Open Access Article
Open Access ArticleIncidental nanoparticles in black tea alleviate DSS-induced ulcerative colitis in BALB/c mice†
Huan
Han
 abc,
Lijing
Ke
abc,
Lijing
Ke
 *cd,
Wei
Xu
bc,
Huiqin
Wang
*cd,
Wei
Xu
bc,
Huiqin
Wang
 c,
Jianwu
Zhou
c and
Pingfan
Rao
c
c,
Jianwu
Zhou
c and
Pingfan
Rao
c
aSchool of Chemical Engineering and Technology, Tianjin University, China
bZhe Jiang Institute of Tianjin University, Shaoxing, China
cFood Nutrition Science Centre, School of Food Science and Biotechnology, Zhejiang Gongshang University, Hangzhou, 310012, China
dSchool of Food Science and Nutrition, University of Leeds, Leeds, LS2 9JT, UK. E-mail: L.Ke@leeds.ac.uk; Tel: +44 113 343 9559
First published on 10th August 2023
Abstract
As the dominant herbal drink consumed worldwide, black tea exhibits various health promoting benefits including amelioration of inflammatory bowel diseases. Despite extensive studies on the tea's components, little is known about the bioactivities of nanoparticles (NPs) which were incidentally assembled in the tea infusion and represent the major components. This study investigated the alleviative effects of black tea infusion, the isolated black tea NPs, and a mixture of caffeine, epigallocatechin-3-gallate, gallic acid and epicatechin gallate on dextran sodium sulfate (DSS)-induced ulcerative colitis. The results showed that both the black tea infusion and the NPs significantly alleviated colitis, suppressed the mRNA levels of pro-inflammatory cytokines TNF-α, IL-6, and IL-1β, and suppressed the DSS-induced loss of cell–cell junction proteins (e.g., E-cadherin, ZO-1, and claudin-1) and increase of p-STAT3. The mixture of four tea components, which is the analogue of bioactive payloads carried by the NPs, was much less effective than the tea infusion and NPs. It shows that the NPs elevate the efficiency of polyphenols and caffeine in black tea in restoring the intercellular connection in the intestine, inhibiting mucosal inflammation, and alleviating ulcerative colitis. This work may inspire the development of tea-based therapeutics for treating inflammatory bowel diseases and have wide influences on value-added processing, quality evaluation, functionalization, and innovation of tea and other plant-based beverages.
1. Introduction
Ulcerative colitis (UC) and Crohn's disease (CD) are common inflammatory bowel diseases (IBD).1,2 In the past 20 years, the incidence and prevalence of IBD have risen sharply in many developed and developing countries including China. Cellular oxidative stresses caused by pathogens and chemical or dietary factors have been found to promote epithelial cell apoptosis and mucosal inflammation in the digestive tract,3,4 thereafter causing various diseases. To date, the drugs used in the treatment of ulcerative colitis, i.e. aminosalicylic acid, glucocorticoids and immunosuppressive agents,5 often come with alarming side effects including steroid dependence,6 lymphoproliferative disorders and lymphoma.7,8 Using antioxidant food, e.g., tea, as a dietary intervention or adjuvant treatment for colitis has been widely explored to overcome the side effects of medicines, in the hope of finding a safer and more accessible solution for the prevention and mitigation of IBD.9Antioxidant food components like tea polyphenols, when used in effective dosages, alleviate ulcerative colitis in animals.10,11 Bitzer et al. demonstrated that oral administration of epigallocatechin-3-gallate (EGCG) at 3.2 mg g−1 for 3 days exerted anti-inflammatory effects on dextran sulfate sodium (DSS)-induced ulcerative colitis in mice.12 Kumar demonstrated that tea GA reduced the expression of inflammatory factors and significantly attenuated the disease activity index in DSS-induced colitis.13 Black tea and its major polyphenol derivates, e.g. thearubigins14 and theaflavins,15 ameliorated mucosal injury in trinitrobenzene sulfonic acid-induced colitis and down-regulated NF-κB activation. Besides the tea polyphenols, other tea components like polysaccharides may work synergically and even more effectively with phenolic compounds to mitigate intestinal inflammation and increase the relative abundance of probiotics in the gut.16 When orally administered at a rather high dosage (200 mg per kg body weight per day for 7 days), the purified polysaccharides from a dark tea (brick tea) attenuated colitis in mice, suppressed pro-inflammatory cytokines, increased the expression of cell junction proteins, and restored the homeostasis of the gut microbiota.17 The prebiotic effects of tea components include increasing the diversity and optimizing the structure of the gut microbiota, and enriching short-chain fatty acids.18,19
The rich content of amphiphilic molecules in tea infusion facilitates the self-assembly of colloidal particles. Evidence is emerging linking the bioactivities of green tea20,21 to these self-assembled particles or engineered nanoparticles of green tea and drugs.22 Previously, we have demonstrated that hybrid nanoparticles of tea polysaccharides (pectin) and tea protein (polyphenol oxidase) were isolated from Chinese black tea infusion. These colloidal nanoparticles carry polyphenols and caffeine, show potent intracellular antioxidant activities on intestinal epithelial cells and macrophages, and protect mitochondrial function against oxidative stress.23 The in vitro cell-protective activities of black tea nanoparticles, together with the co-existence of polyphenols and polysaccharides in the particles, show good potential in regulating intestinal barrier function and mucosal immunity in vivo. We have shown that the ingestion of black tea infusion immediately increased the salivary thiol (SH) and malondialdehyde (MDA) contents while reducing salivary uric acid (UA),24 demonstrating the direct influences of tea infusion on oral mucosal cells. The black tea NPs aggregate under the acidic pH conditions of the stomach to form agglomerates and become more resistance to gastric digestion.25 These agglomerates disassociate under the weakly acidic and neutral pH conditions of the small intestine to facilitate the absorption and release of their components. In addition, the tannic acid-loaded zein/pectin nanoparticles are resistant to gastric digestion and release tannic acid mainly in the small intestine.26 Therefore, black tea nanoparticles would stand a fair chance to survive gastric digestion after oral ingestion, as they are composed of protein, pectin, and polyphenols.23
Here we set off to isolate the incidental nanoparticles from black tea infusion and evaluate the efficacy of black tea infusion and tea NPs for mitigating intestinal inflammation using a DSS-induced colitis mouse model. Meanwhile, the effects of the NPs were compared with those of a mixture of caffeine, EGCG, GA and ECG, at equivalent concentrations, in the hope of distinguishing the contribution of the nanostructure from that of its ‘payload’. The role of black tea nanoparticles in alleviating intestinal inflammation and fortifying cell–cell junctions was explored.
2. Materials and methods
2.1 Reagents
Dried black tea leaves were purchased from Zhengshan Tang Co., Ltd (Fujian, China). The tea infusion was brewed using deionized water from a Millipore Milli-Q water purification system (Millipore, Bedford, MA, USA). DSS (mol wt, 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–50
000–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from MP Biomedicals (Aurora, OH). SASP (0.25 g per pill) was purchased from Xinyi Pharmaceutical Group (Shanghai, China). Gallic acid (GA, ≥99%, CAS: 149-91-7), epigallocatechin gallate (EGCG, ≥95%, CAS: 989-51-5), and epicatechin gallate (ECG, ≥98%, CAS: 1257-08-5) were purchased from Sigma-Aldrich Trading Co., Ltd (Shanghai, PR China), Caffeine (CAF) was obtained from the Tea Research Institute, Chinese Academy of Agricultural Sciences (Hangzhou, PR China).
000) was purchased from MP Biomedicals (Aurora, OH). SASP (0.25 g per pill) was purchased from Xinyi Pharmaceutical Group (Shanghai, China). Gallic acid (GA, ≥99%, CAS: 149-91-7), epigallocatechin gallate (EGCG, ≥95%, CAS: 989-51-5), and epicatechin gallate (ECG, ≥98%, CAS: 1257-08-5) were purchased from Sigma-Aldrich Trading Co., Ltd (Shanghai, PR China), Caffeine (CAF) was obtained from the Tea Research Institute, Chinese Academy of Agricultural Sciences (Hangzhou, PR China).
2.2 Isolation and purification of NPs from black tea
To prepare the black tea infusion, the black tea leaves were extracted with distilled water at a leaf/water ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 (w/v) at 98 ± 2 °C for 10 min using a water bath. The extract was filtered through a 0.45 μm acetate cellulose membrane and the black tea obtained was quickly cooled down to 25 °C.
20 (w/v) at 98 ± 2 °C for 10 min using a water bath. The extract was filtered through a 0.45 μm acetate cellulose membrane and the black tea obtained was quickly cooled down to 25 °C.
The tea NPs were separated with a chromatography column (Sepharose CL-2B, Bio-Rad) attached to a HPLC system (NGC, Bio-Rad). The column was eluted with distilled water at a flow rate of 0.5 mL min−1. The elutes were continuously monitored with a UV detector at 280 nm and a Malvern Nano-ZS (Worcestershire, UK) to determine the light scattering intensity and particle size every 3 seconds. The eluate was collected in 2 mL tubes. Fractions with strong light scattering were collected (retention time 23–32 min) and marked as P1 NPs. The separated components and the black tea infusion were then lyophilized.
The particle size and number of the tea NPs were determined using a NanoSight system (Malvern Instruments, Worcestershire, UK).
2.3 Animal care and experimental design
Female BALB/c mice aged 5 weeks were purchased from Zhejiang Academy of Medical Sciences (Hangzhou, China). After one week of acclimation, the mice were randomly distributed into control and experimental groups. All animals were housed under a controlled atmosphere (22 ± 1 °C at 50% relative humidity) with a 12 h light/12 h dark cycle. Animals had free access to the standard diet and distilled water at all times.The animals were randomly divided into several groups (n = 6 for each group):
(1) Control group: received deionized water for 7 days.
(2) DSS group: 5% DSS was given as the sole source of drinking fluid for 7 days.
(3) Sulfasalazine (SASP) group: 5% DSS was given as the sole source of drinking fluid and SASP (250 mg kg−1) was administered by oral gavage for 7 days.
(4) TEA group: 5% DSS was given as the sole source of drinking fluid and tea infusion (25 mg kg−1) was administered by oral gavage for 7 days.
(5) P1 group: 5% DSS was given as the sole source of drinking fluid and tea NPs (5 mg kg−1) were administered by oral gavage for 7 days.
(6) P1-Simulant group: 5% DSS was given as the sole source of drinking fluid and the P1-Simulant (7.5 μg kg−1 GA, 24 μg kg−1 EGCG, 13 μg kg−1 CAF, 3.5 μg kg−1 ECG) was administered by oral gavage for 7 days.
P1-Simulant: the equivalent concentration of GA, EGCG, CAF, and ECG to P1.
At the end of the experiment, the spleens were removed, rinsed with 0.9% NaCl, and weighed. The colons were obtained, rinsed with ice-cold 0.9% NaCl, and placed on filter paper, and the length was determined using a ruler. The colon was then cut into 4 large sections. After being packed, they were snap frozen at −80 °C. The above process was inspected and approved by the Animal Care & Welfare Committee of Zhejiang Academy of Medical Sciences, China (no. 2019R02004).
2.4 Disease activity index (DAI)
The severity of colitis was assessed by DAI scoring as previously described by Kim.27 DAI is a composite of body weight loss, stool consistency, and fecal breeding scoring. DAI scores were recorded every day from the onset of DSS administration.2.5 Histological colitis score
Colonic tissues were fixed in 4% paraformaldehyde solution and paraffin-embedded after which thin sections (5–8 μm thick) were stained with hematoxylin–eosin according to standard protocols.28 The samples were viewed under a light microscope (200×). Each quarter of the colon was graded using a scale ranging from 0 to 4 by three blinded investigators to examine the extent of inflammation, the depth of inflammation and the amount of crypt damage or regeneration (0: intact recess without inflammation; 1: 1/3 recess damage; 2: 2/3 recess damage and mild inflammation; 3: large recess damage, intact mucosa, and moderate inflammation; and 4: mucosal epithelium loss, erosion, severe inflammation). The average of the four quarters was representative of the whole colon.292.6 Analysis of inflammation factors
Quantitative real-time PCR was performed with whole frozen colon tissues containing the proximal, middle, and rectum sections for each mouse (n = 6 per group). Firstly, total RNA was isolated using the miRNeasy Mini Kit (QIAGEN, 217![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 004).30 Secondly, reverse transcription was performed with the Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, K1622, Waltham, MA, USA). Quantitative real-time PCR (qPCR) was performed in a Rotor-Gene Q PCR cycler with SYBR Select Master Mix (both from Qiagen, Frederick, MD, USA). Finally, dissociation (melting) curve analysis was performed to check for nonspecific amplification and/or primer-dimer formation. The real-time RT-PCR data were obtained and analyzed with the 7300 Real-Time PCR System Sequence Detection Software (v1.4.1, Applied Biosystems) according to default parameters, which generated the cycle threshold (CT) values for each reaction. The PCR amplification efficiency (E) of each primer pair was calculated from the linear regression and the slope of the corresponding standard curves, according to the formula: E (%) = [10 (−1/slope) − 1] × 100. The efficiency of each reference gene was considered in all subsequent statistical analysis.
004).30 Secondly, reverse transcription was performed with the Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, K1622, Waltham, MA, USA). Quantitative real-time PCR (qPCR) was performed in a Rotor-Gene Q PCR cycler with SYBR Select Master Mix (both from Qiagen, Frederick, MD, USA). Finally, dissociation (melting) curve analysis was performed to check for nonspecific amplification and/or primer-dimer formation. The real-time RT-PCR data were obtained and analyzed with the 7300 Real-Time PCR System Sequence Detection Software (v1.4.1, Applied Biosystems) according to default parameters, which generated the cycle threshold (CT) values for each reaction. The PCR amplification efficiency (E) of each primer pair was calculated from the linear regression and the slope of the corresponding standard curves, according to the formula: E (%) = [10 (−1/slope) − 1] × 100. The efficiency of each reference gene was considered in all subsequent statistical analysis.
To achieve this, the raw CT values were transformed into relative quantity using the formula 2(−ΔCT), in which ΔCT corresponds to the highest CT value minus all other CT values for each reference gene measured across all samples.
2.7 Western blot
Proteins were separated on 8% sodium dodecyl sulfate-polyacrylamide running gels and then transferred onto a polyvinylidene fluoride (Φ = 0.45 μm) transfer membrane using the wet transfer method.31 Membranes were blocked with 5% fat free milk in Tris-buffered saline plus 0.1% Tween-20 (TBST) for 1 hour at room temperature. E-cadherin, p-STAT3, Stat3, ZO-1 and claudin-1 primary antibodies were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, 1
1000, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, 1
1000, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, 1
1000, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500, and 1
500, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 in TBST containing 2% milk, and the membranes were incubated with primary antibodies overnight at 4 °C, respectively. The following day, the membranes were washed 4 times (5 min each time) using TBST. Secondary antibodies were diluted 1
500 in TBST containing 2% milk, and the membranes were incubated with primary antibodies overnight at 4 °C, respectively. The following day, the membranes were washed 4 times (5 min each time) using TBST. Secondary antibodies were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000 in TBST containing 2% milk. After 5 minute of washing with TBST, the Pierce ECL Western Blotting Substrate was added onto the membrane and incubated for 5 min to develop the chemiluminescent signal. The SuperSignal® West Dura Extended Duration Substrate was used to capture the intensity of the chemiluminescent signal. The X-ray film was put on the cassette for 5 to 10 min for development and fixing. The intensity of the protein bands was quantified using the ImageJ software.
5000 in TBST containing 2% milk. After 5 minute of washing with TBST, the Pierce ECL Western Blotting Substrate was added onto the membrane and incubated for 5 min to develop the chemiluminescent signal. The SuperSignal® West Dura Extended Duration Substrate was used to capture the intensity of the chemiluminescent signal. The X-ray film was put on the cassette for 5 to 10 min for development and fixing. The intensity of the protein bands was quantified using the ImageJ software.
2.8 Statistical analysis
All the experimental results are expressed as mean ± SEM with at least triplicates and n = 6 for each group. Statistical significance was evaluated using a two-tailed probability test. #: p < 0.05 vs. Control, ##: p < 0.01 vs. Control; *: p < 0.05 vs. DSS, **: p < 0.01 vs. DSS.3. Results
3.1 Black Tea NP separation and characterization
The preparation of tea NPs in black tea infusion has been described previously. The black tea NPs were isolated by size-exclusion chromatography coupled with a dynamic light scattering detector (SEC-DLS), as shown in the ESI.† The average hydrated particle size, ζ-potential, PDI and count rates were 187 ± 7 nm, −32.4 ± 2.5 mV, 0.2 ± 0.01 and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 572 kcps, respectively (Table S1a†). The NP number was counted as 4.0 × 1010 particles per mg. The morphology of black tea NPs was observed by transmission electron microscope (TEM), which showed that they were uniformly spherical (Fig. S1D†). Moreover, as reported earlier,23 the protein, polysaccharide and polyphenol contents of black tea NPs were 86.5%, 8.7% and 4.8%, respectively. It also contains 4.8 μg mg−1 of EGCG, 2.6 μg mg−1 of CAF, 1.5 μg mg−1 of GA and 0.7 μg mg−1 of ECG. SDS-PAGE analysis of the constituent proteins revealed that tea NPs included a band of 66 kDa (Fig. S1B†), which was consistent with the previous report band of polyphenol oxidase.32
800 ± 572 kcps, respectively (Table S1a†). The NP number was counted as 4.0 × 1010 particles per mg. The morphology of black tea NPs was observed by transmission electron microscope (TEM), which showed that they were uniformly spherical (Fig. S1D†). Moreover, as reported earlier,23 the protein, polysaccharide and polyphenol contents of black tea NPs were 86.5%, 8.7% and 4.8%, respectively. It also contains 4.8 μg mg−1 of EGCG, 2.6 μg mg−1 of CAF, 1.5 μg mg−1 of GA and 0.7 μg mg−1 of ECG. SDS-PAGE analysis of the constituent proteins revealed that tea NPs included a band of 66 kDa (Fig. S1B†), which was consistent with the previous report band of polyphenol oxidase.32
3.2 Black tea NPs alleviated the symptoms of DSS induced colitis in mice
Body weight could reflect the health condition intuitively and it is an important index to judge the development of UC. The body weight decrease during the DSS treatment is shown in Fig. 1A. The body weight of healthy mice (black) increased while the DSS-treated mice (grey) suffered continuous weight loss. The positive treatment control group ‘SASP group’ (light blue) did not show significant difference compared with the DSS group. The tea infusion (TEA group) and tea NPs (P1 group) mildly ameliorated the DSS-induced body weight loss. | ||
| Fig. 1 The change in bodyweight (A) and DAI scores (B) during the 7-day DSS treatment. Note: data are expressed as mean ± SEM, n = 6. | ||
DAI is a composite of body weight loss, stool consistency, and fecal breeding scoring. The DAI score increased rapidly since the 5th day of DSS administration, as shown in Fig. 1B. The tea infusion and tea NPs ameliorated the DAI score while SASP failed to do so.
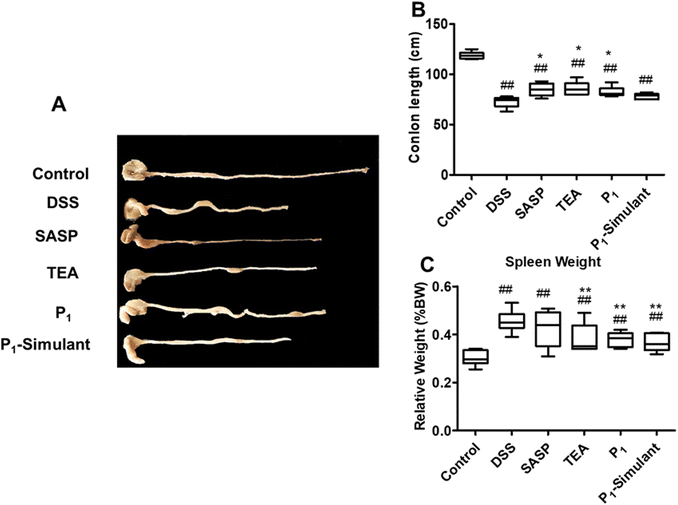
As shown in Fig. 2A, the colon tissue of healthy mice showed normal color, thickness, no signs of edema or congestion, and non-adhesion to the surrounding tissues. The DSS-treated mice showed obvious UC symptoms such as congestion, edema and colon length shortening (p < 0.01, Fig. 2B), as well as ulceration and local necrosis. The SASP-treated mice had a significantly longer colon than the UC mice (p < 0.05), but still showed redness, swelling and hyperemia in colon tissues. The black tea infusion and its P1 NPs significantly alleviated colon shortening (p < 0.05) and redness, while the P1-Simulant did not stop the shortening of the colon.
The relative spleen weight of DSS-treated mice increased significantly (p < 0.01, as shown in Fig. 2C). The tea infusion, P1 NPs and P1-Simulant significantly counteracted the enlargement of the spleen, indicating the suppression of the peripheral inflammation of the colon.
Tea infusion and P1 NPs significantly mitigated the above manifestations of DSS-induced mice; their therapeutic effects were comparable to, if not better than, that of the positive treatment control, SASP. The P1-Simulant showed no significant improvement except the amelioration of spleen weight.
3.3 Black tea NPs alleviated histological changes and pathological scores
The pathological scoring standard of the mouse colon is based on the damage of goblet cells, a type of intestinal epithelial cell, the loss of crypt glands, the infiltration of inflammatory cells, and the occurrence of edema in the mucosal layer. The pathological scores of the tissues are shown in Fig. 3A. Histological changes in the colons of mice are shown in Fig. 3B. The healthy mice showed normal epithelial mucosa and the lowest pathological score, while the mice in the DSS group scored the highest and showed inflammatory infiltration with crypt abscesses, neutrophils infiltrating the glandular epithelium and epithelial hyperplasia. The mice treated with tea infusion and P1 NPs showed significant amelioration of these symptoms (p < 0.05 in the pathological score), while SASP and P1-Simulant treatment showed only a slight but not significant improvement. Despite the dosage of sulfasalazine used here showing good agreement with previous literature,33,34 the outcome is rather disappointing.3.4 Black tea NPs suppressed mRNA levels of pro-inflammatory cytokines
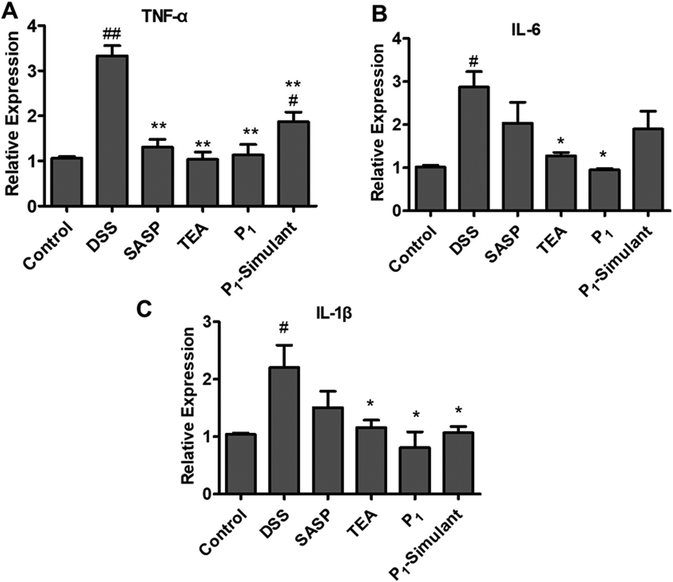
The mRNA levels of pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α, are shown in Fig. 4. These three cytokines are the most important inflammatory mediators that regulate the acute protein expression in inflammation. Their activation produces an inflammatory response, affects the integrity of the colonic mucosa and intensifies intestinal damage, as observed in ulcerative colitis. Substantial increases in the colonic mRNA levels of TNF-α, IL-6 and IL-1β were found in DSS-treated mice. Both tea infusion and P1 NPs significantly inhibited the DSS-induced mRNA levels of these cytokines, while the P1-Simulant only significantly inhibited the mRNA levels of TNF-α and IL-1β, not as strongly as the tea infusion and P1 NPs.3.5 Black tea NPs ameliorated the intercellular connection of intestinal cells
The cell-cell junction is an important structure in mediating cell-cell adhesion and multicellular signal transduction, which are essential for tissue homeostasis and therefore play an important role in intestinal barrier functions.35 Ulcerative colitis can compromise intestinal barrier functions as the pro-inflammatory cytokines often cause damage to the integrity of colonic mucosa. The damage can be indicated by the expression levels of cell junction proteins, e.g. claudin-1, ZO-1, E-cadherin and STAT-3. In this study, DSS significantly downregulated the colonic protein expression of E-cadherin, ZO-1, and claudin-1, and up-regulated the expression of p-STAT3 in the colon (Fig. 5). The intervention using either tea infusion or P1 NPs significantly alleviated the DSS-induced changes on the cell junction proteins. No such alleviation was observed in the animals treated with the P1-Simulant, which is a mixture of constitutive phenolic compounds of P1 NPs and caffeine.There are different types of cell–cell junctions (i.e. adhesion junctions, tight junctions, gap junctions and desmosomes), each with distinct functions and molecular characteristics.36 E-cadherins play a vital role in establishing adhesion junctions, while transmembrane proteins (claudins) and cytoplasmic proteins (ZO-1) comprise the multiple-protein complexes in tight junctions.37,38 The loss of ZO-1 and elevated permeability may occur prior to the development of significant intestinal inflammation.39 Meanwhile, pro-inflammatory cytokines, such as TNF-α and IL-6, can shift the localization of E-cadherins or decrease their expression.40 STAT3 is vital to intercellular signal transmission. Its phosphorylated form (p-STAT3) is an inflammatory indicator,41 expressed in response to IL-6. The IL23R subunit on Th17 cells is known to be induced by IL-6 and transcriptionally regulated, at least partially, by p-STAT3. As mentioned above, the black tea infusion and its NPs restored the protein complexes in the adhesion junction and tight junction, while blocking the intercellular inflammatory signal transmission via the pro-inflammatory cytokines IL-1β, IL-6, and TNF-α. Further studies on the active components of tea NPs and their cellular metabolites are warranted to better understand the mechanism of influence of tea NPs on the mucosal cell junctions and immune responses.
4. Discussion
Oxidative stress plays an important role in the pathology of UC and IBD.42 Antioxidant food components, i.e. tea polyphenols, gallic acid,13 EGCG, etc., have been found to restore endogenous antioxidant enzymes,42 produce anti-inflammatory effects, attenuate colon shortening and disease activity index, mitigate histopathological injury, and reduce IL-6, IL-21, IL-23 and TNF-α in DSS-induced colitis in BALB/c mice43 or C57BL/6 mice. However, as demonstrated previously, a rather high dose of green tea and black tea infusion was acquired to suppress inflammation and mitigate colitis in rats (100 mg kg−1 d−1)43 and in mice44 (0.2% and 1% black tea extract). Oral administration of high dose EGCG in rats (50 mg kg−1 d−1)45 or in mice (3.2 mg g−1 drinking fluid)12 decreases colonic inflammation and permeability in murine model of ulcerative colitis. In this study, the P1 NPs exhibited significantly higher antioxidant activities than the physical mixture of their constituent antioxidants, e.g., EGCG, GA, ECG, CAF, etc. This is the first report that black tea infusion and its incidental nanoparticles effectively alleviated the DSS-induced colitis in vivo at a much lower dosage, 25 mg kg−1 and 5 mg kg−1, respectively.TNF-α, IL-6, and IL-1β are well-known proinflammatory cytokines, which play an important role in various cellular events, including proliferation, differentiation, and death.46 An increase in proinflammatory cytokines can stimulate the production of oxygen free radicals, and vice versa. Oxidative stress can damage the intestinal wall and exacerbate the process of ulcerative colitis. As a major source of proinflammatory cytokines IL-6 and IL-1β, macrophages are an important part of the innate immune response and a key regulator of the homeostasis of the intestinal microenvironment. While our previous study demonstrated the antioxidant activities of black tea nanoparticles in vitro (on intestinal epithelial cells and peritoneal macrophages), this study has explicitly demonstrated their anti-inflammatory effects in vivo, at a higher efficiency than the individual bioactive compounds. The enhancement of the intestinal cell–cell junction protein expression and the suppression of pro-inflammatory cytokines by the black tea NPs show good agreement with their intracellular antioxidant activities in macrophages, epithelial cells, and subcellular organelles.23 Notably, macrophages can engulf tea nanoparticles or bone soup nanoparticles47,48 to effectively protect themselves from oxidative stress-induced cellular malfunction, indicating a possible important mode of food–body interaction occurring in the intestine.
Furthermore, a relative high dose of DSS (5%, w/v) was used in this study. All the samples and the positive-control drug (SASP) were administered simultaneously with DSS, mimicking the possible preventive effects of diet on ulcerative colitis. The therapeutic effects of SASP were marginal, which is possibly attributed to the high dose of DSS and simultaneous administration. SASP needs to be cleaved by colonic bacteria and subsequently release its active moiety.49 The simultaneous administration of SASP and DSS in this study had left little time for SASP to exert its effects.50 However, both tea infusion and its NPs showed satisfactory mitigation of colitis, suggesting the superior efficacy and safety.
Tea NPs are more effective than any other type of tea extracts, which can be well explained either by the enrichment of bioactive components on the water/solid interface of the protein/polysaccharide scaffold of tea NPs,23 or by the accumulation of the NPs in inflamed colon lesions as seen in the self-assembled tannic acid–berberine hybrid particles.51 The mucosal layer of the colon becomes more permeable to nanoparticles in the event of inflammation. In terms of the tea polyphenol enrichment, for example, the content of GA was 1.5 μg mL−1 in an aqueous dispersion of black tea NPs (1 mg mL−1). As the particles only took a small portion of the total volume of dispersion, the average concentration of GA in the NPs would be much higher than in the whole dispersion. The particle number was 4 × 1010 per milliliter, as determined by nanoparticle tracking analysis (NTA). By considering a known mean diameter of 187 nm, the total volume of tea NPs was calculated as 3.5 × 10−15 cm3. Subsequently, the calculated concentration of GA in the particles would be 1.1 × 104 μg mL−1, more than 7000 times its overall concentration in the dispersion. In addition, the protein–polysaccharide scaffold may have also provided resistance to oral and gastric digestion and facilitated the targeted delivery to the intestine. In other words, the NPs could serve as vehicles for the delivery of condensed active components of tea and therefore enhance their bioactivities.
Dysregulated immune responses to gut microbiome dysbiosis are attributed to the occurrence and progression of inflammatory bowel disease.52,53 The consumption of green tea, oolong tea, and black tea substantially increased diversity and altered the structure of gut microbiota.54 The green tea and dark tea extracts, administered daily at 5 mg per kg bodyweight for 4 weeks, exhibited prebiotic effects and produced fecal microbiota that significantly ameliorate symptoms in DSS-induced colitis in mice.18 Wu et al. demonstrated that the oral administration of EGCG (50 mg per kg body weight) attenuated colitis by enriching the gut bacteria Akkermansia and short-chain fatty acids.55 The extracts of aged Pu-erh tea (10 mg per kg body weight) inhibited the intestinal oxidative stress-mediated inflammation, elevated the expression of intestinal cell junction proteins and reshaped the gut microbiota.56 However, there are no reports on the biotransformation of theabrownin, the signature component of Pu-erh tea and black tea, by intestinal microorganisms. The black tea nanoparticles reported here, containing polyphenols, polysaccharides, and proteins, show potential to modulate the gut microbiota. However, the low content of polysaccharides (∼9%) and polyphenols (∼5%) in the tea NPs are dozens of times lower than their effective doses for modulating the gut microbiota, considering that the dose of P1 NPs was only 5 mg kg−1. Therefore, the microbiota-mediate immune modulation is unlikely to play a significant role in the therapeutic effects tea NPs we demonstrated above. Nonetheless, the possibility of black tea NPs influencing gut microorganisms and metabolites still cannot be ruled out without performing a comprehensive experiment.
5. Conclusion
Our results demonstrated that black tea infusion and its self-assembled nanoparticles alleviated DSS-induced ulcerative colitis in mice by suppressing the mRNA levels of pro-inflammatory cytokines (e.g., TNF-α, IL-1β and IL-6) and ameliorating the intercellular connection of intestinal mucosal cells, thus attenuating the severity of colitis. The nanoparticles exhibited almost equivalent anti-inflammatory effects to the full-body infusion of black tea. The activities of the NPs could not be attributed to their constituents EGCG, CAF, ECG and GA. The fortified particle–cell interaction and absorption may play a key role in elevating the therapeutic effects of their constitutive phenolic compounds.This study reinforces our knowledge on the health benefits and underlying mechanisms of tea by providing the first in vivo evidence that self-assembled colloidal particles from a Chinese black tea are the functional units. This new insight might have vast and deep influences on the value-added processing, quality evaluation, function study, and innovation of tea products and many other plant-based beverages, given that black tea is one of the most influential beverages worldwide and self-assembled nanoparticles have been identified in various herbal drinks and medicines. It warrants further studies to reveal how black tea NPs are absorbed, distributed, and transported in the gastrointestinal tract and how they affect the microenvironment and eventually exert therapeutic effects on multiple systems.
Abbreviations
| NPs | Nanoparticles |
| TEA | Tea infusion |
| CAF | Caffeine |
| ECG | Epicatechin gallate |
| EGCG | Epigallocatechin gallate |
| GA | Gallic acid |
| DAI | Disease activity index |
| DSS | Dextran sulfate sodium |
| IBD | Inflammatory bowel disease |
| SASP | Sulfasalazine |
| IL-1β | Interleukin-1-beta |
| IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-α |
| UC | Ulcerative colitis |
| CD | Crohn's disease |
| SEC-DLS | Size exclusion chromatography–dynamic light scattering. |
Author contributions
Huan Han and Dr Lijing Ke conceived the project and planned the study. Huan Han conducted experiments with help from Dr Huiqin Wang and Dr Jianwu Zhou. Material preparation and data collection were carried out with the help of Wei Xu. All authors participated in the data analysis and discussion. Huan Han, Dr Lijing Ke and Dr Pingfan Rao wrote and revised the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This study was supported by the National Key Research and Development Plan (2016YFD0400202) and the National Natural Science Foundation of China (Grant No. 31571803).References
- A. N. Ananthakrishnan, G. G. Kaplan, C. N. Bernstein, K. E. Burke, P. J. Lochhead, A. N. Sasson and M. Agrawal, Lifestyle, behaviour, and environmental modification for the management of patients with inflammatory bowel diseases: an International Organization for Study of Inflammatory Bowel Diseases consensus, Lancet Gastroenterol. Hepatol., 2022, 7, 666–678 CrossRef PubMed.
- S. M. Barbalho, H. Bosso, L. M. Salzedas-Pescinini and R. de Alvares Goulart, Green tea: A possibility in the therapeutic approach of inflammatory bowel diseases?: Green tea and inflammatory bowel diseases, Complement. Ther. Med., 2019, 43, 148–153 CrossRef PubMed.
- A. Tuzun, A. Erdil, V. Inal, A. Aydn, S. Bagc, Z. Yeşilova, A. Sayal, N. Karaeren and K. Dagalp, Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease, Clin. Biochem., 2002, 35, 569–572 CrossRef CAS PubMed.
- A. R. Bourgonje, M. Feelisch, K. N. Faber, A. Pasch, G. Dijkstra and H. van Goor, Oxidative Stress and Redox-Modulating Therapeutics in Inflammatory Bowel Disease, Trends Mol. Med., 2020, 26, 1034–1046 CrossRef CAS PubMed.
- J. Zhang, H. Lei, X. Hu and W. Dong, Hesperetin ameliorates DSS-induced colitis by maintaining the epithelial barrier via blocking RIPK3/MLKL necroptosis signaling, Eur. J. Pharmacol., 2020, 873, 172992 CrossRef CAS PubMed.
- W. A. Faubion, E. V. Loftus, W. S. Harmsen, A. R. Zinsmeister and W. J. Sandborn, The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study, Gastroenterology, 2001, 121, 255–260 CrossRef CAS.
- L. Beaugerie, N. Brousse, A. M. Bouvier, J. F. Colombel, M. Lémann, J. Cosnes, X. Hébuterne, A. Cortot, Y. Bouhnik, J. P. Gendre, T. Simon, M. Maynadié, O. Hermine, J. Faivre and F. Carrat, Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study, Lancet, 2009, 374, 1617–1625 CrossRef CAS PubMed.
- K. Sultan, B. I. Korelitz, D. Present, S. Katz, S. Sunday and I. Shapira, Prognosis of lymphoma in patients following treatment with 6-mercaptopurine/azathioprine for inflammatory bowel disease, Inflamm. Bowel Dis., 2012, 18, 1855–1858 CrossRef.
- A. Y. Miyake, A. Ktdds, B. C. Nagata, C. S. Furukawa, D. A. Andoh, E. T. Yokoyama, F. N. Yoshimura, G. K. Mori, G. T. Ninomiya and H. Y. Yamamoto, Dietary intake of vegetables, fruit, and antioxidants and risk of ulcerative colitis: A case-control study in Japan, Nutrition, 2021, 91–92, 111378 CrossRef PubMed.
- S. M. Karp and T. R. Koch, Oxidative Stress and Antioxidants in Inflammatory Bowel Disease, Disease-a-Month, 2006, 52, 199–207 CrossRef PubMed.
- S. Kanwal, T. P. Joseph, S. Aliya, S. Song, M. Z. Saleem, M. A. Nisar, Y. Wang, A. Meyiah, Y. Ma and Y. Xin, Attenuation of DSS induced colitis by Dictyophora indusiata polysaccharide (DIP) via modulation of gut microbiota and inflammatory related signaling pathways, J. Funct. Foods, 2020, 64, 103641 CrossRef CAS.
- Z. T. Bitzer, R. J. Elias, M. Vijay-Kumar and J. D. Lambert, (-)-Epigallocatechin-3-gallate decreases colonic inflammation and permeability in a mouse model of colitis, but reduces macronutrient digestion and exacerbates weight loss, Mol. Nutr. Food Res., 2016, 60, 2267–2274 CrossRef CAS PubMed.
- A. K. Pandurangan, Gallic acid attenuates dextran sulfate sodium-induced experimental colitis in BALB/c mice, Drug Des., Dev. Ther., 2015, 9, 3923–3934 CrossRef PubMed.
- E. Haslam, Thoughts on thearubigins, Phytochemistry, 2003, 64, 61–73 CrossRef CAS.
- A. Ukil, S. Maity and P. K. Das, Protection from experimental colitis by theaflavin-3,3′-digallate correlates with inhibition of IKK and NF-kappaB activation, Br. J. Pharmacol., 2006, 149, 121–131 CrossRef CAS PubMed.
- C. Chen, H. Wang, T. Hong, X. Huang, S. Xia, Y. Zhang, X. Chen, Y. Zhong and S. Nie, Effects of tea polysaccharides in combination with polyphenols on dextran sodium sulfate-induced colitis in mice, Food Chem.: X, 2022, 13, 100190 CAS.
- Z. Zeng, Z. Xie, G. Chen, Y. Sun, X. Zeng and Z. Liu, Anti-inflammatory and gut microbiota modulatory effects of polysaccharides from Fuzhuan brick tea on colitis in mice induced by dextran sulfate sodium, Food Funct., 2022, 13, 649–663 RSC.
- Y. Liu, L. Luo, Y. Luo, J. Zhang, X. Wang, K. Sun and L. Zeng, Prebiotic Properties of Green and Dark Tea Contribute to Protective Effects in Chemical-Induced Colitis in Mice: A Fecal Microbiota Transplantation Study, J. Agric. Food Chem., 2020, 68, 6368–6380 CrossRef CAS PubMed.
- Z. Liu, J.-P. Vincken and W. J. C. d. Bruijn, Tea phenolics as prebiotics, Trends Food Sci. Technol., 2022, 127, 156–168 CrossRef CAS.
- J. Gopal, M. Muthu, D. Paul, D. H. Kim and S. Chun, Bactericidal activity of green tea extracts: the importance of catechin containing nano particles, Sci. Rep., 2016, 6, 19710 CrossRef CAS PubMed.
- S. Yi, Y. Wang, Y. Huang, L. Xia, L. Sun, S. C. Lenaghan and M. Zhang, Tea Nanoparticles for Immunostimulation and Chemo-Drug Delivery in Cancer Treatment, J. Biomed. Nanotechnol., 2014, 10, 1016–1029 CrossRef CAS PubMed.
- P. Prema, T. Boobalan, A. Arun, K. Rameshkumar, R. S. Babu, V. Veeramanikandan, V.-H. Nguyen and P. Balaji, Green tea extract mediated biogenic synthesis of gold nanoparticles with potent anti-proliferative effect against PC-3 human prostate cancer cells, Mater. Lett., 2022, 306, 130882 CrossRef CAS.
- H. Han, L. Ke, H. Wang, G. Gao, Y. zhang, P. Rao, J. Zhou, O. Tirosh and B. Schwartz, Incidental Nanoparticles in Black Tea Infusion: Carriers of Bioactives Fortifying Protection on Intestinal Mucosal Cells Against Oxidative Stresses, Food Biophys., 2022, 17, 1–12 CrossRef.
- X. Meng, P. H. Chong, B. Song, P. Zhang, L. Li, P. Rao, Z. Yu and L. Ke, Distinguishable Shot-term Effects of Tea and Water Drinking on Human Saliva Redox, Res. Square, 2023, 1, 1–21 Search PubMed.
- H. Han, H. Wang, G. Gao, P. Rao, J. Zhou, L. Ke and Y. Xu, pH effect on colloidal characteristics of micro-nano particles in lapsang souchong black tea infusion, Food Control, 2022, 133, 108643 CrossRef CAS.
- X. Liang, K. Cao, W. Li, X. Li, D. J. McClements and K. Hu, Tannic acid-fortified zein-pectin nanoparticles: Stability, properties, antioxidant activity, and in vitro digestion, Food Res. Int., 2021, 145, 110425 CrossRef CAS.
- K. M. Kim, Y. S. Kim, J. Y. Lim, S. J. Min, J. H. Shin, H. C. Ko, S. J. Kim, Y. Lim and Y. Kim, Sasa quelpaertensis leaf extract suppresses dextran sulfate sodium–induced colitis in mice by inhibiting the proinflammatory mediators and mitogen-activated protein kinase phosphorylation, Nutr. Res., 2014, 34, 894–905 CrossRef CAS.
- Z. Zheng, Z. Dai, Y. Cao, Q. Shen and Y. Zhang, Docosapentaenoic acid (DPA, 22:5n-3) ameliorates inflammation in an ulcerative colitis model, Food Funct., 2019, 10, 4199–4209 RSC.
- M. Bruckner, S. Westphal, W. Domschke, T. Kucharzik and A. Lugering, Green tea polyphenol epigallocatechin-3-gallate shows therapeutic antioxidative effects in a murine model of colitis, J. Crohn’s Colitis, 2012, 6, 226–235 CrossRef PubMed.
- A. É. I. Gomes, L. P. Stuchi, N. M. G. Siqueira, J. B. Henrique, R. Vicentini, M. L. Ribeiro, M. Darrieux and L. F. C. Ferraz, Selection and validation of reference genes for gene expression studies in Klebsiella pneumoniae using Reverse Transcription Quantitative real-time PCR, Sci. Rep., 2018, 8, 1–14 Search PubMed.
- B. Chen, L. Carr and X. P. Dun, Dynamic expression of Slit1–3 and Robo1-2 in the mouse peripheral nervous system after injury, Neural Regener. Res., 2020, 15, 948–958 CrossRef CAS.
- L. Ke, W. Xu, J. Gao, G. Gao, H. Wang, J. Zhou, J. Liu, P. Rao and Y. Xu, Isolation and characterization of thermo-tolerant polyphenol oxidases in a black tea infusion, Food Control, 2021, 119, 107465 CrossRef CAS.
- A. Yousefi-Ahmadipour, S. Ebrahimi-Barough, S. Niknia, A. Allahverdi, A. Mirzahosseini-pourranjbar, M. Tashakori, S. K. Ravari, F. Asadi, R. H. B. Nezhad, N. Lotfibakhshaiesh and M. R. Mirzaei, Therapeutic effects of combination of platelet lysate and sulfasalazine administration in TNBS-induced colitis in rat, Biomed. Pharmacother., 2020, 125, 109949 CrossRef CAS PubMed.
- M. R. Shin, K. J. Kim, S. H. Kim, S. J. Kim, B. I. Seo, H. J. An and S. S. Roh, Comparative Evaluation between Sulfasalazine Alone and in Combination with Herbal Medicine on DSS-Induced Ulcerative Colitis Mice, BioMed Res. Int., 2017, 2017, 1–11 Search PubMed.
- A. K. Kieninger and I. Maldener, Cell–cell communication through septal junctions in filamentous cyanobacteria, Curr. Opin. Microbiol., 2021, 61, 35–41 CrossRef CAS.
- A. Angulo-Urarte, T. van der Wal and S. Huveneers, Cell-cell junctions as sensors and transducers of mechanical forces, Biochim. Biophys. Acta, Biomembr., 2020, 1862, 183316 CrossRef CAS PubMed.
- G. Laurent, The cytoplasmic plaque of tight junctions: a scaffolding and signalling center, Biochim. Biophys. Acta, 2008, 3, 601–613 Search PubMed.
- C. M. Van Itallie and J. M. Anderson, Architecture of tight junctions and principles of molecular composition, Semin. Cell Dev. Biol., 2014, 36, 157–165 CrossRef CAS.
- L. S. Poritz, K. I. Garver, C. Green, L. Fitzpatrick, F. Ruggiero and W. A. Koltun, Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis, J. Surg. Res., 2007, 140, 12–19 CrossRef CAS.
- T. Saito, K. Yoshida, K. Matsumoto, K. Saeki, Y. Tanaka, S.-M. Ong, N. Sasaki, R. Nishimura and T. Nakagawa, Inflammatory cytokines induce a reduction in E-cadherin expression and morphological changes in MDCK cells, Res. Vet. Sci., 2014, 96, 288–291 CrossRef CAS PubMed.
- X. Chen, X. Lv and S. Liu, Baitouweng decoction alleviates dextran sulfate sodium-induced ulcerative colitis by regulating intestinal microbiota and the IL-6/STAT3 signaling pathway, J. Ethnopharmacol., 2021, 265, 113357 CrossRef.
- Q. Zhang, H. Tao, Y. Lin, Y. Hu, H. An, D. Zhang, S. Feng, H. Hu, R. Wang, X. Li and J. Zhang, A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease, Biomaterials, 2016, 105, 206–221 CrossRef CAS PubMed.
- H. S. Oz, T. S. Chen, D. Flomenhoft and W. J. de Villiers, Tu1644 Green Tea Polyphenols and Sulfasalazine Have Parallel Anti-Inflammatory Properties in Colitis Models, Gastroenterology, 2013, 144, S-813 CrossRef.
- Y. Song, Y. Park, K. Kim, C. Chung, G. Lee, D. Cho, H. Ki, K. Park, S. Cho, W. Lee, N. Kim, B. Ahn and Y. Joo, Black tea extract prevents lipopolysaccharide-induced NF-κB signaling and attenuates dextran sulfate sodium-induced experimental colitis, BMC Complementary Altern. Med., 2011, 11, 1–9 CrossRef.
- B. Xue, X. Liu, W. Dong, L. Liang and K. Chen, EGCG Maintains Th1/Th2 Balance and Mitigates Ulcerative Colitis Induced by Dextran Sulfate Sodium through TLR4/MyD88/NF- κ B Signaling Pathway in Rats, Can. J. Gastroenterol. Hepatol., 2017, 2017, 1–10 Search PubMed.
- R. Francescone, V. Hou and S. Grivennikov, Cytokines, IBD, and colitis-associated cancer, Inflamm. Bowel Dis., 2015, 21, 409–418 CrossRef PubMed.
- H. Wang, G. Gao, L. Ke, J. Zhou, P. Rao, Y. Jin, L. He, J. Wan and Q. Wang, Isolation of colloidal particles from porcine bone soup and their interaction with murine peritoneal macrophage, J. Funct. Foods, 2019, 54, 403–411 CrossRef CAS.
- L. Ke, H. Wang, G. Gao, P. Rao, L. He and J. Zhou, Direct interaction of food derived colloidal micro/nano-particles with oral macrophages, NPJ Sci. Food, 2017, 1, 1–9 CrossRef PubMed.
- R. Deshmukh, R. K. Harwansh, S. D. Paul and R. Shukla, Controlled release of sulfasalazine loaded amidated pectin microparticles through Eudragit S 100 coated capsule for management of inflammatory bowel disease, J. Drug Delivery Sci. Technol., 2020, 55, 101495 CrossRef CAS.
- C. Burrello, F. Garavaglia, F. M. Cribiu, G. Ercoli, G. Lopez, J. Troisi, A. Colucci, S. Guglietta, S. Carloni, S. Guglielmetti, V. Taverniti, G. Nizzoli, S. Bosari, F. Caprioli, M. Rescigno and F. Facciotti, Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells, Nat. Commun., 2018, 9, 5184 CrossRef PubMed.
- S. Chen, Z. Chen, Y. Wang, W. Hao, Q. Yuan, H. Zhou, C. Gao, Y. Wang, X. Wu and S. Wang, Targeted delivery of Chinese herb pair-based berberine/tannin acid self-assemblies for the treatment of ulcerative colitis, J. Adv. Res., 2022, 40, 263–276 CrossRef CAS PubMed.
- J. Halfvarson, C. J. Brislawn, R. Lamendella, Y. Vázquez-Baeza, W. A. Walters, L. M. Bramer, M. D’Amato, F. Bonfiglio, D. McDonald, A. Gonzalez, E. E. McClure, M. F. Dunklebarger, R. Knight and J. K. Jansson, Dynamics of the human gut microbiome in inflammatory bowel disease, Nat. Microbiol., 2017, 2, 1–7 Search PubMed.
- M. G. Rooks, P. Veiga, L. H. Wardwell-Scott, T. Tickle, N. Segata, M. Michaud, C. A. Gallini, C. Beal, J. E. v. Hylckama-Vlieg, S. A. Ballal, X. C. Morgan, J. N. Glickman, D. Gevers, C. Huttenhower and W. S. Garrett, Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission, ISME J., 2014, 8, 1403–1417 CrossRef CAS PubMed.
- Z. Liu, Z. Chen, H. Guo, D. He, H. Zhao, Z. Wang, W. Zhang, L. Liao, C. Zhang and L. Ni, The modulatory effect of infusions of green tea, oolong tea, and black tea on gut microbiota in high-fat-induced obese mice, Food Funct., 2016, 7, 4869–4879 RSC.
- Z. Wu, S. Huang, T. Li, N. Li, D. Han, B. Zhang, Z. Z. Xu, S. Zhang, J. Pang, S. Wang, G. Zhang, J. Zhao and J. Wang, Gut microbiota from green tea polyphenol-dosed mice improves intestinal epithelial homeostasis and ameliorates experimental colitis, Microbiome, 2021, 9, 184–191 CrossRef CAS PubMed.
- S. Hu, S. Li, Y. Liu, K. Sun, L. Luo and L. Zeng, Aged Ripe Pu-erh Tea Reduced Oxidative Stress-Mediated Inflammation in Dextran Sulfate Sodium-Induced Colitis Mice by Regulating Intestinal Microbes, J. Agric. Food Chem., 2021, 69, 10592–10605 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo00641g |
| This journal is © The Royal Society of Chemistry 2023 |