycosides and polymethoxylated flavones in citrus juices using liquid chromatography, J. Chromatogr. A, 1998, 171–179 CrossRef CAS PubMed.
- N. Rani, S. Bharti, B. Krishnamurthy, J. Bhatia, C. Sharma, M. Amjad Kamal, S. Ojha and D. Singh Arya, Pharmacological Properties and Therapeutic Potential of Naringenin: A Citrus Flavonoid of Pharmaceutical Promise, Curr. Pharm. Des., 2016, 4341–4359 CrossRef CAS PubMed.
- E. Hernández-Aquino and P. Muriel, Beneficial effects of naringenin in liver diseases: Molecular mechanisms, World J. Gastroenterol., 2018, 1679 CrossRef PubMed.
- E. E. Mulvihill, J. M. Assini, B. G. Sutherland, A. S. Dimattia, M. Khami, J. B. Koppes, C. G. Sawyez, S. C. Whitman and M. W. Huff, Naringenin decreases progression of atherosclerosis by improving dyslipidemia in high-fat-fed low-density lipoprotein receptor-null mice, Arterioscler., Thromb., Vasc. Biol., 2010, 742–748 CrossRef CAS PubMed.
- D. J. D. Hartogh and E. Tsiani, Antidiabetic properties of naringenin: A citrus fruit Polyphenol, Biomolecules, 2019, 99 CrossRef PubMed.
- C. Manach, C. Morand, A. Gil-Izquierdo, C. Bouteloup-Demange and C. Remesy, Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice, Eur. J. Clin. Nutr., 2003, 57, 235–242 CrossRef CAS PubMed.
- M. Dall'Asta, E. Derlindati, V. Curella, P. Mena, L. Calani, S. Ray, I. Zavaroni, F. Brighenti and D. Del Rio, Effects of naringenin and its phase II metabolites on in vitro human macrophage gene expression, Int. J. Food Sci. Nutr., 2013, 64, 843–849, DOI:10.3109/09637486.2013.804039.
- F. A. Tomás-Barberán, M. V. Selma and J. C. Espín, Interactions of gut microbiota with dietary polyphenols and consequences to human health, Curr. Opin. Clin. Nutr. Metab. Care, 2016, 19, 471–476 CrossRef PubMed . Available from: https://journals.lww.com/co-clinicalnutrition/Fulltext/2016/11000/Interactions_of_gut_microbiota_with_dietary.12.aspx.
- D. Carregosa, C. Pinto, M.Á Ávila-Gálvez, P. Bastos, D. Berry and C. N. Santos, A look beyond dietary (poly)phenols: The low molecular weight phenolic metabolites and their concentrations in human circulation, Compr. Rev. Food Sci. Food Saf., 2022, 21, 3931–3962, DOI:10.1111/1541-4337.13006.
- L. Bresciani, L. Calani, L. Bocchi, F. Delucchi, M. Savi, S. Ray, F. Brighenti, D. Stilli and D. Del Rio, Bioaccumulation of resveratrol metabolites in myocardial tissue is dose-time dependent and related to cardiac hemodynamics in diabetic rats, Nutr., Metab. Cardiovasc. Dis., 2014, 24, 408–415, DOI:10.1016/j.numecd.2013.09.008.
- C. R. Lanzi, D. J. Perdicaro, A. Antoniolli, P. Piccoli, M. A. V. Prieto and A. Fontana, Phenolic metabolites in plasma and tissues of rats fed with a grape pomace extract as assessed by liquid chromatography-tandem mass spectrometry, Arch. Biochem. Biophys., 2018, 651, 28–33 CrossRef PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0003986118302522.
- J. A. Novotny, T. Y. Chen, A. I. Terekhov, S. K. Gebauer, D. J. Baer, L. Ho, G. M. Pasinetti and M. G. Ferruzzi, The effect of obesity and repeated exposure on pharmacokinetic response to grape polyphenols in humans, Mol. Nutr. Food Res., 2017, 61, 1700043, DOI:10.1002/mnfr.201700043.
- B. W. Redan, K. K. Buhman, J. A. Novotny and M. G. Ferruzzi, Altered Transport and Metabolism of Phenolic Compounds in Obesity and Diabetes: Implications for Functional Food Development and Assessment, Adv. Nutr., 2016, 7, 1090–1104 CrossRef CAS PubMed . Available from: https://academic.oup.com/advances/article/7/6/1090/4568666.
- R. Estruch, E. Ros, J. Salas-Salvadó, M. I. Covas, D. Corella, F. Arós, E. Gómez-Gracia, V. Ruiz-Gutiérrez, M. Fiol and J. Lapetra, et al., Primary prevention of cardiovascular disease with a Mediterranean diet, N. Engl. J. Med., 2013, 1279–1290 CrossRef CAS PubMed.
- C. Dallas, A. Gerbi, G. Tenca, F. Juchaux and F. X. Bernard, Lipolytic effect of a polyphenolic citrus dry extract of red orange, grapefruit, orange (SINETROL) in human body fat adipocytes, Mechanism of action by inhibition of cAMP-phosphodiesterase (PDE), Phytomedicine, 2008, 783–792 CrossRef CAS PubMed.
- C. Dallas, A. Gerbi, Y. Elbez, P. Caillard, N. Zamaria and M. Cloarec, Clinical study to assess the efficacy and safety of a citrus polyphenolic extract of red orange, grapefruit, and orange (sinetrol-xpur) on weight management and metabolic parameters in healthy overweight individuals, Phytother. Res., 2014, 212–218 CrossRef CAS PubMed.
- J. Cases, C. Romain, C. Dallas, A. Gerbi and J. M. Rouanet, A 12-week randomized double-blind parallel pilot trial of Sinetrol XPur on body weight, abdominal fat, waist circumference, and muscle metabolism in overweight men, Int. J. Food Sci. Nutr., 2015, 471–477 CrossRef CAS PubMed.
- J. M. Yoo, M. Lee, H. O. Kwon, S. G. Choi, M. H. Bae and O. K. Kim, Effects of Sinetrol-XPur on leptin-deficient obese mice and activation of cAMP-dependent UCP-2, J. Korean Soc. Food Sci. Nutr., 2016, 484–491 CrossRef.
- G. Pereira-Caro, I. A. Ludwig, T. Polyviou, D. Malkova, A. García, J. M. Moreno-Rojas and A. Crozier, Identification of Plasma and Urinary Metabolites and Catabolites Derived from Orange Juice (Poly)phenols: Analysis by High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry, J. Agric. Food Chem., 2016, 64, 5724–5735, DOI:10.1021/acs.jafc.6b02088.
- S. Kim, M. J. Lee, J. Hong, C. Li, T. J. Smith, G. Y. Yang, D. N. Seril and C. S. Yang, Plasma and Tissue Levels of Tea Catechins in Rats and Mice During Chronic Consumption of Green Tea Polyphenols, Nutr. Cancer, 2009, 37, 41–48, DOI:10.1207/S15327914NC3701_5.
- World Health Organisation, Declaration of Helsinki World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects, J. Am. Med. Assoc., 2013, 310, 2191–2194 CrossRef PubMed.
- A. Vijayananthan and O. Nawawi, The importance of Good Clinical Practice guidelines and its role in clinical trials, Biomed. Imaging Intervention J., 2008, 4, e5 CAS.
- A. M. Roza and H. M. Shizgal, The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass, Am. J. Clin. Nutr., 1984, 168–182 CrossRef CAS PubMed.
- F. Castello, G. Costabile, L. Bresciani, M. Tassotti, D. Naviglio, D. Luongo, P. Ciciola, M. Vitale, C. Vetrani and G. Galaverna, et al., Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans, Arch. Biochem. Biophys., 2018, 646, 1–9 CrossRef CAS PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0003986118301450.
- R. Feliciano, E. Mecha, M. Bronze and A. Rodriguez-Mateos, Development and validation of a high-throughput micro solid-phase extraction method coupled with ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid identification and quantification of phenolic metabolites in human plasma and urine, J. Chromatogr. A, 2016, 1464, 21–31 CrossRef CAS PubMed.
- N. Brindani, P. Mena, L. Calani, I. Benzie, S. W. Choi, F. Brighenti, F. Zanardi, C. Curti and D. Del Rio, Synthetic and analytical strategies for the quantification of phenyl-γ-valerolactone conjugated metabolites in human urine, Mol. Nutr. Food Res., 2017, 61, 1700077, DOI:10.1002/mnfr.201700077.
- G. Bianco, S. Abate, C. Labella and T. R. I. Cataldi, Identification and fragmentation pathways of caffeine metabolites in urine samples via liquid chromatography with positive electrospray ionization coupled to a hybrid quadrupole linear ion trap (LTQ) and Fourier transform ion cyclotron resonance mass spectrometry and tandem mass spectrometry, Rapid Commun. Mass Spectrom., 2009, 23, 1065–1074, DOI:10.1002/rcm.3969.
- S. Martínez-López, B. Sarriá, G. Baeza, R. Mateos and L. Bravo-Clemente, Pharmacokinetics of caffeine and its metabolites in plasma and urine after consuming a soluble green/roasted coffee blend by healthy subjects, Food Res. Int., 2014, 64, 125–133 CrossRef PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0963996914003585.
- Y. Zhang, M. Huo, J. Zhou and S. Xie, PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel, Comput. Methods Programs Biomed., 2010, 99, 306–314 CrossRef PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0169260710000209.
- G. Pereira-Caro, B. Fernández-Quirós, I. A. Ludwig, I. Pradas, A. Crozier and J. M. Moreno-Rojas, Catabolism of citrus flavanones by the probiotics Bifidobacterium longum and Lactobacillus rhamnosus, Eur. J. Nutr., 2016, 57, 231–242, DOI:10.1007/s00394-016-1312-z.
- X. Zeng, Y. Bai, W. Peng and W. Su, Identification of Naringin Metabolites in Human Urine and Feces, Eur. J. Drug Metab. Pharmacokinet., 2017, 42, 647–656, DOI:10.1007/s13318-016-0380-z.
- F. Castello, G. Costabile, L. Bresciani, M. Tassotti, D. Naviglio, D. Luongo, P. Ciciola, M. Vitale, C. Vetrani and G. Galaverna, et al., Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans, Arch. Biochem. Biophys., 2018, 646, 1–9 CrossRef CAS PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0003986118301450.
- X. Zeng, H. Yao, Y. Zheng, T. Chen, W. Peng, H. Wu and W. Su, Metabolite Profiling of Naringin in Rat Urine and Feces Using Stable Isotope-Labeling-Based Liquid Chromatography-Mass Spectrometry, J. Agric. Food Chem., 2020, 68, 409–417, DOI:10.1021/acs.jafc.9b06494.
- I. Erlund, E. Meririnne, G. Alfthan and A. Aro, Plasma Kinetics and Urinary Excretion of the Flavanones Naringenin and Hesperetin in Humans after Ingestion of Orange Juice and Grapefruit Juice, J. Nutr., 2001, 131, 235–241 CrossRef CAS PubMed . Available from: https://academic.oup.com/jn/article/131/2/235/4686993.
- C. Manach, C. Morand, A. Gil-Izquierdo, C. Bouteloup-Demange and C. Rémésy, Bioavailability in humans of the flavanones hesperidin and narirutin after the ingestion of two doses of orange juice, Eur. J. Clin. Nutr., 2003, 57, 235–242 CrossRef CAS PubMed . Available from: https://www.nature.com/articles/1601547.
- P. Mena, L. Bresciani, M. Tassotti, A. Rosi, D. Martini, M. Antonini, A. D. Cas, R. Bonadonna, F. Brighenti and D. Del Rio, Effect of different patterns of consumption of coffee and a cocoa-based product containing coffee on the nutrikinetics and urinary excretion of phenolic compounds, Am. J. Clin. Nutr., 2021, 114, 2107–2118 CrossRef PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S000291652200541X.
- M. N. Clifford, E. L. Copeland, J. P. Bloxsidge and L. A. Mitchell, Hippuric acid as a major excretion product associated with black tea consumption, Xenobiotica, 2000, 30, 317–326, DOI:10.1080/004982500237703.
- H. L. Self, R. R. Brown and J. M. Price, Quantitative Studies on the Metabolites of Tryptophan in the Urine of Swine, J. Nutr., 1960, 70, 21–25, DOI:10.1093/jn/70.1.21.
- J. W. Bridges, M. R. French, R. L. Smith and R. T. Williams, The fate of benzoic acid in various species, Biochem. J., 1970, 118, 47–51 CrossRef CAS PubMed.
- C. D. Kay, G. Pereira-Caro, I. A. Ludwig, M. N. Clifford and A. Crozier, Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence, Annu. Rev. Food Sci. Technol., 2017, 8, 155–180, DOI:10.1146/annurev-food-030216-025636.
- L. N. Fraga, C. P. Coutinho, A. C. Rozenbaum, E. D. C. Tobaruela, F. M. Lajolo and N. M. A. Hassimotto, Blood pressure and body fat % reduction is mainly related to flavanone phase II conjugates and minor extension by phenolic acid after long-term intake of orange juice, Food Funct., 2021, 12, 11278–11289, 10.1039/D1FO02664J.
- R. C. Barnes, H. Kim, C. Fang, W. Bennett, M. Nemec, M. A. Sirven, J. S. Suchodolski, N. Deutz, R. A. Britton and S. U. Mertens-Talcott, et al., Body Mass Index as a Determinant of Systemic Exposure to Gallotannin Metabolites during 6-Week Consumption of Mango (Mangifera indica L.) and Modulation of Intestinal Microbiota in Lean and Obese Individuals, Mol. Nutr. Food Res., 2019, 63, 1800512, DOI:10.1002/mnfr.201800512.
- O. D. Rangel-Huerta, C. M. Aguilera, M. V. Martin, M. J. Soto, M. C. Rico, F. Vallejo, F. Tomas-Barberan, A. J. Perez-de-la-Cruz, A. Gil and M. D. Mesa, Normal or High Polyphenol Concentration in Orange Juice Affects Antioxidant Activity, Blood Pressure, and Body Weight in Obese or Overweight Adults1, 2, 3, 4, J. Nutr., 2015, 145, 1808–1816 CrossRef CAS PubMed https://www.sciencedirect.com/science/article/pii/S0022316622088265 .
- Y. Fukuchi, M. Hiramitsu, M. Okada, S. Hayashi, Y. Nabeno, T. Osawa and M. Naito, Lemon Polyphenols Suppress Diet-induced Obesity by Up-Regulation of mRNA Levels of the Enzymes Involved in β-Oxidation in Mouse White Adipose Tissue, J. Clin. Biochem. Nutr., 2008, 43, 201–209 CrossRef PubMed.
- A. Serra, C. Bladé, L. Arola, A. Macià and M. J. Motilva, Flavanol metabolites distribute in visceral adipose depots after a long-term intake of grape seed proanthocyanidin extract in rats, Br. J. Nutr., 2013, 110, 1411–1420 CrossRef CAS PubMed . Available from: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/flavanol-metabolites-distribute-in-visceral-adipose-depots-after-a-longterm-intake-of-grape-seed-proanthocyanidin-extract-in-rats/B815EC0512ACFC0071576DB5A2A55B8F.
- C. Andres-Lacueva, M. T. MacArulla, M. Rotches-Ribalta, M. Boto-Ordóñez, M. Urpi-Sarda, V. M. Rodríguez and M. P. Portillo, Distribution of resveratrol metabolites in liver, adipose tissue, and skeletal muscle in rats fed different doses of this polyphenol, J. Agric. Food Chem., 2012, 60, 4833–4840, DOI:10.1021/jf3001108.
- J. Pérez-Jiménez, J. Hubert, L. Hooper, A. Cassidy, C. Manach, G. Williamson and A. Scalbert, Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review, Am. J. Clin. Nutr., 2010, 92, 801–809 CrossRef PubMed . Available from: https://academic.oup.com/ajcn/article/92/4/801/4597530.
- J. I. Ottaviani, R. Y. Fong, G. Borges, H. Schroeter and A. Crozier, Use of LC-MS for the quantitative analysis of (poly)phenol metabolites does not necessarily yield accurate results: Implications for assessing existing data and conducting future research, Free Radicals Biol. Med., 2018, 124, 97–103 CrossRef CAS PubMed . Available from: https://www.sciencedirect.com/science/article/pii/S0891584918309717.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo02820h |
| This journal is © The Royal Society of Chemistry 2023 |