Open Access Article
Open Access ArticleCaffeic acid supplementation ameliorates intestinal injury by modulating intestinal microbiota in LPS-challenged piglets†
Xiaobin
Wen‡
 a,
Fan
Wan‡
a,
Fan
Wan‡
 ac,
You
Wu
bd,
Lei
Liu
a,
Yueping
Liu
b,
Ruqing
Zhong
*a,
Liang
Chen
a and
Hongfu
Zhang
*a
ac,
You
Wu
bd,
Lei
Liu
a,
Yueping
Liu
b,
Ruqing
Zhong
*a,
Liang
Chen
a and
Hongfu
Zhang
*a
aState Key Laboratory of Animal Nutrition, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, 100193, China. E-mail: zhongruqing@caas.cn; zhanghongfu@caas.cn
bCollege of Biological Science and Engineering, Beijing University of Agriculture, Beijing 102206, China
cState Key Laboratory of Grassland Agro-Ecosystem, Key Laboratory of Grassland Livestock Industry Innovation, Ministry of Agriculture and Rural Affairs, College of Pastoral Agriculture Science and Technology, Lanzhou University, Lanzhou 730020, China
dCollege of Animal Science and Technology, China Agricultural University, Beijing 100193, China
First published on 21st July 2023
Abstract
During weaning, piglets are susceptible to intestinal injuries caused by a range of infections, which result in serious economic losses for pig producers. Caffeic acid (CA) is a plant-derived phenolic acid that exhibits potential as a dietary supplement for enhancing intestinal health. There is, however, limited information available about the potential benefits of CA supplementation on intestinal injury and growth performance in piglets. A 28-day study was conducted to examine the effectiveness of CA supplementation in protecting against intestinal injury induced by intraperitoneal injection of Escherichia coli lipopolysaccharide (LPS) in piglets. Twenty-four piglets (7.43 ± 0.79 kg body weight; Duroc × Landrace × Large White; barrows) were randomly divided into 4 groups: the control group, the LPS group, the LPS + CA group, and the CA group. Piglets were administered with LPS or saline on d21 and d28 of the experiment. Supplementation with CA improved intestinal barrier function in LPS-challenged piglets by enhancing intestinal morphology and integrity, as well as increasing the expression of Claudin-1 and ZO-1. Meanwhile, CA supplementation improved the systemic and colonic inflammation responses, oxidative stress, and apoptosis induced by LPS. CA supplementation improved the alpha diversity and structure of the intestinal microbiota by increasing the abundance of beneficial microbiota. Additionally, it was found that it improves metabolic disorders of colonic bile acids (BAs) and short-chain fatty acids (SCFAs) in LPS-challenged piglets, including an increase in primary BAs and isovalerate. In conclusion, CA supplementation could enhance intestinal integrity and barrier function by modifying intestinal microbiota and its metabolites, which could lead to a reduction in inflammatory responses and oxidative stress and ultimately enhanced growth performance in piglets.
1. Introduction
The intestine is the primary place for digestion and absorption, and also the largest immune organ, which plays an essential role in maintaining normal immune defense function. However, young animals are susceptible to intestinal injury as a result of their compromised immune function and intestinal dysfunction, ultimately elevating their susceptibility to intestinal diseases.1,2 During weaning, piglets face various physiological and environmental challenges, which make them susceptible to intestinal injury and diarrhoea. This vulnerability can lead to reduced growth performance and even death, resulting in significant economic losses for the breeding industry annually.3 Thus, the preservation of intestinal integrity and functionality is imperative for the well-being of animals and the optimization of pig farming.4 Previous studies have proved that the intestinal microbiota and its metabolites, including BAs and SCFAs, are essential in the regulation of intestinal health and are essential for the physiology, metabolism, and immune system development of hosts.5 Additionally, as the most extensive and intricate micro-ecological system in the body, the intestinal microbiota and its metabolites serve as a critical connection between diet and the host. Accordingly, intestinal microbial modulation may be a potential new strategy for preventing intestinal injury and its related disorders. Recent studies have provided mounting evidence that polyphenolic plant extracts can exert a beneficial impact on the metabolic functions of certain microorganisms, ultimately leading to an enhancement of host health.6–8Caffeic acid (3,4-dihydroxycinnamic acid, CA) is a polyphenol compound that is extensively present in plants, including fruits and vegetables, and exhibits various promising biological activities.9 Numerous pharmacological activities and physiological functions of CA have been reported, including antioxidative, anti-inflammatory, anti-apoptotic, and antibacterial activities, and it has the potential to attenuate intestinal injury.10–12 Meanwhile, we have demonstrated that plant polyphenols modulate the intestinal microbiota and its metabolites in mice, resulting in beneficial biological effects.8,13 Furthermore, a recent study found that CA can mitigate non-alcoholic fatty liver disease in mice by modulating the gut microbiota.14 However, there exists a dearth of information concerning the precise mechanisms through which CA supplementation can enhance intestinal health in piglets, particularly concerning the protective function of intestinal barriers. Hence, this investigation aims to examine and clarify the potential mechanism by which CA supplementation ameliorates intestinal injury and promotes intestinal health in a piglet model.
Herein, we hypothesized that CA supplementation could potentially modulate intestinal microbiota and its metabolites, thereby improving intestinal barrier function and mitigating intestinal injury. To test this hypothesis, a series of experiments were conducted using the piglet model to study the effect of CA supplementation on the intestinal microbiota and its metabolites. Furthermore, a lipopolysaccharide (LPS)-induced intestinal injury model was used to evaluate the potential of CA supplementation to modulate the intestinal microbiota and its metabolites and improve intestinal injury. Collectively, this study provides valuable insights into the regulation of CA supplementation on intestinal barrier function and intestinal microecology.
2. Materials and methods
2.1 Ethics statement
All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Chinese Academy of Agriculture Sciences and approved by the Animal Ethics Committee of the Institute of Animal Science, the Chinese Academy of Agricultural Sciences (Ethics Code Permit IAS2021-227).2.2 Animals and experiments
Twenty-four piglets (7.43 ± 0.79 kg body weight (BW); Duroc × Landrace × Large White; barrows) were randomly divided into 4 groups after adaptive feeding for 4 days: (1) the control group (CON), (2) the LPS group (LPS), (3) the LPS + CA group (CAL), and (4) the CA group (CA). All piglets in each group were provided with basal diets with or without 500 mg per kg of CA, and the experimental period was 28 days. On the 21st and 28th days, piglets were weighed to calculate average daily gain (ADG), and piglets in the LPS and CAL groups were administered intraperitoneal injections of LPS (80 μg per kg of BW, Escherichia coli O55:B5, Sigma), while piglets in the CON and CA groups received the same volume of saline. All piglets were able to drink and eat freely during the experiment. The basal diet (Table S1†) was formulated to meet the nutrient requirements for piglets as recommended by the NRC 2012.15 CA (purity ≥ 99%) was obtained from Hangzhou Viablife Biotech Co., Ltd. The experimental scheme is shown in Fig. S1.†2.3 Blood and intestinal sample collection
On the 28th day, three hours after administering LPS or saline, blood was collected from the anterior vena cava and serum was obtained through centrifugation at 1800g for 10 min. After the collection of blood, all piglets were subjected to anaesthesia with pentobarbital sodium and euthanized through exsanguination. A 2 cm colonic segment was preserved for morphological analysis in a solution of 4% paraformaldehyde or 2.5% glutaraldehyde. The colonic mucosa or digesta was collected, immediately flash-frozen in liquid nitrogen, and then stored at −80 °C until detection.2.4 Intestinal morphology
The colonic segments fixed in 4% paraformaldehyde were used to determine morphology using the H&E staining kit and to determine goblet cells using PAS-AB staining kits. The H&E and PAS-AB staining kits were purchased from Beijing Solarbio Science & Technology Co., Ltd. Following the process of dehydration, embedding, sectioning, and staining, the colonic sections were examined using a Leica microscope. The crypt depth and the goblet cell number were quantified with ImageJ software. Additionally, the colonic segments fixed in 2.5% glutaraldehyde solution were prepared for transmission electron microscopy (TEM) analysis. Ultrathin sections were made by professionals from Wuhan Servicebio Technology Co., Ltd.2.5 Serum cytokines, antioxidants, and biochemical indexes
Serum cytokines (IL-1β, IL-6, TNF-α, IL-10), antioxidants (T-AOC, SOD, GSH-Px, CAT, MDA, H2O2), permeability indicators (D-Lac, DAO), and LPS were measured using biochemical assay kits (Jiancheng Biochemical, Nanjing, China).2.6 RNA extraction and quantitative real-time (qRT)-PCR
Total RNA was extracted from the colonic mucosa with an RNeasy kit (Genebetter, Beijing, China), and the same amount of RNA was used for reverse transcription into cDNA with a PrimeScript RT reagent kit (Takara, Shiga, Japan). qRT-PCR was performed using a TB Green kit (Takara, Shiga, Japan) on an ABI Q7 Real-Time PCR system. Gene expression was calculated using the 2−ΔΔCT method with β-actin and GAPDH as internal reference genes. The primer sequences are shown in Table S2.†2.7 Bacterial 16S rRNA gene amplicon sequencing
Total bacterial DNA was extracted from the colonic digesta with a MagPure Soil DNA LQ kit (Magen, Guangdong, China). To analyze bacterial diversity, the V3–V4 variable regions of 16S rRNA genes were amplified using primers 343F (5′-TACGGRAGGCAGCAG-3′) and 798R (5′-AGGGTATCTAATCCT-3′), followed by sequencing on the Illumina NovaSeq 6000 (OE Biotech Company; Shanghai, China). Bioinformatics analysis was conducted using OECloud tools at https://cloud.oebiotech.cn. The raw reads were deposited in the Sequence Read Archive (SRA) database (accession number: PRJNA960710) of the NCBI.2.8 Quantification of bile acids (BAs) and short chain fatty acids (SCFAs)
The BAs were extracted from colonic digesta according to the method used in the previous study.2 Briefly, approximately 50 mg of lyophilized colonic digesta was suspended in 50 mM sodium acetate and incubated on an orbital shaker for 1 h (150g, 45 °C). Following centrifugation of the sample at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min, the supernatant was mixed with sodium acetate buffer at a ratio of 1
000g for 15 min, the supernatant was mixed with sodium acetate buffer at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and subsequently passed through a Bond Elute C18 cartridge (Agilent, CA, USA). Then the cartridge was washed with 25% ethanol, and the BAs were eluted using 5 mL of methanol. It was blow-dried with nitrogen, and the residue was reconstituted with 1 ml of methanol. The quantification of BAs was performed using LC-MS/MS (Waters, Milford, USA) after filtering through a 0.45 μm filter.
3 and subsequently passed through a Bond Elute C18 cartridge (Agilent, CA, USA). Then the cartridge was washed with 25% ethanol, and the BAs were eluted using 5 mL of methanol. It was blow-dried with nitrogen, and the residue was reconstituted with 1 ml of methanol. The quantification of BAs was performed using LC-MS/MS (Waters, Milford, USA) after filtering through a 0.45 μm filter.
Based on our previous studies, we quantified SCFAs using gas chromatography (GC).16 Briefly, approximately 1 g of colonic digesta was dissolved in distilled water, subjected to a 30 min shake, and incubated at 4 °C overnight. The extracted supernatant was obtained through centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 10 min. 25% metaphosphoric acid was added to the extracted supernatant at a ratio of 1
000g for 10 min. 25% metaphosphoric acid was added to the extracted supernatant at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 and left for 3 h at room temperature. Following vortexing and centrifugation, the supernatant was filtered through a 0.45 μm filter and subsequently analysed for SCFAs using an Agilent 7890N GC (Agilent, Santa Clara, USA).
9 and left for 3 h at room temperature. Following vortexing and centrifugation, the supernatant was filtered through a 0.45 μm filter and subsequently analysed for SCFAs using an Agilent 7890N GC (Agilent, Santa Clara, USA).
2.9 Statistics
The experimental data were analysed by ANOVA with Tukey's test (JMP 10.0, NC, USA). Different letters represent a significant difference. Significance was declared if P < 0.05, and a tendency was considered if 0.05 < P < 0.10.3. Results
3.1 Caffeic acid supplementation altered the growth performance
During the entire three-week duration of the experiment before the LPS challenge, the D21 body weight (BW) and average daily gain (ADG) were significantly higher in piglets fed the CA diet than in those fed the basal diet (n = 12, P < 0.01, Fig. 1A). From day 21 to 28, piglets in the LPS challenge had significantly lower ADG and D28 BW, whereas piglets treated by CA had significantly higher D28 BW (P < 0.01). There was a significant increase in D28 BW and ADG of piglets in the CAL group compared with the LPS group (P < 0.01).3.2 Caffeic acid supplementation altered colonic morphology and barrier function
The intestinal morphological characteristics are shown in Fig. 1B. In the LPS group, H&E-stained colonic sections demonstrated remarkable destruction of the colonic mucosa and surface epithelium and decreased crypt depth, which was not observed in other groups. Notably, intestinal injuries caused by the LPS challenge were improved in the CAL group (Fig. 1B and C). It was clear that the colonic ultrastructure was broken and damaged after the LPS challenge: the microvilli became shorter or were even broken, the ultrastructure of the tight junction was disrupted, and part of the cell structure was deformed, particularly swelling of the mitochondria. CA supplementation could maintain normal ultrastructural integrity and improve LPS-induced colonic ultrastructure disruption (Fig. 1B). Meanwhile, the LPS challenge caused a significant increase in serum D-Lac and DAO levels compared with the CON group. The increased serum D-Lac and DAO levels induced by the LPS challenge were prevented in the CAL group (P < 0.05; Fig. 1E). Additionally, the LPS-induced decrease in the mRNA expression of tight junction proteins (TJP) ZO-1 and Claudin-1 was restored by CA (P < 0.05; Fig. 1F). Besides, PAS-AB staining results revealed that the LPS challenge decreased goblet cell numbers, but CA supplementation significantly increased goblet cell numbers in the colon (P < 0.05; Fig. 1B and D).3.3 Caffeic acid supplementation altered serum and colonic cytokine levels
The LPS challenge significantly increased the serum concentration of IL-1β, IL-6, and TNF-α, but decreased IL-10 (P < 0.05; Fig. 2A). CA supplementation restored the concentration of IL-1β, IL-6, and TNF-α, and significantly increased IL-10 (P < 0.05; Fig. 2A). The LPS challenge also increased the mRNA expression of IL-1β, IL-6, and TNF-α, and decreased IL-10 (P < 0.05; Fig. 2C), whereas CA supplementation significantly improved the changes of cytokine mRNA expressions induced by LPS in the colon (P < 0.05; Fig. 2C). In addition, the LPS challenge markedly shifted the mRNA expression of TLR4 and NF-κB, which was restored by the CA supplementation (P < 0.05; Fig. 2C). The level of LPS in the LPS group was significantly higher than that in all other groups (P < 0.05; Fig. 2A).3.4 Caffeic acid supplementation reduced oxidative stress
The LPS challenge significantly decreased the levels of T-AOC, CAT, SOD, and GSH-Px, but increased the concentration of H2O2 in the serum (P < 0.05; Fig. 2B). The CA supplementation restored the levels of T-AOC, CAT, SOD, and GSH-Px compared with the LPS group, and the concentration of H2O2 was significantly decreased (P < 0.05; Fig. 2B). Moreover, the LPS challenge decreased the mRNA expression of NQO1 (P = 0.08), SOD1 (P < 0.05), and GPX2 (P = 0.09) but increased HO-1 (P = 0.08) in the colon compared with the CON group, while CA supplementation restored these changes (Fig. 2D).3.5 Caffeic acid supplementation altered the colonic apoptosis-related gene expression
The LPS challenge significantly increased the mRNA expression of Bax and Fas, and Bax/Bcl-2 in the colon of piglets (P < 0.05; Fig. 2E). CA supplementation showed potential in improving the expression disorder of apoptosis-related genes, and significantly increased Bcl-2 and decreased Bax and Fas, and Bax/Bcl-2 in the colon (P < 0.05; Fig. 2E). No significant difference was observed for caspase3 and caspase9 mRNA abundance among the groups.3.6 Caffeic acid supplementation changed colonic microbiota
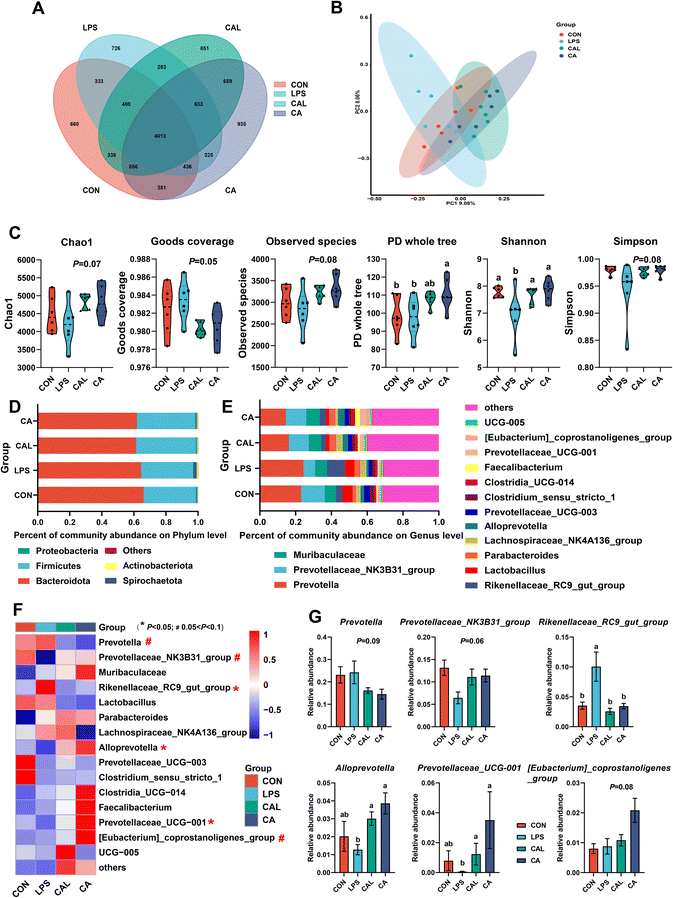
In the CAL group, more OTUs were shared with the CON group than with the LPS group, indicating a structure of intestinal microbiota more similar to that of the CON group (Fig. 3A). Meanwhile, the PCoA plot showed a distinct cluster of the LPS group was formed compared to the CON and CAL groups (Fig. 3B). Furthermore, the LPS challenge significantly decreased the Shannon index, while CA supplementation significantly increased the PD whole tree and restored the Shannon index shift induced by LPS (P < 0.05; Fig. 3C). Approximately 97% of the total colonic bacterial community was made up of Bacteroidota and Firmicutes (Fig. 3D). At the genus level, Prevotella, Prevotellaceae_NK3B31_group, and Muribaculaceae were the three main genera. Furthermore, analysis revealed significant differences in colonic microbiota at the genus level (Fig. 3E). The relative abundance of Rikenellaceae_RC9_gut_group was significantly increased, but Alloprevotella and Prevotellaceae_UCG-001 were significantly decreased by the LPS challenge (P < 0.05; Fig. 3F and G). Meanwhile, the LPS challenge tended to decrease the relative abundance of Prevotellaceae_NK3B31_group (P = 0.06; Fig. 3F and G). Additionally, CA supplementation showed a tendency to increase the relative abundances of Alloprevotella, Prevotellaceae_UCG-001, and [Eubacterium]_coprostanoligenes_group, and decrease the relative abundance of Prevotella (0.05 < P < 0.1; Fig. 3F and G). Compared with the LPS group, the relative abundance of Rikenellaceae_RC9_gut_group was lower in the CAL group, whereas the relative abundances of Alloprevotella and Prevotellaceae_UCG-001 were greater (P < 0.05; Fig. 3F and G).3.7 Caffeic acid supplementation altered BA and SCFA levels
A total of 22 BAs were detected in the colonic digesta of piglets, of which about 96% BAs existed in free form (Fig. 4A). BAs in the secondary BAs (SBAs) or primary BAs (PBAs) were, respectively, hyodeoxycholic acid (HDCA, 83.79%) or hyocholic acid (HCA, 7.07%), which consisted of about 97% of total colonic digesta BAs with chenodeoxycholic acid (CDCA, 1.82%), LCA, lithocholic acid (LCA, 1.37%), glycol-ursodeoxycholic acid (GUDCA, 1.27%), and glycol-CDCA (GCDCA, 1.02%). Overall, compared with other groups, the concentration of PBAs in the LPS group increased significantly (P < 0.05), while total BAs (TBAs) and SBAs did not significantly differ among the groups (Fig. 4C). Eleven highly differentially abundant BAs among the groups of piglets were further identified (Fig. 4B and D). Specifically, the LPS challenge decreased the concentration of tauroursodeoxycholic acid (TUDCA), taurohyodeoxycholic acid (THDCA), tauro-ω-muricholic acid (Tω-MCA), taurocholic acid (TCA) and LCA, but increased ursodeoxycholic acid (UDCA), β-muricholic acid (β-MCA), HCA, CDCA, and cholic acid (CA*; P < 0.05). The LPS-induced increased concentrations of UDCA, β-MCA, HCA, CDCA, and CA* were alleviated by the CA supplementation. The concentrations of UDCA, β-MCA, HCA, CDCA, and CA* in the CAL group were similar to those in the CON group (Fig. 4D). Moreover, an additional assessment of SCFA levels in the colon was conducted, revealing a noteworthy elevation of colonic isovalerate in the LPS group, while piglets treated with CA displayed a reduced concentration of isovalerate (P < 0.05; Fig. 4E).3.8 Correlation analysis between colonic microbiota and its metabolites and biochemical parameters
We furthermore performed correlation analyses to elucidate the potential associations between colonic microbiota and its metabolites and biochemical parameters, encompassing intestinal barrier indicators (D-Lac, DAO, and TJP), inflammatory cytokines, oxidative status indices, and apoptosis-related genes in weaned piglets (Fig. 5). In terms of intestinal barrier indicators, the results indicate a negative correlation between TUDCA and serum DAO, while UDCA and β-MCA show a positive correlation. Additionally, Rikenellaceae_RC9_gut_group, HCA, and isovalerate exhibited a negative correlation with colonic ZO-1, whereas Alloprevotella was positively but UDCA was negatively correlated with colonic Claudin-1 (P < 0.05). In terms of inflammatory cytokines, Rikenellaceae_RC9_gut_group, UDCA, and isovalerate were positively correlated with serum IL-1β. Additionally, Rikenellaceae_RC9_gut_group and UDCA were positively correlated, while Alloprevotella was negatively correlated with serum IL-6. Furthermore, Rikenellaceae_RC9_gut_group, UDCA, and β-MCA were positively correlated, while Alloprevotella was negatively correlated with serum TNF-α. The study also observed a negative correlation between Alloprevotella and colonic IL-1β and a positive correlation between HCA and colonic IL-1β. UDCA was positively correlated with colonic IL-6. Finally, Rikenellaceae_RC9_gut_group and HCA were negatively correlated but Alloprevotella and Prevotellaceae_UCG-001 were positively correlated with colonic IL-10 (P < 0.05). In terms of oxidative indexes, LCA exhibited a positive association, whereas UDCA, β-MCA, CDCA, and isovalerate exhibited a negative association with serum T-AOC. Additionally, THDCA exhibited a positive association, while UDCA and β-MCA exhibited a negative association with serum CAT. Furthermore, UDCA, HCA, and CA* exhibited a negative association, whereas Prevotellaceae_UCG-001, TUDCA, and TCA exhibited a positive association with serum SOD. Lastly, CDCA and CA* exhibited a negative association with serum GSH-Px (P < 0.05). In terms of colonic apoptosis-related genes, Rikenellaceae_RC9_gut_group and UDCA exhibited a positive correlation, while Alloprevotella, TUDCA, and THDCA displayed a negative correlation with colonic Bax. Additionally, Prevotellaceae_UCG-001 was positively associated with colonic Bcl-2, HCA demonstrated a positive correlation with colonic Fas, and TUDCA exhibited a negative correlation with colonic caspase3 (P < 0.05). | ||
| Fig. 5 Correlation analysis between colonic microbiota and its metabolisms and biochemical parameters. Significance is presented as *P < 0.05 and **0.05 < P < 0.01. | ||
4. Discussion
During weaning, piglets are vulnerable to intestinal injury caused by incomplete intestinal function development and alterations in their nutritional and environmental conditions. This can result in substantial growth retardation and consequential economic losses in pig production. Consequently, it is crucial to identify effective therapeutic drugs to improve intestinal injury. The present study used LPS to construct a piglet intestinal injury model. CA is a kind of natural plant polyphenol, which has the potential effect of reducing intestinal injury, but its mechanism is still unclear. Here, we showed that CA supplementation significantly improved the growth performance, immune response, and oxidative stress while enhancing intestinal integrity in LPS-challenged piglets. Furthermore, the administration of CA was found to ameliorate the dysbiosis of intestinal microbiota and modulate the microbial metabolites. More specifically, CA supplementation promoted the contents of beneficial bacteria and metabolites to maintain intestinal microecology, and further improved intestinal barrier function. In general, we showed that CA has the potential to serve as a viable nutritional intervention for mitigating intestinal injury in piglets.CA is renowned for its exceptional therapeutic properties, such as its efficacy as a hepatoprotective, neuroprotective, and antidiabetic agent. However, there is a dearth of information about the effect of CA supplementation on the growth performance of piglets. As far as we know, this is the first study to demonstrate this effect in piglets. Our findings showed that CA supplementation significantly improves the growth performance of piglets before the LPS challenge. Other studies on plant polyphenols have demonstrated the same results in piglets, such as chlorogenic acid17 and ellagic acid.18 In that regard, the findings from a recent study on the growth performance of beluga are very interesting. Ahmadifar et al. (2022) found significantly higher digestive enzyme activity, such as amylase, lipase, and pepsin activities, and better growth performance in the CA-treated group.19 Concurrently, our findings suggest that the provision of CA supplementation yields advantageous effects on intestinal health status, thereby implying a potential correlation between enhanced growth performance in piglets and an improved intestinal milieu, leading to heightened intestinal digestion and absorption, ultimately resulting in improved growth performance. It should be noted that these digestive enzymes in piglets could be further studied in the future to confirm our views. Moreover, our data illustrated that CA supplementation alleviated the LPS-induced decline in growth performance in piglets.
In this study, histomorphometry analyses showed that CA treatment significantly improved intestinal injury in LPS-induced piglets, such as intestinal rupture and shedding, destruction of microvilli and organelles, and changes in goblet cells and crypts. Interestingly, we found that the CD of piglets fed with CA increased compared with that of the control group, which may be related to the increase in feed intake.20 Previous research has indicated a positive correlation between food intake and colonic recess depth. However, since our study did not measure food intake, it remains uncertain whether this relationship holds true in our specific investigation. It is speculated that CA promotes the development of the colonic crypt. Serum D-Lac and DAO can be used as indicators of intestinal integrity, as they are normally present in very small amounts in blood circulation, but their amounts are enhanced when the mucosa is damaged. The present study demonstrated that the LPS challenge led to a significant increase in DAO and D-Lac levels, which were subsequently restored by supplementing with CA. These findings indicate that CA may have protective effects against intestinal barrier injury in response to LPS stimulation. Additionally, we observed that the mRNA expression of TJP also changed significantly. Consistent with previous research, CA supplementation has been shown to enhance Occludin, Claudin-1, and ZO-1 abundance in the DSS-induced colitis mouse model.8 These results indicate that CA could partially protect intestinal barrier function by enhancing TJP expression. Taken together, CA supplementation has a protective effect on the intestinal integrity and barrier function of piglets.
In young animals and infants, the etiology of intestinal injury is attributed to the activation of inflammatory responses by various factors, including oxidative stress, toxic compounds, and pathogenic bacteria.21 Polyphenolic compounds, such as flavonoids, phenolic acids, and phenolic alcohols, have been proven to mitigate inflammation by regulating the TLR4/NF-κB signalling pathway.22 The present study revealed that the administration of LPS resulted in an up-regulation of TLR4 and NF-κB, whereas CA supplementation led to a down-regulation of NF-κB expression. Based on this, we speculate that CA may confer beneficial effects on intestinal health by mitigating the inflammatory response. Subsequently, it was observed that IL-1β, IL-6, and TNF-α were up-regulated following the LPS challenge in piglets, which could be indicative of compromised intestinal integrity. However, CA supplementation ameliorated the deleterious effects of LPS and promoted IL-10 expression, aligning with the alteration trend of inflammatory cytokines in the serum. It has been found that when the level of intestinal inflammation increases, the intestinal mucosal barrier is damaged,23 which promotes endotoxins and inflammatory cytokines to enter the bloodstream, which was confirmed by the increase of serum cytokines and LPS in this study. Taken together, weaned piglets with CA supplementation showed significant improvement in intestinal inflammation.
Intestinal injury is often linked to oxidative stress, as seen in weaned piglets who are exposed to various harmful stimuli that cause intestinal injury and post-weaning diarrhoea in pigs.24 Our data indicate that a challenge with LPS increases H2O2 levels and decreases T-AOC, CAT, SOD, and GSH-Px. However, CA supplementation has been shown to improve the levels of these antioxidant enzymes, which is in agreement with the findings of Wan et al.8 A recent study also found that CA can protect HTR-8/SVneo cells from oxidative stress and genotoxicity induced by H2O2.25 The obtained data revealed their promising antioxidant behaviour. In terms of mechanism, it has been shown that CA alleviates oxidative stress by regulating the Nrf2/HO-1 pathway, thus preventing colitis.8 We noticed that CA caused up-regulation of the downstream genes NQO1, SOD1, and GPX2, although it did not reach a significant level. Interestingly, we observed that LPS caused an increase in the expression of HO-1, while CA restored HO-1 in the colon. HO-1 is an enzyme that can be induced and is recognized for its anti-inflammatory and antioxidant properties. Recent research has revealed that exposure to an inflammatory stimulus increased microglial HO-1, leading to oxidative stress and increased inflammatory markers, including IL-1β. Notably, these changes were prevented in aged HMOX1M-KO and WT mice treated with the HO-1 inhibitor.26 This suggests that the possible relationship between LPS/CA and HO-1 requires further studies. Collectively, CA supplementation serves as a protective measure against LPS-induced intestinal injury in piglets by augmenting antioxidant functions.
Recent research has demonstrated that alterations in apoptosis, whether increased or decreased, can result in heightened intestinal permeability and compromised barrier function, ultimately leading to intestinal dysfunction.27 Past studies have revealed that CA can mitigate the apoptosis of hematopoietic stem cells induced by ionizing radiation by preventing the up-regulation of caspase3 and the down-regulation of anti-apoptotic factors Bcl-2.28 The current investigation revealed that the administration of LPS resulted in an elevation of pro-apoptotic factors Bax and Fas, as well as Bax/Bcl-2. Conversely, the supplementation of CA led to a reduction in these pro-apoptotic genes and an increase in Bcl-2. Furthermore, similar results have been found in other plant polyphenols, such as tea polyphenols.29 These findings suggest that CA may mitigate LPS-induced apoptosis of intestinal epithelial cells by down-regulating apoptosis-related gene expression in the colon of piglets. Overall, our study provides the first evidence in a piglet model that CA possesses the ability to suppress excessive apoptosis of intestinal epithelial cells.
The present study revealed that CA could improve intestinal injury in a piglet model, but the potential mechanism remains unclear. Many studies have shown that intestinal microbiota and its metabolites play a crucial role in health and diseases. A recent review has indicated that the health benefits of plant polyphenols are contingent upon the composition of the intestinal microbiota.30 In our previous investigation, we observed that CA mitigated inflammation by regulating the colonic microbiota composition in mice with DSS-induced colitis.8 Consequently, we hypothesized that the intestinal microbiota and its metabolites may mediate the advantageous effects of CA on intestinal health. As anticipated, our findings indicate that CA supplementation effectively restored the LPS-induced alterations in the intestinal microbiota, encompassing microbial composition and alpha diversity. Specifically, we observed a surge in Rikenellaceae_RC9_gut_group following LPS exposure, which was subsequently mitigated by CA supplementation. Our investigation further revealed that the increase of Rikenellaceae_RC9_gut_group was accompanied by the aggravation of intestinal inflammation and injury, as evidenced by the correlation between IL-10, ZO-1, and Bax with Rikenellaceae_RC9_gut_group. A positive correlation was also found between Rikenellaceae_RC9_gut_group and inflammatory cytokines. It appears that the current studies on Rikenellaceae_RC9_gut_group mostly focused on glycolipid metabolism. However, there is a lack of consensus on its impact on the intestinal barrier. For instance, previous research has demonstrated that the prevalence of Rikenellaceae_RC9_gut_group increases in mice fed a high-fat diet, and this has been linked to abnormal glucose and lipid metabolism.31 In summary, the present study reveals that Rikenellaceae_RC9_gut_group serves as a biomarker of intestinal injury and is most prevalent in the LPS group. Furthermore, our findings indicate that the abundance of Alloprevotella and Prevotellaceae_UCG-001 is lower in LPS-challenged piglets, while CA supplementation has the opposite effect. It has been proved that Alloprevotella is negatively correlated with inflammation, insulin resistance, and obesity.32 Additionally, Alloprevotella correlated positively with Claudin-1 expression but negatively with pro-inflammatory cytokines, indicating that CA supplementation may have beneficial properties for intestinal barrier and inflammation. So far, the role played by Prevotellaceae_UCG-001 remains controversial. One study showed that in colitis mice, the abundance of Prevotellaceae_UCG-001 decreased significantly.33 In the present study, Prevotellaceae_UCG-001 had a positive correlation with the level of SOD, IL-10, and Bax, indicating that Prevotellaceae_UCG-001 was an important beneficial player in intestinal health. Consequently, CA supplementation may reduce intestinal inflammation and injury by regulating intestinal microbiota balance.
The intestinal microbiota has been found to exert an influence on host intestinal homeostasis, primarily through metabolic pathways involving BAs and SCFAs. Recent studies have shown that BAs function as signalling molecules and are integral to regulating intestinal homeostasis and overall host health. For instance, a recent review posits that the intestinal microbiota–BAs axis represents a promising therapeutic target for addressing inflammatory bowel diseases (IBD) and colorectal cancer (CRC).34 Moreover, some studies have shown that plant polyphenols could regulate the composition of BAs and metabolic diseases.35 We found that metabolism of intestinal BAs was disordered in the case of intestinal injury such as primary BAs being significantly increased, and CA treatment restored intestinal BA composition. Compared to healthy individuals, the primary BA level of IBD patients is higher, and the secondary BA level is lower, which led to a more pronounced inflammatory response in Caco-2 cells.36 Notably, HCA, CDCA, and UDCA increased, while LCA and TUDCA decreased after LPS stimulation. CDCA, a prevalent primary BA in mammals, has been previously linked to pro-inflammatory properties.37 HCA, which is converted by CDCA in the liver and specific primary BAs in pigs, has been shown to be critically involved in maintaining glucose homeostasis.38 Yet, there are few reports on its regulation of intestinal injury. Our study found that the increase of HCA in the colon was positively correlated with IL-1β and Fas, showing pro-inflammatory and pro-apoptotic properties. UDCA is a secondary BA converted by intestinal microbiota metabolizing CDCA, although UDCA supplementation has already been reported to improve the disruption of intestinal barrier function and colonic inflammation during colitis.39 Meanwhile, currently available evidence in this study suggests that UDCA may be related to apoptosis, oxidative stress, and inflammation. UDCA is conveyed from the intestine to the liver via enterohepatic circulation, where it undergoes conjugation with taurine to generate TUDCA, which is subsequently transported back into the intestine. The primary BAs are converted into LCA by intestinal bacterial 7α-dehydroxylation. TUDCA and LCA exhibit antagonistic properties towards intestinal inflammation and demonstrate an inhibitory effect on experimental colitis.40,41 It is thus possible that CA alleviates intestinal injury by regulating the bacterial transformation of BAs. In addition, SCFAs have been demonstrated to regulate the immune response and have therapeutic potential for protection against various inflammatory diseases. Our previous research showed that CA supplementation elevated butyrate levels, thereby mitigating DSS-induced colitis in mice.8 Butyrate has been shown to exert anti-inflammatory effects in multiple disease models.42 However, the current study observed notable alterations in the level of isovalerate, but not in the level of butyrate. At present, there has been less research into the effects of isovalerate on intestinal health. It is known that the main factor of isovaleric acidaemia in children is the severe metabolic disorder caused by the accumulation of isovalerate due to the lack of isovaleryl-CoA dehydrogenase.43 The present study revealed that isovalerate exhibited a positive correlation with pro-inflammatory cytokines and a negative correlation with antioxidant enzymes. These findings suggest that the administration of CA may enhance intestinal barrier function and growth performance by modulating intestinal microbiota and its metabolites.
5. Conclusions
In summary, dietary CA supplementation could augment intestinal barrier function, ameliorate intestinal injury and enhance the growth performance in LPS-challenged piglets. Furthermore, dietary CA supplementation could improve the intestinal microbiota dysbiosis and abnormal metabolism of BAs and SCFAs caused by the LPS challenge, thereby contributing to a reduction in inflammatory responses and oxidative stress in piglets. This study offers a novel approach for utilizing CA as a functional food ingredient and enhancing intestinal health and growth performance through dietary interventions.Author contributions
Xiaobin Wen: conceptualization, methodology, software, and writing – original draft. Fan Wan: methodology and formal analysis. You Wu: writing – review & editing. Lei Liu: resources. Yueping Liu: writing – review & editing. Ruqing Zhong: conceptualization, writing – original draft, and resources. Liang Chen: conceptualization, writing – review & editing, and supervision. Hongfu Zhang: conceptualization, writing – review & editing, and supervision.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was funded by the National Key Research and Development Program of China [2022YFD1300705], Science and Technology Program of Guizhou Province ([2021]Genaral149), and Agricultural Science and Technology Innovation Program (cxgc-ias-16, ASTIPIAS07).Notes and references
- X. Wen, R. Zhong, G. Dang, B. Xia, W. Wu, S. Tang, L. Tang, L. Liu, Z. Liu, L. Chen and H. Zhang, Pectin supplementation ameliorates intestinal epithelial barrier function damage by modulating intestinal microbiota in lipopolysaccharide-challenged piglets, J. Nutr. Biochem., 2022, 109, 109107 CrossRef CAS PubMed.
- S. Tang, Y. Chen, F. Deng, X. Yan, R. Zhong, Q. Meng, L. Liu, Y. Zhao, S. Zhang, L. Chen and H. Zhang, Xylooligosaccharide-mediated gut microbiota enhances gut barrier and modulates gut immunity associated with alterations of biological processes in a pig model, Carbohydr. Polym., 2022, 294, 119776 CrossRef CAS PubMed.
- N. Ma, P. Guo, J. Chen, Z. Qi, C. Liu, J. Shen, Y. Sun, X. Chen, G. Q. Chen and X. Ma, Poly-β-hydroxybutyrate alleviated diarrhea and colitis via Lactobacillus johnsonii biofilm-mediated maturation of sulfomucin, Sci. China: Life Sci., 2022, 1569–1588 Search PubMed.
- R. Hu, Z. He, M. Liu, J. Tan, H. Zhang, D. X. Hou, J. He and S. Wu, Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets, J. Anim. Sci. Biotechnol., 2020, 11, 92 CrossRef CAS PubMed.
- L. M. Petersen, E. J. Bautista, H. Nguyen, B. M. Hanson, L. Chen, S. H. Lek, E. Sodergren and G. M. Weinstock, Community characteristics of the gut microbiomes of competitive cyclists, Microbiome, 2017, 5, 98 CrossRef PubMed.
- J. Chen, Y. Luo, Y. Li, D. Chen, B. Yu and J. He, Chlorogenic Acid Attenuates Oxidative Stress-Induced Intestinal Epithelium Injury by Co-Regulating the PI3K/Akt and IκBα/NF-κB Signaling, Antioxidants, 2021, 10, 1915 CrossRef CAS PubMed.
- M. Park, J. Choi and H. J. Lee, Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study, Nutrients, 2020, 12, 1815 CrossRef CAS PubMed.
- F. Wan, R. Zhong, M. Wang, Y. Zhou, Y. Chen, B. Yi, F. Hou, L. Liu, Y. Zhao, L. Chen and H. Zhang, Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice, Front. Microbiol., 2021, 12, 784211 CrossRef PubMed.
- S. Mirzaei, M. H. Gholami, A. Zabolian, H. Saleki, M. V. Farahani, S. Hamzehlou, F. B. Far, S. O. Sharifzadeh, S. Samarghandian, H. Khan, A. R. Aref, M. Ashrafizadeh, A. Zarrabi and G. Sethi, Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer, Pharmacol. Res., 2021, 171, 105759 CrossRef CAS PubMed.
- R. Hao, J. Ge, Y. Ren, X. Song, Y. Jiang, D. Sun-Waterhouse, F. Li and D. Li, Caffeic acid phenethyl ester mitigates cadmium-induced hepatotoxicity in mice: Role of miR-182-5p/TLR4 axis, Ecotoxicol. Environ. Saf., 2021, 207, 111578 CrossRef CAS PubMed.
- M. Kolgazi, S. Cilingir, O. Yilmaz, M. Gemici, H. Yazar, S. Ozer, M. Acikel-Elmas, S. Arbak and G. G. Suyen, Caffeic acid attenuates gastric mucosal damage induced by ethanol in rats via nitric oxide modulation, Chem.-Biol. Interact., 2021, 334, 109351 CrossRef CAS PubMed.
- M. F. Vera Castro, C. E. Assmann, N. Stefanello, K. P. Reichert, T. V. Palma, A. D. da Silva, V. V. Miron, V. B. Mostardeiro, V. M. M. Morsch and M. R. Chitolina Schetinger, Caffeic acid attenuates neuroinflammation and cognitive impairment in streptozotocin-induced diabetic rats: pivotal role of the cholinergic and purinergic signaling pathways, J. Nutr. Biochem., 2023, 115, 109280 CrossRef.
- H. Han, R. Zhong, S. Zhang, M. Wang, X. Wen, B. Yi, Y. Zhao, L. Chen and H. Zhang, Hydroxytyrosol attenuates diquat-induced oxidative stress by activating Nrf2 pathway and modulating colonic microbiota in mice, J. Nutr. Biochem., 2023, 113, 109256 CrossRef CAS PubMed.
- H. N. Mu, Q. Zhou, R. Y. Yang, W. Q. Tang, H. X. Li, S. M. Wang, J. Li, W. X. Chen and J. Dong, Caffeic acid prevents non-alcoholic fatty liver disease induced by a high-fat diet through gut microbiota modulation in mice, Food Res. Int., 2021, 143, 110240 CrossRef CAS PubMed.
- N. R. Council, Nutrient Requirements of Swine: Eleventh Revised Edition, The National Academies Press, Washington, DC, 2012 Search PubMed.
- S. Tang, R. Zhong, C. Yin, D. Su, J. Xie, L. Chen, L. Liu and H. Zhang, Exposure to High Aerial Ammonia Causes Hindgut Dysbiotic Microbiota and Alterations of Microbiota-Derived Metabolites in Growing Pigs, Front. Nutr., 2021, 8, 689818 CrossRef PubMed.
- Y. Zhang, Y. Wang, D. Chen, B. Yu, P. Zheng, X. Mao, Y. Luo, Y. Li and J. He, Dietary chlorogenic acid supplementation affects gut morphology, antioxidant capacity and intestinal selected bacterial populations in weaned piglets, Food Funct., 2018, 9, 4968–4978 RSC.
- Y. Lu, M. Zhao, J. Mo, G. Lan and J. Liang, Dietary supplementation ellagic acid on the growth, intestinal immune response, microbiota, and inflammation in weaned piglets, Front. Vet. Sci., 2022, 9, 980271 CrossRef PubMed.
- E. Ahmadifar, S. Mohammadzadeh, N. Kalhor, F. Salehi, M. Eslami, A. Zaretabar, M. S. Moghadam, S. H. Hoseinifar and H. Van Doan, Effects of caffeic acid on the growth performance, growth genes, digestive enzyme activity, and serum immune parameters of beluga (Huso huso), J. Exp. Zool., Part A, 2022, 337, 715–723 CrossRef CAS.
- B. Keimer, S. Kröger, I. Röhe, R. Pieper, A. Simon and J. Zentek, Influence of differently processed yeast (Kluyveromyces fragilis) on feed intake and gut physiology in weaned pigs, J. Anim. Sci., 2018, 96, 194–205 CrossRef CAS PubMed.
- C. Huang, Z. Fan, D. Han, L. J. Johnston, X. Ma and F. Wang, Pyrroloquinoline quinone regulates the redox status in vitro and in vivo of weaned pigs via the Nrf2/HO-1 pathway, J. Anim. Sci. Biotechnol., 2021, 12, 77 CrossRef CAS PubMed.
- M. Rahimifard, F. Maqbool, S. Moeini-Nodeh, K. Niaz, M. Abdollahi, N. Braidy, S. M. Nabavi and S. F. Nabavi, Targeting the TLR4 signaling pathway by polyphenols: A novel therapeutic strategy for neuroinflammation, Ageing Res. Rev., 2017, 36, 11–19 CrossRef CAS PubMed.
- H. Z. Zhu, Y. D. Liang, Q. Y. Ma, W. Z. Hao, X. J. Li, M. S. Wu, L. J. Deng, Y. M. Li and J. X. Chen, Xiaoyaosan improves depressive-like behavior in rats with chronic immobilization stress through modulation of the gut microbiota, Biomed. Pharmacother., 2019, 112, 108621 CrossRef CAS PubMed.
- D. Wang, Y. Kuang, Q. Lv, W. Xie, X. Xu, H. Zhu, Y. Zhang, X. Cong, S. Cheng and Y. Liu, Selenium-enriched Cardamine violifolia protects against sepsis-induced intestinal injury by regulating mitochondrial fusion in weaned pigs, Sci. China: Life Sci., 2023, Published online Search PubMed.
- S. Kostić, A. Vilotić, A. Pirković, D. Dekanski, S. Borozan, M. Nacka-Aleksić, S. Vrzić-Petronijević and M. Jovanović Krivokuća, Caffeic acid protects human trophoblast HTR-8/SVneo cells from H2O2-induced oxidative stress and genotoxicity, Food Chem. Toxicol., 2022, 163, 112993 CrossRef PubMed.
- C. Fernández-Mendívil, E. Luengo, P. Trigo-Alonso, N. García-Magro, P. Negredo and M. G. López, Protective role of microglial HO-1 blockade in aging: Implication of iron metabolism, Redox Biol., 2021, 38, 101789 CrossRef.
- X. Chen, Y. Wu, Y. Hu, Y. Zhang and S. Wang, Lactobacillus rhamnosus GG Reduces β-conglycinin-Allergy-Induced Apoptotic Cells by Regulating Bacteroides and Bile Secretion Pathway in Intestinal Contents of BALB/c Mice, Nutrients, 2020, 13, 55 CrossRef PubMed.
- X. Wang, W. Liao, J. Chen, Y. Wu, C. Liu, S. Chen, Y. Xu, S. Wang, Y. Su, C. Du and J. Wang, Caffeic acid attenuates irradiation-induced hematopoietic stem cell apoptosis through inhibiting mitochondrial damage, Exp. Cell Res., 2021, 409, 112934 CrossRef CAS PubMed.
- Y. Chen, R. Luo, J. Li, S. Wang, J. Ding, K. Zhao, B. Lu and W. Zhou, Intrinsic Radical Species Scavenging Activities of Tea Polyphenols Nanoparticles Block Pyroptosis in Endotoxin-Induced Sepsis, ACS Nano, 2022, 16, 2429–2441 CrossRef CAS PubMed.
- A. D. Inchingolo, G. Malcangi, A. M. Inchingolo, F. Piras, V. Settanni, G. Garofoli, G. Palmieri, S. Ceci, A. Patano, N. De Leonardis, C. Di Pede, V. Montenegro, D. Azzollini, M. G. Garibaldi, Z. Kruti, A. Tarullo, G. Coloccia, A. Mancini, B. Rapone, A. Semjonova, D. Hazballa, M. T. D'Oria, M. Jones, L. Macchia, I. R. Bordea, A. Scarano, F. Lorusso, G. M. Tartaglia, C. Maspero, M. Del Fabbro, L. Nucci, K. Ferati, A. B. Ferati, N. Brienza, A. Corriero, F. Inchingolo and G. Dipalma, Benefits and Implications of Resveratrol Supplementation on Microbiota Modulations: A Systematic Review of the Literature, Int. J. Mol. Sci., 2022, 23, 4027 CrossRef CAS.
- Z. Wang, X. Li, L. Zhang, J. Wu, S. Zhao and T. Jiao, Effect of Oregano Oil and Cobalt Lactate on Sheep In Vitro Digestibility, Fermentation Characteristics and Rumen Microbial Community, Animals, 2022, 12, 118 CrossRef.
- J. Wang, P. Wang, D. Li, X. Hu and F. Chen, Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice, Eur. J. Nutr., 2020, 59, 699–718 CrossRef CAS PubMed.
- L. Hu, L. Jin, D. Xia, Q. Zhang, L. Ma, H. Zheng, T. Xu, S. Chang, X. Li, Z. Xun, Y. Xu, C. Zhang, F. Chen and S. Wang, Nitrate ameliorates dextran sodium sulfate-induced colitis by regulating the homeostasis of the intestinal microbiota, Free Radicals Biol. Med., 2020, 152, 609–621 CrossRef CAS PubMed.
- J. Cai, L. Sun and F. J. Gonzalez, Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis, Cell Host Microbe, 2022, 30, 289–300 CrossRef CAS.
- Z. Wang, M. Zeng, Z. Wang, F. Qin, J. Chen and Z. He, Dietary Polyphenols to Combat Nonalcoholic Fatty Liver Disease via the Gut-Brain-Liver Axis: A Review of Possible Mechanisms, J. Agric. Food Chem., 2021, 69, 3585–3600 CrossRef CAS PubMed.
- S. L. Collins, J. G. Stine, J. E. Bisanz, C. D. Okafor and A. D. Patterson, Bile acids and the gut microbiota: metabolic interactions and impacts on disease, Nat. Rev. Microbiol., 2023, 21, 236–247 CrossRef CAS PubMed.
- W. Jia, G. Xie and W. Jia, Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis, Nat. Rev. Gastroenterol. Hepatol., 2018, 15, 111–128 CrossRef CAS PubMed.
- X. Zheng, T. Chen, R. Jiang, A. Zhao, Q. Wu, J. Kuang, D. Sun, Z. Ren, M. Li, M. Zhao, S. Wang, Y. Bao, H. Li, C. Hu, B. Dong, D. Li, J. Wu, J. Xia, X. Wang, K. Lan, C. Rajani, G. Xie, A. Lu, W. Jia, C. Jiang and W. Jia, Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism, Cell Metab., 2021, 33, 791–803 CrossRef CAS PubMed .e797.
- J. B. J. Ward, N. K. Lajczak, O. B. Kelly, A. M. O’Dwyer, A. K. Giddam, N. J. Gabhann, P. Franco, M. M. Tambuwala, C. A. Jefferies, S. Keely, A. Roda and S. J. Keely, Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon, Am. J. Physiol.: Gastrointest. Liver Physiol., 2017, 312, G550–G558 CrossRef PubMed.
- N. K. Lajczak-McGinley, E. Porru, C. M. Fallon, J. Smyth, C. Curley, P. A. McCarron, M. M. Tambuwala, A. Roda and S. J. Keely, The secondary bile acids, ursodeoxycholic acid and lithocholic acid, protect against intestinal inflammation by inhibition of epithelial apoptosis, Physiol. Rep., 2020, 8, e14456 CAS.
- S. R. Sinha, Y. Haileselassie, L. P. Nguyen, C. Tropini, M. Wang, L. S. Becker, D. Sim, K. Jarr, E. T. Spear, G. Singh, H. Namkoong, K. Bittinger, M. A. Fischbach, J. L. Sonnenburg and A. Habtezion, Dysbiosis-Induced Secondary Bile Acid Deficiency Promotes Intestinal Inflammation, Cell Host Microbe, 2020, 27, 659–670 CrossRef CAS .e655.
- M. R. Couto, P. Gonçalves, F. Magro and F. Martel, Microbiota-derived butyrate regulates intestinal inflammation: Focus on inflammatory bowel disease, Pharmacol. Res., 2020, 159, 104947 CrossRef CAS.
- J. Vockley and R. Ensenauer, Isovaleric acidemia: new aspects of genetic and phenotypic heterogeneity, Am. J. Med. Genet., Part C, 2006, 142C, 95–103 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: the experimental scheme, Fig. S1; composition and nutrient levels of the basal diet (air-dry-basis), Table S1; primers for qRT-PCR analysis, Table S2. See DOI: https://doi.org/10.1039/d3fo02286b |
| ‡ These authors equally contributed to the study. |
| This journal is © The Royal Society of Chemistry 2023 |