Open Access Article
Open Access ArticleTrait stacking simultaneously enhances provitamin A carotenoid and mineral bioaccessibility in biofortified Sorghum bicolor†
Michael P.
Dzakovich
 *ab,
Hawi
Debelo
*ab,
Hawi
Debelo
 a,
Marc C.
Albertsen
a,
Marc C.
Albertsen
 c,
Ping
Che
c,
Ping
Che
 c,
Todd J.
Jones
c,
Todd J.
Jones
 c,
Marissa K.
Simon
c,
Marissa K.
Simon
 c,
Zuo-Yu
Zhao
c,
Kimberly
Glassman
c and
Mario G.
Ferruzzi
c,
Zuo-Yu
Zhao
c,
Kimberly
Glassman
c and
Mario G.
Ferruzzi
 *ad
*ad
aPlants for Human Health Institute, North Carolina State University, 600 Laureate Way, Kannapolis, North Carolina 28081, USA
bUSDA-ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, 1100 Bates Ave., Houston, TX 77030, USA. E-mail: Michael.Dzakovich@usda.gov
cCorteva Agriscience, 8305 NW 62nd Ave., Johnston, IA 50131, USA
dArkansas Children's Nutrition Center, Section of Developmental Nutrition, University of Arkansas for Medical Sciences, 15 Children's Way, Little Rock, AR 72202, USA. E-mail: MFerruzzi@uams.edu
First published on 14th July 2023
Abstract
Vitamin A, iron, and zinc deficiencies are major nutritional inadequacies in sub-Saharan Africa and disproportionately affect women and children. Biotechnology strategies have been tested to individually improve provitamin A carotenoid or mineral content and/or bioaccessibility in staple crops including sorghum (Sorghum bicolor). However, concurrent carotenoid and mineral enhancement has not been thoroughly assessed and antagonism between these chemical classes has been reported. This work evaluated two genetically engineered constructs containing a suite of heterologous genes to increase carotenoid stability and pathway flux, as well as phytase to catabolize phytate and increase mineral bioaccessibility. Model porridges made from transgenic events were evaluated for carotenoid and mineral content as well as bioaccessibility. Transgenic events produced markedly higher amounts of carotenoids (26.4 μg g−1 DW) compared to null segregants (4.2 μg g−1 DW) and wild-type control (Tx430; 3.7 μg g−1 DW). Phytase activation by pre-steeping flour resulted in significant phytate reduction (9.4 to 4.2 mg g−1 DW), altered the profile of inositol phosphate catabolites, and reduced molar ratios of phytate to iron (16.0 to 4.1), and zinc (19.0 to 4.9) in engineered material, suggesting improved mineral bioaccessibility. Improved phytate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mineral ratios did not significantly affect micellarization and bioaccessible provitamin A carotenoids were over 23 times greater in transgenic events compared to corresponding null segregants and wild-type controls. A 200 g serving of porridge made with these transgenic events provide an estimated 53.7% of a 4–8-year-old child's vitamin A estimated average requirement. These data suggest that combinatorial approaches to enhance micronutrient content and bioaccessibility are feasible and warrant further assessment in human studies.
mineral ratios did not significantly affect micellarization and bioaccessible provitamin A carotenoids were over 23 times greater in transgenic events compared to corresponding null segregants and wild-type controls. A 200 g serving of porridge made with these transgenic events provide an estimated 53.7% of a 4–8-year-old child's vitamin A estimated average requirement. These data suggest that combinatorial approaches to enhance micronutrient content and bioaccessibility are feasible and warrant further assessment in human studies.
1. Introduction
Incidence of food insecurity is unusually high in sub-Saharan Africa where diets are dominated by carbohydrate-rich cereal grains.1,2 As a result of food scarcity and regional dietary patterns, vitamin A, iron, and zinc deficiencies remain prominent.3,4 Chronic vitamin A deficiency can result in xerophthalmia; the leading cause of preventable blindness in children.5,6 Likewise, iron and zinc are required for normal growth and development and their deficiencies are associated with an increased risk of death from other diseases such as diarrhea.7,8 Considering social, economic, and environmental factors, food choice and availability are unlikely to markedly change in sub-Saharan Africa in the near future. Improvement of staple foods already predominant in the broader diet of at-risk populations is a viable strategy to ameliorate malnutrition.Sorghum (Sorghum bicolor (L.) Moench) is a close relative of maize (Zea mays) and one of the most popular cereal grains consumed in sub-Saharan Africa.9 Sorghum is often processed into porridges (e.g. tô) by mixing flour with boiling water.10 However, sorghum and subsequent food products are generally low in micronutrients such as iron and zinc as well as provitamin A carotenoids.11,12 The nutritional impacts of low mineral contents are exacerbated by the presence of high levels of phytate (inositol hexakisphosphate), a major phosphorous storage molecule that strongly chelates divalent metals like iron and zinc, and substantially reduces their bioavailability in humans.13 Previous research using labeled mineral elements estimate that molar ratios of phytate to iron or zinc above 10–15 indicate poor bioavailability.14–17 Depending on sorghum genetic background and environmental conditions, reported molar mineral/phytate ratios are overwhelmingly above these thresholds and vary between 6–55 for phytate to iron and 30–40 for phytate to zinc.11,18,19 Given the low levels of carotenoids, high concentration of phytate, and dietary significance of the crop, sorghum is an ideal candidate for biofortification efforts.
Biofortification strategies seeking to simultaneously improve delivery of provitamin A and key shortfall minerals such as iron and zinc are rare. Improvements in carotenoid content, stability, and reducing phytate concentration have been tested individually by ectopic expression of non-codon optimized genes including homogentisate geranylgeranyl transferase (HGGT), phytoene synthase (PSY1), phytoene desaturase (CRTI), phytoene synthase (CRTB), deoxy-xylulose-phosphate synthase (DXS), and phytase (PhyA).20–24 These studies demonstrated that not only could carotenoids concentrations be significantly increased in sorghum grain (e.g. CRTB), but their stability could be substantially enhanced by increasing antioxidant tocopherol and tocotrienol species (e.g. HGGT). Through modern biotechnological approaches, it is theoretically possible to address both provitamin A carotenoid and mineral content as well as minimizing factors that negatively impact bioavailability. However, potential for antagonism between carotenoid bioavailability and divalent minerals has been reported in in vitro and in vivo studies.25–29 These reported effects are concentration dependent and their relationship in the context of sorghum biofortification remains unexplored, but critical to define.
To investigate potential antagonism between carotenoid bioavailability and divalent minerals released by enzymatic phytate degradation, codon optimized vectors (ABS4-1 and ABS4-2) were designed and used to develop multiple transgenic events (Table 1). These events were engineered to simultaneously improve provitamin A carotenoid biosynthetic capacity, stability, and increased ability to degrade phytate compared to previous efforts.21,22 Sorghum events were processed into model porridges and a three-stage in vitro digestion model was utilized to evaluate carotenoid and mineral bioaccessibility. Due to the intrinsically low mineral content of sorghum, we hypothesized that increased mineral release from phytate degradation would not significantly counteract the delivery of provitamin A carotenoids. Our results suggest that these transgenic sorghum events provide dramatically higher amounts of bioaccessible provitamin A carotenoids compared to previous efforts and exhibit altered phytate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) iron/zinc molar ratios suggestive of enhanced mineral bioaccessibility.
iron/zinc molar ratios suggestive of enhanced mineral bioaccessibility.
| Transgene combinationa | Event identifier | Genetic background |
|---|---|---|
| a Enzyme abbreviations are defined as follows: PSY1, phytoene synthase 1, CRTB, bacterial phytoene synthase, CRTI, bacterial phytoene desaturase, HGGT, homogentisate geranylgeranyl transferase, PhyA, phytase, and PSY1, phytoene synthase 1. | ||
| ABS4-1 = HGGT + PSY1 + CRTI + PhyA | ABS4-1A | Tx430 |
| ABS4-1B | ||
| ABS4-1C | ||
| ABS4-2 = HGGT + CRTB + CRTI + PhyA | ABS4-2A | Tx430 |
| ABS4-2B | ||
| ABS4-2C | ||
| ABS4-2D | ||
| NA | Control | Tx430 |
2. Materials and methods
2.1 Chemicals and reagents
Reagents sourced from Sigma Aldrich (Sigma Chemical Co., St Louis, MO, USA) included mucin (M2378), α-amylase (A3176 (lot: SLCF0615)), pepsin (P7125 (lot: SLBW6671)), lipase (L3126 (lot: SLBX2124)), pancreatin (P7545 (lot: SLBV6830)), bile (B8631 (lot: SLBX1760) and authentic standards of α-carotene, β-carotene, lutein, lycopene, trans-β-apo-8′-carotenal, retinyl palmitate, and zeaxanthin. Urea (U15-500) and uric acid (A13346-14) as well as components for the oral phase base solution (potassium chloride, sodium phosphate, sodium sulfate, sodium chloride, and sodium bicarbonate) were purchased from Fisher Scientific (Fisher Scientific, Waltham, MA, USA). Reagents used for the extraction and analysis of carotenoids and inositol phosphates included LC-MS grade water, methanol, and acetonitrile, HPLC grade ammonium acetate, dihexylammonium acetate, ethyl acetate, and glacial acetic acid, as well as ACS grade acetone, ethanol, hexanes, isopropanol, methyl tert-butyl ether, and petroleum ether purchased from Fisher Scientific.2.2 Sorghum transformation, plant material, and harvest conditions
Immature embryo explants isolated from greenhouse grown sorghum plants were transformed with Agrobacterium auxotrophic strain LBA4404 Thy-carrying a ternary vector transformation system to generate transgenic sorghum plants as previously described.30 Transgenic grains used in this study were generated from two transformation vectors: ABS4-1 and ABS4-2 (Table 1). ABS4-1 carried the maize codon-optimized phytoene desaturase CRTI22,31 gene from Pantoea ananatis to increase provitamin A biosynthesis, the maize codon-optimized phytoene synthase PSY122,32 gene from Zea mays L. to modulate flux through the carotenoid pathway, the homogentisate geranylgeranyl transferase HGGT22,33 gene from H. vulgare to increase vitamin E accumulation, the maize codon optimized phytase PhyA34 gene from Aspergillus niger used for phytate metabolism, and the phosphomannose isomerase PMI30,35 gene from Escherichia coli as selectable marker. ABS4-2 carried all the identical genes as described in ABS4-1 except PSY1 was replaced by maize codon-optimized CRTB,36 another phytoene synthase gene from Erwinia uredovora. In both constructs, CRTB, fused to a maize codon optimized delta-4-palmitoyl-ACP desaturase gene transit peptide (Cs-DPAD),37 and PSY1 were driven with the same sorghum α-kafirin promoter.38CRTI was fused to a maize codon optimized ribulose-1,5-bisphosphate carboxylase small subunit transit peptide (PS SSU TP)37,39 and was driven by the sorghum β-kafirin promoter.38PhyA was fused to a H. vulgare alpha amylase signal peptide (BAASS)40 and was driven by the endosperm-specific ZM-LEG1A promoter. HGGT was driven by the Zm-WS1 whole seed promoter (seed-specific KG86 promoter41), and PMI was driven with the Zm-UBI1 promoter.30,35Transgenic events derived from the same transformation vector represent independent transgenic events (Table 1). All transgenic events used in this study were homozygous with a single T-DNA insertion. Both null segregants isolated through segregation of corresponding transgenic events and wild-type Tx430 were used as controls. Panicles were collected from sorghum plants 45-day after pollination, air dried at 24 °C for 2 weeks before threshing, and then stored at −80 °C.
2.3 High-throughput sorghum flour and experimental porridge preparation
Whole sorghum kernels were added to fill approximately half of a 15 mL polycarbonate vial (SPEX Sample Prep, Metuchen, NJ, USA; UX-04545-71) containing two ¼′′ 440C stainless steel balls (Grainger, Minooka, IL, USA; 4RJJ1). Samples were milled into flour using a Geno/Grinder® 2010-115 (SPEX Sample Prep) operated at 1400 RPM for 2 cycles of 2.5 minutes with samples cooled between cycles. Sorghum flour was stored at −40 °C until analysis.Steeped samples underwent the following pre-treatment: sorghum flour (0.4 g) was suspended in 0.8 mL of doubly distilled water and incubated at 37 °C for 3 hours at 120 oscillations per minute (OPM). After incubation, an additional 0.8 mL of doubly distilled water was added to each tube and samples were agitated by hand. Samples were then cooked and processed as described below.
Porridges were formulated based on a traditional Burkina Faso style tô containing 20% sorghum flour and 80% water by weight10 and scaled to a 10 mL volume to align with the cooking method outlined by Lipkie and others.21 Briefly, 0.4 g of sorghum flour was weighed into 15 mL flip-cap tubes and 1.6 mL of doubly distilled water was added. Each tube was agitated by hand until flour was fully dispersed. Tubes were transferred with their lids open to metal racks and placed into an induction heated stock pot submerging only the portion of the tubes that contained suspended flour. The stockpot lid was replaced to maintain temperature at 100 °C and high humidity while the samples cooked for 10 minutes. After cooking, racks were removed and 100 mg (± 5 mg) of canola oil was added to each tube (5% lipid by porridge mass) to promote micellarization during the intestinal phase and incorporated by manual mixing. After the addition of canola oil (∼30 min), sample tubes were transferred to a −80 °C freezer and subjected to a simulated digestion within 12 hours. All sorghum samples were processed in triplicate to account for experimental variation.
2.4 High-throughput in vitro digestions of sorghum porridge
A previously described three-stage high-throughput in vitro digestion method that utilizes a Tecan Freedom EVO 150 liquid handling system (Tecan; Mannedorf, Switzerland) was adapted for sorghum porridge.21,42 Briefly, thawed porridge samples (2 g FW) were mixed with 1.2 mL of oral phase solution (containing 31.8 mg mL−1 of α-amylase; 380 units) and incubated at 37 °C for 10 minutes at 120 OPM. Samples were diluted with a 0.4 mL of 10 mg mL−1 pepsin solution (in 0.1 M HCl; 270 units) and 2.7 mL of saline (0.9% NaCl) solution and then adjusted to pH 2.5 by addition of 1.0 M HCl. Samples were diluted with additional saline to 8 mL, blanketed with nitrogen gas, and incubated at 37 °C for 1 hour at 120 OPM. Samples were then adjusted to pH 5.0 using 1.0 M NaHCO3. Then, 0.4 mL of pancreatin-lipase solution (20 mg mL−1 of each enzyme in 0.1 M NaHCO3; 3520 units lipase) and 0.6 mL of bile extract (30 mg mL−1) in 0.1 M NaHCO3 were added to each sample. Samples were adjusted to pH 7.0 using 1.0 M NaHCO3 and saline was added to dilute samples to a final volume of 10 mL. Nitrogen gas was added to each tube and samples were incubated at 37 °C for 2 hours at 120 OPM. After incubation, samples were hand-agitated and 4 mL of digesta was removed, aliquoted, and stored at −80 °C for future analysis. The remaining samples were blanketed with nitrogen gas and centrifuged at 3428 × g for 75 minutes in an Eppendorf 5920R centrifuge (Eppendorf, Hamburg, Germany) maintained at 4 °C. Following centrifugation, 4 mL aliquots of the aqueous fraction were filtered through 0.22 μm cellulose acetate filters. Samples were blanketed with nitrogen gas and stored at −80 °C until analysis.2.5 High-throughput extraction and analysis of carotenoids
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 acetone
2 acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate + 0.01% BHT (w/v). Tubes were shaken at 1400 RPM for 45 seconds in a Geno/Grinder® 2010-115. Samples were centrifuged at 20
ethyl acetate + 0.01% BHT (w/v). Tubes were shaken at 1400 RPM for 45 seconds in a Geno/Grinder® 2010-115. Samples were centrifuged at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 3 minutes and the supernatant was collected into borosilicate tubes. The remaining sample pellets were twice extracted as outlined above with methyl tert-butyl ether + 0.01% BHT (w/v) or until pellet was colorless. Combined supernatants were dried using a RapidVap (Labconco, Kansas City, MO, USA), blanketed with nitrogen, and stored at −80 °C. Samples were redissolved in 2 mL of 1
000 × g for 3 minutes and the supernatant was collected into borosilicate tubes. The remaining sample pellets were twice extracted as outlined above with methyl tert-butyl ether + 0.01% BHT (w/v) or until pellet was colorless. Combined supernatants were dried using a RapidVap (Labconco, Kansas City, MO, USA), blanketed with nitrogen, and stored at −80 °C. Samples were redissolved in 2 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate
1 ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol and filtered through 0.45 μm PTFE syringe filters prior to analysis. Extractions were conducted in triplicate and under low-light to minimize photoisomerization.
methanol and filtered through 0.45 μm PTFE syringe filters prior to analysis. Extractions were conducted in triplicate and under low-light to minimize photoisomerization.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 acetone
3 acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether + 0.01% BHT (w/v). Samples were vortexed for 1 minute, centrifuged for 2 minutes at 4000 RPM, and the supernatant was collected. Combined supernatants were dried in a RapidVap and stored at −80 °C until analysis (<24 hours later). Digesta and aqueous fraction samples were resolubilized in 200 or 100 μL of 1
petroleum ether + 0.01% BHT (w/v). Samples were vortexed for 1 minute, centrifuged for 2 minutes at 4000 RPM, and the supernatant was collected. Combined supernatants were dried in a RapidVap and stored at −80 °C until analysis (<24 hours later). Digesta and aqueous fraction samples were resolubilized in 200 or 100 μL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ethyl acetate
3 ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol + 0.01% BHT (w/v), respectively, and filtered with a 0.22 μm PTFE filter prior to analysis.
methanol + 0.01% BHT (w/v), respectively, and filtered with a 0.22 μm PTFE filter prior to analysis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 HPLC grade methanol: 1.0 M ammonium acetate adjusted to pH 4.6 (A) and ethyl acetate (B). Solvent flow rate was maintained at 0.45 mL per minute and carotenoids were separated on a 2.0 mm × 150 mm YMC C30 column with 3 μm particle size (YMC America, Devens, MA) using the following gradient: 100% A to 20% A over 4.93 minutes, 20% A to 0% A over 1.65 minutes, 0% A held for 1.46 minutes, a return to 100% A over 1.0 minute, and a hold on 100% A for 3.46 minutes to recondition the column. The column compartment and autosampler were maintained at 35 and 15 °C, respectively. Carotenoids were quantified at 450 nm using a Waters 2998 PDA detector based on response curves of authentic standards. For analytes quantified without authentic standards, an adjusted slope was calculated using a ratio of molar extinction coefficients with the most structurally related carotenoid available. The internal standard RP was quantified at 325 nm. Limits of detection determined by least squares regression for lutein, zeaxanthin, α-carotene, β-carotene, and lycopene were 0.28, 0.03, 0.11, 0.09, and 0.21 picomoles on column, respectively.
2 HPLC grade methanol: 1.0 M ammonium acetate adjusted to pH 4.6 (A) and ethyl acetate (B). Solvent flow rate was maintained at 0.45 mL per minute and carotenoids were separated on a 2.0 mm × 150 mm YMC C30 column with 3 μm particle size (YMC America, Devens, MA) using the following gradient: 100% A to 20% A over 4.93 minutes, 20% A to 0% A over 1.65 minutes, 0% A held for 1.46 minutes, a return to 100% A over 1.0 minute, and a hold on 100% A for 3.46 minutes to recondition the column. The column compartment and autosampler were maintained at 35 and 15 °C, respectively. Carotenoids were quantified at 450 nm using a Waters 2998 PDA detector based on response curves of authentic standards. For analytes quantified without authentic standards, an adjusted slope was calculated using a ratio of molar extinction coefficients with the most structurally related carotenoid available. The internal standard RP was quantified at 325 nm. Limits of detection determined by least squares regression for lutein, zeaxanthin, α-carotene, β-carotene, and lycopene were 0.28, 0.03, 0.11, 0.09, and 0.21 picomoles on column, respectively.
2.6 Extraction and analysis of minerals from sorghum flour
Minerals were hot acid extracted from 0.2 g of sorghum flour using an open-vessel microwave system, then diluted and analyzed using ICP-OES. All extractions and analyses were conducted by A&L Great Lakes Laboratories (Fort Wayne, IN, USA).2.7 Extraction and analysis of inositol phosphates
Extraction of inositol phosphates from raw and steeped sorghum samples was carried as described previously with minor modifications.43 Briefly, 50 mg of ground sorghum samples were defatted using 1.5 mL of hexane. The hexane layer was carefully removed after centrifuging (3600 rpm, 4 min) and the pellet was dried under nitrogen. The dried samples were then resuspended with 1 mL of HCl (0.4 M) and sonicated for 1 h at room temperature. Samples were centrifuged, supernatants were transferred into culture tubes and dried using RapidVap vacuum evaporator. Dried samples were then resolubilized with 500 μl of 5% acetonitrile, filtered using 0.45 μm PES syringe filter prior to LC-MS analysis.Inositol phosphate content was determined using a Waters UPLC Acquity I Class system coupled with a Xevo TQ-S triple quadrupole mass spectrometer. Separation was performed on a BEH C18 column (2.1 mm × 50 mm, 1.7 μm) at a flow rate of 0.5 mL min−1 using an adapted gradient as follows: 70% A to 62% A over 2.04 minutes, 62% A to 60% A over 0.03 minutes, 60% A to 44% A over 0.72 minutes, 44% A to 20% A over 0.61 minutes, 20% A to 70% A over 0.03 minutes, and a hold of 70% A for an additional 1.67 minutes.43 Mobile phases consisted of (A) 5% aqueous acetonitrile containing ion pairing agents dihexylammonium acetate (5 mM) and ammonium acetate (5 mM) and (B) 100% acetonitrile. Calibration curves of D-myo-inositol-1-monophosphate dipotassium salt (IP1), D-myo-inositol-1,4-diphosphate sodium salt (IP2), D-myo-inositol-1,4,5-triphosphate, sodium salt (IP3), D-myo-inositol-1,3,4,5-tetraphosphate, sodium salt (IP4), D-myo-inositol-1,3,4,5,6-pentaphosphate, sodium salt (IP5) and myo-inositol-hexakis (dihydrogen phosphate) (IP6) were used to quantify inositol phosphate species using optimized multiple reaction monitoring (MRM) experiments. Details regarding MRM experiments can be found in the ESI.† Analytical conditions were as follows: electrospray ionization in negative mode; capillary voltage: 3.0 kV; probe temp: 150 °C; source temp: 600 °C; desolvation gas flow: 1000 L h−1; cone gas flow: 5 L h−1.
2.8 Statistical analysis and data visualization
Statistical analyses and data visualization were conducted using R version 4.1.1.44 Box and whisker plots were generated using ggplot2 using the Wes Anderson color palette generator.45,46 Fixed-effect analysis of variance models were generated to determine if genetic background, transgene state of the transgenic construct, or phytase activation pre-treatment significantly affected outcomes measured in our experiments. For the analysis of raw material carotenoid, mineral, and phytate content, (excluding pre-treated phytase activated samples) the following model was used:| Yijk = μ + Ei + Tj + Rk(Ei) + εijk |
To isolate the effect of transgenic construct transgene state or phytase activation pre-treatment on relative and absolute bioaccessibility, the following simplified models were used:
| Yi = μ + Ti + εi |
| Yi = μ + Pi + εi |
To determine significance among the interactions between transgenic construct transgene state and phytase activation pre-treatment, the following model was used:
| Yij = μ + Ti + Pj + TiPj + εij |
3. Results
3.1 Carotenoid content increased in transgenic sorghum events
Sorghum flours were analyzed for several major carotenoids including those with provitamin A activity (Table 2). A comprehensive summary and statistical analysis of all carotenoids and their geometric isomers is detailed in Table S1.† Carotenoid values are presented on a “per serving” basis to reflect the amount present in a standard 200 g serving of porridge. Additionally, we report multiple aggregate values including xanthophylls (lutein, zeaxanthin, α-cryptoxanthin, and β-cryptoxanthin), carotenes (α-carotene, cis-β-carotene, β-carotene, lycopene, and any monitored cis isomers), and provitamin A content (1/2(α-cryptoxanthin + β-cryptoxanthin + α-carotene + any monitored cis isomers) + β-carotene). Overwhelmingly, transgenic material outperformed null segregants and the wild-type control in terms of carotenoid content. On average, transgenic events contained between 2.7 to 32 times more carotenoids than their non-transgenic counterparts and wild-type control, depending on the carotenoid. A 200 g porridge serving made with the null segregants or wild type control used in this study contained on average 0.15 mg of xanthophylls, 0.03 mg of carotenes, and 0.02 mg of provitamin A content while a serving of porridge made with transgenic events contained on average 0.35 mg of xanthophylls, 0.70 mg of carotenes, and 0.67 mg of provitamin A content. By design, transgenic sorghum events were particularly high in β-carotene with an average content of 0.63 mg per 200 g serving of porridge.| Event identifier | Transgene statea | Lutein | Zeaxanthin | α-Crypb | β-Cryp. | α-Carotene | β-Carotene | cis-β-Carotene | Lycopene | Xanthoc | Carotenesd | Provitamin Ae |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Transgene state indicates if a transgenic construct is present or absent (Null). b Sum of all-trans and cis isomers of α-cryptoxanthin. c Xantho represent the sum of xanthophyll carotenoids (lutein, zeaxanthin, and α and β-cryptoxanthin). d Carotenes represent the sum of α and β-carotene, cis-β-carotene isomers, and lycopene. e Provitamin A was calculated as the sum of β-carotene plus half of the sum of α and β-cryptoxanthin, α-carotene, 15-cis-β-carotene, 13-cis-β-carotene, and 9-cis-β-carotene. f Not detected. Values with unique letters are statistically significant as determined by a Tukey's HSD (α = 0.05) post hoc test. | ||||||||||||
| ABS4-1A | Present | 192.2 ± 3.6ab | 194.2 ± 2.7a | 18.1 ± 1.7abc | 9.9 ± 2.2a | 17.7 ± 2.0ab | 581.2 ± 6.1c | 36.70 ± 8.77a | 10.3 ± 1.1d | 414.4 ± 9.4a | 646.0 ± 6.8c | 622.4 ± 5.3b |
| ABS4-1B | Present | 201.3 ± 6.9a | 152.7 ± 3.3bc | 19.5 ± 1.6a | 11.5 ± 1.9a | 16.1 ± 0.5b | 614.7 ± 13.6b | 37.35 ± 3.01a | 7.6 ± 0.7e | 385.1 ± 6.7ab | 675.8 ± 17.0bc | 657.0 ± 13.8b |
| ABS4-1C | Present | 193.6 ± 4.9ab | 162.2 ± 7.0b | 10.6 ± 7.2abcde | 9.4 ± 0.7a | 15.5 ± 0.6b | 624.9 ± 22.6b | 37.23 ± 1.43a | 8.5 ± 1.1e | 375.8 ± 12.1b | 686.1 ± 22.7bc | 661.3 ± 22.4b |
| ABS4-2A | Present | 183.3 ± 8.6b | 126.3 ± 4.2d | 20.6 ± 1.0a | 8.4 ± 4.0a | 36.7 ± 1.4a | 606.4 ± 20.9bc | 26.88 ± 4.00ab | 24.7 ± 0.6b | 338.7 ± 15.5c | 694.7 ± 24.4b | 652.7 ± 24.0b |
| ABS4-2B | Present | 189.2 ± 6.6ab | 149.9 ± 0.4c | 18.4 ± 14.8ab | 10.1 ± 7.9a | 28.6 ± 24.9ab | 850.7 ± 13.4a | 31.94 ± 15.13ab | 28.6 ± 0.7a | 366.7 ± 30.5bc | 939.5 ± 42.1a | 894.6 ± 32.7a |
| ABS4-2C | Present | 144.0 ± 4.2c | 127.9 ± 5.7d | 15.9 ± 1.0abcd | 8.2 ± 0.5a | 22.3 ± 1.3ab | 505.5 ± 14.6d | 16.75 ± 1.01bcd | 22.1 ± 0.2c | 295.9 ± 10.0d | 566.7 ± 16.9d | 537.1 ± 15.9c |
| ABS4-2D | Present | 133.5 ± 8.8c | 134.2 ± 2.3d | 13.7 ± 0.9abcde | 10.2 ± 0.6a | 23.9 ± 0.3ab | 599.5 ± 12.3bc | 22.67 ± 4.42abc | 25.7 ± 1.0b | 291.7 ± 12.5d | 671.7 ± 7.7bc | 634.7 ± 10.6b |
| ABS4-1A | Null | 72.6 ± 2.8d | 93.2 ± 3.6e | 4.8 ± 3.0cde | ndf | nd | 20.8 ± 0.4e | 6.91 ± 0.88d | nd | 170.2 ± 8.3e | 28.1 ± 1.2e | 26.6 ± 1.1d |
| ABS4-1B | Null | 73.6 ± 2.2d | 75.9 ± 2.3f | 5.0 ± 0.3bcde | nd | nd | 21.3 ± 0.5e | 6.35 ± 0.52d | nd | 154.6 ± 3.8ef | 28.2 ± 0.7e | 27.2 ± 0.7d |
| ABS4-1C | Null | 59.9 ± 0.9d | 76.9 ± 2.4f | 3.7 ± 2.1de | nd | nd | 17.8 ± 2.4e | 7.33 ± 4.70cd | nd | 140.3 ± 5.0ef | 25.4 ± 3.2e | 23.3 ± 1.2d |
| ABS4-2A | Null | 61.6 ± 5.1d | 64.2 ± 5.2g | 3.9 ± 0.4de | nd | nd | 15.5 ± 1.4e | 5.43 ± 0.77d | nd | 129.8 ± 10.6f | 21.1 ± 0.8e | 20.3 ± 1.2d |
| ABS4-2B | Null | 65.2 ± 3.8d | 77.5 ± 3.4f | 4.7 ± 0.3cde | nd | nd | 19.3 ± 2.6e | 5.89 ± 1.16d | nd | 147.3 ± 6.3ef | 25.5 ± 4.1e | 24.8 ± 3.4d |
| ABS4-2C | Null | 73.7 ± 4.7d | 84.0 ± 1.4ef | 2.4 ± 1.8e | nd | nd | 23.7 ± 2.8e | 7.10 ± 4.77cd | nd | 159.2 ± 8.6ef | 31.2 ± 7.2e | 28.2 ± 4.3d |
| ABS4-2D | Null | 58.2 ± 1.2d | 80.5 ± 1.5f | 5.9 ± 0.5bcde | nd | nd | 17.9 ± 1.7e | 4.66 ± 1.48d | nd | 144.6 ± 3.0ef | 23.0 ± 2.9e | 23.4 ± 2.3d |
| Control | NA | 64.9 ± 5.6d | 60.9 ± 1.3g | 4.8 ± 0.5abc | nd | nd | 11.0 ± 0.9e | 6.30 ± 1.15d | nd | 130.6 ± 6.5f | 18.0 ± 1.6e | 16.9 ± 1.0d |
3.2 Relative and absolute bioaccessibility of carotenoids varied in transgenic and non-transgenic sorghum events
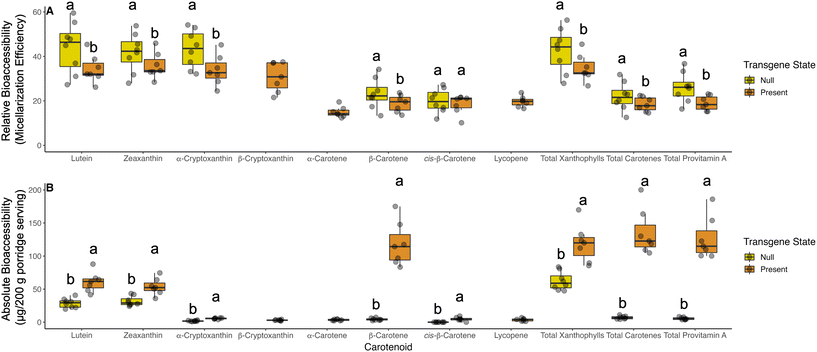
In our germplasm, relative bioaccessibility (micellarization efficiency) was higher for almost all carotenoids in the non-transgenic material (Fig. 1A). On average, 23.5% of β-carotene in null segregants and wild type control was bioaccessible compared to 18.8% in transgenic lines. A detailed breakdown of relative bioaccessibility values for all carotenoids and respective geometric isomers quantified are reported in Table S2.† β-Cryptoxanthin, α-carotene, and lycopene were low or not detectable in non-transgenic material (Fig. 1 and Table 2).For all carotenoids measured in our study, transgenic material had substantially higher absolute bioaccessibility (quantity of carotenoids available for absorption; Fig. 1B). On average, transgenic events released 0.118 mg of β-carotene while non-transgenic material released 4.57 μg per 200 g serving of porridge. This finding represents an approximate 26-fold increase in absorbable β-carotene from porridges made using transgenic sorghum events. Individual transgenic and null segregants exhibited differences in bioaccessibility within their respective groups. Notably, ABS4-2A released the most β-carotene, total xanthophylls, carotenes, and provitamin A carotenoids (Table S2†).
3.3 Mineral profiles were similar in all sorghum lines studied
Mineral elements common to cereal grains were analyzed in sorghum samples to better understand the potential interactions between their presence and carotenoid bioaccessibility and to determine if specific events caused alterations in mineral homeostasis (Table 3 and Table S3†). Mineral profiles of all sorghum lines did not significantly vary regardless of transgene state. It is important to emphasize that these constructs were not engineered to differentially accumulate minerals and all germplasm studied here were grown in a controlled environment.| Event identifier | Transgene statea | Zinc | Iron | Calcium | Magnesium |
|---|---|---|---|---|---|
| a Transgene state indicates if a transgenic construct is present or absent (null). b Values with unique letters are statistically significant as determined by a Tukey's HSD (α = 0.05) post hoc test. | |||||
| ABS4-1A | Present | 1.3 ± 0.0abcb | 1.4 ± 0.2b | 14.7 ± 2.3ab | 58.7 ± 6.1abc |
| ABS4-1B | Present | 1.6 ± 0.3abc | 1.5 ± 0.1b | 16.0 ± 0.0ab | 68.0 ± 13.9ab |
| ABS4-1C | Present | 1.5 ± 0.2abc | 1.8 ± 0.6ab | 17.3 ± 2.3ab | 65.3 ± 6.1ab |
| ABS4-2A | Present | 2.0 ± 0.2a | 2.1 ± 0.7ab | 17.3 ± 6.1ab | 73.3 ± 4.6a |
| ABS4-2B | Present | 1.6 ± 0.4ab | 1.3 ± 0.1b | 16.0 ± 0.0ab | 68.0 ± 0.0ab |
| ABS4-2C | Present | 1.6 ± 0.2abc | 1.7 ± 0.3ab | 16.0 ± 0.0ab | 66.7 ± 12.9ab |
| ABS4-2D | Present | 1.5 ± 0.1abc | 1.6 ± 0.2ab | 17.3 ± 2.3ab | 70.7 ± 4.6ab |
| ABS4-1A | Null | 1.4 ± 0.1abc | 1.3 ± 0.1b | 13.3 ± 2.3b | 61.3 ± 2.3abc |
| ABS4-1B | Null | 1.3 ± 0.2abc | 1.5 ± 0.2b | 21.3 ± 6.1ab | 62.7 ± 11.5ab |
| ABS4-1C | Null | 1.3 ± 0.2abc | 1.4 ± 0.5b | 13.3 ± 2.3b | 57.3 ± 6.1abc |
| ABS4-2A | Null | 1.5 ± 0.2abc | 2.5 ± 0.7a | 22.7 ± 2.3a | 68.0 ± 4.0ab |
| ABS4-2B | Null | 1.4 ± 0.1abc | 1.5 ± 0.1b | 16.0 ± 0.0ab | 65.3 ± 2.3ab |
| ABS4-2C | Null | 1.2 ± 0.1bc | 1.4 ± 0.1b | 13.3 ± 2.3b | 56.0 ± 8.0bc |
| ABS4-2D | Null | 1.8 ± 0.2ab | 2.0 ± 0.2ab | 17.3 ± 2.3ab | 64.0 ± 8.0ab |
| Control | NA | 1.0 ± 0.3c | 1.3 ± 0.2b | 13.3 ± 2.3b | 45.3 ± 2.3c |
3.4 Total inositol phosphate pools decreased as phytate (IP6) was metabolized into other inositol phosphate forms (IP1–IP5) in steeped transgenic sorghum events
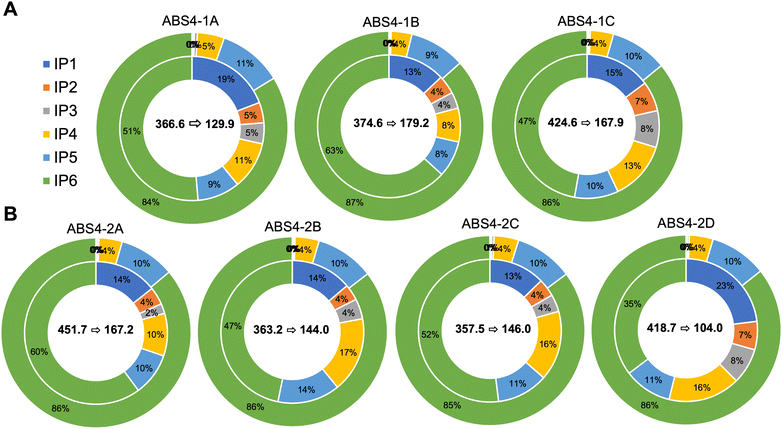
The effects of activating both native and heterologous phytase enzymes on inositol phosphate (IP1–IP6) profiles and carotenoid bioaccessibility were also examined in this study. Without steeping prior to porridge production and digestion, transgenic and non-transgenic events did not substantially differ from one another in terms of their phytate catabolite (IP1–IP5) profiles (Table S4†). However, large statistically significant differences were observed when flours were steeped prior to cooking (Fig. 2 and Table S4†). Phytate catabolites (IP1–IP5) were significantly higher in steeped transgenic material compared to their non-steeped counterparts as well as both steeped and non-steeped null segregants and non-transgenic controls (Table S4†). In non-steeped transgenic material, IP1–IP3 represented 0.6% of the total inositol phosphate pool on average whereas IP1–IP3 comprised 25.7% of the total inositol phosphate pool in steeped transgenic events. In non-transgenic material, changes in inositol phosphate profiles due to steeping were less pronounced, but statistical differences were observed for IP2–IP6 (Table S4†). Additionally, a significant reduction in total inositol phosphate species was observed in transgenic material after steeping compared to non-steeped counterparts as well as both steeped and non-steeped null segregants and wild-type control (Fig. 2 and Table S4†). More precisely, steeped transgenic material contained, on average, 370.8 mg of total inositol phosphates whereas non-steeped events contained 937.1 mg per 200 g of porridge.3.5 The molar ratios of phytate to iron and zinc were reduced in steeped transgenic sorghum events
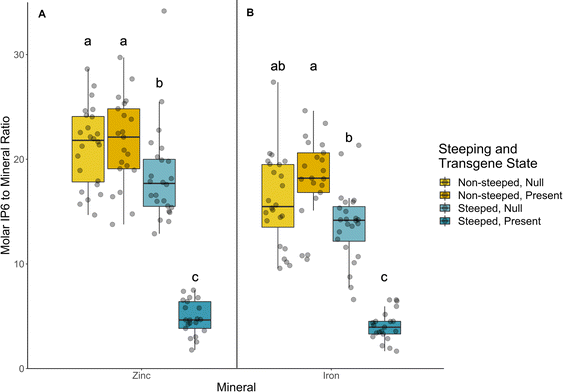
We calculated molar ratios of phytate (IP6) to zinc and iron as a proxy for mineral bioaccessibility. In this analysis, both steeped and non-steeped transgenic and non-transgenic material were compared to determine phytate degradation efficiency among wild-type, transgenic and corresponding nulls. As shown in Fig. 3 and Table S5,† no significant differences in the phytate to zinc (21.6 and 21.2; transgenic and non-transgenic) and phytate to iron (18.2 and 16.4; transgenic and non-transgenic) ratios were observed for all the materials tested without steeping. However, a significant reduction in phytate to zinc (4.9 and 17.6; transgenic and non-transgenic) and phytate to iron (4.1 and 13.6; transgenic and non-transgenic) ratios was observed for all the sorghum lines after steeping, indicating that the steeping treatment was able to activate not only the heterologous phytase, but also naturally occurring phytase enzymes. Although steeping reduced the phytate to iron and zinc ratios in both transgenic and non-transgenic material, transgenic material exhibited the most dramatic reduction falling below 5.0 for both minerals (Fig. 3 and Table S5†).3.6 Relative and absolute bioaccessibility of carotenoids were not affected by phytase activation in transgenic sorghum events
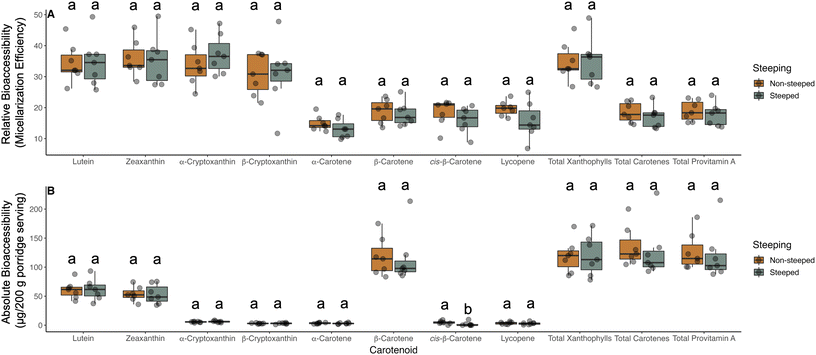
We focused our remaining analysis on the transgenic events given the notable differences in their total phytate, likely due to the heterologous phytase activated by steeping (Fig. 2 and Table S4†). Relative bioaccessibility was not significantly impacted for any of the carotenoids measured in this study by phytase activation (Fig. 4A). In terms of absolute bioaccessibility, cis-β-carotene isomers were statistically more bioaccessible from non-steeped wild-type and null controls, but no other individual or aggregate measurements of carotenoids were affected (Fig. 4B and Table S2†). A modest and consistent pattern can be seen in Fig. 4B and Table S2† suggesting a slight downward trend in deliverable carotenoids as a function of steeping and phytase activation, but these differences did not reach a level of statistical significance. Therefore, delivery of carotenoids and provitamin A carotenoids were found to be similar between steeped and non-steeped transgenic material and not significantly impacted by the presumed increase of divalent cations liberated through phytase activation.4. Discussion
Among the leading causes of death for children under 5 years of age in sub-Saharan Africa are vitamin A and mineral deficiencies.3 The sorghum germplasm used in this study were specifically engineered to address how modifying the carotenoid biosynthetic pathway in endosperm tissue impacts carotenoid profiles and bioaccessibility. Secondarily, the interactions among carotenoid bioaccessibility, phytase activation, and the presence of liberated divalent cations was also studied by including PhyA in these constructs. The two constructs used in this study differed only in that ABS4-1 contained PSY1 while ABS4-2 contained CRTB (Table 1). In both cases, carotenoid profiles in the germplasm represent improvements over previous iterations with prominent increases in β-carotene.21–23 However, concentrations of xanthophylls such as lutein and zeaxanthin were slightly lower in this germplasm likely due to increased pathway flux towards β-carotene. Given that PSY1 and CRTB both encode phytoene synthase and catalyze the same reaction through conserved prenyl transferase domains, changes in gene expression through codon optimization (codon optimized PSY1 was used in this study compared to previous iterations22) and/or difference in the enzyme catalytic activity differences may have contributed to variation in xanthophyll content as well as provitamin A carotenoids in different transgenic constructs.48 Regardless, xanthophyll concentrations in transgenic events were between ∼3–4× higher than our non-transgenic control (Tx430) and may provide greater long term benefits related to eye health (Table 2).49,50Depending on factors such as age and sex, the estimated average requirement (EAR) of retinol activity equivalents (RAE) for vitamin A can range from 275 μg up to 885 μg for children 4–8 and lactating mothers, respectively.51 The reported conversion of dietary β-carotene to retinol is 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7 However, biofortified cereals like maize and Golden rice have been shown to have more effective conversion rates (6
1.7 However, biofortified cereals like maize and Golden rice have been shown to have more effective conversion rates (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3.8
1 and 3.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively).52,53 Given the similarity of sorghum flour's composition to maize, retinol conversion rates may be closer to those reported for other cereals. A recent report using a Mongolian gerbil model suggests that the conversion efficiency of β-carotene to retinol from similar transgenic sorghum events is 4.5
1, respectively).52,53 Given the similarity of sorghum flour's composition to maize, retinol conversion rates may be closer to those reported for other cereals. A recent report using a Mongolian gerbil model suggests that the conversion efficiency of β-carotene to retinol from similar transgenic sorghum events is 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.54 Based on the 4.5
1.54 Based on the 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 conversion rate, a 200 g porridge serving made with the transgenic events used in this study contains on average 665.69 μg of β-carotene equivalents (147.93 RAE); fulfilling 53.7% of a 4–8-year-old child's vitamin A EAR (275 μg RAE). However, the efficiency of provitamin A carotenoid conversion to retinol will be affected by many other factors, such as porridge matrix, preparation method, carotenoid bioaccessibility, and interindividual differences in absorption and metabolism capabilities.55
1 conversion rate, a 200 g porridge serving made with the transgenic events used in this study contains on average 665.69 μg of β-carotene equivalents (147.93 RAE); fulfilling 53.7% of a 4–8-year-old child's vitamin A EAR (275 μg RAE). However, the efficiency of provitamin A carotenoid conversion to retinol will be affected by many other factors, such as porridge matrix, preparation method, carotenoid bioaccessibility, and interindividual differences in absorption and metabolism capabilities.55
Consistent with previous reports, relative bioaccessibility was lower in transgenic events with high concentrations of carotenoids (Fig. 1A).21,56,57 This trend could not be observed in β-cryptoxanthin, α-carotene, and lycopene as these carotenoids were low or not detectable in non-transgenic material. We hypothesize that due to the prominently higher carotenoid concentrations in transgenic events, and the process by which carotenoids partition into water-soluble micelles may have been approaching saturation. Regardless, the transgenic events used in this study have the potential to deliver significantly more total and provitamin A carotenoids than their non-transgenic counterparts (Fig. 1B), with a particular emphasis on β-carotene. Despite the modestly lower relative bioaccessibility in these transgenic sorghum events, higher absolute bioaccessibility indicates these lines would have potential for public health impact to consumers of traditional porridges.
It has been previously estimated that addressing zinc deficiencies could prevent 4.4% of childhood deaths.8 Biofortification strategies have sought to improve mineral content in crops through enhanced uptake and/or partitioning in edible tissues.20,24,58 However, mineral content is poorly associated with bioavailability.59 Mineral elements co-localize in sorghum tissues (e.g. pericarp) with phytate which reduces bioavailability due to its ability to chelate metal ions.60
We explored the effects of activating both naturally occurring and heterologous phytase enzymes on the profiles of inositol phosphates as well as carotenoid bioaccessibility. Phytase has long been known to catabolize phytate into various inositol phosphate products in legumes61 as well as cereals including sorghum, corn, rice, and wheat.62 Similar trends were observed in the current study as phytase activation shifted inositol phosphate profiles, normally dominated by phytate (IP6), to smaller catabolites (IP1–5; Fig. 2 and Table S4†). Notably, the heterologous PhyA in our transgenic events more effectively reduced total inositol phosphate pools in sorghum flours. This finding was evident in significant shifts in calculated molar ratios of phytate to zinc and iron (Fig. 3). Lower molar ratios of phytate to zinc and iron are associated with higher mineral bioaccessibility.16,63 While estimates vary, critical values for molar phytate to zinc or iron are generally believed to fall between 10–15.14–17 Above these thresholds, bioaccessibility is impaired due to the chelating activity of phytate. Our study demonstrated that phytase activated transgenic material exhibited the most drastic reduction of phytate to iron ratio from 16.0 to 4.1 and phytate to zinc ratio from 19.0 to 4.9 (Fig. 3), far below reported critical thresholds. However, a potential challenge emerges due to the known antagonistic relationship between carotenoid micellarization and minerals.
Previous studies have shown that divalent cations in concentrations above certain thresholds are able to precipitate bile salts64 as well as fatty acids.65 The resulting formation of insoluble soaps has been hypothesized to be a potential mechanism by which carotenoid bioaccessibility is negatively impacted by minerals.26,27,66,67 In this study, total phytate remaining in steeped, transgenic events was on average 22.9% of the original amount and additional minerals were ostensibly released through phytate degradation. However, no significant effect was observed on relative or absolute carotenoid bioaccessibility in our study as a function of pre-steeping and phytase activation. This outcome may be due to the inherently low concentrations of mineral elements in sorghum relative to the treatments tested in other bodies of work in which concentrations of minerals tended to fall within the mmol range.26,27,29,66,67 When prepared as a traditional porridge, an average 200 g serving of porridge (40 g of flour) from all germplasm studied in this work contains 1.62 and 1.47 mg (29.01 and 22.48 μmol L−1) of iron and zinc, respectively. Given the effects of divalent minerals on micellarization are concentration dependent,26,27,67 we hypothesize that the low mineral content of sorghum may obscured any significant effects of steeping, phytase activation, and subsequent liberation of minerals on micellarization efficiency. Critical values above which micellarization is negatively affected for divalent cations such as zinc, magnesium, and calcium have previously been reported at 100, 300, and 500 mg L−1, respectively.28 While iron and zinc both fell below 40 mg L−1 in the sorghum material studied here, magnesium was present at 1584.4 mg L−1 on average. The relatively higher concentrations of magnesium likely contributed to modest reductions observed. However, the modest decreases in micellarization efficiency observed in steeped transgenic sorghum porridges were not statistically significant. This finding is consistent with studies conducted by our group in other crops such as spinach.68 Regardless, the quantity of carotenoids available for absorption was not significantly affected by steeping and the subsequent release of divalent cations. Should future iterations of this material generate transgenic events capable of delivering larger quantities of minerals per serving, it would be prudent to ensure micellarization efficiency and deliverable carotenoids are not significantly impacted. Given the chemical aspects of the sorghum germplasm in this study, our findings indicate that the transgenic events reported here can effectively deliver both carotenoids and divalent minerals simultaneously and more efficiently than non-transgenic controls.
The present study sought to leverage biotechnology for the purpose of generating nutritionally enhanced cultivars of sorghum. We utilized these events to study interactions between divalent minerals and carotenoid bioaccessibility through the activation of native or heterologous phytase. Data generated in this study indicate that transgenic events reported here can simultaneously deliver significant quantities of provitamin A carotenoids, carotenoids associated with eye and brain development, and divalent minerals required for normal growth and development. Importantly, increasing the deliverability of mineral elements by enzymatically degrading phytate did not significantly compromise carotenoid bioavailability. While human trials are needed to determine the clinical efficacy of these sorghum events, our data suggest that simultaneously addressing multiple (and competing) nutrient deficiencies is feasible and warrants additional attention.
Disclaimer statement
The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. The USDA is an equal opportunity provider and employer.Data availability
Novel biological materials described in this publication may be available to the academic community and other not-for-profit institutions solely for non-commercial research purposes upon acceptance and signing of a material transfer agreement between the author's institution and the requestor. In some cases, such materials may contain genetic elements described in the manuscript that were obtained from a third party(s) (e.g., Zm-WS1 whole seed promoter41) and the authors may not be able to provide materials including third party genetic elements to the requestor because of certain third-party contractual restrictions placed on the author's institution. In such cases, the requester will be required to obtain such materials directly from the third party. The author's and authors’ institution do not make any express or implied permission(s) to the requester to make, use, sell, offer for sale, or import third party proprietary materials. Obtaining any such permission(s) will be the sole responsibility of the requestor. Corteva Agriscience™ proprietary plant germplasm and any transgenic material will not be made available except at the discretion of the owner and then only in accordance with all applicable governmental regulations.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We thank Sydney Corbin, Jacob Fitzgerald, Micaela Hayes, Zulfiqar Mohamedshah, and Candace Nunn for their assistance during in vitro digestion and carotenoid extraction experiments. This work was supported by a gift from the Pioneer Foundation (to MGF) and in part by the U.S. Department of Agriculture, Agricultural Research Service (USDA-ARS Project 6026-51000-012 and 3092-51000-061-000-D).References
- S. Fraval, J. Hammond, J. R. Bogard, M. Ng’endo, J. van Etten, M. Herrero, S. J. Oosting, I. J. M. de Boer, M. Lannerstad, N. Teufel, C. Lamanna, T. S. Rosenstock, T. Pagella, B. Vanlauwe, P. M. Dontsop-Nguezet, D. Baines, P. Carpena, P. Njingulula, C. Okafor, J. Wichern, A. Ayantunde, C. Bosire, S. Chesterman, E. Kihoro, E. J. O. Rao, T. Skirrow, J. Steinke, C. M. Stirling, V. Yameogo and M. T. van Wijk, Food Access Deficiencies in Sub-saharan Africa: Prevalence and Implications for Agricultural Interventions, Front. Sustain. Food Syst., 2019, 3, 104 CrossRef.
- M. Xu, Y. Luan, Z. Zhang and S. Jiang, Dietary pattern changes over Africa and its implication for land requirements for foodMitig Adapt Strateg Glob Change 2021, vol. 26, p. 13 Search PubMed.
- R. E. Black, L. H. Allen, Z. A. Bhutta, L. E. Caulfield, M. de Onis, M. Ezzati, C. Mathers and J. Rivera, Maternal and child undernutrition: global and regional exposures and health consequences, Lancet, 2008, 371, 243–260 CrossRef PubMed.
- R. Harika, M. Faber, F. Samuel, J. Kimiywe, A. Mulugeta and A. Eilander, Micronutrient Status and Dietary Intake of Iron, Vitamin A, Iodine, Folate and Zinc in Women of Reproductive Age and Pregnant Women in Ethiopia, Kenya, Nigeria and South Africa: A Systematic Review of Data from 2005 to 2015, Nutrients, 2017, 9, 1096 CrossRef PubMed.
- J. H. Humphrey, K. P. West and A. Sommer, Vitamin A deficiency and attributable mortality among under-5-year-olds., Bull. W. H. O., 1992, 70, 225–232 CAS.
- J. P. Whitcher, M. Srinivasan and M. P. Upadhyay, Corneal blindness: a global perspective, Bull. W. H. O., 2001, 79, 214–221 CAS.
- P. Trumbo, A. A. Yates, S. Schlicker and M. Poos, Dietary Reference Intakes: Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc, J. Am. Diet. Assoc., 2001, 101, 294–301 CrossRef CAS PubMed.
- C. L. Fischer Walker, M. Ezzati and R. E. Black, Global and regional child mortality and burden of disease attributable to zinc deficiency, Eur. J. Clin. Nutr., 2009, 63, 591–597 CrossRef CAS PubMed.
- Y. Peng, K. F. Schertz, S. Cartinhour and G. E. Hart, Comparative genome mapping of Sorghum bicolor (L.) Moench using an RFLP map constructed in a population of recombinant inbred lines, Plant Breed., 1999, 118, 225–235 CrossRef CAS.
- S. Da, J. O. Akingbala, L. Rooney, J. F. Scheuring and F. R. Miller, in Evaluation of Tô Quality in a Sorghum Breeding Program, ed. L. W. Rooney and D. S. Murty, International Crops Research Institute for the SemiArid Tropics, Patancheru, A.P., India, 1981, pp. 11–23 Search PubMed.
- A. P. P. Kayodé, A. R. Linnemann, J. D. Hounhouigan, M. J. R. Nout and M. A. J. S. van Boekel, Genetic and Environmental Impact on Iron, Zinc, and Phytate in Food Sorghum Grown in Benin, J. Agric. Food Chem., 2006, 54, 256–262 CrossRef PubMed.
- E. G. Kean, N. Bordenave, G. Ejeta, B. R. Hamaker and M. G. Ferruzzi, Carotenoid bioaccessibility from whole grain and decorticated yellow endosperm sorghum porridge, J. Cereal Sci., 2011, 54, 450–459 CrossRef CAS.
- Ael-M Afify, H. S. El-Beltagi, S. M. A. El-Salam and A. A. Omran, Bioavailability of Iron, Zinc, Phytate and Phytase Activity during Soaking and Germination of White Sorghum Varieties, PLoS One, 2011, 6, e25512 CrossRef CAS PubMed.
- D. Oberleas and B. E. Harland, Phytate content of foods: Effect on dietary zinc bioavailability, J. Am. Diet. Assoc., 1981, 79, 433–436 CrossRef CAS.
- J. R. Turnlund, J. C. King, W. R. Keyes, B. Gong and M. C. Michel, A stable isotope study of zinc absorption in young men: effects of phytate and a-cellulose, Am. J. Clin. Nutr., 1984, 40, 1071–1077 CrossRef CAS PubMed.
- E. R. Morris and R. Ellis, Usefulness of the dietary phytic acid/zinc molar ratio as an index of zinc bioavailability to rats and humans, Biol. Trace Elem. Res., 1989, 19, 107–117 CrossRef CAS PubMed.
- P. R. Saha, C. M. Weaver and A. C. Mason, Mineral Bioavailability in Rats from Intrinsically Labeled Whole Wheat Flour of Various Phytate Levels, J. Agric. Food Chem., 1994, 42, 2531–2535 CrossRef CAS.
- A. P. Kayodé, A. R. Linnemann, M. J. Nout and M. A. Van Boekel, Impact of sorghum processing on phytate, phenolic compounds and in vitro solubility of iron and zinc in thick porridges, J. Sci. Food Agric., 2007, 87, 832–838 CrossRef.
- J. Kruger, A. Oelofse and J. R. N. Taylor, Effects of aqueous soaking on the phytate and mineral contents and phytate:mineral ratios of wholegrain normal sorghum and maize and low phytate sorghum, Int. J. Food Sci. Nutr., 2014, 65, 539–546 CrossRef CAS PubMed.
- G. S. Khush, S. Lee, J.-I. Cho and J.-S. Jeon, Biofortification of crops for reducing malnutrition, Plant Biotechnol. Rep., 2012, 6, 195–202 CrossRef.
- T. E. Lipkie, F. F. De Moura, Z.-Y. Zhao, M. C. Albertsen, P. Che, K. Glassman and M. G. Ferruzzi, Bioaccessibility of Carotenoids from Transgenic Provitamin A Biofortified Sorghum, J. Agric. Food Chem., 2013, 61, 5764–5771 CrossRef CAS PubMed.
- P. Che, Z.-Y. Zhao, K. Glassman, D. Dolde, T. X. Hu, T. J. Jones, D. F. Gruis, S. Obukosia, F. Wambugu and M. C. Albertsen, Elevated vitamin E content improves all-trans β-carotene accumulation and stability in biofortified sorghum, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11040–11045 CrossRef CAS PubMed.
- P. Che, Z.-Y. Zhao, M. Hinds, K. Rinehart, K. Glassman and M. Albertsen, in Sorghum: Methods and Protocols, ed. Z.-Y. Zhao and J. Dahlberg, Springer, New York, NY, 2019, pp. 209–220 Search PubMed.
- J. Díaz-Gómez, R. M. Twyman, C. Zhu, G. Farré, J. C. Serrano, M. Portero-Otin, P. Muñoz, G. Sandmann, T. Capell and P. Christou, Biofortification of crops with nutrients: factors affecting utilization and storage, Curr. Opin. Biotechnol., 2017, 44, 115–123 CrossRef PubMed.
- E. Biehler, A. Kaulmann, L. Hoffmann, E. Krause and T. Bohn, Dietary and host-related factors influencing carotenoid bioaccessibility from spinach (Spinacia oleracea), Food Chem., 2011, 125, 1328–1334 CrossRef CAS.
- E. Biehler, L. Hoffmann, E. Krause and T. Bohn, Divalent Minerals Decrease Micellarization and Uptake of Carotenoids and Digestion Products into Caco-2 Cells, J. Nutr., 2011, 141, 1769–1776 CrossRef CAS PubMed.
- J. Corte-Real, M. Iddir, C. Soukoulis, E. Richling, L. Hoffmann and T. Bohn, Effect of divalent minerals on the bioaccessibility of pure carotenoids and on physical properties of gastro-intestinal fluids, Food Chem., 2016, 197, 546–553 CrossRef CAS PubMed.
- J. Corte-Real, M. Bertucci, C. Soukoulis, C. Desmarchelier, P. Borel, E. Richling, L. Hoffmann and T. Bohn, Negative effects of divalent mineral cations on the bioaccessibility of carotenoids from plant food matrices and related physical properties of gastro-intestinal fluids, Food Funct., 2017, 8, 1008–1019 RSC.
- R. E. Kopec, C. Caris-Veyrat, M. Nowicki, J.-P. Bernard, S. Morange, C. Chitchumroonchokchai, B. Gleize and P. Borel, The Effect of an Iron Supplement on Lycopene Metabolism and Absorption During Digestion in Healthy Humans, Mol. Nutr. Food Res., 2019, 63, 1900644 CrossRef CAS PubMed.
- P. Che, A. Anand, E. Wu, J. D. Sander, M. K. Simon, W. Zhu, A. L. Sigmund, G. Zastrow-Hayes, M. Miller, D. Liu, S. J. Lawit, Z.-Y. Zhao, M. C. Albertsen and T. J. Jones, Developing a flexible, high-efficiency Agrobacterium-mediated sorghum transformation system with broad application, Plant Biotechnol. J., 2018, 16, 1388–1395 CrossRef CAS PubMed.
- X. Ye, S. Al-Babili, A. Klöti, J. Zhang, P. Lucca, P. Beyer and I. Potrykus, Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm, Science, 2000, 287, 303–305 CrossRef CAS PubMed.
- J. A. Paine, C. A. Shipton, S. Chaggar, R. M. Howells, M. J. Kennedy, G. Vernon, S. Y. Wright, E. Hinchliffe, J. L. Adams, A. L. Silverstone and R. Drake, Improving the nutritional value of Golden Rice through increased pro-vitamin A content, Nat. Biotechnol., 2005, 23, 482–487 CrossRef CAS PubMed.
- E. B. Cahoon, S. E. Hall, K. G. Ripp, T. S. Ganzke, W. D. Hitz and S. J. Coughlan, Metabolic redesign of vitamin E biosynthesis in plants for tocotrienol production and increased antioxidant content, Nat. Biotechnol., 2003, 21, 1082–1087 CrossRef CAS PubMed.
- Y. Han, D. B. Wilson and X. G. Lei, Expression of an Aspergillus niger phytase gene (phyA) in Saccharomyces cerevisiae, Appl. Environ. Microbiol., 1999, 65, 1915–1918 CrossRef CAS PubMed.
- D. Negrotto, M. Jolley, S. Beer, A. R. Wenck and G. Hansen, The use of phosphomannose-isomerase as a selectable marker to recover transgenic maize plants (Zea mays L.) via Agrobacterium transformation, Plant Cell Rep., 2000, 19, 798–803 CrossRef CAS PubMed.
- U. Neudert, I. M. Martínez-Férez, P. D. Fraser and G. Sandmann, Expression of an active phytoene synthase from Erwinia uredovora and biochemical properties of the enzyme, Biochim. Biophys. Acta, 1998, 1392, 51–58 CrossRef CAS PubMed.
- World Intellectual Property Organization, WO2014070646A1, 2014.
- United States, US20090049571A1, 2009.
- G. Coruzzi, R. Broglie, A. Cashmore and N. H. Chua, Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide., J. Biol. Chem., 1983, 258, 1399–1402 CrossRef CAS PubMed.
- S. Park, R. G. Ong and M. Sticklen, Strategies for the production of cell wall–deconstructing enzymes in lignocellulosic biomass and their utilization for biofuel production, Plant Biotechnol. J., 2016, 14, 1329–1344 CrossRef CAS PubMed.
- World Intellectual Property Organization, WO2010122110A1, 2010.
- M. Hayes, M. Pottorff, C. Kay, A. Van Deynze, J. Osorio-Marin, M. A. Lila, M. Iorrizo and M. G. Ferruzzi, In Vitro Bioaccessibility of Carotenoids and Chlorophylls in a Diverse Collection of Spinach Accessions and Commercial Cultivars, J. Agric. Food Chem., 2020, 68, 3495–3505 CrossRef CAS PubMed.
- S. Zhang, W. Yang, Q. Zhao, X. Zhou, Y. Fan and R. Chen, Rapid Method for Simultaneous Determination of Inositol Phosphates by IPC-ESI–MS/MS and Its Application in Nutrition and Genetic Research, Chromatographia, 2017, 2, 275–286 CrossRef.
- R Development Core Team, R: A language and environment for statistical computing, 2018 Search PubMed.
- K. Ram, H. Wickham, C. Richards and A. Baggett, wesanderson: A Wes Anderson Palette Generator, 2018 Search PubMed.
- H. Wickham, ggplot2 Elegant Graphics for Data Analysis, Springer-Verlag, New York, NY, 2016 Search PubMed.
- F. de Mendiburu and M. Yanseen, Agricolae: Statistical Procedures for Agricultural Research, 2021 Search PubMed.
- F. X. Cunningham and E. Gantt, Genes and Enzymes of Carotenoid Biosynthesis in Plants, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1998, 49, 557–583 CrossRef CAS PubMed.
- J. Mares, Lutein and Zeaxanthin Isomers in Eye Health and Disease, Annu. Rev. Nutr., 2016, 36, 571–602 CrossRef CAS PubMed.
- S. E. Thomas and E. J. Johnson, Xanthophylls, Adv. Nutr., 2018, 9, 160–162 CrossRef PubMed.
- Institute of Medicine (US) Committee on Nutrition Standards for National School Lunch and Breakfast Programs, Nutrition Standards and Meal Requirements for National School Lunch and Breakfast Programs: Phase I. Proposed Approach for Recommending Revisions, National Academies Press (US), Washington (DC), 2008 Search PubMed.
- G. Tang, J. Qin, G. G. Dolnikowski, R. M. Russell and M. A. Grusak, Golden Rice is an effective source of vitamin A, Am. J. Clin. Nutr., 2009, 89, 1776–1783 CrossRef CAS PubMed.
- S. Li, A. Nugroho, T. Rocheford and W. S. White, Vitamin A equivalence of the β-carotene in β-carotene–biofortified maize porridge consumed by women, Am. J. Clin. Nutr., 2010, 92, 1105–1112 CrossRef CAS PubMed.
- H. You, Doctor of Philosophy, Iowa State University, Digital Repository, 2016 Search PubMed.
- D. Weber and T. Grune, The contribution of β-carotene to vitamin A supply of humans, Mol. Nutr. Food Res., 2012, 56, 251–258 CrossRef CAS PubMed.
- M. L. Failla, C. Chitchumroonchokchai, D. Siritunga, F. F. De Moura, M. Fregene, M. J. Manary and R. T. Sayre, Retention during processing and bioaccessibility of β-carotene in high β-carotene transgenic cassava root, J. Agric. Food Chem., 2012, 60, 3861–3866 CrossRef CAS PubMed.
- I. J. Aragón, H. Ceballos, D. Dufour and M. G. Ferruzzi, Pro-vitamin A carotenoids stability and bioaccessibility from elite selection of biofortified cassava roots (Manihot esculenta, Crantz) processed to traditional flours and porridges, Food Funct., 2018, 9, 4822–4835 RSC.
- H. Debelo, M. Albertsen, M. Simon, P. Che and M. Ferruzzi, Identification and Characterization of Carotenoids, Vitamin E and Minerals of Biofortified Sorghum, Curr. Dev. Nutr., 2020, 4, 1792 Search PubMed.
- R. P. Glahn and H. Noh, Redefining Bean Iron Biofortification: A Review of the Evidence for Moving to a High Fe Bioavailability Approach, Front. Sustain. Food Syst., 2021, 5, 682130 CrossRef.
- M. G. Ferruzzi, J. Kruger, Z. Mohamedshah, H. Debelo and J. R. N. Taylor, Insights from in vitro exploration of factors influencing iron, zinc and provitamin A carotenoid bioaccessibility and intestinal absorption from cereals, J. Cereal Sci., 2020, 96, 103126 CrossRef CAS.
- J. Frias, R. Doblado, J. R. Antezana and C. Vidal-Valverde, Inositol phosphate degradation by the action of phytase enzyme in legume seeds, Food Chem., 2003, 81, 233–239 CrossRef CAS.
- M. A. Azeke, S. J. Egielewa, M. U. Eigbogbo and I. G. Ihimire, Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum), J. Food Sci. Technol., 2011, 48, 724–729 CrossRef CAS PubMed.
- G. Ma, Y. Li, Y. Jin, F. Zhai, F. J. Kok and X. Yang, Phytate intake and molar ratios of phytate to zinc, iron and calcium in the diets of people in China, Eur. J. Clin. Nutr., 2007, 61, 368–374 CrossRef CAS PubMed.
- J. Gu, A. Hofmann, H. Ton-Nu, C. Schteingart and K. Mysels, Solubility of calcium salts of unconjugated and conjugated natural bile acids., J. Lipid Res., 1992, 33, 635–646 CrossRef CAS.
- J. O. Atteh and S. Leeson, Influence of Age, Dietary Cholic Acid, and Calcium Levels on Performance, Utilization of Free Fatty Acids, and Bone Mineralization in Broilers, Poult. Sci., 1985, 64, 1959–1971 CrossRef CAS PubMed.
- D. Y. Graham and J. W. Sackman, Solubility of calcium soaps of long-chain fatty acids in simulated intestinal environment, Dig. Dis. Sci., 1983, 28, 733–736 CrossRef CAS PubMed.
- J. Corte-Real, C. Desmarchelier, P. Borel, E. Richling, L. Hoffmann and T. Bohn, Magnesium affects spinach carotenoid bioaccessibility in vitro depending on intestinal bile and pancreatic enzyme concentrations, Food Chem., 2018, 239, 751–759 CrossRef CAS PubMed.
- M. Hayes, S. Corbin, C. Nunn, M. Pottorff, C. D. Kay, M. A. Lila, M. Iorrizo and M. G. Ferruzzi, Influence of simulated food and oral processing on carotenoid and chlorophyll in vitro bioaccessibility among six spinach genotypes, Food Funct., 2021, 12, 7001–7016 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo03606a |
| This journal is © The Royal Society of Chemistry 2023 |