Open Access Article
Open Access ArticleMaternal vitamin D deficiency and brain functions: a never-ending story
Lidia
Saidi
 ab,
Habib
Hammou
b,
Flavie
Sicard
ac,
Jean-François
Landrier
a and
Lourdes
Mounien
ab,
Habib
Hammou
b,
Flavie
Sicard
ac,
Jean-François
Landrier
a and
Lourdes
Mounien
 *a
*a
aAix Marseille Univ, INSERM, INRA, C2VN, Marseille, France. E-mail: lourdes.mounien@univ-amu.fr; Tel: +33 4 91 32 42 84
bUniversité Oran 1, Ahmed Ben Bella, Laboratoire de Physiologie de la Nutrition et Sécurité Alimentaire, Département de Biologie, Faculté des Sciences de la Nature et de la Vie, Oran, Algeria
cPhenoMARS Aix-Marseille Technology Platform, CriBiom, Marseille, France
First published on 31st May 2023
Abstract
A large number of observational studies have highlighted the prevalence rates of vitamin D insufficiency and deficiency in many populations including pregnant women. Vitamin D is well known to have a crucial role in differentiation and proliferation, as well as neurotrophic and neuroprotective actions in the brain. It has been observed that this micronutrient can modulate neurotransmission and synaptic plasticity. Recent results from animal and epidemiological studies indicated that maternal vitamin D deficiency is associated with a wide range of neurobiological diseases including autism, schizophrenia, depression, multiple sclerosis and developmental defects. The aim of this review is to summarize the current state of knowledge on the effect of maternal vitamin D deficiency on brain functions and development.
1. Introduction
Challenges during pre-conception, foetal and early postnatal periods of life, such as nutritional and lifestyle imbalances, can cause the onset of diseases during adulthood; this concept is known as the Developmental Origins of Health and Disease (DOHaD).1,2 These changes can occur via various mechanisms including the disruption of the placental function as well as epigenetic modifications such as histone modification, DNA methylation or non-coding RNAs, the consequences of which induce an alteration in the expression of key genes.2,3The perinatal period including gestation and lactation is considered a critical period with high epigenome plasticity and sensitivity, during which time the mother is the only source of nutrients for the foetus.4 Macronutrients like carbohydrates or proteins as well as micronutrients like minerals and vitamins are essential for the developing foetus and any imbalance induces functional changes and has long-lasting health consequences for the offspring.1,2,5 It is also interesting to note that several studies have reported that the foetus responds to external signals such as maternal nutritional stimuli in a gender-dependent manner, suggesting that perinatal reprogramming is characterized by sexual dimorphism.1,2,6
In this context, the role of vitamin D (VD) in foetal development is widely studied. VD is not only known for its role in calcium homeostasis and foetal bone development, but also for its implication in many biological functions as a hormone.7 Due to this, many studies have highlighted its skeletal and non-skeletal roles during pregnancy such as foetal implantation as well as placental formation and function8 or lung activity.9–11 Interestingly, VD is also important for brain development, and particularly, in the dopamine system ontogeny, axonal connectivity and neuronal differentiation.12 In the last decade, several studies have exposed the adverse consequences of gestational VD deficiency (VDD) on brain development and the onset of neurological diseases in the later stages of life. The aim of this review is to assess the existing evidence of the impact of maternal VDD on the neurodevelopment of offspring and its long-term neurological consequences.
2. Vitamin D
2.1. Biosynthesis, degradation and mechanisms of action of vitamin D
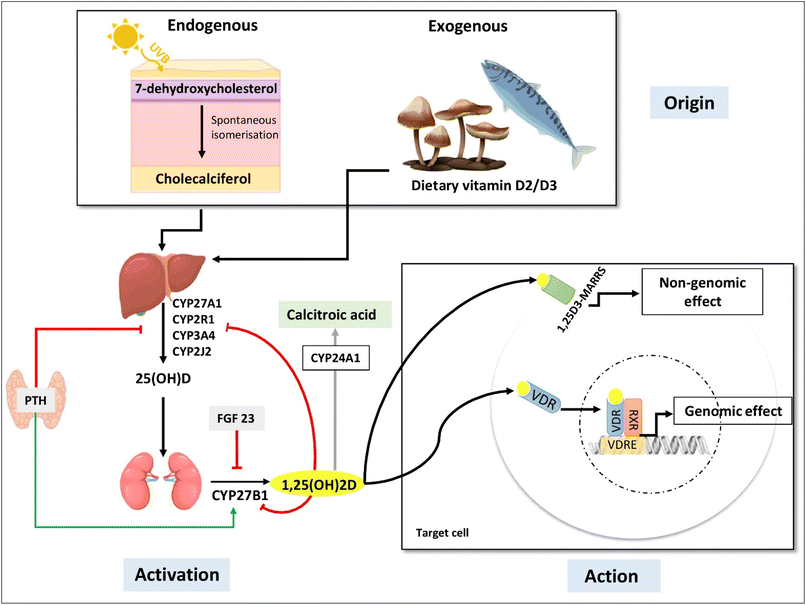
VD is a fat-soluble hormone that has both exogenous and endogenous origins. During endogenous synthesis, VD is produced in the skin under the action of ultraviolet B rays (UVB) and is known as cholecalciferol or vitamin D3 (VD3). For the exogenous origin, VD can be found in different foodstuff; in particular, in animal food sources such as fish, fish oils, egg yolks, and beef liver in the form of VD3, and also in vegetables and mushrooms under the name of ergocalciferol or vitamin D2 (VD2)13 (Fig. 1).VD requires two hydroxylation processes in order to be biologically active.13–15 The first step of VD activation takes place in the liver and generates 25-hydroxyvitamin D (25(OH)D), which represents the main circulating but inactive form of VD with a half-life of three to four weeks.7,13,14 The adipose tissue is considered as a storage site for 25(OH)D16–18 as reported in rats,19 mice20 and humans.21 Then, 25(OH)D is transported to the kidneys, where the second step of VD activation will take place, leading to the formation of 1,25-dihydroxyvitamin D (1,25(OH)2D), also called calcitriol, which is the main active form of VD.13 Kidney production of 1,25(OH)2D is highly regulated by calcium and phosphorus levels, PTH (parathyroid hormone) and FGF-23 (fibroblast growth factor 23).7,13 1,25(OH)2D is involved in the activation of its own catabolism by inducing the expression of the ubiquitous enzyme: CYP24A1, which converts 1,25(OH)2D to 1,24,25(OH)3D, resulting in the inactive form of VD called calcitroic acid (Fig. 1).7,13 In the context of this review, it has also been reported that there is a local activation and catabolism of VD in the central nervous system where CYP27B1 and CYP24A1 are expressed in several cell types such as neurons, astrocytes and other glial cells.22
The active form of VD is known to have both genomic and non-genomic effects.7,22,23 The genomic effects result from the binding of active VD to its nuclear receptor VDR (vitamin D receptor), which induces either the activation or repression of the target gene (Fig. 1).7,23 This gene regulation of VD via VDR has epigenetic effects as it modulates the expression of enzymes involved in methylation and acetylation.23 In addition, VD also regulates the expression of micro-RNAs (miRs), which are small non-coding RNAs that are known for their role in post-transcriptional regulation of gene expression and gene silencing.23–25 For the non-genomic effects, VD acts via a membrane receptor named Pdia3 (Fig. 1). Binding and activation of this receptor by 1,25(OH)2D induces the activation of signal transduction pathways which will elicit rapid responses.23,26–29
2.2. Biological roles of vitamin D
In addition to its primary role in the regulation and maintenance of phosphocalcic homeostasis, VD is known to have several other physiological functions in the immune system,13,30–32 cancer27,31–33 and adipose tissue biology,14,15,17,34–36 cardiovascular function and arterial pressure.31,32,37 More interestingly, VD is also considered an essential micronutrient for brain development during foetal life.38 In accordance with its involvement in foetal neurodevelopment, the lack of VD in later life stages seems to be associated with an increased risk and course of many neurodegenerative and neuroinflammatory diseases as described below.3. Vitamin D deficiency
3.1. Vitamin D status
25(OH)D represents the most abundant circulating form of VD. In addition to being in balance with the spare 25(OH)D, only a fraction of this metabolite is converted to active VD. Thus, the serum concentration of 25(OH)D is the best indicator of VD status.17 A serum level greater than 30 ng ml−1 (75 nmol L−1) is considered optimal; therefore, the VD standards have been established as follows: sufficiency >30 ng ml−1 (75 nmol L−1), insufficiency 10–30 ng ml−1 (25–75 nmol L−1), and deficiency <10 ng ml−1 (25 nmol L−1). VD deficiency (VDD) or hypovitaminosis D is considered as a risk factor for many diseases such as cardiovascular39 and autoimmune diseases,40 type 2 diabetes41 and cancer.42 VDD is now regarded as a global epidemic induced by many factors including a diet low in VD, inadequate exposure to the sun or obesity.13,143.2. Vitamin D deficiency during the perinatal period
The mother is the primary source of VD for the fetus and the placenta is a key structure in fetal development because of its involvement in the diffusion of nutrients such as 1,25(OH)2D and 25(OH)D from the mother to the fetus. Due to the presence of 1α-hydroxylase, 25(OH)D can be activated in both the trophoblast and the decidua. In addition to this enzyme, VDR is also found in the placenta, indicating that this structure not only produces but also responds to 1,25(OH)2D.43,44 It has been shown that VD modulates the synthesis of placental hormones involved in pregnancy such as human chorionic gonadotropin (hCG) and also plays an important role in endometrial decidualization and thus foetal implantation.44,45A large proportion of pregnant women exhibit VDD (8% to 100% in some parts of the world).46 Several studies showed that maternal VDD induced foetal growth restriction. A prospective cohort that examined the impact of maternal VDD in a multi-ethnic population of 7098 women found that low maternal 25(OH)D levels were associated with foetal growth restriction, low birth weight and increased risk of premature birth.47 These results are correlated with several other studies.48,49 Maternal VDD during pregnancy predisposes the offspring to several diseases such as childhood rickets, and severe VDD in the early stages of pregnancy was linked to an increased risk of childhood obesity.5 In our group, we showed that maternal VDD in mice was associated with a small birth weight in juvenile 5-week-old male offspring, which was related to an increase in spontaneous activity and energy expenditure.6 Interestingly, in adulthood, a High Fat Diet (HFD) combined with maternal VDD disrupted glucose homeostasis and adiposity in male offspring but not in females.6 Under the same experimental conditions, maternal VDD coupled with a HFD induced an increase in white adipose tissue inflammation as well as increased adiposity in male offspring only, thus highlighting the sexual dimorphism in response to developmental VDD.50 Maternal VDD is also associated with an increased risk of type 1 diabetes in the offspring.51,52
Maternal VD status also affects the epigenome of the foetus via different epigenetic mechanisms, particularly in the early stages of gestation, which can contribute to the onset and progression of diseases in adulthood.53 VD also exerts a genomic effect in the developing brain by modulating the proliferation and differentiation of neural stem cells, regulating the development of the dopaminergic system and the release of neurotransmitters such as dopamine. It can also exhibit non-genomic actions by inducing an increase in extracellular Ca2+ uptake or by modulating kinase-activated signalling pathways in the developing cortex. Taken together, these data highlight the importance of VD and the fact that exposure to low levels of this micronutrient during foetal life alters brain development and predisposes offspring to various neurological disorders.22,54,55
In the next sections of this review, an update on the impact of maternal VDD on the onset of neurological and psychological disorders in the offspring has been presented.
4. The impact of maternal vitamin D deficiency on cerebral functions
4.1. Maternal vitamin D deficiency and brain development
VD is considered as a potent neurosteroid that acts via genomic and non-genomic effects.56 Interestingly, the brain has the ability to metabolize VD due to the presence of 1α-hydroxylase and CYP24A1 in neurons and glial cells.57 VDR is highly expressed in different areas of the mesencephalon during embryonic development as well as in the hippocampus, thalamus, hypothalamus, cortex and substantia nigra, suggesting that VD can modulate brain development and maturation.57–59During brain development, VD inhibits proliferation and potentiates neuronal cell differentiation and apoptosis. In accordance with these observations, it has been demonstrated that VDD in embryonic rats induced an increase in brain cell proliferation and a decrease in apoptosis with dysregulation of pro-apoptotic genes such as Bak (BCL2 antagonist/killer 1).12,60 From a morphological point of view, maternal VDD in rats resulted in a longer and larger brain, a longer and thinner cortex and enlarged lateral ventricles,12,60 which persist into adulthood.61 However, the brains of maternal VDD mice were reduced in length with reduced lateral ventricle volume.62 The absence of VD during foetal development also appears to affect brain development in C57BL/6J female mice at P0 with a reduction in their hippocampal volume that did not persist in adulthood. In addition, a volumetric decrease in the lateral ventricle and an increase in striatum volume were observed in adult male mice.63 Altogether, these observations strongly suggest the involvement of VD in the release of many neurotransmitters. In the context of the role of VD in the onset of neurotransmitter systems, it has been shown in a Sprague–Dawley rat maternal VDD model that VD depletion during development reduced factors essential for the onset of dopaminergic phenotypes such as Nurr1 (nuclear receptor-related 1) and p57Kip2 (p57 kinase inhibitory protein 2).64 The tyrosine hydroxylase (TH) gene expression was reduced in the foetal brain of female offspring from a mouse model of maternal VDD with a decrease of TH protein in the substantia nigra.62 Regarding the GABAergic system, it has been shown that maternal VD deficiency is associated with a decrease in the expression of GABA-A alpha4 (gamma-aminobutyric acid-Aα4), a gene involved in GABA neurotransmission, in the male offspring brains.61 This micronutrient also promotes the release and expression of neurotrophic factors such as nerve growth factor (NGF),12,65 glial-derived neurotrophic factor (GDNF) and brain-derived neurotrophic factor (BDNF)12,66 in different neuronal and glial cell models. Interestingly, a decrease in the synthesis of NGF as well as the expression of genes involved in the neuronal structure such as MAP-2 (microtubule associated protein-2) was observed in 10-week-old male rats born from VDD dams.61
Maternal VDD affects brain development during foetal life and causes alterations that may persist into adulthood, which can lead to the onset of many neurological disorders and diseases.
4.2. Maternal vitamin D deficiency and brain pathologies
| Growth stage | Experimental findings | Ref. |
|---|---|---|
| BDNF: brain-derived growth factor; DVD: developmental vitamin D deficiency; Foxp2: forkhead box protein P2; GABA-Aα4: gamma-amino butyric acid A alpha 4; GDNF: glial-derived neurotrophic factor; MAP-2: microtubule associated protein-2; NGF: nerve growth factor; Nurr1: nuclear receptor-related 1; p57Kip2: p57 kinase inhibitory protein 2; p75NTR: p75 neurotrophin receptor; TgfB1: transforming growth factor beta 1; TH: tyrosine hydroxylase. | ||
| Morphological and functional changes | ||
| E12–E15 | Reduced Nurr1 levels at E12 and E15 | 65 |
| Reduced p57Kip2 expression at E12 | ||
| Reduced TH expression at E12 | ||
| E14.5–E17.5 | Reduced lateral ventricle volume | 63 |
| Decrease in the expression of BDNF at E14.5 and increase in BDNF and TgfB1 at E17.5 | ||
| Reduced TH gene expression in females at E17.5 | ||
| Reduced TH protein localization in the substantia nigra of female fetuses | ||
| E19–P7 | Enlarged lateral ventricles | 61 |
| Down-regulation of pro-apoptotic gene expression with reduced apoptosis | ||
| Up-regulation of pro-mitotic gene expression with increased cell proliferation | ||
| P0 | Increased number of mitotic cells | 12 |
| Longer and larger hemispheres | ||
| Elongated and thin cortex | ||
| Enlarged lateral ventricles | ||
| Decreased expression of NGF, GDNF and p75NTR | ||
| P0–P70 | Reduction of the hippocampal volume in P0 female mice | 64 |
| Volumetric decrease in lateral ventricle and increased striatum volume in adult male mice | ||
| 10 weeks | Decrease in the synthesis of neurotrophic factors such as NGF, the expression of genes involved in the neuronal structure such as MAP-2 and neurotransmission with decreased levels of GABA-Aα4 | 62 |
| Enlarged lateral ventricles in adult brains | ||
| Behavioural impairment/disorders | ||
| E14.5–E17.5 | Decreased expression levels of Foxp2 and reduced Foxp2 protein expression in the cortex of females at E17.5 | 62 |
| E15 | Decreased levels of Nurr1 and TH in the embryonic mesencephalon | 93 |
| P12 | Decreased cortical FoxP2 | 70 |
| Increased vocalization | ||
| Post weaning (P21) | Increased impulsive behavior | 68 |
| Decreased inhibitory control | ||
| P27–P80 | Less social interaction observed in adolescents | 81 |
| Altered stereotyped repetitive behavior in adolescent and young adult rats | ||
| Hyperactivity in adults | ||
| 10 weeks | Hyperlocomotion | 69 |
| 10 weeks | Dysregulated genes and proteins – identified in multiple sclerosis – in the prefrontal cortex and hippocampus of the DVD-deficient progeny | 111 and 112 |
| 4 months | Increased grooming behaviour | 71 |
| Impaired social, learning and memory behaviour | ||
| 5–6 months | Spontaneous locomotor hyperactivity | 70 |
| 30 weeks | Impaired learning | 72 |
Some behavioural defects were also observed in humans as it has been reported in an Australian cohort that there was a 2-fold increase in the risk of language disorder at ages of 5 and 10 years in the offspring of mothers with rates of 25(OH)D ≤ 46 nmol L−1 during the second trimester.72 The Spanish INMA cohort also showed that there was a positive association between maternal VD levels and social competence in 5-year-old offspring.73
Using data from the Stockholm youth cohort, Magnusson et al.78 examined a population of 4–17-year-old children exposed to low levels of VD during gestation and were able to report a positive association between maternal VDD and ASD.78 Analysing the same cohort, Lee et al.79 suggested that high levels of VD during pregnancy were associated with a moderate decrease in the risk of ASD in the offspring.79 A prospective study of a multi-ethnic cohort in the Netherlands (generation R study) has also shown an association between maternal mid-gestation VDD and a two-fold increase in the risk of autism in children80 (Table 2). Interestingly, VD supplementation seems to clinically improve ASD symptoms in affected children.81,82
| Cohort | Offspring age | Experimental findings | Ref. |
|---|---|---|---|
| ASD: autism spectrum disorder; VD: vitamin D; VDD: vitamin D deficiency; MS: multiple sclerosis; UV: ultraviolet. | |||
| Autism/developmental disorders | |||
| Australian cohort | 5–10 years old | Language impairment | 73 |
| Stockholm Youth Cohort | 4–17 years old | Positive association between maternal VD deficiency and ASD | 79 |
| Stockholm Youth Cohort | 0–17 years old | Association between high maternal levels of 25OHD and modestly lower risk of ASD | 80 |
| Generation R study | 6 years old | Two-fold increased risk of ASD in children of VDD mothers (mid-gestation) | 81 |
| Schizophrenia | |||
| Danish case–control study | Significant increase of schizophrenia for new-borns with 25OHD levels below 20.4 nmol L−1 | 95 | |
| Finnish birth cohort | 31 years (follow-up from pregnancy) | VD supplement in the first year of life is associated with a decreased risk of schizophrenia in males | 96 |
| Depression | |||
| DaFO88 cohort | 22 years (follow-up from birth) | Direct association between maternal VDD and offspring depression | 105 |
| The Avon Longitudinal Study of Parents and Children (ALSPAC) | Children: 10.6 years old (mean age) | No risk of developing depression during childhood/adolescence was assessed | 106 |
| Adolescents: 13.8 years old (mean age) | |||
| Multiple sclerosis | |||
| Women in the Finnish Maternity Cohort | Exposure to maternal 25(OH)D levels <30 nmol L−1 during early gestational stages increases the risk of developing MS | 113 | |
| Cohort study (Nurses’ Health Study II) | Higher maternal VD dietary intake and higher 25(OH) D levels may be associated with a decreased risk of developing MS | 114 | |
| Longitudinal analysis | Low maternal exposure to UV is associated with an increased risk of MS in offspring | 117 | |
| Population-based study | Association between the month of birth of offspring and an increased risk of MS | 118 | |
In humans, the neonatal status of 25(OH)D from dried blood samples demonstrated that newborns with VD levels below 20.4 nmol L−1 had a significant increase in the risk of schizophrenia.94 Interestingly, a prospective Finnish cohort has shown that VD supplementation in males during the first year of life reduced the risk of developing schizophrenia in adulthood95 (Table 2).
In humans, by using data from a pre-birth cohort with up to 22 years of follow-up, Strøm et al.104 tried to find an association between maternal 25(OH)D levels and the neurodevelopmental outcomes of the offspring, taking into consideration the first admission diagnosis of the offspring or the prescription of medication for depression. A direct association between maternal 25(OH)D levels and offspring depression was observed.104 However, the Avon Longitudinal Study of Parents and Children (ALSPAC) showed contradictory results. This study included 2938 children (mean age: 10.6) and 2485 adolescents (mean age: 13.8) and had their short moods and feelings questionnaire (SMFQ) assessed at least once, which indicated that maternal VDD during pregnancy did not increase the risk of developing childhood/adolescent depression105 (Table 2). Several studies96,97,106 have explored the link between VDD and depression as well as the impact of VD supplementation on reducing depression symptoms.107,108 However, the exact mechanism by which VD contributes to the aetiology and treatment of depression needs further study.
Similar observations were made in many human studies. A Finnish study reported an increased risk of developing MS in the offspring of VD deficient mothers with 25(OH)D levels <30 nmol L−1 early in their pregnancy.112 Another study assessing the diet of 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 794 pregnant mothers and the follow-up of their daughters found that the offspring of mothers with higher VD dietary intake and higher 25(OH)D predicted levels during pregnancy had a lower risk of developing MS during adulthood.113 Thus, MS patients with low VD levels have been associated with higher disease activity.114,115 Many studies on the time and region of birth have found associations between low maternal exposure to ultraviolet rays (UV),116 the month of birth117 and the risk of MS in offspring (Table 2). As VD seems to be a risk factor for MS, several studies have tried to investigate the potential therapeutic role of VD supplementation in MS. To address this question, a rodent model of MS was used to explore the impact of VD supplementation during different developmental stages, including supplementation prior to and during gestation and lactation, after weaning, throughout the juvenile/adolescence period and during adulthood.118 This study highlighted that VD supplementation was associated with a decrease in central nervous system inflammation and demyelination. However, these results were dependent on the developmental stage during which this supplementation occurred.118 However, the benefits of VD supplementation on the disease course of MS are yet to be established as many clinical studies have shown mixed results for the effectiveness of VD supplementation in MS, and the appropriate dose of supplementation still remains undetermined.119–121
794 pregnant mothers and the follow-up of their daughters found that the offspring of mothers with higher VD dietary intake and higher 25(OH)D predicted levels during pregnancy had a lower risk of developing MS during adulthood.113 Thus, MS patients with low VD levels have been associated with higher disease activity.114,115 Many studies on the time and region of birth have found associations between low maternal exposure to ultraviolet rays (UV),116 the month of birth117 and the risk of MS in offspring (Table 2). As VD seems to be a risk factor for MS, several studies have tried to investigate the potential therapeutic role of VD supplementation in MS. To address this question, a rodent model of MS was used to explore the impact of VD supplementation during different developmental stages, including supplementation prior to and during gestation and lactation, after weaning, throughout the juvenile/adolescence period and during adulthood.118 This study highlighted that VD supplementation was associated with a decrease in central nervous system inflammation and demyelination. However, these results were dependent on the developmental stage during which this supplementation occurred.118 However, the benefits of VD supplementation on the disease course of MS are yet to be established as many clinical studies have shown mixed results for the effectiveness of VD supplementation in MS, and the appropriate dose of supplementation still remains undetermined.119–121
5. Conclusions and perspectives
Many animal and epidemiological studies have been conducted to explore the implication of VDD during foetal life on the developing brain and many have found associations between low gestational VD levels and the onset of many neurological diseases in adulthood. Different animal studies described changes in the expression of many genes related to overall brain development and functions, but the molecular mechanisms by which maternal VDD alters brain development remain poorly understood (Fig. 2).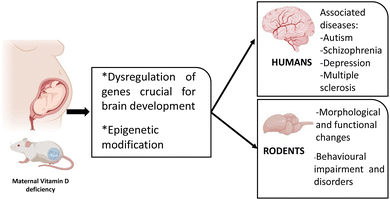 | ||
| Fig. 2 The impact of maternal VDD on the development of neurological diseases in rodents and humans. | ||
However, recent research has generated interesting data on the role of epigenetic modifications during gestational VD restriction. For instance, the group of Eyles observed an upregulation of miR-181c-5p that suppressed neurite outgrowth of DA neurons from the ventral mesencephalon.122 These data suggested that miRNAs can be involved in the onset of psychological disorders such as schizophrenia. In addition, it has been shown that VD plays a role in the DNA methylation state in the germline and soma cells, suggesting a role of this mechanism in brain development.53 In their preclinical study, Liang et al. showed that VDD potentiates maternal diabetes-induced autism-related phenotypes in offspring by epigenetic mechanisms.123 In the same way, epigenetic modifications were observed in a population-based mother–offspring cohort, in which a negative association between the free maternal 25(OH)D index and RXR alpha (retinoic X receptor) methylation at a specific site was detected.124 Altogether, these data indicate that maternal VDD induces epigenetic modifications in the offspring. However, more studies are needed to identify the exact mechanisms underlying the changes and outcomes that developmental VDD has on the brain. Many clinical studies show that supplementation with VD during pregnancy has beneficial effects on both the mother and the offspring.125 These studies suggest that maintaining optimal VD levels might reduce the risk of developing a wide range of disorders. Additional rigorous and larger randomised trials are required to evaluate the effects of VD supplementation in pregnancy, particularly in relation to the onset of brain disorders.
Author contributions
Writing—original draft preparation, L. S. and L. M. Writing—review and editing, H. H., F. S., J.-F. L. and L. M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
AMU, INRAE, and INSERM are acknowledged for their financial support. The authors are grateful to O. Knowles for critical reading of the manuscript.References
- C. N. Hsu and Y. L. Tain, The Good, the Bad, and the Ugly of Pregnancy Nutrients and Developmental Programming of Adult Disease, Nutrients, 2019, 11, 894 CrossRef CAS PubMed.
- D. Vieau, Perinatal nutritional programming of health and metabolic adult disease, WJG, 2011, 2, 133 CrossRef PubMed.
- M. A. Hanson and P. D. Gluckman, Early Developmental Conditioning of Later Health and Disease: Physiology or Pathophysiology?, Physiol. Rev., 2014, 94, 1027–1076 CrossRef CAS PubMed.
- D. J. Hoffman, T. L. Powell, E. S. Barrett and D. B. Hardy, Developmental origins of metabolic diseases, Physiol. Rev., 2021, 101, 739–795 CrossRef CAS PubMed.
- D. H. Elsori and M. S. Hammoud, Vitamin D deficiency in mothers, neonates and children, J. Steroid Biochem. Mol. Biol., 2018, 175, 195–199 CrossRef CAS PubMed.
- E. M. Seipelt, F. Tourniaire, C. Couturier, J. Astier, B. Loriod, H. Vachon, M. Pucéat, L. Mounien and J. Landrier, Prenatal maternal vitamin D deficiency sex–dependently programs adipose tissue metabolism and energy homeostasis in offspring, FASEB J., 2020, 34, 14905–14919 CrossRef CAS PubMed.
- S. Christakos, A. Verstuyf, L. Verlinden and G. Carmeliet, Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects, Physiol. Rev., 2016, 96, 365–408 CrossRef CAS PubMed.
- A. Olmos-Ortiz, E. Avila, M. Durand-Carbajal and L. Díaz, Regulation of Calcitriol Biosynthesis and Activity: Focus on Gestational Vitamin D Deficiency and Adverse Pregnancy Outcomes, Nutrients, 2015, 7, 443–480 CrossRef PubMed.
- A. T. Kho, S. Sharma, W. Qiu, R. Gaedigk, B. Klanderman, S. Niu, C. Anderson, J. S. Leeder, S. T. Weiss and K. G. Tantisira, Vitamin D related genes in lung development and asthma pathogenesis, BMC Med. Genomics, 2013, 6, 47 CrossRef PubMed.
- R. E. Foong, A. Bosco, A. C. Jones, A. Gout, S. Gorman, P. H. Hart and G. R. Zosky, The Effects of In Utero Vitamin D Deficiency on Airway Smooth Muscle Mass and Lung Function, Am. J. Respir. Cell Mol. Biol., 2015, 53, 664–675 CrossRef CAS PubMed.
- H. Papalia, A. Samonini, C. Buffat, E. Gras, C. Robert, J.-F. Landrier, V. Pauly and F. Boubred, Low Vitamin D Levels at Birth and Early Respiratory Outcome in Infants With Gestational Age Less Than 29 Weeks, Front. Pediatr., 2022, 9, 790839 CrossRef PubMed.
- D. Eyles, J. Brown, A. Mackay-Sim, J. McGrath and F. Feron, Vitamin d3 and brain development, Neuroscience, 2003, 118, 641–653 CrossRef CAS PubMed.
- L. J. Dominguez, M. Farruggia, N. Veronese and M. Barbagallo, Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment, Metabolites, 2021, 11, 255 CrossRef CAS PubMed.
- I. Bennour, N. Haroun, F. Sicard, L. Mounien and J.-F. Landrier, Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies, Nutrients, 2022, 14, 2049 CrossRef CAS PubMed.
- I. Bennour, N. Haroun, F. Sicard, L. Mounien and J.-F. Landrier, Recent insights into vitamin D, adipocyte, and adipose tissue biology, Obes. Rev., 2022, 23, e13453 CAS.
- J. Marcotorchino, F. Tourniaire and J.-F. Landrier, Vitamin D, adipose tissue, and obesity, Horm. Mol. Biol. Clin. Invest., 2013, 15, 123–128 CAS.
- J.-F. Landrier, E. Karkeni, J. Marcotorchino, L. Bonnet and F. Tourniaire, Vitamin D modulates adipose tissue biology: possible consequences for obesity?, Proc. Nutr. Soc., 2016, 75, 38–46 CrossRef CAS PubMed.
- L. Bonnet, E. Karkeni, C. Couturier, J. Astier, C. Defoort, L. Svilar, F. Tourniaire, L. Mounien and J.-F. Landrier, Four days high fat diet modulates vitamin D metabolite levels and enzymes in mice, J. Endocrinol., 2021, 248, 87–93 CAS.
- M. Blum, G. Dolnikowski, E. Seyoum, S. S. Harris, S. L. Booth, J. Peterson, E. Saltzman and B. Dawson-Hughes, Vitamin D3 in fat tissue, Endocrine, 2008, 33, 90–94 CrossRef CAS PubMed.
- L. Bonnet, M. A. Hachemi, E. Karkeni, C. Couturier, J. Astier, C. Defoort, L. Svilar, J.-C. Martin, F. Tourniaire and J.-F. Landrier, Diet induced obesity modifies vitamin D metabolism and adipose tissue storage in mice, J. Steroid Biochem. Mol. Biol., 2019, 185, 39–46 CrossRef CAS PubMed.
- A. Carrelli, M. Bucovsky, R. Horst, S. Cremers, C. Zhang, M. Bessler, B. Schrope, J. Evanko, J. Blanco, S. J. Silverberg and E. M. Stein, Vitamin D Storage in Adipose Tissue of Obese and Normal Weight Women, J. Bone Miner. Res., 2017, 32, 237–242 CrossRef CAS PubMed.
- X. Cui, H. Gooch, A. Petty, J. J. McGrath and D. Eyles, Vitamin D and the brain: Genomic and non-genomic actions, Mol. Cell. Endocrinol., 2017, 453, 131–143 CrossRef CAS PubMed.
- F. Y. Ideraabdullah, A. M. Belenchia, C. S. Rosenfeld, S. W. Kullman, M. Knuth, D. Mahapatra, M. Bereman, E. D. Levin and C. A. Peterson, Maternal vitamin D deficiency and developmental origins of health and disease (DOHaD), J. Endocrinol., 2019, 241, R65–R80 CAS.
- E. Karkeni, L. Bonnet, J. Marcotorchino, F. Tourniaire, J. Astier, J. Ye and J.-F. Landrier, Vitamin D limits inflammation-linked microRNA expression in adipocytes in vitro and in vivo: A new mechanism for the regulation of inflammation by vitamin D, Epigenetics, 2018, 13, 156–162 CrossRef PubMed.
- A. A. Giangreco and L. Nonn, The sum of many small changes: microRNAs are specifically and potentially globally altered by vitamin D3 metabolites, J. Steroid Biochem. Mol. Biol., 2013, 136, 86–93 CrossRef CAS PubMed.
- L. Vuolo, C. Di Somma, A. Faggiano and A. Colao, Vitamin D and Cancer, Front. Endocrinol., 2012, 3, 58 CAS.
- S.-J. Lim and S. H. Kim, Vitamin D in cancer: effects of pharmaceutical drugs on the vitamin D pharmacokinetics, J. Pharm. Invest., 2014, 44, 317–328 CrossRef CAS.
- M. R. Walters and I. Nemere, Receptors for steroid hormones: membrane-associated and nuclear forms, CMLS, Cell. Mol. Life Sci., 2004, 61(18), 2309–2321 CrossRef CAS PubMed.
- C. Turano, E. Gaucci, C. Grillo and S. Chichiarelli, ERp57/GRP58: A protein with multiple functions, Cell. Mol. Biol. Lett., 2011, 16, 539 CAS.
- A.-S. Vanherwegen, C. Gysemans and C. Mathieu, Regulation of Immune Function by Vitamin D and Its Use in Diseases of Immunity, Endocrinol. Metab. Clin. North Am., 2017, 46, 1061–1094 CrossRef PubMed.
- D. D. Bikle, Extraskeletal actions of vitamin D, Ann. N. Y. Acad. Sci., 2016, 1376, 29–52 CrossRef CAS PubMed.
- R. Bouillon, C. Marcocci, G. Carmeliet, D. Bikle, J. H. White, B. Dawson-Hughes, P. Lips, C. F. Munns, M. Lazaretti-Castro, A. Giustina and J. Bilezikian, Skeletal and Extraskeletal Actions of Vitamin D: Current Evidence and Outstanding Questions, Endocr. Rev., 2018, 40, 1109–1151 CrossRef PubMed.
- M. de La Puente-Yagüe, M. A. Cuadrado-Cenzual, M. J. Ciudad-Cabañas, M. Hernández-Cabria and L. Collado-Yurrita, Vitamin D: And its role in breast cancer, Kaohsiung J. Med. Sci., 2018, 34, 423–427 CrossRef PubMed.
- J. Marcotorchino, E. Gouranton, B. Romier, F. Tourniaire, J. Astier, C. Malezet, M.-J. Amiot and J.-F. Landrier, Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes, Mol. Nutr. Food Res., 2012, 56, 1771–1782 CrossRef CAS PubMed.
- C. Y. Park and S. N. Han, The Role of Vitamin D in Adipose Tissue Biology: Adipocyte Differentiation, Energy Metabolism, and Inflammation, J. Lipid Atheroscler., 2021, 10, 130–144 CrossRef CAS PubMed.
- E. Karkeni, J. Marcotorchino, F. Tourniaire, J. Astier, F. Peiretti, P. Darmon and J.-F. Landrier, Vitamin D limits chemokine expression in adipocytes and macrophage migration in vitro and in male mice, Endocrinology, 2015, 156, 1782–1793 CrossRef CAS PubMed.
- F. de la Guía-Galipienso, M. Martínez-Ferran, N. Vallecillo, C. J. Lavie, F. Sanchis-Gomar and H. Pareja-Galeano, Vitamin D and cardiovascular health, Clin. Nutr., 2021, 40, 2946–2957 CrossRef PubMed.
- X. Cui and D. W. Eyles, Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders, Nutrients, 2022, 14, 4353 CrossRef CAS PubMed.
- A. Zhou, J. B. Selvanayagam and E. Hyppönen, Non-linear Mendelian randomization analyses support a role for vitamin D deficiency in cardiovascular disease risk, Eur. Heart J., 2022, 43, 1731–1739 CrossRef CAS PubMed.
- A. Mazur, P. Frączek and J. Tabarkiewicz, Vitamin D as a Nutri-Epigenetic Factor in Autoimmunity—A Review of Current Research and Reports on Vitamin D Deficiency in Autoimmune Diseases, Nutrients, 2022, 14, 4286 CrossRef CAS PubMed.
- A. G. Pittas, R. Jorde, T. Kawahara and B. Dawson-Hughes, Vitamin D Supplementation for Prevention of Type 2 Diabetes Mellitus: To D or Not to D?, J. Clin. Endocrinol. Metab., 2020, 105, dgaa594 CrossRef PubMed.
- B. N. Ames, W. B. Grant and W. C. Willett, Does the High Prevalence of Vitamin D Deficiency in African Americans Contribute to Health Disparities?, Nutrients, 2021, 13, 499 CrossRef CAS PubMed.
- A. Ganguly, J. A. Tamblyn, S. Finn-Sell, S.-Y. Chan, M. Westwood, J. Gupta, M. D. Kilby, S. R. Gross and M. Hewison, Vitamin D, the placenta and early pregnancy: effects on trophoblast function, J. Endocrinol., 2018, 236, R93–R103 Search PubMed.
- N. Q. Liu and M. Hewison, Vitamin D, the placenta and pregnancy, Arch. Biochem. Biophys., 2012, 523, 37–47 CrossRef CAS PubMed.
- S. Agarwal, O. Kovilam and D. K. Agrawal, Vitamin D and its impact on maternal-fetal outcomes in pregnancy: A critical review, Crit. Rev. Food Sci. Nutr., 2018, 58, 755–769 CrossRef CAS PubMed.
- A. Hossein-nezhad and M. F. Holick, Vitamin D for Health: A Global Perspective, Mayo Clin. Proc., 2013, 88, 720–755 CrossRef CAS PubMed.
- K. Miliku, A. Vinkhuyzen, L. M. E. Blanken, J. J. McGrath, D. W. Eyles, T. H. Burne, A. Hofman, H. Tiemeier, E. A. P. Steegers, R. Gaillard and V. W. V. Jaddoe, Maternal Vitamin D Concentrations During Pregnancy, Fetal Growth Patterns and Risks of Adverse Birth Outcomes, Am. J. Clin. Nutr., 2016, 103, 1514–1522 CrossRef CAS PubMed.
- V. Daraki, T. Roumeliotaki, G. Chalkiadaki, M. Katrinaki, M. Karachaliou, V. Leventakou, M. Vafeiadi, K. Sarri, M. Vassilaki, S. Papavasiliou, M. Kogevinas and L. Chatzi, Low maternal vitamin D status in pregnancy increases the risk of childhood obesity: Maternal 25(OH)D and child obesity outcomes, Pediatr. Obes., 2018, 13, 467–475 CrossRef CAS PubMed.
- E. Morales, A. Rodriguez, D. Valvi, C. Iñiguez, A. Esplugues, J. Vioque, L. S. Marina, A. Jiménez, M. Espada, C. R. Dehli, A. Fernández-Somoano, M. Vrijheid and J. Sunyer, Deficit of vitamin D in pregnancy and growth and overweight in the offspring, Int. J. Obes., 2015, 39, 61–68 CrossRef CAS PubMed.
- N. Haroun, I. Bennour, E. Seipelt, J. Astier, C. Couturier, L. Mounien and J.-F. Landrier, Maternal Vitamin D Deficiency in Mice Increases White Adipose Tissue Inflammation in Offspring, Cells, 2022, 11, 2024 CrossRef CAS PubMed.
- I. M. Sørensen, G. Joner, P. A. Jenum, A. Eskild, P. A. Torjesen and L. C. Stene, Maternal Serum Levels of 25-Hydroxy-Vitamin D During Pregnancy and Risk of Type 1 Diabetes in the Offspring, Diabetes, 2012, 61, 175–178 CrossRef PubMed.
- G. Tapia, K. Mårild, S. R. Dahl, N. A. Lund-Blix, M. K. Viken, B. A. Lie, P. R. Njølstad, G. Joner, T. Skrivarhaug, A. S. Cohen, K. Størdal and L. C. Stene, Maternal and Newborn Vitamin D–Binding Protein, Vitamin D Levels, Vitamin D Receptor Genotype, and Childhood Type 1 Diabetes, Diabetes Care, 2019, 42, 553–559 CrossRef CAS PubMed.
- J. Xue, S. A. Schoenrock, W. Valdar, L. M. Tarantino and F. Y. Ideraabdullah, Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations, Clin. Epigenet., 2016, 8, 107 CrossRef PubMed.
- X. Cui, J. J. McGrath, T. H. J. Burne, A. Mackay-Sim and D. W. Eyles, Maternal vitamin D depletion alters neurogenesis in the developing rat brain, Int. J. Dev. Neurosci., 2007, 25, 227–232 CrossRef CAS PubMed.
- J. O'Loan, D. W. Eyles, J. Kesby, P. Ko, J. J. McGrath and T. H. J. Burne, Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring, Psychoneuroendocrinology, 2007, 32, 227–234 CrossRef PubMed.
- N. J. Groves, J. J. McGrath and T. H. J. Burne, Vitamin D as a Neurosteroid Affecting the Developing and Adult Brain, Annu. Rev. Nutr., 2014, 34, 117–141 CrossRef CAS PubMed.
- D. W. Eyles, S. Smith, R. Kinobe, M. Hewison and J. J. McGrath, Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain, J. Chem. Neuroanat., 2005, 29, 21–30 CrossRef CAS PubMed.
- R. Moretti, M. E. Morelli and P. Caruso, Vitamin D in Neurological Diseases: A Rationale for a Pathogenic Impact, Int. J. Mol. Sci., 2018, 19(8), 2245 CrossRef PubMed.
- X. Cui, M. Pelekanos, P.-Y. Liu, T. H. J. Burne, J. J. McGrath and D. W. Eyles, The vitamin D receptor in dopamine neurons; its presence in human substantia nigra and its ontogenesis in rat midbrain, Neuroscience, 2013, 236, 77–87 CrossRef CAS PubMed.
- P. Ko, R. Burkert, J. McGrath and D. Eyles, Maternal vitamin D3 deprivation and the regulation of apoptosis and cell cycle during rat brain development, Dev. Brain Res., 2004, 153, 61–68 CrossRef CAS PubMed.
- F. Féron, T. H. J. Burne, J. Brown, E. Smith, J. J. McGrath, A. Mackay-Sim and D. W. Eyles, Developmental Vitamin D3 deficiency alters the adult rat brain, Brain Res. Bull., 2005, 65, 141–148 CrossRef PubMed.
- J. E. Hawes, D. Tesic, A. J. Whitehouse, G. R. Zosky, J. T. Smith and C. S. Wyrwoll, Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse, Behav. Brain Res., 2015, 286, 192–200 CrossRef CAS PubMed.
- L. R. Harms, G. Cowin, D. W. Eyles, N. D. Kurniawan, J. J. McGrath and T. H. J. Burne, Neuroanatomy and psychomimetic-induced locomotion in C57BL/6J and 129/X1SvJ mice exposed to developmental vitamin D deficiency, Behav. Brain Res., 2012, 230, 125–131 CrossRef CAS PubMed.
- X. Cui, M. Pelekanos, T. H. J. Burne, J. J. McGrath and D. W. Eyles, Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon, Neurosci. Lett., 2010, 486, 220–223 CrossRef CAS PubMed.
- J. Brown, J. I. Bianco, J. J. McGrath and D. W. Eyles, 1,25-Dihydroxyvitamin D3 induces nerve growth factor, promotes neurite outgrowth and inhibits mitosis in embryonic rat hippocampal neurons, Neurosci. Lett., 2003, 343, 139–143 CrossRef CAS PubMed.
- H. A. Shirazi, J. Rasouli, B. Ciric, A. Rostami and G. Zhang, 1,25-dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation, Exp. Mol. Pathol., 2015, 98, 240–245 CrossRef CAS PubMed.
- K. M. Turner, J. W. Young, J. J. McGrath, D. W. Eyles and T. H. J. Burne, Cognitive performance and response inhibition in developmentally vitamin D (DVD)-deficient rats, Behav. Brain Res., 2013, 242, 47–53 CrossRef CAS PubMed.
- T. H. J. Burne, A. Becker, J. Brown, D. W. Eyles, A. Mackay-Sim and J. J. McGrath, Transient prenatal Vitamin D deficiency is associated with hyperlocomotion in adult rats, Behav. Brain Res., 2004, 154, 549–555 CrossRef CAS PubMed.
- J. P. Kesby, J. C. O'Loan, S. Alexander, C. Deng, X.-F. Huang, J. J. McGrath, D. W. Eyles and T. H. J. Burne, Developmental vitamin D deficiency alters MK-801-induced behaviours in adult offspring, Psychopharmacology, 2012, 220, 455–463 CrossRef CAS PubMed.
- N. J. Yates, D. Tesic, K. W. Feindel, J. T. Smith, M. W. Clarke, C. Wale, R. C. Crew, M. D. Wharfe, A. J. O. Whitehouse and C. S. Wyrwoll, Vitamin D is crucial for maternal care and offspring social behaviour in rats, J. Endocrinol., 2018, 237, 73–85 CAS.
- D. A. Fernandes de Abreu, E. Nivet, N. Baril, M. Khrestchatisky, F. Roman and F. Féron, Developmental vitamin D deficiency alters learning in C57Bl/6J mice, Behav. Brain Res., 2010, 208, 603–608 CrossRef CAS PubMed.
- A. J. O. Whitehouse, B. J. Holt, M. Serralha, P. G. Holt, M. M. H. Kusel and P. H. Hart, Maternal Serum Vitamin D Levels During Pregnancy and Offspring Neurocognitive Development, Pediatrics, 2012, 129, 485–493 CrossRef PubMed.
- M. López-Vicente, J. Sunyer, N. Lertxundi, L. González, C. Rodríguez-Dehli, M. Espada Sáenz-Torre, M. Vrijheid, A. Tardón, S. Llop, M. Torrent, J. Ibarluzea and M. Guxens, Maternal circulating Vitamin D3 levels during pregnancy and behaviour across childhood, Sci. Rep., 2019, 9, 14792 CrossRef PubMed.
- H. Hodges, C. Fealko and N. Soares, Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation, Transl. Pediatr., 2020, 9, S55–S65 CrossRef PubMed.
- H. Mazahery, C. A. Camargo Jr., C. Conlon, K. L. Beck, M. C. Kruger and P. R. von Hurst, Vitamin D and Autism Spectrum Disorder: A Literature Review, Nutrients, 2016, 8(4), 236 CrossRef PubMed.
- J. J. Cannell, Vitamin D and autism, what's new?, Rev. Endocr. Metab. Disord., 2017, 18, 183–193 CrossRef CAS PubMed.
- A. Ali, S. Vasileva, M. Langguth, S. Alexander, X. Cui, A. Whitehouse, J. J. McGrath and D. Eyles, Developmental Vitamin D Deficiency Produces Behavioral Phenotypes of Relevance to Autism in an Animal Model, Nutrients, 2019, 11(5), 1187 CrossRef CAS PubMed.
- C. Magnusson, M. Lundberg, B. K. Lee, D. Rai, H. Karlsson, R. Gardner, K. Kosidou, S. Arver and C. Dalman, Maternal vitamin D deficiency and the risk of autism spectrum disorders: population-based study, BJPsych Open, 2016, 2, 170–172 CrossRef PubMed.
- B. K. Lee, D. W. Eyles, C. Magnusson, C. J. Newschaffer, J. J. McGrath, D. Kvaskoff, P. Ko, C. Dalman, H. Karlsson and R. M. Gardner, Developmental vitamin D and autism spectrum disorders: findings from the Stockholm Youth Cohort, Mol. Psychiatry, 2021, 26(5), 1578–1588 CrossRef CAS PubMed.
- A. A. E. Vinkhuyzen, D. W. Eyles, T. H. J. Burne, L. M. E. Blanken, C. J. Kruithof, F. Verhulst, T. White, V. W. Jaddoe, H. Tiemeier and J. J. McGrath, Gestational vitamin D deficiency and autism spectrum disorder, BJPsych Open, 2017, 3, 85–90 CrossRef PubMed.
- J. Feng, L. Shan, L. Du, B. Wang, H. Li, W. Wang, T. Wang, H. Dong, X. Yue, Z. Xu, W. G. Staal and F. Jia, Clinical improvement following vitamin D3 supplementation in Autism Spectrum Disorder, Nutr. Neurosci., 2017, 20, 284–290 CrossRef CAS PubMed.
- Z. Javadfar, H. Abdollahzad, J. Moludi, S. Rezaeian, H. Amirian, A. A. Foroughi, S. M. Nachvak, N. Goharmehr and R. Mostafai, Effects of vitamin D supplementation on core symptoms, serum serotonin, and interleukin-6 in children with autism spectrum disorders: A randomized clinical trial, Nutrition, 2020, 79–80, 110986 CrossRef CAS PubMed.
- J. S. Kim, C. M. Park, J. A. Choi, E. Park, H. J. Tchoe, M. Choi, J. K. Suh, Y. H. Kim, S. H. Won, Y. C. Chung, K. Y. Bae, S. K. Lee, S. C. Park and S. H. Lee, The association between season of birth, age at onset, and clozapine use in schizophrenia, Acta Psychiatr. Scand., 2017, 136, 445–454 CrossRef CAS PubMed.
- E. Cantor-Graae and J.-P. Selten, Schizophrenia and Migration: A Meta-Analysis and Review, Am. J. Psychiatry, 2005, 162, 12–24 CrossRef PubMed.
- D. St Clair, Rates of Adult Schizophrenia Following Prenatal Exposure to the Chinese Famine of 1959–1961, J. Am. Med. Assoc., 2005, 294, 557 CrossRef CAS PubMed.
- X. Cui, J. J. McGrath, T. H. J. Burne and D. W. Eyles, Vitamin D and schizophrenia: 20 years on, Mol. Psychiatry, 2021, 26, 2708–2720 CrossRef PubMed.
- K. R. Patel, J. Cherian, K. Gohil and D. Atkinson, Schizophrenia: Overview and Treatment Options, P T, 2014, 39, 638–645 Search PubMed.
- D. W. Eyles, F. Feron, X. Cui, J. P. Kesby, L. H. Harms, P. Ko, J. J. McGrath and T. H. J. Burne, Developmental vitamin D deficiency causes abnormal brain development, Psychoneuroendocrinology, 2009, 34, S247–S257 CrossRef CAS PubMed.
- M. Di Forti, J. M. Lappin and R. M. Murray, Risk factors for schizophrenia—All roads lead to dopamine, Eur. Neuropsychopharmacol., 2007, 17, S101–S107 CrossRef CAS PubMed.
- M. J. Owen, A. Sawa and P. B. Mortensen, Schizophrenia, Lancet, 2016, 388, 86–97 CrossRef PubMed.
- J. P. Kesby, X. Cui, J. O'Loan, J. J. McGrath, T. H. J. Burne and D. W. Eyles, Developmental vitamin D deficiency alters dopamine-mediated behaviors and dopamine transporter function in adult female rats, Psychopharmacology, 2010, 208, 159–168 CrossRef CAS PubMed.
- W. Luan, L. A. Hammond, E. Cotter, G. W. Osborne, S. A. Alexander, V. Nink, X. Cui and D. W. Eyles, Developmental Vitamin D (DVD) Deficiency Reduces Nurr1 and TH Expression in Post-mitotic Dopamine Neurons in Rat Mesencephalon, Mol. Neurobiol., 2018, 55, 2443–2453 CrossRef CAS PubMed.
- H. Gooch, X. Cui, V. Anggono, M. Trzaskowski, M. C. Tan, D. W. Eyles, T. H. J. Burne, S. E. Jang, M. Mattheisen, D. M. Hougaard, B. N. Pedersen, A. Cohen, P. B. Mortensen, P. Sah and J. J. McGrath, 1,25-Dihydroxyvitamin D modulates L-type voltage-gated calcium channels in a subset of neurons in the developing mouse prefrontal cortex, Transl. Psychiatry, 2019, 9(1), 281 CrossRef PubMed.
- D. W. Eyles, M. Trzaskowski, A. A. E. Vinkhuyzen, M. Mattheisen, S. Meier, H. Gooch, V. Anggono, X. Cui, M. C. Tan, T. H. J. Burne, S. E. Jang, D. Kvaskoff, D. M. Hougaard, B. Nørgaard-Pedersen, A. Cohen, E. Agerbo, C. B. Pedersen, A. D. Børglum, O. Mors, P. Sah, N. R. Wray, P. B. Mortensen and J. J. McGrath, The association between neonatal vitamin D status and risk of schizophrenia, Sci. Rep., 2018, 8(1), 17692 CrossRef CAS PubMed.
- J. McGrath, K. Saari, H. Hakko, J. Jokelainen, P. Jones, M.-R. Järvelin, D. Chant and M. Isohanni, Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study, Schizophr. Res., 2004, 67, 237–245 CrossRef PubMed.
- F. M. Moy, V. C. Hoe, N. N. Hairi, S. R. Vethakkan and A. Bulgiba, Vitamin D deficiency and depression among women from an urban community in a tropical country, Public Health Nutr., 2017, 20, 1844–1850 CrossRef PubMed.
- J. H. Jhee, H. Kim, S. Park, H.-R. Yun, S.-Y. Jung, Y. K. Kee, C.-Y. Yoon, J. T. Park, S. H. Han, S.-W. Kang and T.-H. Yoo, Vitamin D deficiency is significantly associated with depression in patients with chronic kidney disease, PLoS One, 2017, 12, e0171009 CrossRef PubMed.
- L. Fu, Y.-H. Chen, X. Chen, S. Xu, Z. Yu and D.-X. Xu, Vitamin D deficiency impairs neurobehavioral development in male mice, Physiol. Behav., 2017, 179, 333–339 CrossRef CAS PubMed.
- Y. He, Z. Wu, T. Lan, Y. Wang, Y. Tian, X. Chen, Y. Li, M. Bai, J. Liu, X. Gong, K. Cheng and P. Xie, The 25(OH)D/VDR signaling may play a role in major depression, Biochem. Biophys. Res. Commun., 2020, 523, 405–410 CrossRef CAS PubMed.
- R. P. Patrick and B. N. Ames, Vitamin D hormone regulates serotonin synthesis. Part 1: relevance for autism, FASEB J., 2014, 28, 2398–2413 CrossRef CAS PubMed.
- M. Berger, J. A. Gray and B. L. Roth, The Expanded Biology of Serotonin, Annu. Rev. Med., 2009, 60, 355–366 CrossRef CAS PubMed.
- C. B. Nemeroff and M. J. Owens, The Role of Serotonin in the Pathophysiology of Depression: As Important As Ever, Clin. Chem., 2009, 55, 1578–1579 CrossRef CAS PubMed.
- F. Kazemi, S. Babri, P. Keyhanmehr, M. Farid-Habibi, S. N. Rad and F. Farajdokht, Maternal vitamin D supplementation and treadmill exercise attenuated vitamin D deficiency-induced anxiety-and depressive-like behaviors in adult male offspring rats, Nutr. Neurosci., 2022, 1–13 Search PubMed.
- M. Strøm, T. I. Halldorsson, S. Hansen, C. Granström, E. Maslova, S. B. Petersen, A. S. Cohen and S. F. Olsen, Vitamin D Measured in Maternal Serum and Offspring Neurodevelopmental Outcomes: A Prospective Study with Long-Term Follow-Up, Ann. Nutr. Metab., 2014, 64, 254–261 CrossRef PubMed.
- M.-J. Wang, E. C. Dunn, O. I. Okereke, P. Kraft, Y. Zhu and J. W. Smoller, Maternal vitamin D status during pregnancy and offspring risk of childhood/adolescent depression: Results from the Avon Longitudinal Study of Parents and Children (ALSPAC), J. Affective Disord., 2020, 265, 255–262 CrossRef CAS PubMed.
- W. J. G. Hoogendijk, P. Lips, M. G. Dik, D. J. H. Deeg, A. T. F. Beekman and B. W. J. H. Penninx, Depression Is Associated With Decreased 25-Hydroxyvitamin D and Increased Parathyroid Hormone Levels in Older Adults, Arch. Gen. Psychiatry, 2008, 65, 508–512 CrossRef CAS PubMed.
- F. Vellekkatt and V. Menon, Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials, J. Postgrad. Med., 2019, 65, 74–80 CAS.
- M. Kaviani, B. Nikooyeh, H. Zand, P. Yaghmaei and T. R. Neyestani, Effects of vitamin D supplementation on depression and some involved neurotransmitters, J. Affective Disord., 2020, 269, 28–35 CrossRef CAS PubMed.
- C. Pierrot-Deseilligny and J.-C. Souberbielle, Vitamin D and multiple sclerosis: An update, Mult. Scler. Relat. Disord., 2017, 14, 35–45 CrossRef PubMed.
- D. Eyles, L. Almeras, P. Benech, A. Patatian, A. Mackay-Sim, J. McGrath and F. Féron, Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain, J. Steroid Biochem. Mol. Biol., 2007, 103, 538–545 CrossRef CAS PubMed.
- L. Almeras, D. Eyles, P. Benech, D. Laffite, C. Villard, A. Patatian, J. Boucraut, A. Mackay-Sim, J. McGrath and F. Féron, Developmental vitamin D deficiency alters brain protein expression in the adult rat: Implications for neuropsychiatric disorders, Proteomics, 2007, 7, 769–780 CrossRef CAS PubMed.
- K. L. Munger, J. Åivo, K. Hongell, M. Soilu-Hänninen, H.-M. Surcel and A. Ascherio, Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort, JAMA Neurol., 2016, 73, 515–519 CrossRef PubMed.
- F. Mirzaei, K. B. Michels, K. Munger, E. O'Reilly, T. Chitnis, M. R. Forman, E. Giovannucci, B. Rosner and A. Ascherio, Gestational Vitamin D and the Risk of Multiple Sclerosis in the Offspring, Ann. Neurol., 2011, 70, 30–40 CrossRef CAS PubMed.
- J. Smolders, P. Menheere, A. Kessels, J. Damoiseaux and R. Hupperts, Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis, Mult. Scler., 2008, 14, 1220–1224 CrossRef CAS PubMed.
- A. Ascherio, K. L. Munger, R. White, K. Köchert, K. C. Simon, C. H. Polman, M. S. Freedman, H.-P. Hartung, D. H. Miller, X. Montalbán, G. Edan, F. Barkhof, D. Pleimes, E.-W. Radü, R. Sandbrink, L. Kappos and C. Pohl, Vitamin D as an early predictor of multiple sclerosis activity and progression, JAMA Neurol., 2014, 71, 306–314 CrossRef PubMed.
- J. Staples, A.-L. Ponsonby and L. Lim, Low maternal exposure to ultraviolet radiation in pregnancy, month of birth, and risk of multiple sclerosis in offspring: longitudinal analysis, Br. Med. J., 2010, 340, c1640 Search PubMed.
- C. J. Willer, D. A. Dyment, A. D. Sadovnick, P. M. Rothwell, T. J. Murray and G. C. Ebers, Timing of birth and risk of multiple sclerosis: population based study, Br. Med. J., 2005, 330, 120 CrossRef PubMed.
- M. Z. Adzemovic, M. Zeitelhofer, S. Hochmeister, S. A. Gustafsson and M. Jagodic, Efficacy of vitamin D in treating multiple sclerosis-like neuroinflammation depends on developmental stage, Exp. Neurol., 2013, 249, 39–48 CrossRef CAS PubMed.
- P. Bhargava, K. C. Fitzgerald, P. A. Calabresi and E. M. Mowry, Metabolic alterations in multiple sclerosis and the impact of vitamin D supplementation, JCI Insight, 2017, 2, e95302 CrossRef PubMed.
- M. T. Kampman, L. H. Steffensen, S. I. Mellgren and L. Jørgensen, Effect of vitamin D 3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial, Mult. Scler., 2012, 18, 1144–1151 CrossRef PubMed.
- E. James, R. Dobson, J. Kuhle, D. Baker, G. Giovannoni and S. V. Ramagopalan, The effect of vitamin D-related interventions on multiple sclerosis relapses: a meta-analysis, Mult. Scler., 2013, 19, 1571–1579 CrossRef PubMed.
- R. Aparecida Nedel Pertile, D. Kiltschewskij, M. Geaghan, M. Barnett, X. Cui, M. J. Cairns and D. Eyles, Developmental vitamin D-deficiency increases the expression of microRNAs involved in dopamine neuron development, Brain Res., 2022, 1789, 147953 CrossRef CAS PubMed.
- Y. Liang, H. Yu, X. Ke, D. Eyles, R. Sun, Z. Wang, S. Huang, L. Lin, J. J. McGrath, J. Lu, X. Guo and P. Yao, Vitamin D deficiency worsens maternal diabetes induced neurodevelopmental disorder by potentiating hyperglycemia–mediated epigenetic changes, Ann. N. Y. Acad. Sci., 2021, 1491, 74–88 CrossRef CAS PubMed.
- N. C. Harvey, A. Sheppard, K. M. Godfrey, C. McLean, E. Garratt, G. Ntani, L. Davies, R. Murray, H. M. Inskip, P. D. Gluckman, M. A. Hanson, K. A. Lillycrop and C. Cooper, Childhood bone mineral content is associated with methylation status of the RXRA promoter at birth, J. Bone Miner. Res., 2014, 29, 600–607 CrossRef CAS PubMed.
- C. Palacios, L. K. Kostiuk and J. P. Peña-Rosas, Vitamin D supplementation for women during pregnancy, Cochrane Database Syst. Rev., 2019, 7(7), CD008873 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |