Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The Maillard reaction end product Nε-carboxymethyllysine is metabolized in humans and the urinary levels of the microbial metabolites are associated with individual diet†
Silvia
Tagliamonte
,
Antonio Dario
Troise‡
,
Rosalia
Ferracane
and
Paola
Vitaglione
 *
*
Department of Agricultural Sciences, University of Naples Federico II, 80055 Portici, Italy. E-mail: paola.vitaglione@unina.it
First published on 27th January 2023
Abstract
During food processing most of the thermally-driven chemical reactions start off on the side chain amino group of lysine generating structurally modified compounds with specific metabolic routes. Upon human digestion, dietary Nε-carboxymethyllysine (CML) may enter the colon and undergo gut microbial metabolism. However, little is known about the in vivo metabolic fate of dietary CML and its relationship with the habitual diet. We explored by hydrophilic interaction liquid chromatography tandem mass spectrometry the metabolites of CML in urine samples collected from 46 healthy subjects and studied the associations with diet. Mean concentration of N-carboxymethylcadaverine (CM-CAD), N-carboxymethylaminopentanoic acid (CM-APA), N-carboxymethylaminopentanol (CM-APO), and the N-carboxymethyl-Δ1-piperideinium ion were 0.49 nmol mg−1 creatinine, 1.45 nmol mg−1 creatinine, 4.43 nmol mg−1 creatinine and 4.79 nmol mg−1 creatinine, respectively. The urinary concentration of CML, its metabolites and lysine were positively correlated. Dietary intake of meat products negatively correlated with urinary excretion of CML and CM-APA; conversely dietary plant-to-animal proteins ratio positively correlated with urinary CML and its metabolites. The identification and quantification of CML metabolites in urine and the associations with diet corroborate the hypothesis that CML, an advanced glycation end-product, can undergo further biochemical transformations in vivo. The gut microbiome may have a major role in human metabolism of dietary CML.
1. Introduction
Several observational studies suggest a positive association between consumption of some processed foods rich in sugars, fats and salts, and the prevalence of obesity and related chronic diseases.1,2 Chronic consumption of high energy dense processed foods impairs intestinal barrier permeability and complement pathway activation increasing the risk of microvascular disease.3 This evidence pinpointed a key role of food processing in the occurrence of metabolic diseases, even if a causative relationship between processed foods and pathophysiological outcomes has not yet been demonstrated at molecular level.4 On the contrary, food processing is fundamental to guarantee safety, prolong preservation and improve nutritional aspects of foods by facilitating nutrient digestion and bioaccessibility.5 Recent trends in structural design of foods use processing and physiochemical modifications of food components to develop a new generation of desirable, tasty, convenient, healthy, bio-functional and sustainable products.6Within the debate on the beneficial and adverse effects of food processing, the Maillard reaction plays a decisive role. The reaction between reducing sugars and amino groups generates post-translational protein modifications that oversee the formation of dietary advanced glycation end-products (d-AGEs).7 Glycated proteins impair protein digestibility, decreasing the release of free amino acids and small peptides, thus potentially activating metabolic routes leading to d-AGEs accumulation in target organs.8
Glycated amino acid such as Amadori and Heyns compounds and their Maillard reaction late stage products as pyrraline, pentosidine, Nε-carboxymethyllysine (CML), Nε-carboxyethyllysine (CEL), methylglyoxal-hydroimidazolone (MG-H1, an end-product of arginine modifications) are excreted in urine.9 However, most of d-AGEs are poorly absorbed and enter the colon where they interact with the microbiota, undergo specific metabolic processes10,11 and generate metabolites that could affect human health.12
Whether the gut microbiota adapts to utilize the d-AGEs, the underneath chemical nature of metabolites formed and their absorption into the systemic circulation remain unresolved questions in Maillard biochemistry and in human physiology.13
The amino side chain of lysine represents one of the most prominent targets for chemical and enzymatic modifications rendering modified lysine an invaluable source to decipher how metabolic pathways change when exposed to non-canonical and modified amino acids.14 Indeed, focusing on glycation reactions, the degradation of Nε-fructosyllysine by Intestinimonas AF211 to form butyrate has been demonstrated15 and fecal suspensions obtained from human volunteers also degraded at different extent CML and pyrraline in vitro.16 Moving to the Maillard late stage compounds, probiotic strains of Escherichia coli metabolized CML in aerobic conditions producing N-carboxymethylcadaverine (CM-CAD), N-carboxymethylaminopentanoic acid (CM-APA), and the N-carboxymethyl-Δ1-piperideinium ion.17 The formation of these metabolites has been confirmed in anaerobic conditions using human feces and Oscillibacter and Cloacibacillus evryensis have been suggested to greatly contribute to such transformation.18
However, the fate of CML in humans in relationship with its metabolites is a challenging topic that can potentially implement metabolization pathways of modified lysine. In this study we hypothesized that CML, CM-CAD, CM-APA, N-carboxymethylaminopentanol (CM-APO), and the N-carboxymethyl-Δ1-piperideinium ion are absorbed and excreted in the urines and we deepened the correlation between urinary excretion of CML, its microbial metabolites, CML intake and some aspects of the diet in a cohort of healthy subjects.
2. Materials and methods
2.1 Chemicals
Acetonitrile and water of mass spectrometry grade were obtained from Merck (Darmstadt, Germany). Formic acid, and the analytical standard lysine hydrochloride were purchased from Sigma-Aldrich (Merck, St Louis, MO). Pyrraline, CML and its deuterated standard Nε-carboxy[2H4]methyllysine (d4-CML) were obtained from Toronto Research Chemicals (North York, Canada).2.2 Subjects and study design
Subjects were recruited based on a medical and nutritional anamnesis performed by a physician and a nutritionist, respectively. Subjects suffering with gastrointestinal disorder of any kind, relevant organic, systemic or metabolic diseases, food intolerance or alcohol abuse were excluded. Eligible subjects were healthy men or non-pregnant/non-lactating women aged between 19 ≤ age 65 ≤ years, with BMI ≤29.9 kg m−2, not taking medications, no consumption of probiotics, laxatives and/or antibiotics. They participated into the study after signing a written informed consent.The study was conducted according to the Declaration of Helsinki and approved by the Ethics Committee of the University of Naples Federico II (protocol number 419/20).
Participants self-recorded their diet by a 7-day weighed diary and data were extracted as previously reported.19 The day after the diary completion, participants fasting from 10 h reached the Food and Nutrition Laboratory of the Department of Agricultural sciences at University of Naples, collected a urine sample and provided the diary. Urine samples were rapidly frozen and stored at −80 °C until analysis.
2.3 Liquid chromatography tandem mass spectrometry (LC-MS/MS)
Free lysine, pyrraline, CML and its metabolites CM-CAD, CM-APA, CM-APO, and the N-carboxymethyl-Δ1-piperideinium ion were simultaneously analyzed according to Bui et al.18 and Hellwig et al.17 adapting analytical protocols to urine samples. Aliquots of urine (200 μL) were gently mixed with a mixture (1790 μL) of acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (50/50, v/v) with 0.1% formic acid, spiked with 10 μL of d4-CML internal standards (final concentration 200 ng mL−1) and vortexed at 500 rpm, 5 min. The mixture was centrifuged (4 °C, 10 min, 2500g), then filtered by hydrophilic regenerated cellulose syringe filters (RC, 0.22 μm Phenomenex, Torrance, CA). Without any further dilution, 5 μL of each sample were injected by using an autosampler (Series 200, PerkinElmer, Carlsbad, CA) and two micro-pumps (Series 200, PerkinElmer). Analyte separation was achieved on a silica sulfobetaine zwitterionic modified HILIC column (50 × 2.1 mm, 3.0 μm, Thermo Fisher, Bremen, Germany) at 35 °C, equipped with a security guard cartridge packed with the same stationary phase. Mobile phases for zwitterionic column were 0.1% formic acid in acetonitrile (solvent A) and 0.1% formic acid in water (solvent B). The flow rate was set at 200 μL min−1 with the following linear gradient of solvent B (minutes/%B): (0/10), (0.80/10), (3.5/75), (5.5/75); the system was equilibrated for 5 min before each injection. The chromatographic profile was recorded in multiple reaction monitoring mode (MRM) by using an API 3000 triple quadrupole (ABSciex, Carlsbad, CA). Positive electrospray ionization was used for detection and the source parameters were selected as follows: spray voltage 5.0 kV, capillary temperature 350 °C, dwell time 100 ms, cad gas and curtain gas were set to 45 and 5 (arbitrary units). The target analytes and their labeled internal standards were analyzed using the mass transitions given in parentheses along with collision energy (CE) and in bold the transition used for the quantitation: lysine (m/z 147 → 84, 130, CE: 25, 18), pyrraline (m/z 255 → 84, 148, 175 CE: 40, 25, 18), CML (m/z 205 → 84, 130, CE: 29, 17), d4-CML (m/z 209 → 88, 134, CE: 29, 17), CM-CAD (m/z 161 → 86, 69, 98 CE: 10, 20, 10), CM-APA (m/z 176 → 84, 101, 112 CE: 20, 10, 10) CM-APO (m/z 162 → 116, 98, 69, CE: 10, 10, 10), and the N-carboxymethyl-Δ1-piperideinium ion (m/z 142 → 114, 96 CE: 14, 18). Analytical performance robustness, sensitivity, reproducibility, repeatability, linearity, accuracy, carry over and matrix effects were evaluated by following the procedures previously reported focusing on CML, d4-CML and lysine.20
water (50/50, v/v) with 0.1% formic acid, spiked with 10 μL of d4-CML internal standards (final concentration 200 ng mL−1) and vortexed at 500 rpm, 5 min. The mixture was centrifuged (4 °C, 10 min, 2500g), then filtered by hydrophilic regenerated cellulose syringe filters (RC, 0.22 μm Phenomenex, Torrance, CA). Without any further dilution, 5 μL of each sample were injected by using an autosampler (Series 200, PerkinElmer, Carlsbad, CA) and two micro-pumps (Series 200, PerkinElmer). Analyte separation was achieved on a silica sulfobetaine zwitterionic modified HILIC column (50 × 2.1 mm, 3.0 μm, Thermo Fisher, Bremen, Germany) at 35 °C, equipped with a security guard cartridge packed with the same stationary phase. Mobile phases for zwitterionic column were 0.1% formic acid in acetonitrile (solvent A) and 0.1% formic acid in water (solvent B). The flow rate was set at 200 μL min−1 with the following linear gradient of solvent B (minutes/%B): (0/10), (0.80/10), (3.5/75), (5.5/75); the system was equilibrated for 5 min before each injection. The chromatographic profile was recorded in multiple reaction monitoring mode (MRM) by using an API 3000 triple quadrupole (ABSciex, Carlsbad, CA). Positive electrospray ionization was used for detection and the source parameters were selected as follows: spray voltage 5.0 kV, capillary temperature 350 °C, dwell time 100 ms, cad gas and curtain gas were set to 45 and 5 (arbitrary units). The target analytes and their labeled internal standards were analyzed using the mass transitions given in parentheses along with collision energy (CE) and in bold the transition used for the quantitation: lysine (m/z 147 → 84, 130, CE: 25, 18), pyrraline (m/z 255 → 84, 148, 175 CE: 40, 25, 18), CML (m/z 205 → 84, 130, CE: 29, 17), d4-CML (m/z 209 → 88, 134, CE: 29, 17), CM-CAD (m/z 161 → 86, 69, 98 CE: 10, 20, 10), CM-APA (m/z 176 → 84, 101, 112 CE: 20, 10, 10) CM-APO (m/z 162 → 116, 98, 69, CE: 10, 10, 10), and the N-carboxymethyl-Δ1-piperideinium ion (m/z 142 → 114, 96 CE: 14, 18). Analytical performance robustness, sensitivity, reproducibility, repeatability, linearity, accuracy, carry over and matrix effects were evaluated by following the procedures previously reported focusing on CML, d4-CML and lysine.20
All the acquisition parameters are summarised in Table S1.† CML was measured by using internal standard technique. Because of the absence of analytical standards, CM-APA, CM-APO, CM-CAD and piperideinium ion calibration curves were performed by using CML/d4-CML ratio as reference, while for free pyrraline and free lysine, external calibration curve with reference standards was used. The monitored compounds were adjusted for urinary creatinine concentrations. Creatinine in urine samples was measured according to Larsen.21
Concentration of analytes was reported as nmol mg−1 creatinine, samples were analyzed from two independent replicates.
2.4 Statistical analysis
After being checked for normality with Shapiro–Wilk test, variables showing a significant positive skewness, were transformed in ln (x). On the normally-distributed data, One-way ANOVA with post hoc Tukey was performed to check differences among monitored compounds. Pearson/Spearman correlation and Tukey test were performed in GraphPad Prism (v. 6.01, GraphPad software, San Diego, CA).The heatmap correlation of log transformed variables was visualized in R (version 4.0.3) using the Hmisc package and the function heatmap.2. Data are expressed as means ± SEM.
3. Results and discussion
3.1 Subjects and diets
Forty-six healthy subjects, 21 males and 25 females, mean age 29.6 ± 9.9 years (range:19–61 years), mean BMI 23.0 ± 2.8 kg m−2 participated in the study. Table 1 reports the nutritional composition of the diet, the contribution from each food category and other aspects including the average CML concentration, the intake of plant proteins, animal proteins and their ratio and the study participants’ adherence to the Mediterranean diet (assessed by Italian Mediterranean Index22) as an index of healthy diet. The Italian Mediterranean Index scores range from 0 to 11: scores in the range 0–3 indicates a low, in 4–5 a medium, and in 6–11 a high adherence to the Mediterranean diet.22 The cohort of participants analyzed in this study presented an average score corresponding to a medium adherence to the Mediterranean diet. CML concentration in the diet was estimated by using publicly available databases as Dresden AGE database and the values reported in the paper by Scheijen and coworkers that monitored CML, Nε-carboxyethyllysine (CEL), methylglyoxal-hydroimidazolone (MG-H1) in several food items by stable isotope dilution, liquid chromatography, derivatization, and tandem mass spectrometry.23 The average intake of CML was 3.28 ± 0.21 mg per day.| Nutritional composition | |
|---|---|
| Data are expressed as mean ± SEM. PP: plant proteins; AP: animal proteins; PP/AP, plant proteins/animal proteins; CML, carboxymethyllysine; IMI: italian mediterranean index. | |
| Energy, kcal | 1628.15 ± 73.60 (748.7–3010.2) |
| Carbohydrates, g | 175.89 ± 8.36 (73.5–310.7) |
| Dietary fiber, g | 13.30 ± 0.94 (4.4–33.8) |
| Proteins, g | 62.32 ± 3.78 (30.4–155.8) |
| Lipids, g | 66.74 ± 3.77 (30.2–150.9) |
| Food categories | |
| Fruit, g | 166.74 ± 18.60 (0.0–573.4) |
| Legumes, g | 23.43 ± 4.57 (0.0–130.0) |
| Vegetables, g | 148.72 ± 11.25 (37.1–340.9) |
| Wholegrain products, g | 23.28 ± 3.38 (0.0–82.9) |
| Refined grain products, g | 141.76 ± 9.38 (17.1–287.0) |
| Eggs, g | 20.32 ± 2.76 (0.0–85.7) |
| Meat products, g | 85.65 ± 8.86 (0.0–307.1) |
| Fish, g | 49.70 ± 5.24 (0.0–150.3) |
| Milk and dairy products, g | 136.72 ± 12.14 (17.1–340.9) |
| Fatty condiments, g | 23.20 ± 1.97 (7.9–92.1) |
| Snacks, g | 88.04 ± 7.97 (0.0–260.3) |
| Other aspects of diet | |
| CML, mg | 3.28 ± 0.21 (1.0–7.8) |
| PP, g | 17.92 ± 1.21 (6.0–49.7) |
| AP, g | 44.40 ± 3.32 (13.4–136.5) |
| PP/AP | 0.47 ± 0.04 (0.1–1.8) |
| Mediterranean diet adherence (IMI) | 4.67 ± 0.22 (2.0–8.0) |
We performed a Pearson correlation analysis to evaluate possible correlations between the CML content and the consumption of specific food categories or nutrient in the diet. Data showed that estimated daily intake of CML was positively associated with the energy intake (r 0.74, p-value <0.001). The intake of carbohydrates and lipids contributed the most to that correlation being positively correlated to CML intake (r 0.66, p-value <0.0001 and r 0.72, p-value <0.0001, respectively), whereas protein intake showed a significant lower association (r 0.44, p-value 0.0020). As expected, CML was poorly correlated with the intake of dietary fiber.
3.2 CML and CML microbial metabolites in urine samples
Due to the lack of authentic reference standards for CM-CAD, CM-APA, CM-APO and the N-carboxymethyl-Δ1-piperideinium ion, mass spectrometry transitions in MRM were preliminarily optimized by injecting diluted urine samples and scanning the ion in full scan mode; then precursor ions (namely m/z 161, 176, 162, 142) were scanned in single ion monitoring mode (SIM) and precursors were fragmented in product ion scan mode by tuning collision energies in parallel with chromatographic acquisition. Finally experimental MRM were setup and included in HILIC profiles. Fig. S1† reports MRM chromatograms of all monitored compounds identified in urines. To determine the concentration of CML and its metabolites in urines, analytical strategy was based on hydrophilic interaction chromatography by taking advantage of analyte positive charge in acidic conditions: zwitterionic stationary phase effectively separated polar charged analytes and improved response and robustness in positive ion mode confirming analytical performances for lysine and CML.18,20 Indeed, quality control samples (pooled urine samples spiked with internal standards) showed a relative standard deviation of less than 12% between sample batches and calibration curve batches.Fig. 1 reports urinary excretions of CML, pyrraline, CM-CAD, CM-APO, CM-APA and N-carboxymethyl-Δ1-piperideinium ion in all the subjects.
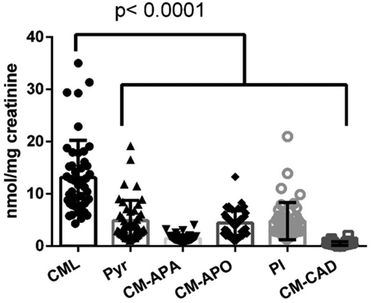 | ||
| Fig. 1 Scatter plot reporting concentrations (nmol mg−1 creatinine) of CML, pyrraline (Pyr) and CML metabolites in urines from the 46 study participants. | ||
As expected, CML concentration was higher than pyrraline and higher than its metabolite concentration: indeed, mean urinary CML was 2.69, 9.02, 2.96, 26.52 and 2.74-times higher than pyrraline, CM-APA, CM-APO, CM-CAD and N-carboxymethyl-Δ1-piperideinium ion, respectively. Focusing on commonly measured markers of lysine modifications, the mean concentration of lysine (145.33 ± 112.81 nmol mg−1 creatinine), pyrraline (4.87 ± 3.86 nmol mg−1 creatinine) and CML (13.12 ± 7.13 nmol mg−1 creatinine) agreed with previous works reporting the urinary excretion of d-AGEs in healthy subjects.24,25
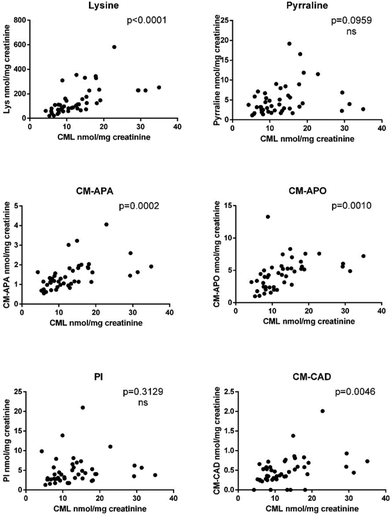
Fig. 2 shows the correlations between CML, and the other six compounds measured in urine samples.
Interestingly, a significant positive correlation was observed for lysine (CML precursor), CM-APA, CM-APO and CM-CAD with a Pearson coefficient (r) of 0.59, 0.25, 0.52 and 0.47, respectively; for pyrraline and piperideinium ion correlation was not significant, however both reached the significance by calculating the Spearman rho coefficient (0.36 and 0.38, respectively) indicating a poor monotonic association (Table S2†).
Previous in vitro studies used model systems where pure CML was incubated in probiotic cultures or fecal suspensions.17,18 When CML was incubated in aerobic conditions, CM-CAD was the key metabolization product with a conversion rate higher than CM-APA.17 Conversely, when CML was incubated in anaerobic conditions, similarly to what occurs in vivo, CM-APA was the most abundant metabolite.18 Considering a mass balance between precursor (CML) and its metabolites, we observed that the sum of all metabolites covers up to 85% of CML, but we cannot exclude that CML is subjected to other biotransformation pathways leading to metabolites that have a chemical behavior different from polar carboxymethylated biogenic amines and alcohols. Indeed, we did not target non-polar and volatile compounds in this study. Because of the similarities in the chemical structures of Nε-fructosyllysine and CML or in the carboxymethylated residues of CM-APO, CM-APA and CM-CAD, we hypothesize that branched chain small acids can be formed. However, specific untargeted and targeted in vitro procedures using carbon module labeled analysis are mandatory to disentangle the whole CML metabolic profile.
CML metabolization and the kind and quantity of compounds formed are a direct consequence of the microbial populations colonizing the gut. The composition of the gut microbiota oversees the quantity, the chemical nature and the distribution of glycation compound metabolites.26 Here, we hypothesize that the gut microbiome and the interindividual variability play a major role in the metabolization of CML leading to individualized metabolites’ profile, that can potentially represent the target for an intervention study.27
The effect of specific dietary patterns differing for poorly bioavailable glycation compounds on the gut microbiome and the relation with CML metabolites are still obscure. While some evidence highlighted that d-AGEs can modulate the gut microbiome28 towards changes that are detrimental for the host's health,29 recent evidence attributes those changes to dicarbonyls in abdominally obese humans.30 Considering the high variability and the possibility that other factors influence the absorption of CML metabolites, we also postulated a role of the endogenous source of CML in combination with dietary CML. Endogenous CML formation can be one of the consequences of aging, of the individual health status, that in turn are tightly connected to the protein turnover and to the renal function.31 Even if proteins undergo spontaneous glycation and oxidation, adducts as carboxymethylated residues are formed in low quantity in tissues and biofluids and wherever cellular repair is not possible, structurally modified residues are excreted in the urines.32 This specific route and the related cellular pathways should be studied in detail to demonstrate an interconnection between CML metabolites and enzymatic repair and metabolization in cells. Considering the body of evidence present in literature, we assumed that CML degradation can arise only from microbial fermentation.
Moving to N-substituted pyrrole formed via Maillard reaction of lysine with glucose and its degradation product 3-deoxyglucosone, pyrraline can potentially minimize the dichotomy between exogenous and endogenous sources of glycation compounds as it is exclusively formed in foods. The lack of an exhaustive reference database on pyrraline concentration in foods represents a major limitation for the association between pyrraline intake and pyrraline urinary excretion. Remarkably, it has been observed that up to 50% of dietary pyrraline is recovered in urine and 50% accumulates within the body likely being degraded to unknown metabolites; this observation points out the necessity to investigate metabolization processes on pyrraline residues.33,34
3.3 Associations between habitual diet, urinary CML and its microbial metabolites
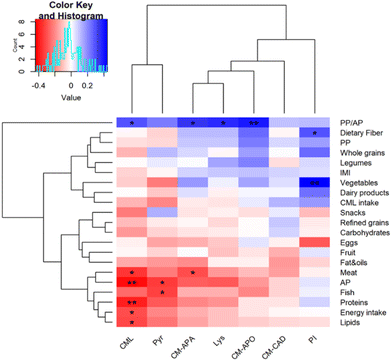
To explore the role of the diet on CML and CML metabolite excretion, we correlated the characteristics of individual diet with the urinary concentrations of monitored compounds (Fig. 3). Data outlined a positive correlation between urinary CML, CM-APA, lysine and CM-APO with plant protein to animal protein ratio intake, whereas N-carboxymethyl-Δ1-piperideinium ion in urine was positively associated with habitual dietary fiber and vegetable intake. This divergent role of protein source in affecting CML and its metabolite excretion could arise from a different microbial fermentation, that possibly mirror differences in the gut microbiome.35 Indeed, plant proteins are less digestible than animal proteins thus providing a higher quantity of undigested proteins entering the colon and shaping the gut microbiome with the contribution of other typical plant food components, e.g. dietary fiber.36 This hypothesis must be tested in future studies.Moreover, urinary CML was negatively associated with habitual energy, protein and lipid intake and AP, while CML and CM-APA showed a negative association with meat product intake. Finally, pyrraline was negatively associated with fish intake. Similarly, Semba and colleagues showed a positive association between urinary excretion of CML with AGE-low foods such as starchy vegetables and whole grains, a negative association with red meat and fast food and no association with AGE-rich food.37 No correlations were found between urinary CML or metabolites with IMI and CML intake as was previously shown in young volunteers receiving a weekly high d-AGEs diet and a low d-AGEs diet.38 Conversely, CML intake and free CML urinary excretion were positively correlated in individuals with an elevated risk for type 2 diabetes and cardiovascular disease.39 Therefore, our data suggest that the consumption of foods that are high in AGEs is not a major determinant for the urinary CML levels in healthy subjects.40 The gut microbiome may have a major role in human metabolism of dietary CML as it was recently established the capability of intestinal microorganisms in metabolizing dietary CML in vitro.18 However, in vivo factors like the gut permeability affects the amount of nutrient and metabolites absorbed and/or excreted. Therefore, an increased gut permeability, that is a characteristic of both human type 2 diabetes41 and cardiovascular disease,42 could explain the difference found between healthy subjects and patients.
Future in vivo studies will assess the putative involvement of microorganisms harbored in the human gut in affecting the dietary CML metabolism and the contribution of gut permeability in the bioavailability of dietary CML and its metabolites.
This study has the limitation that the habitual diet was estimated by self-reported data from participants using previously validated survey instrument, which is not as reliable as direct observations.
Conclusions
We implemented a targeted HILIC-tandem mass spectrometry technique to quantify glycation compounds as pyrraline, CML and its metabolites in human urine samples. The availability of an accurate analytical technique is fundamental to assess the causative role of d-AGEs in pathophysiological outcomes. Furthermore, we demonstrated for the first time that CML metabolites can pass into the systemic circulation and are excreted in urines of healthy subjects confirming preliminary evidence on the biochemical modifications occurring on CML. No correlation between the intake of dietary CML and metabolites excretion was found. However, we showed that the higher the habitual consumption of plant proteins at the expense of animal proteins the higher the urinary excretion of CML and its metabolites. We hypothesized that diet could affect CML metabolites formation by modulating the gut microbiome. An intervention study is needed to evaluate the causal role of dietary intake of glycation compounds on gut microbial metabolization and urinary excretion of the metabolites.Author contributions
Silvia Tagliamonte: formal analysis, methodology, data curation, visualization, writing – review & editing. Rosalia Ferracane: methodology. Antonio Dario Troise: conceptualization, methodology, formal analysis, writing – original draft, data curation, visualization. Paola Vitaglione: conceptualization, supervision, project administration, funding acquisition, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.References
- T. Fiolet, B. Srour, L. Sellem, E. Kesse-Guyot, B. Allès, C. Méjean, M. Deschasaux, P. Fassier, P. Latino-Martel, M. Beslay, S. Hercberg, C. Lavalette, C. A. Monteiro, C. Julia and M. Touvier, Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort, Br. Med. J., 2018, 360, k322 CrossRef PubMed.
- L. Schnabel, E. Kesse-Guyot, B. Allès, M. Touvier, B. Srour, S. Hercberg, C. Buscail and C. Julia, Association between ultraprocessed food consumption and risk of mortality among middle-aged adults in France, JAMA Intern. Med., 2019, 179, 490 CrossRef PubMed.
- M. Snelson, S. M. Tan, R. E. Clarke, C. de Pasquale, V. Thallas-Bonke, T.-V. Nguyen, S. A. Penfold, B. E. Harcourt, K. C. Sourris, R. S. Lindblom, M. Ziemann, D. Steer, A. El-Osta, M. J. Davies, L. Donnellan, P. Deo, N. J. Kellow, M. E. Cooper, T. M. Woodruff, C. R. Mackay, J. M. Forbes and M. T. Coughlan, Processed foods drive intestinal barrier permeability and microvascular diseases, Sci. Adv., 2021, 7, eabe4841 CrossRef CAS PubMed.
- D. Knorr and M. A. Augustin, Food processing needs, advantages and misconceptions, Trends Food Sci. Technol., 2021, 108, 103–110 CrossRef CAS.
- M. van Boekel, V. Fogliano, N. Pellegrini, C. Stanton, G. Scholz, S. Lalljie, V. Somoza, D. Knorr, P. R. Jasti and G. Eisenbrand, A review on the beneficial aspects of food processing, Mol. Nutr. Food Res., 2010, 54, 1215–1247 CrossRef CAS PubMed.
- D. J. McClements, Future foods: A manifesto for research priorities in structural design of foods, Food Funct., 2020, 11, 1933–1945 RSC.
- C. Delgado-Andrade and V. Fogliano, Dietary advanced glycosylation end-products (dages) and melanoidins formed through the Maillard reaction: Physiological consequences of their intake, Annu. Rev. Food Sci. Technol., 2018, 9, 271–291 CrossRef CAS PubMed.
- F. J. Tessier, C. Niquet-Léridon, P. Jacolot, C. Jouquand, M. Genin, A.-M. Schmidt, N. Grossin and E. Boulanger, Quantitative assessment of organ distribution of dietary protein-bound13C-labeled Nε-carboxymethyllysine after a chronic oral exposure in mice, Mol. Nutr. Food Res., 2016, 60, 2446–2456 CrossRef CAS PubMed.
- M. W. Poulsen, J. M. Andersen, R. V. Hedegaard, A. N. Madsen, B. N. Krath, R. Monošík, M. J. Bak, J. Nielsen, B. Holst, L. H. Skibsted, L. H. Larsen and L. O. Dragsted, Short-term effects of dietary advanced glycation end products in rats, Br. J. Nutr., 2015, 115, 629–636 CrossRef PubMed.
- K. M. Tuohy, D. J. Hinton, S. J. Davies, M. J. Crabbe, G. R. Gibson and J. M. Ames, Metabolism of Maillard reaction products by the human gut microbiota – implications for health, Mol. Nutr. Food Res., 2006, 50, 847–857 CrossRef CAS PubMed.
- R. N. Carmody, J. E. Bisanz, B. P. Bowen, C. F. Maurice, S. Lyalina, K. B. Louie, D. Treen, K. S. Chadaideh, V. M. Rekdal, E. N. Bess, P. Spanogiannopoulos, Q. Y. Ang, K. C. Bauer, T. W. Balon, K. S. Pollard, T. R. Northen and P. J. Turnbaugh, Cooking shapes the structure and function of the gut microbiome, Nat. Microbiol., 2019, 4, 2052–2063 CrossRef PubMed.
- A. R. Wolf, D. A. Wesener, J. Cheng, A. N. Houston-Ludlam, Z. W. Beller, M. C. Hibberd, R. J. Giannone, S. L. Peters, R. L. Hettich, S. A. Leyn, D. A. Rodionov, A. L. Osterman and J. I. Gordon, Bioremediation of a common product of food processing by a human gut bacterium, Cell Host Microbe, 2019, 26, 463–477 CrossRef CAS PubMed.
- M. Snelson and M. Coughlan, Dietary advanced glycation end products: Digestion, metabolism and modulation of Gut Microbial Ecology, Nutrients, 2019, 11, 215 CrossRef CAS PubMed.
- J. Lassak, A. Sieber and M. Hellwig, Exceptionally versatile take II: Post-translational modifications of lysine and their impact on bacterial physiology, Biol. Chem., 2022, 403, 819–858 CrossRef CAS PubMed.
- T. P. Bui, J. Ritari, S. Boeren, P. de Waard, C. M. Plugge and W. M. de Vos, Production of butyrate from lysine and the Amadori product fructoselysine by a human gut commensal, Nat. Commun., 2015, 6, 10062 CrossRef CAS.
- M. Hellwig, D. Bunzel, M. Huch, C. M. Franz, S. E. Kulling and T. Henle, Stability of individual Maillard reaction products in the presence of the human colonic microbiota, J. Agric. Food Chem., 2015, 63, 6723–6730 CrossRef CAS PubMed.
- M. Hellwig, C. Auerbach, N. Müller, P. Samuel, S. Kammann, F. Beer, F. Gunzer and T. Henle, Metabolization of the advanced glycation end productn-ε-carboxymethyllysine (CML) by different probiotic E. Colistrains, J. Agric. Food Chem., 2019, 67, 1963–1972 CrossRef CAS.
- T. P. Bui, A. D. Troise, V. Fogliano and W. M. de Vos, Anaerobic degradation of n-ε-carboxymethyllysine, a major glycation end-product, by human intestinal bacteria, J. Agric. Food Chem., 2019, 67, 6594–6602 CrossRef CAS PubMed.
- P. Vitaglione, I. Mennella, R. Ferracane, A. A. Rivellese, R. Giacco, D. Ercolini, S. M. Gibbons, A. La Storia, J. A. Gilbert, S. Jonnalagadda, F. Thielecke, M. A. Gallo, L. Scalfi and V. Fogliano, Whole-grain wheat consumption reduces inflammation in a randomized controlled trial on overweight and obese subjects with unhealthy dietary and lifestyle behaviors: role of polyphenols bound to cereal dietary fiber, Am. J. Clin. Nutr., 2015, 101, 251–261 CrossRef CAS PubMed.
- A. D. Troise, A. Fiore, M. Wiltafsky and V. Fogliano, Quantification of nε-(2-furoylmethyl)-l-lysine (furosine), nε-(carboxymethyl)-l-lysine (CML), nε-(carboxyethyl)-l-lysine (CEL) and total lysine through stable isotope dilution assay and tandem mass spectrometry, Food Chem., 2015, 188, 357–364 CrossRef CAS PubMed.
- K. Larsen, Creatinine assay by a reaction-kinetic principle, Clin. Chim. Acta, 1972, 41, 209–217 CrossRef CAS.
- C. Agnoli, V. Krogh, S. Grioni, S. Sieri, D. Palli, G. Masala, C. Sacerdote, P. Vineis, R. Tumino, G. Frasca, V. Pala, F. Berrino, P. Chiodini, A. Mattiello and S. Panico, A priori–defined dietary patterns are associated with reduced risk of stroke in a large Italian cohort, J. Nutr., 2011, 141, 1552–1558 CrossRef CAS PubMed.
- J. L. J. M. Scheijen, E. Clevers, L. Engelen, P. C. Dagnelie, F. Brouns, C. D. A. Stehouwer and C. G. Schalkwijk, Analysis of advanced glycation endproducts in selected food items by ultra-performance liquid chromatography tandem mass spectrometry: Presentation of a dietary age database, Food Chem., 2016, 190, 1145–1150 CrossRef CAS PubMed.
- J. Masania, G. Faustmann, A. Anwar, H. Hafner-Giessauf, N. Rajpoot, J. Grabher, K. Rajpoot, B. Tiran, B. Obermayer-Pietsch, B. M. Winklhofer-Roob, J. M. Roob, N. Rabbani and P. J. Thornalley, Urinary metabolomic markers of protein glycation, oxidation, and nitration in early-stage decline in metabolic, vascular, and renal health, Oxid. Med. Cell. Longevity, 2019, 2019, 1–15 CrossRef.
- C. Virgiliou, G. Theodoridis, I. D. Wilson and H. G. Gika, Quantification of endogenous aminoacids and aminoacid derivatives in urine by hydrophilic interaction liquid chromatography tandem mass spectrometry, J. Chromatogr. A, 2021, 1642, 462005 CrossRef CAS PubMed.
- N. ALjahdali and F. Carbonero, Impact of maillard reaction products on nutrition and Health: Current Knowledge and need to understand their fate in the human digestive system, Crit. Rev. Food Sci. Nutr., 2017, 59, 474–487 CrossRef PubMed.
- K. C. W. van Dongen, M. van der Zande, B. Bruyneel, J. J. M. Vervoort, I. M. C. M. Rietjens, C. Belzer and K. Beekmann, An in vitro model for microbial fructoselysine degradation shows substantial interindividual differences in metabolic capacities of human fecal slurries, Toxicol. in Vitro, 2021, 72, 105078 CrossRef CAS PubMed.
- I. Seiquer, L. A. Rubio, M. J. Peinado, C. Delgado-Andrade and M. P. Navarro, Maillard reaction products modulate gut microbiota composition in adolescents, Mol. Nutr. Food Res., 2014, 58, 1552–1560 CrossRef CAS PubMed.
- M. Murray, M. T. Coughlan, A. Gibbon, V. Kumar, F. Z. Marques, S. Selby-Pham, M. Snelson, K. Tsyganov, G. Williamson, T. M. Woodruff, T. Wu and L. E. Bennett, Reduced growth, altered gut microbiome and metabolite profile, and increased chronic kidney disease risk in young pigs consuming a diet containing highly resistant protein, Front. Nutr., 2022, 9, 816749 CrossRef PubMed.
- A. M. Linkens, N. van Best, P. M. Niessen, N. E. Wijckmans, E. E. de Goei, J. L. Scheijen, M. C. van Dongen, C. C. van Gool, W. M. de Vos, A. J. Houben, C. D. Stehouwer, S. J. Eussen, J. Penders and C. G. Schalkwijk, A 4-week diet low or high in advanced glycation endproducts has limited impact on gut microbial composition in abdominally obese individuals: The deageing trial, Int. J. Mol. Sci., 2022, 23, 5328 CrossRef CAS PubMed.
- E. D. Schleicher, E. Wagner and A. G. Nerlich, Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging, J. Clin. Invest., 1997, 99, 457–468 CrossRef CAS PubMed.
- P. J. Thornalley and N. Rabbani, Detection of oxidized and glycated proteins in clinical samples using mass spectrometry—a user's perspective, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840, 818–829 CrossRef CAS PubMed.
- A. Foerster and T. Henle, Glycation in food and metabolic transit of dietary ages (advanced glycation end-products): Studies on the urinary excretion of pyrraline, Biochem. Soc. Trans., 2003, 31, 1383–1385 CrossRef CAS PubMed.
- I. Nowotny, D. Schröter, M. Schreiner and T. Grune, Dietary advanced glycation end products and their relevance for human health, Ageing Res. Rev., 2018, 47, 55–66 CrossRef.
- I. J. Portune, M. Beaumont, A.-M. Davila, D. Tomé, F. Blachier and Y. Sanz, Gut microbiota role in dietary protein metabolism and health-related outcomes: The two sides of the coin, Trends Food Sci. Technol., 2016, 57, 213–232 CrossRef.
- S. Wu, Z. F. Bhat, R. S. Gounder, I. A. M. Ahmed, F. Y. Al-Juhaimi, Y. Ding and A. E. Bekhit, Effect of dietary protein and processing on gut microbiota a systematic review, Nutrients, 2022, 14, 453 CrossRef CAS PubMed.
- R. D. Semba, A. Ang, S. Talegawka, C. Crasto, M. Dalal, P. Jardack, M. G. Traber, L. Ferrucci and L. Arab, Dietary intake associated with serum versus urinary carboxymethyl-lysine, a major advanced glycation end product, in adults: the Energetics Study, Eur. J. Clin. Nutr., 2012, 66, 3–9 CrossRef CAS PubMed.
- C. Delgado-Andrade, F. J. Tessier, C. Niquet-Leridon, I. Seiquer and M. P. Navarro, Study of the urinary and faecal excretion of N ε-carboxymethyllysine in young human volunteers, Amino Acids, 2011, 43, 595–602 CrossRef PubMed.
- J. L. J. M. Scheijen, N. M. J. Hanssen, M. M. van Greevenbroek, C. J. Van der Kallen, E. J. M. Feskens, C. D. A. Stehouwer and C. G. Schalkwijk, Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study, Clin. Nutr., 2018, 37, 919–925 CrossRef CAS PubMed.
- J. M. Ames, Evidence against dietary advanced glycation endproducts being a risk to human health, Mol. Nutr. Food Res., 2007, 51, 1085–1090 CrossRef CAS.
- M. Gurung, Z. Li, H. You, R. Rodrigues, D. B. Jump, A. Morgun and N. Shulzhenko, Role of gut microbiota in type 2 diabetes pathophysiology, EBioMedicine, 2020, 51, 102590 CrossRef PubMed.
- V. C. Lewis and W. R. Taylor, Intestinal barrier dysfunction as a therapeutic target for cardiovascular disease, Am. J. Physiol.: Heart Circ. Physiol., 2020, 319, H1227–H1233 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo03480h |
| ‡ Present address: Proteomics, Metabolomics & Mass Spectrometry Laboratory, Institute for the Animal Production System in the Mediterranean Environment, National Research Council, 80055, Portici, Italy. |
| This journal is © The Royal Society of Chemistry 2023 |