Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Casein–phenol interactions occur during digestion and affect bioactive peptide and phenol bioaccessibility†
Aytul
Hamzalioglu
*a,
Silvia
Tagliamonte
b,
Vural
Gökmen
 a and
Paola
Vitaglione
a and
Paola
Vitaglione
 *b
*b
aFood Quality and Safety (FoQuS) Research Group, Department of Food Engineering, Hacettepe University, 06800 Beytepe, Ankara, Turkey. E-mail: aytulhamzalioglu@hacettepe.edu.tr
bDepartment of Agricultural Sciences, University of Naples, 80055 Portici, Naples, Italy. E-mail: paola.vitaglione@unina.it
First published on 4th October 2023
Abstract
Casein (CN) represents many proline residues that may bind polyphenols. Some evidence exists of CN-polyphenols interaction in model systems. The formation of such interactions upon digestion and the effects on CN digestibility and potential functionality due to the release of bioactive peptides are obscure. This study aimed to explore the interactions of CN with different phenol compounds under digestive conditions and monitor how they affect the bioaccessibility of phenol compounds and bioactive peptides. CN or CN hydrolysate and phenol compounds such as chlorogenic acid, ellagic acid, catechin, green tea extract, and tea extract, singularly or in combination with CN were digested in vitro. Total antioxidant capacity (TAC), degree of hydrolysis, and bioactive peptide formation were assessed in the samples collected through the digestion. The results showed that bioaccessible TAC was 1.17 to 1.93-fold higher in CN co-digested with phenol compounds than initially due to a higher release of antioxidant peptides in the presence of phenolic compounds. However, TAC values in the intestinal insoluble part of CN–phenol digests were higher than the initial, indicating that such interactions may be functional to transport phenols to the colon. Bioactive peptide release was affected by the phenol type (catechins were the most effective) as well as phenol concentration. As an opioid peptide released from β-CN, β-casomorphin formation was significantly influenced by the co-digestion of CN with phenol compounds. This study confirmed the possible CN–phenol interaction during digestion, affecting bioactive peptide release.
Introduction
Due to their nature, proteins have reactive sites that are susceptible to phenol attacks leading to protein–phenol interactions. They are mostly non-covalent interactions such as hydrophobic, van der Waals, hydrogen bonding, and ionic interactions, therefore they are weak and reversible.1 However, covalent interactions have also been reported to take place between reactive sites of proteins and quinone forms of oxidized phenols.2 Typically, non-covalent interactions take place in mild conditions. For instance, at neutral pH, hydrogen bonds and hydrophobic interactions dominated the non-covalent interactions between soy protein and tea polyphenols.3 However, it was also found that hydrophobic interactions and hydrogen bonds were involved in the binding of soy protein to EGCG under acidic conditions.4 When the pH is neutral or alkaline (pH > pI), anionic side groups of protein can encourage the hydrophobic areas that can subsequently hydrophobically engage with nonpolar aromatic rings in polyphenols. On the other hand, covalent interactions mostly take place when the pH is alkaline.5 Alkaline pH conditions are important for stimulating such interactions, as pH highly changes the charge of reactive sites and the stability of phenol compounds.6 Tryptophan, cysteine, methionine, histidine, tyrosine, and proline are examples of nucleophilic residues on protein side chains that covalently cross-link with polyphenol compounds in the presence of oxygen.7 Indeed, at an alkaline pH with oxygen, phenol compounds are oxidized to quinone forms, which are highly electrophilic compounds. Owing to this high electrophilicity, they become prone to react with nucleophilic residues of protein or peptides via Schiff base (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and Michael addition mechanisms (C–NH) and may induce protein crosslinking.8
N) and Michael addition mechanisms (C–NH) and may induce protein crosslinking.8
To be formed the protein–phenol complexes take advantage of the vast amount of residual reactive groups present in proteins, especially proline residues.9 Casein (CN), which comprises 80% of the proteins in cow's milk, has a large proportion of proline residues equally dispersed across their amino acid sequences and generally open structural characteristics. Therefore, CNs are available for protein–phenol interactions as has been demonstrated using purified CNs and flavan-3-ols or tea phenolics in model solutions.10 Moreover, the interactions of CNs with tea catechins were demonstrated capable of reducing the astringency of those polyphenols11,12 whereas interactions of CN with naringenin were indicated as a potential nano-carrier system for naringenin.13 Therefore, clarifying the interactions of CN with phenol compounds can shed light on both sensory and functional aspects of food.
From a biological perspective, protein–phenol interactions might affect the bioaccessibility of both protein and phenol compounds. There is a debate about how milk digestion is affected by protein–phenol interactions. Some studies report that the in vivo antioxidant activity of green and black tea is negatively affected by the combined consumption of milk.14,15 On the contrary, other studies find that the addition of milk to green and black tea does not lead to any differences in plasma antioxidant activity.16,17 Additionally, it is reported that catechin availability after in vitro digestion was higher in green and black tea with milk compared to tea alone.18,19 These studies report how the extent of protein bioaccessibility was affected by monitoring the inhibition/promotion of the amino acid release. However, dipeptides, tripeptides, and larger peptides may be absorbed besides amino acids through a paracellular path under certain conditions, and the formation of these peptides might be affected by protein–phenol interactions. For instance, the allergen peptide formation was influenced by the protein–phenol interactions.20,21 Covalent binding of dietary polyphenols to peanut allergen peptide, Ara h1, significantly reduced its immunoglobulin E binding capacity. Similarly, the conjugation of lactoferrin protein with EGCG reduced the binding capacity of lactoferrin to IgE and immunoglobulin G. In addition to these, some of the peptides released during the digestion of proteins might be bioactive peptides (BAPs), i.e. they are physiologically active.22 Milk proteins, including CNs, are an important source of BAPs23 that are available in circulation after consuming milk as we have recently demonstrated in humans.24 Among CN-derived BAPs, the opioid β-casomorphins (BCMs) were the most studied.25 BCMs have been found to prolong gastrointestinal transit time, exert antidiarrheal action,26 and possibly contribute with other BAPs to milk-related gastro-intestinal discomfort.24,27,28 Indeed, Tagliamonte et al.24 recently found that the circulating higher relative concentration of BAPs opioid agonists-to-antagonists one hour after consuming milk was concomitant with the onset of gastrointestinal discomfort in a group of subjects suffering from this condition. Therefore, protein digestion and the type and time-concentration of BAPs in the alimentary canal and/or in circulation (beyond the amino acids profile) may be crucial for proteins to elicit some physiological BAP-mediated effects.
The digestion process includes the different compartments such as the mouth, stomach, and intestine where the structural alterations take place because of different pH and enzyme activities. The stomach consists of an acidic pH (approx. pH 2–3), whereas it subsequently reaches pH 7–8 in the small intestine. pH as well as enzymatic activity provides the structural opening of proteins and more available reactive sites. In this context, protein–phenol or peptide–phenol interactions that could occur during digestion and influence BAPs bioaccessibility, maybe fundamental in clarifying the functional effects of proteins beyond nutritional ones.
Few studies showed only the BAP profiles of some plant-based proteins rich in phenol compounds29,30 such as glutelin isolated from cocoa seeds31 whereas the effects of protein–phenol interaction during digestion on the BAPs profile have been under investigated. This study aims to explore the interactions of CN with different phenol compounds under digestive conditions and monitor how they affect the bioaccessibility of phenol compounds and bioactive peptide release in vitro.
Experimental
Chemicals
CN from bovine milk, ellagic acid (≥95%), catechin hydrate (>97%), and chlorogenic acid (≥95%) were obtained from Sigma-Aldrich (Diesenhofen, Germany). Potassium chloride, sodium chloride, magnesium chloride, ammonium bicarbonate, and potassium dihydrogen phosphate were purchased from Merck (Darmstadt, Germany). The enzymes pepsin (≥250 U mg−1 solid) from porcine gastric mucosa, pancreatin (8 × USP) from porcine pancreas, and the bile salts were also purchased from Sigma Aldrich (Deisenhofen, Germany). o-Phenylenediamine (OPA), dithiothreitol (DTT), amino acid standard solution, and L-Proline – 2,5,5-d3 hydrochloride were purchased from Merck (Darmstadt, Germany).All solvents used were of MS grade; formic acid (98%), methanol (98%), and acetonitrile (98%) were from Merck (Darmstadt, Germany). Syringe filters (nylon, 0.45 μm) and Strata (1 mL, 30 mg) solid-phase extraction cartridges were supplied by Phenomenex (Torrance, CA). 6-Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) and 2,2-diphenyl-1-picrylhydrazyl (95%) were purchased from Sigma-Aldrich (Deisenhofen, Germany). Cellulose (powder from spruce) was purchased from Fluka (Buchs, Switzerland). Green tea and tea leaves (La via del Te) were purchased from a local market in Naples.
Preparation of phenol extract from green tea and black tea leaves
Green tea and black tea extracts used in this study were obtained by the extraction of green tea and black tea leaves. For the extraction, 6 g of green tea or black tea was infused with 200 mL of hot distilled water at 85 °C for 15 min. Following infusion, black or green tea leaves were filtered using filter paper, and the resulting extracts were frozen before freeze-drying. Green tea and black tea extracts (200 mL) contained 1.2–1.6 g of dry matter. Finally, the green tea and black tea extract powders were obtained for the preparation of co-digestion samples as well as for the phenolic profile analysis.Preparation of CN hydrolysate
Three grams of CN from bovine milk were dissolved in 30 mL of 0.1 M NaOH (pH 7.0–7.5). 600 μL of Alcalase (2.4–4.0 AU g−1, Novozyme, Denmark) was added and kept at 62 °C for 30 min. Then, 150 μL of Flavorzyme (500–1000 LAPU g−1, Novozyme, Denmark) was added and kept at 62 °C for 30 min. After the hydrolysis procedure was completed, enzymes were inactivated by heating the mixture at 95 °C for 10 min. The hydrolysate obtained was frozen and freeze-dried.Preparation of co-digestion samples
For the preparation of the CN or CN hydrolysate (H) model system, 300 mg of CN or H were suspended in 2.5 mL of distilled water and mixed in a vortex shaker at room temperature for 5 min. Phenol compounds catechin (C), chlorogenic acid (CG), green tea extract (G), black tea extract (T), and ellagic acid (E) were used in this study at a concentration of 10%. Phenol control samples were prepared to comprehend any potential alterations or losses in phenol components. They were combined with 300 mg of cellulose to recreate the same conditions as the CN–phenol model systems. Cellulose was chosen because it is an inert compound, and it is undigestible.To have a co-digestion model system, 300 mg of CN was mixed with 30 mg of phenol compound in 2.5 mL of distilled water (CN–phenol). Similarly, 300 mg of H was mixed with 30 mg of phenol compound in 2.5 mL of distilled water (H-phenol model system). These mixtures were then shaken in a vortex shaker for 5 min. The study design was schematized in Fig. S1 in ESI.†
In vitro digestion of samples
The samples were subjected to INFOGEST in vitro digestion protocol.32 Simulating salivary fluid (SSF), simulating gastric fluid (SGF), and simulating intestinal fluid (SIF) were prepared according to INFOGEST, and then these simulating gastrointestinal fluids were used in the in vitro digestion. Pepsin and pancreatin were the enzymes used during gastric and intestinal digestion, respectively. At the end of the gastric phase, digests were centrifuged at 7000g for 5 min to separate the supernatant, bioaccessible part. It was collected and frozen at −20 °C prior to analysis. Intestinal enzymes and fluids were added to the remaining pellet for the intestinal phase of digestion. At the end of the intestinal phase, digests were centrifuged at 7000g for 5 min and the supernatant was collected as a soluble part of the intestinal phase. The remaining pellet from the intestinal phase, which corresponds to the undigestible fraction that is delivered to the colon, was frozen and freeze-dried for further analysis.Measurement of total antioxidant capacity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) until an absorbance of 0.9 ± 0.02 at 517 nm was obtained. The sample was incubated with DPPH radical in agitation for 30 min; subsequently, the mixture was centrifuged at 7000g for 5 min at 4 °C and the absorbance of the supernatant was measured at a wavelength of 517 nm.
50 v/v) until an absorbance of 0.9 ± 0.02 at 517 nm was obtained. The sample was incubated with DPPH radical in agitation for 30 min; subsequently, the mixture was centrifuged at 7000g for 5 min at 4 °C and the absorbance of the supernatant was measured at a wavelength of 517 nm.
Analysis of the degree of hydrolysis in digested samples
To determine how the hydrolysis of CN is affected, the amount of hydrolysis during digestion was observed. OPA a well-known reagent for amino group determination was prepared according to Nielsen, Petersen, and Dambmann35 and used for the analysis degree of hydrolysis (DH).Three milliliters of OPA were added to test tubes and then 400 μL of sample/standard or blank were added and mixed for 5 seconds. The mixture stood for exactly 2 min before being read at 340 nm in the spectrophotometer. Glutamine (0.9516 meqv L−1) was used as the standard solution for the calculation of DH.
Determination of h:
h is then:
| h = (glutamine-NH2 − β)/α meqv per g protein; |
Calculation of DH:
| DH = h/htot × 100%; |
Analysis of BAPs by UHPLC/HRMS
Analysis of BAPs was performed as reported in Tagliamonte et al.24 Briefly, samples added with internal standard (13C-labelled BCM7, 2.5 μg mL−1) clean-up was performed by using Strata C18-E (55 μm, 70 Å) cartridges (50 mg/1 mL) (Phenomenex, USA) and eluted with 1 mL of acetonitrile/water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) 0.1% formic acid. Thereafter, eluates were dried under nitrogen and reconstituted in water with 0.1% formic acid prior to LC-HRMS analysis. An ultra-high-performance liquid chromatography (UHPLC) Accela system (Thermo Fisher Scientific, San Jose, CA, USA) consisting of a degasser, a quaternary pump, an autosampler, and a column oven was used. The UHPLC was directly interfaced with an Exactive Orbitrap MS (Thermo Fisher Scientific, San Jose, CA, USA). BAPs were separated on a Luna Omega 1.6 μm Polar C18 100 (50 × 2.1 mm) column (Phenomenex, USA) with a setting temperature of 40 °C. The mobile phases consisted of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The elution gradient was set as follows: 5% di B (0.5 min), 5–80% B (0.5–9 min), constant at 80% B (3 min), 80–5% B (12–15 min). The flow rate was set at 100 μL min−1. Analytes were detected in positive ionization mode by using an ESI source and scanning the ions in the range of 150 and 1500 m/z range. Peptide identifications were obtained using the milk bioactive peptide database (MBPDB).36 The tolerance range for mass accuracy of BAPs was fixed at ±5 ppm. Peptides were partially quantified according to BCM-7 and expressed as ng g−1. Free and bound amino acid analysis by UHPLC/HRMS.
20) 0.1% formic acid. Thereafter, eluates were dried under nitrogen and reconstituted in water with 0.1% formic acid prior to LC-HRMS analysis. An ultra-high-performance liquid chromatography (UHPLC) Accela system (Thermo Fisher Scientific, San Jose, CA, USA) consisting of a degasser, a quaternary pump, an autosampler, and a column oven was used. The UHPLC was directly interfaced with an Exactive Orbitrap MS (Thermo Fisher Scientific, San Jose, CA, USA). BAPs were separated on a Luna Omega 1.6 μm Polar C18 100 (50 × 2.1 mm) column (Phenomenex, USA) with a setting temperature of 40 °C. The mobile phases consisted of 0.1% formic acid in water (solvent A) and 0.1% formic acid in acetonitrile (solvent B). The elution gradient was set as follows: 5% di B (0.5 min), 5–80% B (0.5–9 min), constant at 80% B (3 min), 80–5% B (12–15 min). The flow rate was set at 100 μL min−1. Analytes were detected in positive ionization mode by using an ESI source and scanning the ions in the range of 150 and 1500 m/z range. Peptide identifications were obtained using the milk bioactive peptide database (MBPDB).36 The tolerance range for mass accuracy of BAPs was fixed at ±5 ppm. Peptides were partially quantified according to BCM-7 and expressed as ng g−1. Free and bound amino acid analysis by UHPLC/HRMS.
Free and bound amino acid analysis by UHPLC/HRMS
For the analysis of all free amino acids, supernatants collected during digestion were diluted with a mixture of water and acetonitrile (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v) and centrifuged at 7000g for 3 min. The extracts were passed through a syringe filter (0.45 μm) and collected into an autosampler vial. For the analysis of bound amino acids, a hydrolysis procedure was applied first. Two hundred microliter of supernatants collected during digestion were mixed with 1.8 mL of 8 N HCl in a glass tube, and tightly closed after the tubes were flushed with nitrogen. They were hydrolyzed for 23 h at 110 °C. The hydrolysates were filtered through filter paper, and then 200 μL filtrate was put into a glass vial. It was evaporated to dryness under nitrogen gas and dissolved in 1 mL of a mixture of water and acetonitrile (20
80 v/v) and centrifuged at 7000g for 3 min. The extracts were passed through a syringe filter (0.45 μm) and collected into an autosampler vial. For the analysis of bound amino acids, a hydrolysis procedure was applied first. Two hundred microliter of supernatants collected during digestion were mixed with 1.8 mL of 8 N HCl in a glass tube, and tightly closed after the tubes were flushed with nitrogen. They were hydrolyzed for 23 h at 110 °C. The hydrolysates were filtered through filter paper, and then 200 μL filtrate was put into a glass vial. It was evaporated to dryness under nitrogen gas and dissolved in 1 mL of a mixture of water and acetonitrile (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v). An isotope labeled d3 proline was added as an internal standard. The analysis of amino acids in the samples was performed according to Tagliamonte et al.24 Chromatographic separation was performed on a Synchronis HILIC column (250 × 4.6 mm, 5 μm) (Thermo Fisher Scientific, San Jose, CA, USA). A linear gradient mixture of acetonitrile (A) and 0.1% formic acid in water (B) was used as the mobile phase at a flow rate of 400 μl min−1 at 45 °C. The gradient elution was programmed as follows: 95% B (0–1.5 min), 95–10% B (1.5–4 min), 10% B (4–7 min), 10–95% B (7–9 min) and then constant at 95% B (9–12 min).
80 v/v). An isotope labeled d3 proline was added as an internal standard. The analysis of amino acids in the samples was performed according to Tagliamonte et al.24 Chromatographic separation was performed on a Synchronis HILIC column (250 × 4.6 mm, 5 μm) (Thermo Fisher Scientific, San Jose, CA, USA). A linear gradient mixture of acetonitrile (A) and 0.1% formic acid in water (B) was used as the mobile phase at a flow rate of 400 μl min−1 at 45 °C. The gradient elution was programmed as follows: 95% B (0–1.5 min), 95–10% B (1.5–4 min), 10% B (4–7 min), 10–95% B (7–9 min) and then constant at 95% B (9–12 min).
The Exactive Orbitrap MS equipped with a heated electrospray interface was operated in the positive mode, scanning the ions in the m/z range of 60–220. The resolving power was set to 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 full widths at half maximum resulting in a scan time of 0.5 s. The automatic gain control target was set into a high dynamic range; the maximum injection time was 100 ms. The interface parameters were as follows: the spray voltage of 4.8 kV, the capillary voltage of 25 V, the capillary temperature of 295 °C, a sheath gas 30, and an auxiliary gas 5 arbitrary units, respectively.
000 full widths at half maximum resulting in a scan time of 0.5 s. The automatic gain control target was set into a high dynamic range; the maximum injection time was 100 ms. The interface parameters were as follows: the spray voltage of 4.8 kV, the capillary voltage of 25 V, the capillary temperature of 295 °C, a sheath gas 30, and an auxiliary gas 5 arbitrary units, respectively.
Total phenol content analysis
The analysis was performed according to Altunkaya & Gökmen.37 The total phenol content (TPC) of the samples that contain protein and phenol compounds was determined using Folin–Ciocalteu reagent. Insoluble material obtained at the end of digestion was freeze-dried and the powders were mixed with 0.8 mL of 0.2 N Folin–Ciocalteu reagent and 0.8 mL of 20% Na2CO3 solution. The reaction mixture was incubated at 25 °C for 2 h and the absorbance was measured at a wavelength of 765 nm. The TPC was calculated from a standard curve prepared using gallic acid at a concentration range of 0–100 mg L−1. The samples were read in triplicate, and the results were presented as mg of gallic acid equivalent (GAE) per g of the sample.Free and bound phenol analysis by HPLC
The analysis was performed according to Chiacchio et al.38 For the analysis of free phenol compounds, 600 μL of supernatants collected during digestion were diluted with 1400 μL of methanol and centrifuged at 7000g for 3 min. The extracts were passed through a syringe filter (0.45 μm) and collected into an autosampler vial.For the analysis of bound phenol compounds, a hydrolysis procedure was applied first; 250 μL of supernatants collected during digestion were mixed with 5 mL of 4 N NaOH in a glass tube, and tightly closed after the tubes were flushed with nitrogen. They were hydrolysed for 4 h at room temperature. The pH of the hydrolysates was then adjusted to 2.0 with 6 N HCl. The hydrolysates were filtered through filter paper, and then 1 mL of filtrate was put into a glass vial and extracted with a mixture of ethyl acetate and diethyl ether (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v) for 4 times. It was evaporated to dryness under nitrogen gas and dissolved in 1 mL of a mixture of water and methanol (30
50 v/v) for 4 times. It was evaporated to dryness under nitrogen gas and dissolved in 1 mL of a mixture of water and methanol (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v).
70 v/v).
Chromatographic separation was performed in Prodigy ODS3 100 Å (250 mm × 4.6 mm, particle size 5 μm) column (Phenomenex, CA, USA). The analysis was performed by using a Shimadzu HPLC coupled to a degasser, SIL-20A autosampler, and a binary pump equipped with a UV/VIS SPD-20° (Prominence, CA, USA) as a detector set at 280 nm. An injection volume of 20 μL was used for each run at a constant flow of 1 mL min−1. A gradient mixture of methanol (A) and 0.1% formic acid in water (B) was used as the mobile phase at a flow rate of 1 mL min−1 at 30 °C. The gradient program was set as follows: 20% B (0–2 min), 20–30% B (6 min), 30–40% B (10 min); 40–50% of B (8 min), 50–90% of B (8 min), constant flow to 90% of B (3 min); and, to rebalance the column, 90–20% of B for 2 min and 20–20% of B for 4 min.
Statistical analysis
Data were statistically analyzed by ANOVA and the Low Significant Differences (LSD) method with 95% significance was applied using the statistic software XLStat (Lumivero, France). Principal Component Analysis (PCA) was performed using XLStat (Lumivero France).Results and discussion
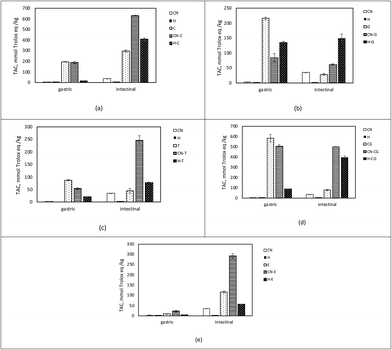
In this study, pure phenol compounds C, CG, and E as well as a mix of different phenol compounds as present in G and T were tested. During production, green tea leaves are heated and dried, and most of the oxidative enzymes are inhibited. Therefore, green tea leaves mainly contain monomeric flavan-3-ols, and catechins.39 However, a certain amount of procyanidins were also found in green tea leaves.40 On the other hand, upon black tea production, flavan-3-ols are mainly oxidized by the oxidative enzymes during fermentation. This process results in the formation of a complex mixture of dimeric, oligomeric, and polymeric flavan-3-ols.41 Major phenol compounds in G and T were catechin, epicatechin, and epigallocatechin gallate. G and T were found to contain 19.95 and 9.49 g total catechins/100 g dry extract, respectively.Possible interactions between CN and phenol compounds during in vitro digestion were monitored by TAC analysis. It is possible that phenol compounds are simultaneously oxidized as well as polymerized during digestion. In addition to these structural alterations, they could also be covalently or non-covalently bound to protein residues, which makes monitoring individual phenol compounds during digestion difficult. However, thanks to the antioxidant properties of phenol compounds, TAC was measured during digestion as a measure of phenol bioaccessibility, as reported by others.33,42
Initially, TAC was measured prior to digestion, and the initial TACs of CN and H were found to be 1.14 ± 0.08 and 2.27 ± 0.80 mmol TE per kg, respectively (Table 1). Even though proteins exert antioxidant activity proportionally to their reactive sites, they are not considered important antioxidant compounds.43 Phenol compounds alone showed a comparably higher initial antioxidant capacity, as expected. CG had the highest antioxidant capacity (662.443 ± 12.146 mmol TE per kg) of the phenol compounds, while T had the lowest (110.793 ± 2.666 mmol TE per kg).
| TAC (mmol TE per kg) | |||
|---|---|---|---|
| Initial | Bioaccessible | Colon | |
| The abbreviations of the samples indicate; CN: casein alone, H: casein hydrolysate alone, C: catechin alone, CN-C: casein-%10 catechin, H-C: casein hydrolysate-%10 catechin; G: green tea extract alone, CN-G: casein-%10 green tea extract, H-G: casein hydrolysate-%10 green tea extract, T: tea extract alone, CN-T: casein-%10 tea extract, H-T: casein hydrolysate-%10 tea extract, CG: chlorogenic acid alone, CN-CG: casein-%10 chlorogenic acid, H-CG: casein hydrolysate-%10 chlorogenic acid, E: ellagic acid alone, CN-E: casein-%10 ellagic acid, H-E: casein hydrolysate-%10 ellagic acid. *Values with different lowercase superscript letters within the same column, and uppercase superscript letters within the same row are significantly different (p < 0.05). | |||
| CN | 1.142 ± 0.08g,B | 37.375 ± 1.457gh,A | 1.229 ± 0.105g,B |
| H | 2.943 ± 0.039g,B | 4.419 ± 0.463h,A | 0.000 ± 0.000g,C |
| C | 406.211 ± 1.925d,B | 494.007 ± 13.895d,A | 42.628 ± 3.277fg,C |
| CN-C | 422.548 ± 5.713d,B | 818.319 ± 12.478b,A | 105.354 ± 1.017def,C |
| H-C | 425.490 ± 14.120d,A | 421.504 ± 16.299d,A | 176.260 ± 32.770cd,B |
| G | 188.477 ± 1.854e,B | 244.454 ± 7.743e,A | 1.214 ± 0.156g,C |
| CN-G | 125.385 ± 1.136f,C | 146.016 ± 16.158f,B | 295.610 ± 2.313b,A |
| H-G | 128.330 ± 11.368f,C | 282.949 ± 18.825e,A | 146.374 ± 6.937cde,B |
| T | 110.793 ± 2.666f,B | 130.580 ± 12.149f,A | 2.526 ± 0.083g,C |
| CN-T | 75.124 ± 2.726f,C | 299.774 ± 20.359e,A | 184.359 ± 0.997cd,B |
| H-T | 82.637 ± 2.726f,C | 98.874 ± 3.430fg,B | 178.357 ± 36.965cd,A |
| CG | 662.443 ± 12.146a,A | 658.101 ± 43.563c,A | 1.430 ± 0.077g,B |
| CN-CG | 600.694 ± 1.969b,B | 1008.034 ± 17.343a,A | 72.377 ± 1.164efg,C |
| H-CG | 620.717 ± 6.103ab,A | 483.152 ± 14.191d,B | 0.000 ± 0.000g,C |
| E | 460.917 ± 9.724cd,A | 124.925 ± 4.735fg,C | 419.856 ± 1.622a,B |
| CN-E | 512.080 ± 18.105c,A | 314.034 ± 13.452e,B | 112.610 ± 22.059cdef,C |
| H-E | 518.020 ± 28.200c,A | 59.788 ± 1.715fgh,C | 189.265 ± 25.690c,B |
The total TAC released during the gastric and intestinal phases of digestion corresponds to the bioaccessible TAC. In the bioaccessible fraction of CN and H, thanks to the CN hydrolysis, TAC was higher. According to Elias et al.,43 protein hydrolysis increases reactive sites, resulting in more radical scavenging capability. Furthermore, peptides produced by CN hydrolysis have been found to possess antioxidant activities. Amino acids with antioxidant characteristics include histidine, glutamic acid, proline, tyrosine, cysteine, methionine, and phenylalanine. Moreover, CN hydrolysates from bovine milk were reported to exert antioxidant activity via radical scavenging properties in both aqueous and lipid model systems.44,45
Similarly, the bioaccessible TAC of the CN and H samples co-digested with phenol compounds was comparably higher than that of CN or phenol compounds digested alone. Bioaccessible TAC was higher than initial in CN samples digested with phenol compounds; it was respectively 1.68, 1.93, 1.17, and 3.98-fold higher in the samples of CN co-digested with CG, C, G, and T. The H samples that were digested with the phenol compounds G and T showed a similar pattern. Such a high TAC indicated the CN–phenol interaction during digestion, as also reported by others. Similar research found that covalently linked conjugates of soy protein isolate, and black rice anthocyanins showed increased antioxidant potential after gastric and intestinal digestion.5 In a recent study, it was reported that the interactions of catechins with proteins improved the soluble TAC.46
As illustrated in Fig. 1, the TAC was different in the bioaccessible fraction of phenol, CN–phenol, and H-phenol-containing samples. Throughout the first 2 hours of digestion (gastric phase), most of the TAC became accessible, resulting in 88% and 79% of the TAC being released from the CG and T samples, respectively (Fig. 1d and c). These findings might suggest that the phenol compounds become easily available just after ingestion. Similarly, in vivo studies report that TAC in blood plasma after consumption of phenolic compounds starts to increase in 1 h and peaks in 2 h, pointing out the possible absorption through gastric phase.47 On the other hand, TAC released in the samples of E during the gastric phase was comparably lower (Fig. 1e), which was also reported by Gonzalez-Sarrias et al.48 Nevertheless, when phenol compounds were co-digested with CN or H, TAC release in the gastric phase was lower compared to TAC released from the phenol samples. These results might indicate that phenol compounds are mostly transported to the intestinal phase and co-digested with CN, whereas they are more bioaccessible in the gastric phase if they are digested alone.
As mentioned above, bioaccessible TAC corresponds to soluble TAC released in the gastric and intestinal phases, whereas the insoluble part contains the remaining components of the digest, i.e. the part that in vivo would enter the colon (colon fraction). The TAC of the colon fraction is reported in Table 1.
On the other hand, a significant amount of TAC was observed in the colon fraction in the samples of CN co-digested with phenol compounds. In the CN samples co-digested with CG, C, and E, it was discovered that 12%, 25%, and 21% of the initial TAC were maintained, respectively. Interestingly, 2.36-fold and 2.45-fold TAC in proportion to initial TAC were observed in the CN samples digested with G and T, respectively. TPC analysis also proved the presence of such high phenol content in the residual intestinal digestion of the samples containing G (95.44 ± 1.47 GAE per kg) and T (75.52 ± 1.61 GAE per kg). This might indicate that catechins remain undigested in polymerized forms (procyanidins), which are found in G and T formed from flavan-3-ols by oxidation and polymerization. Green tea extracts are found to contain 0.3–1.89 g procyanidin/100 g green tea, whereas tea extracts contain 0.10–0.98 g procyanidin/100 g tea.40 A model system containing roughly equal amounts of procyanidin structures in both G and T was subjected to digestion to determine the contribution of procyanidins of G and T. CN was co-digested with 5% of G and 7.5% of T, and initial TAC was 78.13 ± 0.67 and 69.39 ± 5.07 mmol TE per kg, respectively. Interestingly, remaining TACs in the intestinal residues were approximately similar (179.56 ± 1.52 in CN-G and 174.22 ± 2.23 mmol TE per kg in CN-T), indicating a significant contribution of procyanidins to TAC in the insoluble part. These results might suggest the stabilization of the phenol compounds49 and their better delivery to the colon when they are digested together with CN. In this respect, thanks to the possible interactions between CN and phenol compounds during digestion, CN acted as a “carrier” of phenol compounds. The carrier role of proteins for polyphenol compounds is a well-known strategy for their delivery50–53 and is important for colon health. Recent studies on tea and pomegranate phenolic compounds report their benefits to gut microbiota.48,54–56 Especially procyanidins that have a degree of polymerization >3 pass through the gastrointestinal tract in a stable manner, thus being accumulated in the colon and metabolized by the gut microbiota.57 As a result, aromatic acids and valerolactones accumulate in the colon and/or are absorbed into the bloodstream58 to exhibit biological activities on the colonic epithelium or in extra-intestinal tissues and, therefore, contribute to the beneficial effects of dietary procyanidins.59
Phenol compounds bound to protein fragments and peptides because of CN–phenol interaction may account for the higher TAC in the bioaccessible fraction of CN–phenol and H-phenol co-digests. However, it might also be due to the better hydrolysis of CN in the presence of phenol compounds. As it is well known, under the acidic conditions of the gastric phase, proteins are subjected to structural changes, especially the destruction of secondary structures. In the case of CN, the structural opening might be encouraged in the presence of phenol compounds, improving the hydrolysis rate of CN.
To understand how phenol compounds affect CN digestibility, DH in the CN samples digested with phenol compounds was monitored and compared with the CN samples digested alone. The total number of free amino groups present in the bioaccessible fraction, as measured in terms of peptides and amino acids, correlates to the DH. As given in Table 2, there was no significant difference in the degree of gastric hydrolysis between the CN samples and the CN samples co-digested with phenol compounds (p > 0.05). However, the presence of G and T along with CN reduced the degree of CN hydrolysis in the intestinal phase. In the H samples, protein hydrolysis was much higher when it was digested with phenol compounds than when it was digested alone. The effect of protein–phenol interaction on protein digestibility has generated some debate in the literature. Certain digestive proteases are inhibited by polyphenols according to some studies,60 while protein digestion is stimulated by polyphenols in other studies.61,62 In a recent study, intestinal β-CN hydrolysis was highly inhibited by tea catechins in a milk-tea beverage system.46
| Degree of hydrolysis (%) | ||
|---|---|---|
| Gastric | Intestinal | |
| The abbreviations of the samples indicate; CN: casein alone, H: casein hydrolysate alone, C: catechin alone, CN-C: casein-%10 catechin, H-C: casein hydrolysate-%10 catechin; CN-G: casein-%10 green tea extract, H-G: casein hydrolysate-%10 green tea extract, CN-T: casein-%10 tea extract, H-T: casein hydrolysate-%10 tea extract, CN-CG: casein-%10 chlorogenic acid, H-CG: casein hydrolysate-%10 chlorogenic acid, CN-E: casein-%10 ellagic acid, H-E: casein hydrolysate-%10 ellagic acid. *Values with different superscript letters within the same column are significantly different (p < 0.05). | ||
| CN | 4.70 ± 0.48d | 36.13 ± 1.27c |
| H | 37.66 ± 6.94c | 54.97 ± 1.73b |
| CN-C | 5.17 ± 1.08d | 37.68 ± 1.35c |
| H-C | 39.25 ± 5.43c | 59.87 ± 2.83ab |
| CN-G | 4.95 ± 0.76d | 28.79 ± 0.92d |
| H-G | 56.55 ± 3.70a | 60.39 ± 0.27ab |
| CN-T | 4.65 ± 0.94d | 29.78 ± 0.42d |
| H-T | 32.37 ± 3.83c | 65.68 ± 0.46a |
| CN-CG | 6.02 ± 0.48d | 35.08 ± 4.02c |
| H-CG | 46.25 ± 1.10b | 63.23 ± 1.00a |
| CN-E | 3.58 ± 0.02d | 39.47 ± 6.42c |
| H-E | 37.57 ± 0.50c | 65.68 ± 0.46a |
In addition to DH, total free amino acids were evaluated in the bioaccessible fractions of CN–phenol co-digestion samples. Table S1† gives the total free amino acids in the bioaccessible fraction. The amount of free amino acids was consistent with the DH of the protein, while free amino acid release from protein digestion was enhanced by the presence of phenol compounds. Similar results were obtained with different proteins in the literature.63,64 Intestinal digestion was promoted from the digestion of lysozyme-derivatized with chlorogenic acid because derivatization causes structural and conformational changes in the lysozyme.63 Research conducted with soybean proteins and different phenolic compounds found that intestinal hydrolysis was encouraged but simulated gastric digestion using pepsin was less or unaffected.64
These results suggested that CN–phenol interaction takes place during digestion. To clarify this hypothesis bioaccessible fractions were analyzed in terms of bound amino acids and bound phenols. Results given in Table S2† might clearly indicate the occurrence of CN–phenol interactions during the digestion of CN with phenol compounds. Moreover, G and T were the phenol compounds that were effective in these interactions, and they were followed by C, CG, and E. Green tea and tea extracts are known as good sources of different types of catechins. It was reported in the literature that catechins readily interact with proteins rich in proline, with an open and flexible structure.65,66 Furthermore, the structure of polyphenol compounds (such as whether they are glycosylated or not), their molecular size, protein structure, and amino acid composition all have a significant impact on protein–phenol interactions.67,68 Particularly, it was reported that the binding affinity of polyphenols to proteins increased with the molecular size of polyphenols.69 This might be the possible reason for the better interaction of G and T with CN during in vitro digestion.
As previously mentioned, a higher bioaccessible TAC might also be due to the enhanced release of antioxidant peptides from CN, as well as antioxidant amino acids such as histidine, glutamic acid, proline, tyrosine, cysteine, methionine, and phenylalanine.70,71 Compared to protein digestion, more antioxidant amino acids were released into the bioaccessible fraction when they were co-digested with phenol compounds (Table S1†). The amount of antioxidant amino acids was 2.92-fold in the CN samples digested with T (CN-T), whereas it was 1.74-fold in the CN samples digested with G (CN-G). Similarly, total free antioxidant amino acids were 1.99-fold in the H samples digested with G (H-G).
Along with antioxidant amino acids, CN digestion may also release peptides with antioxidant characteristics. Currently, CNs from milk are regarded as a good source of antioxidant peptides.72
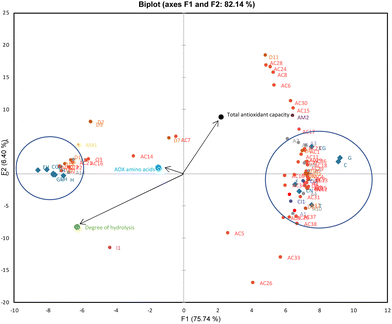
There are 32 antioxidant peptides isolated and identified from bovine CN.36 HRMS analysis was carried out to monitor the antioxidant peptides as well as other BAPs in the bioaccessible fraction of the digested samples. Results showed the presence of 10 (A1–A10 in Table S3†) of 32 antioxidant peptides.
Peptide data collected from HRMS analysis in the gastric and intestinal phases of CN/H and their co-digestions with phenol compounds were normalized (Table S4†) and analyzed by PCA to get a general overview of the peptide data. PCA in Fig. S2† could differentiate the BAPs profile of the samples composed of CN and H after gastric and intestinal digestion. In the gastric fraction, only 3 antioxidant peptides (A2, A3, and A5) could be observed in CN-containing samples, whereas 5 antioxidant peptides (A4, A9, A10, A13 and A14) could be found in H-containing samples.
Local PCAs were also applied to see the clusters in the intestinal fraction of the samples (Fig. 2). F1 and F2, explaining 82.14% of the data variability, show that the BAPs of CN (CN), as well as CN–phenol co-digestion samples (C, G, T, CG, E), form a distinct cluster to the right side, while BAPs from H, as well as H-phenol co-digestion samples (CH, GH, TH, CGH, EH) samples, are placed to the left (Fig. 2). Looking at PCA in Fig. 2, vectors indicated that TAC was highly correlated with CN samples (CN and CN–phenol) and antioxidant peptides. However, the vectors on the left showed a correlation between H samples and antioxidant amino acids as well as the DH (H and H-phenol). These findings indicated that the bioaccessible fraction of the samples containing CN may have a higher TAC release due to the formation of antioxidant peptides; in addition, the digested samples containing H may have a higher TAC release due to a higher DH and the release of antioxidant amino acids.
As given in Fig. 2, the antioxidant peptides with the sequences YPEL (A3), AYFYPE (A6), and AYFYPEL (A7) were closer to the TAC than the other antioxidant peptides clustered on the right. These peptides are derived from α-s1-CN and have both antioxidant and ACE-inhibitory activity.36
CN was in vitro digested with phenol compounds up to a concentration of 20% to see whether the release of antioxidant peptides is affected by the phenol concentration. Fig. S3† demonstrates that increasing the phenol concentration to 20% had a significant impact on the amount of antioxidant peptides produced from CN in the intestinal phase. The amount of antioxidant peptides and phenol concentration were highly correlated, according to linear regression coefficients that ranged from 0.951 to 0.999.
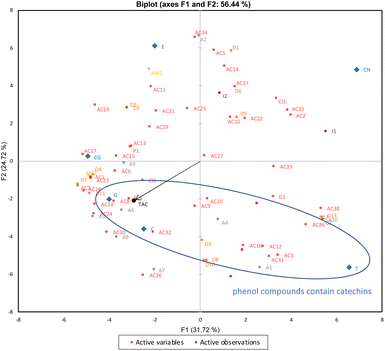
In addition to antioxidant peptides, there are more than 200 BAPs isolated from bovine CN.36 Formation of DPP-IV inhibitory (D), ACE-inhibitory (AC), immune-modulatory (I), growth-promoting (G), opioid (O), antimicrobial (AM), pepsin inhibitory (PI) and cathepsin-inhibitory (CI) peptides during the digestion of bovine CN was reported to date.36 The BAPs found in the CN and H-containing samples during digestion are listed in Table S3.† The differences between the BAP profiles of CN and H are evident in Fig. 2. Commercial enzymes are employed in the preparation of H, resulting in the aggressive hydrolysis of CN and the formation of smaller quantities of BAPs. Because the hydrolysates have a vast number of shorter peptides (di-tri peptides), amino acids might be favorably formed during their digestion.
As shown in Fig. 2, most BAPs DPP-IV inhibitors tended to group together on the left, indicating a positive correlation with the samples that included H. The ACE-inhibitory peptides, on the other hand, grouped on the right side, demonstrating a favorable correlation with the CN-containing samples. The data acquired from in vitro digestion of CN alone or combination with phenol compounds were subjected to local PCA to gain an overview of the BAPs produced in the presence of five different phenol compounds (Fig. 3). Both the F2 and F1 axes distinguished the digested samples including phenol compounds (C, G, T, CG, and E) from CN alone, as predicted by PCA in Fig. 3. Interestingly, the F2 axis also separated phenol compounds (C, G, and T) that contain catechins from the other phenol compounds (CG and E) and grouped on the negative side. The release of antioxidant peptides was positively correlated with the presence of phenol compounds that contain catechins, which is why antioxidant peptides also grouped with these phenol compounds.
The heatmap shown in Fig. S4† was created using BAPs data to provide a final comparison between the CN and H samples. The map reveals a distinct clustering (indicating a lesser similarity) between the CN and H samples. The samples of CN showed that the difference varied most when it was co-digested with C, G, and T, whereas the difference varied least when it was co-digested with E and CG. Additionally, the concentration of BAPS produced is shown by an increase in the intensity of light green color in the CN samples co-digested with the phenolic compounds C, G, and T.
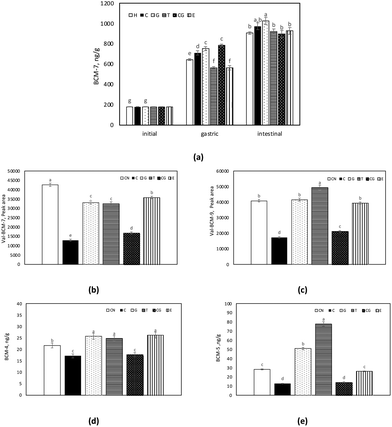
In recent years, opioid peptides, particularly BCMs, have been the most studied BAPs and related to some diseases and disorders including autism, cardiovascular disease, and diabetes24 as well as delayed gastrointestinal transit, looser stools, and occurrence of discomfort after consuming milk. Therefore, in the present study, BCMs were monitored in the gastric and intestinal digests of CN and H-containing samples.
The concentration of BCM-7 during in vitro digestion of H samples is shown in Fig. 4a. In the gastric fraction, the formation of BCM-7 was affected by the presence of phenol compounds, whereas no significant change was observed in the intestinal digests. In contrast to H, BCM-7 could not be detected over the in vitro digestion of CN samples. In a similar study, only small amounts of BCM-7 could be detected in the milk CN digests, but only after 4 h of in vitro digestion.73 On the other hand, in the intestinal phase, in vitro digestion of CN by pancreatin resulted in the release of BCM-7 and BCM-9 peptides with a valine residue present at the N-terminal Val-BCM-7 (VYPFPGPI) and Val-BCM-9 (VYPFPGPIPN). Comparably higher amounts of Val-BCM-7 and Val-BCM-9 were detected in CN samples (Fig. 4b and c). The peak area of Val-BCM-7 and Val-BCM-9 were 12–50 times higher in CN samples than in H samples. This was also reported by Edwards et al.73 The signal intensity of Val-BCM-7 and Val-BCM-9 was about 50-fold higher compared to the corresponding peptides without valine. These results pointed out that digestive enzymes, pancreatin, and pepsin, used in in vitro digestion were not capable of cleaving the bond between BCM-7 and Val, whereas leucine amino peptidase, a brush border enzyme, easily cleaves it during in vivo digestion in the human body.73,74 However, Val-BCM-7 and Val-BCM-9 levels could indicate the possible formation of BCM-7 and BCM-9 from CN. The release of Val-BCM-7 was affected by the presence of phenol compounds, as it was comparably lower in the CN co-digested with phenol compounds. Similarly, 40% of Val-BCM-9 was found in the CN samples co-digested with C, whereas the addition of T to CN led to a significant increase in Val-BCM-9.
In addition to Val-BCM-7 and Val-BCM-9, BCM-4 and BCM-5 were also detected in CN intestinal digests (Fig. 4d and e). Similarly, significantly lower amounts of BCM-4 and BCM-5 were detected in CN digests with C. Nonetheless, the presence of G and T stimulated the release of BCM-4 and BCM-5.
In this study, the formation of BAPs is stimulated in the presence of phenol compounds. In particular, BAPs as well as BCM formation from both CN and H were induced in the presence of G and T. However, C and CG provided a slight but significant reduction in the BCM formation.
Conclusions
This study clarified the reactions of phenol compounds with CN during digestion and their effects on BAPs release. The results of this study pointed out that protein–phenol interactions take place during digestion, mostly in the intestinal phase, encouraged by the pH conditions. Among the phenol compounds, phenol compounds that contain catechins are the most effective in protein–phenol interaction. G and T were more promising for the interaction with CN thanks to their wide variety of catechins and polymerized catechin contents, as the molecular size of phenol compounds has a high impact on protein–phenol interactions. Interestingly, the findings of this study show that the bioaccessibility of phenol compounds is improved when they are co-digested with CN. Protected by protein–phenol interaction in the digestive tract, phenol compounds could be delivered to the colon in a more stable manner. The release of BAPs, especially the antioxidant peptides from CN, is stimulated by the presence of phenol compounds in the digestive tract. On the other hand, the formation of BCMs, which may affect gastrointestinal transit, is influenced as well.These results support further investigations of protein–phenol interactions as potential delivery systems of phenol compounds. However, investigation of the role of phenol compounds on colon health is an emerging area, and the carrier role of proteins seems to be crucial for their stable transfer. On the other hand, there is still little knowledge about how protein–phenol interactions affect the formation of peptides. As protein–polyphenol interactions influence the bioactivity of both phenol compounds and proteins in relation to antioxidant, anti-inflammatory, and anti-cancer activities, future studies are required to clarify the effects of protein–phenol interactions for improving bioavailability, specific-target delivery, and biological activity.
Author contributions
Conceptualization, A. H., V. G. and P. V.; methodology, P. V.; validation, S. T.; investigation, A. H.; resources, P. V.; data curation, A. H. and S. T.; writing—original draft preparation, A. H.; writing—review and editing, P. V. and V. G.; visualization, A. H.; supervision, P. V.; project administration, P. V. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by The Scientific and Technological Research Council of Turkey in the frame of 2219-International Postdoctoral Research Fellowship Program for Turkish Citizens.References
- K. Nagy, M. C. Courtet-Compondu, G. Williamson, S. Rezzi, M. Kussmann and A. Rytz, Non-covalent binding of proteins to polyphenols correlates with their amino acid sequence, Food Chem., 2012, 132, 1333–1339 CrossRef CAS PubMed.
- C. Le Bourvellec and C. M. Renard, Interactions between polyphenols and macromolecules: quantification methods and mechanisms, Crit. Rev. Food Sci. Nutr., 2012, 52, 213–248 CrossRef CAS PubMed.
- S. Dai, Z. Lian, W. Qi, Y. Chen, X. Tong, T. Tian, B. Lyu, M. Wang, H. Wang and L. Jiang, Non-covalent interaction of soy protein isolate and catechin: Mechanism and effects on protein conformation, Food Chem., 2022, 384, 132507 CrossRef CAS PubMed.
- X. Tong, J. Cao, T. Tian, B. Lyu, L. Miao, Z. Lian, W. Cui, S. Liu, H. Wang and L. Jiang, Changes in structure, rheological property and antioxidant activity of soy protein isolate fibrils by ultrasound pretreatment and EGCG, Food Hydrocolloids, 2022, 122, 107084 CrossRef CAS.
- L. Jiang, Y. Liu, L. Li, B. Qi, M. Ju, Y. Xu, Y. Zhang and X. Sui, Covalent conjugates of anthocyanins to soy protein: Unravelling their structure features and in vitro gastrointestinal digestion fate, Food Res. Int., 2019, 120, 603–609 CrossRef CAS PubMed.
- J. E. Beart, T. H. Lilley and E. Haslam, Plant polyphenols—secondary metabolism and chemical defence: Some observations, Phytochemistry, 1985, 24, 33–38 CrossRef CAS.
- T. g. Pan, Y. n. Wu, S. He, Z. Wu and R. Jin, Food allergenic protein conjugation with plant polyphenols for allergenicity reduction, Curr. Opin. Food Sci., 2022, 43, 36–42 CrossRef CAS.
- M. Li, C. Ritzoulis, Q. Du, Y. Liu, Y. Ding, W. Liu and J. Liu, Recent Progress on Protein-Polyphenol Complexes: Effect on Stability and Nutrients Delivery of Oil-in-Water Emulsion System, Front. Nutr., 2021, 8, 765589 CrossRef PubMed.
- N. J. Baxter, T. H. Lilley, E. Haslam and M. P. Williamson, Multiple Interactions between Polyphenols and a Salivary Proline-Rich Protein Repeat Result in Complexation and Precipitation, Biochemistry, 1997, 36, 5566–5577 CrossRef CAS PubMed.
- L. A. Kartsova and A. V. Alekseeva, Effect of milk caseins on the concentration of polyphenolic compounds in tea, J. Anal. Chem., 2008, 63, 1107–1111 CrossRef CAS.
- T. Hofmann, A. Glabasnia, B. Schwarz, K. N. Wisman, K. A. Gangwer and A. E. Hagerman, Protein Binding and Astringent Taste of a Polymeric Procyanidin, 1,2,3,4,6-Penta-O-galloyl-β-d-glucopyranose, Castalagin, and Grandinin, J. Agric. Food Chem., 2006, 54, 9503–9509 CrossRef CAS PubMed.
- I. Lesschaeve and A. C. Noble, Polyphenols: factors influencing their sensory properties and their effects on food and beverage preferences, Am. J. Clin. Nutr., 2005, 81, 330s–335s CrossRef CAS PubMed.
- A.-A. Moeiniafshari, A. Zarrabi and A.-K. Bordbar, Exploring the interaction of naringenin with bovine beta-casein nanoparticles using spectroscopy, Food Hydrocolloids, 2015, 51, 1–6 CrossRef CAS.
- A. Rietveld and S. Wiseman, Antioxidant Effects of Tea: Evidence from Human Clinical Trials, J. Nutr., 2003, 133, 3285S–3292S CrossRef CAS PubMed.
- M. Serafini, A. Ghiselli and A. Ferro-Luzzi, In vivo antioxidant effect of green and black tea in man, Eur. J. Clin. Nutr., 1996, 50, 28–32 CAS.
- R. Leenen, A. J. Roodenburg, L. B. Tijburg and S. A. Wiseman, A single dose of tea with or without milk increases plasma antioxidant activity in humans, Eur. J. Clin. Nutr., 2000, 54, 87–92 CrossRef CAS PubMed.
- V. C. Reddy, G. V. Vidya Sagar, D. Sreeramulu, L. Venu and M. Raghunath, Addition of milk does not alter the antioxidant activity of black tea, Ann. Nutr. Metab., 2005, 49, 189–195 CrossRef CAS PubMed.
- R. J. Green, A. S. Murphy, B. Schulz, B. A. Watkins and M. G. Ferruzzi, Common tea formulations modulate in vitro digestive recovery of green tea catechins, Mol. Nutr. Food Res., 2007, 51, 1152–1162 CrossRef CAS PubMed.
- M. C. van der Burg-Koorevaar, S. Miret and G. S. Duchateau, Effect of milk and brewing method on black tea catechin bioaccessibility, J. Agric. Food Chem., 2011, 59, 7752–7758 CrossRef CAS PubMed.
- W. He, T. Zhang, T. C. Velickovic, S. Li, Y. Lyu, L. Wang, J. Yi, Z. Liu, Z. He and X. Wu, Covalent conjugation with (−)-epigallo-catechin 3-gallate and chlorogenic acid changes allergenicity and functional properties of Ara h1 from peanut, Food Chem., 2020, 331, 127355 CrossRef CAS PubMed.
- X. Li, M. Li, T. Zhang, D. J. McClements, X. Liu, X. Wu and F. Liu, Enzymatic and Nonenzymatic Conjugates of Lactoferrin and (−)-Epigallocatechin Gallate: Formation, Structure, Functionality, and Allergenicity, J. Agric. Food Chem., 2021, 69, 6291–6302 CrossRef CAS PubMed.
- C. C. Udenigwe and V. Fogliano, Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin?, J. Funct. Foods, 2017, 35, 9–12 CrossRef CAS.
- M. Naeem, M. I. Malik, T. Umar, S. Ashraf and A. Ahmad, A Comprehensive Review About Bioactive Peptides: Sources to Future Perspective, Int. J. Pept. Res. Ther., 2022, 28, 155 CrossRef CAS.
- S. Tagliamonte, R. Barone Lumaga, F. De Filippis, V. Valentino, R. Ferracane, M. Guerville, I. Gandolfi, G. Barbara, D. Ercolini and P. Vitaglione, Milk protein digestion and the gut microbiome influence gastrointestinal discomfort after cow milk consumption in healthy subjects, Food Res. Int., 2023, 170, 112953 CrossRef CAS PubMed.
- R. Nagpal, P. Behare, R. Rana, A. Kumar, M. Kumar, S. Arora, F. Morotta, S. Jain and H. Yadav, Bioactive peptides derived from milk proteins and their health beneficial potentials: an update, Food Funct., 2011, 2, 18–27 RSC.
- H. Daniel, M. Vohwinkel and G. Rehner, Effect of casein and beta-casomorphins on gastrointestinal motility in rats, J. Nutr., 1990, 120, 252–257 CrossRef CAS PubMed.
- M. P. Barnett, W. C. McNabb, N. C. Roy, K. B. Woodford and A. J. Clarke, Dietary A1 β-casein affects gastrointestinal transit time, dipeptidyl peptidase-4 activity, and inflammatory status relative to A2 β-casein in Wistar rats, Int. J. Food Sci. Nutr., 2014, 65, 720–727 CrossRef CAS PubMed.
- S. Pal, K. Woodford, S. Kukuljan and S. Ho, Milk Intolerance, Beta-Casein and Lactose, Nutrients, 2015, 7, 7285–7297 CrossRef CAS PubMed.
- R. Pérez-Gregorio, S. Soares, N. Mateus and V. de Freitas, Bioactive Peptides and Dietary Polyphenols: Two Sides of the Same Coin, Molecules, 2020, 25, 3443 CrossRef PubMed.
- X. Yan, Z. Zeng, D. J. McClements, X. Gong, P. Yu, J. Xia and D. Gong, A review of the structure, function, and application of plant-based protein–phenolic conjugates and complexes, Compr. Rev. Food Sci. Food Saf., 2023, 22, 1312–1336 CrossRef CAS PubMed.
- E. G. Tovar-Pérez, L. Guerrero-Becerra and E. Lugo-Cervantes, Antioxidant activity of hydrolysates and peptide fractions of glutelin from cocoa (Theobroma cacao L.) seed, CyTA – J. Food, 2017, 15, 489–496 CrossRef.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carriere, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, S. Marze, D. J. McClements, O. Menard, I. Recio, C. N. Santos, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and A. Brodkorb, A standardised static in vitro digestion method suitable for food - an international consensus, Food Funct., 2014, 5, 1113–1124 RSC.
- V. A. Papillo, P. Vitaglione, G. Graziani, V. Gokmen and V. Fogliano, Release of Antioxidant Capacity from Five Plant Foods during a Multistep Enzymatic Digestion Protocol, J. Agric. Food Chem., 2014, 62, 4119–4126 CrossRef CAS PubMed.
- V. Gökmen, A. Serpen and V. Fogliano, Direct measurement of the total antioxidant capacity of foods: the ‘QUENCHER’ approach, Trends Food Sci. Technol., 2009, 20, 278–288 CrossRef.
- P. M. Nielsen, D. Petersen and C. Dambmann, Improved method for determining food protein degree of hydrolysis, J. Food Sci., 2001, 66, 642–646 CrossRef CAS.
- S. D. Nielsen, R. L. Beverly, Y. Qu and D. C. Dallas, Milk bioactive peptide database: A comprehensive database of milk protein-derived bioactive peptides and novel visualization, Food Chem., 2017, 232, 673–682 CrossRef CAS PubMed.
- A. Altunkaya and V. Gökmen, Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa), Food Chem., 2008, 107, 1173–1179 CrossRef CAS.
- M. F. Chiacchio, S. Tagliamonte, A. Visconti, R. Ferracane, A. Mustafa and P. Vitaglione, Baobab-Fruit Shell and Fibrous Filaments Are Sources of Antioxidant Dietary Fibers, Molecules, 2022, 27, 5563 CrossRef CAS PubMed.
- H. N. Graham, Green tea composition, consumption, and polyphenol chemistry, Prev. Med., 1992, 21, 334–350 CrossRef CAS PubMed.
- U. H. Engelhardt, C. Lakenbrink and O. Pokorny, in Nutraceutical Beverages, American Chemical Society, 2003, vol. 871, ch. 19, pp. 254–264 Search PubMed.
- M. E. Harbowy, D. A. Balentine, A. P. Davies and Y. Cai, Tea Chemistry, Crit. Rev. Plant Sci., 1997, 16, 415–480 CrossRef CAS.
- S. Pastoriza, C. Delgado-Andrade, A. Haro and J. A. Rufián-Henares, A physiologic approach to test the global antioxidant response of foods. The GAR method, Food Chem., 2011, 129, 1926–1932 CrossRef CAS.
- R. J. Elias, S. S. Kellerby and E. A. Decker, Antioxidant activity of proteins and peptides, Crit. Rev. Food Sci., 2008, 48, 430–441 CrossRef CAS PubMed.
- B. Petrat-Melin, P. Andersen, J. T. Rasmussen, N. A. Poulsen, L. B. Larsen and J. F. Young, In vitro digestion of purified beta-casein variants A(1), A(2), B, and I: Effects on antioxidant and angiotensin-converting enzyme inhibitory capacity, J. Dairy Sci., 2015, 98, 15–26 CrossRef CAS PubMed.
- K. Rossini, C. P. Z. Norena, F. Cladera-Olivera and A. Brandelli, Casein peptides with inhibitory activity on lipid oxidation in beef homogenates and mechanically deboned poultry meat, LWT – Food Sci. Technol., 2009, 42, 862–867 CrossRef CAS.
- X. Qie, Y. Wu, Y. Chen, C. Liu, M. Zeng, F. Qin, Z. Wang, J. Chen and Z. He, Competitive interactions among tea catechins, proteins, and digestive enzymes modulate in vitro protein digestibility, catechin bioaccessibility, and antioxidant activity of milk tea beverage model systems, Food Res. Int., 2021, 140, 110050 CrossRef CAS PubMed.
- R. L. Prior, L. Gu, X. Wu, R. A. Jacob, G. Sotoudeh, A. A. Kader and R. A. Cook, Plasma Antioxidant Capacity Changes Following a Meal as a Measure of the Ability of a Food to Alter In Vivo Antioxidant Status, J. Am. Coll. Nutr., 2007, 26, 170–181 CrossRef CAS PubMed.
- A. Gonzalez-Sarrias, R. Garcia-Villalba, M. A. Nunez-Sanchez, J. Tome-Carneiro, P. Zafrilla, J. Mulero, F. A. Tomas-Barberan and J. C. Espin, Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts, J. Funct. Foods, 2015, 19, 225–235 CrossRef CAS.
- S. Lamothe, N. Azimy, L. Bazinet, C. Couillard and M. Britten, Interaction of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment, Food Funct., 2014, 5, 2621–2631 RSC.
- B. Chima, P. Mathews, S. Morgan, S. A. Johnson and C. B. Van Buiten, Physicochemical Characterization of Interactions between Blueberry Polyphenols and Food Proteins from Dairy and Plant Sources, Foods, 2022, 11, 2846 CrossRef CAS PubMed.
- J. Ge, X. Yue, S. Wang, J. Chi, J. Liang, Y. Sun, X. Gao and P. Yue, Nanocomplexes composed of chitosan derivatives and β-Lactoglobulin as a carrier for anthocyanins: Preparation, stability and bioavailability in vitro, Food Res. Int., 2019, 116, 336–345 CrossRef CAS PubMed.
- Y. Wang, J. Zhang and L. Zhang, Anthocyanin-Dietary Proteins Interaction and Its Current Applications in Food Industry, Food Rev. Int., 2023, 39, 3301–3313 CrossRef CAS.
- H. Wu, G. Oliveira and M. A. Lila, Protein-binding approaches for improving bioaccessibility and bioavailability of anthocyanins, Compr. Rev. Food Sci. Food Saf., 2023, 22, 333–354 CrossRef CAS PubMed.
- S. M. Henning, J. Yang, M. Hsu, R.-P. Lee, E. M. Grojean, A. Ly, C.-H. Tseng, D. Heber and Z. Li, Decaffeinated green and black tea polyphenols decrease weight gain and alter microbiome populations and function in diet-induced obese mice, Eur. J. Nutr., 2018, 57, 2759–2769 CrossRef CAS PubMed.
- Z. Liu, Z. Chen, H. Guo, D. He, H. Zhao, Z. Wang, W. Zhang, L. Liao, C. Zhang and L. Ni, The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice, Food Funct., 2016, 7, 4869–4879 RSC.
- Z. Liu, W. J. C. De Bruijn, M. E. Bruins and J. P. Vincken, Microbial Metabolism of Theaflavin-3,3′-digallate and Its Gut Microbiota Composition Modulatory Effects, J. Agric. Food Chem., 2021, 69, 232–245 CrossRef CAS PubMed.
- Y. Y. Choy, G. K. Jaggers, P. I. Oteiza and A. L. Waterhouse, Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract, J. Agric. Food Chem., 2013, 61, 121–127 CrossRef CAS PubMed.
- C. Li, M.-J. Lee, S. Sheng, X. Meng, S. Prabhu, B. Winnik, B. Huang, J. Y. Chung, S. Yan, C.-T. Ho and C. S. Yang, Structural Identification of Two Metabolites of Catechins and Their Kinetics in Human Urine and Blood after Tea Ingestion, Chem. Res. Toxicol., 2000, 13, 177–184 Search PubMed.
- M. J. Cires, X. Wong, C. Carrasco-Pozo and M. Gotteland, The Gastrointestinal Tract as a Key Target Organ for the Health-Promoting Effects of Dietary Proanthocyanidins, Front. Nutr., 2016, 3, 57 Search PubMed.
- D. Tagliazucchi, E. Verzelloni and A. Conte, Effect of Some Phenolic Compounds and Beverages on Pepsin Activity during Simulated Gastric Digestion, J. Agric. Food Chem., 2005, 53, 8706–8713 CrossRef CAS PubMed.
- H. M. Rawel, J. Kroll and S. Rohn, Reactions of phenolic substances with lysozyme—physicochemical characterisation and proteolytic digestion of the derivatives, Food Chem., 2001, 72, 59–71 CrossRef CAS.
- H. M. Rawel, D. Czajka, S. Rohn and J. Kroll, Interactions of different phenolic acids and flavonoids with soy proteins, Int. J. Biol. Macromol., 2002, 30, 137–150 CrossRef CAS PubMed.
- H. M. Rawel, J. Kroll and B. Riese, Reactions of chlorogenic acid with lysozyme: Physicochemical characterization and proteolytic digestion of the derivatives, J. Food Sci., 2000, 65, 1091–1098 CrossRef CAS.
- H. M. Rawel, S. Rohn, H. P. Kruse and J. Kroll, Structural changes induced in bovine serum albumin by covalent attachment of chlorogenic acid, Food Chem., 2002, 78, 443–455 CrossRef CAS.
- S. Soares, N. Mateus and V. de Freitas, Interaction of Different Polyphenols with Bovine Serum Albumin (BSA) and Human Salivary α-Amylase (HSA) by Fluorescence Quenching, J. Agric. Food Chem., 2007, 55, 6726–6735 CrossRef CAS PubMed.
- C. Poncet-Legrand, C. Gautier, V. Cheynier and A. Imberty, Interactions between Flavan-3-ols and Poly(l-proline) Studied by Isothermal Titration Calorimetry: Effect of the Tannin Structure, J. Agric. Food Chem., 2007, 55, 9235–9240 CrossRef CAS PubMed.
- A. Papadopoulou, R. J. Green and R. A. Frazier, Interaction of Flavonoids with Bovine Serum Albumin: A Fluorescence Quenching Study, J. Agric. Food Chem., 2005, 53, 158–163 CrossRef CAS PubMed.
- P. Bandyopadhyay, A. K. Ghosh and C. Ghosh, Recent developments on polyphenol–protein interactions: effects on tea and coffee taste, antioxidant properties and the digestive system, Food Funct., 2012, 3, 592–605 RSC.
- F. Liu, C. Sun, W. Yang, F. Yuan and Y. Gao, Structural characterization and functional evaluation of lactoferrin–polyphenol conjugates formed by free-radical graft copolymerization, RSC Adv., 2015, 5, 15641–15651 RSC.
- A. G. P. Samaranayaka and E. C. Y. Li-Chan, Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications, J. Funct. Foods, 2011, 3, 229–254 CrossRef CAS.
- B. H. Sarmadi and A. Ismail, Antioxidative peptides from food proteins: A review, Peptides, 2010, 31, 1949–1956 CrossRef CAS PubMed.
- E. Haque and R. Chand, Antihypertensive and antimicrobial bioactive peptides from milk proteins, Eur. Food Res. Technol., 2008, 227, 7–15 CrossRef CAS.
- T. S. Edwards, K. L. Dawson, J. I. Keenan and A. S. Day, A simple method to generate β-casomorphin-7 by in vitro digestion of casein from bovine milk, J. Funct. Foods, 2021, 85, 104631 CrossRef CAS.
- Y. Jinsmaa and M. Yoshikawa, Enzymatic release of neocasomorphin and β-casomorphin from bovine β-casein, Peptides, 1999, 20, 957–962 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo02630b |
| This journal is © The Royal Society of Chemistry 2023 |