Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metabolic and nutritional biomarkers in adults consuming lacto-ovo vegetarian, vegan and omnivorous diets in Spain. A cross-sectional study
Elena
García-Maldonado
 ,
Belén
Zapatera
,
Belén
Zapatera
 ,
Alexandra
Alcorta
and
M. Pilar
Vaquero
,
Alexandra
Alcorta
and
M. Pilar
Vaquero
 *
*
Department of Metabolism and Nutrition, Institute of Food Science, Technology and Nutrition (ICTAN), CSIC, José Antonio Novais 10, 28040 Madrid, Spain. E-mail: mpvaquero@ictan.csic.es
First published on 13th January 2023
Abstract
Knowledge on the characteristics of consumers who choose plant-based diets and the relationship with nutritional status and disease risk is needed. In the present study, 207 Spanish adults participated in a cross-sectional study, and were classified in three groups: lacto-ovo vegetarian (LOV), vegan (VEG), and omnivore (OMN). Dietary intake, anthropometry, body composition, haematology, and metabolic markers were evaluated. Body composition and body weight did not vary among groups. The majority of these adults performed moderate-vigorous physical activity, and LOV performed more moderate activity than OMN. Total energy intake (En) was similar in the three groups. However, cholesterol and fat intakes (%En) were higher in the order OMN, LOV, VEG, fibre and carbohydrate intakes showed the opposite trend, and protein intake (%En) was higher in OMN than both LOV and VEG (all p < 0.001). Systolic blood pressure (p = 0.04), erythrocytes (p < 0.001), and haematocrit (p < 0.001) were lower in LOV and VEG than OMN, and lymphocyte count was lower in LOV than OMN (p < 0.01). There were marked differences between groups in serum total-cholesterol and LDL-cholesterol that were lower in LOV and VEG than OMN (both p < 0.001). However, glucose, insulin and insulin resistance did not show group differences. Leptin and adiponectin were related with gender and body fat but not with diet. The inflammation marker interleukin-1β was lower in LOV than OMN but TNF-α did not show differences. All levels were within normal ranges. Conclusion: consumption of plant-based diets compared to omnivorous diets in combination with moderate-high physical activity appears to protect similarly from cardiometabolic diseases in Spanish adults.
1. Introduction
Promotion of sustainable and healthy diets leads to a reduction of red meat and its products and an increase in vegetables intake. This trend is aimed at reducing cardiometabolic diseases, such as obesity, diabetes, and hypertension, preserving at the same time the planet.1 In this line, consumption of plant-based diets is increasing enormously and the extreme is to follow a diet where meat and fish is totally absent.The American Academy of Nutrition and Dietetic stated in 2016 that appropriately planned vegetarian diets are healthful and nutritionally adequate.2 When planning vegetarian diets, the use of supplements to prevent micronutrient deficiencies should be considered.3,4
As a result of these recommendations and the various social awareness campaigns, the number of vegetarians is intensely increasing all over the world.5–7 Vegetarianism and veganism in Spain coexist with the culture of the Mediterranean diet and current estimations indicate that approximately 10% of the population follow these diets.8 While the dietary intake and cardiovascular health of vegetarians have been addressed in a number of studies,7,9,10 data from Spain are very scarce. Generally, intake of saturated fats and cholesterol is reported to be lower in vegetarians compared to omnivores. Therefore, vegetarian diets are associated with low cardiovascular risk.11 Other data indicate that plant-based diets favour insulin sensitivity.12 We investigated the dietary intake and nutritional status of Spanish lacto-ovo vegetarian and vegans and observed that one third did not use any food supplements. If they did, they predominantly selected a vitamin B12 supplement that is recommended in all guides, while the use of other supplements such as vitamin D or omega-3 fatty acids was very low.13 Consequently, 11% of the sample presented vitamin B12 deficiency or inadequacy. More recently we observed that the ratio of fatty acids omega-6/omega-3 was unbalanced even through vegetarians try to avoid saturated fat and ingest a high proportion of polyunsaturated fatty acids.14 However, in those studies a comparable group of omnivorous subjects was not available.
Therefore, in the present study we aimed to know the dietary intake, body composition, physical activity, and nutritional status of Spanish vegetarians, lacto-ovo vegetarians and vegans, compared to omnivorous subjects of similar characteristics. The hypothesis was that the health status evaluated by haematological and cardiometabolic biomarkers was higher in vegetarians compared to omnivores.
2. Materials and methods
2.1. Experimental design and setting
The design was a controlled cross-sectional study. Volunteers were selected and classified according to their usual diet in three groups: lacto-ovo vegetarian (LOV), vegan (VEG), and omnivore (OMN, reference group).The study is part of a larger project investigating the health status of a vegetarian population and the effects of an intervention with a new nutritional supplement of omega-3 fatty acids through a randomized controlled trial. For this purpose, based on serum docosahexaenoic acid, a sample size of n = 90 was needed, but due to the global pandemic the clinical assay had to be cancelled in 2020 and was taken up again in 2021.15 Therefore, there were two recruitment periods and this cross-sectional study was performed with the total sample size of the participants who started the intervention (n = 102 and n = 105, in 2020 and 2021 respectively). It was carried out in Madrid (Spain) at the Human Nutrition Unit of the Institute of Food Science, Technology and Nutrition of the Spanish CSIC (ICTAN-CSIC) according to the guidelines established in the Helsinki declaration and was approved by the Ethical Committee of the Hospital Puerta del Hierro, Majadahonda (Ref. PI176/19, Acta n° 20/2019, 18 November 2019) and the Ethics Committee of CSIC (Ref. 104/2019, 19 December 2019). In addition, it was registered at ClinicalTrials.gov (ID: NCT04278482).
2.2. Subjects
All volunteers were recruited in the Madrid region (Spain). Recruitment was done through advertisements published on websites inviting healthy omnivores and vegetarians to participate in the study. Inclusion criteria were: adult, age 18 years or older, healthy, following omnivorous diets with a maximum fish intake of 2 portions per week or vegetarian diets for at least 6 months. Exclusion criteria were: eating disorders, diagnosis of digestive, renal, haematological, endocrine or oncological diseases, breastfeeding, pregnancy, menopause, having donated blood in the last 3 months, and for the vegetarian subjects, occasional consumption of meat or fish. Potential participants were asked about these criteria by an on-line questionnaire that included specific questions on food habits, disease diagnosis and drug treatments.Before the study, each volunteer signed an informed consent form accepting the participation in the study. All data collection and blood sampling were performed in winter time (February 2020 and March 2021).
2.3. Dietary intake and physical activity assessments
The habitual diet intake was assessed by 72-hour dietary records. Each volunteer filled an on-line questionnaire during the week prior to blood extraction at the Human Nutrition Unit of the ICTAN-CSIC. Details of all foods eaten, the type or brand of the products and the portion or weight of the food had to be specified. Dietary intake was analysed using the DIAL software (Alce Ingeniería, Madrid, Spain). This software allows the addition of the nutritional composition of products that are not included in the data base, as was the case for many vegan foods. For this purpose, data from the manufacturer was used. Additionally, there were questions about the consumption of supplements aimed at collecting information on the use of vitamin B12, proteins, multivitamins and others that could increase total daily intake.Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ) short version. Data are presented as vigorous, moderate, walking and sitting activities in min per week and total Metabolic Equivalent of Task (MET-minutes per week). The physical activity categories, low, moderate or high, for each subject were also calculated.16
2.4. Anthropometric, body composition, and blood pressure measurements
Height, body weight, and waist and hip perimeters were measured by standardised procedures and performed by a trained member of the research group. Waist and hip perimeters were measured for each volunteer, fasting and in underwear, using an anthropometric tape measure and height was assessed by a measuring rod. Body mass index (BMI) and body composition were calculated by bioimpedance (Tanita BC-601, Tanita Ltd, Amsterdam, The Netherlands). Systolic and diastolic blood pressure was measured using a validated automatic digital blood pressure monitor (IHEalth KN-550BT, iHealthLabs Europe, Paris) and the mean of two consecutive reading was recorded.2.5. Analytical determinations
After an overnight fast (at least 10 h), blood sampling was performed. Total blood was collected for haematological analyses and serum was separated by centrifugation (centrifuge Jouan CR-312, Ilkeston, UK) during 15 min at 1000g. Aliquots of serum were stored at −80° for further analyses.Haematocrit, erythrocytes, haemoglobin, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC), platelets, mean platelet volume, leukocytes, neutrophils, lymphocytes, monocytes, eosinophils, and basophils were measured using an automatic analyser.
Total cholesterol (chol), HDL-chol, LDL-chol, triglycerides, glucose and insulin were determined in serum by autoanalyzer. Serum leptin, adiponectin, TNF-α and IL-1β were determined in the subsample of subjects recruited in 2021 (n = 98) by enzyme immunoassay (DRG Instruments, Marburg, Germany).
2.6. Statistical analyses
The data were analysed using SPSS for Windows version 26.0 (IBM SPSS Statistics for Windows Armonk, NY, USA). Results are expressed as Mean ± SEM unless otherwise specified.Variable distributions were checked by the Kolmogorov–Smirnov test and visually, leptin and adiponectin were log-transformed before statistical analyses but IL-1β could not be normalized and was studied by non-parametric tests. General linear models were applied for normal variables with diet (OMN, LOV, VEG) and gender (women, men) as the fix factors. The effects of diet, gender and diet × gender interaction were studied. In addition, the possible effect of the year in which the data were taken was initially tested (2020 and 2021), however because this factor did not modify the effects of the fix factors or presented any interactions with them, it was not included in further models.
Qualitative variables were analysed by Pearson Chi square. The effects on IL-1β and the physical activity variables were studied by Kruskal–Wallis and Mann–Whitney U tests. The Bonferroni correction for multiple comparations was used.
To study the relationship between selected variables, Pearson or Spearman (if non-normal variables were included) correlations were performed.
The level of significance was set at p < 0.05.
3. Results
The Strobe guidelines are used to report this cross-sectional study (https://www.strobe-statement.org).From a total of 285 subjects interested in the study, 231 were assessed for eligibility, 13 were excluded due to different reasons, and finally 207 were selected and participated in the study. The distribution among diet groups was: OMN, n = 93; LOV, n = 55; VEG, n = 59. Women represented 62% of the total. Ethnicity was >98% Caucasian.
The characteristics of the participants are presented in Table 1. There were no significant differences among diet groups, except for age (p = 0.016) that was higher in VEG than OMN. Women tended to have lower BMI than men (p = 0.06), had lower waist perimeter but similar hip perimeter, and it was shown that the parameters related to body muscle and water contents were higher in men while those of fat were higher in women (all p < 0.001).
| Omnivore | Lacto-ovo vegetarian | Vegan | p gender | ||||
|---|---|---|---|---|---|---|---|
| Man | Woman | Man | Woman | Man | Woman | ||
| Values are Mean ± SEM. Differences due to diet group were no significant, except for age that was higher in Vegan than Omnivore (p < 0.05). There interaction diet × gender was not significant for any variable. Gender p values are shown (multivariate general linear model). | |||||||
| Age (year) | 26 ± 1 | 25 ± 1 | 24 ± 1 | 26 ± 1 | 29 ± 1 | 27 ± 1 | 0.801 |
| BMI (kg m−2) | 23.0 ± 0.0 | 22.0 ± 0.0 | 22.0 ± 1.0 | 22.0 ± 0.0 | 23.0 ± 1.0 | 22.0 ± 1.0 | 0.065 |
| Waist perimeter (cm) | 81.6 ± 1.3 | 74.2 ± 0.9 | 80.2 ± 1.5 | 74.7 ± 1.4 | 83.8 ± 1.9 | 76.3 ± 1.6 | <0.001 |
| Hip perimeter (cm) | 94.5 ± 1.1 | 95.9 ± 1.0 | 94.0 ± 1.4 | 95.6 ± 1.1 | 95.2 ± 1.2 | 96.0 ± 1.3 | 0.201 |
| Body weight (kg) | 71.3 ± 1.9 | 59.1 ± 1.1 | 69.7 ± 2.2 | 57.8 ± 1.2 | 72.2 ± 2.1 | 59.6 ± 1.7 | <0.001 |
| Abdominal muscle (kg) | 30.7 ± 0.7 | 23.8 ± 0.3 | 30.7 ± 0.9 | 23.2 ± 0.3 | 30.9 ± 0.8 | 23.9 ± 0.5 | <0.001 |
| Body muscle mass (kg) | 56.9 ± 1.2 | 41.7 ± 0.4 | 56.3 ± 1.7 | 40.4 ± 0.5 | 57.4 ± 1.3 | 41.2 ± 1.0 | <0.001 |
| Body bone mass (kg) | 3.0 ± 0.1 | 2.2 ± 0.0 | 3.0 ± 0.1 | 2.2 ± 0.0 | 3.0 ± 0.1 | 2.2 ± 0.0 | <0.001 |
| Body mass fat (%) | 15.5 ± 0.8 | 25.0 ± 0.9 | 14.2 ± 1.6 | 25.8 ± 0.9 | 15.7 ± 1.3 | 25.6 ± 1.1 | <0.001 |
| Abdominal fat (%) | 16.1 ± 1.0 | 20.1 ± 1.1 | 14.1 ± 1.6 | 21.4 ± 1.0 | 17.0 ± 1.5 | 21.7 ± 1.3 | <0.001 |
| Body water (%) | 61.1 ± 0.7 | 55.4 ± 0.6 | 62.0 ± 1.2 | 54.7 ± 0.6 | 60.2 ± 1.0 | 54.8 ± 0.8 | <0.001 |
Physical activity varies between diet groups but not between genders (Table 2). Moderate activity (min per week) was significantly higher in LOV compared to OMN. The analysis of physical activity categories showed that 6%, 35% and 59% of the volunteers in whole sample had low, moderate, and high activity levels, respectively without significant differences between diet groups.
| Omnivore | Lacto-ovo vegetarian | Vegan | p diet | ||||
|---|---|---|---|---|---|---|---|
| Man | Woman | Man | Woman | Man | Woman | ||
| Values are median (Q1–Q3) for n = 202. Diet effects were analysed by the Kruskal–Wallis test and pair differences by the Mann–Whitney U test. There were no significant gender effects (Mann–Whitney). *Significantly different compared to Omnivore.a Metabolic equivalent of task. | |||||||
| Vigorous activity (min per week) | 140 (60–270) | 90 (0–180) | 180 (83–270) | 120 (0–270) | 70 (0–225) | 45 (0–225) | 0.286 |
| Moderate activity (min per week) | 60 (0–150) | 45 (0–120) | 150 (78–180)* | 90 (0–210)* | 100 (0–180) | 60 (20–180) | 0.008 |
| Walking (min per week) | 180 (80–420) | 280 (140–420) | 298 (195–578) | 350 (120–630) | 205 (100–630) | 280 (180–490) | 0.485 |
| Sitting (min per week) | 385 (240–540) | 360 (300–480) | 450 (370–570) | 420 (240–570) | 390 (240–600) | 480 (300–600) | 0.284 |
| Total activity (METa-min per week) | 2571 (1428–5815) | 2453 (1146–3486) | 3554 (2086–4017) | 3198 (1746–4297) | 2431 (1188–6452) | 2657 (1387–3275) | 0.254 |
Table 3 shows the haematological and clinical parameters of the volunteers. Men had higher blood pressure than women (SBP, p < 0.001 and DBP, p = 0.011) and OMN had higher SBP than LOV and VEG (p = 0.04). Red blood cells and haematocrit were lower in women (both p < 0.001) than men and in the two vegetarian groups compared to OMN. This was reflected also in the haemoglobin values that were significantly lower in LOV and VEG than in OMN (p < 0.05), and the prevalence of anaemia (haemoglobin lower that 12.5 g dL−1) was 10% independently of diet group (data not shown). Although total leucocytes did not vary among diet groups, there were differences in lymphocyte counts, with LOV and VEG presenting lower values than OMN (p < 0.001), and a tendency to lower % lymphocytes and higher % monocytes in these two groups (p = 0.022 and 0.049, respectively).
| Omnivore | Lacto-ovo vegetarian | Vegan | p gender | p diet | ||||
|---|---|---|---|---|---|---|---|---|
| Man | Woman | Man | Woman | Man | Woman | |||
| SBP, systolic blood pressure; DBP, diastolic blood pressure. Values are Mean ± SEM. There were no significant diet × gender interactions, p values for gender and diet are shown (multivariate general linear model). * Significantly different compared to Omnivore (Bonferroni test). | ||||||||
| SBP (mmHg) | 134.7 ± 1.9 | 119.2 ± 1.4 | 130.0 ± 1.5* | 117.6 ± 1.8* | 128.0 ± 1.9* | 117.0 ± 2.0* | <0.001 | 0.040 |
| DBP (mmHg) | 81.4 ± 1.6 | 76.3 ± 1.2 | 80.4 ± 2.1 | 76.2 ± 1.3 | 77.8 ± 2.0 | 76.4 ± 1.7 | 0.011 | 0.541 |
| Heart rate (bpm) | 70.5 ± 2.1 | 77.2 ± 1.9 | 76.3 ± 4.5 | 75.0 ± 2.0 | 72.5 ± 2.6 | 76.2 ± 1.7 | 0.135 | 0.751 |
| Red blood cells (106 mm−3) | 5.3 ± 0.1 | 4.6 ± 0.0 | 5.2 ± 0.1* | 4.5 ± 0.1* | 5.1 ± 0.1* | 4.4 ± 0.1* | <0.001 | 0.035 |
| Haematocrit (%) | 46.4 ± 0.4 | 40.9 ± 0.4 | 45.6 ± 0.6* | 39.7 ± 0.4* | 45.3 ± 0.7* | 39.7 ± 0.6* | <0.001 | 0.039 |
| Leucocytes (×103 mm−3) | 5.6 ± 0.2 | 5.9 ± 0.2 | 5.8 ± 0.3 | 5.3 ± 0.2 | 5.2 ± 0.2 | 5.4 ± 0.2 | 0.949 | 0.142 |
| Neutrophils (×103 mm−3) | 3.0 ± 0.1 | 3.3 ± 0.2 | 3.4 ± 0.4 | 3.3 ± 0.2 | 2.9 ± 0.2 | 3.2 ± 0.2 | 0.827 | 0.771 |
| Lymphocytes (×103 mm−3) | 2.0 ± 0.1 | 2.0 ± 0.1 | 1.7 ± 0.1* | 1.6 ± 0.1* | 1.7 ± 0.1* | 1.6 ± 0.1* | 0.809 | <0.001 |
| Monocytes (×103 mm−3) | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.078 | 0.160 |
| Eosinophils (×103 mm−3) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.195 | 0.554 |
| Neutrophils (%) | 53.7 ± 1.0 | 54.8 ± 1.2 | 58.3 ± 2.6 | 56.2 ± 1.7 | 55.4 ± 2.5 | 57.4 ± 1.5 | 0.816 | 0.156 |
| Lymphocytes (%) | 34.9 ± 0.9 | 33.9 ± 1.1 | 30.1 ± 2.2 | 31.7 ± 1.5 | 32.2 ± 2.2 | 30.8 ± 1.3 | 0.838 | 0.022 |
| Monocytes (%) | 7.8 ± 0.3 | 7.6 ± 0.2 | 8.8 ± 0.5 | 8.2 ± 0.3 | 8.7 ± 0.4 | 7.9 ± 0.4 | 0.086 | 0.049 |
| Eosinophils (%) | 2.8 ± 0.3 | 2.9 ± 0.3 | 2.2 ± 0.4 | 3.1 ± 0.5 | 3.1 ± 0.5 | 3.0 ± 0.3 | 0.359 | 0.734 |
Daily intake of energy, fibre, total fat, proteins, and carbohydrates was significantly higher in men than women (all p < 0.001). Fig. 1 shows the dietary intake of the volunteers according to diet group and gender. Men ingested slightly more cholesterol than women but the differences were only significant within OMN (p = 0.008). Macronutrient profiles were similar in men and women (values as % energy). Although total energy intake was similar in the three diet groups, there were marked differences in the rest of variables (p < 0.001). Cholesterol intake declined in the order OMN, LOV, and VEG, and it was higher intake of fibre and carbohydrates and lower intake of proteins and fat in the two vegetarian groups compared to OMN.
Concerning the use of supplements, vitamin B12 supplements were used by 81% of VEG, 54% of LOV, and 5% of OMN (p < 0.001). Protein supplements were used by less than 10% of the subjects (6% OMN, 9%LOV, 3% VEG), without significant differences between diet groups, and the use of other supplements such as iron, calcium or vitamin D was much lower (<3%).
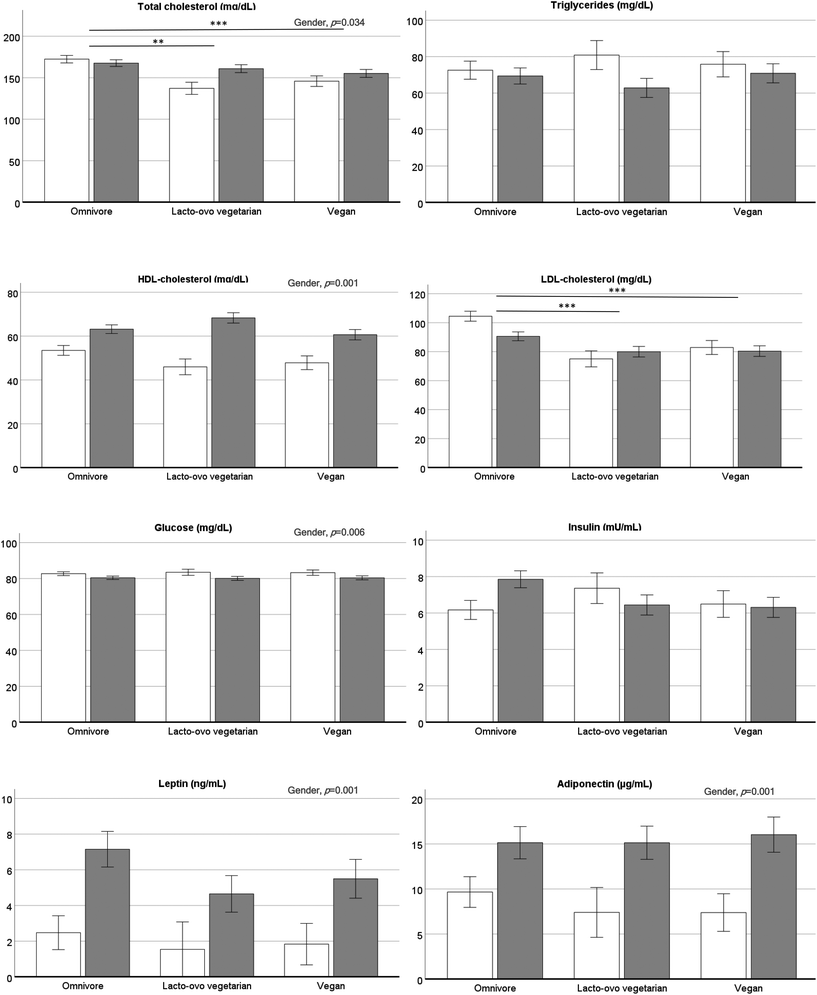
Serum levels of lipids, glucose and the hormones insulin, leptin and adiponectin are presented in Fig. 2. There were gender differences in total-chol (p = 0.034), HDL-chol (p < 0.001), glucose (p = 0.006), leptin (p < 0.001), and adiponectin (p < 0.001). Diet significantly affected total-chol and LDL-chol (both p < 0.001), that were lower in LOV and VEG compared to OMN.
The risk indexes (Table 4) total-chol/HDL-chol, LDL-chol/HDL, and the molar ratio triglycerides/HDL-chol were significantly lower in women than men, but there were no differences due to diet, except for LDL-chol/HDL-chol that was significantly lower in LOV and VEG compared to OMN (p = 0.010). The insulin resistance (HOMA-IR) and insulin sensitivity (QUICKI) indexes did not show significant differences. Concerning the inflammation biomarkers, TNF-α was slightly higher in men than women (p = 0.055) and was unaffected by diet. In contrast, IL-1β showed no gender effect and a significant diet effect (p = 0.010), with lower values in LOV than OMN.
| Omnivore | Lacto-ovo vegetarian | Vegan | p gender | p diet | ||||
|---|---|---|---|---|---|---|---|---|
| Man | Woman | Man | Woman | Man | Woman | |||
| Values are Mean ± SEM for n = 207, except TNF-α and IL-1β which are median (Q1–Q3) for n = 98. The effects of gender, diet and interaction gender × diet were tested by general linear models, except for # that were studied by Kruskal–Wallis and Mann–Whitney U tests. *Significantly different compared to Omnivore. | ||||||||
| Total chol/HDL | 3.36 ± 0.14 | 2.74 ± 0.08 | 3.11 ± 0.25 | 2.43 ± 0.07 | 3.14 ± 0.18 | 2.63 ± 0.08 | <0.001 | 0.073 |
| LDL-chol/HDL-chol | 2.06 ± 0.12 | 1.51 ± 0.07 | 1.73 ± 0.20* | 1.24 ± 0.07* | 1.81 ± 0.15* | 1.37 ± 0.06* | <0.001 | 0.010 |
| Triglycerides/HDL-chol (mol mol−1) | 0.65 ± 0.07 | 0.50 ± 0.03 | 0.84 ± 0.15 | 0.43 ± 0.03 | 0.74 ± 0.09 | 0.56 ± 0.05 | <0.001 | 0.373 |
| HOMA-IR | 1.15 ± 0.07 | 1.42 ± 0.11 | 1.37 ± 0.15 | 1.15 ± 0.09 | 1.25 ± 0.21 | 1.14 ± 0.10 | 0.838 | 0.747 |
| QUICKI | 0.38 ± 0.00 | 0.37 ± 0.00 | 0.36 ± 0.01 | 0.38 ± 0.01 | 0.38 ± 0.01 | 0.38 ± 0.01 | 0.934 | 0.132 |
| TNF-α (pg mL−1) | 11.6 ± 0.8 | 9.9 ± 0.4 | 10.9 ± 1.0 | 9.4 ± 0.7 | 10.9 ± 0.7 | 10.7 ± 0.5 | 0.055 | 0.645 |
| IL-1β (pg mL−1)# | 14.5 (9.8–26.2) | 17.4 (11.1–21.9) | 9.5 (3.9–20.3)* | 9.7 (7.6–15.4)* | 11.5 (10.1–15.0) | 14.1 (9.3–18.9) | 0.940 | 0.010 |
Insulin and leptin were highly correlated (r = 0.376, p < 0.001) and both show positive correlations with % body fat mass (r = 0.258, p < 0.001 and r = 0.686, p < 0.001) and abdominal fat (r = 0.255, p < 0.001 and r = 0.630, p < 0.001). Leptin was also negatively correlated with body muscle mass (r = −0.292, p < 0.001), abdominal muscle (r = −0.238, p < 0.001) and % body bone mass (r = 0.295, p < 0.001). Adiponectin showed positive correlations with % body fat mass (r = 0.321, p = 0.001) and abdominal fat (r = 0.228, p = 0.023), and negative correlations with body muscle mass, abdominal muscle, and bone mass (r = −0.340, r = −0.219, r = −0.339, all p < 0.001). TNF-α was negatively correlated with % neutrophils (Spearman rho = −0.212, p = 0.034) and IL-1β was positively correlated with lymphocytes count (Spearman rho = 0.248, p = 0.014) and % lymphocytes (Spearman rho = 0.643, p < 0.001).
4. Discussion
This cross-sectional study is the first one performed in Spain and presents body characteristics, nutritional intake, usual physical activity, as well as different haematological and biochemical biomarkers, including selected hormones and inflammatory cytokines, in vegetarians, LOV and VEG, compared to omnivores.Participants were young adults who lived in an urban environment and did not declare any disease. As expected, the two vegetarian diets involved lower intake of cholesterol and fat and higher intake of fibre and carbohydrates than the omnivorous diet. Protein intake was also lower in these groups compared to the control. There were marked differences in dietary intake between groups, indeed, VEG ingested virtually no cholesterol and their diet differed from that of LOV in macronutrient profile with more energy from carbohydrates and less from fat (Fig. 1). But being vegan instead of lacto-ovo vegetarian appears to lead to minor changes in the study biomarkers. This could be partly explained because VEG seemed to be more conscious than LOV of the limitations of their diets, for instance, the majority of VEG took vitamin B12 supplements, while only about half of LOV took them (81% versus 54%).
This behaviour of LOV is was not expected as the consumption of vitamin B12 supplements resulted lower than in our previous study, in which we found that 75% of lacto-ovo vegetarians and 70% of vegans were vitamin B12 users, and it was confirmed that consumption of this supplement was clearly associated with a higher cobalamin status.17 The different figures in the two studies could be explained by the way the volunteers were recruited, in the previous survey advertisements were done through a dietitian clinic and in the current study it was through university campus and vegetarian associations. Therefore, vegetarians but particularly LOV should be encouraged to consume vitamin B12 supplements as all information on nutritional recommendations for them emphasize the importance of taking vitamin B12 supplements.
Concerning the biomarkers analysed in this study, and consistent with different findings in vegetarians,7,14,18 total-chol and LDL-chol levels were lower in LOV and VEG than in OMN and were similar in the two vegetarian groups, thus avoiding consumption of eggs and dairy products does not involve further cholesterol reductions. Results clearly show that there is no parallelism between cholesterol intake and serum levels, and that there is a threshold around 150 mg dL−1 that represents endogenous cholesterol production. In fact, VEG with negligible cholesterol intake, had similar serum total-chol and LDL-chol than LOV and in turn OMN, whose estimated cholesterol intake was between 300 and 400 mg day−1, presented serum total-chol below the lower recommended level of 200 mg dL−1. Therefore, taking into account the values of triglycerides, glucose and the very well-known cardiovascular indexes (LDL-chol/HDL-chol and T-chol/HDL-chol) and those related with insulin resistance (HOMA-IR and triglycerides/HDL-chol) and sensitivity (QUICKI),19 we can conclude that all three diet groups had low cardiometabolic risk.
In this work there were no differences in leptin and adiponectin between vegetarians and non-vegetarians (Fig. 2). It was confirmed that both hormones were higher in women as it is known that there is a relationship with body fat.20,21 When lower leptin levels have been observed in vegetarians than omnivores, there were also differences in body weight between the test groups.21 In the present study, OMN, LOV and VEG consumed isocaloric diets and presented similar BMI and body composition, which explained the obtained leptin and adiponectin results. Clearly, our correlation results confirmed the relationship of these two hormones with body composition but not with the type of diet consumed.
In addition to the usual diet, other lifestyle factors such as physical activity, were evaluated, and the obtained results are in line to the WHO guidelines on physical activity and sedentary behaviour, with LOV subjects having more moderate activity than OMN although total METs-min per week did not differ between groups. Current guidelines recommend 150–300 min per week of moderate-intensity or 75–150 min per week of vigorous-intensity physical activity or a combination of both.16 Therefore, volunteers performed weekly more physical activity than the minimum recommended. Indeed, nearly 60% of them, independently of their usual diet, were classified in the high activity category.
With regard to haematological results and in agreement with our previous observations, red blood cells and haematocrit were in the normal range but at the lowest level22 and both parameters were lower in vegetarians compared to non-vegetarians. Men had more erythrocytes than women, as expected considering the age of the volunteers, and all subjects with low haemoglobin were women. In this regard, in addition to the intake of iron and its bioavailability, it was previously reported that iron status is inversely related to the intensity of menstrual blood losses.22,23 The use of oral contraceptives, by reducing menstrual losses, have been also shown to improve iron status in vegetarian and non-vegetarian women.23,24 Therefore, vegetarian women should be aware of the risks of iron deficiency and apply dietary strategies to increase iron bioavailability or use appropriate supplements.
Generally, decreases in total white blood cell counts or in neutrophils, lymphocytes and monocytes in vegetarians compared to meat eaters have been observed.25–27 This has been attributed to the low intake of branched-chain amino acids from plant-based diets, which are involved in the differentiation of these blood cells. Consistently, in our study lymphocyte counts were lower with a tendency to lower % lymphocyte and higher % monocyte in the two vegetarian groups compared to OMN. Low leukocyte numbers are associated with low inflammation and decrease risk of chronic diseases but in contrast can compromise immunity. In this line, a direct correlation was observed between lymphocytes and the inflammatory cytokine IL1-β, that was lower in LOV than OMN. However, TNF-α was similar in the three diet groups and it should be noticed than mean values were all in the normal reference ranges including those of TNF-α and IL1-β.
In recent years there has been a growing communication on the beneficial effects of plant-based diets and in parallel the number of vegetarians is increasing. This leads to the development of a huge variety of new food formulations and ingredients, as well as food supplements. This cross-sectional study was the first one performed in Spain and describes the nutritional intake, body composition, haematological and cardiometabolic biomarkers, physical activity, and use of supplements of consumers of these diets. It should be pointed out that mean/median values of all parameters were in the normal range, and considering the different charts of cardiovascular disease risk,28–30 the studied population should be classified at low risk.
The strengths of the study are the sample size, with three diet groups that resulted homogeneous in BMI, energy intake and lifestyle habits apart from the diet; the subjects were self-classified in one of the diet groups (omnivore, lacto-ovo vegetarian or vegan) and the classification was validated; both genders were included; and the analytical determinations were carried out under strict methodological conditions. However, there were several limitations, mainly due to the study design where exposure and outcome measures were collected at the same time, thus it is impossible to infer any cause and effect relationship. It should be also recognized as a limitation that the diet groups did not have the same number of participants.
Present results constitute the first report on biomarker levels of apparently healthy consumers of plant-based diets in Spain. Of note, these consumers follow vegetarian diets in a country where the Mediterranean diet is widespread followed and promoted. Nevertheless, some individuals should be at risk of nutrient deficiencies, and for those the use of specific supplements are recommended. Further long-term studies on biomarker changes and disease incidence are needed to confirm these results.
Abbreviations
| BMI | Body mass index |
| Chol | Cholesterol |
| DBP | Diastolic blood pressure |
| HDL-chol | High density cholesterol |
| HOMA-IR | Homeostatic model assessment-insulin resistance |
| IL-1β | Interleukin-1β |
| LDL-chol | Low density-cholesterol |
| MET | Metabolic equivalent task |
| QUICKI | Quantitative insulin sensitivity check index |
| SBD | Systolic blood pressure |
| TNF-α | Tumour necrosis factor-alpha |
Author contributions
MPV, conceptualization; all authors, investigation; EG-M, data curation; MPV and EG-M, formal statistical analyses; EG-M and MPV, writing – original draft; MPV, funding acquisition; MPV, supervision; all authors revised and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by a Development of Industrial Doctorate project from the Community of Madrid (IND2018/BIO-9554). Recipients were CSIC and Zamdeh Laboratories and E.G.-M. was funded for her industrial doctorate. Miriam Martínez is thanked for helping with volunteer's management and data files and Ignacio de Tomas is thanked for helping with dietary assessment. The authors are grateful to all volunteers for their collaboration. Open Access APCs supported by CSIC agreement with Royal Society of ChemistryReferences
- FAO and WHO, Sustainable healthy diets: guiding principles, Rome, 2019 Search PubMed.
- V. Melina, C. Winston and L. Susan, Position of the Academy of Nutrition and Dietetics: Vegetarian Diets, Acad. Nutr. Diet., 2016, 116, 11 Search PubMed.
- M. E. Nelson, M. W. Hamm, F. B. Hu, S. A. Abrams and T. S. Griffin, Alignment of Healthy Dietary Patterns and Environmental Sustainability: A Systematic Review, Adv. Nutr., 2016, 7, 1005–1025 CrossRef PubMed.
- D. R. Bakaloudi, A. Halloran, H. L. Rippin, A. C. Oikonomidou, T. I. Dardavesis, J. Williams, K. Wickramasinghe, J. Breda and M. Chourdakis, Intake and adequacy of the vegan diet. A systematic review of the evidence, Clin. Nutr., 2021, 40, 3503–3521 CrossRef CAS PubMed.
- J. Aschemann-Witzel, R. F. Gantriis, P. Fraga and F. J. A. Perez-Cueto, Plant-based food and protein trend from a business perspective: markets, consumers, and the challenges and opportunities in the future, Crit. Rev. Food Sci. Nutr., 2020, 1–10 Search PubMed.
- A. Alcorta, A. Porta, A. Tárrega, M. D. Alvarez and M. P. Vaquero, Foods for Plant-Based Diets: Challenges and Innovations, Foods, 2021, 10, 293 CrossRef CAS PubMed.
- A. Del Re and K. Aspry, Update on Plant-Based Diets and Cardiometabolic Risk, Curr. Atheroscler. Rep., 2022, 24, 173–183 CrossRef PubMed.
- Lantern, The Green Revolution 2019, https://www.lantern.es/papers/the-green-revolution-2019, (accessed November 30, 2020).
- A. L. Elorinne, G. Alfthan, I. Erlund, H. Kivimäki, A. Paju, I. Salminen, U. Turpeinen, S. Voutilainen and J. Laakso, Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians, PLoS One, 2016, 11, e0148235 CrossRef PubMed.
- W. J. Craig, A. R. Mangels, U. Fresán, K. Marsh, F. L. Miles, A. V. Saunders, E. H. Haddad, C. E. Heskey, P. Johnston, E. Larson-Meyer and M. Orlich, The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals, Nutrients, 2021, 13, 4144 CrossRef CAS PubMed.
- Y. Yokoyama, S. M. Levin and N. D. Barnard, Association between plant-based diets and plasma lipids: a systematic review and meta-analysis, Nutr. Rev., 2017, 75, 683–698 CrossRef PubMed.
- H. Kahleova, M. Matoulek, H. Malinska, O. Oliyarnik, L. Kazdova, T. Neskudla, A. Skoch, M. Hajek, M. Hill, M. Kahle and T. Pelikanova, Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes, Diabetic Med., 2011, 28, 549–559 CrossRef CAS PubMed.
- A. Gallego-Narbon, B. Zapatera, L. Barrios and M. P. Vaquero, Vitamin B-12 and folate status in Spanish lacto-ovo vegetarians and vegans, J. Nutr. Sci., 2019, 8, e7, DOI:10.1017/jns.2019.2.
- A. M. Salvador, E. García-Maldonado, A. Gallego-Narbón, B. Zapatera and M. P. Vaquero, Fatty Acid Profile and Cardiometabolic Markers in Relation with Diet Type and Omega-3 Supplementation in Spanish Vegetarians, Nutrients, 2019, 11, 1659 CrossRef CAS PubMed.
- E. García-Maldonado, A. Alcorta, B. Zapatera and M. P. Vaquero, Changes in fatty acid levels after consumption of a novel docosahexaenoic supplement from algae: a crossover randomized controlled trial in omnivorous, lacto-ovo vegetarians and vegans, Eur. J. Nutr., 2022, 23, 1–15 Search PubMed.
- World Health Organization, WHO guidelines on physical activity and sedentary behaviour, Geneva, 2020 Search PubMed.
- A. Gallego-Narbón, B. Zapatera, I. Álvarez and M. P. Vaquero, Methylmalonic Acid Levels and their Relation with Cobalamin Supplementation in Spanish Vegetarians, Plant Foods Hum. Nutr., 2018, 73, 166–171 CrossRef PubMed.
- M. Dinu, R. Abbate, G. F. Gensini, A. Casini and F. Sofi, Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies, Crit. Rev. Food Sci. Nutr., 2017, 57, 3640–3649 CrossRef PubMed.
- M. P. Vaquero, M. Martínez-Suárez, Á. García-Quismondo, F. J. del Cañizo and F. J. Sánchez-Muniz, Diabesity negatively affects transferrin saturation and iron status. The DICARIVA study, Diabetes Res. Clin. Pract., 2021, 172, 108653 CrossRef PubMed.
- M. Vučić Lovrenčić, M. Gerić, I. Košuta, M. Dragičević, V. Garaj-Vrhovac and G. Gajski, Sex-specific effects of vegetarian diet on adiponectin levels and insulin sensitivity in healthy non-obese individuals, Nutrition, 2020, 79–80, 110862 CrossRef PubMed.
- P. Gogga, A. Śliwińska, E. Aleksandrowicz-Wrona and S. Małgorzewicz, Association between different types of plant-based diets and leptin levels in healthy volunteer, Acta Biochim. Pol., 2019, 66, 77–82 CAS.
- A. Gallego-Narbón, B. Zapatera and M. P. Vaquero, Physiological and Dietary Determinants of Iron Status in Spanish Vegetarians, Nutrients, 2019, 11, 1734 CrossRef PubMed.
- R. Blanco-Rojo, L. Toxqui, A. M. López-Parra, C. Baeza-Richer, A. M. Pérez-Granados, E. Arroyo-Pardo and M. P. Vaquero, Influence of Diet, Menstruation and Genetic Factors on Iron Status: A Cross-Sectional Study in Spanish Women of Childbearing Age, Int. J. Mol. Sci., 2014, 15, 4077–4087 CrossRef CAS PubMed.
- L. Toxqui, A. M. Pérez-Granados, R. Blanco-Rojo, I. Wright and M. P. Vaquero, A simple and feasible questionnaire to estimate menstrual blood loss: relationship with hematological and gynecological parameters in young women, BMC Women's Health, 2014, 14, 71 CrossRef PubMed.
- J. C. Craddock, E. P. Neale, G. E. Peoples and Y. C. Probst, egetarian-Based Dietary Patterns and their Relation with Inflammatory and Immune Biomarkers: A Systematic Review and Meta-Analysis, Adv. Nutr., 2019, 10, 433–451 CrossRef PubMed.
- T. Y. N. Tong, T. J. Key, K. Gaitskell, T. J. Green, W. Guo, T. A. Sanders and K. E. Bradbury, Hematological parameters and prevalence of anemia in white and British Indian vegetarians and nonvegetarians in the UK Biobank, Am. J. Clin. Nutr., 2019, 110, 461–472 CrossRef PubMed.
- A.-K. Lederer, A. Maul-Pavicic, L. Hannibal, M. Hettich, C. Steinborn, C. Gründemann, A. M. Zimmermann-Klemd, A. Müller, B. Sehnert, U. Salzer, R. Klein, R. E. Voll, Y. Samstag and R. Huber, Vegan diet reduces neutrophils, monocytes and platelets related to branched-chain amino acids – A randomized, controlled trial, Clin. Nutr., 2020, 39, 3241–3250 CrossRef CAS PubMed.
- WHO CVD Risk Chart Working Group, World Health Organization cardiovascular disease risk charts: revised models to estimate risk in 21 global regions, Lancet Global Health, 2019, 7, e1332–e1345 CrossRef PubMed.
- R. M. Conroy, K. Pyörälä, A. P. Fitzgerald, S. Sans, A. Menotti, G. De Backer, D. De Bacquer, P. Ducimetière, P. Jousilahti, U. Keil, I. Njølstad, R. G. Oganov, T. Thomsen, H. Tunstall-Pedoe, A. Tverdal, H. Wedel, P. Whincup, L. Wilhelmsen, I. M. Graham and on behalf of the SCORE project group, Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE Project, Eur. Heart J., 2003, 24, 987–1003 CrossRef CAS PubMed.
- S. Sans, A. P. Fitzgerald, D. Royo, R. Conroy and I. Graham, Calibrating the SCORE Cardiovascular Risk Chart for Use in Spain, Rev. Esp. Cardiol., 2007, 60, 476–485 CrossRef.
| This journal is © The Royal Society of Chemistry 2023 |