Open Access Article
Open Access ArticlePyrroloquinoline quinone disodium salt improves brain function in both younger and older adults†
Masanori
Tamakoshi
a,
Tomomi
Suzuki
a,
Eiichiro
Nishihara
b,
Shinichiro
Nakamura
 b and
Kazuto
Ikemoto
b and
Kazuto
Ikemoto
 *c
*c
aDepartment of Life Science, Mitsubishi Gas Chemical Company, Inc., Mitsubishi building, 2-5-2 Chiyoda-ku, Tokyo 100-8324, Japan
bNakamura Laboratory, RIKEN Cluster for Science, Technology and Innovation Hub, 2-1 Hirosawa, Wako, Saitama 351-0198, Japan
cNiigata Research Laboratory, Mitsubishi Gas Chemical Company, Inc., 182 Tayuhama, Kita-ku, Niigata-city, Niigata 950-3112, Japan. E-mail: kazuto-ikemoto@mgc.co.jp
First published on 15th February 2023
Abstract
Brain function is important for a good quality of life. Pyrroloquinoline quinone disodium salt (PQQ) has been proven to improve brain function and cognition in older adults (above 45 years). In this double-blind, placebo-controlled study, we investigated the effects of PQQ on cognitive function in adults aged between 20 and 65 years. PQQ (20 mg per day) was administered for 12 weeks to the participants. After 12 weeks, the participants showed improvements in composite memory and verbal memory. A further age-stratified analysis was performed. In younger adults (aged 20–40 years), PQQ improved cognitive function (cognitive flexibility, processing speed, and execution speed) after 8 weeks. Only older adults (aged 41–65 years) showed improvements in complex and verbal memory after 12 weeks. In the logistic regression analysis that included the results of all cognitive tests, the changes due to PQQ intake were observed at 8 and 12 weeks in the young and old groups, respectively.
1. Introduction
The brain has advanced functions that are essential for life support. Cognitive function includes a variety of processes such as perception, attention, memory, decision-making, and language comprehension. It is a higher-order brain function. However, cognitive function must be maintained and improved as it deteriorates with age. Improvement of cognitive function is also essential in young people with conditions such as attention deficit hyperactivity disorder, to improve their quality of life. Various food items have been shown to improve cognitive function.1 One of them, discussed herein, is pyrroloquinoline quinone disodium salt (disodium 9-carboxy-4,5-dioxo-4,5-dihydro-1H-pyrrolo[2,3-f]quinoline-2,7-dicarboxylate: PQQ), which is a water-soluble salt of the coenzyme pyrroloquinoline quinone.2,3 Products with PQQ have been registered for use in the USA, the EU, and Japan (BioPQQ in Japan and the USA; MGCPQQ in the EU).4 Pyrroloquinoline quinone disodium salt is obtained using fermentation and purification. It has been subjected to multiple safety tests. Various functions of PQQ have been reported, including mitochondrial biogenesis, promoting longevity, antioxidant activity, and improving skin texture.5–8In vitro studies have reported that PQQ has a strong neuroprotective effect against neurotoxicity and enhances the expression of nerve growth factor (NGF).9,10 In addition, water maze tests in rats have shown that oral ingestion of PQQ promotes memory learning and suppresses memory loss caused by oxidative stress.11 These studies indicate that PQQ could be used as a brain food, and clinical trials related to brain function have been conducted. In healthy adults, PQQ reportedly improves attention, information identification and processing, language memory, immediate memory, working memory, and sleep as well as reduces stress and fatigue.12–16 However, most studies involved older individuals, and very few studies included young adults. Furthermore, only a few studies have evaluated the effects of PQQ in a wide range of age groups. In recent years, data analysis technologies are being extensively used and machine learning is widely applied. Several clinical trials have been conducted using newly developed technologies, including logistic regression.17,18 This technique can be used to classify groups based on highly variable data. Wide age groups are expected to have variability scores in brain function tests. We used all data from the Cognitrax test to determine whether we could distinguish between placebo and PQQ recipients, that is, to determine whether there were changes in cognitive function with PQQ intake. Herein, we report the effects of PQQ on brain function in individuals aged between 20 and 65 years. Cognitive function improvement is necessary for all individuals irrespective of sex. We conducted this research without considering sex as a factor.2. Materials and methods
2.1 Clinical test
This study has been registered at the UMIN Clinical Trials Registry System under UMIN ID UMIN000034459 (https://www.umin.ac.jp/icdr/index.html). The study was performed with a double-blind placebo control. Informed consent was obtained from all participants. Mitsubishi Gas Chemical Company Inc. commissioned a contractor of Huma R & D Co., Ltd to perform this clinical test. The pyrroloquinoline quinone disodium salt used in this study was BioPQQ, manufactured by Mitsubishi Gas Chemical Company (Tokyo, Japan). The capsules used in the test group contained 20 mg PQQ, 224 mg starch, and 5 mg Ca stearate. The capsules used in the placebo group contained 224 mg starch and 5 mg Ca stearate. One capsule was taken with water after breakfast, every day.The exclusion criteria were individuals with serious liver/kidney disease, heart disease, respiratory disorder, an endocrine disorder, diabetes, food allergy, drug allergy, cancer, dementia and mental illness, and allergy to gelatin; pregnant women/nursing women; those who wished to become pregnant during the test period; those who had taken PQQ within 3 months before the study; and those who were deemed unsuitable by the physician for participation. Blood and urine tests were performed in advance at weeks 8 and 12.
2.2 Software
All software used Python (https://www.python.org/). Statistical analyses were performed using the “stats” module in the library scipy1.5.2 of python3.8.5. Machine learning used the library scikit-learn 0.23.2.2.3 Ethics review
Yoga Allergy Clinic Clinical Research Ethics Review Board (Installation location: 4-32-16 Yoga, Setagaya-ku, Tokyo Establisher: Tadao Kawamura) and Nihonbashi Egawa Clinic Clinical Research Ethics Review Board reviewed and approved the study; approval number RD10010TS04. This study complied with the ethical principles of the Declaration of Helsinki (adopted in 1964, and revised in October 2013). Furthermore, the study was based on the Japanese law “Ethical Guidelines for Medical Research for Humans (2014 Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labor and Welfare Notification No. 3)” and “Law Concerning the Protection of Personal Information (May 30, 2003, Japanese Law No. 57)”.3. Results and discussion
3.1 Clinical study
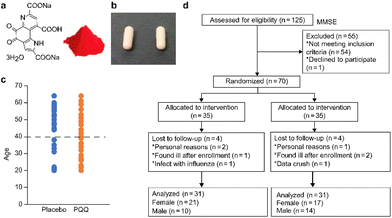
Pyrroloquinoline quinone (4,5-dihydro-4,5-dioxo-1H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid) was fermented by bacteria and purified under good manufacturing practice (GMP, food grade). Currently (2022), only MGCPQQ (BioPQQ in the US and Japan), which was used in this study, is permitted as a food in the EU. The product is highly pure as determined using HPLC analysis (ESI Fig. S1 and S2†). The commercial product of PQQ is a red trihydrate crystal (Fig. 1 and Fig. S3†).20 This product is safe, as it involves the use of a non-genetically modified organism (non-GMO), and is usable by vegetarians. Hard capsules made of cellulose contained 20 mg PQQ, and they were ingested daily by the participants. The placebo group received capsules without PQQ (Fig. 1). The placebo and PQQ capsules were similar in terms of appearance.This study was conducted in a double-blind manner to assess cognitive and brain functions in healthy Japanese adults aged between 20 and 65 years. The Cognitrax test is used for various kinds of cognitive functions.21 This is a computerized neurocognitive test. The measurement of brain function of a food component using the Cognitrax test has also been reported.22 We analyzed 15 kinds of brain function. Each participant visited a medical institution (testing institution) and underwent the tests and diagnoses prescribed. With respect to the schedule, inspections were carried out at 0 weeks (0 w: first inspection day), 8 weeks (8 w), and 12 weeks (12 w) of ingestion.
The person responsible for test food allocation adopted the Cognitrax task as a comprehensive cognitive function assessment, because the original cognitive function may be a confounding factor, and used the standardized score results and age structure of the Cognitrax Neurocognitive Index task. Randomization was performed via dynamic allocation, and the participants were assigned to the following two groups: a test food group and a control food group.
The test food allocation manager supervised the food allocation table and ensured that blinding was maintained for all other parties involved. The test period was 12 weeks. No adverse events were observed after the ingestion of the test food. Of the 70 participants, 66 completed the test, and 4 dropped out. In addition, the data of one participant were corrupted. The age distribution of the participants is shown in Fig. 1 and Table 1, and the data were found to be continuous.
| Age (years) | Number | ||||
|---|---|---|---|---|---|
| Average | SD | Placebo | PQQ | ||
| Total (age 20–65 years) | 41.5 | 13.7 | 62 | 31 | 31 |
| Old (age 41–65 years) | 52.9 | 6.6 | 33 | 18 | 15 |
| Young (age 20–40 years) | 28.8 | 6.7 | 29 | 13 | 16 |
Three types of analysis were performed. The group named “All” included participants of all ages. The “Old” group included individuals aged 41–65 years. The “Young” group included individuals aged 20–40 years. The mean, standard deviation, and P-value of the changes before and after ingestion of the test food were calculated in the PQQ and placebo groups.
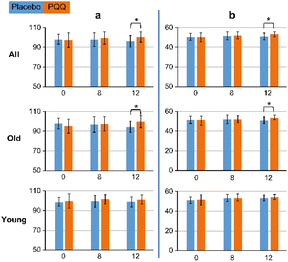
Data analysis was performed using Python statistical software. All results are shown in ESI Tables S1–S3.† The cognitive function showed an improvement in score with the administration of PQQ and an overall significant difference in composite memory (P = 0.003) and verbal memory (P = 0.001). Fig. 2 (ESI Fig. S4†) shows the results, along with an age-specific analysis, of this function. In the Old group, composite memory (P = 0.011) and verbal memory (P = 0.002) improved at 12 weeks of PQQ ingestion. No differences were observed in the Young group in terms of verbal memory and composite memory.
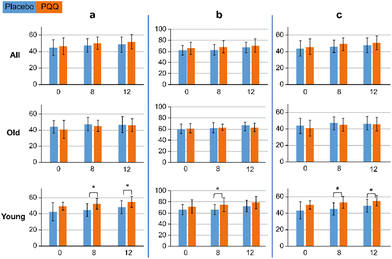
In the age-specific analysis, some functions improved upon PQQ ingestion, whereas some functions did not show a significant difference among the groups. There was a significant difference in cognitive flexibility, processing speed, and executive speed in the Young group at 8 weeks. The results are shown in Fig. 3 (ESI Fig. S5†). There was no significant difference in these functions between the other two groups.
3.2 Logistic regression
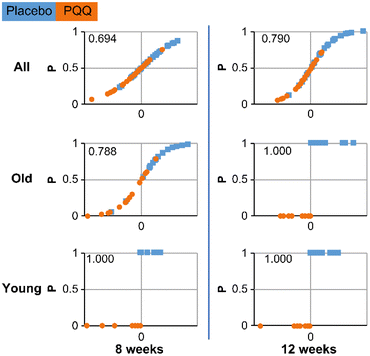
Logistic regression was used for distinguishing the PQQ and placebo groups. This analysis differentiates between placebo and PQQ intake groups across all data, rather than individual trial items. In other words, it determines whether the two groups can be separated from the overall cognitive function test results. It is a method to determine the effect of PQQ intake with high sensitivity. PQQ was considered to affect cognitive function if the effects of its intake and non-intake could be distinguished. Data from the 15 cognitive functions, divided into training and testing data, were used in the analysis (ESI Scheme S1†), and the results are presented in Table 2. The correct answer rate indicated differences between the groups. The logistic regression analysis was based on the premise that unavoidable errors and mistakes are mixed in any measurement. In other words, we determined whether the results were valid probabilistically; if the probability is ≤0.5, it is difficult to conclude, but if it is >0.5, a conclusion can be drawn. Furthermore, as the period of ingesting PQQ increased from 8 weeks to 12 weeks, the differences between the groups, as determined using a double-blind test, increased. This showed that the efficacy increased as a function of the ingestion time. The correct answer rate for 8 weeks was slightly more than 0.5, and it increased to 0.67 at 12 weeks. At 12 weeks, PQQ intake affected cognitive function. There was a difference in the duration of PQQ intake. Thus, the results of the double-blind study showed that the effectiveness of PQQ increased as a function of time. Here, the logistic regression analysis showed the distribution of the two groups, placebo, and PQQ, and this finding is consistent with the above results. Cognitive function improves after 12 weeks of PQQ intake.| Results Correct rate | Analysis condition | ||
|---|---|---|---|
| 8 weeks | 12 weeks | Learning data number | Test data number |
| a 5-fold stratified cross-validation. | |||
| 0.57 | 0.67 | 50 | 12 |
Fig. 4 shows the results of assessing the distinction between the placebo and PQQ groups by machine learning after training all data (ESI Scheme S2†). In the case of logistic regression, the correct answer rate of the result of learning with data (100%) and testing with the same data (100%) is shown as a score. The age-specific analysis showed improvements in the Young group in 8 weeks, with a score of 1.00. Thus, the cognitive function test results show that PQQ is effective compared with the placebo. At 12 weeks, the correct answer rate of both age groups was 0.67, whereas the score of the Old group was 1.00. The analysis of data by age strongly showed the effect of PQQ. Additionally, the results of the logistic regression analysis demonstrated that as the age distribution of the group becomes smaller, the effect of PQQ becomes clearer.
3.3 Effect of PQQ
In the present study, the effects of PQQ on the cognitive function of old and young adults were evaluated. A wide range of age (20–65 years) was considered in the present study, in contrast to that in previous studies on PQQ cognitive function.12–15 The average age group of 41.5 ± 13.7 years comprised several age groups. This average age is close to the average age of individuals in most stable countries, but less than that in Japan.23 We have tabulated the cognition-related studies using PQQ (Table 3).| Cognitive function | Age | Ref. | ||
|---|---|---|---|---|
| 1 | Composite memory, verbal memory (cognitive flexibility, processing speed, executive speed) | 41.5 ± 13.7 (20–40) | Present study | |
| 2 | Attention, information identification, processing ability | 45–65 | 12 | |
| 3 | Immediate memory | 50–70 | Lower score is strong effect | 14 |
| 4 | Attention, working memory | 58.6 ± 5.1 | Lower score is strong effect (attention) | 15 |
| 5 | Language memory | 50–71 | 13 | |
| 6 | Composite memory, verbal memory cognitive flexibility, processing speed, executive speed reaction time, complex attention, motor speed | 72.10 ± 3.77 | 24 |
These trials involved only elderly individuals, targeting people over 45 years of age. This group comprises individuals older than normal citizens. Therefore, the effects of PQQ on young people remained largely elusive until the present study was conducted.
In our research, the functions that were related by PQQ are as follows: verbal memory is the memory of words. Complex memory is the sum of verbal and visual memories. Cognitive flexibility is the ability to respond to changes in instructions. Processing speed is the ability to process information quickly. Executive speed is understanding and making decisions about rules and concepts. These functions are necessary for leading a social life.
We believe that composite and verbal memories are functions that are strongly affected by PQQ intake in ALL groups. As our study covers a wide age range, it is anticipated that the effect of PQQ intake is unlikely to appear. However, in fact, PQQ intake at a sharp age (72.10 ± 3.77) reportedly improves various brain functions, including attention, information identification, processing ability, immediate memory, working memory, language memory, composite memory, verbal memory, cognitive flexibility, processing speed, executive speed, reaction time, complex attention, and motor speed.24 Furthermore, composite and verbal memories are effective in older adults. In contrast, the young group did not show a significant difference plausibly because this group's composite memory was good from the beginning.
Age-based analysis indicated that the improvements in cognitive flexibility and executive speed are more effective in the young group. The processing speed showed a significant difference at 8 weeks and no effect at 12 weeks because of the weak function. The improvements in the young group were unexpected and significant. In previous studies, cognitive function was found to be high in groups with low scores after the intake of PQQ.14,15 Therefore, it was speculated that the effect of PQQ would be small because the brain functions in young adults were high. In fact, the score was not low for younger individuals. These features are unique to young people, unlike the old group. Cognitive function changed faster in the young group than in the old group, as determined by the logistic regression analysis. In this study, we were able to observe differences in the effects of PQQ by age.
Our hypothesis is as follows: cognitive function improves faster in the young group than in the old group. It is consistent with the fact that aging is known to lower basal metabolism. Moreover, the effect of PQQ is closely related to the metabolism of mitochondrial neoplasia and activation. Therefore, active metabolism in young individuals could contribute to the differential improvement in cognitive function by age. It is expected that young people with a high metabolism will show improved brain function early by mitochondrial activation. Increased metabolism results in an increase in the production of reactive oxygen species. Thus, the high antioxidant activity of PQQ could contribute to improved cognitive function, especially in younger individuals. Additionally, the cognitive areas differ between young and elderly individuals. The characteristic cognitive area of young people is focused on processing speed, and not memory. Thus, PQQ may also improve functions that have not declined with age.
Cognitive flexibility scores are associated with ADHD.25 Cognitive flexibility is improved by training.26 Sports have a positive influence on cognitive function.27 PQQ increases the expression of PGC-1α, whose levels are elevated during sports activities.3 In the future, determining the influence on learning and sports activities is a challenge in PQQ studies.
4. Conclusions
In summary, PQQ improves cognitive functions of cognitive flexibility and executive speed within 8 weeks in young adults aged 20–40 years. Cognitive functions of composite and verbal memories are improved after 12 weeks in adults aged 20–65 years. In the logistic regression analysis, 100% of learning data could determine PQQ intake from the cognitive test results. It was observed that cognitive function improved after 8 weeks in the younger generation. It was found that younger people are more likely to be effective even if different analysis methods are used. The areas of cognitive function are also different. PQQ is a functional food that improves brain function at any age. Our research is expected to expand the area where PQQ is used as a functional food from the elderly to all generations.Author contributions
T. S. developed the clinical plan; M. T. analyzed the study results; K. I. wrote the article; and E. N. and S. N. conducted the statistical analysis.Conflicts of interest
RIKEN's joint research is sponsored by Mitsubishi Gas Chemical Company, Inc. This research was completely funded by Mitsubishi Gas Chemical Company, Inc.References
- D. Swanson, R. Block and S. Mousa, Omega-3 fatty acids EPA and DHA: health benefits throughout life, Adv. Nutr., 2012, 3, 1–7, DOI:10.3945/an.111.000893.
- K. Jonscher, W. Chowanadisai and B. Rucker, Pyrroloquinoline-quinone Is more than an antioxidant: A vitamin-like accessory factor important in health and disease prevention, Biomolecules, 2021, 11, 1441, DOI:10.3390/biom11101441.
- M. Akagawa, M. Nakano and K. Ikemoto, Recent progress in studies on the health benefits of pyrroloquinoline quinone, Biosci. Biotechnol. Biochem., 2016, 80, 13–22, DOI:10.1080/09168451.2015.1062715.
- D. Turck, J. Bresson, B. Burlingame, T. Dean, S. Fairweather–Tait, M. Heinonen and K. Hirsch-Ernst, et al., Safety of pyrroloquinoline quinone disodium salt as a novel food pursuant to Regulation (EC) No 258/97, EFSA J., 2017, 15, e05058, DOI:10.2903/j.efsa.2017.5058.
- W. Chowanadisai, K. Bauerly, E. Tchaparian, A. Wong, G. Cortopassi and R. Rucker, Pyrroloquinoline quinone stimulates mitochondrial biogenesis through cAMP response element-binding protein phosphorylation and increased PGC-1alpha expression, J. Biol. Chem., 2010, 285, 142–152, DOI:10.1074/jbc.M109.030130.
- H. Sasakura, H. Moribe, M. Nakano, K. Ikemoto, K. Takeuchi and I. Mori, Lifespan extension by peroxidase and dual oxidase-mediated ROS signaling through pyrroloquinoline quinone in C. elegans, J. Cell Sci., 2017, 130, 2631–2643, DOI:10.1242/jcs.202119.
- A. Ouchi, M. Nakano, S. Nagaoka and K. Mukai, Kinetic study of the antioxidant activity of pyrroloquinoline quinol (PQQH2, a reduced form of Pyrroloquinoline quinone) in micellar solution, J. Agric. Food Chem., 2009, 57, 450–456, DOI:10.1021/jf802197d.
- M. Nakano, A. Kamimura, F. Watanabe, T. Kamiya, D. Watanabe, E. Yamamoto, M. Fukagawa, K. Hasumi and E. Suzuki, Effects of orally administered pyrroloquinoline quinone disodium salt on dry skin conditions in mice and healthy female subjects, J. Nutr. Sci. Vitaminol., 2015, 61, 241–246, DOI:10.3177/jnsv.61.241.
- K. Yamaguchi, T. Tsuji, D. Uemura and K. Kondo, Cyclooxygenase induction is essential for NGF synthesis enhancement by NGF inducers in L-M Cells, Biosci. Biotechnol. Biochem., 1996, 60, 92–94, DOI:10.1271/bbb.60.92.
- S. Guan, J. Xu, Y. Guo, D. Ge, T. Liu, X. Ma and Z. Cui, Pyrroloquinoline quinone against glutamate-induced neurotoxicity in cultured neural stem and progenitor cells, Int. J. Dev. Neurosci., 2015, 42, 37–45, DOI:10.1016/j.ijdevneu.2015.02.008.
- K. Ohwada, H. Takeda, M. Yamazaki, H. Isogai, M. Nakano, M. Shimomura, K. Fukui and S. Urano, Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by oxidative stress in rats, J. Clin. Biochem. Nutr., 2008, 42, 29–34, DOI:10.3164/jcbn.2008005.
- M. Nakano, K. Ubukata, T. Yamamoto and H. Yamaguchi, Effect of pyrroloquinoline quinone (PQQ) on mental status of middle-aged and elderly persons, Food Style 21, 2009, 13, 50–53 CAS.
- Y. Yamada, K. Nishii, K. Kuwata, M. Nakamichi, K. Nakanishi, A. Sugimoto and K. Ikemoto, Effects of pyrroloquinoline quinone and imidazole pyrroloquinoline on biological activities and neural functions, Heliyon, 2020, 6, e03240, DOI:10.1016/j.heliyon.2020.e03240.
- T. Koikeda, M. Nakano and K. Masuda, Pyrroloquinoline quinone disodium salt improves higher brain function, Med. Consult. New Remedies, 2011, 48, 519–527 Search PubMed.
- Y. Itoh, K. Hine, H. Miura, T. Uetake, M. Nakano, N. Takemura and K. Sakatani, Effect of the antioxidant supplement pyrroloquinoline quinone disodium salt (BioPQQ™) on cognitive functions, Adv. Exp. Med. Biol., 2016, 876, 319–325, DOI:10.1007/978-1-4939-3023-4_40.
- M. Nakano, T. Yamamoto, H. Okamura, A. Tsuda and Y. Kowatari, Effect of oral supplementation with pyrroloquinoline quinone on stress, fatigue, and sleep, Funct. Foods Health Dis., 2012, 8, 307–324 Search PubMed.
- M. Talaei, E. Sdona, P. Calder, L. Jones, P. Emmett, R. Granell, A. Bergström, E. Melén and S. Shaheen, Intake of n-3 polyunsaturated fatty acids in childhood, FADS genotype and incident asthma, Eur. Respir. J., 2021, 58, 2003633, DOI:10.1183/13993003.03633-2020.
- M. Yazdy, S. Tinker, A. Mitchell, L. Demmer and M. Werler, Maternal tea consumption during early pregnancy and the risk of spina bifida, Birth Defects Res., Part A, 2012, 94, 756–761, DOI:10.1002/bdra.23025.
- CNS vital Signs, https://www.cnsvs.com/WhitePapers/CNS_Vital_Signs_Brochure.pdf.
- K. Ikemoto, H. Sakamoto and N. Masahiko, Crystal structure and characterization of pyrroloquinoline quinone disodium trihydrate, Chem. Cent. J., 2012, 6, 57 CrossRef CAS PubMed.
- T. Gualtieri and L. Johnson, Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs, Arch. Clin. Neuropsychol., 2006, 21, 623–643, DOI:10.1016/j.acn.2006.05.007.
- T. Sekikawa, Y. Kizawa, Y. Li and T. Takara, Cognitive function improvement with astaxanthin and tocotrienol intake: a randomized, double-blind, placebo-controlled study, J. Clin. Biochem. Nutr., 2020, 67, 307–316, DOI:10.3164/jcbn.19-116.
- Average age by country. https://www.worlddata.info/average-age.php.
- Y. Shiojima, M. Takahashi, R. Takahashi, H. Moriyama, D. Bagchi, M. Bagchi and M. Akanuma, Effect of dietary Pyrroloquinoline Quinone Disodium Salt on Cognitive Function in healthy volunteers: A randomized, double-blind, placebo-controlled, parallel-group study, J. Am. Coll. Nutr., 2021, 20, 1–14, DOI:10.1080/07315724.2021.1962770.
- G. Iverson, B. Brooks, D. Weiss, L. Johnson and T. Gualtieri, Clinical usefulness of CNS vital signs for assessing neurocognition in ADHD, The Clin. Neuropsychol., 2007, 21, 686–686 Search PubMed.
- S. Mekari, H. Neyedli, S. Fraser, M. O'Brien, R. Martins, K. Evans, M. Earle, R. Aucoin, J. Chiekwe, Q. Hollohan, D. Kimmerly and O. Dupuy, High-intensity interval training improves cognitive flexibility in older adults, Brain Sci., 2020, 10, 796, DOI:10.3390/brainsci10110796.
- I. Bidzan-Bluma and M. Lipowska, Physical activity and cognitive functioning of children: A systematic review, Int. J. Environ. Res Public Health, 2018, 15, 800, DOI:10.3390/ijerph15040800.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo01515c |
| This journal is © The Royal Society of Chemistry 2023 |