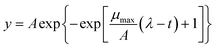Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Application of pre-adaptation strategies to improve the growth of probiotic lactobacilli under food-relevant stressful conditions†
Giulia
Bisson
 ,
Michela
Maifreni
,
Nadia
Innocente
and
Marilena
Marino
,
Michela
Maifreni
,
Nadia
Innocente
and
Marilena
Marino
 *
*
Department of Agricultural, Food, Animal and Environmental Sciences, University of Udine, via Sondrio 2/A, 33100 Udine, Italy. E-mail: marilena.marino@uniud.it
First published on 31st January 2023
Abstract
While formulating a probiotic food, it is mandatory to make sure that the viability of probiotics is adequate at the point of consumption, which can be strongly compromised by stressful conditions due to low pH and high osmolarity. In this study, three probiotic lactobacilli were subjected to different pre-adaptation conditions, and the turbidimetric growth kinetics in challenging conditions (pH 4.0–6.5, NaCl 1–7%, sucrose 0.1–0.7 M) were evaluated. Different effects were observed for Lactobacillus acidophilus, Lacticaseibacillus casei, and Lactiplantibacillus plantarum. Indeed, pre-exposition to sub-optimal conditions in terms of pH and % NaCl significantly improved the ability of L. acidophilus and L. casei to overcome the osmotic stress due to salt or sucrose, and similar effects were observed for acidic stress. L. plantarum showed to be more tolerant to the challenging conditions applied in this study. Anyway, the pre-adaptation at conditions SUB_1 (pH 4.5 and NaCl 4%) and SUB_2 (pH 5 and NaCl 2%) speeded-up its growth kinetics by reducing the length of the lag phase under sucrose stress and enhancing the maximum growth rate at the highest pH tested. Moreover, an improvement in biomass amount was observed under sucrose stress. The whole data evidenced that the application of the appropriate pre-adaptation condition could contribute to making probiotics more robust towards challenging conditions due to food matrix, processing, and storage as well as gastrointestinal transit. Further studies will be necessary to gain insight into the proteomics and metabolomics responsible for increased tolerance to stressful conditions.
Introduction
The growing interest in beneficial microorganisms has increased the demand for foods containing probiotics which are known as “living microorganisms that, when administrated in adequate amounts, confer benefits on the host”.1 Following this definition, a microorganism must be alive and present in the food in a sufficient amount by the end of its shelf-life to pass through the gastrointestinal tract and reach the target site, i.e., the gut. To be effective, a probiotic food should contain at least 107 cfu g−1 or mL−1 at the time of consumption to ensure the daily recommended intake that should be near 109 cfu per die.2,3Often the food doesn't represent the optimum environment for the survival of probiotic microorganisms. Both matrix and processing parameters (e.g., pH, temperature, dissolved oxygen, sugar or salt concentration, nutrients, …) could compromise probiotics’ survival.4,5 The presence of an oxygenic environment in yogurt during manufacture and storage negatively affected the survival of Lactobacillus acidophilus and Bifidobacterium spp.6 Also, the acidic environment in fruit juices (orange and apple) reduced the viability of some Lactobacillus and Bifidobacterium strains during refrigerated storage.7 Moreover, a significant loss of viability can occur during the gastrointestinal transit (GIT), both due to the acidic gastric environment and the antimicrobial activity of bile salts.8 For this reason, scientific research has focused on the study of different strategies to improve probiotics’ stability and functionality.5,9
Strain selection allows the identification of the best-performing strains showing stress resistance during food production, storage, and GIT.10 However, this technique has some drawbacks due to the need to characterize a large panel of strains by using phenotypical tests, which is quite time-consuming.11
The encapsulation processes consist in segregating a sensitive component (i.e., probiotic cells) from the surrounding environment by enclosing it within a proper substance that will protect and release it at the target site.12 This approach has some disadvantages due to the possible negative effects on the texture and sensory properties of the food product.13
The improvement of stress tolerance can also be obtained through gene modification, inducing the expression of genes already present on the strain, or by introducing genes from other microbial species. However, genetic modification of organisms is not well accepted by consumers and is not allowed in many countries.14
The cultivation of probiotics under sub-lethal conditions before exposing them to a challenging environment has shown to increase their survival. Lactobacilli after a pre-treatment under sub-lethal conditions can develop adaptive stress responses which result in an improvement of their ability to grow under severe levels of the same stress.15–17 Moreover, bacterial strains can develop cross-tolerance, which means that cells preadapted under mild stress conditions increased their tolerance towards diverse harsher conditions such as high temperatures, acidity, as well as oxygen presence.18–22
The improvement of viability after pre-adaptation has been demonstrated also in foods, e.g., yogurt and skimmed milk.23,24 The bacterial response to sublethal stress is strictly related to the microbial strain and to the growth limits for each parameter (sublethal level).25 Very recently, a turbidimetric study of the growth kinetic parameters, in terms of length of the lag phase, maximum growth rate, as well as maximum growth achieved during the stationary phase has been reported as a useful tool to identify growth limits that can be used as a starting point for an adaptation strategy.26 Despite its rapidity, a turbidimetric approach has never been applied to explore the effect of pre-adaptation strategies on probiotic growth. Thus, this study aimed to assess the consequences of the pre-adaptation on the growth kinetics of three probiotic lactobacilli strains challenged under stressful conditions (pH, % NaCl, and sucrose).
Materials and methods
Media and reagents
Lactobacillus acidophilus Lyofast LA3, Lacticaseibacillus casei Lyofast BGP93, and Lactiplantibacillus plantarum Lyofast BG112 were purchased from Sacco s.r.l. (Como, Italy). De Man Rogosa Sharpe broth (MRS), technical agar, and Maximum Recovery Diluent (MRD) were from Oxoid (Milan, Italy). D-(+)-sucrose, glycerol, 1 M hydrochloric acid (HCl), sodium hydroxide (NaOH), and sodium chloride (NaCl) were from Sigma-Aldrich (Milan, Italy).Inoculum preparation
L. acidophilus, L. casei, and L. plantarum were stored at −80 °C in MRS broth with 30% (v/v) glycerol. Before each analysis, one loopful of each culture was inoculated into 10 mL of MRS broth and incubated at 37 °C overnight in anaerobiosis. Cells were washed twice (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g at 4 °C for 10 min) and resuspended in MRD (final concentration of about 108 cfu mL−1, as evaluated in a preliminary step by viable counts on MRS agar).
000g at 4 °C for 10 min) and resuspended in MRD (final concentration of about 108 cfu mL−1, as evaluated in a preliminary step by viable counts on MRS agar).
Pre-adaptation of probiotics to sub-optimal conditions
According to previous data,26 for each strain two different sub-optimal conditions were chosen (SUB_1 and SUB_2; Table 1). 3 mL of overnight cultures were diluted in MRD 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (v/v) and added to 300 mL of MRS modified, when necessary, with HCl 1 M, NaCl, or sucrose. Then cultures were incubated at 30 °C for 48 h under stirring at 100 rpm. During incubation, the pH of the medium was maintained at the pre-set value by adding 0.5 M NaOH. At the end of the fermentation (stationary phase monitored by optical density; data not shown) cells were recovered by centrifugation at 13
100 (v/v) and added to 300 mL of MRS modified, when necessary, with HCl 1 M, NaCl, or sucrose. Then cultures were incubated at 30 °C for 48 h under stirring at 100 rpm. During incubation, the pH of the medium was maintained at the pre-set value by adding 0.5 M NaOH. At the end of the fermentation (stationary phase monitored by optical density; data not shown) cells were recovered by centrifugation at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 2 min at 4 °C, washed twice, and resuspended in MRD at approximately 106 cfu mL−1.
000g for 2 min at 4 °C, washed twice, and resuspended in MRD at approximately 106 cfu mL−1.
| Strain | Condition | pH | NaCl (%) | Sucrose (M) |
|---|---|---|---|---|
| L. acidophilus | SUB_1 | 4.5 | 4.0 | — |
| SUB_2 | 5.0 | 2.0 | — | |
| L. casei | SUB_1 | 5.0 | — | 0.3 |
| SUB_2 | 5.0 | 2.0 | — | |
| L. plantarum | SUB_1 | 4.5 | 4.0 | — |
| SUB_2 | 5.0 | 2.0 | — |
Growth of probiotics under optimal conditions
3 mL of the overnight cultures were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 (v/v) in MRD and added to 300 mL of MRS broth incubated at 37 °C for 24 h. Cells were recovered, washed as described above and resuspended in MRD at approximately 106 cfu mL−1 (control cells, i.e., CTRL).
100 (v/v) in MRD and added to 300 mL of MRS broth incubated at 37 °C for 24 h. Cells were recovered, washed as described above and resuspended in MRD at approximately 106 cfu mL−1 (control cells, i.e., CTRL).
Growth kinetic determination
Growth curves were obtained in MRS broth modified at pH 4.0–6.5 (whit 0.5-intervals), with 1–7% NaCl (1%-intervals) or 0.1–0.7 M (0.1-intervals) sucrose. Duplicate wells of U-bottomed 96-well microtiter plates (Corning Life Science, Corning, NY) were added with 190 μl of modified MRS and inoculated with 10 μl of the cell cultures. Microplates were incubated at 37 °C for 48 h and optical density at 630 nm (OD630) was determined at 30 min intervals by a Sunrise microplate reader (Tecan, Cernusco sul Naviglio, Milan, Italy).26 The kinetic data were modelled using the online tool GCAT27 according to the Gompertz equation:28where t is time (h), y is response (i.e., the log-transformed OD value), A (amplitude) is the upper asymptote, μmax is the maximum specific growth rate (log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OD per h), and λ is the lag time (h).
OD per h), and λ is the lag time (h).
Statistical analysis
All trials were carried out in two biological replicates, and for each replicate, at least two technical replicates were prepared. Data were expressed as the mean (n ≥ 4). t-Test (p < 0.05) was performed using R v. 3.1.1. for Windows (https://www.r-project.org/).Results
All the turbidimetric growth curves were modelled using the GCAT online tool and fitted well with the Gompertz model, with coefficients of determination (R squared) in the range 0.997–1.000, and RSS (Residual Sum of Squares) between 0.01 and 2.39. An example of the GCAT output is reported in Fig. S1.†Effect of pre-adaptation on L. acidophilus growth
Pre-adaptation to both sub-optimal conditions (SUB_1 and SUB_2) significantly improved the growth rate of L. acidophilus under NaCl, sucrose, and acidic stress. As an example, Fig. 1 shows the growth of L. acidophilus in presence of NaCl 2%. As reported in Table 2, non-pre-adapted L. acidophilus didn't grow at all at NaCl concentrations >1%, whereas pre-adapted cells did.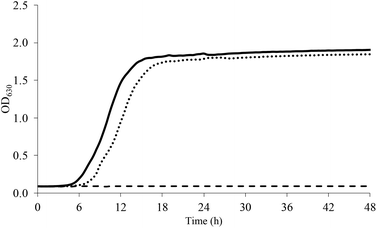 | ||
| Fig. 1 Growth curve of L. acidophilus in presence of NaCl 2% (dashed line, CTRL; continuous line, SUB_1; dotted line, SUB_2). | ||
| Condition | Lag phase (h) |
μ
max (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OD per h) OD per h) |
Amplitude (OD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CTRL | SUB_1 | SUB_2 | CTRL | SUB_1 | SUB_2 | CTRL | SUB_1 | SUB_2 | |
| NaCl 1% | 23.57 (0.13) | 5.09* (0.16) | 6.20* (0.13) | 0.12 (0.50) | 0.41* (0.07) | 0.38* (0.05) | 1.53 (0.06) | 2.32* (0.10) | 2.24* (0.17) |
| NaCl 2% | NG | 4.98* (0.06) | 6.72* (0.09) | NG | 0.37* (0.06) | 0.36* (0.14) | NG | 2.35* (0.05) | 2.30* (0.09) |
| NaCl 3% | NG | 3.91* (0.13) | 7.08* (0.07) | NG | 0.27* (0.06) | 0.33* (0.11) | NG | 2.33* (0.05) | 2.31* (0.13) |
| NaCl 4% | NG | 5.93* (0.08) | 7.63* (0.04) | NG | 0.33* (0.09) | 0.28* (0.10) | NG | 2.31* (0.03) | 2.26* (0.04) |
| NaCl 5% | NG | 6.57* (0.09) | 8.49* (0.09) | NG | 0.21* (0.17) | 0.24* (0.06) | NG | 2.30* (0.01) | 2.25* (0.16) |
| NaCl 6% | NG | 7.93* (0.03) | 10.72* (0.05) | NG | 0.22* (0.14) | 0.23* (0.06) | NG | 2.34* (0.10) | 2.26* (0.09) |
| NaCl 7% | NG | 9.73* (0.01) | 13.00* (0.08) | NG | 0.16* (0.19) | 0.15* (0.06) | NG | 2.25* (0.02) | 2.26* (0.14) |
| Sucrose 0.1 M | 14.12 (0.14) | 5.58* (0.05) | 6.79* (0.12) | 0.20 (0.05) | 0.45* (0.08) | 0.46* (0.09) | 1.20 (0.04) | 2.30* (0.10) | 2.27* (0.15) |
| Sucrose 0.2 M | 10.79 (0.19) | 5.88* (0.10) | 6.97* (0.13) | 0.04 (0.10) | 0.45* (0.04) | 0.43* (0.11) | 1.23 (0.03) | 2.32* (0.10) | 2.26* (0.10) |
| Sucrose 0.3 M | 18.29 (0.14) | 6.22* (0.16) | 7.38* (0.07) | 0.06 (0.28) | 0.45* (0.04) | 0.45* (0.05) | 1.26 (0.06) | 2.29* (0.02) | 2.26* (0.16) |
| Sucrose 0.4 M | 16.68 (0.18) | 6.58* (0.12) | 7.81* (0.13) | 0.07 (0.14) | 0.42* (0.03) | 0.42* (0.10) | 1.18 (0.05) | 2.28* (0.06) | 2.24* (0.18) |
| Sucrose 0.5 M | 17.66 (0.28) | 6.84* (0.07) | 8.14* (0.12) | 0.04 (0.35) | 0.38* (0.04) | 0.37* (0.11) | 0.85 (0.07) | 2.28* (0.03) | 2.23* (0.17) |
| Sucrose 0.6 M | 16.70 (0.12) | 6.84* (0.13) | 8.04* (0.06) | 0.04 (0.07) | 0.32* (0.09) | 0.31* (0.15) | 0.70 (0.05) | 2.28* (0.12) | 2.24* (0.02) |
| Sucrose 0.7 M | 19.46 (0.07) | 7.25* (0.08) | 8.66* (0.07) | 0.03 (0.17) | 0.29* (0.11) | 0.29* (0.15) | 0.61 (0.15) | 2.27* (0.08) | 2.21* (0.09) |
| pH 4.0 | NG | 5.93* (0.07) | 7.49* (0.13) | NG | 0.17* (0.14) | 0.17* (0.29) | NG | 2.24* (0.02) | 2.16* (0.19) |
| pH 4.5 | NG | 4.59* (0.13) | 6.93* (0.12) | NG | 0.21* (0.06) | 0.24* (0.21) | NG | 2.31* (0.06) | 2.24* (0.16) |
| pH 5.0 | 26.52 (0.08) | 4.77* (0.03) | 5.99* (0.12) | 0.03 (0.07) | 0.31* (0.10) | 0.26* (0.14) | 1.40 (0.04) | 2.31* (0.06) | 2.25* (0.12) |
| pH 5.5 | 18.61 (0.16) | 4.51* (0.07) | 5.41* (0.07) | 0.07 (0.06) | 0.34* (0.03) | 0.28* (0.09) | 1.45 (0.07) | 2.30* (0.07) | 2.23* (0.17) |
| pH 6.0 | 10.63 (0.09) | 4.53* (0.04) | 5.35* (0.06) | 0.06 (0.02) | 0.37* (0.07) | 0.29* (0.15) | 1.46 (0.05) | 2.35* (0.07) | 2.28* (0.19) |
| pH 6.5 | 6.59 (0.12) | 4.72* (0.11) | 5.49* (0.09) | 0.04 (0.08) | 0.38* (0.09) | 0.30* (0.10) | 1.64 (0.03) | 2.35* (0.11) | 2.29* (0.18) |
At NaCl 2% the stationary phase was reached after 16 and 18 h for SUB_1 and SUB_2, respectively. Moreover, the pre-adaptation to both sub-optimal conditions allowed the growth of L. acidophilus at the highest NaCl concentration used in this study (7%).
In presence of sucrose, non-pre-adapted L. acidophilus grew also at the highest concentration tested (0.7 M) although the lag phase values ranged from about 14 h (0.1 M) to about 19 h (0.7 M), the maximum growth rate was ≤0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OD per h and amplitude ≤1.2 OD. The pre-adaptation to both the sub-optimal conditions significantly reduced the lag phase at all the tested concentrations with a maximum lag phase value of about 8 hours for the SUB_2 cells exposed to the highest sucrose concentration tested. In this condition, the maximum growth rate significantly increased up to ten times while the amplitude almost tripled.
OD per h and amplitude ≤1.2 OD. The pre-adaptation to both the sub-optimal conditions significantly reduced the lag phase at all the tested concentrations with a maximum lag phase value of about 8 hours for the SUB_2 cells exposed to the highest sucrose concentration tested. In this condition, the maximum growth rate significantly increased up to ten times while the amplitude almost tripled.
As for acidic stress, the control culture of L. acidophilus was able to grow, although very slowly, starting from pH 5, with a lag phase of more than 26 h. As the pH increased, the growth kinetics shortened. At both the sub-optimal conditions applied, this species grew even at pH < 5 with very short lag times (between 4.59 and 7.49 h). Furthermore, at all the conditions tested, the pre-adaptation of the cells led to an increase in the maximum growth rate and amplitude, and the latter value increased by over 60% in nearly all conditions.
Effect of pre-adaptation on L. casei growth
Control L. casei cells could grow up to NaCl concentrations of 5% with a lag phase of about 26 h. In this condition, SUB_1 and SUB_2 cultures showed a 4-times shorter lag phase and increased maximum growth rate and amplitude values (Table 3).| Condition | Lag phase (h) |
μ
max (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OD per h) OD per h) |
Amplitude (OD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CTRL | SUB_1 | SUB_2 | CTRL | SUB_1 | SUB_2 | CTRL | SUB_1 | SUB_2 | |
| NaCl 1% | 15.46 (0.13) | 5.12* (0.12) | 4.96* (0.12) | 0.18 (0.02) | 0.45* (0.06) | 0.42* (0.08) | 2.24 (0.09) | 2.37 (0.13) | 2.34 (0.05) |
| NaCl 2% | 11.85 (0.25) | 5.57* (0.13) | 5.18* (0.10) | 0.11 (0.18) | 0.42* (0.11) | 0.38* (0.06) | 2.31 (0.03) | 2.33 (0.07) | 2.33 (0.08) |
| NaCl 3% | 13.94 (0.07) | 6.18* (0.13) | 5.62* (0.05) | 0.10 (0.07) | 0.37* (0.03) | 0.35* (0.14) | 2.20 (0.05) | 2.28 (0.14) | 2.29 (0.10) |
| NaCl 4% | 18.90 (0.16) | 6.91* (0.14) | 6.13* (0.16) | 0.08 (0.09) | 0.33* (0.06) | 0.30* (0.13) | 2.21 (0.03) | 2.27 (0.03) | 2.26 (0.03) |
| NaCl 5% | 26.86 (0.09) | 7.86* (0.32) | 6.49* (0.23) | 0.05 (0.18) | 0.26* (0.16) | 0.21* (0.15) | 1.71 (0.09) | 2.25* (0.10) | 2.25* (0.09) |
| NaCl 6% | NGa | 10.30* (0.10) | 8.57* (0.12) | NG | 0.19* (0.15) | 0.18* (0.26) | NG | 2.32* (0.16) | 2.24* (0.14) |
| NaCl 7% | NG | 13.42* (0.03) | 10.09* (0.04) | NG | 0.14* (0.20) | 0.12* (0.19) | NG | 2.24* (0.14) | 2.25* (0.06) |
| Sucrose 0.1 M | 11.99 (0.17) | 5.44* (0.04) | 5.05* (0.16) | 0.20 (0.02) | 0.47* (0.05) | 0.44* (0.10) | 2.26 (0.03) | 2.33 (0.13) | 2.29 (0.04) |
| Sucrose 0.2 M | 9.90 (0.10) | 6.00* (0.10) | 5.65* (0.16) | 0.13 (0.08) | 0.49* (0.08) | 0.45* (0.04) | 2.33 (0.11) | 2.33 (0.09) | 2.27 (0.10) |
| Sucrose 0.3 M | 11.63 (0.09) | 6.24* (0.06) | 5.88* (0.17) | 0.13 (0.11) | 0.46* (0.03) | 0.44* (0.04) | 2.31 (0.03) | 2.29 (0.06) | 2.26 (0.09) |
| Sucrose 0.4 M | 14.49 (0.07) | 6.51* (0.05) | 6.13* (0.08) | 0.13 (0.13) | 0.42* (0.11) | 0.41* (0.05) | 2.28 (0.09) | 2.28 (0.14) | 2.25 (0.07) |
| Sucrose 0.5 M | 17.01 (0.12) | 6.89* (0.04) | 6.62* (0.09) | 0.14 (0.14) | 0.39* (0.03) | 0.38* (0.05) | 2.26 (0.10) | 2.27 (0.17) | 2.27 (0.09) |
| Sucrose 0.6 M | 18.30 (0.15) | 7.06* (0.02) | 6.50* (0.18) | 0.14 (0.04) | 0.33* (0.11) | 0.32* (0.10) | 2.19 (0.11) | 2.25 (0.08) | 2.29 (0.10) |
| Sucrose 0.7 M | 22.09 (0.09) | 7.55* (0.05) | 7.22* (0.08) | 0.15 (0.06) | 0.31* (0.13) | 0.29* (0.07) | 2.17 (0.02) | 2.28 (0.12) | 2.23 (0.09) |
| pH 4.0 | 28.95 (0.10) | 5.71* (0.09) | 5.41* (0.15) | 0.09 (0.11) | 0.18* (0.07) | 0.17* (0.05) | 1.83 (0.13) | 2.20* (0.09) | 2.23* (0.14) |
| pH 4.5 | 17.53 (0.06) | 5.33* (0.09) | 5.21* (0.10) | 0.13 (0.03) | 0.24* (0.05) | 0.23* (0.10) | 2.10 (0.11) | 2.29* (0.02) | 2.28* (0.04) |
| pH 5.0 | 9.15 (0.11) | 4.94* (0.12) | 4.34* (0.14) | 0.10 (0.09) | 0.32* (0.04) | 0.28* (0.03) | 2.21 (0.09) | 2.28* (0.03) | 2.29* (0.09) |
| pH 5.5 | 7.71 (0.09) | 4.86* (0.06) | 4.27* (0.16) | 0.12 (0.07) | 0.35* (0.04) | 0.33* (0.14) | 2.25 (0.11) | 2.27 (0.12) | 2.29 (0.06) |
| pH 6.0 | 7.81 (0.17) | 4.67* (0.06) | 4.04* (0.15) | 0.13 (0.03) | 0.38* (0.07) | 0.31* (0.05) | 2.28 (0.05) | 2.29 (0.06) | 2.29 (0.09) |
| pH 6.5 | 7.95 (0.19) | 4.75* (0.02) | 4.84* (0.10) | 0.14 (0.06) | 0.38* (0.10) | 0.35* (0.10) | 2.31 (0.13) | 2.31 (0.16) | 2.31 (0.04) |
At NaCl concentrations >5% control cells didn't grow at all while SUB_1 and SUB_2 cells were able to grow relatively fast.
As for sucrose, non-preadapted L. casei grew at all the concentrations tested. The pre-exposition to both SUB_1 and SUB_2 led to a two- and three-times shorter lag phase and to a two-times faster growth rate in the exponential phase. No effect on the amplitude was observed.
As for acidic stress, non-pre-adapted L. casei grew at all the pH tested even though growth kinetics were generally slower as the pH decreased. The application of sub-optimal pre-adaptation significantly reduced the lag phase with a more appreciable effect at pH < 5. As an example, Fig. 2 shows the growth of L. casei at pH 4.
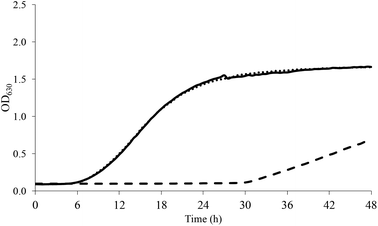 | ||
| Fig. 2 Growth curve of L. casei at pH 4 (dashed line, CTRL; continuous line, SUB_1; dotted line, SUB_2). | ||
Effect of pre-adaptation on L. plantarum growth
L. plantarum control cultures well-tolerated salt stress, as they grew up to the maximum NaCl concentration tested (7%), despite the lag phase lasting about 32 h (Table 4). Anyway, at all the NaCl concentrations tested, the pre-adaptation led to a significantly shorter lag phase and higher maximum growth rate with respect to CTRL cells with a more evident effect for SUB_1 cells. As for SUB_2 cells, the shortening of the lag phase was observed starting from NaCl 4%. No effect was observed on the maximum growth rate.| Condition | Lag phase (h) | μ max (logOD per h) | Amplitude (OD) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CTRL | SUB_1 | SUB_2 | CTRL | SUB_1 | SUB_2 | CTRL | SUB_1 | SUB_2 | |
| NaCl 1% | 6.56 (0.05) | 5.81* (0.17) | 6.83 (0.04) | 0.27 (0.11) | 0.42* (0.09) | 0.26 (0.02) | 2.33 (0.12) | 2.29 (0.16) | 2.25 (0.05) |
| NaCl 2% | 7.33 (0.14) | 5.55* (0.18) | 7.04 (0.07) | 0.25 (0.16) | 0.36* (0.10) | 0.23 (0.11) | 2.36 (0.09) | 2.27 (0.06) | 2.29 (0.03) |
| NaCl 3% | 8.56 (0.18) | 6.13* (0.33) | 7.90 (0.08) | 0.24 (0.25) | 0.35* (0.11) | 0.22 (0.12) | 2.27 (0.07) | 2.23 (0.11) | 2.29 (0.10) |
| NaCl 4% | 10.44 (0.19) | 6.85* (0.22) | 8.60* (0.08) | 0.22 (0.14) | 0.32* (0.11) | 0.20 (0.07) | 2.32 (0.09) | 2.27 (0.12) | 2.28 (0.09) |
| NaCl 5% | 12.96 (0.08) | 7.23* (0.14) | 9.45* (0.08) | 0.19 (0.26) | 0.26* (0.10) | 0.16 (0.10) | 2.36 (0.06) | 2.30 (0.02) | 2.26 (0.03) |
| NaCl 6% | 20.69 (0.05) | 8.77* (0.09) | 12.50* (0.02) | 0.14 (0.43) | 0.18 (0.18) | 0.12 (0.14) | 2.23 (0.01) | 2.28 (0.07) | 2.16 (0.13) |
| NaCl 7% | 32.01 (0.09) | 11.59* (0.17) | 18.90* (0.01) | 0.12 (0.33) | 0.15 (0.26) | 0.10 (0.08) | 2.22 (0.07) | 2.19 (0.15) | 2.03 (0.07) |
| Sucrose 0.1 M | 7.00 (0.04) | 5.86* (0.15) | 7.48 (0.11) | 0.36 (0.03) | 0.45* (0.07) | 0.33 (0.04) | 1.95 (0.14) | 2.31* (0.10) | 2.23* (0.14) |
| Sucrose 0.2 M | 8.17 (0.12) | 6.31* (0.16) | 7.65 (0.05) | 0.37 (0.08) | 0.47* (0.05) | 0.32 (0.04) | 1.98 (0.04) | 2.32* (0.13) | 2.24* (0.12) |
| Sucrose 0.3 M | 8.87 (0.06) | 6.69* (0.15) | 8.21 (0.07) | 0.42 (0.05) | 0.49 (0.05) | 0.33* (0.08) | 1.98 (0.01) | 2.31* (0.16) | 2.28* (0.05) |
| Sucrose 0.4 M | 9.74 (0.10) | 6.98* (0.06) | 8.86 (0.21) | 0.40 (0.05) | 0.45 (0.02) | 0.29* (0.10) | 1.72 (0.15) | 2.29* (0.06) | 2.24* (0.09) |
| Sucrose 0.5 M | 10.43 (0.10) | 7.23* (0.17) | 9.07* (0.12) | 0.38 (0.10) | 0.40 (0.03) | 0.27* (0.10) | 1.72 (0.12) | 2.29* (0.05) | 2.23* (0.11) |
| Sucrose 0.6 M | 10.73 (0.09) | 7.42* (0.04) | 8.82* (0.08) | 0.32 (0.12) | 0.36 (0.05) | 0.22* (0.02) | 1.75 (0.09) | 2.28* (0.11) | 2.21* (0.12) |
| Sucrose 0.7 M | 11.70 (0.17) | 7.68* (0.07) | 9.37* (0.05) | 0.29 (0.04) | 0.29 (0.08) | 0.20* (0.12) | 1.63 (0.05) | 2.20* (0.04) | 2.20* (0.11) |
| pH 4.0 | 5.81 (0.14) | 5.58 (0.07) | 5.36 (0.19) | 0.15 (0.13) | 0.19 (0.06) | 0.11 (0.22) | 2.29 (0.12) | 2.20 (0.15) | 2.12 (0.10) |
| pH 4.5 | 5.87 (0.15) | 6.12 (0.16) | 6.61 (0.14) | 0.16 (0.18) | 0.30* (0.04) | 0.17 (0.09) | 2.34 (0.05) | 2.30 (0.17) | 2.26 (0.10) |
| pH 5.0 | 6.01 (0.22) | 5.59 (0.02) | 6.39 (0.13) | 0.23 (0.07) | 0.38* (0.02) | 0.23 (0.13) | 2.38 (0.04) | 2.29 (0.08) | 2.26 (0.08) |
| pH 5.5 | 5.80 (0.19) | 5.24 (0.03) | 5.77 (0.07) | 0.25 (0.10) | 0.43* (0.03) | 0.28 (0.03) | 2.36 (0.10) | 2.31 (0.10) | 2.25 (0.16) |
| pH 6.0 | 5.99 (0.17) | 5.36 (0.09) | 6.23 (0.08) | 0.27 (0.08) | 0.47* (0.02) | 0.35* (0.01) | 2.33 (0.01) | 2.33 (0.04) | 2.29 (0.15) |
| pH 6.5 | 6.26 (0.10) | 5.27* (0.08) | 6.19 (0.19) | 0.28 (0.10) | 0.44* (0.04) | 0.33* (0.08) | 2.37 (0.09) | 2.32 (0.14) | 2.24 (0.02) |
On the other hand, the SUB_1 condition seems to positively affect L. plantarum increasing the maximum growth rate up to NaCl 5%. The amplitude was not affected at all by pre-exposition to sub-optimal conditions.
L. plantarum well tolerated a sucrose-rich environment and grew up to 0.7 M (Fig. 3). The pre-exposition to SUB_1 however reduced the lag phase by up to 4 h and increased the amplitude at all the concentrations tested, while the maximum growth rate was enhanced only at sucrose ≤0.2 M. Instead, SUB_2 reduced the lag phase of L. plantarum at sucrose ≥0.5 M, while the maximum growth rate decreased starting from 0.3 M sucrose. As opposed to SUB_2, SUB_1 improved the maximum growth rate up to 0.2 M sucrose.
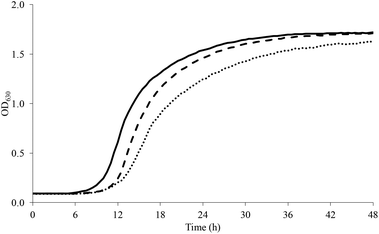 | ||
| Fig. 3 Growth curve of L. plantarum in presence of sucrose 0.7 M (dashed line, CTRL; continuous line, SUB_1; dotted line, SUB_2). | ||
A different effect between SUB_1 and SUB_2 was also observed in the case of acidic stress. SUB_1 demonstrated to shorten the lag phase by about 1 h at pH 6.5 while starting from pH 4.5 the maximum growth rate almost doubled. As for SUB_2, no effect on lag phase and amplitude was observed, and only an increase in growth rate was observed at pH ≥ 6.
Discussion
To investigate the occurrence and magnitude of adaptive stress responses to a challenging environment in probiotics, lactobacilli were pre-adapted to different sub-optimal conditions, then kinetic parameters under challenging conditions were assessed. The data obtained were compared with that of control cultures (i.e., non-preadapted cell cultures). In many cases, pre-adaptation induced a modification of the growth kinetics both in terms of shortening of the lag phase and increase of the maximum growth rate and the biomass obtained in the exponential phase.The probiotic strain for which pre-adaptation proved to be most effective was L. acidophilus. L. acidophilus is a probiotic widely used in dietary supplements, however, its addition to foods is only related to dairy foods, especially yogurt and fermented milk.29,30 One reason for the limited use of this probiotic species in foods is its low tolerance to osmotic stress. In the presence of osmotic stress, probiotics can suffer in terms of loss of cell turgor and changes in solute concentration, as well as changes in cell volume.17
In this study, control cells of L. acidophilus were able to grow only up to 1% NaCl. Instead, in presence of sucrose it grew up to the maximum concentration tested (0.7 M), even if as the sucrose concentration increased, the length of the lag phase increased, and the growth rate decreased. This different behavior under osmotic stress may be due to the nature of the solute. Indeed, both NaCl and sucrose generate osmotic stress, however, the cell damage due to NaCl is more severe than that of sucrose. High saline concentrations can increase the production of ROS, inhibit DNA replication and transcription, and damage proteins. Instead, in presence of a sucrose-rich environment, LAB can reduce the osmotic pressure differences between the external environment and the cytoplasm by activating transport systems on the cell membrane.31 The response mechanism of L. acidophilus to osmotic stress could also involve the increase of production of S-layer proteins that are linked to a protective role, forming stealth that protects the cell from environmental stresses.32 For example, the exposition of L. acidophilus ATCC4356 to NaCl 0.6 M increased the amount of S-layer proteins on the cell surface.33
Pre-adaptation conditions applied to L. acidophilus differed for pH and NaCl concentration (i.e., SUB_1, pH 4.5 and NaCl 4%, and SUB_2, pH 5 and NaCl 2%). Regardless of the pre-adaptation conditions, SUB_1 and SUB_2 stimulated the growth in challenging conditions induced by high NaCl and sucrose concentrations, revealing that this strategy could lead to the development of cross-resistance mechanisms.19,34 As an example, pre-adaptation at pH 4.5 and 4% NaCl significantly speeded up the growth of L. acidophilus in presence of high sucrose concentrations. Similar behavior was observed for osmotic stress induced by NaCl. Indeed, control cells did not grow at all at NaCl ≥ 2%, whereas SUB_1 and SUB_2 cultures grew very quickly up to 7%. These results are of relevance for probiotic foods having high NaCl or sucrose content, which could widen the application of L. acidophilus in foods.35 Indeed, in products such as white brined and long ripened-cheese, ice cream, and confectionery products this species hardly survives due to low aw, freezing, high NaCl and sucrose concentrations, and spray drying.11,36,37
L. casei possesses interesting commercial, industrial, and health-promoting characteristics. This species is commonly used for the fermentation of dairy products, but it is of interest also thanks to its ability to produce bioactive metabolites with health benefits.38,39 For this reason, some L. casei strains are considered probiotics, and their effects on human health (anti-obesity, anticancer activity, diarrhea prevention, etc.) are widely reported.40 In this study, L. casei was more tolerant to osmotic stress than L. acidophilus, growing up to 7% NaCl. This tolerance is expressed also in foods. Indeed, despite L. casei is not intentionally added to milk for cheesemaking, it becomes one of the most prevalent species at the end of cheese ripening, when salt-in-moisture reached the maximum concentration.41–43 The pre-adaptation with different combinations of osmotic and acidic stress (i.e., SUB_1, pH 5 and sucrose 0.3 M, SUB_2, pH 5 and NaCl 2%) allowed L. casei to grow with a shorter lag phase, increased maximum growth rate and increased biomass amount at the end of the stationary phase.
Similar results on the lag phase and maximum growth rate were observed for L. casei challenged with sucrose. At the highest sucrose concentration used (0.7 M), pre-adapted L. casei had a three-times shorter lag phase and a doubled maximum growth rate than control cells. Only a few data are available on the response of L. casei to osmotic stress. It has been evidenced that prior exposition of L. casei ATCC 393 to hypertonic conditions (NaCl 1 M) increased cell hydrophobicity allowing it to better face the stress of a hypertonic environment.44 Several pathways are involved in the physiological stress response in L. casei, leading to cell protection, modification of the cell membrane, and key components of central metabolism.45 The data obtained in this study are of relevance since for this species it is worth looking for strategies to preserve viability in foods. For example, the viability of L. casei in ice cream with different sugar concentrations (14–18%) significantly decreased during storage as the sugar content increased.46
L. plantarum is characterized by great adaptability and it is found in various ecological niches. Furthermore, it is of interest to researchers thanks to its health-related properties such as cholesterol-lowering effects, management of gastrointestinal disorders, diarrhea prevention, as well as its ability to produce bacteriocins.47 This species is commonly found at the end of the fermentation of plant foods in which a selective pressure on native microflora is applied by adding salt.48–50 In this study the pre-adaptation strategy speeded up the growth of L. plantarum by increasing the maximum growth rate and decreasing the lag time at all the NaCl concentrations tested. The preadaptation in the condition SUB_1 (pH 4.5, NaCl 4%, 24 h) determined a decrease of the lag phase in the whole range of NaCl and sucrose concentrations tested. On the other hand, the pre-exposition to sublethal conditions SUB_2 (pH 5, NaCl 2%, 25 °C, for 48 h) reduced the lag phase starting from 4% NaCl ≥ 0.5 M. The ability of this species to face the osmotic stress was attributed to its capability to produce extracellular polymeric substances such as exopolysaccharides, which form a layer that protects the cells from water loss increasing the cell resistance to osmosis.51,52 The exposition of L. plantarum to NaCl-induced osmotic stress can improve the autoaggregation and the adhesion as well as the pathogen adhesion inhibition, thus strengthening the probiotic.53
The tolerance to acid stress is relevant for probiotic bacteria, as acidity is commonly encountered during food fermentation and in the gastrointestinal tract, due to the production of organic acids and gastric fluids, respectively.54 As for L. acidophilus, the pre-exposition of cells to sub-optimal conditions allowed them to grow even at pH 4, whereas control cells didn't grow at all when pH was lower than 5. In orange juice having pH 3.8 viability of L. acidophilus rapidly decreased (viability loss 4.08 log CFU mL−1) during 35-days storage at 4 °C.55 For all the pH conditions tested, pre-adapted cells grew faster due to the shortening of the lag phase and the increase in maximum growth rate. Also, the amplitude was affected as an increase in biomass production was observed.
Our results agree with Lorca et al.,56 who observed that also a very short (1 h) pre-exposition to pH 5 improved the survival of L. acidophilus to pH 3. Once challenged with acid stress, microorganisms can activate various mechanisms, such as pH homeostasis, enhanced protein synthesis, and increased protein stability due to the production of acid-stress proteins and the reduction of protein degradation rate.54,57 The authors also hypothesized that acid-stress proteins could increase the stability of the pre-existing proteins. Furthermore, in suboptimal conditions, ATPase activity increases, which can counteract acidic stress.56,58 The results of this study are of interest since they can extend the use of L. acidophilus in acidic foods such as fruit juices, where pH could be a limiting factor for the survival of the cells.59 Moreover, the application of such a strategy could avoid the use of more invasive techniques such as encapsulation which could negatively affect the texture and sensory features of the product.13
L. casei tends to be more tolerant than L. acidophilus and more adaptable to different acidic fermented products such as dairy foods, fermented meat and vegetables, and sourdough.60–62 The resistance of this species to acidic stress could be attributed to different mechanisms (modulation of membrane fluidity, fatty acids composition, and cell integrity).45 Indeed, not-pre-adapted L. casei grew throughout the whole pH range (4.0–6.5). However, at pH 4 it grew very slowly, and the lag time was about 29 hours. In this study, both suboptimal conditions tested (SUB_1, pH 5 and sucrose 0.3 M, and SUB_2, pH 5 and NaCl 2%) speeded up the growth of L. casei at all the conditions. For example, at pH 4, pre-adapted cells had a six-times shorter lag phase and doubled the maximum growth rate with respect to control. Moreover, at pH ≤ 5 a gain in terms of final biomass amount was observed.
It has been hypothesized that greater resistance may be caused by the increased activity of the proton-translocating ATPase enzyme which allows the maintenance of pH homeostasis.17 Moreover, it was observed that the pre-adaptation to acidic stress modified the composition of the cell membrane both in terms of the percentage of saturated and cyclopropane fatty acids, indicating an increase in the fluidity of the cell membrane and decrease in rigidity.63 The possibility to obtain more acid-resistant probiotics is relevant for their addition to foods that might represent a stressful environment.4 For example, Olivares et al.64 observed that the viability of L. casei decreased in orange and raspberry juice during 28 days of shelf life at 4 °C and attributed this loss of vitality to the low pH. The application of a pre-adaptation strategy could be useful also to improve bacterial tolerance to the transit through the gastrointestinal tract.63
L. plantarum showed to tolerate acidic stress better than L. casei and L. acidophilus. Indeed, many authors already reported a high tolerance to acidic stress in this probiotic species.65,66 Anyway, the pre-exposure of both SUB_1 and SUB_2 conditions allowed to significantly increase in the maximum growth rate in most pH conditions applied. The speed-up of bacterial growth could be useful at the industrial level since the microbial biomass production step can become less time-consuming, and at risk of contamination.67
Conclusion
The optimization of cultural conditions by applying a pre-adaptation under sub-optimal conditions could contribute to enhancing the viability of probiotic lactobacilli in adverse conditions, avoiding more invasive techniques such as encapsulation that could negatively affect the sensory features of food products. The application of prolonged exposure to sub-optimal conditions showed to be very effective in enhancing the ability of L. acidophilus to overcome the osmotic stress due to high salt concentration, allowing it to grow up to 7% NaCl in a short time. Similar effects were observed in L. casei, and also in the case of acidic stress. L. plantarum was instead stimulated mainly in terms of the increase of growth rate during the exponential phase and final biomass amount. This could significantly make the biomass production step more time-effective and safe in terms of risk of contamination. Despite each strain requiring an individual evaluation of growth conditions, it is envisioned that the data obtained could be applied to enhance the viability of probiotic lactobacilli in challenging conditions due to food formulation, processing, and storage, as well as during GIT. It would be particularly worthwhile to carry out studies to gain insight into the proteomics and metabolomics responsible for increased tolerance to stressful conditions.Conflicts of interest
There are no conflicts to declare.References
- C. Hill, F. Guarner, G. Reid, G. R. Gibson, D. J. Merenstein, B. Pot, L. Morelli, R. B. Canani, H. J. Flint, S. Salminen, P. C. Calder and M. E. Sanders, The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic, Nat. Rev. Gastroenterol. Hepatol., 2014, 11, 506–514 CrossRef PubMed.
- A. de Prisco and G. Mauriello, Probiotication of foods: a focus on microencapsulation tool, Trends Food Sci. Technol., 2016, 48, 27–39 CrossRef CAS.
- A. Homayouni, A. Azizi, M. R. Ehsani, M. S. Yarmand and S. H. Razavi, Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of symbiotic ice cream, Food Chem., 2008, 111, 50–55 CrossRef CAS.
- K. Papadimitriou, Á. Alegría, P. A. Bron, M. de Angelis, M. Gobbetti, M. Kleerebezem, J. A. Lemos, D. M. Linares, P. Ross, C. Stanton, F. Turroni, D. van Sinderen, P. Varmanen, M. Ventura, M. Zúñiga, E. Tsakalidou and J. Kok, Stress physiology of lactic acid bacteria, Microbiol. Mol. Biol. Rev., 2016, 80, 837–890 CrossRef CAS PubMed.
- M. K. Tripathi and S. K. Giri, Probiotic functional foods: survival of probiotics during processing and storage, J. Funct. Foods, 2014, 9, 225–241 CrossRef CAS.
- R. I. Dave and N. P. Shah, Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures, Int. Dairy J., 1997, 7, 31–41 CrossRef.
- W. K. Ding and N. P. Shah, Survival of free and microencapsulated probiotic bacteria in orange and apple juices, Int. Food Res. J., 2008, 15, 219–232 Search PubMed.
- G. B. Brinques and M. A. Z. Ayub, Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration and yogurt, J. Food Eng., 2011, 103, 123–128 CrossRef CAS.
- A. Terpou, A. Papadaki, I. K. Lappa, V. Kachrimanidou, L. A. Bosnea and N. Kopsahelis, Probiotics in food systems: significance and emerging strategies towards improved viability and delivery of enhanced beneficial value, Nutrients, 2019, 11, 1591 CrossRef CAS PubMed.
- A. Upadrasta, C. Stanton, C. Hill, G. F. Fitzgerald and R. P. Ross, Improving the stress tolerance of probiotic cultures: recent trends and future directions, in Stress Responses of Lactic Acid Bacteria, ed. E. Tsakalidou and K. Papadimitriou, Springer, Boston, MA, 2011, pp. 395–438 Search PubMed.
- M. Marino, N. Innocente, S. Melchior, S. Calligaris and M. Maifreni, Main technological challenges associated with the incorporation of probiotic cultures into foods, in Advances in Probiotics, ed. D. Dhanasekaran and A. Sankaranarayanan, Academic Press, Cambridge, MA, 2021, pp. 479–495 Search PubMed.
- G. K. Gbassi and T. Vandamme, Probiotic encapsulation technology: from microencapsulation to release into the gut, Pharmaceutics, 2012, 4, 149–163 CrossRef CAS PubMed.
- K. B. Vivek, Use of encapsulated probiotics in dairy based foods, Int. J. Food Agric. Vet. Sci., 2013, 3, 188–199 Search PubMed.
- M. Gueimonde and B. Sánchez, Enhancing probiotic stability in industrial processes, Microb. Ecol. Health Dis., 2012, 23, 2–5 Search PubMed.
- J. Barbosa, S. Borges and P. Teixeira, Influence of sub-lethal stresses on the survival of lactic acid bacteria after spray-drying in orange juice, Food Microbiol., 2015, 52, 77–83 CrossRef CAS PubMed.
- C. Zhang, J. Lu, D. Yang, X. Chen, Y. Huang and R. Gu, Stress influenced the aerotolerance of Lactobacillus rhamnosus hsryfm, Biotechnol. Lett., 2018, 40, 729–735 CrossRef PubMed.
- M. de Angelis and M. Gobbetti, Environmental stress response in Lactobacillus: a review, Proteomics, 2004, 4, 106–122 CrossRef CAS PubMed.
- H. Yang, S. Yao, M. Zhang and C. Wu, Heat adaptation induced cross protection against ethanol stress in Tetragenococcus halophilus: physiological characteristics and proteomic analysis, Front. Microbiol., 2021, 12, 686672 CrossRef PubMed.
- J. Ma, C. Xu, F. Liu, J. Hou, H. Shao and W. Yu, Stress adaptation and cross-protection of Lactobacillus plantarum KLDS 1.0628, CyTA–J. Food, 2021, 19, 72–80 CrossRef CAS.
- M. Matsumoto, H. Ohishi and Y. Benno, H+-ATPase activity in Bifidobacterium with special reference to acid tolerance, Int. J. Food Microbiol., 2004, 93, 109–113 CrossRef CAS PubMed.
- L. Ruiz, P. Ruas-Madiedo, M. Gueimonde, C. G. de Los Reyes-Gavilán, A. Margolles and B. Sánchez, How do bifidobacteria counteract environmental challenges? Mechanisms involved and physiological consequences, Genes Nutr., 2011, 6, 307–318 CrossRef CAS PubMed.
- L. Ruiz, M. Gueimonde, R. M. Patricia, A. Ribbera, C. G. de los Reyes-Gavilán, M. Ventura, A. Margolles and B. Sánchez, Molecular clues to understand the aerotolerance phenotype of Bifidobacterium animalis subsp. lactis, Appl. Environ. Microbiol., 2012, 78, 644–650 CrossRef CAS PubMed.
- S. Settachaimongkon, H. J. F. van Valenberg, V. Winata, X. Wang, M. J. R. Nout, T. C. M. van Hooijdonk, M. H. Zwietering and E. J. Smid, Effect of sublethal preculturing on the survival of probiotics and metabolite in set-yogurt, Food Microbiol., 2015, 49, 104–115 CrossRef CAS PubMed.
- C. Desmond, C. Stanton, G. F. Fitzgerald, K. Collins and R. Paul Ross, Environmental adaptation of probiotic lactobacilli towards improvement of performance during spray drying, Int. Dairy J., 2002, 12, 183–190 CrossRef CAS.
- V. Ferrando, A. Quiberoni, J. Reinhemer and V. Suárez, Resistance of functional Lactobacillus plantarum strains against food stress conditions, Food Microbiol., 2015, 48, 63–71 CrossRef CAS PubMed.
- G. Bisson, M. Marino, D. Poletti, N. Innocente and M. Maifreni, Turbidimetric definition of growth limits in probiotic Lactobacillus strains from the perspective of an adaptation strategy, J. Dairy Sci., 2021, 104, 12236–12248 CrossRef CAS PubMed.
- Y. v. Bukhman, N. W. di Piazza, J. Piotrowski, J. Shao, A. G. W. Halstead, M. D. Bui, E. Xie and T. K. Sato, Modeling the microbial growth curve with GCAT, BioEnergy Res., 2015, 8, 1022–1030 CrossRef CAS.
- M. H. Zwietering, I. Jongenburger, F. M. Rombouts and K. Van't Riet, Modeling of the bacterial growth curve, Appl. Environ. Microbiol., 1990, 56, 1875–1881 CrossRef CAS PubMed.
- S. J. Masoumi, D. Mehrabani, M. Saberifiroozi, M. R. Fattahi, F. Moradi and M. Najafi, The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance, Food Sci. Nutr., 2021, 9, 1704–1711 CrossRef CAS PubMed.
- M. Bull, S. Plummer, J. Marchesi and E. Mahenthiralingam, The life history of Lactobacilus acidophilus as probiotic: a tale of revisionary taxonomy, misidentification and commercial success, FEMS Microbiol. Lett., 2013, 349, 77–87 CrossRef CAS PubMed.
- E. Glaasker, F. S. B. Tjan, P. F. ter Steeg, W. N. Konings and B. Poolman, Physiological response of Lactobacillus plantarum to salt and nonelectrolyte stress, J. Bacteriol., 1998, 180, 4718–4723 CrossRef CAS PubMed.
- E. Gerbino, P. Carasi, P. Mobili, M. A. Serradell and A. Gómez-Zavaglia, Role of S-layer proteins in bacteria, World J. Microbiol. Biotechnol., 2015, 31, 1877–1887 CrossRef CAS PubMed.
- M. M. Palomino, P. M. Waehner, J. Fina Martin, P. Ojeda, L. Malone, C. Sánchez Rivas, M. Prado Acosta, M. C. Allievi and S. M. Ruzal, Influence of osmotic stress on the profile and gene expression of surface layer proteins in Lactobacillus acidophilus ATCC 4356, Appl. Microbiol. Biotechnol., 2016, 100, 8475–8484 CrossRef CAS PubMed.
- H. Yang, M. He and C. Wu, Cross-protection of lactic acid bacteria during environmental stresses: stress response and underlying mechanisms, LWT–Food Sci. Technol., 2021, 144, 111203 CrossRef CAS.
- D. Żyżelewicz, E. Nebesny, G. Budryn and W. Krysiak, Technology and stability of probiotic and prebiotic confectionery products, in Probiotic and prebiotic foods: technology, stability and benefits to human health, ed. N. P. Shah, A. G. Da Cruz and J. A. F. Faria, Nova Science Publishers, Inc., New York, 2011, p. 295 Search PubMed.
- M. Yilmaztekin, B. H. Özer and F. Atasoy, Survival of Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-02 in white-brined cheese, Int. J. Food Sci. Nutr., 2004, 55, 53–60 CrossRef CAS PubMed.
- S. Calligaris, M. Marino, M. Maifreni and N. Innocente, Potential application of monoglyceride structured emulsions as delivery systems of probiotic bacteria in reduced saturated fat ice cream, LWT–Food Sci. Technol., 2018, 96, 329–334 CrossRef CAS.
- C. G. Dietrich, T. Kottmann and M. Alavi, Commercialy available probiotic drinks containing Lactobacillus casei DN-114001, World J. Gastroenterol., 2014, 20, 15837–15844 CrossRef PubMed.
- L. Alonso, E. P. Cuesta and S. E. Gilliland, Production of free conjugated linoleic acid by Lactobacillus acidophilus and Lactobacillus casei of human intestinal origin, J. Dairy Sci., 2003, 86, 1941–1946 CrossRef CAS PubMed.
- D. Hill, I. Sugrue, C. Tobin, C. Hill, C. Stanton and R. P. Ross, The Lactobacillus casei group: history and health related application, Front. Microbiol., 2018, 9, 2107 CrossRef PubMed.
- M. Marino, M. Maifreni and G. Rondinini, Microbiological characterization of artisanal Montasio cheese: analysis of its indigenous lactic acid bacteria, FEMS Microbiol. Lett., 2003, 229, 133–140 CrossRef CAS PubMed.
- L. Carraro, M. Maifreni, I. Bartolomeoli, M. E. Martino, E. Novelli, F. Frigo, M. Marino and B. Cardazzo, Comparison of culture-dependent and indipendent methods for bacterial community monitoring during Montasio cheese manufacturing, Res. Microbiol., 2011, 162, 231–239 CrossRef CAS PubMed.
- B. Bottari, A. Levante, E. Neviani and M. Gatti, How the fewest become the greatest. L. casei's impact on long ripened cheeses, Front. Microbiol., 2018, 9, 2014–2019 CrossRef PubMed.
- M. C. Machado, C. S. López, H. Heras and E. A. Rivas, Osmotic response in Lactobacillus casei ATCC 393: biochemical and biophysical characteristics of membrane, Arch. Biochem. Biophys., 2004, 422, 61–70 CrossRef CAS PubMed.
- C. Wu, J. Zhang, M. Wang, G. Du and J. Chen, Lactobacillus casei combats acid stress by maintaining cell membrane functionality, J. Ind. Microbiol. Biotechnol., 2012, 39, 1031–1039 CrossRef CAS PubMed.
- A. Shahsavan, R. Pourahmad and P. Rajaei, Effect of different amounts of sugar and fat on the viability of Lactobacillus casei, physical, chemical and sensory properties of probiotic ice cream, Int. J. Biol. Biotechnol., 2018, 15, 63–69 CAS.
- H. A. Seddik, F. Bendali, F. Gancel, I. Fliss, G. Spano and D. Drider, Lactobacillus plantarum and its probiotic and food potentialities, Probiotics Antimicrob. Proteins, 2017, 9, 111–122 CrossRef PubMed.
- Z. Yu, X. Zhang, S. Li, C. Li, D. Li and Z. Yang, Evaluation of probiotic properties of Lactobacillus plantarum strains isolated from Chinese sauerkraut, World J. Microbiol. Biotechnol., 2013, 29, 489–498 CrossRef CAS PubMed.
- Y. Alan, Z. Topalcengiz and M. Dığrak, Biogenic amine and fermentation metabolite production assessments of Lactobacillus plantarum isolates for naturally fermented pickles, LWT–Food Sci. Technol., 2018, 98, 322–328 CrossRef CAS.
- M. Ziadi, T. Bouzaiene, S. Lakhal, K. Zaafouri, S. Massoudi, X. Dousset and M. Hamdi, Screening of lactic starter from Tunisian fermented vegetables and application for the improvement of caper (Capparis spinosa) fermentation through an experimental factorial design, Ann. Microbiol., 2019, 69, 1373–1385 CrossRef CAS.
- P. T. Nguyen, T. T. Nguyen, T. T. U. Nguyen, Q. K. Hoang and H. T. Nguyen, Food Bioprod. Process., 2022, 131, 149–155 CrossRef CAS.
- P. T. Nguyen, T. T. Nguyen, T. N. T. Vo, T. T. X. Nguyen, Q. K. Hoang and H. T. Nguyen, Response of Lactobacillus plantarum VAL6 to challenges of pH and sodium choloride stresses, Sci. Rep., 2021, 11, 1–9 CrossRef PubMed.
- J. Ma, W. Wang, C. Sun, L. Gu, Z. Liu, W. Yu, L. Chen, Z. Jiang and J. Hou, Effects of environmental stresses on the physiological characteristics, adhesion ability and pathogen adhesion inhibition of Lactobacillus plantarum KLDS 1.0328, Proc. Biochem., 2020, 92, 426–436 CrossRef CAS.
- N. Guan and L. Liu, Microbial response to acid stress: mechanisms and applications, Appl. Microbiol. Biotechnol., 2020, 104, 51–65 CrossRef CAS PubMed.
- A. Sohail, M. S. Turner, E. K. Prabawati, A. G. A. Coombes and B. Bhandari, Evaluation of Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM encapsulated using a novel impinging aerosol method in fruit food products, Int. J. Food Microbiol., 2012, 157, 162–166 CrossRef CAS PubMed.
- G. L. Lorca, G. Font De Valdez and Å. Ljungh, Characterization of the protein-synthesis dependent adaptive acid tolerance in Lactobacillus acidophilus, J. Mol. Microbiol. Biotechnol., 2002, 4, 525–532 CAS.
- S. Mills, C. Stanton, G. F. Fitzgerald and R. P. Ross, Enhancing the stress responses of probiotics for a lifestyle from gut to product and back again, Microb. Cell Fact., 2011, 10, S19 CrossRef PubMed.
- G. L. Lorca and G. F. de Valdez, A low-pH inducible, stationary-phase acid tolerance response in Lactobacillus acidophilus CRL 639, Curr. Microbiol., 2001, 42, 21–25 CrossRef CAS PubMed.
- T. Marques da Silva, V. Sonza Pinto, V. Ramires Fonseca Soares, D. Marotz, A. J. Cichoski, L. Queiroz Zepka, E. Jacob Lopes, C. de Bona da Silva and C. R. de Menezes, Viability of microencapsulated Lactobacillus acidophilus by complex coacervation associated with enzymatic crosslinking under application in different fruit juices, Food Res. Int., 2021, 141, 110190 CrossRef CAS PubMed.
- N. Ullah, X. Wang, J. Wu, Y. Guo, H. Ge, T. Li, S. Khan, Z. Li and X. Feng, Purification and primary characterization of a novel bacteriocin, LiN333, from Lactobacillus casei, LWT–Food Sci. Technol., 2017, 84, 867–875 CrossRef CAS.
- B. Haghshenas, Y. Nami, M. Haghshenas, N. Abdullah, R. Rosli, D. Radiah and A. Yari Khosroushahi, Bioactivity characterization of Lactobacillus strains isolated from dairy products, Microbiologyopen, 2015, 4, 803–813 CrossRef CAS PubMed.
- E. Bartkiene, V. Lele, M. Ruzauskas, K. J. Domig, V. Starkute, P. Zavistanaviciute, V. Bartkevics, I. Pugajeva, D. Klupsaite, G. Juodeikiene, R. Mickiene and J. M. Rocha, Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation, Microorganisms, 2019, 8, 64 CrossRef PubMed.
- J. R. Broadbent, R. L. Larsen, V. Deibel and J. L. Steele, Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress, J. Bacteriol., 2010, 192, 2445–2458 CrossRef CAS PubMed.
- A. Olivares, C. Soto, E. Caballero and C. Altamirano, Physiological and transcriptional response of Lactobacillus casei ATCC 334, Electron. J. Biotechnol., 2019, 42, 42–48 CrossRef CAS.
- M. M. Ayyash, A. K. Abdalla, N. S. AlKalbani, M. A. Baig, M. S. Turner, S. Q. Liu and N. P. Shah, Characterization of new probiotics from dairy and nondairy products – inshights into acid tolerance, bile metabolism and tolerance, and adhesion capability, J. Dairy Sci., 2021, 104, 8363–8379 CrossRef CAS PubMed.
- E. Parente, F. Ciocia, A. Ricciardi, T. Zotta, G. E. Felis and S. Torriani, Diversity of stress tolerance in Lactobacillus plantarum, Lactobacillus pentosus and Lactobacillus paraplantarum: a multivariate screening study, Int. J. Food Microbiol., 2010, 144, 270–279 CrossRef CAS PubMed.
- M. R. Ladisch, E. Ximenes, N. Mosier, A. S. Engelberth, K. Solomon and R. Binkley, Bioprocess engineering, in Industrial Microbiology, ed. D. B. Wilson, H. Sahm, K.-P. Stahmann and M. Koffas, Wiley, Weinheim, 2020, pp. 23–58 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo03215e |
| This journal is © The Royal Society of Chemistry 2023 |