Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metabolism disturbance by light/dark cycle switching depends on the rat health status: the role of grape seed flavanols†
Jorge R.
Soliz-Rueda
 abc,
Raúl
López-Fernández-Sobrino
a,
Cristina
Torres-Fuentes
abc,
Raúl
López-Fernández-Sobrino
a,
Cristina
Torres-Fuentes
 abc,
Francisca I.
Bravo
abc,
Francisca I.
Bravo
 abc,
Manuel
Suárez
abc,
Manuel
Suárez
 abc,
Miquel
Mulero
abc,
Miquel
Mulero
 abc and
Begoña
Muguerza
abc and
Begoña
Muguerza
 *abc
*abc
aUniversity Rovira i Virgili, Biochemistry and Biotechnology Department, Nutrigenomics Research Group, Tarragona, 43007, Spain. E-mail: begona.muguerza@urv.cat
bPere Virgili Institute for Health Research (IISPV), Tarragona 43007, Spain
cCenter of Environmental, Food and Toxicological Technology (TecnATox), University Rovira i Virgili, 43007 Tarragona, Spain
First published on 28th June 2023
Abstract
Changes in light/dark cycles and obesogenic diets are related to the disruption of circadian rhythms and metabolic disorders. Grape seed flavanols have shown beneficial effects on metabolic diseases and, recently, a circadian system modulation has been suggested to mediate their health-enhancing properties. Therefore, the aim of this study was to evaluate the grape seed (poly)phenol extract (GSPE) effects in healthy and obese rats after a light/dark cycle disruption. Forty-eight rats were fed a standard (STD) or cafeteria (CAF) diet for 6 weeks under STD conditions of a light/dark cycle (12 h light per day, L12). Then, animals were switched to a long (18 h light per day, L18) or short (6 h light per day, L6) photoperiod and administered a vehicle (VH) or GSPE (25 mg kg−1) for 1 week. The results showed changes in serum lipids and insulin and metabolomic profiles dependent on the photoperiod and animal health status. GSPE administration improved serum parameters and increased the Nampt gene expression in CAF rats and modified the metabolomic profile in a photoperiod-dependent manner. Metabolic effects of light/dark disturbance depend on the health status of the rats, with diet-induced CAF-induced obese rats being more affected. Grape seed flavanols improve the metabolic status in a photoperiod-dependent manner and their effects on the circadian system suggest that part of their metabolic effects could be mediated by their action on biological rhythms.
Introduction
Living organisms are able to detect modifications in the external environment and promote physiological and metabolic changes to adapt their biological rhythms to the external cues and to be more energetically efficient.1,2 Light/dark cycles generated by the rotation of the Earth around its axis and its translation around the sun reset the rhythmicity of processes such as blood pressure, body temperature and the sleep–wake cycle, among others.3,4 The central clock, located in the hypothalamic suprachiasmatic nucleus (SCN), is the pacemaker of the body rhythms and sends signals to the rest of the oscillators present in different tissues. In this context, light, which is captured by the retina through the retinohypothalamic tract (RHT), is the most important external synchronizer or zeitgeber of biological rhythms.2,5 In addition to light, food can also modulate and reprogram the diurnal oscillations of the body.2,6,7 Nevertheless, misaligning cues, such as alterations in light/dark cycles and consumption of calorie-dense foods, can induce the disruption of diurnal rhythmicity and trigger the appearance of metabolic disorders.8 The disruption of the hypothalamic–pituitary (HP) axis by alteration of the normal light/dark cycle has also been related to detrimental metabolic consequences.9 The HP axis plays a crucial role in the control of the endocrine system crucial to maintain homeostasis of metabolism and synchrony of timing across and within tissues. Hypothalamic–pituitary–adrenal (HPA), –gonadal (HPG) and –thyroid (HPT) are three main endocrine axes, which regulate the functionality of the adrenal gland, the gonads, and the thyroid gland, respectively.10–12Metabolomics studies allow establishing relationships between metabolic disease and serum levels of particular metabolites.13 The study of metabolite concentrations in plasma from the Framingham Heart Study (n = 1015) and the Malmö Diet and Cancer Study (n = 746) revealed that metabolic risk factors were associated with multiple metabolites such as branched-chain amino acids (BCAAs) and other hydrophobic amino acids, tryptophan breakdown products, and nucleotide metabolites.14 Also, other studies have also reported a strong association between cardiometabolic risk factors and plasma metabolite levels such as high levels of pro-inflammatory mediators and metabolic disease with increased BCAA and aromatic amino acid levels,15 insulin resistance with high plasma alanine concentration13 or inflammation, oxidative stress, and mortality in patients which chronic kidney disease with low levels of histidine.16 Succinate has also been recently proposed as a biomarker of metabolic dysfunction since metabolic diseases such as diabetes and obesity are associated with chronic high levels of this metabolite.17 In addition, metabolomics studies highlight the role of biological rhythms in metabolism and allow the investigation of the molecular mechanisms involved in the regulation of metabolism by the clock system.18 In fact, the majority of the detected metabolites in serum show diurnal rhythmicity under normal physiological conditions.19 This rhythmicity has been associated with the coherent time of day communication to different tissues, maintenance of specific synchronization of peripheral clocks, and promotion of time-efficient activation of diurnal metabolic pathways.20 Nevertheless, the metabolome is very susceptible to diurnal disruption by nutrient challenge, and, for instance, changes at some time point over half of the serum metabolites have been observed in mice fed a high fat diet (HFD).21 In this context, it has been reported that diet composition may cause misalignment of the clock system, which may lead to metabolic disorders.20,22
Flavanols are a sub-class of flavonoids extensively studied for their beneficial effects.23 Interestingly, a modulating effect of grape seed flavanols on both central and peripheral biological rhythms has been demonstrated by our group in healthy Wistar rats.24 Moreover, different doses of grape seed flavanols administered to Wistar rats fed a cafeteria (CAF) diet for four weeks exerted a positive dose-dependent modulation of some components of the peripheral clock in liver, gut and white adipose tissue, including Bmal1, Nampt, important genes in the clock machinery and its integration in metabolism, SIRT1, and NAD+.25,26 A CAF diet, which consists of highly palatable, calorie-dense and unhealthy human food, is considered a robust model of human metabolic syndrome.27 Therefore, grape seed flavanols could modulate physiology and metabolism by adjusting the biological oscillation by clock system modulation in CAF-fed animals, improving the alteration caused by metabolic syndrome.28
Nevertheless, laboratory rats are generally unresponsive to light/dark shifts, although Fischer 344 (F344) rats have shown to be affected by photoperiods.29 F344 rats fed a CAF diet have also shown metabolic disorders and alteration in their biological rhythms,30,31 and the administration of grape seed flavanols to CAF diet-fed F344 rats resulted in diurnal modulation of hepatic mitochondrial homeostasis.32 In addition, we have also recently evidenced the protective effect of grape seed flavanols on the diurnal disruption caused by an abrupt light/dark shift in healthy and CAF-diet obese F344 rats.33 The study showed the impact of a CAF diet and photoperiod on body weight (BW), food intake, locomotor activity, and diurnal hormonal oscillation of the animals, and how flavanol administration contributed to the adaptation of the rats to the new light/dark cycles.33 Therefore, grape seed flavanols may act as zeitgebers for the molecular clock and contribute to restoring alterations caused by both light/dark shifts and diet-induced obesity.
Therefore, the aim of this study was to evaluate whether grape seed (poly)phenol extract (GSPE) rich in flavanols can ameliorate the metabolic disorders caused by a sudden change of the light/dark cycle in both healthy and CAF-induced obese rats. For this purpose, F344 rats fed a standard diet (STD) and CAF were abruptly transferred from STD conditions of a light/dark cycle (12 h of light per day, L12) to a long (18 h of light per day, L18) or short (6 h of light per day, L6) photoperiod, and the phenotypic biorhythm was evaluated after 1 week by measuring the serum melatonin, serum corticosterone, triiodothyronine (T3), thyroxine (T4), testosterone levels and metabolite levels. In addition, the gene expression of the central clock of these rats was studied.
Materials and methods
Grape seed (poly)phenol extract
The grape seed extract used in this study was provided by Les Dérives Résiniques et Terpéniques (Dax, France) and was obtained from white grape seeds. The phenolic profile of this extract is mainly composed of catechin, epicatechin, gallic acid, epicatechin gallate, and dimers, trimers and tetramers of proanthocyanidins.34Animal procedures
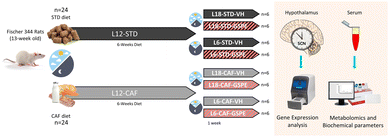
Forty-eight 13-week-old male F344/IcoCrl rats from Charles River Laboratories (Barcelona, Spain) were housed under the STD conditions at 22 °C and L12 (light density: 350 lux) with ad libitum access to food and drinking water. The F344/IcoCrl rats were seen to be responsive to photoperiods.31,35–38 After two weeks of the acclimatization period, rats were weighted and randomly divided into two groups (n = 24): one group (L12-STD) was fed a STD diet (72% carbohydrates, 8% lipids, and 19% protein; Safe-A04c, Scientific Animal Food and Engineering, Barcelona, Spain), and the other group (L12-CAF) was fed a CAF diet for 6 weeks. After this time, the animals were switched from a L12 to L18 or L6 photoperiod for 1 week. When the animals were transferred to the L18 photoperiod or L6 photoperiod, they received a daily vehicle (VH), which consisted of condensed milk diluted in water (1/5 v/v), or 25 mg kg−1 of BW of GSPE (Fig. 1). The VH and extract were orally administered by allowing rats to drink them from the tip of a syringe. This GSPE dose has been widely used by our group and has been shown to be the lowest and most effective dose for modulating many central metabolic pathways.25 In addition, using a translation of animal to human doses, corresponds to a daily intake of almost 300 mg of GSPE per day for a 70 kg human, which can be easily achieved by people who adhere to polyphenol-rich diets, such as a Mediterranean diet.39 The onset of light was at 8:00 a.m. and defined as zeitgeber time 0 (ZT0). When rats were transferred to each photoperiod, the time to turn off the lights was changed at 14:00 p.m. and 2:00 a.m. for L6 and L18, respectively, keeping the lights on at the same time. The VH and GSPE were administered at ZT0. Thus, the animals were finally grouped into 8 different groups (n = 6). The sacrifice of the rats was 3 h after the last dose (ZT3). Animals fed a STD diet were considered healthy groups for each photoperiod, as they had no metabolic alterations prior to the photoperiod change. The CAF diet was prepared every day and contained bacon, cookie with paté, cookie with cheese, carrots, ensaïmada (pastry), STD chow and sweetened milk (22% sucrose, w/v), and its caloric distribution was 56.43% carbohydrates, 45.72% lipids and 9.5% protein.40 After the experimental period, the animals were deprived of food for 3 hours before being sacrificed by decapitation. The hypothalamus samples were rapidly removed after death, frozen in liquid nitrogen and stored at −80 °C until further analyses. Blood was collected in non-heparinized tubes, incubated for 1 h at room temperature and immediately centrifuged at 1200g for 15 min at 4 °C to collect the serum.The animal procedures were approved by The Animal Ethics Committee of University Rovira i Virgili (Tarragona, Spain) and the Generalitat de Catalunya (reference number 9495, 18/09/19) and were carried out in accordance with Directive 86/609EEC of the Council of the European Union and the procedure established by the Departament d'Agricultura, Ramaderia i Pesca of the Generalitat de Catalunya.
Serum biochemical parameters and hormone levels
Enzymatic colorimetric assays were used for the analysis of serum glucose, total cholesterol (TC) and triglycerides (TAG) (QCA, Amposta, Tarragona, Spain) and non-esterified free fatty acids (NEFAs) (WAKO, Neuss, Germany) according to the manufacturer's instructions.Serum hormone concentrations were measured by liquid chromatography coupled to triple quadrupole mass spectrometry (LC-QqQ). Serum samples were thawed at 4 °C and 50 μL were mixed with 250 μL of methanol containing the internal STD (2 ng mL−1). Then, the mixture was vortexed and centrifuged for 5 minutes at 4 °C and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200g. The supernatant was transferred to a new tube and mixed with 700 μL of 0.1% formic acid in water. The sample was loaded onto an SPE cartridge previously conditioned with methanol and 0.1% formic acid in water. The cartridge was washed with 0.1% formic acid in water and dried under high vacuum. The compounds were eluted with 500 μL of methanol. The samples were evaporated in a SpeedVac at 45 °C and reconstituted with 50 μL of water
200g. The supernatant was transferred to a new tube and mixed with 700 μL of 0.1% formic acid in water. The sample was loaded onto an SPE cartridge previously conditioned with methanol and 0.1% formic acid in water. The cartridge was washed with 0.1% formic acid in water and dried under high vacuum. The compounds were eluted with 500 μL of methanol. The samples were evaporated in a SpeedVac at 45 °C and reconstituted with 50 μL of water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol (60
methanol (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, v/v) and transferred to a glass vial for analysis. The hormones detected were melatonin, corticosterone, T3, T4 and testosterone.
40, v/v) and transferred to a glass vial for analysis. The hormones detected were melatonin, corticosterone, T3, T4 and testosterone.
Metabolomics analysis
Metabolomics analysis of the serum samples was performed using gas chromatography coupled to quadrupole time-of-flight mass spectrometry (GC-qTOF). The extraction was performed by adding 400 μL of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (8
water (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) containing the internal STD mixture to the serum samples (approx. 100 μL). Then, the samples were mixed, incubated at 4 °C for 10 min, centrifuged at 25
2) containing the internal STD mixture to the serum samples (approx. 100 μL). Then, the samples were mixed, incubated at 4 °C for 10 min, centrifuged at 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200g and 4 °C for 10 min and the supernatant was evaporated to dryness before compound derivatization (methoximation and silylation). The derivatized compounds were analyzed by GC-qTOF (model 7200 of Agilent, USA). The chromatographic separation was based on the Fiehn method41 using a J&W Scientific HP5-MS (30 m × 0.25 mm i.d., 0.25 μm film capillary column and helium as the carrier gas using an oven program from 60 to 325 °C). Ionization was done by electronic impact (EI), with an electron energy of 70 eV and by operating in the full scan mode. Identification of metabolites was performed using commercial STDs and by matching their EI mass spectrum and retention time to the metabolomics Fiehn library (from Agilent), which contains more than 1400 metabolites. After the putative identification of metabolites, they were semi-quantified in terms of the internal STD response ratio.
200g and 4 °C for 10 min and the supernatant was evaporated to dryness before compound derivatization (methoximation and silylation). The derivatized compounds were analyzed by GC-qTOF (model 7200 of Agilent, USA). The chromatographic separation was based on the Fiehn method41 using a J&W Scientific HP5-MS (30 m × 0.25 mm i.d., 0.25 μm film capillary column and helium as the carrier gas using an oven program from 60 to 325 °C). Ionization was done by electronic impact (EI), with an electron energy of 70 eV and by operating in the full scan mode. Identification of metabolites was performed using commercial STDs and by matching their EI mass spectrum and retention time to the metabolomics Fiehn library (from Agilent), which contains more than 1400 metabolites. After the putative identification of metabolites, they were semi-quantified in terms of the internal STD response ratio.
Gene expression analysis
The total RNA was extracted from the hypothalamus using the E.Z.N.A.® Micro RNA Kit (Omega Bio-tek, Inc., Norcross, GA, USA) according to the manufacturer's protocol. The RNA yield was quantified using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). cDNA was synthesized using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Barcelona, Spain) for analyzing the expression of the samples. A Labnet MultiGene Gradient PCR Thermal Cycler (Sigma-Aldrich, Madrid, Spain) was used for reverse transcription. The reaction was performed according to the instructions of the manufacturer. The cDNA was subjected to quantitative reverse transcriptase polymerase chain reaction amplification using the iTaq Universal SYBR Green Supermix (Bio-Rad, Madrid, Spain) in a CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Madrid Spain). The thermal cycle conditions used for the qPCR were 30 s at 90 °C, 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The primers used for the different genes are described in Table S1† and were obtained from Biomers.net (Ulm, Germany). The fold changes in the mRNA levels were calculated by normalizing to the L18-STD-VH group using the 2−ΔΔCt method with the Ppia gene as an endogenous control, as reported by Schmittgen and Livak.42Statistical analysis
Data were represented as means ± STD error of mean (SEM) of each group, and for this, data normality as well as homogeneity of variance were tested by the Shapiro–Wilk test and the Levene test, respectively, and the differences between groups were assessed by 2-way and 3-way ANOVA, followed by the LSD post hoc test. The Kruskal–Wallis test or Mann–Whitney test was used to analyze the metabolomics data, as indicated in the respective figure legend, to explore the origin of outcomes. These statistical analyses were performed using the statistical software package SPSS Statistics 22 (SPSS Inc., Chicago, IL, USA). The overall effects of changing the photoperiod and the effect of diet and treatments on the serum metabolomic profile were analyzed by principal component analysis (PCA) and the sparse Partial Least Squares Discriminant Analysis (sPLS-DA) using MetaboAnalyst v.4.0 (McGill University, Montreal, Canada).Results
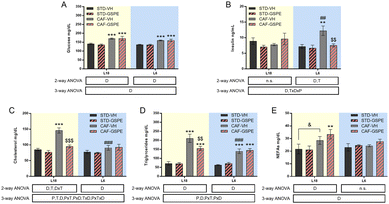
GSPE administration to CAF-fed rats under conditions of photoperiod change improved the insulin sensitivity and serum lipid profile under L6 and L18 conditions, respectively
Serum glucose of rats showed no day length-dependent changes, but an increase in this parameter was observed in CAF-fed animals under both photoperiods with respect to their respective counterparts (Fig. 2A). Although no changes in serum glucose were found, photoperiod-dependent changes in insulin levels were detected (Fig. 2B). While CAF-fed rats transferred to the L6 photoperiod showed a significant increase in this hormone compared to the L6-STD fed rats (p = 0.002), CAF-fed rats under L18 conditions did not show changes in the insulin values compared to the STD-fed rats. In fact, differences in the values of the concentration of this hormone were detected between both CAF-fed groups (p = 0.005, L18-CAF-VH vs. L6-CAF-VH). The increased insulin levels observed in the CAF-fed animals under the L6 photoperiod were reversed by GSPE administration (p = 0.005), returning to similar values to those of healthy rats.Regarding the lipid profile, total cholesterol of rats fed a CAF diet increased significantly under L18 conditions compared to the STD-fed animals (p < 0.001), and GSPE administration decreased their levels (p < 0.001), returning to values of the STD-fed rats. However, under L6 conditions, the CAF-fed rats showed similar values to their corresponding healthy rats (Fig. 2C). In fact, a photoperiod effect on this parameter was observed in the CAF-fed rats, as the effects of the diet were dependent on the light/dark conditions (p < 0.001). In the case of triglycerides (Fig. 2D), the CAF-fed rats under L18 conditions also showed elevated levels compared to the healthy rats (p < 0.001), while the GSPE-treated rats showed significantly lower values (p = 0.004). Under the L6 photoperiod, the CAF-fed rats showed an increase in serum triglycerides compared to the healthy rats (p < 0.001), although this increase was lower than that seen in the CAF-fed rats under L18 conditions, showing a photoperiod effect (p < 0.001). The triglyceride values in the CAF-VH and CAF-GSPE rats were similar under L6 conditions. Regarding serum NEFA levels (Fig. 2E), no differences were found in the animals under L6 conditions. However, CAF-fed animals administered VH and GSPE showed a tendency and significant differences, respectively, compared to their healthy counterparts under L18 conditions, although no differences were found between VH and GSPE under this photoperiod.
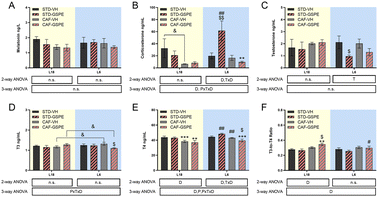
GSPE administration under the conditions of photoperiod change affected the hypothalamic–pituitary–adrenal, –gonadal and –thyroid axes
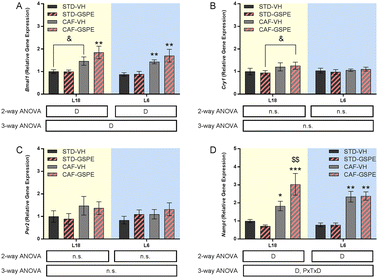
No changes in serum melatonin levels were observed in any photoperiod, neither by diet nor by treatment (Fig. 3A). For corticosterone and testosterone, no significant differences were found under the L18 photoperiod, although their levels in the CAF-fed animals administered VH showed a tendency to decrease with respect to their levels in healthy rats. Regarding the corticosterone levels under L6 conditions, an interaction among the photoperiod, diet, and treatment effect was observed, since in the healthy animals, the GSPE administration resulted in an increase of this hormone levels compared to the rats administered VH (p = 0.004). This increment was also significant with respect to the GSPE-administered animals under L18 conditions (p = 0.004) (Fig. 3B). Regarding the testosterone levels, the administration of GSPE to STD-fed rats under the L6 photoperiod resulted in a decrease compared to the healthy rats administered VH (p = 0.047) (Fig. 3C). Thyroid hormones were also studied, and in the case of the T3 hormone, an interaction between the effect of the photoperiod, treatment and diet was observed (Fig. 3D). The levels of this hormone showed a tendency to increase in the control CAF-fed rats under the L6 photoperiod compared to the control CAF-fed rats under the L18 photoperiod (p = 0.092). However, a significant reduction of these levels was observed after GSPE administration under a short photoperiod since the T3 levels of the L6-CAF-GSPE group were lower than those obtained in the L6-CAF-VH animals (p = 0.029). In addition, these T3 levels of the L6-CAF-GSPE group showed a tendency to decrease when compared to those obtained for the L18-CAF-GSPE group (p = 0.082). For the T4 hormone, a significant effect of diet was observed under L18 conditions, in which both CAF-fed groups showed a decrease in T4 concentrations (p < 0.001) with respect to their corresponding STD-fed groups (Fig. 3E). In addition, an increase in T4 concentrations was observed in rats fed a CAF diet under the L6 photoperiod compared to the CAF-fed rats under L18 conditions (p = 0.001). However, this increase was mitigated by GSPE administration (p = 0.041). These results were reflected in the T3-to-T4 ratio (Fig. 3F), since an increase in the proportion was observed in the CAF-fed rats administered GSPE under L18 conditions compared to the CAF-fed rats administered VH (p = 0.044). Additionally, this effect was not observed under L6 conditions, where the GSPE treatment in the CAF-fed rats resulted in a lower T3-to-T4 ratio than L18-CAF-GSPE (p = 0.043).GSPE administration to CAF-fed rats under the conditions of photoperiod change increased the expression of the clock gene Nampt under L18 conditions
The expression of Bmal1 was upregulated by a CAF diet administered with VH and GSPE in both photoperiods with respect to their respective STD groups (Fig. 4A), although only a tendency to increase Bmal1 expression was observed under L18 conditions in the CAF-CH group (p = 0.074). However, no significant differences were found neither in Cry1 (Fig. 4B) nor in Per2 (Fig. 4C) expressions, although an incremental tendency of Cry1 expression was observed in the L18-CAF-GSPE rats compared to the L18-STD-GSPE animals (p = 0.080). Regarding Nampt (Fig. 3D), a significant increase in gene expression was observed in rats fed a CAF diet in both photoperiods compared to their respective STD controls (p = 0.039 and p = 0.001 for L18-CAF-VH and L6-CAF-VH, respectively). Interestingly, in the CAF-fed rats, GSPE administration under L18 conditions resulted in an increase of the expression of this gene compared to the corresponding CAF-fed rats administered with VH (p = 0.004).GSPE administration to CAF-fed rats under the conditions of photoperiod change modified the metabolomic profile in a photoperiod-dependent manner
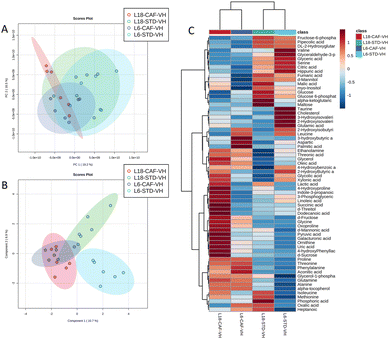
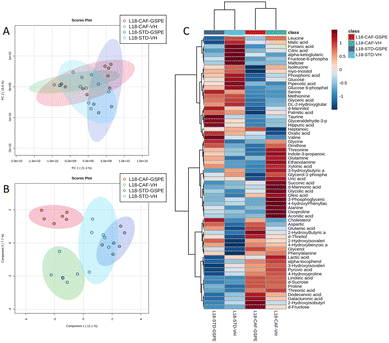
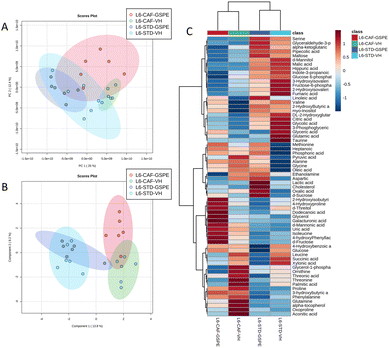
A total of 66 metabolites were identified in the serum samples and annotated (Table S2†). The analysis of the metabolite profile using PCA, and sPLS-DA of the STD and CAF diet fed animals under L6 and L18 photoperiods administered with VH, is shown in Fig. 5. PCA did not show clustering by photoperiod or diet (Fig. 5A). However, sPLS-DA did show clustering by photoperiod with a clear separation between L6 and L18 conditions for the STD-fed rats administered VH (Fig. 5B). The metabolomic profile was clearly different between the STD and CAF-fed groups, determined by heatmap analysis (Fig. 5C). Regarding the differences between photoperiods, more differences were observed between the CAF-fed groups under different photoperiods than between the L18-STD-VH and L6-STD-VH groups. In addition, the L18-CAF-VH group showed greater differences compared to the other groups, indicating that the effects were more pronounced when the CAF diet-induced obese animals were subjected to a long photoperiod. The effects of diet and treatment on the metabolomic profile were also analyzed independently in both the long and short photoperiod groups (Fig. 6 and 7, respectively). Regarding the L18 conditions, no clustering by treatment or diet was observed in the PCA (Fig. 6A). However, the groups did show clustering by diet and treatment in the sPLS-DA, showing a clear separation among the L18-CAF-VH, L18-CAF-GSPE and STD-fed groups (Fig. 6B). The heatmap analysis also revealed changes in the serum metabolite profile according to the diet composition in the animals under the long photoperiod (Fig. 6C), and clear differences were observed between the groups fed a CAF diet and those fed STD diets. Interestingly, the L18-CAF-GSPE group showed a different metabolomic profile compared to their corresponding VH rats.Regarding the animals under L6 conditions, the PCA showed a clustering by diet with a slight separation between the CAF-fed groups and the STD-fed groups (Fig. 7A). In addition, sPLS-DA showed the same clustering by diet and, in addition, a clear separation between the L6-CAF-GSPE and L6-CAF-VH groups (Fig. 7B). Diet and treatment effects were also observed by the heatmap analysis (Fig. 7C), where clearly different metabolomic profiles were observed between the CAF and STD-fed groups and between L6-CAF-VH and L6-CAF-GSPE.
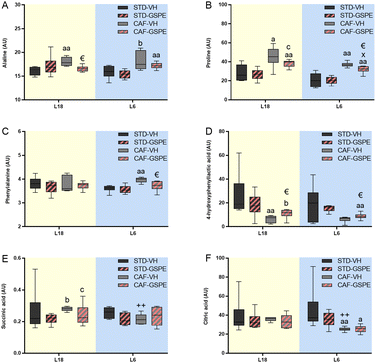
Statistical analysis between the groups of 66 metabolites by the Kruskal–Wallis test and the Mann–Whitney test showed that the concentration of 16 metabolites from the animals under L18 conditions were different depending on the diet and treatment. Additionally, under L6 conditions, 18 metabolites were detected that varied by diet and treatment (Table S2†). In this regard, remarkable changes in amino acids such as alanine, proline, phenylalanine and 4-hydroxyphenyllactic acid, as well as energy-metabolism metabolites such as succinic and citric acids (Fig. 8) were found in rats administered GSPE. In particular, a CAF diet increased and tended to increase the alanine levels under L18 (p = 0.004) and L6 (p = 0.065) conditions, respectively, compared to their corresponding STD-fed rats (Fig. 8A). GSPE administration resulted in a lower alanine level only under L18 conditions (p = 0.016). Regarding proline (Fig. 8B), a CAF diet resulted in a higher concentration of this amino acid in L18 (p = 0.016) and L6 (p = 0.002) groups, and this increment was reversed by GSPE administration under both L18 (p = 0.055) and L6 (p = 0.009) conditions. In the case of phenylalanine levels (Fig. 8C), a CAF diet promoted an increase in the concentration of this amino acid in serum only under L6 conditions (p = 0.002), and this increase was reversed by GSPE (p = 0.026). Furthermore, a CAF diet reduced the levels of 4-hydroxyphenyllactic acid only in the animals under the L18 photoperiod compared to their respective control group (p = 0.004) (Fig. 8D). However, the concentrations of this amino acid were increased with GSPE treatment under both photoperiods (p = 0.037 and p = 0.041 for L18 and L6 conditions, respectively). Succinic acid was also altered by diet in a photoperiod-dependent manner (Fig. 8E). Thus, the CAF-fed rats tended to show higher levels of this metabolite under L18 conditions (p = 0.055) compared to their corresponding STD-fed rats and these levels were also higher compared to the L6-CAF-VH group (p = 0.004). The GSPE administration tended to lower levels under L18 conditions (p = 0.078). No differences were found for this metabolite under L6 conditions. Finally, citric acid was only affected under L6 conditions. This metabolite reduced its concentration levels in the CAF-fed rats compared to the L6-STD-VH (p = 0.002) and L18-CAF-VH groups (p = 0.002), showing a diet and photoperiod effect (Fig. 8F). The administration of GSPE did not produce changes in the levels of this metabolite.
Discussion
Biological rhythms play a crucial role in the regulation and efficiency of metabolism and their disruption has been linked to the appearance of several diseases. Indeed, shift or disturbances of the normal light/dark cycle have been associated with metabolic disorders that could lead to the development of metabolic syndrome.43 In this context, flavanols are phenolic compounds that have demonstrated beneficial effects on metabolism-related pathologies.23 Different molecular mechanisms have been involved in the healthy properties of grape seed flavanols, including their interaction with the biological rhythms.28,44,45 According to this, we have recently reported that GSPE could mitigate, in healthy and CAF-fed rats, disturbances caused by a sudden photoperiod change, restoring the diurnal oscillations of their locomotor activity and some key metabolic regulators such as corticosterone or testosterone.33 Therefore, the aim of this study was to evaluate the impact of GSPE on the metabolite profile and hypothalamic clock genes under diurnal disruption conditions caused by an obesogenic diet and changes in the light/dark cycle.The effect of a CAF diet on the metabolism in rats under L12 conditions has been extensively studied since this animal model mimics the classical human metabolic syndrome.46 In fact, the animals in this study showed hyperphagia and increased BW compared to the animals fed a STD diet.33 Nevertheless, the effect of this diet on animals subjected to different photoperiods has not been so widely explored. Our findings clearly showed that the serum biochemical parameters were affected by the abrupt change of the light–dark cycle in a photoperiod-dependent manner under conditions of CAF diet-induced obesity. An altered serum lipid profile was found only in CAF-fed rats under L18 conditions, while animals fed a CAF diet under the L6 photoperiod showed elevated insulin concentrations, although no differences in glucose levels were found in these animals. Disturbances in serum lipid and insulin sensitivity under photoperiod change conditions have been previously reported.47 However, while some studies have found similar disturbances in animals subjected to a long or short photoperiod in terms of serum lipid or insulin levels,31,48,49 a worse lipid profile in rats exposed to a short photoperiod compared to those exposed to a long photoperiod was reported in another study.47 These discrepancies between studies could be due to the different times of animal exposure to the different photoperiods, only one week in our study to avoid a potential adaptation of the animal to the new light/dark cycle.
The main marker of the light/dark cycle, melatonin, was not affected by the diet and photoperiod, as expected, since the samples were obtained at a single time point during the light phase. These findings are in agreement with those obtained previously by our group,33 since in the light phase no melatonin variations were observed after the change of the photoperiod. There was an increase in the concentration of this hormone during the dark phase, which was an important signal for the organism to recognize the photoperiod to which it was exposed.50 Interestingly, exogenous melatonin administration has been shown to reduce serum triglyceride and cholesterol levels, improving the serum lipid profile in rats.51,52 Therefore, the higher dark melatonin levels in rats under short light conditions33 could explain the improved lipid profile observed in the L6-CAF-VH group compared to the L18-CAF-VH group.
Interestingly, GSPE administration under L18 conditions resulted in a reduction of cholesterol levels to the values of the STD-fed rats. Additionally, the administration of GSPE resulted in a reduction of triglyceride levels in the CAF-fed rats under L18 conditions, although no effects of GSPE administration were observed in the L6 photoperiod. According to this, photoperiod-dependent GSPE effects on CAF-fed rats, more significant under L18 conditions, have been recently reported.36 Differences in energy intake in CAF-fed rats depending on the photoperiod have also been reported. Specifically, a tendency to increase the energy intake under short compared to long light conditions was observed.33 Interestingly, this difference in energy intake was mainly due to an increase in carbohydrate intake, which could be related to the higher insulin levels observed in CAF-fed rats under L6 conditions compared to those exposed to the L18 photoperiod. Interestingly, GSPE administration to the L6-CAF-group resulted in a decrease of insulin levels, the values reaching those similar to the L6-STD group. The insulin-lowering effect of GSPE at the same dose of 25 mg kg−1 conditions has been previously reported in CAF rats, although in animals under L12 conditions.23,53 Regarding the components of grape seed extract responsible for this effect, although this extract is rich in proanthocyanidins, it has to be noted that monomers also participate in this effect because they appear in considerable quantities. In fact, it has been reported that some of the effects of this grape seed extract in relation to the metabolic syndrome, such as the antihypertensive effect, are mediated by the monomers in rats.54
Corticosterone hormone plays a key role in the HPA axis.10 In this study, although their values in all the groups were below 100 ng mL−1, which are in concordance with those obtained in our previous study for this phase of the day,33 some differences in their levels could be observed between the groups. Notably, the STD-GSPE group under the L6 photoperiod displayed the highest levels for this hormone. This increase could be due to that GSPE administered rats were quicker to adapt to a long dark cycle than the corresponding STD-VH rats, since a clear corticosterone peak during the dark phase has been reported in F344 rats under a short photoperiod.55 The HPG and HPT axes are important in processes such as lipid and carbohydrate metabolism and are closely related to biological rhythms. Testosterone and thyroid hormones, respectively, act as modulators of these axes.11,12 In this study, only STD-fed rats administered GSPE under L6 conditions showed a decrease in testosterone levels compared to their control. Taking into account that, in rats, the lowest testosterone levels are detected during the light phase,56 the reduction of this hormone in L6 animals administered GSPE could be related to a better adaptation to the new photoperiod, characterized by only 6 h of light, because testosterone levels would decrease faster in a shorter light period to adapt to the new light/dark cycle. Regarding thyroid hormones, an incremental tendency of T3 hormone levels was observed in the CAF-fed rats under L6 conditions compared to the CAF-fed rats under L18 conditions. Interestingly, this CAF diet effect was reversed by GSPE treatment, where an incremental tendency of T3 levels was observed in the GSPE-treated rats under L18 conditions compared to the GSPE-treated rats under L6 conditions. Elevated T3 levels have been associated with reduced cholesterol and triglyceride levels.11 In addition, higher levels of thyroid hormones and the T3-to-T4 ratio are associated with a loss of BW.57 Therefore, the improvement in cholesterol and triglyceride levels found in the CAF-fed animals administered GSPE under L18 conditions could be attributed to an increase in the T3 levels and thyroid hormone ratio, which were also increased in this group.
The most important controller of the circadian system is the central clock; this pacemaker is in the hypothalamus and maintains the synchronization of the peripheral clocks with the environment and its oscillators through hormone signals and neural connections.5 To study the status of the central clock in the animals, we analyzed the gene expression of Bmal1, Per2, Cry1 and Nampt. In this regard, Bmal1 and Nampt were affected by the CAF diet, increasing their expression in both photoperiods. In contrast, no differences were found in the expression of Per2 and Cry1 genes. A high-fat diet has shown the ability to modify the expression of clock genes in peripheral tissues.6,58,59 Additionally, our previous findings showed that a CAF diet was able to modify hypothalamic Bmal1, the central clock regulatory gene.60 Although no significant effects were found by GSPE administration on Bmal1, a slight increase in this gene expression was observed in the CAF-fed rats under the L18 photoperiod. In addition, according to this, GSPE administration caused an increase in Nampt expression under L18 conditions in CAF-fed rats compared to their corresponding CAF-VH administered rats. Nampt is an important clock gene, whose expression is related to energy metabolism.61–63 In fact, NAMPT is the rate-limiting enzyme in NAD biosynthesis through its salvage pathway.64 NAD homeostasis is related to free radical-mediated reactive oxygen species production, which has been linked to innumerable pathologies, including metabolic diseases.65 In addition, this metabolite activates several NAD-dependent deacetylases, including SIRT1, controlling the activity of many cellular pathways linked to lipid and glucose metabolism. Thus, the improved lipid profile of the CAF-induced obese rats under L18 conditions could be modulated by the increased Nampt gene expression in addition to the increased levels of T3 and T3-to-T4 ratio, as previously mentioned.
Regarding the serum metabolites, the metabolomics data showed changes between the groups in a diet- and photoperiod-dependent manner when the normal light–dark cycle was altered. Overall, the STD-fed rats showed a different metabolomic profile depending on the photoperiod and clustered differently from the CAF-fed groups. The CAF diet caused significant changes in 14 metabolites. Specifically, a strong increase in 2-hydroxyisobutyric acid, glycolic acid, and proline concentrations under L18 conditions was observed. In addition, in this photoperiod, the CAF diet tended to increase the levels of succinic acid, a metabolite whose elevated levels have been associated with mitochondrial stress under diabetic conditions.17 Interestingly, an increase in Krebs cycle metabolites, such as malic acid, succinic acid, citric acid or fumaric acid, was observed in the CAF-fed rats under L18 conditions compared to those exposed under L6 conditions. It has been reported that the activity of Krebs cycle enzymes, including citric acid synthase, is reduced in mice under conditions of excess of nutrients and this fact is associated with obesity.66,67 Therefore, the increase in these metabolites in animals subjected to a long photoperiod could be related to the improvement of the lipid profile showed in the CAF-fed rats under L18 conditions. Additionally, phosphoric acid, 4-hydroxyphenyllactic acid, maltose and hippuric acid were significantly reduced in all CAF-fed rats compared to the STD-fed rats. Interestingly, hippuric acid has been negatively associated with BW gain.68 In CAF-fed rats, increased alanine and phenylalanine concentrations were observed in both photoperiods. The increase of these amino acids has been related to obesity and the development of metabolic syndrome.13 Under L6 conditions, a significant increase in the levels of proline, glycerol, d-sucrose, and alanine due to the CAF diet was observed, while the levels of d-mannitol, hippuric acid, 4-hydroxyphenyllactic acid and citric acid were reduced. Interestingly, the metabolomic profile of the CAF-fed rats was different between those administered with GSPE and VH under the conditions of each photoperiod for metabolites associated with the conditions of metabolic syndrome.13,17,66,67 Remarkably, L18 conditions significantly mitigated these variations for 4-hydroxyphenyllactic acid and alanine, and tended to restore the normal values for glycolic acid, succinic acid, and proline. On the other hand, GSPE administration mitigated the variations of proline, phenylalanine and 4-hydroxyphenyllactic acid levels in the CAF-fed rats, while no effects were observed in the STD-fed rats by GSPE treatment under L6 conditions.
Conclusions
In summary, the alteration in the light/dark cycle by suddenly changing the photoperiod in CAF-fed rats caused worsening of the serum lipid profile under L18 conditions, while a reduced insulin sensitivity was observed under L6 conditions. The difference in the metabolic status between photoperiods was also reflected in the serum metabolomic profile. An increase in metabolites involved in the Krebs cycle was observed in CAF-fed rats under L18 conditions compared to those exposed under the L6 photoperiod. Additionally, photoperiod-dependent changes of metabolite levels associated with obesity and metabolic syndrome such as alanine, phenylalanine or hippuric acid were observed in the CAF-fed rats. These disturbances were improved by GSPE administration, resulting in significant changes in the serum metabolite profiles. Furthermore, the results suggest that an increase in metabolic rate through the regulation of the HPG and HPT axes could be mediated by GSPE, since these flavanols were able to modify the concentration of hormones such as T3, T4, and testosterone. In addition, Nampt gene expression, closely related to energy metabolism, was stimulated by GSPE administration. Therefore, these results indicate the ability of GSPE to modify the metabolic profile in a photoperiod-dependent manner in CAF-induced obese rats subjected to an alteration of the light/dark cycle. Additionally, these findings suggest that the effect of GSPE on metabolism could be mediated by their potential effect as a modulator of biological rhythms. However, further studies are needed to elucidate the metabolic pathways and processes involved in these events.Author contributions
Conceptualization: J. R. S.-R., C. T.-F., F. I. B., M. S., M. M. and B. M.; formal analysis: J. R. S.-R. and R. L.-F.-S.; data curation: J. R. S.-R. and R. L.-F.-S.; investigation: J. R. S.-R. and R. L.-F.-S.; methodology: J. R. S.-R. and R. L.-F.-S.; funding acquisition: C. T.-F., F. I. B., M. S., M. M. and B. M.; project administration: C. T.-F., F. I. B., M. S., M. M. and B. M.; resources: C. T.-F., F. I. B., M. S., M. M. and B. M.; software: J. R. S.-R.; visualization: J. R. S.-R.; supervision: B. M.; validation: J. R. S.-R. and B. M.; writing – original draft: J. R. S.-R.; and writing – review and editing: B. M.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This project was funded by the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033/FEDER “Una manera de hacer Europa” (Grant numbers: AGL2016-77105-R and PID2021-128813OB-I00). J. R. S.-R. is the recipient of a grant for the hiring of predoctoral research staff (Grant number: BES-2017-080919) from the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033 and FSE “El FSE invierte en tu futuro”. F. I. B. and M. M. are Serra Húnter Fellows. Special thanks go to Niurka Llópiz and Rosa M. Pastor for their assistance and technical support. We thank Pol Herrero and Antonio del Pino of the Centre for Omic Sciences (COS) Joint Unit of the Universitat Rovira i Virgili-Eurecat, for their contribution to the hormone and metabolomics analyses.References
- G. R. McGinnis and M. E. Young, Circadian regulation of metabolic homeostasis: Causes and consequences, Nat. Sci. Sleep, 2016, 8, 163–180 Search PubMed.
- S. M. Reppert and D. R. Weaver, Coordination of circadian timing in mammals, Nature, 2002, 418, 935–941 CrossRef CAS PubMed.
- B. Claustrat, J. Brun and G. Chazot, The basic physiology and pathophysiology of melatonin, Sleep Med. Rev., 2005, 9, 11–24 CrossRef PubMed.
- F. Levi and U. Schibler, Circadian Rhythms: Mechanisms and Therapeutic Implications, Annu. Rev. Pharmacol. Toxicol., 2007, 47, 593–628 CrossRef CAS PubMed.
- C. Dibner, U. Schibler and U. Albrecht, The mammalian circadian timing system: Organization and coordination of central and peripheral clocks, Annu. Rev. Physiol., 2009, 72, 517–549 CrossRef PubMed.
- A. Kohsaka, et al., High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice, Cell Metab., 2007, 6, 414–421 CrossRef CAS PubMed.
- K. L. Eckel-Mahan, et al., Reprogramming of the circadian clock by nutritional challenge, Cell, 2013, 155, 1464–1478 CrossRef CAS PubMed.
- J. Laermans and I. Depoortere, Chronobesity: role of the circadian system in the obesity epidemic, Obes. Rev., 2016, 17, 108–125 CrossRef CAS PubMed.
- N. Gotlieb, J. Moeller and L. J. Kriegsfeld, Circadian control of neuroendocrine function: implications for health and disease, Curr. Opin. Physiol., 2018, 5, 133–140 CrossRef PubMed.
- N. Nader, G. P. Chrousos and T. Kino, Interactions of the circadian CLOCK system and the HPA axis, Trends Endocrinol. Metab., 2010, 21, 277–286 CrossRef CAS PubMed.
- R. Mullur, Y. Y. Liu and G. A. Brent, Thyroid hormone regulation of metabolism, Physiol. Rev., 2014, 94, 355–382 CrossRef CAS PubMed.
- X. W. Lin, I. D. Blum and K. F. Storch, Clocks within the Master Gland: Hypophyseal Rhythms and Their Physiological Significance, J. Biol. Rhythms, 2015, 30, 263–276 CrossRef CAS PubMed.
- D. Lent-Schochet, M. McLaughlin, N. Ramakrishnan and I. Jialal, Exploratory metabolomics of metabolic syndrome: A status report, World J. Diabetes, 2019, 10, 23–36 CrossRef PubMed.
- S. Cheng, et al., Metabolite profiling identifies pathways associated with metabolic risk in humans, Circulation, 2012, 125, 2222–2231 CrossRef CAS PubMed.
- C. B. Newgard, Interplay between lipids and branched-chain amino acids in development of insulin resistance, Cell Metab., 2012, 15, 606–614 CrossRef CAS PubMed.
- M. Watanabe, et al., Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality, Am. J. Clin. Nutr., 2008, 87, 1860–1866 CrossRef CAS PubMed.
- S. Fernández-Veledo, V. Ceperuelo-Mallafré and J. Vendrell, Rethinking succinate: an unexpected hormone-like metabolite in energy homeostasis, Trends Endocrinol. Metab., 2021, 32, 680–692 CrossRef PubMed.
- R. Ch, O. Chevallier and C. T. Elliott, Metabolomics reveal circadian control of cellular metabolism, TrAC, Trends Anal. Chem., 2020, 130, 115986 CrossRef CAS.
- S. K. Davies, et al., Effect of sleep deprivation on the human metabolome, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 10761–10766 CrossRef CAS PubMed.
- K. A. Dyar and K. L. Eckel-Mahan, Circadian Metabolomics in Time and Space, Front. Neurosci., 2017, 11, 369 CrossRef PubMed.
- S. Abbondante, K. L. Eckel-Mahan, N. J. Ceglia, P. Baldi and P. Sassone-Corsi, Comparative circadian metabolomics reveal differential effects of nutritional challenge in the Serum and Liver, J. Biol. Chem., 2016, 291, 2812–2828 CrossRef CAS PubMed.
- A. Ribas-Latre and K. Eckel-Mahan, Interdependence of nutrient metabolism and the circadian clock system: Importance for metabolic health, Mol. Metab., 2016, 5, 133–152 CrossRef CAS PubMed.
- C. Bladé, et al., Proanthocyanidins in health and disease, BioFactors, 2016, 42, 5–12 Search PubMed.
- A. Ribas-Latre, et al., Dietary proanthocyanidins modulate melatonin levels in plasma and the expression pattern of clock genes in the hypothalamus of rats, Mol. Nutr. Food Res., 2015, 59, 865–878 CrossRef CAS PubMed.
- G. Aragonès, et al., Dietary proanthocyanidins boost hepatic NAD+ metabolism and SIRT1 expression and activity in a dose-dependent manner in healthy rats, Sci. Rep., 2016, 6, 1–12 CrossRef PubMed.
- A. Ribas-Latre, et al., Chronic consumption of dietary proanthocyanidins modulates peripheral clocks in healthy and obese rats, J. Nutr. Biochem., 2015, 26, 112–119 CrossRef CAS PubMed.
- B. P. Sampey, et al., Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet, Obesity, 2011, 19, 1109–1117 CrossRef CAS PubMed.
- J. Ávila-Román, et al., Phenolic compounds and biological rhythms: Who takes the lead?, Trends Food Sci. Technol., 2021, 113, 77–85 CrossRef.
- P. D. Heideman and C. J. Sylvester, Reproductive Photoresponsiveness in Unmanipulated Male Fischer 344 Laboratory Rats, Biol. Reprod., 1997, 57, 134–138 CrossRef CAS PubMed.
- H. Palacios-Jordan, et al., The Disruption of Liver Metabolic Circadian Rhythms by a Cafeteria Diet Is Sex-Dependent in Fischer 344 Rats, Nutrients, 2020, 12, 1085 CrossRef CAS PubMed.
- R. Mariné-Casadó, et al., Intake of an Obesogenic Cafeteria Diet Affects Body Weight, Feeding Behavior, and Glucose and Lipid Metabolism in a Photoperiod-Dependent Manner in F344 Rats, Front. Physiol., 2018, 9, 1639 CrossRef PubMed.
- R. M. Rodríguez, et al., Time-of-Day Circadian Modulation of Grape-Seed Procyanidin Extract (GSPE) in Hepatic Mitochondrial Dynamics in Cafeteria-Diet-Induced Obese Rats, Nutrients, 2022, 14, 774 CrossRef PubMed.
- J. R. Soliz-Rueda, et al., Grape Seed Proanthocyanidins Mitigate the Disturbances Caused by an Abrupt Photoperiod Change in Healthy and Obese Rats, Nutrients, 2022, 14, 1834 CrossRef CAS PubMed.
- M. Quiñones, et al., Involvement of nitric oxide and prostacyclin in the antihypertensive effect of low-molecular-weight procyanidin rich grape seed extract in male spontaneously hypertensive rats, J. Funct. Foods, 2014, 6, 419–427 CrossRef.
- R. de Azua and M. J, et al., Fatty acid metabolism in liver and muscle is strongly modulated by photoperiod in Fischer 344 rats, J. Photochem. Photobiol., B, 2023, 238, 112621 CrossRef PubMed.
- R. M. Rodríguez, et al., Grape-Seed Procyanidin Extract (GSPE) Seasonal-Dependent Modulation of Glucose and Lipid Metabolism in the Liver of Healthy F344 Rats, Biomolecules, 2022, 12, 839 CrossRef PubMed.
- A. Gibert-Ramos, A. Crescenti and M. Salvadó, Consumption of Cherry out of Season Changes White Adipose Tissue Gene Expression and Morphology to a Phenotype Prone to Fat Accumulation, Nutrients, 2018, 10, 1102 CrossRef PubMed.
- A. Gibert-Ramos, M. Ibars, M. J. Salvadó and A. Crescenti, Response to the photoperiod in the white and brown adipose tissues of Fischer 344 rats fed a standard or cafeteria diet, J. Nutr. Biochem., 2019, 70, 82–90 CrossRef CAS PubMed.
- A. Mas-Capdevila, et al., Changes in arterial blood pressure caused by long-term administration of grape seed proanthocyanidins in rats with established hypertension, Food Funct., 2020, 11, 8735–8742 RSC.
- Z. Pons, M. Margalef, F. I. Bravo, A. Arola-Arnal and B. Muguerza, Acute administration of single oral dose of grape seed polyphenols restores blood pressure in a rat model of metabolic syndrome: role of nitric oxide and prostacyclin, Eur. J. Nutr., 2015, 55, 749–758 CrossRef PubMed.
- T. Cajka and O. Fiehn, Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics, Anal. Chem., 2016, 88, 524–545 CrossRef CAS PubMed.
- K. J. Livak and T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method, Methods, 2001, 25, 402–408 CrossRef CAS PubMed.
- P. Zimmet, et al., The Circadian Syndrome: is the Metabolic Syndrome and much more!, J. Intern. Med., 2019, 286, 181–191 CrossRef CAS PubMed.
- C. Torres-Fuentes, et al., Cardioprotective Properties of Phenolic Compounds: A Role for Biological Rhythms, Mol. Nutr. Food Res., 2022, 2100990 CrossRef CAS PubMed.
- N. Ibarz-Blanch, et al., Role of Chrononutrition in the Antihypertensive Effects of Natural Bioactive Compounds, Nutrients, 2022, 14, 1920 CrossRef CAS PubMed.
- Z. Pons, M. Margalef, F. I. Bravo, A. Arola-Arnal and B. Muguerza, Chronic administration of grape-seed polyphenols attenuates the development of hypertension and improves other cardiometabolic risk factors associated with the metabolic syndrome in cafeteria diet-fed rats, Br. J. Nutr., 2017, 117, 200–208 CrossRef CAS PubMed.
- X. Xie, et al., Effects of altered photoperiod on circadian clock and lipid metabolism in rats, Chronobiol. Int., 2017, 34, 1094–1104 CrossRef CAS PubMed.
- R. Mariné-Casadó, et al., Cherry consumption out of season alters lipid and glucose homeostasis in normoweight and cafeteria-fed obese Fischer 344 rats, J. Nutr. Biochem., 2019, 63, 72–86 CrossRef PubMed.
- A. W. Ross, et al., Photoperiod regulates lean mass accretion, but not adiposity, in growing F344 rats fed a high fat diet, PLoS One, 2015, 10, e0119763 CrossRef PubMed.
- R. J. Reiter, The pineal and its hormones in the control of reproduction in mammals, Endocr. Rev., 1980, 1, 109–131 CrossRef CAS PubMed.
- M. Navarro-Alarcón, et al., Melatonin and metabolic regulation: a review, Food Funct., 2014, 5, 2806–2832 RSC.
- S. A. R. Hussain, Effect of melatonin on cholesterol absorption in rats, J. Pineal Res., 2007, 42, 267–271 CrossRef CAS PubMed.
- G. Montagut, et al., Effects of a grapeseed procyanidin extract (GSPE) on insulin resistance, J. Nutr. Biochem., 2010, 21, 961–967 CrossRef CAS PubMed.
- M. Quiñones, et al., The blood pressure effect and related plasma levels of flavan-3-ols in spontaneously hypertensive rats, Food Funct., 2015, 6, 3479 RSC.
- T. Otsuka, et al., Photoperiod Regulates Corticosterone Rhythms by Altered Adrenal Sensitivity via Melatonin-Independent Mechanisms in Fischer 344 Rats and C57BL/6J Mice, PLoS One, 2012, 7, e39090 CrossRef CAS PubMed.
- U. Lang, et al., Diurnal rhythm of melatonin action on sexual maturation of male rats, Neuroendocrinology, 1984, 38, 261–268 CrossRef CAS.
- G. Liu, et al., Thyroid Hormones and Changes in Body Weight and Metabolic Parameters in Response to Weight-Loss Diets: The POUNDS LOST Trial, Int. J. Obes., 2017, 41, 878 CrossRef CAS PubMed.
- M. Barnea, Z. Madar and O. Froy, High-fat diet followed by fasting disrupts circadian expression of adiponectin signaling pathway in muscle and adipose tissue, Obesity, 2010, 18, 230–238 CrossRef CAS PubMed.
- M. Barnea, Z. Madar and O. Froy, High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver, Endocrinology, 2009, 150, 161–168 CrossRef CAS.
- J. Hirayama, et al., CLOCK-mediated acetylation of BMAL1 controls circadian function, Nature, 2007, 450, 1086–1090 CrossRef CAS PubMed.
- R. M. de Guia, et al., Fasting- and ghrelin-induced food intake is regulated by NAMPT in the hypothalamus, Acta Physiol., 2020, 228, e13437 CAS.
- A. Tran, W. He, N. Jiang, J. T. C. Chen and D. D. Belsham, NAMPT and BMAL1 Are Independently Involved in the Palmitate-Mediated Induction of Neuroinflammation in Hypothalamic Neurons, Front. Endocrinol., 2020, 11, 351 CrossRef PubMed.
- K. Tokizane and S. Imai, NAD+ oscillation and hypothalamic neuronal functions, Fac. Rev., 2021, 10, 42 CAS.
- G. Magni, A. Amici, M. Emanuelli, N. Raffaelli and S. Ruggieri, Enzymology of NAD+ synthesis, Adv. Enzymol. Relat. Areas Mol. Biol., 1999, 73, 135–182 CAS.
- S. J. Forrester, D. S. Kikuchi, M. S. Hernandes, Q. Xu and K. K. Griendling, Reactive Oxygen Species in Metabolic and Inflammatory Signaling, Circ. Res., 2018, 122, 877 CrossRef CAS PubMed.
- S. Cho, N. Song, J. Y. Choi and A. Shin, Effect of Citric Acid Cycle Genetic Variants and Their Interactions with Obesity, Physical Activity and Energy Intake on the Risk of Colorectal Cancer: Results from a Nested Case-Control Study in the UK Biobank, Cancers, 2020, 12, 1–22 Search PubMed.
- T. D. Cummins, et al., Metabolic remodeling of white adipose tissue in obesity, Am. J. Physiol. Endocrinol. Metab., 2014, 307, E262–E277 CrossRef CAS PubMed.
- B. Lanzon, et al., Lipidomic and metabolomic signature of progression of chronic kidney disease in patients with severe obesity, Metabolites, 2021, 11, 836 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo00260h |
| This journal is © The Royal Society of Chemistry 2023 |