Open Access Article
Open Access ArticleOral intake of collagen peptide NS improves hydration, elasticity, desquamation, and wrinkling in human skin: a randomized, double-blinded, placebo-controlled study
Miyeong
Lee
a,
Eunjoung
Kim
b,
Hyunwoo
Ahn
 c,
Seokjun
Son
d and
Hyunjun
Lee
c,
Seokjun
Son
d and
Hyunjun
Lee
 *d
*d
aMariedm Co., Ltd., 14, Pungseong-ro, Gangdong-gu, Seoul, Republic of Korea
bCorederm Co., Ltd., 56, Jungdae-ro, Songpa-gu, Seoul, Republic of Korea
cDepartment of Food Science and Biotechnology, Dongguk University-Seoul, 32, Dongguk-ro, Ilsandong-gu, Goyang-si, Gyeonggi-do 410-820, Republic of Korea
dResearch & Development Center, Nong Shim Co., Ltd., 112, Yeouidaebang-ro, Dongjak-gu, Seoul, Republic of Korea. E-mail: lee33@nongshim.com; Tel: +82-10-9139-4280
First published on 1st March 2023
Abstract
Collagen hydrolysate, which contains bioactive peptides, is used as a dietary supplement for the refinement of elasticity, hydration, desquamation, and wrinkling of aging human skin. Here, we conducted a double-blind, randomized, and placebo-controlled oral administration study on the effects of a collagen peptide (CPNS) containing dipeptides, including Gly-Pro and Pro-Hyp, on skin wrinkling, desquamation, elasticity, and hydration. Our results show that an intake of 1650 mg per day of CPNS for 12 weeks had beneficial effects on skin health in a cohort of women aged from 30 to 60 years (n = 100). Compared with the placebo group, skin desquamation, hydration, skin wrinkling, and elasticity were significantly improved after 4, 4, 12, and 12 weeks of administration, respectively. In a safety test of CPNS ingestion, none of the participants showed any side effects during the clinical study period. These results demonstrate that the low molecular weight bioactive peptides contained in CPNS, such as Gly-Pro and Pro-Hyp, exert positive effects on skin hydration, elasticity, desquamation, and wrinkling.
1. Introduction
Various environmental and endogenous factors influence skin damage and aging. The two main layers of human skin are the dermis and the epidermis, constituting connective tissue and stratified squamous epithelium, respectively. The extracellular matrix (ECM) elements of the dermis layer, such as hydroxyapatite, collagen, glycosaminoglycan (GAG), and enzymes are mostly generated by fibroblasts.1–3 Wrinkling, coarse skin, dryness, telangiectasia, pigmental degradation, and decrease of tensile strength due to photoaging are visible because of a decrease in metabolic reactions and include various changes in dermal ceramide and collagen.4,5 A recently emerging trend in the management of skin care is the intake of dietary supplements to enhance the arrangement and appearance of the skin “from the inside out”.Previous studies have demonstrated that oral intake of enzymatically hydrolyzed collagen peptides can have positive effects on skin health, such as dermal ECM generation, fibroblast growth, antioxidation, recovery, elasticity, and wrinkle reduction.6–10 Particularly, gelatin- and collagen-oriented hydrolysates are widely applied these days and are produced in a variety of fields, such as functional foods, nutraceuticals, medical supplies, and the biotech industry.11–13 The molecular weight of gelatin, a water-soluble polymer, is considerably higher than that of collagen hydrolysates, and it is produced by enzymatically hydrolyzing collagen that is normally used as a food ingredient and delivery carrier for the regulated distribution of bioactivities.14–17 When the high molecular weight gelatin is hydrolyzed by treating with a particular fungal or bacterial protease, the outputs, small molecular weight peptides, are conveniently called collagen hydrolysates.11,12,18 There is growing evidence indicating that collagen peptides improve not only human skin indicators associated with aging, such as hydration, wrinkles, and elasticity, but also the antihypertensive effect in spontaneously hypertensive rats (SHRs).19 They also reduce skin cellulite20 and osteoporosis and joint pain21–23 and have a positive effect on hair follicles and cell proliferation.24 The effectiveness of collagen hydrolysates in improving damaged skin has been demonstrated, yet the acknowledgment of the beneficial effects and functions of low molecular weight bioactive proteins and peptides from collagen hydrolysates is still low.14,25 Enzymatically produced bioactive peptides are characterized as a combination of short sequences of amino acid monomers bound via peptide bonds, which invigorate functional effects and may control the homeostasis system.26,27 After oral administration of collagen hydrolysate, the bioactive peptides hydroxypropyl-glycine (Hyp-Gly) and prolyl-hydroxyproline (Pro-Hyp) are distributed at high levels throughout the bloodstream, indicating that they play a crucial role in the improvement of skin status.28,29 The Gly-Pro dipeptide is mainly produced as an end product of enzymatic hydrolysis of Gly-Pro, alongside other peptide products (Gly-Pro-X).30 The Gly-Pro dipeptide is highly resistant to proteases of the intestine and hydrolysis by enterocytes, which result in part permeation into the human bloodstream.31 However, particular receptors for Gly-Pro have not been identified, and the functional efficacy of Gly-Pro or Gly-Pro-containing collagen peptides has not been thoroughly studied so far.32
As previously mentioned, lots of research related to collagen hydrolysates and bioactive peptides is being actively conducted. However, in vivo testing has limitations, and the difference in physiological function between mice and humans is difficult to prove. More research is needed to verify the efficacy and safety of collagen hydrolysate intake by conducting controlled clinical trials.4,33,34 In some clinical studies, the oral administration of collagen hydrolysates was ineffective against photoaging skin,35,36 and the number of volunteers or the completeness of the design was insufficient.37 Other clinical trials were also conducted with collagen hydrolysates extracted from pigs24,38–40 or chickens,41,42 not from fish. In addition, some clinical studies have proved that simply enzymatically hydrolyzed collagen products without revealing the bioactive peptide component exert beneficial effects on skin hydration.36,38,43,44 Above all, most clinical studies conducted oral supplementation with physiologically active ingredients such as antioxidants, vitamins, and collagen hydrolysates rather than collagen hydrolysate intake alone.37,45–48 To the best of our knowledge, this is the first study to estimate the photoaged skin improvement efficacy through a randomized, double-blinded, and placebo-controlled clinical study design using fish-derived collagen hydrolysates containing specific bioactive dipeptide forms, Gly-Pro, and Pro-Hyp, not combined with any antioxidants and vitamins.
Here, a collagen peptide product produced by Nongshim Co., Ltd, Seoul, Korea, CPNS, was used to demonstrate the physiochemical properties of collagen hydrolysates. The main source of CPNS is fish scales, and it is manufactured by employing specific fungal proteases. The composition of CPNS, with Pro-Hyp and Gly-Pro as its main components, distinguishes it from other commercial collagen peptides produced using collagenase. In an earlier in vivo study, hairless mice with photoaging induced by ultraviolet ray exposure for 12 weeks were orally administered with CPNS and showed improvements in wrinkle-related indicators, such as wrinkle formation and epidermal thickness, and moisturization-related indicators, such as moisture loss and moisture content, which surpassed the results observed in control animals.10 In addition, in an in vitro test, the CPNS spurred hyaluronic acid (HA) production in human dermal fibroblasts (HDF) by stimulation of HAS2 transcription.10 The purpose of this clinical study was thus to estimate the anti-photoaging efficacy of CPNS in the human dermis and determine its effect on the process of skin aging.
2. Materials and methods
2.1 Preparation of collagen hydrolysate and determination of the dose
The fish collagen hydrolysate CPNS was manufactured by the Research and Development (R&D) center of Nongshim Co., Ltd (Seoul, Korea). Concisely, CPNS was derived from tilapia (Oreochromis niloticus) fish scales that were enzymatically degraded with multiple fungal proteases (Amano Enzyme Inc., Nagoya, Japan). In a previous study, the main collagen peptides of CPNS were identified by utilizing a dual-piston pump preparative high-pressure liquid chromatography system (prep-HPLC, Gilson, Inc., Middleton, USA). Moreover, the low molecular weight peptide fractions below 500 Da were analyzed by liquid chromatography/tandem mass spectrometry (LC-MS/MS, Thermo Fisher Scientific, Waltham, USA) to identify the specific peptide sequences. Unlike other studied collagen hydrolysates,12,49–51 CPNS contains 4% Gly-Pro as its representative peptide10, whereas the other short-chain peptides existed in minor quantities (data not shown). Based on the efficient dose determined in a previous study,10 the titrated dose for humans was estimated utilizing the body surface area (BSA) normalization method.52,53 Based on a dose of 300 or 500 mg per kg per day used in mice, 1650 mg per day for humans was determined as the titrated dose for clinical trials, adapted to 60 kg, the average body weight of an adult.2.2 Clinical study design
This study was conducted to evaluate human skin hydration, desquamation, wrinkling, and elasticity after 12 weeks of daily intake of CPNS, following a randomized, double-blind, placebo-controlled supplementation design. All study procedures were in line with the applicable Good Clinical Practice guidelines and the standard operating procedures of COREDERM Inc, the skin research center (Seoul, Korea), and clinical research organizations in the bio-food sector.54 The clinical protocol was recognized by the institutional review board of COREDERM Inc. Skin Research Center in Seoul, Korea. For reference, the approval number is CDE-1700-020. All participants were informed about the study details and provided written informed consent. Participants were assigned to two groups based on block randomization, the CPNS group and the placebo group. A 3300 mg test product (tablet) contained 1650 mg of CPNS along with vehicle ingredients. The placebo product (tablet) had the same cellulose, flavor, and excipients for tablet figuration as the test formulation, except for CPNS (Table 1).| Ingredients | Test | Placebo | ||
|---|---|---|---|---|
| Content (mg) | Content (%) | Content (mg) | Content (%) | |
| CPNS | 1650 | 50 | 0 | 0 |
| Cellulose | 1485 | 45 | 3135 | 95 |
| Excipients | 132 | 4 | 132 | 4 |
| Flavor | 33 | 1 | 33 | 1 |
| Total | 3300 | 100 | 3300 | 100 |
2.3 Study participants
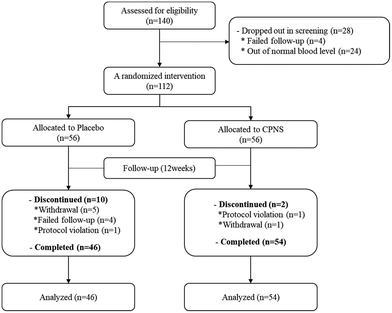
We recruited a total of 140 women (aged from 30 to 60 years) who were mentally and physically healthy, had dry facial skin (water content ≤ 49) and crow's feet, which refer to wrinkles around the eyes greater than grade 3, and satisfied all inclusion and exclusion criteria (Table 2). Ahead of the intervention, we confirmed that the participants were willing and able to visit the center on the prescribed days and follow the schedule and all regulations. During the screening phase, 28 volunteers were excluded because the levels of blood parameters were not in the normal range or because they withdrew consent. A total of 112 participants were administered either the test product (with CPNS included) or the placebo (without CPNS included), and among the 112 participants, 12 participants were withdrawn from the study later because of either being lost to follow-up or due to protocol violations. Finally, 100 participants completed the study in good condition (Fig. 1). From the aspect of safety, before and 12 weeks after administration, all of the participants took a blood test (WBC, RBC, hemoglobin, platelet, T Protein, albumin, T Bil, SGOT, SGPT, total cholesterol, triglyceride (TG), glucose, BUN, creatinine), and we surveyed concomitant medication and medical history (Table 3). Also, to verify that the diet was maintained as usual during the study period, we recorded that the subjects were required to bring a self-assessment of their usual diet to the dietary recording sheet provided 3 days before (−3 days), 4, 8, and 12 weeks after the product intake. In addition, physical examination and body weight analysis and analysis of BMI at baseline (0 W) and 12 weeks after the product intake were performed. We confirmed that the results of the blood test were in normal ranges of all blood parameters in all the participants even though some parameters had a significant difference between baseline and 12 weeks after administration. And weight, BMI, and the diet of the participants were not a significant difference between the test and placebo group after administration (Table 4). Moreover, participants were advised not to alter their daily skincare routine, not to undergo dermatological procedures on the test areas of their face, and not to make changes to their lifestyle and dietary habits, daily skincare products, or dietary supplements. They were also told not to be exposed to excessive sunlight (ex: outdoor activities such as outdoor swimming, skiing, hiking, and long-term travel) during the study periods (Table 3).| Inclusion criteria |
|---|
| • Female or male subjects aged between 25 and 60 with eye wrinkles and pigmentation on the face that began or had already been conducted (visual assessment grade 3 or higher). |
| • Healthy subjects who are free from acute and chronic diseases including skin ailment. |
| • Those who were briefed in detail by the investigator about the purpose and contents of the test and possible occurrences of adverse reactions and who willingly signed the informed consent form. |
| • Those who can do a follow-up observation during the testing period. |
| Exclusion criteria |
| • Those who are pregnant and feed or have a pregnancy plan within 24 weeks. |
| • Those who take oral contraceptives, hormones, and diuretics. |
| • Those who have received treatment for oral steroids or retinoids, topical steroids ointment within 24 weeks before the start of the study. |
| • Those who have participated in a similar test within 24 weeks before the start of the study. |
| • Those who have skin matters on the test site like moles, pimples, red spots, scalds (burns), or scars. |
| • Those who have used or taken similar effective cosmetic and topical products or supplements on the test site within 12 weeks before starting this study. |
| • Those who got a treatment of surgery (skin dermabrasion, Botox, laser, skincare, etc.) on test site within 24 weeks. |
| • Those who have a chronic or wasting disease (asthma, diabetes, high blood pressure, etc.) |
| • Those who smoke or quit smoking for less than a year. |
| • Those who have a psychiatric disease and an infectious skin disease. |
| • Those who have a specific allergic reaction to foods that are related to the test product. |
| • Those who have experience in drug-sensitive and allergic reactions. |
| • Those who have cardiovascular, endocrine, digestive, and urinary diseases. |
| • Those who are employed in this center. |
| • Those who are considered as a nonqualified person by the judge of the investigator and ineligible for the blood test. |
| Parameter | Mean ± S.D | P-Value | |
|---|---|---|---|
| Placebo group (n = 46) | Test group (n = 54) | ||
| Student's t-test for continuous variables and chi-square or Fisher's exact test for categorical variables were used to compare the difference between the groups. | |||
| Age | 44.9 ± 5.2 | 45.7 ± 7.5 | 0.530 |
| Weight (kg) | 59.98 ± 1.47 | 57.60 ± 1.07 | 0.185 |
| BMI (kg m−2) | 23.04 ± 0.56 | 22.46 ± 0.42 | 0.400 |
| Exposure hours to UV (daily/%) | |||
| Less than 1h | 32.61 | 14.81 | 0.115 |
| 1–3 h | 60.87 | 77.78 | |
| More than 3 h | 6.52 | 7.41 | |
| Sleeping hours (daily/%) | |||
| Less than 5 h | 8.70 | 5.56 | 0.367 |
| 5–8 h | 76.09 | 87.04 | |
| More than 8 h | 15.22 | 7.41 | |
| Hydration (A.U) | |||
| Cheek | 38.80 ± 0.87 | 36.29 ± 1.05 | 0.075 |
| Volar forearm | 32.23 ± 1.01 | 32.01 ± 0.97 | 0.871 |
| TEWL (g m −2 h −1 ) | |||
| Cheek | 13.92 ± 0.61 | 13.08 ± 0.51 | 0.290 |
| Volar forearm | 7.27 ± 0.30 | 7.69 ± 0.23 | 0.265 |
| Desquamation (%) | |||
| Cheek | 14.69 ± 0.50 | 16.04 ± 0.48 | 0.056 |
| Volar forearm | 18.81 ± 0.64 | 20.10 ± 0.58 | 0.139 |
| Wrinkling (μm) | |||
| R a | 12.85 ± 0.33 | 13.05 ± 0.32 | 0.663 |
| R max | 80.46 ± 2.13 | 82.71 ± 1.97 | 0.440 |
| R z | 59.65 ± 1.50 | 61.08 ± 1.45 | 0.499 |
| R p | 40.04 ± 0.99 | 41.15 ± 1.03 | 0.447 |
| R v | 45.31 ± 1.38 | 46.42 ± 1.23 | 0.548 |
| Elasticity | |||
| K (mm) | 1.140 ± 0.007 | 1.139 ± 0.005 | 0.833 |
| Indent (mm) | 0.557 ± 0.015 | 0.563 ± 0.013 | 0.767 |
| Alpha (ratio) | 0.027 ± 0.001 | 0.027 ± 0.001 | 0.823 |
| CoR (ratio) | 0.682 ± 0.010 | 0.686 ± 0.009 | 0.744 |
| Area (mm2) | 71.319 ± 3.293 | 71.854 ± 2.942 | 0.891 |
| Parameter | Mean±S.D (P-Value1) | P-Value2 | ||||
|---|---|---|---|---|---|---|
| Placebo group (n = 46) | Test group (n = 54) | |||||
| WBC: white blood cell, RBC: red blood cell, T Protein: total protein, T bil: total bilirubin, SGOT: serum glutamic oxaloacetic transaminase, SGPT: serum glutamic-pyruvic transaminase, BUN: blood urea nitrogen, (1) paired t-test or Wilcoxon signed-rank test, *P < 0.05 it means that there is a significant difference when compared to the baseline (0 W) (2) independent t-test, R RM-ANOVA, *P < 0.05 it means that there is a significant difference between the groups. | ||||||
| Diet | −3 days | 2.01 ± 0.05 | — | 2.00 ± 0.04 | — | — |
| 4 weeks | 2.01 ± 0.06 | (0.205) | 1.99 ± 0.09 | (0.628) | 0.594R | |
| 8 weeks | 2.00 ± 0.14 | (0.556) | 1.99 ± 0.07 | (0.315) | 0.863R | |
| 12 weeks | 2.00 ± 0.05 | (0.265) | 2.00 ± 0.06 | (0.354) | 0.879R | |
| Weight | 0 week | 59.98 ± 9.98 | — | 57.60 ± 7.87 | — | — |
| 12 weeks | 60.41 ± 9.76 | (0.024*) | 58.04 ± 8.07 | (0.016*) | 0.968 | |
| BMI (kg m−2) | 0 weeks | 23.04 ± 3.80 | — | 22.46 ± 3.06 | — | — |
| 12 weeks | 23.20 ± 3.64 | (0.025*) | 22.64 ± 3.19 | (0.015*) | 0.891 | |
| Blood test | ||||||
| WBC | 0 week | 6.01 ± 1.41 | — | 5.73 ± 1.33 | — | — |
| 12 weeks | 6.26 ± 1.49 | (0.195) | 6.02 ± 1.46 | (0.113) | 0.863 | |
| RBC | 0 week | 4.34 ± 0.29 | — | 4.34 ± 0.35 | — | — |
| 12 weeks | 4.38 ± 0.26 | (0.140) | 4.37 ± 0.31 | (0.322) | 0.745 | |
| Hemoglobin | 0 week | 12.87 ± 0.96 | — | 12.74 ± 1.19 | — | — |
| 12 weeks | 13.07 ± 0.84 | (0.006*) | 12.88 ± 0.96 | (0.049*) | 0.571 | |
| Platelet | 0 week | 269.35 ± 46.58 | — | 277.80 ± 55.81 | — | — |
| 12 weeks | 271.50 ± 50.23 | (0.615) | 280.04 ± 62.26 | (0.622) | 0.989 | |
| T Protein | 0 week | 7.34 ± 0.35 | — | 7.35 ± 0.37 | — | — |
| 12 weeks | 7.36 ± 0.38 | (0.684) | 7.35 ± 0.33 | (0.972) | 0.735 | |
| Albumin | 0 week | 4.63 ± 0.21 | — | 4.63 ± 0.23 | — | — |
| 12 weeks | 4.64 ± 0.25 | (0.942 | 4.63 ± 0.22 | (0.907) | 0.896 | |
| T Bil | 0 week | 0.65 ± 0.21 | — | 0.61 ± 0.23 | — | — |
| 12 weeks | 0.57 ± 0.24 | (0.016*) | 0.54 ± 0.22 | (0.030*) | 0.737 | |
| SGOT | 0 week | 18.74 ± 4.29 | — | 19.96 ± 5.21 | — | — |
| 12 weeks | 20.39 ± 6.76 | (0.102) | 20.44 ± 5.67 | (0.496) | 0.327 | |
| SGPT | 0 week | 13.24 ± 5.23 | — | 15.06 ± 6.48 | — | — |
| 12 weeks | 16.09 ± 7.40 | (0.023*) | 15.52 ± 6.14 | (0.144) | 0.112 | |
| Total cholesterol | 0 week | 186.67 ± 29.97 | — | 186.85 ± 28.93 | — | — |
| 12 weeks | 184.89 ± 32.56 | (0.564) | 192.72 ± 29.72 | (0.021*) | 0.052 | |
| Triglyceride | 0 week | 89.30 ± 40.05 | — | 85.37 ± 35.50 | — | — |
| 12 weeks | 92.61 ± 40.15 | (0.475) | 94.20 ± 55.34 | (0.188) | 0.509 | |
| Glucose | 0 week | 89.67 ± 6.18 | — | 90.22 ± 8.55 | — | — |
| 12 weeks | 91.17 ± 8.25 | (0.177) | 91.39 ± 7.45 | (0.330) | 0.839 | |
| BUN | 0 week | 11.41 ± 2.75 | — | 11.56 ± 2.86 | — | — |
| 12 weeks | 12.27 ± 2.74 | (0.035*) | 13.27 ± 10.20 | (0.246) | 0.602 | |
| Creatinine | 0 week | 0.67 ± 0.09 | — | 0.67 ± 0.08 | — | — |
| 12 weeks | 0.65 ± 0.09 | (0.067) | 0.65 ± 0.07 | (0.004*) | 0.843 | |
2.4 Study schedule
All participants were asked to ingest four tablets containing 1650 mg of CPNS once a day. Moreover, they agreed to only use cosmetics provided by the center for a wash-out period of 2 weeks and the intervention period to ensure a normal skin status. All participants visited the center five times in total during the clinical trial period to estimate the measuring parameters: before administration formulation at baseline (0 week), at 4, 8, and 12 weeks after the start of the administration of the prepared formulation for the assessment of effects, and 2 days after the end of the ingestion period at 12 weeks for a safety test. Participants were told not to wear make-up for 12 h before visiting the assessment center. At each visit, participants washed their entire face with a foam cleanser named ‘Facial Care Smart Whip’ of Welcos Co., Ltd, which has no functional ingredients that affect the skin and is available on the market, and waited for 20 min at a controlled temperature (22 ± 2 °C) and humidity (50 ± 5%) before the evaluations.2.5 Measurement of skin hydration
Skin moisture was measured on the face and the volar forearm, 5 cm distal to the elbow, using a Corneometer®CM 825 (C+K, Germany). This device is well known for being able to estimate the moisture content in the stratum corneum (SC).55 All electrical phenomena are caused by electric charges, with capacitance indicating the ability to store these charges, and when the anode plate of a probe is charged, it creates an electric field in between. The principle of the storing of generated charges can thus be used to measure the moisture content in the SC of the epidermis that has high resistance to electricity. Since the measured capacitance is relatable to the water content of the SC, the higher the reading, the higher the moisture content; moreover, the more hydrated the SC is, and the more intensive the electronic conductivity. The measured output levels are represented in arbitrary units (au).2.6 Measurement of trans-epidermal water loss (TEWL)
TEWL is one of the well-known factors for the estimation of the moisture barrier capacity of the skin.56 It was measured on the participant's face and their volar forearm using a Tewameter® TM 300 (C+K, Germany). The device's “open chamber” principle represents the most common method for the estimation of TEWL. The Tewameter® probe gauges the density gradient of moisture vaporization from the specific skin area indirectly with the double sensors (relative humidity and temperature) inside the hollow cylinder. This is the only method that allows for assessing the TEWL continuously without influencing its microenvironment. The measured values express the evaporation rate in g m−2 h−1.2.7 Measurement of skin desquamation
Desquamation on the face and the volar forearm was assessed with a Standard D-Squame® disc (Heiland electronic GmbH, Wetzlar, Germany). When assessing the desquamation of the skin, a D-Squame pressure instrument (D500; Heiland electronic GmbH) was used to apply a constant pressure of 225 g cm−2. The obtained skin desquamation was analyzed using a SquameScan® 850 (Heiland electronic GmbH) which measures the total protein (%) of corneocytes on disc images to assess desquamation on the disc. The skin desquamation on the disc was assessed with an optical microscope with a 10-fold magnification lens (SOPTOP MDX320, China).2.8 Measurement of skin wrinkling
Crow's feet were evaluated using two different methods, visual assessments, and instrumental measurements. For the visual assessments, a dermatologist and an investigator, who has over 10 years of experience in the skin clinical research field, assessed the participant's skin, following a double-blinded method, and recorded grades according to the wrinkle scoring criteria of the MFDS (Ministry of Food and Drug Safety, KOREA) guidelines. Instrumental measurements were conducted using a PRIMOS® lite (Canfield, USA). Based on digital micromirror devices (DMDs), the principle of this method is to measure the digital stripe projection. The device can take a photo and transform it into a 3D image. The analyzed value of the same region was determined by comparing two images (before and after measurements) using the matching and overlay system, and the result gave the following parameters: Ra represents the arithmetic average value of profile peaks within the total length; Rmax is the maximum of all peak-to-valley values; Rz is the average maximum height of the profile; Rp is the maximum profile peak height; and Rv is the maximum profile valley height.9,572.9 Measurement of skin elasticity
The elasticity of the dermis was estimated by assessing the participant's cheek with a Ballistometer BLS780 (Dia-Stron Ltd, United Kingdom). The measuring principle is based on the traditional ballistometric method of impacting force on the skin surface.58 When placed on the surface of the skin and applying vibrational energy, the waveform of the vibration (reduction) is analyzed and expressed as the value of each parameter and the bounce profile. We obtained the following four parameters: K represents skin firmness or softness; indent indicates skin softness; alpha is the rate of energy breaking; CoR is the coefficient of restoration; and the area is the area between the zero datum and under the curve of the bounce profile.2.10 Statistical analysis
The statistical analysis was conducted using SPSS 12.0. (IBM, USA) and SAS 9.4 (SAS Institute, USA). All variables of measurement at the baseline were assessed for normal distributions using the Shapiro–Wilk test and kurtosis & skewness measurements. All continuous data were analyzed using paired and independent t-tests if normality was satisfied and the Wilcoxon signed-rank test and the Mann–Whitney U test if distributions were not normal. Categorical data were analyzed using chi-square tests or Fisher's exact tests. All data are presented as the mean ± standard error (S.E.), and P < 0.05 was considered to indicate statistical significance.3. Results and discussion
3.1 Participant baseline characteristics
A total of 100 participants (CPNS group = 54; placebo group = 46) completed the clinical study, and their data were entered into the analyses. Homogeneity tests between groups did not reveal significant differences in age, body weight, TEWL, desquamation, wrinkling, or elasticity at the baseline (Table 3).3.2 Effect of CPNS on skin hydration and TEWL
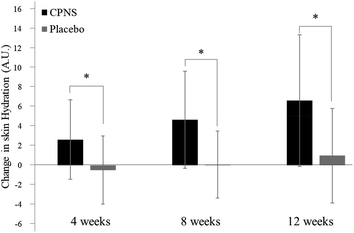
Skin hydration on the face was significantly ameliorated at 4 weeks (P = 0.000), 8 weeks (P = 0.000), and 12 weeks (P = 0.000) after the start of CPNS administration, compared to the baseline, whereas no variation from the baseline was observed in the placebo group after 4, 8, and 12 weeks of administration (P = 0.370, P = 0.861, and P = 0.169, respectively). When comparing the two groups directly, the skin was significantly more hydrated in the CPNS group than in the placebo group at 4, 8, and 12 weeks (Table 5 and Fig. 2) (P = 0.000, respectively). Improvements in skin hydration were also greater in the CPNS group than in the placebo group (5.19-fold at 4 weeks, 52.00-fold at 8 weeks, and 6.63-fold at 12 weeks). Skin hydration on the forearm did not show a significant difference between the groups (data not shown). Furthermore, TEWL and the forearm skin tended to show greater improvements in the CPNS group than in the placebo group; however, the difference between the groups was not statistically significant (data not shown).| Time | CPNS Group (n = 54) | Placebo Group (n = 46) | CPNS/Placebo P-Value | ||
|---|---|---|---|---|---|
| Mean (S.D.) | P-Value | Mean (S.D.) | P-Value | ||
| P-Values for test comparison with baseline values; *indicates P < 0.05. | |||||
| 0 week | 36.29 (7.75) | 38.80 (5.92) | |||
| 4 weeks | 38.93 (7.84) | 0.000* | 38.33 (5.22) | 0.370 | 0.000* |
| 8 weeks | 40.97 (8.17) | 0.000* | 38.89 (5.74) | 0.861 | 0.000* |
| 12 weeks | 42.92 (7.66) | 0.000* | 39.80 (6.74) | 0.169 | 0.000* |
3.3 Effect of CPNS on skin desquamation
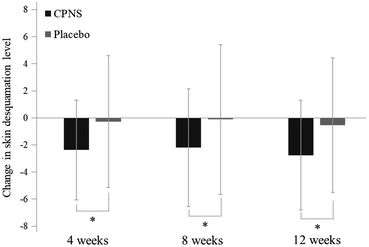
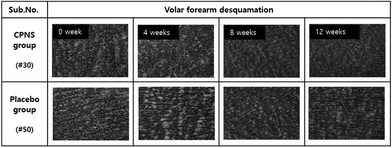
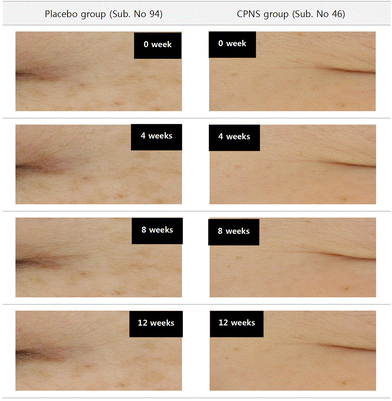
Forearm desquamation was significantly ameliorated in the CPNS group at 4 weeks (P = 0.000), 8 weeks (P = 0.001), and 12 weeks (P = 0.000) compared to the baseline, while no significant difference was observed in the placebo group at 4, 8, and 12 weeks (P = 0.708, P = 0.892, and P = 0.474). The comparison between groups showed that the desquamation level was significantly lower in the CPNS group than in the placebo group at 4, 8, and 12 weeks (P = 0.017, P = 0.039, and P = 0.016) (Fig. 3 and 4). The CPNS group showed an 8.74-fold improvement compared to the placebo group at 4 weeks, a 19.82-fold improvement at 8 weeks, and a 5.19-fold improvement at 12 weeks. An improvement in skin desquamation on the face was observed at 12 weeks in the CPNS group. However, there was no significant difference between the two groups (data not shown).3.4 Effect of CPNS on skin
According to the visual grading of the participants’ crow's feet wrinkles, the CPNS group showed significant improvements compared to the placebo group at 12 weeks (P = 0.039) (Table 6 and Fig. 5), with the CPNS group's appearance level being 4.67-fold higher than that of the placebo group. The assessment of instrumental measurements showed significant improvements in the CPNS group compared to the placebo group in all wrinkle parameters (Ra, Rmax, Rz, Rp, and Rv) after 4 weeks (P = 0.006, P = 0.006, P = 0.004, P = 0.003, and P = 0.028, respectively) and 12 weeks of administration (P = 0.028, P = 0.015, P = 0.011, P = 0.020, and P = 0.044) (Table 7). Moreover, the differences in variance values (baseline–after administration) of wrinkle indicators (Ra, Rmax, Rz, Rp, and Rv) showed 3.26-fold, 5.04-fold, 4.07-fold, 7.73-fold, and 4.11-fold improvements compared to the placebo group at 4 weeks and 2.68-fold, 4.10-fold, 3.71-fold, 3.58-fold, and 3.83-fold improvements at 12 weeks of administration. None of the parameters showed significant improvements at 8 weeks.| Time (week) | CPNS group (n = 54) | Placebo group (n = 46) | CPNS/placebo P-Value | ||
|---|---|---|---|---|---|
| Mean (S.D.) | P-Value | Mean (S.D.) | P-Value | ||
| 0 | 4.48 (1.09) | 4.33 (1.07) | |||
| 4 | 4.48 (1.09) | 1.000* | 4.30 (1.05) | 0.323 | 0.323 |
| 8 | 4.39 (1.06) | 0.006* | 4.30 (1.05) | 0.323 | 0.074 |
| 12 | 4.34 (1.08) | 0.003* | 4.29 (1.04) | 0.183 | 0.039* |
| Parameter | Time (week) | Variance value | CPNS/placebo P-value | |
|---|---|---|---|---|
| CPNS group (n = 54) | Placebo group (n = 46) | |||
| Skin-wrinkling parameters: R1, skin roughness; R2, maximum roughness; R3, average roughness; R4, smoothness depth; R5, arithmetic average roughness. P-Values for independent t-test and Mann–Whitney U test comparisons between values in the CPNS group and the placebo group; *indicates P < 0.05. | ||||
| R a | 4 | −1.337 ± 1.668 | −0.410 ± 1.631 | 0.006* |
| 8 | −0.646 ± 1.480 | −0.314 ± 1.571 | 0.281 | |
| 12 | −1.173 ± 1.442 | −0.437 ± 1.853 | 0.028* | |
| R max | 4 | −7.993 ± 11.594 | −1.585 ± 10.875 | 0.006* |
| 8 | −3.741 ± 10.069 | −0.944 ± 9.920 | 0.166 | |
| 12 | −7.029 ± 9.652 | −1.713 ± 11.761 | 0.015* | |
| R z | 4 | −6.019 ± 7.711 | −1.478 ± 7.615 | 0.004* |
| 8 | −3.317 ± 7.182 | −1.147 ± 6.873 | 0.128 | |
| 12 | −5.443 ± 6.964 | −1.469 ± 8.399 | 0.011* | |
| R p | 4 | −3.974 ± 5.890 | −0.514 ± 5.419 | 0.003* |
| 8 | −2.428 ± 6.221 | −0.823 ± 4.701 | 0.155 | |
| 12 | −3.738 ± 5.852 | −1.043 ± 5.516 | 0.020* | |
| R v | 4 | −4.529 ± 7.887 | −1.102 ± 7.364 | 0.028* |
| 8 | −1.626 ± 6.154 | −0.507 ± 7.360 | 0.409 | |
| 12 | −3.780 ± 5.987 | −0.987 ± 7.724 | 0.044* | |
3.5 Effect of CPNS on skin elasticity
Some measured parameters of elasticity were significantly ameliorated in the CPNS group after 8 weeks of oral intake compared to the baseline, and some (K, alpha, CoR, and area) also after 12 weeks of administration (P < 0.05 in all cases). However, the placebo group did not show any improvements in skin elasticity from baseline values during the entire study period, except for the parameter K at 8 and 12 weeks after the start of placebo administration. In comparison with the placebo group, the CPNS group showed significant improvements in most skin elasticity parameters (K, alpha, and CoR) after 12 weeks (P = 0.031, P = 0.012, and P = 0.013,), while the area parameter was improved at 8 and 12 weeks (P = 0.005, and P = 0.000). K, alpha, and CoR showed 1.89-fold, 2.00-fold, and 1.60-fold improvements in the CPNS group compared to the placebo group at 12 weeks, and the area parameter showed a 13.09-fold improvement at 8 weeks and a 36.56-fold improvement at 12 weeks, compared to the placebo group (Table 8).| Parameter | Time (weeks) | Variance value | CPNS/placebo P-value | |
|---|---|---|---|---|
| CPNS group (n = 54) | Placebo group (n = 46) | |||
| Skin-elasticity parameters: K, start height; indent, indentation depth; alpha, rate of exponential decay; CoR, coefficient of restitution; area, area under the curve. P-Values for independent t-test comparisons between values in the CPNS group and the placebo group; *indicates P < 0.05. | ||||
| K | 4 | −0.007 ± 0.034 | −0.005 ± 0.027 | 0.761 |
| 8 | 0.026 ± 0.041 | 0.019 ± 0.040 | 0.239 | |
| 12 | 0.034 ± 0.039 | 0.018 ± 0.034 | 0.031* | |
| Indent | 4 | 0.005 ± 0.043 | 0.003 ± 0.047 | 0.820 |
| 8 | 0.027 ± 0.042 | 0.008 ± 0.045 | 0.038* | |
| 12 | 0.039 ± 0.044 | 0.013 ± 0.054 | 0.012* | |
| Alpha | 4 | 0.000 ± 0.004 | 0.000 ± 0.003 | 0.731 |
| 8 | −0.001 ± 0.004 | 0.000 ± 0.005 | 0.260 | |
| 12 | −0.002 ± 0.004 | 0.001 ± 0.005 | 0.012* | |
| CoR | 4 | 0.003 ± 0.020 | 0.002 ± 0.021 | 0.726 |
| 8 | 0.004 ± 0.021 | −0.004 ± 0.023 | 0.060 | |
| 12 | 0.008 ± 0.027 | −0.005 ± 0.026 | 0.013* | |
| Area | 4 | 0.535 ± 7.466 | −1.236 ± 7.933 | 0.253 |
| 8 | 4.280 ± 7.816 | −0.327 ± 8.103 | 0.005* | |
| 12 | 6.325± 7.871 | −0.173 ± 8.431 | 0.000* | |
4. Discussion
Dryness leads to skin aging due to skin barrier dysfunction and skin-related disease development, such as winter xerosis, atopic dermatitis, and eczema.59–61 Dry skin becomes more notable with age and is related to a reduction in ceramide contents and natural moisturizing factors,62 and skin moisture content is influenced by water attached to HA in the epidermis and the dermis.63 Koyama et al.64 reported that in Japanese women, the ingestion of hydrolyzed collagen peptides enhanced the absorption rate of water content in the SC. Moreover, the intake of bioactive peptides improves skin conditions such as moisture, sebum, and gloss.65,66Collagen, which is an abundant protein and a major element of connective and structural tissue in mammals, is found in cartilage, bones, and skin, as well as joints, hair, cornea, and blood vessels.67 Collagen is widely known to help prevent aging and improve bone, ligament, and cartilage health when taken orally.25,68,69 Due to its excellent moisture retention capability, it has been widely used as a cosmetic material for enhancing skin elasticity and moisture. However, because collagen is a large-size protein with a high molecular weight of about 300 kDa, there is an ongoing controversy over its absorption rate; the latest trend in related research has thus been to develop functional materials with collagen peptides whose bioavailability has been enhanced by reducing their molecular weight using enzymatic hydrolysis. For the investigation of physicochemical properties of collagen hydrolysates, the CPNS used here was prepared from fish scales, and manufactured by hydrolyzing the fish scale collagen by treatment with fungal proteases.
This clinical study found that oral administration of CPNS significantly improved skin hydration parameters after 4 weeks of intake, while no changes from baseline were observed in the placebo group at 4, 8, and 12 weeks. However, we did not observe a significant difference in TEWL between the test and placebo groups. In our in vitro study, we aimed to estimate the beneficial effects of CPNS on the generation of HA as well as the generation of related proteins and genes associated with HA synthesis using human adult low-calcium high-temperature (HaCaT) keratinocytes.70 The results of real-time PCR expression tests demonstrated that CPNS dose- and time-dependently induces the up-regulation of the HAS2 gene, which plays a crucial role in the down-regulation of the HYAL1 gene, related to HA degradation as well as HA synthesis. Moreover, western blotting results showed that the CPNS treatment led to improvements in parameters related to HAS2. These results demonstrate that in HaCaT cells, CPNS accelerates the synthesis of HA via the down-regulation of HYAL1 and the up-regulation of HAS2, which results in the effects of CPNS supplementation on skin moisture contents.70
Excessive UV irradiation causes skin photoaging and is responsible for the generation of MMP-1 followed by the disassembly of type 1 and 3 collagen in the skin.71–74 The effect of CPNS on the concentration of MMP-1 induction in the supernatants of HDF cell cultures under ultraviolet B (UVB)-irradiation has been estimated, and it has been found that excessive UVB irradiation causes MMP-1 synthesis while CPNS considerably reduces the concentration of MMP-1 in a dose-dependent manner. In our skin wrinkling evaluation, we also verified if the response to the administration of CPNS were in line with the findings of an earlier in vivo study where oral ingestion of CPNS in UVB-irradiated mice inhibited MMP-1 and resulted in improvements in skin moisture contents and wrinkle formation.10
Type 1 collagen is the core component in the skin dermis, the massive fibers generally known as collagen.75 Other previous clinical studies showed that collagen hydrolysate intake for 4 to 12 weeks affects skin elasticity.4,66 In this study, CPNS enhanced skin elasticity and wrinkles after 12 weeks of administration. These distinct time points of efficacy might be explained by various factors, such as the collagen hydrolysate constituents of the peptide sequences, physical elements, environmental variables, and the plan of study. Our previous studies have shown that long-term administration of collagen hydrolysate is related to beneficial effects on skin health. Moreover, other collagen-related research has shown that nutraceutical supplements should be used to acquire notable efficacy, given that their bioactive ingredients can be fortified and thereby control the transduction of several cell signals in marked tissues.65 The CPNS product administered here is suitable for increasing absorption rates and functional properties caused by the specific bioactive peptide Gly-Pro. After peroral administering CPNS to Sprague-Dawley rats, the plasma concentrations of dipeptide forms, Gly-Pro and Pro-Hyp, increased dramatically. Particularly, the Gly-Pro dipeptide is absorbed considerably faster than other CPNS-originated bioactive peptides such as Gly-Pro-Hyp tripeptide and Pro-Hyp dipeptide. According to Yamamoto et al., Gly-Pro detected a notably faster Tmax than the other bioactive peptides of collagen hydrolysate, such as Pro-Ala, Pro-Hyp, Gly-Pro-Ala, and Gly-Pro-Hyp with intraperitoneal administration.71 Moreover, the absorption of bioactive dipeptides from the collagen hydrolysate into the skin may activate the movement and synthesis of fibroblasts.7,76 These findings indicate that the rapid absorption of the Gly-Pro dipeptide occurs in the bloodstream and that it is promptly distributed to the organs because of the much smaller size of Gly-Pro compared to other peptides. Moreover, the Gly-Pro dipeptide has been found to increase the generation of type 1 procollagen as well as to strongly inhibit MMP-1 production in HDF cells.10 Collectively, the high concentrations of both Gly-Pro and Pro-Hyp in the bloodstream after oral intake of CPNS could protect against skin photoaging. Our earlier in vitro study showed that CPNS treatment restricted MMP-1 production and procollagen synthesis, and the current clinical study shows that CPNS administration improves skin moisture, desquamation, elasticity, and wrinkle formation in women.10 Skin hydration significantly influences skin desquamation and elasticity, because the structural alterations in elastin formation only occur in the water-bound protein form.77 The improved skin hydration parameters that we investigated following the administration of CPNS may therefore have affected the decrease in skin desquamation and increase in skin elasticity that we observed. No side effects of CPNS administration were observed, and the vital signs in the CPNS group were in the normal range.
5. Conclusions
Oral ingestion of CPNS, 1650 mg per day for 12 weeks, significantly ameliorated several skin parameters in women aged 30–60 years. We observed a significant increase in skin hydration and a reduction in skin desquamation after 4 weeks of administration and reduced wrinkling after 12 weeks, as well as improved elasticity after 8 weeks. Since this clinical study only included women between the ages of 30 and 60 years, further studies are required for males of different ages. The cosmetic functional food market has been expanding rapidly, and CPNS may be used as a safe, economical, and efficient substance for nutraceutical foods, to ameliorate skin wrinkling, hydration, desquamation, and elasticity. Synthetically, these results suggest that Gly-Pro- and Pro-Hyp-containing CPNS may be an alternative supplement for anti-photoaging in the nutraceutical or nutricosmetic industry.Author contributions
Conceptualization: Hyunjun Lee, Eunjoung Kim, and Miyeong Lee; methodology: Miyeong Lee; validation: Hyunjun Lee and Miyeong Lee; formal analysis: Miyeong Lee; writing–original draft preparation: Hyunjun Lee and Miyeong Lee; writing–review and editing: Hyunwoo Ahn and Seokjun Son; visualization: Hyunjun Lee and Miyeong Lee; supervision: Hyunjun Lee; project administration: Eunjoung Kim; funding acquisition: Seokjun Son.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by Nongshim Co., Ltd. Grant.References
- L. Cen, W. Liu, L. Cui, W. Zhang and Y. Cao, Collagen tissue engineering: development of novel biomaterials and applications, Pediatr. Res., 2008, 63, 492–496 CrossRef CAS PubMed.
- T. Okada, T. Hayashi and Y. Ikada, Degradation of collagen suture in vitro and in vivo, Biomaterials, 1992, 13, 448–454 CrossRef CAS PubMed.
- R. H. Champion, J. L. Burton and F. Ebling, Rook/Wilkinson/Ebling-Textbook of Dermatology, 1992, pp. xvi, 3160, lvii–xvi, 3160, lvii Search PubMed.
- E. Proksch, M. Schunck, V. Zague, D. Segger, J. Degwert and S. Oesser, Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis, Skin Pharmacol. Physiol., 2014, 27, 113–119 CrossRef CAS PubMed.
- I. Sjerobabski Masnec and S. Poduje, Photoaging, Coll. Antropol., 2008, 32, 177–180 Search PubMed.
- E. C. Li-Chan, S. L. Hunag, C. L. Jao, K. P. Ho and K. C. Hsu, Peptides derived from atlantic salmon skin gelatin as dipeptidyl-peptidase IV inhibitors, J. Agric. Food Chem., 2012, 60, 973–978 CrossRef CAS PubMed.
- Y. Shigemura, K. Iwai, F. Morimatsu, T. Iwamoto, T. Mori, C. Oda, T. Taira, E. Y. Park, Y. Nakamura and K. Sato, Effect of Prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin, J. Agric. Food Chem., 2009, 57, 444–449 CrossRef CAS PubMed.
- M. Tanaka, Y. Koyama and Y. Nomura, Effects of collagen peptide ingestion on UVB-induced skin damage, Biosci., Biotechnol., Biochem., 2009, 73, 930–932 CrossRef CAS PubMed.
- S. Yotsawimonwat, J. Rattanadechsakul, P. Rattanadechsakul and S. Okonogi, Skin improvement and stability of Echinacea purpurea dermatological formulations, Int. J. Cosmet. Sci., 2010, 32, 340–346 CrossRef CAS PubMed.
- H. J. Lee, H. L. Jang, D. K. Ahn, H. J. Kim, H. Y. Jeon, D. B. Seo and S. S. Kang, Orally administered collagen peptide protects against UVB-induced skin aging through the absorption of dipeptide forms, Gly-Pro and Pro-Hyp, Biosci., Biotechnol., Biochem., 2019, 83, 1146–1156 CrossRef CAS PubMed.
- H. Song and B. Li, Beneficial effects of collagen hydrolysate: a review on recent developments, Biomed. J. Sci. Tech. Res., 2017, 1, 458–461 Search PubMed.
- M. Harris, J. Potgieter, K. Ishfaq and M. Shahzad, Developments for collagen hydrolysate in biological, biochemical, and biomedical domains: A comprehensive review, Materials, 2021, 14, 2806 CrossRef CAS PubMed.
- F. D. Choi, C. T. Sung, M. Juhasz and N. A. Mesinkovsk, Oral collagen supplementation: a systematic review of dermatological applications, J. Drugs Dermatol., 2019, 18, 9–16 Search PubMed.
- V. B. Djagny, Z. Wang and S. Xu, Gelatin: a valuable protein for food and pharmaceutical industries: review, Crit. Rev. Food Sci. Nutr., 2001, 41, 481–492 CrossRef CAS PubMed.
- J. Wasswa, J. Tang and X. Gu, Utilization of fish processing by-products in the gelatin industry, Food Rev. Int., 2007, 23, 159–174 CrossRef CAS.
- M. Furtado, L. Chen, Z. Chen, A. Chen and W. Cui, Development of fish collagen in tissue regeneration and drug delivery, Engineered Regeneration, 2022, 3, 217–231 CrossRef.
- B. Sahithi, S. Ansari, S. Hameeda, G. Sahithya, D. M. Prasad and Y. Lakshmi, A review on collagen based drug delivery systems, Indian J. Res. Pharm. Biotechnol., 2013, 1, 461 CAS.
- H. Hong, H. Fan, M. Chalamaiah and J. Wu, Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives, Food Chem., 2019, 301, 125222 CrossRef CAS PubMed.
- T. Ichimura, A. Yamanaka, T. Otsuka, E. Yamashita and S. Maruyama, Antihypertensive effect of enzymatic hydrolysate of collagen and Gly-Pro in spontaneously hypertensive rats, Biosci., Biotechnol., Biochem., 2009, 73, 2317–2319 CrossRef CAS PubMed.
- M. Schunck, V. Zague, S. Oesser and E. Proksch, Dietary Supplementation with Specific Collagen Peptides Has a Body Mass Index-Dependent Beneficial Effect on Cellulite Morphology, J. Med. Food, 2015, 18, 1340–1348 CrossRef CAS PubMed.
- K. L. Clark, W. Sebastianelli, K. R. Flechsenhar, D. F. Aukermann, F. Meza, R. L. Millard and J. R. Deitch, 24-week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain, Curr. Med. Res. Opin., 2008, 24, 1485–1496 CrossRef CAS PubMed.
- R. W. Moskowitz, Role of collagen hydrolysate in bone and joint disease, Semin. Arthritis Rheum., 2000, 30, 87–99 CrossRef CAS PubMed.
- C. Bongers, D. Ten Haaf, M. Catoire, B. Kersten, J. A. Wouters, T. M. H. Eijsvogels and M. T. E. Hopman, Effectiveness of collagen supplementation on pain scores in healthy individuals with self-reported knee pain: a randomized controlled trial, Appl. Physiol., Nutr. Metab., 2020, 45, 793–800 CrossRef CAS PubMed.
- S. Oesser, The oral intake of specific Bioactive Collagen Peptides has a positive effect on hair thickness, Int. J. Nutraceuticals, Func. Foods Nov. Foods, 2020, 1, 134–138 Search PubMed.
- B. Walther and R. Sieber, Bioactive proteins and peptides in foods, Int. J. Vitam. Nutr. Res., 2011, 81, 181–192 CrossRef CAS PubMed.
- J. Liang, X. Pei, Z. Zhang, N. Wang, J. Wang and Y. Li, The protective effects of long–term oral administration of marine collagen hydrolysate from chum salmon on collagen matrix homeostasis in the chronological aged skin of Sprague–Dawley male rats, J. Food Sci., 2010, 75, H230–H238 CrossRef CAS PubMed.
- L. Wang, Y. Jiang, X. Wang, J. Zhou, H. Cui, W. Xu, Y. He, H. Ma and R. Gao, Effect of oral administration of collagen hydrolysates from Nile tilapia on the chronologically aged skin, J. Funct. Foods, 2018, 44, 112–117 CrossRef CAS.
- K. Iwai, T. Hasegawa, Y. Taguchi, F. Morimatsu, K. Sato, Y. Nakamura and A. Higashi, Identification of food-derived collagen peptides in human blood after oral ingestion of gelatin hydrolysates, J. Agric. Food Chem., 2005, 53, 6531–6536 CrossRef CAS PubMed.
- Y. Shigemura, S. Akaba, E. Kawashima, E. Y. Park, Y. Nakamura and K. Sato, Identification of a novel food-derived collagen peptide, hydroxyprolyl-glycine, in human peripheral blood by pre-column derivatisation with phenyl isothiocyanate, Food Chem., 2011, 129, 1019–1024 CrossRef CAS PubMed.
- G. Samonina, I. Ashmarin and L. Lyapina, Glyproline peptide family: review on bioactivity and possible origins, Pathophysiology, 2002, 8, 229–234 CrossRef CAS PubMed.
- F. R. Backwell, D. Wilson and A. Schweizer, Evidence for a glycyl-proline transport system in ovine enterocyte brush-border membrane vesicles, Biochem. Biophys. Res. Commun., 1995, 215, 561–565 CrossRef CAS PubMed.
- J. Asselin, C. G. Knight, R. W. Farndale, M. J. Barnes and S. P. Watson, Monomeric (glycine-proline-hydroxyproline)10 repeat sequence is a partial agonist of the platelet collagen receptor glycoprotein VI, Biochem. J., 1999, 339(Pt 2), 413–418 CrossRef CAS PubMed.
- C. Li, Y. Fu, H. Dai, Q. Wang, R. Gao and Y. Zhang, Recent progress in preventive effect of collagen peptides on photoaging skin and action mechanism, Food Sci. Hum. Wellness, 2022, 11, 218–229 CrossRef CAS.
- M. Borumand and S. Sibilla, Daily consumption of the collagen supplement Pure Gold Collagen® reduces visible signs of aging, Clin. Interventions Aging, 2014, 9, 1747 Search PubMed.
- E. Sumida, The effect of oral ingestion of collagen peptide on skin hydration and biochemical data of blood, J. Nutr. Food, 2004, 7, 45–52 Search PubMed.
- H. Ohara, K. Ito, H. Iida and H. Matsumoto, Improvement in the moisture content of the stratum corneum following 4 weeks of collagen hydrolysate ingestion, J. Jpn. Soc. Food Sci. Technol., 2009, 56, 137–145 CrossRef CAS.
- H. Matsumoto, Clinical effects of fish type I collagen hydrolysate on skin properties, ITE. Lett. Batteries, New Technol. Med., 2006, 7, 386–390 Search PubMed.
- J. Asserin, E. Lati, T. Shioya and J. Prawitt, The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: evidence from an ex vivo model and randomized, placebo–controlled clinical trials, J. Cosmet. Dermatol., 2015, 14, 291–301 CrossRef PubMed.
- E. Proksch, D. Segger, J. Degwert, M. Schunck, V. Zague and S. Oesser, Oral supplementation of specific collagen peptides has beneficial effects on human skin physiology: a double-blind, placebo-controlled study, Skin Pharmacol. Physiol., 2014, 27, 47–55 CrossRef CAS PubMed.
- M. Igase, K. Kohara, Y. Okada, M. Ochi, K. Igase, N. Inoue, T. Kutsuna, H. Miura and Y. Ohyagi, A double-blind, placebo-controlled, randomised clinical study of the effect of pork collagen peptide supplementation on atherosclerosis in healthy older individuals, Biosci., Biotechnol., Biochem., 2018, 82, 893–895 CrossRef CAS PubMed.
- A. G. Schauss, J. Stenehjem, J. Park, J. R. Endres and A. Clewell, Effect of the novel low molecular weight hydrolyzed chicken sternal cartilage extract, BioCell Collagen, on improving osteoarthritis-related symptoms: a randomized, double-blind, placebo-controlled trial, J. Agric. Food Chem., 2012, 60, 4096–4101 CrossRef CAS PubMed.
- S. R. Schwartz and J. Park, Ingestion of BioCell Collagen®, a novel hydrolyzed chicken sternal cartilage extract; enhanced blood microcirculation and reduced facial aging signs, Clin. Interventions Aging, 2012, 7, 267 CAS.
- M. Evans, E. D. Lewis, N. Zakaria, T. Pelipyagina and N. Guthrie, A randomized, triple–blind, placebo–controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity, J. Cosmet. Dermatol., 2021, 20, 825–834 CrossRef PubMed.
- N. Inoue, F. Sugihara and X. Wang, Ingestion of bioactive collagen hydrolysates enhance facial skin moisture and elasticity and reduce facial ageing signs in a randomised double–blind placebo–controlled clinical study, J. Sci. Food Agric., 2016, 96, 4077–4081 CrossRef CAS PubMed.
- A. Czajka, E. M. Kania, L. Genovese, A. Corbo, G. Merone, C. Luci and S. Sibilla, Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing, Nutr. Res., 2018, 57, 97–108 CrossRef CAS PubMed.
- L. Genovese, A. Corbo and S. Sibilla, An insight into the changes in skin texture and properties following dietary intervention with a nutricosmeceutical containing a blend of collagen bioactive peptides and antioxidants, Skin Pharmacol. Physiol., 2017, 30, 146–158 CrossRef CAS PubMed.
- D.-U. Kim, H.-C. Chung, J. Choi, Y. Sakai and B.-Y. Lee, Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: a randomized, double-blind, placebo-controlled study, Nutrients, 2018, 10, 826 CrossRef PubMed.
- K. Jung, S.-H. Kim, K.-M. Joo, S.-H. Lim, J.-H. Shin, J. Roh, E. Kim, C. W. Park and W. Kim, Oral Intake of Enzymatically Decomposed AP Collagen Peptides Improves Skin Moisture and Ceramide and Natural Moisturizing Factor Contents in the Stratum Corneum, Nutrients, 2021, 13, 4372 CrossRef CAS PubMed.
- T. F. Juher and E. B. Perez, An overview of the beneficial effects of hydrolysed collagen intake on joint and bone health and on skin ageing, Nutr. Hosp., 2015, 32(Suppl 1), 62–66 Search PubMed.
- M. Nasri, Bioactive peptides from fish collagen byproducts: A review, Byprod. Agric. Fish., 2019, 309–333 Search PubMed.
- N. Halim, H. Yusof and N. Sarbon, Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review, Trends Food Sci. Technol., 2016, 51, 24–33 CrossRef CAS.
- S. Reagan-Shaw, M. Nihal and N. Ahmad, Dose translation from animal to human studies revisited, FASEB J., 2008, 22, 659–661 CrossRef CAS PubMed.
- S. S. AbuMweis, S. Jew and P. J. Jones, Optimizing clinical trial design for assessing the efficacy of functional foods, Nutr. Rev., 2010, 68, 485–499 CrossRef PubMed.
- H. Daba, S. Pandian, J. F. Gosselin, R. E. Simard, J. Huang and C. Lacroix, Detection and activity of a bacteriocin produced by Leuconostoc mesenteroides, Appl. Environ. Microbiol., 1991, 57, 3450–3455 CrossRef CAS PubMed.
- M.-M. Constantin, E. Poenaru, C. Poenaru and T. Constantin, Skin hydration assessment through modern non-invasive bioengineering technologies, Maedica, 2014, 9, 33 Search PubMed.
- J. D. Plessis, A. Stefaniak, F. Eloff, S. John, T. Agner, T. C. Chou, R. Nixon, M. Steiner, A. Franken and I. Kudla, International guidelines for the in vivo assessment of skin properties in non–clinical settings: Part 2. transepidermal water loss and skin hydration, Skin Res. Technol., 2013, 19, 265–278 CrossRef PubMed.
- E. Hwang, Z. W. Sun, T. H. Lee, H. S. Shin, S. Y. Park, D. G. Lee and B. G. Cho, Enzyme-processed Korean red ginseng extracts protects against skin damage induced by UVB irradiation in hairless mice, J. Ginseng Res., 2013, 37, 425–434 CrossRef CAS PubMed.
- G. B. Jemec, E. Selvaag, M. Ågren and H. C. Wulf, Measurement of the mechanical properties of skin with ballistometer and suction cup, Skin Res. Technol., 2001, 7, 122–126 CrossRef CAS PubMed.
- B. A. Gilchrest, A review of skin ageing and its medical therapy, Br. J. Dermatol., 1996, 135, 867–875 CrossRef CAS PubMed.
- G. Imokawa, A. Abe, K. Jin, Y. Higaki, M. Kawashima and A. Hidano, Decreased level of ceramides in stratum corneum of atopic dermatitis: an etiologic factor in atopic dry skin?, J. Invest. Dermatol., 1991, 96, 523–526 CrossRef CAS PubMed.
- B. P. Vickery, Skin barrier function in atopic dermatitis, Curr. Opin. Pediatr., 2007, 19, 89–93 CrossRef PubMed.
- I. Horii, Y. Nakayama, M. Obata and H. Tagami, Stratum corneum hydration and amino acid content in xerotic skin, Br. J. Dermatol., 1989, 121, 587–592 CrossRef CAS PubMed.
- E. Papakonstantinou, M. Roth and G. Karakiulakis, Hyaluronic acid: A key molecule in skin aging, Dermatoendocrinol, 2012, 4, 253–258 CrossRef CAS PubMed.
- N. Matsuda, Y. Koyama, Y. Hosaka, H. Ueda, T. Watanabe, T. Araya, S. Irie and K. Takehana, Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in the dermis, J. Nutr. Sci. Vitaminol., 2006, 52, 211–215 CrossRef CAS PubMed.
- D. U. Kim, H. C. Chung, C. Kim and J. K. Hwang, Oral intake of Boesenbergia pandurata extract improves skin hydration, gloss, and wrinkling: A randomized, double-blind, and placebo-controlled study, J. Cosmet. Dermatol., 2017, 16, 512–519 CrossRef PubMed.
- S. Y. Choi, E. J. Ko, Y. H. Lee, B. G. Kim, H. J. Shin, D. B. Seo, S. J. Lee, B. J. Kim and M. N. Kim, Effects of collagen tripeptide supplement on skin properties: a prospective, randomized, controlled study, J. Cosmet. Laser Ther., 2014, 16, 132–137 CrossRef PubMed.
- M. D. Shoulders and R. T. Raines, Collagen structure and stability, Annu. Rev. Biochem., 2009, 78, 929–958 CrossRef CAS PubMed.
- F. Shahidi and Y. Zhong, Bioactive peptides, J. AOAC Int., 2008, 91, 914–931 CrossRef CAS PubMed.
- S. Yamamoto, K. Deguchi, M. Onuma, N. Numata and Y. Sakai, Absorption and urinary excretion of peptides after collagen tripeptide ingestion in humans, Biol. Pharm. Bull., 2016, 39, 428–434 CrossRef CAS PubMed.
- H. Kim, B. Jeon, H.-J. Lee and D. K. Chung, Evaluation of the Skin Moisturizing Efficacy of a Collagen Peptide Isolated from Fish Scales, Using HaCaT Keratinocytes, J. Korean Soc. Food Sci. Nutr., 2020, 49, 454–461 CrossRef CAS.
- S. Yamamoto, F. Hayasaka, K. Deguchi, T. Okudera, T. Furusawa and Y. Sakai, Absorption and plasma kinetics of collagen tripeptide after peroral or intraperitoneal administration in rats, Biosci., Biotechnol., Biochem., 2015, 79, 2026–2033 CrossRef CAS PubMed.
- M. Brennan, H. Bhatti, K. C. Nerusu, N. Bhagavathula, S. Kang, G. J. Fisher and J. Varani, Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin, Photochem. Photobiol., 2003, 78, 43–48 CrossRef CAS PubMed.
- H. G. Welgus, J. J. Jeffrey and A. Z. Eisen, The collagen substrate specificity of human skin fibroblast collagenase, J. Biol. Chem., 1981, 256, 9511–9515 CrossRef CAS PubMed.
- M. Vaalamo, L. Mattila, N. Johansson, A. L. Kariniemi, M. L. Karjalainen-Lindsberg, V. M. Kahari and U. Saarialho-Kere, Distinct populations of stromal cells express collagenase-3 (MMP-13) and collagenase-1 (MMP-1) in chronic ulcers but not in normally healing wounds, J. Invest. Dermatol., 1997, 109, 96–101 CrossRef CAS PubMed.
- N. Matsuda, Y. Koyama, Y. Hosaka, H. Ueda, T. Watanabe, T. Araya and S. Irie, Effects of ingestion of collagen peptide on collagen fibrils and glycosaminoglycans in the dermis, J. Nutr. Sci. Vitaminol., 2006, 52, 211–215 CrossRef CAS PubMed.
- M. Yazaki, Y. Ito, M. Yamada, S. Goulas, S. Teramoto, M.-A. Nakaya, S. Ohno and K. Yamaguchi, Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin, J. Agric. Food Chem., 2017, 65, 2315–2322 CrossRef CAS PubMed.
- J. Gosline, M. Lillie, E. Carrington, P. Guerette, C. Ortlepp and K. Savage, Elastic proteins: biological roles and mechanical properties, Philos. Trans. R. Soc., B, 2002, 357, 121–132 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |