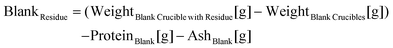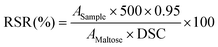Open Access Article
Open Access ArticleImpact of different fibre ingredients on a low-FODMAP biscuit model system†
Aylin W.
Sahin‡
 a,
Jonas J.
Atzler‡
a,
Emily
Crofton
b,
Eimear
Gallagher
b,
Emanuele
Zannini
a,
Jonas J.
Atzler‡
a,
Emily
Crofton
b,
Eimear
Gallagher
b,
Emanuele
Zannini
 ac,
Jens
Walter
de and
Elke K.
Arendt
ac,
Jens
Walter
de and
Elke K.
Arendt
 *ad
*ad
aUniversity College Cork, School of Food and Nutritional Sciences, College Road, Cork, Ireland. E-mail: aylin.sahin@ucc.ie; j.atzler@umail.ucc.ie; e.arendt@ucc.ie; Tel: +353 21 490 2064
bTeagasc Food Research Centre Ashtown, Dublin, D15 KN3K, Ireland. E-mail: emily.crofton@teagasc.ie; eimear.gallagher@teagasc.ie
cDepartment of Environmental Biology, “Sapienza” University of Rome, Italy. E-mail: e.zannini@ucc.ie
dAPC Microbiome Ireland, Cork, Ireland
eSchool of Microbiology and Department of Medicine, University College Cork, Ireland
First published on 10th July 2023
Abstract
Fermentable oligo-, di-, monosaccharides and polyols (FODMAPs) are carbohydrates which can cause symptoms of irritable bowel syndrome (IBS). Cereal-based products are high in FODMAPs, as they are part of the carbohydrate fraction in flour. Low-FODMAP products are starch-based which leads to a low dietary fibre content. Hence, the fortification with dietary fibre ingredients low in FODMAPs is essential. This study reveals the impact of three different fibre ingredients, resistant starch, cellulose, and arabinoxylan, and their interactions with each other in a low-FODMAP biscuit model system using response surface methodology. All fibre ingredients have an affinity to water which was further increased by their coexistence in the model system. Fibersym RW affected the biscuit hardness by its morphology and potential to recrystallise leading to a maximum inclusion level of 40%. VITACEL L 600-30 also increased biscuit hardness due to its plasticising character leading to a maximum inclusion of 20%. AgriFiber BFG mainly impacted the colour of the product restricting its inclusion to 2.3%. Additionally, it reduced the degree of starch digestibility of the biscuit by the formation of a film imbedding the starch granules and reducing enzyme attack. This research provides an in-depth insight into the integration potential of these fibre ingredients into a low-FODMAP biscuit, their interactions within the system and inclusion levels which allow their coexistence.
1. Introduction
FODMAPs are carbohydrates which can cause digestive discomfort and further symptoms of irritable bowel syndrome.1,2 Wheat flour is one of many sources of these FODMAPs and hence are consumed when eating cereal-based products.3 Furthermore, the increasing consumer trend towards gut-health-oriented diets has led to research investigating the dietary fibres (DF) fortification in biscuit production.4–6Low-FODMAP bakery products are mainly represented by gluten-free goods which substitute flour by starch, leading to low levels of dietary fibre.2 Therefore, there is a high demand for low FODMAP products, which have both an acceptable quality and contain high amounts of DFs beneficial for irritable bowel syndrome (IBS) patients. DF ingredients used for low-FODMAP products should contain at least one of the following characteristics: low fermentability, insolubility and high viscosity.1 Resistant starch (RS) is a non-digestible carbohydrate that has been intensively researched regarding its potential for treating gastrointestinal disorders and its overall health benefits.7 These advances include slow fermentability for most RS-types, the improvement of insulin response, an increase in microbiome diversity, and a rise in the production of butyric and propionic acid, which are commonly reported to promote gut health.8,9 Cellulose is an insoluble DF which increases stool frequency and is not fermentable. These characteristics make this DF beneficial for IBS patients as they can support relieving constipation and bloating.1 Arabinoxylans are a diverse group of dietary fibres consisting of xylose and arabinose monomers. Arabinoxylans with a degree of polymerisation (DP) of 10 or above is reported to have an increased viscosity and lower fermentability rate with a high potential in lowering sugar release during digestion.10–13 Therefore, this group of arabinoxylan fulfils the criteria of DFs beneficial for IBS patients.
Research on the interactions of fibre ingredients in biscuits and the determination of addition levels which allow coexistence are scarce. In this study, response surface methodology (RSM) was used for the experimental design to investigate the effect of single fibre and fibre combinations on the quality of a low-FODMAP biscuit model system. This includes dough properties and biscuit characteristics, such as texture and colour, as well as effect on in vitro digestibility and sensory evaluation.
2. Materials and methods
2.1. Raw materials
All biscuit recipes included sucrose (Siucra, Dublin Ireland, UK), shortening (Stork, Sussex, UK), diacetyl tartaric acid ester of mono- and diglycerides (DATEM; Danisco, Copenhagen, Denmark), salt (Glacia British Salt Limited, Cheshire, UK), baking powder (ValeoFood, Dublin, Ireland) and tap water. As the base, biscuit flour (East End Foods PLC, West Bromwich, UK) and wholemeal flour (Odlum, Dublin, Ireland) were used to produce two control biscuits, BiFC and WMC, respectively. Biscuits low in FODMAPs included a combination of wheat starch (Roquette, Lestrem, France) and vital gluten (Roquette, Lestrem, France) instead of flour, which represents control 3 (LFC). Fibre-fibre interactions between Fibersym RW (MGP, Atchinson, Kansas, USA), powdered cellulose VITACEL L 600-30 (J.Rettenmaier GmBH, Germany), and AgriFiber BFG (Agrifiber, Mundelein, Illinois, USA) in a low-FODMAP biscuit model system were investigated. All chemicals were purchased from Sigma-Aldrich if not specified differently.2.2. Compositional analysis
Compositional analysis of the ingredients and the biscuits were performed, which includes protein content, fat content, ash, moisture, digestible starch and FODMAPs, total dietary fibre and arabinoxylan content.2.2.2.1. Digestible starch and FODMAPs. The contents of digestible starch were determined using the enzymatic assay K-RAPRS provided by Megazyme. FODMAPs were extracted and quantified as described by Ispiryan et al. (2019)17via high performance anionic exchange chromatography with coupled pulsed amperometric detection (HPAEC-PAD). For the calculation of FODMAPs per serving, a serving size of 55 g of biscuits was used.
2.2.2.2. Dietary fibre (DF). Total dietary fibre was determined using the K-RINTDF enzyme assay kit (Megazyme, Bray, Ireland), based on AOAC Method 2022.01.18 One gram of freeze-dried sample was suspended in 2 mL of 95% ethanol, followed by the addition of 35 mL of sodium maleate buffer (0.2 M, pH 6.0, 0.05 M CaCl2). After equilibration for 10 min at incubation temperature (37 °C), 5 mL of enzyme mixture (pancreatic α-amylase (0.8 kU mL−1) and amyloglucosidase (0.34 kU mL−1)) were added, the sample was incubated in a shaking water bath (250 strokes per min) at 37 °C for 4 h, and the reaction was stopped by adding 3 mL of tris buffer (0.1 M, pH 11.2) and incubating the sample in a water bath at 95 °C for 20 min. The temperature was adjusted to 60 °C, 0.1 mL of protease (350 tyrosine units per mL; in 3.2 M ammonium sulphate buffer) were added, and the sample was incubated in a shaking water bath (250 strokes per min) at 60 °C for 30 min.
Subsequently, the reaction was terminated by adding 4 mL of 2 M acetic acid and 1 mL of glycerol (10 mg mL−1), which is used as an internal standard for the quantification of SDFS via HPLC. The sample suspensions and the blanks were then filtered using filter crucibles (50 mL; coarse) under vacuum. Crucibles were washed twice with 15 mL 95% EtOH, 15 mL 70% EtOH, and 15 mL acetone. The filtrate was then transferred into a 100 mL volumetric flask and filled up to the mark. Afterwards, the contents were transferred into a 500 ml bottle containing 290 mL of 95% EtOH and precipitation was carried out overnight at room temperature. Precipitates were filtered as previously described. Crucibles were dried in an air oven at 130 °C until a constant weight was reached, and the contents were used to determine ash and protein as described in section 2.2.1. The values obtained from the first filtration are used to calculate insoluble dietary fibre (IDF), and the ones obtained after the second filtration are used to calculate soluble high molecular weight dietary fibre (SDFP). The following equations were used for calculation:
After the second filtration, filtrates were reduced in volume by evaporating EtOH using a vacuum centrifuge (ScanVac, Lynge, Denmark), and the following conditions were applied: (1.) 1500 rpm, 45 °C, 1 h; (2.) 2000 rpm, 50 °C, 2 h; (3.) 2000 rpm, 50 °C, 2 h.
Samples were then transferred into a 20 mL volumetric flask and filled to volume with sodium azide (50 ppm). Afterwards, samples were filtered through a 0.45 mm PPE syringe filter. The determination of soluble low molecular weight fibre (SDFS) was achieved via HPLC using an Agilent Infinity 1260 attached to a refractive index detector (RFID). Separation of SDFS from mono- and disaccharides was performed on the following column set-up: Biorad H+ Cartridge, Biorad CO3-cartridge (Biorad Laboratories Inc., Berkeley, California, USA), TSK guard column PWXL and two analytical columns TSKgel G2500 PWXL (Tosoh Bioscience, Tokyo, Japan). An isocratic method with 100% of ultrapurified water as eluent, a run time of 60 min and a temperature of 80 °C was used. Detection via RFID was carried out at 50 °C.
SDFS contents were determined by measurement of the total area of peaks eluting between 20 min and 33 min. A mixture containing glucose (10 mg mL−1; 1 mL measured) and glycerol (10 mg mL−1; 1 mL measured) was measured to determine the response factor needed for calculating the SDFS content. The following two equations were used:
Total dietary fibre (TDF) is defined as the sum of IDF, SDFP and SDFS.
2.2.2.3. Arabinoxylan. Arabinoxylan are a specific type of dietary fibre. Analysis of the total arabinoxylan content (TAC), the content of water-extractable arabinoxylans (WEAX) and water-unextractable arabinoxylans (WUAX) is based on the complete hydrolysis of these components by trifluoric acetic acid. The extraction and quantification were performed as reported by Lynch et al. (2021).19 The amount of WUAX was calculated by subtracting the amount of WEAX from the TAC.
2.3. Biscuit dough preparation
The biscuit flour control recipe (BiFC) is illustrated in Table 1. Biscuit dough was prepared using a Kenwood chef mixer (Kenwood Ltd, New Hampshire, UK). Dry ingredients were premixed using a whisk attachment at minimum speed for 1 min. Following, shortening and water (adjusted as outlined in section 2.5) were added, and the dough was mixed for 3 min at speed 1 with a K-beater attachment. After mixing, the dough was covered in cling film and rested at room temperature for 15 minutes. The wholemeal flour control (WMC) was prepared by replacing the biscuit flour with wholemeal flour. The low-FODMAP control (LFC) was prepared by replacing all biscuit flour (w/w) with a mixture of 90% wheat starch and 10% vital gluten. Fibre-enriched, low-FODMAP biscuits were prepared by replacing wheat starch, as the carbohydrate source, with the fibre ingredient according to the experimental design by Design Expert outlined in the next section and illustrated in Table 2.| BiFC (% based on flour) | |
|---|---|
| Flour | 100.0 |
| Water | 20.0 |
| Shortening | 44.4 |
| Sugar | 40.4 |
| Baking powder | 0.5 |
| DATEM | 0.5 |
| Salt | 0.7 |
| Run | Fibersym RW [%] | VITACEL L 600-30 [%] | AgriFiber BFG [%] | Linear regression | RSQ | Adjusted water contenta [%] |
|---|---|---|---|---|---|---|
| a Based on flour/starch + vital gluten. | ||||||
| 1 | 25 | 10 | 5 | y = 5.92x − 46.88 | 0.9906 | 18.1 ± 0.2f |
| 2 | 25 | 10 | 5 | y = 5.83x − 46.89 | 0.9910 | 18.2 ± 0.2f |
| 3 | 10 | 20 | 0 | y = 7.72x − 58.38 | 0.9949 | 15.3 ± 0.2b |
| 4 | 10 | 0 | 0 | y = 7.35x − 60.87 | 0.9879 | 16.4 ± 0.4cde |
| 5 | 25 | 0 | 5 | y = 3.75x − 16.99 | 0.9914 | 20.5 ± 0.3g |
| 6 | 10 | 20 | 10 | y = 6.37x − 45.82 | 0.9937 | 16.6 ± 0.4de |
| 7 | 40 | 0 | 0 | y = 6.70x − 52.58 | 0.9912 | 16.8 ± 0.3e |
| 8 | 25 | 10 | 5 | y = 5.92x − 46.87 | 0.9906 | 18.1 ± 0.4f |
| 9 | 25 | 10 | 10 | y = 10.72x − 83.32 | 0.9938 | 13.4 ± 0.4a |
| 10 | 25 | 10 | 5 | y = 5.99x − 45.86 | 0.9902 | 17.9 ± 0.3f |
| 11 | 40 | 20 | 10 | y = 6.09x − 49.02 | 0.9907 | 18.1 ± 0.4f |
| 12 | 40 | 10 | 5 | y = 7.75x − 64.59 | 0.9885 | 16.1 ± 0.2bcde |
| 13 | 10 | 10 | 5 | y = 8.11x − 67.48 | 0.9899 | 15.7 ± 0.3bc |
| 14 | 25 | 20 | 5 | y = 7.63x − 66.73 | 0.9899 | 16.6 ± 0.3de |
| 15 | 40 | 0 | 10 | y = 8.43x − 72.04 | 0.9848 | 15.7 ± 0.3bc |
| 16 | 40 | 20 | 0 | y = 8.04x − 62.97 | 0.9953 | 15.3 ± 0.3b |
| 17 | 10 | 0 | 10 | y = 8.59x − 75.21 | 0.9782 | 15.7 ± 0.3bcd |
| 18 | 25 | 10 | 0 | y = 5.71x − 43.96 | 0.9810 | 18.2 ± 0.3f |
| BiFC | 0 | 0 | 0 | — | — | 20.0g |
| WMC | 0 | 0 | 0 | y = 6.90x − 46.17 | 0.9923 | 19.4 ± 1.1fg |
| LFC | 0 | 0 | 0 | y = 9.13x − 51.23 | 0.9887 | 14.1 ± 0.8b |
| LFP | 40 | 10 | 2.3 | 18.1 ± 0.4f | ||
2.4. Experimental design using response surface methodology
Response surface methodology (RSM) was used to design the fibre inclusion levels and combinations for the investigation of fibre-fibre interactions in a low-FODMAP biscuit model system. The RSM was performed using the software Design Expert 9 (StatEase, Minneapolis, MN, USA). A three-factorial, face-centred, central composite design was chosen with single factorial points and 4 repetitions of the centre point. Concentrations of Fibersym RW (10–40%), VITACEL L 600-30 (0–20%) and AgriFiber BFG (0–10%) were used as variable parameters of the experiment. The maximum concentrations of each fibre were chosen based on preliminary experiments at which biscuit quality significantly decreased (data not shown). All concentrations were based on the starch replacement, and the minimum and maximum addition levels of the single fibre ingredients were chosen based on results of pre-liminary trials (data not shown). The replacement level of starch by the single fibres used for the 18 different factorial points are shown in Table 2. Models were produced applying backward elimination regression of insignificant model terms with α to exit of 0.1. For significant models with an insignificant lack of fit (LOF), 3D-response surface plots were produced. The water content of all recipes was adjusted using the Farinograph-TS with the Farino-AddS300 attachment as reported in section 2.5. The impact of the fibre ingredients, singly and in different combinations, on biscuit quality was evaluated by the determination of dough hardness, dough stickiness, biscuit hardness, spreadability/shrinkage, L*-value of colour, and the water activity as described in the following sections.2.5. Water adjustment
The water content of the recipes was adjusted using the Farinograph-TS with the FarinoAdd-S300 attachment (Brabender GmbH & Co. KG, Duisburg, Germany). The sample size for each measurement was 200 g flour/starch-vital gluten mix and fibre ingredients based on 14% total moisture. First, the torque of the biscuit flour control recipe (BiFC) (biscuit flour + water) was measured to determine the target torque of all other recipes. A target torque of 180 BU was detected. The water adjustment of all recipes was performed by linear regression based on torque responses to 8%, 12%, 16% and 20% water addition, with an R2 > 0.98. Measurements were monitored for 3 min after premixing for one minute, which mimicked the mixing conditions during biscuit production. The standard conditions of chamber temperature of 30 °C and a mixing speed of 63 rpm were chosen.2.6. Biscuit dough characteristics
Dough hardness and dough stickiness were measured using a texture analyser TA-XT2i (Stable Micro Systems, Godalming, UK) attached to a 30 kg load cell.Dough hardness was determined by applying a compression test. Per measurement, 110 g of dough were transferred into the test cell, and the dough was distributed evenly using a flattening plunger. A cylindrical probe (6 mm diameter), a test speed of 3 mm s−1 and a performance distance of 20 mm were used. Dough hardness was evaluated in Newton [N]. Each sample was measured five times.
Dough stickiness was analysed using a Chen-Hoseney dough stickiness rig combined with an acrylic cylinder probe (diameter 25 mm). The test speed was 5 mm s−1. Dough stickiness was expressed in Newton [N]. The stickiness of five replicates per batch were measured.
2.7. Biscuit baking procedure
After the dough resting time, the biscuit dough was sheeted to a thickness of 3 mm using a laminator (Rondo, Chessington, UK). The sheeted dough was then cut with a biscuit cutter (diameter 71 mm), and the cut biscuits were placed on a baking tray lined with a baking sheet and baked in a deck oven (MIWE condo, Armstein, Germany) at 220 °C (upper and lower heat) for 12 min. Biscuits were cooled for one hour at room temperature and analysed regarding their physicochemical properties.2.8. Biscuit properties
2.9. Combination of fibre ingredients
The combination of all three fibre ingredients and the addition level at which they can co-exist in the low-FODMAP biscuit model system without impacting the biscuit and biscuit dough quality was determined using the computer simulation software Design Expert. This resulted in the replacement of 52.3% wheat starch by 40% Fibersym RW, 10% VITACEL L 600-30, and 2.3% AgriFiber BFG. The model showed a significant response in biscuit hardness; thus, a desired biscuit hardness of 40 N, which resembles the hardness of the biscuit flour control biscuit (BiFC), was set as a desirability factor in the software. Desirability ranges from 0 to 1, with 0 resembling the least desirable and 1 representing the most acceptable outcome, meaning the closest to 40 N.In addition to the dough characteristics and the biscuit properties, microstructure, in vitro starch digestibility, and a quantitative descriptive sensory analysis were performed on BiFC, WMF, LFC and the low-FODMAP prototype (LFP).
where Asample represents sample absorbance at 546 nm; AMaltose represents absorbance value of a solution containing 1 mg of maltose per millilitre phosphate buffer; DSC represents the amount (in milli-grams) of digestible carbohydrates in the sample; 500 is the total volume of solution in millilitre and 0.95 is the conversion factor from maltose to starch.
The 4 biscuit samples (BiFC, WMF, LFC, LFP) were presented one at a time and evaluated in triplicate (three sessions) according to a balanced experimental design. The intensities of the sensory attributes were rated on a 10 cm line scale anchored at the extremities with ‘weak’ and ‘strong’. The quality of appearance, flavour and texture of each biscuit sample was rated on a ten-point scale where 1 = poor and 10 = excellent. Sensory testing was conducted under white light in the sensory testing facility (ISO 8589:2007) at Teagasc Food Research Centre, Dublin, Ireland, using Compusense Cloud Software (Compusense Inc., Ontario, Canada). Unsalted crackers and filtered water were used as palate cleansers during testing. The sensory panel evaluated ‘evenness of colour’, ‘hardness of first bite’, ‘sound at break of first break’, hardness during chewing’, ‘graininess’, ‘dryness’, ‘buttery flavour’, ‘sweetness’ and ‘aftertaste’. The explanation of the descriptors is illustrated in the ESI content (Table S1†).
2.10. Statistical analysis
All measurements were performed in triplicate. Statistical analysis was performed using SPSS version 28.01 (SPSS Inc., Chicago, IL) and Microsoft Excel version 2207 (Microsoft Redmond, WA), with statistical significance set to α ≤ 0.05. A one-way Analysis of Variance (ANOVA) was used for the statistical evaluation and determination of significant differences among the techno-functional and nutritional properties. To investigate fibre–fibre interactions, the different models for the corresponding response factor were evaluated using both linear and 2-factorial-interaction in Design Expert. For each model both significance of the model itself and each factor/interaction were analysed. The model needs to be significant, and the lack-of-fit has to be insignificant in order to determine the inclusion levels of fibre ingredients able to coexist in the low-FODMAP biscuit system.A two-way ANOVA was applied for the sensory data, considering samples, assessors, and their interaction. Significant differences were determined using Tukey's post hoc test with α = 0.05 was used. Furthermore, a Pearson correlation analysis was carried out to correlate the techno-functional data regarding dough and biscuit texture with the sensory data.
3. Results
3.1. Compositional analysis of raw ingredients
Biscuit flour, wholemeal flour, wheat starch, vital gluten, and the fibre ingredients Fibersym RW, VITACEL L 600-30 and AgriFiber BFG were analysed regarding their nutritional constituents. The results are illustrated in Table 4 and the statistical evaluation of the models is shown in the ESI (Table S2).†| Biscuit flour | Wholemeal flour | Wheat starch | Vital gluten | Fibersym RW | VITACEL L 600-30 | AgriFiber BFG | |
|---|---|---|---|---|---|---|---|
| Mean ± standard deviation with different letters in row are significantly different (p ≤ 0.05); all contents based on ‘as is’ and as used for biscuit production, n.d. – non determinable as below limit of quantification; IDF – insoluble dietary fibre; SDFP – soluble dietary fibre precipitated in 95% ethanol; SDFS – soluble dietary fibre soluble in 95% ethanol; TAC – total arabinoxylan content; WEAX – water extractable arabinoxylans, WUAX – water unextractable arabinoxylans. | |||||||
| Moisture content [g per 100 g] | 12.12 ± 0.32bc | 9.23 ± 0.06ab | 14.98 ± 0.14d | 7.18 ± 0.08a | 11.17 ± 1.1bc | 14.53 ± 1.47cd | 14.59 ± 1.63cd |
| Ash [g per 100 g] | 0.256 ± 0.085ab | 0.454 ± 0.071b | 0.050 ± 0.001a | 1.331 ± 0.192c | 1.211 ± 0.080c | 0.102 ± 0.013a | 4.458 ± 0.012d |
| Protein [g per 100 g] | 9.29 ± 0.1c | 13.41 ± 0.24d | 0.22 ± 0.01a | 76.74 ± 1.47e | 0.07 ± 0.01a | 0.08 ± 0.02a | 5.48 ± 0.5b |
| Fat [g per 100 g] | 1.77 ± 0.29b | 2.05 ± 0.18bc | 0.20 ± 0.01a | 1.65 ± 0.24b | 0.64 ± 0.04a | 0.25 ± 0.01a | 2.37 ± 0.36c |
| Digestible starch [g per 100 g] | 83.23 ± 1.67e | 67.66 ± 1.79d | 75.51 ± 8.20de | 2.01 ± 0.21a | 41.83 ± 4.68c | n.d. | 6.32 ± 0.42b |
| Total FODMAPs [g per 100 g] | 2.38 ± 0.31c | 2.94 ± 0.18d | 0.58 ± 0.02b* | 0.21 ± 0.01a* | n.d. | n.d. | n.d. |
| Total dietary fibre [g per 100 g] | 6.16 ± 0.95b | 18.13 ± 0.67d | 0.68 ± 0.09a | 12.82 ± 1.75c | 40.74 ± 3.22e | 96.62 ± 1.22f | 42.07 ± 4.24e |
| IDF [g per 100 g] | 3.65 ± 0.42a | 9.66 ± 0.22b | 0.06 ± 0.01a | 8.59 ± 0.69b | 40.74 ± 3.22c | 96.62 ± 1.22d | 1.25 ± 0.12a |
| SDFP [g per 100 g] | 1.09 ± 0.12a | 5.64 ± 0.53b | 0.62 ± 0.11a | 3.51 ± 0.23ab | n.d. | n.d. | 40.46 ± 4.22c |
| SDFS [g per 100 g] | 1.42 ± 0.08c | 2.83 ± 0.33d | n.d. | 0.72 ± 0.11b | n.d. | n.d. | 0.36 ± 0.06ab |
| Arabinoxylan [g per 100 g] | |||||||
| TAC [g per 100 g] | 0.73 ± 0.02a | 2.32 ± 0.17b | n.d. | n.d. | n.d. | n.d. | 33.93 ± 1.09c |
| WEAX [g per 100 g] | 0.22 ± 0.01a | n.d. | n.d. | n.d. | n.d. | n.d. | 31.29 ± 1.01b |
| WUAX [g per 100 g] | 0.54 ± 0.04a | 2.28 ± 0.17b | n.d. | n.d. | n.d. | n.d. | 2.63 ± 0.08c |
| Run | Fibersym RW [%] | VITACEL L 600-30 [%] | AgriFiber BFG [%] | Dough hardness [N] (n = 5) | Dough stickiness [N] (n = 5) | Biscuit diameter [mm] (n = 10) | Biscuit hardness [N] (n = 10) | L*-value (n = 10) | a w [−] (n = 3) |
|---|---|---|---|---|---|---|---|---|---|
| BiFC – biscuit flour control; WMC – wholemeal flour control; LFC – low-FODMAP control; LFP – low-FODMAP prototype. | |||||||||
| 1 | 25 | 10 | 5 | 3.46 ± 0.24 | 0.26 ± 0.02 | 70.61 ± 0.15 | 56.79 ± 6.77 | 63.06 ± 2.85 | 0.171 ± 0.002 |
| 2 | 25 | 10 | 5 | 3.98 ± 0.36 | 0.24 ± 0.03 | 70.48 ± 0.27 | 48.44 ± 5.9 | 63.67 ± 3.15 | 0.184 ± 0.001 |
| 3 | 10 | 20 | 0 | 8.55 ± 0.97 | 0.20 ± 0.05 | 70.40 ± 0.30 | 42.33 ± 3.15 | 74.59 ± 7.47 | 0.211 ± 0.002 |
| 4 | 10 | 0 | 0 | 1.88 ± 0.12 | 0.27 ± 0.03 | 70.64 ± 0.29 | 23.87 ± 10.63 | 75.13 ± 3.71 | 0.220 ± 0.003 |
| 5 | 25 | 0 | 5 | 1.73 ± 0.09 | 0.33 ± 0.03 | 70.89 ± 0.16 | 36.76 ± 8.31 | 64.73 ± 2.13 | 0.204 ± 0.006 |
| 6 | 10 | 20 | 10 | 4.85 ± 0.40 | 0.14 ± 0.01 | 67.67 ± 0.42 | 64.63 ± 3.74 | 49.53 ± 3.74 | 0.211 ± 0.003 |
| 7 | 40 | 0 | 0 | 1.95 ± 0.14 | 0.21 ± 0.05 | 69.00 ± 0.49 | 18.92 ± 9.53 | 68.94 ± 2.83 | 0.197 ± 0.002 |
| 8 | 25 | 10 | 5 | 2.72 ± 0.35 | 0.20 ± 0.03 | 70.96 ± 0.19 | 62.69 ± 23.88 | 62.30 ± 3.29 | 0.190 ± 0.001 |
| 9 | 25 | 10 | 10 | 4.56 ± 0.34 | 0.20 ± 0.02 | 70.67 ± 0.27 | 70.69 ± 20.08 | 49.62 ± 1.83 | 0.217 ± 0.004 |
| 10 | 25 | 10 | 5 | 4.13 ± 0.36 | 0.25 ± 0.01 | 70.61 ± 0.18 | 51.17 ± 4.98 | 61.81 ± 2.66 | 0.234 ± 0.006 |
| 11 | 40 | 20 | 10 | 8.39 ± 0.64 | 0.16 ± 0.02 | 69.69 ± 0.49 | 78.88 ± 11.45 | 52.50 ± 3.54 | 0.203 ± 0.004 |
| 12 | 40 | 10 | 5 | 6.12 ± 0.22 | 0.17 ± 0.03 | 70.71 ± 0.23 | 54.43 ± 6.22 | 59.82 ± 3.15 | 0.251 ± 0.003 |
| 13 | 10 | 10 | 5 | 4.07 ± 0.31 | 0.16 ± 0.04 | 70.72 ± 0.17 | 45.56 ± 11.6 | 59.04 ± 2.81 | 0.170 ± 0.006 |
| 14 | 25 | 20 | 5 | 8.98 ± 0.54 | 0.15 ± 0.02 | 70.63 ± 0.27 | 51.58 ± 9.94 | 59.49 ± 4.02 | 0.180 ± 0.002 |
| 15 | 40 | 0 | 10 | 4.56 ± 0.24 | 0.17 ± 0.01 | 72.46 ± 0.32 | 46.37 ± 5.71 | 48.76 ± 2.34 | 0.092 ± 0.005 |
| 16 | 40 | 20 | 0 | 1.48 ± 0.04 | 0.22 ± 0.03 | 70.72 ± 0.19 | 37.40 ± 6.48 | 62.02 ± 4.23 | 0.114 ± 0.006 |
| 17 | 10 | 0 | 10 | 2.47 ± 0.08 | 0.21 ± 0.02 | 70.93 ± 0.37 | 65.13 ± 7.28 | 54.12 ± 8.09 | 0.109 ± 0.003 |
| 18 | 25 | 10 | 0 | 3.13 ± 0.18 | 0.24 ± 0.02 | 68.62 ± 0.67 | 35.93 ± 7.70 | 78.26 ± 2.62 | 0.189 ± 0.004 |
| BiFC | 0 | 0 | 0 | 2.71 ± 0.32 | 0.34 ± 0.02 | 69.51 ± 0.35 | 41.05 ± 2.74 | 78.45 ± 3.25 | 0.520 ± 0.010 |
| WMC | 0 | 0 | 0 | 3.32 ± 0.26 | 0.23 ± 0.03 | 66.97 ± 0.48 | 40.65 ± 2.14 | 62.97 ± 10.74 | 0.220 ± 0.010 |
| LFC | 0 | 0 | 0 | 2.45 ± 0.47 | 0.26 ± 0.04 | 69.9 ± 0.18 | 32.54 ± 3.48 | 74.89 ± 2.61 | 0.180 ± 0.010 |
| LFP | 40 | 10 | 2.3 | 3.20 ± 0.29 | 0.19 ± 0.02 | 69.83 ± 0.17 | 44.85 ± 3.76 | 68.36 ± 3.05 | 0.170 ± 0.010 |
Ash contents of all ingredients ranged from 0.05 g per 100 g in wheat starch to 4.46 g per 100 g in AgriFiber BFG. The ash levels in biscuit flour (0.256 ± 0.085 g per 100 g) did not differ significantly from wholemeal flour (0.454 ± 0.071 g per 100 g) and VITACEL L 600-30 (0.102 ± 0.013 g per 100 g).
The ingredients differed in protein content significantly from each other. Vital gluten showed the highest protein content (76.74 ± 8.20 g per 100 g), followed by wholemeal flour (13.41 ± 0.24 g per 100 g) and biscuit flour (9.29 ± 0.10 g per 100 g). The lowest protein content was determined in Fibersym RW (0.07 ± 0.01 g per 100 g), VITACEL L 600-30 (0.08 ± 0.02 g per 100 g) and wheat starch (0.22 ± 0.01 g per 100 g).
The highest fat content was determined in AgriFiber BFG (2.37 ± 0.36 g per 100 g). The fat level in biscuit flour (1.77 ± 0.29 g per 100 g), wholemeal flour (2.05 ± 0.18 g per 100 g) and vital gluten (1.65 ± 0.24 g per 100 g) did not differ significantly from each other. Wheat starch, Fibersym RW and VITACEL L 600-30 contained less than 0.70 g per 100 g fat.
The carbohydrate analysis included the determination of digestible starch, FODMAPs, dietary fibre, and specifically arabinoxylan. The highest digestible starch was determined in biscuit flour (83.23 ± 1.67 g per 100 g), followed by wheat starch (75.51 ± 8.20 g per 100 g) and wholemeal flour (67.66 ± 1.79 g prr 100 g). Amongst the fibre ingredients, Fibersym RW showed the highest amount of digestible starch (41.83 ± 4.68 g per 100 g), followed by AgriFiber BFG (6.32 ± 0.42 g per 100 g). FODMAP determination showed the highest FODMAP content in wholemeal flour (2.49 ± 0.18 g per 100 g) and biscuit flour (2.38 ± 0.31 g per 100 g), followed by wheat starch (0.58 ± 0.02 g per 100 g) and vital gluten (0.21 ± 0.01 g per 100 g). All three fibre ingredients did not contain any FODMAPs. Regarding dietary fibre, wheat starch contained the lowest amount consisting of 0.06 ± 0.01 g per 100 g IDS and 0.62 ± 0.11 g per 100 g SDFP. Biscuit flour and wholemeal flour contained 6.16 ± 0.95 g per 100 g and 18.13 ± 0.67 g per 100 g dietary fibre, respectively. The proportion between IDF, SDFP and SDFS in biscuit four was 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18
18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 and in wholemeal flour 53
23 and in wholemeal flour 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16. Interestingly, vital gluten contained 12.82 ± 1.75 g per 100 g dietary fibre, mainly IDF. VITACEL L 600-30 showed the highest dietary fibre value (96.62 g per 100 g), which was identified as insoluble dietary fibre (IDF). Arabinoxylan (AX) was present in biscuit flour, wholemeal flour and AgriFiber BFG. AgriFiber BFG showed the highest AX-content 33.93 ± 1.09 g per 100 g, of which 92% were WEAX, followed by wholemeal flour containing 2.32 ± 0.17 g per 100 g AX, which was determined to be WUAX. Biscuit flour showed significantly lower amounts of total AX (0.73 ± 0.02 g per 100 g), of which around 30% are WEAX and 70% are WUAX.
16. Interestingly, vital gluten contained 12.82 ± 1.75 g per 100 g dietary fibre, mainly IDF. VITACEL L 600-30 showed the highest dietary fibre value (96.62 g per 100 g), which was identified as insoluble dietary fibre (IDF). Arabinoxylan (AX) was present in biscuit flour, wholemeal flour and AgriFiber BFG. AgriFiber BFG showed the highest AX-content 33.93 ± 1.09 g per 100 g, of which 92% were WEAX, followed by wholemeal flour containing 2.32 ± 0.17 g per 100 g AX, which was determined to be WUAX. Biscuit flour showed significantly lower amounts of total AX (0.73 ± 0.02 g per 100 g), of which around 30% are WEAX and 70% are WUAX.
3.2. Water adjustment
The water levels of all recipes were adjusted using the Farinograph-TS using linear regression aiming for a dough torque of 180 BU, the torque of the BiFC. The linear regression of 100% flour replacement by wholemeal flour was y = 6.90x − 46.17 (R2 = 0.99) leading to an adjusted water content of 19.4 ± 1.1% based on flour to reach the target torque. The low-FODMAP control recipe (LFC) resulted in the linear regression model y = 9.13x − 51.23 (R2 = 0.99), hence, 14.1 ± 0.8% water based on wheat starch and vital gluten (=100%) was required to reach 180 BU.The water adjustment results of the trials including fibre ingredients are illustrated in Table 2. The highest amount of water was required in the biscuit recipe including 25% Fibersym RW and 5% AgriFiber BFG (20.5% water). Interestingly, the recipe with the highest fibre inclusion (40% Fibersym RW, 20% VITACEL L 600-30, 10% AgriFiber BFG) only required a water content of 18.1%.
All recipes which contained 25% Fibersym RW, 10% VITACEL L 600-30 and not more than 5% AgriFiber BFG did not differ in adjusted water content compared to the recipe with the highest fibre inclusion. The lowest water addition was determined when wheat starch was replaced by 25% Fibersym RW, 10% VITACEL L 600-30, and 10% AgriFiber BFG (13.4% water).
3.3. Impact of fibre ingredients and fibre-fibre interaction on a low-FODMAP biscuit
The impact of fibre ingredients on biscuit dough and biscuit quality was investigated. A minimum concentration of 10% Fibersym RW was chosen as the low-FODMAP biscuit system is starch based.Dough hardness of all trials ranged between 1.48 N and 8.98 N. The LFC had a dough hardness of 2.45 N. The inclusion of the maximum amount of Fibersym RW (40%) and the maximum amount of VITACEL L 600-30 (20%) led to the softest dough. Moreover, the lowest and highest inclusion level of Fibersym RW as a single fibre ingredient in the matrix resulted in dough softening, with a dough hardness of 1.88 N and 1.95 N, respectively. The hardest dough occurred in the combination of 25% Fibersym RW, 20% VITACEL L 600-30 and 5% AgriFiber BFG, which also showed a relatively low dough stickiness (0.15 N), considering the lowest stickiness value detected was 0.14 N and the highest was 0.33 N.
During baking a shrinkage of biscuit dough occurred resulting in a decrease in diameter. The combination of 20% VITACEL L 600-30 and 10% AgriFiber BFG resulted in the highest degree of biscuit shrinkage. The smallest diameter was 67.67 mm and was determined in biscuits containing 10% Fibersym RW, 20% VITACEL L 600-30 and 10% AgriFiber BFG. The fibre combination 40% Fibersym RW, 0% VITACEL L 600-30 and 10% AgriFiber BFG caused a spreading of the biscuit dough during baking by 1.45 mm.
The L*-value of the biscuits represents the lightness in colour. The BiFC had a L*-value of 78.45 ± 3.25 and was not significantly different from the LFC (74.89 ± 2.61). The use of wholemeal flour instead of biscuit flour caused a decrease in biscuit lightness (62.97 ± 10.74).
The inclusion of fibre ingredients showed a significant impact on biscuit lightness depending on the type and the addition level of the fibre. The lowest L*-value was determined in biscuits with the highest addition of AgriFiber BFG (10% addition) which ranged from 48.76 ± 2.34 to 54.12 ± 8.09. Furthermore, the addition of 5% AgriFiber BFG decreased the biscuit lightness as well, but to a lesser degree (between 59.04 ± 2.81 and 64.73 ± 2.13). Trials with no addition of AgriFibre BFG were not significantly different from LFC.
The water activity represents the amount of free water in the system. The highest water activity amongst the trials was determined in the BiFC (0.520 ± 0.010). The WMC showed aw-value of 0.220 ± 0.010, and the LFC biscuit had a water activity of 0.180 ± 0.010. The inclusion of fibre ingredients did not influence the aw-value significantly.
3.4. Co-existence of fibre ingredients in a low-FODMAP biscuit model system
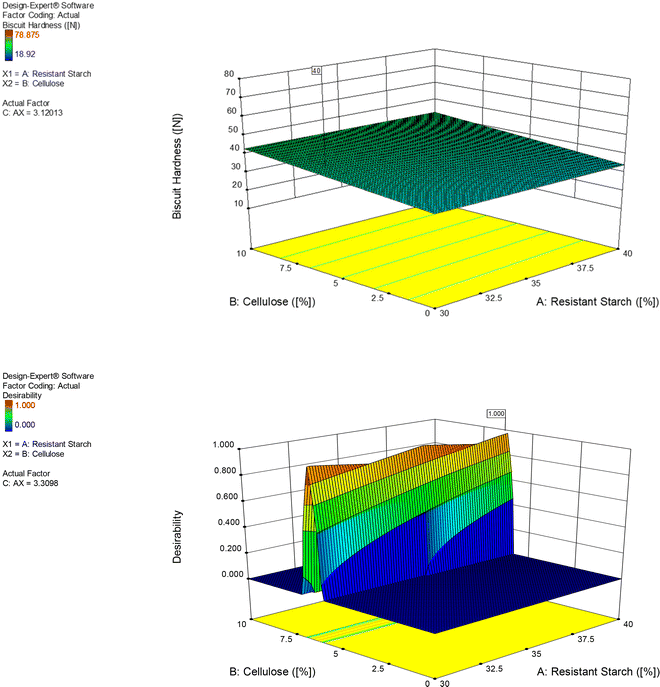
To determine the combination of the fibre ingredients, including their concentration, at which they can co-exist in the low-FODMAP biscuit system, the proposed model by Design Expert needs to be significant, and the lack-of-fit has to be insignificant. The model of each parameter was analysed for its significance (ESI Tables S2 and S3†) Biscuit hardness was the only parameter which showed a linear model as illustrated in Fig. 1. The statistical evaluation of the trials provided the significance of fibre–fibre interactions. Both, VITACEL L 600-30 and AgriFiber BFG increased biscuit hardness. Furthermore, the Two-factorial interaction model of biscuit hardness showed significant interaction between Fibersym RW and VITACEL L 600-30, however, the overall model was not significant. It is noteworthy that VITACEL L 600-30 was found to be significant for the linear model of dough stickiness but overall linear model insignificant.Based on the data input of the RSM trials and the statistical evaluation, a low-FODMAP prototype (LFP) which included 40% Fibersym RW, 10% VITACEL L600-30 and 2.3% AgriFiber BFG, resulted.
Table 4 illustrates the results of LFP. The dough hardness was 3.20 ± 0.29 which was slightly higher compared to the BiFC and LFC. The dough stickiness of LFP was significantly lower (0.19 ± 0.02) compared to the controls which is generally more desirable in biscuit production due to easier processibility on-line. As all control biscuits, LFP showed a shrinkage during baking which is comparable with BiFC and LFC. Furthermore, the biscuit hardness of LFP was 44.85 ± 3.76, higher compared to LFC biscuit but not significantly different from BiFC. LFP showed a L*-value of 68.36 ± 3.05, resulting in a darker biscuit. However, as demonstrated in Fig. 2, the difference occurred rather on the edge of the biscuit than in the centre. The aw-value of LFP 0.170 ± 0.010 showed no significant difference to all controls.
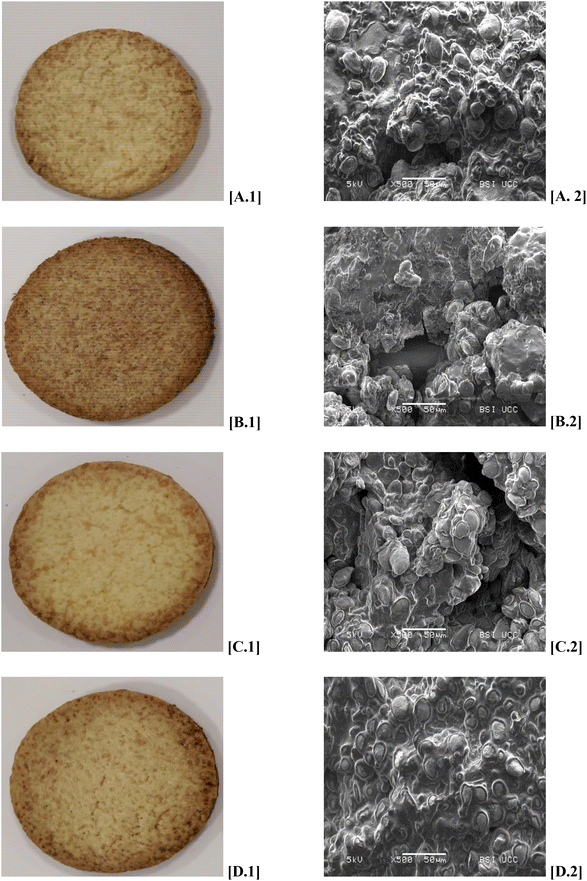 | ||
| Fig. 2 Photographs [.1] and SEM [.2] images of Biscuit flour control [A], wholemeal flour control [B], low FODMAP control biscuit [C] and low-FODMAP prototype [D]. | ||
Fig. 2 also shows the ultrastructure of the biscuits BiFC, WMC, LFC and LFP. All four biscuit samples mainly consist of starch granules embedded in a protein matrix. However, WMC and LFP differed from the BiFC and LFC: less starch granules were visible in WMC, and the LFC biscuit showed an alteration of the morphology of the starch granules. The granules are noticeably flatter and have a shape of elongated drops with a visible outer layer which is darker in colour.
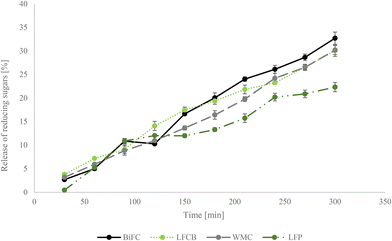
Sugar release during digestion was measured using an in vitro starch digestibility model and provides a prediction of the glycaemic index. The release of sugars over time is illustrated in Fig. 3. In the first 100 min of biscuit digestion the samples BiFC, WMC, LFC and LFP did not differ from each other. After 150 min the BiFC and the LFC biscuit showed the highest sugar release while WMC and LFP were significantly lower. At time point 250 min, however, a rapid increase in sugar release was detected during WMC digestion resulting in the same values as LFC; starch digestion of BiFC caused the highest sugar release amongst all biscuits. Throughout the in vitro starch digestibility, the lowest sugar release over time was detected in LFP. At the end of the digestion trial, the BiFC caused a release of 32.7% reducing sugars, and WMC and LFC biscuits released 30% of the sugars. LFP, on the other hand, showed in the lowest sugar release during digestion with a max amount of 22% after 300 min.
These four biscuit types were evaluated by a trained sensory panel regarding their sensory properties to determine the co-existence potential of Fibersym RW, VITACEL L 600-30 and AgriFiber BFG. The results of the Quantitative Descriptive Analysis (QDA) are demonstrated in Table 5. Regarding the evenness of colour, The BiFC showed the highest value (5.94 ± 1.8) followed by the LFP (5.22 ± 1.72), while WMC had the most uneven colour distribution (4.36 ± 1.99). The sensory panel determined the LFP biscuit as the hardest amongst all four biscuit types (8.65 ± 1.59), followed by the LFC (7.78 ± 1.42). The BiFC received the lowest hardness value from the panel (5.97 ± 1.31). The same trend was observed for the descriptors ‘sound at break after first bite’ and ‘hardness during chewing’. Regarding ‘graininess’ the WMC received the highest score with 5.32 ± 1.69, followed by LFC (4.03 ± 1.49) and LFP (3.95 ± 1.57) which did not differ significantly from each other. The lowest graininess was detected by the panel in BiFC (2.96 ± 1.19), which was not significantly different from the LFP. The sensory panel evaluated the dryness of the biscuits and did not determine any difference amongst the four samples. In terms of flavour and taste, the intensity of the descriptors ‘buttery’, ‘sweetness’ and ‘aftertaste’ were determined. No significant difference in buttery flavour between BiFC, WMC and LFP occurred, while LFC showed a significantly lower intensity. Regarding sweetness, BiFC was perceived as the sweetest with an intensity score of 6.75 ± 1.06, which did not differ significantly from the LFP (5.86 ± 1.4). WMC and LFC received lower sweetness scores. The aftertaste of the biscuits ranged between 4.97 and 5.83, however, no significant differences between the different biscuits occurred. The panel also evaluated the overall quality of the biscuits. The best overall quality was determined in BiFC (6.18 ± 1.57), followed by the LFC (5.16 ± 1.68). The LFP received the lowest overall quality score with 4.26 ± 1.62, which, however, was not significantly different from LFC and WMC.
| BiFC | WMC | LFC | LFP | |
|---|---|---|---|---|
| Appearance | ||||
| Evenness of colour | 5.94 ± 1.8b | 4.36 ± 1.99a | 4.95 ± 1.87ab | 5.22 ± 1.72ab |
| Texture | ||||
| Hardness of first bite | 5.97 ± 1.31a | 6.18 ± 1.52a | 7.78 ± 1.42b | 8.65 ± 1.59b |
| Sound at break after first bite | 5.78 ± 1.47a | 5.84 ± 1.27a | 7.97 ± 1.07b | 8.53 ± 0.85b |
| Hardness during chewing | 4.39 ± 1.38a | 4.6 ± 0.98a | 6.71 ± 1.6b | 7.85 ± 1.68c |
| Graininess | 2.96 ± 1.19a | 5.32 ± 1.69c | 4.03 ± 1.49b | 3.95 ± 1.57ab |
| Dryness | 4 ± 1.61a | 4.58 ± 1.68a | 4.33 ± 1.47a | 4.8 ± 1.88a |
| Flavour/taste | ||||
| Buttery | 5.38 ± 2.09b | 5.32 ± 1.81b | 3.43 ± 1.51a | 5.08 ± 2.05b |
| Sweetness | 6.75 ± 1.06b | 5.69 ± 1.58a | 5.06 ± 1.36a | 5.86 ± 1.45ab |
| Aftertaste | 4.97 ± 2.05a | 4.78 ± 2.04a | 5.83 ± 2.42a | 5.18 ± 2.15a |
| Quality | ||||
| Overall quality | 6.18 ± 1.57b | 4.81 ± 1.91a | 5.16 ± 1.68ab | 4.26 ± 1.62a |
A Pearson correlation analysis was conducted to identify interactions between the techno-functional parameters which analyse the structure of the dough and biscuits (dough hardness, dough stickiness, biscuit hardness) and the sensory properties describing biscuit texture. Significant correlations were only found for the dough hardness, dough stickiness, ‘sound at break after the first bite’ and ‘hardness during chewing’ (p > 0.05 and correlation above 0.5). However, no correlations were found between the texture of the biscuits measured via TPA and the sensory properties.
The nutritional composition of all four biscuits, BiFC, WMC, LFC and LFP, is demonstrated in Table 6. Significantly lower contents of digestible starch were quantified in LFP and WMC with 42.73 ± 4.88 g per 100 g and 38.06 ± 0.93 g per 100 g of digestible starch, respectively, while LFC and the BiFC had digestible starch contents above 50 g per 100 g.
| BiFC | WMC | LFC | LFP | |
|---|---|---|---|---|
| Ash [g per 100 g] | 0.92 ± 0.24a | 1.56 ± 0.01b | 1.42 ± 0.04b | n.d. |
| Fat [g per 100 g] | 21.5 ± 2.53 | 20.75 ± 3.22 | 19.02 ± 3.62 | 22.74 ± 3.73 |
| Protein [g per 100 g] | 4.74 ± 0.15a | 7.72 ± 0.51c | 5.67 ± 0.06b | 4.71 ± 0.14a |
| Carbohydrates | ||||
| Digestible starch [g per 100 g] | 51.77 ± 5.73ab | 38.06 ± 0.93a | 56.12 ± 4.99b | 42.73 ± 4.88ab |
| TAC [g per 100 g] | 0.652 ± 0.076a | 3.046 ± 0.471b | n.d. | 0.658 ± 0.042a |
| WEAX [g per 100 g] | 0.011 ± 0.001ab | 0.032 ± 0.004b | n.d. | 0.591 ± 0.063c |
| WUAX [g per 100 g] | 0.641 ± 0.076b | 3.014 ± 0.373c | n.d. | 0.067 ± 0.009a |
| Total dietary fibre (TDF) [g per 100 g] | 2.3 ± 0.18a | 10.05 ± 1.55b | 1.54 ± 0.13a | 14.60 ± 1.84c |
| IDF [g per 100 g] | 1.28 ± 0.11a | 8.57 ± 1.42b | 0.79 ± 0.05a | 13.18 ± 1.84c |
| IDF/TDF (average) | 0.56 | 0.85 | 0.51 | 0.90 |
| SDFP [g per 100g] | 0.75 ± 0.03a | 0.87 ± 0.08b | n.d. | 0.66 ± 0.05a |
| SDFS [g per 100 g] | 0.33 ± 0.06a | 1.48 ± 0.13c | 0.65 ± 0.05b | 0.56 ± 0.08b |
| SDFP/SDFS (average) | 2.27 | 0.59 | — | 1.18 |
| Total FODMAP [g per serving] | 0.13 ± 0.02a | 0.97 ± 0.07b | 0.12 ± 0.01a | 0.06 ± 0.01a |
BiFC, LFC and LFP contained a total FODMAP content of ∼0.10 g per serve size and were below the cut-off value of 0.30 g per serving. The total FODMAP content of WMC exceeded this cut-off value with ∼1.00 g per serving size.2
The analysis of the total dietary fibre content (TDF) in the biscuits revealed significant differences. The highest DF content was determined for the LFP (14.60 ± 1.84 g per 100 g), followed by WMC (10.05 ± 1.55 g per 100 g). In contrast, the DF contents of LFC and BiFC were significantly lower, ranging between 0.8 and 1.3 g per 100 g.
Additionally, variances were determined in the type of DF in the biscuits. The LFP was found to have a significantly higher IDF content compared to LFC. Although SDFS contents of ∼0.7 g per 100 g were observed in both, LFP and the LFC, these samples differed in their SDFS proportion due to vast discrepancies in the TDF contents. SDFS proportion of 5% and 50%, respectively, were detected. Moreover, LFP and the WMC had similar insoluble to soluble dietary fibre ratios with IDF contributing to more than 80% of the total dietary fibre content. However, LFP and WMC differed in the amount of SDFP and SDFS leading to a different composition in soluble dietary fibre. While WMC contained 0.87 ± 0.08 g per 100 g SDFP and 1.48 ± 0.13 g per 100 g SDFS, a ratio of 0.58 SDFP/SDFS, LFP contained higher amounts of SDFP (ratio of 1.18 SDFP/SDFS).
The LFP and the BiFC showed similarities regarding the TAC; in both samples, a TAC of ∼0.7 g per 100 g was determined. No arabinoxylans were quantifiable in the LFC. The TAC of the WMC was significantly higher (3.02 g per 100 g), of which more than 95% are WUAX.
4. Discussion
The investigation of the impact of dietary fibre on simple cereal-based food systems is essential for both academia and industry. Studies on fibre coexistence in food and the impact on low-FODMAP food systems are scarce. Furthermore, the selection of the ‘right’ fibre ingredients for the development of low-FODMAP food products is vital since some polysaccharides can cause IBS symptoms. This study provides firstly an in-depth investigation of interactions between resistant starch (Fibersym RW), a purified insoluble fibre ingredient (VITACEL L 600-30) and a soluble fibre (AgriFiber BFG). Secondly, the use of response surface methodology resulted in a low-FODMAP biscuit prototype formulation which is high in dietary fibre, a market gap.Each fibre ingredient used in this study resulted in an addition range in which biscuit quality was not affected. High amounts of Fibersym RW caused a significant increase in biscuit hardness. Resistant starch, especially of the type RS4, is known to recrystallise faster than the digestible parts of starch due to differences in morphology.22 Therefore, accelerated crystallisation due to a higher percentage of resistant starch granules can increase biscuit hardness. Furthermore, dietary fibre ingredients, compete with other macromolecules, such as protein, starch, and sugar, for water.23–26 This results in a restriction of gluten network development and starch gelatinisation27 and inhibits biscuit spreading usually caused by solubilized sugar.28,29 In this study a shrinkage of biscuits was observed, which led to an increase in biscuit height. This, in turn, increases the biscuit hardness. The biscuit shrinkage is putatively, due to sugar not being dissolved in the water phase due to hydrophilic fibre ingredients present. This led to the caramelisation of solid sugar crystals on the surface causing an increase in biscuit hardness.29–31 Fibersym RW and VITACEL L 600-30 are insoluble in water,32 which most likely led to an increase in biscuit hardness,33 putatively due to insoluble character of the ingredient causing higher compression forces during the measurement.32,33 Although Fibersym RW and VITACEL L600-30 are insoluble in water, these ingredients absorb water and swell. Hence, the combination of Fibersym RW and VITACEL L 600-30 amplified biscuit hardening, putatively due to a higher total water affinity of fibre in the system. Moreover, cellulose, the main compound in VITACEL L 600-30, has the potential to absorb fat. During the baking process of biscuits, shortening melts and contributes to the softening of biscuits. This phenomenon is restricted by the presence of VITACEL L 600-30 absorbing the melted fat.32 Moreover, VITACEL L 600-30 might contribute to a plasticising effect resulting in an increased biscuit hardness.34 Plasticisers can relocate the water and therefore accelerate the water absorption by compounds with a higher water-holding capacity.34 This plasticising phenomenon can be visually interpreted in Fig. 2D.2, illustrating the starch granules imbedded in a film-like matrix. AgriFiber BFG is a soluble fibre ingredient which contributes to water retention and recrystalises after baking. AgriFibre BFG is mainly water-extractable arabinoxylan (Table 3), which are known to increase biscuit hardness by interfering with gluten network development and starch crystallisation.10 Moreover, arabinoxylans of a higher DP crystallise at higher temperatures as occur during baking, leading to a rise in the biscuit hardness.35,36 AgriFibre BFG was the only ingredient influencing the colour of the biscuits which is due to the colour of the ingredient itself appearing like golden dust.
The combination of all three fibre ingredients resulted in a biscuit dough of higher quality (lower dough stickiness). The investigated fibre ingredients have a high affinity to water leading to an increased water-retention within the dough system, which is proven by the higher water absorption of the formulation compared to the LFC (Table 2). Especially insoluble dietary fibre ingredients, in this study Fibersym RW and VITACEL L 600-30, causes a decrease in deformable (viscous) proportion of the dough.37 This, in turn, causes an increase in (non-deformable) elastic parts in the dough system leading to reduced dough stickiness and increased dough stability as indicated by the higher dough hardness of LFP.37
The effect of the fibre ingredients on biscuit dough and biscuit quality are most likely an explanation for the impact on sugar release during starch digestion. Food structure influences the susceptibility of substrate, in this case starch, to digestive enzymes.38 As mentioned above, the ultrastructure of LFP showed imbedded starch granules covered by a film-like matrix, that, putatively reduced the accessibility of starch to the amylase. Furthermore, a reduction of the predicted glycaemic index (pGI) can be linked to the use of high amounts of Fibersym RW, a resistant starch RS4. Resistant starch RS4 has been reported to significantly lower the pGI of biscuits and cookies.6–8,39 This is most likely due to the lower degree of gelatinisation; an essential process starch needs to undergo to be digestible. Biscuit formulations include low amounts of water, which is necessary for gelatinisation, and thus, restricting this process.29,40 Furthermore, the amounts of fat in the formulation, cover starch granules and decrease the degree of gelatinisation. The difference between WMC and LFP can be explained by the type of fibre present in the system, in particular, the high amounts of resistant starch RS4 and the presence of soluble arabinoxylan. RS4 resistant starch granules are different in morphology, less susceptible towards enzyme attack,41 and AgriFiber BFG, putatively, creates a protective layer around the starch granules (Fig. 2D.2).
The incorporation of resistant starch alone does not affect sensory properties regarding taste, flavour, appearance, texture, and overall acceptance.6,42,43 It is most likely the combination of the fibre ingredients which impacted the biscuit hardness. It is noteworthy to mention that according to Porter (2021)44 the addition of 20% arabinoxylan from corn results in a desirable biscuit quality.
5. Conclusion
Maintaining an adequate fibre supply in a low FODMAP diet can be challenging, which leads to the need for fortification with low-FODMAP fibre ingredients in food products for patients with IBS. Ideally, a range of different types of fibre are used to provide the consumer with benefits for digestive wellbeing. This study reveals the impact of three different types of dietary fibre ingredients on a low-FODMAP biscuit model system, and, further, highlights the interactions between them leading to inclusion levels which allows their coexistence. These ingredients did not only differ in their source but also in their chemical composition and structure, and functionality. Fibersym RW is an ingredient which can be very easily incorporated in biscuits in high amounts without impacting the quality. However, its recrystallization properties increase hardness. This phenomenon will be amplified when combined with VITACEL L 600-30 due to the plasticising effect of cellulose. AgriFiber BFG is a soluble fibre, and has a major impact on colour, but also on starch digestibility, as it forms a film around starch granules restricting enzyme attack. The combination of these three dietary fibre ingredients in the determined concentration by RSM resulted in a fibre rich low-FODMAP biscuit with comparable quality to control biscuits made of biscuit flour, and superior quality compared to non-fibre low-FODMAP biscuits.Ethical approval
This work was following the guidelines for ethical approval in Teagasc Ashtown, Ireland.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Irish Department of Agriculture, Food and the Marine. Project Acronym: TALENTFOOD – Project: code 15F602. The authors would like to thank Mr Tom Hannon for technical support.References
- J. J. Atzler, A. W. Sahin, E. Gallagher, E. Zannini and E. K. Arendt, Characteristics and properties of fibres suitable for a low FODMAP diet- an overview, Trends Food Sci. Technol., 2021, 112, 823–836 CrossRef CAS.
- L. Ispiryan, E. Zannini and E. K. Arendt, FODMAP modulation as a dietary therapy for IBS: Scientific and market perspective, Compr. Rev. Food Sci. Food Saf., 2022, 21, 1491–1516 CrossRef PubMed.
- L. Ispiryan, E. Zannini and E. K. Arendt, Characterization of the FODMAP-profile in cereal-product ingredients, J. Cereal Sci., 2020, 92, 102916 CrossRef CAS.
- A. Raymundo, P. Fradinho and M. C. Nunes, Effect of Psyllium fibre content on the textural and rheological characteristics of biscuit and biscuit dough, Bioact. Carbohydr. Diet. Fibre, 2014, 3, 96–105 CrossRef CAS.
- A. W. Sahin, T. Rice, E. Zannini, K. M. Lynch, A. Coffey and E. K. Arendt, The incorporation of sourdough in sugar-reduced biscuits: a promising strategy to improve techno-functional and sensory properties, Eur. Food Res. Technol., 2019, 245, 1841–1854 CrossRef CAS.
- M. Cervini, A. Frustace, G. D. Garrido, G. Rocchetti and G. Giuberti, Nutritional, physical and sensory characteristics of gluten-free biscuits incorporated with a novel resistant starch ingredient, Heliyon, 2021, 7, 1–7 CrossRef PubMed.
- S. K. Walsh, A. Lucey, J. Walter, E. Zannini and E. K. Arendt, Resistant starch—An accessible fiber ingredient acceptable to the Western palate, Compr. Rev. Food Sci. Food Saf., 2022, 21, 2930–2955 CrossRef CAS PubMed.
- E. Fuentes-Zaragoza, M. J. Riquelme-Navarrete, E. Sánchez-Zapata and J. A. Pérez-Álvarez, Resistant starch as functional ingredient: A review, Food Res. Int., 2010, 43, 931–942 CrossRef CAS.
- B. A. Ashwar, A. Gani, A. Shah, I. A. Wani and F. A. Masoodi, Preparation, health benefits and applications of resistant starch - A review, Starch/Staerke, 2016, 68, 287–301 CrossRef CAS.
- C. M. Courtin and J. A. Delcour, Arabinoxylans and Endoxylanases in Wheat Flour Bread-making, J. Cereal Sci., 2002, 35, 225–243 CrossRef CAS.
- S. Bengtsson and P. Åman, Isolation and chemical characterization of water-soluble arabinoxylans in rye grain, Carbohydr. Polym., 1990, 12, 267–277 CrossRef CAS.
- V. van Craeyveld, J. A. Delcour and C. M. Courtin, Extractability and chemical and enzymic degradation of psyllium (Plantago ovata Forsk) seed husk arabinoxylans, Food Chem., 2009, 112, 812–819 CrossRef CAS.
- J. J. Atzler, A. W. Sahin, E. Gallagher, E. Zannini and E. K. Arendt, Characteristics and properties of fibres suitable for a low FODMAP diet- an overview, Trends Food Sci. Technol., 2021, 112, 823–836 CrossRef CAS.
- AACC International, Method 44–15.02. Moisture - Air-Oven Methods, in Approved Methods of Analysis, 11th edn, 2000, pp. 3–6 Search PubMed.
- Aoac, Ash of Flour (Direct Method), Method 923.03, in Official Methods of Analysis, AOAC International Publisher, Gaithersburg, 18th edn, 2005. References - Scientific Research Publishing, https://scirp.org/reference/referencespapers.aspx?referenceid=1123249, (accessed 4 December 2022) Search PubMed.
- Food and Agriculture Organization of the United Nations, Food energy: Methods of analysis and conversion factors, Food and Agriculture Organization of the United Nations, 2002, vol. 77, pp. 7–17 Search PubMed.
- L. Ispiryan, M. Heitmann, A. Hoehnel, E. Zannini and E. K. Arendt, Optimization and Validation of an HPAEC-PAD Method for the Quantification of FODMAPs in Cereals and Cereal-Based Products, J. Agric. Food Chem., 2019, 67, 4384–4392 CrossRef CAS PubMed.
- AOAC, AOAC Official Method 2022.01-2022 Insoluble, Soluble, and Total Dietary Fiber, AOAC International, 17th edn, 2000 Search PubMed.
- K. M. Lynch, C. R. Strain, C. Johnson, D. Patangia, C. Stanton, F. Koc, J. Gil-Martinez, P. O'Riordan, A. W. Sahin, R. P. Ross and E. K. Arendt, Extraction and characterisation of arabinoxylan from brewers spent grain and investigation of microbiome modulation potential, Eur. J. Nutr., 2021, 60, 4393–4411 CrossRef CAS PubMed.
- C. S. Brennan and C. M. Tudorica, Evaluation of potential mechanisms by which dietary fibre additions reduce the predicted glycaemic index of fresh pastas, Int. J. Food Sci. Technol., 2008, 43, 2151–2162 CrossRef CAS.
- H. Stone, J. Sidel, S. Oliver, A. Woosley and R. C. Singleton, Sensory evaluation by quantitative descriptive analysis, Food Technol., 1974, 28, 24–34 Search PubMed.
- E. Fuentes-Zaragoza, M. J. Riquelme-Navarrete, E. Sánchez-Zapata and J. A. Pérez-Álvarez, Resistant starch as functional ingredient: A review, Food Res. Int., 2010, 43, 931–942 CrossRef CAS.
- A. W. Sahin, J. J. Atzler, D. Valdeperez, S. Münch, G. Cattaneo, P. O'Riordan and E. K. Arendt, Rejuvenated brewer's spent grain: Evervita ingredients as game-changers in fibre-enriched bread, Foods, 2021, 10, 1–23 CrossRef PubMed.
- A. W. Sahin, K. Hardiman, J. J. Atzler, M. Vogelsang-O'Dwyer, D. Valdeperez, S. Münch, G. Cattaneo, P. O'Riordan and E. K. Arendt, Rejuvenated Brewer's Spent Grain: The impact of two BSG-derived ingredients on techno-functional and nutritional characteristics of fibre-enriched pasta, Innovative Food Sci. Emerging Technol., 2021, 68, 102633 CrossRef CAS.
- A. W. Sahin, A. Coffey and E. Zannini, Functionalisation of wheat and oat bran using single-strain fermentation and its impact on techno-functional and nutritional properties of biscuits, Eur. Food Res. Technol., 2021, 247, 1825–1837 CrossRef CAS.
- B. Pareyt, K. Brijs and J. A. Delcour, Sugar-Snap Cookie Dough Setting: The Impact of Sucrose on Gluten Functionality, J. Agric. Food Chem., 2009, 57, 7814–7818 CrossRef CAS PubMed.
- A. W. Sahin, C. Axel and E. K. Arendt, Understanding the function of sugar in burger buns: a fundamental study, Eur. Food Res. Technol., 2017, 243, 1905–1915 CrossRef CAS.
- A. W. Sahin, T. Rice, E. Zannini, K. M. Lynch, A. Coffey and E. K. Arendt, The incorporation of sourdough in sugar-reduced biscuits: a promising strategy to improve techno-functional and sensory properties, Eur. Food Res. Technol., 2019, 245, 1841–1854 CrossRef CAS.
- B. Pareyt and J. A. Delcour, The role of wheat flour constituents, sugar, and fat in low moisture cereal based products: A review on sugar-snap cookies, Crit. Rev. Food Sci. Nutr., 2008, 48, 824–839 CrossRef CAS PubMed.
- R. G. M. van der Sman and S. Renzetti, Understanding functionality of sucrose in biscuits for reformulation purposes, Crit. Rev. Food Sci. Nutr., 2018, 8398, 1–15 Search PubMed.
- R. W. Hartel and A. V. Shastry, Sugar crystallization in food products, Crit. Rev. Food Sci. Nutr., 1991, 30, 49–112 CrossRef CAS PubMed.
- X. He, W. Lu, C. Sun, H. Khalesi, A. Mata, R. Andaleeb and Y. Fang, Cellulose and cellulose derivatives: Different colloidal states and food-related applications, Carbohydr. Polym., 2021, 255, 1–13 Search PubMed.
- H. Chen, G. L. Rubenthaler and E. G. Schanus, Effect of Apple Fiber and Cellulose on the Physical Properties of Wheat Flour, J. Food Sci., 1988, 53, 304–305 CrossRef CAS.
- J. J. Atzler, A. W. Sahin, E. Gallagher, E. Zannini and E. K. Arendt, Investigation of different dietary-fibre-ingredients for the design of a fibre enriched bread formulation low in FODMAPs based on wheat starch and vital gluten, Eur. Food Res. Technol., 2021, 247, 1939–1957 CrossRef CAS.
- T. B. Qaisrani, M. M. Qaisrani and T. M. Qaisrani, Arabinoxylans from psyllium husk: A review, J. Environ. Agric. Sci., 2016, 6, 33–39 Search PubMed.
- A. Pollet, V. van Craeyveld, T. van de Wiele, W. Verstraete, J. A. Delcour and C. M. Courtin, In vitro fermentation of arabinoxylan oligosaccharides and low molecular mass arabinoxylans with different structural properties from wheat (Triticum aestivum L.) bran and psyllium (Plantago ovata Forsk) seed husk, J. Agric. Food Chem., 2012, 60, 946–954 CrossRef CAS PubMed.
- L. Laguna, M. J. Hernández, A. Salvador and T. Sanz, Study on Resistant Starch Functionality in Short Dough Biscuits by Oscillatory and Creep and Recovery Tests, Food Bioprocess Technol., 2012, 6, 1312–1320 CrossRef.
- D. J. A. Jenkins, M. J. Thorne, T. M. S. Wolever, A. V. Rao and L. U. Thompson, The effect of starch-protein interaction in wheat on the glycemic response and rate of in vitro digestion, Am. J. Clin. Nutr., 1987, 45, 946–951 CrossRef CAS PubMed.
- T. Gelencsér, V. Gál, M. Hódsági and A. Salgó, Evaluation of quality and digestibility characteristics of resistant starch-enriched pasta, Food Bioprocess Technol., 2008, 1, 171–179 CrossRef.
- R. G. M. van der Sman and S. Renzetti, Understanding functionality of sucrose in biscuits for reformulation purposes, Crit. Rev. Food Sci. Nutr., 2018, 1–15 Search PubMed.
- S. R. Falsafi, Y. Maghsoudlou, M. Aalami, S. M. Jafari and M. Raeisi, Physicochemical and morphological properties of resistant starch type 4 prepared under ultrasound and conventional conditions and their in-vitro and in-vivo digestibilities, Ultrason. Sonochem., 2019, 53, 110–119 CrossRef CAS PubMed.
- M. di Cairano, M. C. Caruso, F. Galgano, F. Favati, N. Ekere and F. Tchuenbou-Magaia, Effect of sucrose replacement and resistant starch addition on textural properties of gluten-free doughs and biscuits, Eur. Food Res. Technol., 2021, 247, 707–718 CrossRef CAS.
- L. Laguna, A. Salvador, T. Sanz and S. M. Fiszman, Performance of a resistant starch rich ingredient in the baking and eating quality of short-dough biscuits, LWT–Food Sci. Technol., 2011, 44, 737–746 CrossRef CAS.
- R. O. Porter, in A Glance at Food Processing Applications, ed. I. Var and S. Uzunlu, IntechOpen, 2021, vol. 1, pp. 9–45 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo00830d |
| ‡ Joint first authorship. |
| This journal is © The Royal Society of Chemistry 2023 |