Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The association of serum serine levels with the risk of incident cancer: results from a nested case–control study
Tong
Liu†
abc,
Chenan
Liu†
abc,
Mengmeng
Song†
abc,
Yaping
Wei
d,
Yun
Song
e,
Ping
Chen
e,
Lishun
Liu
e,
Binyan
Wang
*ef and
Hanping
Shi
 *abc
*abc
aDepartment of Gastrointestinal Surgery/Clinical Nutrition, Capital Medical University Affiliated Beijing Shijitan Hospital, Beijing, 100038, China. E-mail: shihp@ccmu.edu.cn
bBeijing International Science and Technology Cooperation Base for Cancer Metabolism and Nutrition, Beijing, 100038, China
cKey Laboratory of Cancer FSMP for State Market Regulation, Beijing, 100038, China
dKey Laboratory of Precision Nutrition and Food Quality, Ministry of Education, Department of Nutrition and Health, College of Food Sciences and Nutritional Engineering, China Agricultural University, Beijing 100083, China
eShenzhen Evergreen Medical Institute, Shenzhen, China. E-mail: binyanwang163@163.com
fInstitute for Biomedicine, Anhui Medical University, Hefei, China
First published on 14th August 2023
Abstract
Background: Cancer is associated with the dysregulation of serum serine levels, and tumor growth is supported by increased serine biosynthesis. This study aims to explore the association of serum serine levels with incident cancer risk in Chinese hypertensive adults. Materials and methods: 1391 patients with incident cancer and 1391 matched controls in terms of age, sex, and residence with cases in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio were included in this nested case–control study. The serum serine concentrations were determined by liquid chromatography with tandem quadrupole mass spectrometry (LC-MS/MS) at the baseline. The associations of serum serine levels with the risk of overall, digestive system, non-digestive system, and lung cancers (the most common type) were assessed by conditional logistic regression. Results: When serum serine concentration was assessed as quartiles, a significantly higher risk of total cancer (OR = 1.32; 95% CI: 1.01–1.71; P = 0.038) was found in participants in the highest quartile (≥17.68 μg mL−1) compared with participants in the lowest quartile (<13.27 μg mL−1). Similar results were also observed for non-digestive system and lung cancers, but not for digestive system cancers. Significant associations of serum with overall cancer risk were found among all age subgroups, men, non-smokers, non-drinkers, and individuals with lower folic acid levels. Conclusion: High serum serine concentrations were associated with an increased risk of overall, non-digestive system, and lung cancers among Chinese hypertensive adult patients.
1 ratio were included in this nested case–control study. The serum serine concentrations were determined by liquid chromatography with tandem quadrupole mass spectrometry (LC-MS/MS) at the baseline. The associations of serum serine levels with the risk of overall, digestive system, non-digestive system, and lung cancers (the most common type) were assessed by conditional logistic regression. Results: When serum serine concentration was assessed as quartiles, a significantly higher risk of total cancer (OR = 1.32; 95% CI: 1.01–1.71; P = 0.038) was found in participants in the highest quartile (≥17.68 μg mL−1) compared with participants in the lowest quartile (<13.27 μg mL−1). Similar results were also observed for non-digestive system and lung cancers, but not for digestive system cancers. Significant associations of serum with overall cancer risk were found among all age subgroups, men, non-smokers, non-drinkers, and individuals with lower folic acid levels. Conclusion: High serum serine concentrations were associated with an increased risk of overall, non-digestive system, and lung cancers among Chinese hypertensive adult patients.
Introduction
Cancer is a focal clinical and public health issue worldwide. In 2020, in over 185 countries, it was estimated that there were 19.3 million new cancer cases and 10.0 million cancer deaths.1 According to the Global Cancer Statistics (2002), the top three most frequently diagnosed cancers are as follows: lung cancer, breast cancer, and colorectal cancer.2 It is estimated that 4.3 million new cases of cancer and 2.8 million cancer deaths occurred in China in 2015.3 Identifying important and valid risk factors is urgently needed for curbing the rising trend of cancer. Cancer emergence is associated with a variety of factors including genetics, family history, lifestyle, and microbial infection. Recent studies have found that cancer is also a metabolic disease, which mainly manifests as cell metabolic reprogramming, a hallmark of malignancy.4 Cancer cells require more nutriment than normal cells, such as glucose, glutamine, and serine, to synthesize the substances necessary to sustain rapid cell growth and proliferation.5In addition to being a non-essential amino acid, serine can be synthesized by cells utilizing their serine synthesis pathway (SSP). Serine plays a critical, pivotal role in intermediary metabolism, and links biosynthetic flux from glycolysis to purine synthesis, glutathione synthesis, folate-mediated one-carbon metabolism, and lipid metabolism.6 The dysregulation of plasma serine is associated with various diseases, including metabolic syndrome,7 schizophrenia and major depression,8,9 cystathionine beta-synthase deficiency,10 fatty liver,11 type I diabetes,12 hypertensive nephrosclerosis,13 primary biliary cholangitis,14 and cancer. As the third highest metabolite consumed by cancer cells in nucleotide biosynthesis, serine is also a carbon donor and a building block for proteins.15 Cancer cells depend on endogenous and exogenous serine to enhance their metabolism and proliferation. In addition, studies have found that a decrease in serine biosynthesis leads to an increase in tumor growth in several different cancers.5,16
A recent study revealed that serum serine was higher among smokers with bladder cancer compared to non-smokers,17 and another study found that high levels of serine were associated with decreased overall survival of patients with head and neck cancer.18 Intriguingly, researchers found that alterations of some free amino acids, such as serine, occur in the serum of non-small-cell lung cancer (NSCLC) patients in the early stage of the disease, and this might serve as a valuable component of a blood multi-marker panel for NSCLC detection.19 All of these studies indicated that patients with cancer showed dysregulation of serum serine. To date, no study has been conducted to explore the association of serum serine concentrations with subsequent cancer risk. Our study aims to examine the relationship between serum serine concentrations and cancer risk using a case–control study nested in a prospective cohort study.
Materials and methods
Study population and design
The participants for this nested, case–control study were obtained from the China H-Type Hypertension Registry Study (CHHRS; https://www.chictr.org.cn/showproj.html?proj=28262; unique identifier: ChiCTR1800017274). In brief, the CHHRS was a community-based, observational, real-world registry study including 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492 participants, conducted primarily in Rongcheng city, Shandong Province, China, from July 2018 to July 2023. Participants eligible for the study were men and women over the age of 18 with hypertension systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and without a history of cancer at the screening visit. In the current study, two phases are involved: recruitment (screening) and a three-year observation follow-up. Follow-ups were scheduled every three months to collect measurement data, and to record blood pressure, medication usage, and study outcomes, such as cardiovascular disease, cancer, and all-cause mortality.
492 participants, conducted primarily in Rongcheng city, Shandong Province, China, from July 2018 to July 2023. Participants eligible for the study were men and women over the age of 18 with hypertension systolic blood pressure (SBP) ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and without a history of cancer at the screening visit. In the current study, two phases are involved: recruitment (screening) and a three-year observation follow-up. Follow-ups were scheduled every three months to collect measurement data, and to record blood pressure, medication usage, and study outcomes, such as cardiovascular disease, cancer, and all-cause mortality.
Outcome ascertainment
From 2016 to 2019, cancer cases were identified based on certain clinical criteria including operative recordings, imaging results, and serum tumor markers or positive histopathology data from hospitals that had treated cancer patients. In the absence of pathological data, two oncologists assessed potential cases. To identify cancer cases, the two professionals had to diagnose it at the same time and code it using the International Classification of Diseases, 10th Revision (ICD-10).Nested case–control study
As a result of the CHHRS follow-up, 1419 cancer cases were identified among 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 492 participants in the Rongcheng District. Within this cohort, 1419 incident cancer cases and 1419 matched controls were included in a nested case–control study. Participants belonging to the control group were those who were still alive and had not developed cancer during the follow-up, and matched in terms of age, sex, and residence with cases in a 1
492 participants in the Rongcheng District. Within this cohort, 1419 incident cancer cases and 1419 matched controls were included in a nested case–control study. Participants belonging to the control group were those who were still alive and had not developed cancer during the follow-up, and matched in terms of age, sex, and residence with cases in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. After excluding those without data on serine and unpaired individuals, 1391 incident cases and 1391 matched controls were analyzed (Fig. 1). Participants were further divided according to the quartiles of serine concentration with the cutoffs 13.27 μg mL−1, 15.27 μg mL−1, and 17.67 μg mL−1, respectively.
1 ratio. After excluding those without data on serine and unpaired individuals, 1391 incident cases and 1391 matched controls were analyzed (Fig. 1). Participants were further divided according to the quartiles of serine concentration with the cutoffs 13.27 μg mL−1, 15.27 μg mL−1, and 17.67 μg mL−1, respectively.
The CHHRS and the present study were approved by the ethics committee of the Institute of Biomedicine, Anhui Medical University in Hefei, China, and adhered to the Declaration of Helsinki. Each participant gave written, informed consent before participating in the study.
Exposure and potential confounders
Data on socioeconomic status, lifestyle behaviors, and medical history of the participants and their families were collected using a standard questionnaire.20 Participants’ height, weight, and waist and hip circumference were measured by trained medical staff. EDTA-containing vacuum tubes were used to collect overnight fasted venous blood samples. Serum folate levels were measured by a commercial laboratory using a chemiluminescent immunoassay (New Industrial, Shenzhen, China). Liquid chromatography with tandem quadrupole mass spectrometry (LC-MS/MS) was used in a commercial laboratory (Beijing DIAN Medical Laboratory, China) to determine serum serine concentrations. At the Shenzhen Tailored Medical Laboratory, serum levels of biochemical indexes including triglycerides (TG), total cholesterol (TC), albumin (ALB), high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), uric acid (UA), and creatinine were measured using automatic clinical analyzers (Beckman Coulter).Statistical analysis
Normally distributed variables, skewed distributed variables, and categorical variables were expressed as mean ± standard deviation, median with interquartile range, and n (%), respectively, with the differences between cases and controls compared by paired Student's t-tests, non-parametric Kruskal–Wallis tests, and chi-square tests. Restricted cubic spline regression (RCS) was used to calculate the dose–response association of serine with the risk of overall, digestive system, and non-digestive system cancers. Odds ratios (ORs) of incident cancer in terms of serum serine concentrations (continuous and quartiles) were calculated using conditional logistic regression models. Multivariate regression models were adjusted for body mass index (BMI), smoking status, alcohol consumption, sleep quality, antihypertensive drug usage, SBP (as continuous), FBG (as continuous), TC (as continuous), TG (as continuous), UA (as continuous), creatinine (as continuous), folate (as continuous), alanine aminotransferase (ALT, as continuous), ALB (as continuous), and the family history of cancer.Age, sex, BMI, smoking and drinking are strongly associated with the risk of cancer development and may influence the role of serum serine with tumor development.21 Studies have shown that the metabolism of folic acid and the interconversion of serine in the folate cycle may play a role in tumor prevention before precancerous lesions form, and once precancerous lesions are formed, the administration of folate and serine and other nutrients may provide DNA precursors for tumor cell growth to accelerate tumor progression.22 In further hierarchical analyses, potential interaction effects of the association between serum serine concentrations and cancer risk were assessed by dividing all participants into different subgroups including age (median), sex, BMI, smoking and drinking status, and the levels of folate (median). We further explored the effect of serine on the occurrence of digestive, non-digestive system, and lung cancers (the most common cancer type) by using its cutoffs of quartiles. To avoid any possible influence of preclinical disease on the results, we further divided participants by the median follow-up and reanalyzed the association of serum serine with cancer risk.
A two-tailed P < 0.05 was considered statistically significant in all analyses. SAS software (version 9.4) and R software (version 4.0.4, https://www.R-project.org/) were used for all statistical analyses.
Results
Characteristics of the participants
Among the 1391 incident cancer cases, 543 (39.04%) were digestive system cancers and 848 (60.96%) were non-digestive system cancers. Lung cancer was the leading type (n = 361), followed by colorectal cancer (n = 180), gastric cancer (n = 160), liver cancer (n = 107), breast cancer (n = 86), and other types of cancer (n = 497). The mean age of all participants was 69.30 years (SD, 7.77 years). Significant differences were found in the levels of ALB, TC, serine, the percentage of the smoking status, the history of coronary heart disease, the history of stroke, sleep quality, and antihypertensive drug usage across the two groups (all p < 0.05, Table 1).| Variables | Controls (n = 1391) | Cases (n = 1391) | p-Value |
|---|---|---|---|
| BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; ALT: alanine aminotransferase; ALB: albumin; TG: triglycerides; TC: total cholesterol; UA: uric acid; HDL-C: high-density lipoprotein cholesterol; FBG: fasting blood glucose; HCY: homocysteine; CKD: chronic kidney disease; CHD: coronary heart disease. | |||
| Age, years | 69.30 ± 7.77 | 69.30 ± 7.77 | 0.999 |
| Male, n (%) | 779 (56.00) | 779 (56.00) | 1.000 |
| BMI, kg m−2 | 25.73 ± 3.60 | 25.73 ± 3.83 | 0.950 |
| Baseline SBP, mmHg | 148.48 ± 21.25 | 147.51 ± 21.30 | 0.934 |
| Baseline DBP, mmHg | 83.74 ± 11.32 | 83.07 ± 11.75 | 0.129 |
| ALT, U L−1 | 10.0 (7.0, 13.0) | 10.0 (7.0, 14.0) | 0.140 |
| ALB, g L−1 | 45.44 ± 2.45 | 44.75 ± 2.97 | <0.001 |
| TG, mmol L−1 | 1.21 (0.86, 1.77) | 1.19(0.84, 1.80) | 0.641 |
| TC, mmol L−1 | 6.51 ± 1.23 | 6.44 ± 1.30 | 0.045 |
| UA, μmol L−1 | 320.0 (269.0, 371.0) | 314.0 (264.0, 374.0) | 0.389 |
| HDL-C, mmol L−1 | 1.23 ± 0.24 | 1.22 ± 0.27 | 0.243 |
| FBG, mmol L−1 | 6.25 ± 1.71 | 6.29 ± 1.83 | 0.613 |
| Creatinine, μmol L−1 | 51.0 (10.0, 64.0) | 52.0 (10.0, 64.0) | 0.752 |
| Folate, ng ml−1 | 6.14 (3.99, 9.68) | 6.12 (4.23, 10.13) | 0.450 |
| HCY, μmol L−1 | 12.01 (10.31,14.66) | 12.25 (10.06, 15.03) | 0.904 |
| Serine, μg mL−1 | 15.16 (13.11,17.47) | 15.37 (13.41,17.82) | 0.001 |
| Marital status, n (%) | 1146 (82.39) | 1180 (84.83) | 0.354 |
| Educational background, n (%) | 109 (7.84) | 103 (7.40) | 0.668 |
| Current smoker, n (%) | 334 (24.01) | 401 (28.83) | 0.011 |
| Current drinker, n (%) | 388 (27.89) | 370 (26.60) | 0.359 |
| History of CKD, n (%) | 14 (1.01) | 25 (1.80) | 0.076 |
| History of CHD, n (%) | 0 (0) | 165 (11.86) | <0.001 |
| History of stroke, n (%) | 0 (0) | 64 (4.60) | <0.001 |
| Family history of cancer, n (%) | 47 (3.38) | 50 (3.59) | 0.918 |
| Sleep quality, n (%) | 191 (13.73) | 259 (18.62) | 0.002 |
| Antihypertensive drug usage, n (%) | 495 (35.59) | 557 (40.04) | 0.015 |
Association of serum serine concentration with cancer risk
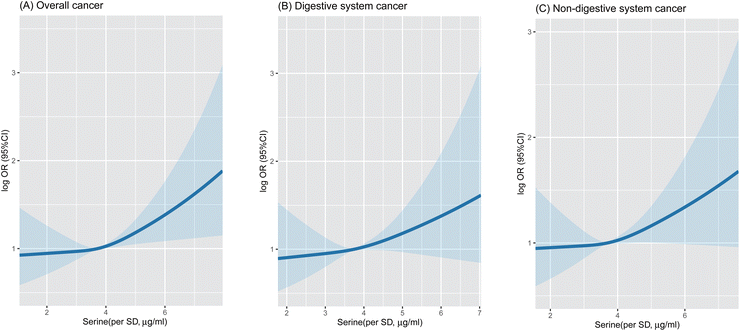
The median follow-up in this current study was 0.85 (0.41, 1.21) years. According to the restricted cubic spline graph, serum serine concentration was positively associated with overall, the digestive system and non-digestive system cancer risk (Fig. 2).Consistently, when serum serine concentration was assessed as a continuous variable, each standard deviation (SD) increment of serum serine levels significantly elevated the risk of overall cancer (OR = 1.12; 95% CI: 1.02–1.23) in the adjusted model (Table 2). When serum serine concentrations were assessed as quartiles, a significantly higher risk of total cancer was found in the participants in the highest quartile (≥17.68 μg mL−1) compared with the participants in the lowest quartile (<13.27 μg mL−1) (OR = 1.32; 95% CI: 1.01–1.71; P = 0.038). Similar results were also observed for non-digestive system and lung cancers, but not for digestive system cancers (Table 3). A higher risk of non-digestive system cancers (OR = 1.29; 95% CI: 1.01–1.82) and lung cancers (OR = 1.61; 95% CI: 1.03–2.88) was found in participants in the highest vs. the lowest quartiles of serum serine.
| Serine (μg mL−1) | Cases/controls (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Crude model | Adjusted model | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | ||
| Models were adjusted for body mass index, smoking status, alcohol drinking, systolic blood pressure, triglycerides, cholesterol, uric acid, fasting blood glucose, high-density lipoprotein cholesterol, creatinine, albumin, alanine aminotransferase, homocysteine, sleep quality, antihypertensive drug usage, and family history of cancer. | |||||
| Serine (per SD) | 1391/1391 | 1.11 (1.03, 1.21) | 0.011 | 1.12 (1.02, 1.23) | 0.014 |
| Quartiles of serine (μg mL −1 ) | |||||
| Q1 (<13.27) | 322/374 | Ref. | Ref. | ||
| Q2 (13.27–15.27) | 354/340 | 1.23 (0.99, 1.53) | 0.062 | 1.14 (0.90, 1.45) | 0.284 |
| Q3 (15.28–17.67) | 349/348 | 1.20 (0.96, 1.50) | 0.102 | 1.10 (0.86, 1.41) | 0.455 |
| Q4 (≥17.68) | 366/329 | 1.33 (1.06, 1.68) | 0.015 | 1.32 (1.01, 1.71) | 0.038 |
| Serine | Digestive system cancera | Non-digestive system cancerb | Lung cancerc | |||
|---|---|---|---|---|---|---|
| Cases/controls | OR (95% CI) | Cases/controls | OR (95% CI) | Cases/controls | OR (95% CI) | |
| Models were adjusted for body mass index, smoking status, alcohol drinking, systolic blood pressure, triglycerides, cholesterol, uric acid, fasting blood glucose, high-density lipoprotein cholesterol, creatinine, albumin, alanine aminotransferase, homocysteine, sleep quality, antihypertensive drug usage, and family history of cancer.a The cutoffs of serine in digestive system cancer were 13.28, 15.41, and 17.72.b The cutoffs of serine in non-digestive system cancer were 13.26, 15.21, and 17.65.c The cutoffs of serine in lung cancer were 13.57, 15.44, and 17.82. | ||||||
| Serine (per SD) | 543/543 | 1.13 (0.96, 1.32) | 848/848 | 1.12 (1.02, 1.26) | 361/361 | 1.17(0.97, 1.42) |
| Quartiles | ||||||
| Q1 | 124/148 | Ref. | 198/226 | Ref. | 84/96 | Ref. |
| Q2 | 139/132 | 1.15(0.78, 1.71) | 214/210 | 1.11(0.81, 1.52) | 92/86 | 1.43(0.84, 2.44) |
| Q3 | 136/135 | 1.16(0.76, 1.76) | 214/209 | 1.12(0.81, 1.53) | 91/92 | 1.19(0.73, 1.96) |
| Q4 | 144/128 | 1.33(0.87, 2.03) | 222/203 | 1.29(1.01, 1.82) | 94/87 | 1.61(1.03, 2.88) |
Subgroup analyses stratified by confounders and follow-up time
Various subgroup analyses were performed to determine whether serum serine concentrations were associated with total cancer risk (Fig. 3). Age, sex, BMI, smoking status, drinking status, and the levels of folate did not modify the association between serine concentrations and the risk of overall cancer (all p for interaction >0.05). Significant associations of serum serine with overall cancer risk were found among all age subgroups, men, non-smokers, non-drinkers, and individuals with lower folic acid levels. Table 4 shows the association of serine with the overall cancer risk stratified by the median follow-up. Elevated concentrations of serum serine were associated with an increased risk of overall cancer that occurred before the median follow-up (per SD increment, OR = 1.16; 95% CI: 1.02–1.33; P = 0.025) and after the median follow-up (Q4 vs. Q1; OR = 1.37; 95% CI: 1.01–1.95; P = 0.048), when serine was assessed as continuous and quartiles, respectively.| Serine (μg mL−1) | Cases/controls (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Before the median follow-up | Cases/controls (ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
After the median follow-up | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |||
| Models were adjusted for body mass index, smoking status, alcohol drinking, systolic blood pressure, triglycerides, cholesterol, uric acid, fasting blood glucose, high-density lipoprotein cholesterol, creatinine, albumin, alanine aminotransferase, homocysteine, sleep quality, antihypertensive drug usage, and family history of cancer. | ||||||
| Serine (per SD) | 699/699 | 1.16 (1.02, 1.33) | 0.025 | 692/692 | 1.10 (0.98, 1.26) | 0.110 |
| Quartiles of serine | ||||||
| Q1 (<13.27) | 156/186 | Ref. | 166/188 | Ref. | ||
| Q2 (13.27–15.27) | 183/165 | 1.22 (0.86, 1.72) | 0.266 | 171/175 | 1.15 (0.81, 1.64) | 0.436 |
| Q3 (15.28–17.67) | 172/181 | 1.16 (0.81, 1.65) | 0.429 | 177/167 | 1.17 (0.82, 1.67) | 0.374 |
| Q4 (≥17.68) | 188/167 | 1.35 (0.92, 1.97) | 0.125 | 178/162 | 1.37 (1.01, 1.95) | 0.048 |
Discussion
In this case–control study, nested in a prospective cohort population of the CHHRS, we found that hypertensive adults with elevated serum serine concentrations were associated with an increased risk of overall, non-digestive system and lung cancers. The results remained stable even after stratifying participants by age, sex, smoking status, drinking status, levels of folic acid, and follow-up time.This is the first study to reveal an association between serum serine concentrations and the incident risk of cancer in a population with hypertension. Cadoni et al. reported that a high level of serum serine was associated with decreased overall survival of head and neck cancer (HNC) patients (hazard ratio, HR = 2.71, 95% CI: 1.39–5.31),18 but was not associated with an increased risk of advanced stage HNC (OR: 1.76, 95% CI: 0.95–3.26). However, the authors did not report on the distribution of serum serine in participants with or without cancer, nor on the association of serum serine with incident cancer risk. Two studies revealed that serum serine is a potential biomarker of colorectal cancer (CRC) (area under curve, AUC = 0.81) and NSCLC (AUC = 0.993), differentiating cancer patients from healthy controls.23,24 However, both studies only reported that serum serine levels were decreased in cancer patients, and did not explore the association of serum serine with incident cancer risk. Conversely, a recent study reported that serum serine concentrations were higher in CRC and colorectal polyp patients than in healthy controls,25 and indicated that abnormal changes in serine metabolism may be an important reason for CRC progression. Notably, a previous study19 reported that some of the free amino acid alterations occurred in the serum of NSCLC patients in the early stage of the disease, and 6 amino acids (including serine) could be used as a blood multi-marker panel for NSCLC detection. However, the results were based on the serum amino acid levels in patients with stage I lung cancer, which only represents the detectability of serum serine combined with other amino acids at the early stage of lung cancer, but cannot predict the risk of incident cancer. Our study illustrated that the level of baseline serum serine was higher for cancer cases than for those without cancer. This signifies that an inchoate serum serine fluctuation indicates a higher risk of cancer. Overall, our study revealed the importance of detecting emergent serum serine fluctuations for predicting cancer risk. Additionally, we found more pronounced positive associations between high serum serine levels and cancer risk in participants who were male, non-smokers, non-drinkers, or had lower folate levels, but the interaction tests for these factors and serum serine were not significant.
The biological mechanism by which serine affects cancer risk is not clear. Serine, a pivotal component of one-carbon metabolism,5 plays an essential role in carbon unit donation and DNA methylation.26 According to genetic and functional evidence, oncogenesis is driven by hyperactivation of the serine biosynthetic pathway. De novo serine biosynthesis was first observed in tumors in 1955.27 Snell et al. demonstrated that serine biosynthesis increases during tumor progression.28 The mechanisms of increased serine synthesis include the overexpression of phosphoglycerate dehydrogenase (PHGDH) in cancer cells,29,30 which is a key enzyme of the serine synthesis pathway. Additionally, peripheral axons secrete amino acids, such as serine, to support the growth and proliferation of pancreatic ductal adenocarcinoma.31 Given the detrimental consequences of increased serine on tumor progression, several studies have attempted to suppress tumor growth by depleting serine or inhibiting the serine synthesis pathway.32,33 As expected, tumor progression can be restrained by these methods. Whether the beginning of abnormal fluctuation in serum serine concentrations occurs before cancer diagnosis remains unclear. Our study revealed that serum serine dysregulation may signify the emergence of inconspicuous precancerous lesions, which is important for cancer screening and prevention.
A novel aspect of our study is that it examines the association of serum serine levels, a component of one-carbon metabolism, with the risk of cancer in hypertensive adults. Hypertensive patients should be monitored for inchoate serum serine concentrations. Furthermore, it has the advantage of being a nested, case–control study that was derived from a large, prospective cohort study, thus avoiding recall bias. In addition, to eliminate the possibility of a causal association between participants’ serum serine concentrations and their cancer diagnosis, baseline blood samples from participants were obtained before any cancer diagnosis could be made.
Several limitations also exist in this study. First, only data of baseline serum serine levels were collected from study participants. It would have been informative to measure serum serine levels regularly to investigate the dynamic relationship between serum serine levels and cancer risk. Second, the small sample size of incident cancer cases and short follow-up time limited any further analysis of different subtypes of cancer; a larger population with longer follow-up is required to validate the results. Third, the findings of this study cannot be extrapolated to individuals without hypertension because it was conducted in hypertensive adults. However, it is important to note that the results of the study were not substantially altered after adjusting for blood pressure measurements at the baseline. It is important to emphasize that this study was a preliminary exploration of the association between one-carbon metabolism components and incident cancer risk. These findings warrant the need for future subsequent studies, including large-scale cohort studies and randomized trials.
Conclusions
This study provides the first prospective evaluation of the independent effect of serum serine concentrations on cancer incidence in a hypertensive adult population. There was a significant increase in the risk of cancer in patients with high serum serine levels. The findings may have clinical and scientific significance in cancer prevention. To confirm these findings in other populations, further studies are needed.Author contributions
Tong Liu: methodology, software, and writing – original draft. Chenan Liu: writing – original draft. Mengmeng Song: methodology and software. Yaping Wei: supervision and validation. Yun Song: investigation and visualization. Ping Chen: supervision. Lishun Liu: supervision and validation. Binyan Wang: validation and supervision. Hanping Shi: conceptualization, supervision, validation, resources and project administration.Data availability
Data will be made available upon reasonable request.Ethics approval and consent to participate
The CHHRS and the present study were approved by the ethics committee of the Institute of Biomedicine, Anhui Medical University in Hefei, China, and adhered to the Declaration of Helsinki. Each participant gave written, informed consent before participating in the study.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank all the staff and participants of the CHHRS for their important contributions. This work was financially supported by grants from the National Key Research and Development Program (2022YFC2009600) and the Beijing Municipal Science and Technology Commission (SCW2018-06) to Prof. Hanping Shi, and grants from the Science, Technology and Innovation Committee of Shenzhen (JSGG20180703155802047) to Prof. Binyan Wang.References
- H. Sung, J. Ferlay, R. L. Siegel, M. Laversanne, I. Soerjomataram and A. Jemal, et al., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA-Cancer J. Clin., 2021, 71(3), 209–249 CrossRef PubMed.
- D. M. Parkin, F. Bray, J. Ferlay and P. Pisani, Global cancer statistics, 2002, CA Cancer J. Clin., 2005, 55(2), 74–108 CrossRef PubMed.
- W. Chen, R. Zheng, P. D. Baade, S. Zhang, H. Zeng and F. Bray, et al., Cancer statistics in China, 2015, CA Cancer J. Clin., 2016, 66(2), 115–132 CrossRef PubMed.
- B. Faubert, A. Solmonson and R. J. DeBerardinis, Metabolic reprogramming and cancer progression, Science, 2020, 368(6487), eaaw5473 CrossRef CAS PubMed.
- I. Amelio, F. Cutruzzolá, A. Antonov, M. Agostini and G. Melino, Serine and glycine metabolism in cancer, Trends Biochem. Sci., 2014, 39(4), 191–198 CrossRef CAS PubMed.
- S. J. Parker and C. M. Metallo, Chasing One-Carbon Units to Understand the Role of Serine in Epigenetics, Mol. Cell, 2016, 61(2), 185–186 CrossRef CAS PubMed.
- A. A. F. Carioca, J. Steluti, A. M. Carvalho, A. M. Silva, I. Silva and R. M. Fisberg, et al., Plasma metabolomics are associated with metabolic syndrome: A targeted approach, Nutrition, 2021, 83, 111082 CrossRef CAS PubMed.
- A. Brouwer, J. J. Luykx, L. van Boxmeer, S. C. Bakker and R. S. Kahn, NMDA-receptor coagonists in serum, plasma, and cerebrospinal fluid of schizophrenia patients: a meta-analysis of case-control studies, Neurosci. Biobehav. Rev., 2013, 37(8), 1587–1596 CrossRef CAS PubMed.
- M. Maes, G. De Backer, E. Suy and B. Minner, Increased plasma serine concentrations in depression, Neuropsychobiology, 1995, 31(1), 10–15 CrossRef CAS PubMed.
- M. Orendác, J. Zeman, S. P. Stabler, R. H. Allen, J. P. Kraus and O. Bodamer, et al., Homocystinuria due to cystathionine beta-synthase deficiency: novel biochemical findings and treatment efficacy, J. Inherited Metab. Dis., 2003, 26(8), 761–773 CrossRef PubMed.
- W. C. Sim, W. Lee, H. Sim, K. Y. Lee, S. H. Jung and Y. J. Choi, et al., Downregulation of PHGDH expression and hepatic serine level contribute to the development of fatty liver disease, Metabolism, 2020, 102, 154000 CrossRef CAS PubMed.
- A. V. Mathew, M. Jaiswal, L. Ang, G. Michailidis, S. Pennathur and R. Pop-Busui, Impaired Amino Acid and TCA Metabolism and Cardiovascular Autonomic Neuropathy Progression in Type 1 Diabetes, Diabetes, 2019, 68(10), 2035–2044 CrossRef CAS PubMed.
- M. A. Øvrehus, P. Bruheim, W. Ju, L. R. Zelnick, K. A. Langlo and K. Sharma, et al., Gene Expression Studies and Targeted Metabolomics Reveal Disturbed Serine, Methionine, and Tyrosine Metabolism in Early Hypertensive Nephrosclerosis, Kidney Int. Rep., 2019, 4(2), 321–333 CrossRef PubMed.
- A. Vignoli, B. Orlandini, L. Tenori, M. R. Biagini, S. Milani and D. Renzi, et al., Metabolic Signature of Primary Biliary Cholangitis and Its Comparison with Celiac Disease, J. Proteome Res., 2019, 18(3), 1228–1236 CrossRef CAS PubMed.
- C. Frezza, Cancer metabolism: Addicted to serine, Nat. Chem. Biol., 2016, 12(6), 389–390 CrossRef CAS PubMed.
- K. R. Mattaini, M. R. Sullivan and M. G. Vander Heiden, The importance of serine metabolism in cancer, J. Cell Biol., 2016, 214(3), 249–257 CrossRef CAS PubMed.
- C. S. Amara, C. R. Ambati, V. Vantaku, D. W. Badrajee Piyarathna, S. R. Donepudi and S. S. Ravi, et al., Serum Metabolic Profiling Identified a Distinct Metabolic Signature in Bladder Cancer Smokers: A Key Metabolic Enzyme Associated with Patient Survival, Cancer Epidemiol. Biomarkers Prev., 2019, 28(4), 770–781 CrossRef CAS PubMed.
- G. Cadoni, L. Giraldi, C. Chiarla, J. Gervasoni, S. Persichilli and A. Primiano, et al., Prognostic Role of Serum Amino Acids in Head and Neck Cancer, Dis. Markers, 2020, 2020, 2291759 Search PubMed.
- A. Klupczynska, P. Dereziński, W. Dyszkiewicz, K. Pawlak, M. Kasprzyk and Z. J. Kokot, Evaluation of serum amino acid profiles’ utility in non-small cell lung cancer detection in Polish population, Lung Cancer, 2016, 100, 71–76 CrossRef PubMed.
- T. Liu, C. Liu, X. Wang, Y. Wei, S. Li and Y. Song, et al., The Association of Serum L-Carnitine Concentrations with the Risk of Cancer in Chinese Adults with Hypertension, Nutrients, 2022, 14(23), 4999 CrossRef CAS PubMed.
- L. A. Torre, R. L. Siegel, E. M. Ward and A. Jemal, Global Cancer Incidence and Mortality Rates and Trends–An Update, Cancer Epidemiol. Biomarkers Prev., 2016, 25(1), 16–27 CrossRef PubMed.
- K. S. Crider, T. P. Yang, R. J. Berry and L. B. Bailey, Folate and DNA methylation: a review of molecular mechanisms and the evidence for folate's role, Adv. Nutr., 2012, 3(1), 21–38 CrossRef CAS PubMed.
- X. Bian, Y. Qian, B. Tan, K. Li, X. Hong and C. C. Wong, et al., In-depth mapping carboxylic acid metabolome reveals the potential biomarkers in colorectal cancer through characteristic fragment ions and metabolic flux, Anal. Chim. Acta, 2020, 1128, 62–71 CrossRef CAS PubMed.
- Y. Mu, Y. Zhou, Y. Wang, W. Li, L. Zhou and X. Lu, et al., Serum Metabolomics Study of Nonsmoking Female Patients with Non-Small Cell Lung Cancer Using Gas Chromatography-Mass Spectrometry, J. Proteome Res., 2019, 18(5), 2175–2184 CrossRef CAS PubMed.
- J. Gu, Y. Xiao, D. Shu, X. Liang, X. Hu and Y. Xie, et al., Metabolomics Analysis in Serum from Patients with Colorectal Polyp and Colorectal Cancer by (1)H-NMR Spectrometry, Dis. Markers, 2019, 2019, 3491852 Search PubMed.
- J. W. Locasale, Serine, glycine and one-carbon units: cancer metabolism in full circle, Nat. Rev. Cancer, 2013, 13(8), 572–583 CrossRef CAS PubMed.
- S. Kit, The biosynthesis of free glycine and serine by tumors, Cancer Res., 1955, 15(11), 715–718 CAS.
- K. Snell, Y. Natsumeda and G. Weber, The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture, Biochem. J., 1987, 245(2), 609–612 CrossRef CAS PubMed.
- M. A. Reid, A. E. Allen, S. Liu, M. V. Liberti, P. Liu and X. Liu, et al., Serine synthesis through PHGDH coordinates nucleotide levels by maintaining central carbon metabolism, Nat. Commun., 2018, 9(1), 5442 CrossRef CAS PubMed.
- M. R. Sullivan, K. R. Mattaini, E. A. Dennstedt, A. A. Nguyen, S. Sivanand and M. F. Reilly, et al., Increased Serine Synthesis Provides an Advantage for Tumors Arising in Tissues Where Serine Levels Are Limiting, Cell Metab., 2019, 29(6), 1410–1421 CrossRef CAS PubMed.
- R. S. Banh, D. E. Biancur, K. Yamamoto, A. S. W. Sohn, B. Walters and M. Kuljanin, et al., Neurons Release Serine to Support mRNA Translation in Pancreatic Cancer, Cell, 2020, 183(5), 1202–1218 CrossRef CAS PubMed.
- M. Tajan, M. Hennequart, E. C. Cheung, F. Zani, A. K. Hock and N. Legrave, et al., Serine synthesis pathway inhibition cooperates with dietary serine and glycine limitation for cancer therapy, Nat. Commun., 2021, 12(1), 366 CrossRef CAS PubMed.
- R. J. DeBerardinis, Serine metabolism: some tumors take the road less traveled, Cell Metab., 2011, 14(3), 285–286 CrossRef CAS PubMed.
Footnote |
| † These authors contributed equally to this work and share the first author. |
| This journal is © The Royal Society of Chemistry 2023 |