Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Theanine maintains sleep quality in healthy young women by suppressing the increase in caffeine-induced wakefulness after sleep onset
Yoshitake
Baba
 *a,
Takanobu
Takihara
a and
Noritaka
Okamura
b
*a,
Takanobu
Takihara
a and
Noritaka
Okamura
b
aCentral Research Institute, ITO EN, Ltd, 21 Mekami, Makinohara, Shizuoka 421-0516, Japan. E-mail: yoshi-baba@itoen.co.jp; t-takihara@itoen.co.jp; Tel: +81-0548-54-1247
bEhime Prefectural University of Health Sciences, 543 Takoda, Tobe-Cho, Iyo-Gun, Ehime 791-2101, Japan. E-mail: okamu@epu.ac.jp
First published on 17th July 2023
Abstract
Energy drinks take advantage of caffeine's effects on wakefulness and performance; however, excessive intake has a negative effect on sleep. Green tea is consumed worldwide and has both a stimulating effect from caffeine and a calming or relaxing effect from theanine. Theanine reduces the excitotoxicity of caffeine. This study evaluated whether theanine improves the sleep quality worsened by caffeine in healthy young women. Sleep latency, sleep time, wake after sleep onset (WASO) time, and the number of WASOs were measured. A crossover study was performed using four treatment groups: theanine (50 mg), caffeine (30 mg), combined theanine and caffeine (TC), and placebo. The sleep stage was determined using electroencephalograms, and cerebral blood flow was measured using near-infrared spectroscopy. The caffeine group showed a significant increase in the WASO time compared with the placebo group; no difference was observed between the theanine or TC group and the placebo group. There were no differences in the sleep-onset latency or number of WASOs between the theanine, caffeine, or TC groups and the placebo group. In combination with theanine, only the caffeine-induced increase in the WASO time was suppressed. Our results suggest that theanine can reduce caffeine's effects on sleep quality.
Introduction
Tea is a widely consumed beverage; a quarter of tea sales are green tea.1 Green tea contains theanine2 and caffeine,3 both of which affect brain function. This study investigated the effects of green tea consumption on sleep and examined whether caffeine and theanine have beneficial effects on sleep.According to the National Health and Nutrition Survey conducted by the Ministry of Health, Labour, and Welfare in 2019, more than half of Japanese males and females sleep less than 7 hours per night.4 Japanese sleep duration compared with the rest of the world is short.5 Females have a shorter total-sleep duration and times-in-bed in their 30 s and older, even among Japanese people.6 Improving sleep quality is an important challenge in improving quality of life, as a lack of sleep can affect attention7 and may lead to traffic accidents.8
Caffeine blocks the adenosine A2A receptor, causing the nerve cells to become excited.3 Furthermore, caffeine excites neural circuits that project from the basal ganglia to the striatum and cerebral cortex.9 The basal ganglia is the center of various functions such as cognitive function, motor function, and emotion. In addition, high concentrations of caffeine inhibit phosphodiesterase activity3 and stimulate dopamine D2 receptors.9 These caffeine-induced changes in the brain affect many psychological functions.3 The combination of caffeine and theanine has been shown to affect brain function; ingesting matcha, which contains both theanine and caffeine, improves attention in humans.10,11 Matcha is powdered tencha, a type of green tea. Tencha has high theanine content because its cultivation and processing methods differ from other green teas.12 Improved attention was observed even when caffeine11 and theanine13 were consumed separately. This finding suggests that caffeine's arousal effect partially contributes to matcha's attention improvement function. Caffeine worsens sleep quality and inhibits sleep when 100 mg or more is consumed at bedtime.14 When this dosage was administered to 8 men with an average age (±standard error of the mean; SEM) of 23.3 ± 0.3 years, it prolonged sleep latency from 10.5 ± 2.7 to 18.8 ± 4.5 min, decreased sleep efficiency from 94.0 ± 0.4% to 91.6 ± 1.0%, and suppressed stage 4 of non-rapid eye movement sleep (NREMS) from 72.9 ± 10.6 to 62.5 ± 10.5 min. In our two previous studies,10,11 2 g of matcha contained 72.5 and 66.2 mg of caffeine. Although this amount of caffeine is less than the threshold for affecting sleep, it is recommended in Japan to avoid consuming this amount before bedtime.
Theanine is a non-proteinogenic amino acid found in the leaves of Camellia sinensis.15 The concentration of theanine in green tea varies depending on the cultivation and manufacturing methods.16 Theanine has a positive effect on sleep and suppresses wakefulness. It inhibits glutamine transport in the neurons and astroglia of rat brains17 and suppresses the release of the excitatory neurotransmitter glutamate.17,18 However, it does not cause daytime sleepiness.19 Ingestion of L-theanine affects the concentration of neurotransmitters such as dopamine, serotonin,20 tryptophan, and γ-aminobutyric acid (GABA).21 It increases GABA, an inhibitory neurotransmitter.22 GABAergic neurons are inhibitory, and theanine is thought to act on GABAergic neurons to promote sleep. Changes in neurotransmitters in the brain produce beneficial effects, including anti-stress effects11,23 and improved sleep. Ingestion of theanine (200 mg) improved sleep and objective sleep quality in 22 men with a mean age of 27.0 ± 0.9 years, according to the Oguri–Shirakawa–Azumi sleep inventory and objective indices, middle age version.24 This questionnaire assesses subjective sleep quality based on five factors: sleepiness on rising, initiation and maintenance of sleep, frequent dreaming, feeling refreshed, and sleep duration. When the participants woke up, theanine significantly improved initiation and maintenance of sleep (p < 0.1), frequent dreaming (p < 0.1), feeling of being refreshed (p < 0.05), and sleep duration (p < 0.05). In addition, an investigation of the body movements of 10 men during sleep using an actigraph showed significant improvements in sleep efficiency (placebo group: 93.8 ± 9.3; theanine group: 96.6 ± 4.1; p < 0.05) and wake after sleep onset (WASO; placebo group: 19.8 ± 24.0; theanine group: 12.6 ± 14.0; p < 0.05).
When cerebral blood flow is measured during non-REM sleep using electroencephalography (EEG), changes in cerebral blood flow dynamics occur when many low-frequency (LF) waves are observed.25 During sleep, cerebral blood flow decreases from onset to light sleep and tends to increase in slow-wave sleep and REM sleep after moderate sleep.26,27 Whether decreased cerebral blood flow causes drowsiness is unclear. However, we hypothesized that if caffeine's action on cerebral arteriole contraction reduces cerebral blood flow, the cerebral hemodynamics of non-REM sleep can be reproduced; thus, sleep onset may be smooth. The binding of adenosine to A2A and A2B receptors on vascular smooth muscles in the brain causes vasodilation.28,29 This hypothesis requires suppressing the excitatory effects of caffeine since it prevents one from falling asleep. However, the combined administration of theanine and caffeine does not show the same excitement demonstrated by administering caffeine alone.30 Caffeine reduces cerebral blood flow and may promote sleep onset by reproducing the cerebral blood flow state in non-REM sleep.
Caffeine acts as a stimulant of neural activity;31L-theanine acts as a suppressant.30 Green tea contains both components; in some cases, they have a beneficial effect due to their interaction, which is enhanced in the wakefulness state.32 The significance of these two components regarding sleep is not clear. In this study, we analyzed sleep quality using EEG, cerebral blood flow using near-infrared spectroscopy (NIRS), and LF and high-frequency (HF) band power values calculated from the pulse wave (LF/HF) to investigate the interaction between a mixture of theanine and caffeine in promoting sleep onset.
Methods
Test food
L-Theanine (trade name: Suntheanine; Taiyo Kagaku Co., Ltd, Mie, Japan) was used as the test food. Suntheanine contains ≥98% L-theanine. A caffeine extract (Shiratori Pharmaceutical Co., Ltd, Chiba, Japan) was used for the caffeine component. Drinks containing caffeine, theanine, or both caffeine and theanine (TC) were mixed using 200 ml of deionized water, adjusted to have the same taste and aroma as placebo drinks, and dispensed in cans of the same shape.In this study, the dose of theanine was 50 mg, which was previously shown to affect brain function when consumed in conjunction with caffeine.10 Furthermore, the theanine content of green tea, gyokuro, and matcha is high, approximately 2%.16 For one cup of tea, 2–3 g of tea leaves were used for theanine extraction. Assuming all the theanine is consumed, the theanine content would be 40–60 mg. Therefore, we set the dosage at 50 mg, assuming a cup of tea is consumed daily.
The amount of caffeine was determined as follows. A crossover study was conducted on five healthy females aged 21–22 years who ingested 0, 15, 30, and 50 mg of caffeine to identify the concentration that does not increase the NIRS signal in the prefrontal cortex. Data were acquired every 10 minutes for 90 minutes after caffeine intake. Individual differences were large, but no significant difference was observed in the mean values. Therefore, based on the changes observed in each participant's NIRS signal, the amount of caffeine was set at 30 mg. Only 1 of the 5 females showed an increased NIRS signal intensity 10–20 minutes after ingesting 30 mg of caffeine (data not shown).
Participants
All participants were university students attending the Ehime Prefectural University of Health Sciences (Ehime, Japan). The principal investigator distributed a paper with the research contents approved by the Ehime Prefectural University of Health Sciences to potential participants and verbally explained the research contents. Those who understood and agreed to participate in the study provided written informed consent. The inclusion criteria were as follows: (1) not suffering from a chronic illness that required regular hospital visits, (2) not taking medication on a daily basis, (3) drinking 2–3 cups of caffeine-containing beverages (e.g., tea and coffee) daily, (4) caffeine sensitivity that is not particularly high (e.g., they never experienced severe insomnia or dizziness after ingesting a caffeine drink), and (5) average daily sleep time of 6–9 h. Participants were not restricted from taking one-time medications for symptoms, such as abdominal pain and fever, that developed during the study period. The demographic characteristics of the participants are listed in Table 1.| Values are presented as the mean ± SD. | |
|---|---|
| Number of participants | 9 |
| Sex (male/female) | 0/9 |
| Age (years, range: 21–22) | 21.3 ± 0.5 |
| Height (cm) | 160.9 ± 3.9 |
| Weight (kg) | 56.0 ± 6.6 |
| Body mass index | 21.6 ± 2.2 |
The number of participants was set based on two previous studies. A study by Unno et al.,33 in which 90% of the participants were female, reported that low-caffeine green tea might improve sleep quality. Another study by Unno et al.34 reported that the stress index of salivary α-amylase activity is high when the sleep period is short, and the ingestion of theanine reduces the stress.
Study design
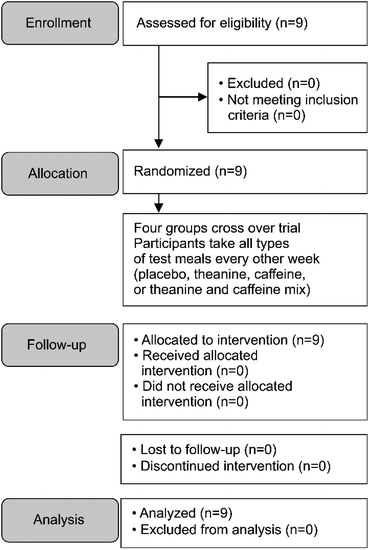
This study had a single-dose, four-treatment double-blind, crossover design. The primary endpoint was the EEG results. The secondary endpoints were the NIRS and LF/HF results. A flow diagram of the study is shown in Fig. 1. The participants adhered strictly to the study schedule (Fig. 2) from the day before the measurement to the end of the measurements. On the day of measurement, participants were fitted with sensors and electrodes at 10:30 pm, the test drink was ingested at 10:50 pm, the measurements were started, and the participants went to bed at 11:00 pm. The measurements were performed until 7:00 am the next morning. Participants ingested four test beverages, including the placebo beverage, with an intervening period of at least 7 days. A volunteer other than the analyst assigned a random number to all test drinks. Each of the four test drinks was placed in a box. For each participant, the tester selected four test drinks, one from each box, and the participants consumed the drinks in ascending order of the assigned numbers. Cigarettes and drinks containing alcohol or caffeine were prohibited the day before the test. Measurements were not performed during menstruation periods. The participants were not restricted from consuming polyphenols (e.g., green tea, black tea, and oolong tea) from the day before the test until the end of the test.Participant evaluations
EEGs were measured using a silver–silver chloride electrode attached to the midline of the head in the Pz electrode mounting position, based on the 10–20 electrode system. The NIRS signal intensity, which depends on the prefrontal cortex cerebral blood flow, was measured using an NIR sensor attached to the forehead. The fingertip pulse volume was measured using a pulse-wave sensor attached to the fingertip. Respiratory movement was measured using a strain gauge breathing sensor attached to the chest. The data were recorded on the hard disk of a laptop computer using a small polygraph system (Vital Monitor ProComp, Thought Technology Ltd, Toronto, Canada) at a sampling frequency of 256 Hz. The measurements were performed at the participant's home using the same indoor environment as when participants went to bed daily.The EEGs were analyzed using a signal analysis program (DADiSP/PRO 2020, CAE Solutions Corporation; Tokyo, Japan). The recorded EEGs were filtered using a high cut-off frequency of 30 Hz and an attenuation characteristic of −24 dB per oct for 2 min per section. The power spectrum was calculated using the fast Fourier transform algorithm. By inspecting the spectrum pattern, the sleep stages were classified into three based on sleep depth: awakening, light sleep, and deep sleep. Awakening was defined as a high peak observed at approximately 9 Hz in the alpha band. Light sleep (stages I and II) was defined as the presence of predominantly slow waves with low amplitude and no peak in the alpha band. Deep sleep (stages III and IV) was defined as the presence of predominantly high-amplitude slow waves. From the sleep depth results, the sleep onset latency, deep sleep latency, light sleep time, deep sleep time, sleep time, WASO time, and number of WASOs were obtained as parameters for evaluating sleep quality.
The NIRS signal strength was calculated using a signal analysis program (DADiSP/PRO 2002, CAE Solutions Corporation; Tokyo, Japan). The integrated NIRS signal strength was calculated using 2 min intervals. The pulse wave was obtained by differentiating the pulse-wave waveform to obtain a spike-like waveform similar to an EEG. The power spectrum was calculated using the fast Fourier transform algorithm, and the heart rate variability was obtained from the differential waveform of the pulse wave as one interval of 64 s, using data analysis software (DADiSP/PRO 2002, CAE Solutions Corporation; Tokyo, Japan). The mean waveform of the power spectrum of the two sections was calculated from 2 min of the heart rate variability power spectrum, and the integrated value from 0.04 to 0.15 Hz was used as the HF component. LF/HF was calculated as a sympathetic nerve activity index using the LF and HF components of the heart rate variability spectrum.
Statistical analysis
All statistical analyses were performed using R software ver 3.2.3 (R Foundation, Vienna, Austria). The EEG, LF/HF, and NIRS signal intensity were compared using two-way repeated measures ANOVA based on group, time, and group × time interactions. Sleep latency, sleep time, and WASO were compared using one-way repeated measures ANOVA and Tukey's post hoc test. All values are reported as the mean ± standard error of the mean (SEM). The level of statistical significance was set at p < 0.05.Results
Sleep quality
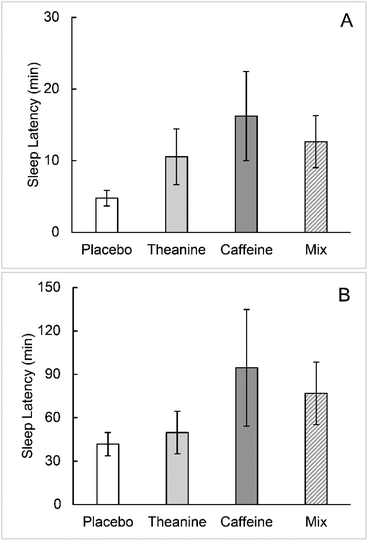
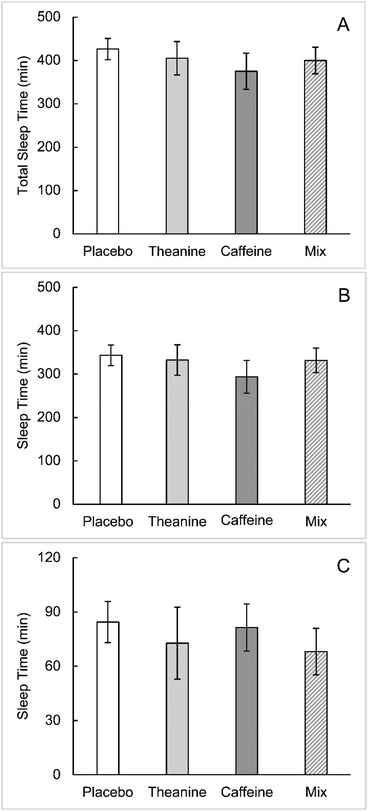
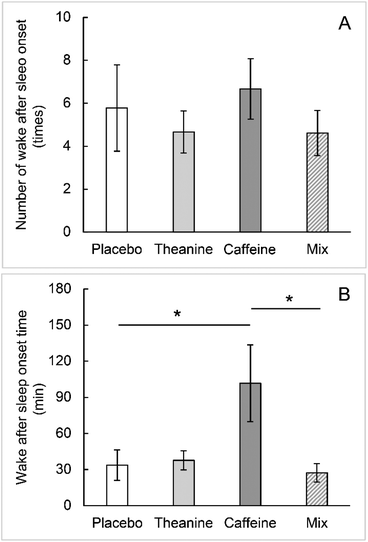
No statistically significant differences were found between the groups for sleep onset latency, sleep time, and number of WASOs, as calculated from the EEG results (Fig. 3–5A). The WASO time was statistically significantly higher in the caffeine group than in the placebo group (Fig. 5B). The WASO time of the TC group was significantly lower than that of the caffeine group (Fig. 5B). There was no statistically significant difference in sleep latency or sleep time between the groups.Cerebral blood flow in the prefrontal cortex
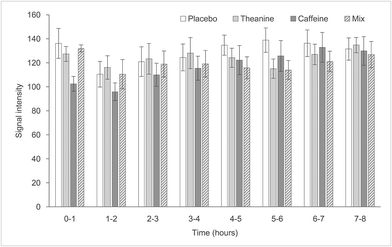
There was no statistically significant difference between the groups in cerebral blood flow in the prefrontal cortex, as measured by NIRS (Fig. 6).LF/HF
The LF/HF calculated from the heart rate variability did not show significant differences between the groups for beverage or time (Fig. 7).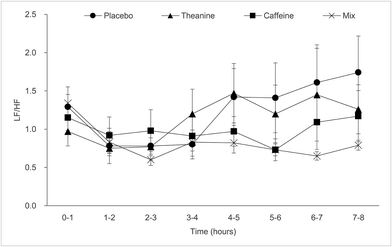 | ||
| Fig. 7 Effects of 30 mg of caffeine and 50 mg of theanine mixture on the ratio of LF to HF (LF/HF). Values are presented as mean ± SEM. | ||
Discussion
This study investigated the effect of combining caffeine with theanine on sleep and found that consuming a mixture of caffeine and theanine suppressed only the WASO time compared with consuming caffeine alone.The NIRS signal intensity in the caffeine group was less than that in the placebo group, but it did not reach statistical significance; however, the WASO time was statistically significantly longer than that in the placebo and TC groups. This result is thought to be caused by the dual effects of caffeine; that is, the sympathetic nervous system-mediated cerebral arteriole contraction and excitatory action in the central nervous system.3 Caffeine ingestion was observed to constrict cerebral blood vessels and reduce cerebral blood flow in the prefrontal cortex. However, since caffeine's central nervous system stimulation effect also occurred, the sleep onset latency in the caffeine group showed a high value. This difference did not reach statistical significance.
The caffeine dose in this study was set to 30 mg based on a preliminary study in which caffeine was administered to healthy male and female college students at 0, 15, 30, and 50 mg; in 4 out of 5 participants, the NIRS signal intensity in the prefrontal cortex decreased 10–20 min after administration, and the sleep stage changed from awakening to stage I. The minimum dose that shifted participants to stage I was 30 mg (data not shown). We enrolled participants who routinely ingest caffeine-containing beverages (2–3 cups of tea or coffee per day); however, some participants experienced wakefulness even after consuming 30 mg of caffeine. This finding was presumed to be caused by differences in individual responses to caffeine.3 In the future, it will be necessary to evaluate participants for the presence or absence of caffeine susceptibility.
The NIRS signal intensity did not decrease in the TC group, and the WASO was significantly reduced compared with that in the caffeine group. We speculate that this is because theanine suppressed both the cerebral arteriole contraction and central nervous system stimulation caused by caffeine. Theanine suppresses the excitatory effects of caffeine.30 Dodd et al.35 evaluated the effect of theanine on cerebral blood flow by measuring oxyhemoglobin and deoxyhemoglobin when cognitive assessment tasks were performed. When a task was performed after ingesting 75 mg of caffeine, oxyhemoglobin decreased and deoxyhemoglobin increased; however, when co-administered with 50 mg of theanine, the effect of caffeine was diminished. This effect depends on the caffeine intake; the amount of caffeine was higher than the amount of theanine in this study, but theanine attenuated the effect of caffeine. How theanine affects cerebral arterioles is a subject for further study, but the results of this study indicate that theanine may antagonize caffeine's effect on cerebral arterioles.
The absorption rates of theanine and caffeine are important for explaining the effects of consuming both simultaneously. The concentration of caffeine peaks in the blood at 30–120 min;36 it peaks at 60 min for theanine.37 When 50 mg of theanine is ingested, alpha waves appear 40 min later.38,39 The blood concentration peak of theanine occurs later than that of caffeine; therefore, after ingesting the test food, caffeine's central nervous system stimulative effect would be temporarily greater than that of theanine. This is considered the reason that the sleep onset latency was not shortened in the TC group compared with the caffeine group. A study that evaluated sleep quality using actigraphs on male college students with good sleeping habits reported that taking 200 mg of theanine for 6 days reduced the WASO.24 The present study's participants were female college students with an average daily sleep time of 6–9 h. The mid-wake time of the placebo group in this study (∼30 min) was longer than that of the previous study (∼20 min). In the future, it will be necessary to conduct tests on participants with similar sleep qualities, such as sleep onset latency and sleep time, to allow for better comparisons.
The LF/HF of the TC group was low but not significantly different among the groups. There were large individual differences, and the fluctuation range within the same section was also large. The low LF/HF values in the TC group are due to theanine suppressing the sympathetic nerve-activating effect of caffeine.
The sleep center in the brain regulates sleep. It is known that heat dissipation, decreased sympathetic nerve activity, and skeletal muscle relaxation occur during sleep. Theanine is known to improve sleep quality, but many aspects of its mechanism remain unclear. The consumption of theanine is reported to alter neurotransmitters such as dopamine, serotonin,20 tryptophan, and γ-aminobutyric acid (GABA),21 suppress the excitotoxicity of glutamate,17 and induce anxiolysis through the induction of alpha waves.38 However, how these effects are related to improving sleep is a topic for future research.
This study has some limitations. Only a limited effect was observed, and all the participants were Japanese females in their twenties; therefore, we cannot deny the possibility that an order-of-time effect occurred in this crossover study. Caffeine dosage, caffeine sensitivity, and sleep habits should be considered when selecting participants. In addition, it is necessary to set optimal concentrations of theanine and caffeine that do not suppress cerebrovascular contractions. Furthermore, the action of theanine on GABAergic neurons and the blocking action of adenosine A2A receptors are cited as the mechanisms of action of the combined effect of theanine and caffeine. However, whether these actions occur simultaneously is unclear, and future studies are needed to verify these actions.
Conclusions
Foods containing caffeine affect sleep quality and should be avoided before bedtime. This study evaluated sleep quality in female participants with a mean age of 21.3 ± 0.5 years who consumed theanine and caffeine together. Theanine and caffeine did not promote sleep onset. However, simultaneous intake of theanine (50 mg) and caffeine (30 mg) before sleep suppressed only the WASO time extension of caffeine. This finding suggests that theanine suppresses the contraction of cerebral arterioles. Although the leaves of Camellia sinensis contain theanine, and theanine can attenuate the effect of caffeine, more attention should be given to their concentrations. This study used test conditions in which the amount of theanine was greater than that of caffeine. Since theanine can potentially be used to treat sleep disorders, further investigations into the positive effects of green tea, which contains both caffeine and theanine, should assess different ratios of the two substances.Ethics statement
The study protocol was approved by the Ethics Committees of Ehime Prefectural University of Health Sciences (Ehime, Japan; approval number: 16-007). The study was conducted in accordance with the tenets of the Helsinki Declaration (as adopted in 1964 and amended in October 2013), the Ethical Guidelines for Medical and Health Research Involving Human Participants (Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare notice no. 3 of 2014), and the Act on the Protection of Personal Information (May 30, 2003; law no. 57). The study was conducted at the Ehime Prefectural University of Health Sciences (Ehime, Japan) from September 2016 to March 2019.Data availability
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to legal restrictions.Author contributions
Conceptualization, Y. B., T. T., and N. O.; data curation, N. O.; investigation, N. O.; methodology, N. O., project administration, Y. B., T. T., and N. O.; resources, Y. B., T. T., and N. O.; visualization, Y. B., and N. O.; writing – original draft, Y. B.; writing – review and editing, Y. B. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
This study was conducted in collaboration with ITO EN, Ltd and the Ehime Prefectural University of Health Sciences.Acknowledgements
We want to express our deep gratitude to the participants who cooperated in this study. We also thank Editage (https://www.editage.com) for English language editing.References
- J. Hu, D. Webster, J. Cao and A. Shao, The safety of green tea and green tea extract consumption in adults – Results of a systematic review, Regul. Toxicol. Pharmacol., 2017, 95, 412–433, DOI:10.1016/j.yrtph.2018.03.019.
- Y. Yoneda, N. Kuramoto and K. Kawada, The role of glutamine in neurogenesis promoted by the green tea amino acid theanine in neural progenitor cells for brain health, Neurochem. Int., 2019, 129, 104505, DOI:10.1016/j.neuint.2019.104505.
- B. B. Fredholm, K. Bättig, J. Holmén, A. Nehlig and E. E. Zvartau, Actions of caffeine in the brain with special reference to factors that contribute to its widespread use, Pharmacol. Rev., 1999, 51, 83–133 CAS . Available: https://www.ncbi.nlm.nih.gov/pubmed/10049999.
- National Health and Nutrition Survey, “Ministry of Health Labour and Welfare, 2019”. Available online: https://www.nibiohn.go.jp/eiken/kenkounippon21/en/eiyouchousa/ (accessed on 2 June 2023).
- Organisation for Economic Co-operation and Development, “Society at a Glance 2009”. Available online: https://www.oecd-ilibrary.org/social-issues-migration-health/society-at-a-glance-2009_soc_glance-2008-en (accessed on 2 June 2023).
- L. Li, T. Nakamura, J. Hayano and Y. Yamamoto, Age and gender differences in objective sleep properties using large-scale body acceleration data in a Japanese population, Sci. Rep., 2021, 11, 9970, DOI:10.1038/s41598-021-89341-x.
- J. C. Lo, J. A. Groeger, N. Santhi, E. L. Arbon, A. S. Lazar, S. Hasan, M. von Schantz, S. N. Archer and D.-J. Dijk, Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase, PLoS One, 2012, 7, e45987, DOI:10.1371/journal.pone.0045987.
- T. Abe, Y. Komada, Y. Nishida, K. Hayashida and Y. Inoue, Short sleep duration and long spells of driving are associated with the occurrence of Japanese drivers’ rear-end collisions and single-car accidents, J. Sleep Res., 2010, 19, 310–316, DOI:10.1111/j.1365-2869.2009.00806.x.
- S. Ferré, An update on the mechanisms of the psychostimulant effects of caffeine, J. Neurochem., 2008, 105, 1067–1079, DOI:10.1111/j.1471-4159.2007.05196.x.
- Y. Baba, T. Takihara, Y. Sagesaka and T. Kaneko, Effects of a daily intake of matcha on cognitive function in middle-aged and older subjects – A placebo-controlled, randomized, double-blind, parallel-group study, Jpn. Pharmacol. Ther., 2019, 47, 1689–1702 CAS.
- Y. Baba, S. Inagaki, S. Nakagawa, M. Kobayashi, T. Kaneko and T. Takihara, Effects of Daily Matcha and Caffeine Intake on Mild Acute Psychological Stress-Related Cognitive Function in Middle-Aged and Older Adults: A Randomized Placebo-Controlled Study, Nutrients, 2021, 13, 1700, DOI:10.3390/nu13051700.
- H. Horie, K. Ema and O. Sumikawa, Chemical Components of Matcha and Powdered Green Tea, J. Cook. Sci. Jpn., 2017, 50, 182–188 Search PubMed.
- Y. Baba, S. Inagaki, S. Nakagawa, T. Kaneko, M. Kobayashi and T. Takihara, Effects of l-Theanine on Cognitive Function in Middle-Aged and Older Subjects: A Randomized Placebo-Controlled Study, J. Med. Food, 2021, 24, 333–341, DOI:10.1089/jmf.2020.4803.
- H. Landolt, Caffeine reduces low-frequency delta activity in the human sleep EEG, Neuropsychopharmacology, 1995, 12, 229–238, DOI:10.1016/0893-133X(94)00079-F.
- Y. Sakato, Studies on the Chemical Constituents of Tea. PartIII. On a New Amide Theanine, J. Agric. Chem. Soc. Jpn., 1950, 23, 262–267, DOI:10.1271/nogeikagaku1924.23.262.
- T. Goto, Y. Yoshida, I. Amano and H. Horie, Chemical composition of commercially available Japanese green tea, Foods Food Ingredients J. Jpn., 1996, 170, 46–51 CAS.
- T. Kakuda, E. Hinoi, A. Abe, A. Nozawa, M. Ogura and Y. Yoneda, Theanine, an ingredient of green tea, inhibits [3H]glutamine transport in neurons and astroglia in rat brain, J. Neurosci. Res., 2008, 86, 1846–1856, DOI:10.1002/jnr.21637.
- T. Kakuda, A. Nozawa, A. Sugimoto and H. Niino, Inhibition by theanine of binding of [3H]AMPA, [3H]XXXainite, and [3H]MDL 105,519 to glutamate receptors, Biosci. Biotechnol. Biochem., 2002, 66, 2683–2686, DOI:10.1271/bbb.66.2683.
- M. Ozeki, L. R. Juneja and S. Shirakawa, A study of L-theanine and daytime drowsiness, Jpn. Soc. Phys. Anthropol., 2008, 13, 9–15 Search PubMed.
- H. Yokogoshi and T. Terashima, Effect of theanine, r-glutamylethylamide, on brain monoamines, striatal dopamine release and some kinds of behavior in rats, Nutrition, 2000, 16, 776–777, DOI:10.1016/S0899-9007(00)00384-1.
- H. Shinozaki and M. Ishida, Theanine as a glutamate antagonist at a crayfish neuromuscular junction, Brain Res., 1978, 151, 215–219, DOI:10.1016/0006-8993(78)90967-8.
- R. Kimura and T. Murata, Influence of alkylamides of glutamic acid and related compounds on the central nervous system. I. Central depressant effect of theanine, Chem. Pharm. Bull., 1971, 19, 1257–1261, DOI:10.1248/cpb.19.1257.
- Y. Baba, T. Kaneko and T. Takihara, Matcha consumption maintains attentional function following a mild acute psychological stress without affecting a feeling of fatigue: A randomized placebo-controlled study in young adults, Nutr. Res., 2021, 88, 44–52, DOI:10.1016/j.nutres.2020.12.024.
- M. Ozeki, L. R. Juneja and S. Shirakawa, The effects of L-theanine on sleep using the actigraph, Jpn. Soc. Phys. Anthropol., 2004, 9, 143–150 Search PubMed.
- N. E. Fultz, G. Bonmassar, K. Setsompop, R. A. Stickgold, B. R. Rosen, J. R. Polimeni and L. D. Lewis, Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep, Science, 2019, 366, 628–631, DOI:10.1126/science.aax5440.
- Y. Hoshi, S. Mizukami and M. Tamura, Dynamic features of hemodynamic and metabolic changes in the human brain during all-night sleep as revealed by near-infrared spectroscopy, Brain Res., 1994, 652, 257–262, DOI:10.1016/0006-8993(94)90235-6.
- H. Onoe, Y. Watanabe, M. Tamura and O. Hayaishi, REM sleep-associated hemoglobin oxygenation in the monkey forebrain studied using near-infrared spectrophotometry, Neurosci. Lett., 1991, 129, 209–213, DOI:10.1016/0304-3940(91)90463-4.
- A. M. Coney and J. M. Marshall, Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia, J. Physiol., 1998, 509, 507–518, DOI:10.1111/j.1469-7793.1998.507bn.x.
- A. C. Ngai, E. F. Coyne, J. R. Meno, G. A. West and H. R. Winn, Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles, Am. J. Physiol.: Heart Circ. Physiol., 2001, 280(5), H2329–H2335, DOI:10.1152/ajpheart.2001.280.5.H2329.
- T. Kakuda, A. Nozawa, T. Unno, N. Okamura and O. Okai, Inhibiting Effects of Theanine on Caffeine Stimulation Evaluated by EEG in the Rat, Biosci. Biotechnol. Biochem., 2000, 64, 287–293, DOI:10.1271/bbb.64.287.
- Z. L. Huang, W.-M. Qu, N. Eguchi, J.-F. Chen, M. A. Schwarzschild, B. B. Fredholm, Y. Urade and O. Hayaishi, Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine, Nat. Neurosci., 2005, 8, 858–859, DOI:10.1038/nn1491.
- C. F. Haskell, D. O. Kennedy, A. L. Milne, K. A. Wesnes and A. B. Scholey, The effects of L-theanine, caffeine and their combination on cognition and mood, Biol. Psychol., 2008, 77, 113–122, DOI:10.1016/j.biopsycho.2007.09.008.
- K. Unno, S. Noda, Y. Kawasaki, H. Yamada, A. Morita, K. Iguchi and Y. Nakamura, Ingestion of green tea with lowered caffeine improves sleep quality of the elderly via suppression of stress, J. Clin. Biochem. Nutr., 2017, 61, 210–216, DOI:10.3164/jcbn.17-6.
- K. Unno, N. Tanida, N. Ishii, H. Yamamoto, K. Iguchi, M. Hoshino, A. Takeda, H. Ozawa, T. Ohkubo, L. R. Juneja and H. Yamada, Anti-stress effect of theanine on students during pharmacy practice: positive correlation among salivary α-amylase activity, trait anxiety and subjective stress, Pharmacol., Biochem. Behav., 2013, 111, 128–135, DOI:10.1016/j.pbb.2013.09.004.
- F. L. Dodd, D. O. Kennedy, L. M. Riby and C. F. Haskell-Ramsay, A double-blind, placebo-controlled study evaluating the effects of caffeine and L-theanine both alone and in combination on cerebral blood flow, cognition and mood, Psychopharmacology, 2015, 232, 2563–2576, DOI:10.1007/s00213-015-3895-0.
- J. Blanchard and S. J. Sawers, The absolute bioavailability of caffeine in man, Eur. J. Clin. Pharmacol., 1983, 24, 93–98, DOI:10.1007/BF00613933.
- L. Scheid, S. Ellinger, B. Alteheld, H. Herholz, J. Ellinger, T. Henn, H.-P. Helfrich and P. Stehle, Kinetics of L-theanine uptake and metabolism in healthy participants are comparable after ingestion of L-theanine via capsules and green tea, J. Nutr., 2012, 142, 2091–2096, DOI:10.3945/jn.112.166371.
- K. Kobayashi, Y. Nagato, N. Aoi, L. R. Juneja, M. Kim, T. Yamamoto and S. Sugimoto, Effects of L-theanine on the release of α-brain waves in human volunteers, J. Agric. Chem. Soc. Jpn., 1998, 72, 153–157, DOI:10.1271/nogeikagaku1924.72.153.
- A. C. Nobre, A. Rao and G. N. Owen, L-theanine, a natural constituent in tea, and its effect on mental state, Asia Pac. J. Clin. Nutr, 2008, 17, 167–168, DOI:10.6133/APJCN.2008.17.S1.4.
| This journal is © The Royal Society of Chemistry 2023 |