 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Static in vitro digestion model adapted to the general older adult population: an INFOGEST international consensus
O.
Menard†
a,
U.
Lesmes†
 b,
C. S.
Shani-Levi
b,
A.
Araiza Calahorra
c,
A.
Lavoisier
a,
M.
Morzel
a,
A.
Rieder
b,
C. S.
Shani-Levi
b,
A.
Araiza Calahorra
c,
A.
Lavoisier
a,
M.
Morzel
a,
A.
Rieder
 d,
G.
Feron
ef,
S.
Nebbia
a,
L.
Mashiah
b,
A.
Andres
g,
G.
Bornhorst
d,
G.
Feron
ef,
S.
Nebbia
a,
L.
Mashiah
b,
A.
Andres
g,
G.
Bornhorst
 h,
F.
Carrière
h,
F.
Carrière
 i,
L.
Egger
i,
L.
Egger
 j,
S.
Gwala
k,
A.
Heredia
g,
B.
Kirkhus
d,
A.
Macierzanka
j,
S.
Gwala
k,
A.
Heredia
g,
B.
Kirkhus
d,
A.
Macierzanka
 l,
R.
Portman
l,
R.
Portman
 j,
I.
Recio
j,
I.
Recio
 m,
V.
Santé-Lhoutellier
m,
V.
Santé-Lhoutellier
 n,
C.
Tournier
ef,
A.
Sarkar
n,
C.
Tournier
ef,
A.
Sarkar
 c,
A.
Brodkorb
k,
A.
Mackie
c,
A.
Brodkorb
k,
A.
Mackie
 *c and
D.
Dupont
*c and
D.
Dupont
 *a
*a
aINRAE – Institut Agro, STLO, Rennes, France. E-mail: didier.dupont@inrae.fr
bTechnion Israel Institute of Technology, Israel
cUniv Leeds, Sch Food Sci & Nutr, Leeds LS2 9JT, W Yorkshire, UK
dNofima AS – Norwegian Institute of Food, Fisheries and Aquaculture, PB 210, N-1433 Ås, Norway
eCentre des Sciences du Goût et de l'Alimentation, CNRS, INRAE, Institut Agro, Université de Bourgogne Franche-Comté, F-21000 Dijon, France
fINRAE, PROBE research infrastructure, ChemoSens facility, F-21000 Dijon, France
gInstitute of Food Engineering for Development, Universitat Politecnica de Valencia, Valencia, Spain
hDepartment of Biological and Agricultural Engineering, University of California Davis, Davis, USA
iAix Marseille University, CNRS, UMR7281 Bioenergetics and Protein Engineering, Marseille, France
jAgroscope, Schwarzenburgstr, 161, 3003 Bern, Switzerland
kTeagasc Food Research Centre, Fermoy, Co. Cork, Moorepark, Ireland
lGdansk University of Technology, Faculty of Chemistry, Department of Colloid and Lipid Science, 80-322 Gdansk, Poland
mInstituto de Investigación en Ciencias de la Alimentación, CIAL (CSIC-UAM, CEI UAM+CSIC), Nicolás Cabrera, 9, 28049 Madrid, Spain
nINRAE, France, QuaPA, UR370, Centre de Clermont Auvergne Rhône Alpes, F-63122 Saint Genes Champanelle, France
First published on 25th April 2023
Abstract
Understanding the mechanisms of food digestion is of paramount importance to determine the effect foods have on human health. Significant knowledge on the fate of food during digestion has been generated in healthy adults due to the development of physiologically-relevant in vitro digestion models. However, it appears that the performance of the oro-gastrointestinal tract is affected by ageing and that a model simulating the digestive conditions found in a younger adult (<65 years) is not relevant for an older adult (>65 years). The objectives of the present paper were: (1) to conduct an exhaustive literature search to find data on the physiological parameters of the older adult oro-gastrointestinal tract, (2) to define the parameters of an in vitro digestion model adapted to the older adult. International experts have discussed all the parameters during a dedicated workshop organized within the INFOGEST network. Data on food bolus properties collected in the older adult were gathered, including food particle size found in older adult boluses. In the stomach and small intestine, data suggest that significant physiological changes are observed between younger and older adults. In the latter, the rate of gastric emptying is slowed down, the pH of the stomach content is higher, the amount of secretions and thus the hydrolytic activities of gastric and intestinal digestive enzymes are reduced and the concentration of bile salts lower. The consensus in vitro digestion model of the older adult proposed here will allow significant progress to be made in understanding the fate of food in this specific population, facilitating the development of foods adapted to their nutritional needs. Nevertheless, better foundational data when available and further refinement of the parameters will be needed to implement the proposed model in the future.
1 Introduction
Understanding the fate of food in the oro-gastrointestinal tract has been a topic of growing interest over the last years for the scientific community, and particularly for scientists from the INFOGEST international network on food digestion (https://www.cost-infogest.eu). A quick search on the Web of Science shows that the number of peer-reviewed publications having in any field the words “food” and “digest*” has grown from 2439 in 2009 to 8516 in 2021 (no statistics available for a longer period). Unravelling the mechanisms of food disintegration during digestion is needed to determine how food structure and composition affect the kinetics of nutrient release in the gut lumen (bioaccessibility) and the proportion of nutrients that are absorbed (bioavailability). These questions are also shared by the scientific community working on drugs and the COST Action UNGAP (European Network on Understanding Gastrointestinal Absorption-related Processes, https://gbiomed.kuleuven.be/english/research/50000715/50000716/ungap) has been very active in investigating the release of drugs in the oro-gastrointestinal tract and their subsequent absorption.1In both the food and pharma sectors, the digestive process has been investigated using in vivo models involving either human volunteers or animals. However, there is currently a general trend tending to limit as much as possible studies involving complex living organisms. Furthermore, in vivo studies are cumbersome, time and resource intensive, ethically questionable and exhibit high inter-individual variability. For all these reasons, in vitro digestion models, either static or dynamic, have been the centre of interest of many recent studies.
Static in vitro digestion models consist of a series of bioreactors simulating the physicochemical and enzymatic conditions a food or a drug will meet when entering in the different compartments of the oro-gastrointestinal tract. A first bioreactor mimics the food fragmentation exerted by teeth and mandible, moistening of the food by saliva, initiation of starch hydrolysis and the formation of a food bolus, which is subsequently transferred to a second bioreactor mimicking the stomach, where protein and lipid hydrolysis are initiated, and finally to a third reactor simulating the small intestine. Static models are sequential which means that a phase will only start when the previous one has been fully completed; this is different from the physiology since a proportion of a food can still be in the stomach whereas the other part is already in the small intestine. Furthermore, physicochemical conditions and enzyme activities are kept constant throughout digestion in these models whereas parameters such as the pH or the enzyme activities change over time under the physiological conditions. Static digestion models can be used as a pre-screening method, when a large number of tests need to be performed, or before moving to more complex systems. Since they simplify the digestive process, they can also allow unravelling mechanisms that occur at a molecular scale. For instance, phospholipids such as phosphatidylcholine released by the gastric mucosa have been shown to interact with globular proteins like β-lactoglobulin to harden its structure and make it more resistant to the action of pepsin.2 Finally, static in vitro digestion models can also be relevant to estimate end-point values such as the glycaemic index, protein and lipid digestibility, estimation of micronutrient and secondary plant metabolite release/bioacessibility, among others. Limits and advantages of static in vitro digestion models have been reviewed by INFOGEST scientists.3
A wide variety of static digestion models have been published in the literature, with different parameters (pH and/or ionic strength, duration of each phase, enzymatic activities…) making the results difficult to compare between studies. To overcome the problem, namely the impossibility of comparing the results between different studies and the need to harmonize a digestion protocol that the entire scientific community can use, the international network of researchers INFOGEST, whose objective is to bring together a community of scientists in the field of digestion, has established a consensus around a static digestion protocol for a healthy adult.4,5 Since then, the model has been extensively used to assess food digestibility, nutrient bioaccessibility, food matrix effects, allergen persistence in the GI tract, etc.6 The model is now used all around the world and is about to be recognized as a reference method by International Organization for Standardization (ISO) and International Dairy Federation (IDF) to determine protein digestibility.
Most of the in vitro digestion models developed so far simulate the physiological conditions observed in healthy adults. However, significant changes occur over the life course so in vitro digestion models must be adapted to the different physiological stages.7 Static models mimicking the infant gastrointestinal tract have been proposed;8–11 among those a model has been proposed as an international consensus by INFOGEST participants12 and has been, since then, used in more than a hundred studies published by the scientific community.13–16
Ageing is accompanied by several physiological changes that affect most of the organs of the human body. For example, due to decline in muscular function, impairment in dental status and reduction in salivary flow and modification in composition, there is impairment in oral processing capability which can alter particle size reduction, adversely affecting digestion rate and extent.17,18 However, other studies suggested an adaptation of the oral processing in older adults leading to the formation of similar boli than those made by younger adults.19 Several studies have demonstrated that digestive conditions evolve with age. For example, gastrointestinal motor function, food transit, chemical food digestion, and functionality of the intestinal wall have been previously shown to be affected by ageing.20 This evolution has been considered by different groups who proposed static in vitro digestion models mimicking the oro-gastrointestinal tract of older adults.21–25
The use of different older adult in vitro digestion models varying in parameters such as pH, enzyme activities, duration etc. ends up with data that are not comparable between different studies. Therefore, the objectives of the present work were (1) to conduct an exhaustive literature review in order to find physiological values obtained on older adults for each parameter of the digestion model, (2) to reach an international consensus on the model and propose it to the scientific community. The literature search has been done within the EAT4AGE European project (https://nofima.com/projects/eat4age/) that gathers 6 academic (INRAE, The Norwegian School of Sport Sciences, Nofima, Technion, University of Leeds, and Teagasc Food Research Centre) and 2 industry (Nortura and GatFoods) partners on the development of “Palatable, nutritious and digestible foods for prevention of undernutrition in active ageing”. EAT4AGE aims to prevent undernutrition and avoid impaired muscle function by investigating how age-related changes, such as decline in digestive functions, oral processing, sensory perception, and appetite, can be overcome. Then, based on the proposition made by the EAT4AGE consortium, an international workshop was organised in Cork on the 2–3 of May 2022 gathering 20 experts from 10 countries and 12 institutes. All the parameters of the model have been discussed one by one and only the values for which solid evidence is available has been considered in order to find a consensus based on the existing literature. In the near future, this novel digestion model adapted for the older adult, will help the scientific community and generate comparable data.
In vitro digestion parameters – recommendation and justification
One of the first points was to specify a minimum age to define the starting point of a healthy older adult population. This is a key issue since, for some of the digestion parameters, values at different ages were available in the literature. In 2014, the World Health Organization (WHO) considered that “old people” were over 60–65 years in the developed world.26 Experts in gerontology categorized “old people” into “young old” (60–69 years), the “middle old” (70–79 years) and the “very old” (80 + years)27 whereas others divided the older adults in 3 categories i.e. “young olds” (65–74 years), “middle olds” (75–84 years) and “oldest olds” (85 + years).28 It is common sense that rather than the chronological age, it is the “physiological” age that matters in terms of digestion, and that physiological ageing can proceed at different rates depending on nutrition, environmental factors, physical activity, access to healthcare, etc. Therefore, in the literature search that guided the discussion of the consensus group, articles were utilized when: 1 – age was mentioned (words such as ageing, old, older, elderly…) in the article title or description of participants; and 2 – the lower value of the age range in the group of older adults was at least 65. One can still wonder whether it could be relevant to develop different in vitro digestion models for different age or health categories of older adults but not enough data are currently available to achieve this goal within the scope of the current paper.Based on the available data in the literature, the parameters of the healthy older adult in vitro digestion model will be discussed including:
● (1) Oral phase: simulated salivary fluid (SSF) composition, saliva/food dilution, pH, duration, salivary amylase activity, food bolus particle size,
● (2) Gastric phase: simulated gastric fluid (SGF) composition, food bolus/gastric secretions ratio, pH, duration, pepsin and gastric lipase activities,
● (3) Small intestinal phase: simulated intestinal fluid (SIF) composition, chyme/intestinal secretions ratio, pH, duration, pancreatic lipase and amylase activities, trypsin and chymotrypsin activities, bile salts content.
2 Oral phase
As an introductory note, for the salivary characteristics, we chose whenever possible to select results obtained on stimulated saliva (as opposed to at-rest saliva), which better simulates the situation where food is manipulated in the mouth. The articles quoted below are mostly on saliva obtained after stimulation by chewing parafilm if not stated otherwise.SSF composition
The ionic composition of older adult's saliva is very poorly documented and the only data available are for at-rest saliva. In an article reporting two distinct studies, a significant increase in K+ (by a factor of 1.45) and Cl− concentration (by a factor of 1.50) was observed in old (70–86 years, n = 22) individuals compared to young (20–29 years, n = 23) while the Na+ and Ca2+ concentrations increased, but non-significantly.29 However, in the second study, the concentration of K+ and Ca2+ significantly increased during ageing by a factor of 1.35 and 1.26 respectively, while the Cl− concentration increased in older adults but non-significantly and the Na+ was similar between young (18–24 years, n = 11) and old (60–90 years, n = 18) individuals.29 In contrast, a 27% decrease of calcium concentration was reported between young (20–30 years, n = 20) and old (60–80 years, n = 20) subjects.30Recommendation: all the values found (though limited in literature) are within close limits of adult SSF composition, so it is recommended to use the SSF composition proposed for the young adult INFOGEST model.5
pH
A cross-sectional study was carried out in 139 adults with a mean age of 79.1 ± 9.8 years.31 A slight increase in pH of saliva was observed when the age increased (p = 0.087) with values of 7.76 ± 0.91 for 60–74 years, 7.86 ± 0.67 for 75–84 years and 8.04 ± 0.89 for people over 85 years. In another study,32 forty older adult individuals aged 60–86 were divided into two gender-matched groups of 20, according to the use or non-use (control) of medication and the presence or absence (control) of senile dementia. pH values found in both groups were 6.71 ± 0.55 for the medicated group suffering from dementia (mean age 69.6 years) and 6.95 ± 0.42 for the control group (mean age 68.3 years). In a Swedish study involving 70 years-old 58 men and 53 women, the pH of parafilm-stimulated saliva was found to be 7.2 and 7.1 in males and females respectively.33 Finally, comparing healthy young (20–35 years) and older (>65 years) adults, no significant difference was reported with values in at-rest saliva of 6.58 ± 0.47 vs. 6.74 ± 0.40, respectively.34 Based on these four studies, the pH of saliva of older adult is close to neutral.Recommendation: for all these reasons, it was decided to use a pH of 7.0 for the oral phase in the consensus model of the old adult identically to the one proposed for the consensus in vitro digestion model of the young adult.
Food/saliva ratio
To our knowledge, only two articles report values of the proportion of saliva incorporated into food during bolus formation in older adults. This concerns two versions (control or enriched in proteins) of two cereal products, brioche and sponge cake35,36 tested by 20 subjects with a mean age of 72 years, and four versions of whey-based cheese37 tested by 72 subjects with a mean age of 73.1 years. These results were acquired within the French project ALIMASSENS, where additional insalivation rates i.e. the quantity of saliva incorporated in the food bolus were obtained for cheese, meat products and custard. In addition, in the project REMUS, aiming at designing a dairy product adapted to older adults,38 insalivation rates were recorded for two versions of custard-type dairy desserts. Table 1 provides a summary of values recorded in vivo on older adults for these different products, where it appears that less saliva is incorporated into products with lower dry matter (e.g. custard and meat). Percentage of saliva was calculated as follows:| (bolus weight in g − food weight in g)/bolus weight in g × 100. |
| Mean age (year) | n | Product | Dry matter (%) | Percentage of saliva incorporated (mean ± SD) | Source of data |
|---|---|---|---|---|---|
| 72 | 20 | Sponge cake | 72 | 79 ± 25 | Alimassens (Assad-Bustillos et al. 2019)35 |
| 72 | 20 | Brioche | 70 | 45 ± 11 | |
| 73.1 | 72 | Hard cheese | 50 | 56 ± 29 | Alimassens (Lorieau et al., 2021)37 |
| 73.1 | 72 | Soft cheese | 50 | 45 ± 25 | |
| 73.1 | 72 | Whipped cheese | 50 | 90 ± 35 | |
| 73.1 | 72 | Processed cheese | 50 | 45 ± 30 | |
| 73.2 | 73 | Hard cheese | 50 | 69 ± 6 | Alimassens (unpublished) |
| 73.2 | 73 | Soft cheese | 50 | 65 ± 5 | |
| 73.2 | 73 | Whipped cheese | 50 | 71 ± 6 | |
| 73.2 | 73 | Processed cheese | 50 | 65 ± 6 | |
| 74.0 | 73 | Shredded beef | 25 | 36 ± 10 | |
| 74.0 | 73 | Laminated beef | 30 | 30 ± 12 | |
| 74.0 | 73 | Minced chicken | 30 | 32 ± 10 | |
| 74.0 | 73 | Shredded chicken | 25 | 39 ± 10 | |
| 73 | 76 | Custard-type dairy dessert | 28 | 32 ± 23 | |
| 70.5 | 31 | Commercial custard enriched in proteins | 33 | 26 ± 13 | REMUS (unpublished) |
| 70.5 | 31 | Custard enriched in proteins (reformulated) | 28 | 28 ± 17 |
For cheese, the percentage of saliva incorporated (from 45 to 90%) was higher than in other studies on younger adults, with values of 6 to 19%,39 23 to 52%,40 23 to 46% (ref. 41) or 38 to 50%.42 However, the products used in these different studies were model cheeses with various properties, which makes the comparison of results difficult. For instance, an increase in cheese fat content (from 25% to 50%) induced a decrease (from 41% to 22%) in percentage of saliva incorporated in a middle aged population.40
In the static consensus model of adult digestion,5 a ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (weight of SSF or saliva
1 (weight of SSF or saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) weight of food) is used whereas in the semi-dynamic consensus model of digestion,43 a ratio of 1
weight of food) is used whereas in the semi-dynamic consensus model of digestion,43 a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (weight of SSF or saliva added
1 (weight of SSF or saliva added![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dry weight of food) was proposed. The results in Table 1 support that this proxy seems equally relevant to the older adult population.
dry weight of food) was proposed. The results in Table 1 support that this proxy seems equally relevant to the older adult population.
Recommendation: for studies using the static in vitro model of the older adult and when the objective is to obtain data comparable with those obtained with the consensus adult static model, it is recommended to keep the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (weight of SSF or saliva
1 (weight of SSF or saliva![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) weight of food). Nevertheless, the literature review performed for preparing the present article suggests that the ratio of 1
weight of food). Nevertheless, the literature review performed for preparing the present article suggests that the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (weight of SSF or saliva added
1 (weight of SSF or saliva added![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dry weight of food) is more physiologically relevant, and could be considered in future updated versions of the model of adult digestion.
dry weight of food) is more physiologically relevant, and could be considered in future updated versions of the model of adult digestion.
Salivary amylase
Salivary amylase plays a key role in disintegration of starch-containing foods. It was evidenced in a recent work on bread bolus obtained after deficient mastication and especially in the absence of saliva. Bigger/compacted particles with reduced total and slowly digestible starch were evidenced as demonstrated with FTIR spectroscopy analysis.18 The enzyme starts hydrolysing starch in the mouth but also in the stomach as long as the pH remains higher than the inactivation pH between 3 and 3.5.44 After food ingestion, the gastric pH is close to that of the ingested food and will decrease slowly due to the gastric emptying and the acidic secretion. The decrease in pH will depend on the amount of food, the food buffering capacity, and the subject's physiology, but the pH conditions can remain favourable to the action of amylase for a long time. Indeed, in a recent work where industrial vs. traditional baguette were submitted to dynamic in vitro digestion, the proportion of partially hydrolysed starch after the oral phase (at t = 2 min) was similar for all foods (about 20%) but continued to increase very rapidly during gastric digestion, reaching a plateau after about 20 min of digestion for all breads. The plateau values were very high for all breads, between 63% and 74%, hence confirming the key role of salivary α-amylase's action in the digestion of starch during the gastric phase.45Comparing studies on salivary amylase activity is not straightforward since salivary amylase activity can be determined with different assays. For instance, amylase activity can be assessed by quantifying the reduction of 3,5-dinitrosalicylic acid (DNS). In this method, starch is converted into maltose by α-amylase. Maltose released from starch is measured by the reduction of 3,5-dinitrosalicylic acid. Maltose reduces the pale yellow coloured alkaline DNS to the orange-red colour. The intensity of the colour is proportional to the concentration of maltose present in the sample. Alternatively, amylase activity can be monitored by the CNPG3 Kit. In this test, α-amylase hydrolyses the 2-chloro-4-nitrophenyl maltose trioside leading to the formation of chloro-nitro-phenol that can be measured at 405 nm. Finally, the Phadebas® test is also frequently used by the scientific community. The principle behind the test is that Phadebas®, consisting of starch microspheres with a blue dye cross-linked to the starch, are immobilised on filter paper sheets. In the presence of amylase, the starch is digested, releasing the water-soluble dye, which diffuses through the pores of the filter paper. The resulting blue colour is visually observed on the non-reagent side of the Phadebas® paper.
The INFOGEST young adult model recommends the DNS-based method as a reference to measure amylase activity. In a study involving 169 older adults with a mean age of 81.2 years, salivary amylase activity was measured with the Phadebas test at 212.7 ± 168.1 U ml−1.46 A few years later, the same group analysed the saliva of 175 hospitalized patients (age 82 ± 5.7 years) and 252 outpatients (age 77 ± 5.7 years).47 Mean values were in the range of 202–216 U ml−1 for hospitalized patients and 111–130 U ml−1 for outpatients. However, using the CNPG3 kit, amylase activity in stimulated saliva of the ALIMASSENS participants (66–89 years) was 15.3 ± 11.5 U ml−1. This illustrates the difficulty of comparing results when they are acquired using different methods. One article measured amylase activity in acid-stimulated saliva of younger (21–49 years, n = 13) and older (64–99 years, n = 10) adults using the DNS test, but the assay temperature and the units for expression of results were different from what is advised in the INFOGEST young adult model (ESI of ref. 4). Nevertheless, no significant difference was observed between the younger (583 ± 306 IU × 10−3 ml−1 saliva) and the older group (629 ± 314 IU × 10−3 ml−1 of saliva).48 In at-rest saliva, there was no difference between young (27.8 ± 2.6 years, n = 20) and older (68.6 ± 7.4 years, n = 20) adults30 while an approximate two-fold increase in older (n = 40 in total) compared to younger (n = 34 in total) adults was measured in the two studies reported in ref. 29.
Recommendation: data are scarce, there is limited scientific evidence suggesting that a modification of the INFOGEST young adult model is needed to mimic the healthy older adult conditions. Therefore, it is recommended to use a value of 75 U mL−1 (using the DNS assay) for salivary amylase i.e. the one that is also proposed for the adult model.
Particle size
Ageing is often accompanied by oral deficiencies such as loss of teeth,49 decrease in salivary secretion,50 or decreased masticatory muscle’ strength.51 Oral decline and mastication deficiencies cause alteration of food bolus properties and therefore impact on swallowing.It has been found that individuals differing in age, gender, and ethnicity vary in oral processing time to produce bolus with textural properties optimized to their needs.52 For example, older adults (70 ± 4.3 years, n = 22) produced sausage boli that were softer, more adhesive, less cohesive, and contained more particles than in young adults (22 ± 2.8 years, n = 21). However, ageing did not affect bolus particle size at the swallowing point for this product. In a study comparing 14 young (35.6 ± 10.6 years) to 14 aged dentate individuals (68.1 ± 7.0 year) masticating peanuts and raw carrots, the aged individuals produced boli with similar particle size distribution for peanuts, but the distribution was skewed towards bigger particles for carrots.53
The dental status of the subjects is a critical factor in studying the ability of older adults to fragment the food before swallowing. Indeed, the replacement of the natural teeth by removable full dentures impaired mastication of peanuts and carrots, and food boli containing much coarser particles were observed in the aged complete denture wearers (68.1 ± 7.2 years, n = 14) despite an increased number of chewing cycles and greater electromyographic activity.53 However, in a study investigating the comminution progress in 22 subjects wearing removable denture prosthesis (75.1 ± 5.3 years) and 20 young fully dentate subjects (27.6 ± 1.9 years) consuming a combined test meal (cooked rice, sausage, omelet, raw cabbage, and cucumber), a significant difference in particle size between the two groups was observed at the half-mastication point (mean ± SD = 1.656 ± 0.098 mm for old adults vs. 1.493 ± 0.099 mm for young adults, p < 0.05), but not immediately before swallowing.54
Overall, these studies suggest that older adults tend to adapt their oral processing, in particular by increasing the number of chewing cycles, to form a food bolus containing particles similar in size to that of young adults. Median particle sizes (D50) and particle size range reported in the literature for different foods after mastication by older adults are summarized in Table 2.
| Mean age (year) | n | Product | d 50 (mm) | Particle size range (d 50 mm) | Source of data |
|---|---|---|---|---|---|
| d 50 (mm) corresponds to the median particle diameter (portion of particles with diameters smaller and larger than this value are 50%). | |||||
| 72 | 20 | Sponge cake | 0.3 ± 0.1 | Alimassens (Assad-Bustillos et al. 2019)35 | |
| 72 | 20 | Brioche | 2.9 ± 4.0 | ||
| 75 | 20 | Fortified sponge cake | 0.3 ± 0.1 | 0.14–0.92 | Alimassens (Assad-Bustillos et al., 2020)37 |
| 75 | 20 | Fortified brioche | 0.8 ± 0.6 | 0.17–30.8 | |
| 74.0 | 73 | Minced beef | 1.20 (median) | Alimassens (unpublished) | |
| 74.0 | 73 | Laminated beef | 3.68 (median) | ||
| 74.0 | 73 | Minced chicken | 2.36 (median) | ||
| 74.0 | 73 | Chopped chicken | 3.59 (median) | ||
| 72 | 107 | Carrots | 1.68 (median) | ||
| 68.1 | 14 | Carrots | 1–4 | Mishellany-Dutour et al. (2008)53 | |
| 68.1 | 14 | Peanuts | 0.4–4 | ||
| 70 | 22 | Hotdog sausages | 1.95 ± 0.02 | (Aguayo-Mendoza, Martinez-Almaguer, Piqueras-Fiszman, & Stieger, 2020)52 | |
| 75.1 | 22 | Mixed foods: cooked rice, sausage, Japanese hard omelet, raw cabbage, raw cucumber | 5.5 ± 0.8 | (Sugimoto, Tanaka, Kodama, & Minagi, 2020) | |
For the oral phase of this static in vitro digestion model, what is needed is a robust protocol to grind the food into particles of similar size to those reported in the literature that have been previously recorded in in vivo bolus collection studies. The protocol must be simple and reproducible. After testing several devices, it was decided to use a manual meat mincer to produce food particles; this kind of device was selected since it is easy-to-use, cheap and identical systems are available everywhere in the world. The consortium tested a simple protocol on four food systems: raw carrots, cooked meatballs, roasted peanuts, and sponge cake. Carrots (90% moisture) and meatballs (>50%) were selected as high moisture products, whereas peanuts (∼9%) and sponge cake (∼30%) were chosen as low moisture products. All these products were studied because bolus granulometry data were available. To simulate the oral processing, food boli were prepared by mixing the various samples with distilled water at a final insalivation ratio of 95%, 70%, 40%, and 10%, for peanuts, sponge cake, meatballs, and carrots, respectively to be consistent with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio given above. Samples were then minced using a meat mincer (Kitchen Craft No. 5 meat mincer, Leeds) with a 5 cm mincing disc and a 0.5 cm mesh size for one pass. Once the bolus was recovered, 2 g of carrots, sponge cake, meatballs, and peanuts were suspended in 150 mL glycerol and agitated with a magnetic stirrer for 1 h at 200 rpm to allow particle dispersion without damaging the bolus structure. After this time, the bolus particles were imaged using a ChemiDoc™ XRS + System with image Lab™ Software (Bio Rad Laboratories, Richmond, CA, USA). Images were acquired in greyscale as it offers more contrast between the particles and the background. For each bolus, a minimum of three images per bolus sample were taken to obtain approximately 100 individual particle images from each sample. ImageJ software (version 1.48r, National Institute of Health, Bethesda, USA) was used to determine the area of the different particles. Particles were considered circular to calculate their corresponding D50. Results are presented in Fig. 1. Median particle sizes obtained for the different foods with this simple procedure were fairly similar to the particle sizes reported in the literature (Table 2).
1 ratio given above. Samples were then minced using a meat mincer (Kitchen Craft No. 5 meat mincer, Leeds) with a 5 cm mincing disc and a 0.5 cm mesh size for one pass. Once the bolus was recovered, 2 g of carrots, sponge cake, meatballs, and peanuts were suspended in 150 mL glycerol and agitated with a magnetic stirrer for 1 h at 200 rpm to allow particle dispersion without damaging the bolus structure. After this time, the bolus particles were imaged using a ChemiDoc™ XRS + System with image Lab™ Software (Bio Rad Laboratories, Richmond, CA, USA). Images were acquired in greyscale as it offers more contrast between the particles and the background. For each bolus, a minimum of three images per bolus sample were taken to obtain approximately 100 individual particle images from each sample. ImageJ software (version 1.48r, National Institute of Health, Bethesda, USA) was used to determine the area of the different particles. Particles were considered circular to calculate their corresponding D50. Results are presented in Fig. 1. Median particle sizes obtained for the different foods with this simple procedure were fairly similar to the particle sizes reported in the literature (Table 2).
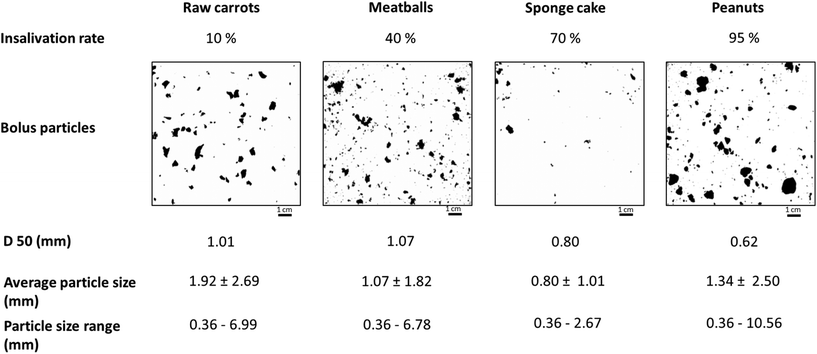 | ||
| Fig. 1 Characteristics of food particles after in vitro oral processing of raw carrots, meatballs, sponge cake and peanuts. | ||
Recommendation: to simulate the oral processing occurring during the first phase of the digestion it is recommended to use of a basic meat mincer (manual, consisting of a mincing disk and a blade, only one pass) to produce food particles and then add SSF at pH 7 (with salivary amylase in case of starch-containing foods) or saliva at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (SSF or saliva added: weight of food or dry weight of food).
1 (SSF or saliva added: weight of food or dry weight of food).
Duration
The time of residence of food in the mouth before swallowing highly depends on the rheological properties of the food and in particular the time needed for the teeth to reduce bites into smaller particles. For cheese products, for instance, the average in-mouth resident time before swallowing ranged from 14 s to 28 s depending on the cheese firmness.19 In case of older adults and for the same type of product, the time of residence observed was slightly longer and ranged from 18 s to 28 s.37 For cereal products, the chewing duration was similar. Older adults are known to adapt their masticatory behaviour by increasing the number of cycles and the chewing duration55 and differences in chewing behaviour between healthy fully dentate young (23.7 ± 1.1 year, n = 10) and older adults (74.1 ± 1.7 year, n = 10) have been found by electromyographic recordings.56 In this paper the chewing duration of carrots was evaluated from 10 s to 33 s depending on the cooking procedure (raw or cooked) and it was slightly but significantly higher for older compared to young adults (10–25 s for young vs. 13–35 s for older adults).In the INFOGEST young adult adult model, the duration of the oral phase has been set to 2 min. This does not reflect the time of residence of the food in the mouth but it is a time long enough to (1) initiate starch hydrolysis as it occurs in vivo and (2) be reproducible when taking samples. Indeed, it has been shown that starch hydrolysis by salivary amylase that starts in the mouth, can continue in the stomach until the gastric pH reaches low values (pH 3–3.5).44 Between 30 to 80% of the starch can be released in the stomach in white bread and pasta, respectively.57 Since the INFOGEST in vitro digestion model is static and the pH is set at 3.0, starch will not be hydrolyzed in the stomach. Setting the duration of the oral phase to 2 min at 37 °C allows hydrolysing a significant proportion of starch like it is done in the stomach in vivo.
Recommendation: since the time of residence in the mouth of foods is not very different between young and older adults except for those equipped with a denture, it is recommended to use the same duration of the oral phase for both populations, i.e. 2 min.
3 Gastric phase
SGF composition
No information regarding possible differences in the gastric fluid composition between old and young adults has been found in the literature despite an exhaustive review. Therefore, the literature was extended to animal models that are traditionally used to mimic what happens in humans i.e. the rat and the pig. Only two references were found on the evolution of gastric secretion output in rats but none of them reported any information on the possible evolution of the composition of gastric fluid that we could use for the present paper.Recommendation: it is recommended to use the same SGF described in the young adult INFOGEST model.5
pH
In humans, the fasted gastric pH ranges between 1 and 2. After food ingestion, pH increases up to 5–7 depending on the type of food ingested and its buffering capacity. Gastric pH then decreases over time due to emptying of the buffering material and addition of acidic gastric secretions. Gastric pH was recorded in 79 healthy, older men and women (71 ± 5 years) under both fasted and fed conditions using the Heidelberg capsule technique.58 The pH was recorded for 1 h in the fasted state, then a standard liquid and solid meal of 1000 kcal was given to the subjects over 30 min and the pH was finally measured for 4 h postprandially. The measured median fasted gastric pH was 1.3 (1.1–1.6). Following the meal, gastric pH decreased from a peak pH of 6.2 (5.8–6.7) to pH 2.0 within 4 h in most subjects with a gastric emptying half-time (T1/2, where 50% of the bolus has been transferred to the small intestine) of 86 min58 The observed rate of return was considerably slower than in young healthy subjects. A significant increase in gastric pH with age has also been observed.59 They measured the gastric pH in 1615 volunteers classified into four categories of age: 50–59 years (n = 769), 60–69 years (n = 643), 70–79 years (n = 188) and >80 (n = 15). They reported a mean gastric pH of 3.5 ± 2.3, 3.7 ± 2.4, 4.4 ± 2.6 and 4.4 ± 2.1, respectively.Recommendation: a strategy to set the gastric pH of the static protocol is to consider the pH at gastric emptying half-time.5 Based on these data, the gastric pH should be set at 3.7, which is higher than the values reported in the literature for younger adults (i.e., pH 3.0).
Bolus/secretions ratio
No specific data were found in the literature about the dilution factor of the bolus by gastric secretions in older adults. So, we used the following indirect approach to estimate this parameter. The pH in the stomach highly depends on the amount of acidic secretions (and buffering capacity of the meal). Based on the gastric pH curve reported by Russell et al. (1993),58 we estimated the amount of secretions needed to reach a pH of 3.7 at T1/2 using the STORM software that monitors the DiDGi® system.60 At 86 min, the ratio meal/secretions was calculated and a value of 47/53 was obtained.Recommendation: for the older adult static in vitro digestion model, a 50/50 dilution of the oral bolus in gastric secretions should be used (i.e. the same dilution used in the young adult model).
Duration
Although the impact of ageing on gastric emptying is still controversial, several studies have shown that gastric emptying slows down with age and possibly motor changes in gastric function may include a delay in gastric emptying of liquids and solids in the older adult. However, these changes are mild.61 Gastric emptying time was assessed on young and older (average 75 years, n = 18) men using ultrasound.62 The two groups of volunteers received a 790 kcal test meal consisting of pasta (80 g), beef (100 g), salad (100 g), olive oil (20 g), bread (80 g) and mineral water (200 ml) representing 18% proteins, 52% carbohydrates, 30% fats and 3.42 g of vegetable fibre. Together with the meal, patients swallowed 20 pieces (2 mm × 5 mm in size) of radiopaque markers to determine the transit time. The final gastric emptying time in older subjects was 335 ± 31 min vs. 245 ± 25 min in young subjects, corresponding to a 36% increase of the gastric emptying time with age. In another study using ultrasound, the gastric emptying of a whole meal by young (32 ± 8 years, n = 9) and old adults (77 ± 3 years, n = 10) was measured.63 The older participants showed a longer gastric emptying time compared to the younger participants (448.6 ± 104 vs. 306.6 ± 57 min, p < 0.002), representing an increase of +46%. Finally, 19 young (23–50 years) and 14 older (70–84 years) volunteers underwent measurements of gastric emptying by scintigraphy after consumption of solid and liquid model meals.64 Data showed an increase of +43% of the T1/2 for the solid meal and a +34% for the liquid one, when older subjects were compared to the younger group. Based on these data, it appears that the duration of the gastric phase increases by 34–46% with ageing.Recommendation: to make the protocol simpler, the duration of the gastric phase should be increased by 50% to 3 h in the older adult model.
Gastric enzymes
Studies on enzymatic activity in the ageing stomach are scarce and there is a marked lack of knowledge about pepsin and gastric lipase activities in the postprandial state in older adult populations. Nevertheless, the atrophy of gastric mucosa with age results in a gradual loss of secretory cells (chief cells for pepsin and gastric lipase; parietal cells for gastric acid secretion) that results in the reduced secretion of both enzymes and gastric acid.65,66Recommendation: based on these results, the consortium proposes to set the pepsin activity at 1200 U ml−1 of gastric content in the older adult model (i.e., 60% of the recommended value in the young adult model of Brodkorb et al.5).
Recommendation: for this reason, it is recommended to reduce the gastric lipase activity by 40% in the older adult model compared to the young adult digestion model from Brodkorb et al.,5 identically to the recommendation for pepsin, i.e. 36 U of lipase per ml of gastric content.
4 Intestinal phase
SIF composition
Electrolyte composition of intestinal fluids in older adults has not been reported precisely so far. However, in a study of the pancreatic exocrine secretions of 180 patients aged from 16 to 83 years, it was shown that calcium concentration was lowest around 41 years and then increased over time,68 following the equation:| [Ca2+] = 0.01 × age + 0.35 |
Recommendation: if we consider a 65 years old person, the calcium concentration in the SIF should be set at 1 mM rather than 0.6 mM in the young adult INFOGEST model.
Chyme/secretions ratio
This parameter is extremely difficult to assess. Nevertheless, one thing to consider is the decrease of pancreatic secretions with age that, if demonstrated, could eventually limit the dilution of the gastric chyme. Here again, data from the literature are controversial. Fikry (1968) investigated pancreatic exocrine secretions in 23 healthy males aged from 60 to 72 years using the intravenous secretin test considered by investigators in the field of pancreatic diseases as the gold standard.69 The data collected showed a two-third reduction in the volume of pancreatic secretions in older compared to young adults.69 The mean volume of secretions produced over 80 min by the older adults was 55.5 mL (30–81.5 mL) whereas it was around 193 mL (123–310 mL) for the younger group (the mean age of the control population was not provided in this study). This reduced volume of secretions might be attributed to a sole or combined action of three factors: (1) the ageing process itself, (2) the frequency of the chronic fibrosing pancreatitis in the aged population, favoured by increased incidence of gallstone formation, and (3) impairment of the vascular supply to the pancreas. A reduction in pancreatic secretions with age was also observed in another study that reported a linear decrease in secretory volume after 60 years,70 following this equation:| Pancreatic secretion volume (mL) = −6.5 × age + 620.9 |
In contrast, other robust studies using similar methodologies reported no differences in the volume of pancreatic secretions with age. Dreiling et al. (1985) found no significant changes in the volume of pancreatic secretions after 50 years on a large group of 1615 subjects.59 Similarly, in another study involving three groups of volunteers with a mean age of 40 years (n = 30), 64 years (n = 15) and 74 years (n = 10), no significant differences in the volume of pancreatic secretions were observed between the groups.71
Recommendation: based on these controversial data, it is difficult to determine whether pancreatic secretions tend to decrease during ageing or not. Since the study by Dreiling et al. (1985) involved the highest number of volunteers (i.e., 1615), including 1034 volunteers over 60 years, the recommendation to keep the gastric chyme/secretions ratio as proposed in the young adult INFOGEST model is based on these results i.e. the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) dilution of the gastric content with SIF.
1 (v/v) dilution of the gastric content with SIF.
Duration
While the duration of the gastric phase can be assessed by recording gastric emptying, the duration of the intestinal phase is not often monitored. An indication of the duration of the intestinal phase of digestion can be obtained by looking at the oro-cecal transit time that corresponds to the sum of the gastric, small and large intestinal phases or at the whole gut transit time. In a recent study, 111 healthy volunteers (21–88 years) had a 602 kcal breakfast consisting in oats/cornflakes, 1 tablespoon raisins/2 teaspoons sugar, skimmed milk, 1 slice wholegrain bread with plant-based margarine and 1 portion jam or ham.72 Immediately after having the breakfast, volunteers ingested a 3D-Transit system (Motilis Medica SA, Lausanne, Switzerland) consisting of ingestible electromagnetic capsules which when activated and swallowed emitted an electromagnetic tracking signal that was detected by an external detector plate positioned over the abdomen. The progress of the capsules in the gastrointestinal tract allowed measurement of gastric emptying and small intestinal, colonic and whole gut transit times. Results showed an increase of the gastric emptying time (as already discussed in the Gastric Phase section) and colonic transit time leading to an overall increase of the whole gut transit time (p < 0.01) with age. However, no significant change in small intestinal transit time was observed with age.Clarkston et al. (1997) measured (1) gastric emptying (by scintigraphy), (2) orocecal transit (through breath hydrogen) and (3) total gut transit (with radiopaque markers) in 19 younger (23–50 years) and 14 older (70–84 years) volunteers.64 Gastric emptying (T1/2) for solid (182 ± 26 vs. 127 ± 13 min, p < 0.05) and liquid (47 ± 4 vs. 35 ± 3 min, p < 0.05) meal components was slower in the older adults. However, there were no significant differences in the orocecal and total gut transit times between the two groups. Ageing seems to be associated with slowing of solid and liquid gastric content emptying (see paragraph on gastric phase duration) but no change in orocecal and total gut transit was observed.64
Finally, another study investigating lactose malabsorption did not find any statistical difference in orocecal transit time between three groups of subjects, aged <65 years (45 ± 15 years, n = 33), 65–74 years (69 ± 3 years, n = 17) and >74 years (81 ± 4 years, n = 34).73
Recommendation: based on the data, the duration of the intestinal phase of the older adult in vitro digestion model should be set at 2 h, which is the same as in the INFOGEST younger adult model.
Pancreatic enzymes
On the contrary, two other studies found a significant decrease in pancreatic lipase activity with age. In a study involving 180 volunteers (102 males, 78 females) aged 16–83 years, Laugier et al. found that the decrease in lipase activity as a function of age was following the equation:68
| lipase = −8.4 × age + 1603 |
By dividing the volunteers into two groups (younger and older than 65 years), they reported values of 1256 IU mL−1 and 994 IU mL−1 of pancreatic juice respectively, indicating a 21% decrease in intestinal lipase activity with age. These activities were measured with olive oil as substrate according to the US and European Pharmacopeia assay for pancreatic lipase. INFOGEST recommends another assay with tributyrin as substrate for pancreatic lipase.5,74 Nevertheless, a conversion factor of 2.8 allows converting USP lipase units into tributyrin units was recently proposed.75 The values reported by Laugier et al. for the two groups correspond to 3517 and 2783 U mL−1 of pancreatic juice, and these activity values are in the same range as the activity (4000 U mL−1) recently reported for human pancreatic juice.75 They can be compared with the 2000 U mL−1 currently recommended by INFOGEST for pancreatic lipase in the intestinal phase for healthy adults, i.e. a dilution of pancreatic juice by around a factor 2. Vellas et al. (1988) also observed a decrease in intestinal lipase activity with age.76 Twenty-seven subjects (36 ± 7.8 years) and 28 subjects (72 ± 3.2 years), with no clinical or radiological evidence of digestive disease, were selected. Duodenal aspirates (over a 60 min period) were obtained during continuous infusion of secretin (0.5 U kg−1 h−1) and caerulein (75 ng kg−1 h−1). Both lipase output and concentration, measured with olive oil as substrate (Pharmacopeia assay), were significantly reduced in the older adult group by 15.6% and 43.3%, respectively.
Furthermore, in a rather early study, two groups of healthy men were examined (mean age of the first group 24.7 ± 3.6 years, n = 10, mean age of the second group 67.2 ± 6.3 years, n = 10). In all subjects the exocrine pancreatic secretion was examined after repeated stimulation of the pancreas.77 No significant difference in pancreatic amylase output was observed between the two groups after one stimulation of the pancreas. However, repeated stimulations resulted in a significant decrease of about 35% in amylase output in the older group. These findings suggest some exhaustion of the pancreatic secretion function in old age. Finally, Dreiling et al. (1985) did not find any statistically significant difference in duodenal amylase activity with age, although a 25% difference in amylase activity was observed between volunteers in the 50–59 years group compared to volunteers in the 70–79 years group.59
Recommendation: since pancreatic enzymes are provided by the addition of pancreatin, it is recommended to decrease the amount of pancreatin (expressed by trypsin activity) in the older adult model by 20% compared to the young adult in vitro digestion model, i.e. 80 U trypsin per mL of intestinal content (or 1600 U pancreatic lipase per mL).
Bile
Only two studies investigating the effect of ageing on bile concentration and providing interpretable values were found in the literature. In the first one, a 38% decrease in bile acids synthesis with age was reported (1.32 mM per day under 40 years and 0.81 mM per day over 65 years, n = 60).78In another study involving only 24 subjects, a 33% decrease in postprandial conjugated and unconjugated serum bile acids levels was observed with ageing.79
Recommendation: based on these limited data, it is recommended to reduce the amount of bile salts in the intestinal phase by 33% in the older adult model compared to the young adult INFOGEST model, i.e. the reduction to 6.7 mM bile salts.
5 Conclusion
In conclusion, the exhaustive literature search that was conducted within the EAT4AGE consortium, as well as the exchanges that were held in the framework of the INFOGEST international network on food digestion have allowed for design of a static in vitro digestion model representative of bolus properties after oral processing and of the physiology of the gastrointestinal tract of an older, healthy adult. The most important differences relative to the INFOGEST young adult model correspond to different pH and duration of the gastric phase, different activities of the digestive enzymes in the stomach and small intestine and the concentration of bile salts (Table 3). The oral phase might also be different especially for denture wearers or people suffering from xerostomia or dysphagia. Nevertheless, it has to be noted that for some parameters, the values considered in the proposed model were based on a limited number of rather old publications and new data would be of paramount importance to refine the model in future studies. In the coming months, EAT4AGE partners will apply the proposed in vitro digestion model of the older adult to three types of food matrices (cereal-based, dairy and meat products) and compare the data with those obtained with the young adult in vitro model as well as with already published in vivo data, when available.| Phase | Parameter | Adult | Elderly |
|---|---|---|---|
| SSF: simulated salivary fluid, SGF: simulated gastric fluid, SIF: simulated intestinal fluid. DNS: 3,5-dinitrosalicyclic acid. TAME: p-toluene-sulfonyl-L-arginine methyl ester. | |||
| Oral | SSF composition | See Brodkorb et al. (2019)5 | Same |
Food![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SSF dilution SSF dilution |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1 1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 according to DM 1 according to DM |
|
| pH | 7.0 | 7.0 | |
| Duration | 2 min | 2 min | |
| Chewing protocol | Dilute food with SSF at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (wt/wt) to achieve a swallowable bolus with a paste-like consistency. If necessary, simulate mastication by mincing the food in an electric or manual mincer 1 (wt/wt) to achieve a swallowable bolus with a paste-like consistency. If necessary, simulate mastication by mincing the food in an electric or manual mincer |
Use of a basic meat mincer (manual, consisting of a 5 cm mincing disk, a 0.5 cm mesh size and a blade, only one pass) to produce food particles, then add SSF at pH 7 (with salivary amylase in case of starch-containing foods) or saliva at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
| Amylase | 75 U ml−1 (using DNS as substrate, see Brodkorb et al. 2019)5 | 75 U ml−1 (using DNS as substrate, see Brodkorb et al. 2019)5 | |
| Gastric | SGF composition | See Brodkorb et al. (2019)5 | Same |
Bolus![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SGF dilution SGF dilution |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
| pH | 3.0 | 3.7 | |
| Duration | 2 h | 3 h | |
| Pepsin | 2000 U ml−1 of gastric content (using haemoglobin as substrate, see Brodkorb et al. 2019)5 | 1200 U ml−1 of gastric content (using haemoglobin as substrate, see Brodkorb et al. 2019)5 | |
| Gastric lipase | 60 U ml−1 of gastric content (using tributyrin as substrate, see Brodkorb et al. 2019)5 | 36 U ml−1 of gastric content (using tributyrin as substrate, see Brodkorb et al. 2019)5 | |
| Intestinal | SIF composition | See Brodkorb et al. (2019)5 | Same but with [Ca2+] = 1 mM |
Chyme![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SIF dilution SIF dilution |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|
| pH | 7.0 | 7.0 | |
| Duration | 2 h | 2 h | |
| Pancreatin | 100 U ml−1 trypsin (using TAME as substrate, see Brodkorb et al. 2019)5 | 80 U ml−1 trypsin (using TAME as substrate, see Brodkorb et al. 2019)5 | |
| Bile salts | 10 mM bile salts | 6.7 mM bile salts | |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The present work was initiated within the research project EAT4AGE “Palatable, nutritious and digestible foods for prevention of undernutrition in active aging” (https://nofima.com/projects/eat4age/). The authors are all participants of the INFOGEST network on Food Digestion (https://www.cost-infogest.eu) and wish to thank INRAE for financially supporting the network.References
- Z. Vinarov, B. Abrahamsson, P. Artursson, H. Batchelor, P. Berben and A. Bernkop-Schnurch, et al., Current challenges and future perspectives in oral absorption research: An opinion of the UNGAP network, Adv. Drug Delivery Rev., 2021, 171, 289–331 CrossRef CAS PubMed.
- G. Mandalari, A. M. Mackie, N. M. Rigby, M. S. J. Wickham and E. N. C. Mills, Physiological phosphatidylcholine protects bovine b-lactoglobulin from simulated gastrointestinal proteolysis, Mol. Nutr. Food Res., 2009, 53, 131–139 CrossRef PubMed.
- T. Bohn, F. Carriere, L. Day, A. Deglaire, L. Egger and D. Freitas, et al., Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models?, Crit. Rev. Food Sci. Nutr., 2018, 58(13), 2239–2261 CrossRef CAS PubMed.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn and C. Bourlieu, et al. A standardised static in vitro digestion method suitable for food - an international consensus, Food Funct., 2014, 5(6), 1113–1124 RSC.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assuncao and S. Ballance, et al., INFOGEST static in vitro simulation of gastrointestinal food digestion, Nat. Protoc., 2019, 14(4), 991–1014 CrossRef CAS PubMed.
- R. Colombo, L. Ferron, I. Frosi and A. Papetti, Advances in static in vitro digestion models after the COST action Infogest consensus protocol, Food Funct., 2021, 12(17), 7619–7636 RSC.
- C. Shani-Levi, P. Alvito, A. Andres, R. Assuncao, R. Barbera and S. Blanquet-Diot, et al., Extending in vitro digestion models to specific human populations: Perspectives, practical tools and bio-relevant information, Trends Food Sci. Technol., 2017, 60, 52–63 CrossRef CAS.
- D. Dupont, G. Mandalari, D. Molle, J. Jardin, O. Rolet-Repecaud and G. Duboz, et al., Food processing increases casein resistance to simulated infant digestion, Mol. Nutr. Food Res., 2010, 54(11), 1677–1689 CrossRef CAS PubMed.
- R. Lacomba, J. Salcedo, A. Alegría, R. Barberá, P. Hueso and E. Matencio, et al., Effect of Simulated Gastrointestinal Digestion on Sialic Acid and Gangliosides Present in Human Milk and Infant Formulas, J. Agric. Food Chem., 2011, 59(10), 5755–5762 CrossRef CAS PubMed.
- T. T. P. Nguyen, B. Bhandari, J. Cichero and S. Prakash, Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study, Food Res. Int., 2015, 76, 348–358 CrossRef CAS PubMed.
- Y. Wada and B. Lonnerdal, Effects of Industrial Heating Processes of Milk-Based Enteral Formulas on Site-Specific Protein Modifications and Their Relationship to in Vitro and in Vivo Protein Digestibility, J. Agric. Food Chem., 2015, 63(30), 6787–6798 CrossRef CAS PubMed.
- O. Menard, C. Bourlieu, S. C. de Oliveira, N. Dellarosa, L. Laghi and F. Carriere, et al., A first step towards a consensus static in vitro model for simulating full-term infant digestion, Food Chem., 2018, 240, 338–345 CrossRef CAS PubMed.
- R. Doerfler, J. R. Melamed and K. A. Whitehead, The Effect of Infant Gastric Digestion on Human Maternal Milk Cells, Mol. Nutr. Food Res., 2022, 66(19), 2200090 CrossRef CAS PubMed.
- J. Calvo-Lerma, P. Bueno-Llamoga, C. Bauerl, E. Cortes-Macias, M. Selma-Royo and F. Perez-Cano, et al., Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion, Nutrients, 2022, 14(10), 2117 CrossRef CAS PubMed.
- J. Tang, H. J. Wichers and K. A. Hettinga, Glycation of soy and pea proteins influences infant gastric digestibility more than intestinal digestibility, Food Hydrocolloids, 2023, 136(A), 108251 CrossRef CAS.
- Q. Ren, M. Boiani, T. He, H. J. Wichers and K. A. Hettinga, Heating affects protein digestion of skimmed goat milk under simulated infant conditions, Food Chem., 2023, 402, 134261 CrossRef CAS PubMed.
- A. Sarkar, Oral processing in elderly: understanding eating capability to drive future food texture modifications, Proc. Nutr. Soc., 2019, 78(3), 329–339 CrossRef PubMed.
- S. Ribes, M. Genot, L. Aubry, P. Talens, A. Vénien and V. Santé-Lhoutellier, et al. Oral impairments decrease the nutrient bioaccessibility of bread in the elderly, Food Hydrocolloids, 2023, 135, 108202 CrossRef CAS.
- C. Yven, J. Patarin, A. Magnin, H. Laboure, M. Repoux and E. Guichard, et al., Consequences of individual chewing strategies on bolus rheological properties at the swallowing threshold, J. Texture Stud., 2012, 43(4), 309–318 CrossRef.
- D. Remond, D. R. Shahar, D. Gille, P. Pinto, J. Kachal and M. A. Peyron, et al., Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition, Oncotarget, 2015, 6(16), 13858–13898 CrossRef PubMed.
- C. S. Levi and U. Lesmes, Bi-compartmental elderly or adult dynamic digestion models applied to interrogate protein digestibility, Food Funct., 2014, 5(10), 2402–2409 RSC.
- E. Hernandez-Olivas, S. Munoz-Pina, A. Andres and A. Heredia, Impact of elderly gastrointestinal alterations on in vitro digestion of salmon, sardine, sea bass and hake: Proteolysis, lipolysis and bioaccessibility of calcium and vitamins, Food Chem., 2020, 326, 127024 CrossRef CAS PubMed.
- A. M. Plante, A. L. McCarthy and F. O'Halloran, The effect of elderly simulated gastrointestinal in vitro digestion (SGID) on the antioxidant properties of different cheese matrices, Proc. Nutr. Soc., 2020, 79(OCE2), E412 CrossRef.
- K. Aalaei, B. Khakimov, C. De Gobba and L. Ahrne, Digestion patterns of proteins in pasteurized and ultra-high temperature milk using in vitro gastric models of adult and elderly, J. Food Eng., 2021, 292, 786 CrossRef.
- S. Y. Lee, D. Y. Lee, J. H. Kang, J. H. Kim, H. W. Kim and D. H. Oh, et al., Effect of age-related in vitro human digestion with gut microbiota on antioxidative activity and stability of vitamins, LWT – Food Sci. Technol., 2022, 159, 113243 CrossRef CAS.
- WHO, World report on ageing and health, 2014, vol. 260 Search PubMed.
- D. E. Forman, A. D. Berman, C. H. McCabe, D. S. Baim and J. Y. Wei, PTCA in the elderly: the “young-old” versus the “old-old”, J. Am. Geriatr. Soc., 1992, 40(1), 19–22 CrossRef CAS PubMed.
- C. A. Zizza, K. J. Ellison and C. M. Wernette, Total water intakes of community-living middle-old and oldest-old adults, J. Gerontol., Ser. A, 2009, 64(4), 481–486 CrossRef PubMed.
- R. Nagler and O. Hershkovich, Relationships between age, drugs, oral sensorial complaints and salivary profile, Arch. Oral Biol., 2005, 50(1), 7–16 CrossRef PubMed.
- M. Nassar, N. Hiraishi, M. Islam, M. Otsuki and J. Tagami, Age-related changes in salivary biomarkers, J. Dent. Sci., 2014, 9(1), 85–90 CrossRef.
- H. Islas-Granillo, S. A. Borges-Yanez, C. E. Medina-Solis, C. A. Galan-Vidal, J. J. Navarrete-Hernandez and M. Escoffie-Ramirez, et al., Salivary Parameters (Salivary Flow, pH and Buffering Capacity) in Stimulated Saliva of Mexican Elders 60 Years Old and Older, West Indian Med. J., 2014, 63(7), 758–765 CAS.
- S. C. Leal, J. Bittar, A. Portugal, D. P. Falcao, J. Faber and P. Zanotta, Medication in elderly people: its influence on salivary pattern, signs and symptoms of dry mouth, Gerodontology, 2010, 27(2), 129–133 CrossRef PubMed.
- T. Osterberg, S. Landahl and B. Hedegård, Salivary flow, saliva, pH and buffering capacity in 70-year-old men and women. Correlation to dental health, dryness in the mouth, disease and drug treatment, J. Oral Rehabil., 1984, 11(2), 157–170 CrossRef CAS PubMed.
- M. Shaila, G. P. Pai and P. Shetty, Salivary protein concentration, flow rate, buffer capacity and pH estimation: A comparative study among young and elderly subjects, both normal and with gingivitis and periodontitis, J. Indian Soc. Periodontol., 2013, 17(1), 42–46 CrossRef PubMed.
- M. Assad-Bustillos, C. Tournier, C. Septier, G. Della-Valle and G. Feron, Relationships of oral comfort perception and bolus properties in the elderly with salivary flow rate and oral health status for two soft cereal foods, Food Res. Int., 2019, 118(SI), 13–21 CrossRef CAS PubMed.
- M. Assad-Bustillos, C. Tournier, J. Palier, C. Septier, G. Feron and G. Della-Valle, Oral processing and comfort perception of soft cereal foods fortified with pulse proteins in the elderly with different oral health status, Food Funct., 2020, 11(5), 4535–4547 RSC.
- L. Lorieau, J. Floury, C. Septier, A. Laguerre, L. Le Roux and E. Hazart, et al., Relationship among oral health status, bolus formation and food comfortability during consumption of model cheeses in elderly, Food Funct., 2021, 12(16), 7379–7389 RSC.
- C. Tournier, C. L. Goff, C. Septier, M. Saint-Jalmes, G. Feron and C. Bonhomme, et al., Formulation d'aliments à destination des séniors : exemple d'une approche in vivo multidisciplinaire intégrant physiologie orale, perception sensorielle, prise alimentaire et digestion, Nutr. Clin. Metab., 2020, 34(1), 55–56 CrossRef.
- S. R. Drago, M. Panouille, A. Saint-Eve, E. Neyraud, G. Feron and I. Souchon, Relationships between saliva and food bolus properties from model dairy products, Food Hydrocolloids, 2011, 25(4), 659–667 CrossRef CAS.
- M. Repoux, H. Laboure, P. Courcoux, I. Andriot, E. Semon and C. Yven, et al., Combined effect of cheese characteristics and food oral processing on in vivo aroma release, Flavour Fragrance J., 2012, 27(6, SI), 414–423 CrossRef CAS.
- M. Doyennette, I. Deleris, G. Feron, E. Guichard, I. Souchon and I. C. Trelea, Main individual and product characteristics influencing in-mouth flavour release during eating masticated food products with different textures: Mechanistic modelling and experimental validation, J. Theor. Biol., 2014, 340, 209–221 CrossRef CAS PubMed.
- A. Saint-Eve, M. Panouillé, C. Capitaine, I. Déléris and I. Souchon, Dynamic aspects of texture perception during cheese consumption and relationship with bolus properties, Food Hydrocolloids, 2015, 46, 144–152 CrossRef CAS.
- A. I. Mulet-Cabero, L. Egger, R. Portmann, O. Menard, S. Marze and M. Minekus, et al., A standardised semi-dynamic in vitro digestion method suitable for food - an international consensus, Food Funct., 2020, 11(2), 1702–1720 RSC.
- D. Freitas, S. Le Feunteun, M. Panouille and I. Souchon, The important role of salivary alpha-amylase in the gastric digestion of wheat bread starch, Food Funct., 2018, 9(1), 200–208 RSC.
- D. Freitas, F. Boue, M. Benallaoua, G. Airinei, R. Benamouzig and E. Lutton, et al., Glycemic response, satiety, gastric secretions and emptying after bread consumption with water, tea or lemon juice: a randomized crossover intervention using MRI, Eur. J. Nutr., 2022, 61(3), 1621–1636 CrossRef CAS PubMed.
- H. Pajukoski, J. H. Meurman, S. Snellman-Gröhn, S. Keinänen and R. Sulkava, Salivary flow and composition in elderly patients referred to an acute care geriatric ward, Oral Surg., Oral Med., Oral Pathol., 1997, 84(3), 265–271 CrossRef CAS PubMed.
- H. Pajukoski, J. H. Meurman, P. Halonen and R. Sulkava, Prevalence of subjective dry mouth and burning mouth in hospitalized elderly patients and outpatients in relation to saliva, medication, and systemic diseases, Oral Surg., Oral Med., Oral Pathol., 2001, 92(6), 641–649 CrossRef CAS PubMed.
- P. M. Helfman and P. A. Price, Human parotid α-amylase—A test of the error theory of aging, Exp. Gerontol., 1974, 9(5), 209–214 CrossRef CAS PubMed.
- T. Mueller, M. Naharro and G. E. Carlsson, What are the prevalence and incidence of tooth loss in the adult and elderly population in Europe?, Clin. Oral Implants Res., 2007, 18(3), 2–14 CrossRef PubMed.
- M. Vandenberghe-Descamps, H. Laboure, A. Prot, C. Septier, C. Tournier and G. Feron, et al., Salivary flow decreases in healthy elderly people independently of dental status and drug intake, J. Texture Stud., 2016, 47(4), 353–360 CrossRef.
- F. Fontijn-Tekamp, A. van der Bilt, J. Abbink and F. Bosman, Swallowing threshold and masticatory performance in dentate adults, Physiol. Behav., 2004, 83(3), 431–436 CrossRef CAS PubMed.
- M. G. Aguayo-Mendoza, E. F. Martinez-Almaguer, B. Piqueras-Fiszman and M. Stieger, Differences in oral processing behavior of consumers varying in age, gender and ethnicity lead to changes in bolus properties but only to small differences in dynamic texture perception of sausages, Food Funct., 2020, 11(11), 10022–10032 RSC.
- A. Mishellany-Dutour, J. Renaud, M. A. Peyron, F. Rimek and A. Woda, Is the goal of mastication reached in young dentates, aged dentates and aged denture wearers?, Br. J. Nutr., 2008, 99(1), 121–128 CrossRef CAS PubMed.
- H. Sugimoto, Y. Tanaka, N. Kodama and S. Minagi, Differences in comminution progress during mastication in healthy dentate and denture-wearing elderly people, J. Prosthodont Res., 2020, 64(3), 257–263 CrossRef PubMed.
- M. A. Peyron, A. Woda, P. Bourdiol and M. Hennequin, Age-related changes in mastication, J. Oral Rehabil., 2017, 44(4), 299–312 CrossRef CAS PubMed.
- Y. Zhu and J. H. Hollis, Differences in chewing behaviors between healthy fully dentate young and older adults assessed by electromyographic recordings, Int. J. Food Sci. Nutr., 2015, 66(4), 452–457 CrossRef PubMed.
- D. Freitas, I. Souchon and S. Le Feunteun, The contribution of gastric digestion of starch to the glycaemic index of breads with different composition or structure, Food Funct., 2022, 13(4), 1718–1724 RSC.
- T. L. Russell, R. R. Berardi, J. L. Barnett, L. C. Dermentzoglou, K. M. Jarvenpaa and S. P. Schmaltz, et al., Upper Gastrointestinal pH in Seventy-Nine Healthy, Elderly, North American Men and Women, Pharm. Res., 1993, 10(2), 187–196 CrossRef CAS PubMed.
- D. A. Dreiling, A. T. Triebling and M. Koller, The effect of age on human exocrine pancreatic secretion, Mt. Sinai J. Med., 1985, 52(5), 336–339 CAS.
- O. Menard, T. Cattenoz, H. Guillemin, I. Souchon, A. Deglaire and D. Dupont, et al., Validation of a new in vitro dynamic system to simulate infant digestion, Food Chem., 2014, 145, 1039–1045 CrossRef CAS PubMed.
- M. Firth and C. Prather, Gastrointestinal motility problems in the elderly patient, Gastroenterology, 2002, 122(6), 1688–1700 CrossRef PubMed.
- A. Brogna, R. Ferrara, A. M. Bucceri, E. Lanteri and F. Catalano, Influence of aging on gastrointestinal transit time. An ultrasonographic and radiologic study, Invest Radiol., 1999, 34(5), 357–359 CrossRef CAS PubMed.
- V. Di Francesco, M. Zamboni, A. Dioli, E. Zoico, G. Mazzali and F. Omizzolo, et al., Delayed postprandial gastric emptying and impaired gallbladder contraction together with elevated cholecystokinin and peptide YY serum levels sustain satiety and inhibit hunger in healthy elderly persons, J. Gerontol., Ser. A, 2005, 60(12), 1581–1585 CrossRef PubMed.
- W. K. Clarkston, M. M. Pantano, J. E. Morley, M. Horowitz, J. M. Littlefield and F. R. Burton, Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults, Am. J. Physiol., 1997, 272(1 Pt 2), R243–R248 CAS.
- M. Feldman, B. Cryer, K. E. McArthur, B. A. Huet and E. Lee, Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study, Gastroenterology, 1996, 110(4), 1043–1052 CrossRef CAS PubMed.
- E. Lahner, M. Carabotti and B. Annibale, Atrophic Body Gastritis: Clinical Presentation, Diagnosis, and Outcome, EMJ Gastroenterol., 2017, 6, 75–82 Search PubMed.
- H. Moreau, J. F. Sauniere, Y. Gargouri, G. Pieroni, R. Verger and H. Sarles, Human gastric lipase: variations induced by gastrointestinal hormones and by pathology, Scand. J. Gastroenterol., 1988, 23(9), 1044–1048 CrossRef CAS PubMed.
- R. Laugier, J. P. Bernard, P. Berthezene and P. Dupuy, Changes in Pancreatic Exocrine Secretion with Age: Pancreatic Exocrine Secretion Does Decrease in the Elderly, Digestion, 1991, 50(3–4), 202–211 CAS.
- M. E. Fikry, Exocine pancreatic functions in the aged, J. Am. Geriatr. Soc., 1968, 16(4), 463–467 CrossRef CAS PubMed.
- T. Ishibashi, S. Matsumoto, H. Harada, K. Ochi, J. Tanaka and T. Seno, et al., Aging and exocrine pancreatic function evaluated by the recently standardized secretin test, Jpn. J. Geriatr., 1991, 28(5), 599–605 CrossRef CAS PubMed.
- L. Gullo, P. Priori, C. Daniele, M. Ventrucci, G. Gasbarrini and G. Labò, Exocrine pancreatic function in the elderly, Gerontology, 1983, 29(6), 407–411 CrossRef CAS PubMed.
- G. K. Nandhra, E. B. Mark, G. L. Di Tanna, A. M. Haase, J. Poulsen and S. Christodoulides, et al., Normative values for region-specific colonic and gastrointestinal transit times in 111 healthy volunteers using the 3D-Transit electromagnet tracking system: Influence of age, gender, and body mass index, Neurogastroenterol. Motil., 2020, 32(2), e13734 CrossRef PubMed.
- M. Di Stefano, G. Veneto, S. Malservisi, A. Strocchi and G. R. Corazza, Lactose malabsorption and intolerance in the elderly, Scand. J. Gastroenterol., 2001, 36(12), 1274–1278 CrossRef CAS PubMed.
- M. M. L. Grundy, E. Abrahamse, A. Almgren, M. Alminger, A. Andres and R. M. C. Ariens, et al., INFOGEST inter-laboratory recommendations for assaying gastric and pancreatic lipases activities prior to in vitro digestion studies, J. Funct. Foods, 2021, 82, 104497 CrossRef CAS.
- A. Salhi, S. Amara, P. Mansuelle, R. Puppo, R. Lebrun and B. Gontero, et al., Characterization of all the lipolytic activities in pancreatin and comparison with porcine and human pancreatic juices, Biochimie, 2020, 169(SI), 106–120 CrossRef CAS PubMed.
- B. Vellas, D. Balas, J. Moreau, M. Bouisson, F. Senegas-Balas and M. Guidet, et al., Exocrine pancreatic secretion in the elderly, Int. J. Pancreatol., 1988, 3(6), 497–502 CrossRef CAS PubMed.
- V. Bartos and J. Groh, The effect of repeated stimulation of the pancreas on the pancreatic secretion in young and aged men, Gerontol. Clin., 1969, 11(1), 56–62 CrossRef CAS PubMed.
- K. Einarsson, K. Nilsell, B. Leijd and B. Angelin, Influence of Age on Secretion of Cholesterol and Synthesis of Bile Acids by the Liver, N. Engl. J. Med., 1985, 313(5), 277–282 CrossRef CAS PubMed.
- J. M. J. I. Salemans, F. M. Nagengast, A. Tangerman, A. V. Schaik, W. P. M. Hopman and A. F. J. D. Haan, et al., Effect of ageing on postprandial conjugated and unconjugated serum bile acid levels in healthy subjects, Eur. J. Clin. Invest., 1993, 23(3), 192–198 CrossRef CAS PubMed.
Footnote |
| † Co first-authors who contributed equally to the present work. |
| This journal is © The Royal Society of Chemistry 2023 |
